Abstract
A main group-catalyzed method for the synthesis of aryl- and heteroarylamines by intermolecular C–N coupling is reported. The method employs a small-ring organophosphorus-based catalyst (1,2,2,3,4,4-hexamethylphosphetane) and a terminal hydrosilane reductant (phenylsilane) to drive reductive intermolecular coupling of nitro(hetero)arenes with boronic acids. Applications to the construction of both Csp2–N (from arylboronic acids) and Csp3–N bonds (from alkylboronic acids) are demonstrated; the reaction is stereospecific with respect to Csp3–N bond formation. The method constitutes a new route from readily available building blocks to valuable nitrogen-containing products with complementarity in both scope and chemoselectivity to existing catalytic C–N coupling methods.
Graphical Abstract

Aryl- and heteroarylamines comprise a diverse class of organic compounds with significant value as pharmaceuticals, agrochemicals, fine chemicals, and optoelectronic materials. The prevailing strategy for the preparation of these useful compounds—N-arylation of the parent aniline through carbon-nitrogen (C–N) coupling (Figure 1A)—is currently shaped by transition metal catalyzed methods (e.g. Buchwald-Hartwig, Ullmann, Chan-Lam couplings).1,2 Herein, we describe an alternative main group approach to catalytic intermolecular C–N bond construction that does not rely on transition metals, enabling a complementary route from readily accessible components to (hetero)arylamines. Specifically, we show that a redox active organophosphorus-based catalyst operating in the PIII/Pv=O manifold drives reductive coupling of nitroarenes and boronic acids with C–N bond formation to give (hetero)arylamine products (Figure 1B) in a manner functionally distinct from current catalytic practice.
Figure 1.
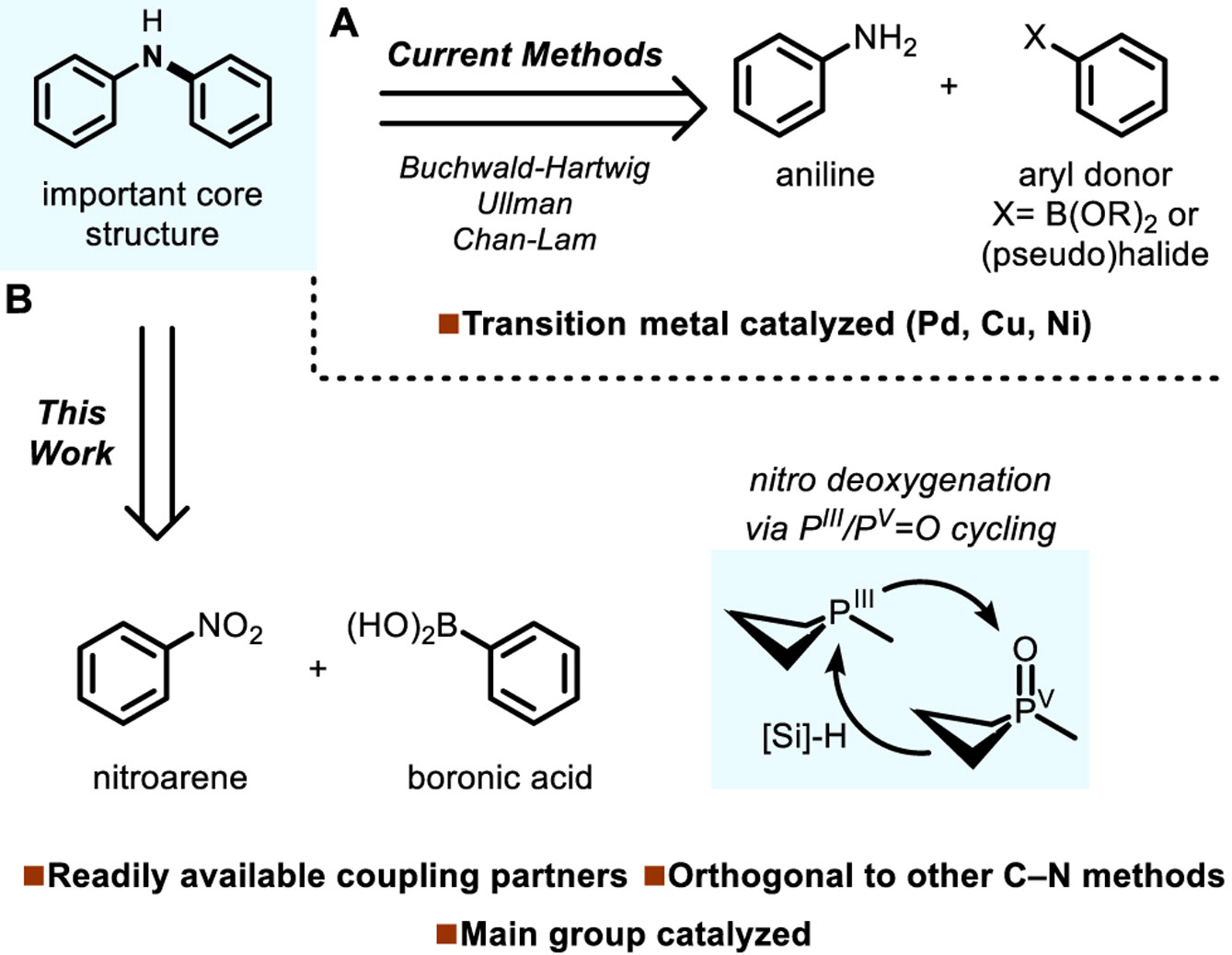
A) Established methods for intermolecular C–N coupling. B) This work: PIII/Pv=O-catalyzed reductive C–N coupling of nitroarenes and boronic acids.
Nitroarenes are common intermediates in synthesis (most typically as aniline precursors), but are relatively underutilized for direct catalytic C–N bond forming reactions.3 Notable exceptions include the work of Nicholas4 and Baran,5 who have reported iron-catalyzed reductive C–N bond construction by reaction of nitroarenes with alkynes and alkenes, respectively. Hu has reported a related iron-catalyzed reductive C–N bond formation by reaction of nitroarenes with alkyl6 and acyl7 electrophiles. Stoichiometric main group metal approaches have also been described; Knochel,8 Kürti,9 and Niggemann10 have demonstrated reductive conversion of nitroarenes to N-arylanilines.
Our entry into nitroarene functionalization has centered on the use of a redox-active small-ring phosphorus-based compound. We have previously reported that a simple trialkylphosphine catalyst containing a core four-membered ring, in combination with phenylsilane as a terminal reductant, constitutes a competent system for the catalytic transformation of nitroaromatic substrates into azaheterocycles through intramolecular C–N bond forming Cadogan cyclization.11,12 In this chemistry, the phosphacyclic catalyst promotes reductive O-atom transfer from the nitroarene substrates by cycling in the PIII/Pv=O catalytic couple.13-16 We considered whether introduction of a suitable exogenous coupling partner to the PIII/Pv=O catalytic conditions might enable the construction of C–N bonds in an intermolecular manner.
The reaction of nitrobenzene (1) and phenylboronic acid (2a) to give diphenylamine (3) was chosen for discovery and optimization studies (Table 1). An initial attempt at reductive coupling using conditions previously reported for Cadogan cyclization proved promising, providing diphenylamine in an unoptimized 59% yield (Fig. S1). Using the Design of Experiments approach to evaluate the impact of temperature, concentration, and reagent equivalencies on the reaction outcome (Fig. S2), optimization studies converged on the conditions outlined in Table 1 (entry 1, 1.1 equiv of 2a, 15 mol % of 4•[O], 0.5 M in m-xylene, 120 °C). Under these conditions, the organophosphorus-catalyzed reductive coupling of nitrobenzene and phenylboronic acid gave diphenylamine in 86% GC yield, and 80% isolated yield on a one millimole scale. A comparable performance is observed if the corresponding tricoordinate phosphacycle 4 is employed as catalyst (entry 2), consistent with the interconversion of PIII and Pv=O oxidation states by catalytic cycling. Relatedly, a stoichiometric implementation of the reductive coupling of 1 and 2a employing 3 equivalents of phosphetane 4 is successful (89% yield) (Table S2).17 Control experiments omitting either the phosphorus catalyst (entry 3) or the terminal silane reductant (entry 4) do not give the desired product. The reaction performed well in a variety of nonpolar solvents (entries 1,5,6), but was less efficient in a solvent of high donicity (entry 7). The identity of the boron reagent was found to play a significant role in the success of the reaction (Table 1); both phenylboronic acid (2a) and phenylboroxine (2b, entry 8) were successfully aminated by nitrobenzene to give diphenylamine 3 under standard catalytic conditions. However, other common phenylboronic esters are either less productive (catecholatoboronate 2c – entry 9) or unproductive (pinacolato-boronate 2d – entry 10) when employed as the aryl donor in the catalytic C–N coupling reaction, suggesting the possibility of chemoselective differentiation of boryl moieties (vide infra).
Table 1.
Discovery and optimization of the organophosphorus-catalyzed reductive C–N coupling reaction.a

| ||||
|---|---|---|---|---|

| ||||
|
| ||||
| Entry | [B] | Solvent | R3P=O | Yield (%) |
| 1 | 2a | m-xylene | 4•[O] | 86% (80%) |
| 2 | 2a | m-xylene | 4 | 82% |
| 3 | 2a | m-xylene | none | 0% |
| 4 | 2a | m-xylene | 4•[O]; no silane | 0% |
| 5 | 2a | dibutyl ether | 4•[O] | 86% |
| 6 | 2a | toluene | 4•[O] | 80% |
| 7 | 2a | DMF | 4•[O] | 17% |
| 8b | 2b | m-xylene | 4•[O] | 74% |
| 9 | 2c | m-xylene | 4•[O] | 54% |
| 10 | 2d | m-xylene | 4•[O] | 2% |
Yields were determined through analysis by gas chromatography (GC) with the aid of an internal standard. Yield in parenthesis (entry 1) is for isolated material from a 1.0 mmol reaction scale.
0.37 mmol of 2b was used. See SI for additional optimization experiments.
A qualitative assessment of the electronic demand of the reaction was undertaken (Figure 2A). For a series of differentially para-substituted nitroarenes, an empirical electronic trend is observed where increasingly electron-withdrawing para substituents lead to faster qualitative rates and higher yields of C–N coupling (cf. 5-8). Complementarily, the inverse empirical trend with respect to electron demand of the arylboronic acid moiety is observed, where increasingly electron-donating para substituents result in higher yields of C–N coupling (cf. 9-12). The consequence of these two differing trends is that the organophosphorus-catalyzed C–N coupling reaction is most productive for union of electron deficient nitroarenes with electron rich arylboronic acids, as illustrated in the synthesis of 15 in 88% by the coupling of electron-deficient nitroarene 13 with electronic-rich boronic acid 14 (Figure 2A). This observed electronic preference serves as a point of distinction with respect to palladium-catalyzed C–N coupling, where the arylation of electron-deficient arylamine substrates are among the most persistently challenging.18 The current organophosphorus-catalyzed method may therefore provide a route to construction of otherwise electronically deactivated C–N bonds.
Figure 2.
Examples of catalytic reductive C–N coupling. (A) Electronic effects on C–N coupling. (B) Synthetic examples of C–N coupling. See SI for full experimental details and conditions. Yields are reported for isolated material following purification on a 1 mmol scale, except as noted. Compounds 5-12 were prepared on 2 mmol scale; compound 26 was prepared on a 3 mmol scale; and compounds 34-39 were prepared on 1 gram scale. Preparation of 22 used 1.3 equivalents of boronic acid. Compound 36 was isolated as its hydrochloride salt.
Additional synthetic examples illustrating the reaction scope are collected in Figure 2B. The main group-catalyzed conditions for the C–N coupling method show good functional group compatibility and provide complementary chemoselectivities with respect to established transition metal coupling. Since phosphines do not readily undergo oxidative addition to Csp2–X bonds, halogen substitution is well-tolerated on both the nitroarene component (7, 19, 20) and the boronic acid (11, 23, 24) component. Even very electrophilic 2-chloro and 2-bromopyridyl substrates (26, 27), which are known to be excellent electrophiles for both SNAr and transition metal-catalyzed substitution, are carried through the phosphine-catalyzed reductive C–N coupling without undesired cleavage. Protic functional groups such as anilines (18, 24) and phenols (25) are orthogonal in reactivity to the nitro group and are therefore tolerated in the coupling chemistry without explicit protection. Even multiple distinct nitro or boryl moieties within a single reaction pair can be differentiated in select circumstances. Due to the aforementioned electronic trends in C–N coupling (Figure 2A), 1,4-dinitrobenzene becomes electronically deactivated following an initial reductive C–N coupling event, such that selective mono-coupling product 28 may be isolated in good yield. And notably, only selective C–N cross coupling product 29 is observed in the reaction of 4-pinacolatoborylnitrobenzene and phenylboronic acid; the Bpin moiety is inert to the main group-catalyzed conditions, allowing for further functionalization by known transition metal-catalyzed chemistry if so desired.
The reaction is not limited to Csp2–N bond formation; indeed, the amination of nitrobenzene with Csp3 boronic acid reagents including methyl (30), primary alkyl (31), secondary alkyl (32), and strained cycloalkyl (33) provide serviceable yields of the desired Csp3–N cross coupling products. As a further illustration of the synthetic utility of the transformation, a number of heteroarylamine structures displaying varied substitution on both the nitro and boronic acid components were synthesized. Carboxyesters on either the nitroarene or boronic acid reaction component were well tolerated (34, 35), and both π-deficient (37) and π-excessive (39) heterocycles are readily employed. As a general point, since the organophosphorus catalyst is only weakly Lewis acidic, substrates and products with Lewis basic functionalities (amines, pyridines, sulfides) are not inherently inhibitory under these main group coupling conditions.
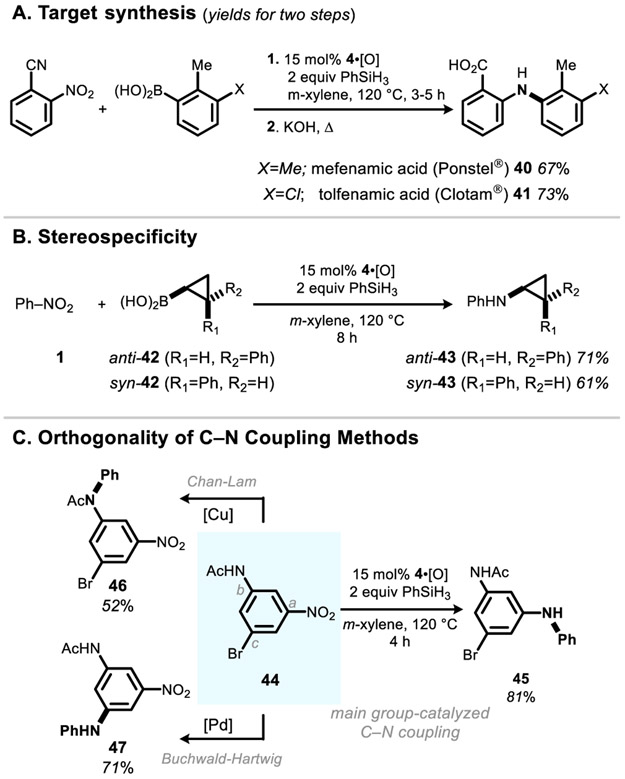
To demonstrate the potential synthetic utility of this methodology in the context of pharmaceutical chemistry, mefenamic acid (40) and tolfenamic acid (41) (Figure 3A)—members of the fenamate group of nonsteroidal anti-inflammatory drugs (NSAIDs) marketed under the tradenames Ponstel and Clotam, respectively—were synthesized with 67% and 73% yields on 1 millimole scale in one-pot by organophosphorus-catalyzed reductive C–N cross coupling of 2-nitrobenzonitrile and either 2,3-dimethylphenylboronic acid or 3-chloro-2-methylphenylboronic a cid, followed by alkaline nitrile hydrolysis according to a known procedure.19
Figure 3.
Synthetic applications of the catalytic reductive C–N coupling reaction. (A) Synthesis of mefenamic acid and tolfenamic acid. (B) Demonstration of the stereospecificity of C–N coupling. Yields determined by NMR spectroscopy. (C) Selectivity and complementarity in the functionalization of 44 by C–N coupling methods. See SI for full experimental details and conditions.
The stereospecificity of the catalytic C–N coupling reaction was evaluated by amination of stereochemical probe molecules (Fig. 3B). Reductive coupling of anti-phenylcyclopropylboronic acid (anti-42) with nitrobenzene under standard main group-catalyzed conditions gave the N-phenyl tranylcypromine derivative anti-43 in 71% NMR yield with retention of configuration. Relatedly, reductive coupling of the syn-cyclopropane epimer (syn-42) with nitrobenzene furnished syn-43, in 61% NMR yield with stereochemical retention. Consistent with related protocols for amination of boron derivatives,20 the reductive C–N coupling reaction is stereospecific with respect to the boronic acid component, permitting its potential implementation in stereoselective synthesis.
The complementarity of the current main group method for C–N coupling with respect to existing transition metal strategies is exemplified by the diversification of 1,3,5-trisubstituted arene 44 (Figure 3C). Whereas C–N coupling under existing Cu-mediated (Chan-Lam) or Pd-catalyzed (Buchwald-Hartwig) methods permits chemoselective functionalization at the anilide (site b, 46) or arylbromide (site c, 47) positions, respectively, catalytic arylamination by the newly developed organophosphorus-catalyzed coupling approach results in selective functionalization at the nitro moiety (site a, 45) in 81% yield. These results suggest a strategic orthogonality between the bond constructions possible with the various C–N coupling methods. Viewed in this light, we envision that the main group method will augment technical capacity by providing new freedom to synthesize valuable arylamine products from diverse and readily available building blocks.
The foregoing results constitute a practical, scalable, and operationally robust organophosphorus-catalyzed protocol for intermolecular C–N coupling of nitroarenes and boronic acid partners. These findings expand the biphilic reactivity of phosphetanes as platforms for catalytic reductive O-atom transfer operating in the PIII/Pv=O redox couple, providing further precedent for the catalytic potential of main group compounds in reaction classes heretofore dominated by transition metal catalysis.
Supplementary Material
ACKNOWLEDGMENT
Funding was provided by NIH NIGMS (GM114547), MIT, and Bristol-Myers Squibb. We thank Prof. Stephen Buchwald for valuable discussions and for access to equipment and chemicals.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Additional optimization results and synthetic procedures (.pdf) 1H, 13C, and 31P NMR spectra (.pdf)
Notes
The authors declare no competing financial interests.
REFERENCES
- 1 (a).Hartwig JF Transition Metal Catalyzed Synthesis of Arylamines and Aryl Ethers from Aryl Halides and Triflates: Scope and Mechanism. Angew. Chem. Int. Ed 1998, 37, 2046. [DOI] [PubMed] [Google Scholar]; (b) Jiang L; Buchwald SL Palladium-Catalyzed Aromatic Carbon-Nitrogen Bond Formation, in Metal-Catalyzed Cross-Coupling Reactions. De Meijere A; Diderich F, Eds.; Wiley-Blackwell: Hoboken, NJ, 2008; ed. 2, pp. 699–760. [Google Scholar]; (c) Jiang Y; Ma D Copper - Catalyzed Ligand Promoted Ullmann - type Coupling Reactions in Catalysis without Precious Metals. Bullock RM Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, 2010; pp 213–233. [Google Scholar]; (d) Bariwal J; Eycken E. V. der. C–N bond forming cross-coupling reactions: an overview Chem. Soc. Rev 2013, 42, 9283. [DOI] [PubMed] [Google Scholar]; (e) Corcoran EB; Pirnot MT; Lin S; Dreher SD; DiRocco DA; Davies IW; Buchwald SL; MacMillan DWC Aryl amination using ligand-free Ni(II) salts and photoredox catalysis. Science 2016, 353, 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2 (a).Schlummer B; Scholz U Palladium-Catalyzed C-N and C-O Coupling–A Practical Guide from an Industrial Vantage Point. Adv. Synth. Catal 2004, 346, 1599. [Google Scholar]; (b) Torborg C; Beller M Recent Applications of Palladium-Catalyzed Coupling Reactions in the Pharmaceutical, Agrochemical, and Fine Chemical Industries. Adv. Synth. Catal 2009, 351, 3027. [Google Scholar]; (c) Yin J “Selected Applications of Pd- and Cu-Catalyzed Carbon–Heteroatom Cross Coupling Reactions in the Pharmaceutical Industry.” In Applications of Transition Metal Catalysis in Drug Discovery and Development: An Industrial Perspective, Crawley ML; Trost BM Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, 2012; pp 97–163. [Google Scholar]; (d) Ruiz-Castillo P; Buchwald SL Chem. Rev 2016, 116, 12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Lower oxygenates of nitrogen or alternative nitrogen reagents are more common in C-N bond formation with boronic acids. Nitrosoarenes: Yu Y; Srogl J; Liebeskind LS Cu(I)-Mediated Reductive Amination of Boronic Acids with Nitroso Aromatics. Org. Lett 2004, 6, 2631. [DOI] [PubMed] [Google Scholar]; (b) Roscales S; Csákÿ AG Synthesis of Di(hetero)arylamines from Nitrosoarenes and Boronic Acids: A General, Mild, and Transition-Metal-Free Coupling. Org. Lett 2018, 20, 1667; N-Alkyl hydroxylamines: [DOI] [PubMed] [Google Scholar]; (c) Sun H-B; Gong L; Tian Y-B; Wu J-G; Zhang X; Liu J; Fu Z; Niu D Metal- and Base-Free Room-Temperature Amination of Organo-boronic Acids with N-Alkyl Hydroxylamines. Angew. Chem. Int. Ed 2018, 57, 9456.; Azides: [DOI] [PubMed] [Google Scholar]; (d) Ou. L; Shao J; Zhang G; Yu Y Metal-Free Carbon-nitrogen Bond-Forming Coupling Reaction between Arylboronic Acids and Organic Azides. Tetrahedron Lett. 2011, 52, 1430. Tosyl triazenes: [Google Scholar]; (e) Sarma MJ; Phukan P Metal-Free Synthesis of Secondary Amines by the Reaction of Tosyl Triazene and Aryl Boronic Acid. Synth. Commun 2018, 48, 656. [Google Scholar]
- 4.Srivastava RS; Nicholas KM Kinetics of the Allylic Animation of Olefins by Nitroarenes Catalyzed by [CpFe(CO)2]2. Organometallics 2005, 24, 1563. [Google Scholar]
- 5.Gui J; Pan C-M; Jin Y; Qin T; Lo JC; Lee BJ; Spergel SH; Mertzman ME; Pitts WJ; La Cruz TE; Schmidt MA; Darvatkar N; Natarajan SR; Baran PS Practical olefin hydroamination with nitroarenes. Science 2015, 348, 886. [DOI] [PubMed] [Google Scholar]
- 6.Cheung CW; Hu X Amine synthesis via iron-catalysed reductive coupling of nitroarenes with alkyl halides. Nat. Commun 2016, 7, 12494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung CW; Hu X Nickel-Catalyzed Reductive Transamidation of Secondary Amides with Nitroarenes. ACS Catal. 2017, 7, 7092. [Google Scholar]
- 8 (a).Sapountzis I; Knochel P A New General Preparation of Polyfunctional Diarylamines by the Addition of Functionalized Arylmagnesium Compounds to Nitroarenes. J. Am. Chem. Soc 2002, 124, 9390. [DOI] [PubMed] [Google Scholar]; (b) Doyle W; Staubitz A; Knochel P Mild Synthesis of Polyfunctional Benzimidazoles and Indoles by the Reduction of Functionalized Nitroarenes with Phenylmagnesium Chloride. Chem. Eur. J 2003, 9, 5323. [DOI] [PubMed] [Google Scholar]; (c) Kopp F; Sapountzis I; Knochel P Preparation of Polyfunctionalized Amines by the Addition of Functionalized Organomagnesium Reagents to Nitrosoarenes Synlett, 2003, 885. [Google Scholar]; (d) Sapountzis I; Knochel P A New Method for the Selective Amination of 1,3- and 1,4-Dinitrobenzenes and Protected Nitroanilines Leading to Polyfunctional 1,3- and 1,4- Disubstituted Anilines. Synlett 2004, 955. [Google Scholar]; (e) Dhayalan V; Saemann C; Knochel P Synthesis of polyfunctional secondary amines by the addition of functionalized zinc reagents to nitrosoarenes. Chem. Commun 2015, 51, 3239. [DOI] [PubMed] [Google Scholar]
- 9.Gao H; Xu Q-L; Ess DH; Kürti L Transition-Metal-Free, Low-Temperature Intramolecular Amination of Aromatic C-H Bonds: Rapid Synthesis of Fused Heterocycles.” Angew. Chem. Int. Ed 2014, 53, 2701. [DOI] [PubMed] [Google Scholar]
- 10.Rauser M; Ascheberg C; Niggemann M Electrophilic Amination with Nitroarenes. Angew. Chem. Int. Ed 2017, 56, 11570. [DOI] [PubMed] [Google Scholar]
- 11 (a).Nykaza TV; Harrison TS; Ghosh A; Putnik RA; Radosevich AT A Biphilic Phosphetane Catalyzes N-N Bond-Forming Cadogan Heterocyclization via PIII/Pv=O Redox Cycling. J. Am. Chem. Soc 2017, 139, 6839. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Nykaza TV; Ramirez A; Harrison TS; Luzung MR; Radosevich AT Biphilic Organophosphorus-Catalyzed Intramolecular Csp2-H Amination: Evidence for a Nitrenoid in Catalytic Cadogan Cyclizations. J. Am. Chem. Soc 2018, 140, 3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12 (a).Cadogan JIG; Cameron-Wood M; Mackie RK; Searle RJG 896. The Reactivity of Organophosphorus Compounds. Part XIX. Reduction of Nitro-Compounds by Triethyl Phosphite: A Convenient New Route to Carbazoles, Indoles, Indazoles, Triazoles, and Related Compounds. J. Chem. Soc 1965, 0, 4831. [Google Scholar]; (b) Cadogan JIG Reduction of Nitro- and Nitroso-Compounds by Tervalent Phosphorus Reagents. Q. Rev. Chem. Soc 1968, 22, 222. [Google Scholar]
- 13.For a review of PIII/Pv=O redox cycling, see: Marsden SP Catalytic Variants of Phosphine Oxide-Mediated Organic Transformations in Sustainable Catalysis; Dunn PJ, Hii KK, Krische MJ, Williams MT, Eds.; John Wiley & Sons, Inc.: New York, 2013; pp 339–361. [Google Scholar]
- 14.(a) For recent examples of PIII/Pv=O redox cycling, see: O’Brien CJ; Tellez JL; Nixon ZS; Kang LJ; Carter AL; Kunkel SR; Przeworski KC; Chass GA Recycling the Waste: The Development of a Catalytic Wittig Reaction Angew. Chem. Int. Ed 2009, 48, 6836. [DOI] [PubMed] [Google Scholar]; (b) O’Brien CJ; Lavigne F; Coyle EE; Holohan AJ; Doonan BJ Breaking the Ring through a Room Temperature Catalytic Wittig Reaction Chem. Eur. J 2013, 19, 5854. [DOI] [PubMed] [Google Scholar]; (c) O’Brien CJ; Nixon ZS; Holohan AJ; Kunkel SR; Tellez JL; Doonan BJ; Coyle EE; Lavigne F; Kang LJ; Przeworski KC Part I: The Development of the Catalytic Wittig Reaction Chem. Eur. J 2013, 19, 15281. [DOI] [PubMed] [Google Scholar]; (d) Coyle EE; Doonan BJ; Holohan AJ; Walsh KA; Lavigne F; Krenske EH; O’Brien CJ Catalytic Wittig Reactions of Semi- and Nonstabilized Ylides Enabledby Ylide Tuning Angew. Chem. Int. Ed 2014, 53, 12907. [DOI] [PubMed] [Google Scholar]; (e) van Kalkeren HA; Leenders SHAM; Hommersom CRA; Rutjes FPJT; van Delft FL In Situ Phosphine Oxide Reduction: A Catalytic Appel Reaction Chem. Eur. J 2011, 17, 11290. [DOI] [PubMed] [Google Scholar]; (f) van Kalkeren HA; Bruins JJ; Rutjes FPJT; van Delft FL Organophosphorus-Catalysed Staudinger Reduction Adv. Synth. Catal 2012, 354, 1417. [Google Scholar]; (g) Zhao W; Yan PK; Radosevich AT Phosphetane catalyzes deoxy-genative condensation of α-keto esters and carboxylic acids via PIII/Pv=O redox cycling. J. Am. Chem. Soc 2015, 137, 616. [DOI] [PubMed] [Google Scholar]; (h) Lee C; Chang T; Yu J; Reddy GM; Hsiao M; Lin W Synthesis of functionalized furans via chemoselective reduction/Wittig reaction using catalytic tri-ethylamine and phosphine. Org. Lett 2016, 18, 3758. [DOI] [PubMed] [Google Scholar]; (i) Saleh N; Voi-turiez A Synthesis of 9H-pyrrolo[1,2-a]indole and 3H-pyrrolizine derivatives via a phosphine-catalyzed umpolung addition/intramolecular Wittig reaction. J. Org. Chem 2016, 81, 4371. [DOI] [PubMed] [Google Scholar]; (j) Saleh N; Blanchard F; Voi-turiez A Synthesis of nitrogen containing heterocycles and cyclopente-none derivatives via phosphine catalyzed Michael addition/intramolecular Wittig reaction. Adv. Synth. Catal 2017, 359, 2304. [Google Scholar]; (k) Zhang K; Cai L; Yang Z; Houk KN; Kwon O Bridged [2.2.1] bicyclic phosphine oxide facilitates catalytic γ-umpolung addition–Wittig olefination. Chem. Sci 2018, 9, 1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.(a) For redox-neutral phosphine oxide organophosphorus-catalyzed methods: Aza-Wittig Campbell TW; Monagle JJ A New Synthesis of Mono- and Polycarbodiimides. J. Am. Chem. Soc 1962, 84, 1493. [Google Scholar]; (b) Marsden SP; McGonagle AE; McKeever-Abbas B Catalytic aza-Wittig Cyclizations for Heteroaromatic Synthesis. Org. Lett 2008, 10, 2589. [DOI] [PubMed] [Google Scholar]; (c) Wang L; Qin R-Q; Yan H-Y; Ding M-W New Efficient Synthesis of 1,4-Benzodiazepin-5-Ones by Catalytic Aza-Wittig Reaction. Synthesis 2015, 47, 3522. Appel: [Google Scholar]; (d) Denton RM; An J; Adeniran B Phosphine Oxide-Catalysed Chlorination Reactions of Alcohols under Appel Conditions. Chem. Commun 2010, 46, 3025. [DOI] [PubMed] [Google Scholar]; (e) Denton RM; An J; Adeniran B; Blake AJ; Lewis W; Poulton AM Catalytic Phosphorus(V)-Mediated Nucleophilic Substitution Reactions: Development of a Catalytic Appel Reaction. J. Org. Chem 2011, 76, 6749. [DOI] [PubMed] [Google Scholar]; (f) An J; Tang X; Moore J; Lewis W; Denton RM Phosphorus(V)-Catalyzed Deoxydichlorination Reactions of Aldehydes. Tetrahedron 2013, 69, 8769. [Google Scholar]; (g) Yu T-Y; Wang Y; Xu P-F An Unusual Tri-phenylphosphine Oxide Catalyzed Stereoselective 1,3-Dichlorination of Unsaturated Ketoesters. Chem. Eur. J 2014, 20, 98. [DOI] [PubMed] [Google Scholar]
- 16.(a) For electrophilic phosphonium-catalyzed methods, see: Caputo CB; Hounjet LJ; Dobrovetsky R; Stephan DW Lewis Acidity of Organofluorophosphonium Salts: Hydrodefluorination by a Saturated Acceptor. Science 2013, 341, 1374. [DOI] [PubMed] [Google Scholar]; (b) Bayne JM; Stephan DW Phosphorus Lewis Acids: Emerging Reactivity and Applications in Catalysis. Chem. Soc. Rev 2016, 45, 765. [DOI] [PubMed] [Google Scholar]
- 17.Attempted use of other reported phosphacyclic catalysts in the pIII/pv=O literature proved less effective (Table S3).
- 18.Pompeo M; Farmer JL; Froese RDJ; Organ MG Room-Temperature Amination of Deactivated Aniline and Aryl Halide Partners with Carbonate Base Using a Pd-PEPPSI-IPentcl- o-Picoline Catalyst. Angew. Chem. Int. Ed 2014, 126, 3287. [DOI] [PubMed] [Google Scholar]
- 19 (a).Trinus FP; Mokhort NA; Yagupol'skii LM; Fadeicheva AG; Danilenko VS; Ryabukha TK; Fialkov YA; Kirichek LM; Endel'man é. S.; Get'man GA Mefenamic acid—A nonsteroid anti-inflammatory agent. Pharm. Chem. J 1977, 11, 1706. [Google Scholar]; (b) Rao B; Zeng X Aminocyanation by the Addition of N–CN Bonds to Arynes: Chemoselective Synthesis of 1,2-Bifunctional Aminobenzonitriles. Org. Lett 2014, 16,314. [DOI] [PubMed] [Google Scholar]
- 20 (a).Brown HC; Kim KW; Cole TE; Singaram B J. Am. Chem. Soc 1986, 108, 6761. [Google Scholar]; (b) Matteson DS; Kim GY Asymmetric Alkyldifluoroboranes and Their Use in Secondary Amine Synthesis. Org. Lett 2002, 4, 2153. [DOI] [PubMed] [Google Scholar]; (c) Bagutski V; Elford TG; Aggarwal VK Synthesis of Highly Enantioenriched C-Tertiary Amines from Boronic Esters: Application to the Synthesis of Igmesine. Angew. Chem. Int. Ed 2011, 50, 1080. [DOI] [PubMed] [Google Scholar]; (d) Mlynarski SN; Karns AS; Morken JP Direct Steros-pecific Amination of Alkyl and Aryl Pinacol Boronates J. Am. Chem. Soc 2012, 134, 16449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.