Abstract
Objective
Isotretinoin is a notorious teratogen otherwise used for the treatment of acne vulgaris. Some countries, including those in North America and the European Union, implemented the pregnancy prevention program (PPP); however, no PPP has yet been established in South Korea. So the aim of this study was to evaluate the rate of pregnant women exposed to isotretinoin among the callers of the Korean Mother Safe Counseling Center.
Methods
This is a prospective cohort study. We evaluated the demographic characteristics, obstetric history, and isotretinoin exposure of pregnant women based on the mother safe registry from April 2010 to July 2016.
Results
Among 22,374 callers, 650 (2.9%) pregnant women were exposed to isotretinoin. The mean age was 29.0±4.4 years in the isotretinoin-exposed group and 32.0±4.2 years in the unexposed group (P<0.001). Moreover, the incidence of pregnancies within 30 days after isotretinoin discontinuation or during isotretinoin intake was 78.9% (513/650). The median duration of isotretinoin exposure was 18 (1–4,231) days. Furthermore, from 2011 to 2015, the incidence of isotretinoin exposure was 2.9±1.2 pregnancies per 10,000 births in South Korea.
Conclusion
Approximately 80% of pregnant women are exposed to isotretinoin within the recommended 30 days of contraception or during pregnancy. Therefore, the PPP has to be established in South Korea.
Keywords: Pregnancy, Isotretinoin, Teratogen
Introduction
Isotretinoin was approved by the United States Food and Drug Administration (US FDA) in 1982 for the treatment of severe acne [1]. Furthermore, the Korea Food and Drug Administration (KFDA) approved the use of isotretinoin for the treatment of nodular and cystic acne that are refractory to other treatments [2]. However, isotretinoin is a widely prescribed notorious fatal teratogen in North America [3,4] and Korea.
As a teratogen, isotretinoin is associated with a congenital defect risk of 20–35% and causes craniofacial, cardiovascular, neurologic, and thymic malformations in the exposed intrauterine fetus [5]. In addition, neurocognitive impairment was observed in 30–60% of children who were exposed to isotretinoin before birth [5]. Therefore, the US FDA prohibited isotretinoin use during pregnancy and labelled it as a pregnancy category X drug. Moreover, the KFDA labeled isotretinoin as a drug contraindicated during pregnancy (grade 1).
Nevertheless, isotretinoin is highly effective in reducing severe cystic acne. It is reported that approximately 20% of the patients achieved remission after taking isotretinoin for 20 weeks [6]; thus, isotretinoin is available for use. In Canada, it is estimated that 3 in 10,000 women between 13 and 45 years are taking this drug [3], which is 10 times higher (3 in 1,000) in the United States [4]. In 2016, the total sales in Korea reached to 5 billion won (approximately 5 million US dollars) [7].
The estimated incidence of unplanned pregnancies in Korea is about 48%, and in such cases, the mothers are likely to be exposed to teratogens such as medications, alcohol, cigarette smoke, and X-ray radiation [8]. Considering that 76% of women who became pregnant within 30 days of isotretinoin intake experienced artificial abortion [9], the pregnancy prevention program (PPP) for women taking isotretinoin is necessary.
The PPP run by North American and European Union countries includes education of both physicians and patients. Such programs address the possible teratogenicity of isotretinoin, need for contraception while taking isotretinoin, and requirement for a pregnancy test before drug prescription [10]. The United States has a more active and strict PPP called iPLEDGE. This program requires the dermatologists to register their patients to the iPLEDGE system before prescribing isotretinoin and the pharmacists to confirm the prescription in the dedicated website before dispensing the drug. In addition, women of childbearing age who are taking isotretinoin are required to use two different types of contraception before and after 1 month of drug use. Moreover, two consecutive pregnancy tests with a 30-day interval should both yield negative results [11].
However, even after introducing these programs, a few women still became pregnant while taking isotretinoin. A recent population-based study in the Netherlands on isotretinoin exposure during pregnancy revealed that 2.5 of 10,000 pregnant women were exposed to isotretinoin [12].
In Korea, some pregnant women who were exposed to isotretinoin following its wide prescription decided to terminate their pregnancy because of the fear of fetal malformations. Therefore, there is a need to introduce the PPP for women who are using isotretinoin in Korea. To do this, it is necessary to evaluate the isotretinoin exposure of pregnant women. Despite the need for a population-based survey, the introduction of the PPP is not easy in terms of the time, cost, and methodology. Therefore, in this study, we aimed to evaluate the isotretinoin exposure of pregnant women in Korea.
Materials and methods
We performed a prospective cohort study. From April 2010 to July 2016, a total of 22,374 pregnant women from the Korean Mother Safe Counseling Center registry were evaluated for demographic characteristics, obstetric history, and exposure to medications. This registry is an online database of pregnant women exposed to medications, who were counseled at the Korean Mother Safe Counseling Center. This center comprises a network of different centers located in Seoul, Busan, Gwangju, Daejeon, Daegu, and Ulsan and is a teratogen information service operating under the support of the Ministry of Health and Welfare since 2010.
In this study, we investigated the total number of pregnant women with isotretinoin exposure based on the Korean Mother Safe Counseling Center registry, stages of pregnancy during the exposure, amount of exposure, and number of isotretinoin-exposed women versus the number of births by year. The number of births by year was obtained from the National Statistics Office. From 2011 to 2015, the number of births was 471,265, 484,550, 436,455, 435,435, and 438,420, respectively [13]. The Student's t-test was used to compare continuous variables while the χ2-test was used to compare categorical variables. A P-value <0.05 was considered to be statistically significant.
Results
Based on the results of this study, among 22,374 callers, 650 (2.9%) pregnant women were exposed to isotretinoin. The mean age of the isotretinoin-exposed group was 29.0±4.4 years (range, 18.1–46.0 years).
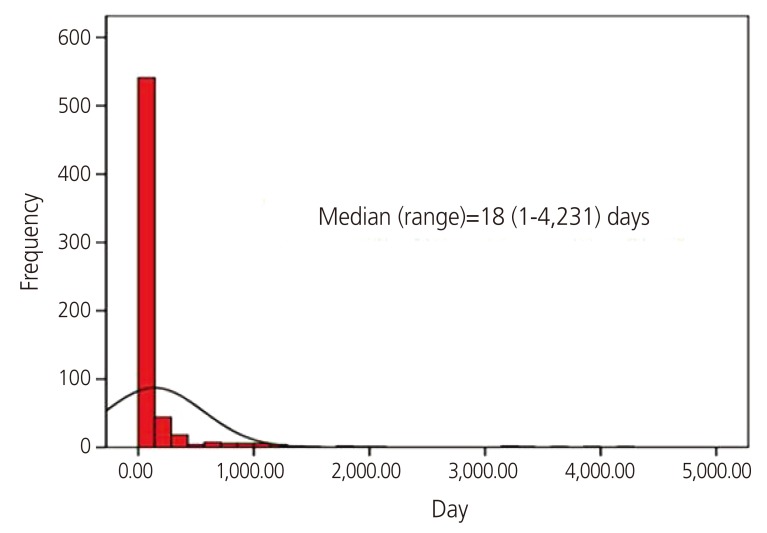
However, the mean age of women in the non-exposed group (32.0±4.2 years) was significantly higher than those in the exposed group (P<0.001) (Table 1). Moreover, the gravidity, parity, and number of spontaneous abortions were significantly higher in the non-exposed group. However, the number of therapeutic abortions was higher in the isotretinoin-exposed group, although no significant difference was noted. The median duration of isotretinoin exposure was 18 days (range, 1–4,231 days) (Fig. 1).
Table 1. Characteristics of the isotretinoin exposure and non-exposure groups.
| Characteristics | Exposure | Non-exposure | P-valuea) | ||
|---|---|---|---|---|---|
| Pregnancies (n=22,374) | 650 (2.9) | 21,724 (97.1) | |||
| Maternal age (yr) | |||||
| Mean±SD | 29.0±4.4 | 32.0±4.2 | <0.001 | ||
| Range | 18.1–46.0 | 15.2–51.3 | |||
| No. of subjects by ages (%) | |||||
| <20 | 4 (0.7) | 16 (0.1) | <0.001 | ||
| ≥20–24 | 65 (11.9) | 575 (3.1) | |||
| ≥25–29 | 246 (45.1) | 3,876 (21.1) | |||
| ≥30–34 | 171 (31.1) | 8,944 (48.7) | |||
| ≥35 | 60 (11.0) | 4,954 (27.0) | |||
| Gravidity | 1.1±0.9 | 1.4±1.1 | <0.001 | ||
| Parity | 0.2±0.5 | 0.4±0.6 | <0.001 | ||
| Spontaneous abortion | 0.03±0.17 | 0.07±0.32 | 0.001 | ||
| Therapeutic abortion | 0.12±0.50 | 0.11±0.44 | 0.443 | ||
| Duration of isotretinoin exposure (day), median (range) | 18 (1–4,231) | - | |||
| Alcohol | |||||
| No | 543 (83.5) | 18,686 (86.0) | 0.073 | ||
| Yes | 107 (16.5) | 3,038 (14.0) | |||
| Cigarette smoking | |||||
| No | 622 (95.7) | 21,052 (96.9) | 0.080 | ||
| Yes | 28 (4.3) | 672 (3.1) | |||
Values are presented as number (%) or mean±standard deviation.
SD, standard deviation.
a)P<0.05, t-test or χ2 test.
Fig. 1.
Duration of isotretinoin exposure.
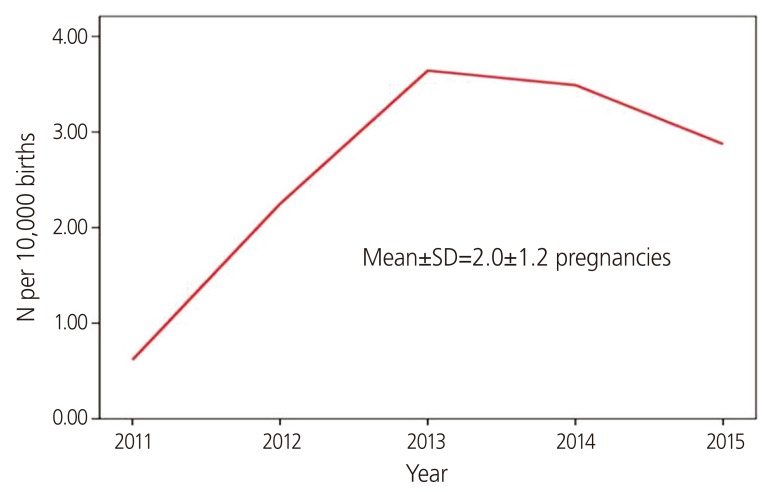
The incidence of pregnancy within 30 days after isotretinoin discontinuation or during isotretinoin intake was 78.9% (513/650) (Table 2). Moreover, the mean number of isotretinoin-exposed pregnant women from 2011 to 2015 was 2.9±1.2 per 10,000 births in Korea (Fig. 2).
Table 2. Frequency of isotretinoin exposure according to period (stages) of pregnancy.
| Period | Frequency | Exposure duration (day), median (range) | Termination rate of pregnancy [15] | |
|---|---|---|---|---|
| Before pregnancy | ||||
| >30 days discontinuation | 137 (21.1%) | 30 (1–4,231) | 12.9% (4/31) | |
| ≤30 days discontinuation | 104 (16.0%) | 41 (1–3,226) | 17.6% (3/17) | |
| During pregnancy | ||||
| <4 wk | 221 (34.0%) | 13 (1–3,653) | 50% (5/10) | |
| ≥4 wk | 188 (28.9%) | 21 (1–1,461) | 40% (4/10) | |
Fig. 2.
Number of isotretinoin-exposed pregnant women versus number of Korean births by year (2011–2015).
SD, standard deviation.
Discussion
Isotretinoin is widely prescribed in Korea, although it is a fatal teratogenic drug and therefore, is prohibited during pregnancy. Contraception is recommended for at least 1 month after isotretinoin discontinuation. However, it is inevitable that pregnant women become exposed to isotretinoin because 50% of pregnancies are unplanned [8].
The results of this study showed that the incidence of isotretinoin exposure in Korea is 2.9 pregnancies per 10,000 births, which is similar to the incidence of 2.5 pregnancies per 10,000 births reported in a Dutch population-based study [12]. The incidence of pregnancy within 30 days after isotretinoin discontinuation or during isotretinoin intake accounted for 78.9% of the total exposure. The median duration of exposure was 18 days.
However, this study is limited to the Korean Mother Safe Counseling Center, rather than a population-based study, as in the Netherlands and the United States. In the United States, where a strict PPP for isotretinoin is practiced, a population-based study revealed that 3.4 pregnancies occur per 1,000 courses of isotretinoin intake [14]. Considering that the incidence in the United States is 10 times higher than that found in this study, the actual isotretinoin exposure in Korea is estimated to be much higher.
In this study, the median duration of isotretinoin exposure during pregnancy was 18 days (range, 1–4,231 days). By contrast, in the United States, the average duration was 140 days [14]. Isotretinoin is often used for the long-term treatment of acne. Some individuals reported that they were able to obtain some isotretinoin capsules from another patient. In this regard, patients should be given guidance to refrain from sharing their prescribed isotretinoin to others.
It is postulated that some pregnant women who are exposed to isotretinoin consider having an abortion because of the fear of fetal malformations. In another study involving this cohort, 50% and 40% of women exposed to isotretinoin within and after 4 weeks of gestation, respectively, terminated their pregnancy [15]. Likewise, in a population-based study conducted in Canada between 1984 and 2002, 90 of 8,609 women aged 13–45 years who took isotretinoin became pregnant and 76 (84%) women chose to terminate their pregnancy [16]. In the United States, 72% of the women who became pregnant while taking isotretinoin terminated their pregnancy [14]. The rate of pregnancy termination in these countries is higher than that in Korea because artificial abortion is legal in Canada and the United States. Furthermore, pregnancy termination is recommended to women exposed to isotretinoin during pregnancy by the Motherisk Program in Canada, which is a teratogen information service.
The mean age of women in the isotretinoin-exposed group during pregnancy (29 years) was significantly lower than those in the non-exposed group (32 years) (P<0.001). In addition, a normal distribution pattern from 18–46 years was noted based on the age of isotretinoin-exposed women. In Canada and the United States, The median age of isotretinoin-exposed pregnant women was 26 years (range, 18–34 years and 17–39 years, respectively) [14,16]. The mean and highest ages in those countries were lower than those in Korea. Acne affects approximately 90% of individuals during puberty [17] and moderate-to-severe acne occurs in about 20% of individuals during puberty, 64% in their 20s, and 43% in their 30s [18]. Considering the age of onset and acne improvement, isotretinoin exposure would be higher in patients in their early 20s, and the incidence of isotretinoin exposure during pregnancy would be lower in older persons. However, in this study, the mean age of isotretinoin-exposed pregnant women was 29 years, showing a normal distribution pattern from 18 to 46 years old. This means that isotretinoin is widely used in Korea for acne treatment regardless of the patient's age and disease severity. Its use is permitted by the KFDA for the treatment of severe acne (nodular and cystic acne or acne conglobata) that is unresponsive to other therapies, especially when occurring in the trunk [2].
According to the Korean Health Insurance Review and Assessment Service in 2016, isotretinoin with insurance was prescribed in about 25,000 women of childbearing age (20–44 years) whereas isotretinoin without insurance was prescribed in 170,000 women for 6 months [19].
Approximately 400,000 isotretinoin prescriptions are given to women of childbearing age per year[19]. According to the above data, the rate of prescription with insurance was only 15% in Korea, and the remaining 85% were prescriptions without insurance. This suggests that isotretinoin is used for mild acne and sebum control, which is not covered by the insurance, rather than for severe acne, which is covered by insurance, in Korea.
Fetal malformations have been reported to occur in about 25–30% of isotretinoin-exposed pregnant women. Such malformations include craniofacial, heart, and central nervous system defects [5]. PPPs have been implemented in developed countries to avoid the teratogenic effects of isotretinoin. Since the first documentation of isotretinoin teratogenicity in 1983, the US FDA has established a sponsor-developed PPP. Since the introduction of the drug, a total of 1,995 isotretinoin-exposed pregnant women have been reported in the PPP from 1982 to 2000; however, the actual number is expected to be higher [16]. Therefore, the FDA Advisory Committee adopted a new restriction program called System to Manage Accutane® Related Teratogenicity™ or SMART™ [16]. Nevertheless, generic isotretinoin compounds were introduced in the market in 2002, and the risk management program was not centralized then [16]. Since 2006, the US FDA has been strictly managing isotretinoin use though iPLEDGE (https://www.ipledgeprogram.com/), an integrated management system that includes all isotretinoin compounds [16]. The European Union also maintains a policy to prevent pregnancy while taking isotretinoin by conducting the PPP.
According to this study, a few women became pregnant during isotretinoin intake and opted for pregnancy termination. Therefore, guidelines are required to provide both health-care providers and patients with various contraception methods during isotretinoin intake in Korea. In addition, laws and regulations should be enacted.
As this study was not a population-based survey (the sample is rather limited to pregnant women registered in the Korean Mother Safe Counseling Center), the incidence of isotretinoin exposure during pregnancy might be underestimated in this study. Moreover, the frequency of pregnancy termination in this study was based on a limited data. Despite these limitations, however, this is the first prospective study to provide data on isotretinoin-exposed pregnant women. Our findings suggest that the PPP for women of childbearing age is required.
In conclusion, approximately 80% of pregnant women are exposed to isotretinoin within the recommended 30 days of contraception or during pregnancy. Therefore, there is an urgent need to introduce the PPP in Korea.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1:162–169. doi: 10.4161/derm.1.3.9364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korea Pharmaceutical Information Center. Isotretinoin information [Internet] Seoul: Korea Pharmaceutical Information Center; c2018. [cited 2018 Feb 19]. Available from: http://www.health.kr/searchDrug/result_drug.asp?drug_cd=A11AHHHHH0304. [Google Scholar]
- 3.Azoulay L, Oraichi D, Bérard A. Patterns and utilization of isotretinoin for acne from 1984 to 2003: is there need for concern? Eur J Clin Pharmacol. 2006;62:667–674. doi: 10.1007/s00228-006-0151-x. [DOI] [PubMed] [Google Scholar]
- 4.Honein MA, Paulozzi LJ, Erickson JD. Continued occurrence of Accutane-exposed pregnancies. Teratology. 2001;64:142–147. doi: 10.1002/tera.1057. [DOI] [PubMed] [Google Scholar]
- 5.Lammer EJ, Chen DT, Hoar RM, Agnish ND, Benke PJ, Braun JT, et al. Retinoic acid embryopathy. N Engl J Med. 1985;313:837–841. doi: 10.1056/NEJM198510033131401. [DOI] [PubMed] [Google Scholar]
- 6.Stern RS. When a uniquely effective drug is teratogenic. The case of isotretinoin. N Engl J Med. 1989;320:1007–1009. doi: 10.1056/NEJM198904133201510. [DOI] [PubMed] [Google Scholar]
- 7.Health Insurance Review & Assessment service. Health insurance statistical information [Internet] Wonju: Health Insurance Review & Assessment Service; c2017. [cited 2018 Feb 19]. Available from: http://opendata.hira.or.kr/op/opc/olapGnlInfo.do. [Google Scholar]
- 8.Han JY, Nava-Ocampo AA, Koren G. Unintended pregnancies and exposure to potential human teratogens. Birth Defects Res A Clin Mol Teratol. 2005;73:245–248. doi: 10.1002/bdra.20132. [DOI] [PubMed] [Google Scholar]
- 9.Dathe K, Schaefer C. Drug safety in pregnancy: the German Embryotox institute. Eur J Clin Pharmacol. 2018;74:171–179. doi: 10.1007/s00228-017-2351-y. [DOI] [PubMed] [Google Scholar]
- 10.Abroms L, Maibach E, Lyon-Daniel K, Feldman SR. What is the best approach to reducing birth defects associated with isotretinoin? PLoS Med. 2006;3:e483. doi: 10.1371/journal.pmed.0030483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.iPLEDGE. iPLEDGE information [Internet] Silver Spring (MD): U.S. Food and Drug Administration; c2016. [cited 2018 Feb 19]. Available from: https://www.ipledgeprogram.com. [Google Scholar]
- 12.Zomerdijk IM, Ruiter R, Houweling LM, Herings RM, Sturkenboom MC, Straus SM, et al. Isotretinoin exposure during pregnancy: a population-based study in the Netherlands. BMJ Open. 2014;4:e005602. doi: 10.1136/bmjopen-2014-005602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Statistics Korea. Newborn statistical information [Internet] Daejeon: Statistics Korea; c2014. [cited 2018 Feb 19]. Available from: http://m.kosis.kr/mobService/jipyolist/ModDataD.do?parmTitleYear=0&parmMainJipyo=601&parmPrdSe=&parmPrdDe=&parmAreaType=1&parmTypeGubun=D&preCode=A&preListNm=%EC%9D%B8%EA%B5%AC%EA%B0%80%EA%B5%AC&rn=3. [Google Scholar]
- 14.Mitchell AA, Van Bennekom CM, Louik C. A pregnancy-prevention program in women of childbearing age receiving isotretinoin. N Engl J Med. 1995;333:101–106. doi: 10.1056/NEJM199507133330206. [DOI] [PubMed] [Google Scholar]
- 15.Yook JH, Han JY, Choi JS, Ahn HK, Lee SW, Kim MY, et al. Pregnancy outcomes and factors associated with voluntary pregnancy termination in women who had been treated for acne with isotretinoin. Clin Toxicol (Phila) 2012;50:896–901. doi: 10.3109/15563650.2012.739287. [DOI] [PubMed] [Google Scholar]
- 16.Bérard A, Azoulay L, Koren G, Blais L, Perreault S, Oraichi D. Isotretinoin, pregnancies, abortions and birth defects: a population-based perspective. Br J Clin Pharmacol. 2007;63:196–205. doi: 10.1111/j.1365-2125.2006.02837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stathakis V, Kilkenny M, Marks R. Descriptive epidemiology of acne vulgaris in the community. Australas J Dermatol. 1997;38:115–123. doi: 10.1111/j.1440-0960.1997.tb01126.x. [DOI] [PubMed] [Google Scholar]
- 18.Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474–485. doi: 10.1111/bjd.12149. [DOI] [PubMed] [Google Scholar]
- 19.Health Insurance Review & Assessment Service. Health insurance statistical information [Internet] Wonju: Health Insurance Review & Assessment Service; c2017. [cited 2018 Feb 19]. Available from: http://opendata.hira.or.kr/home.do. [Google Scholar]