Abstract
Following spinal cord injury (SCI), function is lost below the level of injury due to axon damage and demyelination. Spinal progenitors, and more broadly neural stem cells, can promote the growth of axons through multiple mechanisms, yet their poor survival following transplantation has been limiting the ability to obtain functional effects. In this study, we investigated multichannel poly(lactide-co-glycolide) bridges, which reduce inflammation and promote axon regrowth, as a support for spinal progenitor survival and function at the injury epicenter. Specifically, we hypothesized that mouse embryonic day 14 (E14) spinal progenitors expressing enhanced green fluorescent protein (EGFP) would lead to regenerative gains compared to age-matched adult progenitor controls, which are expected to have similar regenerative capacity to the endogenous progenitors. E14spinal EGFP progenitors were transplanted into a lateral T9-10 hemisection and EGFP+ cells were evident in the bridge and contralateral tissue 8 weeks postinjury, with enhanced survival of E14 compared to adult transplants. Only E14 progenitor-loaded bridges increased axon regrowth compared to blank bridges, resulting in a 3.3-fold increase in axon density (1674 v 497 axons/mm2) and 3.6-fold increase in myelination (∼30% of axons) after 8 weeks. By 6 months, NeuN+ neural bodies were increased within the bridge region of mice transplanted with E14 progenitors. Mice receiving E14 transplants exhibited modest improvements in locomotion, including an earlier ability to perform ipsilateral stepping. The combination of bridges with E14 progenitors produced synergistic reparative gains, with early axon growth and remyelination followed by latent increases in neural bodies within the bridge.
Keywords: : spinal progenitor cells, spinal cord injury, biomaterial, axon elongation
Impact Statement
Spinal cord injury (SCI) results in loss of tissue innervation below the injury. Spinal progenitors have a greater ability to repair the damage and can be injected into the injury, but their regenerative potential is hampered by their poor survival after transplantation. Biomaterials can create a cell delivery platform and generate a more hospitable microenvironment for the progenitors within the injury. In this work, polymeric bridges are used to deliver embryonic spinal progenitors to the injury, resulting in increased progenitor survival and subsequent regeneration and functional recovery, thus demonstrating the importance of combined therapeutic approaches for SCI.
Introduction
Spinal cord injury (SCI) results in primary nerve damage, in addition to axonal dieback and de-myelination that ensue after the initial injury. Axon regrowth and myelination into and through the injury are necessary for restoring functional deficits; however, there are several challenges to regeneration. Infiltrating immune cells and astrocytes, as well as the presence of excitotoxic factors and myelin debris contribute to the highly inflammatory milieu and glial scar formation that present obstacles to nerve regeneration.1,2 Delivery of a number of neurotrophic and anti-inflammatory factors is needed to overcome these barriers to regeneration with transplanted cells providing one potential source of exogenous factors to regulate the biochemical milieu.3–6
Neural stem cells (NSCs) actively promote nerve regeneration through a variety of mechanisms, including release of neurotrophic factors, repopulation of beneficial neurons and oligodendrocytes, and immune cell modulation. Numerous growth factors,7–11 extracellular matrix proteins,12–16 and proteoglycans13,15,17–22 are released by NSCs in vitro, with observable increases in these factors in the tissue following delivery of NSCs to a central nervous system (CNS) injury site.4,6,23–25 While NSCs offer numerous proneurogenic advantages, a number of caveats associated with using NSCs currently limit their efficacy. Careful selection of the NSC source is required, as NSCs can be used to broadly define a number of neural stem and progenitor populations that have different neurogenic potentials.26 While it is widely accepted that NSCs within the adult brain maintain some degree of neurogenic potential,27,28 it is highly contentious whether adult spinal progenitors are capable of forming new neurons following injury.29–31 To that end, we will refer to transplanted cells within our studies as neural or spinal progenitors to denote the combined stem and progenitor population within the embryonic spinal cord, while primarily progenitor cells are present within the adult spinal cord. Cell source, including the age, tissue location, and species used for isolation, as well as the ex vivo expansion techniques employed to generate sufficient cells for transplantation will also influence survival and the subsequent regenerative potential of the transplants.26,32 Generally, neural stem and progenitor survival without co-delivery of other pharmacological or biochemical factors have been reported as high as 12% in mice based on bioluminescence assessment,33 but is typically less than 0.5–2% when quantifying through flow cytometry or histology, if quantified at all.26,34–36 Cell survival as high as 5% has been reported following SCI transplant; however, this study failed to discern between exogenous transplants and infiltrating endogenous progenitors.37 Typically, poor survival is attributed to the shear forces exerted on cells during the initial injection, if applicable, and to the highly cytotoxic milieu,38,39 as most of the cells die within the first few days after transplantation.
The method of delivery for cell transplants may affect survival, as most methods deliver the cells through direct injection. Direct injection of cells into a highly inflammatory injury epicenter results in a further 50% reduction in survival of transplanted cells,40 and increasing the dose of neural progenitor cells does not result in a commensurate increase in survival and proliferation.41 Increased neural progenitor cell delivery to compensate for transplant death would require more delivery sites rostral and caudal to the injury. Alternatively, utilizing prenatal or embryonic progenitor populations may have greater survival and subsequent regenerative potential than postnatal or adult progenitors due to the increased immunomodulatory capabilities of younger cells upon both the innate and adaptive immunity.10,11,36,38 Coupling this technique with a biomaterial as a platform for cell delivery could provide a substrate for cell attachment, leading to an upregulation of β1integrins triggering the MAPK signaling that leads to activation of downstream survival and proliferation pathways.12,42 Activation of cell adhesion pathways has long been reported to result in enhanced transplant survival39; thus, early attachment of spinal progenitors to substrates offers great promise. Biomaterial delivery of spinal progenitors may also be beneficial to cell survival and subsequent engraftment as these materials limit inflammation and scarring following SCI by filling the injury and preventing cavity formation.
Biomaterial platforms such as soft hydrogels and highly organized bridges have been evaluated for progenitor cell delivery following SCI. Hydrogels can conform to the shape of the injury site to promote regeneration and limit scar formation after SCI.35,43,44 Current hydrogel technologies offer a vehicle to deliver progenitors in high doses; however, most hydrogels employed in spinal cord repair lack topographical cues to guide axon extension. Neural progenitors within hydrogels are typically injected directly into the injury, at which point the hydrogel will polymerize or crosslink. During this process, the cells undergo shear stresses that can reduce survival similar to direct injection methods. Cell survival may also be limited by insufficient time to spread and proliferate within the hydrogel as more stable integrin binding reduces apoptosis and increases survival by inhibiting the Rho/ROCK pathway after transplantation.45 It is likely these factors contributed to the low survival (1.2%) reported following injections of hyaluronan-based hydrogels.35
Alternatively, bridges can be used to fill the gap between the tissue rostral and caudal to the injury, limit scar formation, and readily guide axons extending through the injury site.46–51 As the shape of a bridge would be predetermined, spinal progenitors can be cultured on these substrates in advance, allowing the cells to spread throughout the material, thus permitting them to acclimate to the substrate before exposure to the elevated levels of inflammatory cytokines after SCI. Poly(lactide-co-glycolide) (PLG) bridges have been a promising biomaterial platform with limited scar formation and guidance of axon elongation through the injury.46,47,52,53 The high degree of porosity within these bridges also allows for cell infiltration and integration of the biomaterial with the surrounding tissue. Increased surface area through porosity and channel design readily facilitates delivery of therapeutic factors,54,55 such as spinal progenitors that can enhance the regenerative potential of unmodified bridges. Moreover, a bridge used as a progenitor delivery system would result in viable transplanted cells directly in the injury epicenter. While many bridge materials have been able to control stem and progenitor proliferation and differentiation in vitro, few have been employed to deliver these cells following SCI.56–58
In this work, we hypothesize that embryonic day 14 (E14) mouse spinal progenitors would promote increased regeneration after a lateral hemisection injury in the mouse spinal cord when delivered on PLG bridges, with regeneration by the E14 spinal progenitors surpassing age-matched (adult) mouse spinal progenitor controls that are representative of the endogenous progenitors that would traffic to the injury. Spinal progenitors have been shown to elicit less of an immune response than terminally differentiated cells due to low antigen expression.38,59 The PLG bridges have also been shown to attenuate the immune response46,60 and, in this work, we evaluate whether the E14 spinal progenitors would further alter immune cell infiltration into the bridge, resulting in greater spinal progenitor transplant survival. To investigate this potential immunomodulatory synergy, immunosuppressants that would reduce inflammation were not used in this study. We did not evaluate glial scar formation, as previous reports have demonstrated that bridge implants, but not the progenitor cells, alter scar formation.53,61,62 A complementary increase in regeneration is expected in the event of increased E14 progenitor survival within the bridge, as more proneurogenic cells would be in the injury. Synergistic regenerative improvements due to the presence of E14 spinal progenitors in the bridges were evaluated in the context of neuron and oligodendrocyte repopulation, axon growth and myelination, and ipsilateral hindlimb stepping. Combining bridges that mimic the architecture of the spinal cord with proneurogenic E14 spinal progenitors delivered to the injury epicenter was thought to lead to regenerative gains.
Materials and Methods
Multichannel bridge fabrication
Multichannel bridges with 90% porosity were generated with final dimensions of 2.25 × 0.75 × 1.25 mm for use in mouse spinal cord hemisection lesion as previously described.47 Briefly, sugar strands drawn from caramelized sucrose (Sigma, St. Louis, MO), dextran (Sigma), and glucose (Sigma) were coated with a 1:1 mixture of PLG microspheres (75:25 ratio of D,L-lactide to L-glycolide, inherent viscosity 0.76 dL g−1; Lakeshore Biomaterials, Birmingham, AL) and NaCl with an average granule size of 63–106 μm. Sugar strands were packed into a mold, equilibrated to 800 psi of CO2 for 16 h, and subsequently released at 60 psi/min to foam into the final structure. The bridges were cut to size leached in distilled water, dried, and stored in a desiccator at room temperature until sterilized. Immediately before loading with cells or implantation into the injury site, the bridges were rehydrated in water and then disinfected with 70% ethanol for 2 min. Bridges were then rinsed in water and blotted twice on sterile filter paper to remove excess water.
Spinal progenitor isolation
Adult and embryonic spinal progenitors were isolated from the spinal cords of C57BL/6-Tg(CAG-EGFP)10sb/J mice (Jackson Laboratory, Bar Harbor, ME), enzymatically dissociated into single cells, and expanded as neurospheres in ultralow attachment flasks (Corning, Corning, NY). For adult spinal progenitors, the spinal cord was isolated from 6- to 8-week-old female mice. Spinal cords were dissociated with 1 U/mL liberase (Roche, Basel, Switzerland) in Hank's balanced salt solution (Gibco, Carlsbad, CA) for 30 min, triturated, and centrifuged in an 4-morpholinepropanesulfonic acid:Opti-prep gradient (Sigma) to remove myelin. Adult spinal progenitors were expanded in Dulbecco's Modified Eagle Medium (DMEM; Gibco) mixed 1:1 with F12 medium supplemented with 1X B27(Gibco), 1X N2 (Gibco), 100 μg/mL heparin (Sigma), and 20 ng/mL basic fibroblast growth factor (FGF2; Peprotech, Rocky Hill, NJ) and epidermal growth factor (Peprotech). Adult cell colonies were passaged with liberase as needed and not used beyond the second passage. For embryonic spinal progenitors, spinal cords were isolated from E14 mouse pups and dissociated with 10 U/mL papain (Worthington, Lakewood, NJ), 37 μg/mL DNase (Sigma), and N-acetyl cysteine (NAC; Sigma) in DMEM for 20 min. E14 tissue was then triturated and centrifuged, and E14 progenitors were expanded in DMEM supplemented with 1X B27, 1X N2, 1X NAC, and 20 ng/mL of FGF-2, and leukemia inhibitory factor (Peprotech). E14 cell colonies were passaged with papain as needed and not used beyond the second passage.
Loading of bridges with progenitors
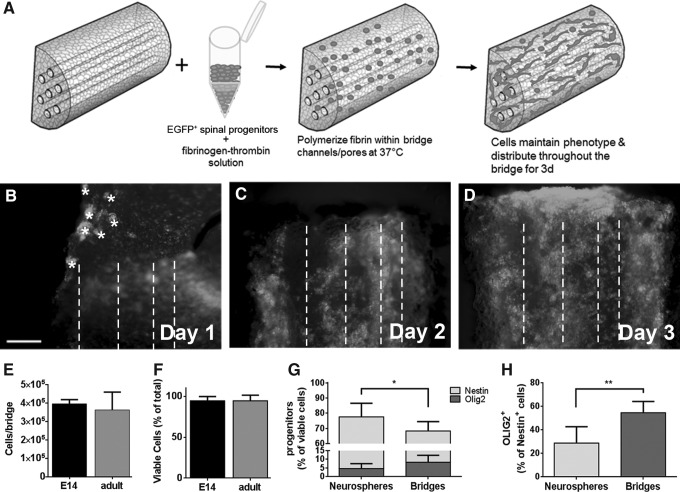
Neurospheres were collected, dissociated with 10 U/mL papain for 10 min in thermomixer (Thermo Scientific, Waltham, MA) at 1400 rpm at 37°C, and centrifuged for 5 min at 300g. Dissociated progenitors were resuspended at 1.25e5 cells/μL in fibrin solution [2 mg/mL fibrinogen (Millipore, Darmstadt, Germany), 5 U/mL thrombin (Sigma), and 2.5 mM CaCl2 in 1 × tris buffered saline (Sigma)]. Fibrin was used as it provides an initial substrate for attachment on the PLG bridges during the initial culture phase, is biocompatible, and has been used extensively for stem and progenitor cell transplantation.63,64 Cell-laden fibrin solution was pipetted onto bridges that had been disinfected in 70% ethanol. Bridges were semiwet, thus allowing for the cell solution to be drawn into the pores as described previously with lentiviral loading onto the PLG bridges.53,65 Fibrin was polymerized for 5 min at 37°C and then the culture medium was added as described in Figure 1A. Loading of the 2.5 e5 enhanced green fluorescent protein (EGFP)+ progenitors onto the bridge was confirmed using an Axiovert LED fluorescent microscope (Zeiss, Oberkochen, Germany) with 5 × dry objective. The medium was supplemented with appropriate growth factors on day 2. After 3 days of culture on the bridge, the cell-laden bridges were implanted into a mouse hemisection injury or evaluated with flow cytometry.
FIG. 1.
Spinal progenitors can be cultured on PLG bridges. (A) A solution of EGFP+ spinal progenitor cells in a fibrinogen-thrombin mix is pipetted onto bridges and the fibrin solution polymerizes at 37°C before media are added. (B–D) EGFP+ cells are cultured on the PLG bridges for 3 days to allow cells to disperse throughout the material, at which time the presence of channels becomes visible (channel midline denoted by dashed lines) and small clusters of spheres (denoted by *) have resolved onto the bridge. After 3 days of culture on bridges, there was no significant difference in cell number per bridge (E) or the percentage of these cells that were viable (F), as assessed by flow cytometry. Further evaluation with flow cytometry of the E14 cells on the bridges revealed that greater than 70% of the E14 cells on the bridges are Nestin+ (G), a modest decrease compared to neurosphere cultures (n = 6 samples per condition, *p < 0.05). Nestin+OLIG2+ cells increase in the bridges, but are not significantly different from E14 neurosphere cultures. (H) Total percent OLIG2+ cells are significantly increased in bridges compared to neurosphere cultures (n = 6 samples per condition, **p < 0.001). Data are represented as mean ± standard deviation. Scale bar = 200 μm. PLG, poly(lactide-co-glycolide); E14, embryonic day 14; EGFP, enhanced green fluorescent protein.
Flow cytometry
Neurospheres or spinal progenitor-loaded bridges were dissociated into a single cell suspension with 1 U/mL liberase at 37°C for 6 min at 1400 RPM. Live cells were detected with a violet fix exclusion dye, after which cells were fixed with 4% paraformaldehyde and permeabilized. Cells were stained with mouse anti-Nestin (1:200; Millipore) and rabbit anti-OLIG2 (1:500; Millipore) for 30 min at 4°C, rinsed, and incubated for 30 min with species-specific secondary antibodies. Cells were analyzed on a Cyan5 flow cytometer using appropriate excitation lasers and emission filters (Beckman Coulter, Brea, CA). Data were analyzed with FlowJo software (FlowJo, Ashland, OR).
Transplantation of progenitor cells
All animal work was performed with prior approval and in accordance with the Animal Care and use Committee guidelines at the University of Michigan. A T9–10 lateral hemisection SCI was created in adult C57BL/6J female mice 6–8 weeks of age, as previously described.47,53,65 Briefly, mice were anesthetized with 2% isoflurane and provided preemptive pain management (1 mg/kg bupivacaine). After confirmation of sufficient anesthesia, a 2 cm incision was made in the skin between the scapula. A laminectomy was performed between T9–10 with a 2 mm lateral hemisection excised. Blank or cell-laden (E14 or adult) bridges were implanted into the injury site. Injury site was secured with gelfoam and then the muscles were sutured and skin stapled. Mice were immediately provided postoperative antibiotics (enrofloxacin 2.5 mg/kg once a day for 2 weeks), analgesics (buprenorphine 0.1 mg/kg twice a day for 3 days), and supportive care (saline 1 mL/20 g once a day for 5 days). Bladders were expressed twice daily until function recovered and staples were removed after 10 days. Mice were euthanized and spinal cord segments (T8–11) were collected after 1, 8, or 24 weeks.
Immunohistochemistry analysis
Isolated spinal cords were flash frozen and then cryosectioned transversely in 18 μm sections. Sections were used throughout the length of the bridge for analysis, with care taken to use tissues from the rostral, middle, and caudal regions of the injury site (approximately three sections 0.75 mm in length), as indicated for each figure. Samples were fixed, permeabilized as necessary, and incubated for 4 h in 0.3% wt/vol Sudan Black (ACROS, Geel, Belgium) in 70% ethanol to reduce background autofluorescence. The following antibodies were used for primary detection: chicken anti-GFP (1:200; Aves Labs, Tigard, OR), rat anti-CD45 (1:500; Abcam, Cambridge, United Kingdom), rat anti-F4/80 (1:200; Abcam), rabbit anti-CD4 (1:300; Abcam), goat anti-arginase I (1:100; Santa Cruz, Dallas, TX), rabbit anti-neurofilament-200 (1:200; Sigma), goat anti-myelin basic protein (MBP; 1:500; Santa Cruz), mouse anti-O4 (1:100; Millipore), rabbit anti-Tuj1 (1:500; Sigma), and mouse anti-NeuN (1:500; Millipore). Species-specific secondary antibodies were used for detection at 1:1000 (Life Technologies, Carlsbad, CA). Hoechst 33342 (Life Technologies) was used as a counterstain in all tissue sections.
Immunostained tissue sections were imaged using an Axio Observer inverted fluorescence microscope (Zeiss) using a 10 × dry objective. All quantification of EGFP+ cells, cell phenotypes, or axon/myelin quantification was confined to the bridge region. Bridge area exhibited some residual autofluorescence due to the presence of the polymer with randomized cellular distribution within channels and pores, while contralateral tissue had even cellular distribution, although at lower densities than the bridge. Based on these criteria, the bridge area was defined conservatively to avoid inclusion of tissue at the interface of the intact tissue and bridge.
Semiautomated counting software, previously described by McCreedy et al.,66 was used to quantify axons and the co-localization of myelin with axons in transverse sections taken from the rostral, middle, and caudal regions of the injury. Briefly, the software was calibrated using manual NF-200+ and NF-200+MBP+ counts from a subset of transverse images taken from different animals and regions of the implant. The software then used a series of Hessian filters and threshold functions within the bridge region to reduce noise for selected NF-200 and MBP images.66 The software then output total axon counts, as well as the myelinated axon counts based on the curvilinear MBP co-localizing with axons. ImageJ (NIH, Bethesda, MD) was used to analyze all other fluorescent images and define the bridge area. The bridge was divided into these three segments to delineate between the rostral, middle, and caudal regions of the bridge, as axons will grow from the rostral and caudal ends into the bridge and will therefore have higher densities than the middle of the bridge at 8 weeks. Three tissues were used for each region (rostral, middle, and caudal) of each animal. For 6-month tissue, there was no difference between these three regions, and thus, the axon densities were averaged across all regions within the bridge resulting in nine tissues used for quantification.
For other cell phenotype markers, cells can enter the bridge from the lateral and medial edges, and thus, this regional distinction was unnecessary. EGFP+ cell survival was quantified in two ways. In the first, the cells were quantified and divided by the bridge area (region criteria described above) for nine tissue sections per animal to determine cell density. This value was for an 18 μm tissue section, which was extrapolated across the 2.25 mm bridge length to obtain the cell number for the bridge volume. The cell number/bridge volume was then divided by the number of cells delivered on the bridge to arrive at the percent EGFP survival. Transplanted cells identified by EGFP+ cells as well as mature neurons (NeuN) containing Hoechst+ nuclei were counted manually by two blinded researchers independently. Similarly, cells positive for CD45+ (leukocytes), CD45+CD4+ (helper T cells), F4/80+ (macrophages), and F4/80+arginaseI+ (M2 macrophages) containing Hoechst+ nuclei were also counted manually by two blinded researchers to quantify infiltration of immune cells.
Basso Mouse scale
Basso Mouse scale (BMS) was used to evaluate mice in an open-field locomotion test. Two researchers scored the mice on ankle movement, hindlimb placement, hindlimb stepping, and trunk stability and must reach a consensus to ensure accuracy. Mice were acclimated to the open field before testing and for the raters to become familiar with the normal gait pattern of the mice. Mice were scored weekly for the first month and then every other week for 6 months after surgery.
Statistics
Multiple comparisons pairs were analyzed using a one-way analysis of variance (ANOVA) with Tukey post-hoc test. For all conditions, n = 6 mice per condition per time point for histological analysis. BMS data were analyzed using two methods. In the first, a one-way ANOVA with repeated measures was used with a Tukey post-hoc text. In the second, a chi-square test was used to evaluate the initial binary ability of each mouse to perform hindlimb stepping (BMS score 4) with data plotted as a contingency graph indicating the percentage of the population that could hindlimb step. For hindlimb stepping, n = 11 mice for each condition. Significance was defined at a level of p < 0.05 unless otherwise noted. All data are reported as mean ± standard deviation.
Results
Development of an EGFP-spinal progenitor delivery paradigm
Dissociated EGFP+ spinal progenitors from either E14 or age-matched adult spinal cords were suspended in a fibrinogen solution, mixed with thrombin, and pipetted onto bridges. The fibrin gel was retained within the pores and functioned to enhance the seeding efficiency (Fig. 1A). EGFP+ cell occupancy was evaluated over 3 days (Fig. 1B–D) to ensure a well-distributed population before transplanting the cell-laden bridges. There was no significant difference in cell number or viability between the adult and E14 cells after culture on the bridges in vitro as assessed by flow cytometry (Fig. 1). We tested the maintenance of the E14 progenitor phenotype on bridges in comparison to neurosphere colonies and demonstrated that greater than 70% of the cells maintained a Nestin+ phenotype with an increase in OLIG2+ cells in the bridges compared to neurosphere controls (Fig. 1; Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea).
EGFP-spinal progenitors exhibit source-dependent survival
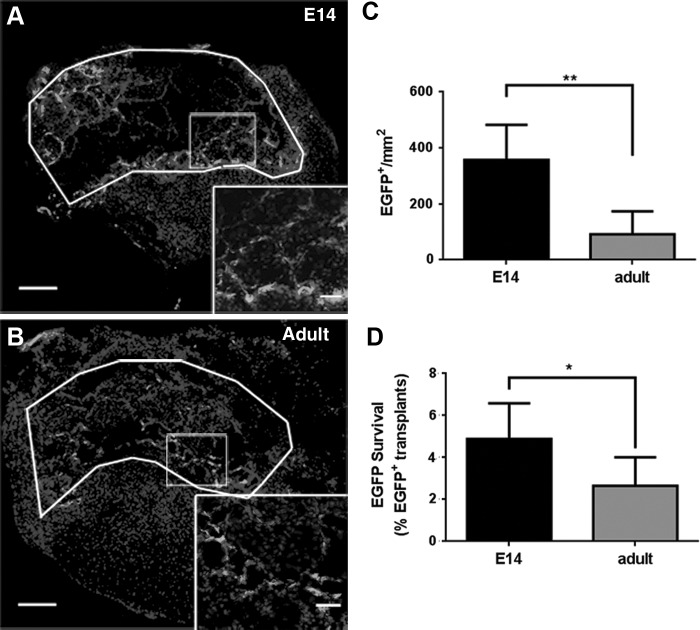
We subsequently investigated survival of the spinal progenitors that were transplanted on bridges into a lateral T9–10 hemisection spinal cord defect. Spinal progenitors from two different sources were investigated, using E14 progenitors that were posited to enhance survival and regeneration compared to age-matched (adult) progenitor controls that likely exhibit limited survival and regenerative potential comparable to the endogenous progenitor population. Cords were collected 7 days postinjury and evaluated histologically for E14 EGFP+ cells (Fig. 2A) and age-matched adult EGFP+ transplant control (Fig. 2B) survival. EGFP+ cells were evident throughout the bridge on day 7, with cells along both the lateral and midline edges of the bridge. The overall survival of the transplanted cells was low for both conditions, although a 3.9-fold increase in EGFP+ cell density (Fig. 2C, p = 0.0015, n = 6) and an increased percentage survival (Fig. 2D) were observed for E14 transplants (4.9%) compared to adult transplants (2.6%). At 8 weeks, EGFP+ cell survival had dropped to 0.3% (E14) and 0.17% (adult) of the initial transplant population (Supplementary Fig. S2). Furthermore, of the surviving cell population at week 8, 35% (E14) and 46% (adult) of the remaining cells had migrated into the contralateral tissue (Supplementary Fig. S2). By 6 months, only 0.42–2.1 EGFP+ cells/mm2 cells were observed across both spinal progenitor conditions (data not shown).
FIG. 2.
EGFP+ spinal progenitors survive in the bridge 7 days postinjury and implantation. EGFP+ spinal progenitor cells isolated from (A) E14 or (B) adult spinal cords survive postinjury inflammation. White lines delineate bridge area, while rectangles denote higher magnification insets for Hoechst33342 and EGFP. Increased cell density (C) and percent EGFP+ survival from the total cell number implanted (D) of E14 EGFP+ spinal progenitors compared to adult EGFP+ spinal progenitors suggest improved survival of E14 cell transplants (*p < 0.05, **p < 0.005, n = 6 mice per bridge condition), although the overall percent survival is low. Data are represented as mean ± standard deviation. Scale bar = 200 or 20 μm (inset).
Spinal progenitor-laden bridge transplants do not affect leukocyte infiltration
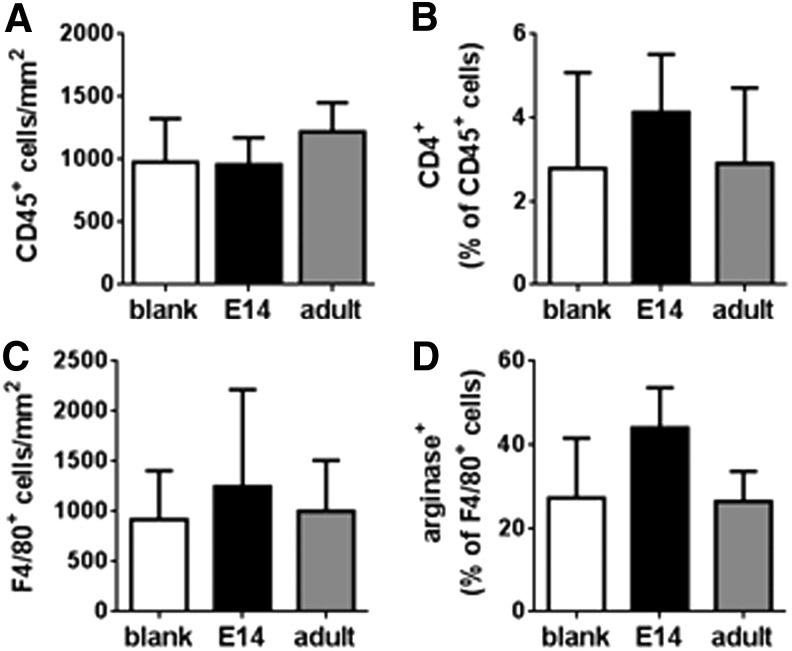
Stem and progenitor populations have been implicated in modulating the inflammatory response, and we subsequently investigated the host response to blank bridges or cell-loaded (E14 or adult) bridges. No difference in CD45+ leukocytes (Fig. 3A; Supplementary Fig. S3) or CD45+CD4+ helper T cells (Fig. 3B; Supplementary Fig. S3) was detected in the blank bridges or EGFP-spinal progenitor (E14 or adult)-loaded bridges 7 days postinjury. Furthermore, the macrophage density was not changed (Fig. 3D; Supplementary Fig. S4), nor was the percentage of macrophages that were arginase I+ (p = 0.052, Fig. 3C; Supplementary Fig. S4).
FIG. 3.
Immune cells infiltrate blank and spinal progenitor-loaded bridges at 7 days postinjury. (A) Total CD45+ leukocyte infiltration is not altered due to the presence of transplanted stem cells. (B) The percentage of the total leukocyte population that is CD45+CD4+ T cells is not significantly different between conditions, even with the presence of exogenous spinal progenitors. No significant difference in total macrophages (C) or arginase+ (M2) macrophages (D) was detected in histological sections. There was a trend toward increased M2 macrophages in bridges loaded with E14 transplant condition; however, this increase was not statistically significant (p = 0.8, n = 6 mice per bridge condition). Full immunohistochemistry of CD45 with CD4 and F4/80 with arginase can be seen in Supplementary Figures S3 and S4. Data are represented as mean ± standard deviation.
E14 spinal progenitors enhance early axon elongation through the injury
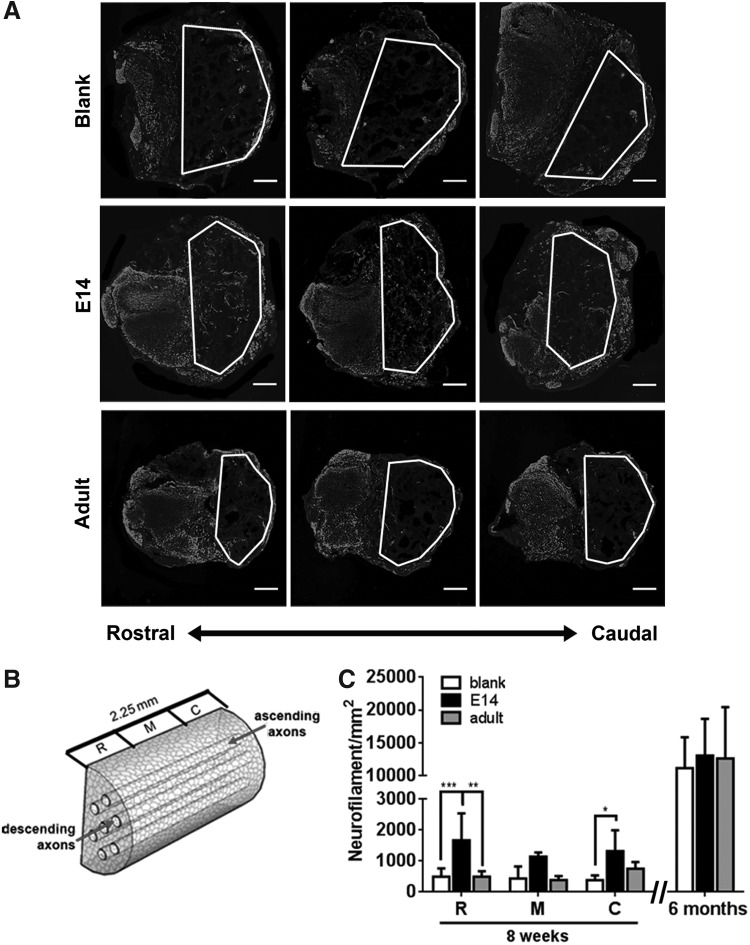
We next investigated the extent to which the severed ascending and descending axons elongate through the bridge from the caudal or rostral ends of the injury. Axons were identified by neurofilament staining, and 0.18 μm transverse sections were separated into three 0.75 mm segments of the bridge: rostral (R), middle (M), and caudal (C), for tissue at 8 weeks (Fig. 4). NF-200+ axons are present in all sections of bridge in each condition. For blank bridges, axon regrowth appears confined to the channels, whereas bridges transplanted with adult or E14 spinal progenitors had axon growth throughout the bridge cross-section (Fig. 4A). Bridges transplanted with E14 spinal progenitors had an increased axon number relative to blank and adult spinal progenitor bridge controls, with the greatest increase resulting primarily from axon numbers in the rostral and caudal sections of the bridge (Fig. 4C). There was no observable co-localization of EGFP and NF-200, suggesting that the transplanted cells did not directly contribute to the increase in axonal density within the bridge.
FIG. 4.
Bridges loaded with E14 EGFP+ spinal progenitors enhance axon elongation at 8 weeks after transplantation in T9-10 hemisection. (A) Qualitatively, NF-200+ axon expression is greater in bridges containing E14 EGFP+ spinal progenitors compared to adult EGFP+ spinal progenitors and empty bridges. (B) Quantification of regenerating axons was binned into three 0.75 mm transverse sections of the bridge: R-rostral, M-middle of the injury, and C-caudal. (C) Axon density is increased in E14 EGFP+ spinal progenitor-loaded bridges compared to adult EGFP+ spinal progenitor-loaded and empty bridges at 8 weeks postinjury. This increase is most dramatic in the rostral and caudal sections of the bridges. By 6 months, axon density is similar for all conditions. Data are represented as mean ± standard deviation. n = 6 mice per bridge condition, *p < 0.05, **p < 0.01, ***p < 0.001. Scale bar 200 μm.
Axon elongation through the injury was evaluated at 6 months to discern if the difference in axon regrowth at 8 weeks was maintained. Regrowth was averaged through all sections (rostral, middle, and caudal) of the bridge at 6 months, as no discernable location-based differences in axon counts were observed. After 6 months, axon density increased ∼10-fold compared to 8-week data, indicating robust axon regeneration is possible with PLG bridges. Axon density within the bridge across all conditions was approximately half of that observed in the tissue contralateral to the bridge. The initial enhancement in axon numbers between E14 cell-loaded bridges and the adult cell-loaded and empty bridge controls observed at 8 weeks was not evident at 6 months. The high degree of axon regrowth suggests a supportive regenerative environment is created using the bridge alone, yet with more rapid regrowth evident upon inclusion of E14 spinal progenitors (Fig. 4C).
E14 spinal progenitors improve myelination of axons within the bridge
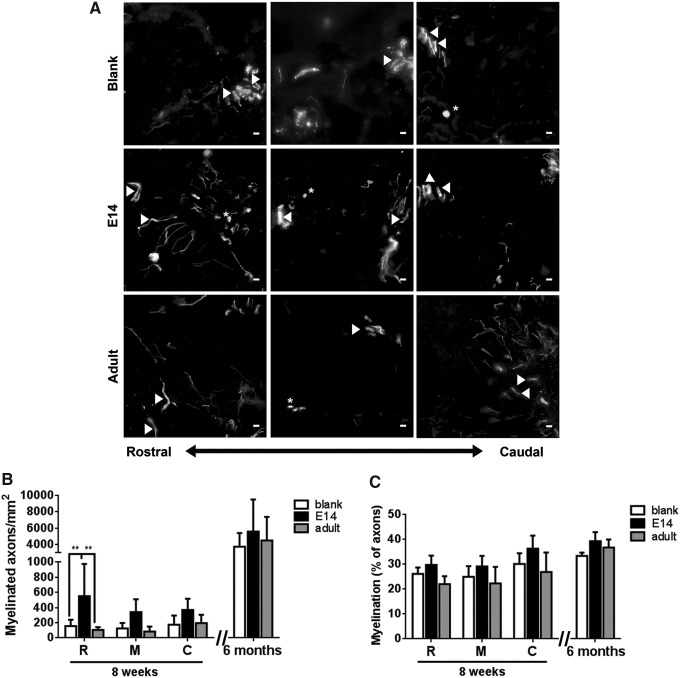
The extent of myelination of the regenerating axons was subsequently characterized, which is necessary for functional improvements. MBP+ myelin, which does not make a distinction between cell source (oligodendrocytes or Schwann cells) or myelin quality (compact vs. noncompact, internodal distance), was found to co-localize with the regenerating axons in the bridge (Fig. 5A). Myelinated axons were seen throughout the progenitor-loaded bridges, localizing within channels and along the midline of the tissue, while myelinated axons within the blank bridges were predominantly found within channels. An increase in myelinated axon density for bridges transplanted with E14 spinal progenitors compared to adult spinal progenitor and blank bridges was observed at 8 weeks, with significant increases observed in the rostral tissue yielding 4.8-fold and 3.5-fold more myelinated axons relative to adult and blank bridges, respectively (Fig. 5B). The average percent myelination throughout the bridge for each condition was similar for each condition (Fig. 5C), however, a trend toward increased myelination was observed for E14 spinal progenitor loaded (31.6%) relative to adult spinal progenitor loaded (23.7%) and blank (27.1%) bridges (p = 0.13) at 8 weeks.
FIG. 5.
Bridges loaded with E14 EGFP+ spinal progenitors enhance myelination of axons at 8 weeks after spinal cord injury. (A) Qualitatively, myelination (MBP) of NF-200+ axon expression is greater in E14 bridges compared to adult progenitor and empty bridges. Quantification of myelination was binned into three 0.75 mm sections of the bridge: R-rostral, M-middle of the injury, and C-caudal. Arrowheads indicate axons wrapped in myelin that traverse the tissue longitudinally. Asterisks indicate myelinated axon cross-sections. Increased myelination (B), but not overall percent myelination (C), was observed in E14 bridges compared to blank and adult progenitor bridges at 8 weeks. Differences in myelinated axon density or percent were no longer evident 6 months postinjury. Data are represented as mean ± standard deviation. n = 6 mice per bridge condition, **p < 0.01, scale bar 10 μm.
At 6 months, the density of myelinated axons increased ∼10-fold across all conditions (Fig. 5B), eliminating the initial difference seen in myelination at 8 weeks. No significant difference was observed between myelination percentage at 8 weeks and 6 months; however, the average percent of myelinated axons did trend higher in the spinal cord at 6 months with 34–40% of all axons being myelinated (Fig. 5C). By contrast, 45–58% of the intact contralateral spinal cord tissue was myelinated at 6 months, which was significantly higher for the injured tissue receiving blank (p = 0.0028) and E14 transplants (p = 0.0473), but not adult transplant (p = 0.0973) bridges.
E14 spinal progenitor-loaded bridges increase neural bodies within the bridge
We have demonstrated that progenitor-loaded bridges result in early differences in axon elongation through the bridge, but it is unclear if some of these axons are a result of newly formed neuronal cells. For this reason, we evaluated the early neuronal marker Tuj1 and early oligodendrocyte marker O4 at 8 weeks, which presumably arose from either the endogenous or exogenous (EGFP+) progenitors. After 8 weeks, there was a significant increase in both Tuj1+ and O4+ cells observed within both E14 and age-matched control transplants compared to the blank bridges (Supplementary Fig. S5). Less than 5% of the differentiated cells within the bridge co-expressed EGFP. Of the EGFP+ cells, less than 40% of the cells exhibited Tuj1 or O4 markers, either within (Supplementary Fig. S5C) or outside of (Supplementary Fig. S5D) the bridge.
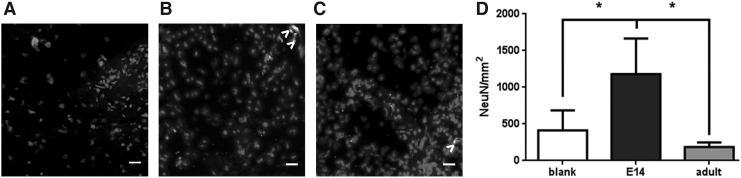
The mature neuronal marker NeuN localizes to nuclei of cells committed to a neuron lineage and was used to further evaluate neurons within the bridge at 6 months. NeuN+ neurons were evident in each of the bridge conditions, including bridges that were not loaded with exogenous spinal progenitors (Fig. 6A–C). A significant increase in NeuN+ neurons was observed in E14 progenitor-loaded bridges, with densities exceeding 1000 NeuN+ cells/mm2 (Fig. 6D). Sparingly few (0.42–2.1 cells/mm2) EGFP+ cells were observed within the bridge or adjacent tissue of mice receiving spinal progenitor transplants. Transplanted EGFP+ cells accounted for 1.6% ± 1.4% and 7.6% ± 6.7% of the NeuN+ cells within the bridges of E14 and adult cell transplants, respectively. The high variability of the NeuN+EGFP+ population is likely due to the low 6-month survival rate of the transplanted cells. Together these data suggest that the E14 spinal progenitor cells support robust infiltration of endogenous progenitor cells, leading to new neurons within the bridge that likely arises from both exogenous and endogenous progenitor populations. Moreover, the EGFP+NeuN+ cells within bridges receiving the age-matched transplants exhibit newly formed neurons, demonstrating that adult spinal progenitors may indeed be capable of neuronal differentiation, but to a lesser extent than the younger progenitor populations.
FIG. 6.
Increased neural bodies are present within E14 loaded bridges after 6 months. (A) Blank, (B) E14 spinal progenitor-loaded, and (C) adult spinal progenitor-loaded bridges 6 months after transplantation in a spinal cord hemisection stained for NeuN and EGFP+ cell transplants (>) with Hoechst nuclear counterstain. Quantification of histological sections exhibits a significant increase in NeuN+ neurons in E14 spinal progenitor-loaded bridges (D). Data are represented as mean ± standard deviation. n = 6 mice per bridge condition, *p < 0.01, scale bar 20 μm.
Modest improvements in locomotion present in mice with no difference in long-term regrowth
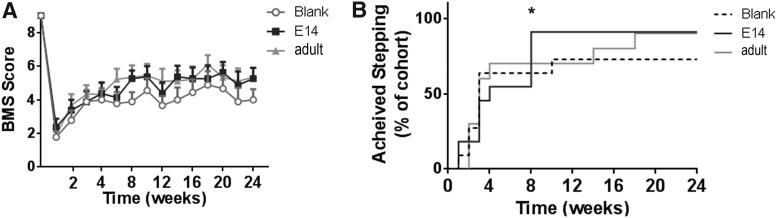
An open field locomotor BMS test was performed to assess coordination and hindlimb motor function. Average BMS scores were observed to increase across all conditions over time (Fig. 7A). With 90% of the mice that received E14 spinal progenitor-loaded bridges recovering hindlimb stepping abilities by 8 weeks postinjury, these mice demonstrated a significantly (Fig. 7B, p < 0.01) earlier recovery compared to adult spinal progenitor-loaded or blank bridges. Ninety percent of mice receiving adult spinal progenitors were able to perform hindlimb stepping by week 18, whereas only 73% of mice receiving blank bridges achieved stepping by 6 months.
FIG. 7.
Faster ability of hindlimb stepping through inclusion of E14 spinal progenitor cells. Average BMS scores are observed to increase over time for each condition; however, no significant difference between groups over time was observed as determined by one-way analysis of variance with repeated measures using a Tukey post-hoc test (A). By 8 weeks, significantly more mice that received bridges with E14 transplants were able to perform stepping (BMS = 4) on ipsilateral hindlimb affected by the lateral hemisection as determined by chi-squared test [(B); *p < 0.01, n = 11 mice per bridge condition]. By 6 months, 90% of mice receiving either E14 or adult transplants achieved stepping compared to 70% transplanted with blank bridges. ANOVA, analysis of variance; BMS, Basso Mouse scale.
Discussion
In this work, we provided a conservative estimation of exogenous spinal progenitor cell survival from bridges, resulting in a modest increase in transplanted EGFP+ cell survival (4.9%) at the injury epicenter compared to the 0.5–2% reported in the literature.26,34–36 Exogenous stem and progenitor cells have the potential to repopulate an injury through expansion, and can also promote the recruitment of endogenous progenitor populations. The benefits of exogenous cells, whether multipotent NSCs or more differentiated progenitor cells, are contingent upon their ability to survive the highly inflammatory microenvironment at the injury. In this work, E14 spinal progenitors were able to survive transplantation following SCI and a subset of the population migrated into the intact contralateral tissue. Survival of spinal progenitor cells at 1 week was ∼5% of the initial cells seeded on the bridge and less than 1% after 8 weeks; however, these numbers may underestimate cell density due to potential silencing of the EGFP gene. Butenschön et al. reported inadequate EGFP expression for detection due to silencing of EGFP by 5 weeks after transplantation of progenitors and used BrdU labeling as an alternative method to quantify the progenitors within the injury.37 They found that 4% of the cells co-expressed BrdU and markers for neuronal lineages, but did not delineate between exogenous and infiltrating endogenous progenitors. Nevertheless, proliferative markers may be poor indicators of exogenous cell transplants, as proliferation has been shown to be limited to the first few weeks after transplantation.67 While EGFP expression may be silenced over time, quantification by EGFP provides a conservative assessment of survival of exogenous cells for our studies compared to the use of proliferative markers.
The level of engraftment for spinal progenitors within the bridge is consistent with the upper limits reported by others that have transplanted neural stem or progenitor cells isolated from either the brain or spinal cord into an injured spinal cord without immunosuppression. Studies delivering progenitor cells within a hydrogel without immunosuppression in mice have previously seen only 1.2% cell survival 1 week after transplant.35 Similarly, cell survival following direct injection methods without immunosuppression in immune-competent animals results in poor survival (0.5–2%) when characterized by histology.34,36,37 Utilizing a NOD-SCID model of SCI to evaluate spinal progenitor transplant survival after direct injection increased survival to ∼10–20% after 1 week,67,68 with no further therapeutic advantage in co-delivery of immunosuppressants in the NOD-SCID model.69 Reported differences in survival may be attributed to the delivery and quantification methods across these studies. For example, quantification of survival using bioluminescence imaging tends to obtain higher cell densities up to 10%33 compared to flow cytometry or histology. Regardless of these methods, it is likely that survival is limited without active control of inflammation. PLG bridges have been shown to attenuate inflammation by limiting glial scar formation and immune cell infiltration compared to no treatment injured controls, yet do not shift the phenotype toward anti-inflammatory.46,60 Delivery of interleukin-10 from the bridges produced a more regenerative microenvironment even in the presence of immune cells due to the shift toward anti-inflammatory immune phenotypes.65 The delivery of immunomodulatory factors that limit inflammation may have the potential to further enhance cell transplant survival when delivered within bridges as a localized strategy, rather than systemic immunosuppression.
In this work, bridge-mediated delivery of E14 derived spinal progenitor cells attained greater survival relative to the age-matched adult spinal progenitors. Survival of transplanted cells in an SCI model has been reported to be dependent on injection dose,26,41 location,26,40 and cell source.26,70 Optimal injection dosages of 104–105 cells/animal are preferable as increasing the dosage has a negative impact on survival and proliferation of the transplanted cells.41 Injections of these cells into the rostral and caudal tissue rather than the injury epicenter will also increase cell survival twofold and allow the cells to migrate into the injury over time as inflammation dissipates.40
The largest source of survival variability can be attributed to the cell source, such as species, tissue location, and host age.26 Immature stem cells express fewer major antigens than terminally differentiated cells,59,71,72 a characteristic that is increasingly prominent with stem cells sourced from embryonic tissue,38 such as the E14 spinal progenitors when compared to adult spinal progenitors. Interestingly, no significant differences in infiltration of the immune cell phenotypes evaluated within spinal progenitor-loaded bridges were observed compared to blank bridges. One possible explanation for this result is that enhanced survival of E14 spinal progenitors was due to decreased susceptibility to apoptosis through increased activation of proliferation and survival pathways in the inflammatory milieu compared to adult spinal progenitors.73 However, the degree of survival was likely insufficient to further modulate infiltrating immune phenotypes to the extent observed in vitro, where there is a more robust ratio of progenitor cells to immune cells.9–11
E14 spinal progenitor cells exhibit increased survival compared to the adult spinal progenitor cells, suggesting that they may also make larger contributions to the biochemical milieu. Neural stem and progenitor cells secrete numerous neurotrophic and immunomodulatory factors, a subset of which mediates endogenous progenitor recruitment. Neural stem and progenitor cells have been reported to express extracellular matrix constituents, such as glycoproteins and12–16 proteoglycans.13,15,17–22 These cells can also produce endogenous neurotrophins6–8,23–25 immunomodulatory cytokines.9–11,21,25 Furthermore, cell-cell adhesion molecules9,14 are upregulated by progenitor populations when transplanted into an injury and may foster more attachment sites for growing axons. A number of these factors have only been demonstrated with in vitro release by neural progenitor cells6–8,12–15,17–25 or in co-cultures with immune cells,9–11 while in vivo release of these factors has been attributed to progenitor transplants, but has not been directly demonstrated.6,12,16,17,23
Transplantation of spinal progenitors into a CNS injury resulting in increased concentrations of neurotrophic or immunomodulatory factors compared to the injury alone suggests that transplanted cells may release these factors or encourage other cells to release these factors to promote regeneration, as few spinal progenitors survive after transplant. The proteins secreted by the adult and E14 sourced progenitor cells may be variable in composition and concentration due to inherent differences in the cell phenotypes.73–77 These differences would be pronounced immediately after bridge implantation, as the transplanted cells were cultured for 3 days on the bridges before implanting, thus allowing the spinal progenitor cells to deposit numerous factors onto the bridges. Potential differences in the biochemical milieu of adult and E14 cells would likely continue due to differences in survival rates, with greater expression of survival genes in cells sourced from younger tissues,73 thus allowing more cells to make biochemical contributions to the injury microenvironment.
Potential differences in the biochemical milieu may contribute to observed increases in axon elongation and myelination at 8 weeks. Delivery of E14 spinal progenitors promotes faster axon regrowth and myelination than adult spinal progenitors or bridges alone. The initial increase in regrowth may also be indicative of increased plasticity outside of the bridge that contributed to the observed ability for 90% of the mice to perform stepping earlier in E14 progenitor-loaded bridges. The ability of mice in the other two groups eventually develops a similarly large percent (70–90%) of the population that can achieve stepping at 6 months and this corresponds to a commensurate increase in axon regrowth through the bridge. Quantification of axon density in the contralateral tissue demonstrated no difference between conditions at 6 months (data not shown). Axon density within the bridge was approximately half the density of axons within the contralateral tissue at 6 months, suggesting robust regrowth through the bridge in all conditions that may mask the early benefits to axon regrowth as a result of transplanting E14 spinal progenitors.
Robust axon regeneration observed in this study had not previously been observed between 9 weeks and 6 months postinjury within a rat model46 and may be due to the combination of fewer channels in the bridge, decreased porosity, and quantification of axons being limited to the channels within the rat bridges compared to the mouse bridges. While the robust axon growth is promising, future studies will investigate strategies to increase E14 spinal progenitor survival through the use of delayed delivery methods and co-administration of localized anti-inflammatory factors. Increased survival will likely lead to enhanced biochemical contributions and subsequent regeneration and this will be increasingly evident at later time points when the E14 cells no longer offer an advantage to axon regrowth.
Interestingly, the 10-fold increase in axon density within the bridge at 6 months, which approached contralateral axon density, did not correlate to significantly higher average BMS scores over time, suggesting that the axons may not have formed or may have incorrectly formed synapses with intact tissue, and thus did not contribute to enhanced stepping abilities, such as hindlimb coordination and trunk stability. Retrograde tracing techniques would be necessary to discern whether synapses are forming between axons growing through the bridge with intact motor circuitry or whether the improved recovery is due to increased sparing of axons outside of the bridge. Ultimately, axon regrowth is necessary to rebuild circuits; however, limited data have shown a direct correlation between improved BMS and axon density following SCI due to limited in vivo imaging techniques for axon density over time. A positive correlation of spared axon area with locomotor function recovery using diffusion tensor imaging has been made at the individual animal level; however, this correlation was not extended to axon density.78
We report that roughly 30% of the axons are myelinated across all conditions and previous reports state that 40–60% of spinal cord axons are myelinated in healthy rodents,79,80 yet this relatively high density of axons did not correlate with an improved locomotor recovery. The average BMS score did not significantly improve after week 8 even with substantial axon growth and 40% myelination at 6 months. As discussed previously, a number of factors would explain the lack of correlation between histological improvements and behavioral improvements. Not only is the axon regrowth and correct synapse formation necessary but also myelination of these axons will play a role in restoration of motor function. It is possible that the myelin quality may be inferior, thus requiring higher levels of myelination within the expected healthy range79,80 to achieve higher functional benefits. Furthermore, myelination was not quantified in continuous transverse tissue sections; thus, axons may not be myelinated along their full length, which could also contribute to reduced functional recovery relative to the report myelinated axon density.
Appreciable differences in the density of neural cell bodies within the bridges at 8 weeks and 6 months were observed, despite the modest survival of the exogenous progenitors. Neurogenesis following SCI has been reported previously with both exogenous26,32,63,81 and endogenous29,30 progenitors. Both native and transplanted progenitors that give rise to new neurons have the potential to integrate onto preexisting circuits, forming functional synapses, as demonstrated by electrophysiology30,63 or monosynaptic rabies tracing.82 By 6 months, neurons were evident in all bridge conditions, even those devoid of initial cellular transplants, suggesting a basal level of neurogenesis by local progenitors that migrate into the bridges, although there is little evidence in the literature that endogenous progenitors in the spinal cord are capable of neurogenesis.29,30 An increase in neural body density was observed within the E14 spinal progenitor-loaded bridges, with few of these NeuN+ cells co-expressing EGFP+. NeuN+EGFP+ neurons originating from exogenous cells were also observed with the adult transplants, although to a lesser extent than that observed with the E14 transplants.
The presence of EGFP+NeuN+ cells within adult transplants suggests that the adult spinal cord progenitors are capable of neurogenesis; however, it is significantly reduced compared to prenatal spinal progenitors. While the remaining neurons within the bridge were not EGFP+, it is possible that the EGFP was silenced and that exogenous cells are indeed contributing to the increased NeuN+ cell density. However, it is also likely that these newly formed neurons are originating from endogenous cell populations given that NeuN+ cells were observed in the blank bridges. It is unlikely that mature neurons migrated into these bridges, as primarily progenitors and neuroblasts are thought to be capable of migration within the adult CNS.83 However, interneurons within the hippocampus can migrate short distances and maintain their synaptic connections.84 Additional studies would be necessary to determine the source of these cells, and the critical assessment of neural phenotype and electrophysiology would be required to discern if these cells have integrated into host circuitry.
Conclusion
In summary, we investigated the transplantation of E14 spinal progenitors on multichannel PLG bridges with the goal of enhancing the number and extent of myelination for axons growing into and through the injury. We successfully delivered a modest spinal progenitor population and surviving cells were correlated with a commensurate increase in the density of axons and myelin at 8 weeks within the injury for mice receiving E14 transplants compared to age-matched adult transplants. This early regrowth of axons and their myelination were correlated with a more rapid functional recovery relative to the control conditions, although by 6 months, the axon density and extent of myelination were similar between all conditions as was the functional recovery This work uses aligned bridges to deliver stem cells and, in the future, can be combined with other therapeutics, such as the delivery of anti-inflammatory cytokines, to further enhance transplant engraftment along with nerve plasticity and repair.
Supplementary Material
Acknowledgment
This work was supported by the National Institutes of Health [RO1EB005678].
Disclosure Statement
No competing financial interests exist.
References
- 1.Sroga J.M., Jones T.B., Kigerl K.A., McGaughy V.M., and Popovich P.G. Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. J Comp Neurol 462, 223, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Donnelly D.J., and Popovich P.G. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol 209, 378, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dumont C.M., Margul D.J., and Shea L.D. Tissue engineering approaches to modulate the inflammatory milieu following spinal cord injury. Cells Tissues Organs 202, 52, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hawryluk G.W., Mothe A., Wang J., Wang S., Tator C., and Fehlings M.G. An in vivo characterization of trophic factor production following neural precursor cell or bone marrow stromal cell transplantation for spinal cord injury. Stem Cells Dev 21, 2222, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu P., Jones L.L., Snyder E.Y., and Tuszynski M.H. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol 181, 115, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Lladó J., Haenggeli C., Maragakis N.J., Snyder E.Y., and Rothstein J.D. Neural stem cells protect against glutamate-induced excitotoxicity and promote survival of injured motor neurons through the secretion of neurotrophic factors. Mol Cell Neurosci 27, 322, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Hsieh F.Y., Lin H.H., and Hsu S.H. 3D bioprinting of neural stem cell-laden thermoresponsive biodegradable polyurethane hydrogel and potential in central nervous system repair. Biomaterials 71, 48, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Li Q., Ford M.C., Lavik E.B., and Madri J.A. Modeling the neurovascular niche: VEGF- and BDNF-mediated cross-talk between neural stem cells and endothelial cells: an in vitro study. J Neurosci Res 84, 1656, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Nazmi A., Mohamed Arif I., Dutta K., Kundu K., and Basu A. Neural stem/progenitor cells induce conversion of encephalitogenic T cells into CD4+-CD25+- FOXP3+ regulatory T cells. Viral Immunol 27, 48, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Bonnamain V., Mathieux E., Thinard R., et al. . Expression of heme oxygenase-1 in neural stem/progenitor cells as a potential mechanism to evade host immune response. Stem Cells 30, 2342, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Wang L., Shi J., van Ginkel F.W., et al. . Neural stem/progenitor cells modulate immune responses by suppressing T lymphocytes with nitric oxide and prostaglandin E2. Exp Neurol 216, 177, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Campos L.S., Leone D.P., Relvas J.B., et al. . Beta1 integrins activate a MAPK signalling pathway in neural stem cells that contributes to their maintenance. Development 131, 3433, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Chaerkady R., Letzen B., Renuse S., et al. . Quantitative temporal proteomic analysis of human embryonic stem cell differentiation into oligodendrocyte progenitor cells. Proteomics 11, 4007, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gurok U., Steinhoff C., Lipkowitz B., Ropers H.H., Scharff C., and Nuber U.A. Gene expression changes in the course of neural progenitor cell differentiation. J Neurosci 24, 5982, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abaskharoun M., Bellemare M., Lau E., and Margolis R.U. Glypican-1, phosphacan/receptor protein-tyrosine phosphatase-zeta/beta and its ligand, tenascin-C, are expressed by neural stem cells and neural cells derived from embryonic stem cells. ASN Neuro 2, e00039, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakaguchi M., Shingo T., Shimazaki T., et al. . A carbohydrate-binding protein, Galectin-1, promotes proliferation of adult neural stem cells. Proc Natl Acad Sci U S A 103, 7112, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Q., Yang L., Alexander C., and Temple S. The niche factor syndecan-1 regulates the maintenance and proliferation of neural progenitor cells during mammalian cortical development. PLoS One 7, e42883, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu W.L., Fu S.L., Wang Y.X., et al. . Chondroitin sulfate proteoglycans regulate the growth, differentiation and migration of multipotent neural precursor cells through the integrin signaling pathway. BMC Neurosci 10, 128, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sirko S., von Holst A., Wizenmann A., Götz M., and Faissner A. Chondroitin sulfate glycosaminoglycans control proliferation, radial glia cell differentiation and neurogenesis in neural stem/progenitor cells. Development 134, 2727, 2007 [DOI] [PubMed] [Google Scholar]
- 20.von Holst A., Sirko S., and Faissner A. The unique 473HD-Chondroitinsulfate epitope is expressed by radial glia and involved in neural precursor cell proliferation. J Neurosci 26, 4082, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tham M., Ramasamy S., Gan H.T., et al. . CSPG is a secreted factor that stimulates neural stem cell survival possibly by enhanced EGFR signaling. PLoS One 5, e15341, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taupin P., Ray J., Fischer W.H., et al. . FGF-2-responsive neural stem cell proliferation requires CCg, a novel autocrine/paracrine cofactor. Neuron 28, 385, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Chirasani S.R., Sternjak A., Wend P., et al. . Bone morphogenetic protein-7 release from endogenous neural precursor cells suppresses the tumourigenicity of stem-like glioblastoma cells. Brain 133, 1961, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Kamei N., Tanaka N., Oishi Y., et al. . NT-3, and NGF released from transplanted neural progenitor cells promote corticospinal axon growth in organotypic cocultures. Spine Phila Pa 1976 32, 1272, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Laterza C., Merlini A., De Feo D., et al. . iPSC-derived neural precursors exert a neuroprotective role in immune-mediated demyelination via the secretion of LIF. Nat Commun 4, 2597, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Tetzlaff W., Okon E.B., Karimi-Abdolrezaee S., et al. . A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma 28, 1611, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kernie S.G., Erwin T.M., and Parada L.F. Brain remodeling due to neuronal and astrocytic proliferation after controlled cortical injury in mice. J Neurosci Res 66, 317, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Ramaswamy S., Goings G.E., Soderstrom K.E., Szele F.G., and Kozlowski D.A. Cellular proliferation and migration following a controlled cortical impact in the mouse. Brain Res 1053, 38, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Fan C., Li X., Xiao Z., et al. . A modified collagen scaffold facilitates endogenous neurogenesis for acute spinal cord injury repair. Acta Biomater 51, 304, 2017 [DOI] [PubMed] [Google Scholar]
- 30.Yang Z., Zhang A., Duan H., et al. . NT3-chitosan elicits robust endogenous neurogenesis to enable functional recovery after spinal cord injury. Proc Natl Acad Sci U S A 112, 13354, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabelström H., Stenudd M., and Frisén J. Neural stem cells in the adult spinal cord. Exp Neurol 260, 44, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Lowry N., Goderie S.K., Adamo M., et al. . Multipotent embryonic spinal cord stem cells expanded by endothelial factors and Shh/RA promote functional recovery after spinal cord injury. Exp Neurol 209, 510, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Nishimura S., Yasuda A., Iwai H., et al. . Time-dependent changes in the microenvironment of injured spinal cord affects the therapeutic potential of neural stem cell transplantation for spinal cord injury. Mol Brain 6, 3, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parr A.M., Kulbatski I., Zahir T., et al. . Transplanted adult spinal cord-derived neural stem/progenitor cells promote early functional recovery after rat spinal cord injury. Neuroscience 155, 760, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Mothe A.J., Tam R.Y., Zahir T., Tator C.H., and Shoichet M.S. Repair of the injured spinal cord by transplantation of neural stem cells in a hyaluronan-based hydrogel. Biomaterials 34, 3775, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Cusimano M., Biziato D., Brambilla E., et al. . Transplanted neural stem/precursor cells instruct phagocytes and reduce secondary tissue damage in the injured spinal cord. Brain 135, 447, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butenschön J., Zimmermann T., Schmarowski N., et al. . PSA-NCAM positive neural progenitors stably expressing BDNF promote functional recovery in a mouse model of spinal cord injury. Stem Cell Res Ther 7, 11, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonnamain V., Neveu I., and Naveilhan P. Neural stem/progenitor cells as a promising candidate for regenerative therapy of the central nervous system. Front Cell Neurosci 6, 17, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cooke M.J., Vulic K., and Shoichet M.S. Design of biomaterials to enhance stem cell survival when transplanted into the damaged central nervous system. Soft Matter 6, 4988, 2010 [Google Scholar]
- 40.Piltti K.M., Salazar D.L., Uchida N., Cummings B.J., and Anderson A.J. Safety of epicenter versus intact parenchyma as a transplantation site for human neural stem cells for spinal cord injury therapy. Stem Cells Transl Med 2, 204, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piltti K.M., Avakian S.N., Funes G.M., et al. . Transplantation dose alters the dynamics of human neural stem cell engraftment, proliferation and migration after spinal cord injury. Stem Cell Res 15, 341, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzuki Y., Yanagisawa M., Yagi H., Nakatani Y., and Yu R.K. Involvement of beta1-integrin up-regulation in basic fibroblast growth factor- and epidermal growth factor-induced proliferation of mouse neuroepithelial cells. J Biol Chem 285, 18443, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Belkas J.S., Munro C.A., Shoichet M.S., Johnston M., and Midha R. Long-term in vivo biomechanical properties and biocompatibility of poly(2-hydroxyethyl methacrylate-co-methyl methacrylate) nerve conduits. Biomaterials 26, 1741, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Tsai E.C., Dalton P.D., Shoichet M.S., and Tator C.H. Matrix inclusion within synthetic hydrogel guidance channels improves specific supraspinal and local axonal regeneration after complete spinal cord transection. Biomaterials 27, 519, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Koyanagi M., Takahashi J., Arakawa Y., et al. . Inhibition of the Rho/ROCK pathway reduces apoptosis during transplantation of embryonic stem cell-derived neural precursors. J Neurosci Res 86, 270, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Tuinstra H.M., Margul D.J., Goodman A.G., et al. . Long-term characterization of axon regeneration and matrix changes using multiple channel bridges for spinal cord regeneration. Tissue Eng Part A 20, 1027, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas A.M., Kubilius M.B., Holland S.J., et al. . Channel density and porosity of degradable bridging scaffolds on axon growth after spinal injury. Biomaterials 34, 2213, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao M., Lu P., Bednark B., et al. . Templated agarose scaffolds for the support of motor axon regeneration into sites of complete spinal cord transection. Biomaterials 34, 1529, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gros T., Sakamoto J.S., Blesch A., Havton L.A., and Tuszynski M.H. Regeneration of long-tract axons through sites of spinal cord injury using templated agarose scaffolds. Biomaterials 31, 6719, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Fan J., Zhang H., He J., et al. . Neural regrowth induced by PLGA nerve conduits and neurotrophin-3 in rats with complete spinal cord transection. J Biomed Mater Res B Appl Biomater 97, 271, 2011 [DOI] [PubMed] [Google Scholar]
- 51.Günther M.I., Weidner N., Müller R., and Blesch A. Cell-seeded alginate hydrogel scaffolds promote directed linear axonal regeneration in the injured rat spinal cord. Acta Biomater 27, 140, 2015 [DOI] [PubMed] [Google Scholar]
- 52.Pawar K., Cummings B.J., Thomas A., et al. . Biomaterial bridges enable regeneration and re-entry of corticospinal tract axons into the caudal spinal cord after SCI: association with recovery of forelimb function. Biomaterials 65, 1, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomas A.M., Seidlits S.K., Goodman A.G., et al. . Sonic hedgehog and neurotrophin-3 increase oligodendrocyte numbers and myelination after spinal cord injury. Integr Biol (Camb) 6, 694, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomas A.M., and Shea L.D. Polysaccharide-modified scaffolds for controlled lentivirus delivery in vitro and after spinal cord injury. J Control Release 170, 421, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tuinstra H.M., Aviles M.O., Shin S., et al. . Multifunctional, multichannel bridges that deliver neurotrophin encoding lentivirus for regeneration following spinal cord injury. Biomaterials 33, 1618, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nomura H., Zahir T., Kim H., et al. . Extramedullary chitosan channels promote survival of transplanted neural stem and progenitor cells and create a tissue bridge after complete spinal cord transection. Tissue Eng Part A 14, 649, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Madigan N.N., McMahon S., O'Brien T., Yaszemski M.J., and Windebank A.J. Current tissue engineering and novel therapeutic approaches to axonal regeneration following spinal cord injury using polymer scaffolds. Respir Physiol Neurobiol 169, 183, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olson H.E., Rooney G.E., Gross L., et al. . Neural stem cell- and Schwann cell-loaded biodegradable polymer scaffolds support axonal regeneration in the transected spinal cord. Tissue Eng Part A 15, 1797, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mammolenti M., Gajavelli S., Tsoulfas P., and Levy R. Absence of major histocompatibility complex class I on neural stem cells does not permit natural killer cell killing and prevents recognition by alloreactive cytotoxic T lymphocytes in vitro. Stem Cells 22, 1101, 2004 [DOI] [PubMed] [Google Scholar]
- 60.Yang Y., De Laporte L., Zelivyanskaya M.L., et al. . Multiple channel bridges for spinal cord injury: cellular characterization of host response. Tissue Eng Part A 15, 3283, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Teng Y.D., Lavik E.B., Qu X., et al. . Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci U S A 99, 3024, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cummings B.J., Uchida N., Tamaki S.J., et al. . Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci U S A 102, 14069, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu P., Wang Y., Graham L., et al. . Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 150, 1264, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taylor S.J., Rosenzweig E.S., McDonald J.W., 3rd, and Sakiyama-Elbert S.E. Delivery of neurotrophin-3 from fibrin enhances neuronal fiber sprouting after spinal cord injury. J Control Release 113, 226, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Margul D.J., Park J., Boehler R.M., et al. . Reducing neuroinflammation by delivery of IL-10 encoding lentivirus from multiple-channel bridges. Bioeng Transl Med 1, 136, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McCreedy D.A., Margul D.J., Seidlits S.K., et al. . Semi-automated counting of axon regeneration in poly(lactide co-glycolide) spinal cord bridges. J Neurosci Methods 263, 15, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sontag C.J., Uchida N., Cummings B.J., and Anderson A.J. Injury to the spinal cord niche alters the engraftment dynamics of human neural stem cells. Stem Cell Rep 2, 620, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yasuda A., Tsuji O., Shibata S., et al. . Significance of remyelination by neural stem/progenitor cells transplanted into the injured spinal cord. Stem Cells 29, 1983, 2011 [DOI] [PubMed] [Google Scholar]
- 69.Sontag C.J., Nguyen H.X., Kamei N., Uchida N., Anderson A.J., and Cummings B.J. Immunosuppressants affect human neural stem cells in vitro but not in an in vivo model of spinal cord injury. Stem Cells Transl Med 2, 731, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anderson A.J., Piltti K.M., Hooshmand M.J., Nishi R.A., and Cummings B.J. Preclinical efficacy failure of human CNS-derived stem cells for use in the pathway study of cervical spinal cord injury. Stem Cell Rep 8, 249, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hori J., Ng T.F., Shatos M., Klassen H., Streilein J.W., and Young M.J. Neural progenitor cells lack immunogenicity and resist destruction as allografts. Stem Cells 21, 405, 2003 [DOI] [PubMed] [Google Scholar]
- 72.Klassen H., Schwartz M.R., Bailey A.H., and Young M.J. Surface markers expressed by multipotent human and mouse neural progenitor cells include tetraspanins and non-protein epitopes. Neurosci Lett 312, 180, 2001 [DOI] [PubMed] [Google Scholar]
- 73.McGinn M.J., Colello R.J., and Sun D. Age-related proteomic changes in the subventricular zone and their association with neural stem/progenitor cell proliferation. J Neurosci Res 90, 1159, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Piccin D., Tufford A., and Morshead C.M. Neural stem and progenitor cells in the aged subependyma are activated by the young niche. Neurobiol Aging 35, 1669, 2014 [DOI] [PubMed] [Google Scholar]
- 75.Bouab M., Paliouras G.N., Aumont A., Forest-Bérard K., and Fernandes K.J. Aging of the subventricular zone neural stem cell niche: evidence for quiescence-associated changes between early and mid-adulthood. Neuroscience 173, 135, 2011 [DOI] [PubMed] [Google Scholar]
- 76.Gordon R.J., Mehrabi N.F., Maucksch C., and Connor B. Chemokines influence the migration and fate of neural precursor cells from the young adult and middle-aged rat subventricular zone. Exp Neurol 233, 587, 2012 [DOI] [PubMed] [Google Scholar]
- 77.Sun F., Mao X., Xie L., Ding M., Shao B., and Jin K. Notch1 signaling modulates neuronal progenitor activity in the subventricular zone in response to aging and focal ischemia. Aging Cell 12, 978, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tu T.W., Kim J.H., Yin F.Q., Jakeman L.B., and Song S.K. The impact of myelination on axon sparing and locomotor function recovery in spinal cord injury assessed using diffusion tensor imaging. NMR Biomed 26, 1484, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chung K., and Coggeshall R.E. Numbers of axons in lateral and ventral funiculi of rat sacral spinal cord. J Comp Neurol 214, 72, 1983 [DOI] [PubMed] [Google Scholar]
- 80.Chung K., and Coggeshall R.E. Propriospinal fibers in the rat. J Comp Neurol 217, 47, 1983 [DOI] [PubMed] [Google Scholar]
- 81.Ogawa Y., Sawamoto K., Miyata T., et al. . Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J Neurosci Res 69, 925, 2002 [DOI] [PubMed] [Google Scholar]
- 82.Adler A.F., Lee-Kubli C., Kumamaru H., Kadoya K., and Tuszynski M.H. Comprehensive monosynaptic rabies virus mapping of host connectivity with neural progenitor grafts after spinal cord injury. Stem Cell Rep 8, 1525, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Doetsch F., and Alvarez-Buylla A. Network of tangential pathways for neuronal migration in adult mammalian brain. Proc Natl Acad Sci U S A 93, 14895, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morozov Y.M., Ayoub A.E., and Rakic P. Translocation of synaptically connected interneurons across the dentate gyrus of the early postnatal rat hippocampus. J Neurosci 26, 5017, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.