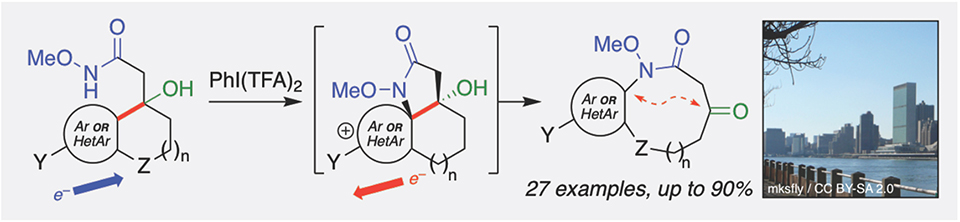
Abstract
Medium-ring natural products exhibit diverse biological activities but such scaffolds are underrepresented in probe and drug discovery efforts due to the limitations of classical macrocyclization reactions. We report herein a tandem oxidative dearomatization-ring-expanding rearomatization (ODRE) reaction that generates benzannulated medium-ring lactams directly from simple bicyclic substrates. The reaction accommodates diverse aryl substrates (haloarenes, aryl ethers, aryl amides, heterocycles) and strategic incorporation of a bridgehead alcohol generates a versatile ketone moiety in the products amenable to downstream modifications. Cheminformatic analysis indicates that these medium rings access regions of chemical space that overlap with related natural products and are distinct from synthetic drugs, setting the stage for their use in discovery screening against novel biological targets.
Keywords: medium ring, tandem reaction, oxidative dearomatization, ring expansion, umpolung
East River Reactivity:
Reversal of electron flow during a tandem oxidative dearomatization-ring-expanding rearomatization reaction (left) was inspired by the East River in New York City, a tidal estuary that reverses direction with each tide (right). The tandem reaction provides rapid and efficient access to a wide range of medium-ring products that probe natural product-like regions of chemical space.
Introduction
Medium-ring structures (8–11-membered rings)[1] are found in diverse biologically-active natural products[2] and are attractive scaffolds for use in discovery libraries.[3–16] Such scaffolds have also been leveraged in structure-based drug design.[17–19] The cyclic constraint imparts conformational restriction that is associated with favorable pharmacological properties, including increased binding affinity,[20] cell permeability,[21,22] and oral bioavailability.[23] However, medium rings remain severely underrepresented in screening collections and approved drugs,[24] likely due to the well-known synthetic challenges in accessing these structures.[25] Conventional cyclization-based approaches to medium rings are highly substrate-dependent[26–29] and suffer from the lowest cyclization rates among all ring sizes due to unfavorable transannular interactions.[25] To address this synthetic challenge, we have been developing alternative synthetic approaches based on ring expansion to access medium-ring and macrocyclic structures.[15,16] Herein, we report a new tandem oxidative dearomatization-ring expansion (ODRE) reaction that provides efficient access to a wide range of medium-ring lactams in a single step from readily available bicyclic substrates (Fig. 1a). We demonstrate the scope of this tandem ODRE across 31 substrates, and downstream reactions of the resulting scaffolds to introduce additional functionalities. Cheminformatic analysis confirms that the resulting medium-ring compounds access regions of chemical space that overlap with related natural products and are distinct from synthetic drugs and conventional drug-like libraries.
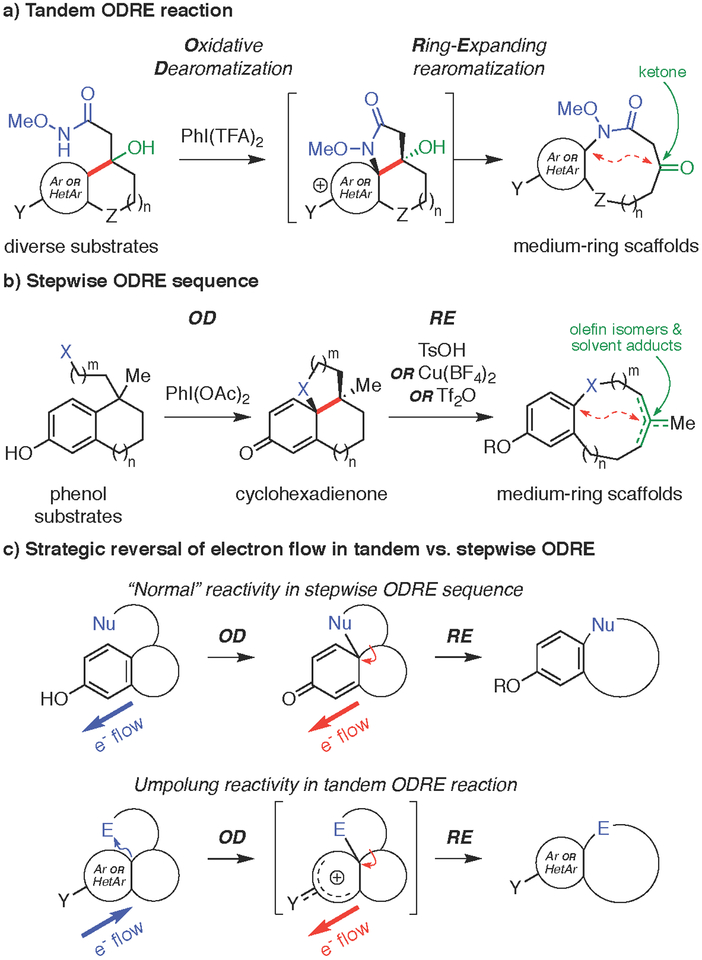
Figure 1. Oxidative dearomatization-ring-expanding re aromatization (ODRE) approaches to medium ring synthesis.
(a) Tandem ODRE provides medium-ring scaffolds from bicyclic substrates having an electron-rich aromatic ring. (b) Stepwise ODRE sequence is limited to phenol substrates and provides mixtures of products. (c) Umpolung reversal of electron flow enables new tandem ODRE reaction.
In recent years, there has been growing interest in developing creative synthetic strategies to access medium-ring compounds.[30–33] Tandem cyclization/ring expansion approaches[34–40] are particularly useful as they offer greater efficiency and flexibility compared to conventional direct cyclization methods. However, despite these advances, several limitations are commonly observed, such as tedious multistep substrate preparation and narrow tolerance of functional groups and ring sizes found in natural products and bioactive pharmacophores.[41–43]
We recently reported a biomimetic, stepwise ODRE sequence to access diverse, natural product-based benzannulated medium rings (Fig. 1b).[16] This synthetic approach was inspired by Barton’s seminal proposal for the biosynthesis of the alkaloid protostephanine,[44] which was later reduced to practice in several biomimetic syntheses.[45–49] Initial oxidative dearomative of a bicyclic phenol substrate forms an electrophilic cyclohexadienyl cation intermediate, which is then attacked by a side chain nucleophile to generate a tricyclic cyclohexadienone. This intermediate is then activated with a Brønsted acid, Lewis acid, or triflic anhydride to induce ring expansion with rearomatization of the phenol ring. While this stepwise ODRE sequence provided a variety of ring linkages found in medium-ring natural products, including aryl ethers, diaryl ethers, lactones, and biaryls, it was restricted to phenolic substrates and often led to mixtures of olefin isomers and solvent adducts, arising from various termination reactions of a penultimate tertiary carbocation intermediate.
To overcome these limitations, we envisioned a new umpolung approach in which the initial oxidative dearomatization step would instead proceed via attack of an electron-rich aromatic ring on an electrophilic side chain (Fig. 1c).[50–56] This would allow a wider range of non-phenolic substrates to be used and might also allow direct ring expansion from the nascent reactive intermediate in a tandem reaction (Fig. 1a). This umpolung ODRE strategy would also allow installation of a tertiary alcohol in the substrate to terminate the cationic cascade via formation of a ketone product, avoiding various other termination pathways as well as providing a versatile handle for further functionalization. Notably, Kikugawa has leveraged hypervalent iodine activation of N-methoxyamides as nitrenium electrophiles in such umpolung reactivity,[54] which was used by Wardrop in ipso-cyclization reactions to synthesize spirolactams.[57–59] Thus, we set out to investigate the utility of such N-methoxyamide side chains in an ODRE approach to medium-ring synthesis.
Results and Discussion
Development of tandem ODRE with haloaromatic substrates
In initial studies, we investigated the reactivity of bromotetralin 1a to access medium-ring lactam 3a (Fig. 2a). The phenol in the original substrate system (Fig. 1b) was replaced with an aryl bromide[60,61] to avoid competing oxidative activation of the phenol. Indeed, Ciufolini has reported extensive studies of such oxidative dearomatization reactions of phenols in the presence of nitrogen nucleophiles under the ‘normal’ polarity reaction manifold.[62–65] We postulated that umpolung oxidative dearomatization would provide a cyclohexadienyl bromonium intermediate 2a in situ,[60] which could then undergo spontaneous ring-expanding rearomatization to form a C1 tertiary carbocation, providing the olefin 3a after E1 elimination.[16] We recognized that a potential undesired pathway could involve solvolysis of the bromonium intermediate 2a to form a stable cyclohexadienone intermediate that would not be expected to undergo spontaneous ring expansion.[16,60] Moreover, the penultimate C1 tertiary carbocation could also form other endo- and exocyclic olefin regioisomers or solvent adducts.[16]
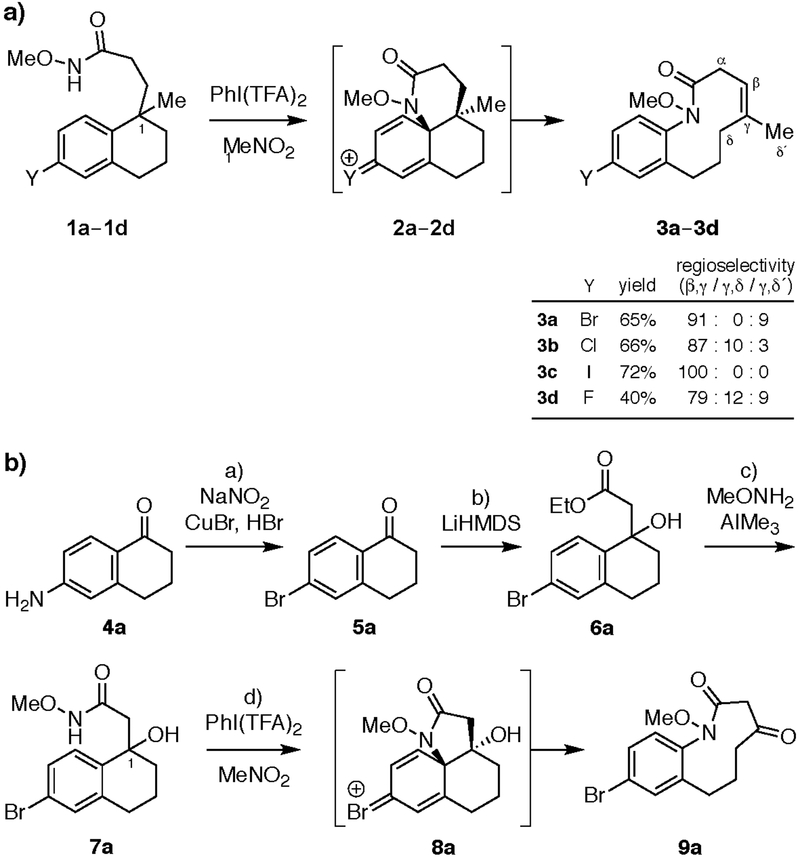
Figure 2. Tandem ODRE reactions of haloaromatic substrates to form medium-ring lactam products.
(a) Synthesis of 10-membered ring haloaromatics 3a–d (major isomer shown). Reagents and conditions: PhI(TFA)2 (2.0 equiv), MeNO2, 0 °C to 24 °C, 14 h. (b) Synthesis of 9- membered ketolactam 9a. Reagents and conditions: a) NaNO2 (1.1 equiv),CuBr (2.2 equiv), HBr (aq), 85%. b) LiHMDS (3.0 equiv), EtOAc (3.0 equiv), THF, –78 °C, 3 h, 93%. c) AlMe3 (3.0 equiv), NH2(OMe)・HCl (3.0 equiv), THF, 0 °C to 24 °C, 16 h, 96%. d) PhI(TFA)2 (1.5 equiv), MeNO2, 0 °C to 24 °C, 1 h, 73%. HMDS = hexamethyldisilazide; TFA = trifluoroacetate; THF = tetrahydrofuran.
Treatment of bromotetralin 1a with (diacetoxyiodo)benzene in trifluoroethanol led to a mixture of olefin regioisomers (70 : 28 : 2 β,γ / γ,δ / γ,δ) for the desired medium-ring lactam product 3a. Use of PhI(TFA)2 in trifluoroethanol improved the ratio of olefin isomers (75 : 18 : 7). Moreover, changing the solvent to nitromethane provided 3a in 65% yield as a 91:9 mixture of β,γ and γ,δ’ olefin regioisomers, without formation of the γ,δ isomer. Similarly, PhI(TFA)2-mediated tandem ODRE of chlorotetralin 1b, iodotetralin 1c, and fluorotetralin 1d afforded the corresponding medium-ring lactams 3b, 3c, and 3d in modest to good yields, with varying regioselectivity favoring endocyclic β,γ olefins.
Strategic installation of tertiary alcohol to generate ketone products
Next, to avoid formation of mixtures of olefin regioisomers, we replaced the C1-methyl group in the tetralin substrate with a hydroxyl group, which would lead to a single ketone product (Fig. 2b). We reasoned that the lone-pair electrons of the hydroxyl group would also potentiate the ring-expanding rearomatization step, by analogy to previous biomimetic syntheses of protostephanine and related alkaloids, in which an endocyclic amine nitrogen likely plays a similar role in driving ring-expanding rearomatization.[45–49] Moreover, the resulting ketone motif would serve as a versatile handle for further chemical diversification of the medium-ring scaffolds. Notably, this approach was not feasible in the original stepwise ODRE sequence with phenolic substrates[16] due to competing Adler–Becker oxidation.[66,67] Thus, reversing the electron flow in the oxidative dearomatization step of this umpolung tandem ODRE reaction was critical to this approach.
Accordingly, bromotetralol 7a was prepared from commercially available 6-amino-1-tetralone (4a) in 3 steps and 76% overall yield. Treatment of 7a with PhI(TFA)2 in nitromethane then afforded the desired β-ketolactam 9a in 73% yield. The corresponding chloro-, iodo-, and fluorotetralol substrates 7b-d were also obtained using the same scalable, efficient synthetic sequence and converted via tandem ODRE to medium rings 9b-d in good yields (Fig. 3). The aryl fluoride product 9d was obtained in the highest yield in this series (81%), consistent with improved “back-donation” of the fluorine lone pairs to stabilize the cyclohexadienyl halonium intermediate.[60,68]
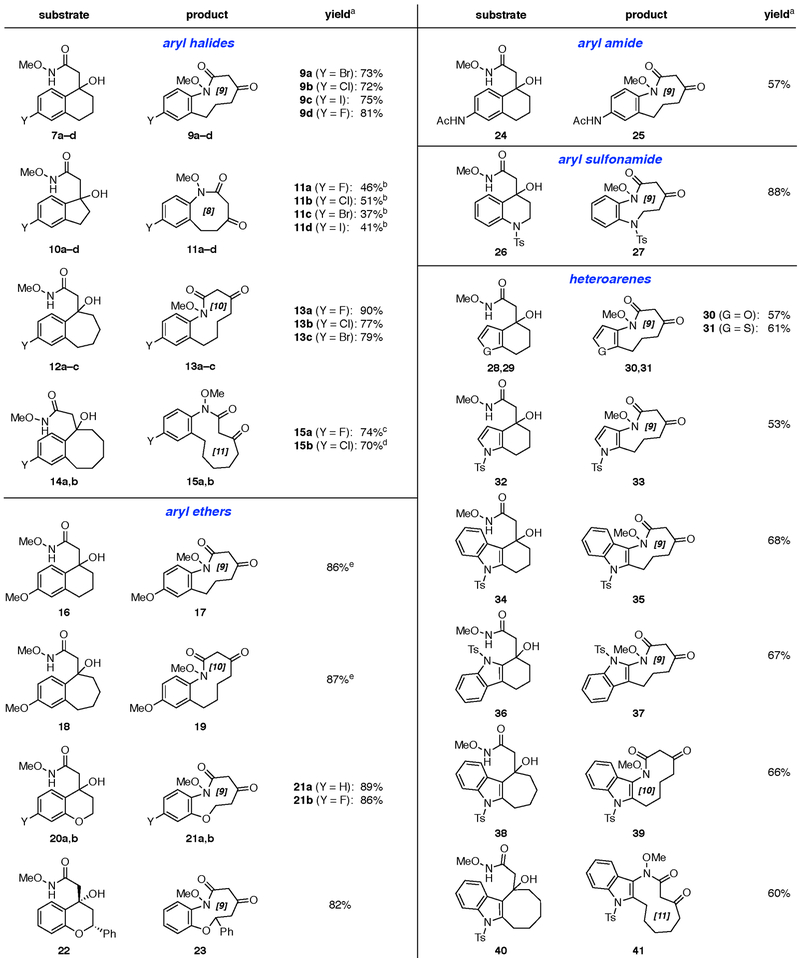
Figure 3. Scope of tandem ODRE reaction.
a Reagents and conditions: PhI(TFA)2 (1.0—1.5 equiv), M eNO2 , 0 °C to 24 °C, 0.5 −2 h. bRemainder indene byproducts resulting from dehydration of 10a -d and unidentified side products, based on 1H-NMR analysis of crude product. cIsolated as a mixture of amide rotamers (3:2 E/Z). dIsolated as a mixture of amide rotamers (2:1 E/Z). eReaction performed in MeOH at 0 °C.
Scope of the tandem ODRE reaction
We next investigated the effects of varying ring size in the substrates (Fig. 3). In tandem ODRE reactions of haloindanol substrates 10a-d , the corresponding 8-membered ring lactam products 11a-d were recovered in moderate 37–51% yields. This decreased efficiency may be attributed to competing formation of indene side products via dehydration of the indanol substrates, or poor orbital overlap of the scissile bond with the nascent aromatic p-system in the rearomatization reaction.[16] In contrast, the corresponding halobenzosuberanols 12a-c and halobenzocyclooctanols 14a,b afforded 10- and 11-membered benzannulated lactams 13a-c and 15a,b, respectively, in 70–90% yields.
Next, we explored the use of aryl ether substrates in the tandem ODRE. Anisoles have been used previously in such umpolung oxidative dearomatization spirocyclization reactions of N-methoxyamides.[57–59] However, reaction of the anisole 16 led to only a 15% yield of the desired medium-ring lactam 17. From this complex mixture, we also recovered a cyclohexadienone side product (≈20%), presumably resulting from hydrolysis or demethylation of the corresponding O-methyl oxocarbenium intermediate (Supplementary Fig. 1a). We posited that this unproductive pathway could be avoided by stabilizing the O-methyloxocarbenium intermediate with a temporary nucleophile. Thus, when the reactions were conducted in methanol instead of nitromethane, the 9- and 10-membered medium-ring products 17 and 19 were obtained in 86% and 87% yields, respectively.
Notably, subjecting 16 to the same conditions in CD3OD did not result in deuterium incorporation in the methyl ether moiety of the product 17, suggesting that methanol may undergo a 1,4-addition to the O-methyloxocarbenium intermediate, followed by elimination during, or en route to, anisole rearomatization (Supplementary Fig. 1b).
In contrast, chromanone-derived substrates 20a,b and flavanone derivative 22, both having an endocyclic ether moiety, did not suffer from the dealkylation pathway in nitromethane, and proceeded to the medium-ring products 21a,b and 23, respectively, in excellent yields (82–89%). Similarly, acetanilide substrate 24 and quinolone derivative 26 provided the corresponding acetamidoaryl lactam and sulfonamidoaryl lactam products 25 and 27 respectively, under the standard reaction conditions. Sulfanilides have been used previously in hypervalent iodine-induced oxidative dearomatization spirocyclization reactions under the ‘normal’ polarity reaction manifold.[69]
We next sought to extend the tandem ODRE reaction to heteroaromatic ring systems found in natural products and drug pharmacophores. Commercially available furano-, thiopheno-, and pyrrolocyclohexanones were transformed into the corresponding b-hydroxy-N-methoxyamide substrates 28, 29, and 32, respectively, then converted via tandem ODRE to 9-membered ring lactam products 30, 31, and 33 in serviceable yields. Hypervalent iodine-induced oxidative dearomatization reactions have been reported under the ‘normal’ polarity reaction manifold for furans.[70,71] and under the umpolung reaction manifold for thiophenes and pyrroles.[72,73] Further, indoles 34 and 36 proved to be reactive as nucleophiles at both the C3-and C2-positions, respectively, providing the corresponding regioisomeric indole products 35 and 37. Hypervalent iodine-induced oxidative dearomatization reactions of indoles with nitrogen nucleophiles or electrophiles have been reported under the ‘normal’[74,75] and umpolung[76–78] reaction manifolds, respectively, the larger indole-fused 7- and 8-membered ring substrates 38 and 40, readily accessed from the corresponding cyclic ketones via Fischer indole synthesis followed by a DDQ oxidation sequence, were also converted to the corresponding 10- and 11 -membered lactam products 39 and 41 in good yields. Taken together, these results demonstrate the excellent scope of the tandem ODRE reaction in providing access to a wide variety of benzannulated medium-ring lactam products.
Mechanistic proposal
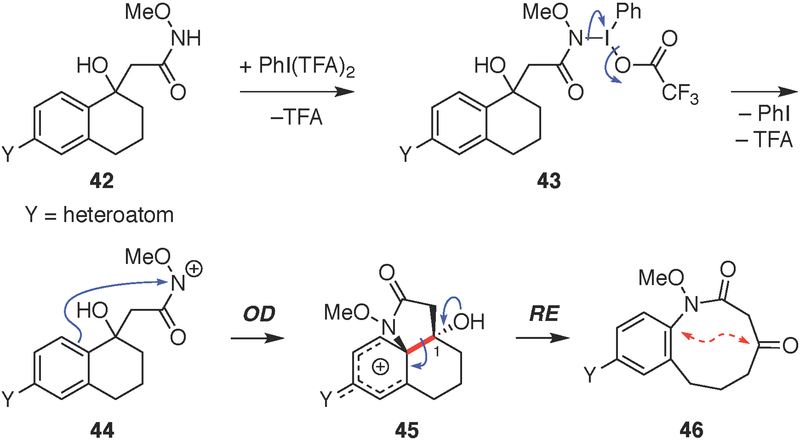
A proposed mechanism for the tandem ODRE reaction involves initial PhI(TFA)2 activation of the N-methoxyamide side chain in 42, in the presence of the charge-stabilizing solvent nitromethane, to form a nitrenium ion intermediate[57,79–81] 44 (Fig. 4). Ensuing intramolecular electrophilic substitution through ipso-attack of the tethered arene generates a cationic tricyclic intermediate 45 poised for ring expansion. Rearomatization of the arene then drives spontaneous C-C bond cleavage, facilitated by the lone-pair electrons on the C1-hydroxyl group, to afford the benzannulated medium ring-lactam product 46.
Figure 4. Proposed mechanism of tandem ODRE reaction.
Functionalization of medium-ring scaffolds
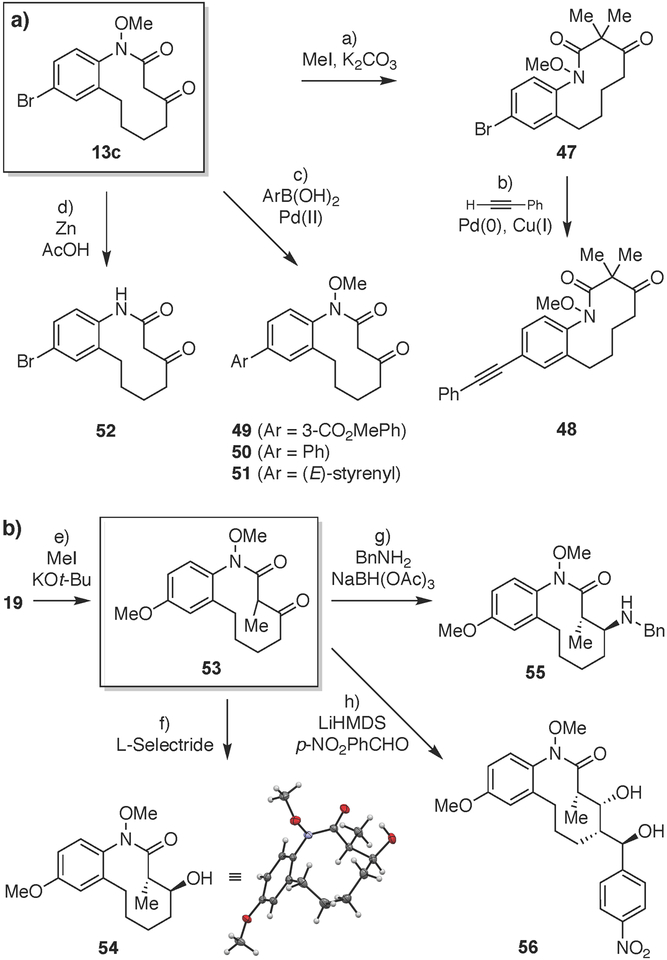
With access to a variety of medium-ring scaffolds established, we next investigated downstream synthetic modifications to introduce additional structural diversity. Thus, bromoaryllactam 13c and methoxyaryllactam 53 were scaled up to explore these transformations (Fig. 5a). The acidity of the 1,3-dicarbonyl methylene protons in 13c enabled conversion to α,α-dimethylketone 47 in a one-pot geminal dimethylation.[82] Sonogashira coupling of the aryl bromide moiety in 47 with phenyl acetylene then afforded aryl alkyne 48 in 65% yield.[83] From the parent aryl bromide 13c, several boronic acids were also coupled under standard Suzuki-Miyaura conditions[84] to provide cross-coupling products 49–51 in good yields. Reductive cleavage of the N-O bond in 13c with zinc metal afforded corresponding secondary lactam 52 without affecting the bromide.[85]
Figure 5. Downstream modification reactions of medium-ring scaffolds 13c and 53.
Reagents and conditions: a) Mel (4.0 equiv), K2CO3 (4.0 equiv), DMF, 24 °C, 48 h, 68%. b) phenylacetylene (10.0 equiv), Pd(PPh3)4 (20 mol%), Cul (25 mol%), Et3 N, DMF, 60 °C, 16 h, 65%. c) ArB(OH)2 (1.1 equiv), Pd(OAc)2 (20 mol%), K2CO3 (2.5 equiv), TBAB (1.1 equiv), H2O, 70 °C, 2 h, 65–77%. d) Zn (40.0 equiv), AcOH/H2O (1:1), 24 °C, 24 h, 85%. e) Mel (3.0 equiv), KOt-Bu (1.05 equiv), THF, 0 °C, 4 h, 72%. f) L-Selectride (2.0 equiv), THF, 0 °C to 24 °C, 3 h. 94%, >99:1 dr anti/syn. g) BnNH2 (1.1 equiv), AcOH (1.0 equiv), 4A MS, toluene, 90 °C, 2 h, then NaBH(OAc)3 (4.0 equiv), DCE, 24 °C, 16 h, 76%, 94:6 dr ant//syn. h) LiHMDS (3.0 equiv), THF, −78 °C, 1 h; then p-NO2 PhCHO (2.5 equiv), −78 °C to 24 °C, 16 h, 59%, 99:1 dr. DCE = 1,2-dichloroethane; DMF = N,N-dim ethylform amide; L-Selectride = lithium tri-s-butylborohydride; TBAB = tetrabutylamm onium bromide.
In a second series, β-ketolactam 19 (Fig. 5b) was first converted to α-methyl-β-ketolactam 53 using potassium tert- butoxide and methyl iodide. The ketone moiety of 53 was then reduced to afford anti-α-methyl-β-hydroxylactam 54 in 94% yield and >99:1 dr.[86] The stereochemical configuration of 54 was assigned based on X-ray crystallographic analysis (see Electronic Supplementary Information). Analogously, reductive amination of the ketone in scaffold 53 with benzyl amine provided β-aminolactam 55 in 76% yield and 94:6 dr, favoring the anti diastereomer, assigned based on extensive 2D NMR studies.[87] Finally, a one-pot aldol-Tishchenko reaction generated anti-1,3-diol 56 in good yield as a single diastereomer with four contiguous stereocenters.[88] Relative stereochemistry was assigned via conversion to the corresponding acetonide and 1D and 2D NMR studies (see Electronic Supplementary Information).
Cheminformatic analysis of medium-ring scaffolds
To assess the structural features of the tandem ODRE products, we carried out a cheminformatic analysis of these 41 compounds in comparison to 47 accessed by the original stepwise ODRE sequence, 20 benzannulated medium-ring natural products, and our previously established reference sets of 60 diverse natural products, 40 top-selling brand-name drugs, and 20 commercial drug-like library compounds.[15,16,89,90] We analyzed each compound based on our established set of 20 structural and physicochemical parameters, then used principal component analysis (PCA) to identify correlations between parameters, reducing the dimensionality of the complete 20-dimensional dataset to enable convenient visualization (Supplementary Fig. 2).[89] The first three principal components (PC1-PC3) accounted for 75% of the variance represented in the complete 20-dimensional dataset.
In this analysis, visualization of the first two principal components (PC1, PC2) was insufficient to differentiate the medium-ring ODRE libraries and natural products clearly from the synthetic drugs and drug-like libraries. In contrast, the reference set of 60 diverse natural products occupied a larger, distinct region of the plot. Examination of component loadings (Supplementary Fig. 3) indicated that positioning along PC1 was dominated by parameters that correlate with molecular size (e.g., molecular weight, van der Waals surface area). Along PC2, parameters that correlate with hydrophobicity (logP, logD) shifted molecules down (negative) while those that correlate with polarity (logS, relative polar surface area) shift molecules up (positive). Thus, the overlap of the medium-ring and drug/drug-like sets is likely due to their relatively small and hydrophobic nature. Further, the broader range of this plot covered by the diverse natural product reference set is primarily due to the larger size of these molecules and, in some cases, high polarity.
In contrast, when PC3 was plotted, the medium-ring libraries and natural products diverged from the synthetic drugs and drug-like libraries. Along PC3, parameters that correlate with three-dimensional structure (stereochemical density, sp3 content) shifted molecules positively while aromatic ring content shifted molecules negatively. Thus, the relatively three-dimensional structures of the medium-ring compounds effectively differentiated them from the relatively flat, highly aromatic structures of the drugs and drug-like molecules. Notably, the former parameters have been associated with increased target specificity[91] and progression through preclinical and clincial development[92,93] while the latter has been associated with preclinical toxicity.[94,95] Moreover, both of the ODRE libraries overlapped well with bonafide medium-ring natural products across all three principal components.
Conclusions
Despite their prevalance in natural products and attractive pharmacological properties, medium-ring structures are underrepresented in current discovery libraries due to the challenges associated with classical cyclization-based synthetic approaches. To address this limitation, we have developed a novel tandem ODRE reaction that provides flexible, efficient access to diverse medium-ring scaffolds in 3 steps from readily available cyclic ketone precursors. In contrast to our previously reported stepwise ODRE sequence,[16] this tandem reaction provides medium-ring scaffolds directly from simple bicyclic precursors through an umpolung strategy. Conceptual reversal of electron flow in the initial oxidative dearomatization step leads to a cationic tricyclic intermediate that undergoes spontaneous ring-expanding rearomatization. Moreover, this umpolung strategy enables strategic installation of an adjacent hydroxyl group to prevent formation of olefin regioisomers and other cation termination products, while also providing a versatile ketone motif for further transformations. This was not feasible in the original stepwise ODRE sequence due to competing Adler–Becker reaction of phenolic substrates. Finally, the umpolung strategy enables use of a much wider array of arene substrates to provide haloaryl, aryl ether, acetanilide, aryl sulfonamide and heteroaromatic medium-ring products found in numerous natural and synthetic pharmacophores. The resulting natural product-based medium ring scaffolds are amenable to scale-up and a variety of downstream modifications. Cheminformatic analysis indicates that the tandem ODRE library overlaps with medium-ring natural products and is distinct from conventional synthetic drugs and drug-like libraries, accessing regions of chemical space that are underrepresented in probe and drug discovery. Notably, related benzannulated medium-ring lactam scaffolds have also been used in structure-based designed of angiotensin-converting enzyme inhibitors.[17–19] While the immediate applications of these molecules lie in efforts to discover novel biological probes, both β-ketolactam and N-alkoxyamide motifs are found in approved and investigational drugs, suggesting that these motifs are also compatible with eventual translational applications[96–100] Biological evaluation of this tandem ODRE library is ongoing and will provide insights into its utility in identifying novel probes and therapeutic leads.
Experimental Section
See Supporting Information for complete experimental protocols and analytical data, as well as PCA and X-ray crystallographic datafiles.
General procedure for tandem ODRE reaction
The β-hydroxy-N-methoxyamide substrate (0.32 mmol, 1.0 equiv) was dissolved in nitrom ethane (3.2 mL) and cooled to 0 °C. [Bis(trifluoroacetoxy)iodo]benzene (0.48 mmol, 1.5 equiv) was added as a solid at 0 °C and the reaction was slowly warmed to 24 °C and stirred for 0.5–2 h and monitored by TLC until complete cosumption of the starting material was observed. The reaction was then quenched with satdaq NaHCO3. The mixture was extracted with CH2Cl2 (4’ 10 mL). The combined organic extracts were washed with brine, dried (Na2SO4), filtered, and concentrated by rotary evaporation to afford the crude product. Purification by silica flash chromatography (0% → 5% MeOH in CH2Cl2) provided the corresponding medium-ring lactam. In the case of anisole substrates (16, 18), the general procedure was modified by using methanol instead of nitromethane as the solvent and keeping the reaction at 0 °C.
Principal component analysis
PCA of the 41 resulting benzannulated medium-ring lactams, 47 medium-ring products synthesized previously by the stepwise ODRE sequence,[16] and our previously established reference sets of 40 drugs, 20 commercial drug-like library members, and 60 natural products (Supplementary Figs. S4–S10 and Supplementary Table S1) was conducted using R, an open-source statistical computing package.[89] A set of 20 physicochemical descriptors (Supplementary Tables 2 and 3) for all compounds was obtained from PubChem and/or calculated using cheminformatics tools (Instant JChem and VCCLab [101]) or ChemDraw and uploaded to R for the study. The first three principal components (PC1-PC3) were obtained using R, which accounted for 74.6% of the cumulative variance in the complete data set (Supplementary Table 4). They were then plotted on newly generated, unitless, orthogonal axes (principal components) based on linear combinations of the original 20 parameters (Supplementary Fig. 3 and Supplementary Data Set 1). The PCA graphs shown in Supplementary Fig. 2 were generated using the data visualization softw are Prism.
Supplementary Material
Acknowledgements
We thank Prof. Marco Ciufolini (University of British Columbia) for helpful discussions, Dr. George Sukenick, Rong Wang, and Dr. Sylvi Rusli (MSKCC) for expert NMR and mass spectral assistance, and Dr. Kristin Kirschbaum and Kelly Lambright (University of Toledo) for X-ray crystallographic analysis. Instant JChem was generously provided by ChemAxon. Financial support from the NIH (P41 GM076267 to D.S.T., T32 CA062948-Gudas to T.A.W., CCSG P30 CA008748 to C. B. Thompson), William and Alice Goodwin and the Commonwealth Foundation for Cancer Research, and MSKCC Experimental Therapeutics Center is gratefully acknowledged.
References
- [1].Eliel EL, Wilen SH, Stereochemistry of Organic Compounds, John Wiley, New York, 1994. [Google Scholar]
- [2].Hussain A, Yousuf SK, Mukherjee D, RSC Adv. 2014, 4, 43241–43257. [Google Scholar]
- [3].Spring DR, Krishnan S, Schreiber SL, J. Am. Chem. Soc 2000, 122, 5656–5657. [Google Scholar]
- [4].Spring DR, Krishnan S, Blackwell HE, Schreiber SL, J. Am. Chem. Soc 2002, 124, 1354–1363. [DOI] [PubMed] [Google Scholar]
- [5].Krishnan S, Schreiber SL, Org. Lett 2004, 6, 4021–4024. [DOI] [PubMed] [Google Scholar]
- [6].Brown N, Gao G, Minatoya M, Xie B, VanderVelde D, Lushington GH, Perchellet J-PH, Perchellet EM, Crow KR, Buszek KR, J. Comb. Chem 2008, 10, 628–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Marcaurelle LA, Comer E, Dandapani S, Duvall JR, Gerard B, Kesavan S, Lee MD, Liu H, Lowe JT, Marie J-C, Mulrooney CA, Pandya BA, Rowley A, Ryba TD, Suh B-C, Wei J, Young DW, Akella LB, Ross NT, Zhang Y-L, Fass DM, Reis SA, Zhao W-N, Haggarty SJ, Palmer M, Foley MA, J. Am. Chem. Soc 2010, 132, 16962–16976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gerard B, Duvall JR, Lowe JT, Murillo T, Wei J, Akella LB, Marcaurelle LA, ACS Comb. Sci 2011, 13, 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chou DH-C, Duvall JR, Gerard B, Liu H, Pandya BA, Suh B-C,Forbeck EM, Faloon P, W agner BK, Marcaurelle LA, ACS Med. Chem. Lett 2011, 2, 698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schaefer GI, Perez JR, Duvall JR, Stanton BZ, Shamji AF, Schreiber SL, J. Am. Chem. Soc 2013, 135, 9675–9680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zheng S, Laraia L, O’Connor CJ, Sorrell D, Tan YS, Xu Z, Venkitaraman AR, Wu W, Spring DR, Org. Biomol. Chem 2012, 10, 2590–2593. [DOI] [PubMed] [Google Scholar]
- [12].Lambu MR, Kumar S, Yousuf SK, Sharma DK, Hussain A, Kumar A, Malik F, Mukherjee D, J. Med. Chem 2013, 56, 6122–6135. [DOI] [PubMed] [Google Scholar]
- [13].Zhang J, Wu JB, Hong BK, Ai WY, Wang XM, Li HH, Lei XG, Nat. Commun 2014, 5, 4614. [DOI] [PubMed] [Google Scholar]
- [14].W elford AJ, Caldwell JJ, Liu M, Richards M, Brown N, Lomas C, Tizzard GJ, Pitak MB, Coles SJ, Eccles SA, Raynaud FI, Collins I, Chem. Eur. J 2016, 22, 5657–5664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kopp F, Stratton CF, Akella LB, Tan DS, Nat. Chem. Biol 2012,8, 358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bauer RA, W enderski TA, Tan DS, Nat. Chem. Biol 2013, 9, 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Parsons WH, Davidson JL, Taub D, Aster SD, Thorsett ED, Patchett AA, Ulm EH, Lamont BI, Biochem. Biophys. Res. Commu 1983, 117, 108–113. [DOI] [PubMed] [Google Scholar]
- [18].Robl JA, Simpkins LM, Sulsky R, Sieber-McMaster E, Stevenson J, Kelly YF, Sun C-Q, Misra RN, Ryono DE, Asaad M, Bird MJE, Trippodo NC, Karanewsky DS, Bioorg. Med. Chem. Lett 1994, 4, 1795–1800. [Google Scholar]
- [19].Bohacek R, De Lombaert S, McMartin C, Priestle J, Gruetter M, J. Am. Chem. Soc 1996, 118, 8231–8249. [Google Scholar]
- [20].Khan AR, Parrish JC, Fraser ME, Smith WW, Bartlett PA, James MNG, Biochem istry 1998, 37, 16839–16845 [DOI] [PubMed] [Google Scholar]
- [21].Reza i T, Yu B, M illhau ser GL, Jacobson MP ,Lokey RS, J. Am. Chem. Soc 2006. , 128,2510–2511. [DOI] [PubMed] [Google Scholar]
- [22].Kwon Y-U, Kodadek T, Chem. Biol 2007, 14, 671–677. [DOI] [PubMed] [Google Scholar]
- [23].Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, Kopple KD, J. Med. Chem 2002, 45, 2615–2623. [DOI] [PubMed] [Google Scholar]
- [24].McGrath NA, Brichacek M, Njardarson JT, J. Chem. Educ 2010, 87, 1348–1349, see also: http://njardarson.lab.arizona.edu/content/top-pharmaceuticals-poster. [Google Scholar]
- [25].Illuminati G, Mandolini L, Acc. Chem. Res 1981, 14, 95–102 [Google Scholar]
- [26].Marti-Centelles V, Pandey MD, Burguete MI, Luis SV, Chem. Rev 2015, 115, 8736–8834. [DOI] [PubMed] [Google Scholar]
- [27].Pospisil J, Mueller C, Fuerstner A, Chem. Eur. J 2009, 15, 5956–5968. [DOI] [PubMed] [Google Scholar]
- [28].Deb I, John S, Namboothiri INN, Tetrahedron 2007, 63, 11991–11997. [Google Scholar]
- [29].Andrus MB, Argade AB, Tetrahedron Lett. 1996, 37, 5049–5052. [Google Scholar]
- [30].Zhou B, Li L, Zhu XQ, Yan JZ, Guo YL, Ye LW, Angew. Chem. Int. Ed 2017, 56, 4015–4019. [DOI] [PubMed] [Google Scholar]
- [31].Huang L, Dai LX, You SL, J. Am. Chem. Soc 2016, 138, 5793–5796. [DOI] [PubMed] [Google Scholar]
- [32].Xu Z, Chen HY, Wang ZX, Ying AG, Zhang LM, J. Am. Chem. Soc 2016, 138, 5515–5518. [DOI] [PubMed] [Google Scholar]
- [33].Vo CVT, Luescher MU, Bode JW, Nat. Chem 2014, 6, 310–314. [DOI] [PubMed] [Google Scholar]
- [34].Li L, Li ZL, Wang FL, Guo Z, Cheng YF, Wang N, Dong XW, Fang C, Liu JJ, Hou CH, Tan B, Liu XY, Nat. Commun 2016, 7, 13852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Li ZL, Li XH, Wang N, Yang NY, Liu XY, Angew. Chem. Int. Ed 2016, 55, 15100–15104. [DOI] [PubMed] [Google Scholar]
- [36].Hall JE, Matlock JV, Ward JW, Gray KV, Clayden J, Angew. Chem. Int. Ed 2016, 55, 11153–11157. [DOI] [PubMed] [Google Scholar]
- [37].Kitsiou C, Hindes JJ, I’Anson P, Jackson P, Wilson TC, Daly EK, Felstead HR, Hearnshaw P, Unsworth WP, Angew. Chem. Int. Ed 2015, 54, 15794–15798. [DOI] [PubMed] [Google Scholar]
- [38].Stephens TC, Lodi M, Steer AM, Lin Y, Gill MT, Unsworth WP, Chem. Eur. J 2017, 23, 13314–13318. [DOI] [PubMed] [Google Scholar]
- [39].Mendoza-Sanchez R, Corless VB, Nguyen QNN, Bergeron- Brlek M, Frost J, Adachi S, Tantillo DJ, Yudin AK, Chem. Eur. J 2017, 23, 13319–13322. [DOI] [PubMed] [Google Scholar]
- [40].Loya DR, Jean A, Cormier M, Fressigne C, Nejrotti S, Blanchet J, Maddaluno J, De Paolis M, Chem. Eur. J 2018, 24, 2080–2084. [DOI] [PubMed] [Google Scholar]
- [41].Donald JR, Unsworth WP, Chem. Eur. J 2017, 23, 8780–8799. [DOI] [PubMed] [Google Scholar]
- [42].Roxburgh CJ, Tetrahedron 1993, 49, 10749–10784. [Google Scholar]
- [43].Hesse M, Ring Enlargem ent in Organic Chemistry, VCH, Weinheim, 1991. [Google Scholar]
- [44].Barton DHR, Pure Appl. Chem 1964, 9, 35–48. [Google Scholar]
- [45].Kupchan SM, Dhingra OP, Kim CK, J. Chem. Soc., Chem. Commun 1977, 847–848. [Google Scholar]
- [46].Marino JP, Samanen JM, J. Org. Chem 1976, 41, 179–180. [Google Scholar]
- [47].Kupchan SM, Kim C-K, Lynn JT, J. Chem. Soc., Chem. Comm 1976, 86. [Google Scholar]
- [48].Marino JP, Samanen JM, Tetrahedron Lett. 1973, 46, 4553–4556. [Google Scholar]
- [49].Battersby AR, Bhatnaga AK, Hackett P, Thornber CW, Staunton J, Chem. Commun 1968, 1214–1215. [Google Scholar]
- [50].Kikugawa Y, Kawase M, J. Am. Chem. Soc 1984, 106, 5728–5729. [Google Scholar]
- [51].Kawase M, Kitamura T, Kikugawa Y, J. Org. Chem 1989, 54, 3394–3403. [Google Scholar]
- [52].Glover SA, Goosen A, McCleland CW, Schoonraad JL, J. Chem. Soc., Perkin Trans 1 1984, 2255–2260. [Google Scholar]
- [53].Glover SA, Goosen A, McCleland CW, Schoonraad JL, Tetrahedron 1987, 43, 2577–2592. [Google Scholar]
- [54].Kikugawa Y, Kawase M, Chem. Lett 1990, 581–582. [Google Scholar]
- [55].Telma D, Clemente V, Lobo AM, Prabhakar S, Marcelo-Curto MJ, Tetrahedron Lett. 1994, 35, 2043–2046. [Google Scholar]
- [56].Prata JV, Clemente D-TS, Prabhakar S, Lobo AM, Mourato I, Branco PS, J. Chem. Soc., Perkin Trans 1 2002, 513–528. [Google Scholar]
- [57].W ardrop DJ, Basak A, Org. Lett 2001, 3, 1053–1056. [DOI] [PubMed] [Google Scholar]
- [58].W ardrop DJ, Zhang W, Org. Lett 2001, 3, 2353–2356. [DOI] [PubMed] [Google Scholar]
- [59].Miyazawa ES, T.; Kikugawa Y, Heterocycles 2003, 59, 149–160. [Google Scholar]
- [60].Miyazawa E, Sakamoto T, Kikugawa Y, J. Org. Chem 2003, 68, 5429–5432. [DOI] [PubMed] [Google Scholar]
- [61].Hassall CH, Lewis JR, J. Am. Chem. Soc 1961, 2312–2315. [Google Scholar]
- [62].Braun NA, Ciufolini MA, Peters K, Peters E-M, Tetrahedron Lett. 1998, 39, 4667–4670. [Google Scholar]
- [63].Canesi S, Belmont P, Bouchu D, Rousset L, Ciufolini MA, Tetrahedron Lett. 2002, 43, 5193–5195. [Google Scholar]
- [64].Liang H, Ciufolini MA, Chem. Eur. J 2010, 16, 13262–13270. [DOI] [PubMed] [Google Scholar]
- [65].Liang H, Ciufolini MA, Tetrahedron 2010, 66, 5884–5892. [Google Scholar]
- [66].Morton JGM, Kwon LD, Freeman JD, Njardarson JT, Tetrahedron Lett. 2009, 50, 1684–1686. [Google Scholar]
- [67].Becker HD, Bremholt T, Adler E, Tetrahedron Lett. 1972, 4205–4208. [Google Scholar]
- [68].Olah GA, Singh BP, Liang G, J. Org. Chem 1984, 49, 2922–2925. [Google Scholar]
- [69].Zawada PV, Banfield SC, Kerr MA, Synlett 2003, 971–974. [Google Scholar]
- [70].Acharya A, Eickhoff JA, Chen K, Catalano VJ, Jeffrey CS, Org. Chem. Front 2016, 3, 330–334. [Google Scholar]
- [71].Anumandla D, Littlefield R, Jeffrey CS, Org. Lett 2014, 16, 5112–5115. [DOI] [PubMed] [Google Scholar]
- [72].Correa A, Herrero MT, Tellitu I, Dominguez E, Moreno I, SanMartin R, Tetrahedron 2003, 59, 7103–7110. [Google Scholar]
- [73].Mulcahy JV, Du Bois J, J. Am. Chem. Soc 2008, 130, 12630–12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Braun NA, Bray JD, Ciufolini MA, Tetrahedron Lett. 1999, 40, 4985–4988. [Google Scholar]
- [75].Braun NA, Ousmer M, Bray JD, Bouchu D, Peters K, Peters E-M, Ciufolini MA, J. Org. Chem 2000, 65, 4397–4408. [DOI] [PubMed] [Google Scholar]
- [76].Padwa A, Stengel T, Org. Lett 2002, 4, 2137–2139. [DOI] [PubMed] [Google Scholar]
- [77].Padwa A, Flick AC, Leverett CA, Stengel T, J. Org. Chem 2004, 69, 6377–6386. [DOI] [PubMed] [Google Scholar]
- [78].Misu Y, Togo H, Org. Biomol. Chem 2003, 1, 1342–1346. [DOI] [PubMed] [Google Scholar]
- [79].Dohi T, Maruyama A, Minamitsuji Y, Takenaga N, Kita Y, Chem.Commun. 2007, 1224–1226. [DOI] [PubMed] [Google Scholar]
- [80].Correa A, Tellitu I, Dominguez E, Moreno I, SanMartin R, J. Org. Chem 2005, 70, 2256–2264. [DOI] [PubMed] [Google Scholar]
- [81].Chang CY, Yang TK, Tetrahedron Asymm. 2003, 14, 2081–2085. [Google Scholar]
- [82].Zhang ZG, Zhang Q, Sun SG, Xiong T, Liu Q, Angew. Chem. Int. Ed 2007, 46, 1726–1729. [DOI] [PubMed] [Google Scholar]
- [83].McAllister LA, McCormick RA, James KM, Brand S, Willetts N,Procter DJ, Chem. Eur. J 2007, 13, 1032–1046. [DOI] [PubMed] [Google Scholar]
- [84].Badone D, Baroni M, Cardamone R, Ielmini A, Guzzi U, J. Org. Chem 1997, 62, 7170–7173. [DOI] [PubMed] [Google Scholar]
- [85].Miyabe H, Matsumura A, Moriyama K, Takemoto Y, Org. Lett 2004,6, 4631–4634. [DOI] [PubMed] [Google Scholar]
- [86].Grainger RS, Betou M, Male L, Pitak MB, Coles SJ, Org. Lett 2012, 14, 2234–2237. [DOI] [PubMed] [Google Scholar]
- [87].B o rch RF, Bernstein MD , Durst HD, J. A m. Chem. Soc 1971, 93, 2897–2904. [Google Scholar]
- [88].Bodnar PM, Shaw JT, W oerpel KA, J. Org. Chem 1997, 62, 5674–5675. [Google Scholar]
- [89].W enderski TA, Stratton CF, Bauer RA, Kopp F, Tan DS, Chemical Biology: M ethods and Protocols 2015, 1263, 225–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Moura-Letts G, DiBlasi CM, Bauer RA, Tan DS, Proc. Natl. Acad. Sci. USA 2011, 108, 6745–6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Clemons PA, Bodycombe NE, Carrinski HA, Wilson JA, Shamji AF, W agner BK, Koehler AN, Schreiber SL, Proc. Natl. Acad. Sci. USA 2010, 107, 18787–18792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Luker T, Alcaraz L, Chohan KK, Blomberg N, Brown DS, Butlin RJ, Elebring T, Griffin AM, Guile S, St-Gallay S, Swahn BM, Swallow S, Waring MJ, Wenlock MC, Leeson PD, Bioorg. Med. Chem. Lett 2011, 21, 5673–5679. [DOI] [PubMed] [Google Scholar]
- [93].Lovering F, Bikker J, Humblet C, J. Med. Chem 2009, 52, 6752–6756. [DOI] [PubMed] [Google Scholar]
- [94].Ritchie TJ, Macdonald SJF, Young RJ, Pickett SD, Drug Discov. Today 2011, 16, 164–171. [DOI] [PubMed] [Google Scholar]
- [95].Ritchie TJ, Macdonald SJF, Drug Discov. Today 2009, 14, 1011–1020. [DOI] [PubMed] [Google Scholar]
- [96].Markham A, Drugs 2017, 77, 923–927. [DOI] [PubMed] [Google Scholar]
- [97].Sartori A, Carle D, Freedman MS, Expert Opin. Pharmacother 2014, 15, 1019–1027. [DOI] [PubMed] [Google Scholar]
- [98].Xu S, Rouzer CA, Marnett LJ, IUBMB Life 2014, 66, 803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Alonso-Alvarez S, Caballero MD, Mateos MV, Martin A, Pardal E,Sanchez-Nieto D, Navarro M, Drug. Des. Devel. Ther 2017, 11, 253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Wu P-K, Park J-I, Semin. Oncol 2015, 42, 849–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Tetko IV, Tanchuk VY, Kasheva TN, Villa AEP, J. Chem. Inf. Comput. Sci 2001, 41, 246–252, see also: http://www.vcclab.org/. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.