Abstract
Background
Sarcopenia, characterized by low muscle mass, associates with mortality in patients with cirrhosis. Skeletal muscle area in a single computed tomography image at the level of the third lumbar vertebrate (L3) is a valid representative of whole body muscle mass. Controversy remains regarding applicability of psoas muscle to identify patients at greater risk of mortality. We aimed to determine psoas muscle index (PMI) association with skeletal muscle index (SMI) and to evaluate the capacity of PMI to predict liver transplant waitlist mortality.
Methods
We evaluated listed adult patients with cirrhosis from 2012 to 2013 at four North American liver transplant centres. From L3 computed tomography images within 3 months of listing, we determined SMI and PMI expressed by cm2/m2. Low SMI was defined as SMI <39 cm2/m2 in women and <50 cm2/m2 in men as published by us earlier. Cut‐offs for PMI to predict mortality were established using a receiver‐operating characteristic analysis. Mortality predictors were determined using competing‐risk analysis with reported results as subdistribution hazard ratios (sHRs).
Results
Of 353 waitlist candidates, 68% were men, mean age 56 ± 9 years, and Model for End‐stage Liver Disease of 16 ± 8 points. Low SMI was present more frequently in men than women (51 vs. 36%, P = 0.02). Moderately strong correlation between SMI and PMI was observed (r > 0.7, P < 0.001). Low PMI (males < 5.1 cm2/m2; females < 4.3 cm2/m2) yielded poor and moderate concordance with low SMI in men and women, respectively (Kappa coefficient 0.31 and 0.63). SMI (39 ± 9 vs. 43 ± 7 cm2/m2; P = 0.009) and PMI (4.4 ± 1.3 vs. 5.2 ± 1.1 cm2/m2; P = 0.001) were lower in women who died and/or were delisted (compared with non‐deceased patients) whereas men who died and/or were delisted had only lower SMI (47 ± 7 vs. 51 ± 9 cm2/m2; P = 0.003), but not PMI compared with non‐deceased patients. In women, both SMI (sHR 0.94, P = 0.048) and PMI (sHR 0.58, P = 0.002) were predictors of mortality, while in men, SMI was significant (sHR 0.95, P = 0.001) and PMI showed a trend to be (sHR 0.85, P = 0.09) associated with mortality. Overall, 104 patients (29%) were misclassified between SMI and PMI categories. Using PMI cut‐offs, 66% and 28% of low SMI men and women, who have a higher risk of mortality, were incorrectly classified as low risk.
Conclusions
Skeletal muscle index is a more complete and robust measurement than PMI, especially in men with cirrhosis. Low PMI identifies an incomplete subset of patients at increased risk of mortality indicated by low SMI. Given the poor performance of PMI, SMI should not be substituted by PMI.
Keywords: Sarcopenia, Prognosis, Computed tomography, End‐stage liver disease
Introduction
Sarcopenia, characterized by low muscle mass, is independently associated with elevated mortality in end‐stage liver disease (ESLD) patients.1, 2, 3, 4 Higher waitlist mortality and longer hospital stay following liver transplantation (LT) have been reported in patients with ESLD and sarcopenia.5 In most studies, sarcopenia was more prevalent in male patients with cirrhosis compared with female patients.1, 5, 6
Various approaches to assess body composition such as body mass index (BMI, kg/m2), bioelectrical impedance analysis, and dual‐energy X‐ray absorptiometry6, 7 have been used to patients with cirrhosis. Non‐invasive imaging with computed tomography (CT) has been applied as a precise technique for muscle and adipose tissue quantification.8 Diagnostic CT imaging is a routine part of LT assessment in most LT centres, to screen for hepatocellular carcinoma (HCC) and evaluate the vascular and biliary anatomy.
A comprehensive definition of sarcopenia has been recently developed in a population of cirrhotic patients listed for LT at four North American LT centres.1 CT‐determined skeletal muscle index (SMI) was used to discriminate waitlist mortality in these patients.1 SMI in a single CT image at the level of the third lumbar vertebrate (L3) is a reliable and valid representative of whole body muscle mass.8, 9 Besides SMI, individual muscle measurements, i.e. psoas muscle thickness,10 psoas muscle index (PMI),11 and psoas muscle cross‐sectional area (PMA),12, 13 have been used as indicators of increased risk of mortality in later literature. Psoas muscle assessment is easy and rapid,11 and not influenced by abdominal swelling,10 but is affected by long‐term chronic disease.12 Controversy remains regarding the use of PMI instead of SMI for the purposes of mortality risk prediction. We therefore first determined PMI association with SMI as continuous and dichotomous variables in patients with ESLD. Second, we evaluated the capability of PMI to predict mortality in patients with ESLD awaiting LT.
Methods
Study population
Included were adult (≥18 years) patients with cirrhosis newly listed for LT (n = 353) from 1 January through 31 December 2012 at four North American LT centres: (i) University of California, San Francisco (141); (ii) University of Pittsburgh (n = 83); (iii) Mayo Clinic in Arizona (n = 52); and (iv) University of Alberta (77). Patients with an abdominal CT scan within 3 months of listing were included. All four centres are part of the Fitness, Life Enhancement, and Exercise in Transplantation (FLEXIT) Consortium with the ultimate goal to better understand muscle abnormalities, frailty, and their association with outcome in patients listed for LT. The study protocol was reviewed and approved by the institutional research ethics board at each centre. Clinical and demographic characteristics of the patients were collected from the medical charts at each centre. Coded, de‐identified clinical data and CT images were shared among consortium members under the terms of a formal data use agreement among the institutions.
Computed tomography image analysis
Skeletal muscle areas were quantified analysing L3‐CT scans by Slice‐O‐Matic software (V4.2; Tomovision, Montreal, QC, Canada) or Advantage Windows 2.2 Volume Viewer (GE Healthcare, Waukesha, WI), depending on the centre.1 Two collaborators blinded to patient outcome read the CT images. Correlation (r) between areas measured by two independent observers was 0.98 for both psoas and total skeletal muscle. Standard Hounsfield Unit thresholds of −29 to 15014 were used to estimate muscle cross‐sectional areas. By applying tissue‐specific radiodensity, muscle area assessments were not affected by ascites. Muscles visualized at L3 include the psoas, erector spinae, quadratus lumborum, transversus abdominis, external and internal obliques, and rectus abdominis. Cross‐sectional areas of these muscles were calculated by summing tissue pixels followed by multiplying by the pixel surface areas. Total abdominal muscle area was quantified using semi‐automatic measurement of all these muscle areas. For psoas evaluation, bilateral psoas muscle area was quantified. From L3‐CT images, we determined two muscularity variables: total skeletal muscle cross‐sectional area to establish the SMI, and psoas muscle area to determine the PMI, each normalized to the squared patient height (cm2/m2).
Statistical analysis
Continuous variables are reported as mean and standard deviation, and independent t‐test was used to compare means of two independent groups. Descriptive statistics for categorical variables are presented as frequency and percentages, and Pearson χ 2 test was conducted to compare study groups. Correlation between SMI and PMI was evaluated using Pearson's correlation coefficient (r) analysis. C‐statistics analysis was used to evaluate the performance of muscle cross‐sectional area, SMI, PMA, and PMI for waitlist mortality prediction.
The primary outcome of the study was waitlist mortality, described as death or delisting for being too sick for LT. In patients listed for LT, death and LT, the two competing outcomes of interest, were evaluated using competing‐risk analysis.15 Significant predictors of mortality in patients listed for LT were determined using univariate and multivariate Fine and Gray subdistribution hazards models, and the results were reported as subdistribution hazard ratios (sHRs) with 95% confidence intervals (CI). Variables with the P < 0.10 in the univariate analysis were included in the multivariate model.
Cut‐offs for PMI to predict mortality were established using a receiver‐operating characteristic analysis. Model ability to differentiate between outcome groups was assessed using the area under the curve. Sex‐specific potential cut‐offs with the highest Youden's Index (sensitivity + specificity −1) were included in adjusted competing risk models, and the value with a highest significant P‐value was considered as the optimal cut‐off point. Low SMI was defined using pre‐established mortality‐associated cut‐offs in ESLD patients waiting for LT as SMI <39 cm2/m2 in women and <50 cm2/m2 in men.1 Potential cut‐offs for SMI in Carey et al.1 was calculated based on a log‐rank and Wilcoxon test statistics, followed by combination of maximized power and a significant P‐value. It was mentioned by previous researchers15, 16 that methods for the estimation of area under the receiver‐operating characteristic curve are primarily based on the Wilcoxon rank‐sum test and this relationship could offer a preferred level of statistical power (low type II error) in comparative experiments.
Overall survival estimates, 6 month, 1 year, and 2 year survival probabilities were obtained using Kaplan–Meier curves, and comparison between low and high PMI patients was conducted using the log‐rank test. The Cohen's kappa coefficient was applied to obtain agreement between SMI and PMI categories.
Results
Characteristics of listed patients
Characteristics of patients with cirrhosis listed for LT are presented in Table 1. Of 353 waitlist candidates, 70% were male with the mean age of 56 ± 9 years and Model for End‐stage Liver Disease (MELD) score of 16 ± 8 points. Mean BMI was 28 ± 6 kg/m2 with the majority (67%) of the patients being overweight or obese (BMI ≥ 25). Concomitant HCC was present in 41% of patients. Patients were followed to death (101), LT (170), or censoring (82).
Table 1.
Patient clinical characteristics by sex at the time of listing
| Characteristics | All Patients (n = 353) | Male (n = 246) | Female (n = 107) | P value |
|---|---|---|---|---|
| Age (years) | 56 ± 9 | 56 ± 9 | 56 ± 9 | 0.74 |
| Albumin (g/dL, 3.5–5.5) | 3 ± 2 | 3 ± 1 | 3 ± 3 | 0.12 |
| Sodium (mmol/L, 133–146) | 135 ± 5 | 134 ± 5 | 134 ± 6 | 0.35 |
| MELD score | 16 ± 8 | 16 ± 8 | 17 ± 9 | 0.29 |
| Hepatic encephalopathy | 0.73 | |||
| None | 157 (48) | 112 (49) | 45 (45) | |
| Well controlled | 156 (48) | 105 (46) | 51 (51) | |
| Poorly controlled | 14 (4) | 10 (4) | 4 (4) | |
| Ascites | 0.04 | |||
| None | 131 (40) | 98 (43) | 33 (33) | |
| Mild | 130 (40) | 80 (35) | 50 (50) | |
| Refractory | 66 (20) | 49 (22) | 17 (17) | |
| HCC | 144 (41) | 111 (44) | 33 (30) | 0.02 |
| Body composition characteristics | ||||
| BMI (kg/m2) | 28 ± 6 | 28 ± 5 | 27 ± 6 | 0.19 |
| PMA (cm2) | 18 ± 6 | 20 ± 5 | 13 ± 3 | <0.001 |
| PMI (cm2/m2) | 6 ± 1.7 | 6.5 ± 1.6 | 4.9 ± 1.3 | <0.001 |
| SMA (cm2) | 129 ± 31 | 154 ± 27 | 109 ± 22 | <0.001 |
| SMI (cm2/m2) | 47 ± 9 | 50 ± 8 | 42 ± 8 | <0.001 |
| VATI | 35 ± 27 | 37 ± 28 | 29 ± 23 | 0.009 |
| SATI | 60 ± 40 | 57 ± 37 | 69 ± 44 | 0.004 |
| Muscle attenuation (HU) | 36 ± 9 | 37 ± 9 | 33 ± 9 | <0.001 |
| Low SMIa | 165 (47) | 126 (51) | 39 (36) | 0.02 |
Numbers in parentheses are percentages.
BMI, body mass index; HCC, hepatocellular carcinoma; MELD, Model for End‐stage Liver Disease; PMA, psoas muscle area; PMI, psoas muscle index; SATI, subcutaneous adipose tissue index; SMA, skeletal muscle area; SMI, skeletal muscle index; VATI, visceral adipose tissue index.
Low SMI was defined using established cut‐offs in patients with cirrhosis listed for LT.1
Overall mean SMI was 47 ± 9 cm2/m2 (range 23–81), and PMI was 6 ± 1.7 cm2/m2 (range 2–12). Despite similar BMI between men and women, SMI (50 ± 8 vs. 42 ± 8 cm2/m2) and PMI (6.5 ± 1.6 vs. 4.9 ± 1.3 cm2/m2) were higher in men (P < 0.001 for each; Table 1). Low SMI was more frequent in men compared with women (51 vs. 36%; P = 0.02). SMI (39 ± 9 vs. 43 ± 7 cm2/m2; P = 0.009) and PMI (4.4 ± 1.3 vs. 5.2 ± 1.1 cm2/m2; P = 0.001) were lower in women who died and/or were delisted (compared with non‐deceased patients) whereas men who died and/or were delisted had only lower SMI (47 ± 7 vs. 51 ± 9 cm2/m2; P = 0.003), but not PMI compared with non‐deceased patients (Table 2). Higher proportion of the patients who died and/or were delisted had HCC at the time of listing (50% vs. 36%; P = 0.02). Considering differences in SMI and PMI between men and women, mortality analysis was conducted by sex.
Table 2.
Clinical characteristics of listed patients according to the mortality status
| Characteristics | Died/Delisted (n = 101) | All others (n = 252) | P value |
|---|---|---|---|
| Age (years) | 57 ± 9 | 55 ± 9 | 0.18 |
| Albumin (g/dL, 3.5–5.5) | 3 ± 3 | 3 ± 1 | 0.53 |
| Sodium (mmol/L, 133–146) | 134 ± 5 | 135 ± 6 | 0.30 |
| MELD score | 17 ± 8 | 16 ± 9 | 0.38 |
| Hepatic encephalopathy | 0.74 | ||
| None | 48 (50) | 107 (47) | |
| Well controlled | 43 (45) | 111 (49) | |
| Poorly controlled | 5 (5) | 9 (4) | |
| Ascites | 0.69 | ||
| None | 35 (36) | 94 (42) | |
| Mild | 41 (43) | 87 (38) | |
| Refractory | 20 (21) | 46 (20) | |
| HCC | 50 (50) | 91 (36) | 0.02 |
| Body composition characteristics | |||
| BMI (kg/m2) | 27 ± 6 | 28 ± 5 | 0.07 |
| PMI (cm2/m2) | 5.5 ± 1.7 | 6.2 ± 1.6 | <0.001 |
| SMI (cm2/m2) | 44 ± 9 | 49 ± 9 | <0.001 |
| Males | |||
| PMI (cm2/m2) | 6.2 ± 1.6 | 6.6 ± 1.6 | 0.12 |
| SMI (cm2/m2) | 47 ± 7 | 51 ± 9 | 0.003 |
| Females | |||
| PMI (cm2/m2) | 4.4 ± 1.3 | 5.2 ± 1.1 | 0.001 |
| SMI (cm2/m2) | 39 ± 9 | 43 ± 7 | 0.009 |
Numbers in parentheses are percentages.
BMI, body mass index; HCC, hepatocellular carcinoma; MELD, Model for End‐stage Liver Disease; PMI, psoas muscle index; SMI, skeletal muscle index.
Figure 1 highlights the total skeletal muscle and psoas muscle quantification at L3 from two male patients. Figure 1A,B presents a patient who had low SMI (40 cm2/m2) but high PMI (6.6 cm2/m2). Figure 1C,D shows a patient with high SMI (54 cm2/m2) but low PMI (4.8 cm2/m2). This example illustrates that psoas muscle quantification may not necessarily reflect total muscle area assessment.
Figure 1.
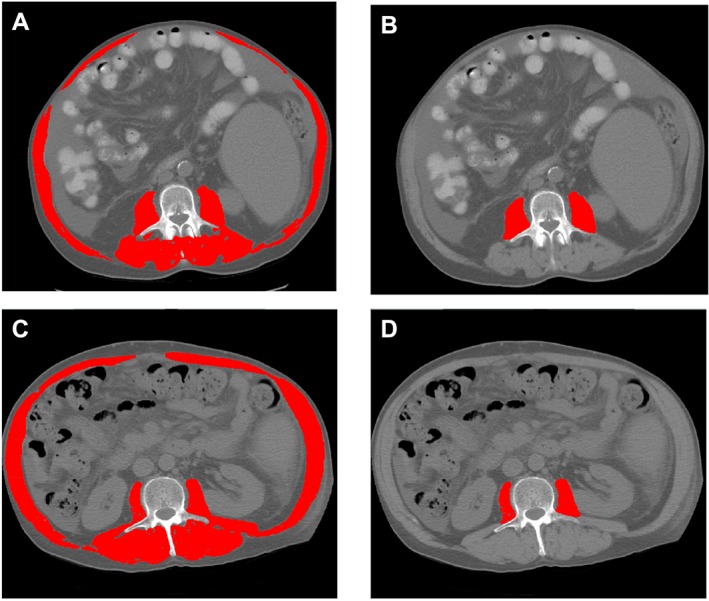
Abdominal computed tomography images taken at the third lumbar vertebra of two male patients with cirrhosis applied to quantify total muscle and psoas muscle areas. Panels (A) and (B) present a patient who had low skeletal muscle index (SMI) (40 cm2/m2) but high psoas muscle index (PMI) (6.6 cm2/m2). Panels (C) and (D) show a patient with high SMI (54 cm2/m2) and low PMI (4.8 cm2/m2).
Association between skeletal muscle index, psoas muscle index, and waitlist mortality
There was a significant linear (P < 0.001) and moderately strong (r = 0.73) relationship between SMI and PMI in male patients (Figure 2A). Waitlist mortality prediction yielded a C‐statistic of 0.62 (95% CI 0.55–0.70, P = 0.004) for SMI and 0.56 (95% CI 0.48–0.65, P = 0.13) for PMI. The same results were obtained using areas rather than indices to predict waitlist mortality, again with a limited ability for psoas to predict mortality (Table 3). However, indices were selected as the standardized muscle values.1, 17
Figure 2.
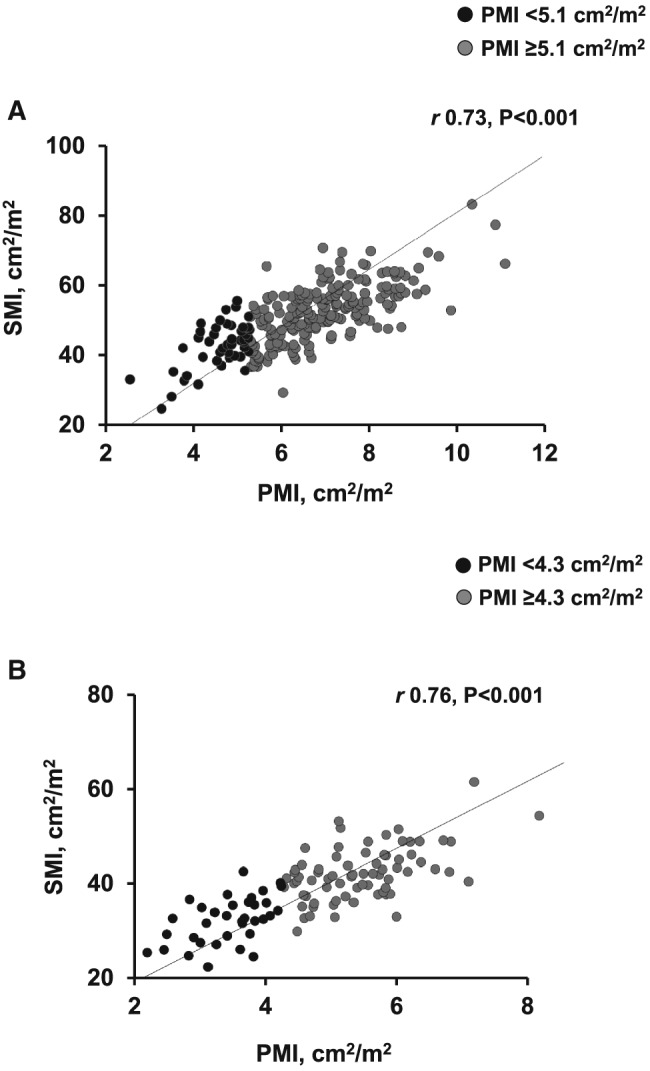
Scatter graph depicting correlations between skeletal muscle index and psoas muscle index in (A) male and (B) female patients. Using Pearson's correlation coefficient, moderately strong association between psoas muscle index (PMI) and skeletal muscle index (SMI) was observed in men (r = 0.73) and women (r = 0.76).
Table 3.
Discriminatory value of different muscle measurements in predicting waitlist mortality
| Variable | AUC | 95% CI | P value |
|---|---|---|---|
| Men | |||
| PMA | 0.56 | 0.48–0.65 | 0.15 |
| PMI | 0.56 | 0.48–0.65 | 0.13 |
| SMA | 0.64 | 0.56–0.71 | 0.002 |
| SMI | 0.62 | 0.55–0.70 | 0.004 |
| Women | |||
| PMA | 0.67 | 0.56–0.78 | 0.004 |
| PMI | 0.68 | 0.57–0.79 | 0.002 |
| SMA | 0.65 | 0.53–0.76 | 0.01 |
| SMI | 0.66 | 0.55–0.77 | 0.007 |
AUC, area under the ROC curve; CI, confidence interval; PMA, psoas muscle area; PMI, psoas muscle index; SMA, skeletal muscle area; SMI, skeletal muscle index.
In univariate analysis, assessing the association between clinical parameters and outcome, SMI, as a continuous variable, was significantly associated with waitlist mortality (sHR 0.96, 95% CI 0.93–0.98, P < 0.001; Table 4A). Considering LT as the competing event, SMI was independently associated with mortality after adjusting for MELD score and HCC (sHR 0.95, 95% CI 0.92–0.98, P = 0.001; SMI‐Model, Table 4A). Using pre‐established mortality‐associated cut‐offs for SMI, male patients with low SMI, as a categorical variable, had more than two‐fold higher mortality risk after adjusting for recognized mortality predictors (sHR 2.46, 95% CI 1.38–4.39, P = 0.002; SMI‐Model, Table 5A).
Table 4.
Clinical parameters associated with mortality in listed patients according to sex
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| SMI‐Model | PMI‐Model | |||||
| Characteristic | sHR (95% CI) | P value | sHR (95% CI) | P value | sHR (95% CI) | P value |
| A. Male Patients | ||||||
| Age (years) | 1.02 (0.99–1.06) | 0.21 | ||||
| Albumin (g/dL) | 0.80 (0.51–1.25) | 0.32 | ||||
| MELD score | 1.01 (0.98–1.04) | 0.45 | 1.01 (0.98–1.05) | 0.52 | 1.02 (0.98–1.05) | 0.32 |
| Ascites | 1.22 (0.87–1.70) | 0.25 | ||||
| Sodium (mmol/L) | 0.97 (0.93–1.01) | 0.13 | ||||
| HCC | 1.76 (1.07–2.90) | 0.03 | 2.16 (1.19–3.91) | 0.01 | 1.93 (1.08–3.44) | 0.03 |
| Hepatic encephalopathy | 1.05 (0.67–1.65) | 0.82 | ||||
| SMI (cm2/m2) | 0.96 (0.93–0.98) | <0.001 | 0.95 (0.92–0.98) | 0.001 | ||
| PMI (cm2/m2) | 0.86 (0.73–1.02) | 0.08 | 0.85 (0.70–1.03) | 0.09 | ||
| B. Female patients | ||||||
| Age (years) | 1.01 (0.98–1.05) | 0.55 | ||||
| Albumin (g/L) | 1.02 (0.997–1.05) | 0.07 | 0.74 (0.42–1.31) | 0.30 | 0.75 (0.44–1.27) | 0.29 |
| MELD score | 1.02 (0.99–1.05) | 0.25 | 1.00 (0.97–1.04) | 0.69 | 1.00 (0.97–1.04) | 0.73 |
| Ascites | 0.90 (0.63–1.30) | 0.59 | ||||
| Sodium (mmol/L) | 0.99 (0.95–1.04) | 0.82 | ||||
| HCC | 1.45 (0.80–2.65) | 0.22 | ||||
| Hepatic encephalopathy | 0.93 (0.51–1.68) | 0.80 | ||||
| SMI (cm2/m2) | 0.94 (0.90–0.99) | 0.01 | 0.94 (0.89–1.00) | 0.048 | ||
| PMI (cm2/m2) | 0.59 (0.44–0.79) | <0.001 | 0.58 (0.41–0.82) | 0.002 | ||
sHRs and P values were estimated using Fine‐Gray subdistribution hazard model.
CI, confidence interval; HCC, hepatocellular carcinoma; MELD, Model for End‐stage Liver Disease; PMI, psoas muscle index; sHR, subdistribution hazard ratio; SMI, skeletal muscle index.
Table 5.
Association of low SMI and low PMI with mortality in listed patients according to sex
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| SMI‐Model | PMI‐Model | |||||
| Characteristic | sHR (95% CI) | P value | sHR (95% CI) | P value | sHR (95% CI) | P value |
| A. Male patients | ||||||
| MELD score | 1.01 (0.98–1.04) | 0.45 | 1.02 (0.99–1.06) | 0.22 | 1.02 (0.99–1.05) | 0.26 |
| HCC | 1.76 (1.07–2.90) | 0.03 | 2.18 (1.22–3.89) | 0.008 | 1.98 (1.12–3.49) | 0.02 |
| Low SMIa | 2.17 (1.29–3.66) | 0.003 | 2.46 (1.38–4.39) | 0.002 | ||
| Low PMIb | 1.85 (1.05–3.24) | 0.03 | 2.04 (1.13–3.68) | 0.02 | ||
| B. Female patients | ||||||
| MELD score | 1.02 (0.99–1.05) | 0.25 | 1.00 (0.97–1.04) | 0.82 | 1.01 (0.98–1.05) | 0.47 |
| Albumin (g/dL) | 1.02 (0.997–1.05) | 0.07 | 0.79 (0.44–1.41) | 0.42 | 0.77 (0.43–1.36) | 0.36 |
| Low SMIa | 2.12 (1.14–3.93) | 0.02 | 2.05 (1.00–4.21) | 0.050 | ||
| Low PMIb | 2.45 (1.32–4.58) | 0.005 | 2.47 (1.24–4.95) | 0.01 | ||
sHRs and P values were estimated using Fine‐Gray subdistribution hazard model.
Variables with a P < 0.10 of Table 2 are included in multivariable model.
CI, confidence interval; MELD, Model for End‐stage Liver Disease; PMI, psoas muscle index; sHR, subdistribution hazard ratio; SMI, skeletal muscle index.
Low SMI was defined using established cut‐offs in patients with cirrhosis.1
Defined as PMI < 5.1 cm2/m2 in men and PMI < 4.3 cm2/m2 in women.
Psoas muscle index (cm2/m2), as a continuous variable, demonstrated a trend towards negative association with mortality in both univariate (sHR 0.86, 95% CI 0.73–1.02, P = 0.08) and multivariate models (sHR 0.85, 95% CI 0.70–1.03, P = 0.09; Table 4A). Every 1 cm2/m2 increase in PMI tended to be associated with 15% decreased risk of death. Applying the highest Youden's Index, PMI of 5.1 cm2/m2 was found to be independently associated with mortality with a sensitivity of 84%. Male patients with PMI <5.1 cm2/m2 had a two times higher risk of mortality (HR 2.04, 95% CI 1.13–3.68, P = 0.02; PMI‐Model, Table 5A) compared with the patients with high PMI (≥5.1 cm2/m2). The 6 month, 1 year, and 2 year probabilities of survival were 80%, 59%, and 54% in patients with low PMI compared with 88%, 83%, and 68% in men with high PMI (log rank = 0.02) (Figure 3A).
Figure 3.
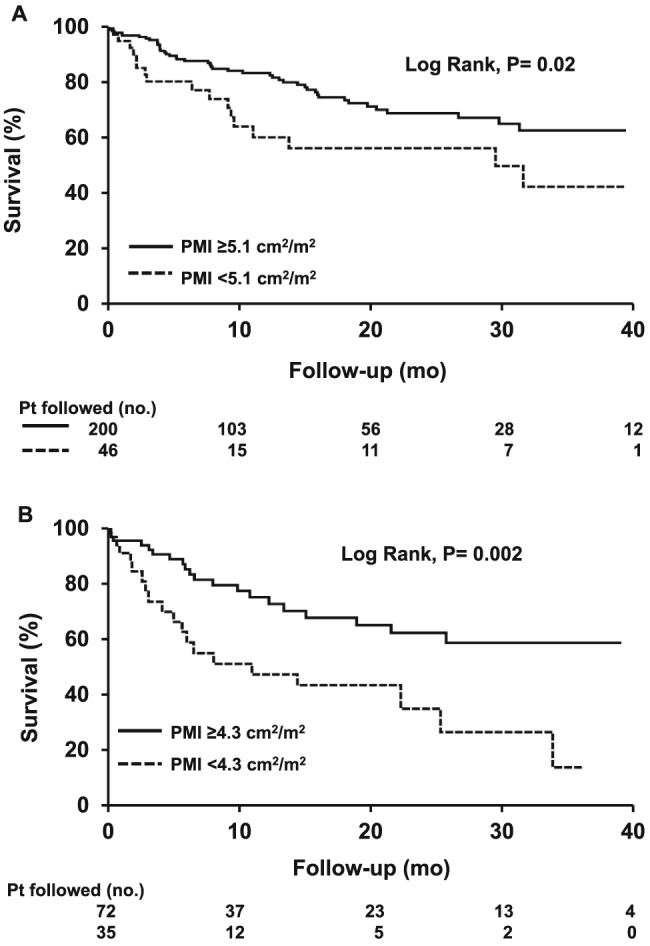
Survival curves in (A) male and (B) female patients with high and low psoas muscle index. Survival over time was estimated using Kaplan–Meier curves, and the curves were compared using the log‐rank test. Longer survival was observed in patients with high psoas muscle index (PMI) compared with the patients with low PMI (log rank, P = 0.02 and P = 0.002 in men and women, respectively).
We observed a moderately strong (r = 0.76) linear (P < 0.001) correlation between SMI and PMI in female patients (Figure 2B). The ability of the SMI and PMI to rank patients according to waitlist mortality (i.e. C‐statistic) were 0.66 (95% CI 0.55–0.77, P = 0.007) and 0.68 (95% CI 0.57–0.79, P = 0.002), respectively (Table 3).
Univariate and multivariate competing risk analysis of features associated with mortality were conducted in female patients listed for LT. Albumin, MELD score, SMI, and PMI were predictors of mortality in women in the univariate analysis (Table 4B). There was a negative association between SMI (cm2/m2) and mortality in multivariate models (sHR 0.94, 95% CI 0.89–1.00, P = 0.048). PMI (cm2/m2) was significantly negatively associated with mortality in multivariate models (sHR 0.58, 95% CI 0.41–0.82, P = 0.002) (Table 4B). In a multivariate model including confounding clinical variables, low SMI increased by two‐fold the risk of mortality in female patients (sHR 2.05, 95% CI 1.00–4.21, P = 0.05; SMI‐Model, Table 5B) compared with patients with high SMI. In women, PMI of 4.3 cm2/m2 was found to yield the highest Youden's Index, with the sensitivity of 78% and area under the curve of 0.68 (95% CI 0.57–0.79). Low PMI (PMI <4.3 cm2/m2) was an independent predictor of elevated mortality (sHR 2.47, 95% CI 1.24–4.95, P = 0.01), after adjusting for other survival predictors (PMI‐Model, Table 5B). Kaplan–Meier analysis to calculate survival probability based on psoas muscle cut‐offs revealed that the 6 month, 1 year, and 2 year probabilities of survival were 63%, 47%, and 34% in patients with low PMI compared with 85%, 75%, and 62% in women with high PMI (log rank = 0.002) (Figure 3B).
Concordance between skeletal muscle index and psoas muscle index categories
Of all male patients with low SMI, only 34% were categorized as low based on the PMI (Table 6). The Kappa coefficient for the agreement between low SMI and low PMI was 0.31 (P < 0.001; Table 6), demonstrating a poor agreement between the two assessments. In women, 72% of those classified as low SMI were classified in low PMI group. The agreement estimated by the Kappa coefficient was 0.63 in women indicating a moderate agreement between the two assessments. Overall, 104 patients (29%) were misclassified between SMI and PMI categories (Table 6). No significant difference regarding age (56 ± 9 vs. 55 ± 10; P = 0.42), HCC (34% vs. 41%, P = 0.19), ascites (62% vs. 59%, P = 0.13), and albumin (3 ± 2 vs. 3 ± 1 g/dL; P = 0.65) was observed between a misclassified group of patients and those who were correctly classified. Male patients were more likely to be misclassified (35% vs. 17%, P = 0.001), and MELD score was lower in misclassified patients (16 ± 7 vs. 18 ± 10 cm2/m2; P = 0.03).
Table 6.
Concordance between SMI and PMI categories
| Male patients | Female patients | |||
|---|---|---|---|---|
| Low SMI | High SMI | Low SMI | High SMI | |
| Low PMI | 43 (34) | 3 (3) | 28 (72) | 7 (10) |
| High PMI | 83 (66) | 117 (97) | 11 (28) | 61 (90) |
| Kappa coefficient (95% CI) | 0.31 | 0.63 | ||
| 0.22–0.41 | 0.47–0.77 | |||
| P value | <0.001 | <0.001 | ||
CI, confidence interval; PMI, psoas muscle index; SMI, skeletal muscle index.
Kappa coefficient was used to assess the agreement between two assessments.
Low PMI was defined as PMI < 5.1 cm2/m2 in men and PMI < 4.3 cm2/m2 in women.
Numbers in parentheses are percentages.
Discussion
The prevalence of sarcopenia, defined as low SMI, in cirrhosis ranges between 22% and 70% according to CT studies,18 with higher prevalence in men.4, 6 SMI has been shown to be a reliable indicator of total body muscle mass,8, 9 and predictor of mortality in patients with cirrhosis,4, 6 and in patients listed for LT.1 Currently, there is a discrepancy in the literature regarding sarcopenia quantification and definition in cirrhosis. This demonstrates the necessity to develop a standardized definition of sarcopenia that can be implemented in other LT centres. The authors have started a consortium of LT centres to develop a comprehensive data set and reach consensus to perform the muscularity assessment in a uniform fashion. To date, this data set is the only North American study to define sarcopenia using data derived from a diverse population of LT candidates, which has been implemented in this study.
Sarcopenia associates with various clinical complications in aging19, 20, 21, 22 and cachexia‐associated chronic diseases3, 23, 24, 25, 26; therefore, sensitive and reproducible measures of muscle mass are required to assess the evolution of muscle loss and to follow outcomes of therapeutic interventions26, 27 directed at attenuation of tissue loss in order to improve outcome of patients. Considering availability of diagnostic CT scans in the majority of cachexia‐associated disease such as cancer and cirrhosis, as well as high accuracy and time efficacy of different software programmers28 for body composition measurements, finding an appropriate indicator of muscle mass, i.e. total muscle or psoas, should be the priority in cachexia context.
This study aimed to investigate whether low PMI can be applied as a representative surrogate of low SMI for predicting waitlist mortality in patients with cirrhosis. A moderately strong correlation between SMI and PMI as continuous variables was observed; however, categorizing PMI yielded poor and moderate concordance with SMI in men and women, respectively. Our data show that PMI has a poor capacity to identify patients with higher waitlist mortality in cirrhosis. A significant proportion of patients with higher waitlist mortality risk might be underestimated in men using PMI cut‐offs, as more than half of men with low SMI (66%) were categorized as having high PMI with an implied lower mortality risk. Poor agreement (Kappa coefficient = 0.31) between the two assessments accounted for the different proportions of patients in each category. Moreover, among men, the cut‐off for SMI values identified a 51% of participants into the low muscle mass group. Conversely, the threshold that was identified as most statistically optimal for PMI only classifies 19% of the men as low muscle, which is a large discrepancy, and results are in poor concordance. Moreover, it is not only the selected PMI cut‐offs that perform poorly, but also when PMI was evaluated as a continuous variable in relation to mortality risk and the mean of died/delisted group was compared with all others, it was not as effective as SMI in men. As a consequence, it is not only the stratification strategy used that is flawed but also that analysis of PMI is not as good a metric as SMI, in men. Therefore, patients with a higher risk of mortality would incorrectly be classified as low risk and given the importance of sarcopenia in predicting mortality of men with cirrhosis; PMI should not be used to substitute SMI to predict mortality. Greater predictive accuracy and robustness of SMI compared with that of PMI appears to be biologically plausible because SMI takes into account a larger proportion of total muscle mass than does PMI.
Although sarcopenia is not a current criterion for expedited LT, giving some priority to those patients with sarcopenia may help to decrease mortality in a subgroup of patients with cirrhosis.3, 29 Sarcopenia is one of the major manifestations of protein‐energy malnutrition in cachexia‐associated diseases, and to prevent adverse clinical implications, early and proper identification of patients with sarcopenia should be implanted and intense nutritional and physical interventions should be included to improve patient conditions and to lessen mortality risk in patients with higher risk as determined by low SMI.
In women, the cut‐off for PMI‐associated mortality was at the 33rd percentile of calculated PMI, and subsequently, 33% of women fell into the high‐risk group. This result is similar to 33% of women with low SMI, below the established cut‐off previously reported by our group.1 Although Kappa coefficient showed good agreement between two assessments in women (0.63), there might still be some misclassification as 28% of women with low SMI and higher mortality risks were placed in the high‐PMI category. We speculate that psoas muscle in women with cirrhosis is preferentially depleted in sarcopenia, whereas muscles other than psoas might be affected at greater intensity in men. The exact mechanism behind this association has not been elucidated. However, a study in post‐menopausal women enrolled in a weight loss training programme showed that muscle was mainly lost from psoas and erector spine areas but not from lateral abdominal area and rectus abdominis.30 Moreover, it has been described by other researchers that noticeable decline in female psoas muscle area may be due to post‐menopausal hormonal changes.31 This emphasizes the necessity for longitudinal studies to investigate potential loss of PMI and SMI stratified by sex.
The psoas is a key muscle for posture and core strength,32 which might represent sarcopenia better than other individual anthropometric muscle measurements, such as triceps thickness;17 however, it only accounts for approximately 13% of total skeletal muscle area at the level of L3 in our population. Psoas muscle mass in an individual patient might also be affected by other conditions such as degenerative spine disease and chronic low back pain,33 both common conditions in an older population and potentially selective for psoas muscle loss compared with loss of total musculature.
Studies evaluating the association between psoas muscle and mortality in various populations have yielded inconsistent results.10, 11, 12, 34, 35 Discrepancy may arise because of disparate techniques and measurements of psoas muscle, such as PMA, PMI (normalized to patient squared height),11 right psoas thickness at the level of umbilicus,10 short‐axis to long‐axis ratios of the right and left psoas muscles36 that have been applied to predict clinical outcomes. Because of the asymmetrical shape of psoas muscle, validity and reliability of some of those approaches could be questioned.37 Despite a strong association between sarcopenia, measured by total psoas area, with post‐transplant mortality, variations in total psoas areas in patients undergoing LT led to extreme variation in mortality risk following LT.12
Overall, data regarding the use of PMI instead of SMI for the purposes of mortality risk prediction in cirrhosis are limited. Among various muscle measurements, i.e. PMA, PMI, and SMI at L3, to predict post‐LT mortality in 256 transplanted patients, PMA was selected as the simplest measure with the highest accuracy.13 Using sex‐specific cut‐off values for PMA (1561 mm2 in men, 1464 mm2 in women), 22% of the patients were identified as sarcopenic (below cut‐off for PMA) with poorer survival,13 whereas in our previous experience1 using SMI cut‐offs (50 cm2/m2 in men, 39 cm2/m2 in women), 50% of men and 33% of women exhibited higher waitlist mortality associated with low SMI. Therefore, comparison between studies is limited by divergent outcomes, post‐LT mortality in patients undergoing LT12, 13 vs. waitlist mortality in listed patients,1 which may contribute to inconsistent results.
Abdominal total skeletal muscle and psoas muscle assessment by CT images in patients with ESLD listed for LT is the main strength of this multicentre study. Moreover, we evaluated mortality risk by competitive risk analysis with LT as the competitive event. Applying competing‐risk analysis, rather than conventional survival analysis, is a more robust approach in the presence of competing event.38 Patients were included from four North American LT centres with standardized inclusion criteria to ensure homogeneous patient population. We acknowledge limitations of the present study, as we could not include patients without an available CT for muscularity assessment. Also, we were unable to evaluate muscle quality and muscle strength because of the retrospective nature of the study.
In conclusion, CT‐defined PMI cut‐off predicts waitlist mortality in patients with cirrhosis but identifies only an incomplete subset of those at increased risk of mortality indicated by low SMI. Therefore, we propose that SMI is a more complete and robust measurement, especially in men with cirrhosis, and given the poor performance of PMI, SMI should not be substituted by PMI. Transferability of the results to clinical settings requires consensus and more specific definition of muscle abnormalities including both muscle mass and quality in ESLD patients.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
The authors certify that they comply with the ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia, and Muscle.39
Ebadi, M. , Wang, C. W. , Lai, J. C. , Dasarathy, S. , Kappus, M. R. , Dunn, M. A. , Carey, E. J. , Montano‐Loza, A. J. , and From the Fitness, Life Enhancement, and Exercise in Liver Transplantation (FLEXIT) Consortium (2018) Poor performance of psoas muscle index for identification of patients with higher waitlist mortality risk in cirrhosis. Journal of Cachexia, Sarcopenia and Muscle, 9: 1053–1062. 10.1002/jcsm.12349.
References
- 1. Carey EJ, Lai JC, Wang CW, Dasarathy S, Lobach I, Montano‐Loza AJ, et al. A multicenter study to define sarcopenia in patients with end‐stage liver disease. Liver Transpl 2017;23:625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Montano‐Loza AJ, Angulo P, Meza‐Junco J, Prado CMM, Sawyer MB, Beaumont C, et al. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle 2016;7:126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Montano‐Loza AJ, Duarte‐Rojo A, Meza‐Junco J, Baracos VE, Sawyer MB, Pang JX, et al. Inclusion of sarcopenia within MELD (MELD‐Sarcopenia) and the prediction of mortality in patients with cirrhosis. Clin Transl Gastroenterol 2015;6:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Montano‐Loza AJ, Meza‐Junco J, Prado CMM, Lieffers JR, Baracos VE, Bain VG, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol H 2012;10:166–U4. [DOI] [PubMed] [Google Scholar]
- 5. Montano‐Loza AJ. Skeletal muscle abnormalities and outcomes after liver transplantation. Liver Transpl 2014;20:1293–1295. [DOI] [PubMed] [Google Scholar]
- 6. Tandon P, Low G, Mourtzakis M, Zenith L, Myers RP, Abraldes JG, et al. A model to identify sarcopenia in patients with cirrhosis. Clin Gastroenterol Hepatol 2016;14:1473–1480, e3. [DOI] [PubMed] [Google Scholar]
- 7. Romeiro FG, Augusti L. Nutritional assessment in cirrhotic patients with hepatic encephalopathy. World J Hepatol 2015;7:2940–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shen W, Punyanitya M, Wang Z, Gallagher D, St‐Onge MP, Albu J et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross‐sectional image. J Appl Physiol (1985). 2004;97(6):2333–8. [DOI] [PubMed] [Google Scholar]
- 9. Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- 10. Durand F, Buyse S, Francoz C, Laouenan C, Bruno O, Belghiti J, et al. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J Hepatol 2014;60:1151–1157. [DOI] [PubMed] [Google Scholar]
- 11. Izumi T, Watanabe J, Tohyama T, Takada Y. Impact of psoas muscle index on short‐term outcome after living donor liver transplantation. Turk J Gastroenterol 2016;27:382–388. [DOI] [PubMed] [Google Scholar]
- 12. Englesbe MJ, Patel SP, He K, Lynch RJ, Schaubel DE, Harbaugh C, et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg 2010;211:271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Golse N, Bucur PO, Ciacio O, Pittau G, Sa Cunha A, Adam R, et al. A new definition of sarcopenia in patients with cirrhosis undergoing liver transplantation. Liver Transpl 2017;23:143–154. [DOI] [PubMed] [Google Scholar]
- 14. Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985). 1998;85(1):115–22. [DOI] [PubMed] [Google Scholar]
- 15. Mason SJ, Graham NE. Areas beneath the relative operating characteristics (ROC) and relative operating levels (ROL) curves: statistical significance and interpretation. Q J Roy Meteorol Soc 2002;128:2145–2166. [Google Scholar]
- 16. Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983;148:839–843. [DOI] [PubMed] [Google Scholar]
- 17. Park SY, Yoon JK, Lee SJ, Haam S, Jung J. Postoperative change of the psoas muscle area as a predictor of survival in surgically treated esophageal cancer patients. J Thorac Dis 2017;9:355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Vugt JL, Levolger S, de Bruin RW, van Rosmalen J, Metselaar HJ, JN IJ. Systematic review and meta‐analysis of the impact of computed tomography‐assessed skeletal muscle mass on outcome in patients awaiting or undergoing liver transplantation. Am J Transplant 2016;16:2277–2292. [DOI] [PubMed] [Google Scholar]
- 19. Tyrovolas S, Koyanagi A, Olaya B, Ayuso‐Mateos JL, Miret M, Chatterji S, et al. Factors associated with skeletal muscle mass, sarcopenia, and sarcopenic obesity in older adults: a multi‐continent study. J Cachexia Sarcopenia Muscle 2016;7:312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dodds RM, Granic A, Davies K, Kirkwood TB, Jagger C, Sayer AA. Prevalence and incidence of sarcopenia in the very old: findings from the Newcastle 85+ Study. J Cachexia Sarcopenia Muscle 2017;8:229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Makizako H, Shimada H, Doi T, Tsutsumimoto K, Lee S, Lee SC, et al. Age‐dependent changes in physical performance and body composition in community‐dwelling Japanese older adults. J Cachexia Sarcopenia Muscle 2017;8:607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fabbri E, Chiles Shaffer N, Gonzalez‐Freire M, Shardell MD, Zoli M, Studenski SA, et al. Early body composition, but not body mass, is associated with future accelerated decline in muscle quality. J Cachexia Sarcopenia Muscle 2017;8:490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Choi MH, Oh SN, Lee IK, Oh ST, Won DD. Sarcopenia is negatively associated with long‐term outcomes in locally advanced rectal cancer. J Cachexia Sarcopenia Muscle 2018;9:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lewis A, Lee JY, Donaldson AV, Natanek SA, Vaidyanathan S, Man WD, et al. Increased expression of H19/miR‐675 is associated with a low fat‐free mass index in patients with COPD. J Cachexia Sarcopenia Muscle 2016;7:330–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kazemi‐Bajestani SM, Becher H, Ghosh S, Montano‐Loza AJ, Baracos VE. Concurrent depletion of skeletal muscle, fat, and left ventricular mass in patients with cirrhosis of the liver. J Cachexia Sarcopenia Muscle 2016;7:97–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tournadre A, Pereira B, Dutheil F, Giraud C, Courteix D, Sapin V, et al. Changes in body composition and metabolic profile during interleukin 6 inhibition in rheumatoid arthritis. J Cachexia Sarcopenia Muscle 2017;8:639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stewart Coats AJ, Ho GF, Prabhash K, von Haehling S, Tilson J, Brown R, et al. Espindolol for the treatment and prevention of cachexia in patients with stage III/IV non‐small cell lung cancer or colorectal cancer: a randomized, double‐blind, placebo‐controlled, international multicentre phase II study (the ACT‐ONE trial). J Cachexia Sarcopenia Muscle 2016;7:355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Vugt JL, Levolger S, Gharbharan A, Koek M, Niessen WJ, Burger JW, et al. A comparative study of software programmes for cross‐sectional skeletal muscle and adipose tissue measurements on abdominal computed tomography scans of rectal cancer patients. J Cachexia Sarcopenia Muscle 2017;8:285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van Vugt JLA, Alferink LJM, Buettner S, Gaspersz MP, Bot D, Darwish Murad S, et al. A model including sarcopenia surpasses the MELD score in predicting waiting list mortality in cirrhotic liver transplant candidates: a competing risk analysis in a national cohort. J Hepatol 2017. [DOI] [PubMed] [Google Scholar]
- 30. Ryan AS, Harduarsingh‐Permaul AS. Effects of weight loss and exercise on trunk muscle composition in older women. Clin Interv Aging 2014;9:395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ilayperuma I, Nanayakkara BG. Gross anatomical characterization of the psoas major muscle: a cadaver study. Galle Medical Journal 2008;13. [Google Scholar]
- 32. Sheetz KH, Zhao L, Holcombe SA, Wang SC, Reddy RM, Lin J, et al. Decreased core muscle size is associated with worse patient survival following esophagectomy for cancer. Dis Esophagus 2013;26:716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sebro R, O'Brien L, Torriani M, Bredella MA. Assessment of trunk muscle density using CT and its association with degenerative disc and facet joint disease of the lumbar spine. Skeletal Radiol 2016;45:1221–1226. [DOI] [PubMed] [Google Scholar]
- 34. Heberton GA, Nassif M, Bierhals A, Novak E, LaRue SJ, Lima B, et al. Usefulness of psoas muscle area determined by computed tomography to predict mortality or prolonged length of hospital stay in patients undergoing left ventricular assist device implantation. Am J Cardiol 2016;118:1363–1367. [DOI] [PubMed] [Google Scholar]
- 35. Rutten IJG, Ubachs J, Kruitwagen R, Beets‐Tan RGH, Olde Damink SWM, Van Gorp T. Psoas muscle area is not representative of total skeletal muscle area in the assessment of sarcopenia in ovarian cancer. J Cachexia Sarcopenia Muscle 2017;8:630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hanaoka M, Yasuno M, Ishiguro M, Yamauchi S, Kikuchi A, Tokura M, et al. Morphologic change of the psoas muscle as a surrogate marker of sarcopenia and predictor of complications after colorectal cancer surgery. Int J Colorectal Dis 2017;32:847–856. [DOI] [PubMed] [Google Scholar]
- 37. Baracos VE. Psoas as a sentinel muscle for sarcopenia: a flawed premise. J Cachexia Sarcopenia Muscle 2017;8:527–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation 2016;133:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the journal of cachexia, sarcopenia and muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]


