SUMMARY
Bovine herpesvirus 1 (BoHV-1), a significant viral pathogen of cattle, causes inflammation in affected tissue during acute infection. Consequently, we tested whether productively infected bovine cells stimulate inflammasome formation. Expression of two components required for inflammasome formation, the DNA sensor IFI16 (gamma-interferon-inducible protein 16) and NLRP3 (NOD-like receptor family, pyrin domain containing 3), were induced in bovine kidney cells by eight hours after infection. IFI16 was detected in punctate granules localized to the cytoplasm and nucleus. During productive infection, more than ten times more cells were caspase 1 positive, which is activated following inflammasome formation.Two caspase 1 inhibitors had no effect on productive infection. Conversely, another caspase 1 inhibitor, glyburide, significantly inhibited virus infection suggesting it had off-target effects on related enzymes or interfered with infection via non-enzymatic mechanisms. Collectively, these studies demonstrated that BoHV-1 infection stimulated inflammasome formation, which we predict is important for clinical symptoms in cattle.
Keywords: BoHV-1, inflammasome, DNA sensor IFI16, NLRP3, caspase 1
Bovine herpesvirus 1 (BoHV-1) is an alpha-herpesvirinae subfamily member that causes significant economical losses to the cattle industry. Infection of cattle with BoHV-1 can lead to conjunctivitis, genital disorders, abortions and bovine respiratory disease complex, a life threatening respiratory tract infection, reviewed by (Jones, 2009; Jones and Chowdhury, 2007).The ability of BoHV-1 to induce immune suppression in cattle is important for its pathogenic potential, reviewed in (Jones, 2009). For example, infection inhibits cell-mediated immunity (Carter et al., 1989; Griebel et al., 1990; Griebel et al., 1987a; Griebel et al., 1987b), CD8+ T cell recognition of infected cells (Hariharan et al., 1993; Hinkley et al., 1998; Koppers-Lalic et al., 2005; Nataraj et al., 1997), and induces apoptosis in CD4+ T cells (Eskra and Splitter, 1997; Winkler et al., 1999). Furthermore, two viral regulatory proteins, bICP0 and bICP27, inhibit interferon dependent transcription (da Silva and Jones, 2012; Henderson et al., 2005; Jones, 2009; Saira et al., 2007; Saira and Jones, 2009). Finally, infection erodes mucosal surfaces within the upper respiratory tract, and consequently promotes establishment of bacterial pathogens in the lower respiratory tract (Highlander, 2000, Highlander et al., 2001; Zecchinon and Desmecht, 2005).
The focus of these studies was to examine the effect that BoHV-1 had on inflammasome formation. The rational for these studies stem from observations demonstrating that acute infection of calves leads to inflammation in the trachea, trigeminal ganglia, tonsils, and tissues within the ocular cavity (Perez et al., 2005, 2006; Winkler et al., 2002; Winkler et al., 2000). It is also clear that other viruses, including Epstein Barr virus and herpes simplex virus type 1 (HSV-1), activate the inflammasome during productive infection (Gastaldello et al., 2013; Johnson et al., 2013). For these studies, bovine kidney cells (CRIB) were infected with BoHV-1 and at various times after infection expression of cellular proteins that promote inflammasome formation were examined (IFI16 and NLRP3) (Latz et al., 2013). Polyclonal antibodies that recognize human, mouse, and rat proteins were used for these studies to ensure the possibility that they recognized the respective bovine protein. IFI16 and NLRP3 were consistently induced by 8 hours after infection (Figure 1A). The IFI16 antibody detected three prominent proteins, which stages o are consistent with the size of IFI16 proteins expressed in rodents and humans (68, 75,95 kD). The bovine IFI16 ORF is nearly the same size as other mammalian IFI16 proteins, and has several conserved domains (C Jones, unpublished data). The NLRP3 antibody detected a prominent 72 kD band after infection, also consistent with the size of the NLRP3 protein expressed in humans and rodents. Expression of the bICP0 protein was readily detected at 2 hours after infection as well as 1 hour after infection when the blot was exposed for longer periods of time (data not shown). Although bICP0 was only detected in infected cells, β-actin protein expression was detected at similar levels in all samples. At 16, 24, and 32 hours after infection, the levels of IFI16 and NLRP3 were similar to 8 hours after infection (Figure 1B). In summary, these results suggested that accumulation of late viral gene products triggered inflammasome formation or a viral-encoded or –induced factor interfered with inflammasome formation during early stages of infection.
Figure 1. BoHV-1 infection induces IFI16 and NLRP3 protein expression.
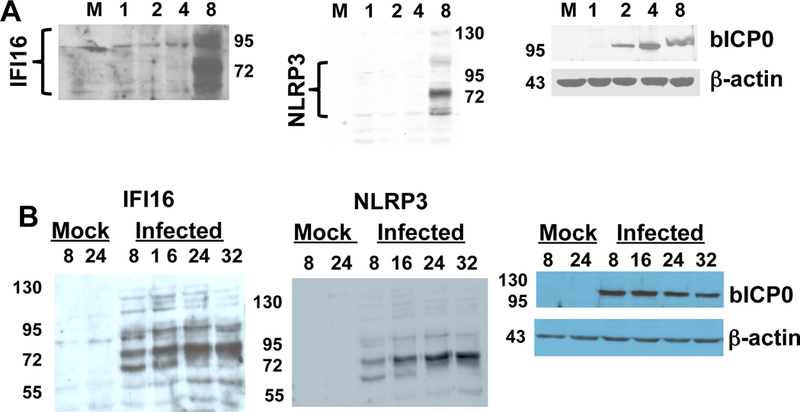
Panel A: CRIB cells were infected with BoHV-1 (1 plaque forming unit/cell of the CooperStrain) for 1, 2, 4, or 8 hours after infection. Total cell lysate was prepared as previously described (Workman et al., 2012; Workman et al., 2011). As a control, cell lysate from mock-infected cells was used (lane M).
Panel B: CRIB cells were infected as described in panel A for 8, 16, 24, or 32 hours after infection. As controls, cell lysate from mock-infected cells that were cultured for 8 or 24 hours were examined (Mock lane).
Proteins from each sample in Panels A and B (100 ug protein) were separated on a SDS-polyacrylamide gel and proteins subsequently transferred to a membrane. Western blot analysis was performed using antibodies directed against IFI16 (sc-6050, Santa Cruz Biotechnology), NLRP3 (ab4207, Abcam), bICP0 (peptide specific antibody), and β-actin as previously described (Workman et al., 2011, 2012; Workman and Jones, 2010). The results in panels A and B are consistent with 5 independent experiments.
Confocal microscopy was performed to determine the localization of IFI16 in CRIB cells after infection. Several studies demonstrated IFI16 is present in the nucleus; conversely other studies concluded IFI16 is localized to the cytoplasm (Duan et al., 2011; Gariano and Gariglio, 2012; Orzalli et al., 2012; Unterholzner et al., 2010; Veeranki and Choubey, 2012). At 16 hours after infection, most of the IFI16 was detected in granules localized to areas surrounding the nucleus (Figure 2). Close inspection of the images also suggested that a small percentage of these granules were present in a subset of nuclei. As expected, IFI16 and bICP0 were not detected in mock-infected cells, and bICP0 was primarily localized to the nucleus. Biochemical fractionation of infected cells further suggested that IFI16 may be present in the nuclear fraction of infected cells at 16 hours after infection (Figure 2B). The cytoplasmic fraction also contained IFI16, which was consistent with the confocal microscopy studies. Histone 3 (H3) was primarily detected in the nuclear extract at 16 hours after infection as expected. As previously demonstrated in primary bovine cells infected with BHV-1 (Frizzo da Silva and Jones, 2011), a subset of bICP0 was detected in the cytoplasm; however most of the bICP0 protein was detected in the nuclear fraction. Evidence is accumulating for a functional role of IFI16 in controlling HSV-1 infection because IFI16 requires an intact nuclear localization signal to bind to HSV-1 DNA in infected cells and activate IFN-β expression (Li et al., 2012). An independent study demonstrated that IFI16 sensing of HSV-1 DNA is nuclear in human foreskin fibroblast cells (Orzalli et al., 2012). It will be of interest to determine whether IFI16 interacts with BoHV-1 DNA during productive infection and influences viral replication.
Figure 2. Localization of IFI16 after BoHV-1 infection.
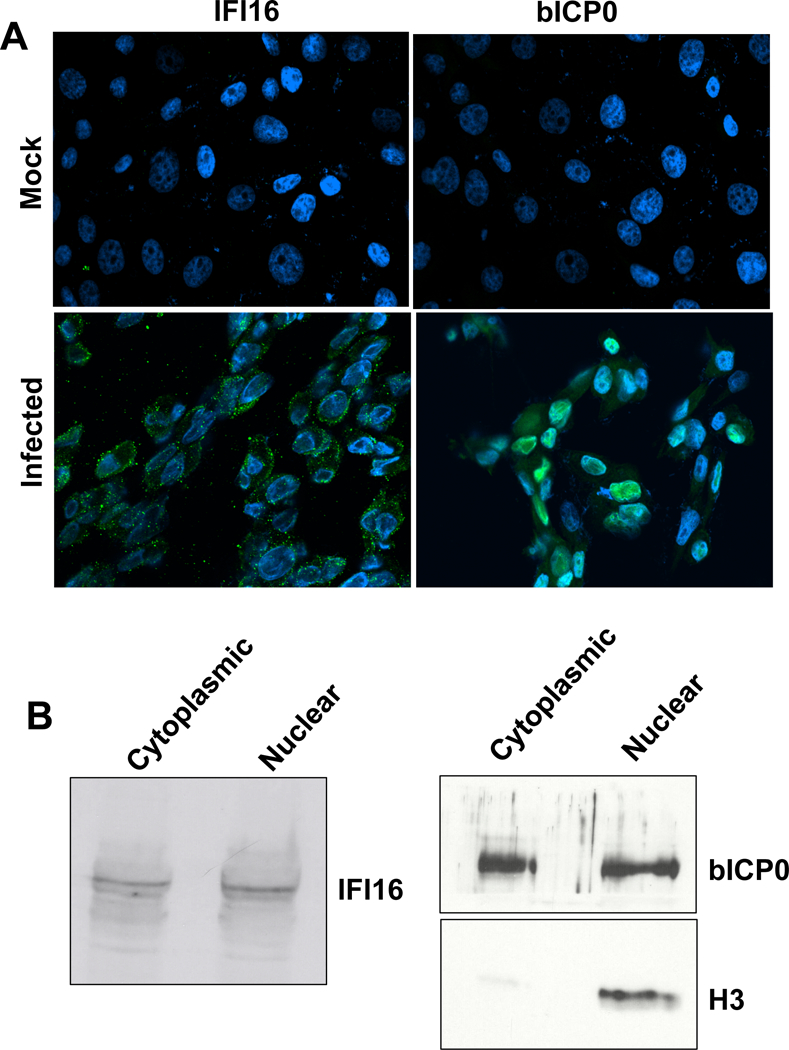
Panel A: CRIB cells were infected with BoHV-1 (1 plaque forming unit/cell) for 16 hours (infected panels). As a control, mock-infected cells were also examined. Confocal microscopy was performed to test whether IFI16 and bICP0 (rabbit-specific sera) were expressed (green staining) using previously published results (Sinani and Jones, 2011). The secondary antibody, goat anti-rabbit/Alexa Fluor 488 (Invitrogen/Molecular Probes A11008) (1:100) was added and cells were incubated for 1 hour at room temperature in the dark. DAPI (Thermo Scientific 46190) (1:1000) was used to stain nuclear DNA (blue). An Olympus IX 81 Inverted confocal laser-scanning microscope was used to collect images (excitation/emission at 488/520 nm and 633/660 nm). The merged images are shown.
Panel B: Biochemical fractionation of IFI16 following infection. Cytoplasmic and nuclear extracts were prepared from CRIB cells that were infected for 16 hours as previously described (Workman and Jones, 2011). In brief, CRIB cells were harvested by centrifugation at 1,000 rpm for 5 minutes and washed twice in cold phosphate buffered saline (PBS). Cells were then suspended in buffer A (50 mM NaCl, 10 mM HEPES pH8, 500 mM sucrose, 1 mM EDTA, 0.5% NP40), vortexed briefly, and centrifuged at 5,000 X g for 2 minutes. The supernatant was collected and saved as the cytoplasmic fraction. The nuclear pellet was washed twice with low salt buffer B (50 mM NaCl, 10 mM HEPES pH8, 25% glycerol, 0.1 mM EDTA). The pellet was then suspended in high salt lysis buffer C (350 mM NaCl, 10 mM HEPES pH8, 25% glycerol, 0.1mM EDTA). Lysis was carried out at 4 °C for 30 min. Supernatant was cleared by centrifuging at 13,000 rpm for 15 minutes at 4 °C. Similar amounts of protein (100 ug/lane) were used for Western blot analysis. The histone 3- (H3) specific antibody (ab1191, Abcam) was diluted 1:500 and was used as a control for proteins that are present in the nucleus.
Caspase 1 cleavage and activation is the hallmark of inflammasome formation, reviewed by (Grant and Dixit, 2013; Latz et al., 2013; Stutz, 2009). Therefore, it was of interest to determine whether caspase 1 was cleaved and activated following infection. We initially tested whether caspase 1 was cleaved using polyclonal antibodies. These antibodies did not yield reliable results: perhaps because they do not recognize bovine caspase 1 (data not shown). Consequently, we measured caspase 1 activity in infected cells using a fluorescent peptide (Immunochemistry Technologies, Bloomington, MN) that specifically and covalently binds activated caspase 1. Approximately 10 times more fluorescent positive cells were observed at 16 hours after infection when compared to uninfected cells (Figure 3A). To confirm these studies, flow cytometry was used to quantify the number of caspase 1-activated cells after infection relative to mock-infected cells. In agreement with studies in Figure 3A, there was a significant difference between the number of caspase 1 positive cells at 16 hours after infection relative to mock-infected cells (Figure 3B).
Figure 3. Analysis of caspase 1 following infection of CRIB cells.
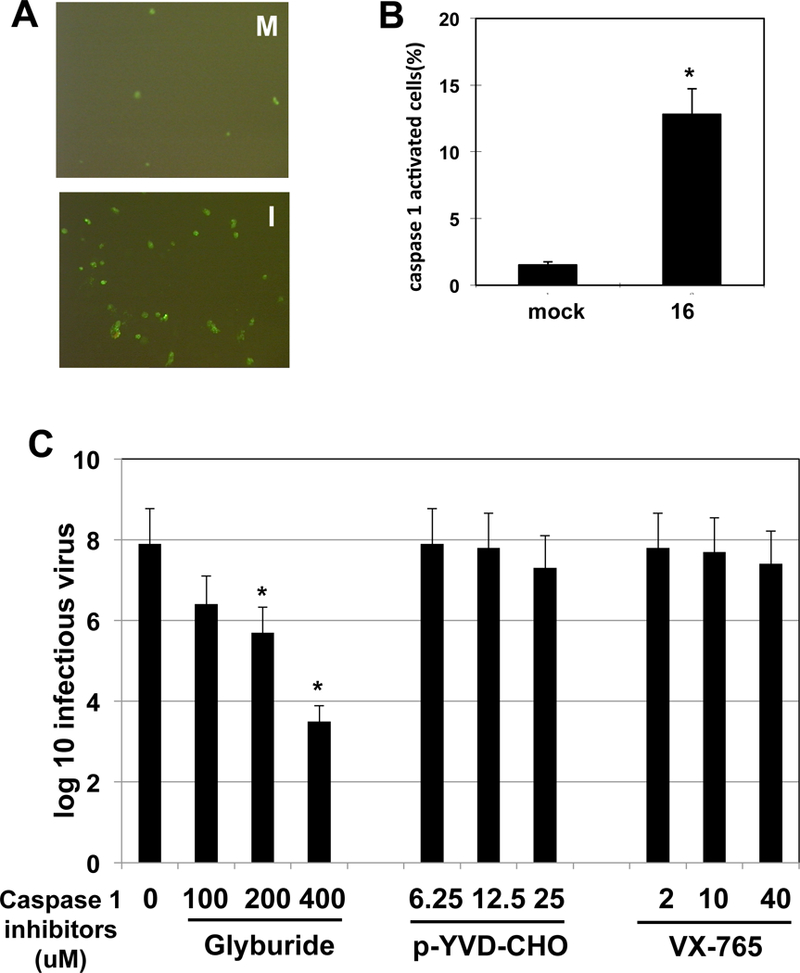
Panel A: CRIB cells were infected with BoHV-1 (1 plaque forming unit/cell) and at 16 hours after infection (panel I) caspase 1+ cells were identified using a specific peptide that binds to active caspase 1 (FAM FLICA Caspase 1 Assay Kit, Immunochemistry Technologies, Bloomington, MN) as described by the manufacturer. Fluorescent microscopy was used to detect caspase 1+ cells. Cells from mock-infected (panel M) cells served as a control.
Panel B: Analysis of caspase 1+ cells at 16 hours after infection by flow cytometry. Flow cytometry was used to quantify the number of caspase 1+ cells as described by the manufacturer. The asterisk denotes significant differences (P < 0.05) in caspase 1+ cells at 16 hours after infection relative to mock-infected cells, as determined by the Student t test.
Panel C: CRIB cell were infected with BoHV-1 (1 pfu/ml). After 1-hour adsorption at 37 °C, the respective caspase 1 inhibitors were added (uM concentration) and at 24 hours after infection total virus was prepared from the infected cells. Plaque assays were then performed, and the amount of infectious virus in the lysate calculated (values presented as log 10). The values shown are the average of three independent studies. An asterisk denotes significant differences (P < 0.05) in virus production in CRIB cells not treated with glyburide, as determined by the Student t test.
To test whether caspase 1 activation influences BoHV-1 productive infection, we examined the effect that caspase 1 inhibitors have on virus production. Three specific caspase 1 inhibitors were used for these studies: 1) glyburide, a sulfonylurea drug (Lamkani et al., 2009),2) YVDA-CHO a peptide based inhibitor (ENZO Life Sciences), and VX-765 (Cellagen Technology) (Gastaldello et al., 2013; Lamkani et al., 2009). YVDA-CHO and VX-765 had little or no effect on virus yield in CRIB cells (Figure 3C). In contrast, increasing concentrations of glyburide decreased the levels of infectious virus in CRIB cells suggesting glyburide has unknown off-target effects on BoHV-1 replication. Glyburide is widely used for type 2 diabetes in the United States, and has few side-effects on patients (Riddle , 2003). Consequently, it is of interest to understand its mechanism of action against BoHV-1 and whether it has anti-viral activities directed against human alpha-herpesviruses. Interestingly, a recent study demonstrated that caspase 1 inhibitors, including glyburide, inhibited Epstein Barr virus replication, in part by blocking the cleavage of a large tegument protein (BPLF1) that has deneddylase activity (Gastaldello et al., 2013). BPLF1 is reported to be the homologue of HSV VP16 (Schmaus et al., 2004). However, it is not known whether the BoHV-1 VP16 protein has deneddylase activity. At the concentration used for the respective drugs, no major effects on cell growth or morphology were observed (data not shown). The concentrations used for the respective drugs are in the range known to inhibit caspase 1 activity (Gastaldello et al., 2013; Lamkani et al., 2009). In keeping with this published study, we found that treating infected cells with 25 uM YVDA-CHO, 40 uM VX-765, or 400 uM glyburide reduced the number of caspase 1 activated cells (data not shown).
In conclusion, these studies provided evidence that BoHV-1 stimulates inflammasome formation and consequently caspase 1 activation. Although caspase 1 had no obvious effect on productive infection in CRIB cells, we predict that inflammasome formation mediates certain aspects of BoHV-1 induced pathogenesis following infection. Studies designed to address the effect of the inflammasome in cattle and identification of viral factors that regulate inflammasome formation is underway.
Highlights.
Bovine herpesvirus 1 stimulates inflammasome formation during productive infection.
The cellular DNA sensor, IFI16, is induced and localizes primarily to the cytoplasm after infection.
Caspase 1 activity is induced following infection of cultured cells.
Inhibiting caspase 1 does not have a dramatic effect on virus growth in cultured cells.
ACKNOWLEDGEMENTS
This research was supported by grants from the Nebraska Research Initiative and Agriculture and Food Research Initiative Competitive Grants Program (2013–01041) from the USDA, National Institute of Food and Agriculture. A grant to the Nebraska Center for Virology (1P20RR15635), in particular the Microscopy and FACS core facilities were also essential for certain aspects of these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Carter JJ, Weinberg AD, Pollard A, Reeves R, Magnuson JA, and Magnuson NS, 1989Inhibition of T-lymphocyte mitogenic responses and effects on cell functions by bovineherpesvirus 1. J Virol 63, 1525–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva LFand Jones C, 2012. The ICP27 protein encoded by bovine herpesvirus type 1 (bICP27) interferes with promoter activity of the bovine genes encoding beta interferon 1 (IFN-β1) and IFN-β3. Virus Res, 162–168. [DOI] [PMC free article] [PubMed]
- Duan X, P.L., Veeranki S, Panchanathan R, Dickerson E, and Choubey D, 2011. Differential roles for the interferon-inducible IFI16 and AIM2 innate immune sensors for cytosolic DNA in cellular senescence of human fibroblasts. Mol Cancer Res 5, 589–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskra L, and Splitter GA, 1997. Bovine herpesvirus-1 infects activated CD4+ lymphocytes. J Gen Virol 78, 2159–2166. [DOI] [PubMed] [Google Scholar]
- Frizzo da Silva Land Jones C, 2011. Infection of cultured bovine cells with bovine herpesvirus1 (BHV-1) or Sendai virus induces different beta interferon subtypes. Virus Res 157, 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariano GR, Dell’Oste V, Bronzini M, Gatti D, Luganini A, De Andrea M, Gribaudo G, Gariglio M, and Landolfo S ., 2012. The intracellular DNA sensor IFI16 gene acts as restriction factor for human cytomegalovirus replication. PloS Pathogens 8, e1002498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastaldello S, Chen X, Callegari S, and Masucci MG, 2013. Caspase-1 promotes Epstein-Barr virus replication by targeting the large tegument protein deneddylase to the nucleus of productively infected cells. PloS Pathogens 9, e1003664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant RW and Dixit VD, 2013. Mechanisms of disease: inflammasome activation and the development of type 2 diabetes. Frontiers in Immunology 4, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel P, Ohmann HB, Lawman MJ, and Babiuk LA, 1990. The interaction between bovine herpesvirus type 1 and activated bovine T lymphocytes. J Gen Virol 71, 369–377. [DOI] [PubMed] [Google Scholar]
- Griebel P, Qualtiere L,Davis WC Gee A, Ohmann H Bielefeldt, Lawman MJ, and Babiuk LA,1987a. T lymphocyte population dynamics and function following a primary bovine herpesvirus type-1 infection. Viral Immunol 1, 287–304. [DOI] [PubMed] [Google Scholar]
- Griebel PJ, Qualtiere L, Davis WC, Lawman MJ, and Babiuk LA, 1987b. Bovine peripheral blood leukocyte subpopulation dynamics following a primary bovine herpesvirus-1 infection. Viral Immunol 1, 267–286. [DOI] [PubMed] [Google Scholar]
- Hariharan MJ, Nataraj C, and Srikumaran S, 1993. Down regulation of murine MHC class I expression by bovine herpesvirus 1. Viral Immunol 6, 273–284. [DOI] [PubMed] [Google Scholar]
- Henderson G, Zhang Y, Jones C, 2005. The bovine herpesvirus 1 gene encoding infected cell protein 0 (bICP0) can inhibit interferon-dependent transcription in the absence of other viral genes. J Gen Virol 86, 2697–2702. [DOI] [PubMed] [Google Scholar]
- Highlander SK, 2001. Molecular genetic analysis of virulence in Mannheimia (Pasteurella) haemolytica.. Front Biosci, D1128–1150. [DOI] [PubMed]
- Highlander SK, Fedorova ND, Dusek DM, Panciera R, Alvarez LE, and Renehart C,2000. Inactivation of Pasteurella (Mannheimia) haemolytica leukotoxin causes partial attenuation of virulence in a calf challenge model. Infect Immun 68, 3916–3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkley S, Hill AB, and Srikumaran S, 1998. Bovine herpesvirus-1 infection affects the peptide transport activity in bovine cells. Virus Res 53, 91–96. [DOI] [PubMed] [Google Scholar]
- Johnson KE, Chikoti L, and Chandran B, 2013. Herpes simplex virus 1 infection induces activationa and subsequent inhibition of the IFI16 and NLRP3 inflammasomes. J Virol 87, 5005–5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C, 2009. Regulation of innate immune responses by bovine herpesvirus 1 and infected cell protein 0. Viruses 1, 255–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones Cand Chowdhury S, 2007. A review of the biology of bovine herpesvirus type 1 (BHV-1), its role as a cofactor in the bovine respiratory disease complex, and development of improved vaccines. Adv in Anim Health 8, 187–205. [DOI] [PubMed] [Google Scholar]
- Koppers-Lalic EA, Reits EAJ, Ressing ME, Lipinska AD, Abele R, Koch J, Rezende MM, Admiraal P, van Leeuwen D, Bienkowsaka-Szewczyc K, Mettenleiter TC, Rijsewijk FAM, Tampe R, Neefjes J, H.E.J. and Wiertz J, 2005. Varicelloviruses avoid T cell recognition by UL49.5-mediated inactivation of the transporter associated with antigen processing. Proc. Natl. Acad. Sci. USA 102, 5144–5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkani M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, Deshayes K, Lee WP, Hoffman HM, and Dixit VM, 2009. Glyburide inhibits the cryopyrin/Nalp3 inflammasome. J Cell Biol 187, 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latz E, Sam Xiao T, and Stutz A, 2013. Activation and regulation of the inflammasomes. Nature Reviews Immunol 13, 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Diner BA, Chen J, and Cristea IM., 2012. Acetylation modulates cellular distribution and DNA sensing ability of interferon-inducible protein IFI16. Proc Natl Acad Sci U S A 109, 10558–10563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataraj C, Eidmann S, Hariharan MJ, Sur JH, Perry GA, and Srikumaran S, 1997. Bovine herpesvirus 1 downregulates the expression of bovine MHC class I molecules. Viral Immunol 10, 21–34. [DOI] [PubMed] [Google Scholar]
- Orzalli MJH, DeLuca NA, and Knipe DM, 2012. Nuclear IFI16 induction of IRF-3 Signaling during herpesviral infection: Degradation of IFI16 by nuclear HSV ICPO protein. Proc Natl Acad Sci U S A 109, E3008–E3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez S, Lovato L, Zhou J, Doster A, and Jones C, 2006. Comparison of inflammatory infiltrates in trigeminal ganglia of cattle infected with wild type BHV-1 versus a virus strain containing a mutaition in the LR (latency-related) gene. J Neurovirol, 12:392–397. [DOI] [PubMed] [Google Scholar]
- Perez S, Inman M, Doster A, and Jones C, 2005. Latency-related gene encoded by bovine herpesvirus 1 promotes virus growth and reactivation from latency in tonsils of infected calves. J Clin Micro 43, 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle MC, 2003. Editorial: sulfonylureas differ in effects on ischemic preconditioning-is it time to retire glyburide? J Clin Endocrinol Metab 88, 528–530. [DOI] [PubMed] [Google Scholar]
- Saira K, Zhou Y, and Jones C, 2007. The infected cell protein 0 encoded by bovine herpesvirus 1 (bICP0) induces degradation of interferon response factor 3 (IRF3), and consequently inhibits beta interferon promoter activity. J Virol 81, 3077–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saira K and Jones C, 2009. The infected cell protein 0 encoded by bovine herpesvirus 1 (bICP0) associates with interferon regulatory factor 7 (IRF7), and consequently inhibits beta interferon promoter activity. J Virol 83, 3977–3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaus S, Wolf H, and Schwarzmann F, 2004. The reading frame BPLF1 of epstein Barr virus: a homologue of herpes simplex virus protein VP16. Virus Genes 29, 267–277. [DOI] [PubMed] [Google Scholar]
- Sinani D and Jones C, 2011. Localization of sequences in a protein encoded by the latency related gene of bovine herpesvirus 1 (ORF2) that inhibits apoptosis and interferes with Notch1 mediated trans-activation of the bICP0 promoter. J Virol 85, 12124–12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutz A, Golenbock DT, and Latz E, 2009. Inflammasomes: too big to miss. J of Clinical Investigations 119, 3502–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterholzner L KS, Baran M, Horan KA, Jensen SB, Sharma S, Sirois CM, Jin T, Latz E, Xiao TS, Fitzgerald KA, Paludan SR, and Bowie AG, 2010. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol 11, 997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeranki S and Choubey D, 2012. Interferon-inducible p200-family protein IFI16, an innate immune sensor for cytosolic and nuclear double-stranded DNA: regulation of subcellular localization. Mol Immunol 49, 567–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler MT, Doster A, Sur JH, and Jones C, 2002. Analysis of bovine trigeminal ganglia following infection with bovine herpesvirus 1. Vet Microbiol 86, 139–155. [DOI] [PubMed] [Google Scholar]
- Winkler MT, Doster A, and Jones C, 1999. Bovine herpesvirus 1 can infect CD4(+) T lymphocytes and induce programmed cell death during acute infection of cattle. J Virol 73, 8657–8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler MTC, Doster A, and Jones C, 2000. Persistence and reactivation of bovine herpesvirus 1 in the tonsil of latently infected calves. J Virol 74, 5337–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman A, Eudy J, Smith L, Frizzo da Silva L, Sinani D, Bricker H, Cook E, Doster A, and Jones C, 2012. Cellular transcription factors induced in trigeminal ganglia during dexamethasone-induced reactivation from latency stimulate bovine herpesvirus 1 productive infection and certain viral promoters. J Virol 86, 2459–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman A and Jones C, 2010. Bovine herpesvirus 1 productive infection and bICP0 early promoter activity are stimulated by E2F1. J Virol 84, 6308–6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman A and Jones C, 2011. Analysis of the cell cycle regulatory protein (E2F1) after infection of cultured cells with bovine herpesvirus 1 (BHV-1) or herpes simplex virus type 1 (HSV-1). Virus Res 160, 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecchinon L, T.F., and Desmecht D 2005. How Mannheimia haemolytica defeats host defense through a kiss of death mechanism. Vet Res 36, 133–156. [DOI] [PubMed] [Google Scholar]


