ABSTRACT
The pro-inflammatory cytokine interleukin-15 (IL15) and its receptor α (IL15RA) participate in the regulation of musculoskeletal function and metabolism. Deletion of the Il15ra gene in mice increases spontaneous activity, improves fatigue resistance in the glycolytic extensor digitorum longus (EDL) and protects from diet-induced obesity. In humans, IL15RA single-nucleotide polymorphisms (SNPs) have been linked to muscle strength, metabolism and performance in elite endurance athletes. Taken together, these features suggest a possible role for IL15RA in muscle mitochondrial structure and function. Here, we have investigated the consequences of loss of IL15RA on skeletal muscle fiber-type properties and mitochondrial ultrastructure. Immunostaining of the EDL for myosin heavy chain (MyHC) isoforms revealed no significant changes in fiber type. Electron microscopy (EM) analysis of the EDL indicated an overall higher mitochondria content, and increased cristae density in subsarcolemmal and A-band mitochondrial subpopulations. The higher cristae density in Il15ra−/− mitochondria was associated with higher OPA1 and cardiolipin levels. Overall, these data extend our understanding of the role of IL15RA signaling in muscle oxidative metabolism and adaptation to exercise.
KEY WORDS: Myokine, Fatigue, Interleukin, IL15, IL15RA, Mitochondria, Ultrastructure, Microscopy, Fiber type, OPA1, Cardiolipin
Summary: Lack of IL15RA causes ultrastructural changes in muscle mitochondrial cristae to support the energy demand associated with increased activity.
INTRODUCTION
Physical activity induces significant metabolic and molecular changes in order to support the energy demand associated with increased muscle work. Most of the energy requirements during moderate activity are met by mitochondria. These highly dynamic organelles are distributed all over the muscle fiber and are capable of undergoing significant adaptations in response to exercise (Lundby and Jacobs, 2016). Two main mitochondria subpopulations can be distinguished in muscle: subsarcolemmal (SS) and intermyofibrillar (IMF). In a cross-section, IMF mitochondria are typically smaller than SS. It is unclear what functional differences exist between the SS and IMF mitochondria (Ferreira et al., 2010; Kuznetsov et al., 2006; Lombardi et al., 2000). Metabolically, SS mitochondria respond to endurance exercise by activating fatty acid oxidation to a greater extent than IMF mitochondria (Koves et al., 2005), and these mitochondria have been shown to be compromised in obese and diabetic patients (Ritov et al., 2005). In a fiber cross-section, the IMF subpopulation can be further separated into two additional subgroups. At the I-band, elongated and interconnected mitochondria lie in close proximity to the Ca2+-releasing units (triads/dyads). At the A-band, small and rounded mitochondria are often located near capillaries (Franzini-Armstrong and Boncompagni, 2011). The amount and distribution of SS and IMF mitochondria vary between different fiber types. In fast glycolytic IIb fibers mitochondria are localized close to the I-bands, while in IIx fibers they are also found at the A-bands and occasionally in small subsarcolemmal clusters. Slower, oxidative type IIa and type I fibers have large amounts of IMF and SS mitochondria located at both I- and A-bands. In these fibers, clusters of SS mitochondria distribute along the perimeter, often in the proximity of capillaries, and have numerous contacts and interconnections (Kuznetsov et al., 2006; Franzini-Armstrong and Boncompagni, 2011; Boncompagni et al., 2012).
By controlling fusion or fission events at the level of their inner and outer membranes, mitochondria modulate their respiratory capacity and at the same time perform quality control to preserve their integrity or, in extreme situations, activate apoptosis. Mitochondrial dynamics and function respond to the energetic status of the cell. Low-energy conditions, such as occur during exercise (Picard et al., 2013) or fasting (Gomes et al., 2011; Rambold et al., 2015), promote mitochondrial interactions, fusion and elongation. An excess of nutrients, on the other hand, leads to fragmentation (Liesa and Shirihai, 2013). Inner mitochondrial membrane invaginations, called cristae, orchestrate mitochondrial function and control the release of pro-apoptotic stimuli by reorganizing in response to stressors or changes in energy balance (Cipolat et al., 2006; Frezza et al., 2006; Cogliati et al., 2013). In conditions of low ATP availability, proteins such as OPA1 promote cristae fusion (Detmer and Chan, 2007; Mannella, 2006) in order to increase the efficiency of assembly of the respiratory chain supercomplexes (Cogliati et al., 2013). Disruption of the cristae architecture is associated with severe disease conditions (Spinazzi et al., 2008; Tezze et al., 2017; Yu-Wai-Man et al., 2010).
The cytokine/myokine IL15 and its primary binding partner IL15RA, besides their well-studied role in T and natural killer (NK) cell physiology, participate in the regulation of energy processes in a variety of other tissues (He et al., 2010), including brain (Wu et al., 2010; Nguyen et al., 2017), bone (Djaafar et al., 2010; Loro et al., 2017) and muscle (Quinn et al., 2013; Pistilli et al., 2011; Loro et al., 2015). Fast muscles of mice lacking IL15RA (Il15ra−/−) are fatigue resistant (Pistilli et al., 2011) and their mitochondria more efficiently oxidize fatty acids as primary energy fuel (Loro et al., 2015). Polarographic analysis of isolated SS and IMF Il15ra−/− muscle mitochondria showed higher state 3 respiration and a tendency towards uncoupled oxygen consumption, especially in the IMF fraction (O'Connell et al., 2015).
In this study, we have analyzed the effect of lack of IL15RA in skeletal muscle by undertaking a quantitative morphometric analysis of fiber type distribution and mitochondrial ultrastructure. Using immunofluorescence and electron microscopy (EM) techniques, we have assessed the quantity, morphology and internal architecture of the different mitochondrial subpopulations. Our findings suggest that Il15ra−/− mitochondria (particularly SS and IMF A-band) undergo significant ultrastructural changes to meet the energy requirements associated with increased activity.
RESULTS
Increased spontaneous activity and AMPK activation in Il15ra−/− mice
Il15ra−/− mice had significantly higher spontaneous locomotor activity than age-matched controls over a 24-h period, as measured by in-cage beam breaks analysis (Fig. 1A). Increased activity was associated with a 3.5-fold higher basal phosphorylation of the energy sensor AMPK in Il15ra−/− tibialis anterior (TA) muscles (Fig. 1B). This finding is consistent with previous reports of increased activity (He et al., 2010; Pistilli et al., 2011) and AMP-activated protein kinase (AMPK) and acetyl-CoA carboxylase (ACC) phosphorylation in ex vivo stimulated Il15ra−/− extensor digitorum longus (EDL) (Loro et al., 2015). The intramuscular NAD and NADH levels in quadriceps muscle were not significantly different between control and Il15ra−/− mice (Fig. 1C,D). The NAD:NADH ratio trended towards a reduced redox state, consistent with increased activity of NADH-generating pathways such as β-oxidation and tricarboxcylic acid cycle (TCA). The higher in-cage spontaneous activity was not sufficient to cause changes in basal lactate levels in the serum (Fig. 1F) and in quadriceps muscle (Fig. 1E). Taken together, these data confirm that lack of IL15RA increases overall activity and activates muscle energy metabolism.
Fig. 1.
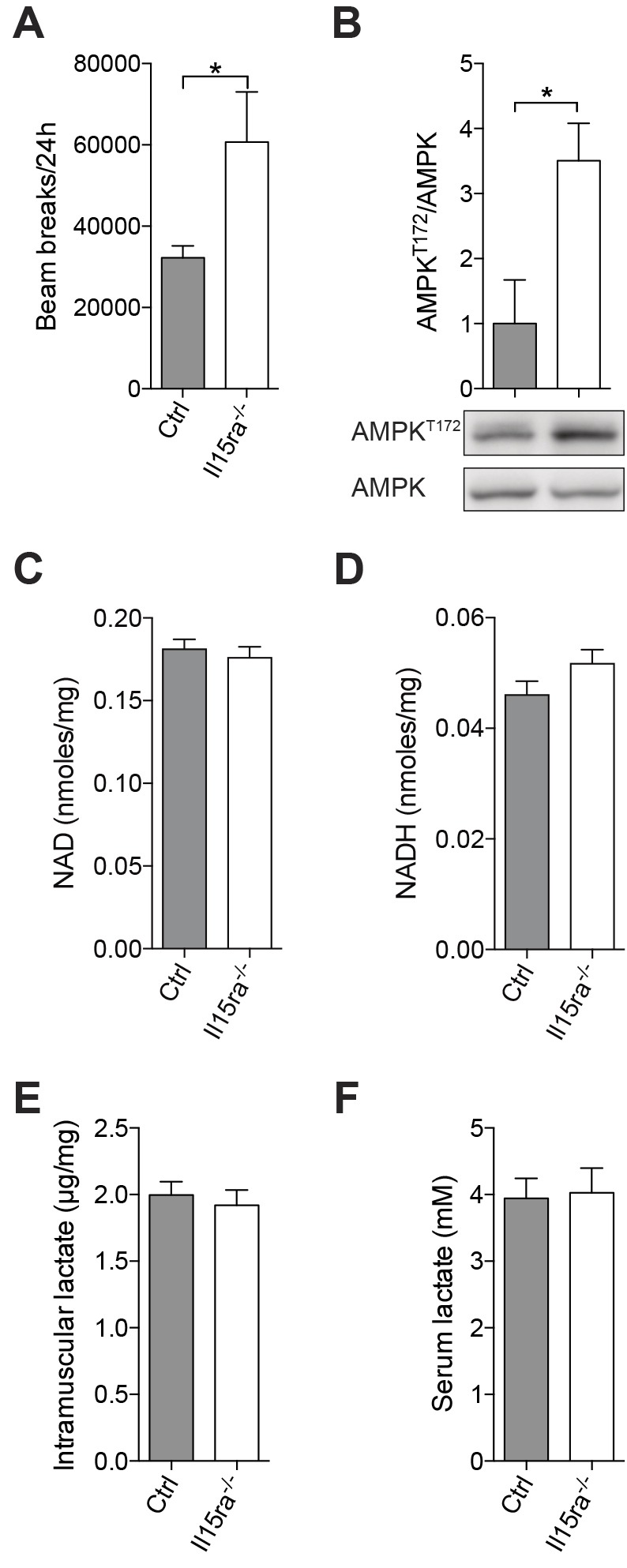
Increased spontaneous activity and AMPK phosphorylation in Il15ra−/− mice. (A) Beam breaks accumulated over 24 h (n=5, unpaired t-test, *P<0.05). (B) AMPK phosphorylation at T172 in TA muscle of mice undertaking normal in-cage activity (n=3, unpaired Mann–Whitney, *P<0.05). (C) NAD, (D) NADH, (E) intramuscular lactate dosages from quadriceps muscle (n=10–12). (F) Serum lactate dosage (n=6). Results are mean±s.e.m.
Effect of lack of IL15RA on muscle fiber type
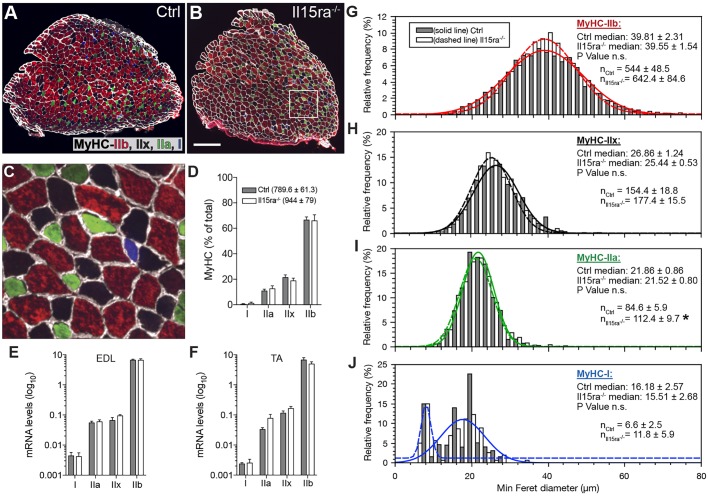
A more-oxidative muscle metabolism is often associated with a switch in fiber type, from fast/glycolytic to slow/oxidative. To test whether the increased spontaneous activity resulted in changes in fiber type composition, we immunostained EDL sections with antibodies specific for the different isoforms of myosin heavy chain (MyHC) (Fig. 2A–C). While the slow/oxidative type IIa fibers were significantly more abundant in Il15ra−/− than in controls (112.4±9.7 versus 84.6±5.9; mean±s.e.m.) (Fig. 2I), the overall fiber-type distribution was unaltered (Fig. 2D). qPCR analysis of MyHC isoforms in EDL (Fig. 2E) and tibialis anterior (TA) (Fig. 2F), as well as western blots for MyHC IIa (Myh2) and IIb (Myh4) in TA (Fig. S1) revealed that there was no significant differences between control and Il15ra−/− mice. We then tested whether, despite the unaltered fiber-type distribution, the increased activity of Il15ra−/− mice caused changes in fiber size. The median values of minimum Feret fiber diameter decreased, as expected, in the order IIb>IIx>IIa>I, but were not different between control and Il15ra−/− muscles (Fig. 2G–J). Differences in the size of type I fibers (Fig. 2J) were not informative due to the low number of fibers encountered (∼4% of the total) in the muscle sections examined.
Fig. 2.
Effect of lack of IL15RA on muscle fiber type. (A–C) MyHC staining in control (A) and Il15ra−/− (B) EDL muscles. The area enclosed in the white box is shown at 6× magnification in C. Scale bar: 200 µm (D) Relative fiber-type composition of EDL (n=5, counting all the fibers in each section). The mean±s.e.m. number of fibers per section is shown in the legend. Quantifications of MyHC isoforms by qPCR in EDL (E) and TA (F) samples (n=4). (G–J) Frequency histograms of the quantification of minimum Feret diameter of EDL fibers distinguishing between IIb (G), IIx (H), IIa (I), I (J) fibers (n=5 mice per genotype, measuring all the fibers in each section, *P<0.05).
Il15ra−/− muscle fibers have higher mitochondrial content
It was previously shown that Il15ra−/− muscles have higher mitochondrial content (Pistilli et al., 2011, 2013). To independently verify that mitochondrial content was higher, we measured the area occupied by mitochondria in low-magnification (3000×) electron micrographs of EDL type IIa fibers. We specifically focused on type IIa fibers because of the abundant mitochondrial content and because the limiting size of the hexagonal grids used for mounting the samples did not allow to image bigger fibers entirely. By first creating the outline of the fiber (Fig. 3A,B), and then applying an automated thresholding step (Fig. 3C), we measured the area occupied by the mitochondria, which was expressed as a percentage of the total area of the fiber. Il15ra−/− fibers had significantly more mitochondria than control fibers (32.5%±1.14 versus 27.9%±1.2; mean±s.e.m.) (Fig. 3D–F).
Fig. 3.
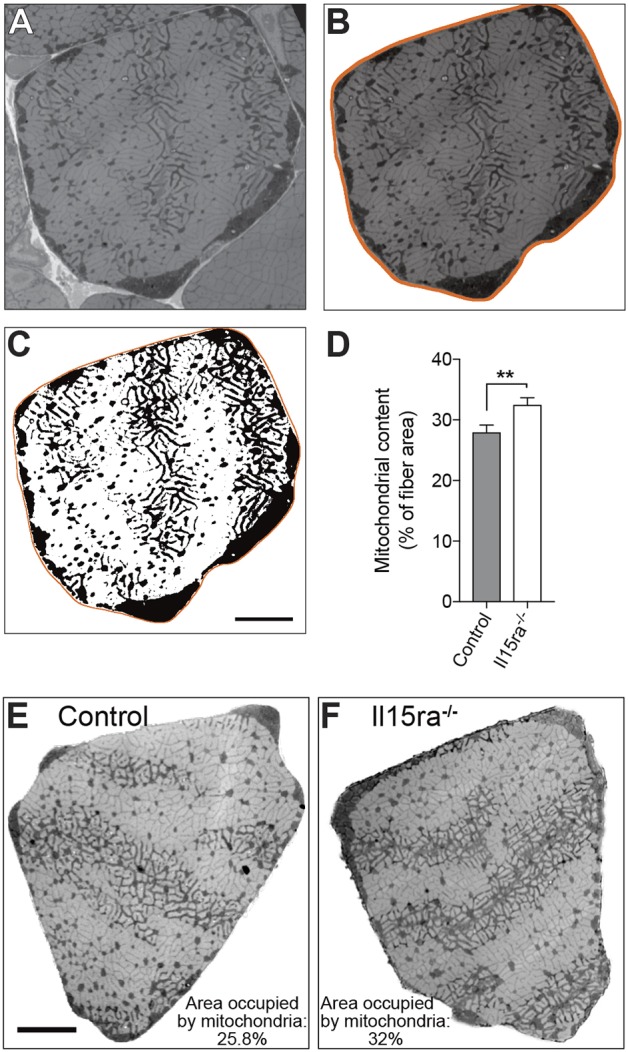
Il15ra−/− muscle fibers have higher mitochondrial content. (A) Representative EM picture (taken at 3000× magnification) of a type IIa EDL fiber used for the quantification of mitochondrial content. (B) Same IIa fiber isolated from the surrounding image. (C) Threshold mask identifying the mitochondria populations for quantification. (D) Quantification of percentage area occupied by mitochondria in every fiber analyzed (n=20, unpaired t-test, **P<0.01). Representative images of type IIa EDL fibers from control (E) and Il15ra−/− (F) muscles used for the analysis. Scale bars: 5 µm.
Il15ra−/− muscle mitochondria have increased cristae content
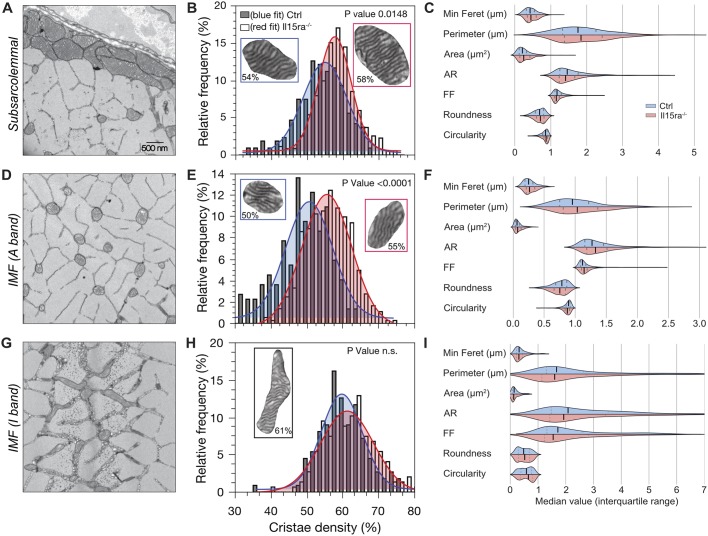
Changes in muscle mitochondrial ultrastructure reflect the metabolic state of the cell and influence the efficiency of energy-generating and -consuming reactions (Cogliati et al., 2013; Zick et al., 2009; Detmer and Chan, 2007). To analyze the ultrastructure of Il15ra−/− EDL muscle mitochondria, we undertook a systematic quantitative analysis of mitochondrial morphology and cristae content using EM. The different fiber types can be distinguished based on the amount and subcellular distribution of their mitochondria (Boncompagni et al., 2012). We focused on type IIa and type IIx fibers because of the presence of mitochondria in all three major subcellular regions (SS, A-band and I-band; Fig. 4A,D,G). The area occupied by cristae (cristae density) was expressed as a percentage of the total area enclosed by the outer mitochondrial membrane (OMM). Il15ra−/− mitochondria in the SS (Fig. 4B) and A-band (Fig. 4E) regions had a significantly higher cristae density (6.2% more for subsarcolemmal and 11.5% more for A-band), while the differences in density for the I-band mitochondria were minimal (2%, Fig. 4H). A trend for higher mitochondrial area and perimeter was noted in Il15ra−/− SS (Fig. 4C) and A-band mitochondria (Fig. 4F) compared to controls. Other descriptors of mitochondria morphology (minimum Feret diameter, aspect ratio, form factor, roundness and circularity) were distinguishable between mitochondrial subpopulations, but were not different between genotypes (Fig. 4C,F,I), suggesting that the increase in cristae content was not accompanied by a significant increase in inter-mitochondrial fusion events, which would be predicted to increase mitochondrial size.
Fig. 4.
Il15ra−/− muscle mitochondria have increased cristae content. (A) Representative EM picture of subsarcolemmal mitochondria in EDL. (B) Histogram of cristae density of SS mitochondria (3 animals/genotype; n=210–350). (C) Grouped violin plots of mitochondrial shape descriptors (see Materials and Methods). (D–I) The same analysis was repeated for intermyofibrillar mitochondria on the A-band (D–F) (3 animals/genotype; n=220-361) and on the I-band (panels G–I) (3 animals/genotype; n=110-150). Statistical analysis performed by applying a three-way mixed model with interaction followed by Tukey's multiple comparisons test. n.s., not significant.
Biochemical features of Il15ra−/− muscle mitochondria
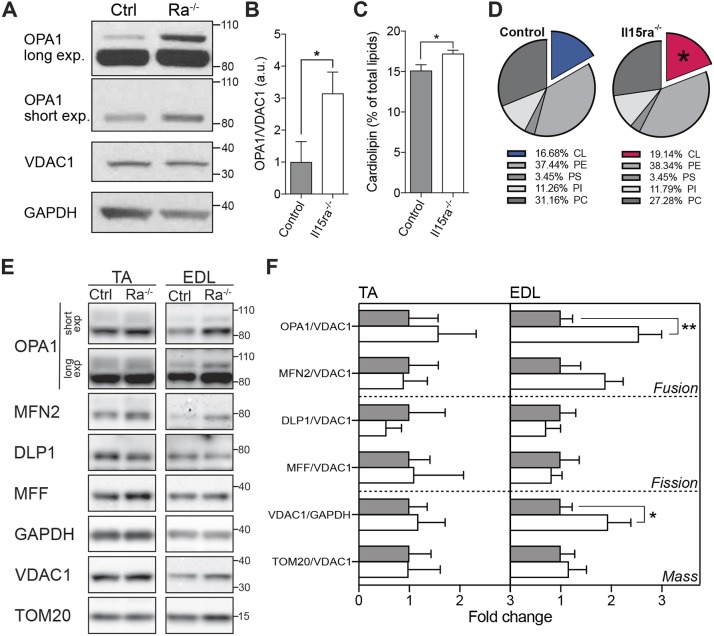
Higher cristae density is usually accompanied by increased levels of the inner mitochondrial membrane fusion protein OPA1, which confers stability to the cristae and ensures the maintenance of mitochondrial activity during energy-demanding conditions, such as muscle activity (Patten et al., 2014; Tezze et al., 2017). OPA1 protein levels, normalized to the level of the mitochondrial marker VDAC1, were 3.1 times higher in Il15ra−/− than control EDL muscles (Fig. 5A,C), consistent with the increased cristae content.
Fig. 5.
Biochemical features of Il15ra−/− muscle mitochondria. (A) Western blot analysis of OPA1, VDAC1 and GAPDH on EDL muscles from control and Il15ra−/− (Ra–/–) mice (n=4, unpaired Mann–Whitney test, *P<0.05), with bar chart presenting a quantification of total OPA1 (B). (C) Bar chart of relative cardiolipin content in mitochondrial membranes as determined by TLC analysis (unpaired Mann–Whitney test, *P<0.05). (D) Pie charts of relative lipid composition of mitochondrial membranes obtained by TLC analysis (n=6). (E) Western blot analysis of markers of mitochondrial fusion (OPA1 and MFN2), fission (DLP1 and MFF) and mass (VDAC1 and TOM20) in TA and EDL muscles, with relative quantifications (F) (n=8 for EDL and 4 for TA, unpaired Mann–Whitney test, *P<0.05; **P<0.01). a.u., arbitrary units.
The amount of mitochondrial cristae also correlates with the levels of cardiolipin, a major polyglycerophospholipid synthesized in the mitochondria and incorporated primarily in the inner mitochondrial membrane (Mejia et al., 2014), where it has both structural and functional roles (Paradies et al., 2014). Cardiolipin levels increase with exercise and play an important role in energy metabolism (Menshikova et al., 2006, 2007). The relative content of cardiolipin in isolated Il15ra−/− muscle mitochondria, as quantified by thin-layer chromatography (TLC), increased by a small but significant fraction (Fig. 5C,D; Fig. S2). Western blot analysis of mitochondrial markers comparing EDL and TA samples showed that OPA1 and VDAC1 elevation was more prominent in EDL than TA muscle (Fig. 5E,F).
DISCUSSION
In this study, we have applied biochemical techniques, immunofluorescence and electron microscopy to examine the effects of lack of IL15RA on mitochondrial organization and ultrastructure within the muscle fiber. The changes we have documented are supportive of a role for IL15RA in the determination of cellular energy metabolic processes (Loro et al., 2015, 2017; Pistilli et al., 2011, 2013). We and others have previously reported that Il15ra−/− mice have increased locomotor activity (Pistilli et al., 2011; He et al., 2010), higher mitochondrial content (Pistilli et al., 2013), and a shift towards increased muscle oxidative metabolism, which contributes to decreased ability to show fatigue and resistance to diet-induced obesity (Loro et al., 2015). All these features point towards the involvement of mitochondria as mediators of the adaptation to increased energy demand in Il15ra−/− muscles.
Previous studies focusing on the functional effects of lack of IL15RA in isolated muscle mitochondria fractions suggested that Il15ra−/− mitochondria are capable of a higher state 3 respiration (ADP-stimulated in presence of excess substrate), but also are in a partially uncoupled state (O'Connell et al., 2015). In addition, enhanced contraction-induced AMPK and ACC phosphorylation promotes mitochondrial uptake and oxidation of fatty acids in Il15ra−/− muscles (Loro et al., 2015). Since mitochondria are highly dynamic organelles in which function and structure readily adapt to the metabolic requirements of the tissue, we hypothesized that the Il15ra−/− phenotype would cause changes in mitochondrial distribution and ultrastructural organization within the muscle fiber. Consistent with previous reports (Pistilli et al., 2011; He et al., 2010), we have found that the Il15ra−/− mice were more active than controls, and that AMPK phosphorylation levels were increased in the basal state (Fig. 1). The active phenotype was not associated with changes in fiber type composition of EDL muscles (Fig. 2A–F). Because we did not use any marker specific for type IIx fibers, with our approach we were not able to quantify the occurrence of smaller intermediate changes such as in the proportion of double-positive IIa-IIx fibers. Previous publications showed that Il15ra−/− TA muscles had more fibers than controls (Pistilli et al., 2011). Our data is consistent with this, with Il15ra−/− EDL sections showing a trend towards more fibers (Fig. 2D,G–J). The higher mitochondrial density in EDL, as measured by EM (Fig. 3) and western blotting of VDAC1 (Fig. 5E,F), confirmed that Il15ra−/− muscle mitochondria undergo adaptive changes to support the increased muscle activity. To further characterize these changes and increase the resolution of our analysis, we then focused on ultrastructural features of the different subpopulations of mitochondria. Using EM, we measured shape descriptors and internal features of subsarcolemmal and intermyofibrillar (A- and I-band) mitochondria (Fig. 4). From this analysis, lack of IL15RA was associated with a significant increase in cristae density in SS and A-band mitochondria (Fig. 4A,B,D,E). These two subpopulations tend to localize in close proximity to capillaries and have lower OPA1-uptake capacity than I-band mitochondria (Franzini-Armstrong and Boncompagni, 2011). The higher cristae density in SS and A-band mitochondria in the active Il15ra−/− mice is particularly interesting since mitochondrial cristae are essential contributors to the metabolic adaptation to exercise. Together with higher intracellular lipids [also found in Il15ra−/− muscles (Loro et al., 2015)], increased mitochondria number and cristae density are well documented in muscles of endurance-trained humans and rodents (Holloszy and Booth, 1976; Gollnick and King, 1969; Hoppeler et al., 1973). Recently, Nielsen and colleagues showed that the ratio of cristae per mitochondrial volume increases significantly in endurance-trained athletes (20–30% on average in cross-country skiers and soccer players) and positively correlates with muscle mitochondria content and whole-body oxygen consumption (Nielsen et al., 2017). The increase in cristae density we measured in Il15ra−/− mitochondria (6 and 11% in SS and A-band) was quantitatively less than that reported in human endurance-trained athletes; however, it should be noted that the Il15ra−/− mice in our study were never subjected to any kind of training other than normal in-cage activity. It would be interesting to repeat this analysis in Il15ra−/− mice after endurance training, as well as analyzing mitochondrial ultrastructure in athletes carrying the rs2228059 IL15RA SNP, which we have previously shown to be associated with elite endurance performance (Pistilli et al., 2011). An increase in cristae density has important translational implications. By controlling the assembly of the complexes of the respiratory chain, mitochondrial cristae have a mechanism of modulating the extent and efficiency of mitochondrial respiration (Cogliati et al., 2013). The higher cristae density associated with endurance exercise contributes to rescuing the progeroid phenotype and the mitochondrial dysfunctions in the mtDNA mutator mouse model (Safdar et al., 2011). On the other hand, loss of cristae complexity is a typical ultrastructural defect in mitochondrial myopathies (Vincent et al., 2016). Consistent with the higher cristae density, an increase in VO2 has been previously reported in 2–3-month-old Il15ra−/− mice (He et al., 2010) and in 6–7-month-old mice on a high-fat diet (Loro et al., 2015).
Cristae composition, morphology and functionality are precisely regulated by a series of factors, among which OPA1 plays a key role (Patten et al., 2014; Frezza et al., 2006). OPA1 controls the fusion dynamics of the inner mitochondrial membrane, consequently influencing ATP synthesis and regulation of apoptosis. Mutations of OPA1 cause multi-system neurological diseases such as dominant optic atrophy (DOA) (Yu-Wai-Man et al., 2010; Carelli et al., 2015), and age-associated loss of OPA1 affects muscle mass and metabolism (Tezze et al., 2017). Conversely, OPA1 overexpression improves the phenotype of mitochondrial diseases (Civiletto et al., 2015). Western blot analysis of Il15ra−/− EDL muscles showed a significant increase of OPA1 levels (Fig. 5A,B). In addition, the amount of the inner mitochondrial membrane phospholipid cardiolipin (Mejia et al., 2014) (Fig. 5C,D), as quantified TLC, was enriched in Il15ra−/− mitochondria. Cardiolipin content positively correlates with cristae content and mitochondrial ATP production and is required to stabilize the interactions between respiratory supercomplexes (Claypool et al., 2008).
The western blot comparison of EDL and TA showed that the elevation of OPA1 and VDAC1 was more pronounced in EDL than TA Il15ra−/− muscles. Despite having similar histological (Schiaffino and Reggiani, 2011) and contractile properties (Lieber, 1997), these two muscles undergo differential recruitment during spontaneous ambulation. The EDL tendons insert at the level of the phalanges of the lateral four digits, while the TA tendon inserts on the metatarsal bone. As a consequence, in rodents, the electromyographic activation of EDL precedes that of TA under normal in-cage activity (Slawinska and Kasicki, 2002). It would be interesting to test whether increased TA load by exercise activity will reflect in an OPA1 and VDAC1 elevation similar to what found in EDL.
Together, our findings highlight an important adaptive response of Il15ra−/− mitochondria to meet the energetic requirements of the active phenotype. Because mitochondria occupy 20–40% of the total muscle volume, it is interesting that even such small changes at the level of a single organelle can translate to substantial effects on overall muscle energy metabolism. The physiological and molecular mechanisms connecting IL15RA, activity and mitochondria, as well as the possibility of applying this knowledge to treat various neuromuscular disorders (e.g. mitochondrial myopathies) are currently being investigated.
MATERIALS AND METHODS
Mice
All animal experiments were conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. 10-week-old male Il15ra−/− (Jackson Laboratory, stock No. 003723) and control B6129SF2/J (Jackson Laboratory, stock No. 101045) mice were housed at 22°C under a 12-h-light–12-h-dark cycle with food and water provided ad libitum. The 24 h ambulatory activity of single-caged animals was assessed using an Oxymax/Comprehensive Laboratory Animal Monitoring System (CLAMS; Columbus Instruments).
Tissue and serum biochemistry
Skeletal muscles were dissected and immediately frozen in liquid nitrogen. 50 mg of quadriceps muscle was homogenized in 0.6 M ice-cold perchloric acid using a bead homogenizer (Qiagen) and centrifuged at 13,400 g for 15 min. The supernatant was collected and the pH was neutralized using 1.8 M potassium carbonate. Samples were centrifuged, and the supernatant was used for lactate measurement with a commercial lactate assay kit (Trinity Biotech). The same prepared samples were used for ATP quantification with a commercially available ATP assay (Thermo Fisher). NAD and NADH content was measured as previously described. Briefly, for NAD determination, 50 mg of quadriceps muscle was homogenized in 0.6 M ice-cold perchloric acid, while for NADH samples of similar size were homogenized in ice-cold 0.25 M KOH in 50% ethanol. An enzymatic cycling assay was used as previously described to determine the content of NAD and NADH in the two extracts (Frederick et al., 2015, 2016). Blood lactate was measured from tail blood using a Lactate Pro handheld meter (Arkray, Japan).
Immunofluorescence
EDL muscles for immunofluorescence were dissected, embedded in tissue freezing medium (Electron Microscopy Science, US) and frozen in isopentane cooled with liquid nitrogen. Fiber typing immunofluorescence was performed on 10 µm frozen sections using antibodies specific for MyHC subtypes deposited to the DSHB by Prof. Schiaffino, University of Padova, Italy (DSHB Hybridoma Products BF-F3, SC-71 and BA-D5, dilution 1:100) (Schiaffino et al., 1989). Wheat germ agglutinin conjugated to Alexa Fluor 633 (Life Technologies, dilution 1:500) was used to stain the sarcolemma. Sections were imaged using an EVOS FL Auto Cell Imaging System (Life Technologies). To estimate the fiber composition of EDL muscles, all the fibers in each section (750–950) were counted and grouped according to the predominant MyHC expressed. Analysis of fiber minimum Feret diameter was performed using Fiji software (Schindelin et al., 2012, 2015).
EM and image analysis
EDL muscles were maintained at the physiological length, fixed with 4% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) at room temperature and stored in fixative at 4°C until processed by the Electron Microscopy Resource Laboratory at University of Pennsylvania for standard thin-section EM. Central cross-sectional muscle segments were post-fixed in 2% OsO4 in cacodylate buffer and embedded. Sections were stained with lead citrate solutions and imaged using a Jeol-1010 transmission electron microscope. Morphometric analysis was performed with Fiji software by manually outlining the perimeter of the fiber (at a low magnification of 3000× for the quantification of mitochondrial density in each fiber) or the outer mitochondrial membrane (at a high magnification of 30,000× for detection of mitochondrial cristae) and applying an automatic Otsu threshold. The minimum Feret diameter is the shortest distance between two lines tangential to the shape measured, and is preferable over fiber diameter or area because this method is not influenced by the angle of the section. The aspect ratio (AR), describing the length of mitochondria, is the value given by (major axis)/(minor axis) of the mitochondrion. Form factor (FF), describing the branching of mitochondria, is the value given by (4π area)/(perimeter2). Roundness [4·(surface area)/(π·major axis2)] and circularity [4π·(surface area/perimeter2)] have maximum values of 1 for a perfect sphere.
Western blotting and qPCR
Muscles were homogenized in a buffer containing 50 mM Tris-HCl pH 7.5, 150 mM NaCl, 10 mM MgCl2, 0.5 mM DTT, 1 mM EDTA, 10% glycerol, 2% SDS, 1% Triton X-100, protease and phosphatase inhibitors (Roche). After protein quantification via a bicinchoninic acid assay (BCA), the samples were run on Bis-Tris or Tris-acetate NuPage precast gels (ThermoFisher Scientific) and blotted onto PVDF membranes (Bio-Rad). Antibodies for myosin heavy chains were the same as used for immunofluorescence. Antibodies were purchased from Santa Cruz Biotechnology (TOM20, FL-145, 1:500), Cell Signaling Technologies (GAPDH D16H11, 1:5000), BD Biosciences (OPA1 18/OPA1, 1:1000; DLP1 8/DLP1, 1:1000), Sigma-Aldrich (MFN2, 1:500) or AbCam (VDAC1 20B12AF2, 1:1000; MFF, 1:1000).
RNA extraction was performed with Trizol® Reagent (Life Technologies) and was followed by cDNA synthesis with SuperScript III Reverse Transcriptase (Life Technologies). SYBR Green qPCR was run using a QuantStudio3 machine (Applied Biosystems). mRNA expression was normalized to that of the housekeeping gene Rplp0 (forward 5′-GGGCATCACCACGAAAATCTC-3′, reverse 5′- CTGCCGTTGTCAAACACCT-3′). Primers for MyHC isoforms were previously described (Sartorius et al., 1998)
TLC
Phospholipid extraction, staining and quantification were performed as previously described (Claypool et al., 2008, 2006). Briefly, phospholipids from 0.9 mg of mitochondria were extracted with chloroform/methanol. Chloroform-resuspended samples were loaded onto TLC plates and resolved using chloroform:ethanol:water:triethylamine (30:35:7:35). Phospholipids were visualized with 1.3% Molybdenum Blue spray reagent (Sigma-Aldrich), imaged and quantified with Fiji. Cardiolipin content was reported as percentage of total lipid in the mitochondrial membrane preparation.
Statistics
Data are presented as mean±s.e.m. Data were processed with GraphPad Prism 7 (GraphPad Software, La Jolla, CA) or QtiPlot. Grouped violin plots in Fig. 4C,F,I were obtained using the Seaborn 0.8.1 package for Python 3. Statistical analysis was performed with GraphPad Prism 7 or R. Unless specified differently, a two-tailed Mann–Whitney test was used for statistical analysis of nonparametric data. Data in Figs 2G and 4 was analyzed by applying a three-way mixed-effects model with interactions analysis (mouse ID: random effect; genotype and mitochondria subpopulation: fixed effects) followed by Tukey's multiple comparison test using the R (R Development Core Team, 2014) package lme4 (Bates et al., 2015). P<0.05 was considered statistically significant.
Supplementary Material
Acknowledgements
We would like to thank Prof. Clara Franzini-Armstrong (University of Pennsylvania) for the guidance with performing and interpreting the electron microscopy analyses.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: E.L.; Methodology: E.L., S.B.; Formal analysis: E.L.; Investigation: E.L., S.B.; Resources: T.S.K.; Data curation: E.L.; Writing - original draft: E.L.; Writing - review & editing: E.L., S.B., T.S.K.; Supervision: E.L.; Funding acquisition: T.S.K.
Funding
This work was supported in part by the Institute for Translational Medicine and Therapeutics of the Perelman School of Medicine at the University of Pennsylvania. Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under Award Number UL1TR000003. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.218313.supplemental
References
- Bates D., Mächler M., Bolker B. and Walker S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Soft. 67, 1-48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Boncompagni S., Protasi F. and Franzini-Armstrong C. (2012). Sequential stages in the age-dependent gradual formation and accumulation of tubular aggregates in fast twitch muscle fibers: SERCA and calsequestrin involvement. Age 34, 27-41. 10.1007/s11357-011-9211-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli V., Musumeci O., Caporali L., Zanna C., La Morgia C., Del Dotto V., Porcelli A. M., Rugolo M., Valentino M. L., Iommarini L. et al. (2015). Syndromic parkinsonism and dementia associated with OPA1 missense mutations. Ann. Neurol. 78, 21-38. 10.1002/ana.24410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolat S., Rudka T., Hartmann D., Costa V., Serneels L., Craessaerts K., Metzger K., Frezza C., Annaert W., D'Adamio L. et al. (2006). Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell 126, 163-175. 10.1016/j.cell.2006.06.021 [DOI] [PubMed] [Google Scholar]
- Civiletto G., Varanita T., Cerutti R., Gorletta T., Barbaro S., Marchet S., Lamperti C., Viscomi C., Scorrano L. and Zeviani M. (2015). Opa1 overexpression ameliorates the phenotype of two mitochondrial disease mouse models. Cell Metab. 21, 845-854. 10.1016/j.cmet.2015.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claypool S. M., McCaffery J. M. and Koehler C. M. (2006). Mitochondrial mislocalization and altered assembly of a cluster of Barth syndrome mutant tafazzins. J. Cell Biol. 174, 379-390. 10.1083/jcb.200605043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claypool S. M., Oktay Y., Boontheung P., Loo J. A. and Koehler C. M. (2008). Cardiolipin defines the interactome of the major ADP/ATP carrier protein of the mitochondrial inner membrane. J. Cell Biol. 182, 937-950. 10.1083/jcb.200801152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogliati S., Frezza C., Soriano M. E., Varanita T., Quintana-Cabrera R., Corrado M., Cipolat S., Costa V., Casarin A., Gomes L. C. et al. (2013). Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell 155, 160-171. 10.1016/j.cell.2013.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detmer S. A. and Chan D. C. (2007). Functions and dysfunctions of mitochondrial dynamics. Nat. Rev. Mol. Cell Biol. 8, 870-879. 10.1038/nrm2275 [DOI] [PubMed] [Google Scholar]
- Djaafar S., Pierroz D. D., Chicheportiche R., Zheng X. X., Ferrari S. L. and Ferrari-Lacraz S. (2010). Inhibition of T cell-dependent and RANKL-dependent osteoclastogenic processes associated with high levels of bone mass in interleukin-15 receptor-deficient mice. Arthritis Rheum. 62, 3300-3310. 10.1002/art.27645 [DOI] [PubMed] [Google Scholar]
- Ferreira R., Vitorino R., Alves R. M. P., Appell H. J., Powers S. K., Duarte J. A. and Amado F. (2010). Subsarcolemmal and intermyofibrillar mitochondria proteome differences disclose functional specializations in skeletal muscle. Proteomics 10, 3142-3154. 10.1002/pmic.201000173 [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C. and Boncompagni S. (2011). The evolution of the mitochondria-to-calcium release units relationship in vertebrate skeletal muscles. J. Biomed. Biotechnol. 2011, 830573 10.1155/2011/830573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick D. W., Davis J. G., Dávila A., Agarwal B., Michan S., Puchowicz M. A., Nakamaru-Ogiso E. and Baur J. A. (2015). Increasing NAD synthesis in muscle via nicotinamide phosphoribosyltransferase is not sufficient to promote oxidative metabolism. J. Biol. Chem. 290, 1546-1558. 10.1074/jbc.M114.579565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick D. W., Loro E., Liu L., Davila A., Chellappa K., Silverman I. M., Quinn W. J., Gosai S. J., Tichy E. D., Davis J. G. et al. (2016). Loss of NAD homeostasis leads to progressive and reversible degeneration of skeletal muscle. Cell Metab. 24, 269-282. 10.1016/j.cmet.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frezza C., Cipolat S., Martins de Brito O., Micaroni M., Beznoussenko G. V., Rudka T., Bartoli D., Polishuck R. S., Danial N. N., De Strooper B. et al. (2006). OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 126, 177-189. 10.1016/j.cell.2006.06.025 [DOI] [PubMed] [Google Scholar]
- Gollnick P. D. and King D. W. (1969). Effect of exercise and training on mitochondria of rat skeletal muscle. Am. J. Physiol. 216, 1502-1509. 10.1152/ajplegacy.1969.216.6.1502 [DOI] [PubMed] [Google Scholar]
- Gomes L. C., Di Benedetto G. and Scorrano L. (2011). During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat. Cell Biol. 13, 589-598. 10.1038/ncb2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Wu X., Khan R. S., Kastin A. J., Cornelissen-Guillaume G. G., Hsuchou H., Robert B., Halberg F. and Pan W. (2010). IL-15 receptor deletion results in circadian changes of locomotor and metabolic activity. J. Mol. Neurosci. 41, 315-321. 10.1007/s12031-009-9319-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloszy J. O. and Booth F. W. (1976). Biochemical adaptations to endurance exercise in muscle. Annu. Rev. Physiol. 38, 273-291. 10.1146/annurev.ph.38.030176.001421 [DOI] [PubMed] [Google Scholar]
- Hoppeler H., Lüthi P., Claassen H., Weibel E. R. and Howald H. (1973). The ultrastructure of the normal human skeletal muscle. Pflügers Arch. 344, 217-232. 10.1007/BF00588462 [DOI] [PubMed] [Google Scholar]
- Koves T. R., Noland R. C., Bates A. L., Henes S. T., Muoio D. M. and Cortright R. N. (2005). Subsarcolemmal and intermyofibrillar mitochondria play distinct roles in regulating skeletal muscle fatty acid metabolism. Am. J. Physiol. Cell Physiol. 288, C1074-C1082. 10.1152/ajpcell.00391.2004 [DOI] [PubMed] [Google Scholar]
- Kuznetsov A. V., Troppmair J., Sucher R., Hermann M., Saks V. and Margreiter R. (2006). Mitochondrial subpopulations and heterogeneity revealed by confocal imaging: possible physiological role? Biochim. Biophys. Acta 1757, 686-691. 10.1016/j.bbabio.2006.03.014 [DOI] [PubMed] [Google Scholar]
- Lieber R. L. (1997). Muscle fiber length and moment arm coordination during dorsi- and plantarflexion in the mouse hindlimb. Acta Anat. 159, 84-89. 10.1159/000147970 [DOI] [PubMed] [Google Scholar]
- Liesa M. and Shirihai O. S. (2013). Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 17, 491-506. 10.1016/j.cmet.2013.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi A., Damon M., Vincent A., Goglia F. and Herpin P. (2000). Characterisation of oxidative phosphorylation in skeletal muscle mitochondria subpopulations in pig: a study using top-down elasticity analysis. FEBS Lett. 475, 84-88. 10.1016/S0014-5793(00)01633-1 [DOI] [PubMed] [Google Scholar]
- Loro E., Seifert E. L., Moffat C., Romero F., Mishra M. K., Sun Z., Krajacic P., Anokye-Danso F., Summer R. S., Ahima R. S. et al. (2015). IL-15Rα is a determinant of muscle fuel utilization, and its loss protects against obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309, R835-R844. 10.1152/ajpregu.00505.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loro E., Ramaswamy G., Chandra A., Tseng W.-J., Mishra M. K., Shore E. M. and Khurana T. S. (2017). IL15RA is required for osteoblast function and bone mineralization. Bone 103, 20-30. 10.1016/j.bone.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundby C. and Jacobs R. A. (2016). Adaptations of skeletal muscle mitochondria to exercise training. Exp. Physiol. 101, 17-22. 10.1113/EP085319 [DOI] [PubMed] [Google Scholar]
- Mannella C. A. (2006). Structure and dynamics of the mitochondrial inner membrane cristae. Biochim. Biophys. Acta 1763, 542-548. 10.1016/j.bbamcr.2006.04.006 [DOI] [PubMed] [Google Scholar]
- Mejia E. M., Nguyen H. and Hatch G. M. (2014). Mammalian cardiolipin biosynthesis. Chem. Phys. Lipids. 179, 11-16. 10.1016/j.chemphyslip.2013.10.001 [DOI] [PubMed] [Google Scholar]
- Menshikova E. V., Ritov V. B., Fairfull L., Ferrell R. E., Kelley D. E. and Goodpaster B. H. (2006). Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 61, 534-540. 10.1093/gerona/61.6.534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menshikova E. V., Ritov V. B., Ferrell R. E., Azuma K., Goodpaster B. H. and Kelley D. E. (2007). Characteristics of skeletal muscle mitochondrial biogenesis induced by moderate-intensity exercise and weight loss in obesity. J. Appl. Physiol. 103, 21-27. 10.1152/japplphysiol.01228.2006 [DOI] [PubMed] [Google Scholar]
- Nguyen L., Bohlen J., Stricker J., Chahal I., Zhang H. and Pistilli E. E. (2017). Hippocampus-specific deficiency of IL-15Rα contributes to greater anxiety-like behaviors in mice. Metab. Brain Dis. 32, 297-302. 10.1007/s11011-016-9930-y [DOI] [PubMed] [Google Scholar]
- Nielsen J., Gejl K. D., Hey-Mogensen M., Holmberg H.-C., Suetta C., Krustrup P., Elemans C. P. H. and Ørtenblad N. (2017). Plasticity in mitochondrial cristae density allows metabolic capacity modulation in human skeletal muscle. J. Physiol. 595, 2839-2847. 10.1113/JP273040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell G. C., Nichols C., Guo G., Croston T. L., Thapa D., Hollander J. M. and Pistilli E. E. (2015). IL-15Rα deficiency in skeletal muscle alters respiratory function and the proteome of mitochondrial subpopulations independent of changes to the mitochondrial genome. Mitochondrion 25, 87-97. 10.1016/j.mito.2015.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradies G., Paradies V., De Benedictis V., Ruggiero F. M. and Petrosillo G. (2014). Functional role of cardiolipin in mitochondrial bioenergetics. Biochim. Biophys. Acta 1837, 408-417. 10.1016/j.bbabio.2013.10.006 [DOI] [PubMed] [Google Scholar]
- Patten D. A., Wong J., Khacho M., Soubannier V., Mailloux R. J., Pilon-Larose K., MacLaurin J. G., Park D. S., McBride H. M., Trinkle-Mulcahy L. et al. (2014). OPA1-dependent cristae modulation is essential for cellular adaptation to metabolic demand. EMBO J. 33, 2676-2691. 10.15252/embj.201488349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M., Gentil B. J., McManus M. J., White K., St. Louis K., Gartside S. E., Wallace D. C. and Turnbull D. M. (2013). Acute exercise remodels mitochondrial membrane interactions in mouse skeletal muscle. J. Appl. Physiol. 115, 1562-1571. 10.1152/japplphysiol.00819.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistilli E. E., Bogdanovich S., Garton F., Yang N., Gulbin J. P., Conner J. D., Anderson B. G., Quinn L. B. S., North K., Ahima R. S. et al. (2011). Loss of IL-15 receptor α alters the endurance, fatigability, and metabolic characteristics of mouse fast skeletal muscles. J. Clin. Invest. 121, 3120-3132. 10.1172/JCI44945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistilli E. E., Guo G. and Stauber W. T. (2013). IL-15Rα deficiency leads to mitochondrial and myofiber differences in fast mouse muscles. Cytokine 61, 41-45. 10.1016/j.cyto.2012.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn L. B. S., Anderson B. G., Conner J. D. and Wolden-Hanson T. (2013). IL-15 overexpression promotes endurance, oxidative energy metabolism, and muscle PPARδ, SIRT1, PGC-1α, and PGC-1β expression in male mice. Endocrinology 154, 232-245. 10.1210/en.2012-1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. (2014). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rambold A. S., Cohen S. and Lippincott-Schwartz J. (2015). Fatty acid trafficking in starved cells: regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Dev. Cell 32, 678-692. 10.1016/j.devcel.2015.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritov V. B., Menshikova E. V., He J., Ferrell R. E., Goodpaster B. H. and Kelley D. E. (2005). Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes 54, 8-14. 10.2337/diabetes.54.1.8 [DOI] [PubMed] [Google Scholar]
- Safdar A., Bourgeois J. M., Ogborn D. I., Little J. P., Hettinga B. P., Akhtar M., Thompson J. E., Melov S., Mocellin N. J., Kujoth G. C. et al. (2011). Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc. Natl. Acad. Sci. USA 108, 4135-4140. 10.1073/pnas.1019581108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorius C. A., Lu B. D., Acakpo-Sarchivi L., Jacobsen R. P., Byrnes W. C. and Leinwand L. A. (1998). Myosin heavy chains IIa and IId are functionally distinct in the mouse. J. Cell Biol. 141, 943-953. 10.1083/jcb.141.4.943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino S. and Reggiani C. (2011). Fiber types in mammalian skeletal muscles. Physiol. Rev. 91, 1447-1531. 10.1152/physrev.00031.2010 [DOI] [PubMed] [Google Scholar]
- Schiaffino S., Gorza L., Sartore S., Saggin L., Ausoni S., Vianello M., Gundersen K. and Lømo T. (1989). Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J. Muscle Res. Cell Motil. 10, 197-205. 10.1007/BF01739810 [DOI] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B. et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676-682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J., Rueden C. T., Hiner M. C. and Eliceiri K. W. (2015). The ImageJ ecosystem: an open platform for biomedical image analysis. Mol. Reprod. Dev. 82, 518-529. 10.1002/mrd.22489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawinska U. and Kasicki S. (2002). Altered electromyographic activity pattern of rat soleus muscle transposed into the bed of antagonist muscle. J. Neurosci. 22, 5808-5812. 10.1523/JNEUROSCI.22-14-05808.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinazzi M., Cazzola S., Bortolozzi M., Baracca A., Loro E., Casarin A., Solaini G., Sgarbi G., Casalena G., Cenacchi G. et al. (2008). A novel deletion in the GTPase domain of OPA1 causes defects in mitochondrial morphology and distribution, but not in function. Hum. Mol. Genet 17, 3291-3302. 10.1093/hmg/ddn225 [DOI] [PubMed] [Google Scholar]
- Tezze C., Romanello V., Desbats M. A., Fadini G. P., Albiero M., Favaro G., Ciciliot S., Soriano M. E., Morbidoni V., Cerqua C. et al. (2017). Age-associated loss of OPA1 in muscle impacts muscle mass, metabolic homeostasis, systemic inflammation, and epithelial senescence. Cell Metab. 25, 1374-1389.e6. 10.1016/j.cmet.2017.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A. E., Ng Y. S., White K., Davey T., Mannella C., Falkous G., Feeney C., Schaefer A. M., McFarland R., Gorman G. S. et al. (2016). The spectrum of mitochondrial ultrastructural defects in mitochondrial myopathy. Sci. Rep. 6, 30610 10.1038/srep30610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., He Y., Hsuchou H., Kastin A. J., Rood J. C. and Pan W. (2010). Essential role of interleukin-15 receptor in normal anxiety behavior. Brain Behav. Immun. 24, 1340-1346. 10.1016/j.bbi.2010.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu-Wai-Man P., Griffiths P. G., Gorman G. S., Lourenco C. M., Wright A. F., Auer-Grumbach M., Toscano A., Musumeci O., Valentino M. L., Caporali L. et al. (2010). Multi-system neurological disease is common in patients with OPA1 mutations. Brain 133, 771-786. 10.1093/brain/awq007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zick M., Rabl R. and Reichert A. S. (2009). Cristae formation-linking ultrastructure and function of mitochondria. Biochim. Biophys. Acta 1793, 5-19. 10.1016/j.bbamcr.2008.06.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.