Abstract
The study of sexual dimorphism in psychiatric and neurodevelopmental disorders is challenging due to the complex interplay of diverse biological, psychological, and social factors. Males are more susceptible to neurodevelopmental disorders including intellectual disability, autism spectrum disorder, and attention-deficit activity disorder. Conversely, after puberty, females are more prone to major depressive disorder and anxiety disorders compared to males. One major biological factor contributing to sex differences is the sex chromosomes. First, the X and Y chromosomes have unique and specific genetic effects as well as downstream gonadal effects. Second, males have one X chromosome and one Y chromosome, while females have two X chromosomes. Thus, sex chromosome constitution also differs between the sexes. Due to this complexity, determining genetic and downstream biological influences on sexual dimorphism in humans is challenging. Sex chromosome aneuploidies, such as Turner syndrome (X0) and Klinefelter syndrome (XXY), are common genetic conditions in humans. The study of individuals with sex chromosome aneuploidies provides a promising framework for studying sexual dimorphism in neurodevelopmental and psychiatric disorders. Here we will review and contrast four syndromes caused by variation in the number of sex chromosomes: Turner syndrome, Klinefelter syndrome, XYY syndrome, and XXX syndrome. Overall we describe an increased rate of attention-deficit hyperactivity disorder and autism spectrum disorder, along with the increased rates of major depressive disorder and anxiety disorders in one or more of these conditions. In addition to contributing unique insights about sexual dimorphism in neuropsychiatric disorders, awareness of the increased risk of neurodevelopmental and psychiatric disorders in sex chromosome aneuploidies can inform appropriate management of these common genetic disorders.
Subject terms: Genetics research, Neuroimmunology
Introduction
Sex differences in psychiatric disorders are well-recognized yet poorly understood clinical phenomena [1–3]. Neurodevelopmental disorders, including intellectual disability (ID), autism spectrum disorder (ASD), and attention-deficit hyperactivity disorder (ADHD), are more prevalent in males compared to females and have different clinical manifestations in males and in females [4–8]. Conversely, other disorders such as major depressive disorder (“depression”) and anxiety disorders are nearly twice as prevalent in women than in men [9–13]. Females also experience increased symptom severity for depression and anxiety disorders and are diagnosed with more comorbidities than males [9, 12, 14, 15] (see Table 1 for prevalence of ASD, ADHD, depression, and anxiety). Understanding sex differences in these disorders has significant clinical implications for guiding diagnosis, treatment, and prevention in a sex-specific and individualized manner.
Table 1.
Sex differences in lifetime prevalence of psychiatric and neurodevelopmental disorders (with hormonal status)
| TD males | TD females | TS | KS | XYY syndrome | XXX syndrome | |
|---|---|---|---|---|---|---|
| ASD | 2.4% [100] | 0.5% [100] | 5% [65] | 5–10% [43, 102] | 30–40% [74] | 0% [45] |
| ADHD in adolescents | 13% [197] | 4.2% [197] | 25% [62] | 36% [43] | 72% [70] | 49% [102] |
| Depression | 14.2% [11] | 24.2% [11] | 41% [170] | 12% [43] | — | — |
| Anxiety (all anxiety disorders) | 19.2% [11] | 30.5% [11] | 7% [170] | 18% [71] | — | — |
| Hormonal status | Normal hormonal profile (male) | Normal hormonal profile (female) | Early onset ovarian failure (as early as 15 weeks of gestation) Hypogonadism in adulthood (female) Relatively normal hormonal profile from birth until puberty | Varying hypogonadism in adulthood (male) Relatively normal hormonal profile from birth until puberty | Relatively preserved hormonal profile (male) | Relatively preserved hormonal profile (female) |
ASD autism spectrum disorder, ADHD Attention deficit hyperactivity disorder, TD typically developing, TS Turner syndrome, KS Klinefelter syndrome
Sex differences in psychiatric disorders are the result of a complex interplay between genetic, hormonal, immunological, and psychosocial factors [2, 3, 16–23]. Adding to this complexity, the diagnosis of psychiatric disorders is currently based on behaviorally defined DSM criteria (Diagnostic and Statistical Manual 5th edition, DSM-5; [24]), and consequently most diagnoses likely reflect a heterogeneous group of disorders associated with different underlying biological mechanisms. Thus, comparisons of males and females with behaviorally defined DSM-5 disorders are likely confounded by heterogeneity. This heterogeneity further obscures efforts to gain a better understanding of sex differences in neurodevelopmental and psychiatric disorders.
Genetic conditions associated with known genetic risk factors can serve as tractable human models for elucidating the complex interactions between genetic factors, hormonal effects, and environmental influences on brain development and behavior. We posit that this framework bridges the gap between the study of murine models and the study of heterogenic populations with 'idiopathic' psychiatric conditions [25]. This framework has also been shown to allow for the study of complex human behavior such as social cognition and attention in relatively homogeneous populations [25, 26]. Sex chromosome aneuploidies (SCAs) are common genetic conditions, characterized by an abnormal number of X or Y chromosomes, resulting in aberrant gene expression and frequently in an anomalous sex hormone profile. Individuals with SCAs often present with a variable spectrum of cognitive and behavioral phenotype which includes weaknesses in executive functions (EF), social cognition, and learning disabilities [27, 28]. These neurobehavioral phenotypes frequently meet the criteria for DSM-5-based neurodevelopmental and psychiatric diagnoses such as ID, ASD, ADHD, learning disorders, depression, and anxiety disorders. Thus, SCAs serve as a promising model for examining the effects of aberrations in sex chromosome number and changes in hormonal profile on neuropsychiatric phenotype and its neural substrates in humans.
Several review papers from our lab [28, 29] and others [27, 30–33] have examined the contribution of SCAs to our understanding of cognition [28–30, 32, 34–36] and the brain [28, 37–40]. In this review, we will examine neurodevelopmental and psychiatric phenotypes in SCAs, and focus on the contribution of SCAs to our understanding of sex differences in neurodevelopmental and psychiatric disorders. Our first section includes a description of the four most common SCAs—Turner syndrome (X0; TS), Klinefelter syndrome (XXY; KS), XYY syndrome, and XXX syndrome. We also review neuroimaging data providing useful contrasts between SCAs in humans and murine models. We suggest that contrasting between SCAs, in addition to comparing each syndrome to TD controls, adds to our understanding of sex chromosomes effects on brain development. Our second section focuses on male-prevalent neurodevelopmental disorders such as ID, ASD, and ADHD. We review the existing literature on the association of neurodevelopmental disorders with SCAs and compare the rates and the behavioral profile of ASD and ADHD between the assessed SCAs. We also describe findings from existing genome-wide association studies (GWAS) in ADHD and ASD. Our third section focuses on female-prevalent disorders such as depression and anxiety disorders. We examine existing data pertaining to the prevalence of depression and anxiety in SCAs. We also address recent findings on neuroimmunological factors associated with depression and anxiety disorders. We suggest SCAs as promising models for examining the association between depression and anxiety disorders, immune dysfunction, and sex chromosome number and constitution. Finally, we discuss insights and future directions.
Sex chromosome aneuploidies
Clinical characteristics of SCAs include disorder-specific, yet variable physical phenotype, aberrations in sex hormone levels (see Table 1 for hormonal status of the SCAs described in this review), congenital abnormalities, neurological symptoms, and other accompanying conditions such as autoimmune disorders. SCAs also present with an equally variable and somewhat SCA-specific profile of intellectual abilities and cognitive-behavioral phenotype [27–30, 36, 41–47]. The most common SCAs are TS, associated with complete or partial loss of one X chromosome (most commonly 45,X0, one in 1:2000 female livebirths) [48], KS, associated with the addition of an X chromosome (most commonly 47, XXY, 1:450 male livebirths) [49], XYY syndrome, associated with the addition of a single Y chromosome (most commonly 47, XYY, 1:1000 male livebirths) [48], and XXX syndrome, associated with the addition of an X chromosome, (most commonly 47, XXX, 1:1000 female livebirths) [48]. Although an addition or a loss of a complete sex chromosome is the most common chromosomal aberration associated with SCAs, there are often variable karyotypes in each SCA including mosaic forms, ring chromosome (in TS), individuals who carry a higher number of X chromosomes (in KS), and other aberrations [29, 43]. Whereas TS is the only viable human monosomy, KS, XYY syndrome, and XXX syndrome are SCA trisomies. Due to their relatively subtle physical phenotype, trisomic SCAs are often underdiagnosed, with current estimates of only 50% of males with KS [49, 50] and approximately 15% of males with XYY syndrome being clinically identified [51]. While the reported prevalence of XXX syndrome is 1 in 1000 live female births, it was proposed that this syndrome is often underdiagnosed [27, 52].
Each sex chromosome is composed of two pseudoautosomal regions (PAR1 and PAR2), which are conserved homologous sequences on the X and Y chromosomes, in addition to a sex chromosome-specific region which contains a greatly increased amount of genetic material on the X chromosome relative to the Y chromosome [53]. It is assumed that due to the large difference in the amount of genetic material between the X and Y chromosomes, one of two X chromosomes undergoes the process of X-chromosome inactivation in typically developing (TD) females. However, up to 15% of the genes on the inactivated X chromosome escape inactivation, and these genes have been prominent candidates for sexual dimorphism [54]. In addition, the degree of X-chromosome inactivation is thought to vary between individuals and between different tissues, and is dynamic throughout the life span [55, 56].
Therefore, females with TS lack one copy of the PAR1 and PAR2 regions compared to TD males and females, in addition to absent expression of X-chromosome inactivation escape genes compared to TD females [57]. Conversely, males with KS have an extra copy of the PAR1 and PAR2 regions compared to TD males and females, in addition to genes that escape inactivation from the second X chromosome compared to TD males [58, 59]. The additional X chromosome is also inactivated in XXX syndrome [60]. However, it is unclear to what degree this additional X chromosome escapes inactivation [52].
Females with TS are typically characterized by short stature and premature ovarian failure as early as 15 weeks of gestation [61], which results in associated sex hormone deficiencies and absent development of secondary sexual characteristics during puberty. Other prominent features can include congenital renal and cardiovascular defects, webbed neck, sensorineural hearing loss, and short stature [28]. TS is associated with neurodevelopmental and psychiatric disorders including a significantly elevated risk of ADHD [62–64], ASD [65], anxiety, and depression [66].
Males with KS are typically tall, have a genycoid habitus, small testes, and are thought to have similar sex hormone concentrations as TD boys until the onset of puberty (although this notion has been recently challenged) [67, 68]. However, testosterone production stops early or mid-puberty in most boys with KS, resulting in varying degree of hypogonadotropic hypogonadism and infertility. KS is associated with an increased risk of ADHD, ASD, anxiety, and depression [31, 69–72].
Males with XYY syndrome have an increased risk for seizures, are usually tall, and have relatively normal testicular function and sex hormone profile [73, 74]. Like KS and TS, XYY syndrome has been associated with neurodevelopmental disorders, including ADHD and ASD [45, 70, 73, 75, 76].
Females with XXX syndrome usually demonstrate typical growth and development but may present with seizures, genitourinary abnormalities, and moderately tall stature. Pubertal onset and sexual development are normal, but there have been some reports of premature ovarian failure. XXX syndrome has been associated with ADHD, anxiety, and depression [27].
The sex chromosomes and neural structure and function
Neurodevelopmental disorders such as ASD and ADHD have been associated with aberrations in the structure and function of neural circuitry [77–82]. SCAs such as KS and TS have individually been associated with structural and functional aberrations in the neural circuitry of attention, EF, and social cognition/functioning [25, 28, 39, 63, 83–85]. These studies usually compare an SCA (e.g., TS) relative to sex-matched TD controls. However, using this approach, it is difficult to determine specific associations between sex chromosome number/constitution and aberrations in neural structure and function. Studies which directly compare individuals with different SCAs allow us to overcome this limitation.
Hong et al. [84] compared children with KS, TS, and TD males and females in early puberty using volume-based morphometry. Individuals with both SCAs demonstrated highly convergent patterns of brain volumetric differences compared to sex-matched TD controls. In addition, the number of sex chromosomes was associated with increased temporo-insular gray matter volumes in a linear, dose-dependent manner and inversely associated with parieto-occipital volumes. Raznahan et al. [86] compared structural magnetic resonance imaging data between individuals with varied SCAs (KS, XYY syndrome, XXX syndrome, XXYY syndrome) and TD males and females. This study showed that X and Y chromosomes had opposing effects on overall brain size. Y-chromosome number was associated with increased total brain volume, cortical volume, surface area, and cortical thickness, while X-chromosome number was associated with a decrease in all these measures except for cortical thickness, which was unaltered. Conversely, X and Y chromosomes were observed to have a convergent dose-dependent effect on fronto-temporal and occipito-parietal circuitry involved in social perception, emotion, communication, and decision making including reward processing. The authors concluded that these convergent X-chromosome and Y-chromosome effects may result from influences of shared genes. Alternatively, they suggested that a convergent pattern may also be associated with sex chromosome number (in contrast to sex chromosome constitution), as there appeared to be a stepwise correlation between overall sex chromosome number and cortical anatomy in areas of convergent influence. A different comparison of these SCAs by the same group found that while hemispheric lateralization patterns were preserved across all studied SCAs, X-chromosome aneuploidy accentuated normative rightward inferior frontal asymmetries, while Y-chromosome aneuploidy reversed normative rightward medial prefrontal and lateral temporal hemispheric asymmetries [87].
In studies utilizing comparisons between murine models of TS and KS (X0 and XXY mice) and control mice (XX and XY) [88, 89], XY and XXY male mice had increased regional brain volumes in subcortical structures, the amygdala, and the stria terminalis compared to XX female mice. Conversely, XY and XXY male mice had decreased regional volumes in the somatosensory and cerebellar cortices compared to XX female mice. Based on these results, the authors suggest that an extra X chromosome in males does not prevent penetrance of normative sexual dimorphism. An examination of anatomical variation between X0 compared to XX control mice and XXY compared to XY control mice showed a pattern of reciprocal differences in brain volumes between X0 and XXY mice. For example, the amygdala volume was larger in XXY compared to XY mice and smaller in X0 compared to XX mice. This pattern was observed in 70% of all voxels with overlapping XO-XX and XXY-XY differences. Taken together, these findings suggest strong evidence for direct X-chromosome gene-dosage effects on brain volume differences.
Outlining the unique associations between sex chromosome number, sex chromosome constitution, and neurodevelopmental trajectories provides clues regarding the identity and location of sex chromosome genes involved in neurodevelopment. For example, PAR genes and X–Y pair genes are more likely to be associated with neural variation that follows a stepwise pattern when overall sex chromosome number is increased. In addition, the apparent convergence and divergence between X-chromosome and Y-chromosome effects may explain different patterns of neural structure and function in males and females, and may be related to sexual dimorphism in neurodevelopmental disorders.
Neurodevelopmental disorders
The X chromosome contains genes that are associated with intellectual function, and X-chromosome-linked syndromes have been shown to increase risk for ID. ID is more prevalent and more severe in males, who have only one copy of the X chromosome. X-linked ID comprises 5–10% of all ID cases, a disproportionately high fraction of these disorders when considering the size of the X chromosome in the context of the entire genome [90, 91]. Notably, high incidence of neurodevelopmental disorders such as ASD are reported in X-linked ID. As an example, high rates of ASD associated behaviors are observed in X-linked genetic conditions such as fragile X syndrome and Rett syndrome [26, 92, 93].
ID and cognition in SCAs
General intellectual ability seems to be mildly affected in all SCAs, and although general intelligence is relatively preserved and is in the average to low-average range in all SCAs, learning disorders are common [27, 28, 30, 32, 41, 42, 47, 73, 75, 94]. As well, there are many studies pointing to a unique cognitive profile in different SCA subgroups.
Overall, SCAs caused by an addition of a single sex chromosome (KS, XYY syndrome, and XXX syndrome) present with verbal-related and language-related weaknesses [27, 28, 30, 32, 36, 42, 45, 46, 70, 73, 95, 96]. Relatively preserved visuospatial skills are observed in the two male trisomies, KS and XYY syndrome [29, 30, 95]. Conversely, females with TS present with normal or enhanced verbal skills along with significant visuospatial weaknesses [34, 42, 47, 97]. Females with XXX syndrome have been suggested to have a combined deficit pattern with weaknesses in both verbal and visuospatial domains [27, 30, 41]. Notably, cognitive abilities in XXX syndrome have been less extensively studied than in TS, KS, and XYY syndrome, and existing data are based on small sample sizes.
The effects of sex chromosomes on cognition
Relative verbal weaknesses are observed in the three SCA trisomies (KS, XYY syndrome, and XXX syndrome) irrespective of sex, while preserved verbal skills are seen in TS. This suggests that sex chromosome number may exert more influence over the development of verbal cognitive abilities relative to specific sex chromosome constitution or hormonal profile. Conversely, visuospatial abilities, which are adversely affected in females with TS and XXX syndrome but preserved in males with KS and XYY syndrome, may be protected by the Y chromosome or male hormonal profile.
Neurodevelopmental disorders: ASD
ASD represents a group of heterogeneous neurodevelopmental disorders [92, 98] characterized by the early onset of social and communication deficits and a pattern of restricted and repetitive behaviors. ASD prevalence is nearly 2% in the general population [99], and the male-to-female prevalence ratio is 4–5:1 [100, 101].
Genetic and prenatal or perinatal risk factors have been associated with ASD. Genetic heritability has been suggested to account for 37–55% of ASD variance [93, 102, 103]. A significantly increased incidence of ASD has been associated with several relatively well-defined X-linked conditions such as fragile X syndrome and Rett syndrome [26, 92, 93]. In addition, numerous prenatal and perinatal environmental factors have been linked with an increased risk for ASD [93, 104, 105]. However, the etiology of most ASD cases remains unknown.
ASD in SCAs
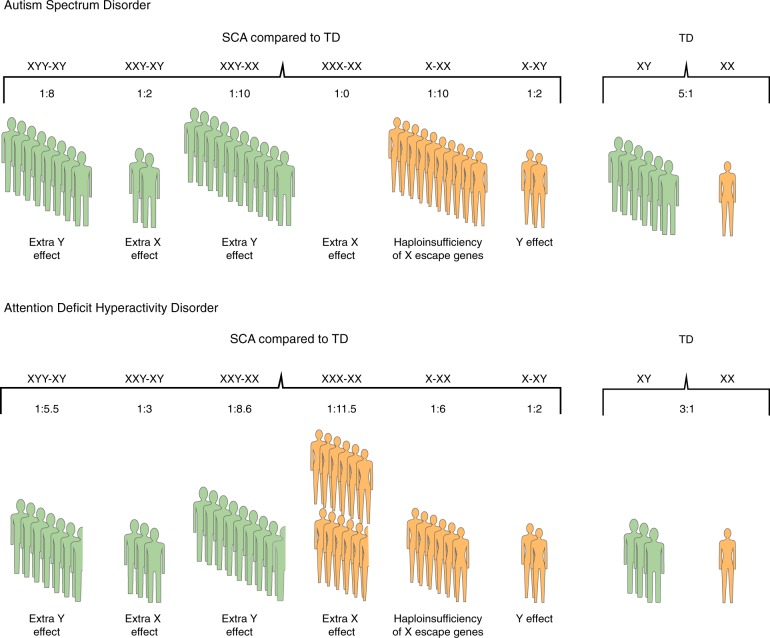
SCAs have been associated with an increased incidence of ASD diagnosis (Fig. 1; see Table 1 for ASD prevalence in SCAs described in this review), and individuals with SCAs often present with ASD traits even when diagnostic criteria for ASD are not fully met.
Fig. 1.
The effects of sex chromosome number and constitution on the rate of autism spectrum disorder and attention-deficit hyperactivity disorder in sex chromosome aneuploidies compared to typically developing males and females. Rate comparisons for a autism spectrum disorder, and b attention-deficit hyperactivity disorder between four sex chromosome aneuploidies (SCAs)—XYY syndrome (XYY), Klinefelter syndrome (XXY), XXX syndrome (XXX), and Turner syndrome (X0), and typically developing males (XY) and females (XX). The text indicates the effects of an extra X or Y chromosome or haploinsufficiency associated with these comparisons. Green represents SCA comparisons to a male sex phenotype; orange represents SCA comparisons to a female sex phenotype. Prevalence data are summarized in Table 1. When a range of prevalence is provided, we use the lower conservative prevalence rate
Approximately 5% of females with TS are diagnosed with ASD [65], and weaknesses in social cognition have been observed in TS [106–109]. Females with TS exhibit weaknesses in face recognition, facial affect discrimination (especially of fear or anger), interpretation of social cues, theory of mind, and problems in social communication. They also may manifest problems in eye tracking and gaze processing, and present with restricted interests or repetitive behaviors [47, 107, 110–115]. An overlap between the TS behavioral phenotype and 'idiopathic ASD' has been previously suggested [110]. However, our study [47] found that although girls (aged 3 to 12 years) with TS (n = 42) had significant social cognition weaknesses and social functioning problems compared to TD girls (n = 32), they had intact social motivation and social appetitive behavior.
Previous studies show that approximately 11–12% of individuals with KS meet various questionnaire-based criteria for ASD or receive a diagnosis of ASD [45, 116]. Males with KS have been previously described as shy, socially anxious, and socially withdrawn [33, 117–120]. Bruining et al. [71] systematically assessed 51 individuals with KS (aged 6 to 19 years) and found that 27% of participants met the criteria for ASD using the Autism Diagnostic Interview-Revised. In comparison, Tartaglia et al. [43] found that 5% (1/20) individuals with KS (aged 6 to 21 years) met the ASD criteria using the Autism Diagnostic Observation Schedule (ADOS).
In systematic assessments of social cognition and functioning domains, men with KS were less accurate in the perception of social-emotional cues such as facial expression. Additionally, they exhibited reduced empathic understanding, weaknesses in identifying and verbalizing emotions, problems in interpreting affective prosody, and reduced eye contact [121–123]. It is important to note that males with KS have well-described verbal difficulties that are likely to interfere with social communication [44], which may, in part, be related to increased risk for ASD in KS.
Studies examining ASD phenotype in XYY syndrome are relatively sparse. Based on several case series reports, it has been suggested that 20–30% of individuals diagnosed with XYY syndrome receive an ASD diagnosis [96]. In a recent systematic study [116] boys (aged 4 to 15 years) with XYY syndrome (n = 26), boys with KS (n = 82) and sex-matched TD controls (n = 50) were screened for ASD using the Social Communication Questionnaire (SCQ). Half of the boys with XYY syndrome vs. 12% of boys with KS and none of the control group scored above the cutoff score on the SCQ, suggesting an increased risk of ASD in XYY syndrome.
Unlike in TS, KS, and XYY syndrome, the available data on XXX syndrome do not find an increased risk of ASD [45], and there are only a few case reports of ASD in girls with this disorder [124].
The effects of the sex chromosomes on ASD
Studies comparing SCAs on social cognition and social functioning provide insights on the effects of sex chromosome number and constitution on ASD risk. Bishop et al. [45] compared parental assessment of socialization and communication skills in the three SCA trisomies (KS, XYY syndrome, and XXX syndrome) using the SCQ, the Vineland Adaptive Behavioral Scale, and the Children’s Communication Checklist-2. They found that none of the 58 girls with XXX syndrome, 11% (2/19) of boys with KS, and 20% (11/58) of boys with XYY syndrome had autistic traits. These findings are consistent with those of Ross et al. [116], who found increased ASD traits in boys with XYY syndrome (50%) relative to boys with KS (12%) and to TD controls. Taken together, these results suggest that the Y chromosome may predispose to ASD-related symptoms, and/or that an extra X chromosome may be protective, even in the context of aberrations in sex chromosome number. A predisposing role for the Y chromosome in ASD is further supported by a study that compared autistic traits in individuals (aged 4 to 18 years) with KS (n = 102), XYY syndrome (n = 40), and XXYY syndrome (n = 32), a rare SCA, using the Social Responsiveness Scale (SRS) [76]. In all three SCA groups, there were significantly more individuals with an SRS score in the severe range, compared to an SRS-normative sample. However, while individuals with XYY syndrome and XXYY syndrome had comparable social problems and autistic symptomatology, individuals with KS were affected to a lesser degree. Further addressing this issue, a recent study [102] compared ASD phenotype in males (aged 3 to 25 years) with KS (n = 20), XYY syndrome (n = 57), and XXYY syndrome (n = 21) using the ADOS and DSM-5 criteria. The authors report an increased rate of ASD diagnosis when Y-chromosome number is increased, 39% (22/57) in XYY syndrome and 52% (11/21) in XXYY syndrome vs. 10% (2/20) in KS. These data suggest that increased Y-chromosome number rather than increased X-chromosome number may be associated with higher ASD risk.
An additional study by Lee et al. [125] compared various supernumerary SCAs using the SRS. This study examined the dose effect of supernumerary X chromosomes and the effects of an additional Y chromosome. Lee et al. [125] compared TD girls to boys with KS and girls with XXX syndrome (a conjoint + 1 X group) and to individuals with rare supernumerary SCAs such as XXXX syndrome and XXXY syndrome (a conjoint + 2/3 X group). They also compared TD boys to boys with XYY syndrome (+1Y). This study confirmed that the addition of a single Y chromosome was associated with more autistic traits. However, while the authors propose that increased X-chromosome number was also associated with more autistic traits, no difference was found between +1X or +2/3X SCAs, suggesting that X-chromosome effects are not necessarily correlated with these traits in a dose-dependent manner. A limitation of Lee et al.’s [125] study design is the utilization of conjoint groups of SCAs (the inclusion of the X and the Y chromosome in the same group, e.g., females with XXX syndrome together with males with KS). This design prevents a more specific examination of the effects of overall sex chromosome number, specific X-chromosome and Y-chromosome effects, and does not specify these from the effects of hormonal profile.
Neurodevelopmental disorders: ADHD
ADHD is the most prevalent psychiatric condition of childhood (~10%) [126] and is characterized by inattention and/or hyperactivity-impulsivity. ADHD has been extensively linked with EF weaknesses such as reasoning, planning, inhibition, set-shifting, and working memory [127].
Differences between males and females in prevalence, comorbidities, and clinical manifestations are hallmarks of ADHD, with a male-to-female prevalence ratio of about 3:1 [7, 8, 128, 129]. Males with ADHD display similar levels of attention problems but increased levels of hyperactivity and impulsivity relative to females with a similar diagnosis [5, 7, 130]. Therefore, the current DSM-5 diagnostic criteria, assigning equal diagnostic importance to attention problems and hyperactivity/impulsivity, may reflect a predominantly male representation of this disorder [7, 130–132], that may result in under-diagnosis and under-treatment in females with ADHD.
ADHD in SCAs
All four SCAs, but especially KS and TS, have been associated with significant ADHD-like symptoms and EF weaknesses (Table 1) [27, 32, 34, 43, 62, 63, 70–73]. Girls with TS have been described as impulsive, hyperactive, and inattentive [106, 108, 133]. Using the DSM-IV diagnostic criteria for ADHD, Russell et al. [62] showed that approximately 25% of 50 girls and adolescents with TS met the diagnostic criteria for ADHD. Similarly, we recently reported that over half of girls (aged 5 to 12 years) with TS (n = 49) had significant ADHD-like symptoms compared to sex-matched TD controls (n = 37). Girls with TS and ADHD-like symptoms were as inattentive and hyperactive as males with idiopathic ADHD and more hyperactive than girls with idiopathic ADHD [63].
With respect to EF, girls with TS exhibit extensive problems including weaknesses in working memory and in the ability to plan, organize, and execute multiple-step problem solving. Additional problems are observed in inhibition, attention, and cognitive flexibility, although results for cognitive flexibility are not consistent across studies [34, 63, 112, 134, 135].
Although it has been noted clinically and in population studies that boys with KS have increased rates of ADHD [71, 72], only a handful of studies systematically assessed attention and hyperactivity in boys with this condition. Tartaglia et al. [43] reported that approximately one-third of 57 males with KS (aged 6 to 21 years) met the DSM-IV diagnostic criteria for ADHD. In a study that compared boys (aged 4 to 15 years) with KS (n = 82), boys with XYY syndrome (n = 26) and TD boys (n = 50) using the Child Behavior Checklist (CBCL) and the Conners’ Parent Rating Scale-Revised (CPRS-R), nearly half of boys and adolescents with KS displayed significantly elevated rates of ADHD compared to TD boys [116]. Males with KS exhibit specific EF weaknesses in working memory, planning and organization skills, response inhibition, and shifting [94, 95, 136–139].
XYY syndrome has also been associated with ADHD [74, 140]. Fifty-two percent of males with XYY syndrome were reported to meet the diagnostic criteria for ADHD based on preexisting psychiatric diagnoses, and 76% met the diagnostic criteria for ADHD based on parent questionnaires (the parent version of the Conners’ Rating Scale, or the Swanson, Nolan, and Pelham Questionnaire—Fourth Edition) [70, 73]. Ross et al. [75] compared boys (aged 4 to 17 years) with KS (n = 93), boys with XYY syndrome (n = 21), and TD boys (n = 36) using the Conners’ Continuous Performance Test (CPT-II or Kiddie CPT) and the Delis-Kaplan Executive Function System Color-Word Interference Test. Overall, males with XYY syndrome and KS had comparable EF weaknesses relative to TD controls on measures of EF and attention, although cognitive flexibility (including inhibition and switching) was more impaired in the XYY syndrome group than the KS group.
XXX syndrome has also been associated with ADHD [141, 142]. More than half of 25 girls and adolescents with XXX syndrome (aged 6 to 20 years) met the criteria for ADHD based on parents’ ratings [70]. However, none of these studies included more detailed rates of attention problems, hyperactivity, and impulsivity, or evaluation of EF skills.
The effects of sex chromosomes on ADHD
Females with TS have been described as hyperactive and impulsive, and hyperactivity levels in this group were comparable to the levels measured in males with idiopathic ADHD, but lower than the hyperactivity levels in females with idiopathic ADHD [63]. In KS, Ross et al. [94] systematically assessed attention and response inhibition in boys and adolescents and showed that impulsivity was not increased in their study group, and attention problems were only seen in children younger than 10 years. An additional study by the same group revealed that only attention problems, but not hyperactivity, were reported by parents using the CPRS-R and CBCL [116]. These findings imply that the number of X chromosomes might affect sexual dimorphism in ADHD profile (e.g., increased attention problems but not hyperactivity in females with idiopathic ADHD and children with KS), consistent with the well-described features of males and females with 'idiopathic' ADHD.
The sex chromosomes and GWAS
Next, we will review the identified effects of SCAs considering recent GWAS of neurodevelopmental disorders. Specifically, we focus on genes identified as linked to specific neurodevelopmental disorders (ADHD, ASD). A review of the literature was conducted with the GWAS catalog (https://www.ebi.ac.uk/gwas/). We used “autism spectrum disorder” and “attention-deficit hyperactivity disorder” as search terms. While 86 SNPs were associated with ASD and 26 SNPs were associated with ADHD (p values <5.0 × 10−8), all of them were located on autosomal genes and none were identified on the X chromosome. These findings are surprising given that ASD and ADHD are sexually dimorphic conditions with respect to prevalence, suggesting that sex chromosomes might exert a significant influence on these conditions [143]. In addition, sex chromosome number and constitution are associated with ASD and ADHD symptoms in SCAs. Therefore, it is plausible that exclusion of the X chromosome from GWAS analyses [143, 144] may contribute to these findings.
Therefore, we examined how many studies of ASD and ADHD in the GWAS catalog included sex chromosomes in their analysis. Our investigation revealed that only half (54%) of the GWAS studies in ASD and only 15% of the GWAS studies in ADHD included the X chromosome in their analyses and reports. Other fields of medicine also report an exclusion of sex chromosomes and unexpected lack of findings for the sex chromosomes in GWAS studies [144]. One possible solution for this issue is re-analysis of existing GWAS data. Indeed, a focused survey of the literature reveals that when re-analyses included the X chromosome, novel and biologically relevant genetic findings were detected for diabetes mellitus type 2 [145], prostate cancer [146], and orofacial clefts [147]. To the best of our knowledge, such re-analyses have not yet been conducted for ASD and ADHD. Such data from GWAS, combined with data from the study of SCAs, could be of great benefit to the understanding of associations between sex chromosome genes and neurodevelopmental disorders.
Depression and anxiety disorders
Depression and anxiety disorders are highly comorbid and share familial risk. Women have a 1.5–2-fold increased lifetime prevalence of depression (24%) and most anxiety disorders (30.5% overall for all anxiety disorders) compared with men [11, 13, 148], with the exception of obsessive-compulsive disorder and bipolar disorder which are similarly prevalent in men and women [10, 13, 149, 150].
Socioeconomic and psychological factors, differential exposure to trauma, and hormonal factors likely contribute to the observed sex differences in depression and anxiety disorders [2, 18, 151–157]. Sex differences in depression and anxiety emerge around puberty [158]. Moreover, women have an elevated risk of depression and anxiety disorders during various stages of their reproductive cycle including pre-menstruation, in the postpartum period, and while entering menopause [18, 153–156, 158–160]. These phenomena have given rise to theories focusing on hormonal factors as major contributors to sex differences in depression and anxiety disorders [158, 161–164]. Nevertheless, there is growing evidence that additional sexually dimorphic biological factors, including immune system function and inflammation responses [153, 165], and sex chromosome factors [166, 167] are associated with observed sex differences in depression and anxiety.
Depression and anxiety in SCAs
Compared to ADHD, ASD, and learning disorders, depression and anxiety disorders have not been as extensively studied amongst individuals with SCAs (Table 1). When assessed, the reported increased prevalence of depression and anxiety disorders has been attributed mostly to the psychosocial burden of having an SCA. For example in TS, increased prevalence of depression and anxiety disorders has been attributed to the lack of secondary sex characteristics [168].
Females with TS have been described as shy and socially anxious [169], and girls and adolescents with TS have been reported to have increased anxiety and depression [66, 107, 109]. In a systematic assessment of current and lifetime psychiatric illness in 100 women with TS, 41% of women with TS met the diagnostic criteria for current or past depressive disorder [170], which is a 1.7-fold increase in depression relative to females in the general population [11, 13, 148].
Increased depression in TS may, in part, be related to hormone deficiency. Estrogen-depleted and progesterone-depleted states such as menopause and postpartum have also been related to onset and exacerbation of depression and anxiety in TD women [153–155, 158–160]. Premature ovarian failure has been shown in female fetuses with TS as early as 15 weeks after gestation [61]. This finding is likely to influence various aspects of early neural development, which might predispose girls with TS to depression. Hormone replacement therapy is commonly initiated in girls with TS around the age of 11 to 14 years, and doses are adjusted later in development. Exogenous hormone replacement therapy is intended to partly mimic the physiological hormonal cycle [165, 171] and may affect mood and anxiety. In a recent case report of a woman with TS, severe postpartum depression was responsive to hormonal supplementation, suggesting a role for hormone replacement therapy in the treatment of depression in women with TS [172]. Depression and anxiety in TS may also be related to effectiveness of social functioning. Social motivation does not seem to be impaired in TS [47], a combination which could predispose to anxiety and depression.
There have also been sporadic reports of increased depression and anxiety in KS [69]. Twenty-four percent of boys (n = 51, aged 6 to 19 years) with KS were diagnosed with depression and 18% were diagnosed with anxiety using the Kiddie-Sads-Present and Lifetime Version [71]. Lower rates, 14% for depression and 12% for anxiety, were reported in boys and adolescents with KS (n = 57, aged 6 to 21 years) using the parent rating scale of the Behavior Assessment System for Children, Second Edition [43]. Nearly 70% of 310 individuals with KS (mostly postpubertal, aged 14 to 75 years) reported clinically significant rates of depression using the Center for Epidemiologic Studies Depression Scale [173], suggesting that males with KS may have an increased risk for depression later in life.
Against this background, exogenous androgen decreased rates of anxiety and depression in pre-pubertal boys with KS in a double-blind controlled study [174], and also in a small cohort of adults with KS who had hypogonadism [118]. However, testosterone administration was not double-blinded or controlled in the study of adults with KS. In addition, testosterone doses administered to pre-pubertal children examined by Ross et al. [174] were higher than physiological testosterone secretion at this age and did not follow physiological secretion patterns. While these data suggest that depression and anxiety in KS may be related to the hormonal aberrations, one cannot infer such relations from treatment response.
Studies assessing depression and anxiety in XYY syndrome are sparse. A study examining various behavioral aspects in boys (aged 4 to 15 years) with XYY syndrome (n = 26) relative to TD boys (n = 50) and boys with KS (n = 82) did not find increased depression and anxiety in this group according to self-report questionnaires (the Children’s depression inventory (CDI), and the Revised Child’s Manifest Anxiety Scale) [116]. Similar results were observed by Bardsley et al. [73], who examined depression and anxiety using the CBCL and CDI-2 in 90 males with XYY syndrome. No increase in depression and anxiety relative to the general population was observed.
There is also a relative paucity of studies on depression and anxiety disorders in XXX syndrome [27]. Yet, an elevated risk of depression/dysthymia and anxiety disorders have been reported in small samples [124, 175].
Taken together, limited results for XYY syndrome, XXX syndrome, and KS suggest that the extra X chromosome and not an extra Y chromosome might be associated with susceptibility to depression and anxiety disorders in these SCAs. Results for individuals with TS are potentially more difficult to interpret, due to the strong moderating effect of hormones on depression and anxiety, and the early onset and continuous aberrations in hormonal profile in TS. More systematic studies in SCAs including studies comparing different SCAs are needed to accurately examine associations between aberrations in the number and constitution of sex chromosomes with depression and anxiety disorders.
The effects of sex chromosomes on autoimmunity, depression, and anxiety
In addition to the association of hormonal abnormalities with depression and anxiety in TS and possibly in KS, the number and constitution of sex chromosomes may also influence the risk of depression and anxiety disorders in SCAs through other biological mechanisms. Recent evidence highlights the role of immune system dysfunction and inflammation in psychiatric disorders, and specifically in the development of depression and anxiety disorders [176–178].
An intriguing subset of immune disorders is autoimmune diseases, a group of heterogeneous disorders in which the immune system attacks the self. There is a high comorbidity between anxiety and depression and autoimmune diseases such as thyroid autoimmune disorders [179], in which thyroid dysregulation can result in anxiety and depressive symptoms in addition to agitation and other neuropsychiatric symptoms. Another example is systemic lupus erythematosus (SLE), in which depression and anxiety are highly prevalent [180].
Immune activation is sexually dimorphic [181], and the sex chromosomes are implicated in immune system function. The Y chromosome contains genes that regulate immune gene expression [182] and the X chromosome contains more immune-related genes than any other chromosome [183]. Indeed, most autoimmune diseases are more prevalent in females than in males, even though there are a few autoimmune diseases that are more prevalent in males such as reactive arthritis and ankylosing spondylitis [181, 184].
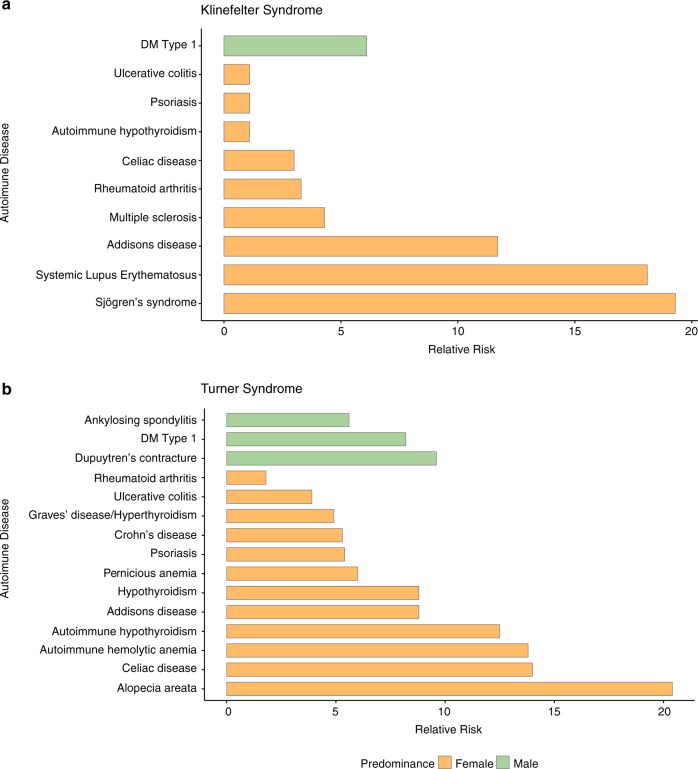
Autoimmune diseases are also generally more prevalent in SCAs than in the general population [183, 185], with variable prevalence amongst different SCAs. Jorgensen et al. [186] found that females with TS have an overall twofold increased risk of autoimmune diseases. Further, they seem to have a particularly increased risk of male-predominant autoimmune diseases (a fourfold increase), relative to female-predominant autoimmune diseases (a 1.7-fold increase) (Fig. 2). Therefore, the authors suggest that autoimmunity pattern in TS may be related to X monosomy.
Fig. 2.
The prevalence of autoimmune diseases among individuals with a Turner syndrome and b Klinefelter syndrome. Green represents male-predominant autoimmune diseases in the general population; orange represents female-predominant autoimmune diseases in the general population. Data retrieved from Seminog et al. [192] for Klinefelter syndrome and Goldacre and Seminog [198] for Turner syndrome
However, the autoimmune disease that has been most extensively associated with TS is Hashimoto’s thyroiditis (12-fold increased risk), a condition that often results in hypothyroidism and is also female-predominant in TD individuals (8:1 female-to-male ratio in the general population) [183, 186–189]. One suggested explanation for the high prevalence of Hashimoto’s thyroiditis in TS is increased production of antithyroid antibodies. Indeed, one study among girls with TS (n = 84) showed that nearly 25% had clinical hypothyroidism and 40–50% had positive thyroid antibodies (antithyroperoxidase and antithyroglobulin antibodies) that are associated with Hashimoto’s thyroiditis and Graves’ disease [190]. The finding of increased rates of Hashimoto’s thyroiditis in TS is not in line with the hypothesis that females with TS show more male-prevalent autoimmune diseases due to their X monosomy and with findings of increased production of antithyroid antibodies in females relative to males in animal models [191].
Males with KS have an increased risk of female-predominant autoimmune diseases such as SLE and Sjogren’s syndrome [192] (Fig. 2). Specifically, a 14-fold increase in SLE prevalence is observed in KS relative to TD males, and SLE is as prevalent in males with KS as in TD females. SLE prevalence is also increased in XXX syndrome [193]. Taken together, the increased risk of SLE in KS and XXX syndrome indicates a link to X-chromosome number [194]. It has recently been shown that specific immune-related genes such as Toll-like receptor 7 (TLR7) gene, a key pathogenic factor in SLE [195], are located on the X chromosome.
In conclusion, data suggest an increased risk of depression and anxiety along with evidence of X-chromosome-associated changes in autoimmunity in KS and TS. In non-syndromic populations, the association between depression and anxiety disorders and the immune system is increasingly described. These studies are limited by the diversity of autoimmune diseases and low rates of specific autoimmune disorders in the general population, as well as by heterogeneity of depression and anxiety disorders in the general population. The study of SCAs not only overcomes these limitations (Figs. 1 and 2), but also offers the opportunity to examine the effects of sex chromosomes on interactions between autoimmunity, depression, and anxiety disorders.
Clinical implications
As described in this review, individuals with SCAs present with an array of neurodevelopmental and psychiatric conditions. Since SCAs are the most common aneuploidy in humans, affecting 1:400 livebirths overall [48], it is essential to recognize psychiatric morbidity as part of SCAs’ clinical presentation. Furthermore, awareness of the increased risk of neurodevelopmental conditions in this population might encourage early intervention that has the potential to improve the developmental trajectories of affected children. In older individuals, increasing our clinical vigilance for depression and anxiety disorders in individuals with SCAs can promote proper treatment of these disabling symptoms.
Insights and future directions
Based on the reviewed literature, we suggest that:
1. Overall sex chromosome number affects behavioral phenotype: Increased ASD rates and social cognition weakness have been described in TS. In addition, increases in sex chromosome number in males appear to be related to a greater risk for ASD-associated symtomes. This suggests that aberrations in overall sex chromosome number may predispose one to an ASD phenotype [102].
2. The Y chromosome is associated with increased susceptibility to ASD traits: In SCAs there are sex chromosome constitutional changes associated with variations in cognitive-behavioral phenotype. Existing data suggest that there is no increase in ASD prevalence in females with XXX syndrome, despite the aberration in sex chromosome number in this condition. In addition, although studies comparing ASD phenotype in KS and XYY syndrome (the two male SCA trisomies) report increased ASD traits in both, rates of ASD are higher in XYY syndrome than in KS. Taken together, these data suggest that the Y chromosome in and of itself may be associated with an increase in ASD susceptibility.
3. Although all SCAs are associated with susceptibility to ADHD, variations in clinical presentation between different SCAs may reflect sexual dimorphism in this disorder: Females with TS have comparable rates of hyperactivity and attention problems to males with idiopathic ADHD. Conversely, several studies suggest that young males with KS present with attention problems, but not with hyperactivity.
4. X-chromosome number can affect behavior through various mechanisms: The immune system is sexually dimorphic, and immune system dysfunction has recently been associated with depression and anxiety. X-chromosome number may be related to sex differences in autoimmunity in SCAs. Thus, these common genetic conditions have potential to serve as intriguing model systems for examining sex chromosome association with immune system dysfunction in depression and anxiety.
5. Direct comparisons between different SCAs provide important clues about sex chromosome effects on neurodevelopment: As evident from reviewed prevalence, behavioral, neuroimaging, and animal-model studies, one of the most powerful tools for examining sex chromosome effects are direct comparisons between multiple SCAs in addition to comparisons with TD controls. These study designs provide a more lucid examination of the effects of sex chromosome number and constitution on neurodevelopment, and supply clues regarding the identity and location of candidate genes affecting neurodevelopmental trajectories and psychopathology.
6. There is a need for inclusion of the sex chromosomes in GWAS of ADHD and ASD; re-analyses of existing GWAS to include the sex chromosomes can potentially provide unique data and new insights: As evident from results of our search of existing GWAS data, a substantial percentage of existing studies in ADHD and ASD did not include the sex chromosomes in their analyses. Based on apparent sexual dimorphism in these neurodevelopmental disorders, it is likely that the exclusion of sex chromosomes from these analyses has hindered the discovery of new candidate genes involved in these conditions.
Several limitations should be considered when attempting to interpret the reviewed studies. One is that the available data come from only a handful of studies on each syndrome. The second limitation is related to ascertainment bias that is inherent in studies of genetically defined conditions. In SCAs, this is even more significant given the variability in phenotype and assumption that many individuals with SCAs (presumably with less affected phenotype) are not diagnosed. The third limitation is related to our choice to use the current psychiatric nosology (DSM-5). SCAs are specifically associated with the absence of an X-chromosome (TS) or additional sex chromosomes (KS, XYY syndrome, XXX syndrome, and other rarer SCAs). This, in turn, is thought to lead to aberrations in cognition and behavior. However, only some of the cognitive-behavioral manifestations in individuals with SCAs can be captured through the current psychiatric nosology. While we believe that characterizing behavior using the psychiatric nosology may allow for generalization of our findings, the symptom threshold required for DSM-5 diagnoses cannot identify the full spectrum of aberrant behaviors in these conditions.
To summarize, the study of SCAs in psychiatry is a clinical research framework that provides us with the opportunity to study sex differences in humans. In this framework, we utilize a unique occurrence in nature, where the number and constitution of the X and the Y chromosomes are the modified factor, an experiment usually only available in the study of animal models [167]. Together, the insight gained from the study of SCAs, animal models [167], and genetic studies of large cohorts with 'idiopathic' psychiatric conditions (that do not omit sex chromosomes from their analyses) [144, 196] have the potential to overcome many challenges in the study of sexual dimorphism in psychiatric and neurodevelopmental disorders.
Acknowledgements
We thank Leah Naomi Slang, BS, and Alexandra Ishak, BS, for assistance with editing. TG was supported by funding from the NICHD (HD090209) and the Stanford WSDM Seed Grants for Biological/Medical Research on Sex Differences and/or Women’s Health. ALR was supported by funding from the NIMH (MH099630), NICHD (HD049653). ALR is an unpaid medical advisor for the Turner Syndrome Society and Turner Syndrome Foundation.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Tamar Green, Shira Flash
References
- 1.Riecher-Rossler A. Sex and gender differences in mental disorders. Lancet Psychiatry. 2017;4:8–9. doi: 10.1016/S2215-0366(16)30348-0. [DOI] [PubMed] [Google Scholar]
- 2.Rutter M, Caspi A, Moffitt TE. Using sex differences in psychopathology to study causal mechanisms: unifying issues and research strategies. J Child Psychol Psychiatry. 2003;44:1092–115. doi: 10.1111/1469-7610.00194. [DOI] [PubMed] [Google Scholar]
- 3.Gobinath AR, Choleris E, Galea LA. Sex, hormones, and genotype interact to influence psychiatric disease, treatment, and behavioral research. J Neurosci Res. 2017;95:50–64. doi: 10.1002/jnr.23872. [DOI] [PubMed] [Google Scholar]
- 4.Mandy W, Chilvers R, Chowdhury U, Salter G, Seigal A, Skuse D. Sex differences in autism spectrum disorder: evidence from a large sample of children and adolescents. J Autism Dev Disord. 2012;42:1304–13. doi: 10.1007/s10803-011-1356-0. [DOI] [PubMed] [Google Scholar]
- 5.Rucklidge JJ. Gender differences in attention-deficit/hyperactivity disorder. Psychiatr Clin N Am. 2010;33:357–73. doi: 10.1016/j.psc.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Werling DM, Geschwind DH. Sex differences in autism spectrum disorders. Curr Opin Neurol. 2013;26:146–53. doi: 10.1097/WCO.0b013e32835ee548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biederman J, Mick E, Faraone SV, Braaten E, Doyle A, Spencer T, et al. Influence of gender on attention-deficit hyperactivity disorder in children referred to a psychiatric clinic. Am J Psychiatry. 2002;159:36–42. doi: 10.1176/appi.ajp.159.1.36. [DOI] [PubMed] [Google Scholar]
- 8.Arnett AB, Pennington BF, Willcutt EG, DeFries JC, Olson RK. Sex differences in ADHD symptom severity. J Child Psychol Psychiatry. 2015;56:632–9. doi: 10.1111/jcpp.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLean CP, Asnaani A, Litz BT, Hofmann SG. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res. 2011;45:1027–35. doi: 10.1016/j.jpsychires.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nolen-Hoeksema S. Sex differences in unipolar depression: evidence and theory. Psychol Bull. 1987;101:259–82. [PubMed] [Google Scholar]
- 11.Patten SB. Accumulation of major depressive episodes over time in a prospective study indicates that retrospectively assessed lifetime prevalence estimates are too low. BMC Psychiatry. 2009;9:19. doi: 10.1186/1471-244X-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angst J, Gamma A, Gastpar M, Lepine JP, Mendlewicz J, Tylee A, et al. Gender differences in depression. Epidemiological findings from the European DEPRES I and II studies. Eur Arch Psychiatry Clin Neurosci. 2002;252:201–9. doi: 10.1007/s00406-002-0381-6. [DOI] [PubMed] [Google Scholar]
- 13.Kessler RC, McGonagle KA, Nelson CB, Hughes M, Swartz M, Blazer DG. Sex and depression in the National Comorbidity Survey. II: Cohort effects. J Affect Disord. 1994;30:15–26. doi: 10.1016/0165-0327(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 14.Bekker MH, van Mens-Verhulst J. Anxiety disorders: sex differences in prevalence, degree, and background, but gender-neutral treatment. Gend Med. 2007;4(Suppl B):S178–193. doi: 10.1016/s1550-8579(07)80057-x. [DOI] [PubMed] [Google Scholar]
- 15.Breslau N, Schultz L, Peterson E. Sex differences in depression: a role for preexisting anxiety. Psychiatry Res. 1995;58:1–12. doi: 10.1016/0165-1781(95)02765-o. [DOI] [PubMed] [Google Scholar]
- 16.Bangasser DA, Valentino RJ. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol. 2014;35:303–19. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solomon MB, Herman JP. Sex differences in psychopathology: of gonads, adrenals and mental illness. Physiol Behav. 2009;97:250–8. doi: 10.1016/j.physbeh.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altemus M. Sex differences in depression and anxiety disorders: potential biological determinants. Horm Behav. 2006;50:534–8. doi: 10.1016/j.yhbeh.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 19.Cahill L. Equal not equal the same: sex differences in the human brain. Cerebrum. 2014;2014:5. [PMC free article] [PubMed] [Google Scholar]
- 20.Cahill L. Fundamental sex difference in human brain architecture. Proc Natl Acad Sci USA. 2014;111:577–8. doi: 10.1073/pnas.1320954111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Vries GJ, Forger NG. Sex differences in the brain: a whole body perspective. Biol Sex Differ. 2015;6:15. doi: 10.1186/s13293-015-0032-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, et al. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22:9005–14. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, et al. Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci USA. 2014;111:823–8. doi: 10.1073/pnas.1316909110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
- 25.Green T, Naylor PE, Davies W. Attention-deficit hyperactivity disorder (ADHD) in phenotypically similar neurogenetic conditions: Turner syndrome and the RASopathies. J Neurodev Disord. 2017;9:25. doi: 10.1186/s11689-017-9205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fung LK, Reiss AL. Moving toward integrative, multidimensional research in modern psychiatry: lessons learned from fragile X syndrome. Biol Psychiatry. 2016;80:100–11. doi: 10.1016/j.biopsych.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tartaglia NR, Howell S, Sutherland A, Wilson R, Wilson L. A review of trisomy X (47,XXX) Orphanet J Rare Dis. 2010;5:8. doi: 10.1186/1750-1172-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong DS, Reiss AL. Cognitive and neurological aspects of sex chromosome aneuploidies. Lancet Neurol. 2014;13:306–18. doi: 10.1016/S1474-4422(13)70302-8. [DOI] [PubMed] [Google Scholar]
- 29.Hong DS, Reiss AL. Cognition and behavior in Turner syndrome: a brief review. Pediatr Endocrinol Rev. 2012;9(Suppl 2):710–2. [PMC free article] [PubMed] [Google Scholar]
- 30.Printzlau F, Wolstencroft J, Skuse DH. Cognitive, behavioral, and neural consequences of sex chromosome aneuploidy. J Neurosci Res. 2017;95:311–9. doi: 10.1002/jnr.23951. [DOI] [PubMed] [Google Scholar]
- 31.Savic I. Advances in research on the neurological and neuropsychiatric phenotype of Klinefelter syndrome. Curr Opin Neurol. 2012;25:138–43. doi: 10.1097/WCO.0b013e32835181a0. [DOI] [PubMed] [Google Scholar]
- 32.Boada R, Janusz J, Hutaff-Lee C, Tartaglia N. The cognitive phenotype in Klinefelter syndrome: a review of the literature including genetic and hormonal factors. Dev Disabil Res Rev. 2009;15:284–94. doi: 10.1002/ddrr.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geschwind DH, Boone KB, Miller BL, Swerdloff RS. Neurobehavioral phenotype of Klinefelter syndrome. Ment Retard Dev Disabil Res Rev. 2000;6:107–16. doi: 10.1002/1098-2779(2000)6:2<107::AID-MRDD4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 34.Hong D, Scaletta Kent J, Kesler S. Cognitive profile of Turner syndrome. Dev Disabil Res Rev. 2009;15:270–8. doi: 10.1002/ddrr.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hutaff-Lee C, Cordeiro L, Tartaglia N. Cognitive and medical features of chromosomal aneuploidy. Handb Clin Neurol. 2013;111:273–9. doi: 10.1016/B978-0-444-52891-9.00030-0. [DOI] [PubMed] [Google Scholar]
- 36.Leggett V, Jacobs P, Nation K, Scerif G, Bishop DV. Neurocognitive outcomes of individuals with a sex chromosome trisomy: XXX, XYY, or XXY: a systematic review. Dev Med Child Neurol. 2010;52:119–29. doi: 10.1111/j.1469-8749.2009.03545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raznahan A, Cutter W, Lalonde F, Robertson D, Daly E, Conway GS, et al. Cortical anatomy in human X monosomy. Neuroimage. 2010;49:2915–23. doi: 10.1016/j.neuroimage.2009.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steinman K, Ross J, Lai S, Reiss A, Hoeft F. Structural and functional neuroimaging in Klinefelter (47,XXY) syndrome: a review of the literature and preliminary results from a functional magnetic resonance imaging study of language. Dev Disabil Res Rev. 2009;15:295–308. doi: 10.1002/ddrr.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giedd JN, Clasen LS, Wallace GL, Lenroot RK, Lerch JP, Wells EM, et al. XXY (Klinefelter syndrome): a pediatric quantitative brain magnetic resonance imaging case–control study. Pediatrics. 2007;119:e232–240. doi: 10.1542/peds.2005-2969. [DOI] [PubMed] [Google Scholar]
- 40.Lenroot RK, Lee NR, Giedd JN. Effects of sex chromosome aneuploidies on brain development: evidence from neuroimaging studies. Dev Disabil Res Rev. 2009;15:318–27. doi: 10.1002/ddrr.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rovet J, Netley C, Bailey J, Keenan M, Stewart D. Intelligence and achievement in children with extra X aneuploidy: a longitudinal perspective. Am J Med Genet. 1995;60:356–63. doi: 10.1002/ajmg.1320600503. [DOI] [PubMed] [Google Scholar]
- 42.Rovet JF. The psychoeducational characteristics of children with Turner syndrome. J Learn Disabil. 1993;26:333–41. doi: 10.1177/002221949302600506. [DOI] [PubMed] [Google Scholar]
- 43.Tartaglia N, Cordeiro L, Howell S, Wilson R, Janusz J. The spectrum of the behavioral phenotype in boys and adolescents 47,XXY (Klinefelter syndrome) Pediatr Endocrinol Rev. 2010;8(Suppl 1):151–9. [PMC free article] [PubMed] [Google Scholar]
- 44.Visootsak J, Graham JM., Jr Social function in multiple X and Y chromosome disorders: XXY, XYY, XXYY, XXXY. Dev Disabil Res Rev. 2009;15:328–32. doi: 10.1002/ddrr.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bishop DV, Jacobs PA, Lachlan K, Wellesley D, Barnicoat A, Boyd PA, et al. Autism, language and communication in children with sex chromosome trisomies. Arch Dis Child. 2011;96:954–9. doi: 10.1136/adc.2009.179747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bishop DV, Scerif G. Klinefelter syndrome as a window on the aetiology of language and communication impairments in children: the neuroligin–neurexin hypothesis. Acta Paediatr. 2011;100:903–7. doi: 10.1111/j.1651-2227.2011.02150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hong DS, Dunkin B, Reiss AL. Psychosocial functioning and social cognitive processing in girls with Turner syndrome. J Dev Behav Pediatr. 2011;32:512–20. doi: 10.1097/DBP.0b013e3182255301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nielsen J, Wohlert M. Sex chromosome abnormalities found among 34,910 newborn children: results from a 13-year incidence study in Arhus, Denmark. Birth Defects Orig Artic Ser. 1990;26:209–23. [PubMed] [Google Scholar]
- 49.Herlihy AS, McLachlan RI. Screening for Klinefelter syndrome. Curr Opin Endocrinol Diabetes Obes. 2015;22:224–9. doi: 10.1097/MED.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 50.Herlihy AS, Halliday JL, Cock ML, McLachlan RI. The prevalence and diagnosis rates of Klinefelter syndrome: an Australian comparison. Med J Aust. 2011;194:24–28. doi: 10.5694/j.1326-5377.2011.tb04141.x. [DOI] [PubMed] [Google Scholar]
- 51.Stochholm K, Juul S, Gravholt CH. Diagnosis and mortality in 47,XYY persons: a registry study. Orphanet J Rare Dis. 2010;5:15. doi: 10.1186/1750-1172-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Otter M, Schrander-Stumpel CT, Curfs LM. Triple X syndrome: a review of the literature. Eur J Hum Genet. 2010;18:265–71. doi: 10.1038/ejhg.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bellott DW, Hughes JF, Skaletsky H, Brown LG, Pyntikova T, Cho TJ, et al. Mammalian Y chromosomes retain widely expressed dosage-sensitive regulators. Nature. 2014;508:494–9. doi: 10.1038/nature13206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Migeon BR. Why females are mosaics, X-chromosome inactivation, and sex differences in disease. Gend Med. 2007;4:97–105. doi: 10.1016/s1550-8579(07)80024-6. [DOI] [PubMed] [Google Scholar]
- 55.Sharp A, Robinson D, Jacobs P. Age- and tissue-specific variation of X chromosome inactivation ratios in normal women. Hum Genet. 2000;107:343–9. doi: 10.1007/s004390000382. [DOI] [PubMed] [Google Scholar]
- 56.Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–4. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- 57.Berletch JB, Yang F, Xu J, Carrel L, Disteche CM. Genes that escape from X-inactivation. Hum Genet. 2011;130:237–45. doi: 10.1007/s00439-011-1011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rocca MS, Pecile V, Cleva L, Speltra E, Selice R, Di Mambro A, et al. The Klinefelter syndrome is associated with high recurrence of copy number variations on the X chromosome with a potential role in the clinical phenotype. Andrology. 2016;4:328–34. doi: 10.1111/andr.12146. [DOI] [PubMed] [Google Scholar]
- 59.Tuttelmann F, Gromoll J. Novel genetic aspects of Klinefelter’s syndrome. Mol Hum Reprod. 2010;16:386–95. doi: 10.1093/molehr/gaq019. [DOI] [PubMed] [Google Scholar]
- 60.Migeon BR. X chromosome inactivation: theme and variations. Cytogenet Genome Res. 2002;99:8–16. doi: 10.1159/000071568. [DOI] [PubMed] [Google Scholar]
- 61.Modi DN, Sane S, Bhartiya D. Accelerated germ cell apoptosis in sex chromosome aneuploid fetal human gonads. Mol Hum Reprod. 2003;9:219–25. doi: 10.1093/molehr/gag031. [DOI] [PubMed] [Google Scholar]
- 62.Russell HF, Wallis D, Mazzocco MM, Moshang T, Zackai E, Zinn AR, et al. Increased prevalence of ADHD in Turner syndrome with no evidence of imprinting effects. J Pediatr Psychol. 2006;31:945–55. doi: 10.1093/jpepsy/jsj106. [DOI] [PubMed] [Google Scholar]
- 63.Green T, Bade Shrestha S, Chromik LC, Rutledge K, Pennington BF, Hong DS, et al. Elucidating X chromosome influences on Attention Deficit Hyperactivity Disorder and executive function. J Psychiatr Res. 2015;68:217–25. doi: 10.1016/j.jpsychires.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Green Tamar, Saggar Manish, Ishak Alexandra, Hong David S, Reiss Allan L. X-Chromosome Effects on Attention Networks: Insights from Imaging Resting-State Networks in Turner Syndrome. Cerebral Cortex. 2017;28(9):3176–3183. doi: 10.1093/cercor/bhx188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marco EJ, Skuse DH. Autism-lessons from the X chromosome. Soc Cogn Affect Neurosci. 2006;1:183–93. doi: 10.1093/scan/nsl028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kilic BG, Ergur AT, Ocal G. Depression, levels of anxiety and self-concept in girls with Turner’s syndrome. J Pediatr Endocrinol Metab. 2005;18:1111–7. doi: 10.1515/jpem.2005.18.11.1111. [DOI] [PubMed] [Google Scholar]
- 67.Manning JT, Kilduff LP, Trivers R. Digit ratio (2D:4D) in Klinefelter’s syndrome. Andrology. 2013;1:94–99. doi: 10.1111/j.2047-2927.2012.00013.x. [DOI] [PubMed] [Google Scholar]
- 68.Ross JL, Samango-Sprouse C, Lahlou N, Kowal K, Elder FF, Zinn A. Early androgen deficiency in infants and young boys with 47,XXY Klinefelter syndrome. Horm Res. 2005;64:39–45. doi: 10.1159/000087313. [DOI] [PubMed] [Google Scholar]
- 69.Mandoki MW, Sumner GS, Hoffman RP, Riconda DL. A review of Klinefelter’s syndrome in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1991;30:167–72. doi: 10.1097/00004583-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 70.Tartaglia NR, Ayari N, Hutaff-Lee C, Boada R. Attention-deficit hyperactivity disorder symptoms in children and adolescents with sex chromosome aneuploidy: XXY, XXX, XYY, and XXYY. J Dev Behav Pediatr. 2012;33:309–18. doi: 10.1097/DBP.0b013e31824501c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bruining H, Swaab H, Kas M, van Engeland H. Psychiatric characteristics in a self-selected sample of boys with Klinefelter syndrome. Pediatrics. 2009;123:e865–870. doi: 10.1542/peds.2008-1954. [DOI] [PubMed] [Google Scholar]
- 72.Cederlof M, Ohlsson Gotby A, Larsson H, Serlachius E, Boman M, Langstrom N, et al. Klinefelter syndrome and risk of psychosis, autism and ADHD. J Psychiatr Res. 2014;48:128–30. doi: 10.1016/j.jpsychires.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 73.Bardsley MZ, Kowal K, Levy C, Gosek A, Ayari N, Tartaglia N, et al. 47,XYY syndrome: clinical phenotype and timing of ascertainment. J Pediatr. 2013;163:1085–94. doi: 10.1016/j.jpeds.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ross JL, Tartaglia N, Merry DE, Dalva M, Zinn AR. Behavioral phenotypes in males with XYY and possible role of increased NLGN4Y expression in autism features. Genes Brain Behav. 2015;14:137–44. doi: 10.1111/gbb.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ross JL, Zeger MP, Kushner H, Zinn AR, Roeltgen DP. An extra X or Y chromosome: contrasting the cognitive and motor phenotypes in childhood in boys with 47,XYY syndrome or 47,XXY Klinefelter syndrome. Dev Disabil Res Rev. 2009;15:309–17. doi: 10.1002/ddrr.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cordeiro L, Tartaglia N, Roeltgen D, Ross J. Social deficits in male children and adolescents with sex chromosome aneuploidy: a comparison of XXY, XYY, and XXYY syndromes. Res Dev Disabil. 2012;33:1254–63. doi: 10.1016/j.ridd.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Assaf M, Jagannathan K, Calhoun VD, Miller L, Stevens MC, Sahl R, et al. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. Neuroimage. 2010;53:247–56. doi: 10.1016/j.neuroimage.2010.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Minshew NJ, Keller TA. The nature of brain dysfunction in autism: functional brain imaging studies. Curr Opin Neurol. 2010;23:124–30. doi: 10.1097/WCO.0b013e32833782d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bush G. Cingulate, frontal, and parietal cortical dysfunction in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69:1160–7. doi: 10.1016/j.biopsych.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Castellanos FX, Proal E. Large-scale brain systems in ADHD: beyond the prefrontal–striatal model. Trends Cogn Sci. 2012;16:17–26. doi: 10.1016/j.tics.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, et al. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am J Psychiatry. 2012;169:1038–55. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Anagnostou E, Taylor MJ. Review of neuroimaging in autism spectrum disorders: what have we learned and where we go from here. Mol Autism. 2011;2:4. doi: 10.1186/2040-2392-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hong DS, Bray S, Haas BW, Hoeft F, Reiss AL. Aberrant neurocognitive processing of fear in young girls with Turner syndrome. Soc Cogn Affect Neurosci. 2014;9:255–64. doi: 10.1093/scan/nss133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hong DS, Hoeft F, Marzelli MJ, Lepage JF, Roeltgen D, Ross J, et al. Influence of the X-chromosome on neuroanatomy: evidence from Turner and Klinefelter syndromes. J Neurosci. 2014;34:3509–16. doi: 10.1523/JNEUROSCI.2790-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brandenburg-Goddard MN, van Rijn S, Rombouts SARB, Veer IM, Swaab H. A comparison of neural correlates underlying social cognition in Klinefelter syndrome and autism. Soc Cogn Affect Neurosci. 2014;9:1926–33. doi: 10.1093/scan/nst190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Raznahan A, Lee NR, Greenstein D, Wallace GL, Blumenthal JD, Clasen LS, et al. Globally divergent but locally convergent X- and Y-chromosome influences on cortical development. Cereb Cortex. 2016;26:70–79. doi: 10.1093/cercor/bhu174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin A, Clasen L, Lee NR, Wallace GL, Lalonde F, Blumenthal J, et al. Mapping the stability of human brain asymmetry across five sex-chromosome aneuploidies. J Neurosci. 2015;35:140–5. doi: 10.1523/JNEUROSCI.3489-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Raznahan A, Lue Y, Probst F, Greenstein D, Giedd J, Wang C, et al. Triangulating the sexually dimorphic brain through high-resolution neuroimaging of murine sex chromosome aneuploidies. Brain Struct Funct. 2015;220:3581–93. doi: 10.1007/s00429-014-0875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Raznahan A, Probst F, Palmert MR, Giedd JN, Lerch JP. High resolution whole brain imaging of anatomical variation in XO, XX, and XY mice. Neuroimage. 2013;83:962–8. doi: 10.1016/j.neuroimage.2013.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lubs HA, Stevenson RE, Schwartz CE. Fragile X and X-linked intellectual disability: four decades of discovery. Am J Hum Genet. 2012;90:579–90. doi: 10.1016/j.ajhg.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tzschach A, Grasshoff U, Beck-Woedl S, Dufke C, Bauer C, Kehrer M, et al. Next-generation sequencing in X-linked intellectual disability. Eur J Hum Genet. 2015;23:1513–8. doi: 10.1038/ejhg.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Caglayan AO. Genetic causes of syndromic and non-syndromic autism. Dev Med Child Neurol. 2010;52:130–8. doi: 10.1111/j.1469-8749.2009.03523.x. [DOI] [PubMed] [Google Scholar]
- 93.Chaste P, Leboyer M. Autism risk factors: genes, environment, and gene–environment interactions. Dialog Clin Neurosci. 2012;14:281–92. doi: 10.31887/DCNS.2012.14.3/pchaste. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ross JL, Roeltgen DP, Stefanatos G, Benecke R, Zeger MP, Kushner H, et al. Cognitive and motor development during childhood in boys with Klinefelter syndrome. Am J Med Genet A. 2008;146A:708–19. doi: 10.1002/ajmg.a.32232. [DOI] [PubMed] [Google Scholar]
- 95.Boone KB, Swerdloff RS, Miller BL, Geschwind DH, Razani J, Lee A, et al. Neuropsychological profiles of adults with Klinefelter syndrome. J Int Neuropsychol Soc. 2001;7:446–56. doi: 10.1017/s1355617701744013. [DOI] [PubMed] [Google Scholar]
- 96.Geerts M, Steyaert J, Fryns JP. The XYY syndrome: a follow-up study on 38 boys. Genet Counsel. 2003;14:267–79. [PubMed] [Google Scholar]
- 97.Martinussen R, Hayden J, Hogg-Johnson S, Tannock R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:377–84. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- 98.DiCicco-Bloom E, Lord C, Zwaigenbaum L, Courchesne E, Dager SR, Schmitz C, et al. The developmental neurobiology of autism spectrum disorder. J Neurosci. 2006;26:6897–906. doi: 10.1523/JNEUROSCI.1712-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim YS, Leventhal BL, Koh YJ, Fombonne E, Laska E, Lim EC, et al. Prevalence of autism spectrum disorders in a total population sample. Am J Psychiatry. 2011;168:904–12. doi: 10.1176/appi.ajp.2011.10101532. [DOI] [PubMed] [Google Scholar]
- 100.Christensen DL, Baio J, Van Naarden Braun K, Bilder D, Charles J, Constantino JN, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2012. MMWR Surveill Summ. 2016;65:1–23. doi: 10.15585/mmwr.ss6503a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fombonne E. Epidemiology of pervasive developmental disorders. Pediatr Res. 2009;65:591–8. doi: 10.1203/PDR.0b013e31819e7203. [DOI] [PubMed] [Google Scholar]
- 102.Tartaglia NR, Wilson R, Miller JS, Rafalko J, Cordeiro L, Davis S, et al. Autism spectrum disorder in males with sex chromosome aneuploidy: XXY/Klinefelter syndrome, XYY, and XXYY. J Dev Behav Pediatr. 2017;38:197–207. doi: 10.1097/DBP.0000000000000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68:1095–102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mandy W, Lai MC. Annual Research Review: the role of the environment in the developmental psychopathology of autism spectrum condition. J Child Psychol Psychiatry. 2016;57:271–92. doi: 10.1111/jcpp.12501. [DOI] [PubMed] [Google Scholar]
- 105.Ornoy A, Weinstein-Fudim L, Ergaz Z. Genetic syndromes, maternal diseases and antenatal factors associated with autism spectrum disorders (ASD) Front Neurosci. 2016;10:316. doi: 10.3389/fnins.2016.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McCauley E, Feuillan P, Kushner H, Ross JL. Psychosocial development in adolescents with Turner syndrome. J Dev Behav Pediatr. 2001;22:360–5. doi: 10.1097/00004703-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 107.McCauley E, Sybert VP, Ehrhardt AA. Psychosocial adjustment of adult women with Turner syndrome. Clin Genet. 1986;29:284–90. doi: 10.1111/j.1399-0004.1986.tb01256.x. [DOI] [PubMed] [Google Scholar]
- 108.Rovet J, Ireland L. Behavioral phenotype in children with Turner syndrome. J Pediatr Psychol. 1994;19:779–90. doi: 10.1093/jpepsy/19.6.779. [DOI] [PubMed] [Google Scholar]
- 109.Lagrou K, Froidecoeur C, Verlinde F, Craen M, De Schepper J, Francois I, et al. Psychosocial functioning, self-perception and body image and their auxologic correlates in growth hormone and oestrogen-treated young adult women with Turner syndrome. Horm Res. 2006;66:277–84. doi: 10.1159/000095547. [DOI] [PubMed] [Google Scholar]
- 110.Mazzola F, Seigal A, MacAskill A, Corden B, Lawrence K, Skuse DH. Eye tracking and fear recognition deficits in Turner syndrome. Soc Neurosci. 2006;1:259–69. doi: 10.1080/17470910600989912. [DOI] [PubMed] [Google Scholar]
- 111.McCauley E, Kay T, Ito J, Treder R. The Turner syndrome: cognitive deficits, affective discrimination, and behavior problems. Child Dev. 1987;58:464–73. [PubMed] [Google Scholar]
- 112.Romans SM, Stefanatos G, Roeltgen DP, Kushner H, Ross JL. Transition to young adulthood in Ullrich–Turner syndrome: neurodevelopmental changes. Am J Med Genet. 1998;79:140–7. [PubMed] [Google Scholar]
- 113.Ross JL, Kushner H, Zinn AR. Discriminant analysis of the Ullrich–Turner syndrome neurocognitive profile. Am J Med Genet. 1997;72:275–80. doi: 10.1002/(sici)1096-8628(19971031)72:3<275::aid-ajmg4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 114.Elgar K, Campbell R, Skuse D. Are you looking at me? Accuracy in processing line-of-sight in Turner syndrome. Proc Biol Sci. 2002;269:2415–22. doi: 10.1098/rspb.2002.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lepage JF, Hong DS, Raman M, Marzelli M, Roeltgen DP, Lai S, et al. Brain morphology in children with 47, XYY syndrome: a voxel- and surface-based morphometric study. Genes Brain Behav. 2014;13:127–34. doi: 10.1111/gbb.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ross JL, Roeltgen DP, Kushner H, Zinn AR, Reiss A, Bardsley MZ, et al. Behavioral and social phenotypes in boys with 47,XYY syndrome or 47,XXY Klinefelter syndrome. Pediatrics. 2012;129:769–78. doi: 10.1542/peds.2011-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ratcliffe S. Long term outcome in children of sex chromosome abnormalities. Arch Dis Child. 1999;80:192–5. doi: 10.1136/adc.80.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Simm PJ, Zacharin MR. The psychosocial impact of Klinefelter syndrome—a 10 year review. J Pediatr Endocrinol Metab. 2006;19:499–505. [PubMed] [Google Scholar]
- 119.van Rijn S, Swaab H, Aleman A, Kahn RS. Social behavior and autism traits in a sex chromosomal disorder: Klinefelter (47XXY) syndrome. J Autism Dev Disord. 2008;38:1634–41. doi: 10.1007/s10803-008-0542-1. [DOI] [PubMed] [Google Scholar]
- 120.Geschwind DH, Dykens E. Neurobehavioral and psychosocial issues in Klinefelter syndrome. Learn Disabil Res Pract. 2004;19:166–73. [Google Scholar]
- 121.van Rijn S, Aleman A, Swaab H, Krijn T, Vingerhoets G, Kahn R. What it is said versus how it is said: comprehension of affective prosody in men with Klinefelter (47,XXY) syndrome. J Int Neuropsychol Soc. 2007;13:1065–70. doi: 10.1017/S1355617707071044. [DOI] [PubMed] [Google Scholar]
- 122.van Rijn S, Barendse M, van Goozen S, Swaab H. Social attention, affective arousal and empathy in men with Klinefelter syndrome (47,XXY): evidence from eyetracking and skin conductance. PLoS ONE. 2014;9:e84721. doi: 10.1371/journal.pone.0084721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.van Rijn S, Swaab H, Aleman A, Kahn RS. X chromosomal effects on social cognitive processing and emotion regulation: a study with Klinefelter men (47,XXY) Schizophr Res. 2006;84:194–203. doi: 10.1016/j.schres.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 124.Otter M, Schrander-Stumpel CT, Didden R, Curfs LM. The psychiatric phenotype in triple X syndrome: new hypotheses illustrated in two cases. Dev Neurorehabil. 2012;15:233–8. doi: 10.3109/17518423.2012.655799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee NR, Wallace GL, Adeyemi EI, Lopez KC, Blumenthal JD, Clasen LS, et al. Dosage effects of X and Y chromosomes on language and social functioning in children with supernumerary sex chromosome aneuploidies: implications for idiopathic language impairment and autism spectrum disorders. J Child Psychol Psychiatry. 2012;53:1072–81. doi: 10.1111/j.1469-7610.2012.02573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wolraich ML, McKeown RE, Visser SN, Bard D, Cuffe S, Neas B, et al. The prevalence of ADHD: its diagnosis and treatment in four school districts across two states. J Atten Disord. 2014;18:563–75. doi: 10.1177/1087054712453169. [DOI] [PubMed] [Google Scholar]
- 127.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 128.Visser SN, Danielson ML, Bitsko RH, Holbrook JR, Kogan MD, Ghandour RM, et al. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003–11. J Am Acad Child Adolesc Psychiatry. 2014;53:34–46 e32. doi: 10.1016/j.jaac.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Willcutt EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics. 2012;9:490–9. doi: 10.1007/s13311-012-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Biederman J, Faraone SV, Mick E, Williamson S, Wilens TE, Spencer TJ, et al. Clinical correlates of ADHD in females: findings from a large group of girls ascertained from pediatric and psychiatric referral sources. J Am Acad Child Adolesc Psychiatry. 1999;38:966–75. doi: 10.1097/00004583-199908000-00012. [DOI] [PubMed] [Google Scholar]
- 131.Martel MM. Research review: a new perspective on attention-deficit/hyperactivity disorder: emotion dysregulation and trait models. J Child Psychol Psychiatry. 2009;50:1042–51. doi: 10.1111/j.1469-7610.2009.02105.x. [DOI] [PubMed] [Google Scholar]
- 132.Biederman J, Petty CR, Doyle AE, Spencer T, Henderson CS, Marion B, et al. Stability of executive function deficits in girls with ADHD: a prospective longitudinal follow up study into adolescence. Dev Neuropsychol. 2008;33:44–61. doi: 10.1080/87565640701729755. [DOI] [PubMed] [Google Scholar]
- 133.Williams JK. Behavioral characteristics of children with Turner syndrome and children with learning disabilities. West J Nurs Res. 1994;16:26–35. doi: 10.1177/019394599401600103. [DOI] [PubMed] [Google Scholar]
- 134.Tamm L, Menon V, Reiss AL. Abnormal prefrontal cortex function during response inhibition in Turner syndrome: functional magnetic resonance imaging evidence. Biol Psychiatry. 2003;53:107–11. doi: 10.1016/s0006-3223(02)01488-9. [DOI] [PubMed] [Google Scholar]
- 135.Lepage JF, Dunkin B, Hong DS, Reiss AL. Impact of cognitive profile on social functioning in prepubescent females with Turner syndrome. Child Neuropsychol. 2013;19:161–72. doi: 10.1080/09297049.2011.647900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Temple CM, Sanfilippo PM. Executive skills in Klinefelter’s syndrome. Neuropsychologia. 2003;41:1547–59. doi: 10.1016/s0028-3932(03)00061-7. [DOI] [PubMed] [Google Scholar]
- 137.Kompus K, Westerhausen R, Nilsson LG, Hugdahl K, Jongstra S, Berglund A, et al. Deficits in inhibitory executive functions in Klinefelter (47, XXY) syndrome. Psychiatry Res. 2011;189:135–40. doi: 10.1016/j.psychres.2011.02.028. [DOI] [PubMed] [Google Scholar]
- 138.Lee NR, Anand P, Will E, Adeyemi EI, Clasen LS, Blumenthal JD, et al. Everyday executive functions in Down syndrome from early childhood to young adulthood: evidence for both unique and shared characteristics compared to youth with sex chromosome trisomy (XXX and XXY) Front Behav Neurosci. 2015;9:264. doi: 10.3389/fnbeh.2015.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lee NR, Wallace GL, Clasen LS, Lenroot RK, Blumenthal JD, White SL, et al. Executive function in young males with Klinefelter (XXY) syndrome with and without comorbid attention-deficit/hyperactivity disorder. J Int Neuropsychol Soc. 2011;17:522–30. doi: 10.1017/S1355617711000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ruud A, Arnesen P, Stray LL, Vildalen S, Vesterhus P. Stimulant medication in 47,XYY syndrome: a report of two cases. Dev Med Child Neurol. 2005;47:559–62. doi: 10.1017/s001216220500109x. [DOI] [PubMed] [Google Scholar]
- 141.Pennington BF, Puck M, Robinson A. Language and cognitive development in 47,XXX females followed since birth. Behav Genet. 1980;10:31–41. doi: 10.1007/BF01067317. [DOI] [PubMed] [Google Scholar]
- 142.Bender Bruce G., Linden Mary G., Robinson Arthur. Neuropsychological impairment in 42 adolescents with sex chromosome abnormalities. American Journal of Medical Genetics. 1993;48(3):169–173. doi: 10.1002/ajmg.1320480312. [DOI] [PubMed] [Google Scholar]
- 143.Nature Medicine Editorial. Accounting for sex in the genome. Nat Med. 2017;23:1243. doi: 10.1038/nm.4445. [DOI] [PubMed] [Google Scholar]
- 144.Wise AL, Gyi L, Manolio TA. eXclusion: toward integrating the X chromosome in genome-wide association analyses. Am J Hum Genet. 2013;92:643–7. doi: 10.1016/j.ajhg.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bonas-Guarch S, Guindo-Martinez M, Miguel-Escalada I, Grarup N, Sebastian D, Rodriguez-Fos E, et al. Re-analysis of public genetic data reveals a rare X-chromosomal variant associated with type 2 diabetes. Nat Commun. 2018;9:321. doi: 10.1038/s41467-017-02380-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hoffmann TJ, Passarelli MN, Graff RE, Emami NC, Sakoda LC, Jorgenson E, et al. Genome-wide association study of prostate-specific antigen levels identifies novel loci independent of prostate cancer. Nat Commun. 2017;8:14248. doi: 10.1038/ncomms14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Skare O, Gjessing HK, Gjerdevik M, Haaland OA, Romanowska J, Lie RT, et al. A new approach to chromosome-wide analysis of X-linked markers identifies new associations in Asian and European case–parent triads of orofacial clefts. PLoS ONE. 2017;12:e0183772. doi: 10.1371/journal.pone.0183772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lim GY, Tam WW, Lu Y, Ho CS, Zhang MW, Ho RC. Prevalence of depression in the community from 30 countries between 1994 and 2014. Sci Rep. 2018;8:2861. doi: 10.1038/s41598-018-21243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gater R, Tansella M, Korten A, Tiemens BG, Mavareas VG, O’latawura MO. Sex differences in the prevalence and detection of depressive and anxiety disorders in general health care settings: report from the World Health Organization Collaborative Study on Psychological Problems in General Health Care. Arch Gen Psychiatry. 1998;55:405–13. doi: 10.1001/archpsyc.55.5.405. [DOI] [PubMed] [Google Scholar]
- 150.Kesler S, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1994;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- 151.Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RA, Updegraff JA. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychol Rev. 2000;107:411–29. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- 152.Tolin DF, Foa EB. Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychol Bull. 2006;132:959–92. doi: 10.1037/0033-2909.132.6.959. [DOI] [PubMed] [Google Scholar]
- 153.Young E, Korszun A. Sex, trauma, stress hormones and depression. Mol Psychiatry. 2010;15:23–28. doi: 10.1038/mp.2009.94. [DOI] [PubMed] [Google Scholar]
- 154.Altemus M, Sarvaiya N, Neill Epperson C. Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol. 2014;35:320–30. doi: 10.1016/j.yfrne.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Freeman MP. Depression: what’s sex got to do with it? J Clin Psychiatry. 2006;67:1610–1. doi: 10.4088/jcp.v67n1017. [DOI] [PubMed] [Google Scholar]
- 156.Hankin BL, Mermelstein R, Roesch L. Sex differences in adolescent depression: stress exposure and reactivity models. Child Dev. 2007;78:279–95. doi: 10.1111/j.1467-8624.2007.00997.x. [DOI] [PubMed] [Google Scholar]
- 157.Kendler KS, Thornton LM, Prescott CA. Gender differences in the rates of exposure to stressful life events and sensitivity to their depressogenic effects. Am J Psychiatry. 2001;158:587–93. doi: 10.1176/appi.ajp.158.4.587. [DOI] [PubMed] [Google Scholar]
- 158.Angold A, Costello EJ, Erkanli A, Worthman CM. Pubertal changes in hormone levels and depression in girls. Psychol Med. 1999;29:1043–53. doi: 10.1017/s0033291799008946. [DOI] [PubMed] [Google Scholar]
- 159.Cohen LS, Altshuler LL, Harlow BL, Nonacs R, Newport DJ, Viguera AC, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295:499–507. doi: 10.1001/jama.295.5.499. [DOI] [PubMed] [Google Scholar]
- 160.Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB, Hollander L. Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch Gen Psychiatry. 2004;61:62–70. doi: 10.1001/archpsyc.61.1.62. [DOI] [PubMed] [Google Scholar]
- 161.O’Hara MW. Social support, life events, and depression during pregnancy and the puerperium. Arch Gen Psychiatry. 1986;43:569–73. doi: 10.1001/archpsyc.1986.01800060063008. [DOI] [PubMed] [Google Scholar]
- 162.O’Hara MW, Schlechte JA, Lewis DA, Varner MW. Controlled prospective study of postpartum mood disorders: psychological, environmental, and hormonal variables. J Abnorm Psychol. 1991;100:63–73. doi: 10.1037//0021-843x.100.1.63. [DOI] [PubMed] [Google Scholar]
- 163.Fernandez-Guasti A, Fiedler JL, Herrera L, Handa RJ. Sex, stress, and mood disorders: at the intersection of adrenal and gonadal hormones. Horm Metab Res. 2012;44:607–18. doi: 10.1055/s-0032-1312592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li SH, Graham BM. Why are women so vulnerable to anxiety, trauma-related and stress-related disorders? The potential role of sex hormones. Lancet Psychiatry. 2017;4:73–82. doi: 10.1016/S2215-0366(16)30358-3. [DOI] [PubMed] [Google Scholar]
- 165.Klein Karen O, Rosenfield Robert L, Santen Richard J, Gawlik Aneta M, Backeljauw Philippe F, Gravholt Claus H, Sas Theo C J, Mauras Nelly. Estrogen Replacement in Turner Syndrome: Literature Review and Practical Considerations. The Journal of Clinical Endocrinology & Metabolism. 2018;103(5):1790–1803. doi: 10.1210/jc.2017-02183. [DOI] [PubMed] [Google Scholar]
- 166.Seney ML, Ekong KI, Ding Y, Tseng GC, Sibille E. Sex chromosome complement regulates expression of mood-related genes. Biol Sex Differ. 2013;4:20. doi: 10.1186/2042-6410-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Arnold AP. A general theory of sexual differentiation. J Neurosci Res. 2017;95:291–300. doi: 10.1002/jnr.23884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rickert VI, Hassed SJ, Hendon AE, Cunniff C. The effects of peer ridicule on depression and self-image among adolescent females with Turner syndrome. J Adolesc Health. 1996;19:34–38. doi: 10.1016/1054-139X(95)00225-H. [DOI] [PubMed] [Google Scholar]
- 169.Schmidt PJ, Cardoso GM, Ross JL, Haq N, Rubinow DR, Bondy CA. Shyness, social anxiety, and impaired self-esteem in Turner syndrome and premature ovarian failure. JAMA. 2006;295:1374–6. doi: 10.1001/jama.295.12.1374. [DOI] [PubMed] [Google Scholar]
- 170.Cardoso G, Daly R, Haq NA, Hanton L, Rubinow DR, Bondy CA, et al. Current and lifetime psychiatric illness in women with Turner syndrome. Gynecol Endocrinol. 2004;19:313–9. [PubMed] [Google Scholar]
- 171.Davenport ML. Approach to the patient with Turner syndrome. J Clin Endocrinol Metab. 2010;95:1487–95. doi: 10.1210/jc.2009-0926. [DOI] [PubMed] [Google Scholar]
- 172.Shea AK, Wolfman W. The role of hormone therapy in the management of severe postpartum depression in patients with Turner syndrome. Menopause. 2017;24:1309–12. doi: 10.1097/GME.0000000000000915. [DOI] [PubMed] [Google Scholar]
- 173.Turriff A, Levy HP, Biesecker B. Prevalence and psychosocial correlates of depressive symptoms among adolescents and adults with Klinefelter syndrome. Genet Med. 2011;13:966–72. doi: 10.1097/GIM.0b013e3182227576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ross JL, Kushner H, Kowal K, Bardsley M, Davis S, Reiss AL, et al. Androgen treatment effects on motor function, cognition, and behavior in boys with Klinefelter syndrome. J Pediatr. 2017;185:193–9 e194. doi: 10.1016/j.jpeds.2017.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Linden MG, Bender BG, Harmon RJ, Mrazek DA, Robinson A. 47,XXX: what is the prognosis? Pediatrics. 1988;82:619–30. [PubMed] [Google Scholar]
- 176.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–41. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Vogelzangs N, Beekman AT, de Jonge P, Penninx BW. Anxiety disorders and inflammation in a large adult cohort. Transl Psychiatry. 2013;3:e249. doi: 10.1038/tp.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dantzer R, O’Connor JC, Lawson MA, Kelley KW. Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology. 2011;36:426–36. doi: 10.1016/j.psyneuen.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Carta MG, Hardoy MC, Carpiniello B, Murru A, Marci AR, Carbone F, et al. A case control study on psychiatric disorders in Hashimoto disease and Euthyroid Goitre: not only depressive but also anxiety disorders are associated with thyroid autoimmunity. Clin Pract Epidemiol Ment Health. 2005;1:23. doi: 10.1186/1745-0179-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ainiala H, Loukkola J, Peltola J, Korpela M, Hietaharju A. The prevalence of neuropsychiatric syndromes in systemic lupus erythematosus. Neurology. 2001;57:496–500. doi: 10.1212/wnl.57.3.496. [DOI] [PubMed] [Google Scholar]
- 181.Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8:737–44. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Case LK, Wall EH, Dragon JA, Saligrama N, Krementsov DN, Moussawi M, et al. The Y chromosome as a regulatory element shaping immune cell transcriptomes and susceptibility to autoimmune disease. Genome Res. 2013;23:1474–85. doi: 10.1101/gr.156703.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bianchi I, Lleo A, Gershwin ME, Invernizzi P. The X chromosome and immune associated genes. J Autoimmun. 2012;38:J187–192. doi: 10.1016/j.jaut.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 184.Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2:777–80. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- 185.Sawalha AH, Harley JB, Scofield RH. Autoimmunity and Klinefelter’s syndrome: when men have two X chromosomes. J Autoimmun. 2009;33:31–34. doi: 10.1016/j.jaut.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jorgensen KT, Rostgaard K, Bache I, Biggar RJ, Nielsen NM, Tommerup N, et al. Autoimmune diseases in women with Turner’s syndrome. Arthritis Rheum. 2010;62:658–66. doi: 10.1002/art.27270. [DOI] [PubMed] [Google Scholar]
- 187.Staii A, Mirocha S, Todorova-Koteva K, Glinberg S, Jaume JC. Hashimoto thyroiditis is more frequent than expected when diagnosed by cytology which uncovers a pre-clinical state. Thyroid Res. 2010;3:11. doi: 10.1186/1756-6614-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Vanderpump MP. The epidemiology of thyroid disease. Br Med Bull. 2011;99:39–51. doi: 10.1093/bmb/ldr030. [DOI] [PubMed] [Google Scholar]
- 189.Witkowska-Sedek E, Borowiec A, Kucharska A, Chacewicz K, Ruminska M, Demkow U, et al. Thyroid autoimmunity in girls with Turner syndrome. Adv Exp Med Biol. 2017;1022:71–76. doi: 10.1007/5584_2017_42. [DOI] [PubMed] [Google Scholar]
- 190.Livadas S, Xekouki P, Fouka F, Kanaka-Gantenbein C, Kaloumenou I, Mavrou A, et al. Prevalence of thyroid dysfunction in Turner’s syndrome: a long-term follow-up study and brief literature review. Thyroid. 2005;15:1061–6. doi: 10.1089/thy.2005.15.1061. [DOI] [PubMed] [Google Scholar]
- 191.Rose NR. The genetics of autoimmune thyroiditis: the first decade. J Autoimmun. 2011;37:88–94. doi: 10.1016/j.jaut.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Seminog OO, Seminog AB, Yeates D, Goldacre MJ. Associations between Klinefelter’s syndrome and autoimmune diseases: English national record linkage studies. Autoimmunity. 2015;48:125–8. doi: 10.3109/08916934.2014.968918. [DOI] [PubMed] [Google Scholar]
- 193.Liu K, Kurien BT, Zimmerman SL, Kaufman KM, Taft DH, Kottyan LC, et al. X chromosome dose and sex bias in autoimmune diseases: increased prevalence of 47,XXX in systemic lupus erythematosus and Sjogren’s Syndrome. Arthritis Rheumatol. 2016;68:1290–1300. doi: 10.1002/art.39560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Scofield RH, Bruner GR, Namjou B, Kimberly RP, Ramsey-Goldman R, Petri M, et al. Klinefelter’s syndrome (47,XXY) in male systemic lupus erythematosus patients: support for the notion of a gene-dose effect from the X chromosome. Arthritis Rheum. 2008;58:2511–7. doi: 10.1002/art.23701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Souyris M, Cenac C, Azar P, Daviaud D, Canivet A, Grunenwald S, et al. TLR7 escapes X chromosome inactivation in immune cells. Sci Immunol. 2018;3. A recent study which examines the link between escape from X chromosome inactivation and increased prevalence of SLE in KS. [DOI] [PubMed]
- 196.Konig IR, Loley C, Erdmann J, Ziegler A. How to include chromosome X in your genome-wide association study. Genet Epidemiol. 2014;38:97–103. doi: 10.1002/gepi.21782. [DOI] [PubMed] [Google Scholar]
- 197.Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication-Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49:980–9. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Goldacre MJ, Seminog OO. Turner syndrome and autoimmune diseases: record-linkage study. Arch Dis Child. 2014;99:71–73. doi: 10.1136/archdischild-2013-304617. [DOI] [PubMed] [Google Scholar]