Abstract
Keratin proteins derived from human hair are known to contain a large number of cysteine residues. In hydrogels composed of oxidatively extracted keratin (keratose, KOS), sulfonic acid on the cysteine residues prevents disulfide cross-linking. Reductively extracted keratin (kerateine, KTN) has thiol groups on cysteine residues that can form disulfide cross-links. The rates of keratin hydrogel erosion and, in some cases, drug release can be tuned by manipulating disulfide cross-linking levels via the KOS:KTN ratio. To investigate the use of these hydrogel mixtures as carriers for growth factors in tissue engineering applications, we fabricated 15% (w/v) hydrogels with KOS:KTN ratios of 100:0, 70:30, 50:50, 30:70, and 0:100 with and without recombinant human bone morphogenetic protein 2 (rhBMP-2). We compared the keratin rhBMP-2 carriers with the clinical system of resorbable collagen sponges, which are known to elicit problems such as edema, inflammation, and ectopic bone growth. In vitro, hydrogels with increasing levels of KOS eroded more rapidly. However, there was little difference among the various keratin formulations in amounts of rhBMP-2 released after 12 h. Collagen had similar total rhBMP-2 release, although with significantly greater release over the first 12 h compared with all keratin formulations, except for 0:100 KOS:KTN. However, increasing levels of KTN led to increasing release of bioactive rhBMP-2 based on the stimulation of alkaline phosphatase activity in MC3T3-E1 cells by the hydrogel releasate. Micro-CT analysis of heterotopic bone growth in a mouse model indicated that with rhBMP-2, the 30:70 formulation led to significant increases in bone volume compared with KOS and collagen, whereas the 50:50 formulation led to significant bone volume increases compared with all of the formulations except for 30:70 KOS:KTN. Histological analysis of images from the heterotopic mouse model indicated differences in the quality and type of bone among the various carriers of rhBMP-2. KOS:KTN in the ratio of 70:30 with rhBMP-2 elicited greater amounts of cortical bone, whereas the ratio 30:70 resulted in greater amounts of marrow, total bone, and residual material. The results indicate that in addition to the rate of rhBMP-2 release, the carrier itself can have important effects on bone volume, type, distribution, and quality.
Keywords: : synthetic bone graft, bone morphogenetic protein 2, collagen, keratose, kerateine, drug delivery
Impact Statement
Recombinant human bone morphogenetic protein 2 (rhBMP-2) delivery from collagen sponges for bone formation is an important clinical example of growth factors in tissue engineering. Side effects from rhBMP-2 burst release and rapid collagen resorption have led to investigation of alternative carriers. Here, keratin carriers with tunable erosion rates were formulated by varying disulfide crosslinking via ratios of oxidatively (keratose) to reductively (kerateine) extracted keratin. In vitro rhBMP-2 bioactivity increased with kerateine content, reaching levels greater than with collagen. Heterotopic bone formation in a mouse model depended on the keratin formulation, highlighting the importance of the growth factor carrier.
Introduction
Keratins are a family of proteins that can be derived from numerous sources. Although soft keratins are found in cells and tissues, hard keratins can be extracted from various extracellular locations, including human hair, the source used in this report. Keratins are also known to possess cell-binding motifs,1 thus promoting cell attachment and making their use for tissue engineering applications of particular interest. Preclinical tissue engineering applications for keratin biomaterials have included nerve,2 skeletal muscle,3,4 skin,5 and bone,6,7 among others.8,9 A salient feature of keratins is their high proportion of cysteine residues. When extracted reductively,10,11 the resulting keratin is known as kerateine (KTN), and the thiol groups are capable of forming disulfide bonds. When extracted by oxidative means,10,12 keratin is referred to as keratose (KOS). The thiol groups of the cysteine residues in KOS are “capped” as sulfonic acid residues and are unable to form disulfide bonds. These chemical differences in the proteins are known to affect the physical properties of biomaterials, particularly hydrogels, derived from keratins. KOS will form hydrogels through physical entanglements, but degrade rapidly due to the lack of covalent disulfide cross-links. KTN persists much longer due to the presence of both physical entanglements and covalent disulfide cross-links.
To bridge these extremes in the physical characteristics of keratin hydrogels, we recently described a straightforward approach to mix KOS and KTN in varying proportions to tune the rates of keratin hydrogel erosion,13 thus mimicking the tunability of synthetic polymers with a natural polymer system. This approach of using mixtures of KOS and KTN to tune hydrogel erosion rates was also shown to achieve tunability in the release of recombinant human insulin-like growth factor 1 (rhIGF-1).13 Furthermore, keratin hydrogels have previously been used for the delivery of various exogenous agents, including small molecule antibiotics14 and growth factors,7,15,16 although these reports are for KOS or KTN individually. These previous results suggest that keratin hydrogels may be a suitable carrier system for various small-molecule drugs and growth factors for tissue engineering applications. As a test bed for this approach, we have explored the tunable KOS–KTN formulations for the delivery of recombinant human bone morphogenetic protein 2 (rhBMP-2) to understand their suitability to induce bone regeneration.
The use of synthetic bone grafts consisting of rhBMP-2 on bovine collagen sponge carriers (resorbable collagen sponge), marketed as Infuse by Medtronic (Minneapolis, MN), has been a clinical commercial success for tissue engineering.17 This product is used as an alternative to autografts and allografts for FDA-approved applications of lumbar spinal fusion (by anterior approach), open tibial fracture, and craniomaxillofacial applications,18 but it has been estimated that up to 85% of its clinical use is for “off-label” applications.19,20 Given that collagen is the main organic constituent of bone and is an osteoconductive material, the use of collagen is rational for bone regeneration. However, collagen is not particularly well suited for a sustained delivery of rhBMP-2, which is rapidly degradable enzymatically in vivo. This rapid degradation and burst release of rhBMP-2 likely contribute to the known clinical side effects of rhBMP-2, such as edema, inflammation, and ectopic bone growth.21 For these reasons, alternative carriers are needed.
Tunable systems, such as keratin, offer the opportunity to (i) investigate the effects of carrier degradation rates and (ii) optimize the system for the most favorable physiological and anatomical outcomes. We hypothesized that the formulation of the tunable degradation keratin carriers would have effects on the bioactivity of rhBMP-2 both in vitro and in vivo. In this report, we describe the in vitro release profile of rhBMP-2 from tunable keratin hydrogels and assess the bioactivity of rhBMP-2 that is released from the hydrogels. We then explore the effects of the keratin carriers of rhBMP-2 on new bone induction in a mouse model of heterotopic bone growth. The results of these studies demonstrate that, in addition to the drug or growth factor being released (in this case, rhBMP-2), the carrier properties must also be considered.
Materials and Methods
Fluorescent labeling of rhBMP-2
To detect the in vitro release of rhBMP-2 (see section “In vitro keratin hydrogel erosion and rhBMP-2 release from keratin hydrogels”), we labeled rhBMP-2 with AlexaFluor 488-succinimidyl ester (AF488-SE; Fisher Scientific) according to the manufacturer's directions. In brief, rhBMP-2 powder was hydrated to 3 mg/mL and then dialyzed against water (6,000–8,000 Da molecular weight; cutoff dialysis tubing) to remove Tris from rhBMP-2, thereby preventing its interference with AF488-SE labeling. After dialysis, AF488-SE was added and reacted for 1 h, followed by an additional dialysis against water. An rhBMP-2 standard curve was prepared to determine the protein extinction coefficient for unlabeled rhBMP-2. The protein concentration of the labeled rhBMP-2 was then determined by measuring absorbance at 280 and 494 nm with the following equation:
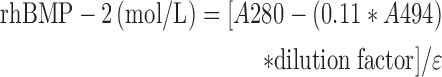 |
where ɛ is the rhBMP-2 molar extinction coefficient and 0.11*A494 subtracts the contribution of AF488 from absorbance measured for the protein at 280 nm. Based on this calculation, the labeling ratio was found to be 1.4 AF488 per rhBMP-2 molecule.
Cell culture
MC3T3-E1 preosteoblast cells were obtained from American Type Culture Collection (Manassas, VA) and were used in these experiments because they exhibit markers of differentiation (e.g., increased alkaline phosphatase [ALP] activity) upon stimulation by rhBMP-2. Cells were cultured according to the supplier's recommendations. In brief, MC3T3-E1 cells were cultured in alpha-minimum essential medium (α-MEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin (P-S; Life Technologies, Carlsbad, CA). Cells were cultured in a humidified atmosphere with 5% CO2 at 37°C. Media were changed every 2–3 days until cells reached 70–80% confluence as recommended to prevent contact-mediated differentiation, at which time cells were subcultured or used for the experiments described hereunder. For subculturing, cells were rinsed with phosphate-buffered saline (PBS) and detached by incubating with 0.25% trypsin for 5 min. Trypsin was deactivated by using four times the volume of α-MEM (with 10% FBS and 1% P-S).
Keratin and collagen carrier formulation and loading of samples with rhBMP-2
KOS and KTN powders, sterilized via 2 MRad gamma irradiation, were purchased from KeraNetics, LLC (Winston-Salem, NC) in lyophilized form and used without further modification. All the experiments were carried out using alpha fractions of keratin (α-keratin). Fifteen percent w/v gels of keratin were used for these experiments. Gels of individual keratins were produced as follows. In a typical 1 mL preparation, 150 mg of total keratin (dry powder) was placed in a 15 mL conical tube. These keratin powders were mixed together in dry form with KOS:KTN (w/w) formulations of 100:0, 70:30, 50:50, 30:70, or 0:100 (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/tea). One milliliter of water (control materials without rhBMP-2) or water containing 100 μg/mL rhBMP-2 (Medtronic) was then added to the powder mixtures. The resulting suspension was immediately mixed vigorously by vortexing and manually (spatula), centrifuged for 1 min at 171 g, and then packed into a 1 mL syringe (no needle). Because keratins are shear thinning materials (flowable), we were able to inject a known volume (100 μL of 15% w/v hydrogels with mass of 100 mg containing 10 μg of rhBMP-2) from the syringe into a 1.5 mL tube. For release experiments, the mass of material added to the tubes was measured to account for any small differences in volumes added to the tube. Because the hydrogels are well mixed during preparation, each sample should contain the same amount of rhBMP-2 (i.e., 10 μg). Typically, we prepared three such tubes from the original 1 mL formulation with 100 μL per tube in three different tubes. The remaining 700 μL of keratin hydrogel was discarded. The keratins in 1.5 mL tubes were then placed at 37°C overnight to allow them to fully gel before use in further experiments as described hereunder.
In the experiments described hereunder, Medtronic's Infuse absorbable collagen sponge was used as an additional control. The collagen sponges were prepared as recommended by the manufacturer with the exception that sponges were cut to a specific length and the dose of rhBMP-2 was 100 μg/mL to match the dose used in the keratin hydrogels and the appropriate dose for rodent experiments (see section “Mouse model for assessing osteoinduction”). A 1 × ¼ cm section (∼6 mg) of the sponge was cut and 100 μL of 100 μg/mL rhBMP-2 was added to give 10 μg of rhBMP-2, which is the same amount as used for keratin samples. rhBMP-2 was allowed to adsorb for 20 min before beginning experiments.
For experimental results, controls indicate that no rhBMP-2 was included in the formulation. These keratin and collagen formulations without rhBMP-2 were used to subtract background levels of signal from fluorescence assays for rhBMP-2 release and ALP activity (see section “In vitro bioactivity of rhBMP-2 released from keratin hydrogels or collagen sponges”).
In vitro keratin hydrogel erosion and rhBMP-2 release from keratin hydrogels
The rate at which keratin hydrogels were eroded (or solubilized) and the rate at which rhBMP-2 was released were each characterized from the same samples. We use the term “erosion” for keratin carriers as we have previously observed that keratin proteins themselves are not degraded, but rather that the bulk material itself is solubilized or eroded.
Keratin hydrogels of known mass (100 μL of 15% w/v, or 100 mg) in 1.5 mL tubes were prepared as described under the section “Keratin and collagen carrier formulation and loading of samples with rhBMP-2”. Control keratin hydrogels (no rhBMP-2) or collagen sponges (no rhBMP-2) were made from only water, whereas experimental groups had 100 μg/mL of AF488-labeled rhBMP-2 (10 μg of rhBMP-2 in 100 μL of keratin in each 1.5 mL tube). One hundred fifty microliters of PBS was placed on top of the gels. At specified time points (1.5, 3, 6, 12, 24 h, and then daily for 7 days), PBS was removed and stored at −20°C until further analysis. One hundred fifty microliters of fresh PBS was added to the samples at each of the time points.
The amount of soluble keratin that had eroded from the hydrogels into PBS was determined using a modified Lowry protein assay (DC Protein Assay; Bio-Rad, Hercules, CA). As shown in the “Results” section, the amount of rhBMP-2 contained in these samples was ∼1/10,000 of the total protein content (10 μg of rhBMP-2 in a 100 mg keratin sample), making the contribution of rhBMP-2 to the assay signal insignificant compared with the amount of keratin. We, therefore, neglected any contribution from rhBMP-2 in the protein assay results and assumed that all measured protein was keratin. The amount of keratin in the samples was determined by comparing with a standard curve of known concentrations of keratin, where KOS was used to generate the standard curve. Differences in the standard curves for KOS and KTN were not noted, so only one standard curve was used. Keratin hydrogels without rhBMP-2 were also analyzed to determine whether the presence of rhBMP-2 had any impact on the rate of keratin hydrogel erosion.
To perform statistical analysis of the rates of keratin erosion, we carried out a nonlinear regression of the data to fit the equation by Ritger and Peppas22 to estimate the rate constant for keratin erosion:
 |
where Mt is the cumulative mass release at time t, M∞ is the complete release, t is time, n is the diffusion exponent, and k is the rate constant. The geometry was dissimilar to the original description of Ritger and Peppas, but the relative values were determined by nonlinear regression analysis.
The amount of rhBMP-2 released from keratin hydrogels was determined by fluorometry of AF488 rhBMP-2 on a Biotek Synergy HT plate reader (Winooski, VT) with excitation/emission wavelengths of 485/528 nm. The amount of AF488-labeled rhBMP-2 released was determined by comparison with a standard curve of known amounts of the labeled rhBMP-2. Autofluorescence due to the keratin or collagen proteins was subtracted based on fluorescence readings from the control keratin hydrogels or collagen sponges that were not loaded with rhBMP-2. Because rhBMP-2 was fluorescently labeled, we could easily detect it above the background contribution of keratin or collagen autofluorescence over the course of the assay (fluorescence in samples containing rhBMP-2 was 8- to 100-fold greater than that of control hydrogels or sponges).
To allow statistical comparison of release rates of rhBMP-2 from the various formulations, we fit the data to a short- and long-term model of diffusion using nonlinear regression according to the equations of Fu and Kao23:
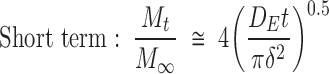 |
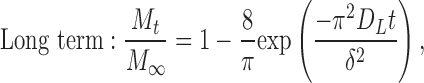 |
where DE is the short-term diffusion coefficient, DL is the long-term diffusion coefficient, and δ is associated with diffusion distance. Because the geometry of the system was irregular as compared with the original description of the equations in the literature, we treated the  and
and 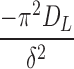 terms as lumped parameters for the nonlinear regression.
terms as lumped parameters for the nonlinear regression.
In vitro bioactivity of rhBMP-2 released from keratin hydrogels or collagen sponges
To assess rhBMP-2 bioactivity with keratin hydrogel or collagen sponge carriers, we collected the releasate at 12, 24, 48, 72, 96, and 120 h and subsequently assayed for ALP activity in MC3T3-E1 cells. For these experiments, keratin hydrogels and collagen sponges were prepared as described in section “Keratin and collagen carrier formulation and loading of samples with rhBMP-2” and had 150 μL of PBS placed on top. The only exception from the rhBMP-2 release experiment was that rhBMP-2 was not labeled with AF488-SE. As before, control keratin hydrogels or collagen sponges with no rhBMP-2 were used. At specified time points, the PBS supernatant was removed, placed in a separate vial, and stored at −80°C until further analysis.
ALP activity in MC3T3-E1 cell culture was assayed by fluorometry according to the manufacturer's instructions (BioVision, Milpitas, CA) and is reported as milliunits of ALP per unit volume of media (100 μL). Five thousand MC3T3-E1 cells were seeded per well of a 96-well plate. Cells were incubated overnight in α-MEM with 10% FBS and 1% P-S. Then, the media were removed, and 25 μL of releasate from the samples in 75 μL of α-MEM with 10% FBS and 1% P-S was added. After 3 days in culture with no media change, the cells were rinsed with PBS and the cell layers were lysed in 1% TritonX100 followed by addition of the ALP assay buffer and incubation with 4-methylumbelliferyl phosphate disodium salt (MUP) substrate to form 4-methylumbelliferone (4-MU). After 30 min of incubation, the stop solution was added and fluorescence was determined at excitation/emission of 360/460 nm on a Bio-Tek plate reader. The relative amount of ALP activity in each sample was determined from a standard curve for the formation of 4-MU from known amounts of MUP in the presence of the alkaline phosphate enzyme (Supplementary Fig. S1). Standards of known amounts of rhBMP-2 starting at 125 ng/mL and in 1:2 serial dilutions to a concentration of 3.9 ng/mL with a blank control of α-MEM with 10% FBS and 1% P-S were assayed for ALP activity in the same manner.
In these assays, we noted that keratin and collagen samples had decreased fluorescence compared with media-only controls or media containing rhBMP-2, possibly due to absorption by the keratin proteins at these wavelengths or due to actual decreases in ALP activity. We, therefore, used releasates from the keratin or collagen controls (without rhBMP-2) to subtract their signal and obtain the relative ALP signal of the keratin or collagen samples that contained rhBMP-2.
We also assessed cell viability in response to the soluble carrier (keratin or collagen) with or without rhBMP-2 to ensure that observations on ALP activity were not associated with cell toxicity. In this experiment, 5,000 MC3T3-E1 cells were seeded in 96-well plates and samples were added as in the ALP assay with 3-day incubation. After 3 days, medium was removed and replaced with 100 μL of fresh medium, including 10% FBS and 1% antibiotic/antimycotic and 25 μL of CellTiter 96 Aqueous Nonradioactive MTS (Promega, Madison, WI). After 1 h, absorbance was measured at 490 nm and values were scaled to cells that were cultured only in medium (100% relative viability).
Mouse model for assessing osteoinduction
We used a well-characterized, heterotopic, osteoinduction mouse model to assess the rhBMP-2 bioactivity in vivo.24 All animal experiments were conducted under the approval and guidance of the Virginia Commonwealth University Institutional Animal Care and Use Committee. KOS:KTN ratios of 100:0, 70:30, 50:50, 30:70, and 0:100 as well as collagen sponges were examined with or without (±) 0.67 μg rhBMP-2/mg material. Hydrogels and collagen with or without (±) rhBMP-2 were formulated as already described. Samples (25 μL gel; 1 cm2 collagen sponge) were placed in gelatin capsules (#5, maximum volume 0.13 mL, body/cap external diameter 4.68 mm/4.981 mm; Torpac, Fairfield, NJ) to prevent the movement of implants away from the implantation site.25 The capsules containing the formulations were then implanted into the gastrocnemius muscle of athymic nude mice, one implant per limb. Thus, each mouse received two implants, both of the same type to reduce any systemic influences, resulting in eight implants per group (n = 8 for each formulation in four different animals implanted bilaterally). The treatments for implantation were randomized.
Eight-week-old athymic male nu/nu mice were anesthetized by inhalation of 5% isoflurane in O2. Hind legs were disinfected using sequential swabs of isopropanol and chlorhexidine. Incisions were made on the skin over the gastrocnemius, and a pouch was prepared in the muscle using blunt dissection. Implants were inserted into the pouch and a single suture was placed to close the pouch. The skin was reapposed and closed using wound clips.
The mice were singly housed in sterile individually ventilated cages. They were given irradiated food and autoclaved water ad libitum and housed in 12 light/12 dark cycle. Implants were harvested after 35 days, which we have previously demonstrated to be sufficient to discriminate between even low levels of osteoinductive activity.26
Micro-CT analysis of heterotopic bone induction
New bone formation was assessed by micro-CT analysis at 35 days postimplantation. Animals were euthanized by CO2 asphyxiation. Hind limbs were disarticulated and fixed in 10% neutral buffered formalin. Bone formation (in mm3) at the implant site was determined by micro-CT (SkyScan 1172; Bruker, Germany) at 55 kVp, 145 μA, integration time of 200 ms, and a voxel size of 10 μm. For evaluation of micro-CT scans, we used a sigma value of 3.3, support value of 2, and threshold of 70, and the same settings were used for each scan.
Histology and histomorphometric analysis of heterotopic bone formation
Subsequent to fixation and acquisition of micro-CT scans, each tissue was decalcified in hydrochloric acid (DeCal; Fisher Scientific). Samples were embedded in paraffin, sectioned to 7 μm thickness, floated in a heated water bath, picked up on slides, dried at 37°C overnight, and stained using hematoxylin and eosin (H&E). Three consecutive cross sections (3–4 mm each) of the implant were obtained on the longitudinal axis of the sample. The slide with the largest cross-sectional implant area was selected for analysis. Qualitative assessment of osteoinduction was performed by two blinded independent observers and calibrated by an expert scorer (Z.S.). Samples were scored according to the following metrics: (i) material present without any bone; (ii) new bone formed at one site within the section that covers <40% of the surface area examined; (iii) new bone formed at more than one site and/or bone that covers >40% but <70% of the cross-sectional area; and (iv) new bone at more than one site that covers >70% of the cross-sectional area.25–27 A score of 0 was used for specimens in which no material or bone was found. When the observers' ratings disagreed, a third observer (S.L.H.) evaluated the disputed sections. Scores were averaged for all implants in each group, and scores >1 were considered osteoinductive. Quantitative histomorphometric analysis was performed on the same histological section as described previously.26,28 Images were captured at 10 × (Zeiss AxioVision Microscope; Carl Zeiss) and evaluated using ZenBlue software. Total bone area, cortical bone area, marrow area, and area of residual material were measured. Our mouse model and histological methods conformed to ASTM standard F2529-13.29
In addition to H&E staining, sections were also stained using Masson's trichrome.30 As described for H&E, samples were embedded in paraffin and sliced to 7 μm thickness. Sections were deparraffinized, rehydrated, and rinsed in distilled water. Bouin's solution was heated to 56°C and then used to mordant the sections for 1 h. Slides were allowed to return to room temperature and then washed with tap water for ∼5 min, and then they were rinsed in distilled water. Sections were then stained in Biebrich's scarlet-acid fuchsin for 5 min and rinsed in distilled water. Phosphomolybdic-phosphotungstic acid was applied to the sections for 1 min. Sections were then stained in aniline blue for 5 min and then rinsed in 1% acetic acid solution for 5 min. Stained sections were dehydrated in 95% and 100% alcohol, twice each, cleared with two changed of xylene and then mounted with a coverslip.
Statistical analysis
For in vitro studies, data were analyzed using one-way analysis of variance (ANOVA) with a post hoc Tukey's test. p-Values <0.05 were considered statistically significant. In vivo data are means ± standard error of the mean, with N being the number of implant sites. Sample size8 was determined assuming alpha of 0.05, power 0.8, and effect size 30%, using of a standard deviation of 0.2 from previous experiments. Thus, a sample size of eight implants was found to be appropriate. Data were analyzed using ANOVA with post hoc Bonferroni's modification of Student's t-test.
Results
In vitro keratin hydrogel erosion and release of rhBMP-2
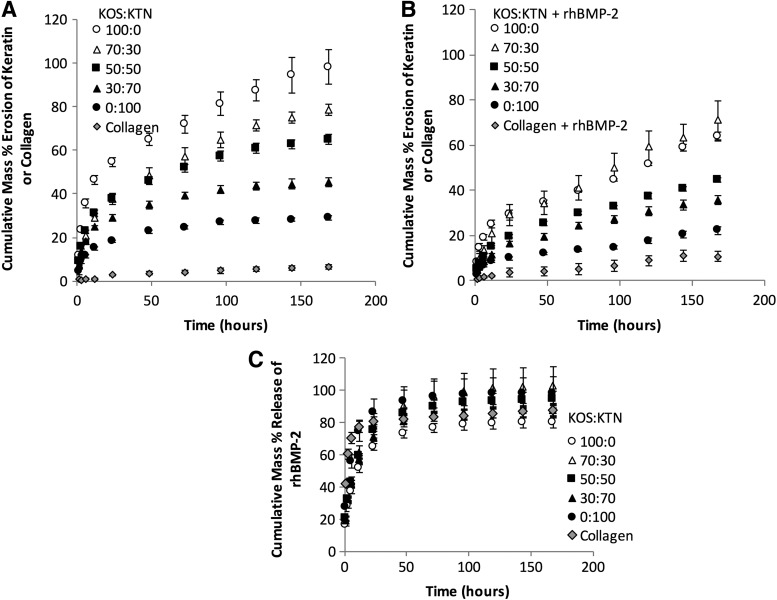
As shown in Figure 1A and B (without or with rhBMP-2, respectively), the erosion profiles of various keratin hydrogel formulations were consistent with our previously published results13 where formulations containing more KTN (higher levels of disulfide cross-linking) degraded more slowly than formulations with more KOS (lower levels of disulfide cross-linking). We analyzed these data based on percentage total erosion at each time point as well as the rate of erosion by fitting the equation of Ritger and Peppas.22 Owing to the large number of time points and samples, we summarize the statistical analysis of keratin percentage erosion in samples without presence of rhBMP-2 as follows: (i) collagen was significantly different than all keratin formulations at all time points; (ii) KOS was significantly different than all other formulations at all time points; (iii) the 50:50 formulation was significantly different than KOS and KTN alone at all time points; (iv) each keratin formulation was significantly different than its closest formulations (e.g., 50:50 compared with 30:70 and 70:30) with the exception that 50:50 and 70:30 formulations did not become statistically different until the 144 h time point. This trend is also reflected in the k values in Table 1 for samples without rhBMP-2 where increasing amounts of KTN led to decreasing k values.
FIG. 1.
Cumulative percentage erosion of keratin hydrogels as a function of time for (A) gels not loaded with rhBMP-2. (B) Cumulative percentage erosion of keratin hydrogels as a function of time for gels loaded with rhBMP-2. (C) Cumulative release of AF488-labeled rhBMP-2 from keratin hydrogel carriers as a function of time. Error bars indicate standard deviation (n = 3). Full statistical description provided in the “Results” section. rhBMP-2, recombinant human bone morphogenetic protein 2.
Table 1.
Nonlinear Regression Parameter Estimates for Keratin and Collagen Erosion in Absence or Presence of rhBMP-2
| Formulation | Rate coefficient parameter, k, ±SEM (1/min) | Diffusion exponent, n, ±SEM | R2value of fit |
|---|---|---|---|
| KOS | 0.178 ± 0.002a | 0.333 ± 0.016# | 0.98 |
| 70:30 KOS:KTN | 0.100 ± 0.013b | 0.414 ± 0.018# | 0.99 |
| 50:50 KOS:KTN | 0.125 ± 0.006b | 0.329 ± 0.003# | 0.99 |
| 30:70 KOS:KTN | 0.108 ± 0.008b | 0.292 ± 0.019# | 0.97 |
| KTN | 0.069 ± 0.007b,c | 0.293 ± 0.018# | 0.97 |
| Collagen | 0.004 ± 0.002d | 0.545 ± 0.040# | 0.97 |
| KOS with rhBMP-2 | 0.08 ± 0.010*,b,c,e | 0.426 ± 0.387# | 0.97 |
| 70:30 KOS:KTN with rhBMP-2 | 0.053 ± 0.014*,c,e,f | 0.544 ± 0.453# | 0.99 |
| 50:50 KOS:KTN with rhBMP-2 | 0.048 ± 0.001*,c,f,g | 0.431 ± 0.433# | 1.0 |
| 30:70 KOS:KTN with rhBMP-2 | 0.039 ± 0.004*,c,f,g | 0.450 ± 0.405# | 1.0 |
| KTN with rhBMP-2 | 0.028 ± 0.004*,d,f,g,h | 0.455 ± 0.385# | 0.97 |
| Collagen with rhBMP-2 | 0.004 ± 0.003d,h | 1.185 ± 0.538* | 0.95 |
Based on model described by Ritger and Peppas.22 Although the geometry is not the same, R2 values indicate very good fit of the data.
p < 0.05 compared with the same formulation without rhBMP-2.
In the second column, the same letters indicate statistical equivalence (p > 0.05; not significantly different).
In the third column, #p < 0.05 compared with collagen with rhBMP-2. ANOVA with Tukey's test was used for all comparisons for n = 3.
ANOVA, analysis of variance; KOS, keratose; KTN, kerateine; rhBMP-2, recombinant human bone morphogenetic protein 2; SEM, standard error of the mean.
We observed a reduction in the rates of erosion in the presence of rhBMP-2 (Fig. 1A, B) compared with samples without rhBMP-2, particularly in 100:0 KOS:KTN (100% KOS). We also used the model of Ritger and Peppas to compare the k values for formulations without and with rhBMP-2, which are also given in Table 1 (R2 values >0.95). Complete statistics are given in Table 1, but the key statistic is that each keratin formulation with rhBMP-2 had a statistically lower rate parameter (k) than its control formulation without rhBMP-2 (indicated by * for p < 0.05). However, the rate constant for collagen was not affected by the presence of rhBMP-2.
The diffusional exponent, n, is provided for completeness and is an indicator of the mechanism of drug release. Because the geometry of our system was irregular, we could not comment on the type of diffusion mechanism, but we observed that none of the keratin formulations (with or without rhBMP-2) were statistically different from each other. The only statistical difference was that all keratin formulations were different than collagen with rhBMP-2.
The release profiles of rhBMP-2 from these materials shown in Figure 1C were well fit (R2 values >0.97 for all keratin formulations and 0.85 for collagen) by the model of Fu and Kao (Table 2) for short- and long-term release. We, therefore, performed statistical analysis on both percentage release of rhBMP-2 and diffusion parameters from the Fu and Kao model. Based on percentage rhBMP-2 release, none of the formulations were statistically different from each other after 24 h. There was some evidence of a more burst release type of behavior for the collagen and KTN (0:100). This is indicated at the early time points (0–12 h) wherein percentage release of rhBMP-2 from collagen was statistically greater than all of the keratin formulations except at 12 h compared with KTN. Also at the early time points (0–-12 hours), the percentage rhBMP-2 release from the KTN formulation was statistically different than the other keratin formulations, with the following exceptions: (i) 50:50 and 70:30 at 3 h; (ii) 70:30 at 6 h; and (iii) 30:70, 50:50, and 70:30 at 12 h. These observations on percentage release match those from the nonlinear regression fit of the data wherein the diffusion parameters of all the keratin formulations were significantly different from those of collagen, but not significantly different from each other in the early (through 60% release) and late phases. The rhBMP-2 diffusion parameter was statistically different for KTN than for the other keratin formulations at the late phase (Table 2).
Table 2.
Nonlinear Regression Fit Parameter Estimates for rhBMP-2 Release
| Formulation |
Short-term diffusion parameter,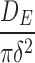 , ±SEM (1/min) , ±SEM (1/min)
|
Long-term diffusion parameter,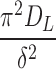 , ±SEM (1/min) , ±SEM (1/min)
|
R2value of fit |
|---|---|---|---|
| KOS with rhBMP-2 | 1.43 × 10−3 ± 1.28 × 10−4*,# | 1.17 × 10−2 ± 4.46 × 10−4*,# | 0.99 |
| 70:30 KOS:KTN with rhBMP-2 | 2.02 × 10−3 ± 2.56 × 10−4* | 9.61 × 10−3 ± 8.01 × 10−4*,# | 0.98 |
| 50:50 KOS:KTN with rhBMP-2 | 1.90 × 10−3 ± 9.33 × 10−5* | 1.08 × 10−2 ± 5.63 × 10−4*,# | 0.99 |
| 30:70 KOS:KTN with rhBMP-2 | 1.79 × 10−3 ± 1.63 × 10−4* | 1.14 × 10−2 ± 3.37 × 10−4*,# | 0.99 |
| KTN with rhBMP-2 | 3.15 × 10−3 ± 4.89 × 10−4* | 9.41 × 10−2 ± 9.93 × 10−3* | 0.97 |
| Collagen with rhBMP-2 | 6.2 × 10−3 ± 5.16 × 10−4# | 0.276 ± 0.011# | 0.85 |
Based on model described by Fu and Kao.23 Owing to geometrical differences where δ is unknown in our system, the short- and long-term diffusion parameters were computed as a lumped parameter.
p < 0.05 compared with collagen with rhBMP-2.
p < 0.05 compared with KTN with rhBMP-2 as determined by ANOVA with Tukey's test for n = 3.
In vitro bioactivity of released rhBMP-2 determined by ALP activity in MC3T3-E1 cells
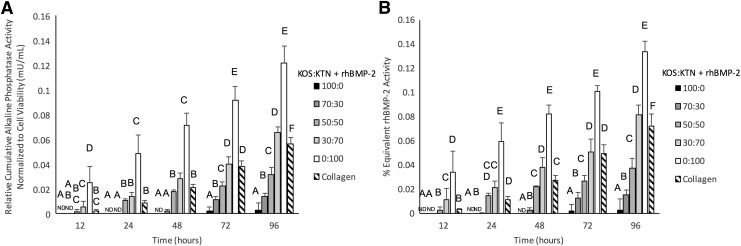
Although release rates were similar, we were interested in determining whether the different formulations had any effect on the bioactivity of the released rhBMP-2 as a function of time. rhBMP-2 released from keratin gels or collagen sponges had higher signal than the control sponges, an indication of its bioactivity (Fig. 2). We observed that the signals for releasates containing no rhBMP-2 for both keratin and collagen were lower than signal obtained from “pristine” (not previously loaded into hydrogels or sponges) rhBMP-2 used to generate the standard curve relating rhBMP-2 concentration to ALP activity, shown in Supplementary Figure S1A. This may be due to absorption of the fluorescence signal by the proteins. It is also possible that these formulations decreased alkaline phosphatase activity as keratins are known to increase Peroxisome proliferator-activated receptor gamma (PPAR-gamma), which could decrease differentiation of the MC3T3-E1 preosteoblasts and their subsequent expression of ALP.31 Because of the lower signals, we subtracted the contribution from the keratin or collagen controls (no rhBMP-2) to determine the contribution of the released rhBMP-2 to the ALP activity. For this reason, the ALP activity shown in Figure 2A is reported as “relative” activity (contribution of controls subtracted). To ensure the observed differences in ALP activity, we normalized to cell viability (cell viability data shown in Supplementary Fig. S2).
FIG. 2.
(A) Relative (to control groups without rhBMP-2) cumulative alkaline phosphatase bioactivity normalized to cell viability after release from keratin hydrogels or collagen sponges as determined by alkaline phosphatase activity in MC3T3-E1 cell line, reported as mU/mL of activity of the alkaline phosphatase enzyme by comparison with a standard curve. (B) Equivalent rhBMP-2 activity indicates the amount of rhBMP-2 that would achieve the levels of alkaline phosphatase activity from (A) (as determined by standard curve shown in Supplementary Fig. S2) divided by the amount of rhBMP-2 release from Figure 1A to give the percentage activity. Percentage activities are very low, indicating that most of the rhBMP-2 released was not bioactive in this assay. Groups indicated with same letters show no statistical difference (p > 0.05); otherwise the groups were statistically different, as determined by Tukey's test. ND indicates that no activity above baseline was detected. Error bars indicate standard error of the mean (n = 3).
We further evaluated the bioactivity of the released rhBMP-2 as shown in Figure 2B. The amount of ALP activity was converted to the equivalent amount of “pristine” (previously unused or not loaded into materials) rhBMP-2 that would achieve the observed signal (Supplementary Fig. S1B). We then divided this by the amount of rhBMP-2 released shown in Figure 1C to estimate the percentage of rhBMP-2 that was bioactive. In this assay, the bioactive rhBMP-2 made up <0.1% of the total rhBMP-2 released at the same time points (Fig. 2B).
A key finding of the results shown in Figure 2 is that the stimulatory effect on ALP activity varied with carrier type. In general, the bioactivity of rhBMP-2 in the gel releasate increased with increasing levels of disulfide bonds (i.e., formulations with greater amounts of KTN). More specifically, the rhBMP-2 releasate from hydrogels containing KTN (0:100) had significantly greater bioactivity than releasate from the other keratin formulations and collagen (with rhBMP-2) at all time points, and the bioactivity of the 30:70 KOS:KTN with rhBMP-2 formulation was higher than that of collagen with rhBMP-2 at 12 h, statistically equivalent through 72 h, and also greater at 96 h. The bioactivity of 50:50 KOS:KTN formulation (with rhBMP-2) was staistically equivalent to that of collagen through 48 h. The ALP signals for KOS and 70:30 KOS:KTN with rhBMP-2 were not detectable (ND in Fig. 2) until 72 h and 48 h, respectively.
Micro-CT analysis of bone volume in mouse heterotopic bone growth model
Micro-CT images of keratin gels with rhBMP-2 at 5 weeks after implantation showed that osteoinduction varied with formulation. Figure 3 shows representative micro-CT images of implant sites of the materials with (+) or without (−) rhBMP-2. Qualitatively, both the amount and appearance of heterotopic bone were different among the collagen and various keratin formulations. KOS with rhBMP-2 resulted in a radioopaque material that is morphologically similar to collagen with rhBMP-2 (Fig. 3A, B). KTN+rhBMP-2 implants (Fig. 3C) generated greater amounts of bone than KOS+rhBMP-2, and the bone was more porous. In contrast, the keratin mixtures containing rhBMP-2 (Fig. 3D–F) produced greater bone volume, which was globular in form. No heterotopic bone was evident in any of the sites implanted with keratin gels or collagen without rhBMP-2 (Fig. 3G–L).
FIG. 3.
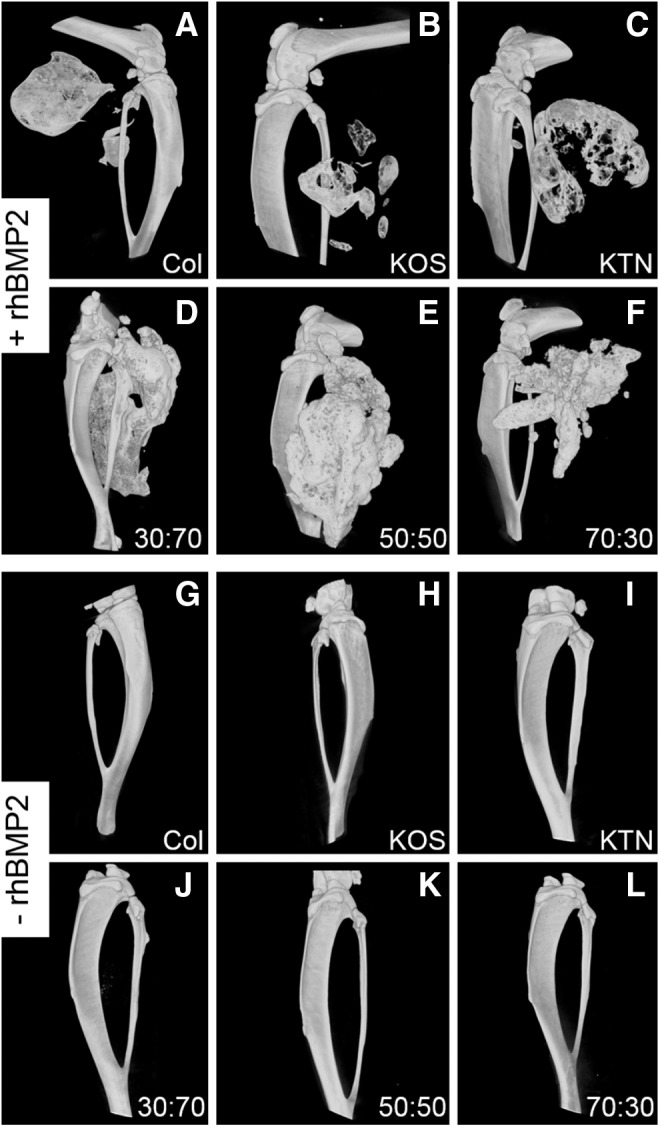
Representative micro-CT images of bone formation in the mouse heterotopic model using keratin hydrogels or collagen sponges with (+) rhBMP-2) or without (−) rhBMP-2). (A) Collagen+rhBMP-2. (B) KOS+rhBMP-2. (C) KTN+rhBMP-2. (D) 30:70 KOS:KTN+rhBMP-2. (E) 50:50 KOS:KTN+rhBMP-2. (F) 70:30 KOS:KTN+rhBMP-2. (G) Collagen only. (H) KOS only. (I) KTN only. (J) 30:70 KOS:KTN only. (K) 50:50 KOS:KTN only. (L) 70:30 KOS:KTN only. Keratin formulations are indicated as KOS:KTN ratio by weight percentage. KOS, keratose; KTN, kerateine.
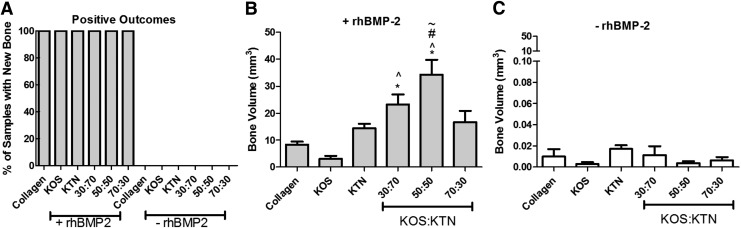
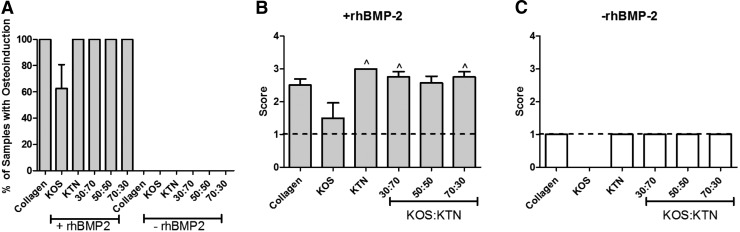
These observations were confirmed by quantitative analysis of the micro-CTs. Only samples containing rhBMP-2 led to bone formation (i.e., positive outcomes), as evidenced by the fact that all samples (eight of eight samples) that carried rhBMP-2 elicited new bone formation, whereas samples that lacked rhBMP-2 had no positive outcomes (no sample of eight samples)(see Fig. 4A). Heterotopic bone formation was dependent on KOS:KTN formulation, with the greatest bone volumes observed in sites implanted with 30:70 and 50:50 KOS:KTN compositions (Fig. 4B). Bone volume at sites implanted with keratin gels that did not contain rhBMP-2 was <1% of the volumes measured in samples that contained rhBMP-2 (note the difference in y-axis of Fig. 4C compared with that of Fig. 4B). Furthermore, there was no statistical difference among any of the formulations without rhBMP-2.
FIG. 4.
Quantitative analysis of micro-CT images showing (A) percentage of samples with new bone, (B) bone volume in samples with (+) rhBMP-2, and (C) bone volume in samples without (−) rhBMP-2. *p < 0.05 versus collagen; ^p < 0.05 versus KOS; #p < 0.05 versus KTN; ∼p < 0.05 versus 30:70 KOS:KTN. There were no significant differences between any of the samples without rhBMP-2. Error bars indicate standard error of the mean (n = 8; bilateral from four animals).
The clinical standard of collagen with rhBMP-2 led to an average bone volume of 8.34 mm3. KOS with rhBMP-2 implants generated 3.05 mm3 average bone volume whereas KTN with rhBMP-2 implants generated 14.41 mm3 average bone volume, but neither of these formulations (KOS or KTN) was significantly different than the collagen with rhBMP-2 formulation. Despite a higher average bone volume of 16.69 mm3 induced by 70:30 KOS:KTN with rhBMP-2, the volume is not statistically different than that of collagen. In contrast, the average bone volume obtained with 30:70 KOS:KTN with rhBMP-2 (23.37 mm3) was significantly higher than that of KOS and collagen with rhBMP-2. Finally, the 50:50 KOS:KTN with rhBMP-2 formulation produced the largest average bone volume (34.32 mm3), and this value was statistically higher than all other formulations, except for the 30:70 KOS:KTN with rhBMP-2 formulation. Our findings are confirmed by qualitative images shown in Figure 3.
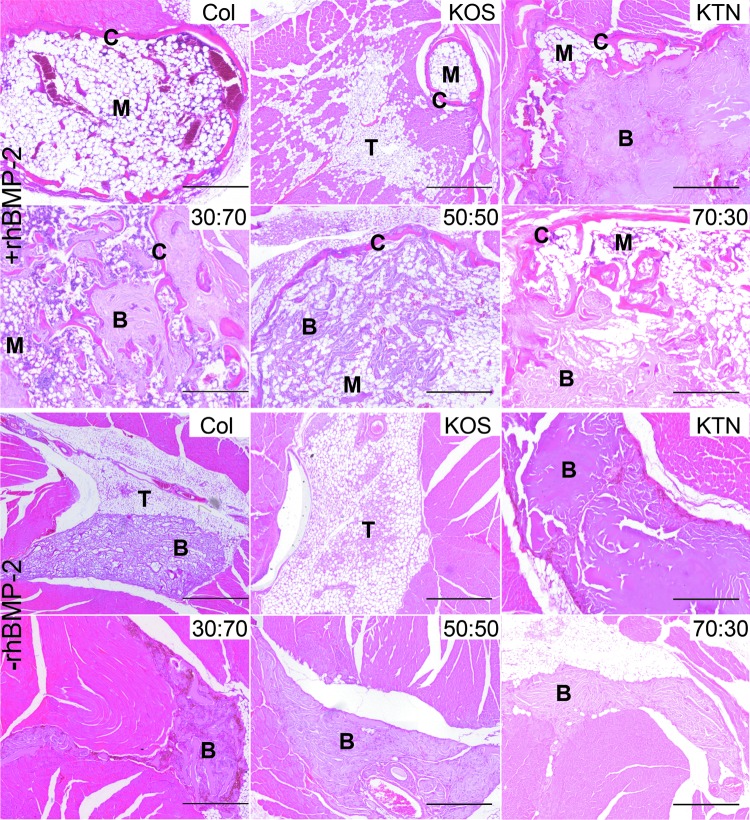
Histological evaluation of heterotopic bone
The H&E images of explanted tissues shown in Figure 5 illustrate that keratin gels containing rhBMP-2 helped in the formation of new bone (see also Supplementary Fig. S3 for Masson's trichrome staining of similar sections after each treatment). However, the appearance of bone tissues differed depending on the carrier system used. For all samples except KOS alone, residual biomaterial (keratin or collagen) was present to some degree and thus, our quantification of new bone formation included residual material where present. When rhBMP-2 was used, regions of marrow and cortical bone were seen, but the amounts of each varied with the formulation. Except for KOS+rhBMP-2, all samples containing rhBMP-2 showed evidence of osteoinduction (Fig. 6A), whereas none of the samples that lacked rhBMP-2 was osteoinductive. Although KOS+rhBMP-2 had an osteoinduction score >1, confirming it had osteoinductive activity, all other keratin formations with rhBMP-2 produced scores >2 (Fig. 6B). KTN, 30:70 and 70:30 implants (with rhBMP-2) resulted in scores of approximately 3. These values were significantly greater than those of the KOS (with rhBMP-2) formulation and were consistent with qualitative findings from micro-CT data. No samples without rhBMP-2 exhibited evidence of heterotopic bone although residual biomaterial was present (Fig. 6C). Although a system was in place for interevaluator variance for scoring, we note that there was no difference in the scoring of the two blinded observers.
FIG. 5.
Representative H&E images of explanted tissues from the mouse heterotopic bone growth model for keratin hydrogels or collagen sponges with (+) rhBMP-2 or without (−) (rhBMP-2). Keratin formulations are indicated as KOS:KTN ratio by weight percentage. B, residual biomaterial (keratin or collagen); C, cortical bone; M, marrow; T, connective tissue; H&E, hematoxylin and eosin. Scalebars indicate 1 mm.
FIG. 6.
Morphometric analysis of H&E images for assessment of bone quality. (A) Percentage of samples showing osteoinduction, (B) bone rating score for samples with (+) rhBMP-2, and (C) bone rating score for samples without (−) rhBMP-2. Error bars indicate standard error of the mean (n = 8; bilateral from four animals) and ^p < 0.05 compared with KOS.
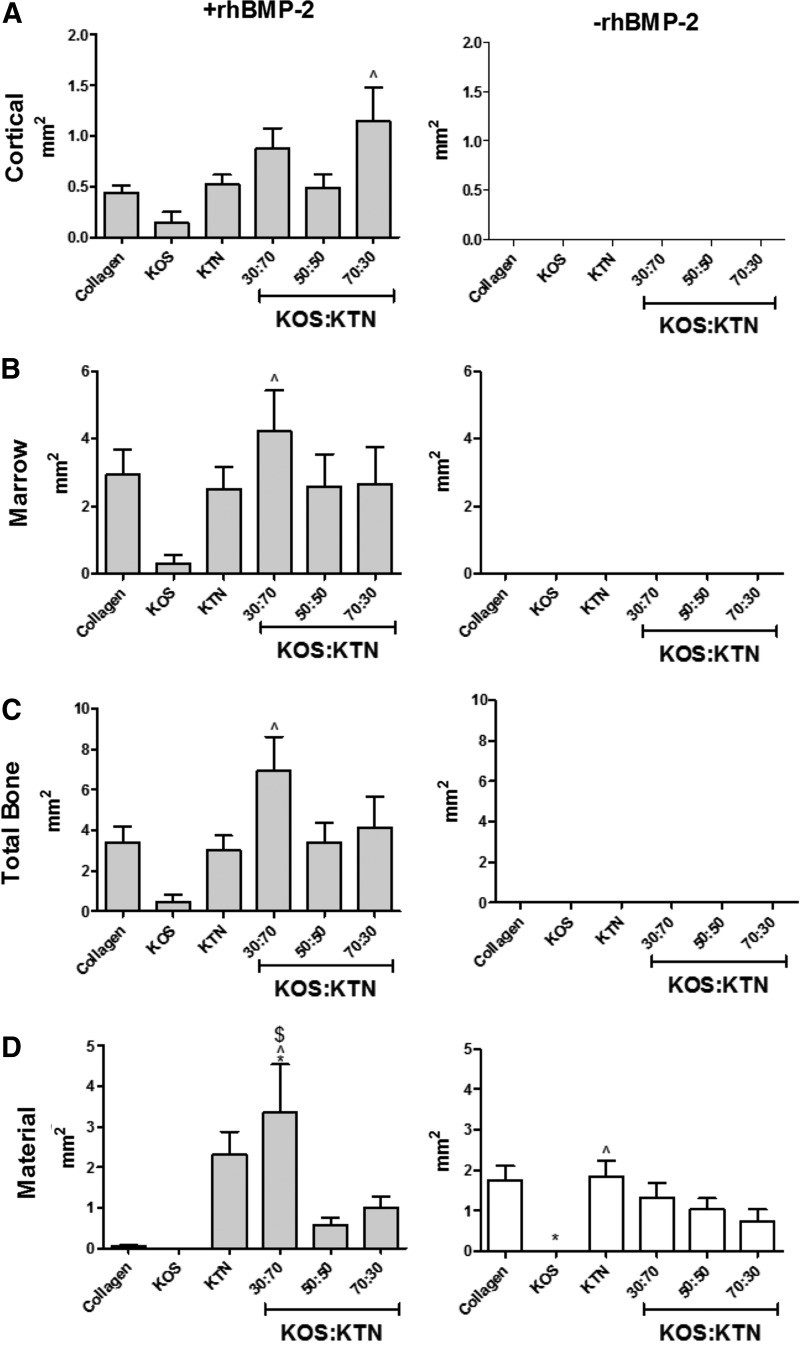
Histomorphometric analyses of the sections from sites implanted with hydrogels + rhBMP-2 showed that the amount of cortical bone was greatest in sites implanted with KOS:KTN 70:30 (Fig. 7A), whereas the amount of marrow was greatest in sites implanted with KOS:KTN 30:70 (Fig. 7B). This resulted in the 30:70 sites having the largest total bone (marrow plus cortical bone; see Fig. 7C). The most residual implant material was also in the 30:70 sites (Fig. 7D). In the absence of rhBMP-2, sites implanted with KTN alone had the greatest amount of residual material, whereas no residual material was evident in sites implanted with KOS alone. As shown in Figure 5 and Supplementary Figure S3 (and discussed hereunder), some regions of bone formation contained residual material (e.g., KTN), which is why total bone exceeded the sum of cortical and marrow bone in some cases. Addition of the residual material leads to the total bone area in these cases.
FIG. 7.
Morphometric analysis of H&E images for determination of the area of (A) cortical bone, (B) marrow-like bone, (C) total bone, and (D) residual biomaterial (keratin or collagen). ^p < 0.05 versus KOS; $p < 0.05 versus 50:50 KOS:KTN; *p < 0.05 versus collagen. Error bars indicate standard error of the mean (n = 8; bilateral from four animals).
Discussion
Spatial and temporal control of growth factor delivery are essential to the success of engineered tissues. A faithful recapitulation of these processes could guide the formation of de novo tissue in a manner reminiscent of bone formation during embryonic development. BMP-2 is both sufficient and necessary for bone tissue formation,32 and the delivery of its recombinant form from collagen carriers is among the best clinical examples of the power of growth factors in tissue engineering strategies.33 The complications with rhBMP-2 delivery from collagen carriers are well documented,21,34 so bone regeneration provides both an excellent system to assess novel delivery carriers and an opportunity to meet a clinical need.
We and other groups have been investigating keratin biomaterials as a carrier system for growth factors in tissue engineering because these natural polymers are biodegradable,35 elicit an M2 macrophage polarization (anti-inflammatory) response,36 and promote cell attachment.37,38 Keratins have been used in bone-related studies, although only as a single form (e.g., KOS or KTN) and either alone39,40 or as a carrier for rhBMP-2.6,7,41 The relatively high presence of cysteine residues also offers an inherent system for tuning the rate of material degradation through disulfide cross links. Chemical methods have been used to “cap” thiol groups on cysteine residues to control disulfide cross-linking and associated material properties.42,43 However, a more straightforward approach is to mix KOS and KTN (obtained by different means of extraction) to tune the disulfide cross-linking levels.13 We hypothesized that the rates of the keratin biomaterial degradation would impact bone formation mediated by rhBMP-2 delivery.
In previous studies, we have observed correlation between the rate of scaffold degradation and growth factor delivery for several growth factors, including IGF-1, basic fibroblast growth factor, and vascular endothelial cell growth factor.15,42 Based on other findings indicating relatively high binding affinity between rhBMP-2 and certain fractions of keratin16,42 and the larger molecular weight of rhBMP-2, we had originally hypothesized that a similar result could be achieved with rhBMP-2. Surprisingly, we found little difference in the rates of rhBMP-2 release from the various keratin formulations in vitro despite significant differences in the rate of keratin hydrogel erosion. Although the reasons for this behavior are unclear, it is possible that interaction of rhBMP-2 in a two-dimensional coating system like those used in previous studies16,42 is different than that in three-dimensional hydrogels used in this report. Differences in the keratin conformation and subsequent changes in binding affinity could explain this observation. Indeed, most of the rhBMP-2 was released from the keratin hydrogels within 48 h, suggesting diffusion-based release, and a nonlinear regression of the data to the model of Fu and Kao23 showed a high goodness of fit. In this study, we used only the alpha fractions of keratin, but future studies to investigate effects of gamma fractions on the release of rhBMP-2 (and other growth factors) may prove useful.
Nonetheless, some role for molecular interactions between rhBMP-2 and keratin is evident in the decreased rates of keratin hydrogel erosion in the presence of rhBMP-2. Keratin erosion was 10% lower in the 70:30 KOS:KTN formulation (with rhBMP-2 compared with without rhBMP-2) but as large as 35% lower in the KOS formulations (with rhBMP-2 compared with without rhBMP-2). The absolute percentage reduction was lowest in the KTN formulations, which interestingly are the more hydrophobic of the formulations. This result is borne out by the estimate of the rate parameter, k, in the nonlinear regression of Ritger and Peppas,22 where the k values for formulations with rhBMP-2 were about half those of the same formulations without rhBMP-2. Unlike keratin, rhBMP-2 had no statistically significant effect on the collagen rate parameter. It seems unlikely that this effect was due to disulfide interactions between rhBMP-2 and KOS because KOS has few thiol groups. Rather, it is more likely that the presence of rhBMP-2 enhanced hydrophobic interactions within the keratin hydrogels.
The differences in the erosion rate of the hydrogels themselves indicated that the release of rhBMP-2 was not dependent simply on the erosion rate of the keratin carrier. Given the unexpected difference in rhBMP-2 release from the keratin hydrogels, we chose to investigate the role of the keratin materials (and collagen sponges) on rhBMP-2 bioactivity in vitro and, later, bone formation in vivo. We hypothesized that different formulations of keratin may have an impact on the bioactivity levels of the released rhBMP-2. More specifically, because rhBMP-2 release rates in vitro were similar, significant differences in bioactivity in vitro may be attributed to the differences in the materials themselves but not to the release rate of the rhBMP-2. Because we used the control keratin hydrogels or collagen sponges without rhBMP-2 to determine the relative ALP activity, differences in the bioactivity of the releasate would represent only the effect of rhBMP-2 but not the erosion byproducts. We do note that although serum can lead to ALP activity, any activity associated with the serum was subtracted out when we indicate data are for relative activity.
The results of the in vitro assay revealed significant differences in the bioactivity of rhBMP-2 for different keratin formulations. Increasing levels of KTN led to higher levels of bioactivity as indicated by ALP activity across times from 12 h to 4 days. To ensure that these differences were not due to toxicity (e.g., from the presence of soluble keratin released from eroding hydrogel samples with more KOS), we normalized the data to cell viability. Indeed, the two- to threefold increase in ALP activity achieved with KTN is not explained by toxicity.
We also used ALP activity (Fig. 2A) in conjunction with the release data (Fig. 1C) to compare the ALP activity of rhBMP-2 released from keratin or collagen to the equivalent amount of previously unused rhBMP-2 required to achieve this level of activity. By using the rhBMP-2 release data, we were able to determine if the differences in ALP activity were due to the small differences in the release. These results demonstrate that differences in ALP activity are not attributable to the small differences in the release of rhBMP-2 from the formulations as a similar pattern emerges even when normalizing by rhBMP-2 release. However, these results show an extremely low level of bioactivity from the rhBMP-2 released not only from keratin but also from the clinical collagen sponges, with only ∼0.13% of the released rhBMP-2 being bioactive for the best formulation (KTN), which was nearly double that of the clinical collagen sponge (0.07% bioactivity).
We have not specifically investigated the mechanisms by which these observations may be explained. It is particularly surprising that although 75–95% of the rhBMP-2 release (depending on the keratin or collagen formulation) has occurred by 72 h, we observed continued bioactivity even after 4 days. Formulations with the most KTN content (0:100 KOS:KTN and 30:70 KOS:KTN) are the most hydrophobic in nature and may, therefore, shield rhBMP-2 from hydrolytic degradation. Although it is also possible that rhBMP-2 remains associated with the keratin after release, we have not found evidence of interaction between keratins and drug molecules after release.44 Based on the low bioactivity, we also cannot rule out the possibility that more bioactive rhBMP-2 remains associated with the keratin and collagen materials for longer periods of time.
The differences in the bioactivity of the releasate may explain, in part, differences in the amount of bone induced based on micro-CT and histology/histomorphometry. Implants that lacked rhBMP-2 did not lead to bone formation, and this result is consistent with previous observations that keratin itself is not osteoinductive.7 Although all formulations with rhBMP-2 induced new bone formation, there was a clear difference in the appearance of the bone in the three-dimensional micro-CT renderings. Differences in the morphology of the heterotopic mineralized tissue reflected differential rates of hydrogel erosion together with retention of residual biomaterial at the implant site. Implants with higher KTN content resulted in greater physical mass on micro-CT, greater osteoinduction scores, and greater retention of carrier at time of harvest.
Histological images also showed differences in the quality of the heterotopic bone. As expected, collagen with rhBMP-2 induced ossicles with a typical morphology: well-developed cortical bone surrounding the marrow. KOS+rhBMP-2 implants also supported formation of a classical ossicle, but it was much smaller than ossicles generated by collagen implants. Implants consisting of KTN alone or KOS:KTN formulations resulted in ossicles that were more irregular in shape, due, in part, to interference with residual material. Those materials that eroded quickly, such as collagen and KOS, were replaced by fatty connective tissue rather than regenerated muscle, whether rhBMP-2 was present or not. Sites implanted with hydrogels containing KTN had residual material. In fact, at higher KTN fractions (and lower KOS fractions), the ossicles formed around the material, whereas at lower KTN fractions (and higher KOS fractions), remnants of hydrogel were incorporated into the ossicle, indicating greater erosion and affecting measures of total bone. These differences in erosion also affected tissues in the absence of rhBMP-2, with fatty connective tissue being present in sites treated with collagen and KOS alone, but accommodation of surrounding muscle in sites implanted with KTN and KTN formulations.
It is interesting to contrast the effects of rhBMP-2 on scaffold erosion in vivo compared with the in vitro situation and consider the resulting amount of bone formation. In vitro studies showed that hydrogels eroded more slowly when rhBMP-2 was present, regardless of the formulation and that erosion was more rapid for formulations containing less KTN (more KOS). However, in vivo, the pattern was much less distinct. The presence of rhBMP-2 led to less residual material for collagen and the 50:50 KOS:KTN formulation (Fig. 7), but higher amounts of the 30:70 formulation. This effect of rhBMP-2 to alter carrier degradation in vivo has been noted previously in studies assessing osteoinduction using a variety of carriers and was attributed to the stimulatory effects of the growth factor on inflammation.45 Here, the presence of more bone tissue (cortical and marrow) around the 30:70 and KTN formulations with rhBMP-2 may have delayed their resorption.
Beyond erosion, there are other properties of the keratin hydrogel materials that could affect ossicle formation. For example, we have previously shown that KOS and keratin materials with lower levels of disulfide cross-linking swell more than KTN in vitro.42 Although this did not impact in vitro rhBMP-2 release, it is conceivable that in vivo rhBMP-2 release differs for different keratin formulations. Conversely, based on the in vitro bioactivity results, it is also possible that the carrier itself affects the rhBMP-2 bioactivity (i.e., carriers with more KTN achieving greater bioactivity), which would impact the amount of ossicle formation. Given that the intermediate formulations (30:70 and 50:50 with rhBMP-2) led to more favorable outcomes than KOS or KTN alone, however, it seems reasonable to conclude that multiple factors are impacting bone formation in this model. We suggest that the observed in vivo effect of rhBMP-2 involves interactions of rhBMP-2 and the material carrier, thereby altering the nonenzymatic, indeed nonbiological, mechanisms of material degradation in an aqueous environment. Our findings are supported by another recent report that showed that the rhBMP-2 carrier has an effect on bone tissue formation.46
We further suggest that optimizing the balance between the rate of bone formation and carrier degradation is an important consideration. For example, although the KTN carrier provides a long-term matrix or “scaffold” upon which bone can form, its slow degradation may impede full formation of the bone, which could be achieved based on the amount of in vitro bioactivity. In contrast, the KOS formulation degrades rapidly such that the material itself does not impede bone formation, but there is a lack of suitable scaffold for cellular growth and infiltration. As such, formulations can be optimized by varying the levels of KOS and KTN (and, thus, disulfide cross-linking) as a means to strike a balance between material degradation and tissue formation rates. Importantly, this approach to mixing KOS and KTN allows modulation of the levels of (disulfide) covalent cross-linking without additional chemical modification after protein extraction.
The implications of these studies in the clinical scenario for bone regeneration are unclear. For example, the larger bone volume achieved with the 50:50 and 30:70 formulations may indicate that these formulations are more susceptible to known clinical problems with collagen carriers (e.g., ectopic bone formation). However, the profile of the resulting bone tissue may indicate that lower doses could be used to achieve similar results as existing clinical products. To better understand these effects, future studies in bone injury models are necessary. Our results also support previous studies that showed that keratin carriers of rhBMP-2 may be suitable alternatives to collagen sponges.6,7 An osteoconductive, proteinaceous natural polymer in combination with flexibility in the formulation of these materials offers a unique carrier platform.
Although the results of this study are specific to keratin carriers for rhBMP-2, there are two broad implications. First, in terms of bone regeneration with rhBMP-2 delivered from polymeric carriers, a key conclusion from these studies is that it is not only the release of the rhBMP-2 itself but also the carrier system that can impact bone regeneration profiles. Second, in the more general sense of growth factor delivery for tissue engineering strategies, these studies indicate that keratin biomaterials have unique features that make them suitable carriers. Namely, the ability to control the rate of scaffold degradation in a manner reminiscent of synthetic polymers allows matching to the rate of tissue regeneration or the size of any defect being treated. Yet, because keratin biomaterials are derived from natural proteins, they can support cell attachment processes through known integrin binding domains.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (J.M.S.; R01AR061391), and the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank Ms. Judy Bohnert for sample preparation and characterization for the in vitro studies, Mr. Lucas Olson for technical assistance with micro-CT scans, and Mr. Illya Kajan for technical assistance with surgical procedures.
Disclosure Statement
No competing financial interests exist.
References
- 1.Verma V., Verma P., Ray P., and Ray A.R. Preparation of scaffolds from human hair proteins for tissue-engineering applications. Biomed Mater 3, 025007, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Pace L.A., Plate J.F., Mannava S., et al. A human hair keratin hydrogel scaffold enhances median nerve regeneration in nonhuman primates: an electrophysiological and histological study. Tissue Eng Part A 20, 507, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker H.B., Passipieri J.A., Siriwardane M., et al. Cell and growth factor-loaded keratin hydrogels for treatment of volumetric muscle loss in a mouse model. Tissue Eng Part A 23, 572, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Passipieri J.A., Baker H.B., Siriwardane M., et al. Keratin hydrogel enhances in vivo skeletal muscle function in a rat model of volumetric muscle loss. Tissue Eng Part A 23, 556, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poranki D., Whitener W., Howse S., et al. Evaluation of skin regeneration after burns in vivo and rescue of cells after thermal stress in vitro following treatment with a keratin biomaterial. J Biomater Appl 29, 26, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Kowalczewski C.J., Tombyln S., Wasnick D.C., et al. Reduction of ectopic bone growth in critically-sized rat mandible defects by delivery of rhBMP-2 from kerateine biomaterials. Biomaterials 35, 3220, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Guzman R.C., Saul J.M., Ellenburg M.D., et al. Bone regeneration with BMP-2 delivered from keratose scaffolds. Biomaterials 34, 1644, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Burnett L.R., Rahmany M.B., Richter J.R., et al. Hemostatic properties and the role of cell receptor recognition in human hair keratin protein hydrogels. Biomaterials 34, 2632, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Reichl S. Films based on human hair keratin as substrates for cell culture and tissue engineering. Biomaterials 30, 6854, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Van Dyke M. Keratin. In: Neves N.M., and Reis R.L., eds. Biomaterials from Nature for Advanced Devices and Therapies. Hoboken, NJ: John Wiley & Sons, Inc., 2016, p. 93 [Google Scholar]
- 11.Hill P., Brantley H., and Van Dyke M. Some properties of keratin biomaterials: kerateines. Biomaterials 31, 585, 2010 [DOI] [PubMed] [Google Scholar]
- 12.de Guzman R.C., Merrill M.R., Richter J.R., Hamzi R.I., Greengauz-Roberts O.K., and Van Dyke M.E. Mechanical and biological properties of keratose biomaterials. Biomaterials 32, 8205, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Ham T.R., Lee R.T., Han S., et al. Tunable keratin hydrogels for controlled erosion and growth factor delivery. Biomacromolecules 17, 225, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roy D., Kowalczewski C., Hall R., et al. Antibiotic-loaded keratin hydrogels prevent infection in a full-thickness porcine excision wound. Tissue Eng Part A 20, S64, 2014 [Google Scholar]
- 15.Tomblyn S., Pettit Kneller E.L., Walker S.J., et al. Keratin hydrogel carrier system for simultaneous delivery of exogenous growth factors and muscle progenitor cells. J Biomed Mater Res B Appl Biomater 104, 864, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Guzman R.C., Tsuda S.M., Ton M.T., Zhang X., Esker A.R., and Van Dyke M.E. Binding interactions of keratin-based hair fiber extract to gold, keratin, and BMP-2. PLoS One 10, e0137233, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lysaght M.J., Jaklenec A., and Deweerd E. Great expectations: private sector activity in tissue engineering, regenerative medicine, and stem cell therapeutics. Tissue Eng Part A 14, 305, 2008 [DOI] [PubMed] [Google Scholar]
- 18.McKay W.F., Peckham S.M., and Badura J.M. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE Bone Graft). Int Orthop 31, 729, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Epstein N.E., and Schwall G.S. Costs and frequency of “off-label” use of INFUSE for spinal fusions at one institution in 2010. Surg Neurol Int 2, 115, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poeran J., Opperer M., Rasul R., et al. Change in off-label use of bone morphogenetic protein in spine surgery and associations with adverse outcome. Global Spine J 6, 650, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Epstein N.E. Complications due to the use of BMP/INFUSE in spine surgery: the evidence continues to mount. Surg Neurol Int 4, S343, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ritger P.L., and Peppas N.A. A simple equation for description of solute release I. Fickian and non-Fickian release from non-swellable devices in teh form of slabs, spheres, cylinders or discs. J Control Release 5, 23, 1987 [PubMed] [Google Scholar]
- 23.Fu Y., and Kao W.J. Drug release kinetics and transport mechanisms from semi-interpenetrating networks of gelatin and poly(ethylene glycol) diacrylate. Pharm Res 26, 2115, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lohmann C.H., Andreacchio D., Koster G., et al. Tissue response and osteoinduction of human bone grafts in vivo. Arch Orthop Trauma Surg 121, 583, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Schwartz Z., Hyzy S.L., Moore M.A., et al. Osteoinductivity of demineralized bone matrix is independent of donor bisphosphonate use. J bone Joint Surg Am 93, 2278, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz Z., Somers A., Mellonig J.T., et al. Ability of commercial demineralized freeze-dried bone allograft to induce new bone formation is dependent on donor age but not gender. J Periodontol 69, 470, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Schwartz Z., Weesner T., van Dijk S., et al. Ability of deproteinized cancellous bovine bone to induce new bone formation. J Periodontol 71, 1258, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Schwartz Z., Mellonig J.T., Carnes D.L., Jr., et al. Ability of commercial demineralized freeze-dried bone allograft to induce new bone formation. J Periodontol 67, 918, 1996 [DOI] [PubMed] [Google Scholar]
- 29.ASTM International. Standard Guide for In Vivo Evaluation of Osteoinductive Potential for Materials Containing Demineralized Bone (DBM). West; Conshohocken, PA: ASTM International, 2013 [Google Scholar]
- 30.Carson F.L. Histotechnology: A Self Instructional Text. Chicago: ASCP Press, 1990 [Google Scholar]
- 31.Lin C.-W., Yang K.-C., Chang N.-C., Tsai W.-B., Lou K.-L., and Yu J. Evaluation of adhesion, proliferation, and differentiation of human adipose-derived stem cells on keratin. J Polym Res 25, 40, 2018 [Google Scholar]
- 32.Wang E.A., Rosen V., D'Alessandro J.S., et al. Recombinant human bone morphogenetic protein induces bone formation. Proc Natl Acad Sci U S A 87, 2220, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mao A.S., and Mooney D.J. Regenerative medicine: current therapies and future directions. Proc Natl Acad Sci U S A 112, 14452, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tannoury C.A., and An H.S. Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine J 14, 552, 2014 [DOI] [PubMed] [Google Scholar]
- 35.Xu S., Sang L., Zhang Y., Wang X., and Li X. Biological evaluation of human hair keratin scaffolds for skin wound repair and regeneration. Mater Sci Eng C Mater Biol Appl 33, 2013, 648 [DOI] [PubMed] [Google Scholar]
- 36.Fearing B.V., and Van Dyke M.E. In vitro response of macrophage polarization to a keratin biomaterial. Acta Biomater 10, 3136, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Richter J.R., de Guzman R.C., and Van Dyke M.E. Mechanisms of hepatocyte attachment to keratin biomaterials. Biomaterials 32, 7555, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Taraballi F., Wang S., Li J., et al. Understanding the nano-topography changes and cellular influences resulting from the surface adsorption of human hair keratins. Adv Healthc Mater 1, 513, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Dias G.J., Mahoney P., Swain M., Kelly R.J., Smith R.A., and Ali M.A. Keratin-hydroxyapatite composites: biocompatibility, osseointegration, and physical properties in an ovine model. J Biomed Mater Res A 95, 1084, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Dias G.J., Peplow P.V., McLaughlin A., Teixeira F., and Kelly R.J. Biocompatibility and osseointegration of reconstituted keratin in an ovine model. J Biomed Mater Res A 92, 513, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Tachibana A., Nishikawa Y., Nishino M., Kaneko S., Tanabe T., and Yamauchi K. Modified keratin sponge: binding of bone morphogenetic protein-2 and osteoblast differentiation. J Biosci Bioeng 102, 425, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Han S., Ham T.R., Haque S., Sparks J.L., and Saul J.M. Alkylation of human hair keratin for tunable hydrogel erosion and drug delivery in tissue engineering applications. Acta Biomater 23, 201, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakata R., Osumi Y., Miyagawa S., Tachibana A., and Tanabe T. Preparation of keratin and chemically modified keratin hydrogels and their evaluation as cell substrate with drug releasing ability. J Biosci Bioeng 120, 111, 2015 [DOI] [PubMed] [Google Scholar]
- 44.Saul J.M., Ellenburg M.D., de Guzman R.C., and Van Dyke M. Keratin hydrogels support the sustained release of bioactive ciprofloxacin. J Biomed Mater Res A 98, 544, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Boyan B.D., Lohmann C.H., Somers A., et al. Potential of porous poly-D,L-lactide-co-glycolide particles as a carrier for recombinant human bone morphogenetic protein-2 during osteoinduction in vivo. J Biomed Mater Res 46, 51, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Krishnan L., Priddy L.B., Esancy C., et al. Delivery vehicle effects on bone regeneration and heterotopic ossification induced by high dose BMP-2. Acta Biomater 49, 101, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.