Abstract
Objective:
Sea cucumbers are considered among the most important functional foods. Following bioassay guided fractionation, we assessed the anti-proliferative and anti-inflammatory activities of Holothuria polii (H. polii) extracts.
Methods:
Sea cucumber ethanolic extract and the partially purified aqueous fractions were assessed for their anti-proliferative activities. These latter bioactivities were evaluated in the highly invasive MDA-MB-231 human breast cancer cells in two-dimensional and three-dimensional cultures using trypan blue exclusion assay. The tumor-suppressive effects of sea cucumber ethanolic extract and aqueous fractions were assayed by measuring the trans-well invasion of MDA-MB-231 cells and the expression of some epithelial mesenchymal transition markers using quantitative reverse-transcription polymerase chain reaction and western blot analysis. The anti-inflammatory activity of the aqueous fraction was tested by measuring the secreted levels of interleukin-6, nitric oxide, and matrix metalloproteinase 9 in endotoxin-induced mammary epithelial SCp2 cells and interleukin-1β in phorbol-12-myristate-13-acetate-activated human monocytic THP-1 cells.
Results:
Sea cucumber ethanolic extract and the aqueous fraction significantly decreased the proliferation of MDA-MB-231 cells by more than 50% at similar and noncytotoxic concentrations and caused an arrest in the S-phase of the cell cycle of treated cells. In contrast, petroleum ether, chloroform, ethyl acetate, and n-butanol organic fractions did not show any significant activity. Furthermore, sea cucumber ethanolic extract and aqueous fraction reduced the proliferation of MDA-MB-231 cells in three-dimensional cultures by more than 60% at noncytotoxic concentrations. In addition, treatment with these concentrations resulted in the loss of stellate outgrowths in favor of spherical aggregates and a 30% decrease in invasive properties. Both sea cucumber ethanolic extract and aqueous decreased the transcription of vimentin and the protein expression levels of vimentin and N-cadherin in three-dimensional cultures. The aqueous fraction decreased the levels of inflammatory markers interleukin-6, nitric oxide, and matrix metalloproteinase 9 in the mouse mammary SCp2 cells, and the level of interleukin-1β produced by phorbol-12-myristate-13-acetate-activated THP-1 human monocytic cells.
Conclusion:
The data reveal for the first time promising anti-proliferative and anti-inflammatory activities in H. polii water extract in two-dimensional and three-dimensional culture models.
Keywords: Sea cucumber, Holothuria polii, anti-proliferative, anti-inflammatory, breast cancer
Introduction
The marine environment harbors a wealth of organisms that produce a wide variety of primary and secondary metabolites with demonstrated significant biological activities. Most drugs against cancer or other diseases are either directly isolated or designed from natural sources. Few marine-derived compounds either made it to the cancer clinic such as Trabectedin1–3 and Eribulin mesylate3–5 or are currently tested in clinical trials against various cancers such as Aplidine4,6,7 and Kahalalide F.8,9
Sea cucumbers are an abundant group of marine invertebrates classified as echinoderms (phylum Echinodermata) belonging to the class Holothuroidea with a total of about 1250 existing species identified so far.10 Besides their nourishing value, sea cucumbers offer an important source of therapeutic and medically valuable metabolites with anti-cancer, anti-coagulant, anti-microbial, anti-oxidant, and anti-viral bioactivities.11–13 A wide range of isolated triterpene glycosides, also known as saponins, from sea cucumbers have shown anti-cancer activities against several cancer models with Frondoside A, isolated from Cucumaria frondosa,14 which is the most studied in animal models of breast,15,16 pancreatic,17 prostate,18 and lung cancers.19 Frondanol, from Cucumaria frondosa, is so far the only patented anti-inflammatory compound extracted from sea cucumbers.20,21
Holothuria polii (H. polii) belongs to the order Aspidochirotida. It prominently inhabits the Mediterranean and is the most abundant among sea cucumbers in Lebanon.22,23 Few studies have been conducted to identify bioactivities from this sea cucumber species. So far, findings include anti-parasitic activities from ethanol extracts,24,25 anti-microbial activities from two ethanolic fractions extracted from the body wall of the animal,26 and cytotoxic activities of a protein-free ethanolic extract against HCT116 and MCF-7 cancer cell lines.27 The objective of this study is to characterize the anti-proliferative bioactivity of H. polii in highly invasive MDA-MB-231 breast cancer cells in two-dimensional (2D) and three-dimensional (3D) cultures and the anti-inflammatory bioactivity in endotoxin (ET)-induced mammary epithelial SCp2 and phorbol-12-myristate-13-acetate (PMA)-activated human monocytic THP-1 cell culture models. Our results demonstrate that the sea cucumber ethanolic extract (SCE) and the partially purified aqueous (Aq) fraction promote a tumor-suppressive phenotype by decreasing proliferation, invasion, and expression of some epithelial mesenchymal transition (EMT) markers. Furthermore, the partially purified Aq fraction showed anti-inflammatory activity by downregulating the levels of some inflammatory mediators. To our knowledge, this is the first study to report anti-proliferative and anti-inflammatory activities in a water-soluble fraction in sea cucumbers.
Materials and methods
Preparation and fractionation of sea cucumber extract
Sample identification, preparation, and ethanol extraction
The identification of the species as H. polii was made in coordination with the American University of Beirut Natural History Museum based on identification keys from Fischer and Bauchot.28 Freshly collected sea cucumbers were handled according to common procedures used to prepare crude extracts (adopted and modified from Husni et al.29). First, the animal samples were rinsed with distilled water, dissected into 2 cm3 pieces and snap frozen in liquid nitrogen and lyophilized for 2 days, pulverized using “A11 basic analytical mill” and stored at −80°C for extraction. Every 1 g of pooled powdered material was reconstituted in 10 mL of 80% ethanol, homogenized with a laboratory Tissue-Tearor for 2 min on ice, and then centrifuged at 700 g for 10 min at 4°C. The supernatant was filtered through 100 µm nylon mesh and lyophilized.
Sea cucumber extract preparation
The lyophilized, ethanol extracted material was reconstituted in phosphate-buffered saline (PBS) and 10% dimethyl sulfoxide (DMSO), vortexed and centrifuged at 17,000 g for 10 min. The supernatant was filtered through 0.2 μm and the resulting extract, referred to as SCE, was used in the study as described.
Sequential solvent fractionation
The lyophilized material was partitioned sequentially in four different organic solvents of increasing polarity and one remaining Aq layer, as described by Riguera.30 About 3–4 g of lyophilized sea cucumber was dissolved in 1:5 ratio of 10% (v/v, 15–20 mL) methanol (MeOH) in water and fractionated twice using a separating funnel against petroleum ether (PE; 30–40 mL) followed by chloroform (CHCl3; 30–40 mL), ethyl acetate (EtAc; 30–40 mL), and n-butanol (BuOH; 30–40 mL). The ratio between the two immiscible solvents was 2:1 in all liquid–liquid fractionation steps. Each fraction was dried using a rotavapor (40°C) to give a solid or an oily residue except for the Aq fraction, which was freeze-dried. A control sample of 10% MeOH free of SCE was fractionated identically in all solvents. The yielded mass from every test and control fraction was formulated similarly to SCE preparation. The resulting five control fractions (four organic and one Aq) were added to the cells in equal volumes to the fractions containing SCE.
The solvent partitioning yielded 10% (±5.1%), 2.6% (±1.2%), 5.3% (±3.6%), 18.2% (±7.2%), and 63.9% (±7.3%) among PE, CHCl3, EtAc, BuOH, and Aq fractions, respectively. The overall loss, due to the fractionation process, from the initial mass of crude tissue was 52.8% (±12.1%) from three independent fractionations.
Treatment
Bioactive and noncytotoxic concentrations were chosen for the crude extract and the purified fractions. The concentrations of the extracts were similar to other studies examining crude and purified extracts from sea cucumbers29 and from H. polii in particular.26 In brief, mass concentration was used to standardize and report the concentrations across different sea cucumber batch preparations and purified fractions. The active mass concentrations against the cell lines tested were reproducible across six different sea cucumber batch preparations. The data provided in this study were generated from a single pool of sea cucumbers.
Cell counting and immunoblotting
MDA-MB-231 (provided by Dr Mina Bissell, Lawrence Berkeley National Lab, Berkeley, CA) human mammary adenocarcinoma cells were grown in 2D on plastic and in 3D on Matrigel (BD Biosciences, San Jose, CA) cultures as described earlier.31 MDA-MB-231 cells were plated in 12-well plates at a density of 4 × 104 cells per well. The cells were treated in triplicates at the indicated concentrations and viable cells were counted daily at 1, 2, and 3 days post treatment in 2D cultures and up to 5 days in 3D cultures. At each time point, for cells cultured in 3D, 2 mL of 2.5 mM PBS-ethylenediaminetetraacetic acid (EDTA) was added to each well and incubated in a shaker at 4°C for 60 min and then left to settle on ice for 10 min. The mixture was centrifuged at 200 g for 5 min at 4°C, the supernatant was removed, and the pellet was washed with 1× PBS, centrifuged, and recovered for cell counting. All cell counts were from three independent experiments.
For western blots analysis, total cellular protein extracts were prepared and resolved on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) as previously described.31 In brief, membranes were blocked at room temperature with 5% skimmed milk in 1% PBS-Tween 20 and incubated overnight with primary antibodies at 4°C (as per suppliers’ recommendations). Secondary antibodies were added at room temperature for 1 h. Proteins were detected using enhanced chemiluminescence (ECL) system. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as housekeeping protein.
3D morphogenesis assay
MDA-MB-231 cells were plated in 12-well plates as described above. Equal number of colonies were counted and scored for the number of spherical and stellate clusters to assess morphology changes. A minimum of 10 fields per well were imaged at 10× magnification. A colony was considered stellate if it displayed at least two extensions from the center of the cluster as described by Talhouk et al.31
RNA extraction and quantitative polymerase chain reaction
Total RNA was extracted from MDA-MB-231 cells using RNeasy Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. In the 3D cultures, the pellet was obtained as described above for cell counting. Reverse-transcription polymerase chain reaction (RT-PCR) was performed using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA). iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA) was used to conduct quantitative PCR (qPCR) in triplicates in a CFX96 Detection System (Bio-Rad), using the protocol and reaction conditions suggested by the manufacturer and tested with each primer pair for optimal temperature and concentration. Data were analyzed using ΔCt method on Bio-Rad CFX Manager Software version 62.1 (Qiagen, Vienna, Austria). Products were amplified using primers for vimentin 5′AAG GTG AAG GTC GGA GTC AAC3′ (forward) and 5′GGT TCC TTT AAG GGC ATC CAC3′ (reverse) and GAPDH 5′AAG GTG AAG GTC GGA GTC AAC3′ (forward) and 5′ GGG GTC ATT GAT GGC AAC AAT A3′ (reverse).
Invasion assay
Trans-well invasion assay was used as previously described.31 In brief, six-well plates were fitted with inserts (8 μm pore size) that have been coated with 400 μL of Matrigel–Media 0% fetal bovine serum (FBS) solution in a ratio of 1:3 or 1:20 (v/v). Enhanced green fluorescent protein (eGFP)-transfected MDA-MB-231 cells were plated at a density of 8 × 104 cells per insert overnight to adhere. On day 1, treatments were added as indicated, and the cells were incubated for an additional 24 h and then fixed using 4% formaldehyde in 1× PBS for 20 min at room temperature. Cell inserts were removed using a cotton swab and were then cut and mounted on a microscopic slide using Vector labs hard-mount fluorescence media and examined by fluorescence microscopy. Fluorescent cells that invaded across the Matrigel layer to the other side of the membrane from 10 random fields per well were counted. Data were collected from three independent experiments.
Cell cycle analysis
Cell cycle analysis was performed as previously described.31 Briefly, on day 3, after treatment with either SCE or Aq fraction at the indicated concentrations, MDA-MB-231 cells were washed with 1× PBS, trypsinized and centrifuged at 200 g for 5 min at 4°C. The pellet was fixed in ice cold 70% ethanol and stored at −20°C overnight. The fixed cells were then centrifuged, and the pellet was washed with 1× PBS to which 150 μL of DNase free RNase A was added at a concentration of 0.2 mg/mL and kept at 37°C for 1.5 h. Samples were then washed twice with 1× PBS, resuspended in 420 μL of 1× PBS and stained (in the dark) with 30 μL of 2 mg/mL propidium iodide. A total of 10,000 cells were then analyzed on FACScan (Becton Dickinson, San Jose, CA). Data were collected from three independent experiments.
Interleukin-6, nitric oxide, and matrix metalloproteinase 9–induced ET treatment
SCp2 mouse mammary epithelial cells (provided by Dr Pierre Desprez, Geraldine Brush Cancer Research Institute, San Francisco, CA) were maintained and induced by a nontoxic dose (10 µg/mL) of bacterial ET to induce inflammation as previously described.32,33 SCp2 cells were grown to 80% confluency after which ET (10 µg/mL) was added 30 min after SCE and Aq treatments were applied and samples were collected 24 h post ET treatment.
Immunoassay of interleukin-6
Interleukin-6 (IL-6) secretion was measured in duplicates by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s protocol (Max Deluxe set mouse IL-6; BioLegend, San Diego, CA) and similar to what was described in Maalouf et al.33 The average of secreted IL-6 (pg/mL) in conditioned media from three independent experiments ± standard deviation (SD) was calculated based on a known standard of IL-6.
Griess reaction assay for nitric oxide
Nitric oxide (NO) was quantified by measuring nitrite using a Griess Reagent Kit according to the manufacturer’s protocol (Molecular Probes, Eugene, OR) and similar to Maalouf et al.33 Samples were assayed in duplicates and an average concentration (μM) in conditioned media of NO2 is shown from three independent experiments ± SD.
SDS-substrate gel electrophoresis (zymography)
Gelatinase activity in conditioned media of ET-induced SCp2 cells was assayed by zymography and visualized as clear white bands on darkly stained blue gels as described earlier.34 Representative zymogram of matrix metalloproteinase 9 (MMP9) is illustrated as a negative image.
ET-induced IL-1β
THP-1 (purchased from the ATCC (American Type Culture Collection)) human monocytic cell line was maintained in RPMI (Roswell Park Memorial Institute) supplemented with 15 mM HEPES, l-glutamine, 1% Pen Strep, and 10% FBS at 37°C (95% air; 5% CO2). For differentiation, 100 ng/mL PMA was added for 48 h. Inflammation in THP-1 cells was induced by application of a nontoxic dose (1 µg/mL) of ET in 0% FBS-RPMI. THP-1 cells were plated at 5 × 105 cells/well in 12-well plates and differentiated as mentioned above. Cells were then washed with 1× PBS and placed in growth medium for 24 h. SCE and Aq treatments were then added to the cultured cells 30 min before ET. Media was collected 24 h post ET-treatment for IL-1β quantification using ELISA (Max Deluxe Set Human IL-1β, BioLegend).
Statistical analysis
Statistical comparisons were performed using paired t-test for comparison of two groups, whereas one-way analysis of variance (ANOVA) was used for three or more groups. p-values less than 0.05 were considered significant. Results are presented as means ± standard error (SE) unless otherwise indicated.
Results
SCE and partially purified Aq fraction decrease MDA-MB-231 cell count, alter cellular morphology in 3D cultures of MDA-MB-231, and arrest cells in the S-phase of the cell cycle
To test whether SCE or any of the sequential fractions possess anti-proliferative bioactivity, MDA-MB- 231 cell count using trypan blue exclusion assay was performed. Treatment up to 3 days in 2D culture revealed that SCE significantly reduced the proliferation of MDA-MB-231 with no apparent cytotoxic effect. SCE treatment at concentrations of 1, 2, and 3 mg/mL decreased cellular proliferation at day 3 by 25%, 55%, and 70%, respectively, compared to control and sham treated cells (Figure 1(a)). PE, CHCl3, EtAc, and BuOH treatments were either ineffective at concentrations up to 1.5, 2, 0.5, and 1.5 mg/mL, respectively, (Figure 1(b)–(e)), or cytotoxic at higher doses in the case of PE and EtAc fractions. In contrast to the narrow margin between the ineffective and cytotoxic concentrations of the above extracts, a dose-dependent growth inhibitory effect was noted in the Aq fraction, suggesting that the inhibitory activity noted in SCE (Figure 1(a)) was retained in the Aq fraction (Figure 1(f)). The Aq fraction, like SCE, significantly reduced the proliferation of MDA-MB-231 cells over a period of 3 days in 2D cultures with no cytotoxic effect. Cell counts showed a decrease by 50%, 60%, and 80% at 2, 4, and 6 mg/mL, respectively, of Aq treatment by day 3 (Figure 1(f)). No significant cell death (⩽10%) was noted across all conditions for SCE and Aq treatments.
Figure 1.
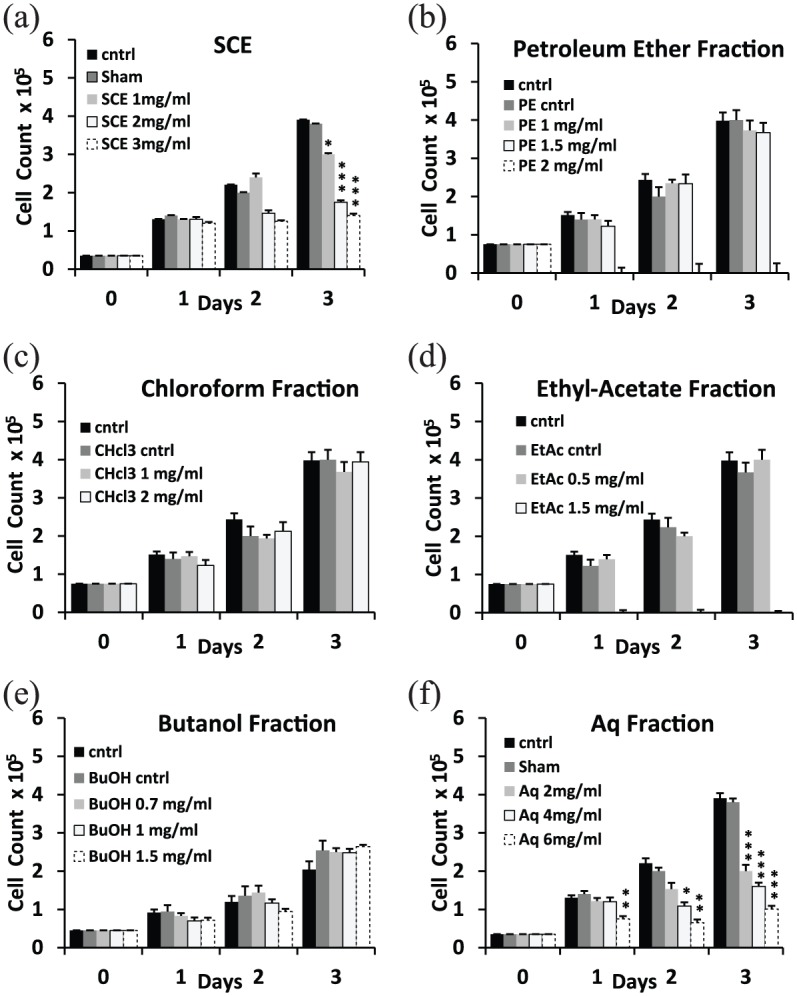
Effect of sea cucumber extract (SCE) and isolated organic fractions on the proliferation of MDA-MB-231 in 2D cultures. Cell count via trypan blue exclusion showed (a) 50% and 60% decrease in cell counts by day 3 at SCE concentrations of 2 and 3 mg/mL, respectively, with no significant cell death (⩽10%) across all conditions. (b) Petroleum ether (PE) fraction did not affect cell proliferation at 1 and 1.5 mg/mL and was cytotoxic at 2 mg/mL. (c) Chloroform (CHCl3) fraction did not inhibit proliferation up to 2 mg/mL. (d) Ethyl acetate (EtAc) fraction did not inhibit proliferation up to 0.5 mg/mL and was cytotoxic on cells at 1.5 mg/mL. (e) n-Butanol (BuOH) fraction showed no effect on proliferation up to 1.5 mg/mL. (f) Aqueous (Aq) concentrations of 2, 4, and 6 mg/mL showed 50%, 60%, and 80% decrease in cell counts by day 3, respectively. Statistical analysis from three independent experiments revealed significant differences represented by (***) asterisks for p < 0.001, (**) asterisks for p < 0.01, and (*) asterisk for p < 0.05.
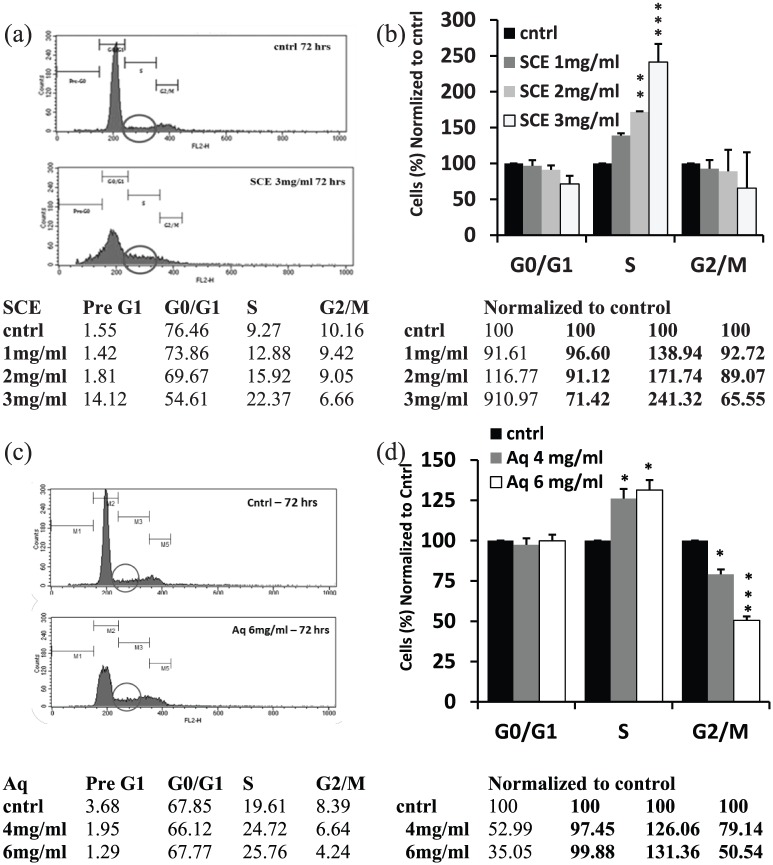
The noted inhibition in proliferation of MDA-MB-231 cells after SCE treatment (3 mg/mL) was accompanied by an arrest in the S-phase of the cell cycle. A 140% increase of cells in the S-phase was observed compared to the control by day 3 (Figure 2(a) and (b)). Analysis of the cell cycle progression of Aq-treated MDA-MB-231 cells in 2D cultures was also performed. A similar pattern to SCE treatment was noted. Compared to the control, 30% and 40% more cells accumulated in the S-phase of the cell cycle when treated with Aq fraction at 4 and 6 mg/mL, respectively, by day 3 in culture. A significant decrease in the percentage of cells in the G2/M phase was also noted at 4 and 6 mg/mL (Figure 2(c) and (d)).
Figure 2.
Effect of SCE and Aq fractions on cell cycle progression of MDA-MB-231. (a) Fluorescence histogram plots showing cell counts (Y-axis) versus fluorescence intensity (X-axis) with circles demonstrating the amount of cells in the S-phase in both control (cntrl) and treated conditions and a table showing percentages of cells in pre-G1, G0/G1, S, and G2/M phases of the cell cycle (left part) and percentages normalized to the control (cntrl), 3 days post SCE treatment (right part). (b) Percentage of cells in different phases of the cell cycle, normalized to cntrl, on day 3 after treatment. SCE delayed the cell cycle by inducing a 140% increase in the number of cells trapped in the S-phase compared to the cntrl at a concentration of 3 mg/mL. (c) Fluorescence histogram plots and a table showing percentages of cells in pre-G1, G0/G1, S, and G2/M phases of the cell cycle (left part) and percentages normalized to the cntrl, 3 days post Aq treatment (right part). (d) Percentage of cells in G0/G1, S, and G2/M phases normalized to cntrl, day 3 post treatment; Aq concentration of 6 mg/mL delayed the cell cycle by increasing the percentage of cells trapped in the S-phase compared to the cntrl by more than 30%. Statistical analysis from three independent experiments revealed significant differences represented by, (***) asterisks for p < 0.001, (**) asterisks for p < 0.01, and (*) asterisk for p < 0.05.
In 3D cultures, SCE treatment at 2 mg/mL showed no effect, while a 30% and 60% decrease in proliferation was observed by day 5 at concentrations of 4 and 6 mg/mL, respectively (Figure 3(a)). Similarly, Aq fraction treatment showed a 40% and 60% decrease in 3D proliferation by day 5 at 6 and 9 mg/mL, respectively (Figure 3(b)).
Figure 3.
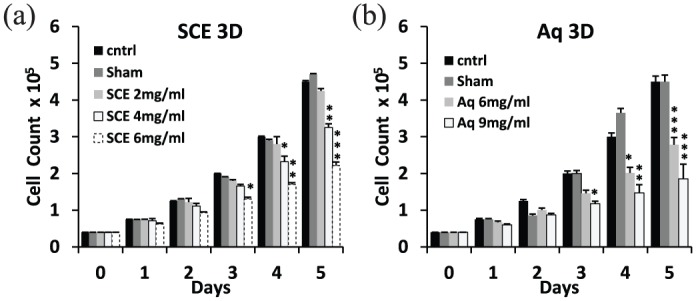
Effect of SCE and Aq fractions on the proliferation of MDA-MB-231 in 3D cultures. (a) 3D-growth analysis by trypan blue counting showed a 30% and 60% growth inhibition by day 5 at 4 and 6 mg/mL of SCE, respectively. (b) Aq treatment showed 40% and 60% growth inhibition by day 5 at concentrations of 6 and 9 mg/mL, respectively. Statistical analysis from three independent experiments revealed significant differences represented by (***) asterisks for p < 0.001, (**) asterisks for p < 0.01, and (*) asterisk for p < 0.05.
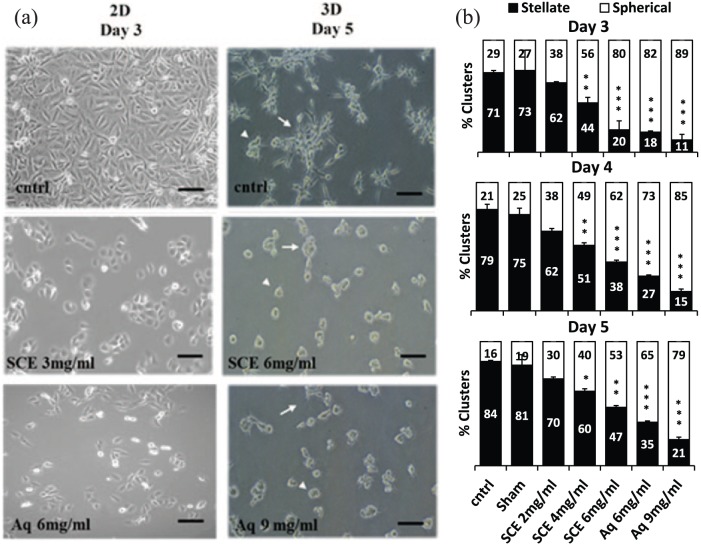
The anti-proliferative bioactivity detected in SCE and the Aq fraction altered cell growth behavior in 2D and 3D cultures. Treated cells grew at a slower rate in 2D conditions with no observed morphological changes by day 3 among control, SCE, and Aq-treated MDA-MB-231 at 3 and 6 mg/mL, respectively (Figure 4(a) left panels). On the contrary, SCE and Aq treatments in 3D cultures reduced stellate outgrowths (white arrows) by more than 60% and increased the abundance of spherical colonies (white arrow heads) when compared to control cultures at a concentration of 6 and 9 mg/mL, respectively, by day 5 (Figure 4(a) right panels and Figure 4(b)).
Figure 4.
Effect of SCE and Aq fractions on the growth morphology of MDA-MB-231. (a) Representative phase contrast images of cntrl and treated (SCE: 3 mg/mL and Aq: 6 mg/mL) cells at day 3 in 2D cultures (left panel) and at day 5 of cntrl and treated (SCE: 6 mg/mL and Aq: 9 mg/mL) in 3D cultures (right panel). White arrows indicate stellate aggregates and white arrow heads point to spherical colonies. (b) Histogram analysis of the percentages of stellate versus spherical clusters on days 3, 4, and 5 post SCE and Aq treatments; both SCE and Aq fractions increased spherical formation and reduced stellate outgrowths by more than 60% at 6 and 9 mg/mL on day 5 of culture, respectively. Scale bars (in black) = 100 μm. Statistical analysis from three independent experiments revealed significant differences represented by (***) asterisks for p < 0.001, (**) asterisks for p < 0.01, and (*) asterisk for p < 0.05.
SCE and Aq treatments inhibit trans-well invasion of MDA-MB-231
A previous study from our laboratory31 showed that eGFP-transfected MDA-MB-231 cells act identically to untransfected ones, whether at the level of proliferation, morphology, or invasion through Matrigel-coated inserts, among other tested parameters.
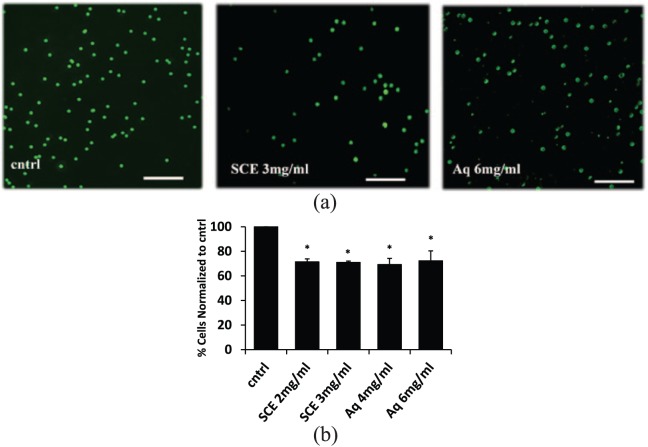
Cell scoring for eGFP-labeled cells that invaded through the Matrigel-coated well (diluted 1:3) showed that SCE and Aq treatments inhibited the invasion of these cells by 30% across all tested conditions (Figure 5(a) and (b)). In 1:20 diluted Matrigel-coated wells, the Aq fraction showed a 70% decrease in invasion compared to untreated cells (Supplemental Figure 1).
Figure 5.
Effect of SCE and Aq fractions on trans-well invasion potential of MDA-MB-231. (a) Fluorescent images of cntrl, SCE, and Aq-treated eGFP-transfected MDA-MB-231 cells that invaded through the Matrigel (diluted 1:3). (b) Histogram analysis of cntrl and treated cells showing a 30% decrease in invasiveness of SCE and Aq-treated cells at all tested concentrations compared to cntrl through Matrigel diluted 1:3. Scale bars (in white) = 200 μm. Statistical analysis from three independent experiments revealed significant differences represented by (*) asterisks for p < 0.05.
SCE and Aq treatments decrease the expression of vimentin and N-cadherin of MDA- MB-231
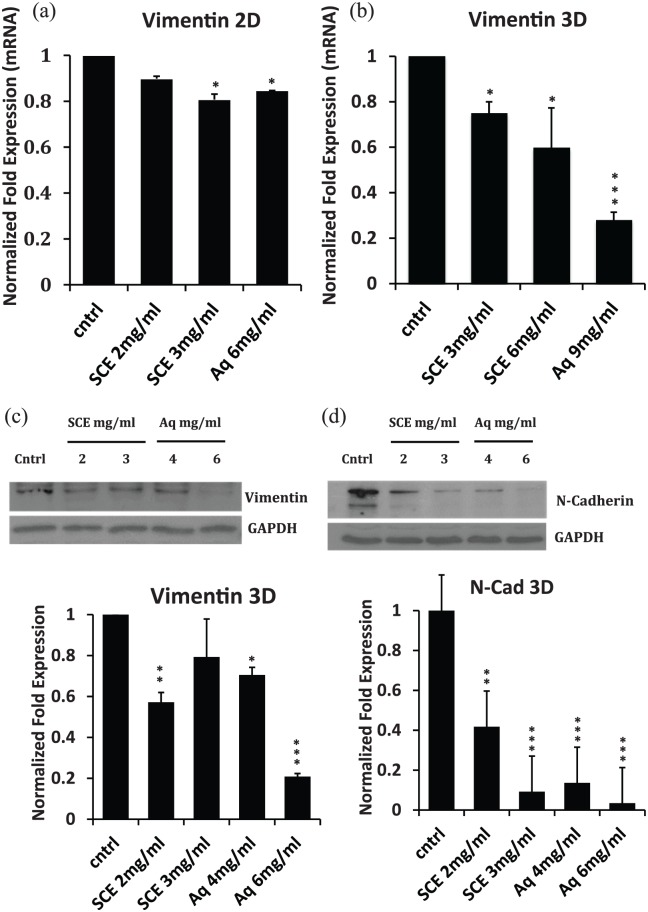
qRT-PCR data showed that SCE and Aq treatments decreased the transcript levels of vimentin in 2D and 3D cultures (Figure 6(a) and (b)). Furthermore, western blot analysis of total cellular extracts showed that both SCE and Aq treatments significantly repressed the expression of both mesenchymal markers, vimentin (Figure 6(c)) and N-cadherin (Figure 6(d)) in 3D cultures.
Figure 6.
Effect of SCE and Aq treatments on mRNA and protein expression of some EMT markers in 2D and 3D cultures of MDA-MB-231. (a, b) Bar diagrams showing the transcript fold change normalized to GAPDH of vimentin in 2D and 3D cultures, respectively. (c) Western blot showing the protein expression of vimentin in cntrl versus SCE/Aq-treated lysates from 3D cultures, with the corresponding densitometric quantification represented in bar diagrams. (d) Western blot showing the protein expression of N-cadherin in cntrl versus SCE/Aq-treated lysates from 3D cultures, with the corresponding densitometric quantification represented in bar diagrams. GAPDH was used for equal loading.
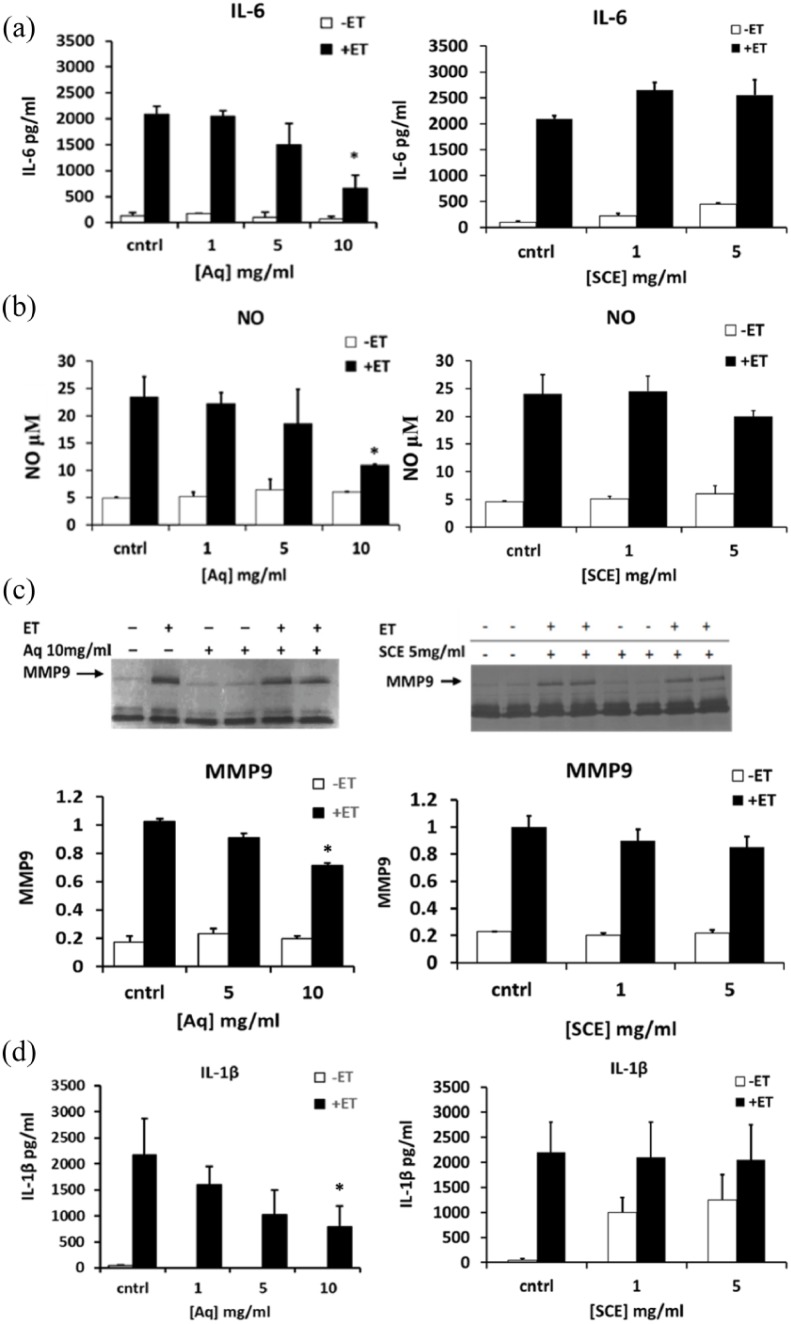
Effect of SCE and Aq fractions on ET-induced IL-6, NO, and MMP9 in SCp2 cells and on ET-induced IL-1β in PMA-activated THP-1 cells
We further tested whether SCE and Aq fractions exhibit anti-inflammatory effects in ET-treated mouse mammary epithelial SCp2 cell culture model previously reported by Safieh-Garabedian et al.34 The Aq fraction, at a noncytotoxic concentration of 10 mg/mL, reduced the levels of secreted IL-6 by about 60% (Figure 7(a) left panel), NO by 50% (Figure 7(b) left panel), and MMP9 by approximately 30% (Figure 7(c) left panel). The effect of the Aq fraction on ET-induced IL-1β in PMA-activated THP-1 cells showed that Aq treatment reduced the level of ET-induced IL-1β secreted from PMA-activated THP-1 cells by 60% at a concentration of 10 mg/mL (Figure 7(d) left panel). Similar to the MDA results where SCE-noted bioactivities were comparable to Aq bioactivities, when the latter was used at twice the concentration of the former, anti-inflammatory acitivities noted in the Aq fraction, at 10 mg/mL, were compared to SCE concentrations at 5 mg/mL. Interestingly, the SCE treatment at this concentration did not show any reduction in the tested anti-inflammatory mediators (Figure 7(a)–(d) right panels).
Figure 7.
Effect of Aq and SCE on endotoxin (ET)-induced interleukin-6 (IL-6), nitric oxide (NO), and matrix metalloproteinase 9 (MMP9) in SCp2 cells and on ET-induced interleukin 1β (IL-1β) in PMA-activated THP-1 cells. Cells were treated with 1, 5, and 10 mg/mL of Aq fraction (left panels) and 1 and 5 mg/mL of SCE (right panels) and media samples were collected 24 h after ET stimulation and analyzed for their (a) IL-6 secretion, (b) NO production, (c) MMP9 activity, and (d) IL-1β secretion using ELISA (for IL-6 and IL-1β), Griess reaction assay (for NO), and zymography (for MMP9 activity). Zymograms were analyzed by gel documentation (Bio-Rad) using the software Quantity One. Statistical significance is represented by (*) asterisk indicating significant difference at p < 0.05
Discussion
The sea cucumber H. polii is the most abundant among sea cucumbers in Lebanon and prominently inhabits the Mediterranean.22,23 Among all classes of echinoderms, sea cucumbers are the most extensively commercialized and fished due to their high pharmacological and nutritious value.35 Sea cucumbers, commonly called “bêche-de-mer,” have long been used in folk medicine in South-East Asia and the Middle East against hypertension, asthma, rheumatism, burns, and constipation, among other conditions.13,36 Extensive studies have been done on sea cucumber extracts to identify potent bioactive compounds with possible anti-inflammatory, immunostimulatory, and anti-cancer properties, yet, very few studies have been conducted to identify bioactivities from H. polii. Given that sea cucumbers offer a large repertoire of bioactive compounds,13,35,37–40 this study is among the first to partially characterize water-soluble bioactive molecules extracted from H. polii.
To investigate whether H. polii harbors any compound(s) with anti-cancer effects, SCE was prepared according to established methodologies for extracting molecules from sea cucumbers and in line with the majority of studies addressing biomass extraction with solvent systems using sea cucumbers.41–46 Treatment with SCE decreased cellular proliferation of MDA-MB-231 cells in a dose-dependent manner on day 3 in 2D cultures without affecting viability. Subsequent sequential gradient partitioning in four organic solvents (PE, CHCl3, EtAc, and BuOH) with increasing polarity yielded four organic and one Aq fraction. None of the organic fractions, at the concentrations tested, maintained the anti-proliferative activity from SCE, yet, the Aq fraction retained it. Contrary to our study, most bioactivities extracted from sea cucumbers resided in the BuOH fraction and were attributed to triterpene glycosides such as frondoside A,14 Holothurin A1,47 and Philinopsides A and E,43 whereas in this study, the noted bioactivity in H. polii resided in the Aq fraction. None of the organic fractions inhibited the proliferation of MDA-MB-231 cells at the tested concentrations. Actually, higher concentrations of the PE and EtAc fractions were cytotoxic, whereas CHCl3 and BuOH fractions were used at the highest available concentrations derived from the adopted fractionation procedure.30 Nevertheless, these results do not necessarily rule out the possibility of the latter two fractions containing a biological activity per se. The observed cytotoxic effects in PE and EtAc fractions do not seem to correlate with the dose-dependent growth inhibitory activity in SCE whereby the inhibition was mediated with minimal cytotoxicity, not exceeding 10% at the highest SCE concentration. Another study of three Malaysian sea cucumbers (Holothuria scabra, Holothuria leucospilota, and Stichopus chloronotus) showed that all organic fractions were able to inhibit the proliferation of A549 non-small cell lung cancer cells and C33A cervical cancer cells while only the Aq fraction from S. chloronotus was shown to have anti-proliferative activity.48 Preliminary data in our laboratory also show that SCE and Aq fractions inhibit the proliferation of colorectal adenocarcinoma and prostate cancer cells (data not shown).
As opposed to the organic fractions, the Aq fraction treatment, similar to SCE treatment, decreased cellular proliferation of MDA-MB-231 cells by 50%, 60%, and 80% at 2, 4, and 6 mg/mL, respectively, in 2D cultures, with no significant cytotoxicity.
This anti-proliferative activity provoked by Aq treatment was accompanied by an arrest of cells in the S-phase and a subsequent shortening in G2/M phase. This is in contrast to the S-phase delay observed after SCE treatment, which was associated with a decrease in both G2/M and G0/G1 phases, albeit insignificant in the latter. This decrease in G0/G1 phase could be accounted for due to the complex and crude nature of the SCE extract. Similar patterns have been observed in the polar fraction of Frondanol A (Frondanol A5 P), a glycolipid extracted from the edible sea cucumber Cucumaria frondosa, from the North Atlantic. In contrast to Frondanol A, Frondanol A5 P induced only a G2/M arrest compared to a dual S and G2/M arrest noted after Frondanol A treatment in human pancreatic cancer cells, AsPC-1 and S2013.49
Treatment with SCE and Aq fractions also decreased cellular proliferation of MDA-MB-231 cells in 3D cultures; however, higher concentrations (1.5- to 2-fold compared to that needed in 2D cultures) of SCE and Aq fractions were required to achieve comparable inhibitory effects. This difference could be ascribed to the nonspecific binding of the Matrigel to bioactive components in the SCE or Aq fractions. In fact, studies on 37 different tumor cell lines treated with 9 chemotherapeutic compounds showed a higher sensitivity of cells to almost all treatments when cultured on flat 2D matrices compared to 3D cultures.50 SCE and Aq treatments of MDA-MB231 cells in 3D cultures resulted in a culture that consists predominantly of spherical clusters over the typical MDA-MB-231 stellate morphology with protruding outgrowth.31 This change in morphology has also been reported in the poorly differentiated Hs578 T breast cancer cell line, in 3D cultures on Matrigel, when treated with geodiamolide H, a depsipeptide isolated from the marine sponge Geodia corticostylifera.51 This was accompanied by an inhibition in cellular motility and invasion and was shown by confocal images to be coupled with a partial acquisition of a polarized phenotype, as monitored by the apical orientation of Golgi apparatus, similar to what was observed in normal human breast cells MCF-10A.52 The morphogenic reversion from stellate into spherical clustering noted in our study, along with the inhibition in trans-well invasion, suggests that SCE holds potential compound(s) that are retained in the Aq fraction, which are able to partially revert the mesenchymal phenotype in MDA-MB-231 cells. As for the enhanced inhibition detected in invasion between different Matrigel dilutions, more cells were able to pass through the more diluted (1:20) Matrigel as compared to the less diluted (1:3) one. As a result, the data observed (Supplemental Figure 1) across the more diluted Matrigel (70%) better correlate with the enrichment of the less aggressive spherical phenotype (60%) observed in Figure 4. This result goes in line with previous studies measuring the invasive potential of tumor cells across different Matrigel dilutions, where the percentage of Hs242/3 T3, T24/3 T3, 3 T3, and MRC-5 cells that invaded the Matrigel was inversely proportional to the matrix amount being used. Moreover, the proper amount of Matrigel used played an important role in representing the true invasive ability of each cell line.53 This inhibition in invasion potential of MDA-MB-231 cells was corroborated by the fact that the expression of both vimentin and N-cadherin decreased in treated cells. Similarly, MMP9 was negatively affected by Aq treatment (Figure 7(c)), suggesting a potential role of the SCE and Aq fractions in inducing a mesenchymal epithelial transition (MET). Interestingly, N-cadherin was shown to enhance the metastatic phenotype of cancer cells by promoting cellular motility and invasion through cooperation with fibroblast growth factor receptor (FGFR), leading to an increase in the expression of MMP9.54,55 When transfected in the weakly metastatic MCF-7 breast cancer cells, N-cadherin expressing cells exhibited increased migratory and invasive abilities, elevated MMP9 secretion, and increased in vivo metastases to the liver, pancreas, salivary gland, omentum, lung, lymph nodes, and lumbar spinal muscle.56 In addition, N-cadherin caused FGFR up-modulation, resulting in EMT and stem/progenitor-like properties involving Snail and Slug upregulation and mammosphere formation in MMTV-Neu mice.57
With the extensive literature documenting the overlap of cellular pathways in cancer progression and chronic inflammation,58 we assessed the ability of the crude and the partially purified Aq fraction to modulate inflammatory phenotypes in already established inflammatory models. ET-induced IL-6, NO, and MMP9 are all common mediators of cancer and inflammation,33,59 and markers of dedifferentiation.31,34,60 The Aq fraction reduced the levels of IL-6, NO, and MMP9 from ET-induced SCp2 cells and the ET-induced IL-1β levels from PMA-activated THP-1 cells, whereas SCE did not at the tested concentrations. The noted decrease in inflammatory mediators after Aq treatment is likely regulated by NFκB as we have demonstrated in earlier studies on SCp2 cells and their parent strain, CID-9 cells,33,34 treated with ET. Whether the Aq fraction is capable of fully reverting the differentiation phenotype, marked by casein expression,34 requires further studies. While SCE decreased the proliferation of SCp2 cells, albeit only by 35% at 10 mg/mL on day 4 of culture, this inhibition in proliferation was totally reversible and achieved growth rates comparable to untreated cells, 24 h after treatment washout (Supplemental Figure 2). This suggests that the anti-proliferative effect of SCE, and possibly that of the Aq fraction, is reversible and does not cause damage to the cells.
The bioactivities noted in the Aq fraction could be attributed to one or more water-soluble compound. Although extracts and isolated molecules of an Aq nature tend to have better bioavailability, especially those with low molecular weights,61 purifying such molecules by “aqueous workup” proved challenging due to poor partitioning and is largely difficult.62 Accordingly, more efficient extraction techniques for marine-derived compounds of similar nature were developed.63–65 In light of the above, one of main the limitations of this study is that we have yet to overcome these purification difficulties and identify the active substance(s) and its structure. Nevertheless, preliminary data suggest that the Aq fraction is stable for several months at −20°C and can withstand several freeze and thaw cycles. In addition, the proposed molecular weight is more than 3 kDa and less than 20 kDa, as the activity was retained on Amicon™ ultra filter tubes (3 kDa cutoff; EMD Millipore Amicon Ultra-0.5 Centrifugal Filter; Merck, Darmstadt, Germany) and a Sephadex® LH-20 column (20 kDa cutoff; Amersham Biosciences, Buckinghamshire, England) (data not shown).
Conclusion
This study is the first to provide evidence that H. polii holds potential anti-proliferative and anti-inflammatory compound(s) that is/are of small molecular weight and is/are water soluble. Further studies are needed to purify and characterize the noted bioactivity.
Supplemental Material
Supplemental material, Supplemental_Figures_(1) for Anti-proliferative and anti-inflammatory activities of the sea cucumber Holothuria polii aqueous extract by Mike Kareh, Rana El Nahas, Lamis Al-Aaraj, Sara Al-Ghadban, Nataly Naser Al Deen, Najat Saliba, Marwan El-Sabban and Rabih Talhouk in SAGE Open Medicine
Acknowledgments
M.K. and R.E.N. completed the experiments; R.T., M.E.-S., and N.S. contributed to designing the experiments and analyzing the data; M.E.-S. directed the primary extraction done at his laboratory by S.A.-G., R.E.N., and M.K.; N.S. directed the sequential extraction procedure done at her laboratory by L.A.-A., R.E.N., and M.K.; M.K. and R.T. wrote the manuscript. Experiments were done in R.T. laboratory. N.N.A.D. contributed to consolidation and data assembly. All authors substantially and thoroughly revised the manuscript. L.A.-A. and S.A.-G. have contributed equally to this work. The authors thank Dr Nadine Darwiche for her contributions and help in reviewing the paper. N.N.A.D. is a recipient of the Joint Lebanese Council for Scientific Research (CNRS-L)/AUB doctoral fellowship.
Footnotes
Animal welfare: Guidelines for humane animal treatment did not apply to this study because no animal subjects were included in the study.
Data availability: All the data used to support the findings of this study are included within the article in addition to two Supplemental figures.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval was not sought for this study because no primary patient cultures or stem cells were used regarding the in vitro work and no vertebrate animals or human subjects were included in the study.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the European Union (grant agreement no. 245137; project acronym: MAREX).
ORCID iD: Talhouk Rabih:  https://orcid.org/0000-0002-1544-1736
https://orcid.org/0000-0002-1544-1736
Supplemental materials: See Supplemental Figures 1 and 2 in the Supplementary Material for invasion analysis and proliferation recovery after treatment washout, respectively.
References
- 1. Tohme R, Darwiche N, Gali-Muhtasib H. A journey under the sea: the quest for marine anti-cancer alkaloids. Molecules 2011; 16: 9665–9696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jimeno J, Lopez-Martin JA, Ruiz-Casado A, et al. Progress in the clinical development of new marine-derived anticancer compounds. Anticancer Drugs 2004; 15: 321–329. [DOI] [PubMed] [Google Scholar]
- 3. Molinski TF, Dalisay DS, Lievens SL, et al. Drug development from marine natural products. Nat Rev Drug Discov 2009; 8: 69–85. [DOI] [PubMed] [Google Scholar]
- 4. Indumathy S, Dass CR. Finding chemo: the search for marine-based pharmaceutical drugs active against cancer. J Pharm Pharmacol 2013; 65: 1280–1301. [DOI] [PubMed] [Google Scholar]
- 5. Cortes J, O’Shaughnessy J, Loesch D, et al. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet 2011; 377: 914–923. [DOI] [PubMed] [Google Scholar]
- 6. Le Tourneau C, Raymond E, Faivre S. Aplidine: a paradigm of how to handle the activity and toxicity of a novel marine anticancer poison. Curr Pharm Des 2007; 13: 3427–3439. [PubMed] [Google Scholar]
- 7. Schwartsmann G, Da Rocha AB, Mattei J, et al. Marine-derived anticancer agents in clinical trials. Expert Opin Investig Drugs 2003; 12: 1367–1383. [DOI] [PubMed] [Google Scholar]
- 8. Newman DJ, Cragg GM. Advanced preclinical and clinical trials of natural products and related compounds from marine sources. Curr Med Chem 2004; 11: 1693–1713. [DOI] [PubMed] [Google Scholar]
- 9. Rademaker-Lakhai JM, Horenblas S, Meinhardt W, et al. Phase I clinical and pharmacokinetic study of kahalalide F in patients with advanced androgen refractory prostate cancer. Clin Cancer Res 2005; 11: 1854–1862. [DOI] [PubMed] [Google Scholar]
- 10. Paulay G. Holothuroidea. World Register of Marine Species, 2014, http://www.marinespecies.org/aphia.php?p=taxdetails&id=123083
- 11. Janakiram NB, Mohammed A, Zhang Y, et al. Chemopreventive effects of Frondanol A5, a Cucumaria frondosa extract, against rat colon carcinogenesis and inhibition of human colon cancer cell growth. Cancer Prev Res 2010; 3: 82–91. [DOI] [PubMed] [Google Scholar]
- 12. Pangestuti R, Arifin Z. Medicinal and health benefit effects of functional sea cucumbers. J Tradit Complement Med 2017; 8: 341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bordbar S, Anwar F, Saari N. High—value components and bioactives from sea cucumbers for functional foods—a review. Marine Drugs 2011; 9: 1761–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jin JO, Shastina VV, Shin SW, et al. Differential effects of triterpene glycosides, frondoside A and cucumarioside A2-2 isolated from sea cucumbers on caspase activation and apoptosis of human leukemia cells. FEBS Lett 2009; 583: 697–702. [DOI] [PubMed] [Google Scholar]
- 15. Ma X, Kundu N, Collin PD, et al. Frondoside A inhibits breast cancer metastasis and antagonizes prostaglandin E receptors EP4 and EP2. Breast Cancer Res Treat 2012; 132: 1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Al Marzouqi N, Iratni R, Nemmar A, et al. Frondoside A inhibits human breast cancer cell survival, migration, invasion and the growth of breast tumor xenografts. Eur J Pharmacol 2011; 668: 25–34. [DOI] [PubMed] [Google Scholar]
- 17. Li X, Roginsky AB, Ding XZ, et al. Review of the apoptosis pathways in pancreatic cancer and the anti-apoptotic effects of the novel sea cucumber compound, Frondoside A. Ann N Y Acad Sci 2008; 1138: 181–198. [DOI] [PubMed] [Google Scholar]
- 18. Dyshlovoy SA, Menchinskaya ES, Venz S, et al. The marine triterpene glycoside frondoside A exhibits activity in vitro and in vivo in prostate cancer. Int J Cancer 2016; 138: 2450–2465. [DOI] [PubMed] [Google Scholar]
- 19. Attoub S, Arafat K, Gelaude A, et al. Frondoside a suppressive effects on lung cancer survival, tumor growth, angiogenesis, invasion, and metastasis. PLoS ONE 2013; 8: e53087. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20. Collin PD. Sea cucumber carotenoid lipid fraction products and methods of use. PCT/US99/01179 Patent, 2002.
- 21. Yang P, Collin P, Madden T, et al. Inhibition of proliferation of PC3 cells by the branched-chain fatty acid, 12-methyltetradecanoic acid, is associated with inhibition of 5-lipoxygenase. Prostate 2003; 55: 281–291. [DOI] [PubMed] [Google Scholar]
- 22. Vergara-Chen C, Gonzalez-Wanguemert M, Marcos C, et al. Genetic diversity and connectivity remain high in Holothuria polii (Delle Chiaje 1823) across a coastal lagoon-open sea environmental gradient. Genetica 2010; 138: 895–906. [DOI] [PubMed] [Google Scholar]
- 23. Shoukr F, Mona M, Abdel–Hamid M. Holothurians (Echinodermata: Holothuroidea) from some Egyptian shores. Bull Fac Sci Zagazig Univ 1984; 6: 662–682. [Google Scholar]
- 24. Mona MH, Omran NE, Mansoor MA, et al. Antischistosomal effect of holothurin extracted from some Egyptian sea cucumbers. Pharmaceut Biol 2012; 50: 1144–1150. [DOI] [PubMed] [Google Scholar]
- 25. Melek FR, Tadros MM, Yousif F, et al. Screening of marine extracts for schistosomicidal activity in vitro. Isolation of the triterpene glycosides echinosides A and B with potential activity from the sea cucumbers Actinopyga echinites and Holothuria polii. Pharmaceut Biol 2012; 50: 490–496. [DOI] [PubMed] [Google Scholar]
- 26. Omran NE, Allam NG. Screening of microbial contamination and antimicrobial activity of sea cucumber Holothuria polii. Toxicol Ind Health 2013; 29: 944–954. [DOI] [PubMed] [Google Scholar]
- 27. Omran NE, Khedr AM. Structure elucidation, protein profile and the antitumor effect of the biological active substance extracted from sea cucumber Holothuria polii. Toxicol Ind Health 2015; 31: 1–8. [DOI] [PubMed] [Google Scholar]
- 28. Fischer W, Bauchot ML. Fiches FAO d’identification des especes pour les besoins de la peche. Mediterranee et mer Noire. Zone de Peche 37: vertebres (Revision 1), vol. 2, 1987, http://www.fao.org/3/a-x0170f.pdf [Google Scholar]
- 29. Husni A, Shin IS, Chung D. Effect of extraction methods on antifungal activity of sea cucumber (Stichopus japonicus). Agritech 2014; 34: 1–7. [Google Scholar]
- 30. Riguera R. Isolating bioactive compounds from marine organisms. J Marine Biotechnol 1997; 5: 187–193. [Google Scholar]
- 31. Talhouk RS, Fares MB, Rahme GJ, et al. Context dependent reversion of tumor phenotype by connexin-43 expression in MDA-MB231 cells and MCF-7 cells: role of beta-catenin/connexin43 association. Exp Cell Res 2013; 319: 3065–3080. [DOI] [PubMed] [Google Scholar]
- 32. Talhouk RS, Nasr B, Fares MB, et al. Anti-inflammatory and cytostatic activities of a parthenolide-like sesquiterpene lactone from Cota palaestina subsp. syriaca. Evid-Based Compl Alt 2015; 2015: 474597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maalouf SW, Talhouk RS, Schanbacher FL. Inflammatory responses in epithelia: endotoxin-induced IL-6 secretion and iNOS/NO production are differentially regulated in mouse mammary epithelial cells. J Inflamm 2010; 7: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Safieh-Garabedian B, Mouneimne GM, El-Jouni W, et al. The effect of endotoxin on functional parameters of mammary CID-9 cells. Reproduction 2004; 127: 397–406. [DOI] [PubMed] [Google Scholar]
- 35. Kiew PL, Don MM. Jewel of the seabed: sea cucumbers as nutritional and drug candidates. Int J Sci Nutr 2012; 63: 616–636. [DOI] [PubMed] [Google Scholar]
- 36. Fredalina BD, Ridzwan BH, Abidin AA, et al. Fatty acid compositions in local sea cucumber, Stichopus chloronotus, for wound healing. Gen Pharmacol 1999; 33: 337–340. [DOI] [PubMed] [Google Scholar]
- 37. Li Q, Qi RR, Wang YN, et al. Comparison of cells free in coelomic and water-vascular system of sea cucumber, Apostichopus japonicus. Fish Shellfish Immunol 2013; 35: 1654–1657. [DOI] [PubMed] [Google Scholar]
- 38. Kim SK, Himaya SW. Triterpene glycosides from sea cucumbers and their biological activities. Adv Food Nutr Res 2012; 65: 297–319. [DOI] [PubMed] [Google Scholar]
- 39. Choi BD, Choi YJ. Nutraceutical functionalities of polysaccharides from marine invertebrates. Adv Food Nutr Res 2012; 65: 11–30. [DOI] [PubMed] [Google Scholar]
- 40. Osbourn A, Goss RJ, Field RA. The saponins: polar isoprenoids with important and diverse biological activities. Nat Prod Rep 2011; 28: 1261–1268. [DOI] [PubMed] [Google Scholar]
- 41. Zhang SL, Li L, Yi YH, et al. Philinopsides E and F, two new sulfated triterpene glycosides from the sea cucumber Pentacta quadrangularis. Nat Prod Res 2006; 20: 399–407. [DOI] [PubMed] [Google Scholar]
- 42. Girard M Bélanger J ApSimon JW et al.Frondoside A.. A novel triterpene glycoside from the holothurian Cucumaria frondosa. Can J Chem 1990; 68: 11–18. [Google Scholar]
- 43. Tian F, Zhang X, Tong Y, et al. PE, a new sulfated saponin from sea cucumber, exhibits anti-angiogenic and anti-tumor activities in vitro and in vivo. Cancer Biol Ther 2005; 4: 874–882. [DOI] [PubMed] [Google Scholar]
- 44. Tong Y, Zhang X, Tian F, et al. Philinopside A, a novel marine-derived compound possessing dual anti-angiogenic and anti-tumor effects. Int J Cancer 2005; 114: 843–853. [DOI] [PubMed] [Google Scholar]
- 45. Tian F, Zhu CH, Zhang XW, et al. Philinopside E, a new sulfated saponin from sea cucumber, blocks the interaction between kinase insert domain-containing receptor (KDR) and alphavbeta3 integrin via binding to the extracellular domain of KDR. Mol Pharmacol 2007; 72: 545–552. [DOI] [PubMed] [Google Scholar]
- 46. Zhang SY, Yi YH, Tang HF. Cytotoxic sulfated triterpene glycosides from the sea cucumber Pseudocolochirus violaceus. Chem Biodivers 2006; 3: 807–817. [DOI] [PubMed] [Google Scholar]
- 47. Zhao Q, Xue Y, Liu ZD, et al. Differential effects of sulfated triterpene glycosides, holothurin A1, and 24-dehydroechinoside A, on antimetastasic activity via regulation of the MMP-9 signal pathway. J Food Sci 2010; 75: H280–H288. [DOI] [PubMed] [Google Scholar]
- 48. Althunibat OY, Hashim RB, Taher M, et al. In vitro antioxidant and antiproliferative activities of three Malaysian sea cucumber species. Eur J Sci Res 2009; 37: 376–387. [Google Scholar]
- 49. Roginsky AB, Ding XZ, Woodward C, et al. Anti-pancreatic cancer effects of a polar extract from the edible sea cucumber, Cucumaria frondosa. Pancreas 2010; 39: 646–652. [DOI] [PubMed] [Google Scholar]
- 50. Fernandez-Fuente G, Mollinedo P, Grande L, et al. Culture dimensionality influences the resistance of glioblastoma stem-like cells to multikinase inhibitors. Mol Cancer Ther 2014; 13: 1664–1672. [DOI] [PubMed] [Google Scholar]
- 51. Rangel M, Prado MP, Konno K, et al. Cytoskeleton alterations induced by Geodia corticostylifera depsipeptides in breast cancer cells. Peptides 2006; 27: 2047–2057. [DOI] [PubMed] [Google Scholar]
- 52. Freitas VM, Rangel M, Bisson LF, et al. The geodiamolide H, derived from Brazilian sponge Geodia corticostylifera, regulates actin cytoskeleton, migration and invasion of breast cancer cells cultured in three-dimensional environment. J Cell Physiol 2008; 216: 583–594. [DOI] [PubMed] [Google Scholar]
- 53. Albini A, Iwamoto Y, Kleinman HK, et al. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res 1987; 47: 3239–3245. [PubMed] [Google Scholar]
- 54. Suyama K, Shapiro I, Guttman M, et al. A signaling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. Cancer Cell 2002; 2: 301–314. [DOI] [PubMed] [Google Scholar]
- 55. Hazan RB, Qiao R, Keren R, et al. Cadherin switch in tumor progression. Ann N Y Acad Sci 2004; 1014: 155–163. [DOI] [PubMed] [Google Scholar]
- 56. Hazan RB, Phillips GR, Qiao RF, et al. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol 2000; 148: 779–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Qian X, Anzovino A, Kim S, et al. N-cadherin/FGFR promotes metastasis through epithelial-to-mesenchymal transition and stem/progenitor cell-like properties. Oncogene 2014; 33: 3411–3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bhatelia K, Singh K, Singh R. TLRs: linking inflammation and breast cancer. Cell Signal 2014; 26: 2350–2357. [DOI] [PubMed] [Google Scholar]
- 59. Schafer ZT, Brugge JS. IL-6 involvement in epithelial cancers. J Clin Invest 2007; 117: 3660–3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jin HZ, Hwang BY, Kim HS, et al. Antiinflammatory constituents of Celastrus orbiculatus inhibit the NF-kappaB activation and NO production. J Nat Prod 2002; 65: 89–91. [DOI] [PubMed] [Google Scholar]
- 61. Savjani KT, Gajjar AK, Savjani JK. Drug solubility: importance and enhancement techniques. ISRN Pharmaceut 2012; 2012: 195727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hyde AM, Zultanski SL, Waldman JH, et al. General principles and strategies for salting-out informed by the Hofmeister series. Org Process Res Dev 2017; 21: 1355–1370. [Google Scholar]
- 63. Grosso C, Valentão P, Ferreres F, et al. Alternative and efficient extraction methods for marine-derived compounds. Marine Drugs 2015; 13: 3182–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hill GB, Sweeney JB. Reaction workup planning: a structured flowchart approach, exemplified in difficult aqueous workup of hydrophilic products. J Chem Educ 2014; 92: 488–496. [Google Scholar]
- 65. Ishikawa M, Hashimoto Y. Improvement in aqueous solubility in small molecule drug discovery programs by disruption of molecular planarity and symmetry. J Med Chem 2011; 54: 1539–1554. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_Figures_(1) for Anti-proliferative and anti-inflammatory activities of the sea cucumber Holothuria polii aqueous extract by Mike Kareh, Rana El Nahas, Lamis Al-Aaraj, Sara Al-Ghadban, Nataly Naser Al Deen, Najat Saliba, Marwan El-Sabban and Rabih Talhouk in SAGE Open Medicine