Abstract
Aims:
Sepsis-caused multiple organ failure remains the major cause of morbidity and mortality in intensive care units. Nicotinamide riboside (NR) is a precursor of nicotinamide adenine dinucleotide (NAD+), which is important in regulating oxidative stress. This study investigated whether administration of NR prevented oxidative stress and organ injury in sepsis.
Methods:
Mouse sepsis models were induced by injection of lipopolysaccharides (LPS) or feces-injection-inperitoneum. NR was given before sepsis onset. Cultured macrophages and endothelial cells were incubated with various agents.
Results:
Administration of NR elevated the NAD+ levels, and elicited a reduction of oxidative stress, in-flammation and caspase-3 activity in lung and heart tissues, which correlated with attenuation of pulmonary microvascular permeability and myocardial dysfunction, leading to less mortality in sepsis models. These protective effects of NR were associated with decreased levels of plasma high mobility group box-1 (HMGB1) in septic mice. Consistently, pre-treatment of macrophages with NR increased NAD+ content and reduced HMGB1 release upon LPS stimulation. NR also prevented reactive oxygen species (ROS) production and apoptosis in endothelial cells induced by a conditioned-medium collected from LPS-treated macrophages. Furthermore, inhibition of SIRT1 by EX527 offset the negative effects of NR on HMGB1 release in macrophages, and ROS and apoptosis in endothelial cells.
Conclusions:
Administration of NR prevents lung and heart injury, and improves the survival in sepsis, likely by inhibiting HMGB1 release and oxidative stress via the NAD+/SIRT1 signaling. Given NR has been used as a health supplement, it may be a useful agent to prevent organ injury in sepsis.
Keywords: HMGB1, Nicotinamide riboside, Oxidative stress, Sepsis, SIRT1
1. Introduction
Sepsis-caused multiple-organ failure is the leading cause of deaths in intensive care unit patients [1,2]. In spite of extensive research, treatment for this fatal condition remains largely supportive without any specifically effective therapies [3].
Acute lung injury (ALI) and myocardial dysfunction frequently occur and significantly contribute to septic death [4–6]. Histopathologic changes of ALI and septic heart include accumulation of activated neutrophils and macrophages, apoptosis, pro-inflammatory response and increased pulmonary capillary permeability [7–9]. High mobility group box 1 protein (HMGB1) has been implicated in sepsis-induced organ injury and represents a therapeutic target for sepsis [10]. The circulating HMGB1 level was reported to reflect the disease severity in the clinical and experimental ALI [11] and HMGB1 directly mediates myocardial dysfunction in a mouse model of sepsis [12]. Nevertheless, the pathogenesis of ALI and myocardial dysfunction in sepsis has not been fully understood and therapeutic approaches are limited. Experimental evidence has shown that approaches targeting to inhibit oxidative stress are beneficial in animal models of sepsis and in clinical trials with septic patients [13], which underscores the importance of oxidative stress in septic organ injury.
Nicotinamide riboside (NR) is a nutrient naturally present in the human diet (e.g. milk) and a widely used health supplement. It is a pyridine-nucleoside form of vitamin B3 that functions as a precursor to nicotinamide adenine dinucleotide (NAD+). NR is converted into NAD+ sequentially by NR kinase 1/2 and nicotinamide mononucleotide adenylyltransferase [14,15]. Thus, administration of NR efficiently boosts intracellular NAD+ levels, and protects against various chronic health issues such as aging and metabolic syndrome [16–19]. It is well known that NAD+ is a co-enzyme in all living cells, which drives poly (ADP-ribose) polymerases (PARPs) and sirtuin (SIRT) reactions [20]. In metabolism, NAD+ is involved in redox reactions. NAD+ accepts electrons and becomes NADH. Both SIRT signaling and redox reactions are important in regulation of cellular oxidative stress. While PARPs consume NAD+ to repair damaged DNA, the SIRT family requires NAD+ for activation. Among seven family members of the SIRT family, SIRT1 is best characterized and controls various functions including cell differentiation, energy metabolism, circadian rhythm, stress responses and cell survival. Emerging evidence has manifested that SIRT1 exerts a protective role in sepsis [21–24]. Interestingly, NAD+ is decreased in sepsis [25,26]. Thus, it is plausible that NR may be beneficial in sepsis.
In this study, we investigated whether treatment with NR prevented oxidative stress and organ injury in sepsis. We demonstrated that administration of NR reduced lung and heart injury, and improved the survival in septic mice, likely by inhibiting high mobility group box-1 (HMGB1) release and oxidative stress through the NAD+/SIRT1 signaling.
2. Materials and methods
2.1. Animals and experimental procedures
This investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23). All experimental procedures were approved by the Animal Use Subcommittee at the Western University, Canada. C57BL/6 mice were purchased from the Jackson Laboratory.
Sepsis was induced in mice (male and aged 2 months) by feces-injection-in-peritoneum (FIP) as previously described [24]. Mice received a same volume of saline as sham controls. A single dose of NR (100, 300 or 500 mg/kg body weight) or vehicle was intraperitoneally injected into mice 30 min before saline or feces administration. These doses of NR were chosen because the lowest-observed-adverse-effect for this formulation was reported to be 1000 mg/kg/day in rodents [27]. A total of 6 groups with 15 mice were included: (1) Saline + Vehicle, (2) Saline + NR (300 mg/kg), (3) FIP + Vehicle, (4) FIP + NR (100 mg/kg), (5) FIP + NR (300 mg/kg), and (6) FIP + NR (500 mg/kg). Doses of 300 and 500 mg/kg for NR were chosen to analyze the dose-dependent response. Septic and their relevant sham mice were killed at 6 h after FIP as we previously described [24], and serum, heart and lung tissues collected for further analyses. No death was observed for the acute study within 6 h after FIP.
For survival analysis, a single dose of NR (300 mg/kg body weight, i.p.) or vehicle was given at 30 min before FIP in mice. Two groups of septic mice (FIP + Vehicle and FIP + NR) with 17 mice in each group were monitored for 24 h after FIP.
Endotoxemia was induced in mice (male and aged 2 months) by intraperitoneal injection of lipopolysaccharides (LPS, 4 mg/kg body weight, Sigma) and sham mice received a same volume of sterile saline. A single dose of NR (300 mg/kg body weight; Biochempartner, China) or vehicle was intraperitoneally injected into mice 30 min before saline and LPS administration. This dose of NR was chosen based on previous reports that showed that a dose of 300 mg/kg body weight was safe [27] and efficiently increased the content of NAD+ in liver and muscle in mice [15,28]. Four groups were included with 10 mice in each group: (1) Saline + Vehicle, (2) Saline + NR, (3) LPS + Vehicle, and (4) LPS + NR. Endotoxemic mice and their relevant sham mice were killed at 4 h after LPS injection as we previously described [24], and serum and lung tissues collected. No death was observed in LPS-injected mice.
All animals were given sterile saline (1 ml) containing the buprenorphine (4 μg/ml) subcutaneously immediately after feces, LPS or saline injection, and then at 6 h intervals as appropriate.
2.2. Echocardiography
Animals were lightly anaesthetized with inhaled isoflurane (1%) and imaged on a warm handling platform using a 40 MHz linear array transducer attached to a preclinical ultrasound system (Vevo 2100, FUJIFILM Visual Sonics, Canada) with nominal in-plane spatial resolution of 40 μm (axial) × 80 μm (lateral) as we described recently [29]. Left ventricular (LV) end-systolic inner diameter (LVIDs), LV enddiastolic inner diameter (LVIDd), fractional shortening (FS), and ejection fraction (EF) were analyzed.
2.3. Cell culture and treatments
Mouse macrophage cell line RAW264.7 was obtained from ATCC (USA) and cultured in DMEM containing 10% fetal bovine serum (FBS). After incubation with NR for 36 h, RAW264.7 cells were exposed to LPS (100 ng/ml in culture medium) for 24 h. The cells and supernatants were assayed for HMGB1 protein.
Mouse cardiac microvascular endothelial cells were purchased from CELLutions Biosystems (Cedarlane Laboratories, Hornby, ON, Canada) and cultured in DMEM containing 5% FBS. Endothelial cells were pretreated with NR for 12 h, and then the culture medium was replaced with a conditioned-medium collected from RAW264.7 cells stimulated with LPS or saline for 24 h. Twelve hours later, the cells were collected for further analysis.
Mouse pulmonary microvascular endothelial cells were obtained from Cell Biologics (Cedarlane Laboratories, Hornby, ON, Canada) and cultured in DMEM containing 20% FBS and growth factors. Endothelial cells were pretreated with NR for 12 h, and then the culture medium was replaced with a conditioned-medium from RAW264.7 cells stimulated with LPS or saline for 24 h. Six hours later, the cells were collected for further analysis.
2.4. Measurement of pulmonary microvascular permeability
Pulmonary microvascular permeability in mice was assessed using Evans blue dye as previously described [24]. Briefly, mice were injected with 0.4% Evans blue solution (50 mg/kg) via caudal vein 30 min before euthanasia. After flashing with 10 ml PBS, the lungs were removed, weighted and homogenized with PBS. The tissue lysates were mixed with 2 ml of formamide, and then incubated at 60 °C for 16 h. The samples were centrifuged at 20,000g at 4°C for 5 min, and the supernatant was used to determine the concentration of Evans blue by spectrophotometry (620, 740 nm). The microvascular permeability was assessed by the leak of Evans blue in lung tissues [Evans Blue (ug)/Lung weight (g) × 10/30] (ug EB/g Lung/min).
2.5. Determination of apoptosis
Caspase-3 activity in endothelial cells, and lung and heart tissues was assessed by using a caspase-3 fluorescent assay kit (BIOMOL Research Laboratories) in accordance with the manufacturer’s instructions. To localize cells undergoing nuclear DNA fragmentation in lung, in situ terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) was performed as described previously [30]. DNA fragmentation was determined using a cellular DNA fragmentation ELISA kit (Roche Applied Science, Germany) in endothelial cells according to the manufacturer’s instructions.
2.6. Measurement of myeloperoxidase (MPO) activity
MPO activity in lung and heart tissues was determined as previously described [31].
2.7. Assessment of MDA production and carbonyl protein content
The levels of malondialdehyde (MDA) and carbonyl protein content in lung and heart tissues were determined using a TBARS assay kit and Protein Carbonyl Colorimetric Assay Kit (Cayman Chemical Company, USA), respectively, following the manufacturer’s instructions.
2.8. Histological examination
Lung tissues were fixed in 4% paraformaldehyde at 4 °C for 48 h, and then embedded in paraffin wax, sectioned (5 μm) and processed for H&E staining.
2.9. NAD+ assay
NAD+ was extracted from cells or tissues and measured by NAD/NADH Microplate Assay Kit (Cohesion Biosciences, UK) according to manufacturer’s instructions.
2.10. Western blotting analysis
Ten microliters of serum or culture medium were assayed for the protein levels of HMGB1 by western blot analysis (1:1000 dilution, Sigma Aldrich, USA). Cells were homogenized and 40 μg of cell lysates were loaded on SDS-PAGE gels. After electrophoresis, the separate proteins were transferred onto Bio-Rad PVDF membranes. The membranes were incubated with antibodies against HMGB1 (1:1000 dilution, Sigma Aldrich, USA), acetylated p65 and p65 (1:1000 dilution, Cell Signaling Technology, USA) or GAPDH (1:5000 dilution, Santa Cruz Biotechnology, USA), respectively, followed by secondary relevant antibodies conjugated with horseradish peroxidase. The signals were then developed using an enhanced version of the chemiluminescence reaction.
2.11. Dihydroethidium staining
After various treatments, mouse cardiac microvascular endothelial cells were incubated with dihydroethidium (DHE, 5 μM, Thermo Fisher Scientific, USA) for 15 min to determine ROS production (red color). Nuclei were stained by Hoechst 33342 (Thermo Fisher Scientific, USA, blue color). ROS production was visualized under fluorescent microscope (red color for ROS and blue color for nucleus). A cell permeable superoxide dismutase (PEG-SOD, 100 units/ml, Sigma, USA) was used to verify ROS production.
2.12. Statistical analysis
All data are presented as mean values ± SD. One-way or two-way analysis of variance followed by Newman–Keuls test was performed for multi-group comparisons, as appropriate. Student t-test was used for 2-group comparison. Survival curves were created by the method of Kaplan and Meier, and compared by the log-rank test. A value of P < 0.05 was considered statistically significant.
3. Results
3.1. Administration of NR prevents oxidative damage and apoptosis, alleviates lung and heart injury, and improves the survival in sepsis
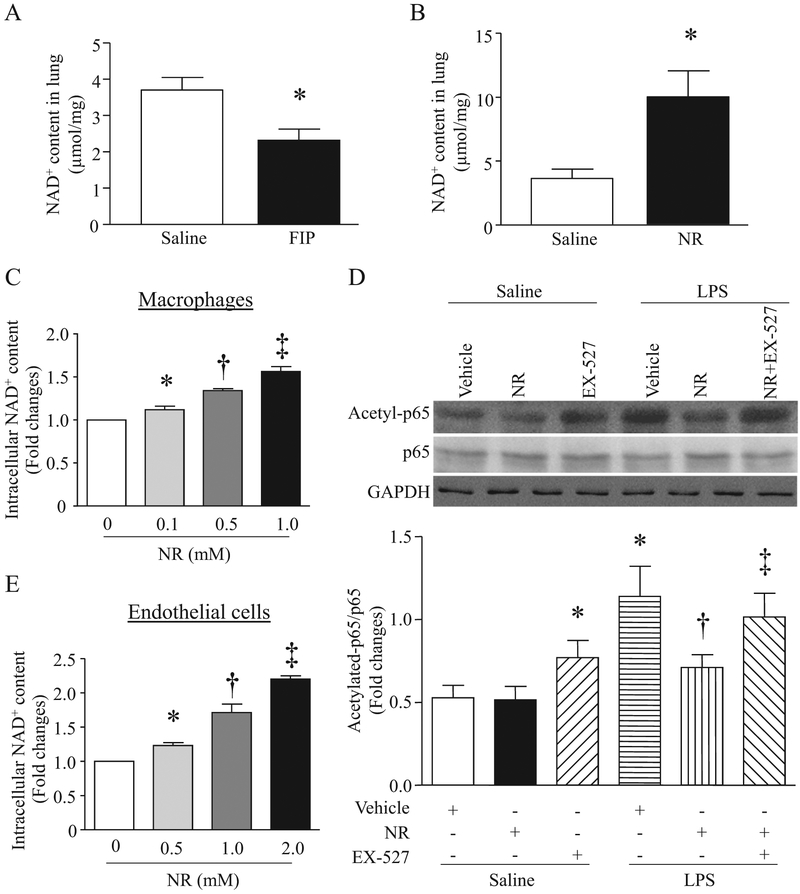
It was reported that the levels of NAD+ were decreased in liver tissues from a mouse model of CLP-induced sepsis [25]. In line with this prior finding, we showed that the levels of NAD+ in lung tissues were also reduced after FIP injection (Fig. 1A). At 6 h after receiving NR, there was about 3-fold increase in NAD+ levels in mouse lung tissues (Fig. 1B).
Fig. 1.
Effects of NR on NAD+ levels and Sirt1 activation. (A) Mice were intraperitoneally injected with feces (3.75 g/kg, n = 6) or saline (n = 5). Six hours later, NAD+ level in lungs were determined. (B) Mice were intraperitoneally administrated with NR (300 mg/kg, n = 5) or saline (n = 5). NAD+ levels in lungs were assessed at 6 h after NR injection. (C) Macrophages (RAW264.7) were incubated with a normal culture medium containing various doses of NR for 36 h and then collected for NAD+ assay. Data are mean ± SD (n = 3). *P<0.05 vs saline, †P<0.05 vs NR (0.1 mM), ‡P<0.05 NR (0.5 mM). (D) Macrophages were pretreated with NR (1 mM) or EX527 (1 μM) alone or in combination, or vehicle, and then incubated with LPS (0.1 μg/ml) or saline. Western Blot analysis was performed to determine acetylated p65, total p65 and GAPDH. Upper panel is representative western blots from 3 independent experiments, and lower panel is the quantification of acetylated p65/p65 ratio. Data are mean ± SD (n = 3). *P<0.05 vs saline + vehicle, †P<0.05 vs LPS + vehicle, ‡P<0.05 vs LPS+NR. (E) Mouse cardiac microvascular endothelial cells were incubated with a normal culture medium containing NR for 12 h, and collected for NAD+ assay. Data are mean ± SD (n = 3).
*P<0.05 vs saline, †P<0.05 vs NR (0.5 mM), ‡P<0.05 vs NR (1.0 mM).
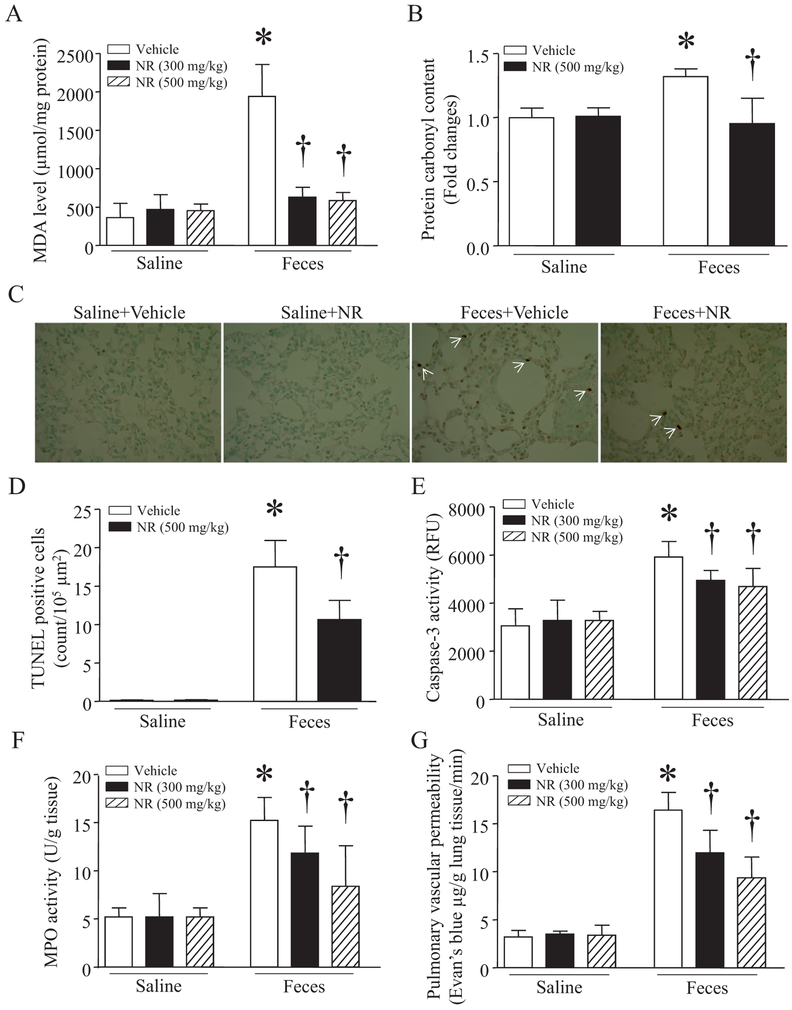
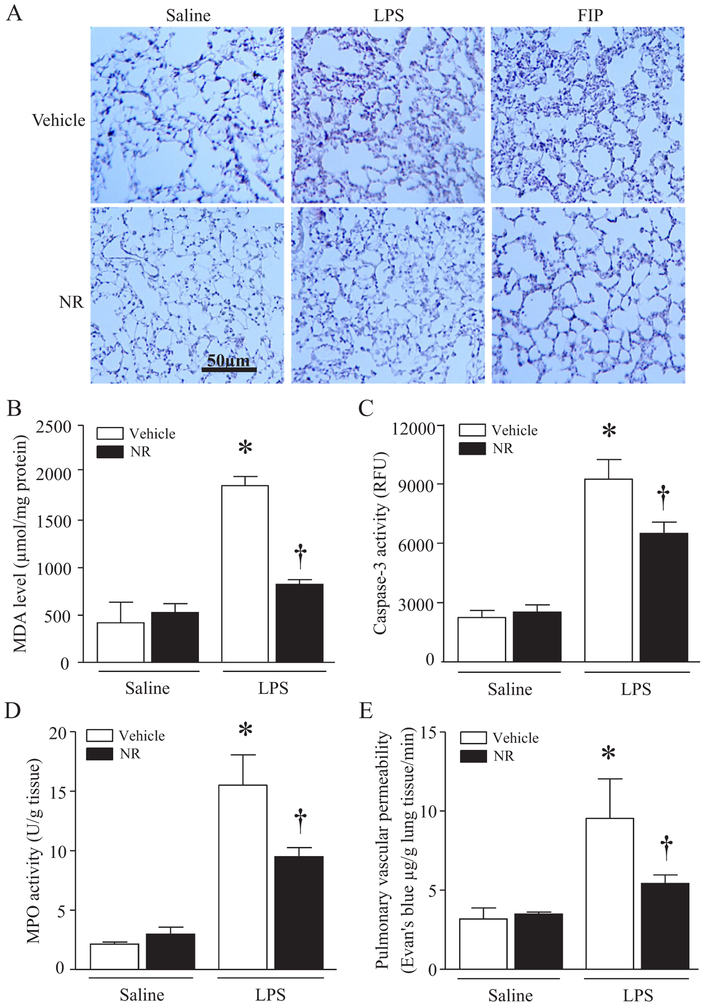
We then determined whether administration of NR reduced lung injury in a mouse model of FIP-induced sepsis, a clinically relevant model. Consistent with previous studies [24,32], sepsis caused oxidative damage as evidenced by elevated MDA production and carbonyl protein content (Fig. 2A and B), induced apoptosis as determined by increases in caspase-3 activity and TUNEL positive cells (Fig. 2D and E), promoted infiltration of neutrophils and/or macrophages as shown by increased MPO activity (Fig. 2F), and increased pulmonary micro-vascular permeability in lung tissues (Fig. 2G), indicative of lung injury. These lung injuries were slightly reduced by a low dose of NR (100 mg/kg, data not shown), but significantly attenuated by NR at 300 mg/kg and further decreased by NR at 500 mg/kg (Fig. 2A–G). Histological analysis manifested highly inflammatory cell infiltration, alveolar wall edema and thickening in lung from septic mice, confirming lung injury (Fig. 3A). Again, these pathological changes were reduced by NR in septic mouse lung. Similarly, administration of NR (300 mg/kg) reduced lung injury in LPS-induced endotoxemic mice (Fig. 3A–E).
Fig. 2.
Protective effects of NR on acute lung injury in septic mice. After receiving an intraperitoneal injection of NR (300 or 500 mg/kg) or vehicle, mice were challenged with feces (3.75 g/kg, i.p.) or saline. Six hours later, MDA level (A), carbonyl protein content (B), TUNEL staining (C, representative micro-pictures with white arrows indicating positive staining; D, quantification of TUNEL positive cells per μm2), caspase-3 activity (E) and MPO activity (F) were determined in lung tissues, and pulmonary microvascular albumin leak was measured (G). Data are mean ± SD (n = 5–7 mice in each group).
*P<0.05 vs sham or vehicle, †P<0.05 vs vehicle + feces.
Fig. 3.
Protective effects of NR on acute lung injury in endotoxemic mice. After receiving an intraperitoneal injection of NR (300 mg/kg) or vehicle, mice were challenged with LPS (4 mg/kg), FIP or saline. (A) Lung tissues were collected, fixed in formalin and embedded in wax. After sectioning and processing, H&E staining was performed. A representative H&E staining for histological changes is presented from 5 different hearts. (B–D) Four hours later after LPS injection, MDA level (B), caspase-3 activity (C) and MPO (D) were analyzed in lung tissues, and pulmonary microvascular albumin leak was determined (E). Data are mean ± SD (n = 5 mice in each group). *P<0.05 vs saline + vehicle, †P<0.05 vs vehicle + LPS.
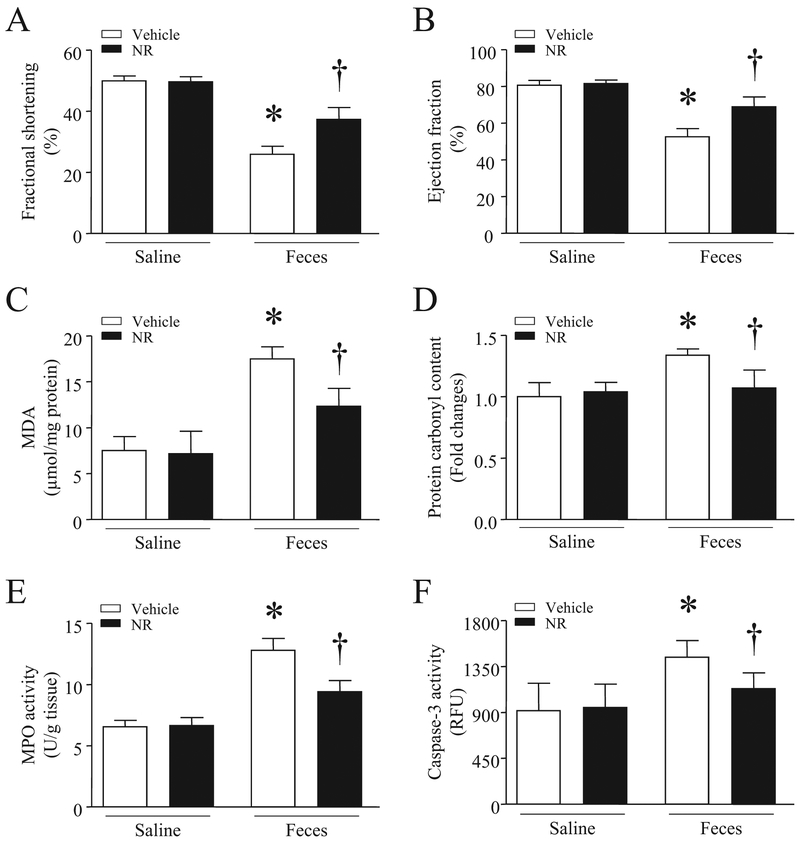
Administration of NR (500 mg/kg) attenuated myocardial dysfunction in septic mice as evidenced by increased FS% and EF% (Fig. 4A and B). The improvement of myocardial function was associated with decreased MDA production, carbonyl protein content, MPO activity and caspase-3 activity in septic mouse hearts (Fig. 4C–F). As a consequence, NR significantly improved the survival in mice at 24 h after onset of sepsis (Fig. 5A). Together, these results demonstrate that administration of NR prevents organ injury (heart and lung) and improves the survival in sepsis.
Fig. 4.
Effects of NR on myocardial injury in septic mice. After receiving an intraperitoneal injection of NR (500 mg/kg) or vehicle, mice were challenged with feces (3.75 g/kg, i.p.) or saline. Six hours later, myocardial function (A and B), MDA level (C), carbonyl protein content (D), MPO activity (E) and caspase-3 activity (F) were measured in heart tissues. Data are mean ± SD (n = 7 mice in each group).
*P<0.05 vs saline + vehicle, †P<0.05 vs vehicle + feces.
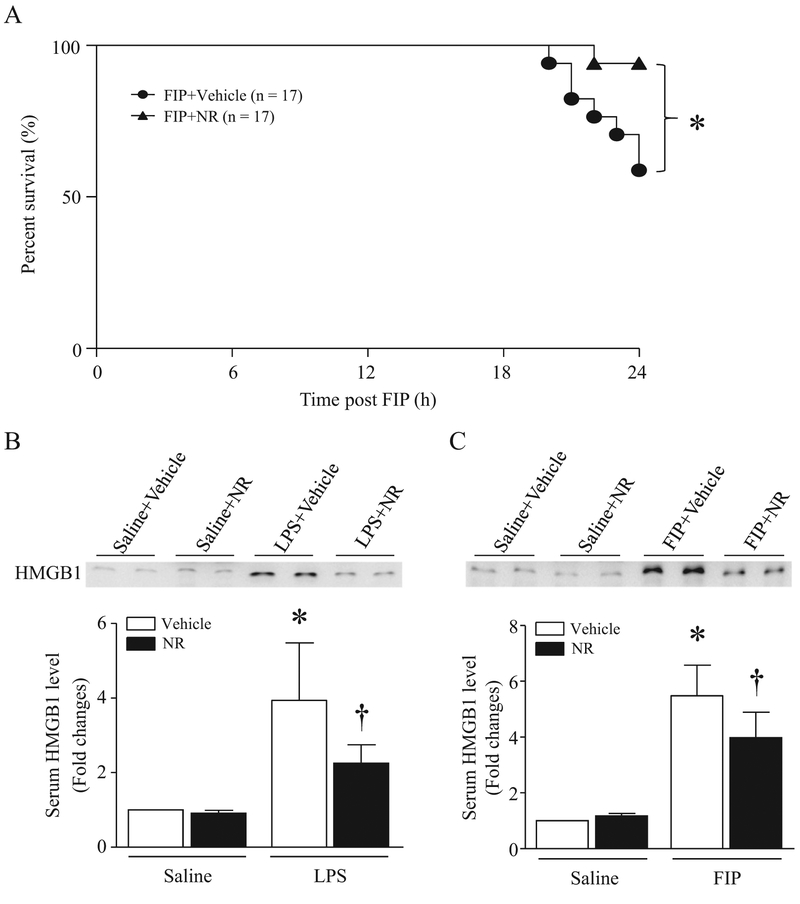
Fig. 5.
Effect of NR on survival in septic mice and serum HMGB1 in endotoxemic and septic mice. (A) After receiving an intraperitoneal injection of NR (300 mg/kg) or vehicle, mice were challenged with feces. Survival was monitored for 24 h in septic mice. *P < 0.05 (n = 17 in each group). (B and C) Mice received NR (300 mg/kg) or vehicle and then LPS, FIP or saline (i.p.). After four hours of LPS injection (B) or six hours of FIP (C), the protein levels of HMGB1 in serum were determined by western blot analysis. Data are mean ± SD (n = 5 mice in each group).
*P<0.05 vs sham + vehicle, †P<0.05 vs vehicle + LPS or vehicle + feces.
3.2. NR reduces serum HMGB1 levels in endotoxemic and septic mice
To explore the mechanisms by which NR provided protective effects, we focused on HMGB1 as the circulating HMGB1 levels were reported to correlate with the disease severity in ALI and HMGB1 directly impaired myocardial function in sepsis [11,12]. Thus, we examined HMGB1 release in endotoxemic and septic mice. The levels of circulating HMGB1 were dramatically increased in LPS-injected mice and FIP-induced septic mice, both of which were prevented by NR pretreatment (Fig. 5B and C). These results suggest that the protective effects of NR may be associated with inhibition of HMGB1 release in sepsis.
3.3. NR inhibits LPS-induced HMGB1 release in macrophages, which is abolished by addition of SIRT1 inhibitor
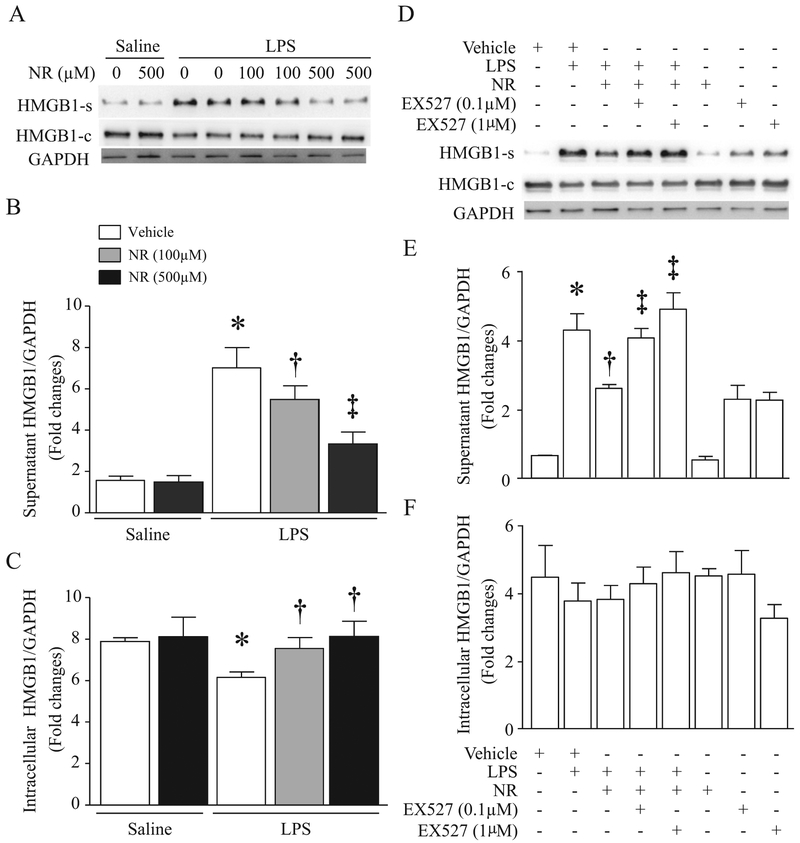
Macrophages are one of major sources for circulating HMGB1 [33]. Therefore, we determined the effect of NR on HMGB1 release directly in cultured macrophages (RAW264.7). LPS dose-dependently induced HMGB1 release in culture medium starting from 4 h after LPS stimulation in macrophages (Supplementary material Fig. 1). NR increased cellular NAD+ content (Fig. 1C). Pre-treatment with NR dose-dependently reduced HMGB1 release in macrophages induced by LPS for 24 h (Fig. 6A and B). Similarly, LPS-induced HMGB1 release was also prevented by a selective inhibitor of cyclooxygenase-2, NS-398 (Supplementary material Fig. 2A). These results suggest that NR and some anti-inflammatory reagents (e.g. cyclooxygenase-2 inhibitor) may share similar mechanisms in reducing septic organ injury.
Fig. 6.
Effects of NR on LPS-induced HMGB1 release in Macrophages. (A–C) Cultured RAW264.7 cells were pretreated with NR (100 μM, 500 μM) or saline for 36 h and then challenged by LPS (0.1 μg/ml). After 24 h of LPS treatment, HMGB1 in supernatants (A, B) and cells (A, C) were determined by western blot analysis. Data are mean ± SD (n = 3). *P<0.05 vs saline + vehicle, †P<0.05 vs vehicle + LPS, and ‡P<0.05 vs vehicle + LPS + NR (100 μM). (D–F) Cultured RAW264.7 cells were treated with NR (500 μM) and/or EX527 (0.1 μM, 1 μM) for 36 h and then challenged with or without LPS (100 ng/ml). Twenty-four hours later, HMGB1 in supernatants (D, E) and cells (D, F) were measured by western blot analysis. Data are mean ± SD (n = 3 independent experiments). *P<0.05 vs vehicle, †P<0.05 vs vehicle + LPS, and ‡P<0.05 vs LPS + NR.
It is well known that HMGB1 is located in the nucleus under physiological conditions. However, it may be modified by acetylation and translocate into the cytoplasm and then released from activated cells [34]. Thus, deacetylation of HMGB1 by SIRT1 prevents its translocation from nucleus to cytoplasm and subsequent release [35]. To examine whether NR inhibited HBGM1 release through SIRT1 signaling, we pre-incubated macrophages with NR or vehicle for 36 h and then exposed them to LPS or saline in the presence or absence of EX527, a specific inhibitor of SIRT1 for 24 h. First, we determined SIRT1 activation by analyzing acetylation of p65 protein. LPS significantly induced acetylation of p65, which was reversed by NR, and this effect of NR on LPS-induced p65 acetylation was offset by EX527 (Fig. 1D). These results suggest that NR activates SIRT1 signaling in macrophages. We then analyzed HMGB1 release. As shown in Fig. 6D–F, the inhibitory effect of NR on HMGB1 release was prevented by EX527 in a dose-dependent manner. Taken together, these results support that NR inhibits LPS-induced HMGB1 release through the SIRT1 signaling.
3.4. NR attenuates LPS-conditioned medium-induced apoptosis in endothelial cells through the SIRT1 signaling
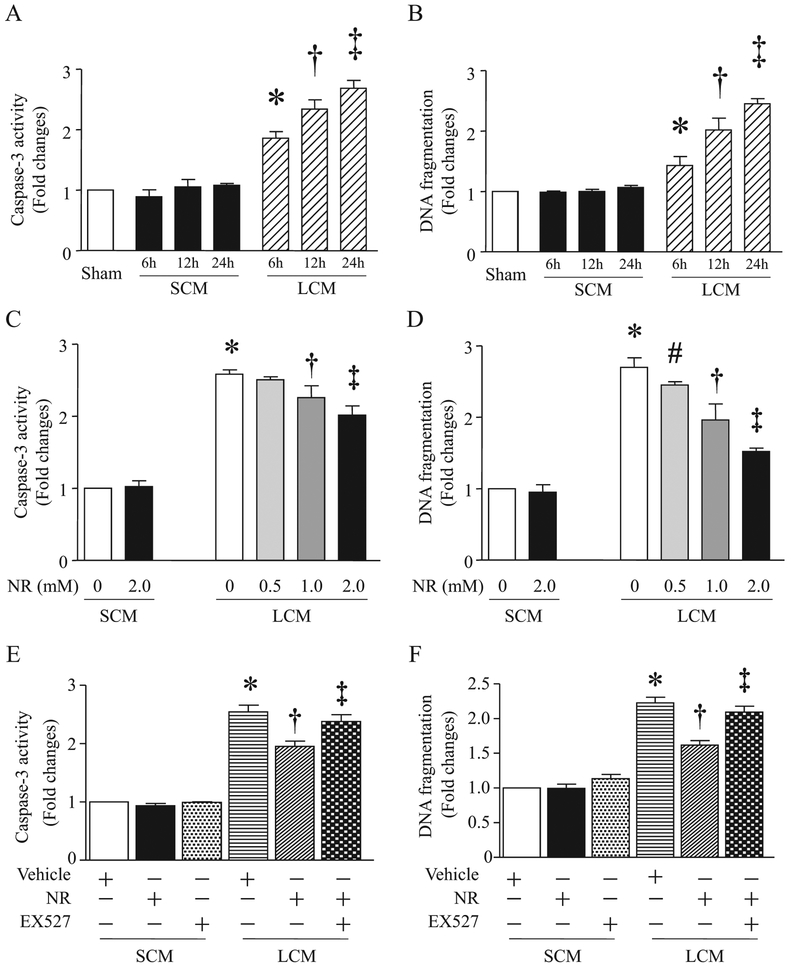
It has been reported that the loss of endothelial cells via apoptosis is implicated in endothelial barrier dysfunction and organ damage during sepsis [36]. Having shown that NR prevented HMGB1 release in macrophages, we would like to answer whether NR protects endothelial cells against inflammatory injury. To address this, we incubated macrophages (RAW264.7) with LPS for 24 h and collected the supernatants as inducers of inflammatory injury as they include a mixture of inflammatory factors including HMGB1. We then exposed cardiac microvascular endothelial cells to the conditioned-medium from LPS-stimulated macrophages. The medium from saline-treated macrophages served as a control. The LPS-conditioned medium time-dependently increased caspase-3 activity and DNA fragmentation (Fig. 7A and B). On the contrary, saline-conditioned medium did not induce apoptosis in endothelial cells (Fig. 7A and B). Administration of NR dose-dependently increased NAD+ content (Fig. 1E), and reduced LPS-conditioned medium-induced caspase-3 activity and DNA fragmentation in endothelial cells (Fig. 7C and D). The inhibitory effect of NR on apoptosis was abolished by pretreatment with EX527 in LPS-conditioned medium-stimulated endothelial cells (Fig. 7E and F). Similarly, inhibition of cyclooxygenase-2 with NS-398 attenuated apoptosis induced by LPS-conditioned medium (Supplementary material Fig. 2B). Although there has been no evidence that cardiac and pulmonary microvascular endothelial cells respond differently to inflammatory injury, we examined such response in pulmonary microvascular endothelial cells. Our result revealed a similar anti-apoptotic effect of NR in pulmonary microvascular endothelial cells stimulated with LPS-conditioned medium (Supplementary material Fig. 3). Thus, our results demonstrate that NR effectively protects against endothelial cell apoptosis under a septic condition through the SIRT1 signaling.
Fig. 7.
Effects of NR on conditioned medium-induced apoptosis in endothelial cells. (A and B) Mouse cardiac microvascular endothelial cells (MCECs) were challenged by a conditioned medium (LCM) from RAW264.7 cells stimulated with LPS or control medium (SCM) from cultured RAW264.7 cells treated with saline for different times. Caspase-3 (A) and DNA fragmentation (B) were analyzed. Data are mean ± SD (n = 3 independent experiments). *P<0.05 vs 0 h, †P<0.05 vs 6 h, ‡P <0.05 vs 12 h. (C and D) MCECs were treated with NR (0.5 mM, 1 mM, 2 mM) or saline for 12 h, and then challenged by LCM or SCM from RAW264.7 cells. Twelve hours after addition of LCM and SCM, caspase-3 activity (C) and DNA fragmentation (D) were measured. Data are mean ± SD (n = 3). *P<0.05 vs 0 + SCM, #P < 0.05 vs 0 + LCM, †P<0.05 vs 0.5 + LCM, ‡P<0.05 vs 1.0 + CM. (E and F) MCECs were pretreated with NR (2 mM) or saline for 12 h, and then stimulated with LCM or SCM in presence of EX527 (1 μM) or vehicle. Twelve hours later, caspase-3 activity (E) and DNA fragmentation (F) were assessed. Data are mean ± SD (n = 3 independent experiments). *P<0.05 vs vehicle + SCM, †P<0.05 vs vehicle + LCM, ‡P<0.05 vs NR + LCM.
3.5. NR reduces LPS-conditioned medium-induced oxidant stress in microvascular endothelial cells, which is reversed by EX527
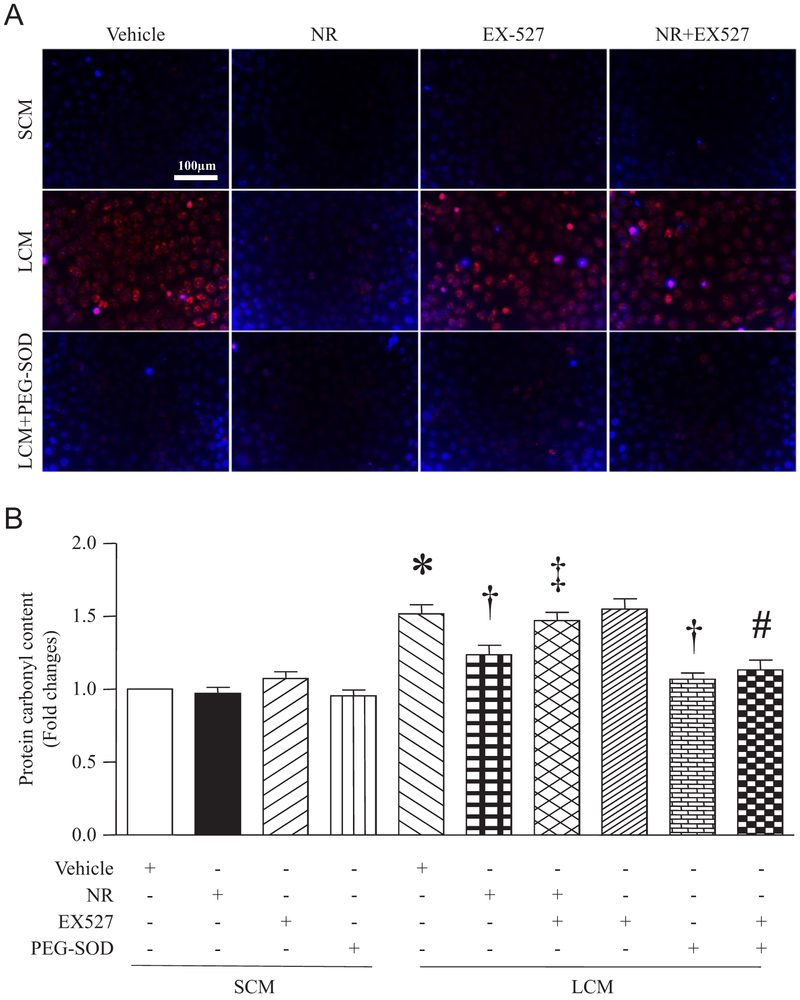
Overproduction of ROS and subsequently oxidative damage are important mechanisms for sepsis-induced organ damage [13]. Our in vivo study has shown that NR prevented MDA production and carbonyl protein content, indicative of decreased oxidative damage. In this study, we determined whether NR also prevents oxidant stress in endothelial cells under a septic condition. We pre-treated cardiac micro-vascular endothelial cells with NR and then incubated them with LPS-conditioned medium or saline-conditioned medium from macrophages in the presence of EX-527 or vehicle in combination with a cell permeable superoxide dismutase, PEG-SOD. Four hours later, the intracellular ROS levels markedly increased in endothelial cells stimulated with LPS-conditioned medium compared with saline-conditioned medium, which was completely inhibited by PEG-SOD, confirming the generation of ROS. NR pretreatment also prevented ROS production in macrophages induced by LPS-conditioned medium. However, the presence of EX527 abolished the inhibitory effect of NR on ROS production. PEG-SOD reversed the effect of EX527 on ROS production (Fig. 8A). Similarly, NR prevented LPS-conditioned medium-induced carbonyl protein content. This inhibitory effect of NR was offset by EX527. In addition, PEG-SOD abrogated the effects of LPS-conditioned medium and EX527 on carbonyl protein content (Fig. 8B). These results indicate that NR inhibits oxidant stress through SIRT1 signaling under a septic condition.
Fig. 8.
Effects of NR on LPS-conditioned medium (LCM)-induced oxidant stress in endothelial cells. After 12 h of treatment with NR (2 mM), EX527 (1 μM), PEGSOD (100 units/ml) or vehicle alone or in combinations, MCECs were challenged by LCM or saline-conditioned medium (SCM) for 12 h. Intracellular ROS production was measured in living cells by DHE staining. (A) A representative fluorescent microscopic picture for DHE staining (red color) and nuclear staining (blue color) from 3 independent experiments. (B) Carbonyl protein content was determined in cell lysates. Data are mean ± SD (n = 3). *P<0.05 vs vehicle + SCM, †P<0.05 vs vehicle + LCM, ‡P<0.05 vs LCM + NR, #P < 0.05 vs EX527 + LCM.
4. Discussion
Currently, therapeutic approaches to reduce lung injury and myocardial dysfunction are limited in sepsis. In this study, we report that pre-administration of NR prevented lung injury and myocardial dysfunction, and improved the survival in septic mice. Mechanistically, we demonstrate that NR inhibited HMGB1 release and prevented oxidative stress under septic conditions through the NAD+/SIRT1 signaling. To the best of our knowledge, this is the first report that NR provides protection against organ injury in sepsis.
NAD+ is required for SIRT1 activation. In sepsis, inhibition of SIRT1 enhanced whereas pharmacological activation of SIRT1 attenuated organ injury in mice [21–24]. Thus, the protection conferred by NR may be attributed to NAD+ biosynthesis and subsequent SIRT1 activation in sepsis. In fact, the present study shows that inhibition of SIRT1 abrogated the protective effects of NR in endothelial cell injury under septic conditions. Furthermore, we discovered that NR pretreatment markedly reduced plasma HMGB1 levels in septic mice, which correlated with an attenuation of lung injury and myocardial dysfunction in septic mice. Studies have demonstrated that hyper-acetylation of HMGB1 promotes its nucleus-to-cytoplasm translocation and release in activated immune cells [37]. SIRT1 induces de-acetylation of HMGB1 and thus, inhibits its release. Thus, we believe that NR/NAD+/SIRT1 signaling contributed to a reduction in plasma HMGB1 levels in septic mice, observed in the present study. This was indeed supported by our in vitro study in macrophages, in which LPS-induced HMGB1 release was inhibited by NR and this negative effect of NR was dose-dependently prevented by SIRT1 inhibitor. Given HMGB1 is an important pro-inflammatory mediator, the present finding suggests that NR provides anti-inflammatory effects in lung and heart under septic conditions. Further evidence in support of this possibility is that infiltration of neutrophils and/or macrophages was significantly attenuated in NR-treated mouse lung and heart tissues during sepsis.
SIRT1 has also been implicated in anti-oxidant stress and anti-apoptosis in different experimental scenarios [38]. In line with this, the present study shows that NR protected endothelial cells against apoptosis and oxidative damage induced by inflammatory response. Several lines of evidence support this finding. Firstly, administration of NR prevented oxidative damage and apoptosis in lung and heart tissues of both endotoxemic and septic mice. Secondly, NR protected endothelial cells against apoptosis induced by a conditioned-medium from LPS-stimulated macrophages. This LPS-conditioned medium contained a mixture of pro-inflammatory factors including HMGB1 and pro-in-flammatory cytokines released from macrophages. Thirdly, NR inhibited ROS production in endothelial cells induced by a conditioned-medium from LPS-stimulated macrophages. Lastly, these inhibitory effects of NR on apoptosis and ROS production were not observed in endothelial cells in the presence of SIRT1 inhibitor. These results provide direct evidence to support that NR protects endothelial cells against oxidative stress and inflammatory injury through SIRT1 signaling. Taken together, our observations are consistent with a model whereby NR induces NAD+/SIRT1 signaling and inhibits oxidative stress, which not only prevents pro-inflammatory response and in-flammation, but also provides protection against inflammatory injury in sepsis.
Previous studies revealed a reduction in NAD+ levels in lung tissues of endotoxemic rats (by about 200%) and livers of septic mice (by about 380%) [25,26]. The present study also shows lower NAD+ levels in lungs of septic mice than those in sham mice. However, a relative less reduction (by about 60%) was observed in our FIP model. This may be due to different models of sepsis (FIP in mice vs LPS in rats or CLP in mice). Several mechanisms have been suggested to contribute to the decline of NAD+ content in sepsis: (a) Depletion of NAD+ by hyper-activation of PARPs enzymes for DNA repairing, in which NAD+ acts as a sole substrate [39]; (b) Block of NAD+ generation through the salvage pathway due to inhibition of NAMPT activity by inflammation [40]; and (c) Reduction of NAD+ by sepsis-induced redox imbalance and mitochondrial damages [41,42]. Since NAD+ plays a critical role in several biological processes, including redox reaction, cellular respiration and energy production, DNA repair and protein de-acetylation [43], it is possible that boosting NAD+ content in tissues may be beneficial to cell function and reduce various injury under pathological conditions. In fact, the present study demonstrates that a single dose of IP NR injection induced a transient increase in NAD+ level (about 3 folds) in lungs of mice, which prevented lung and heart injury, and improved the survival in septic mice. Previous evidence showed that administration of NR effectively elevated NAD+ levels in liver and muscle in mice [15,28]. Thus, boosting NAD+ with NR may also protect other organs in sepsis.
There are other potential mechanisms to boost intracellular NAD+. One immediate option would be NAD+ supplement. Unfortunately, NAD+ is easily degraded by serum hydrolases [17] and it is not readily taken up by cells because of its high polarity [44,45], which makes it difficult to be replenished directly. Alternatively, NAD+ can be biosynthesized from nicotinamide and niacin through the salvage pathway and from tryptophan through the de novo pathway [46]. Indeed, niacin was reported to dose-dependently increase NAD+ levels in lungs and consequently, attenuate lung inflammation and improve the survival in endotoxemic rats [26]. Treatment with nicotinamide protected against acute liver injury and increased the survival in mouse models of endotoxemia and CLP-induced sepsis [47]. These prior studies support the protective effects of NAD+ in sepsis. However, the use of niacin is limited by painful flushing [48], and nicotinamide may inhibit SIRTs [49], a family of de-acetylases required for various essentially cellular functions. On the contrary, administration of NR efficiently boosts intracellular NAD+ levels without negatively affecting SIRT activity, and instead, increasing SIRT activities [16–19]. Thus, NR has the potential to overcome limitations of other precursors while still providing the benefits of elevated NAD+.
It is important to mention that pre-treatment with NR provided protection whereas administration of NR after induction of sepsis did not show any protection in septic mice. This is because sepsis induced a rapid reduction of NR kinase 1/2 in lung and liver (data not shown), essential enzymes responsible for conversion of NR to NAD+ [14,15]. Although the protection conferred by pretreatment with NR may limit the use of NR as a therapeutic agent for sepsis at this moment, administration of NR can provide a preventive approach for septic organ injury as NR has been widely used as a health supplement [27]. It is also worthwhile to mention that TBARS and DHE staining for ROS production may be not very specific. However, measurement of carbonyl protein content provided similar results and the use of PEG-SOD validated the measurement of ROS using DHE staining in the present study.
5. Conclusions
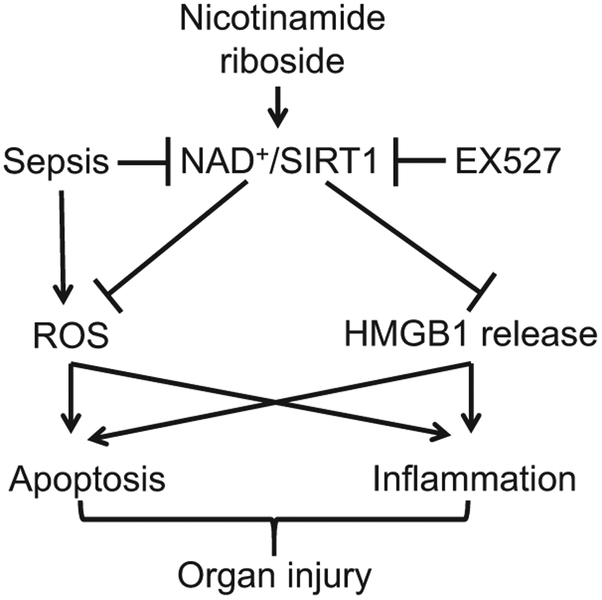
NR reduces lung injury, attenuates myocardial dysfunction and improves the survival in sepsis via the NAD+/SIRT1 signaling (Fig. 9).
Fig. 9.
Schematic of nicotinamide riboside/NAD/SIRT1 pathway in sepsis. Sepsis induces ROS production and inhibits NAD+/SIRT1 signaling, leading to HMGB1 release, apoptosis and inflammation, which contribute to organ injury. Nicotinamide riboside is converted to NAD+ and subsequently activates SIRT1 signaling. Activation of SIRT1 inhibits ROS production and HMGB1 release thereby attenuating apoptosis and inflammation, and finally protecting against organ injury in sepsis. EX527 inhibits SIRT1 activation and reverses the protective effects of nicotinamide riboside on septic organ injury.
Supplementary Material
Acknowledgments
Funding
This work was supported by the Heart and Stroke Foundation of Canada (Grant in aid, G-17–0018361), the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT, IRT1075), the Natural Science Foundation of Zhejiang Province (LY13H150006) and the Funds of 551 Talents Project in Wenzhou.
Abbreviations:
- ALI
acute lung injury
- CLP
cecal ligation and puncture
- EF
ejection fraction
- FBS
fetal bovine serum
- FIP
feces injection in peritoneum
- FS
fractional shortening
- HMGB1
high mobility group box-1
- LPS
lipopolysaccharides
- LV
left ventricular
- LVIDd
left ventricular end-diastolic inner diameter
- LVIDs
left ventricular end-systolic inner diameter
- MDA
malondialdehyde
- MPO
myeloperoxidase
- NAD+
nicotinamide adenine dinucleotide
- NR
nicotinamide riboside
- PARPs
poly (ADP-ribose) polymerases
- ROS
reactive oxygen species
- SIRT
sirtuin
Footnotes
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.freeradbiomed.2018.05.073.
References
- [1].Martin CM, Priestap F, Fisher H, Fowler RA, Heyland DK, Keenan SP, Longo CJ, Morrison T, Bentley D, Antman N, A prospective, observational registry of patients with severe sepsis: the Canadian sepsis treatment and response registry, Crit. Care Med 37 (1) (2009) 81–88. [DOI] [PubMed] [Google Scholar]
- [2].Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC, The third international consensus definitions for sepsis and septic shock (Sepsis-3), JAMA 315 (8) (2016) 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Howell MD, Davis AM, Management of sepsis and septic shock, JAMA 317 (8) (2017) 847–848. [DOI] [PubMed] [Google Scholar]
- [4].Wheeler AP, Bernard GR, Acute lung injury and the acute respiratory distress syndrome: a clinical review, Lancet 369 (9572) (2007) 1553–1564. [DOI] [PubMed] [Google Scholar]
- [5].Micek ST, McEvoy C, McKenzie M, Hampton N, Doherty JA, Kollef MH, Fluid balance and cardiac function in septic shock as predictors of hospital mortality, Crit. Care 17 (5) (2013) R246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sato R, Kuriyama A, Takada T, Nasu M, Luthe SK, Prevalence and risk factors of sepsis-induced cardiomyopathy: a retrospective cohort study, Medicine (Baltimore) 95 (39) (2016) e5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rudiger A, Singer M, Mechanisms of sepsis-induced cardiac dysfunction, Crit. Care Med 35 (6) (2007) 1599–1608. [DOI] [PubMed] [Google Scholar]
- [8].Matuschak GM, Lechner AJ, Acute lung injury and the acute respiratory distress syndrome: pathophysiology and treatment, Mo Med 107 (4) (2010) 252–258. [PMC free article] [PubMed] [Google Scholar]
- [9].Grommes J, Soehnlein O, Contribution of neutrophils to acute lung injury, Mol. Med 17 (3–4) (2011) 293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang H, Ward MF, Sama AE, Targeting HMGB1 in the treatment of sepsis, Expert Opin. Ther. Targets 18 (3) (2014) 257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ueno H, Matsuda T, Hashimoto S, Amaya F, Kitamura Y, Tanaka M, Kobayashi A, Maruyama I, Yamada S, Hasegawa N, Soejima J, Koh H, Ishizaka A, Contributions of high mobility group box protein in experimental and clinical acute lung injury, Am. J. Respir. Crit. Care Med 170 (12) (2004) 1310–1316. [DOI] [PubMed] [Google Scholar]
- [12].Xu H, Su Z, Wu J, Yang M, Penninger JM, Martin CM, Kvietys PR, Rui T, The alarmin cytokine, high mobility group box 1, is produced by viable cardiomyocytes and mediates the lipopolysaccharide-induced myocardial dysfunction via a TLR4/phosphatidylinositol 3-kinase gamma pathway, J. Immunol 184 (3) (2010) 1492–1498. [DOI] [PubMed] [Google Scholar]
- [13].Prauchner CA, Oxidative stress in sepsis: pathophysiological implications justifying antioxidant co-therapy, Burns 43 (3) (2017) 471–485. [DOI] [PubMed] [Google Scholar]
- [14].Bieganowski P, Brenner C, Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans, Cell 117 (4) (2004) 495–502. [DOI] [PubMed] [Google Scholar]
- [15].Ratajczak J, Joffraud M, Trammell SA, Ras R, Canela N, Boutant M, Kulkarni SS, Rodrigues M, Redpath P, Migaud ME, Auwerx J, Yanes O, Brenner C, Canto C, NRK1 controls nicotinamide mononucleotide and nicotinamide riboside metabolism in mammalian cells, Nat. Commun 7 (2016) 13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Srivastava S, Emerging therapeutic roles for NAD(+) metabolism in mitochondrial and age-related disorders, Clin. Transl. Med 5 (1) (2016) 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chi Y, Sauve AA, Nicotinamide riboside, a trace nutrient in foods, is a vitamin B3 with effects on energy metabolism and neuroprotection, Curr. Opin. Clin. Nutr. Metab. Care 16 (6) (2013) 657–661. [DOI] [PubMed] [Google Scholar]
- [18].Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Canto C, Mottis A, Jo YS, Viswanathan M, Schoonjans K, Guarente L, Auwerx J, The NAD (+)/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling, Cell 154 (2) (2013) 430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, Gademann K, Rinsch C, Schoonjans K, Sauve AA, Auwerx J, The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity, Cell Metab. 15 (6) (2012) 838–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sultani G, Samsudeen AF, Osborne B, Turner N, NAD+: a key metabolic regulator with great therapeutic potential, J. Neuroendocrinol (2017). [DOI] [PubMed] [Google Scholar]
- [21].Li T, Zhang J, Feng J, Li Q, Wu L, Ye Q, Sun J, Lin Y, Zhang M, Huang R, Cheng J, Cao Y, Xiang G, Wu Q, Resveratrol reduces acute lung injury in a LPS induced sepsis mouse model via activation of Sirt1, Mol. Med. Rep 7 (6) (2013) 1889–1895. [DOI] [PubMed] [Google Scholar]
- [22].Gao R, Ma Z, Hu Y, Chen J, Shetty S, Fu J, Sirt1 restrains lung inflammasome activation in a murine model of sepsis, Am. J. Physiol. Lung Cell Mol. Physiol 308(8) (2015) L847–L853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Opal SM, Ellis JL, Suri V, Freudenberg JM, Vlasuk GP, Li Y, Chahin AB, Palardy JE, Parejo N, Yamamoto M, Chahin A, Kessimian N, Pharmacological Sirt1 activation improves mortality and markedly alters transcriptional profiles that accompany experimental sepsis, Shock 45 (4) (2016) 411–418. [DOI] [PubMed] [Google Scholar]
- [24].Zheng D, Yu Y, Li M, Wang G, Chen R, Fan GC, Martin C, Xiong S, Peng T, Inhibition of microRNA 195 prevents apoptosis and multiple-organ injury in mouse models of sepsis, J. Infect. Dis 213 (10) (2016) 1661–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liaudet L, Mabley JG, Soriano FG, Pacher P, Marton A, Hasko G, Szabo C, Inosine reduces systemic inflammation and improves survival in septic shock induced by cecal ligation and puncture, Am. J. Respir. Crit. Care Med. 164 (7) (2001) 1213–1220. [DOI] [PubMed] [Google Scholar]
- [26].Kwon WY, Suh GJ, Kim KS, Kwak YH, Niacin attenuates lung inflammation and improves survival during sepsis by downregulating the nuclear factor-kappaB pathway, Crit. Care Med 39 (2) (2011) 328–334. [DOI] [PubMed] [Google Scholar]
- [27].Conze DB, Crespo-Barreto J, Kruger CL, Safety assessment of nicotinamide riboside, a form of vitamin B3, Hum. Exp. Toxicol (2016). [DOI] [PubMed] [Google Scholar]
- [28].Trammell SA, Schmidt MS, Weidemann BJ, Redpath P, Jaksch F, Dellinger RW, Li Z, Abel ED, Migaud ME, Brenner C, Nicotinamide riboside is uniquely and orally bioavailable in mice and humans, Nat. Commun 7 (2016) 12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ni R, Zheng D, Wang Q, Yu Y, Chen R, Sun T, Wang W, Fan GC, Greer PA, Gardiner RB, Peng T, Deletion of capn4 protects the heart against endotoxemic injury by preventing ATP synthase disruption and inhibiting mitochondrial super-oxide generation, Circ. Heart Fail 8 (5) (2015) 988–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shen E, Li Y, Shan L, Zhu H, Feng Q, Arnold JM, Peng T, Rac1 is required for cardiomyocyte apoptosis during hyperglycemia, Diabetes 58 (10) (2009) 2386–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rui T, Feng Q, Lei M, Peng T, Zhang J, Xu M, Abel ED, Xenocostas A, Kvietys PR, Erythropoietin prevents the acute myocardial inflammatory response induced by ischemia/reperfusion via induction of AP-1, Cardiovasc. Res 65 (3) (2005) 719–727. [DOI] [PubMed] [Google Scholar]
- [32].Fisher BJ, Kraskauskas D, Martin EJ, Farkas D, Wegelin JA, Brophy D, Ward KR, Voelkel NF, Fowler AA 3rd, Natarajan R, Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid, Am. J. Physiol. Lung Cell Mol. Physiol 303 (1) (2012) L20–L32. [DOI] [PubMed] [Google Scholar]
- [33].Yamada S, Maruyama I, HMGB1, a novel inflammatory cytokine, Clin. Chim. Acta 375 (1–2) (2007) 36–42. [DOI] [PubMed] [Google Scholar]
- [34].Wu AH, He L, Long W, Zhou Q, Zhu S, Wang P, Fan S, Wang H, Novel mechanisms of herbal therapies for inhibiting HMGB1 secretion or action, Evid.-Based Complement. Altern. Med.: eCAM 2015 (2015) 456305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rabadi MM, Xavier S, Vasko R, Kaur K, Goligorksy MS, Ratliff BB, High-mobility group box 1 is a novel deacetylation target of Sirtuin1, Kidney Int. 87 (1) (2015) 95–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gill SE, Rohan M, Mehta S, Role of pulmonary microvascular endothelial cell apoptosis in murine sepsis-induced lung injury in vivo, Respir. Res 16 (2015) 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hwang JS, Choi HS, Ham SA, Yoo T, Lee WJ, Paek KS, Seo HG, Deacetylation-mediated interaction of SIRT1-HMGB1 improves survival in a mouse model of endotoxemia, Sci. Rep 5 (2015) 15971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Matsushima S, Sadoshima J, The role of sirtuins in cardiac disease, Am. J. Physiol. Heart Circ. Physiol (2015) H1375–H1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Canto C, Menzies KJ, Auwerx J, NAD(+) metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus, Cell Metab. 22 (1) (2015) 31–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Imai S, Yoshino J, The importance of NAMPT/NAD/SIRT1 in the systemic regulation of metabolism and ageing, Diabetes Obes. Metab 15 (Suppl. 3) (2013) S26–S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Misawa T, Takahama M, Kozaki T, Lee H, Zou J, Saitoh T, Akira S, Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 in-flammasome, Nat. Immunol 14 (5) (2013) 454–460. [DOI] [PubMed] [Google Scholar]
- [42].McGettrick AF, O’Neill LA, How metabolism generates signals during innate immunity and inflammation, J. Biol. Chem 288 (32) (2013) 22893–22898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Massudi H, Grant R, Guillemin GJ, Braidy N, NAD+ metabolism and oxidative stress: the golden nucleotide on a crown of thorns, Redox Rep. 17 (1) (2012) 28–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bortell R, Moss J, McKenna RC, Rigby MR, Niedzwiecki D, Stevens LA, Patton WA, Mordes JP, Greiner DL, Rossini AA, Nicotinamide adenine dinucleotide (NAD) and its metabolites inhibit T lymphocyte proliferation: role of cell surface NAD glycohydrolase and pyrophosphatase activities, J. Immunol 167 (4) (2001) 2049–2059. [DOI] [PubMed] [Google Scholar]
- [45].Yang T, Chan NY, Sauve AA, Syntheses of nicotinamide riboside and derivatives: effective agents for increasing nicotinamide adenine dinucleotide concentrations in mammalian cells, J. Med. Chem 50 (26) (2007) 6458–6461. [DOI] [PubMed] [Google Scholar]
- [46].Preyat N, Leo O, Complex role of nicotinamide adenine dinucleotide in the regulation of programmed cell death pathways, Biochem. Pharmacol 101 (2016) 13–26. [DOI] [PubMed] [Google Scholar]
- [47].Yuan H, Wan J, Li L, Ge P, Li H, Zhang L, Therapeutic benefits of the group B3 vitamin nicotinamide in mice with lethal endotoxemia and polymicrobial sepsis, Pharmacol. Res 65 (3) (2012) 328–337. [DOI] [PubMed] [Google Scholar]
- [48].Bogan KL, Brenner C, Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition, Annu. Rev. Nutr 28 (2008) 115–130. [DOI] [PubMed] [Google Scholar]
- [49].Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA, Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1, J. Biol. Chem 277 (47) (2002) 45099–45107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.