Abstract
AIM:
This study aimed to determine the effect of treadmill treatment on oxidative stress markers and endogenous antioxidant status seen from MDA, GSH, MnSOD enzyme specific activity and blood catalase of obese mice.
MATERIALS AND METHODS:
This research is experimental laboratory research using post-test control design group only. The study lasted for 28 days and was divided into 4 groups of study, i.e., group K (normal control), KP (obesity control), P1 (obesity mice with 1 x 10-minute treadmill treatment a day), and P2 (obesity mice with 2 x 10-minute treadmill treatment a day).
RESULTS:
The treadmill treatment had an effect on the improvement of the oxidative status of mice with a decrease of MDA level of obesity mice blood (p ≤ 0.05) compared to KP control. An elevated endogenous antioxidant status of obesity mice was seen from elevated GSH levels, MnSOD specific activity and blood catalase of obesity mice (p ≤ 0.05) compared with KP controls. Treatment of 1 x 10-minute treadmill per day decreased blood MDA level, increased GSH enzyme and increased specific activity of MnSOD enzyme and blood catalase of obese mice.
CONCLUSIONS:
The 2 x 10-minute daily treadmill did not differ significantly in improving the oxidative status and endogenous antioxidant status compared with the treadmill 1 x 10 minutes a day (p ≥ 0.05).
Keywords: Obesity, Treadmill, MDA, GSH, MnSOD, Catalase
Introduction
Obesity is a complex disorder of appetite regulation and energy metabolism that is controlled by some specific biological factors. Physiologically, obesity is defined as a state with excessive fat accumulation [1]. Obesity increases because of a high-fat diet, lack of fibre, and lack of physical activity [2]. The prevalence of obesity around the world is always increasing year by year. The World Health Organization (WHO) in 2013 noted that around one billion people worldwide are overweight and at least 300 million are clinically obese. Obesity and overweight in Indonesia itself are also still high [2] [3].
The state of obesity can trigger the occurrence of oxidative stress conditions due to prooxidant and antioxidant imbalance in the body so that it will form Reactive Oxygen Species (ROS). Obesity occurs excessive lipogenesis and inhibition of lipolysis. Lipogenesis is stimulated by a diet high in carbohydrates. Obesity is closely related to oxidative stress, due to the role of the cyclic AMP (cAMP) in the balance of energy settings in obesity. Markers of oxidative stress include malondialdehyde (MDA) [4] [5].
MDA is a compound resulting from lipid peroxidation. Lipid peroxides are formed as a result of the reaction between free radicals with unsaturated fatty acids or polyunsaturated fatty acids (PUFAs) which are the major elements in cell membranes. Lipid peroxide levels in tissues and blood can be used as an indicator of oxidative stress. Current MDA measurements are often used to determine the extent of damage caused by peroxidation of lipid membranes or lipoproteins [4] [5].
The major antioxidant enzymes that neutralise Reactive Oxygen Species (ROS) are superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx). SOD catalyses superoxide dismutase into H2O2 and oxygen, whereas CAT and GPx neutralize H2O2. The two major SODs in eukaryotes are MnSOD and Cu/ZnSOD found in the cytoplasm. After the increase of adipose tissue, antioxidant enzyme activity such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), were found to be significantly reduced. Finally, high ROS production and decreased antioxidant capacity lead to a variety of disorders, including endothelial dysfunction, characterised by decreased endothelial vasodilation and systemic RBC bioavailability. In the state of obesity can occur chronic inflammatory conditions of low levels with progressive infiltration of immune cells in obese adipose tissue. Cytokines secreted by immune cells and adipokines of adipose tissue promote tissue inflammation [6] [7].
The treadmill has been used as a model for studying aspects of behaviour, physiology, biochemistry and molecular response for acute or chronic physical exercise. The treadmill is one of the tools that can be used to see the physical activity of animal test. The treadmill apparatus in animal testing stamina test has the same working principle as the treadmill used by humans. The test animals ran against the direction of the treadmill at a standardised speed of about 10-18 m/min [8] [9].
Moderate physical exercise in animals with a high-fat diet can provide a protective effect in the development of obesity. Physical exercise triggers adaptation through the defence system against oxidative stress. Obesity has become a global health problem that can cause other health problems. The treadmill is used as a therapy to reduce obesity levels [9].
Figure 1.
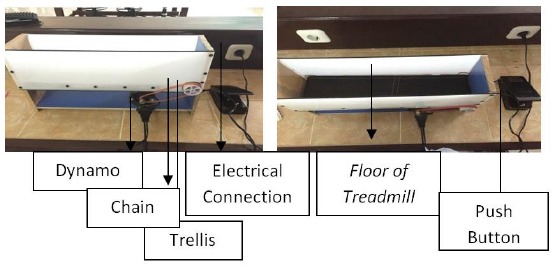
Mice treadmill tool
This study aimed to see the effect of treadmill treatment on the markers of oxidative stress in obese mice, by looking at cell damage markers such as MDA, as well as looking at endogenous antioxidant status by looking at MnSOD, and GSH, MnSOD and specific activity of blood catalase enzymes in obese mice. Expected by treatment of treadmill in obesity mice will be seen changes in oxidative status and endogenous antioxidants with improved oxidation markers and increased antioxidant activity, which has been damaged by the state of obesity.
Material and Methods
This research is experimental research. It was a type of experimental laboratory study using post-test control design group only. In this study, there were 4 study groups, i.e. normal control group (K), obesity control group (KP), treatment group (P1) obesity mice + treadmill treatment 1 time daily for 10 minutes, and treatment group (P2) obesity + mice treadmill 2 times a day every 10 minutes.
The population of this study was male mice (Mus musculus l.) aged 6-8 weeks with an average body weight of normal mice was 20-30 grams, and the average weight of obesity mice was more than 30 grams. The sample used was male mice. The sample size is determined based on the Federer formula - the sample determination formula for the full randomised complete experimental test (RAL). Based on the calculation results obtained the number of samples to be used in each group is 5 male mice (rounding n ≥ 4. 75). The number of samples to be used in the study was 20 male mice.
This study has inclusion criteria for normal mice and obese mice. The inclusion criteria for normal mice consist of this study were male mice, 6-8 weeks old, average weight 20-30 grams, obtained from the same breeding place and maintained at the same place and time. The inclusion criteria for obesity mice were obese male mice, 6-8 weeks old; mean weight > 30 grams were obtained from the same breeding place at the same place and time. Exclusion criteria in this study were weight loss during maintenance process of more than 10%, looked pain during maintenance process (limited motion, dull looking fur, bite wound, liquid faeces) and a dead mouse.
This study used a special treadmill that is connected with electricity. After the treadmill is turned on, the mouse is placed on it and allowed to run for 10 minutes for one treatment in the P1 group and 10 minutes a day twice for the P2 group.
Independent variable (independent variable) in this research is giving a treatment of treadmill and high fat and protein diet to male mouse (Mus musculus l.). The dependent variable in this research is MDA, GSH, MnSOD and catalase levels.
Measurement of MDA content is done by Wills method. The principle is that MDA with thiobarbituric acid will form a pink TBA pigment compound and provide maximum absorption at a wavelength of 530 nm. The absorption of the test solution is compared with the absorption of a standard set of standard solutions that have been known to measure. Measurement of GSH level is done by the Ellman method. The principle is that if glutathione is reacted with the reactant ditiobisnitro benzoate (DTNB), it will produce yellow trinitrobenzene compounds whose absorption can be read by spectrophotometer at 412 nm wavelength. The absorption of the test solution is compared with the uptake of a series of standard GSH solutions that have been identified.
MnSOD activity is determined biochemically using a RanSOD® kit. The reagents in this kit consist of mixed substrates containing xanthine, phosphate buffers to dilute (standard as well as a sample), xanthine oxidase and standard solution to create standard curves. The principle of MnSOD examination using this kit is the measurement of the magnitude of inhibition of superoxide radical formation by MnSOD. Catalase is an enzymatic antioxidant that catalyses the decomposition of H2O2 into H2O and O2 molecules. H2O2 decomposition was observed spectrophotometrically based on decreasing absorption at the maximum wavelength. Measurements of the specific activity of the catalase enzyme are carried out at pH 7 because too acidic or alkaline atmosphere can cause loss of activity of specific catalase enzymes.
Results
This study has received ethical approval from the Medical Research Ethics Commission of the Medical Faculty, University of Lampung No. 2771/UN26/8/DT/2015 dated December 22, 2015. This study is part of another major study on obesity and treadmill.
Before and after treatment, mice were weighed, and body weight data before and after treatment were recorded.
It can be seen that in the treatment of treadmill 1 time a day for 10 minutes can lose weight obesity mice of 6.1 grams and in the treadmill treatment group 2 times a day for 10 minutes can lose weight by 6.8 g (Table 1).
Table 1.
Changes in body weight of mice during the study
| Group K (gr) | Group KP (gr) | Group P1 (gr) | Group P2 (gr) | |
|---|---|---|---|---|
| Average body weight of mice before treatment (D0) | 28.2 | 45.6 | 45.4 | 45.7 |
| Average body weight of mice after treatment (D28) | 32.1 | 50.9 | 39.3 | 38.9 |
| % weight change | +3.9 | +5.3 | -6.1 | -6.8 |
The untreated control group (K) showed a blood MDA level of 4.69 ± 0.19 nmol/mL. In the KP group, there was an elevated blood MDA level of obese mice compared with MDA level of control group mouse blood (K) which was 5.97 ± 1.35 nmol/mL (p ≤ 0,05). In the treatment group P1 and P2, there was a decrease in blood MDA level of obese mice in the KP group, which was 4.82 ± 1.21 nmol/mL and 4.91 ± 0.29 nmol/mL (p ≤ 0.05). More data can be seen in Figure 2.
Figure 2.
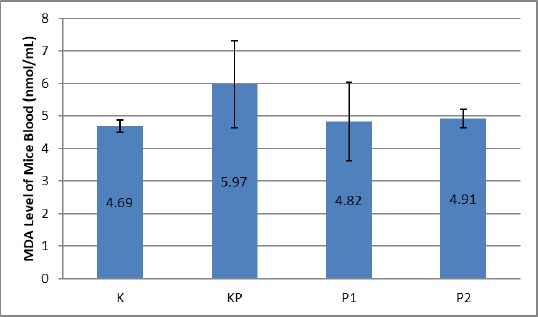
MDA blood levels of obese mice treated treadmill; K: normal control group; KP: obesity control group; P1: treatment group treadmill 1 x 10 minutes daily; P2: Treatment Group Treadmill 2 x 10 minutes daily
Of particular interest were the P1 and P2 groups treated with treadmill 1 x 10 minutes a day and 2 x 10 minutes a day, the examination data showed a significant reduction in mean MDA levels of 4.82 ± 1.21 nmol/mL compared with the control group K and KP p ≤ 0.05). The decrease in blood MDA levels in the treadmill treated group of 1x10 min was almost the same as the P2 group treated with treadmill 2x10 minutes a day. Statistically, they did not show any significant difference (p ≥ 0.05).
Examination of blood GSH levels showed the following results: The untreated control group (K) showed an average blood GSH level of 1.92 ± 0.08 μg/mL. In the positive control group KP, the blood GSH level of obesity mice decreased compared to the K group at 1.42 ± 0.39 μg/mL (p ≤ 0.05). In the P1 and P2 groups, the obese mice treated with treadmill 1 x 10 minutes a day and 2 x 10 minutes a day saw a rise in GSH levels of 1.72 ± 0.44 and 1.69 ± 0.25 μg/mL, which was significantly different (p ≤0.05) compared with the negative control group K and the KP group (p ≤ 0.05).
Figure 3.
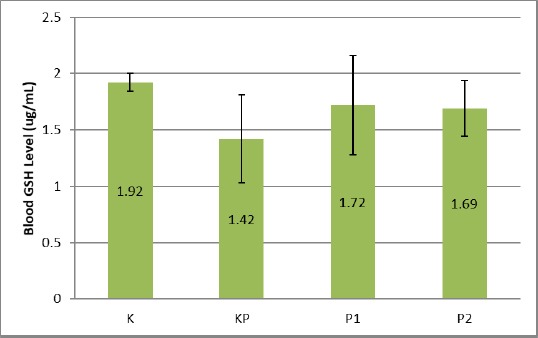
Blood GSH Levels of Obesity Mice; K: Normal Control Group; KP: Obesity Control Group; P1: Treatment Group of Treadmill 1 x 10 minute a day; P2: Treatment Group of Treadmill 2 x 10 minute a day
Interestingly, elevated blood levels of GSH obesity of mice in the P1 and P2 groups did not differ significantly from control group K. The increase in GSH levels in the P1 group was statistically, both of these values, showed significant differences (p ≤ 0.05). Overall GSH levels of Obesity Mice group P1 and P2 were significantly different (p ≤ 0.05) compared to the control group K and KP.
On the examination of specific activity of MnSOD plasma enzyme, it was found that plasma group mice control activity was 0.451 ± 0.321 U/ml, while in control group Obesity Control of MnSOD enzyme activity decreased, by 0.125 ± 0.126 U/ml, but in group P1 and P2 an increase in MnSOD enzyme activity is 0.391 ± 0.095 U/ml, and 0.316 ± 0.233 U/ml. The decrease of specific activity of MnSOD plasma enzyme in the KP group was statistically significant with p-value from 0.05 of 0.000. Increased activity of specific MnSOD enzyme in group P1 and P2 was also statistically significant, that is with p-value of 0.05.
Figure 4.
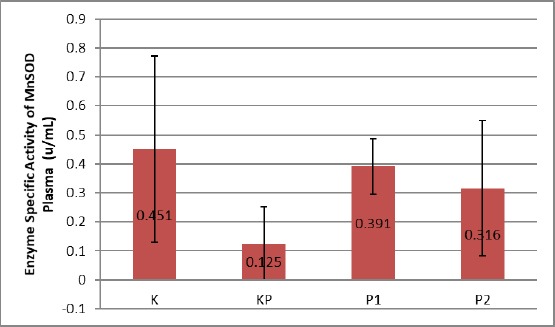
Enzyme Specific Activity of MnSOD Plasma of Obesity Mice who were treated Treadmill; K: Control Group; KP: Obesity Control Group; P1: Treatment Group of Treadmill 1 x 10 minute a day; P2: Treatment Group of Treadmill 2 x 10 minute a day
The specific activity of the MnSOD enzyme in the plasma of obese mice in the KP group decreased compared with control K. The decrease was statistically significant (p ≤ 0.05). In the P1 and P2 groups, the specific activity of the MnSOD plasma enzyme increased compared with the KP group. This increase was also statistically significant compared with control K (p ≤ 0.05) and against the KP group (p ≤ 0.05).
Examination of the specific activity of the blood catalase enzyme of obese mice showed the following results: The untreated normal control group K showed the average activity of blood enzyme catalase 0.96 ± 0.22 U/mg. In the positive control group of KP, the specific activity of blood enzyme catalase enzyme obesity mice decreased compared to group K that is 0.54 ± 0.41 U/mg (p ≤ 0.05). In the P1 and P2 groups, obesity mice treated with treadmill 1 x 10 minutes a day and 2 x 10 minutes a day saw an increase in the specific activity of catalase enzymes by 0.88 ± 0.29 and 0.75 ± 0.46 U/mg, which was significantly different (p ≤ 0.05) compared with the negative control group K and the positive control group KP (p ≤ 0.05).
Figure 5.
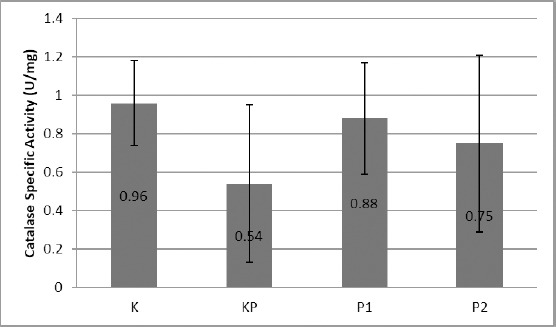
Activity of Catalase Enzyme Specific of Plasma Obesity Mice who were treated of Treadmill; K: Control Group; KP: Obesity Control Group; P1: Treatment Group of Treadmill 1 x 10 minute a day; P2: Treatment Group of Treadmill 2 x 10 minute a day
Discussion
Obesity is associated with a low-level chronic inflammatory condition with progressive infiltration of immune cells in adipose obesity tissue. Cytokines secreted by immune cells and adipokines of adipose tissue promote tissue inflammation. Obesity is a chronic disease with multifactorial causes, and this can be defined as an increase in body fat accumulation. Adipose tissue is not just a triglyceride storage organ, but studies have shown the role of white adipose tissue as a producer of certain bioactive substances called adipokines. These adipokines induce the production of reactive oxygen species (ROS), producing a process known as oxidative stress (OS). One marker of cellular damage due to oxidative stress is malondialdehyde (MDA) [6] [9].
When obesity persists for a long time, the source of endogenous antioxidants may decline, such as enzyme activity such as Glutathione (GSH), superoxide dismutase (MnSOD) and catalase (CAT). SOD and glutathione peroxidase (GPx) activity in individuals with obesity was significantly lower than in healthy people [10] [11].
Inflammation as a manifestation of increased oxidative stress, which increases in a person with obesity. The inflammatory mechanisms of obesity are related to the presence of adipose tissue that produces adipokine and acute phase proteins induced by hypoxia. Hypoxia will be generated during the overgrowth of adipose tissue during obesity. Adipose tissue produces 25% systemic IL-6, so this adipose tissue can cause low-grade systemic inflammation in people with excess body fat [12] [13] [14].
The mechanism of free radical formation in obesity is the increase of proinflammatory cytokines produced by adipocytes and preadipocytes such as TNF-α, IL-1, and IL-6. This cytokine is a potent stimulator for the production of reactive oxygen and nitrogen by macrophages and monocytes. TNF-α increases the interaction of electrons with oxygen to produce superoxide anions. Adipose tissue also has an angiotensin II secretion capacity, which stimulates the oxidation activity of Nicotinamide adenine dinucleotide phosphate (NADPH). NADPH oxidation plays a major role in the production of ROS in adipocytes. Also, through the oxidation of fatty acids and increased oxygen consumption in obesity triggers increased mitochondrial respiration resulting in superoxide, radical peroxide and hydroxyl hydrogen [13] [14].
In obesity, the larger adipose tissue can lead to hypoxia (lack of O2) and chronic inflammation. This can improve the state of oxidative stress by producing excessive ROS as well as decreasing the activity of endogenous antioxidant enzymes. This may increase lipid oxidation markers, such as MDA and carbonyl and decrease endogenous antioxidant enzyme activity such as GSH, MnSOD and catalase. Obesity results in increased inflammatory cytokines in the hypothalamus that increase and activate IL-β, TNF-α, and IL-6 which may affect metabolic processes [15] [16].
Physical exercise such as treadmill is widely used as one of the treatments in losing weight. Based on the results of statistical analysis there was a significant effect of the treadmill on decreasing MDA levels as well as increased endogenous antioxidant activity such as GSH, MnSOD and catalase when compared with control group of obesity (KP). Physical exercise (endurance) such as treadmill can decrease oxidative stress state through the mechanism of increasing mobilisation and oxidation of free fatty acids, affecting leptin signal pathways, hypothalamic inflammatory protection and AMPK activation pathway (AMP-activated protein kinase).
In this study, it is clear that the increase in MDA levels coupled with decreased activity of endogenous antioxidant enzymes such as GSH, MnSOD and catalase enzymes. Lipid peroxidation is a marker of oxidative damage (oxidative injury) presented as malondialdehyde (MDA). MDA levels in plasma in obesity mice can increase, higher than negative control mice. Malondialdehyde is a toxic compound that can disrupt the integrity of the cell membrane, so if the levels are not immediately lowered, it will interfere with the functioning of cells.
The state of oxidative stress can also decrease the activity of endogenous antioxidant enzymes. Several studies have shown that systemic oxidative stress is associated with increased NADPH oxidase 4 (NOX4) enzyme, a member of nitrogen oxides (NOX) that plays an important role in the formation of H2O2 (strong oxidising agents). Therefore, the higher the NOX4 and the formation of H2O2, so that the catalase enzyme work to catalyse the more severe, which eventually suppresses its activity [12] [13].
GSH enzyme activity is much lower than that of the positive control group. GSH enzyme works to convert H2O2 to H2O and O2, but with hypoxia, the availability of O2 is reduced. This is probably because the H2O2 stack cannot be converted to O2 perfectly, due to the low activity of the enzyme. The condition of obesity is independently correlated with high oxidative stress and inflammatory markers. Increased oxidative stress and inflammation in obesity play an important role in the initiation and progression of vascular disease, or it may also lead to the initiation of carcinogenesis in obesity [15] [16]. The mechanisms responsible for the high state of oxidative stress in obesity are not yet known, but clearly, adipose tissue is one of the important oxidative and inflammatory stress mediators as it contributes to the production of free radicals and proinflammatory cytokines, including IL-6, and TNF alpha.
Mitochondria provide the energy needed for virtually all cellular processes that ultimately allow performing physiological functions, besides that mitochondria play a central role in the death of the cell by apoptotic mechanisms. Obesity affects mitochondrial metabolism, which supports the formation of ROS and the development of oxidative stress. On the other hand, other mechanisms have been proposed involving the effects of high triglycerides (TG) on mitochondrial respiratory chain function, where intracellular TG, which is also high, inhibits the translocation of adenine nucleotides and results in superoxide formation. In the P1 and P2 groups, there was an increase in the specific activity of the MnSOD enzyme, presumably because the treadmill treatment could improve exogenous antioxidants. The low activity of MnSOD proves high oxidative stress in the body, so it is not able to eliminate the number of oxidants (free radicals). High oxidative stress is also associated with the condition of patients who are obese [17] [18].
The activity of lipolysis in obesity is impaired because of decreased mRNA expression that regulates lipoprotein lipase activity in adipose tissue and skeletal muscle. Aerobic physical exercise such as treadmill can lower cholesterol and triglyceride levels through the mechanism of improving the cholesterol-back transport process on the transfer of ester cholesterol. Physical exercise such as treadmill is one example of endurance exercise that induces increased AMPK. Improved AMPK stimulates the process of cellular glucose uptake, increased fatty acid oxidation, and decreases fat synthesis [18].
Physical exercise has been shown to increase the phosphorylation of JAK (Janus Kinase 2), activation of STAT3 signal Transducer and Activatortranscription 3 and SOCS3 which can affect appetite and inhibit more food intake. From the results of this study, it can be seen that in the treatment group P1 and P2 there was no significant difference in treadmill 1 x 10 minutes a day and 2 x 10 minutes a day (p ≥ 0,05). Treatment of physical exercise treadmill 2 x 10 minutes a day did not show statistically significant differences in MDA, GSH, MnSOD and catalase levels [18].
Aerobic exercise 30-150 minutes per week is included in the moderate-intensity exercise category. The duration of the treadmill in the treadmill treatment group was both included in moderate intensity exercise and had similar effects at the interval. The intensity of extended physical exercise (150 minutes per week) is more potent than the intensity of 40 minutes of physical exercise performed over 3 times per week. In an extended physical exercise (exercise volume) it is more effective to decrease fatty tissue mass and lipid profile. A short period of physical exercise can be effective if exercise volume is also increased [14] [18] [19] [20]. The reduction in body fat will improve the oxidation marker and increase the antioxidant activity, which has been damaged by obesity. Therefore, weight loss through nutritional and pharmacological therapies, as well as supplementation with antioxidant nutrients such as vitamins E, A, and C, flavonoids, may be key to reducing the risk of developing pathologic conditions associated with OS and obesity such as high blood pressure and metabolic syndrome.
Lipid oxidation is one form of adaptation of hypercholesterolemic conditions to the frequency of physical exercise. This adaptation can occur in moderate physical exercise. This adaptation occurs because energy needs increase in the duration of the treadmill expansion resulting in the release of useful catabolic hormones in lipid degradation to be used as an energy source during physical exercise. Treadmills have protective effects on obesity through increased lipolysis activity, decreased free fatty acids, decreased blood MDA levels, increased GSH levels as well as the specific activity of MnSOD enzymes and catalase compared with positive control groups.
In conclusion, treadmill treatment affects the improvement of oxidative status and endogenous antioxidant status of obese mice. Treatment of 1 x 10-minute treadmill per day decreased blood MDA level, increased GSH enzyme and increased specific activity of MnSOD enzyme and blood catalase of obese mice. The 2 x 10-minute daily treadmill did not differ significantly in improving the oxidative status and endogenous antioxidant status compared with the treadmill 1 x 10 minutes a day (p ≥ 0.05).
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Nugraha GI. Etiology and Pathophysiology of Obesity. In: Soegih RR, Wiramihardja KK, editors. Obesity Problems and Practical Therapy. Sagung Seto: Jakarta; 2009. pp. 9–18. [Google Scholar]
- 2.Soegih R, Wiramihardja KK. Obesity: Problems and practical therapies. Jakarta: Sagung seto: Jakarta; 2009. [Google Scholar]
- 3.Anam MS. Pengaruh intervensi diet dan olahraga terhadap indeks massa tubuh, kesegaran jasmani, hsCRP dan profil lipid pada anak obesitas the effects of diet and exercise on body mass index, physical fitness, hsCRP and lipid profile in obese children (Doctoral dissertation, Universitas Diponegoro) [Google Scholar]
- 4.Rahmawati A. Mechanism of inflammation and oxidative stress in obesity. El-Hayah. 2014;5(1):1–8. https://doi.org/10.18860/elha.v5i1.3034. [Google Scholar]
- 5.Alba F, Eduardo M, Mirandeli B. Inflammation, Oxidative Stress, and Obesity. Int J Mol Sci. 2011;12 (2):3117–3132. doi: 10.3390/ijms12053117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. https://doi.org/10.1172/JCI21625 PMid:15599400 PMCid: PMC535065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Speretta GFF, Rosante MC, Duarte FO, Leite RD, Lino ADS, Andre RA, Silvestre JGO, Araujo HSS, Duarte IACGO. The effects of exercise modalities on adiposity in obese rats. Clinics. 2012;67(12):1469–1477. doi: 10.6061/clinics/2012(12)19. https://doi.org/10.6061/clinics/2012(12)19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carpenter KC, Strohacker K, Breslin WL, Lowder TW, Agha NH, McFarlin BK. Effects of Exercise on Weight Loss and Monocytes in Obese Mice. Comp Med. 2012;62(1):21–26. PMid:22330647 PMCid: PMC3276388. [PMC free article] [PubMed] [Google Scholar]
- 9.Codo-er-Franch P, Boix-García L, Simó-Jordá R, Del Castillo-Villaescusa C, Maset-Maldonado J, Valls-Bellés V. Is obesity associated with oxidative stress in children? Int J Pediatr Obes. 2010;5(1):56–63. doi: 10.3109/17477160903055945. https://doi.org/10.3109/17477160903055945 PMid:19565402. [DOI] [PubMed] [Google Scholar]
- 10.Sankhla M, Sharma Kindergarten Mathur K, Rathor JS, Butolia V, Gadhok AK, Vardey SK, Sinha M, Kaushik GG. Relationship of oxidative stress with obesity and its role in obesity induced metabolic syndrome. Clin Lab. 2012;58(5-6):385–392. PMid:22783566. [PubMed] [Google Scholar]
- 11.Nishimura S, Manabe I, Nagasaki M, Eto K. CD8 +effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15(2):914–920. doi: 10.1038/nm.1964. https://doi.org/10.1038/nm.1964 PMid:19633658. [DOI] [PubMed] [Google Scholar]
- 12.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Sterreicher CHO, Takahashi H, Karin M. Dietary and Genetic Obesity Promote Liver Inflammation and Tumorigenesis by Enhancing IL-6 and TNF Expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. https://doi.org/10.1016/j.cell.2009.12.052 PMid:20141834 PMCid: PMC2836922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vincent HK, Morgan JW, Vincent KR. Obesity exacerbates oxidative stress levels after acute exercise. Med Sci Sports Exerc. 2004;36(5):772–779. doi: 10.1249/01.mss.0000126576.53038.e9. https://doi.org/10.1249/01.MSS.0000126576.53038.E9 PMid:15126709. [DOI] [PubMed] [Google Scholar]
- 14.Wolin KY, Carson K, Colditz GA. Obesity and Cancer. The Oncologist. 2010;15(2):556–565. doi: 10.1634/theoncologist.2009-0285. https://doi.org/10.1634/theoncologist.2009-0285 PMid:20507889 PMCid: PMC3227989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sikaris KA. The clinical biochemistry of obesity. The Clinical Biochemist Reviews. 2004;25(3):165. PMid: 18458706 PMCid: PMC1880830. [PMC free article] [PubMed] [Google Scholar]
- 16.Kurniandari N, Susantiningsih T, Kurniawaty E. Effect of Treadmill Treatment on Lipid Mice Profile (Mus musculus, L) Obesity. Majority. 2017;6(3):25–32. [Google Scholar]
- 17.Bhattacharya A, Rahman MM, Sun D, Lawrence R, Mejia W, McCarter R, O'shea M, Fernandes G. The combination of dietary conjugated linoleic acid and treadmill exercise lowers gain in body fat mass and enhances lean body mass in high fat–fed male balb/c mice. The Journal of nutrition. 2005;135(5):1124–30. doi: 10.1093/jn/135.5.1124. https://doi.org/10.1093/jn/135.5.1124 PMid:15867292. [DOI] [PubMed] [Google Scholar]
- 18.Susantiningsih T. Obesity and stress oxydative. Juke UNILA. 2015;5(9):89–93. [Google Scholar]
- 19.Wilmore HJ, Costill DL, Kenney WL. Physiology of Sport and Exercise. Human Kinetics. 2008;4 (12):110–130. [Google Scholar]
- 20.Ercho NC, Berawi K, Susantiningsih T. The relation of obesity with LDL and HDL levels at preclinic student of medical faculty of lampung university 2013. Majority. 2013;4(1):87–92. [Google Scholar]


