Abstract
Responsiveness to ethanol (EtOH) differs as a function of age. Adolescent rodents are less sensitive than adults to the sedative effects of EtOH, whereas they show enhanced sensitivity to EtOH-induced social facilitation. Late aging is associated with a natural decline in social behavior and aging-related peculiarities in sensitivity to EtOH have been largely unexplored. Whether there are sex differences in the behavioral response to EtOH during late aging remains unknown. Thus, behavioral responses to EtOH in male and female Fischer (F) 344 rats aged 4–5 months (adult) and 19–20 months (aging) were examined. First, the effects of saline and EtOH (0.5 and 0.75 g/kg) on social interaction were assessed. Social investigation and contact behavior were lower in aging animals and higher in females. Interestingly, in aged females, social contact behavior was increased following a 0.5 g/kg EtOH dose, whereas the same dose suppressed social contact in aged males. Behavioral sensitivity to the sedative effects of 3.0 and 3.5 g/kg EtOH was assessed with the loss of righting reflex (LORR) test. Although latency to LORR did not differ as a function of age or sex, aged rats showed significantly greater LORR duration and significantly lower blood ethanol concentrations (BECs) at regaining of the righting reflex relative to adults. In addition, females had a lower LORR duration, regardless of age; no sex differences were evident in BECs at awakening. In a second experiment, blood ethanol concentrations (BECs) over time were assessed following 0.75, 1.5, and 3.0 g/kg EtOH in 3-, 12, and 18-month-old male and female F344 rats. Aged rats had higher peak BECs following 3.0 g/kg EtOH, whereas few age or sex differences were apparent at lower doses. Taken together, these data indicate that late aging is associated with altered sensitivity to the social facilitating effects and sedative effects of EtOH.
Keywords: Aging, Alcohol, LORR, Social Behavior
1.0. Introduction
Alcohol use among the elderly is becoming a significant public health concern, with 42.7% of individuals aged 65 and older reporting alcohol use in the past month and 10% of that population exhibiting patterns of alcohol consumption consistent with binge drinking (Substance Abuse and Mental Health Services Administration, 2015). Although there is a large body of literature describing age differences in ethanol (EtOH) sensitivity between adolescents and adults (Spear, 2015; Varlinskaya & Spear, 2015; Varlinskaya et al., 2014, 2013), relatively little is known about responding to alcohol in aged individuals (Squeglia et al., 2014). Given that the size of the elderly population is expanding rapidly, and rates of drinking among the elderly are increasing (Breslow et al., 2017), a better understanding of how late aging affects the behavioral response to alcohol is needed.
When describing the effects of alcohol across the lifespan, it is helpful to define specific ontogenetic periods. Adolescence is often defined as occurring from 12–18 years of age in humans, although some researchers extend adolescence into the early-mid 20’s (Spear, 2000). In rodents, adolescence can be conservatively defined as occurring from postnatal days (P) 28–42 (Spear, 2000). Adulthood typically begins in the 20’s in humans and after P70 in rats. Aging occurs around 18 months in rats, whereas rats of about 24 months of age are often considered “aged”, although what constitutes aging vs. aged is highly strain-dependent.
It has been repeatedly shown that social behavior declines across the lifespan in humans (Lang, 2001; Schiffman, 1997) and in laboratory rodent models (Hunt et al., 2011; Perkins et al., 2016; Salchner et al., 2004; Shoji and Mizoguchi, 2011). Positive social experiences are associated with significant health benefits such as enhanced resilience (Charuvastra and Cloitre, 2008) and better recovery from illness or trauma (DeVries et al., 2007; Norman et al., 2010), and perceived social isolation and social stress are strong predictors of morbidity and mortality (Bisschop et al., 2003; House et al., 1988; Seeman, 2000). Given relatively high alcohol use among the elderly associated with age-related declines in social behavior, it is critical to understand the relationship between social behavior and alcohol during late aging.
In rats, age-related differences have been observed in the effects of EtOH on social behavior. Specifically, in adult Sprague-Dawley rats, low to moderate doses of EtOH (0.75–1.0 g/kg) produced social inhibition, whereas adolescent Sprague-Dawley rats were not sensitive to these socially suppressing effects of EtOH (Varlinskaya and Spear, 2012). In contrast, adolescent Sprague-Dawley rats demonstrated social facilitation at low EtOH doses, indexed via increases in social behavior, an effect of EtOH not evident in adults under normal conditions (Varlinskaya & Spear, 2002). As mentioned previously, social behavior is suppressed in 18–24 month old F344 rats (Perkins et al., 2016), although whether aged rats are more sensitive to the socially suppressing effects of low-dose EtOH relative to their younger adult counterparts remains to be investigated.
High doses of EtOH produce substantial motor impairment and sedation. Aged Sprague-Dawley rats (18-month-old) are more sensitive to the motor impairing effects of EtOH relative to adolescent and adult Sprague-Dawley rats (Novier et al., 2013; Ornelas et al., 2015), an effect that may be associated with alterations in cerebellar expression of PKCγ (Van Skike et al., 2010). In addition, 18 month old Sprague-Dawley rats are sensitive to acute EtOH-induced cognitive deficits, evidenced by impaired performance on the water maze (Novier et al., 2013). Altered behavior in response to EtOH in late aging could be due simply to age differences in EtOH pharmacokinetics and/or EtOH absorption and distribution. Indeed, there are some studies demonstrating impaired clearance of high doses of EtOH (3.0 g/kg) in 18-month-old Sprague-Dawley rats (Ornelas et al., 2015), although others have shown no age differences in BECs following acute administration of lower doses of EtOH (1.5–2.5 g/kg) (Matthews and Mittleman, 2017; Novier et al., 2016). All the aforementioned studies were performed exclusively in males. Whether aged female rats show altered EtOH pharmacokinetics remains to be determined. The goals of the current study were to: (1) assess behavioral sensitivity to EtOH across a wide range of doses in aged rats, (2) explore age and/or sex differences in the behavioral response (LORR) and physiological response (BEC and CORT) to EtOH, and (3) examine changes in BECs over time following low, moderate, and high doses of EtOH.
2.0. Material and methods
2.1. Subjects
Experiment 1 tested 3- and 18-month-old (at arrival; 4–5 and 19–20 months at testing-referred to as adult and aging, respectively) male and female F344 rats (n = 11–14/group). Experiment 2 tested 3-, 12-, and 18-month-old (at arrival; 4–5, 13–14, and 19–20 months at testing, referred to as adult, middle-aged, and aging, respectively) male and female F344 rats (n = 7–10/group). All animals were obtained from the National Institute of Aging (NIA) colony maintained by Charles River Laboratories. Subjects were given at least 2 weeks to acclimate to the colony before experiments began. Social partners were young adult sex-matched F344 rats obtained from Charles River Laboratories. Colony was maintained at 22 ± 1°C with 12:12 light:dark cycle (lights on 0700). All rats were pair-housed and provided ad libitum access to food and water. On each of two days prior to the onset of behavioral testing, experimental subjects and partners were handled for 3 min. For social interaction testing, subjects were marked on the day prior to testing with a non-toxic permanent marker to allow the experimenter to distinguish subjects from partners. At all times, rats were maintained and treated in accordance with the guidelines set forth by the Institute of Laboratory Rat Resources, (1996) and in accordance with the protocol approved by the IACUC at Binghamton University.
2.2. Experiment 1: Social behavior and loss of righting reflex
To assess the effects of low-dose EtOH on social behavior, a within-subjects design was used in which each rat received vehicle (sterile, pyrogen-free 0.9% saline), 0.5 g/kg EtOH, and 0.75 g/kg EtOH (Figure 1A) with a day off between testing days (Varlinskaya et al., 2015). EtOH solutions (20% v/v) were made with sterile, pyrogen-free 0.9% saline. On each test day, rats were injected intraperitoneally (i.p.) and immediately placed into the testing apparatus for a 30-min acclimation period, after which a novel adult sex-matched conspecific was introduced for a 10-min social interaction test. Social interaction testing occurred in custom-made Plexiglas® chambers lined with clean pine shavings (45 × 30 × 30 cm). Each chamber was divided into two equally sized compartments separated by a clear Plexiglas® partition with an aperture (9 × 7 cm) that allowed the rats to move freely between compartments (Perkins et al., 2017). Males and females were tested in separate rooms with dim lighting (10–20 lux) between 0800 and 1300. Between test subjects, chambers were emptied, wiped with water, and fresh shavings were added. Behavior of the rats was recorded by a camcorder (Sony Model HDR-CX330) and scored by an observer without knowledge of the experimental condition of any given animal.
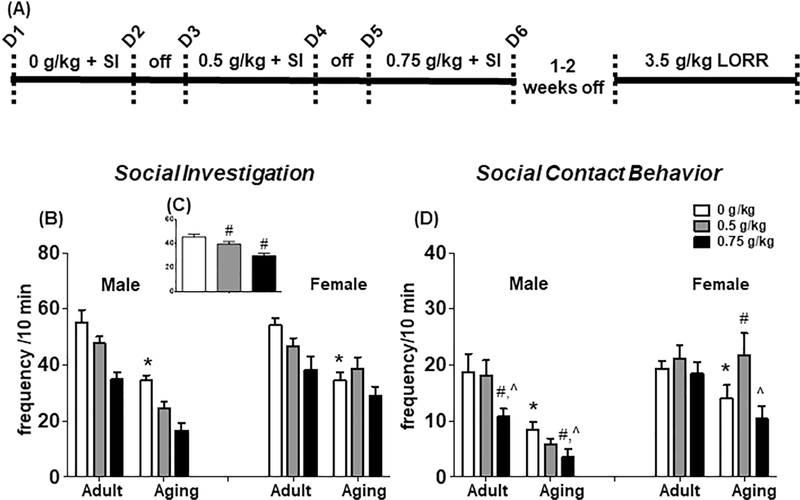
Figure 1.
(A) Timeline for Experiment 1 assessing social behavior and sedation following EtOH. (B) Frequency of social investigation for all groups and collapsed across age and sex (insert; C) and (D) frequency of contact behavior in a 10-min social interaction test with a sex-matched adult conspecific. All data expressed as mean ± SEM. (*) p < 0.05 vs. adult saline-injected rats; (#) p < 0.05 vs. saline-injected vehicle; (^) p < 0.05 vs. 0.5 g/kg EtOH
The total number of crossovers (movement through the aperture) during the 30-min acclimation period was assessed and used as a basic index of locomotor activity. During the social interaction test, the frequency of the following behaviors was scored and assessed: social investigation (sniffing of any part of the body of the partner), contact behavior (sum of crawling over the partner, crawling under the partner, and social grooming). During the social interaction test, movement through the aperture was categorized as toward or away from the partner and used to calculate a coefficient of social preference/avoidance [(total crossovers toward – total crossovers away from/total crossovers) x 100]. Positive values indicate social preference, whereas negative values indicate social avoidance (Varlinskaya et al., 1999, 2015).
After a 1–2-week washout period, behavioral sensitivity to the sedative effects of EtOH was assessed using the Loss of Righting Reflex (LORR) test. All rats were injected (i.p.) with 3.5 g/kg EtOH (20% v/v, made with sterile, pyrogen-free saline). After injection, rats were placed immediately into a fresh cage with pine shavings. A trough was created with the shavings and rats were placed onto their backs. Latency to lose the righting reflex was defined as the inability to regain the righting reflex twice within a 60-sec period (Gano et al., 2017). Rats were observed continually until they regained the righting reflex (RORR). Duration of LORR was calculated as RORR minus the latency of LORR. At the time of RORR, rats were rapidly decapitated and trunk blood was collected into EDTA coated vacutainers (BD Vacutainer, VWR, Radnor, PA). Plasma was separated through refrigerated centrifugation and stored at −20° C until assessment of BECs and corticosterone (CORT).
2.3. Experiment 2: Ethanol pharmacokinetics and loss of righting reflex
To assess whether age and/or sex differences in behavioral sensitivity was associated with EtOH pharmacokinetics, a within-subjects design was used to examine BECs following 0.75 g/kg EtOH (0, 30, 60, 120, and 240 min after injection), 1.5 g/kg EtOH (0, 45, 90, 180, and 360 min after injection) and 3.0 g/kg EtOH (0, 45, 90, 180, and 360 min after injection) (Figure 3A). Rats were given a week off between tests to allow for recovery from multiple blood sampling. Since previous studies have reported age-related differences in EtOH pharmacokinetics at 3.0 g/kg but not 1.5 g/kg in males (Novier et al., 2016; Ornelas et al., 2015), we chose to examine BECs following several doses of EtOH. The low dose of EtOH was utilized previously in the test of social behavior (0.75 g/kg).
Rats were removed from their home cage and placed into a Plexiglas® restraint tube to obtain a baseline blood sample (0 min time point). Blood samples were collected via the tail clip method in which the last 0.5–1.0 mm of the tail was transected and the tail was stroked to collect whole blood (Hueston and Deak, 2014; Vore et al., 2017). Immediately following the baseline sample, rats were injected i.p. with EtOH. Animals were only placed into restraint tubes for the brief blood sampling procedure and remained in their home cages in between time points. Serum was separated through refrigerated centrifugation and stored at −20° C until assessment of BECs.
After a 1–2-week washout period, rats were assessed for behavioral sensitivity to the sedative effects of EtOH. Rats were injected (i.p.) with 3.0 g/kg EtOH (20% v/v, in sterile, pyrogen-free saline) and loss of righting reflex was measured as described in Section 2.2. We chose to assess LORR again at a lower dose for two reasons: (1) a lower dose might allow for the emergence of subtle age and/or sex differences and (2) EtOH pharmacokinetics were examined at this dose. A tail blood sample was obtained at the time of awakening as described above for assessment of BECs (Figure 2).
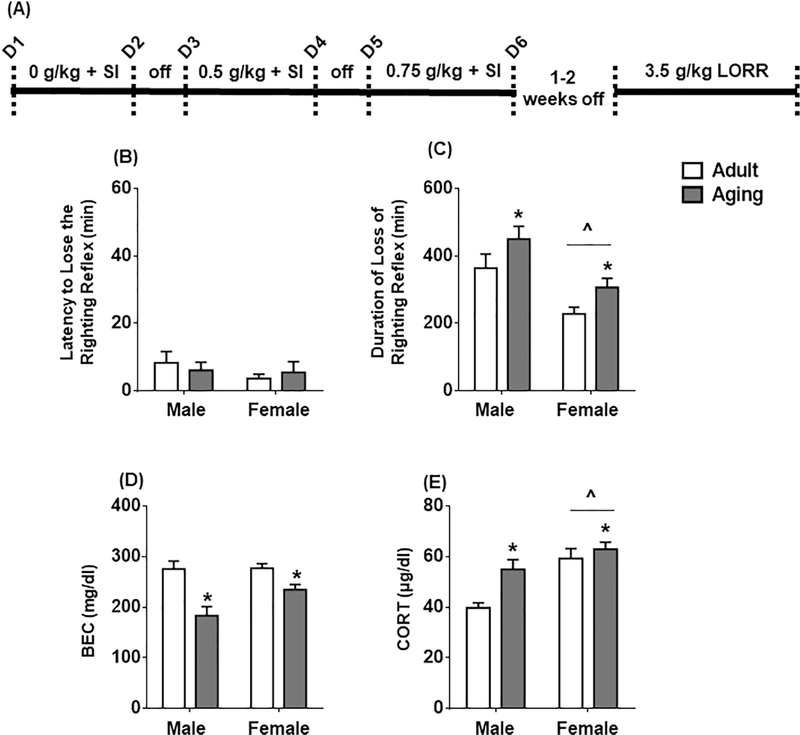
Figure 2.
(A) Timeline for Experiment 1 assessing age and sex differences in social behavior and sedation following EtOH. (B) latency to lose the righting reflex, (C) duration of loss of righting reflex, (D) blood ethanol concentrations (BECs) and (E) corticosterone obtained when rats regained the righting reflex. All data expressed as mean ± SEM. (*) p < 0.05 vs. adult; (^) p < 0.05 vs. males.
2.4. Blood Ethanol Concentrations (BECs)
BECs were measured using an Analox AM-1 alcohol analyzer (Analox Instruments, Lunenburg, MA, USA) in 5 μl aliquots of blood. For samples obtained after 0.75 g/kg EtOH, a 100 mg-% standard was used, whereas for those samples obtained after LORR, 1.5 g/kg, and 3.0 g/kg EtOH, a 300 mg-% standard was used. The Analox machine was calibrated every 15–20 samples to account for drift over time.
2.5. Corticosterone EIA
Assessment of plasma CORT levels was conducted with a commercially available EIA kit (Cat No: ADI-901–097; Enzo Life Sciences, Farmingdale, NY, USA) as described in (Hueston and Deak, 2014). Inter-assay variability was 3.3% and intra-assay variability was 3.5% with a sensitivity of 27 pg/mL. Manufacturer’s instructions were followed with the exception that samples were heat inactivated via immersion in a 75° C water bath for 60 min to denature endogenous CBG.
2.6. Data Analysis
Statistica (Version 7, StatSoft Inc. Tulsa, OK) was used for all analyses. Social behaviors were assessed using individual 2 (Age) x 2 (Sex) x 3 (EtOH Dose), with EtOH dose treated as a repeated measure. LORR data (latency, duration of LORR, BECs at RORR) were analyzed with separate 2 (Age) x 2 (Sex) ANOVAs. To evaluate age and sex differences in EtOH pharmacokinetics, we analyzed BECs for each dose separately (0.75, 1.5, and 3.0 g/kg EtOH). First, a within-subjects ANOVA was used to examine differences in peak BEC levels at the middle time points (30, 60, and 120 min for 0.75 g/kg, 45, 90, and 180 min for 1.5 g/kg and 3.0 g/kg). Secondly, a repeated-measures ANOVA was used to examine differences in clearance by focusing exclusively on the baseline (pre-ethanol) and last time point (240 min for 0.75 g/kg and 360 min for 1.5 g/kg and 3.0 g/kg). We also assessed area under the curve using a 3 × 2 (Age x Sex) ANOVA. Body weights on the first day of the experiment and final day of the experiment were assessed using a repeated-measures ANOVA with day as the repeated measure and Age and Sex as between-subjects factors. For all analyses, post-hoc tests were conducted using Fisher’s Least Significant Difference.
3.0. Results
3.1. Experiment 1: Social behavior and loss of righting reflex
3.1a. Social Interaction Test
The total number of crossovers declined as a function of EtOH dose [F(2,80) = 9.40, p = 0.0002]. Post-hoc tests revealed that crossovers were lower following 0.5 g/kg EtOH, relative to saline, and that they were lower after the 0.75 g/kg EtOH, relative to 0.5 g/kg EtOH (Table 1). Total number of crossovers was higher in females (12.93 ± 1.55), relative to males (7.21 ± 0.84) [main effect of Sex, F(1,40) = 41.13, p < 0.000001], and aging rats (5.00 ± 0.55) showed fewer crossovers relative to adult rats (13.58 ± 1.11) [main effect of Age, F(1,40) = 98.77, p < 0.00001].
Table 1.
Mean social behavior (± SEM) following vehicle or acute ethanol challenge in Experiment 1.
| EtOH Dose (g/kg) | Behavior | Adult | Aging | ||
|---|---|---|---|---|---|
| Males | Females | Males | Females | ||
| 0.0 | Preference | 15.7 ± 8.8 | 5.1 ± 8.5 | -3.9 ± 19.7 | -1.0 ± 12.0 |
| Crosses | 13.0 ± 1.2a | 19.3 ± 1.7 b | 4.8 ± 0.7 c | 8.1 ± 0.9 d | |
| 0.5 | Preference | 23.4 ± 6.8 | 5.7 ± 4.7 | 2.6 ± 14.3 | 4.7 ± 16.5 |
| Crosses | 10.0 ± 0.9*a | 18.0 ± 1.6*b | 3.5 ± 0.6*c | 7.2 ± 0.8*d | |
| 0.75 | Preference | -6.1 ± 12.0 | -26.3 ± 14.3 | -26.7 ± 33.6 | 13.6 ± 23.6 |
| Crosses | 7.9 ± 1.2*a | 17.3 ± 2.5*b | 1.6 ± 0.5*c | 5.9 ± 1.2*d | |
Asterisks (*) indicate a significant difference from saline control (p < 0.05). Significant interactions are indicated by a letter system in which different letters indicate groups that differ from one another.
Given our previous findings of age and/or sex differences in the social interaction test (Perkins et al., 2017, 2016), we first assessed social investigation and contact behavior exclusively in the saline vehicle group. Aging rats had significantly lower social investigation [F(1,37) = 38.44, p < 0.00001] and social contact behavior [F(1,37) = 11.05, p = 0.002] relative to their adult counterparts, whereas no sex differences were evident in either dependent measure (Figure 1 B).
Social investigation varied as a function of EtOH dose [F(2,74) = 25.70, p < 0.00001] and was suppressed by a 0.5 g/kg and 0.75 g/kg dose, relative to saline regardless of age and sex (see Figure 1 C). There was a significant Sex x Age x EtOH dose interaction for social contact behavior [F(2,74) = 3.98, p < 0.05], therefore adult and aging rats were analyzed separately. In adult rats, contact behavior was significantly lower following 0.75 g/kg EtOH, relative to saline and 0.5 g/kg EtOH, although this was likely driven by a significant reduction in males (Figure 1 D). Aging males exhibited a reduction in contact behavior following 0.75 g/kg EtOH, relative to saline, although no decline was evident following 0.5 g/kg EtOH [EtOH dose x Sex interaction, F(2,34) = 5.83, p < 0.01]. In contrast, aging females exhibited an increase in contact behavior following 0.5 g/kg EtOH, relative to saline. In addition, 0.75 g/kg EtOH did not lead to a reduction in social contact in aging females, whereas it did in adult and aging males.
Given the observed age differences in baseline social behavior (both investigation and contact) demonstrated by experimental subjects following saline injection, the percent change from saline was calculated for overall social activity measured as the sum of social investigation and social contact for both EtOH doses. There was a main effect of EtOH Dose [F(1,37) = 24.42, p < 0.0001] indicating that a substantial decrease in social behavior was evident following 0.75 g/kg EtOH, relative to 0.5 g/kg EtOH. In addition, there was a significant Age x Sex interaction [F(1,37) = 7.92, p < 0.01]. Post-hoc tests indicated that adult males and females exhibited similar reductions in social behavior. However, aging males exhibited a reduction in social behavior, whereas aging females exhibited an increase in social behavior. Thus, the increase in social behavior in aged females remains significant after accounting for age differences in baseline social behavior.
3.1b. Loss of Righting Reflex: 3.5 g/kg
To assess sensitivity to the sedative effects of EtOH, LORR was measured after a 3.5 g/kg EtOH dose. There were no age or sex differences in latency to LORR (Figure 2B). However, females exhibited a shorter duration of LORR [F(1,47) = 17.86, p < 0.001]. In addition, aging rats had a longer duration of LORR, relative to their adult counterparts [F(1,47) = 6.18, p = 0.017] (Figure 2C). BECs at RORR were lower in aging rats [F(1,47) = 25.04, p < 0.0001], which is indicative of higher sensitivity to the sedative effects of EtOH (Figure 2D). At RORR, CORT was higher in females relative to males [F(1,47) = 18.25, p < 0.0001] and higher in aging rats relative to adult rats [F(1,47) = 8.74, p = 0.005] (Figure 2E).
3.2. Experiment 2: Ethanol pharmacokinetics and loss of righting reflex
3.2a. Ethanol pharmacokinetics
One possible explanation for differences in social behavior and sedation in late aging is that aging alters EtOH pharmacokinetics. Thus, we examined BECs over time following three doses of EtOH: 0.75 g/kg, 1.5 g/kg, and 3.0 g/kg (Table 2). These doses were chosen to represent low, medium, and high EtOH load, respectively. We were interested in examining age and sex differences in EtOH “peak” and “clearance”. As such, for each time course, we analyzed the data for the middle time points (“peak”) separately than the data for the first and last time point (“clearance”).
Table 2.
Mean BEC (mg/dl ± SEM) following acute EtOH challenge in Experiment 2.
| EtOH Dose (g/kg) | Time (min) | Adult | Middle-Aged | Aging | |||
|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | ||
| 0.75 | 0 | 10.5 ± 2.9 a | 13.4 ± 2.9 a | 13.4 ± 2.5 a | 9.9 ± 2.6 a | 10.6 ± 2.6 a | 13.3 ± 2.8 a |
| 30 | 50.1 ± 4.6 b | 48.7 ± 4.4 b | 55.8 ± 2.6 b | 45.9 ± 3.4 b | 48.6 ± 4.4 b | 55.4 ± 3.9 b | |
| 60 | 46.8 ± 3.2 b | 41.7 ± 4.8 c | 54.5 ± 2.9 b | 39.6 ± 3.3 c | 47.8 ± 3.4 b | 50.4 ± 3.3 c | |
| 120 | 17.5 ± 4.9 c | 11.2 ± 2.1 d | 26.8 ± 5.9 c | 10.2 ± 1.8 d | 19.7 ± 4.8 c | 17.7 ± 4.3 d | |
| 240 | 9.7 ± 2.4 a | 12.6 ± 2.0 a | 10.6 ± 2.3 a | 8.9 ± 2.3 a | 10.1 ± 2.7 a | 14.0 ± 3.1 a | |
| 1.5 | 0 | 14.7 ± 3.3 a | 19.9 ± 3.0 a | 19.3 ± 3.1 a | 17.0 ± 3.2 a | 16.0 ± 3.2 a | 20.3 ± 3.2 a |
| 45 | 92.1 ± 9.8 b | 72.0 ± 8.2 b^ | 90.5 ± 10.2 b | 91.4 ± 8.8 b^ | 96.3 ± 15.3 b | 91.6 ± 9.1 b^ | |
| 90 | 102.0 ± 13.1 b | 96.1 ± 8.9 c^ | 118.2 ± 7.7 b | 89.1 ± 12.4 c^ | 88.5 ± 9.9 b | 109.7 ± 8.1 c^ | |
| 180 | 77.0 ± 17.2 c | 42.4 ± 5.4 d^ | 88.7 ± 6.6 c | 53.4 ± 7.4 d^ | 68.7 ± 6.9 c | 76.3 ± 9.2 d^ | |
| 360 | 26.9 ± 17.1 a | 21.3 ± 4.9 a,d | 21.8 ± 4.9 a | 16.8 ± 4.6 a,d | 16.3 ± 5.3 a | 22.2 ± 4.9 a,d | |
| 3.0 | 0 | 30.0 ± 2.4 a | 27.3 ± 1.9 a* | 27.6 ± 1.3 a* | 31.0 ± 1.5 a | 32.2 ± 2.4 a* | 29.7 ± 1.5 a^* |
| 45 | 200.7 ± 17.6 b | 132.3 ± 14.0 b^* | 184.1 ± 22.7 b* | 191.7 ± 14.0 b^ | 204.0 ± 28.6 b* | 152.6 ± 17.6 b^* | |
| 90 | 165.1 ± 25.7 b | 142.7 ± 2.4 b^* | 175.3 ± 22.5 b* | 200.6 ± 18.8 b^ | 199.6 ± 24.6 b* | 175.3 ± 14.7 b^* | |
| 180 | 183.2 ± 19.0 b | 136.9 ± 13.7 b^* | 188.4 ± 28.5 b* | 178.9 ± 14.8 b^ | 231.0 ± 16.1 b* | 185.2 ± 18.1 b^* | |
| 360 | 130.4 ± 15.8 b | 77.0 ± 9.1 b^* | 133.9 ± 14.7 b* | 110.7 ± 17.9 b^ | 151.9 ± 13.0 b* | 91.6 ± 15.2 b^* | |
Different letters denote significant differences between time points. Significant age differences are denoted by (*). Significant differences between males and females are denoted by (^).
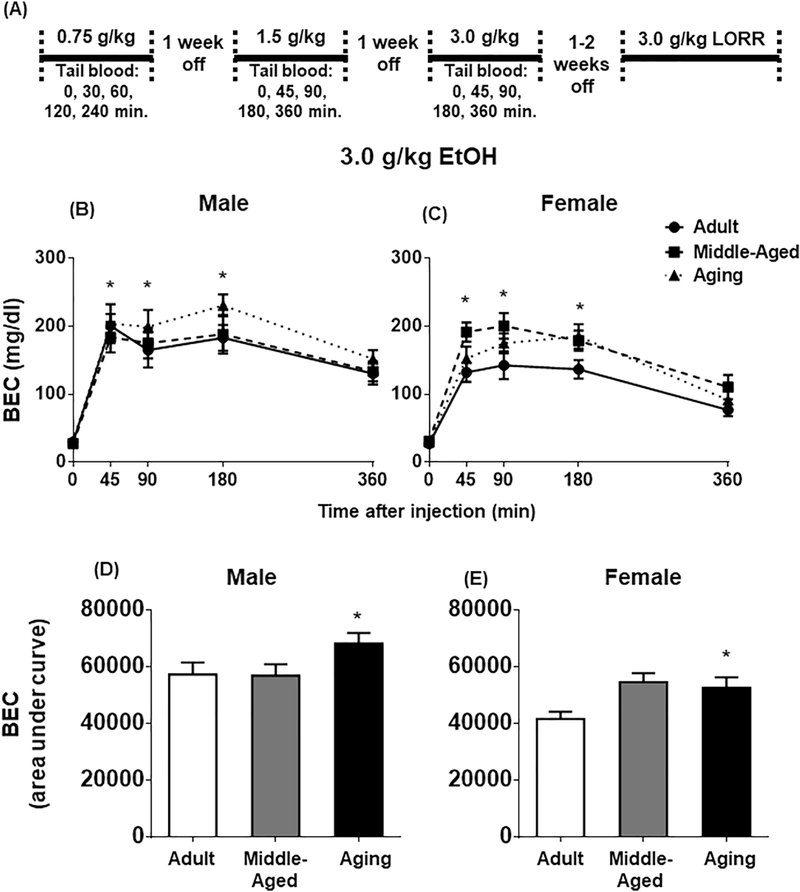
Following a 0.75 g/kg EtOH dose, peak BECs were observed at the 30–60 min time points, with BECs returning to baseline levels by 240 min (Table 2). Females exhibited a significant reduction in BECs from 30 to 60 minutes, with no differences between BECs at the these two time points evident in males (Time Point x Sex interaction, F(2,106) = 4.59, p = 0.01]. There were no age or sex differences in BECs at the last time point; area under the curve was not affected by Age or Sex.
In response to 1.5 g/kg EtOH, there was a significant effect of Time Point [F(2,106) = 19.23, p < 0.00001] that was unaffected by Age or Sex (Table 2). Post-hoc tests revealed a significant increase in BECs from 45 to 90 minutes and a significant decrease in BECs from 90 to 180 minutes. BECs at 360 min were not significantly different from baseline. In addition, regardless of time point, aging females had significantly higher BECs relative to adult females [Age x Sex interaction, F(2,53) = 3.31, p < 0.05]. No such age differences were evident in males. Area under the curve did not differ as a function of Age or Sex.
Males exhibited higher peak BECs in response to a 3.0 g/kg EtOH injection [Main effect of Sex, F(1,51) = 6.41, p < 0.05] (Table 2; Figure 3B). In addition, aging rats had higher BECs at the 45, 90, and 180 min time points, relative to adult and middle-aged rats [F(2,51) = 3.65, p < 0.05] (Figure 3B, C). At the 360 min time point, males had elevated BECs relative to females [Time Point x Sex, F(1,50) = 13.70, p < 0.001]. Analysis of the area under the curve revealed higher BECs overall in males [F(1,52) = 14.36, p < 0.001]. In addition, aging rats had higher BECs, relative to adult rats, as indicated by a larger area under the curve (F(2,52) = 4.67, p < 0.05] (Figure 3D, E).
Figure 3.
(A) Timeline for Experiment 2 assessing EtOH pharmacokinetics and sedation. BECs at 0, 45, 90, 180, and 360 min following a 3.0 g/kg EtOH dose in (B) males and (C) females. BECs calculated as area under the curve in (D) males and (E) females. All data expressed as mean ± SEM. (*) p < 0.05 vs. adult.
3.2b. Loss of Righting Reflex: 3.0 g/kg
One to two weeks after the last BEC time course, rats were injected with 3.0 g/kg EtOH and LORR was assessed (Table 3). There were no group differences in latency to LORR. Similar to what was observed following a 3.5 g/kg EtOH injection, females had a lower duration of LORR [F(1,45) = 43.79, p < 0.00001]. Both middle-aged and aging rats exhibited a higher duration of LORR, relative to adult rats [F(2,45) = 20.50, p < 0.00001]. Females had higher BECs at awakening [F(1,45) = 12.59, p < 0.001]. BECs at awakening also differed as a function of age [F(2,45) = 8.17, p < 0.001]. Post-hoc tests indicated that both middle-aged and aging rats had lower BECs than adult rats. At RORR, CORT was higher in females than in males [F(1,45) = 20.23, p < 0.0001] but did not differ as a function of age.
Table 3.
Loss of Righting Reflex (mean ± SEM) following a 3.0 g/kg EtOH dose in Experiment 2.
| Behavior | Adult | Middle-Aged | Aging | |||
|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | |
| Latency to LORR (min) | 2.8 ± 0.2 | 3.4 ± 1.1 | 4.8 ± 1.4 | 4.4 ± 2.0 | 2.5 ± 0.2 | 2.5 ± 0.2 |
| Duration of LORR (min) | 134.9 ± 16.5 | 74.1 ± 6.5^ | 217.5 ± 24.6* | 135.9 ± 8.6*^ | 245.6 ± 15.8* | 1428 ± 8.7*^ |
| BECs at awakening (mg/dl) | 318.7 ± 10.0 | 336.4 ± 6.3^ | 277.4 ± 13.5* | 306.5 ± 7.0*^ | 272.6 ± 15.9* | 315.1 ± 6.5*^ |
| CORT at awakening (μg/dl) | 39.4 ± 2.0 | 55.2 ± 4.8^ | 40.6 ± 4.2 | 55.1 ± 3.9^ | 44.5 ± 5.6 | 59.7 ± 3.0^ |
Asterisks (*) indicate a significant difference from 3-month-olds. Significant differences between males and females are denoted by (^).
3.3. Body Weights
Body weights were obtained from all rats at several points throughout each experiment but for clarity we are reporting initial body weights and final body weights (Table 4). For Experiment 1, there was a significant Age x Sex x Day interaction [F(1,47) = 22.69, p < 0.0001]. To probe this interaction, males and females were assessed separately. In males, there was a significant Day x Age interaction [F(1,23) = 59.44, p < 0.00001]. Post-hoc tests revealed a slight increase in body weight over the course of the experiment in adult males but a decrease in body weight in aging males. Aging females weighed more than adult females, but there was no interaction with day [F(1,24) = 167.07, p < 0.00001]. For Experiment 2, there was a significant Day x Sex interaction [F(1,49) = 8.02, p = 0.007], whereby males exhibited a slight reduction in body weights over the experiment but females did not. There was also a significant Day x Age interaction [F(2,49) = 20.49, p < 0.00001]. Post-hoc tests revealed that weights did not change over the course of the experiment in adult rats. However, middle-aged and aging rats lost weight over the experiment, regardless of sex. Not surprisingly, in both experiments, males weighed more than females [Exp. 1: F(1,47) = 1774.70, p < 0.000001; Exp. 2: F(1, 49) = 1035.30, p < 0.000001] and aging rats weighed more than adult rats [Exp. 1 & 2] and middle-aged rats [Exp. 2] [Exp. 1: F(1,47) = 193.70, p < 0.000001; Exp. 2: F(2,49) = 68.87, p < 0.00001].
Table 4.
Mean weights (g ± SEM) in Experiments 1 and 2.
| Experiment | Age | Sex | Weight (Initial) | Weight (Final) |
|---|---|---|---|---|
| 1: Social behavior & LORR | Adult | Male | 355.8 ± 3.3 | 364.6 ± 3.8* |
| Aging | 426.5 ± 6.4 | 413.6 ± 6.78* | ||
| Adult | Female | 187.3 ± 1.7 | 186.6 ± 1.9 | |
| Aging | 244.3 ± 4.4 | 241.8 ± 4.2 | ||
| 2: Ethanol pharmacokinetics & LORR | Adult | Male | 317.7 ± 6.6 | 320.5 ± 8.2 |
| Middle-Aged | 399.9 ± 7.2 | 385.2 ± 7.5* | ||
| Aging | 416.3 ± 10.4 | 397.4 ± 7.5* | ||
| Adult | Female | 180.0 ± 3.3 | 185.5 ± 3.5 | |
| Middle-Aged | 210.2 ± 4.1 | 208.8 ± 3.9* | ||
| Aging | 237.8 ± 2.6 | 228.4 ± 2.0* |
Significant changes in body weight are denoted by (*).
4.0. Discussion
These studies demonstrated that late aging results in substantial differences in responsiveness to EtOH. First, aging females exhibited social facilitation, indexed via increased contact behavior following low-dose EtOH (0.5 g/kg EtOH), and were insensitive to the socially suppressing effects of the higher dose of 0.75 g/kg EtOH, with this dose suppressing social behavior in aging males. Second, aging rats overall were more sensitive to the sedative effects of EtOH, exhibiting longer duration of LORR accompanied by lower BECs at RORR. Aging rats also exhibited higher BECs, but only following a high dose of EtOH (3.0 g/kg). No such age differences were evident at low doses (0.75 g/kg & 1.5 g/kg), suggesting that EtOH pharmacokinetics are likely not contributing to age and sex differences in the socially facilitating or socially suppressing effects of EtOH.
Previous studies from our laboratory and others have demonstrated a significant decline in social behavior during late aging (18–30 months old; Wistar, Sprague-Dawley, F344, F344/N) (Hunt et al., 2011; Markel et al., 1995; Perkins et al., 2016; Salchner et al., 2004; Shoji and Mizoguchi, 2011). Consistent with these studies, we provided evidence of decreased social behavior in late aging, observed in saline-injected males and females. Furthermore, in F344 rats, adult females that were single-housed exhibited higher levels of social behavior relative to their adult male counterparts (Perkins et al., 2017). In the present study, however, pair-housing eliminated sex differences observed previously in adult rats, with males and females demonstrating comparable levels of social investigation and contact behavior under 0 g/kg EtOH dose. Thus, it is possible that male and female F344 rats are differentially sensitive to the effects of social deprivation on subsequent tests of social interaction. However, further studies are necessary to determine if this is the case.
Adolescent rats tested at P 35 exhibited increased frequency of social behavior at 0.5 g/kg. This contrasts with adult rats (P 70) that demonstrated suppression of social behavior at low to moderate doses of EtOH (0.75 – 1.0 g/kg) (Trezza et al., 2009; Varlinskaya and Spear, 2007, 2002). Consistent with these studies, we found that adult F344 rats exhibit suppression of social investigation following EtOH (0.75 g/kg) and this was evident in both males and females. However, no study to date has examined whether aging (19–20 month old) rats exhibit altered sensitivity to the socially suppressing effects of EtOH. We demonstrated here for the first time that aged males exhibited social suppression after acute administration of 0.75 g/kg EtOH. Furthermore, pronounced sex differences in sensitivity to the social consequences of EtOH were evident among aging rats, with aging females, in contrast to their male counterparts, exhibiting social facilitation of contact behavior following acute administration of 0.5 g/kg EtOH. The reason for this effect is unknown, although one possible explanation is that suppression of social behavior in late aging, particularly in females, may be due to some stressful or anxiety-provoking aspect of conspecific interaction. Thus, EtOH might have facilitated social behavior in aging females via its anxiolytic properties. Aged (18–22 months old; Wistar, Lewis) rats do exhibit increased anxiety-like behavior (Hovens et al., 2013; Meyza et al., 2011; Pietrelli et al., 2012), although some studies have demonstrated no change (Bergado et al., 2011) or decreased anxietylike behavior (Torras-Garcia et al., 2005) in late aging. It has also been suggested that age-related reductions in social behavior are not a result of increased anxiety (Salchner et al., 2004). However, most of these studies have been conducted in male rats, so it is still unknown whether aged females exhibit alterations in anxiety-like behavior.
Age-related reductions in social behavior have also been attributed to non-specific declines in overall health. We have shown previously that at the age tested here (19–20 months), males and females are not different from adults in sensorimotor function assessed via the forelimb adjusting steps (FAS) and vibrissae-evoked forelimb placing (VEFP) tests (Perkins et al., 2016). It is difficult to separate social motivation from nonspecific debilitation in late aging, but the fact that we observed increased social behavior following low-dose EtOH suggests that aging-related reductions in social behavior may be a function of decreased motivation to engage in social interaction, at least in females, rather than a sign of aging-related debilitation. Another interesting possibility is that late aging-related changes in oxytocin (OT) might alter the response to EtOH. There is an increasing literature relating OT and alcohol consumption in several species including rats (Peters et al., 2017), mice (King et al., 2017), and socially monogamous prairie voles (Stevenson et al., 2017a, 2017b). Furthermore, OT itself has anxiolytic properties (Bahi et al., 2016; Blume et al., 2008; Grund et al., 2017; Klenerova et al., 2009; Van Den Burg et al., 2015), which may be involved in the socially facilitating and/or suppressing effects of EtOH. There have been relatively few studies assessing sex differences in the OT system as a function of late aging. Using ovariectomized females (with or without estradiol treatment), Garcia et al. (2016) demonstrated no age differences in OT or OTR gene expression in PVN, but an increase in OTR expression in SON in aged female rats. Future studies should assess the OT system in late aging as it relates to EtOH-induced changes in social behavior.
Sensitivity to the sedative effects of EtOH increases with age, with young rats being less sensitive to these effects than adults (Silveri and Spear, 1998). Aged male rats (18 months old) exhibit increased sensitivity to EtOH-induced impairment of motor function (Novier et al., 2013; Ornelas et al., 2015). In addition, Ornelas et al. (2015) found that 18-month-old male rats exhibited longer LORR duration at 3.0 g/kg EtOH. Importantly, we also demonstrated enhanced sensitivity to the sedative effects of EtOH, indicating that these changes are likely independent of strain. However, previous studies did not assess BECs at RORR, which is necessary to conclude that aged rats do in fact exhibit enhanced sensitivity to EtOH-associated sedation, rather than differences in EtOH pharmacokinetics. In the current study, which included assessment of EtOH-induced sedation in females, aging rats exhibited increased duration of LORR following acute administration of 3.0 and 3.5 g/kg EtOH, regardless of sex. This was accompanied by lower BECs at RORR, indicating that aging rats are more sensitive to EtOH-induced sedation than their adult (4–5 month) counterparts. One possible explanation for these results is that there are aging-related changes in EtOH pharmacokinetics. Age- and sex-differences in body weight and body composition likely produce differences in EtOH metabolism. Studies have demonstrated that EtOH distributes into water compartments of the body, and that body water composition is inversely related to body weight. Thus, animals with higher body weights (and thus lower body water composition) would be expected to have higher BECs. Interestingly, when EtOH is administered in a manner that accounts for body water composition, aged (27 month) rats did not differ in duration of LORR, although BECs at awakening were significantly reduced relative to adult and middle-aged rats, indicating enhanced sensitivity to the sedative effects of EtOH in late aging (York, 1983). We assessed BECs following acute administration of 0.75 g/kg, 1.5 g/kg, and 3.0 g/kg EtOH. Late aging-related differences were only evident at the highest dose administered, with 19–20-month-old rats exhibiting higher peak BECs relative to young adults. Importantly, sex differences in social behavior induced by EtOH are likely not a result of altered EtOH metabolism, since no age or sex differences in BECs were observed at 0.75 g/kg, whereas age differences in sensitivity to EtOH-induced sedation may be due, in part, to altered EtOH pharmacokinetics. Activity of the hepatic enzyme cytochrome P450 (CYP) 2E1, a key factor in EtOH metabolism, is reduced in the aged (18-month-old) rat (Wauthier et al., 2004), whereas hepatic alcohol dehydrogenase activity is elevated in aged F344 males (24 months old) but is unchanged in aged F344 female (24 months old) rats (Rikans and Kling, 1987). Thus, age-related changes in behavioral sensitivity to high doses of EtOH in particular may be due to several factors, including dose-dependent effects of EtOH that might indicate emergence of liver dysfunction evidenced by reduced clearance of EtOH at high (but not low) doses of EtOH.
There are some limitations and strengths to consider when interpreting the data from the current experiments. First, these studies used F344 rats, which is one of several strains provided by the National Institutes on Aging for use in aging research. However, F344 rats are stress reactive and hyper-adrenergic (Sternberg et al., 1992; Tonelli et al., 2001). Stress reactivity may influence the response to ethanol, although by using a within-subjects design we were able to assess behavior relative to each animal’s own baseline. In fact, when social interaction data were expressed as a percent change from saline, aged females still displayed increased social behavior whereas aged males displayed blunted social behavior. A major strength of the present studies was careful consideration of sex, age, and dose effects. Finally, although it may be seen as a limitation, the use of within-subjects design allowed for tests of EtOH effects (such as LORR) in rats with a recent history of moderate EtOH exposure, which is perhaps a more realistic translation to humans than first-time EtOH effects being assessed in late aging. In addition, we chose to test under conditions of saline first followed by successive doses of ethanol so that any potential development of tolerance effects on social behavior would be uniform across subjects. Additionally, testing ethanol in successive (rather than counter-balanced) fashion allows for a more gradual exposure to ethanol in these aged rats, which have no history of ethanol exposure across the lifespan.
Altogether, these data contribute to a growing literature describing behavioral responses to EtOH in late aging. We demonstrated that late aging is associated with altered sensitivity to the behavioral effects of EtOH that was somewhat sex-specific. Age differences in EtOH sensitivity were evident across a wide range of doses, with aged females, but not aged males, exhibiting social facilitation at the lowest dose of EtOH tested. In response to high sedative doses, aged rats overall were more sensitive, with no differences observed between aged male and female rats. Thus, sex differences in the behavioral response to EtOH were dose-dependent in aged rats. These differences could not be attributed to EtOH pharmacokinetics since no age or sex differences in BECs were observed following low-dose EtOH administration. Future research should explore the mechanisms involved in age and sex differences in behavioral sensitivity to EtOH.
Highlights:
Aging females exhibit social facilitation following low-dose EtOH (0.5 g/kg)
Aging males were sensitive to the socially suppressing effects of EtOH (0.5 & 0.75 g/kg)
Aging rats were more sensitive to the sedative effects of EtOH (3.0 and 3.5 g/kg)
BECs did not vary as a function of age at low and moderate EtOH doses (0.75 & 1.5 g/kg)
Aging rats exhibited elevated BECs following a high EtOH dose (3.0 g/kg)
Acknowledgements:
Research reported in this publication was supported by the National Institute of Health Grants P50AA017823 and RO1AG043467 to T. Deak, and the Center for Development and Behavioral Neuroscience at Binghamton University. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the above stated funding agencies. The authors have no conflicts of interest to declare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bahi A, Al Mansouri S, Al Maamari E, 2016. Nucleus accumbens lentiviral-mediated gain of function of the oxytocin receptor regulates anxiety- and ethanol-related behaviors in adult mice. Physiol. Behav 164, 249–258. https://doi.org/10.1016/j.physbeh.2016.06.009 [DOI] [PubMed] [Google Scholar]
- Bergado JA, Almaguer W, Rojas Y, 2011. Spatial and emotional memory in aged rats: A behavioral-statistical analysis. Neuroscience 172, 256–269. https://doi.org/10.1016/j.neuroscience.2010.10.064 [DOI] [PubMed] [Google Scholar]
- Bisschop MI, Kriegsman DMW, van Tilburg TG, Penninx BWJH, van Eijk JTM, Deeg DJH, 2003. The influence of differing social ties on decline in physical functioning among older people with and without chronic diseases: the Longitudinal Aging Study Amsterdam. Aging Clin. Exp. Res 15, 164–73. [DOI] [PubMed] [Google Scholar]
- Blume A, Bosch OJ, Miklos S, Torner L, Wales L, Waldherr M, Neumann ID, 2008. Oxytocin reduces anxiety via ERK1/2 activation: Local effect within the rat hypothalamic paraventricular nucleus. Eur. J. Neurosci 27, 1947–1956. https://doi.org/10.1111/j.1460-9568.2008.06184.x [DOI] [PubMed] [Google Scholar]
- Breslow RA, Castle IP, Chen CM, Graubard BI, 2017. Trends in Alcohol Consumption Among Older Americans: National Health Interview Surveys , 1997 to 2014. Alcohol. Clin. Exp. Res 41, 976–986. https://doi.org/10.1111/acer.13365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charuvastra A, Cloitre M, 2008. Social Bonds and Posttraumatic Stress Disorder. Annu. Rev. Psychol 59, 301–328. https://doi.org/10.1146/annurev.psych.58.110405.085650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries a. C., Craft TKS, Glasper ER, Neigh GN, Alexander JK, DeVries CA, Craft TKS, Glasper ER, Neigh GN, Alexander JK, 2007. 2006 Curt P. Richter award winner. Social influences on stress responses and health. Psychoneuroendocrinology 32, 587–603. https://doi.org/10.1016/j.psyneuen.2007.04.007 [DOI] [PubMed] [Google Scholar]
- Gano A, Doremus-fitzwater TL, Deak T, 2017. A cross-sectional comparison of ethanol-related cytokine expression in the hippocampus of young and aged Fischer 344 rats. Neurobiol. Aging 54, 40–53. https://doi.org/10.1016/j.neurobiolaging.2017.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AN, Depena CK, Yin W, Gore AC, 2016. Testing the critical window of estradiol replacement on gene expression of vasopressin, oxytocin, and their receptors, in the hypothalamus of aging female rats. Mol. Cell. Endocrinol 419, 102–112. https://doi.org/10.1016/j.mce.2015.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grund T, Goyon S, Li Y, Eliava M, Liu H, Charlet A, Grinevich V, Neumann ID, 2017. Neuropeptide S activates paraventricular oxytocin neurons to induce anxiolysis. J. Neurosci 37, 2161–17. https://doi.org/10.1523/JNEUROSCI.2161-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- House JS, Landis KR, Umberson D, 1988. Social relationships and health. Science 241, 540–5. https://doi.org/10.1126/science.3399889 [DOI] [PubMed] [Google Scholar]
- Hovens IB, Schoemaker RG, Zee EA Van Der, Heineman E, Nyakas C, Van Leeuwen, B.L., 2013. Surgery-induced behavioral changes in aged rats. Exp. Gerontol. 48, 1204–1211. https://doi.org/10.1016/j.exger.2013.07.011 [DOI] [PubMed] [Google Scholar]
- Hueston CM, Deak T, 2014. On the time course, generality, and regulation of plasma progesterone release in male rats by stress exposure. Endocrinology 155, 3527–3537. https://doi.org/10.1210/en.2014-1060 [DOI] [PubMed] [Google Scholar]
- Hunt GE, Van Nieuwenhuijzen PS, Chan-Ling T, McGregor IS, 2011. “When an old rat smells a cat”: A decline in defense-related, but not accessory olfactory, Fos expression in aged rats. Neurobiol. Aging 32, 737–749. https://doi.org/10.1016/j.neurobiolaging.2009.03.014 [DOI] [PubMed] [Google Scholar]
- King CE, Griffin WC, Luderman LN, Kates MM, McGinty JF, Becker HC, 2017. Oxytocin Reduces Ethanol Self-Administration in Mice. Alcohol. Clin. Exp. Res 41, 955–964. https://doi.org/10.1111/acer.13359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenerova V, Krejci I, Sida P, Hlinak Z, Hynie S, 2009. Oxytocin and carbetocin effects on spontaneous behavior of male rats: Modulation by oxytocin receptor antagonists. Neuroendocrinol. Lett 30, 335–342. [PubMed] [Google Scholar]
- Lang FR, 2001. Regulation of social relationships in later adulthood. J. Gerontol. B. Psychol. Sci. Soc. Sci 56, P321–P326. https://doi.org/10.1093/geronb/56.6.P321 [DOI] [PubMed] [Google Scholar]
- Markel E, Felszeghy K, Luiten PGM, Nyakas C, 1995. Beneficial effect of chronic nimodipine treatment on behavioral dysfunctions of aged rats exposed to perinatal ethanol treatment. Arch. Gerontol. Geriatr 21, 75–88. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Mittleman G, 2017. Age-dependent effects of chronic intermittent ethanol treatment: Gross motor behavior and body weight in aged, adult and adolescent rats. Neurosci. Lett 657, 146–150. https://doi.org/10.1016/j.neulet.2017.08.012 [DOI] [PubMed] [Google Scholar]
- Meyza KZ, Boguszewski PM, Nikolaev E, Zagrodzka J, 2011. Age increases anxiety and reactivity of the fear/anxiety circuit in Lewis rats. Behav. Brain Res. 225, 192–200. https://doi.org/10.1016/j.bbr.2011.07.011 [DOI] [PubMed] [Google Scholar]
- Norman GJ, Zhang N, Morris JS, Karelina K, Berntson GG, DeVries a C., 2010. Social interaction modulates autonomic, inflammatory, and depressive-like responses to cardiac arrest and cardiopulmonary resuscitation. Proc. Natl. Acad. Sci. U. S. A 107, 16342–16347. https://doi.org/10.1073/pnas.1007583107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novier A, Ornelas LC, Diaz-Granados JL, Matthews DB, 2016. Differences in Behavioral Responding in Adult and Aged Rats Following Chronic Ethanol Exposure 40, 1462–1472. https://doi.org/10.1111/acer.13098 [DOI] [PubMed] [Google Scholar]
- Novier A, Skike CE Van, Diaz-Granados JL, Mittleman G, Matthews DB, 2013. Acute Alcohol Produces Ataxia and Cognitive Impairments in Aged Animals: A Comparison Between Young Adult and Aged Rats 37, 1317–1324. https://doi.org/10.1111/acer.12110 [DOI] [PubMed] [Google Scholar]
- Ornelas LC, Novier A, Skike CE Van, Diaz-Granados JL, Matthews DB, 2015. The effects of acute alcohol on motor impairments in adolescent , adult , and aged rats. Alcohol 49, 121–126. https://doi.org/10.1016/j.alcohol.2014.12.002 [DOI] [PubMed] [Google Scholar]
- Perkins AE, Doremus-Fitzwater TL, Spencer RL, Varlinskaya EI, Conti MM, Bishop C, Deak T, 2016. A working model for the assessment of disruptions in social behavior among aged rats: The role of sex differences, social recognition, and sensorimotor processes. Exp. Gerontol 76, 46–57. https://doi.org/10.1016/j.exger.2016.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins AE, Woodruff ER, Chun LE, Spencer RL, Varlinskaya E, Deak T, 2017. Analysis of c-Fos induction in response to social interaction in male and female Fisher 344 rats. Brain Res. 1672, 113–121. https://doi.org/10.1016/j.brainres.2017.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters ST, Bowen MT, Bohrer K, McGregor IS, Neumann ID, 2017. Oxytocin inhibits ethanol consumption and ethanol-induced dopamine release in the nucleus accumbens. Addict. Biol 22, 702–711. https://doi.org/10.1111/adb.12362 [DOI] [PubMed] [Google Scholar]
- Pietrelli A, Lopez-costa J, Goñi R, 2012. Aerobic exercise prevents age-dependent cognitive decline and reduces anxiety-related behaviors in middle-aged and old rats. Neuroscience 202, 252–266. https://doi.org/10.1016/j.neuroscience.2011.11.054 [DOI] [PubMed] [Google Scholar]
- Rikans LE, Kling OR, 1987. Effects of aging and testosterone administration on liver alcohol dehydrogenase activity in male Fischer 344 rats. Alcohol. Clin. Exp. Res 11, 562–566. [DOI] [PubMed] [Google Scholar]
- Salchner P, Lubec G, Singewald N, 2004. Decreased social interaction in aged rats may not reflect changes in anxiety-related behaviour. Behav. Brain Res 151, 1–8. https://doi.org/10.1016/j.bbr.2003.07.002 [DOI] [PubMed] [Google Scholar]
- Schiffman SS, 1997. Taste and Smell Losses in Normal Aging and Disease. J. Am. Med. Assoc 278, 1357–1362. [PubMed] [Google Scholar]
- Seeman TE, 2000. Social Health Health Promoting Effects of Friends and Family on Health Outcomes in Older Adults. Am. J. Heal. Promot 14, 362–370. [DOI] [PubMed] [Google Scholar]
- Shoji H, Mizoguchi K, 2011. Aging-related changes in the effects of social isolation on social behavior in rats. Physiol. Behav 102, 58–62. https://doi.org/10.1016/j.physbeh.2010.10.001 [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP, 1998. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol. Clin. Exp. Res 22, 670–676. https://doi.org/10.1111/j.1530-0277.1998.tb04310.x [DOI] [PubMed] [Google Scholar]
- Spear LP, 2015. Adolescent alcohol exposure: Are there separable vulnerable periods within adolescence? Physiol. Behav 148, 122–130. https://doi.org/10.1016/j.physbeh.2015.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, 2000. The adolescent brain and age-related behavioral manifestations, Neuroscience and Biobehavioral Reviews. https://doi.org/10.1016/S0149-7634(00)00014-2 [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Boissoneault J, Van Skike CE, Nixon SJ, Matthews DB, 2014. Age-Related Effects of Alcohol from Adolescent, Adult, and Aged Populations Using Human and Animal Models. Alcohol. Clin. Exp. Res 38, 2509–2516. https://doi.org/10.1111/acer.12531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg EM, Glowa JR, Smith MA, Cologero AE, Listwak SJ, Aksentijevich S, Chrousos GP, Wilder RL, Gold PW, 1992. Corticotropin releasing hormone related behavioral and neuroendocrine responses to stress in Lewis and Fischer rats. Brain Res. 570, 54–60. https://doi.org/10.1016/0006-8993(92)90563-O [DOI] [PubMed] [Google Scholar]
- Stevenson JR, Wenner SM, Freestone DM, Romaine CC, Parian MC, Christian SM, Bohidar AE, Ndem JR, Vogel IR, O’Kane CM, 2017a. Oxytocin reduces alcohol consumption in prairie voles. Physiol. Behav. 179, 411–421. https://doi.org/10.1016/j.physbeh.2017.07.021 [DOI] [PubMed] [Google Scholar]
- Stevenson JR, Young KA, Bohidar AE, Francomacaro LM, Fasold TR, Buirkle JM, Ndem JR, Christian SC, 2017b. Alcohol Consumption Decreases Oxytocin Neurons in the Anterior Paraventricular Nucleus of the Hypothalamus in Prairie Voles. Alcohol. Clin. Exp. Res 41, 1444–1451. https://doi.org/10.1111/acer.13430 [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2015. Results from the 2015 National Survey on Drug Use and Health: Detailed Tables, 2015 National Survey on Drug Use and Health. [Google Scholar]
- Tonelli L, Webster JI, Rapp KL, Sternberg E, 2001. Neuroendocrine responses regulating susceptibility and resistance to autoimmune/inflammatory disease in inbred rat strains. Immunol. Rev 184, 203–211. [DOI] [PubMed] [Google Scholar]
- Torras-Garcia M, Costa-Miserachs D, Coll-Andreu M, Portell-Cortes I, 2005. Decreased anxiety levels related to aging. Exp. Brain Res 164, 177–184. https://doi.org/10.1007/s00221-005-2240-y [DOI] [PubMed] [Google Scholar]
- Trezza V, Baarendse PJJ, Vanderschuren LJMJ, 2009. Prosocial Effects of Nicotine and Ethanol in Adolescent Rats Through Partially Dissociable Neurobehavioral Mechanisms. Neuropsychopharmacology 34, 2560–2573. https://doi.org/10.1038/npp.2009.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Burg EH, Stindl J, Grund T, Neumann ID, Strauss O, 2015. Oxytocin Stimulates Extracellular Ca2+Influx Through TRPV2 Channels in Hypothalamic Neurons to Exert Its Anxiolytic Effects. Neuropsychopharmacology 40, 2938–2947. https://doi.org/10.1038/npp.2015.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Skike CE, Botta P, Chin VS, Tokunaga S, McDaniel JM, Venard J, Diaz-Granados JL, Valenzuela CF, Matthews DB, 2010. Behavioral effects of ethanol in cerebellum are age dependent: Potential system and molecular mechanisms. Alcohol. Clin. Exp. Res 34, 2070–2080. https://doi.org/10.1111/j.1530-0277.2010.01303.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya E, Spear L, Spear N, 1999. Social behavior and social motivation in adolescent rats: role of housing conditions and partner’s activity. Physiol. Behav. Behav 67, 475–482. https://doi.org/10.1016/S0031-9384(98)00285-6 [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP, 2012. Increases in anxiety-like behavior induced by acute stress are reversed by ethanol in adolescent but not adult rats. Pharmacol. Biochem. Behav 100, 440–450. https://doi.org/10.1016/j.pbb.2011.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP, 2007. Chronic tolerance to the social consequences of ethanol in adolescent and adult Sprague-Dawley rats. Neurotoxicol. Teratol. 29, 23–30. https://doi.org/10.1016/j.ntt.2006.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP, 2002. Acute Effects of Ethanol on Social Behavior of Adolescent and Adult Rats: Role of Familiarity of the Test Situation. Alcohol. Clin. Exp. Res 26, 1502–1511. https://doi.org/10.1097/01.ALC.0000034033.95701.E3 [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Truxell E, Spear LP, 2014. Chronic intermittent ethanol exposure during adolescence: effects on social behavior and ethanol sensitivity in adulthood. Alcohol 45, 433–444. https://doi.org/10.1016/j.pestbp.2011.02.012.Investigations [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Truxell EM, Spear LP, 2015. Sex differences in sensitivity to the social consequences of acute ethanol and social drinking during adolescence. Behav. Brain Res 282, 6–13. https://doi.org/10.1016/j.bbr.2014.12.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Vogt B. a., Spear LP, 2013. Social context induces two unique patterns of c-Fos expression in adolescent and adult rats. Dev. Psychobiol 55, 684–697. https://doi.org/10.1002/dev.21064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vore AS, Doremus-Fitzwater T, Gano A, Deak T, 2017. Adolescent Ethanol Exposure Leads to Stimulus-Specific Changes in Cytokine Reactivity and Hypothalamic-Pituitary-Adrenal Axis Sensitivity in Adulthood. Front. Behav. Neurosci 11, 78 https://doi.org/10.3389/fnbeh.2017.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wauthier V, Verbeeck RK, Calderon PB, 2004. Age-related changes in the protein and mRNA levels of CYP2E1 and CYP3A isoforms as well as in their hepatic activities in Wistar rats. What role for oxidative stress? Arch. Toxicol 78, 131–138. https://doi.org/10.1007/s00204-003-0526-z [DOI] [PubMed] [Google Scholar]
- York J, 1983. Increased responsiveness to ethanol with advancing age in rats. Pharmacol. Biochem. Behav 19, 687–691. https://doi.org/10.1016/0091-3057(83)90346-5 [DOI] [PubMed] [Google Scholar]