Abstract
The extracellular matrix (ECM) regulates numerous cellular events in addition to providing structural integrity. Among several protein and enzyme families implicated in functions of the ECM, the lysyl oxidases and ADAMTS proteins are known to participate in microfibril and elastic fiber formation as well as ECM-associated signaling. A yeast two-hybrid screen to identify lysyl oxidase (LOX) binding proteins identified ADAMTSL4 as a potential interactor. We demonstrate here that several members of the LOX and ADAMTS families interact with one another. Upon investigating the interaction between LOX and ADAMTSL2 we found that the absence or inhibition of Lox affected ADAMTSL2 molecular forms and reduced its tissue levels. Thus, ADAMTSL2 stability and inter-molecular complexes may depend on the activity of lysyl oxidases.
Introduction
Complex multicellular organisms require an extracellular matrix (ECM) for structural integrity and for regulating intercellular communication [1]. Assembly of the major ECM supramolecular complexes has been shown to involve several large families of regulatory proteins in addition to structural molecules. A key enzyme family comprises the lysyl oxidases, composed of five members (LOX and LOX-Like 1-4), which share a conserved catalytic domain [2, 3]. These enzymes oxidize lysine residues on collagens, elastin and possibly other substrates, enabling inter- and intramolecular crosslinking and thus contribute to the stability of collagen fibrils, elastic fibers and other macromolecular ECM protein assemblies. LOX inhibition, such as by β-aminopropionitrile (βAPN) reduces cross-links in tissues such as bone and the aorta and is termed lathyrism [4–7]. Lox knockout mice (Lox−/−) demonstrated abnormal respiratory and cardiovascular systems and defective muscle development resulting in neonatal lethality [8–10].
The ADAMTS (A Disintegrin-like and Metalloproteinase domain with Thrombospondin type 1 Motifs) superfamily consists of 19 secreted proteases and 7 ADAMTS-like (ADAMTSL) proteins, which lack a protease domain. ADAMTS proteins are involved in procollagen processing, regulation of microfibril assembly, proteoglycan turnover as well as other processes in the ECM [11–13]. Accordingly, members of the family are widely expressed in multiple tissues (Table 1). Several ADAMTS and ADAMTSL mutations lead to connective tissue or eye disorders affecting fibrillin microfibrils [14] [12] (Table 1). ADAMTSL2, ADAMTSL4 and ADAMTSL6 bind fibrillins and appear to regulate microfibril biogenesis [15–17]. ADAMTSL2 may also be implicated in TGFβ signaling by binding to fibrillin-1 and latent TGFβ-binding protein-1 (LTBP1) [16].
Table 1.
| Gene | Highly expressing tissues | Clinical significance/Genetic disorders |
|---|---|---|
| LOX | Placenta, adipose tissue, breast, smooth muscle, heart muscle, skin, lung, bladder, endometrium | aortic aneurysm, multiple types of cancer [5, 6, 18–20] |
| LOXL1 | Placenta, adipose tissue, seminal vesicle, smooth muscle, heart muscle, bladder, ocular tissue | pseudoexfoliation syndrome [21] |
| LOXL2 | Placenta, adipose tissue, smooth muscle, bladder, appendix, endometrium | multiple types of cancer [22–24] |
| LOXL3 | Placenta, smooth muscle, bladder, spleen, bone marrow, endometrium | Stickler syndrome [25] |
| LOXL4 | Placenta, breast, seminal vesicle, smooth muscle, bone marrow, endometrium | multiple types of cancer [26] |
| ADAMTSL2 | Lung, musculoskeletal tissues, brain, dermis, myocardium | Geleophysic dysplasia [16] |
| ADAMTSL4 | Ocular tissue, vascular smooth muscle | Ectopia lentis [27], ectopia lentis et pupillae [28] |
| ADAMTS10 | Ocular tissue, lung, vascular smooth muscle, musculoskeletal tissues, connective tissue stroma of most organs | Weill-Marchesani syndrome [29] |
Despite potential regulatory roles of lysyl oxidases and ADAMTS proteins, overlapping tissue expression of members of both families and involvement in similar human diseases (e.g., Ehlers Danlos, aortic aneurysm formation; Table 1), no interaction between them has been previously demonstrated. In a yeast two-hybrid screen aimed at identifying novel substrates or regulators of LOX, we identified ADAMTSL4 as a putative interactor. Here we show that the interaction between LOX and ADAMTSL4 is shared between several members of both families. To test the biological relevance of these interactions, we investigated one ADAMTS protein, ADAMTSL2, in Lox mutant mouse embryos [9] and in LOX-inhibited mice. The findings suggest a potential role for lysyl oxidases in modifying and stabilizing ADAMTS proteins, specifically ADAMTSL2.
Results
LOX interacts with ADAMTSL4
Mutations in the human and mouse LYSYL OXIDASE (LOX) gene have devastating consequences largely due to its key roles in ECM deposition and organization [6, 9]. With the aim to better understand the mechanisms underlying its key ECM activities, a yeast 2-hybrid (Y2H) screen was performed using the human LOX cDNA encoding for amino acids 24-417 (i.e., without the signal peptide) as the bait against a human placenta library to identify putative interactors. We reasoned that interacting proteins could be potential substrates for LOX enzymatic reaction or cofactors modulating its activity and/or appropriate ECM localization.
Three distinct ADAMTSL4 cDNA clones were isolated from the Y2H screen as potential LOX interactors (Fig. 1A). Using one such clone for binary analysis with LOX, we confirmed the screen result (Fig. 1B). The overlap between the three ADAMTSL4 clones identified the selected interacting domain (SID) spanning amino acids 367-518 as a LOX-interacting domain. This region essentially comprises the cysteine-rich region and the spacer module, which are present in all ADAMTS proteins; the spacer module is noteworthy for the lack of disulfide bonds [11].
Figure 1. LOX complexes with ADAMTSL4.
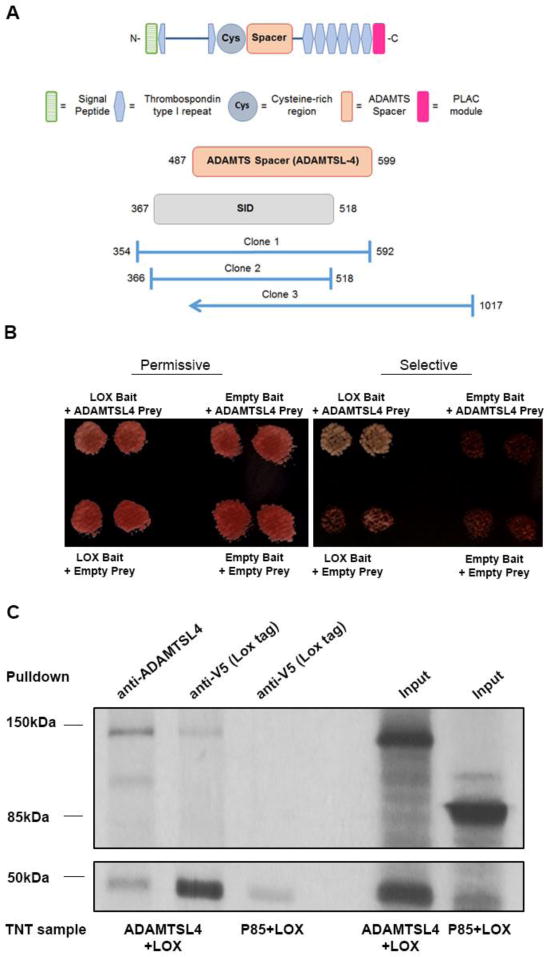
A. A yeast-two-hybrid (Y2H) screen using LOX as the bait identified three distinct ADAMTSL4 clones (clones 1-3). The selective interaction domain (SID) indicates the consensus interacting region of ADAMTSL4 determined by the overlap of the clones. B. Diploid yeast cells containing both a bait vector (LOX) and prey vector (ADAMTSL4 clone 1) were grown on nutrient permissive or selective plates along with negative controls of empty bait vector, empty prey vector and empty bait and prey vectors. The left-hand panel represents yeast colonies grown on (-)Leu (-)Trp permissive medium to maintain the growth of yeast containing both vectors, while the right-hand panel shows a replicate of the same plate on selective medium. Note that only colonies expressing both LOX and ADAMTSL4 grow on selective medium demonstrating that the two proteins interact. C. Autoradiograph of 35S-labeled proteins transcribed in the TNT system. Co-immunoprecipitation of lysates expressing Lox plus ADAMTSL4 or Lox plus P85. A band corresponding to Lox is observed slightly under 50 kDa (bottom panel), while that corresponding for ADAMTSL4 is slightly under 150 kDa (top panel). P85 has a molecular mass of 85 kDa. In the TNT reactions where both proteins were translated (as observed in the input lane, right) bands corresponding to both ADAMTSL4 and LOX are observed upon pull down of either one of the proteins. In contrast, P85, which was co-translated with LOX (right input lane), is not observed following LOX pulldown.
Yeast two-hybrid interactions occur in a reducing environment between proteins that may not carry typical mammalian post-translational modification. Therefore, we sought independent validation of the interactions between LOX and ADAMTSL4. Because attempted expression of ADAMTSL4 in HEK293 or HeLa cells met with limited success (not shown), we expressed murine V5-tagged LOX and FLAG-tagged ADAMTSL4 in the in vitro transcription & translation (TNT) system. Inclusion of 35S-methionine during translation led to radiolabeling of the recombinant proteins prior to co-immunoprecipitation (Fig. 1C). Upon translation, LOX was identified by autoradiography as a band slightly smaller than 50 kDa, whereas ADAMTSL4 expression generated the expected ~150 kDa band. Pull-down of ADAMTSL4 yielded this ~150 kDa band as well as a ~50 kDa band corresponding to the immature unprocessed form of LOX. Reciprocally, LOX pull-down using its C-terminal V5 tag co-precipitated ADAMTSL4. To verify specificity of the LOX interactions in this assay, along with LOX we co-expressed P85, an unrelated protein that is not known to interact with LOX. Immunoprecipitation of LOX did not pull down P85 (Fig. 1C). These results suggested that LOX and ADAMTSL4 interacted specifically with each other.
LOX interacts with additional members of the ADAMTS family
Because interactions between proteins are in many cases mediated by conserved domains we hypothesized that LOX might also interact with other ADAMTS proteins. Towards this end HEK293 cells were stably co-transfected with Lox and murine Adamtsl2 or human ADAMTS10 (Supp. Fig. 1). Since both proteins are secreted, conditioned media from the cells expressing LOX and either ADAMTS protein were used for co-immunoprecipitation with the anti-V5 LOX tag followed by detection of the ADAMTS proteins using their myc tag. ADAMTSL2 was detected as a major species slightly above the 150 kDa mark; ADAMTS10 between 150 kDa and 100 kDa and LOX was detected by a band around 50 kDa. We found that LOX and either ADAMTSL2 or ADAMTS10 complexed in the conditioned media demonstrating these proteins interacted extracellularly (Fig. 2A).
Figure 2. LOX forms a complex with ADAMTSL2 and ADAMTS10.
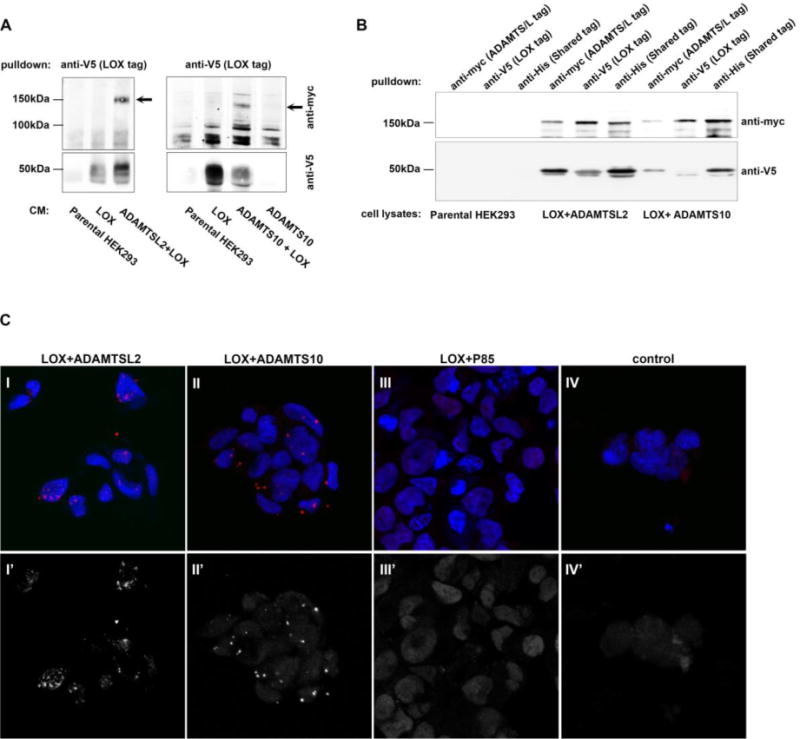
A. Co-immunoprecipitation of 4 day conditioned medium from stably transfected HEK293 cells expressing LOX plus ADAMTSL2, ADAMTSL2 alone (negative control), LOX plus ADAMTS10, or ADAMTS10 alone (negative control). B. Co-immunoprecipitation of stably transfected HEK293 cells expressing LOX plus ADAMTSL2 or LOX plus ADAMTS10 or parental HEK293 lysate (negative control). LOX migrates as two bands at ~50 kDa. ADAMTSL2 at ~150 kDa, and ADAMTS10 at 150 kDa. Membranes (A,B) were incubated with anti-myc (top) and anti-V5 (bottom). C. Proximity ligation assays performed on HEK293 cells expressing LOX+ADAMTSL2 (I, I′ IV, IV′), LOX+ADAMTS10 (II, II′) and LOX+P85 (III, III′). Anti-LOX and anti-myc antibodies were used to target LOX and ADAMTSL2/ADAMTS10 or P85, respectively. Signal amplification, marked by red signal (top, I-IV) or shown in grayscale (bottom, I′–IV′) is observed only in cells expressing LOX and an ADAMTS protein. In panel IV (control), no primary antibodies we added.
We next wished to test whether the interactions between these proteins occurred already within the secretory pathway. Therefore, immunoprecipitation was performed using lysates of the above cells. Immunoprecipitation using anti-His6 tag marking all expressed proteins was used to monitor their expression (Fig. 2B). Upon precipitation with an antibody to the anti-myc tag, present only on the ADAMTS constructs, followed by a blot against V5, the LOX specific tag, a band also corresponding to LOX appeared (Fig. 2B). Reciprocally, immunoprecipitation using the anti-V5 antibody (i.e., pulldown of LOX), followed by a western blot using anti-myc antibodies, revealed that both ADAMTS proteins were co-precipitated (Fig. 2B), confirming the interactions observed above. That complexes of the unprocessed 50 kDa form of LOX are found with ADAMTSL4 and other ADAMTS proteins in cell lysates suggests that these proteins may interact within the secretory pathway.
To further explore these intracellular interactions we performed immunohistochmical assays for LOX and the ADAMTS proteins by monitoring anti-V5 and anti-myc, respectively. Confocal analyses followed by Mander’s correlation to measure colocalization suggests that LOX and either ADAMTS protein are colocalized in HEK-293 cells (Supp. Fig. 2). To confirm these interactions, we carried out proximity ligation assays (PLA) in HEK-293 cells. In the PLA assay, a signal is observed only if the two proteins directly interact or are in the same complex, thus it serves as an in situ interaction assay. We find that LOX and either ADAMTSL2 or ADAMTS10, but not P85, interact in the cells (Fig. 2C).
ADAMTS/L family members interact with additional Lysyl oxidases
The above results demonstrating that LOX could interact with several members of the ADAMTS/L family suggested that a common domain within LOX could mediate these interactions. Since LOX family members share homology within their C-terminal domains, we also tested whether other LOX family members could interact with ADAMTS proteins.
Towards this end, HEK293 cells were stably co-transfected with LOXL3 and ADAMTSL2. Using pull-down assays we detected complexes in the conditioned medium as well as in the cell lysates (Fig. 3A,B). To extend the interactions to other family members. HEK293 were stably co-transfected with LOXL2 and either ADAMTSL2 or ADAMTS10. Conditioned media was used for co-immunoprecipitation assays using anti-myc to identify the ADAMTS proteins and showed that LOXL2 formed complexes with ADAMTSL2 and ADAMTS10 (Fig. 3C).
Figure 3. ADAMTSL2 and ADAMTS10 interact with LOXL2 and LOXL3.
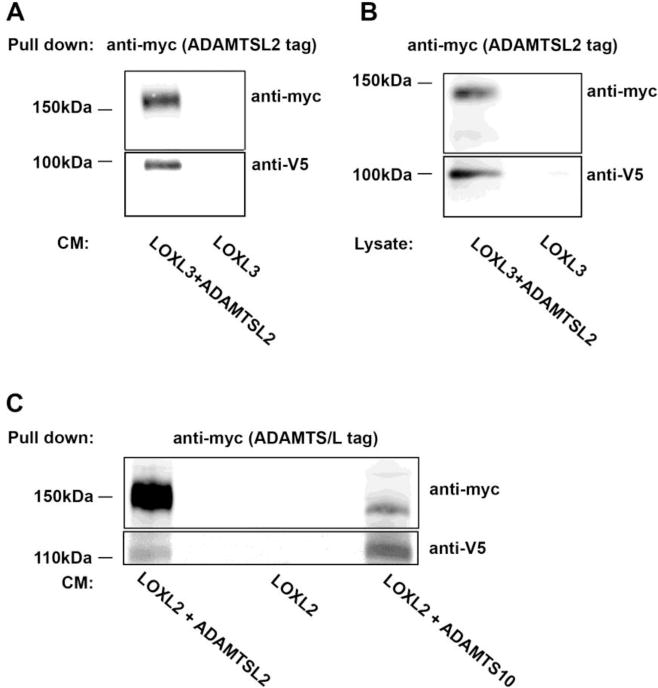
A,B. Co-immunoprecipitation from 4 day conditioned medium (A) or the cell lysate (B) from HEK293 cells stably transfected with ADAMTSL2 plus LOXL3 or LOXL3 alone. C. Co-immunoprecipitation of 4 day conditioned medium from stably transfected HEK293 cells expressing either LOXL2 plus ADAMTSL2, LOXL2 plus ADAMTS10, or LOXL2 alone.
Lox−/− mice have reduced levels of ADAMTSL2
Our observation that LOX and ADAMTS family proteins interacted with one another as a general property raised the possibility that these interactions may have a significant biological role. LOX and ADAMTSL2 were previously implicated in multiple processes including lung development and TGFβ signaling leading us to investigate their interaction further.
An ADAMTSL2 antibody was validated by western blotting of lungs from E18.5 wild-type (wt) and Adamtsl2−/− embryo lungs [30] (Fig. 4A). We used this antibody to determine the impact of LOX inactivation by western blotting of tissue extracts from brains, eyes, lungs and limbs of mutant E18.5 Lox−/− embryos. ADAMTSL2 was identified by one or two bands at around ~120 kDa in wt tissues, likely resulting from variable N-glycosylation (Fig. 4B–E) [30, 31]. Quantification of ADAMTSL2 signal demonstrated that in all these tissues, ADAMTSL2 levels were significantly lower in Lox−/− embryos than the wt littermates (Fig. 4B–E; Supp. Fig. 3). Notably, in the brain, where ADAMTSL2 is abundant, protein levels were reduced by >90% in Lox−/− embryos (Fig. 4E).
Figure 4. ADAMTSL2 content is significantly reduced in mouse Lox−/− tissues.
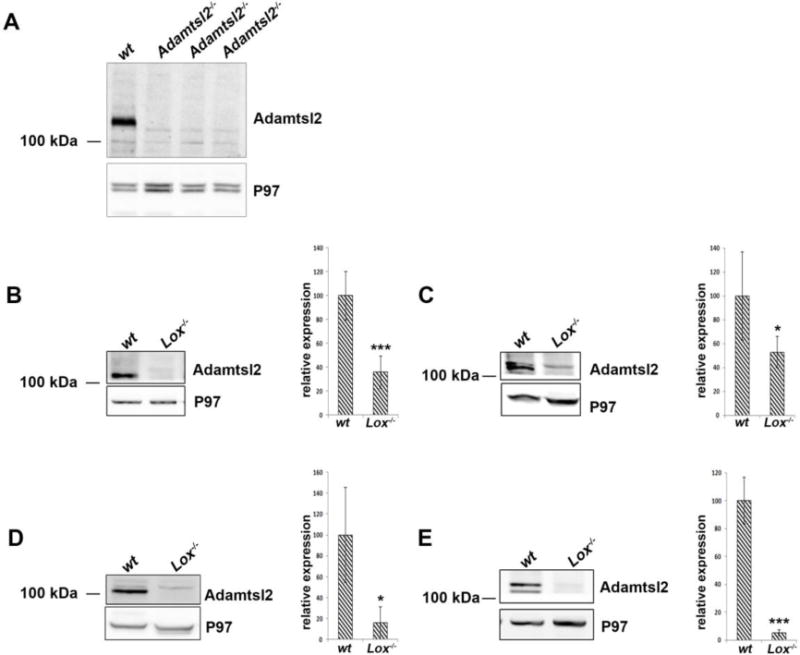
A. Western blot for ADAMTSL2 from wt and Adamtsl2−/− embryos demonstrating ADAMTSL2 antibody specificity (A). B. Western blot analysis from E18.5 wt and Lox−/− mouse eye (B), limb (C), lung (D) and brain (E) lysates. Distinct ADAMTSL2 protein bands, may arise from different glycosylation and/or processing in the different tissues. Molecular mass markers are shown on the left. Quantification of ADAMTSL2 levels measured from western blots (n=4 for each genotype) are shown to the right of each blot. Membranes were incubated with anti-ADAMTSL2 and anti-P97 as a control. * and *** indicate p< 0.05 and p<0.001, respectively.
Quantitative RT-PCR using Taqman probes showed no difference in Adamtsl2 transcript levels between wt and Lox−/− embryos (Supp. Fig. 4). Together with the observed protein-protein interactions between them, these results suggest that LOX may regulate ADAMTSL2 post-translationally.
LOX enzymatic activity is required to maintain appropriate tissue levels and molecular forms of ADAMTSL2
Reduced ADAMTSL2 levels in Lox−/− tissues prompted us to test whether this reduction was a result of a lack of LOX protein acting as a molecular chaperone or due to loss of its enzymatic activity. To distinguish between these possibilities, we treated mice with beta-aminopropionitrile (βAPN), a broad, irreversible inhibitor of all lysyl oxidases [32]. Since daily administration of the inhibitor to pregnant females resulted in embryonic lethality, the impact on embryogenesis was not determined. Therefore, since LOX and ADAMTSL2 are both expressed in the mouse aorta, which depends on its ECM for structural integrity, we chose to focus on this tissue.
Three week old mice were treated with 0.8% βAPN in their drinking water. LOX activity assay using aortic lysates verified LOX inhibition (Supp. Fig. 5). Western blot from these lysates demonstrated that LOX levels were significantly increased in the βAPN treated aortas. In contrast to LOX upregulation, a ~50% reduction in ADAMTSL2 was observed in the treated aortas (Fig. 5, Supp. Fig. 6). Although not as severe as observed in the Lox−/− mice (Fig. 4), these results demonstrate that LOX enzymatic activity is likely required for the stability and maintenance of tissue levels of ADAMTSL2.
Figure 5. βAPN treated mice show reduced ADAMTSL2 protein content.
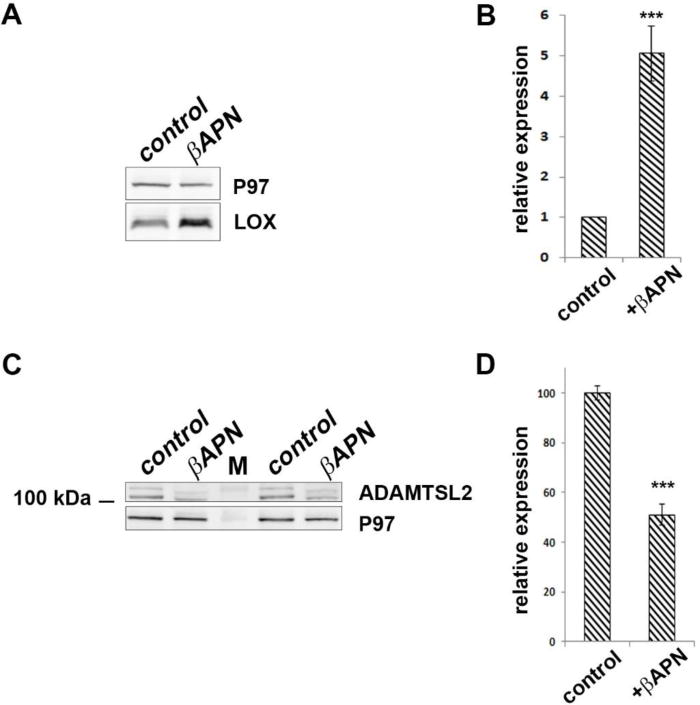
A. Western blot of pooled adult mouse aorta lysate from control and βAPN treated mice using anti-LOX. B. Quantification of LOX levels from control and βAPN treated mouse aortas from western blots (n=5; each lane is a pool of 6 aortas). LOX content is significantly upregulated following βAPN treatment. C. Western blot of pooled adult mouse aorta lysate from control and βAPN treated mice using ADAMTSL2. D. Quantification of ADAMTSL2 levels from control and βAPN treated mouse aortas from western blots (n=3; each lane is a pool of 6 aortas). ADAMTSL2 protein is significantly reduced following βAPN treatment. *** indicates p<0.001. M indicates molecular weight marker.
Western blot analysis of E18.5 wt brain and adult aorta lysates confirmed ADAMTSL2 variants at expected protein weights (120 kDa), although immunoreactive molecular species > 150 kDa were also observed. These protein bands were affected when LOX activity or levels were compromised (i.e., in brains of Lox−/− embryos or βAPN treatment in aortas). We detected three distinct large protein molecular weight bands in E18.5 mouse brains - ~300 kDa, ~200 kDa, and ~180 kDa. The ~200 kDa species was unaffected in Lox−/− brain, whereas the ~300 kDa band was undetectable in Lox−/−. In contrast, the ~180 kDa band was detected in Lox−/− lysate but absent in wt (Fig. 6A).
Figure 6. Alterations in putative ADAMTSL2 complexes following Lox deletion or inhibition.
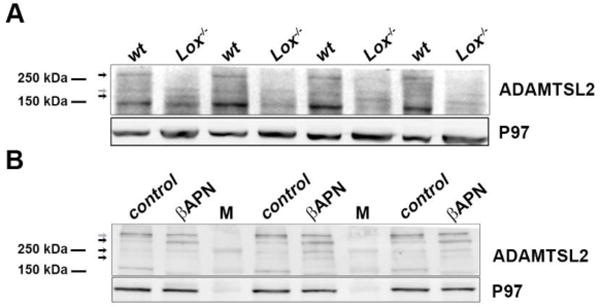
Upper parts of the gels shown in Figs. 4E and 5. A. ADAMTSL2 western blot from E18.5 wild-type and Lox−/− brain lysates. B. Western blot of ADAMTSL2 in adult control and βAPN-treated mouse aorta lysates. Arrows mark high molecular weight bands which are affected (black) or not affected (grey), by the loss of Lox (A) or βAPN treatment (B). Membranes were incubated with anti-ADAMTSL2. M designates molecular weight marker.
Four larger than expected ADAMTSL2 immunoreactive species were identified in adult mouse aorta - ~350 kDa, ~300 kDa, ~230 kDa, and ~200 kDa. Levels of the 350 kDa species were unchanged, but the βAPN treated aorta lysate had higher levels of 300, 230, and 200 kDa species compared to untreated aortas (Fig. 6B). These observations were surprising considering that the lower molecular weight bands of ADAMTSL2 (120kDa) showed reduced expression (Fig. 5). Altogether these results suggest that LOX activity may affect a variety of ADAMTSL2 complexes or cellular processing rather than just protein expression and turnover. To directly test whether lysyl oxidases can oxidize ADAMTSL2, we undertook an in vitro oxidation assay using recombinant LOXL3 [33]. However, we neither detected an enzymatic activity of LOXL3 on ADAMTSL2 (Supp. Fig. 7), nor reduction of ADAMTSL2 in LoxL3 mutant brains (not shown).
Discussion
Protein-protein interactions in the ECM are fundamental for the formation of supramolecular complexes that regulate tissue development, structure and function. To identify how LOX, a key regulator of ECM organization and deposition [3, 34], executes its activities, a yeast 2-hybrid screen was used to detect putative substrates or modifiers and identified ADAMTSL4 as a LOX interactor. Using in vitro pull-down and immunoprecipitation assays we show that the observed interaction between LOX and ADAMTSL4 extends to interactions between other members of both families. Focusing on ADAMTSL2, we find that its levels and molecular species are significantly affected in Lox mutants or enzymatically-inhibited mice suggesting that the interactions between these two protein families are of biological significance.
The more severe reduction of ADAMTSL2 in Lox−/− mice than in the βAPN-treated aortas suggests that whereas LOX enzymatic activity is indeed important for the stability and maintenance of ADAMTSL2, the LOX protein itself, which is otherwise unaffected by βAPN, is necessary for maintaining ADAMTSL2 levels. In this respect, it could act as a molecular chaperone, given the formation of complexes in the secretory pathway, suggested by PLA in the co-transfected HEK293 cells. In vitro, in the TNT system, where no proteolytic activation of LOX occurs, an interaction with ADAMTSL4 is also observed. Since βAPN inhibits lysyl oxidase enzymatic activity in general and not just that of LOX [4], the observed effect could also be caused by inhibited activity of the other LOX family members. Taken together, these observations could thus explain the intermediate reduction of ADAMTSL2 in the βAPN treated animals.
How could LOX activity affect the observed size of the ADAMTSL2 complexes? The 150 kDa ADAMTSL2 band arises from fully glycosylated ADAMTSL2 [31]. The larger species observed in western blots done under reducing conditions could arise from intermolecular crosslinks. Thus, in the absence of the crosslinking activity of LOX, distinct components of the complexes may be lost. Alternatively, LOX could directly oxidize and crosslink ADAMTSL2 itself and the distinct bands observed are fragments of the putative crosslinked protein partner or its proteolytically derived isoforms. Active recombinant LOX that can be used in an oxidation assay is unavailable. Thus although an interaction between LOXL3 and ADAMTSL2 was observed in vitro, determining the mechanistic relationship between these two proteins in vivo may be more complex.
Recent studies demonstrated elevated TGFβ signaling in Lox mutants [8, 35, 36]. Dermal fibroblasts from individuals with geleophysic dysplasia, bearing ADAMTSL2 mutations as well as Adamtsl2 null bronchial epithelial cells demonstrate increase TGFβ signaling [16, 30]. While ADAMTSL2 regulates microfibril assembly and interacts with LTBP1, which sequesters TGFβ [14, 16, 30, 37] interactions between LOX and TGFβ signaling are less clear. LOX has been found to physically interact with TGFβ and to attenuate its activity [35]. It also regulates HTRA1, a protease that cleaves TGFβ ligands [36]. However, could the excessive activation of the cascade be also due to the LOX-dependent reduction of ADAMTSL2? LOX has a central role in ECM stability by its ability to crosslink tropoelastin to generate mature elastic fibers. Elastic fiber deposition relies on microfibril scaffolds as a template [38]. They are dependent on the interaction of fibulin-5 with integrins on one side and the association with fibrillin-1 microfibrils on the other [39]. LOX binds tropoelastin together with fibulin-4. By attaching to fibulin-5, this complex is then relocated to fibrillin microfibrils where LOX catalyzes tropoelastin crosslinking [38, 40–42]. ADAMTSL2 is found on fibrillin microfibrils [43] and interacts with LOX (this work). Through proper regulation of this complex, LTBP1, which binds ADAMTSL2 and is docked on microfibrils, interacts and modulates TGFβ signaling. Thus it is possible that part of the excessive TGFβ signaling observed in Lox−/− embryos is caused by improper microfibril association with LTBP1 via reduced ADAMTSL2.
The building of complex structures such as those in the ECM requires multiple processes to take place in parallel or consecutively. The formation of protein hubs that participate in several complexes is a simple way to orchestrate such tasks. Here we show that the ECM modifiers belonging to the LOX family can interact with several members of the ADAMTS superfamily, themselves matrix modifiers. Future work should be able to delineate how much of the observed activities of LOX protein are mediated via the activities of ADAMTS proteins and whether the latter depend on LOX for carrying out their roles in the ECM.
Materials and Methods
Yeast two-hybrid screen
Yeast two-hybrid screening was performed by Hybrigenics Services, SAS, Paris, France (http://www.hybrigenics-services.com) using the coding sequence for the human LOX protein (accession number AAB23549.1; aa 24–417; without the signal peptide) as a bait. The constructs were sequence-verified and used as a bait to screen a random-primed human placenta cDNA library fused to Gal4. The placental cDNA library, is the richest among the libraries available at Hybrigenics due to the large number of proteins expressed in it.
In vitro transcription and translation
Human ADAMTSL4 and mouse Lox cDNAs were cloned into mammalian expression vectors pcDNA3.1 and pFLAG-CMV9 respectively. Lox was cloned with C-terminal V5+His6 tags, ADAMTSL4 with a C-terminal FLAG tag. Human P85 cDNA was subcloned into pcDNA3.1 with HA tag and kindly provided by Ami Aronheim (Technion, IL). In-vitro transcription and translation reactions were performed using the TNT®-coupled reticulocyte lysate system with T7 RNA polymerase and 35S-labeled methionine (Promega). The reactions were carried out according to manufacturer’s protocols.
Cell culture transfection and lysis
HEK293 cells were used to express selected proteins by transfection of their vectors: mLox in pcDNA3.1 (V5/His tags), mADAMTSL2 and hADAMTS10 in pcDNA3.1 (Myc/His tags), and hLOXL2 in pLenti6 (FLAG tag). Cell cultures were grown on 10 cm plates in Dulbecco’s Modified Eagle Medium (DMEM) containing 1% L-glutamine, 1% penicillin-streptomycin, and 4% horse serum. Lipofectamine® 2000 was used as transfection reagent according to the manufacturer’s protocol. Cell lysates were generated from stably transfected HEK293 cells by scraping the culture with 1 ml immunoprecipitation buffer (20 mM Tris-HCl [pH 7], 0.3 M NaCl, 2 mM EDTA, 1% NP-40 [IGEPAL® CA-630, Sigma], 1:100 Protease inhibitors [AEBSF Hydrochloride, cat#101500, Calbiochem]). The lysate was centrifuged to discard cell debris and to obtain clear supernatant.
HEK293 conditioned medium
Stably transfected HEK293 cells were grown on 10 cm plates. At 80% confluence the plates were gently washed twice with PBS and medium was switched to serum-free DMEM (containing 1% L-glutamine and 1% Penicillin Streptomycin). Conditioned medium was collected after four days incubation.
Immunoprecipitation
Immunoprecipitations from TNT reactions were carried out using 4 μL of the reaction volume incubated with 2.5 μg anti-FLAG (Sigma, F1804), anti-ADAMTSL4 (Proteintech, 15304-1-AP), or anti-V5 (MBL, PM003) in 150 μl immunoprecipitation buffer (20 mM Tris-HCl [pH 7], 0.3 M NaCl, 2 mM EDTA, 1% NP-40 [IGEPAL® CA-630, Sigma] containing 0.2mM AEBSF Hydrochloride, (cat#101500, Calbiochem]) overnight at 4°C and then with 40 μl protein G Dynabeads for one hour. Protein complexes were washed three times in PBS and subsequently extracted with 1X SDS loading buffer for 3 minutes at 95°C.
Pull down of proteins from cell cultures was done by incubating 1 mg cell lysate with 5 μg anti-myc (Santa-Cruz, 9E10, sc-40), anti-V5 (MBL, PM003), or anti-His6 (Santa-Cruz, sc-803) in 1 ml immunoprecipitation buffer as described above.
Immunoprecipitation from conditioned medium was carried out by incubating 8 ml of medium with 5 μg anti-V5 (MBL, PM003), or anti-myc (Santa-Cruz, 9E10, sc-40) for 48 hours at 4°C and then with 40 μl protein G Dynabeads overnight. Protein complexes were washed three times in PBS and subsequently extracted with 1X SDS loading buffer for 3 minutes at 95°C.
Western blotting
Protein lysates harvested from mouse tissue, HEK293 cell lysate and immunoprecipitations were subjected to reducing SDS-PAGE analysis. Proteins in polyacrylamide gels were transferred to nitrocellulose membranes, which were then probed with the following antibodies: anti-myc (Santa-Cruz, 9E10, sc-40, HRP-conjugated, 1:500); anti-V5 (Invitrogen, R960-25, 1:2000); anti-FLAG (Sigma, F1804, 1:500); anti-LOX (LSBio, LS-C143168, 1:3000); and anti-ADAMTSL2 (GeneTex, GTX102069, 1:500). Equal protein loading was determined using an antibody directed against P97 (1:3000; kindly provided by Ariel Stanhill, Technion, IL) or actin (MP Biochemicals; clone C4, 1:5000).
Mice
Experiments involving mice were done in compliance with institutional and national animal welfare laws, guidelines and policies under protocols approved by the Cleveland Clinic or Technion IACUC. Embryonic age was staged according to [44]; midnight of the day a vaginal plug was observed marks the beginning of the embryonic development (E0). Adamtsl2 mutant mice and Lox mutant mice were previously described [9, 30].
βAPN treatment of adult mice
βAPN at a concentration of 0.8% dissolved in 2% sucrose in DDW was administered to the drinking water of 3 week-old mice. Water was changed every other day. After 4 weeks mouse aortas were harvested for analysis. C57Bl/6 mice drink, on average, 6 ml of water/day [45]. Hence, on average each mouse had consumed ~0.06gr of βAPN/day.
Harvest and lysis of mouse tissue
Harvested mouse tissues were placed in RIPA lysis buffer (10 mM Tris-HCl [pH 7], 2 mM EDTA, 1% NP-40 [IGEPAL® CA-630, Sigma], 0.1% sodium deoxycholate [D6750-10G, Sigma], 1:100 protease inhibitors [AEBSF Hydrochloride, cat#101500, Calbiochem] and ground using zirconium oxide beads in a bullet blender. Processed tissue was then centrifuged and the cleared supernatant was retained for analysis.
RNA Isolation and Real-Time PCR
Total RNA was isolated from E18.5 mouse brains using a Nucleo Spin RNA II kit (740955.10; Macherey Nagel) and reverse transcription Real-time qPCR was performed with the following Taqman probes (Life Technologies): Hprt, Mm01256744, ADAMTSL2, Mm01326794_m1.
Proximity Ligation Assay
The assay (Duolink in situ PLA, DUO92101, Sigma) was performed according the manufacturer’s instructions. Briefly, HEK293 cells transfected with LOX-V5 and ADAMTS-myc protein were grown on 13 mm coverslips and fixed using 4% PFA. Antibody staining (anti-LOX or anti-V5 and anti-myc, 9E10) were incubated overnight followed by ligation and amplification according to the protocol. Finally, slides were incubated with DAPI, to mark nuclei, washed and mounted. Stainings were imaged using ZEISS confocal microscope LSM700.
Statistical analyses
Statistical analysis was carried out using GraphPad software. Quantification of western blot protein bands was done with TotalLab Quant software. The unpaired two-tailed Student t test was used in all assays. Significance was reached with a minimum p value of <0.05.
Supplementary Material
Supp. Figure 1. Expression of ADAMTSL2 and ADAMTS10. HEK293 cells were stably transfected with murine myc-tagged ADAMTSL2 plus human V5-tagged LOX, myc-tagged human ADAMTS10 plus V5-tagged LOX, or with empty vector. Western blot of cell lysates is shown for anti-myc (top) and anti-V5 (bottom).
Supp. Figure 2. LOX colocalizes with ADAMTS proteins. Confocal images of HEK293 cells immunostained for V5 and myc highlighting LOX-V5 and ADAMTSL2-myc or ADAMTS10-myc demonstrate that the two proteins colocalize. Note that LOX expression in the cells is wider than that of the ADAMTS protein. Mander’s correlation coefficient measuring the colocalization (ratios of 0.5-1.0 suggest two proteins colocalize) demonstrate a value >0.9 for the correlation of the ADAMTS protein vs. LOX.
Supp. Figure 3. ADAMTSL2 is reduced in Lox mutant tissues. Lysates of E18.5 tissues from wt and Lox−/− embryos were blotted for ADAMTSL2 and used for quantification.
Supp. Figure 4. LOX does not affect Adamtsl2 transcription. No significant changes in Adamtsl2 mRNA expression were identified using Taqman RT-PCR in wt and Lox−/− E18.5 embryonic brains.
Supp. Figure 5. LOX activity is reduced in aortas of βAPN treated mice. Aortas from mice treated for 4 weeks with βAPN or drinking water as control were lysed and processed for a LOX activity assay.
Supp. Figure 6. LOX enzymatic activity is required for maintaining ADAMTSL2 levels. ADAMTSL2 western blot of aortas from mice treated with βAPN-or control (vehicle, distilled, deionized water) that were used for quantification.
Supp. Figure 7. In vitro oxidation assay. In vitro oxidation assay with recombinant LOXL3 and ADAMTSL2. Diaminopentane is used as the control substrate, with or without ADAMTSL2. The blocking of the reaction upon βAPN addition demonstrates the reaction is LOXL3-dependent. Addition of LOXL3 and ADAMTSL2 does not alter the colorimetric reaction.
Research highlights.
Lysyl oxidases and ADAMTS proteins are key ECM modifying proteins.
We screened for LOX interactors and found that ADAMTSL4 complexes with it.
We found that distinct LOX-family members are able to bind several ADAMTS proteins.
In Lox mutant tissues ADAMTSL2 protein levels and molecular species are significantly affected.
The enzymatic activity of LOX is required for the regulation of ADAMTSL2 levels and molecular species.
Acknowledgments
We wish to thank members of our lab for insightful comments and helpful discussions and the personnel of the Animal Core Facilities, Technion for assistance in maintaining the mouse colonies.
Funding
P.H. was supported by grants from the Israeli Science Foundation [1072/13], Binational Science Foundation [2011437] and a Rappaport Family Institute Research Grant. S.A. was supported by funds from the U.S. National Institutes of Health (Award R01-EY021151) and awards from the Marfan Foundation (to S.A., and D.H.). S. A. is also supported by an American Heart Association-Paul G. Allen Frontiers Group Distinguished Investigator Award. J.M. was supported by the Academy of Finland Center of Excellence 2012-2017 Grant 251314 and Academy of Finland Project Grant 296498.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
The authors declare no competing or financial interests.
Author contributions
R.A and S.Z.E. performed the majority of the experiments. D.H. provided the Adamtsl2 mutant mice and carried out the in vitro oxidation assay. R.A., D.H., S.S.A and P.H. planned the study and wrote the manuscript.
References
- 1.Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol. 2010;341(1):126–40. doi: 10.1016/j.ydbio.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Csiszar K. Lysyl oxidases: a novel multifunctional amine oxidase family. Prog Nucleic Acid Res Mol Biol. 2001;70:1–32. doi: 10.1016/s0079-6603(01)70012-8. [DOI] [PubMed] [Google Scholar]
- 3.Mäki JM. Lysyl oxidases in mammalian development and certain pathological conditions. Histol Histopathol. 2009;24(5):651–60. doi: 10.14670/HH-24.651. [DOI] [PubMed] [Google Scholar]
- 4.Dawson DA, Rinaldi AC, Poch G. Biochemical and toxicological evaluation of agent-cofactor reactivity as a mechanism of action for osteolathyrism. Toxicology. 2002;177(2–3):267–84. doi: 10.1016/s0300-483x(02)00233-0. [DOI] [PubMed] [Google Scholar]
- 5.Guo DC, Regalado ES, Gong L, Duan X, Santos-Cortez RL, Arnaud P, Ren Z, Cai B, Hostetler EM, Moran R, Liang D, Estrera A, Safi HJ, Leal SM, Bamshad MJ, Shendure J, Nickerson DA, Jondeau G, Boileau C, Milewicz DM. LOX Mutations Predispose to Thoracic Aortic Aneurysms and Dissections. Circ Res. 2016;118(6):928–34. doi: 10.1161/CIRCRESAHA.115.307130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee VS, Halabi CM, Hoffman EP, Carmichael N, Leshchiner I, Lian CG, Bierhals AJ, Vuzman D, Mecham RP, Frank NY, Stitziel NO. Loss of function mutation in LOX causes thoracic aortic aneurysm and dissection in humans. Proc Natl Acad Sci U S A. 2016;113(31):8759–64. doi: 10.1073/pnas.1601442113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ren W, Liu Y, Wang X, Jia L, Piao C, Lan F, Du J. Beta-Aminopropionitrile monofumarate induces thoracic aortic dissection in C57BL/6 mice. Sci Rep. 2016;6:28149. doi: 10.1038/srep28149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kutchuk L, Laitala A, Soueid-Bomgarten S, Shentzer P, Rosendahl AH, Eilot S, Grossman M, Sagi I, Sormunen R, Myllyharju J, Maki JM, Hasson P. Muscle composition is regulated by a Lox-TGFbeta feedback loop. Development. 2015;142(5):983–93. doi: 10.1242/dev.113449. [DOI] [PubMed] [Google Scholar]
- 9.Mäki JM, Rasanen J, Tikkanen H, Sormunen R, Makikallio K, Kivirikko KI, Soininen R. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation. 2002;106(19):2503–9. doi: 10.1161/01.cir.0000038109.84500.1e. [DOI] [PubMed] [Google Scholar]
- 10.Mäki JM, Sormunen R, Lippo S, Kaarteenaho-Wiik R, Soininen R, Myllyharju J. Lysyl oxidase is essential for normal development and function of the respiratory system and for the integrity of elastic and collagen fibers in various tissues. Am J Pathol. 2005;167(4):927–36. doi: 10.1016/S0002-9440(10)61183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Apte SS. A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms. J Biol Chem. 2009;284(46):31493–7. doi: 10.1074/jbc.R109.052340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Goff C, Cormier-Daire V. The ADAMTS(L) family and human genetic disorders. Hum Mol Genet. 2011;20(R2):R163–7. doi: 10.1093/hmg/ddr361. [DOI] [PubMed] [Google Scholar]
- 13.Porter S, I, Clark M, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. Biochem J. 2005;386(Pt 1):15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hubmacher D, Apte SS. Genetic and functional linkage between ADAMTS superfamily proteins and fibrillin-1: a novel mechanism influencing microfibril assembly and function. Cell Mol Life Sci. 2011;68(19):3137–48. doi: 10.1007/s00018-011-0780-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabriel LA, Wang LW, Bader H, Ho JC, Majors AK, Hollyfield JG, Traboulsi EI, Apte SS. ADAMTSL4, a secreted glycoprotein widely distributed in the eye, binds fibrillin-1 microfibrils and accelerates microfibril biogenesis. Invest Ophthalmol Vis Sci. 2012;53(1):461–9. doi: 10.1167/iovs.10-5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Goff C, Morice-Picard F, Dagoneau N, Wang LW, Perrot C, Crow YJ, Bauer F, Flori E, Prost-Squarcioni C, Krakow D, Ge G, Greenspan DS, Bonnet D, Le Merrer M, Munnich A, Apte SS, Cormier-Daire V. ADAMTSL2 mutations in geleophysic dysplasia demonstrate a role for ADAMTS-like proteins in TGF-beta bioavailability regulation. Nat Genet. 2008;40(9):1119–23. doi: 10.1038/ng.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsutsui K, Manabe R, Yamada T, Nakano I, Oguri Y, Keene DR, Sengle G, Sakai LY, Sekiguchi K. ADAMTSL-6 is a novel extracellular matrix protein that binds to fibrillin-1 and promotes fibrillin-1 fibril formation. J Biol Chem. 2010;285(7):4870–82. doi: 10.1074/jbc.M109.076919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox TR, Bird D, Baker AM, Barker HE, Ho MW, Lang G, Erler JT. LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res. 2013;73(6):1721–32. doi: 10.1158/0008-5472.CAN-12-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cox TR, Erler JT. Lysyl oxidase in colorectal cancer. Am J Physiol Gastrointest Liver Physiol. 2013;305(10):G659–66. doi: 10.1152/ajpgi.00425.2012. [DOI] [PubMed] [Google Scholar]
- 20.Rachman-Tzemah C, Zaffryar-Eilot S, Grossman M, Ribero D, Timaner M, Maki JM, Myllyharju J, Bertolini F, Hershkovitz D, Sagi I, Hasson P, Shaked Y. Blocking Surgically Induced Lysyl Oxidase Activity Reduces the Risk of Lung Metastases. Cell Rep. 2017;19(4):774–784. doi: 10.1016/j.celrep.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thorleifsson G, Magnusson KP, Sulem P, Walters GB, Gudbjartsson DF, Stefansson H, Jonsson T, Jonasdottir A, Jonasdottir A, Stefansdottir G, Masson G, Hardarson GA, Petursson H, Arnarsson A, Motallebipour M, Wallerman O, Wadelius C, Gulcher JR, Thorsteinsdottir U, Kong A, Jonasson F, Stefansson K. Common sequence variants in the LOXL1 gene confer susceptibility to exfoliation glaucoma. Science. 2007;317(5843):1397–400. doi: 10.1126/science.1146554. [DOI] [PubMed] [Google Scholar]
- 22.Barker HE, Chang J, Cox TR, Lang G, Bird D, Nicolau M, Evans HR, Gartland A, Erler JT. LOXL2-mediated matrix remodeling in metastasis and mammary gland involution. Cancer Res. 2011;71(5):1561–72. doi: 10.1158/0008-5472.CAN-10-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, Mikels A, Vaysberg M, Ghermazien H, Wai C, Garcia CA, Velayo AC, Jorgensen B, Biermann D, Tsai D, Green J, Zaffryar-Eilot S, Holzer A, Ogg S, Thai D, Neufeld G, Van Vlasselaer P, Smith V. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med. 2010;16(9):1009–17. doi: 10.1038/nm.2208. [DOI] [PubMed] [Google Scholar]
- 24.Cano A, Santamaria PG, Moreno-Bueno G. LOXL2 in epithelial cell plasticity and tumor progression. Future Oncol. 2012;8(9):1095–108. doi: 10.2217/fon.12.105. [DOI] [PubMed] [Google Scholar]
- 25.Alzahrani F, Al Hazzaa SA, Tayeb H, Alkuraya FS. LOXL3, encoding lysyl oxidase-like 3, is mutated in a family with autosomal recessive Stickler syndrome. Hum Genet. 2015;134(4):451–3. doi: 10.1007/s00439-015-1531-z. [DOI] [PubMed] [Google Scholar]
- 26.Choi SK, Kim HS, Jin T, Moon WK. LOXL4 knockdown enhances tumor growth and lung metastasis through collagen-dependent extracellular matrix changes in triple-negative breast cancer. Oncotarget. 2017;8(7):11977–11989. doi: 10.18632/oncotarget.14450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahram D, Sato TS, Kohilan A, Tayeh M, Chen S, Leal S, Al-Salem M, El-Shanti H. A homozygous mutation in ADAMTSL4 causes autosomal-recessive isolated ectopia lentis. Am J Hum Genet. 2009;84(2):274–8. doi: 10.1016/j.ajhg.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christensen AE, Fiskerstrand T, Knappskog PM, Boman H, Rodahl E. A novel ADAMTSL4 mutation in autosomal recessive ectopia lentis et pupillae. Invest Ophthalmol Vis Sci. 2010;51(12):6369–73. doi: 10.1167/iovs.10-5597. [DOI] [PubMed] [Google Scholar]
- 29.Dagoneau N, Benoist-Lasselin C, Huber C, Faivre L, Megarbane A, Alswaid A, Dollfus H, Alembik Y, Munnich A, Legeai-Mallet L, Cormier-Daire V. ADAMTS10 mutations in autosomal recessive Weill-Marchesani syndrome. Am J Hum Genet. 2004;75(5):801–6. doi: 10.1086/425231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hubmacher D, Wang LW, Mecham RP, Reinhardt DP, Apte SS. Adamtsl2 deletion results in bronchial fibrillin microfibril accumulation and bronchial epithelial dysplasia–a novel mouse model providing insights into geleophysic dysplasia. Dis Model Mech. 2015;8(5):487–99. doi: 10.1242/dmm.017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koo BH, Le Goff C, Jungers KA, Vasanji A, O’Flaherty J, Weyman CM, Apte SS. ADAMTS-like 2 (ADAMTSL2) is a secreted glycoprotein that is widely expressed during mouse embryogenesis and is regulated during skeletal myogenesis. Matrix Biol. 2007;26(6):431–41. doi: 10.1016/j.matbio.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Narayanan AS, Siegel RC, Martin GR. On the inhibition of lysyl oxidase by -aminopropionitrile. Biochem Biophys Res Commun. 1972;46(2):745–51. doi: 10.1016/s0006-291x(72)80203-1. [DOI] [PubMed] [Google Scholar]
- 33.Kraft-Sheleg O, Zaffryar-Eilot S, Genin O, Yaseen W, Soueid-Baumgarten S, Kessler O, Smolkin T, Akiri G, Neufeld G, Cinnamon Y, Hasson P. Localized LoxL3-Dependent Fibronectin Oxidation Regulates Myofiber Stretch and Integrin-Mediated Adhesion. Dev Cell. 2016;36(5):550–61. doi: 10.1016/j.devcel.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Smith-Mungo LI, Kagan HM. Lysyl oxidase: properties, regulation and multiple functions in biology. Matrix Biol. 1998;16(7):387–98. doi: 10.1016/s0945-053x(98)90012-9. [DOI] [PubMed] [Google Scholar]
- 35.Atsawasuwan P, Mochida Y, Katafuchi M, Kaku M, Fong KS, Csiszar K, Yamauchi M. Lysyl oxidase binds transforming growth factor-beta and regulates its signaling via amine oxidase activity. J Biol Chem. 2008;283(49):34229–40. doi: 10.1074/jbc.M803142200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang H, Leung L, Saturno G, Viros A, Smith D, Di Leva G, Morrison E, Niculescu-Duvaz D, Lopes F, Johnson L, Dhomen N, Springer C, Marais R. Lysyl oxidase drives tumour progression by trapping EGF receptors at the cell surface. Nat Commun. 2017;8:14909. doi: 10.1038/ncomms14909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hubmacher D, Apte SS. ADAMTS proteins as modulators of microfibril formation and function. Matrix Biol. 2015;47:34–43. doi: 10.1016/j.matbio.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernandez MR. Ultrastructural immunocytochemical analysis of elastin in the human lamina cribrosa. Changes in elastic fibers in primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 1992;33(10):2891–903. [PubMed] [Google Scholar]
- 39.Hirai M, Ohbayashi T, Horiguchi M, Okawa K, Hagiwara A, Chien KR, Kita T, Nakamura T. Fibulin-5/DANCE has an elastogenic organizer activity that is abrogated by proteolytic cleavage in vivo. J Cell Biol. 2007;176(7):1061–71. doi: 10.1083/jcb.200611026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choudhury R, McGovern A, Ridley C, Cain SA, Baldwin A, Wang MC, Guo C, Mironov A, Jr, Drymoussi Z, Trump D, Shuttleworth A, Baldock C, Kielty CM. Differential regulation of elastic fiber formation by fibulin-4 and -5. J Biol Chem. 2009;284(36):24553–67. doi: 10.1074/jbc.M109.019364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horiguchi M, Inoue T, Ohbayashi T, Hirai M, Noda K, Marmorstein LY, Yabe D, Takagi K, Akama TO, Kita T, Kimura T, Nakamura T. Fibulin-4 conducts proper elastogenesis via interaction with cross-linking enzyme lysyl oxidase. Proc Natl Acad Sci U S A. 2009;106(45):19029–34. doi: 10.1073/pnas.0908268106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, Yanagisawa H, Zuo J, Li T. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet. 2004;36(2):178–82. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- 43.Jensen SA, I, Robertson B, Handford PA. Dissecting the fibrillin microfibril: structural insights into organization and function. Structure. 2012;20(2):215–25. doi: 10.1016/j.str.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 44.Kaufmann MH. In: The Atlas of Mouse Development. Kaufmann MH, editor. 1992. [Google Scholar]
- 45.Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet. 2002;32(6):435–43. doi: 10.1023/a:1020884312053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp. Figure 1. Expression of ADAMTSL2 and ADAMTS10. HEK293 cells were stably transfected with murine myc-tagged ADAMTSL2 plus human V5-tagged LOX, myc-tagged human ADAMTS10 plus V5-tagged LOX, or with empty vector. Western blot of cell lysates is shown for anti-myc (top) and anti-V5 (bottom).
Supp. Figure 2. LOX colocalizes with ADAMTS proteins. Confocal images of HEK293 cells immunostained for V5 and myc highlighting LOX-V5 and ADAMTSL2-myc or ADAMTS10-myc demonstrate that the two proteins colocalize. Note that LOX expression in the cells is wider than that of the ADAMTS protein. Mander’s correlation coefficient measuring the colocalization (ratios of 0.5-1.0 suggest two proteins colocalize) demonstrate a value >0.9 for the correlation of the ADAMTS protein vs. LOX.
Supp. Figure 3. ADAMTSL2 is reduced in Lox mutant tissues. Lysates of E18.5 tissues from wt and Lox−/− embryos were blotted for ADAMTSL2 and used for quantification.
Supp. Figure 4. LOX does not affect Adamtsl2 transcription. No significant changes in Adamtsl2 mRNA expression were identified using Taqman RT-PCR in wt and Lox−/− E18.5 embryonic brains.
Supp. Figure 5. LOX activity is reduced in aortas of βAPN treated mice. Aortas from mice treated for 4 weeks with βAPN or drinking water as control were lysed and processed for a LOX activity assay.
Supp. Figure 6. LOX enzymatic activity is required for maintaining ADAMTSL2 levels. ADAMTSL2 western blot of aortas from mice treated with βAPN-or control (vehicle, distilled, deionized water) that were used for quantification.
Supp. Figure 7. In vitro oxidation assay. In vitro oxidation assay with recombinant LOXL3 and ADAMTSL2. Diaminopentane is used as the control substrate, with or without ADAMTSL2. The blocking of the reaction upon βAPN addition demonstrates the reaction is LOXL3-dependent. Addition of LOXL3 and ADAMTSL2 does not alter the colorimetric reaction.


