Abstract
Objectives
To analyze the influence of thickness and incisal extension of indirect veneers on the stress and strain generated in maxillary canine teeth.
Materials and Methods
A 3-dimensional maxillary canine model was validated with an in vitro strain gauge and exported to computer-assisted engineering software. Materials were considered homogeneous, isotropic, and elastic. Each canine tooth was then subjected to a 0.3 and 0.8 mm reduction on the facial surface, in preparations with and without incisal covering, and restored with a lithium disilicate veneer. A 50 N load was applied at 45° to the long axis of the tooth, on the incisal third of the palatal surface of the crown.
Results
The results showed a mean of 218.16 µstrain of stress in the in vitro experiment, and 210.63 µstrain in finite element analysis (FEA). The stress concentration on prepared teeth was higher at the palatal root surface, with a mean value of 11.02 MPa and varying less than 3% between the preparation designs. The veneers concentrated higher stresses at the incisal third of the facial surface, with a mean of 3.88 MPa and a 40% increase in less-thick veneers. The incisal cover generated a new stress concentration area, with values over 48.18 MPa.
Conclusions
The mathematical model for a maxillary canine tooth was validated using FEA. The thickness (0.3 or 0.8 mm) and the incisal covering showed no difference for the tooth structure. However, the incisal covering was harmful for the veneer, of which the greatest thickness was beneficial.
Keywords: Dental veneer, Finite element analysis, Prosthesis design
INTRODUCTION
Over the years, the demand for aesthetic improvements has increased, as has the demand for aesthetic veneers [1]. Anterior teeth may receive indirect veneers to correct defects such as color modifications and shape abnormalities [2]. Veneers have emerged as an alternative to full crowns [3], since they remove little to no dental structure [4] and minimize the risk of endodontic complications attributed to full-crown preparations [5]. In contrast, approximately 63% to 73% of the healthy dental structure is removed when a full-crown preparation is performed [6]. For this reason, aesthetic veneers have become a favorable alternative because they conserve a greater amount of healthy dental structure (only about 3% to 30% of the structure is removed), and the enamel adhesion is more reliable [4]. The risk of failure for dentin adhesion is higher [7], so the minimum possible thickness should be used to obtain aesthetically appealing results and adequate resistance for restoration durability [4].
Despite the aesthetics and functional success achieved by full-crown treatment, failure may be caused by a fracture in the prosthetic piece [7], as well as for other reasons. More conservative preparations for veneers may be stronger when restricted to the facial surface close to unprepared teeth [8], although this results in thinner restoring material at the incisal margin [9]. The IPS e.Max Press ceramic (Ivoclar Vivadent, Schaan, Liechtenstein) was developed with a structural resistance over 440 MPa [10]. It can be produced through 2 durable manufacturing methods: pressing or machining [11]. After crystallization, lithium metasilicate crystals are transformed into lithium disilicate crystals (70% of the volume), which are responsible for the material's high resistance [12].
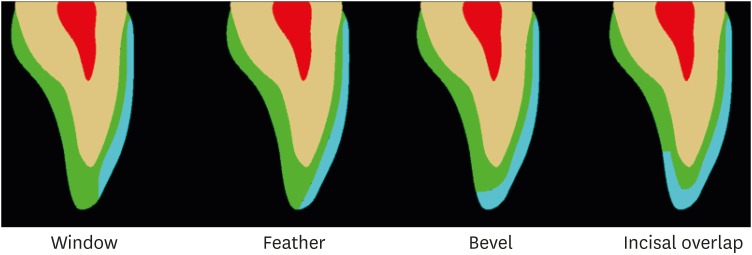
The dental preparation influences the durability, color, translucency, and tone of the ceramic restoration, as it determines the contour from inside the surface and the thickness of the ceramic piece. During the preparation of the cervical third, the gingival margin of the veneer must be lightly subgingival for the anterior teeth, as no difference is found regarding healthy and gingival contours when compared to supragingival margins [13]. For the incisal margin, modifications may be made; in a ‘window’ preparation, there is greater conservation of enamel structure (resulting in a visible line between enamel, cement, and ceramic), while in a ‘feather’ preparation, the veneer extends over the incisal area of the facial surface. Furthermore, in bevel preparations, the incisal region is completely covered, and in the ‘overlap’ preparation (Figure 1), the incisal third of the palatal surface is covered [14]. The choice of the veneer extension type in the incisal third is generally associated with the desired aesthetics, not the resistance of the restoration [15].
Figure 1. Illustration of the window, feather, bevel, and overlap preparations of ceramic veneers.
Finite element analysis (FEA) enables predictions of the stress concentration on structures (in the dental remnant, prosthetic element, and adhesive interface) by means of a quantitative computer simulation, thus providing knowledge about possible fracture origin areas [16,17]. Previous studies have reported divergences between FEA results and those of prospective clinical studies; when compared to an incisal shoulder edge, the palatal chamfer showed more harmonic stress distribution [18] and better resistance to fatigue [19] when tested in FEA, but this difference was not clinically verified [20]. Because of such divergences, it was suggested that quantitative models should be validated prior to stress concentration evaluations [21]. The strain gauge is currently a tool that enables measuring microstrain in the dental element under loading in both in vivo [5] and in vitro studies [22,23]. It consists of sensors fixed to a dental surface capable of recognizing the generated deformation through a process analogical to that of a digital information converter.
This study aimed to validate a 3-dimensional (3D) model of a maxillary canine tooth and to analyze the stress generated when the tooth underwent different types of preparations for ceramic veneers with and without incisal covering with 2 different thicknesses through 3D FEA. The first null hypothesis was that different preparation designs would not influence the stress concentration, and the second null hypothesis was that the thickness would not influence the stress concentration.
MATERIALS AND METHODS
Preparation of specimens
The Ethics Committee (Institute of Science and Technology – UNESP) approved this study under the review protocol approval number 0713616.3.0000.0077. Eight extracted canine teeth were collected, and it was ensured that they did not present caries, restorations, or wear on the cusp tip that could modify their anatomy. Teeth were stored in saline solution for 30 days. The following dimensions of the teeth were measured using a pachymeter: mesiodistal width (10.17 ± 0.58 mm), cervico-occlusal height (7.2 ± 0.58 mm), and tooth length (24.3 ± 1.2 mm).
The teeth were marked 2 mm below the cementoenamel junction, and the root portion apical to demarcation was inserted in fused No. 7 wax, creating enough thickness to simulate the periodontal ligament space. The teeth were inserted in a sufficient amount of polyurethane resin (Polyurethane F160, Axson, Cercy, France) to cover the entire root portion until 2 mm from the cementoenamel junction. A polyether adhesive (3M ESPE, Irvine, CA, USA) was applied over the root portion and over polyurethane. A sufficient amount of polyether (Impregum Soft, 3M ESPE) was proportioned to fill the gap left by the removed wax, and each tooth was repositioned using a dental delineator.
The facial surface of the teeth was marked to locate the central region and then cleaned with isopropyl alcohol. Strain gauges (KFG-1-120-C1-11L1M2R, KYOWA electronic instruments Co., Ltd., Tokyo, Japan: resistance, 119.6 ± 0.4%Ω; gauge length, 1 mm; gauge factor, 2.08% ± 1.0%) were attached with cyanoacrylate adhesive (Super Bonder Loctite, São Paulo, SP, Brazil) [23]. The wires of the strain gauges were welded to a circuit board on which copper wires were also welded, and then attached to a terminal to capture the information generated by the devices (Model 5100B Scanner - System 5000, Instruments Division Measurements Group, Inc., Raleigh, NC, USA). A multimeter was used to verify the absence of electric terminal defects and the soldering points of the wires (Minida ET 2055, Minida, São Paulo, SP, Brazil).
In vitro loading
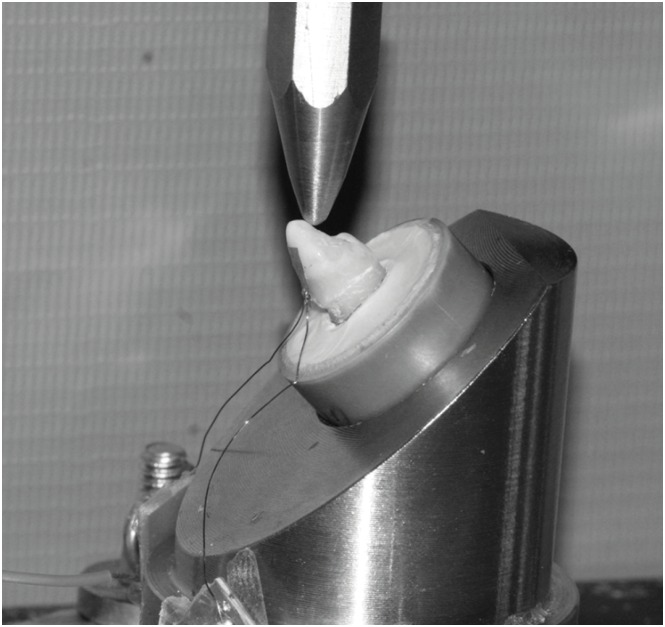
The samples were placed on a steel base inclined at 45°, and a blunt tip of 2 mm in diameter was used to apply a load of 50 N for 20 seconds (Figure 2). With the tip touching the palatal surface in the incisal third at a single point measuring 2 mm in diameter, the generated microdeformations were interpreted by software (StrainSmart, Vishay Measurements Group, Raleigh, NC, USA).
Figure 2. The sample was placed on a metal base with an inclination of 45º and a 50 N load was applied.
Three-dimensional modeling
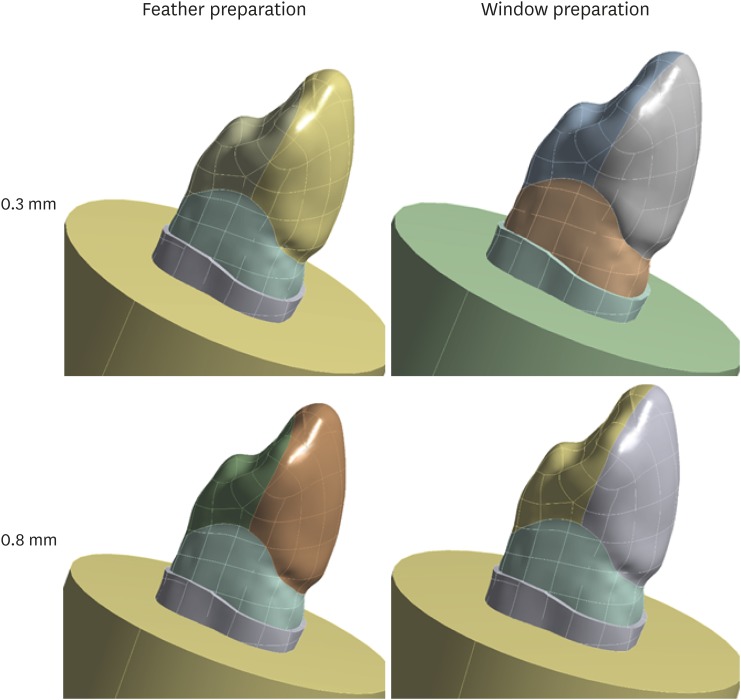
A maxillary canine tooth was scanned (Figures 2 and 3) using stereolithography and exported to CAD software (Rhinoceros 5.0, SR8, McNeel North America, Seattle, WA, USA), in which the external structures were modeled based on the mean values measured in an in vitro study. After the outer surface was finished, the crown contour was outlined in the cervical region in order to discriminate the enamel and dentin structures. The enamel, dentin, and pulp structures were then modeled in depth using the outline obtained in 3D.
Figure 3. Teeth with preparation and restoration according to specifications.
The periodontal ligament external to the root portion, 2 mm below the cementoenamel junction, was modeled with a thickness of 0.1 mm and a resin cylinder (25 mm diameter) was modeled (corresponding to the laboratory specimens). With the rigid structure complete, preparations were made by removing the facial and incisal structures as appropriate for each analyzed group: 0.3 mm with and without incisal covering and 0.8 mm with and without incisal covering. The models then received restorations that returned them to their initial form (Figure 3).
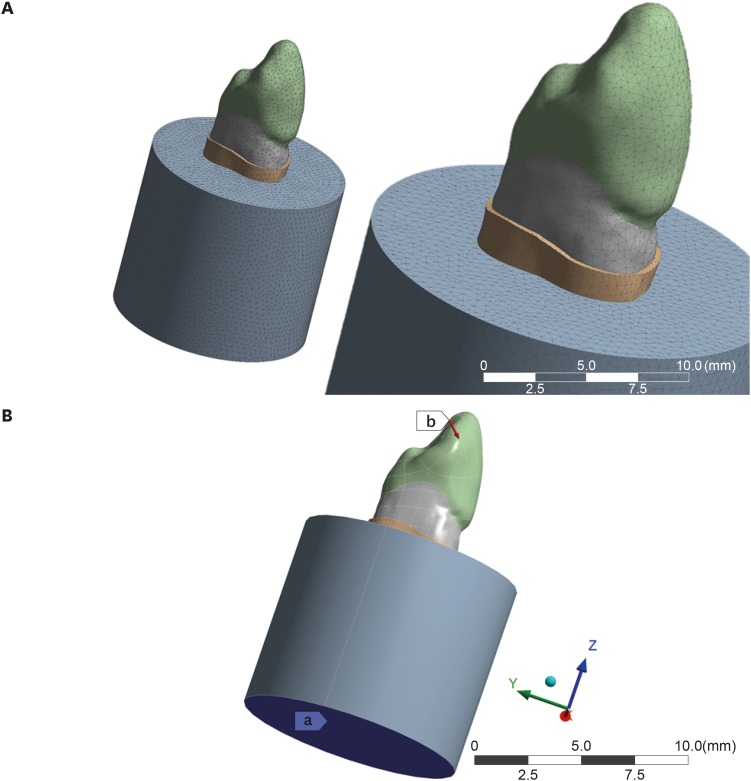
Using analysis software (ANSYS 17.2, ANSYS Inc., Houston, TX, USA) the model was transformed into a mesh with 172,467 tetrahedral elements and 98,467 nodes (Figure 4). The physiological characteristics of the teeth and ideal contacts were considered. The set was fixed by the base of the alveolar bone. The materials were considered isotropic, linearly elastic, and homogeneous [24]. The values of elastic modulus and the Poisson coefficient were chosen based on previous studies and are presented in Table 1.
Figure 4. (A) Generation of mesh with a complex geometry with a finite number of elements; (B) Loading and boundary conditions: a, the fixation area; b, the loading point inclined at 45°.
Table 1. Mechanical properties used in the computational simulation.
RESULTS
Validation of the numeric model by an in vitro strain gauge
In the analysis using a strain gauge, static loading was performed for 20 seconds, and the results are shown in Table 2. For a comparative analysis of the data obtained by the in vitro experiment, the computer model was analyzed for compressive deformation (minimum principal elastic strain) of the area corresponding to the strain gauge area. The analyzed area is highlighted in Figure 5. The theoretical values obtained varied at each node of each element present in the area from 111.87 to 378.66 µstrain, with a mean of 210.63 µstrain. In a comparison with the in vitro values from Table 2 (192.57 µstrain), it can be noted that the theoretically computed value was 8.57% higher than that measured in the laboratory experiment, so the simulation was accepted as valid.
Table 2. Strain values (mm/mm) obtained using strain gauges corresponding to the in vitro generated measurements.
| Tooth | Maximum | Minimum | Mean ± standard deviation |
|---|---|---|---|
| 1 | 237.83 | 90.69 | 180.71 ± 25.39 |
| 2 | 165.43 | 83.69 | 138.01 ± 16.84 |
| 3 | 195.40 | 120.28 | 166.36 ± 14.88 |
| 4 | 182.17 | 106.65 | 154.61 ± 15.16 |
| 5 | 167.72 | 114.83 | 142.04 ± 14.73 |
| 6 | 187.62 | 134.68 | 161.53 ± 14.85 |
| 7 | 186.84 | 136.63 | 162.16 ± 14.79 |
| 8 | 249.51 | 190.34 | 218.16 ± 15.16 |
| Mean | 192.565 | 122.22 | 165.44 ± 16.47 |
Figure 5. Representative area of the strain gauge–bonded area (shown in color) during the analysis of the minimum principal elastic strain.
Analysis of the stress concentration in restored teeth
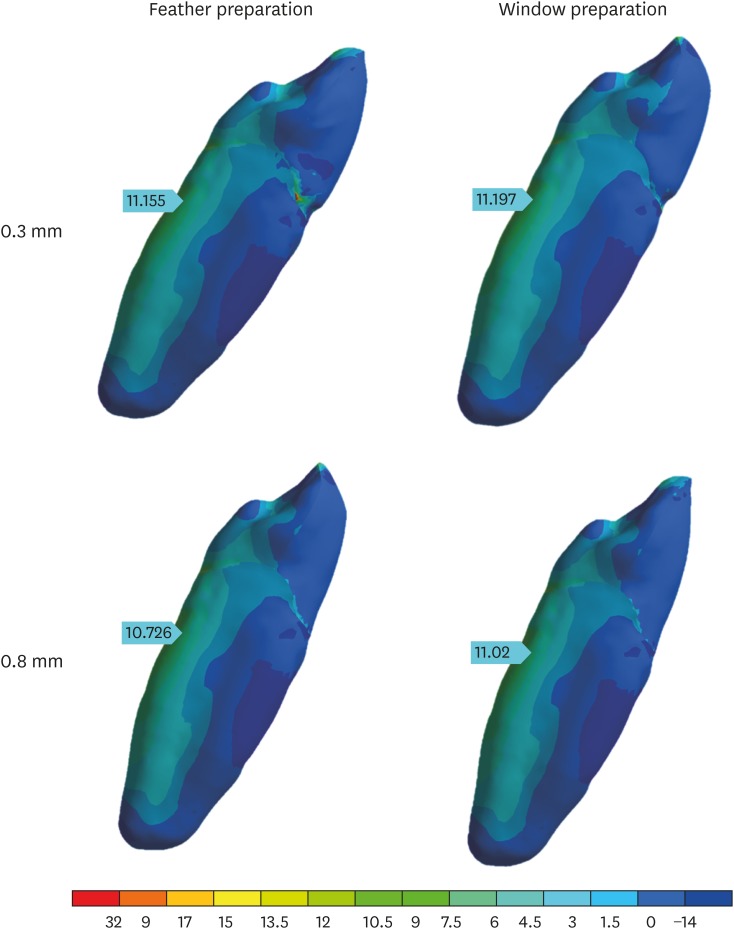
According to the analysis, the palatal surface of the root was the tooth area with the highest tensile stress concentration for all preparation types (Figure 6), with values varying from 10.72 to 11.19 MPa, and variations of less than 3% of the mean value (11.02 MPa).
Figure 6. Lateral view of the tooth with the distribution of tensile stresses in the tooth in each of the specified preparations.
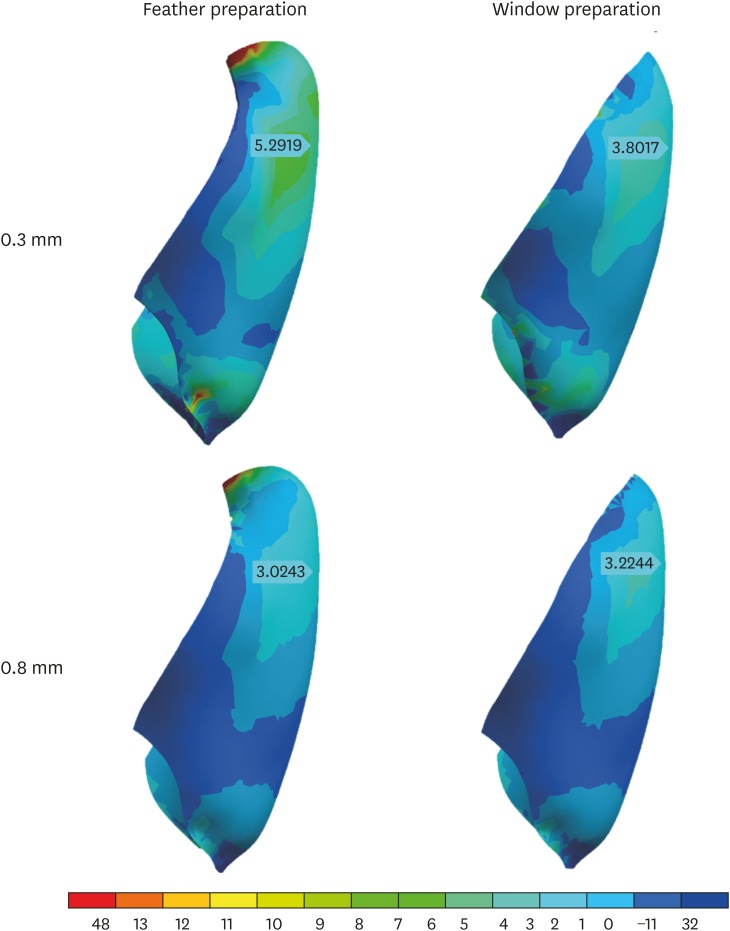
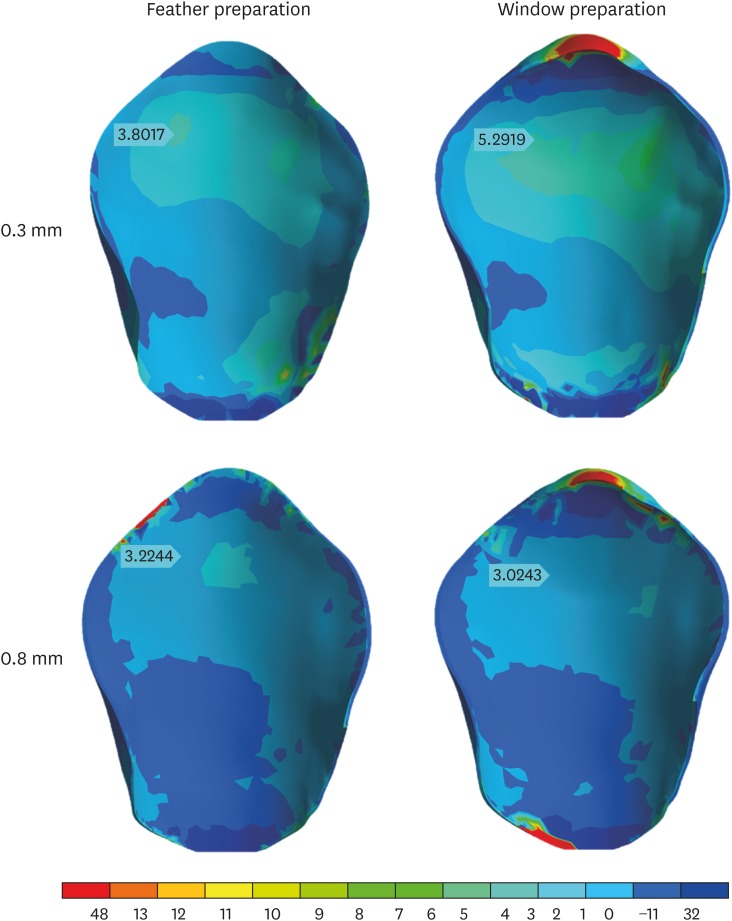
The veneers presented stress concentration in the incisal third, with higher values on the facial side (3.02–5.29 MPa), and variations between preparations reached 40% of the mean value (3.8 MPa). Higher stress concentrations in this area were obtained in thinner veneers (0.3 mm), regardless of the veneer extension. Moreover, the incisal covering led to a new stress concentration zone in the veneer, in the palatal region of the tooth-restoration interface, where the highest values were obtained (48.13 MPa). The results obtained for the veneers are presented in Figures 7 and 8.
Figure 7. Lateral view of the distribution of tensile stress in veneers in each of the specified preparations.
Figure 8. Palatal view of the distribution of tensile stress in veneers in each of the specified preparations.
DISCUSSION
The present study aimed to evaluate the distribution of stress in 2 different types of indirect veneers with 2 different thicknesses. The results demonstrated that both factors influenced the results, so both null hypotheses were rejected. The results were obtained only after the 3D model was validated through an in vitro experiment.
Experimental validation of FEA has been widely performed, and comparable results have been obtained in both in vitro and FEA experiments [21,22,23,27,28,29]. A literature review performed by Chang et al. [30] in 2018 found that among 450 publications over the last 10 years about dental implants using FEA, only 47 articles used some kind of validation (clinical or laboratory experiments, findings in the literature, or another kind of validation), and the strain gauge method of laboratory testing was used in 15 of those 47 articles. That review recognized that simplifying the biomechanical behavior of tissues and ignoring the characteristics of each individual for computer analysis may neglect some aspects, resulting in inaccuracies in the predicted behavior of structures in the studied circumstances. Even though FEA does not provide values that perfectly correspond to reality, it enables observations of the behavior at specific spots of the studied structures, which is impossible in in vivo or in vitro studies. Even so, a study by Stappert et al. [8] showed that, given the limitations of a purely theoretical study, it was possible to validate the model by obtaining close values in both in vivo and in vitro studies. In the present study, the area chosen for analysis was the same area of strain gauge fixation used in the in vitro experiment. In FEA, the obtained values of minimum principal elastic strain varied from 111.87 to 378.66 µstrain, with a mean value of 210.63 µstrain (Figure 5); in the in vitro experiment, the mean values varied from 138 to 218 µstrain (Table 2). Considering the anatomic variations of each tooth, it may be considered possible to obtain results through FEA that are similar to those obtained in vitro.
The use of aesthetic veneers on unprepared teeth does not produce more aesthetically-pleasing results than on prepared teeth; in addition, placing veneers on unprepared teeth is aggressive to soft tissues and inferior in terms of resistance to fracture [31]. When comparing an unprepared tooth restored with ceramic material to teeth that have been subjected to different preparations (strictly facial preparation, facial preparation with incisal covering, and full-crown preparation), Stappert et al. [8] found a higher fracture rate in masticatory movement simulations in the unprepared group, with fractures affecting the remaining dental structure, mainly the root portion, and not the restoring material. Nevertheless, the same study observed 80% more crack formation in the full-crown preparation and restoration group than in the other groups.
Studies have reported highly divergent results regarding the benefits of incisal covering. In a study of incisors, Hui et al. [32] showed negative consequences of this covering in terms of the resistance of the restorative material, while Highton et al. [33] defended this modality by considering the stress that developed in the veneer using photoelastic analysis. Longitudinal studies have reported no difference in the prognosis of indirect veneers depending on the crown preparation extension [8,34]. These studies agreed with those by Stappert et al. [8] and Meijering et al. [34], showing that the tooth was not reinforced by the incisal covering. As in the study by Hui et al. [32], it was found that the incisal covering led to a new area of stress concentration on the palatal side of the restoration.
Thicker veneers generally show more favorable results [35]. In the present study, using colorimetric analysis, we observed a lower stress concentration in thicker veneers (0.8 mm). This difference was observed on both the facial and palatal sides, corroborating the study by Ge et al. [35], in which higher thickness of the material was found to be beneficial for the restoration. By verifying that the stresses generated in the tooth were similar in different-thickness preparations (0.3 and 0.8 mm), this study suggests that there is no damage to the dental element when more invasive preparations are needed (Figure 6). Despite this observation, the veneer thickness presents higher stress concentration values in thinner restorations (0.3 mm) [7,8]. Even though the maximum stress values do not overcome the material's limit to failure, higher thicknesses may contribute to greater longevity of the restoration.
The conducted test exclusively aimed to analyze the stress generated in teeth subjected to different preparation types and different veneers by simulating a single physiological occlusal loading; it was not the goal of this study to analyze material failure, as has been done in previous studies [32,36]. In addition, the load of 50 N to the teeth simulated a physiological load (between 25 N and 50 N) [37], and parafunctional loading, which can reach 6 times over physiological values, was not considered [38].
The data presented by FEA in this study are approximate, which is a limitation of the methodology. Although the dental and periodontal tissues were considered in their entirety, their structures are homogeneous and isotropic, which is not the case in vivo. The veneers were considered to be directly bonded to enamel, disregarding the bonding material between them. The buccal cavity contains specific factors that were not considered in the experiment, such as moisture, which is considered to play an important role in ceramic failure by stimulating the growth of microcracks [36], and temperature changes [4], which are capable of affecting the material and its properties over time.
CONCLUSIONS
Within the limitations inherent to a valid mathematical FEA model of a maxillary canine tooth, there were no significant differences in the stress concentration within the remaining tooth structure between different preparations and veneer extension thicknesses. However, in the veneer, the higher-thickness veneer showed better stress distribution with lower tensile stress concentration, and the more extensive preparations, such as the feather type, exhibited new stress concentration zones on the palatal side of the restoration.
ACKNOWLEDGEMENT
The first author also acknowledges FAPESP for financial support in the form of a scholarship.
Footnotes
Funding: This study was supported by FAPESP (São Paulo Research Foundation), process number 16/20021-0.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: Uemura ES, Borges ALS.
- Data curation: Costa VLS, Tribst JPM, Borges ALS.
- Formal analysis: Tribst JPM.
- Funding acquisition: Costa VLS, Borges ALS.
- Investigation: Costa VLS, Tribst JPM, Morais DC.
- Methodology: Costa VLS, Tribst JPM, Borges ALS.
- Project administration: Borges ALS.
- Resources: Costa VLS.
- Software: Tribst JPM, Borges ALS.
- Supervision: Tribst JPM, Uemura ES, Borges ALS.
- Validation: Borges ALS.
- Visualization: Costa VLS.
- Writing - original draft: Costa VLS, Morais DC.
- Writing - review & editing: Tribst JPM, Borges ALS.
References
- 1.Schmidt KK, Chiayabutr Y, Phillips KM, Kois JC. Influence of preparation design and existing condition of tooth structure on load to failure of ceramic laminate veneers. J Prosthet Dent. 2011;105:374–382. doi: 10.1016/S0022-3913(11)60077-2. [DOI] [PubMed] [Google Scholar]
- 2.Korkut B, Yanıkoğlu F, Günday M. Direct composite laminate veneers: three case reports. J Dent Res Dent Clin Dent Prospect. 2013;7:105–111. doi: 10.5681/joddd.2013.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Re D, Augusti G, Amato M, Riva G, Augusti D. Esthetic rehabilitation of anterior teeth with laminates composite veneers. Case Rep Dent. 2014;2014:849273. doi: 10.1155/2014/849273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magne P, Hanna J, Magne M. The case for moderate “guided prep” indirect porcelain veneers in the anterior dentition. The pendulum of porcelain veneer preparations: from almost no-prep to over-prep to no-prep. Eur J Esthet Dent. 2013;8:376–388. [PubMed] [Google Scholar]
- 5.Schmitz JH, Cortellini D, Granata S, Valenti M. Monolithic lithium disilicate complete single crowns with feather-edge preparation design in the posterior region: a multicentric retrospective study up to 12 years. Quintessence Int. 2017;20:601–608. doi: 10.3290/j.qi.a38678. [DOI] [PubMed] [Google Scholar]
- 6.Edelhoff D, Sorensen JA. Tooth structure removal associated with various preparation designs for anterior teeth. J Prosthet Dent. 2002;87:503–509. doi: 10.1067/mpr.2002.124094. [DOI] [PubMed] [Google Scholar]
- 7.Oztürk E, Bolay S. Survival of porcelain laminate veneers with different degrees of dentin exposure: 2-year clinical results. J Adhes Dent. 2014;16:481–489. doi: 10.3290/j.jad.a32828. [DOI] [PubMed] [Google Scholar]
- 8.Stappert CF, Ozden U, Gerds T, Strub JR. Longevity and failure load of ceramic veneers with different preparation designs after exposure to masticatory simulation. J Prosthet Dent. 2005;94:132–139. doi: 10.1016/j.prosdent.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 9.Andreasen FM, Daugaard-Jensen J, Munksgaard EC. Reinforcement of bonded crown fractured incisors with porcelain veneers. Endod Dent Traumatol. 1991;7:78–83. doi: 10.1111/j.1600-9657.1991.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 10.Spitznagel FA, Boldt J, Gierthmuehlen PC. CAD/CAM ceramic restorative materials for natural teeth. J Dent Res. 2018;97:1082–1091. doi: 10.1177/0022034518779759. [DOI] [PubMed] [Google Scholar]
- 11.Lien W, Roberts HW, Platt JA, Vandewalle KS, Hill TJ, Chu TM. Microstructural evolution and physical behavior of a lithium disilicate glass-ceramic. Dent Mater. 2015;31:928–940. doi: 10.1016/j.dental.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Alkadi L, Ruse ND. Fracture toughness of two lithium disilicate dental glass ceramics. J Prosthet Dent. 2016;116:591–596. doi: 10.1016/j.prosdent.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 13.De Araujo EM, Jr, Fortkamp S, Baratieri LN. Closure of diastema and gingival recontouring using direct adhesive restorations: a case report. J Esthet Restor Dent. 2009;21:229–240. doi: 10.1111/j.1708-8240.2009.00267.x. [DOI] [PubMed] [Google Scholar]
- 14.Haralur SB. Microleakage of porcelain laminate veneers cemented with different bonding techniques. J Clin Exp Dent. 2018;10:e166–e171. doi: 10.4317/jced.53954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pini NP, Aguiar FH, Lima DA, Lovadino JR, Terada RS, Pascotto RC. Advances in dental veneers: materials, applications, and techniques. Clin Cosmet Investig Dent. 2012;4:9–16. doi: 10.2147/CCIDEN.S7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dal Piva AMO, Tribst JPM, Borges ALS, Souza ROAE, Bottino MA. CAD-FEA modeling and analysis of different full crown monolithic restorations. Dent Mater. 2018;34:1342–1350. doi: 10.1016/j.dental.2018.06.024. [DOI] [PubMed] [Google Scholar]
- 17.Tribst JPM, Dal Piva AMO, Madruga CFL, Valera MC, Borges ALS, Bresciani E, de Melo RM. Endocrown restorations: influence of dental remnant and restorative material on stress distribution. Dent Mater. 2018;34:1466–1473. doi: 10.1016/j.dental.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Zarone F, Apicella D, Sorrentino R, Ferro V, Aversa R, Apicella A. Influence of tooth preparation design on the stress distribution in maxillary central incisors restored by means of alumina porcelain veneers: a 3D-finite element analysis. Dent Mater. 2005;21:1178–1188. doi: 10.1016/j.dental.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Chaiyabutr Y, Phillips KM, Ma PS, Chitswe K. Comparison of load-fatigue testing of ceramic veneers with two different preparation designs. Int J Prosthodont. 2009;22:573–575. [PubMed] [Google Scholar]
- 20.Guess PC, Stappert CF. Midterm results of a 5-year prospective clinical investigation of extended ceramic veneers. Dent Mater. 2008;24:804–813. doi: 10.1016/j.dental.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Tribst JPM, Rodrigues VA, Borges ALS, Lima DR, Nishioka RS. Validation of a simplified implant-retained cantilever fixed prosthesis. Implant Dent. 2018;27:49–55. doi: 10.1097/ID.0000000000000699. [DOI] [PubMed] [Google Scholar]
- 22.Tribst JP, Rodrigues VA, Dal Piva AO, Borges AL, Nishioka RS. The importance of correct implants positioning and masticatory load direction on a fixed prosthesis. J Clin Exp Dent. 2018;10:e81–e87. doi: 10.4317/jced.54489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Datte CE, Tribst JP, Dal Piva AO, Nishioka RS, Bottino MA, Evangelhista AM, Monteiro FM, Borges AL. Influence of different restorative materials on the stress distribution in dental implants. J Clin Exp Dent. 2018;10:e439–e444. doi: 10.4317/jced.54554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tribst JPM, Dal Piva AMO, Shibli JA, Borges ALS, Tango RN. Influence of implantoplasty on stress distribution of exposed implants at different bone insertion levels. Braz Oral Res. 2017;31:e96. doi: 10.1590/1807-3107bor-2017.vol31.0096. [DOI] [PubMed] [Google Scholar]
- 25.Monteiro JB, Dal Piva AMO, Tribst JPM, Borges ALS, Tango RN. The effect of resection angle on stress distribution after root-end surgery. Iran Endod J. 2018;13:188–194. doi: 10.22037/iej.v13i2.19089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Souza AC, Xavier TA, Platt JA, Borges AL. Effect of base and inlay restorative material on the stress distribution and fracture resistance of weakened premolars. Oper Dent. 2015;40:E158–E166. doi: 10.2341/14-229-L. [DOI] [PubMed] [Google Scholar]
- 27.Eser A, Akça K, Eckert S, Cehreli MC. Nonlinear finite element analysis versus ex vivo strain gauge measurements on immediately loaded implants. Int J Oral Maxillofac Implants. 2009;24:439–446. [PubMed] [Google Scholar]
- 28.Kheiralla LS, Younis JF. Peri-implant biomechanical responses to standard, short-wide, and mini implants supporting single crowns under axial and off-axial loading (an in vitro study) J Oral Implantol. 2014;40:42–52. doi: 10.1563/AAID-JOI-D-11-00102. [DOI] [PubMed] [Google Scholar]
- 29.Bicalho AA, Valdívia AD, Barreto BC, Tantbirojn D, Versluis A, Soares CJ. Incremental filling technique and composite material--part II: shrinkage and shrinkage stresses. Oper Dent. 2014;39:E83–E92. doi: 10.2341/12-442-L. [DOI] [PubMed] [Google Scholar]
- 30.Chang Y, Tambe AA, Maeda Y, Wada M, Gonda T. Finite element analysis of dental implants with validation: to what extent can we expect the model to predict biological phenomena? A literature review and proposal for classification of a validation process. Int J Implant Dent. 2018;4:7. doi: 10.1186/s40729-018-0119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richter WA, Ueno H. Relationship of crown margin placement to gingival inflammation. J Prosthet Dent. 1973;30:156–161. doi: 10.1016/0022-3913(73)90050-4. [DOI] [PubMed] [Google Scholar]
- 32.Hui KK, Williams B, Davis EH, Holt RD. A comparative assessment of the strengths of porcelain veneers for incisor teeth dependent on their design characteristics. Br Dent J. 1991;171:51–55. doi: 10.1038/sj.bdj.4807602. [DOI] [PubMed] [Google Scholar]
- 33.Highton R, Caputo AA, Mátyás J. A photoelastic study of stresses on porcelain laminate preparations. J Prosthet Dent. 1987;58:157–161. doi: 10.1016/0022-3913(87)90168-5. [DOI] [PubMed] [Google Scholar]
- 34.Meijering AC, Creugers NH, Roeters FJ, Mulder J. Survival of three types of veneer restorations in a clinical trial: a 2.5-year interim evaluation. J Dent. 1998;26:563–568. doi: 10.1016/s0300-5712(97)00032-8. [DOI] [PubMed] [Google Scholar]
- 35.Ge C, Green CC, Sederstrom D, McLaren EA, White SN. Effect of porcelain and enamel thickness on porcelain veneer failure loads in vitro . J Prosthet Dent. 2014;111:380–387. doi: 10.1016/j.prosdent.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 36.Kelly JR. Clinically relevant approach to failure testing of all-ceramic restorations. J Prosthet Dent. 1999;81:652–661. doi: 10.1016/s0022-3913(99)70103-4. [DOI] [PubMed] [Google Scholar]
- 37.De Boever JA, McCall WD, Jr, Holden S, Ash MM., Jr Functional occlusal forces: an investigation by telemetry. J Prosthet Dent. 1978;40:326–333. doi: 10.1016/0022-3913(78)90042-2. [DOI] [PubMed] [Google Scholar]
- 38.Gibbs CH, Mahan PE, Mauderli A, Lundeen HC, Walsh EK. Limits of human bite strength. J Prosthet Dent. 1986;56:226–229. doi: 10.1016/0022-3913(86)90480-4. [DOI] [PubMed] [Google Scholar]