Abstract
Both the ostensibly aversive effects of unpredictable episodes of social stress and the intensely rewarding effects of drugs of abuse activate the mesocorticolimbic dopamine systems. Significant neuroadaptations in interacting stress and reward neurocircuitry may underlie the striking connection between stress and substance use disorders. In rodent models, recurring intermittent exposure to social defeat stress appears to produce a distinct profile of neuroadaptations that translates most readily to the repercussions of social stress in humans. In the present review, preclinical rodent models of social defeat stress and subsequent alcohol, cocaine or opioid consumption are discussed with regard to: (1) the temporal pattern of social defeat stress, (2) male and female protocols of social stress-escalated drug consumption, and (3) the neuroplastic effects of social stress, which may contribute to escalated drug-taking. Neuroadaptations in corticotropin-releasing factor (CRF) and CRF modulation of monoamines in the ventral tegmental area and the bed nucleus of the stria terminalis are highlighted as potential mechanisms underlying stress-escalated drug consumption. However, the specific mechanisms that drive CRF-mediated increases in dopamine require additional investigation as do the stress-induced neuroadaptations that may contribute to the development of compulsive patterns of drug-taking.
Keywords: Social defeat stress, Alcohol, Cocaine, Opioids, CRF, VTA
1. Introduction
The neural mechanisms for coping with social stress and for escalated drug use interact in an intricate manner (Sinha, 2001). How the ostensibly aversive effects of unpredictable episodes of social stress and the intensely rewarding effects of self-administered cocaine infusions concurrently activate the mesocorticolimbic dopamine system remains unresolved. Moreover, this apparent paradox is accentuated by the persistent escalation of cocaine self-administration binges in socially defeated animals that are characterized by elevated dopamine tone (Holly and Miczek, 2016).
Psychomotor stimulants such as amphetamines and cocaine are self-administered at escalated levels after intermittent exposure to various stressors, whereas the stress parameters are much more limited for escalating alcohol and opiate intake (Ahmed et al., 2018; Becker et al., 2011). This review describes the different kinds of social defeat stress and focuses on their cross-species generality and ready translation from experimental animal models to stress-related alcohol and substance use disorders in humans.
Social stress is bi-directional in nature. The ascending limb of this inverted U-shaped curve delineates the energizing, activating effects of mild to moderate stress while the descending limb signifies the potentially debilitating, impairing effects of severe or chronic stress (Sapolsky, 2015). In this review, we discuss how a history of mild, moderate or more severe social defeat stress exposure can ultimately increase or decrease voluntary drug-taking in preclinical animal models.
The neuropeptide, corticotropin-releasing factor (CRF or CRH; encoded by the CRH gene), exerts a salient role in the interacting neural networks for social stress and for drug abuse initiation, escalation and relapse (Bernardi et al., 2017; Sarnyai et al., 2001). Most types of social stress are characterized by hypothalamic CRF activating the hypothalamic pituitary adrenal (HPA) axis in the laboratory and in the field (Covington and Miczek, 2005; Fuchs and Flügge, 2002; Norman et al., 2015; Sapolsky 1990, 1992; Sgoifo et al., 1998). In addition, considerable evidence points to the significance of extrahypothalamic CRF as a modulator of canonical monoamine neurotransmitters (Kelly and Fudge, 2018). We focus on extrahypothalamic CRF signaling due to its critical role in modulating motivation, especially in the context of drug seeking and consumption (Holly and Miczek, 2016; Koob and Volkow, 2010; Wise, 2004).
2. Clinical overview: social stress as a risk factor for drug use
2.1. Alcohol
For some individuals, moderate alcohol consumption increases the desire to engage in prosocial behaviors and renders these social interactions more enjoyable; likewise, social settings can increase total alcohol intake as well as the positive subjective effects of alcohol (de Wit and Sayette, 2018; Van Hedger et al., 2017). Under these conditions, drinking tends to be context-dependent, and cessation of alcohol consumption generally coincides with removal from the social context. While drinking to enhance mood is generally not associated with later, problematic drug use, those who consume alcohol in negative social contexts to reduce social anxiety and stress may be more likely to later meet the Diagnostic and Statistical Manual (DSM-V; American Psychiatric Association, 2013) criteria for an alcohol use disorder (AUD; Cooper et al., 1992b; Sinha, 2001). And in more extreme cases, exposure to moderate or severe psychosocial stressors can further increase the likelihood of developing a sustained pattern of uncontrollable drinking or the probability of future relapse, particularly in those who drink as a coping mechanism (Adinoff et al., 2017; Brown et al. 1990, 1995; Koob and Kreek, 2007). This review will evaluate the characteristics of social stressors that may increase the probability of escalated drinking or drug-taking.
There is substantial evidence pointing to stress exposure and stress responding as predictors of alcohol use in humans (Brown et al. 1990, 1995; Gilpin and Weiner, 2017; Sinha 2001, 2008, 2009; Stewart, 1996; Uhart et al., 2006; Uhart and Wand, 2009). Most clinical experimental studies on social stress and drug-taking use the Trier Social Stress Test (TSST), which can reliably increase circulating cortisol levels and heart rate in response to social examination and performance stress (Allen et al., 2017; Kirschbaum et al., 1993). When asked to complete the TSST, craving and responding for alcohol as well as total alcohol intake increase in participants with an alcohol use disorder compared to healthy controls, suggesting that alcohol-dependent individuals may be more likely to cope with negative affective states by drinking (McCaul et al., 2018; Thomas et al., 2011; Van Hedger et al., 2017). In addition, non-abstaining, alcohol-dependent participants exhibit an augmented stress response compared to abstaining patients or healthy controls (Starcke et al., 2013), indicating that heightened stress reactivity in heavy-drinking individuals may intensify alcohol craving in response to mild stressors.
2.2. Cocaine
Stressful life events can promote excessive cocaine use and increase the probability of relapse in dependent individuals (Sinha, 2001; Wallace, 1989). Cocaine-dependent adults who report higher levels of perceived stress and anxiety appear to take cocaine for more years than those who report less stress and anxiety (Karlsgodt et al., 2003). When asked to imagine and recount a recent stressful life event during a stress imagery task, healthy participants show a characteristic increase in salivary cortisol concentrations which then decline during a subsequent recovery period. However, in cocaine abusers, cortisol levels continue to rise even during the recovery period and these individuals also report increased drug craving as a consequence of performing this task (Sinha et al., 1999, but Harris et al., 2005). During a similar, drug cue imagery procedure, participants recall a recent circumstance that involved drug-related cues and triggered cocaine-taking. As with stress imagery, visualizing drug cues also increases salivary cortisol and drug craving; however, only craving in response to stress imagery, but not drug cue imagery, strongly predicts imminent relapse to cocaine. Likewise, stress-induced increases in cortisol are predictive of the amount of cocaine taken during the relapse (Sinha et al. 2000, 2006; Waldrop et al., 2010). Unlike drug cue imagery, craving in response to direct contact with drug paraphernalia is predictive of relapse in dependent individuals. And, like stress imagery, direct stimulation of the HPA axis with intravenous CRF treatment also produces predictive subjective effects (Back et al., 2010), suggesting that individual differences in HPA axis reactivity and craving in response to stress and drug cues may determine the trajectory of cocaine abuse and treatment outcome.
In an evaluation of recently abstaining cocaine-dependent patients, both stress and drug cue imagery increased negative emotions including feelings of sadness, anger, fear and anxiety. Compared to healthy controls, patients also reported more severe craving for drugs and alcohol in response to stress or drug cue imagery (Fox et al., 2008). For cocaine-dependent outpatients, the effects of stress exposure are potentiated by drug-related cues, and individuals who frequently encounter both environmental stimuli are at the greatest risk of experiencing intense urges to take cocaine (Preston et al., 2018). A similar study using fMRI reported a decrease in anterior cingulate and an increase in dorsal striatal activity during stress imagery in patients compared to healthy individuals, suggesting reduced regulation of brain areas that may exert control over subcortical, reward- and craving-associated regions (Sinha et al., 2005). Together, these findings indicate that stress and drug-related cues can intensify the desire to take cocaine, thereby increasing the risk of relapse in abstinent, cocaine-dependent individuals. In addition, stress-induced neuroadaptations may result in a dysregulation of striatal activity and increased drug abuse risk. Using this information in conjunction with potentially predictive imaging techniques and neuroendocrine measures of HPA axis reactivity, specific therapies could be designed to specifically treat stress-associated relapse to cocaine abuse (Milivojevic and Sinha, 2018; Sinha and Li, 2007).
2.3. Opioids
Lifetime traumatic experiences including childhood maltreatment, assault and domestic violence increase the risk of developing an opioid addiction (Lawson et al., 2013), and opioid abuse may be perpetuated by continued exposure to traumatic events, particularly in the form of physical violence (Cottler et al., 1992). Treatment-seeking, opioid-dependent patients describe greater levels of perceived stress than healthy controls (Hyman et al., 2009). The intensity and frequency of these stressful experiences is associated with more intense drug craving (Kowalczyk et al., 2015; Preston et al., 2017; Preston and Epstein, 2011), while the number and magnitude of life crises increases the likelihood of relapse (Kosten et al., 1986; Krueger, 1981). Greater perceived stress in opioid addicts may result from an amplified sensitivity to stressful stimuli due to a history of traumatic life events; in turn, this may render some individuals more likely to develop an opioid addiction and more vulnerable to relapse (Kosten et al., 1986; McCabe et al., 2016; Sinha 2001, 2007).
In a laboratory setting, opioid-dependent patients report severe drug craving, anxiety and fear during stress or drug cue imagery tasks, whereas only stress imagery escalates heart rate, systolic blood pressure, and self-reported feelings of anger and sadness (Hyman et al., 2007; Sinha et al., 2007). Likewise, during the TSST, opioid-dependent individuals report more negative, subjective effects of social stress compared to healthy controls (Back et al., 2015). Outside of the laboratory, however, stress and drug cues are often experienced in tandem. Faced with concurrent stressful situations and drug-related paraphernalia, heroin-dependent outpatients report an increase in the severity of craving (Preston et al., 2018). In sum, these findings point to a combined role of stressors and drug-related cues as environmental risk factors that may increase the probability of relapse to opioid abuse.
Regarding physiological measures of stress reactivity, heroin-dependent patients exhibit higher circulating cortisol and ACTH concentrations compared to healthy individuals, and an acute dose of heroin can normalize these measures (Schmidt et al., 2014; Walter et al., 2013). Correspondingly, fMRI reveals an augmented left amygdala response to fearful faces in patients; this can also be normalized with acutely administered heroin (Schmidt et al., 2014). Peripheral measures of stress reactivity may correlate with relapse probability (Jaremko et al., 2015). However, a single systemic dose of cortisol does not increase the desire to take heroin (Walter et al., 2015). Deficits in the neural mechanisms that typically provide negative feedback to the HPA axis may ultimately produce both cravings and elevated cortisol levels in heroin users. Together, environmental risk factors like stress and drug-related cues and individual differences in stress reactivity are likely to interact and influence the likelihood of opioid abuse and relapse.
3. Animal models of social stress and pathological patterns of drug-taking
Because clinical experiments are bound by ethical constraints on the type and duration of laboratory stressors, studies aiming to clarify the effects of repeated or continuous social stress exposure predominantly employ rodent models. This review focuses on (1) preclinical models of social stress-heightened alcohol drinking and drug-taking, (2) the possible neurobiological mechanisms underlying interactions between social stress and drugs of abuse, and (3) avenues of future research and promising therapeutic targets for the treatment of drug or alcohol use disorders in individuals with a history of social stress exposure.
3.1. Temporal patterns of social defeat stress and drug-taking
3.1.1. Alcohol
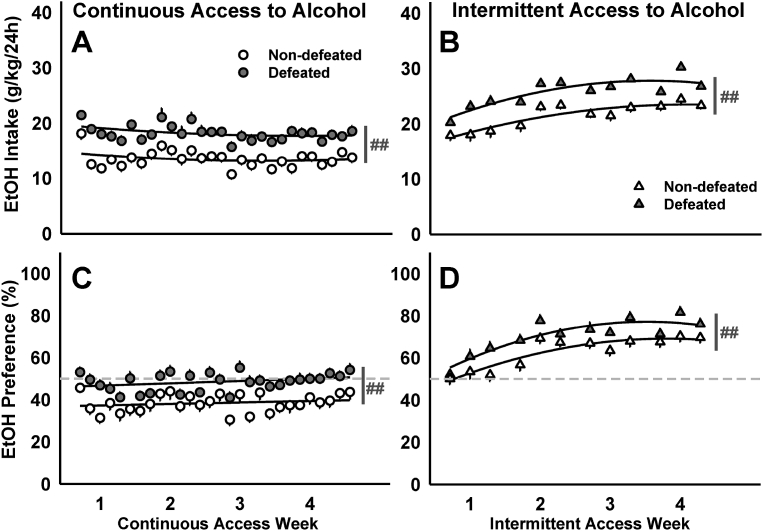
A key feature of experimental protocols that study the link between social stress and escalated alcohol consumption is the timing of the stress episodes. While it is apparent that repeated episodes of social defeat stress are required to produce a pattern of stress-escalated alcohol consumption (Croft et al., 2005), the temporal pattern of these stress episodes differs vastly between protocols, ranging from intermittent to chronic.
Ten consecutive days of intermittent social defeat stress can produce significant, persistent increases in voluntary alcohol consumption in both C57BL/6J (Fig. 1; Albrechet-Souza et al., 2017; Hwa et al., 2016a; Newman et al., 2018) and outbred, Swiss-derived mice (Norman et al., 2015). During intermittent resident-intruder confrontations, an aggressive, physical encounter is terminated after a fixed duration or number of attack bites (e.g., 5 min or 30 bites). Between daily stress episodes, experimental mice are singly housed, which is the primary feature that distinguishes intermittent from continuous social defeat stress protocols.
Fig. 1.
Socially defeated and non-defeated C57BL/6J males received 20% EtOH and water for 4 weeks. Daily mean ± SEM intake (g/kg/24 h) for mice with (A) continuous access to alcohol or (B) intermittent access to alcohol. The amount of alcohol consumed as a percentage of the total daily fluid intake (EtOH Preference) is depicted as daily mean ± SEM for mice with (C) continuous or (D) intermittent access to alcohol. Best-fit curves are in black. ##p < 0.01 non-defeated vs. defeated. Dashed lines mark the preference cutoff: values > 50% indicate a preference for alcohol over water, while values < 50% indicate a preference for water over alcohol (adapted from Newman et al., 2018).
Continuous social defeat stress models in rodents often employ 5–10 min daily resident-intruder confrontations. In the interval between aggressive encounters, defeated animals are housed adjacent to an aggressor, but protected by a perforated partition (Golden et al., 2011; Kudryavtseva et al., 1991). While the physical attack periods remain intermittent, “threatening” housing conditions are continuous. Experimental mice are attacked by a different aggressive resident every day, and subsequently housed next to that aggressor until the following defeat episode. Models of continuous social defeat stress in C57BL/6J (B6) female (Newman et al. in prep) and male mice (Kudryavtseva et al., 2006; Nelson et al., 2018) can lead to persistently escalated alcohol consumption.
In chronic subordinate colony (CSC) stress models, several submissive male mice are housed in the territory of an aggressive resident over the course of days to weeks without the protection of a perforated partition or cage. Following CSC stress, submissive mice voluntarily consume more alcohol compared to either singly-housed controls (Bahi, 2013; Peters et al., 2013) or dominant males (Hilakivi-Clarke and Lister, 1992). Similar findings are reported using CSC protocols in rats (Blanchard et al. 1987, 1992; Ellison, 1987), in evaluations of social dominance among prairie voles (Anacker et al., 2014), and in socially housed squirrel monkeys (McKenzie-Quirk and Miczek, 2008); likewise, social dominance appears to have a protective effect against escalated alcohol intake in cynomolgus monkeys (Helms et al., 2012; Jimenez and Grant, 2017). In rats, pre-stress alcohol intake does not correlate with later hierarchical standing or drinking following CSC stress, indicating that elevated drinking likely results from subordination stress rather than individual differences in alcohol preference (Blanchard et al., 1992; Hilakivi-Clarke and Lister, 1992, but Wolffgramm and Heyne, 1991). While the CSC protocol can reveal stress-escalated alcohol drinking in submissive mice and rats, it is difficult to ensure that defeated animals are stressed equally, and therefore, these models demand large group sizes. In addition, CSC stress can result in severe injuries inflicted by dominant animals onto submissive animals, making it impossible to distinguish between changes in drinking due to psychosocial stress exposure or due to pain associated with injury or infection. In light of these limitations, the neuroadaptations associated with social defeat stress and escalated drug-taking may be better studied in mice or rats with a history of continuous or repeated intermittent social defeat stress rather than CSC stress.
When animals are socially defeated and given alcohol concurrently or provided alcohol access immediately following a defeat episode, mice (Norman et al., 2015) and rats (Van Erp et al., 2001) often consume less alcohol than their non-defeated counterparts. Rats will similarly suppress their operant responding for alcohol when self-administration sessions are conducted soon after the social defeat episode (Funk et al., 2005; Van Erp and Miczek, 2001). Elevated drinking in socially defeated animals develops days or weeks following the final social defeat exposure, but not during or soon after the stress experience (Caldwell and Riccio, 2010; Croft et al., 2005; Lopez et al., 2016; Noori et al., 2014; Norman et al., 2015; Sillaber et al., 2002), suggesting that long-lasting neuroadaptations arise as a consequence of repeated episodes of social defeat stress. In addition, sympathetic activation during the stress exposure may initially suppress drug-taking, making the recovery from intense autonomic activity necessary for escalated drinking or self-administration to become apparent. In terms of the temporal pattern of social stress exposure, the duration and frequency of defeat stress episodes and the post-defeat interval prior to the onset of alcohol access are critical variables that require significant consideration.
3.1.2. Cocaine
In the laboratory, both rat and mouse models of intravenous cocaine self-administration reveal a relationship between social stress exposure and the acceleration towards an addiction-like phenotype that parallels clinical observations. Social stress can accelerate the acquisition of cocaine-taking (Tidey and Miczek, 1997; Arena et al. in prep), promote escalated and dysregulated patterns of drug consumption (Covington and Miczek, 2001; Han et al., 2015), and increase drug-seeking behavior after a drug-free period (Holly et al., 2016; Manvich et al., 2016).
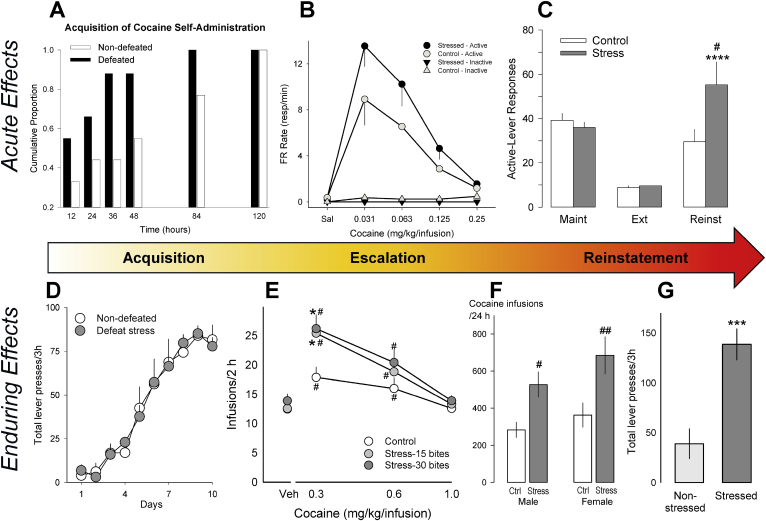
Brief exposure to acute social defeat stress enhances the conditioned effects of psychostimulants, which may in turn promote the acquisition of self-administration behavior. Compared to non-defeated control animals, both mice and rats that are socially defeated immediately before receiving an injection of cocaine subsequently develop a greater preference for the drug-paired environment during conditioned place preference (CPP) testing (McLaughlin et al., 2006; Montagud-Romero et al., 2015; Tovar-Diaz et al., 2018). Likewise, exposure to social defeat stress over five consecutive days can double the rate of cocaine self-administration acquisition (Fig. 2A; Tidey and Miczek, 1997), and rats that receive access to cocaine within 24 h of a stress episode show transiently elevated rates of self-administration (Fig. 2B; Miczek and Mutschler, 1996; Tidey and Miczek, 1997). However, these effects are often absent in studies that examine the more protracted effects of social defeat stress on cocaine self-administration (Fig. 2D; Boyson et al., 2011; Covington and Miczek, 2001; Holly et al., 2016; Kabbaj et al., 2001; but Haney et al., 1995), emphasizing the importance of the duration between stress and the onset of cocaine availability.
Fig. 2.
The acute (A–C) and enduring (D–G) effects of social defeat stress on cocaine self-administration across phases of ‘the addiction cycle’. A cumulative histogram (A) shows increased rates of acquisition of cocaine self-administration (0.25 mg/kg/infusion; FR1) for rats 24 h after defeat (black bars) compared to non-defeated rats (white bars); data are divided into 12 h bins of total drug access (adapted from Tidey and Miczek, 1997). (B) The dose-effect function for cocaine on rate of responding (resp/min) maintained by an FR5 schedule; determined by three sessions per dose, and shown as mean ± SEM. Rats exposed to social threat from an aggressive resident for 60 min immediately prior to each session (black circles) show elevated rates of responding for lower doses in comparison to non-stressed controls (white circles). Triangles depict the response rates on an inactive lever (adapted from Miczek and Mutschler, 1996). (C) Re-exposure to cues predictive of impending social defeat produces reinstatement of cocaine seeking; shown are the active-lever responses during the maintenance phase of self-administration (“Maint”), extinction (“Ext”), and reinstatement (“Reinst”) following defeat-cue re-exposure (grey bars) or a non-stressful control condition (white bars). Data represent the mean ± SEM number of responses during the final three days of self-administration prior to the onset of stress sessions (“Maint”), the final three days of extinction (“Ext”), and a single reinstatement test session (“Reinst”). ****p < 0.0001 compared to extinction; #p < 0.05 compared to the control group (adapted from Manvich et al., 2016). (D) No differences in the acquisition of cocaine self-administration (0.75 mg/kg/infusion) one week after intermittent social defeat (grey circles) or handling (white circles), across ten days of training (adapted from Holly et al., 2016). (E) The dose-effect function for cocaine intake is elevated in mice previously exposed to intermittent social defeat prior to self-administration (grey circles), relative to handled controls (white circles). Individual data were averaged over two sessions; #p < 0.05, ##p < 0.01 compared to the control group (adapted from Han et al., 2015). (F) Total number of infusions during an unlimited access cocaine “binge” (0.3 mg/kg/infusion, FR1) are enhanced by exposure to intermittent social defeat stress (grey bars) prior to self-administration, compared to handled controls (white bars), in male and female rats. “Binge” terminated after 120 min without a cocaine infusion. #p < 0.05, ##p < 0.01 compared to the control group (adapted from Holly et al., 2012). (G) Upon return to the cocaine self-administration chamber after days of forced abstinence, defeated rats (dark grey bar) pressed the previously cocaine-paired lever significantly more than non-stressed animals (light grey bar) over a 3 h session. ***p < 0.001 compared to the control group (adapted from Holly et al., 2016). All data are expressed as mean ± SEM, with the exception of (A).
Just as social stress can potently exacerbate drug-craving in people who are exposed to cocaine-associated cues (Preston et al., 2018; Sinha et al., 1999), acute exposure to social stress can reinstate a previously extinguished preference for drug-paired stimuli in a CPP protocol (Bruchas et al., 2011; Ribeiro Do Couto et al., 2009). However, modeling social stress-mediated reinstatement of operant behavior has proven challenging. Acute exposure to defeat fails to stimulate, and often suppresses conditioned responding on a drug-associated operandum in rodent models of both alcohol and cocaine self-administration (Funk et al., 2005; Manvich et al., 2016). The effects of stress on behavioral arousal adhere to an inverted U-shaped function, and social defeat may represent an especially salient stressor that approaches the descending limb of this curve, possibly resulting in the non-specific suppression of operant behaviors (McEwen et al., 2015; Sapolsky, 2015). Indeed, submissive rats escalate their rates of responding for cocaine immediately after a social threat protocol that prevents physical attack by using a protective barrier to shield the submissive rat from the dominant aggressor (Fig. 2B; Miczek and Mutschler, 1996). While this protocol limits physical injury, the submissive rat is still subjected to threatening auditory, olfactory and visual stimuli generated by the dominant resident. Similarly, re-exposure to stress-associated odor cues in the context of drug self-administration can reinstate previously extinguished cocaine-seeking behavior in rats (Fig. 2C; Manvich et al., 2016).
Intermittent exposure to social defeat can persistently increase and dysregulate typical patterns of cocaine self-administration. Brief, episodic social defeat stress tends to enhance measures of drug self-administration. Across a range of doses, defeat-experienced mice show a robust upward-shift in their rate of cocaine self-administration, indicating a persistent change in the drug's reinforcing effects (Fig. 2E; Han et al., 2015; Arena et al. in prep). In rats, episodic social defeat stress can increase progressive ratio breakpoints (Burke and Miczek, 2015; Covington and Miczek 2001, 2005; Quadros and Miczek, 2009) though this effect is not always evident (Boyson et al. 2011, 2014; Cruz et al., 2011; Miczek et al., 2011; Yap and Miczek, 2007).
Intermittent social defeat stress during adolescence or adulthood consistently intensifies binge-like cocaine consumption during prolonged periods of unlimited access to drug (24–48 h; i.e., the “binge” protocol). During these binges, both male and female rats with a history of episodic social defeat stress often accumulate twice as much cocaine as non-stressed controls (Fig. 2F; Boyson et al., 2011; Boyson et al., 2014; Burke et al., 2016; Burke and Miczek, 2015; Covington et al., 2005; Covington et al., 2008; Covington and Miczek, 2001; Covington and Miczek, 2005; Cruz et al., 2011; Holly et al., 2012; Leonard et al., 2017; Quadros and Miczek, 2009; Yap et al., 2015).
In contrast, under limited access conditions or during the early hours of the binge, many measures of cocaine self-administration are not affected by a history of social defeat stress. For instance, self-administration by defeated and control rats is indistinguishable during the early hours of access, and these animals show precise regulation of drug intake, even in the face of unpredictable changes in unit dose delivery (Covington et al., 2008; Quadros and Miczek, 2009). Likewise, stress exposure does not persistently alter how defeated animals value cocaine under limited access conditions (Leonard et al., 2017). These findings likely reflect a more nuanced disruption in the pattern of cocaine-taking engendered by repeated exposures to social defeat stress. Stress experience appears to disrupt the mechanisms that control the maintenance or termination of cocaine self-administration bouts, resulting in prolonged binges that often persist beyond 24 h. This binge-like phenotype may reflect blunted inhibitory control over continued drug-maintained behavior or deficits in the typical circadian patterns that regulate drug-taking (Covington et al., 2008; Fitch and Roberts, 1993).
In contrast with intermittent social defeat stress, prolonged exposure to social defeat in a continuous stress model can suppress cocaine self-administration in male and female rats (Miczek et al., 2011; Shimamoto et al., 2015). Rats obtain significantly fewer drug infusions under control of a progressive ratio schedule of reinforcement and accumulate less cocaine during a binge. Continuously defeated animals also show a lower saccharin preference compared to non-defeated rats, suggesting that intense, prolonged social stress may suppress a range of reward-related behaviors.
Social adversity can increase the likelihood of relapse and a history of social subordination can engender latent vulnerability to re-engage in drug-seeking behaviors. When historically defeated rats are exposed to a drug-associated environment fifteen days into forced abstinence, they begin responding on a formerly cocaine-paired operandum (Fig. 2F; Holly et al., 2016). In this case, sensitivity to the appetitive effects of drug-associated cues lasts more than a month after the final stress exposure. As such, cumulative prior stress experiences may magnify drug craving during periods of abstinence. This may pose a considerable obstacle for individuals to maintain sobriety, even in the absence of ongoing adversity.
3.1.3. Opioids
Aggressive encounters actively engage and modify endogenous opioid systems in rodents (Chaijale et al., 2013; McLaughlin et al., 2006; Miczek et al., 2008; Nikulina et al., 2005). However, a definitive link between social stress and opioid consumption has yet to be firmly established in preclinical models. Limited evidence in rodent preparations suggests that social defeat stress may transiently enhance opioid-dependent behavior, similar to psychostimulants, though the substrates underlying opioid reinforcement are quite distinct from those of psychostimulants or alcohol (Badiani et al., 2011).
Consistent with the observation that social stress can enhance drug craving in opioid-dependent individuals, acute social stress exposure reinstates morphine-induced CPP, which was previously extinguished (Ribeiro Do Couto et al., 2006). These findings parallel the effects of other stressors (e.g., footshock, tail-pinch), although it has been argued that physical stress modalities may, in part, engage endogenous opioid systems in response to minor injury, producing an increase in subsequent opioid-seeking or intake. A comparison between the effects of social defeat stress and the effects of a non-contact witness stress on morphine intake indicates that social threat may be sufficient to enhance the reinforcing effects of morphine, even in the absence of physical defeat (Cooper et al., 2017). Yet, despite enduring changes in gene expression and neural activity, morphine preference is no longer apparent two weeks after stress exposure. Similarly, intermittent exposure to social defeat does not engender lasting effects on binge-like heroin self-administration (Cruz et al., 2011). Together, these findings suggest that ongoing adversity may significantly increase the likelihood of opioid use.
3.2. Male vs. female social stress and drug-taking
3.2.1. Alcohol
Alcohol use disorders are diagnosed more often in men than in women, possibly due to sex differences in stress reactivity or differences in the mechanisms used by men and women to cope with stressful life events (Cooper et al., 1992a; Greenfield et al., 2010; Nolen-Hoeksema, 2004). However, this gender gap is gradually diminishing (Greenfield et al., 2010; Grucza et al., 2008; Keyes et al., 2010; Wagner and Anthony, 2007) particularly with respect to stress-associated AUDs (Slopen et al., 2011). Some studies even report that women are at a greater risk of developing an AUD after experiencing repeated moderate or severe life stressors than men (Boden et al., 2014; Sannibale and Hall, 2011) and that women are more likely to relapse to heavy drinking in response to interpersonal conflict or unpleasant emotional states (Annis and Graham, 1995). With a significant subset of men and women suffering from stress-related AUDs, it is imperative to develop preclinical models to study the potentially dimorphic mechanisms underlying male and female social stress-escalated alcohol drinking (Becker and Koob, 2016).
In preclinical models of male social defeat stress, B6 or Swiss-derived outbred male mice exposed to repeated attacks from a dominant male resident later consume significantly more alcohol than non-defeated controls (Fig. 1; Albrechet-Souza et al., 2017; Hwa et al., 2016a; Karlsson et al., 2017; Kudryavtseva et al., 2006; Kudryavtseva et al., 1991; Nelson et al., 2018; Newman et al., 2018; Norman et al., 2015). Recently, we developed a model of social stress that promotes inter-female aggression to evaluate the effects of social defeat stress on alcohol consumption in female B6 mice (Newman et al. in prep). Using this protocol, nearly all resident CFW females will rapidly attack a novel B6 intruder female. Unlike other models of female social defeat stress that rely on either aggression from a lactating female conspecific (Brain and Haug 1992; Hashikawa et al., 2017; Holly et al., 2012; Jacobson-Pick et al., 2013; Shimamoto et al. 2011, 2015) or invasive experimental manipulations used to elicit pathological male aggression toward females (Harris et al., 2018; Takahashi et al., 2017), the present social defeat stress model takes an ethological approach by relying on characteristic female-directed female aggression. At present, there is no published model that evaluates the consequences of species-typical physical aggression in male and female mice using identical experimental conditions. Considering the notable differences in male and female patterns of aggressive behavior, employing sex-dependent social defeat stress models may be the ideal experimental approach to characterizing translational phenotypes in defeated animals.
Like B6 males that are intermittently defeated, continuously defeated B6 females consistently consume more 20% EtOH (w/v) than controls, and importantly, continuous social defeat stress does not permanently disrupt estrous cycling, suggesting that escalated drinking is not linked to defeat-induced acyclicity. This preclinical mouse model may be instructive in clarifying the biological mechanisms that contribute to the higher rate of co-occurring mood and substance use disorders in women than in men (Conway et al., 2006) and in screening potential pharmacotherapies for sex-dependent effects.
3.2.2. Cocaine
As with AUDs, more male cocaine-users are likely to meet the DSM-V diagnostic criteria for a substance use disorder (SUD) than women though this gender gap is gradually narrowing (Substance Abuse and Mental Health Services Administration, 2013). Compared to men, women are more likely to rapidly escalate to cocaine dependence (Cotto et al., 2010; McCance-Katz et al., 1999), to use cocaine more frequently (Chen and Kandel, 2002), and to relapse to cocaine use (Anker and Carroll, 2011; Becker and Hu, 2008; Becker and Koob, 2016; Ignjatova and Raleva, 2009). Clinical studies report greater subjective feelings of wellbeing in response to smoked cocaine in women, particularly during the follicular phase of the menstrual cycle when the ratio of estradiol to progesterone is high (Evans et al., 2002; Evans and Foltin, 2006; Sofuoglu et al., 1999). In the context of stress, cocaine-dependent females are often more sensitive to stress cues and imagery than healthy women or dependent men, which may increase relapse probability in these individuals (Back et al., 2005; Fox et al. 2006, 2008; Li et al., 2005; Potenza et al., 2012; Waldrop et al., 2010). Using translational preclinical models, we can investigate how interactions between long-lasting social stress effects and circulating female sex hormones may accelerate the trajectory of cocaine abuse in females compared to males (Lynch and Carroll, 1999; Roth et al., 2004).
Numerous studies illustrate that social defeat stress in male mice and rats can increase measures of cocaine self-administration. In B6 mice, submissive males that are exposed to 15–30 attack bites per day for ten consecutive days escalate their intravenous cocaine self-administration during 2 h sessions and show a heightened accumbal dopamine response to a challenge with a moderate dose of d-amphetamine relative to non-defeated controls (Han et al., 2015; Han et al., 2017; but not in Swiss-derived male mice: Yap and Miczek, 2007). Extensive parametric studies in rats identify four brief episodes of social defeat stress over the course of ten days as necessary and sufficient to prolong cocaine self-administration during a 24 h binge (Boyson et al. 2011, 2014; Covington et al., 2008; Covington and Miczek 2001, 2005; Cruz et al., 2011; Holly et al. 2015, 2016; Leonard et al., 2017; Miczek et al., 2011; Miczek and Mutschler, 1996; Quadros and Miczek, 2009; Yap et al., 2015).
Though several studies characterize the effects of social defeat stress on cocaine-taking in female rats, cocaine-taking has yet to be studied in defeated female mice. Female rats that are intermittently defeated four times over the course of 10 days by an aggressive, lactating female resident show a greater locomotor sensitization to acutely administered cocaine compared to males and a sustained increase in accumbal dopamine in response to cocaine. This effect of cocaine on locomotor sensitization is most apparent in defeated females tested in estrus (Holly et al., 2012). A spike in circulating estradiol during this phase may be associated with increased cocaine-induced dopamine signaling, suggesting that the trajectory of future cocaine-taking may depend on interactions between stress history and the estrous or menstrual cycle phase during the initial drug-taking experience (Calipari et al., 2017; Carroll et al., 2004; Lynch et al. 2000, 2001). Given unlimited access, intermittently defeated female rats take cocaine for a longer duration compared to control females and stressed or non-stressed males (Holly et al., 2012). Importantly, socially defeated males and females self-administer cocaine similarly during 30 min sessions (Haney et al., 1995); dysregulated, persistent self-administration by socially defeated females only becomes apparent when animals are given unlimited access (Holly et al., 2012), indicating that intermittent social defeat stress may impair neural mechanisms that regulate the cessation of drug-taking.
In a continuous defeat protocol, stressed females are defeated by lactating dams during 30 min episodes, occurring twice daily for 21 consecutive days. Stressed animals are then housed in protective cages in an aggressive resident's home cage during the periods between defeat experiences (Shimamoto et al. 2011, 2015). In contrast with the escalation in cocaine-taking observed in intermittently stressed females, a significant subset of continuously defeated females exhibits low saccharin preference and reduced cocaine self-administration compared to controls (i.e., an anhedonic phenotype; Shimamoto et al., 2015). The remaining defeated females display a significant saccharin preference and increases in cocaine self-administration compared to anhedonic individuals. Interestingly, non-defeated and anhedonic females demonstrate the expected cocaine-induced increase in accumbal dopamine whereas non-anhedonic stressed females have a substantially blunted dopamine response to acutely administered cocaine (Shimamoto et al., 2015). While intermittently stressed females likely take more cocaine due to an increase in the drug's rewarding effects (Holly et al., 2012), continuously defeated females may increase their drug-taking due to a suppression in cocaine's rewarding effects (Shimamoto et al., 2015).
These studies provide insight into a possible dopaminergic mechanism that may escalate cocaine self-administration through a shift from positive to negative reinforcement in continuously defeated female rats (Koob and Le Moal, 2008). Due to the prevalence of comorbid substance use and mood disorders in women, potential therapeutic interventions need to be tested using similar translational models of escalated drug self-administration in socially defeated female mice and rats.
3.2.3. Opioids
Among men and women, there has been a rapid increase in the rate of non-medical prescription opioid use and related overdose fatalities as well as a fivefold increase in deaths due to heroin overdoses (Centers for Disease Control and Prevention, 2017; Hall et al., 2008; Paulozzi et al., 2011; Substance Abuse and Mental Health Services Administration, 2013). Women are more likely than men to begin drug use with a valid medical prescription (McHugh et al., 2013), and unlike cocaine, heroin or alcohol, prescription opiate abuse is equally prevalent in men and women (Back et al., 2010; Green et al., 2009). The onset of heroin use among women is strongly associated with heroin use by a sexual partner whereas men tend to receive their first injection from a friend (Frajzyngier et al., 2007). It is apparent that different factors increase the risk of initiating opiate abuse in men and women; likewise, gender may also predict the trajectory of opiate abuse (Back et al., 2011; Hernandez-Avila et al., 2004) and treatment outcome (Greenfield et al., 2010; Jones et al., 2005; Najavits et al., 2007; Unger et al., 2010).
Nearly a quarter of non-medical prescription opioid users report psychological distress, with significantly higher rates among women relative to men (Back et al., 2010) Consistently, women are more likely to have co-occurring psychological and opioid use disorders than men (Back et al., 2010; Green et al., 2009) and women are more likely to abuse prescription opiates due to emotional issues and affective distress (Jamison et al., 2010). While numerous studies report sex-dependent interactions between stress and drugs of abuse (Fox and Sinha, 2009), only a handful of clinical and preclinical studies investigate the sex-dependent effects of social stress on prescription opiate and heroin abuse.
In one of the few related preclinical investigations, Laredo et al. (2015) report that intermittent social defeat stress by a same-sex aggressive resident impedes subsequent behavioral flexibility and downregulates μ-opioid receptor binding in the orbitofrontal cortex of male California mice (Peromyscus californicus). In contrast, female California mice are insensitive to these effects of social defeat stress. Behavioral flexibility, or the capacity for behavioral adjustment in response to changes in external or internal stimuli, may correlate with the initiation and continuation of drug use, the effectiveness of specific interventions and the likelihood of maintained abstinence (Winstanley et al., 2010; Zilverstand et al., 2018). Particularly in males, social stress-induced behavioral inflexibility and changes in μ-opioid receptor expression may maintain drug consumption, and cognitive interventions in conjunction with pharmacotherapy may be ideal for targeting the inflexible behaviors that contribute to compulsive drug use (Konova et al., 2013; Zilverstand et al., 2018). The behavioral and neural mechanisms that promote sex differences in the trajectory of opioid abuse demand additional investigation, particularly in male and female drug users with a significant history of social stress and in male and female rodent social defeat stress models.
4. Stress-induced neuroplasticity and increased drug use
Social stress during acute confrontations persistently activates neural and endocrine processes that promote drug-seeking and taking. Increasing the duration and frequency of stress exposure can yield neuroadaptations that result in long-term changes in neural and behavioral responses to drugs of abuse. While noradrenergic and serotonergic projections have been the focus of many studies (Der-Avakian et al., 2014; Maier and Watkins, 2005; Valentino and Van Bockstaele, 2008), the mesocorticolimbic dopamine system has garnered significant attention because it is both recruited by stress (Imperato et al., 1989) and required for the reinforcing effects of alcohol (Imperato and DiChiara, 1986), cocaine (de Wit and Wise, 1977) and other drugs of abuse (DiChiara and Imperato, 1988). During an aggressive confrontation with a dominant male, subordinate animals show a marked increase in burst firing within a subset of VTA dopamine neurons (Anstrom et al., 2009; Barik et al., 2013). Accordingly, social stress increases extracellular dopamine release within the NAc and mPFC (Barik et al., 2013; Han et al., 2015; Holly et al., 2015; Tidey and Miczek 1996, 1997), and this increase is greatest in rats with prior stress experience. These findings suggest that repeated exposure to social stress can induce adaptive modifications in mesocorticolimbic dopamine activity to escalate alcohol and psychostimulant intake.
An array of peptide signaling systems are mobilized in response to perceived threat and many of these converge on mesocorticolimbic pathways in order to coordinate adaptive coping behaviors. Recurring exposure to social stress persistently alters several peptide and hormone signaling systems that have been implicated in cocaine or alcohol abuse (e.g., opioids, glucocorticoids, orexin, oxytocin, corticotropin releasing factor, urocortin; Golden et al., 2011; Holly et al., 2016; Litvin et al., 2011; Nikulina et al., 1999; Nocjar et al., 2012). Among these, CRF has been comprehensively studied as a candidate target for the interactions between stress and drug abuse (Koob, 2010). Briefly, the CRF/urocortin system mediates several behavioral and physiological effects of psychostimulants (Lodge and Grace, 2005; Lu et al., 2003) and alcohol (Funk et al., 2007; Ryabinin et al., 2002; Valdez et al., 2002; Zorrilla et al., 2014), and may exert control over appetitive behavior through direct modulation of dopamine neurotransmission in both the VTA (Ungless et al., 2003; Wanat et al. 2008, 2013) and NAc (Lemos et al., 2012; Peciña et al., 2006).
4.1. Alcohol
Exposure to repeated stress can increase neural and behavioral responding to future stimuli that similarly engage mesocorticolimbic dopamine circuitry. This mechanism may enhance drug abuse vulnerability in a subset of individuals who are exposed to episodic social defeat stress. In mice, cross-sensitization between defeat stress and alcohol leads to significant changes in accumbal dopamine release; in response to a low dose of alcohol (0.1 g/kg), repeatedly defeated animals show an increase in evoked dopamine release (Yavich and Tiihonen, 2000) while higher doses (2.0 g/kg) reduce dopamine overflow (Deal et al., 2018; Yavich and Tiihonen, 2000). Stress-induced neuroadaptations may alter the rewarding effects of alcohol to increase drinking in socially defeated individuals.
Some neuropeptides, including CRF, can modulate mesocorticolimbic dopamine release, causing lasting neural changes that may lead to stress-escalated alcohol drinking (Hwa et al., 2016a; Ostroumov et al., 2016). Following ten days of continuous social defeat stress, male B6 mice exhibit a unique pattern of chronically elevated regional brain activation along with an increase in repeatedly activated cells, as determined by manganese-enhanced magnetic resonance imaging and ΔFosB immunohistochemistry, respectively. These markers of increased activity are detected in brain areas such as the prefrontal cortex, ventral hippocampus, and also in the bed nucleus of the stria terminalis (BNST; Laine et al., 2017). Crosstalk between neural circuits involved in stress and reward processing may rely on networks in the BNST and central amygdala (CeA; Erb and Stewart, 1999; Lebow and Chen, 2016; Silberman et al., 2013). Novel populations of CRF-expressing, GABAergic spiny projection neurons extend directly from either the CeA or the anterior BNST to the VTA (Dedic et al., 2018). These cells are characterized in part by their expression of calcium/calmodulin-dependent protein kinase 2α, which is involved in synaptic plasticity and may render these projection neurons particularly susceptible to long-lasting effects of social defeat stress on dopamine signaling (Licata and Pierce, 2003; Lisman et al., 2002; Robison, 2014).
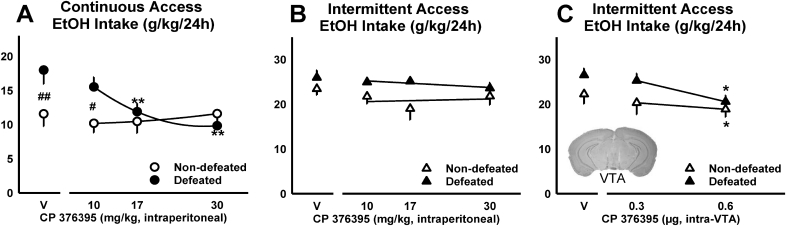
In male B6 mice, systemic or intra-VTA injections of the CRF-R1 antagonist, CP376395, can transiently reduce alcohol consumption in intermittently defeated and non-defeated mice during the initial four hours of intermittent or continuous access to 20% EtOH (Hwa et al. 2016a, 2016b; Newman et al., 2018). In contrast, only systemically administered CP376395 selectively reduces social stress-escalated drinking for 24 h, specifically in mice with continuous access to alcohol (Fig. 3; Newman et al., 2018). These findings reveal that CRF-R1 antagonists likely produce their social stress-specific effects on alcohol intake by acting outside of the VTA (Newman et al., 2018). In addition, the intermittent access to alcohol protocol, which escalates drinking and alcohol preference by alternating between days of alcohol access and days of forced abstinence (Fig. 1; Hwa et al., 2011), seems to prevent CRF-R1 antagonists from producing long-lasting, social defeat stress-specific effects on drinking (Fig. 3).
Fig. 3.
Daily alcohol intake (g/kg/24 h) shown as mean ± SEM for socially defeated and non-defeated C57BL/6J males with either (A) continuous or (B, C) intermittent access to alcohol. Mice were treated with doses of the CRF-R1 antagonist, CP376395, via systemic injections (A, B; adapted from Newman et al., 2018), or via microinfusions, delivered directly into the ventral tegmental area (VTA; C; adapted from Hwa et al., 2016a). Best-fit curves are in black. *p < 0.05, **p < 0.01 vehicle vs. drug; #p < 0.05, ##p < 0.01 non-defeated vs. defeated.
Interestingly, when B6 males are administered CP376395 into the BNST, only non-defeated mice reduce their continuous access alcohol intake and, while a history of intermittent defeat stress increases BNST CRH mRNA, CRF-R1 transcript remains unaltered (Albrechet-Souza et al., 2017). If protein levels parallel mRNA expression data, this could signify a social defeat stress-induced increase in CRF content in GABAergic, VTA-projecting BNST neurons (Dedic et al., 2018). Such mechanisms require additional exploration, as it appears that both social defeat stress and the pattern of alcohol intake produce changes in CRF/CRF-R1 signaling that determine the effectiveness of CRF-R1 antagonists.
Regarding sex differences, studies identify dimorphic patterns of extrahypothalamic CRF signaling in male versus female rats. The extent to which CRF action on CRF-R1 results in receptor internalization is significantly greater in males than in females, indicating that females may be subject to greater levels of basal CRF activity due to a less responsive, cellular negative feedback mechanism (in locus coeruleus: Bangasser et al., 2010; Curtis et al., 2006; Valentino et al., 2013). Acute stress-induced release of CRF prompts CRF-R1 binding with β-arrestin 2, which promotes receptor internalization in males, but not in females. This may serve as an underlying mechanism for increased susceptibility to stress-related psychopathologies in females, and may explain some sex-specific effects of drugs that target the CRF system (Becker and Koob, 2016; Valentino et al., 2013).
Though antagonism of CRF-R1 selectively suppresses social defeat stress-escalated alcohol drinking in male B6 mice (Fig. 3A; Newman et al., 2018), CP376395 does not reduce stress-escalated alcohol consumption in defeated B6 females (Newman et al. in prep). The CRF-R1 antagonist verucerfont was similarly ineffective in suppressing alcohol craving in abstaining, alcohol-dependent women following evaluation stress during the TSST (Schwandt et al., 2016). While CRF-R1 antagonism may be effective in treating ongoing, excessive alcohol consumption in defeated males, it may be more appropriate to address reduced negative feedback of CRF signaling in socially stressed females. Dimorphic patterns of CRF-R1 trafficking in response to stress require further investigation using male and female mouse models of social defeat stress-escalated alcohol drinking, with a focus on brain areas characterized as sexually dimorphic.
4.2. Cocaine
Episodic exposure to social defeat stress results in a profound sensitization to salient behavioral and neurochemical consequences of cocaine administration. An extensive literature demonstrates that social defeat stress can potentiate the motor stimulant effects of cocaine or d-amphetamine for months after the final defeat experience (Boyson et al. 2011, 2014; Covington et al., 2005; Covington and Miczek, 2001; Han et al., 2015; Holly et al., 2012; Miczek et al. 1999a, 1999b; Nikulina et al. 1998, 2004; Yap et al., 2005). Similar effects can be observed after chronic cocaine administration, suggesting that stress and drug exposure may induce behavioral sensitization through similar mechanisms (footshock stress: Herman et al., 1984; MacLennan and Maier, 1983). Indeed, in vivo microdialysis studies indicate that defeat-induced locomotor sensitization parallels changes to the neurochemical response to cocaine within mesolimbic dopamine circuits. Specifically, experience with intermittent social defeat stress potentiates cocaine or amphetamine-induced dopamine release within the NAc of rats and mice (Boyson et al., 2014; Han et al., 2015; Holly et al., 2012; Miczek et al., 2011).
Stressors may facilitate glutamatergic synaptic plasticity within the VTA, thereby strengthening associations between drugs of abuse and drug-predictive cues (Robinson and Berridge, 1993; Vanderschuren and Kalivas, 2000). Intermittent social defeat stress can enhance NMDA receptor-mediated long term potentiation (LTP) by increasing IP3R sensitivity in VTA dopamine neurons (Stelly et al., 2016). Glutamatergic synapses in the VTA are more readily strengthened during a 30-day period after stress exposure, and during this time, rats show enhanced acquisition of cocaine-induced CPP (Stelly et al., 2016). Interestingly, in rats, pharmacological inhibition of VTA NMDA receptors during social defeat can prevent the induction of locomotor sensitization and can block the effects of stress on cocaine self-administration during a binge (Covington et al., 2008). Certain activity-dependent changes in cellular states can increase the probability of LTP (Abraham, 2008); this type of metaplasticity may promote drug-paired contextual cue learning and blocking this phenomenon may serve as a promising direction for therapeutic interventions targeting cocaine addiction and relapse.
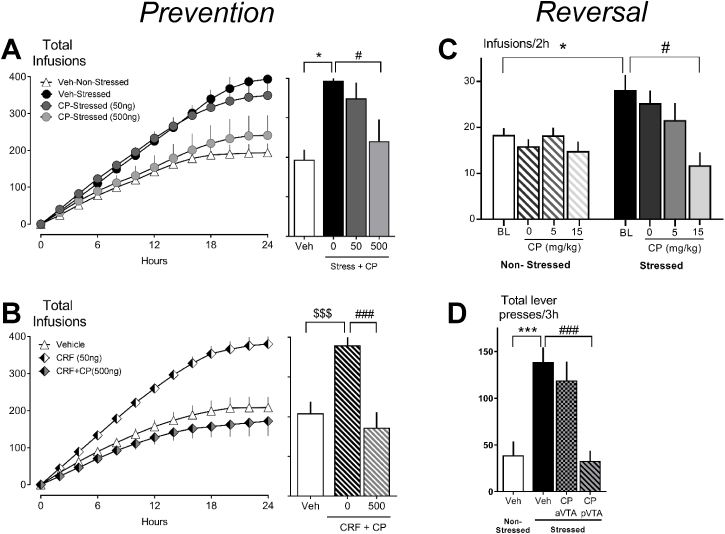
CRF signaling is critical for the long-term maladaptive consequences of social stress on cocaine self-administration and appears to play an important role in the acute initiation of drug-seeking behavior (Blacktop et al., 2011; Specio et al., 2008; Wang et al., 2005). A single social defeat experience elicits a robust but transient phasic increase in extracellular CRF within the posterior VTA. In animals with a history of repeated social defeat stress, the CRF response to social stress or cocaine is enhanced and basal CRF concentrations become and remain tonically elevated throughout the VTA (Han et al., 2017; Holly et al., 2016). Elevated CRF tone may interact with mesolimbic DA outputs to augment later stress- or cocaine-dependent behavioral responses. Indeed, defeat-experienced animals demonstrate escalated rates of cocaine self-administration (Han et al., 2017) and show a robust context-induced reinstatement of cocaine seeking compared to control animals (Fig. 2G; Holly et al., 2016). Importantly, both of these effects can be attenuated with compounds that antagonize CRF signaling (Fig. 4D).
Fig. 4.
The CRF-R1 antagonist, CP376395 prevents escalated cocaine consumption across a 24 h “binge” when administered prior to (A) social defeat stress or (B) prior to intra-VTA microinfusion of CRF; total number of cocaine infusions (0.3 mg/kg) across a 24 h shown as mean ± SEM. (A, adapted from Boyson et al., 2014; B, adapted from Leonard et al., 2017); and acute treatment with CP376395 reverses stress-escalated (C) cocaine intake during a 2 h access session or (D) cocaine-seeking responses during context-induced reinstatement (C, adapted from Han et al., 2017; D, adapted from Holly et al., 2016). *p < 0.05, **p < 0.01, ***p < 0.001 non-defeated vs. defeated; $p < 0.05, $$p < 0.01, $$$p < 0.001 vehicle-microinfusion vs. CRF-microinfusion; #p < 0.05, ##p < 0.01, ###p < 0.001 vehicle vs. CP376395.
These findings collectively suggest that social defeat engenders a neural state that is conducive to relapse-like behavior, and that maladaptive cocaine-dependent behavior can be reversed by the blockade of CRF receptors in the VTA (Fig. 4C-D). In support of this hypothesis, application of CRF receptor antagonists are also sufficient to prevent the behavioral consequences of intermittent stress on subsequent cocaine self-administration (Fig. 4A-B). Intra-VTA microinfusions of either CRF-R1 or CRF-R2 antagonists prior to social stress episodes prevent stress-escalated drug-taking (Boyson et al. 2011, 2014; Burke and Miczek, 2015; Han et al., 2017). On the other hand, exogenous CRF administration in place of social defeat only partially reproduces the behavioral effects of stress on drug-taking (Leonard et al., 2017). Rats that receive repeated deliveries of CRF into the VTA exhibit a pattern of cocaine self-administration that is relatively inelastic to changes in response cost which may reflect more discrete changes in effort-related responding. CRF-treated rats do not, however, engage in prolonged cocaine binges in a pattern that is characteristic of stress-experienced animals. In sum, patterns of cocaine self-administration differ between stress-experienced animals and CRF-treated animals, highlighting the importance of identifying precise behavioral deficits that tie CRF to social defeat stress-induced neuroadaptations.
4.3. Opioids
Stress-sensitive, noradrenergic cells in the locus coeruleus (LC) are regulated, in part, by CRF and the endogenous opioid, enkephalin. Acting via CRF-R1 and μ-opioid receptors, respectively, these neuropeptides have opposing effects; while CRF increases LC discharge rate, enkephalin reduces LC activity (Valentino and Van Bockstaele, 2008). In male rats, exposure to repeated social defeat stress produces a delayed-onset reduction in neural firing of LC cells, indicating a shift from balanced opioid and CRF signaling in favor of preferential opioid receptor activation (Chaijale et al., 2013). For at least two weeks after the final social defeat experience, treatment with the competitive opioid receptor antagonist, naloxone, engenders a significant increase in LC activity, specifically in stressed rats. Through its rapid, competitive binding to opioid receptors, naloxone disinhibits the LC and reveals a substantial upregulation in opioid receptor-mediated suppression of LC discharge (Chaijale et al., 2013). These cellular effects are observed in conjunction with somatic signs of opioid withdrawal including teeth chattering and body shakes. In addition, repeated social defeats can increase LC firing in response to reward, indicating augmented reward salience in stressed animals (Chaijale et al., 2015). These findings suggest that individuals with a history of social defeat stress may experience a withdrawal-like state and may preferentially allocate attentional resources toward rewarding stimuli; future studies will determine whether this phenotype also promotes escalated opiate self-administration (Chaijale et al., 2013, but Cruz et al., 2011).
The development of adaptive coping behaviors likely contributes to the severity of perceived stress, and can reduce the affective consequences of stress-related psychopathologies (i.e. anxiety, depression, posttraumatic stress disorder; Wood and Bhatnagar, 2015). In rats, coping strategy in response to social defeat stress exposure can be distinguished based on the latency to submit by the male intruder during a resident-intruder confrontation. Rats characterized as short-latency (SL) responders exhibit a depressive-like, stress-vulnerable phenotype while long-latency (LL) individuals do not show anhedonic-like behavior and are therefore deemed stress-resilient (Wood et al. 2010, 2012, 2015). Interestingly, all rats are SL responders during their initial defeat experience, suggesting that through repeated stress exposure, a subset of rats eventually shows the resilient-like phenotype (i.e., LL; Reyes et al., 2015). A thorough characterization of LC afferents provides evidence for two, divergent patterns of neuroadaptations in response to repeated social defeat stress exposure. While SL rats display an increase in the activation of central amygdala CRF projections to the LC and a suppression of enkephalin-positive LC afferents, LL rats show the opposite pattern of cellular activation with a suppression in CRF signaling and greater LC innervation by enkephalin-positive projections (Reyes et al., 2015). Though LL rats are stress-resilient in the context of depressive-like behaviors, this subgroup may be more susceptible to increased opiate consumption due to negative reinforcement, resulting from a potential withdrawal-like state (Chaijale et al., 2013; Koob, 2008). Further work should explore whether opioid self-administration selectively increases in SL or LL rats.
5. Conclusions
For a broad range of mammalian species, social stress represents a unique ethological challenge. In rodent models, recurring exposure to social defeat appears to produce a distinct profile of neuroadaptations that translates most readily to the repercussions of social stress in humans (Golden et al., 2011; Miczek et al., 2008). Varying the length of exposure to social threats and attacks can have divergent effects on behavioral and neural outcomes. Brief intermittent episodes of social stress often escalate ethanol and cocaine intake, particularly under extended access conditions. Conversely, continuous exposure to social stress often suppresses cocaine intake in rats, though continuous and chronic stress can escalate alcohol drinking in male and female mice. The direct connection between social stress experience and subsequent changes in drug-taking illustrates a potential role for extrahypothalamic CRF and mesocorticolimbic dopamine.
Stress-induced neuroadaptations in CRF modulation of monoamines are key candidate mechanisms for social defeat stress to escalate alcohol consumption and cocaine self-administration. As demonstrated by the selective effects of CRF-R1 antagonists in socially defeated mice (Fig. 3A; Newman et al., 2018) and rats (Boyson et al. 2011, 2014; Burke and Miczek, 2015; Han et al., 2017), CRF likely plays a critical role in stress-escalated drug-taking. However, presynaptic CRF or urocortin release in conjunction with a primary neurotransmitter, such as glutamate or GABA, may ultimately shape neuroplasticity in postsynaptic monoaminergic cells. Therefore, investigating presynaptic and synaptic interactions between CRF and amino acid neurotransmitters (e.g., co-storage, co-release, modes of synaptic plasticity) may reveal stress-induced changes in co-release mechanisms to yield more effective and targeted therapeutic options.
This review focuses primarily on drug consumption in animals that are repeatedly defeated; however, a growing body of literature also demonstrates the intensely reinforcing effects of winning aggressive encounters (Aleyasin et al., 2018b). Aggressive male mice will work for the opportunity to attack a submissive intruder (Covington et al., 2018; Fish et al. 2002, 2005; Golden et al., 2017b), and will also favor an aggression-paired chamber during CPP testing (Aleyasin et al., 2018a; Golden et al., 2017a). Repeatedly winning aggressive encounters may upregulate dopamine signaling, thereby increasing the incentive to win subsequent confrontations (Becker and Marler, 2015; Couppis and Kennedy, 2008; Filipenko et al., 2001; Kudryavtseva et al., 1999; Schwartzer et al., 2013). Neuroadaptations in dopamine signaling could also render victorious individuals more sensitive to the pro-aggressive effects of alcohol; indeed, aggressive male B6 residents that are repeatedly treated with alcohol show a robust increase in their motivation to engage in future, aggressive encounters (Covington et al., 2018). Additional studies are necessary to illuminate how repeatedly winning aggressive encounters might influence dopaminergic transmission and subsequent, voluntary drug consumption.
While most preclinical studies of social defeat stress evaluate defeated males, recently developed protocols in socially defeated female mice will be instructive for drug self-administration research (Harris et al., 2018; Takahashi et al., 2017; Newman et al. in prep). Considering the overwhelming prevalence of comorbid substance use and mood disorders in women, there is a significant demand for research that investigates the molecular, neural and chemical mechanisms that link sex to stress-related psychopathologies. To advance the targeted manipulation of stress-sensitive cell populations, sex-dependent neuroadaptations to social defeat stress must be thoroughly characterized using cell- and pathway-specific genetic techniques applied to sexually dimorphic brain regions such as the BNST and LC.
Social stress exposure largely increases the risk of drug abuse in both clinical and preclinical populations; however, only a subset of individuals with a history of social stress exhibits stress-escalated drug-taking. Substantial individual variability in stress-related behavioral outcomes suggests that discrete psychobiological factors contribute to the overall pathological impact of a given stressor (de Boer et al., 2017; Feder et al., 2009). While distinct behavioral phenotypes may be useful in predicting or assessing stress vulnerability, stress susceptibility and resilience should be considered in the context of specific dependent variables or experimental endpoints. For instance, although active coping during social stress encounters tends to predict resilience to depressive-like behaviors in rats (Wood et al., 2010), individuals that actively engage with dominant aggressors tend to exhibit increased binge-like cocaine self-administration (Boyson et al., 2016) and more pronounced drug-seeking in response to an acute stress experience (Manvich et al., 2016). Similarly, female rats characterized as stress-susceptible according to their anhedonic-like profile of saccharin preference subsequently self-administer less cocaine than their non-anhedonic counterparts, suggesting a stress-resilient drug-taking phenotype (Shimamoto et al., 2015). In terms of alcohol, socially defeated male mice that display a depressive-like phenotype during social interaction testing subsequently consume more alcohol than stress-resilient animals (Nelson et al., 2018), indicating that stress-escalated drinking and depressive-like behaviors may share some underlying biological mechanisms. These observations highlight the demand for further investigations that characterize the specific behavioral and biological factors that predict vulnerability to distinct, stress-related psychopathologies, including drug and alcohol use disorders.
6. Summary highlights
-
•
Both the pattern of social defeat stress and drug access influence the effects of social stress on drug consumption, and there is strong evidence that these conditions are drug-specific.
-
•
Animal models of repeated or continuous social defeat stress can persistently and preferentially escalate voluntary alcohol consumption when the onset of alcohol access occurs days after the final stress episode.
-
•
Repeated episodic social defeat stress can increase measures of cocaine self-administration in rats given unrestricted access to cocaine during a 24–48 h long binge. By contrast, exposure to continuous stress tends to suppress cocaine-taking in stress-vulnerable individuals.
-
•
The incidence of drug abuse is increasing, particularly among women. Newly developed preclinical models can be used to evaluate the sexually dimorphic effects of social defeat stress on pathological drug consumption.
-
•
Neural adaptations that promote the escalation of drug-taking in the hours, days or weeks following a social defeat experience may involve changes in tonic CRF and in CRF modulation of mesocorticolimbic dopamine signaling.
Funding
This work was supported by the National Institutes of Health [R01AA013983 and R01DA031734 (K.A.M.) and F31AA025827 (E.L.N.)].
Acknowledgements
The authors would like to thank J. Thomas Sopko for his assistance during manuscript preparation.
References
- Abraham W.C. Metaplasticity: tuning synapses and networks for plasticity. Nat. Rev. Neurosci. 2008;9:387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- Adinoff B., Leonard D., Price J., Javors M.A., Walker R., Brown E.S., Xiao H., Rao U. Adrenocortical sensitivity, moderated by ongoing stress, predicts drinking intensity in alcohol-dependent men. Psychoneuroendocrinology. 2017;76:67–76. doi: 10.1016/j.psyneuen.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S., Badiani A., Miczek K.A., Müller C.P. Non-pharmacological factors that determine drug use and addiction. Neurosci. Biobehav. Rev. 2018 doi: 10.1016/j.neubiorev.2018.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrechet-Souza L., Viola T.W., Grassi-Oliveira R., Miczek K.A., de Almeida R.M.M. Corticotropin releasing factor in the bed nucleus of the stria terminalis in socially defeated and non-stressed mice with a history of chronic alcohol intake. Front. Pharmacol. 2017;8:762. doi: 10.3389/fphar.2017.00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleyasin H., Flanigan M.E., Golden S.A., Takahashi A., Menard C., Pfau M.L., Multer J., Pina J., McCabe K.A., Bhatti N., Hodes G.E., Heshmati M., Neve R.L., Nestler E.J., Heller E.A., Russo S.J. Cell-type-specific role of ΔFosB in nucleus accumbens in modulating intermale aggression. J. Neurosci. 2018;38:5913–5924. doi: 10.1523/JNEUROSCI.0296-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleyasin H., Flanigan M.E., Russo S.J. Neurocircuitry of aggression and aggression seeking behavior: nose poking into brain circuitry controlling aggression. Curr. Opin. Neurobiol. 2018;49:184–191. doi: 10.1016/j.conb.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A.P., Kennedy P.J., Dockray S., Cryan J.F., Dinan T.G., Clarke G. The trier social stress test: principles and practice. Neurobiol Stress. 2017;6:113–126. doi: 10.1016/j.ynstr.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . American Psychiatric Publishing; Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition: DSM-5. [Google Scholar]
- Anacker A.M., Smith M.L., Ryabinin A.E. Establishment of stable dominance interactions in prairie vole peers: relationships with alcohol drinking and activation of the paraventricular nucleus of the hypothalamus. Soc. Neurosci. 2014;9:484–494. doi: 10.1080/17470919.2014.931885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker J.J., Carroll M.E. Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr Top Behav Neurosci. 2011;8:73–96. doi: 10.1007/7854_2010_93. [DOI] [PubMed] [Google Scholar]
- Annis H.M., Graham J.M. Profile types on the Inventory of Drinking Situations: implications for relapse prevention counseling. Psychol. Addict. Behav. 1995;9:176–182. [Google Scholar]
- Anstrom K.K., Miczek K.A., Budygin E.A. Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats. Neuroscience. 2009;161:3–12. doi: 10.1016/j.neuroscience.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back S.E., Brady K.T., Jackson J.L., Salstrom S., Zinzow H. Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology. 2005;180:169–176. doi: 10.1007/s00213-004-2129-7. [DOI] [PubMed] [Google Scholar]
- Back S.E., Gros D.F., Price M., LaRowe S., Flanagan J., Brady K.T., Davis C., Jaconis M., McCauley J.L. Laboratory-induced stress and craving among individuals with prescription opioid dependence. Drug Alcohol Depend. 2015;155:60–67. doi: 10.1016/j.drugalcdep.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back S.E., Hartwell K., Desantis S.M., Saladin M., McRae-Clark A.L., Price K.L., Moran-Santa Maria M.M., Baker N.L., Spratt E., Kreek M.J., Brady K.T. Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug Alcohol Depend. 2010;106:21–27. doi: 10.1016/j.drugalcdep.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back S.E., Payne R.L., Wahlquist A.H., Carter R.E., Stroud Z., Haynes L., Hillhouse M., Brady K.T., Ling W. Comparative profiles of men and women with opioid dependence: results from a national multisite effectiveness trial. Am. J. Drug Alcohol Abuse. 2011;37:313–323. doi: 10.3109/00952990.2011.596982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiani A., Belin D., Epstein D., Calu D., Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nat. Rev. Neurosci. 2011;12:685–700. doi: 10.1038/nrn3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahi A. Increased anxiety, voluntary alcohol consumption and ethanol-induced place preference in mice following chronic psychosocial stress. Stress. 2013;16:441–451. doi: 10.3109/10253890.2012.754419. [DOI] [PubMed] [Google Scholar]
- Bangasser D.A., Curtis A., Reyes B.A., Bethea T.T., Parastatidis I., Ischiropoulos H., Van Bockstaele E.J., Valentino R.J. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol. Psychiatr. 2010;15 doi: 10.1038/mp.2010.66. 877, 896–877, 904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barik J., Marti F., Morel C., Fernandez S.P., Lanteri C., Godeheu G., Tassin J.P., Mombereau C., Faure P., Tronche F. Chronic stress triggers social aversion via glucocorticoid receptor in dopaminoceptive neurons. Science. 2013;339:332–335. doi: 10.1126/science.1226767. [DOI] [PubMed] [Google Scholar]
- Becker E.A., Marler C.A. Postcontest blockade of dopamine receptors inhibits development of the winner effect in California mice (peromyscus californicus) Behav. Neurosci. 2015;129:205–213. doi: 10.1037/bne0000043. [DOI] [PubMed] [Google Scholar]
- Becker H.C., Lopez M.F., Doremus-Fitzwater T.L. Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology. 2011;218:131–156. doi: 10.1007/s00213-011-2443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J.B., Hu M. Sex differences in drug abuse. Front. Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J.B., Koob G.F. Sex differences in animal models: focus on addiction. Pharmacol. Rev. 2016;68:242–263. doi: 10.1124/pr.115.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi R.E., Broccoli L., Hirth N., Justice N.J., Deussing J.M., Hansson A.C., Spanagel R. Dissociable role of corticotropin releasing hormone receptor subtype 1 on dopaminergic and D1 dopaminoceptive neurons in cocaine seeking behavior. Front. Behav. Neurosci. 2017;11:221. doi: 10.3389/fnbeh.2017.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacktop J.M., Seubert C., Baker D.A., Ferda N., Lee G., Graf E.N., Mantsch J.R. Augmented cocaine seeking in response to stress or CRF delivered into the ventral tegmental area following long-access self-administration is mediated by CRF receptor type 1 but not CRF receptor type 2. J. Neurosci. 2011;31:11396–11403. doi: 10.1523/JNEUROSCI.1393-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard R.J., Flores T., Magee L., Weiss S., Blanchard D.C. Pregrouping aggression and defense scores influences alcohol consumption for dominant and subordinate rats in visible burrow systems. Aggress. Behav. 1992;18:459–467. [Google Scholar]
- Blanchard R.J., Hori K., Tom P., Blanchard D.C. Social structure and ethanol consumption in the laboratory rat. Pharmacol. Biochem. Behav. 1987;28:437–442. doi: 10.1016/0091-3057(87)90502-8. [DOI] [PubMed] [Google Scholar]
- Boden J.M., Fergusson D.M., Horwood L.J. Associations between exposure to stressful life events and alcohol use disorder in a longitudinal birth cohort studied to age 30. Drug Alcohol Depend. 2014;142:154–160. doi: 10.1016/j.drugalcdep.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Boyson C.O., Holly E.N., Burke A.R., Montagud-Romero S., DeBold J.F., Miczek K.A. Maladaptive choices by defeated rats: link between rapid approach to social threat and escalated cocaine self-administration. Psychopharmacology. 2016;233:3173–3186. doi: 10.1007/s00213-016-4363-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyson C.O., Holly E.N., Shimamoto A., Albrechet-Souza L., Weiner L.A., DeBold J.F., Miczek K.A. Social stress and CRF-dopamine interactions in the VTA: role in long-term escalation of cocaine self-administration. J. Neurosci. 2014;34:6659–6667. doi: 10.1523/JNEUROSCI.3942-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyson C.O., Miguel T.T., Quadros I.M., DeBold J.F., Miczek K.A. Prevention of social stress-escalated cocaine self-administration by CRF-R1 antagonist in the rat VTA. Psychopharmacology. 2011;218:257–269. doi: 10.1007/s00213-011-2266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain P.F., Haug M. Hormonal and neurochemical correlates of various forms of animal aggression. Psychoneuroendocrinology. 1992;17:537–551. doi: 10.1016/0306-4530(92)90014-x. [DOI] [PubMed] [Google Scholar]
- Brown S.A., Vik P.W., McQuaid J.R., Patterson T.L., Irwin M.R., Grant I. Severity of psychosocial stress and outcome of alcoholism treatment. J. Abnorm. Psychol. 1990;99:344–348. doi: 10.1037//0021-843x.99.4.344. [DOI] [PubMed] [Google Scholar]
- Brown S.A., Vik P.W., Patterson T.L., Grant I., Schuckit M.A. Stress, vulnerability and adult alcohol relapse. J. Stud. Alcohol. 1995;56:538–545. doi: 10.15288/jsa.1995.56.538. [DOI] [PubMed] [Google Scholar]
- Bruchas M.R., Schindler A.G., Shankar H., Messinger D.I., Miyatake M., Land B.B., Lemos J.C., Hagan C.E., Neumaier J.F., Quintana A., Palmiter R.D., Chavkin C. Selective p38alpha MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. Neuron. 2011;71:498–511. doi: 10.1016/j.neuron.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke A.R., DeBold J.F., Miczek K.A. CRF type 1 receptor antagonism in ventral tegmental area of adolescent rats during social defeat: prevention of escalated cocaine self-administration in adulthood and behavioral adaptations during adolescence. Psychopharmacology. 2016;233:2727–2736. doi: 10.1007/s00213-016-4336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke A.R., Miczek K.A. Escalation of cocaine self-administration in adulthood after social defeat of adolescent rats: role of social experience and adaptive coping behavior. Psychopharmacology. 2015;232:3067–3079. doi: 10.1007/s00213-015-3947-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell E.E., Riccio D.C. Alcohol self-administration in rats: modulation by temporal parameters related to repeated mild social defeat stress. Alcohol. 2010;44:265–274. doi: 10.1016/j.alcohol.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Calipari E.S., Juarez B., Morel C., Walker D.M., Cahill M.E., Ribeiro E., Roman-Ortiz C., Ramakrishnan C., Deisseroth K., Han M.H., Nestler E.J. Dopaminergic dynamics underlying sex-specific cocaine reward. Nat. Commun. 2017;8:13877. doi: 10.1038/ncomms13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M.E., Lynch W.J., Roth M.E., Morgan A.D., Cosgrove K.P. Sex and estrogen influence drug abuse. Trends Pharmacol. Sci. 2004;25:273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . U.S. Department of Health and Human Services; Bethesda, MD: 2017. Annual Surveillance Report of Drug-related Risks and Outcomes - United States, 2017. [Google Scholar]
- Chaijale N.N., Curtis A.L., Wood S.K., Zhang X.Y., Bhatnagar S., Reyes B.A., Van Bockstaele E.J., Valentino R.J. Social stress engages opioid regulation of locus coeruleus norepinephrine neurons and induces a state of cellular and physical opiate dependence. Neuropsychopharmacology. 2013;38:1833–1843. doi: 10.1038/npp.2013.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaijale N.N., Snyder K., Arner J., Curtis A.L., Valentino R.J. Repeated social stress increases reward salience and impairs encoding of prediction by rat locus coeruleus neurons. Neuropsychopharmacology. 2015;40:513–523. doi: 10.1038/npp.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Kandel D. Relationship between extent of cocaine use and dependence among adolescents and adults in the United States. Drug Alcohol Depend. 2002;68:65–85. doi: 10.1016/s0376-8716(02)00086-8. [DOI] [PubMed] [Google Scholar]
- Conway K.P., Compton W., Stinson F.S., Grant B.F. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J. Clin. Psychiatr. 2006;67:247–257. doi: 10.4088/jcp.v67n0211. [DOI] [PubMed] [Google Scholar]
- Cooper M.L., Russell M., Skinner J.B., Frone M.R., Mudar P. Stress and alcohol use: moderating effects of gender, coping, and alcohol expectancies. J. Abnorm. Psychol. 1992;101:139–152. doi: 10.1037//0021-843x.101.1.139. [DOI] [PubMed] [Google Scholar]
- Cooper M.L., Russell M., Skinner J.B., Windle M. Development and validation of a three-dimensional measure of drinking motives. Psychol. Assess. 1992;4:123–132. [Google Scholar]
- Cooper S.E., Kechner M., Caraballo-Perez D., Kaska S., Robison A.J., Mazei-Robison M.S. Comparison of chronic physical and emotional social defeat stress effects on mesocorticolimbic circuit activation and voluntary consumption of morphine. Sci. Rep. 2017;7:8445. doi: 10.1038/s41598-017-09106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottler L.B., Compton W.M., III, Mager D., Spitznagel E.L., Janca A. Posttraumatic stress disorder among substance users from the general population. Am. J. Psychiatr. 1992;149:664–670. doi: 10.1176/ajp.149.5.664. [DOI] [PubMed] [Google Scholar]
- Cotto J.H., Davis E., Dowling G.J., Elcano J.C., Staton A.B., Weiss S.R. Gender effects on drug use, abuse, and dependence: a special analysis of results from the National Survey on Drug Use and Health. Gend. Med. 2010;7:402–413. doi: 10.1016/j.genm.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Couppis M.H., Kennedy C.H. The rewarding effect of aggression is reduced by nucleus accumbens dopamine receptor antagonism in mice. Psychopharmacology. 2008;197:449–456. doi: 10.1007/s00213-007-1054-y. [DOI] [PubMed] [Google Scholar]
- Covington H.E., III, Kikusui T., Goodhue J., Nikulina E.M., Hammer R.P., Jr., Miczek K.A. Brief social defeat stress: long lasting effects on cocaine taking during a binge and Zif268 mRNA expression in the amygdala and prefrontal cortex. Neuropsychopharmacology. 2005;30:310–321. doi: 10.1038/sj.npp.1300587. [DOI] [PubMed] [Google Scholar]
- Covington H.E., III, Miczek K.A. Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacology. 2001;158:388–398. doi: 10.1007/s002130100858. [DOI] [PubMed] [Google Scholar]
- Covington H.E., III, Miczek K.A. Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacology. 2005;183:331–340. doi: 10.1007/s00213-005-0190-5. [DOI] [PubMed] [Google Scholar]
- Covington H.E., III, Newman E.L., Tran S., Walton L., Hayek W., Leonard M.Z., DeBold J.F., Miczek K.A. The urge to fight: persistent escalation by alcohol and role of NMDA receptors in mice. Front. Behav. Neurosci. 2018 doi: 10.3389/fnbeh.2018.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington H.E., III, Tropea T.F., Rajadhyaksha A.M., Kosofsky B.E., Miczek K.A. NMDA receptors in the rat VTA: a critical site for social stress to intensify cocaine taking. Psychopharmacology. 2008;197:203–216. doi: 10.1007/s00213-007-1024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft A.P., Brooks S.P., Cole J., Little H.J. Social defeat increases alcohol preference of C57BL/10 strain mice; effect prevented by a CCKB antagonist. Psychopharmacology. 2005;183:163–170. doi: 10.1007/s00213-005-0165-6. [DOI] [PubMed] [Google Scholar]
- Cruz F.C., Quadros I.M., Hogenelst K., Planeta C.S., Miczek K.A. Social defeat stress in rats: escalation of cocaine and “speedball” binge self-administration, but not heroin. Psychopharmacology. 2011;215:165–175. doi: 10.1007/s00213-010-2139-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis A.L., Bethea T., Valentino R.J. Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology. 2006;31:544–554. doi: 10.1038/sj.npp.1300875. [DOI] [PubMed] [Google Scholar]
- de Boer S.F., Buwalda B., Koolhaas J.M. Untangling the neurobiology of coping styles in rodents: towards neural mechanisms underlying individual differences in disease susceptibility. Neurosci. Biobehav. Rev. 2017;74:401–422. doi: 10.1016/j.neubiorev.2016.07.008. [DOI] [PubMed] [Google Scholar]
- de Wit H., Sayette M. Considering the context: social factors in responses to drugs in humans. Psychopharmacology. 2018;235:935–945. doi: 10.1007/s00213-018-4854-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H., Wise R.A. Blockade of cocaine reinforcement in rats with the dopamine receptor blocker pimozide, but not with the noradrenergic blockers phentolamine or phenoxybenzamine. Can. J. Psychol. 1977;31:195–203. doi: 10.1037/h0081662. [DOI] [PubMed] [Google Scholar]
- Deal A.L., Konstantopoulos J.K., Weiner J.L., Budygin E.A. Exploring the consequences of social defeat stress and intermittent ethanol drinking on dopamine dynamics in the rat nucleus accumbens. Sci. Rep. 2018;8:332. doi: 10.1038/s41598-017-18706-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedic N., Kuhne C., Jakovcevski M., Hartmann J., Genewsky A.J., Gomes K.S., Anderzhanova E., Pohlmann M.L., Chang S., Kolarz A., Vogl A.M., Dine J., Metzger M.W., Schmid B., Almada R.C., Ressler K.J., Wotjak C.T., Grinevich V., Chen A., Schmidt M.V., Wurst W., Refojo D., Deussing J.M. Chronic CRH depletion from GABAergic, long-range projection neurons in the extended amygdala reduces dopamine release and increases anxiety. Nat. Neurosci. 2018;21:803–807. doi: 10.1038/s41593-018-0151-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A., Mazei-Robison M.S., Kesby J.P., Nestler E.J., Markou A. Enduring deficits in brain reward function after chronic social defeat in rats: susceptibility, resilience, and antidepressant response. Biol. Psychiatr. 2014;76:542–549. doi: 10.1016/j.biopsych.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiChiara G., Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. U. S. A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison G. Stress and alcohol intake: the socio-pharmacological approach. Physiol. Behav. 1987;40:387–392. doi: 10.1016/0031-9384(87)90066-7. [DOI] [PubMed] [Google Scholar]
- Erb S., Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J. Neurosci. 1999;19 doi: 10.1523/JNEUROSCI.19-20-j0006.1999. RC35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans S.M., Foltin R.W. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31:659–674. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Evans S.M., Haney M., Foltin R.W. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Feder A., Nestler E.J., Charney D.S. Psychobiology and molecular genetics of resilience. Nat. Rev. Neurosci. 2009;10:446–457. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipenko M.L., Alekseyenko O.V., Beilina A.G., Kamynina T.P., Kudryavtseva N.N. Increase of tyrosine hydroxylase and dopamine transporter mRNA levels in ventral tegmental area of male mice under influence of repeated aggression experience. Mol. Brain Res. 2001;96:77–81. doi: 10.1016/s0169-328x(01)00270-4. [DOI] [PubMed] [Google Scholar]
- Fish E.W., DeBold J.F., Miczek K.A. Aggressive behavior as a reinforcer in mice: activation by allopregnanolone. Psychopharmacology. 2002;163:459–466. doi: 10.1007/s00213-002-1211-2. [DOI] [PubMed] [Google Scholar]
- Fish E.W., DeBold J.F., Miczek K.A. Escalated aggression as a reward: corticosterone and GABA(A) receptor positive modulators in mice. Psychopharmacology. 2005;182:116–127. doi: 10.1007/s00213-005-0064-x. [DOI] [PubMed] [Google Scholar]
- Fitch T.E., Roberts D.C.S. The effects of dose and access restrictions on the periodicity of cocaine self-administration in the rat. Drug Alcohol Depend. 1993;33:119–128. doi: 10.1016/0376-8716(93)90053-s. [DOI] [PubMed] [Google Scholar]
- Fox H.C., Garcia M., Jr., Kemp K., Milivojevic V., Kreek M.J., Sinha R. Gender differences in cardiovascular and corticoadrenal response to stress and drug cues in cocaine dependent individuals. Psychopharmacology. 2006;185:348–357. doi: 10.1007/s00213-005-0303-1. [DOI] [PubMed] [Google Scholar]
- Fox H.C., Hong K.I.A., Siedlarz K., Sinha R. Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine-dependent individuals compared to social drinkers. Neuropsychopharmacology. 2008;33:796–805. doi: 10.1038/sj.npp.1301470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox H.C., Sinha R. Sex differences in drug-related stress-system changes: implications for treatment in substance-abusing women. Harv. Rev. Psychiatr. 2009;17:103–119. doi: 10.1080/10673220902899680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frajzyngier V., Neaigus A., Gyarmathy V.A., Miller M., Friedman S.R. Gender differences in injection risk behaviors at the first injection episode. Drug Alcohol Depend. 2007;89:145–152. doi: 10.1016/j.drugalcdep.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Flügge G. Social stress in tree shrews: effects on physiology, brain function, and behavior of subordinate individuals. Pharmacol. Biochem. Behav. 2002;73:247–258. doi: 10.1016/s0091-3057(02)00795-5. [DOI] [PubMed] [Google Scholar]
- Funk C.K., Zorrilla E.P., Lee M.J., Rice K.C., Koob G.F. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol. Psychiatr. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D., Harding S., Juzytsch W., Le A.D. Effects of unconditioned and conditioned social defeat on alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology. 2005;183:341–349. doi: 10.1007/s00213-005-0194-1. [DOI] [PubMed] [Google Scholar]
- Gilpin N.W., Weiner J.L. Neurobiology of comorbid post-traumatic stress disorder and alcohol-use disorder. Gene Brain Behav. 2017;16:15–43. doi: 10.1111/gbb.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S.A., Aleyasin H., Heins R., Flanigan M., Heshmati M., Takahashi A., Russo S.J., Shaham Y. Persistent conditioned place preference to aggression experience in adult male sexually-experienced CD-1 mice. Gene Brain Behav. 2017;16:44–55. doi: 10.1111/gbb.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S.A., Covington H.E., III, Berton O., Russo S.J. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S.A., Heins C., Venniro M., Caprioli D., Zhang M., Epstein D.H., Shaham Y. Compulsive addiction-like aggressive behavior in mice. Biol. Psychiatr. 2017;82:239–248. doi: 10.1016/j.biopsych.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T.C., Grimes Serrano J.M., Licari A., Budman S.H., Butler S.F. Women who abuse prescription opioids: findings from the Addiction Severity Index-Multimedia Version Connect prescription opioid database. Drug Alcohol Depend. 2009;103:65–73. doi: 10.1016/j.drugalcdep.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield S.F., Back S.E., Lawson K., Brady K.T. Substance abuse in women. Psychiatr. Clin. 2010;33:339–355. doi: 10.1016/j.psc.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grucza R.A., Norberg K., Bucholz K.K., Bierut L.J. Correspondence between secular changes in alcohol dependence and age of drinking onset among women in the United States. Alcohol Clin. Exp. Res. 2008;32:1493–1501. doi: 10.1111/j.1530-0277.2008.00719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A.J., Logan J.E., Toblin R.L., Kaplan J.A., Kraner J.C., Bixler D., Crosby A.E., Paulozzi L.J. Patterns of abuse among unintentional pharmaceutical overdose fatalities. J. Am. Med. Assoc. 2008;300:2613–2620. doi: 10.1001/jama.2008.802. [DOI] [PubMed] [Google Scholar]
- Han X., Albrechet-Souza L., Doyle M.R., Shimamoto A., DeBold J.F., Miczek K.A. Social stress and escalated drug self-administration in mice II. Cocaine and dopamine in the nucleus accumbens. Psychopharmacology. 2015;232:1003–1010. doi: 10.1007/s00213-014-3734-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., DeBold J.F., Miczek K.A. Prevention and reversal of social stress-escalated cocaine self-administration in mice by intra-VTA CRFR1 antagonism. Psychopharmacology. 2017;234:2813–2821. doi: 10.1007/s00213-017-4676-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M., Maccari S., Le Moal M., Simon H., Piazza P.V. Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain Res. 1995;698:46–52. doi: 10.1016/0006-8993(95)00788-r. [DOI] [PubMed] [Google Scholar]
- Harris A.Z., Atsak P., Bretton Z.H., Holt E.S., Alam R., Morton M.P., Abbas A.I., Leonardo E.D., Bolkan S.S., Hen R., Gordon J.A. A novel method for chronic social defeat stress in female mice. Neuropsychopharmacology. 2018;43:1276–1283. doi: 10.1038/npp.2017.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D.S., Reus V.I., Wolkowitz O.M., Mendelson J.E., Jones R.T. Repeated psychological stress testing in stimulant-dependent patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2005;29:669–677. doi: 10.1016/j.pnpbp.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Hashikawa K., Hashikawa Y., Tremblay R., Zhang J., Feng J.E., Sabol A., Piper W.T., Lee H., Rudy B., Lin D. Esr1(+) cells in the ventromedial hypothalamus control female aggression. Nat. Neurosci. 2017;20:1580–1590. doi: 10.1038/nn.4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms C.M., McClintick M.N., Grant K.A. Social rank, chronic ethanol self-administration, and diurnal pituitary-adrenal activity in cynomolgus monkeys. Psychopharmacology. 2012;224:133–143. doi: 10.1007/s00213-012-2707-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J.P., Stinus L., Le Moal M. Repeated stress increases locomotor response to amphetamine. Psychopharmacology. 1984;84:431–435. doi: 10.1007/BF00555227. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila C.A., Rounsaville B.J., Kranzler H.R. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 2004;74:265–272. doi: 10.1016/j.drugalcdep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Hilakivi-Clarke L., Lister R.G. Social status and voluntary alcohol consumption in mice: interaction with stress. Psychopharmacology. 1992;108:276–282. doi: 10.1007/BF02245112. [DOI] [PubMed] [Google Scholar]
- Holly E.N., Boyson C.O., Montagud-Romero S., Stein D.J., Gobrogge K.L., DeBold J.F., Miczek K.A. Episodic social stress-escalated cocaine self-administration: role of phasic and tonic corticotropin releasing factor in the anterior and posterior ventral tegmental area. J. Neurosci. 2016;36:4093–4105. doi: 10.1523/JNEUROSCI.2232-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly E.N., DeBold J.F., Miczek K.A. Increased mesocorticolimbic dopamine during acute and repeated social defeat stress: modulation by corticotropin releasing factor receptors in the ventral tegmental area. Psychopharmacology. 2015;232:4469–4479. doi: 10.1007/s00213-015-4082-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly E.N., Miczek K.A. Ventral tegmental area dopamine revisited: effects of acute and repeated stress. Psychopharmacology. 2016;233:163–186. doi: 10.1007/s00213-015-4151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly E.N., Shimamoto A., DeBold J.F., Miczek K.A. Sex differences in behavioral and neural cross-sensitization and escalated cocaine taking as a result of episodic social defeat stress in rats. Psychopharmacology. 2012;224:179–188. doi: 10.1007/s00213-012-2846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa L.S., Chu A., Levinson S.A., Kayyali T.M., DeBold J.F., Miczek K.A. Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin. Exp. Res. 2011;35:1938–1947. doi: 10.1111/j.1530-0277.2011.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa L.S., Holly E.N., DeBold J.F., Miczek K.A. Social stress-escalated intermittent alcohol drinking: modulation by CRF-R1 in the ventral tegmental area and accumbal dopamine in mice. Psychopharmacology. 2016;233:681–690. doi: 10.1007/s00213-015-4144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa L.S., Shimamoto A., Kayyali T., Norman K.J., Valentino R.J., DeBold J.F., Miczek K.A. Dissociation of μ-opioid receptor and CRF-R1 antagonist effects on escalated ethanol consumption and mPFC serotonin in C57BL/6J mice. Addiction Biol. 2016;21:111–124. doi: 10.1111/adb.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman S.M., Fox H., Hong K.I., Doebrick C., Sinha R. Stress and drug-cue-induced craving in opioid-dependent individuals in naltrexone treatment. Exp. Clin. Psychopharmacol. 2007;15:134–143. doi: 10.1037/1064-1297.15.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman S.M., Hong K.I., Chaplin T.M., Dabre Z., Comegys A.D., Kimmerling A., Sinha R. A stress-coping profile of opioid dependent individuals entering naltrexone treatment: a comparison with healthy controls. Psychol. Addict. Behav. 2009;23:613–619. doi: 10.1037/a0017324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignjatova L., Raleva M. Gender difference in the treatment outcome of patients served in the mixed-gender program. Bratisl. Lek. Listy. 2009;110:285–289. [PubMed] [Google Scholar]
- Imperato A., DiChiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J. Pharmacol. Exp. Therapeut. 1986;239:219–228. [PubMed] [Google Scholar]
- Imperato A., Puglisi-Allegra S., Casolini P., Zocchi A., Angelucci L. Stress-induced enhancement of dopamine and acetylcholine release in limbic structures: role of corticosterone. Eur. J. Pharmacol. 1989;165:337–338. doi: 10.1016/0014-2999(89)90735-8. [DOI] [PubMed] [Google Scholar]
- Jacobson-Pick S., Audet M.C., McQuaid R.J., Kalvapalle R., Anisman H. Social agonistic distress in male and female mice: changes of behavior and brain monoamine functioning in relation to acute and chronic challenges. PLoS One. 2013;8 doi: 10.1371/journal.pone.0060133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison R.N., Butler S.F., Budman S.H., Edwards R.R., Wasan A.D. Gender differences in risk factors for aberrant prescription opioid use. J. Pain. 2010;11:312–320. doi: 10.1016/j.jpain.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaremko K.M., Sterling R.C., Van Bockstaele E.J. Psychological and physiological stress negatively impacts early engagement and retention of opioid-dependent individuals on methadone maintenance. J. Subst. Abuse Treat. 2015;48:117–127. doi: 10.1016/j.jsat.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez V.A., Grant K.A. Studies using macaque monkeys to address excessive alcohol drinking and stress interactions. Neuropharmacology. 2017;122:127–135. doi: 10.1016/j.neuropharm.2017.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H.E., Fitzgerald H., Johnson R.E. Males and females differ in response to opioid agonist medications. Am. J. Addict. 2005;14:223–233. doi: 10.1080/10550490590949569. [DOI] [PubMed] [Google Scholar]
- Kabbaj M., Norton C.S., Kollack-Walker S., Watson S.J., Robinson T.E., Akil H. Social defeat alters the acquisition of cocaine self-administration in rats: role of individual differences in cocaine-taking behavior. Psychopharmacology. 2001;158:382–387. doi: 10.1007/s002130100918. [DOI] [PubMed] [Google Scholar]
- Karlsgodt K.H., Lukas S.E., Elman I. Psychosocial stress and the duration of cocaine use in non-treatment seeking individuals with cocaine dependence. Am. J. Drug Alcohol Abuse. 2003;29:539–551. doi: 10.1081/ada-120023457. [DOI] [PubMed] [Google Scholar]
- Karlsson C., Schank J.R., Rehman F., Stojakovic A., Bjork K., Barbier E., Solomon M., Tapocik J., Engblom D., Thorsell A., Heilig M. Proinflammatory signaling regulates voluntary alcohol intake and stress-induced consumption after exposure to social defeat stress in mice. Addiction Biol. 2017;22:1279–1288. doi: 10.1111/adb.12416. [DOI] [PubMed] [Google Scholar]
- Kelly E.A., Fudge J.L. The neuroanatomic complexity of the CRF and DA systems and their interface: what we still don't know. Neurosci. Biobehav. Rev. 2018;90:247–259. doi: 10.1016/j.neubiorev.2018.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes K.M., Martins S.S., Blanco C., Hasin D.S. Telescoping and gender differences in alcohol dependence: new evidence from two national surveys. Am. J. Psychiatr. 2010;167:969–976. doi: 10.1176/appi.ajp.2009.09081161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C., Pirke K.M., Hellhammer D.H. The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Konova A.B., Moeller S.J., Goldstein R.Z. Common and distinct neural targets of treatment: changing brain function in substance addiction. Neurosci. Biobehav. Rev. 2013;37:2806–2817. doi: 10.1016/j.neubiorev.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G., Kreek M.J. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am. J. Psychiatr. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F., Le Moal M. Addiction and the brain antireward system. Annu. Rev. Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob G.F., Volkow N.D. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten T.R., Gawin F.H., Rounsaville B.J., Kleber H.D. Cocaine abuse among opioid addicts: demographic and diagnostic factors in treatment. Am. J. Drug Alcohol Abuse. 1986;12:1–16. doi: 10.3109/00952998609083739. [DOI] [PubMed] [Google Scholar]
- Kowalczyk W.J., Phillips K.A., Jobes M.L., Kennedy A.P., Ghitza U.E., Agage D.A., Schmittner J.P., Epstein D.H., Preston K.L. Clonidine maintenance prolongs opioid abstinence and decouples stress from craving in daily life: a randomized controlled trial with ecological momentary assessment. Am. J. Psychiatr. 2015;172:760–767. doi: 10.1176/appi.ajp.2014.14081014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger D.W. Stressful life events and the return to heroin use. J. Hum. Stress. 1981;7:3–8. doi: 10.1080/0097840X.1981.9936820. [DOI] [PubMed] [Google Scholar]
- Kudryavtseva N., Gerrits M.A., Avgustinovich D.F., Tenditnik M.V., van Ree J.M. Anxiety and ethanol consumption in victorious and defeated mice; effect of κ-opioid receptor activation. Eur. Neuropsychopharmacol. 2006;16:504–511. doi: 10.1016/j.euroneuro.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Kudryavtseva N.N., Lipina T.V., Koryakina L.A. Effects of haloperidol on communicative and aggressive behavior in male mice with different experiences of aggression. Pharmacol. Biochem. Behav. 1999;63:229–236. doi: 10.1016/s0091-3057(98)00227-5. [DOI] [PubMed] [Google Scholar]
- Kudryavtseva N.N., Madorskaya I.A., Bakshtanovskaya I.V. Social success and voluntary ethanol consumption in mice of C57BL/6J and CBA/Lac strains. Physiol. Behav. 1991;50:143–146. doi: 10.1016/0031-9384(91)90511-l. [DOI] [PubMed] [Google Scholar]
- Laine M.A., Sokolowska E., Dudek M., Callan S.A., Hyytia P., Hovatta I. Brain activation induced by chronic psychosocial stress in mice. Sci. Rep. 2017;7:15061. doi: 10.1038/s41598-017-15422-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laredo S.A., Steinman M.Q., Robles C.F., Ferrer E., Ragen B.J., Trainor B.C. Effects of defeat stress on behavioral flexibility in males and females: modulation by the mu-opioid receptor. Eur. J. Neurosci. 2015;41:434–441. doi: 10.1111/ejn.12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson K.M., Back S.E., Hartwell K.J., Moran-Santa M.M., Brady K.T. A comparison of trauma profiles among individuals with prescription opioid, nicotine, or cocaine dependence. Am. J. Addict. 2013;22:127–131. doi: 10.1111/j.1521-0391.2013.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebow M.A., Chen A. Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol. Psychiatr. 2016;21:450–463. doi: 10.1038/mp.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos J.C., Wanat M.J., Smith J.S., Reyes B.A., Hollon N.G., Van Bockstaele E.J., Chavkin C., Phillips P.E. Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature. 2012;490:402–406. doi: 10.1038/nature11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard M.Z., DeBold J.F., Miczek K.A. Escalated cocaine “binges” in rats: enduring effects of social defeat stress or intra-VTA CRF. Psychopharmacology. 2017;234:2823–2836. doi: 10.1007/s00213-017-4677-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.S., Kosten T.R., Sinha R. Sex differences in brain activation during stress imagery in abstinent cocaine users: a functional magnetic resonance imaging study. Biol. Psychiatr. 2005;57:487–494. doi: 10.1016/j.biopsych.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Licata S.C., Pierce R.C. The roles of calcium/calmodulin-dependent and Ras/mitogen-activated protein kinases in the development of psychostimulant-induced behavioral sensitization. J. Neurochem. 2003;85:14–22. doi: 10.1046/j.1471-4159.2003.01662.x. [DOI] [PubMed] [Google Scholar]
- Lisman J., Schulman H., Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat. Rev. Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Litvin Y., Murakami G., Pfaff D.W. Effects of chronic social defeat on behavioral and neural correlates of sociality: vasopressin, oxytocin and the vasopressinergic V1b receptor. Physiol. Behav. 2011;103:393–403. doi: 10.1016/j.physbeh.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Lodge D.J., Grace A.A. Acute and chronic corticotropin-releasing factor 1 receptor blockade inhibits cocaine-induced dopamine release: correlation with dopamine neuron activity. J. Pharmacol. Exp. Therapeut. 2005;314:201–206. doi: 10.1124/jpet.105.084913. [DOI] [PubMed] [Google Scholar]
- Lopez M.F., Anderson R.I., Becker H.C. Effect of different stressors on voluntary ethanol intake in ethanol-dependent and nondependent C57BL/6J mice. Alcohol. 2016;51:17–23. doi: 10.1016/j.alcohol.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Liu Z., Huang M., Zhang Z. Dopamine-dependent responses to cocaine depend on corticotropin-releasing factor receptor subtypes. J. Neurochem. 2003;84:1378–1386. doi: 10.1046/j.1471-4159.2003.01635.x. [DOI] [PubMed] [Google Scholar]
- Lynch W.J., Arizzi M.N., Carroll M.E. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology. 2000;152:132–139. doi: 10.1007/s002130000488. [DOI] [PubMed] [Google Scholar]
- Lynch W.J., Carroll M.E. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology. 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Lynch W.J., Roth M.E., Mickelberg J.L., Carroll M.E. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol. Biochem. Behav. 2001;68:641–646. doi: 10.1016/s0091-3057(01)00455-5. [DOI] [PubMed] [Google Scholar]
- MacLennan A.J., Maier S.F. Coping and the stress-induced potentiation of stimulant stereotypy in the rat. Science. 1983;219:1091–1093. doi: 10.1126/science.6681679. [DOI] [PubMed] [Google Scholar]
- Maier S.F., Watkins L.R. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci. Biobehav. Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Manvich D.F., Stowe T.A., Godfrey J.R., Weinshenker D. A method for psychosocial stress-induced reinstatement of cocaine seeking in rats. Biol. Psychiatr. 2016;79:940–946. doi: 10.1016/j.biopsych.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe S.E., Cranford J.A., Boyd C.J. Stressful events and other predictors of remission from drug dependence in the United States: longitudinal results from a national survey. J. Subst. Abuse Treat. 2016;71:41–47. doi: 10.1016/j.jsat.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance-Katz E.F., Carroll K.M., Rounsaville B.J. Gender differences in treatment-seeking cocaine abusers--implications for treatment and prognosis. Am. J. Addict. 1999;8:300–311. doi: 10.1080/105504999305703. [DOI] [PubMed] [Google Scholar]
- McCaul M.E., Wand G.S., Weerts E.M., Xu X. A paradigm for examining stress effects on alcohol-motivated behaviors in participants with alcohol use disorder. Addiction Biol. 2018;23:836–845. doi: 10.1111/adb.12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Bowles N.P., Gray J.D., Hill M.N., Hunter R.G., Karatsoreos I.N., Nasca C. Mechanisms of stress in the brain. Nat. Neurosci. 2015;18:1353–1363. doi: 10.1038/nn.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh R.K., Devito E.E., Dodd D., Carroll K.M., Potter J.S., Greenfield S.F., Connery H.S., Weiss R.D. Gender differences in a clinical trial for prescription opioid dependence. J. Subst. Abuse Treat. 2013;45:38–43. doi: 10.1016/j.jsat.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie-Quirk S.D., Miczek K.A. Social rank and social separation as determinants of alcohol drinking in squirrel monkeys. Psychopharmacology. 2008;201:137–145. doi: 10.1007/s00213-008-1256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J.P., Li S., Valdez J., Chavkin T.A., Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2006;31:1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek K.A., Mutschler N.H. Activational effects of social stress on IV cocaine self-administration in rats. Psychopharmacology. 1996;128:256–264. doi: 10.1007/s002130050133. [DOI] [PubMed] [Google Scholar]
- Miczek K.A., Mutschler N.H., Van Erp A.M.M., Blank A.D., McInerney S.C. d-Amphetamine “cue” generalizes to social defeat stress: sensitization and role of accumbens dopamine. Psychopharmacology. 1999;147:190–199. doi: 10.1007/s002130051160. [DOI] [PubMed] [Google Scholar]
- Miczek K.A., Nikulina E., Kream R.M., Carter G., Espejo E.F. Behavioral sensitization to cocaine after a brief social defeat stress: c- fos expression in the PAG. Psychopharmacology. 1999;141:225–234. doi: 10.1007/s002130050829. [DOI] [PubMed] [Google Scholar]
- Miczek K.A., Nikulina E.M., Shimamoto A., Covington H.E., III Escalated or suppressed cocaine reward, tegmental BDNF and accumbal dopamine due to episodic vs. continuous social stress in rats. J. Neurosci. 2011;31:9848–9857. doi: 10.1523/JNEUROSCI.0637-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek K.A., Yap J.J., Covington H.E., III Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol. Ther. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milivojevic V., Sinha R. Central and peripheral biomarkers of stress response for addiction risk and relapse vulnerability. Trends Mol. Med. 2018;24:173–186. doi: 10.1016/j.molmed.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagud-Romero S., Aguilar M.A., Maldonado C., Manzanedo C., Minarro J., Rodriguez-Arias M. Acute social defeat stress increases the conditioned rewarding effects of cocaine in adult but not in adolescent mice. Pharmacol. Biochem. Behav. 2015;135:1–12. doi: 10.1016/j.pbb.2015.05.008. [DOI] [PubMed] [Google Scholar]
- Najavits L.M., Rosier M., Nolan A.L., Freeman M.C. A new gender-based model for women's recovery from substance abuse: results of a pilot outcome study. Am. J. Drug Alcohol Abuse. 2007;33:5–11. doi: 10.1080/00952990601082597. [DOI] [PubMed] [Google Scholar]
- Nelson B.S., Sequeira M.K., Schank J.R. Bidirectional relationship between alcohol intake and sensitivity to social defeat: association with Tacr1 and Avp expression. Addiction Biol. 2018;23:142–153. doi: 10.1111/adb.12494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E.L., Albrechet-Souza L., Andrew P.M., Auld J.G., Burk K.C., Hwa L.S., Zhang E.Y., DeBold J.F., Miczek K.A. Persistent escalation of alcohol consumption by mice exposed to brief episodes of social defeat stress: suppression by CRF-R1 antagonism. Psychopharmacology. 2018;235:1807–1820. doi: 10.1007/s00213-018-4905-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikulina E.M., Covington H.E., III, Ganschow L., Hammer R.P., Jr., Miczek K.A. Long-term behavioral and neuronal cross-sensitization to amphetamine induced by repeated brief social defeat stress: fos in the ventral tegmental area and amygdala. Neuroscience. 2004;123:857–865. doi: 10.1016/j.neuroscience.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Nikulina E.M., Hammer R.P., Jr., Miczek K.A., Kream R.M. Social defeat stress increases expression of mu-opioid receptor mRNA in rat ventral tegmental area. Neuroreport. 1999;10:3015–3019. doi: 10.1097/00001756-199909290-00026. [DOI] [PubMed] [Google Scholar]
- Nikulina E.M., Marchand J.E., Kream R.M., Miczek K.A. Behavioral sensitization to cocaine after a brief social stress is accompanied by changes in fos expression in the murine brainstem. Brain Res. 1998;810:200–210. doi: 10.1016/s0006-8993(98)00925-1. [DOI] [PubMed] [Google Scholar]
- Nikulina E.M., Miczek K.A., Hammer R.P., Jr. Prolonged effects of repeated social defeat stress on mRNA expression and function of μ-opioid receptors in the ventral tegmental area of rats. Neuropsychopharmacology. 2005;30:1096–1103. doi: 10.1038/sj.npp.1300658. [DOI] [PubMed] [Google Scholar]
- Nocjar C., Zhang J., Feng P., Panksepp J. The social defeat animal model of depression shows diminished levels of orexin in mesocortical regions of the dopamine system, and of dynorphin and orexin in the hypothalamus. Neuroscience. 2012;218:138–153. doi: 10.1016/j.neuroscience.2012.05.033. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Gender differences in risk factors and consequences for alcohol use and problems. Clin. Psychol. Rev. 2004;24:981–1010. doi: 10.1016/j.cpr.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Noori H.R., Helinski S., Spanagel R. Cluster and meta-analyses on factors influencing stress-induced alcohol drinking and relapse in rodents. Addiction Biol. 2014;19:225–232. doi: 10.1111/adb.12125. [DOI] [PubMed] [Google Scholar]
- Norman K.J., Seiden J.A., Klickstein J.A., Han X., Hwa L.S., DeBold J.F., Miczek K.A. Social stress and escalated drug self-administration in mice I. Alcohol and corticosterone. Psychopharmacology. 2015;232:991–1001. doi: 10.1007/s00213-014-3733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroumov A., Thomas A.M., Kimmey B.A., Karsch J.S., Doyon W.M., Dani J.A. Stress increases ethanol self-administration via a shift toward excitatory GABA signaling in the ventral tegmental area. Neuron. 2016;92:493–504. doi: 10.1016/j.neuron.2016.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulozzi L.J., Jones C.M., Mack K.A., Rudd R.A. Vital signs: overdoses of prescription opioid pain relievers — United States, 1999-2008. MMWR Morb. Mortal. Wkly. Rep. 2011;60:1487–1492. [PubMed] [Google Scholar]
- Peciña S., Schulkin J., Berridge K.C. Nucleus accumbens corticotropin-releasing factor increases cue-triggered motivation for sucrose reward: paradoxical positive incentive effects in stress? BMC Biol. 2006;4:8. doi: 10.1186/1741-7007-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S., Slattery D.A., Flor P.J., Neumann I.D., Reber S.O. Differential effects of baclofen and oxytocin on the increased ethanol consumption following chronic psychosocial stress in mice. Addiction Biol. 2013;18:66–77. doi: 10.1111/adb.12001. [DOI] [PubMed] [Google Scholar]
- Potenza M.N., Hong K.I., Lacadie C.M., Fulbright R.K., Tuit K.L., Sinha R. Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. Am. J. Psychiatr. 2012;169:406–414. doi: 10.1176/appi.ajp.2011.11020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston K.L., Epstein D.H. Stress in the daily lives of cocaine and heroin users: relationship to mood, craving, relapse triggers, and cocaine use. Psychopharmacology. 2011;218:29–37. doi: 10.1007/s00213-011-2183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston K.L., Kowalczyk W.J., Phillips K.A., Jobes M.L., Vahabzadeh M., Lin J.L., Mezghanni M., Epstein D.H. Context and craving during stressful events in the daily lives of drug-dependent patients. Psychopharmacology. 2017;234:2631–2642. doi: 10.1007/s00213-017-4663-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston K.L., Kowalczyk W.J., Phillips K.A., Jobes M.L., Vahabzadeh M., Lin J.L., Mezghanni M., Epstein D.H. Exacerbated craving in the presence of stress and drug cues in drug-dependent patients. Neuropsychopharmacology. 2018;43:859–867. doi: 10.1038/npp.2017.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadros I.M., Miczek K.A. Two modes of intense cocaine bingeing: increased persistence after social defeat stress and increased rate of intake due to extended access conditions in rats. Psychopharmacology. 2009;206:109–121. doi: 10.1007/s00213-009-1584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes B.A., Zitnik G., Foster C., Van Bockstaele E.J., Valentino R.J. Social stress engages neurochemically distinct afferents to the rat locus coeruleus depending on coping strategy. eNeuro. 2015;2 doi: 10.1523/ENEURO.0042-15.2015. ENEURO.0042-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro Do Couto B., Aguilar M.A., Lluch J., Rodriguez-Arias M., Minarro J. Social experiences affect reinstatement of cocaine-induced place preference in mice. Psychopharmacology. 2009;207:485–498. doi: 10.1007/s00213-009-1678-1. [DOI] [PubMed] [Google Scholar]
- Ribeiro Do Couto B., Aguilar M.A., Manzanedo C., Rodríguez-Arias M., Armario A., Miñarro J. Social stress is as effective as physical stress in reinstating morphine-induced place preference in mice. Psychopharmacology. 2006;185:459–470. doi: 10.1007/s00213-006-0345-z. [DOI] [PubMed] [Google Scholar]
- Robinson T.E., Berridge K.C. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robison A.J. Emerging role of CaMKII in neuropsychiatric disease. Trends Neurosci. 2014;37:653–662. doi: 10.1016/j.tins.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Roth M.E., Cosgrove K.P., Carroll M.E. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci. Biobehav. Rev. 2004;28:533–546. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Ryabinin A.E., Bachtell R.K., Heinrichs S.C., Lee S., Rivier C., Olive M.F., Mehmert K.K., Camarini R., Kim J.A., Koenig H.N., Nannini M.A., Hodge C.W., Roberts A.J., Koob G.F. The corticotropin-releasing factor/urocortin system and alcohol. Alcohol Clin. Exp. Res. 2002;26:714–722. [PubMed] [Google Scholar]
- Sannibale C., Hall W. Gender-related symptoms and correlates of alcohol dependence among men and women with a lifetime diagnosis of alcohol use disorders. Drug Alcohol Rev. 2011;20:369–383. [Google Scholar]
- Sapolsky R.M. Stress in the wild. Sci. Am. 1990;262:116–123. doi: 10.1038/scientificamerican0190-116. [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M. Neuroendocrinology of the stress response. In: Becker J.B., editor. Behavioral Endocrinology. MIT Press; Cambridge, MA: 1992. pp. 287–325. [Google Scholar]
- Sapolsky R.M. Stress and the brain: individual variability and the inverted-U. Nat. Neurosci. 2015;18:1344–1346. doi: 10.1038/nn.4109. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z., Shaham Y., Heinrichs S.C. The role of corticotropin-releasing factor in drug addiction. Pharmacol. Rev. 2001;53:209–243. [PubMed] [Google Scholar]
- Schmidt A., Borgwardt S., Gerber H., Wiesbeck G.A., Schmid O., Riecher-Rossler A., Smieskova R., Lang U.E., Walter M. Acute effects of heroin on negative emotional processing: relation of amygdala activity and stress-related responses. Biol. Psychiatr. 2014;76:289–296. doi: 10.1016/j.biopsych.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Schwandt M.L., Cortes C.R., Kwako L.E., George D.T., Momenan R., Sinha R., Grigoriadis D.E., Pich E.M., Leggio L., Heilig M. The CRF1 antagonist verucerfont in anxious alcohol-dependent women: translation of neuroendocrine, but not of anti-craving effects. Neuropsychopharmacology. 2016;41:2818–2829. doi: 10.1038/npp.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzer J.J., Ricci L.A., Melloni R.H., Jr. Prior fighting experience increases aggression in syrian hamsters: implications for a role of dopamine in the winner effect. Aggress. Behav. 2013;39:290–300. doi: 10.1002/ab.21476. [DOI] [PubMed] [Google Scholar]
- Sgoifo A., Stilli D., de Boer S.F., Koolhaas J.M., Musso E. Acute social stress and cardiac electrical activity in rats. Aggress. Behav. 1998;24:287–296. [Google Scholar]
- Shimamoto A., DeBold J.F., Holly E.N., Miczek K.A. Blunted accumbal dopamine response to cocaine following chronic social stress in female rats: exploring a link between depression and drug abuse. Psychopharmacology. 2011;218:271–279. doi: 10.1007/s00213-011-2364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamoto A., Holly E.N., Boyson C.O., DeBold J.F., Miczek K.A. Individual differences in anhedonic and accumbal dopamine responses to chronic social stress and their link to cocaine self-administration in female rats. Psychopharmacology. 2015;232:825–834. doi: 10.1007/s00213-014-3725-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y., Matthews R.T., Winder D.G. A corticotropin releasing factor pathway for ethanol regulation of the ventral tegmental area in the bed nucleus of the stria terminalis. J. Neurosci. 2013;33:950–960. doi: 10.1523/JNEUROSCI.2949-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillaber I., Rammes G., Zimmermann S., Mahal B., Zieglgansberger W., Wurst W., Holsboer F., Spanagel R. Enhanced and delayed stress-induced alcohol drinking in mice lacking functional CRH1 receptors. Science. 2002;296:931–933. doi: 10.1126/science.1069836. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R. The role of stress in addiction relapse. Curr. Psychiatr. Rep. 2007;9:388–395. doi: 10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann. N. Y. Acad. Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Stress and addiction: a dynamic interplay of genes, environment, and drug intake. Biol. Psychiatr. 2009;66:100–101. doi: 10.1016/j.biopsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R., Catapano D., O'Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology. 1999;142:343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Sinha R., Fuse T., Aubin L.R., O'Malley S.S. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology. 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Sinha R., Garcia M., Paliwal P., Kreek M.J., Rounsaville B.J. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch. Gen. Psychiatr. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sinha R., Kimmerling A., Doebrick C., Kosten T.R. Effects of lofexidine on stress-induced and cue-induced opioid craving and opioid abstinence rates: preliminary findings. Psychopharmacology. 2007;190:569–574. doi: 10.1007/s00213-006-0640-8. [DOI] [PubMed] [Google Scholar]
- Sinha R., Lacadie C., Skudlarski P., Fulbright R.K., Rounsaville B.J., Kosten T.R., Wexler B.E. Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology. 2005;183:171–180. doi: 10.1007/s00213-005-0147-8. [DOI] [PubMed] [Google Scholar]
- Sinha R., Li C.S. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Slopen N., Williams D.R., Fitzmaurice G.M., Gilman S.E. Sex, stressful life events, and adult onset depression and alcohol dependence: are men and women equally vulnerable? Soc. Sci. Med. 2011;73:615–622. doi: 10.1016/j.socscimed.2011.06.022. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M., Dudish-Poulsen S., Nelson D., Pentel P.R., Hatsukami D.K. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp. Clin. Psychopharmacol. 1999;7:274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Specio S.E., Wee S., O'Dell L.E., Boutrel B., Zorrilla E.P., Koob G.F. CRF(1) receptor antagonists attenuate escalated cocaine self-administration in rats. Psychopharmacology. 2008;196:473–482. doi: 10.1007/s00213-007-0983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starcke K., van Holst R.J., van den Brink W., Veltman D.J., Goudriaan A.E. Physiological and endocrine reactions to psychosocial stress in alcohol use disorders: duration of abstinence matters. Alcohol Clin. Exp. Res. 2013;37:1343–1350. doi: 10.1111/acer.12103. [DOI] [PubMed] [Google Scholar]
- Stelly C.E., Pomrenze M.B., Cook J.B., Morikawa H. Repeated social defeat stress enhances glutamatergic synaptic plasticity in the VTA and cocaine place conditioning. Elife. 2016;5 doi: 10.7554/eLife.15448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart S.H. Alcohol abuse in individuals exposed to trauma: a critical review. Psychol. Bull. 1996;120:83–112. doi: 10.1037/0033-2909.120.1.83. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Department of Health and Human Services, Center for Behavioral Health Statistics and Quality; Rockville, MD: 2013. The NSDUH Report: Substance Use and Mental Health Estimates from the 2013 National Survey on Drug Use and Health: Overview of Findings. [PubMed] [Google Scholar]
- Takahashi A., Chung J.R., Zhang S., Zhang H., Grossman Y., Aleyasin H., Flanigan M.E., Pfau M.L., Menard C., Dumitriu D., Hodes G.E., McEwen B.S., Nestler E.J., Han M.H., Russo S.J. Establishment of a repeated social defeat stress model in female mice. Sci. Rep. 2017;7:12838. doi: 10.1038/s41598-017-12811-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S.E., Bacon A.K., Randall P.K., Brady K.T., See R.E. An acute psychosocial stressor increases drinking in non-treatment-seeking alcoholics. Psychopharmacology. 2011;218:19–28. doi: 10.1007/s00213-010-2163-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey J.W., Miczek K.A. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res. 1996;721:140–149. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- Tidey J.W., Miczek K.A. Acquisition of cocaine self-administration after social stress: role of accumbens dopamine. Psychopharmacology. 1997;130:203–212. doi: 10.1007/s002130050230. [DOI] [PubMed] [Google Scholar]
- Tovar-Diaz J., Pomrenze M.B., Kan R., Pahlavan B., Morikawa H. Cooperative CRF and α1 adrenergic signaling in the VTA promotes NMDA plasticity and drives social stress enhancement of cocaine conditioning. Cell Rep. 2018;22:2756–2766. doi: 10.1016/j.celrep.2018.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhart M., Oswald L., McCaul M.E., Chong R., Wand G.S. Hormonal responses to psychological stress and family history of alcoholism. Neuropsychopharmacology. 2006;31:2255–2263. doi: 10.1038/sj.npp.1301063. [DOI] [PubMed] [Google Scholar]
- Uhart M., Wand G.S. Stress, alcohol and drug interaction: an update of human research. Addiction Biol. 2009;14:43–64. doi: 10.1111/j.1369-1600.2008.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger A., Jung E., Winklbaur B., Fischer G. Gender issues in the pharmacotherapy of opioid-addicted women: buprenorphine. J. Addict. Dis. 2010;29:217–230. doi: 10.1080/10550881003684814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless M.A., Singh V., Crowder T.L., Yaka R., Ron D., Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Valdez G.R., Roberts A.J., Chan K., Davis H., Brennan M., Zorrilla E.P., Koob G.F. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin. Exp. Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Valentino R.J., Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur. J. Pharmacol. 2008;583:194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino R.J., Van B.E., Bangasser D. Sex-specific cell signaling: the corticotropin-releasing factor receptor model. Trends Pharmacol. Sci. 2013;34:437–444. doi: 10.1016/j.tips.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Erp A.M.M., Miczek K.A. Persistent suppression of ethanol self-administration by brief social stress in rats and increased startle response as index of withdrawal. Physiol. Behav. 2001;73:301–311. doi: 10.1016/s0031-9384(01)00458-9. [DOI] [PubMed] [Google Scholar]
- Van Erp A.M.M., Tachi N., Miczek K.A. Short or continuous social stress: suppression of continuously available ethanol intake in subordinate rats. Behav. Pharmacol. 2001;12:335–342. doi: 10.1097/00008877-200109000-00004. [DOI] [PubMed] [Google Scholar]
- Van Hedger K., Bershad A.K., de Wit H. Pharmacological challenge studies with acute psychosocial stress. Psychoneuroendocrinology. 2017;85:123–133. doi: 10.1016/j.psyneuen.2017.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren L.J.M.J., Kalivas P.W. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Wagner F.A., Anthony J.C. Male-female differences in the risk of progression from first use to dependence upon cannabis, cocaine, and alcohol. Drug Alcohol Depend. 2007;86:191–198. doi: 10.1016/j.drugalcdep.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Waldrop A.E., Price K.L., Desantis S.M., Simpson A.N., Back S.E., Mcrae A.L., Spratt E.G., Kreek M.J., Brady K.T. Community-dwelling cocaine-dependent men and women respond differently to social stressors versus cocaine cues. Psychoneuroendocrinology. 2010;35:798–806. doi: 10.1016/j.psyneuen.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace B.C. Psychological and environmental determinants of relapse in crack cocaine smokers. J. Subst. Abuse Treat. 1989;6:95–106. doi: 10.1016/0740-5472(89)90036-6. [DOI] [PubMed] [Google Scholar]
- Walter M., Bentz D., Schicktanz N., Milnik A., Aerni A., Gerhards C., Schwegler K., Vogel M., Blum J., Schmid O., Roozendaal B., Lang U.E., Borgwardt S., de Q.D. Effects of cortisol administration on craving in heroin addicts. Transl. Psychiatry. 2015;5 doi: 10.1038/tp.2015.101. e610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M., Gerber H., Kuhl H.C., Schmid O., Joechle W., Lanz C., Brenneisen R., Schachinger H., Riecher-Rossler A., Wiesbeck G.A., Borgwardt S.J. Acute effects of intravenous heroin on the hypothalamic-pituitary-adrenal axis response: a controlled trial. J. Clin. Psychopharmacol. 2013;33:193–198. doi: 10.1097/JCP.0b013e31828393cb. [DOI] [PubMed] [Google Scholar]
- Wanat M.J., Bonci A., Phillips P.E. CRF acts in the midbrain to attenuate accumbens dopamine release to rewards but not their predictors. Nat. Neurosci. 2013;16:383–385. doi: 10.1038/nn.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat M.J., Hopf F.W., Stuber G.D., Phillips P.E., Bonci A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J. Physiol. (London) 2008;586:2157–2170. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Shaham Y., Zitzman D., Azari S., Wise R.A., You Z.B. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J. Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley C.A., Olausson P., Taylor J.R., Jentsch J.D. Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcohol Clin. Exp. Res. 2010;34:1306–1318. doi: 10.1111/j.1530-0277.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R.A. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wolffgramm J., Heyne A. Social behavior, dominance, and social deprivation of rats determine drug choice. Pharmacol. Biochem. Behav. 1991;38:389–399. doi: 10.1016/0091-3057(91)90297-f. [DOI] [PubMed] [Google Scholar]
- Wood S.K., McFadden K.V., Grigoriadis D., Bhatnagar S., Valentino R.J. Depressive and cardiovascular disease comorbidity in a rat model of social stress: a putative role for corticotropin-releasing factor. Psychopharmacology. 2012;222:325–336. doi: 10.1007/s00213-012-2648-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S.K., Walker H.E., Valentino R.J., Bhatnagar S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology. 2010;151:1795–1805. doi: 10.1210/en.2009-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S.K., Wood C.S., Lombard C.M., Lee C.S., Zhang X.Y., Finnell J.E., Valentino R.J. Inflammatory factors mediate vulnerability to a social stress-induced depressive-like phenotype in passive coping rats. Biol. Psychiatr. 2015;78:38–48. doi: 10.1016/j.biopsych.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S.K., Bhatnagar S. Resilience to the effects of social stress: evidence from clinical and preclinical studies on the role of coping strategies. Neurobiol Stress. 2015;1:164–173. doi: 10.1016/j.ynstr.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap J.J., Chartoff E.H., Holly E.N., Potter D.N., Carlezon W.A., Jr., Miczek K.A. Social defeat stress-induced sensitization and escalated cocaine self-administration: the role of ERK signaling in the rat ventral tegmental area. Psychopharmacology. 2015;232:1555–1569. doi: 10.1007/s00213-014-3796-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap J.J., Covington H.E., III, Gale M.C., Datta R., Miczek K.A. Behavioral sensitization due to social defeat stress in mice: antagonism at mGluR5 and NMDA receptors. Psychopharmacology. 2005;179:230–239. doi: 10.1007/s00213-004-2023-3. [DOI] [PubMed] [Google Scholar]
- Yap J.J., Miczek K.A. Social defeat stress, sensitization, and intravenous cocaine self-administration in mice. Psychopharmacology. 2007;192:261–273. doi: 10.1007/s00213-007-0712-4. [DOI] [PubMed] [Google Scholar]
- Yavich L., Tiihonen J. Ethanol modulates evoked dopamine release in mouse nucleus accumbens: dependence on social stress and dose. Eur. J. Pharmacol. 2000;401:365–373. doi: 10.1016/s0014-2999(00)00456-8. [DOI] [PubMed] [Google Scholar]
- Zilverstand A., Huang A.S., Alia-Klein N., Goldstein R.Z. Neuroimaging impaired response inhibition and salience attribution in human drug addiction: a systematic review. Neuron. 2018;98:886–903. doi: 10.1016/j.neuron.2018.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla E.P., Logrip M.L., Koob G.F. Corticotropin releasing factor: a key role in the neurobiology of addiction. Front. Neuroendocrinol. 2014;35:234–244. doi: 10.1016/j.yfrne.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]