Abstract
Bifidobacterium longum strain BBMN68 is sensitive to low concentrations of oxygen. A transcriptomic study was performed to identify candidate genes for B. longum BBMN68’s response to oxygen treatment (3%, v/v). Expression of genes and pathways of B. longum BBMN68 involved in nucleotide metabolism, amino acid transport, protein turnover and chaperones increased, and that of carbohydrate metabolism, translation and biogenesis decreased to adapt to the oxidative stress. Notably, expression of two classes of ribonucleotide reductase (RNR), which are important for deoxyribonucleotide biosynthesis, was rapidly and persistently induced. First, the class Ib RNR NrdHIEF was immediately upregulated after 5 min oxygen exposure, followed by the class III RNR NrdDG, which was upregulated after 20 min of exposure. The upregulated expression of branched-chain amino acids and tetrahydrofolate biosynthesis-related genes occurred in bifidobacteria in response to oxidative stress. These change toward to compensate for DNA and protein damaged by reactive oxygen species (ROS). In addition, oxidative stress resulted in improved B. longum BBMN68 cell hydrophobicity and autoaggregation. These results provide a rich resource for our understanding of the response mechanisms to oxidative stress in bifidobacteria.
Introduction
Bifidobacteria are Gram-positive, heterofermentative, non-motile, non-spore-forming, anaerobic bacteria that are mainly found in gastrointestinal tract (GIT) of mammals1,2. Some bifidobacteria are considered to be probiotic due to their contribution to the maintenance of gastrointestinal health2,3. Thus, bifidobacteria are incorporated into many food products, such as yogurt, fermented milk and dietary supplements3. However, the efficacy of their probiotic properties can be compromised by their high sensitivity to environmental challenges, especially oxygen-induced oxidative stress4,5; nevertheless, some strains can tolerate 5% to 21% (v/v) oxygen6,7. Incomplete reduction of oxygen forms reactive oxygen species (ROS), which can cause deleterious effects, including protein misfolding and aggregation, DNA damage and lipid peroxidation8.
Enzymes such as NADH oxidase, NADH peroxidase, catalase and superoxide dismutase play key roles in removing ROS in many anaerobic microorganisms9,10. In the more than 50 published genome sequences of bifidobacteria11, no genes encoding NADH peroxidase, catalase or superoxide dismutase have been annotated (with the exception of Bifidobacterium asteroides, which contains a heme-catalase gene12). Previous study suggested that alkyl hydroperoxide reductase is probably the primary scavenger of the endogenous hydrogen peroxide (H2O2) generated during aerobic cultivation of Bifidobacterium longum13. NADH oxidase and oxygen-dependent coproporphyrinogen III oxidase are involved in detoxifying molecular oxygen and/or H2O2 in Bifidobacterium animalis14. Thus, alkyl hydroperoxide reductase, thioredoxin reductase and NADH oxidase are critical in bifidobacteria’s response to oxidative stress2,15, as confirmed by proteomic and transcriptomic analyses14,16–18. On the other hand, bifidobacteria employ a particular set of proteins, mainly molecular chaperones and proteases, to protect the cells from damage caused by the accumulation of unfolded and/or misfolded proteins. These chaperones and proteases play key roles in several post-translational events to prevent protein denaturation, aggregation and misfolding caused by stresses, such as oxidative stress19,20. Induction and assembly of the stress-response system are controlled by a set of complex transcription factors. A report on oxidative responses in Bifidobacterium breve showed that HspR, LexA, HrcA, and Crp regulon are involved in the responses to oxygen, H2O2, and peroxides caused oxidative stress19. Among them, RecA–LexA is the major regulator of the SOS response in bacteria induced by DNA damage21, and HspR regulates dnak, clpB, and clgR, which are involved in heat, osmosis, and solvent stress responses, respectively22.
Despite physiological and biochemical analyses carried out in the last decade and the accumulation of ‘omics’ studies in recent years providing information on oxidative stress responses in bifidobacteria6,14–18,23, the global gene-transcription profile in response to oxygen stress in bifidobacteria has not been well elucidated. B. longum subsp. longum BBMN68 is a gut-inhabiting strain isolated from a healthy centenarian which has a number of probiotic properties24–26. It is very sensitive to low and residual oxygen, and headspace contact with 3% to 6% oxygen yields severe to sublethal growth inhibition16. In the present study, next-generation RNA-sequencing (RNA-Seq) analysis and validation of physiological characteristics were employed to study the oxidative stress response and resistance mechanism in B. longum strain BBMN68.
Materials and Methods
Bacterial strains and growth conditions
B. longum subsp. longum strain BBMN6827 was cultivated under standard anaerobic conditions in de Man Rogosa Sharpe (MRS) broth (Oxoid) with 0.05% (w/v) L-cysteine HCl (MRSC) at 37 °C in Hungate tubes or infusion vials (300 ml capacity) purged with a gas mixture of 10% (v/v) H2, 10% CO2, and 80% N2, unless otherwise noted16.
Oxygen treatment of B. longum BBMN68 culture
Overnight B. longum BBMN68 culture was inoculated (1%, v/v) by syringe into injection vials containing 100 ml pre-warmed MRS medium, and the culture was grown at 37 °C. When growth reached the exponential phase (optical density at 600 nm [OD600] = 0.5, after about 6 h cultivation), 3% (v/v) oxygen was established in the injection vial headspace by previously reported methods16. After the modulation of the headspace gas component, injection vials were incubated at 37 °C with gentle horizontal shaking (100 rpm). Samples used for RNA extraction were collected from six biological replicates after 30 min and 60 min of oxygen treatment by centrifugation at 8,000 × g for 5 min at 4 °C. Culture collected prior to treatment was used as a control.
RNA extraction, sequencing and annotation
The Applied Biosystems (AB) SOLiDTM 4.0 System Sequencing Analyzer was used for the RNA-Seq analyses. Total RNA was isolated from 10 ml bacterial cells (about 1 × 108 CFU ml−1) subjected to the different treatments using TRIzol reagent (Invitrogen, Cat. no. 15596026) according to the manufacturer’s instructions. The mRNA was enriched using a Ribo-minus Kit (Invitrogen, Cat. no. 1083708) that depletes rRNA. A mRNA-Seq library was prepared with the total RNA-Seq Kit (AB) according to the manufacturer’s protocol. cDNA in the 150–200-bp range was selected with Novex precast gel products (Invitrogen, Cat. no. NP0322BOX), amplified by 15 PCR cycles and cleaned with PureLink PCR Micro Kit (Invitrogen, Cat. no. K310250). All sequenced reads were aligned to B. longum subsp. longum BBMN68 (NC_014656.1) using AB’s SOLiD Corona_lite_v4.2 software. We used a recursive strategy to improve the read-mapping ratio: 50mer reads were first mapped to the genome with a tolerance of five mismatches; the reads that failed to be mapped were progressively trimmed—five bases at a time from the 3′ end—and then mapped to the genome again until a match was found (unless the read was trimmed to less than 30 bases). All of these uniquely mapped reads were used to calculate the gene-expression level in RPKM (reads per kilobase of exon per million mapped sequenced reads). We identified differentially expressed genes from the different samples using the R package DEGseq (http://waprna.big.ac.cn/rnaseq/function/degseq.jsp) with statistically significant level set at P < 0.001. The analyzed transcriptomic data were submitted to the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/) with accession number GSE65320.
Real-time quantitative PCR (RT-qPCR) analysis
Reverse transcription was carried out on the total RNA extracted from the treatment and control cultures with M-MLV Reverse Transcriptase (Promega), using 2 µg DNase I-digested total RNA as the template. The absence of residual DNA in the total RNA digested by DNase I was confirmed by PCR. Specific primers for each gene (Table 1) were designed using Primer Premier 5 software. RT-qPCR was performed using SYBR® Premix Ex TaqTM (Takara) and optimized primer concentrations in a LightCycler® 96 Real-Time PCR system (Roche), with cycling and detection of 95 °C for 10 s and 60 °C for 30 s (40 cycles). Gene expression was normalized by the ΔΔCT method28, using 16S rRNA as the reference gene in the calculations14,16. The experiment was performed in triplicate and the average results are reported.
Table 1.
Target gene oligonucleotide primers for RT-PCR.
| Gene (Locus tag) | Primer sequence (5′ → 3′) | Size of product (bp) | |
|---|---|---|---|
| Forward | Reverse | ||
| sufB1 (BBMN68_611) | ACGACGGTGACGCACGACT | AGATGCCGAGCATGTTGAGGT | 243 |
| glycerate kinase (BBMN68_585) | GCCCTCGGCGTTCGTCTTCT | CAATGTGGCGACATCATCTTTGGA | 225 |
| grxC2 (BBMN68_1397) | GCAGTGCGATGCCACCAAG | CAGGAGTTGTCCGGCGTGAT | 147 |
| tatC (BBMN68_1285) | GGAGCCGGACTGGCATGGTATCT | CGTTGCGAGACGCCACTGCTT | 228 |
| hcaD (BBMN68_1524) | ACGCCAGAACCCTCACCTACC | CCGATCACCACTGCCGACTT | 217 |
| 16 S rRNA (BBMN68_rRNA7) | CGTAGGGTGCAAGCGTTATC | GCCTTCGCCATTGGTGTT | 197 |
| nrdI (BBMN68_1398) | GGATGCCGTTTGCAGGAC | TCGTTGAGGAAGCGTTTGAC | 164 |
| nrdE (BBMN68_1399) | CCTGCCGCTCGACAATACT | CTTGAACGCACCAAGGAAAG | 334 |
| nrdF (BBMN68_1401) | CCCTGCTTGACACCATCC | AACTCGTTGTTCTCGCTCC | 199 |
| nrdD (BBMN68_1785) | TGCGGTCAAGTCTGCTTTC | CGAGCCACATCGTACAGGT | 189 |
| nrdG (BBMN68_1786) | TCTTGCCAACGATCCGAAAG | CCGCCAAGGAACGTAATGC | 267 |
| nrdR (BBMN68_197) | GGAGCCATTCAGTAGAGAC | TCCAGACCTGCAAAGTTC | 240 |
Autoaggregation and hydrophobicity assay
B. longum BBMN68 cells grown in MRSC or MRS for 6 h (exponential phase) were harvested and resuspended in phosphate buffer (pH 6.8) to yield an OD600 of 1.0. For the autoaggregation assay, the cell suspension was incubated anaerobically at 37 °C for 3 h and 6 h, and then 0.1 ml of the upper suspension was gently transferred to another tube with 1.9 ml of phosphate buffer and OD600 was measured. The percentage of autoaggregation was expressed as (1 − OD600 of the upper suspension/OD600 of the total bacterial suspension) × 100%29. To determine the hydrophobicity of the bifidobacterial cells, 0.6 ml xylene was added to 3 ml of cell suspension and vortexed for 120 s. The aqueous phase was removed after 1 h of incubation at room temperature and its absorbance at 600 nm was measured. Cell-surface hydrophobicity was calculated as (1 - OD600 of the aqueous phase suspension/OD600 of the total bacterial suspension) × 100%30,31.
Statistical analysis for RT-qPCR and physiological assays results
All of the RT-qPCR and physiological assay data from three independent experiments were analyzed by two-tailed Student’s t test. All analyses were performed using Microsoft Office Excel 2007. Values of P < 0.05 were considered significant.
Results and Discussion
Global transcriptomic analysis of the oxygen response in B. longum BBMN68
A previous study, using a proteomic approach to analyze changes in the cellular protein profiles of BBMN68 exposed to an inhibitory to sublethal concentration of oxygen (3%, v/v), revealed some key proteins involved in the response of BBMN68 to oxygen16. To further understand the mechanism of bifidobacteria’s response to oxidative stress, BBMN68 cells were treated with 3% oxygen and global transcriptional changes were analyzed by SOLiD 4.0 RNA-Seq. A total of 17,539,582, 23,696,766 and 20,913,996 uniquely mapped reads were obtained for the oxygen-challenged samples harvested at two time points (30 and 60 min) after oxygen delivery, and a reference sample taken prior to oxygen delivery (control, 0 min), respectively (Fig. 1). After filtering, the number of effective reads mapped to the genome of BBMN68 was 13,300,802, 18,504,526 and 14,833,647, respectively. Genes that were significantly differentially expressed (based on a fold change of at least two [log2 ratio <−1 or >1] and a t-test P-value < 0.001) in response to oxygen were sorted: expression of 99 genes was downregulated and of 241 genes upregulated after 30 min, and expression of 218 genes was downregulated, 217 upregulated after 60 min of oxygen exposure compared to controls (Tables S1 and S2); expression of 70 genes was downregulated, and 124 upregulated at both time points (Tables S2). Some upregulated genes encoding proteins also induced at protein level by proteomics study, such as AhpC, NrdA, Eno16.
Figure 1.
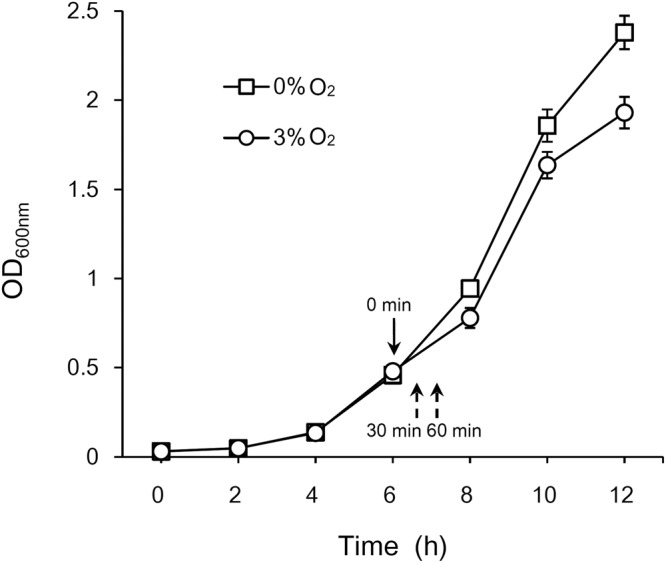
Growth of B. longum BBMN68 in MRS with or without 3% (v/v) oxygen challenge. Samples were collected from the time points indicated by arrows.
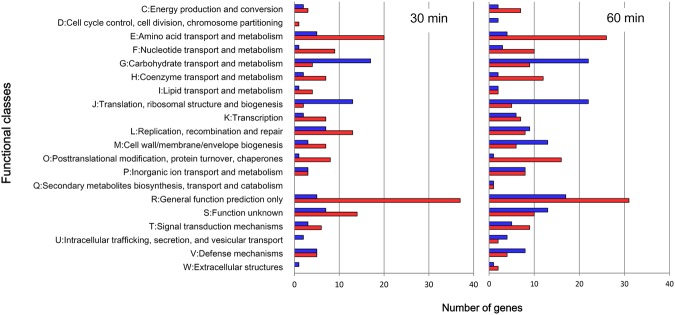
Real-time quantitative PCR (RT-qPCR) analysis of six different genes was performed to validate the transcriptomic data. A strong positive correlation (r2 = 0.92) was found between the fold-change in gene induction or repression obtained from the transcriptomic data and the values determined by RT-qPCR (Fig. 2), suggesting agreement between the two platforms. The differentially expressed genes were grouped into functional categories according to the Clusters of Orthologous Groups (COG) classification system32. COG categories E (amino acid transport and metabolism), R (general function prediction), and O (post-translational modification, protein turnover, chaperones) had a high number of upregulated genes after both 30 min and 60 min oxygen treatment compared to controls (Fig. 3). In addition, many genes in categories F (nucleotide transport and metabolism), H (coenzyme metabolism), T (signal-transduction mechanisms) and L (DNA replication, recombination and repair) were also upregulated, whereas most of the genes belonging to categories G (carbohydrate transport and metabolism) and J (translation, ribosomal structure and biogenesis) were downregulated at both time points after oxygen exposure compared to controls (Fig. 3).
Figure 2.
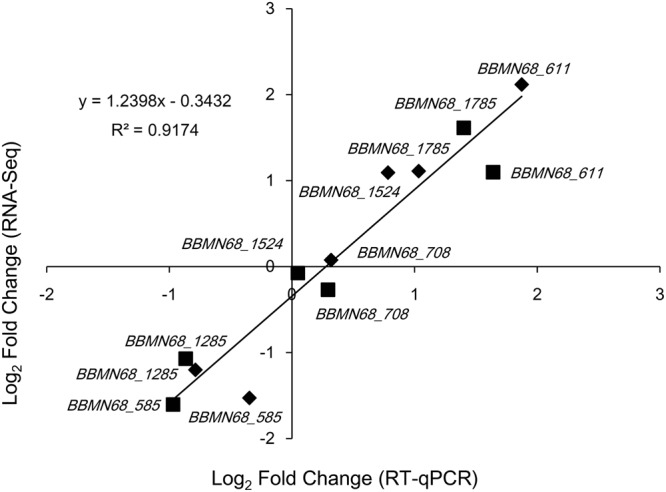
RT-qPCR validation of the RNA-Seq transcriptomic data. Chart shows correlation of fold changes for six genes’ expression from B. longum BBMN68 cells after 30 min (squares) or 60 min (diamonds) exposure to 3% (v/v) oxygen, as derived from the transcriptomic analysis and RT-qPCR. The best fit is shown along with the calculated equation and r2 value.
Figure 3.
Relative abundance of transcripts assigned to COG functional categories. Functional classification of genes with statistically significant increase (red bar) or decrease (blue bar) in mRNA level after 30 min and 60 min exposure to 3% (v/v) oxygen compared to controls.
The results revealed that B. longum BBMN68 cells employ complex defense and adaptation mechanisms to counteract oxygen-driven stresses, including oxygen reduction and ROS detoxification, repair of damaged biomacromolecules, and adaptive modulation of several metabolic processes.
Detoxification and redox homeostasis
Thioredoxin and glutaredoxin make up the thioredoxin- and glutaredoxin-dependent reduction systems in Escherichia coli and many other bacteria, and are responsible for maintaining a reduced environment in the cell cytosol33. However, B. longum BBMN68 has an incomplete glutaredoxin system which lacks any detectable genes for glutathione peroxidase (GPx) or glutathione reductase (GR)27. Two genes encoding glutaredoxin, grxC1 (BBMN68_125) and grxC2 (BBMN68_1397), were upregulated in BBMN68 upon exposure to oxygen. In particular, grxC2, also known as nrdH, was upregulated more than 6-fold after both 30 min and 60 min oxygen exposure (Table 2). A previous study suggested that glutaredoxin encoded by nrdH is reduced by thioredoxin reductase rather than glutathione (GSH)34. Thus, the thioredoxin-dependent antioxidant system might be the major redox homeostasis system in strain BBMN68, as trxB1 (BBMN68_1345) encoding thioredoxin reductase was highly upregulated, and BBMN68_991 encoding the corresponding thioredoxin was also upregulated in BBMN68 after 60 min exposure to oxygen (Table 2). Thioredoxin reductase has been found to respond to oxidative stress at both transcriptional and translational levels in bifidobacteria15,16. The thioredoxin-dependent reduction system plays an important role in the oxidative stress response by reducing a number of proteins including peroxiredoxins, directly reducing H2O2, scavenging hydroxyl radicals, quenching singlet oxygen, and maintaining the intracellular thiol-disulfide balance35. Because of the most bifidobacterial species lacking genes encoding catalase or superoxide dismutase, introduce catalase and/or superoxide dismutase into bifidobacterial cells could dramatically improve their oxidative stress tolerance. We have demonstrated this hypothesis recently36, and the gene encoding catalase has been integrated into the chromosome of bifidobacteria for generating food-grade strain potentially used in food industry (Our unpublished data).
Table 2.
Genes differentially expressed at the transcriptional level in B. longum BBMN68 exposed to 3% (v/v) oxygen.
| Proposed function | Gene name | Log2 ratioa | Locus tagb | |
|---|---|---|---|---|
| 30 min vs. Cont | 60 min vs. Cont | |||
| Oxidative response (detoxification) | ||||
| Thioredoxin reductase | trxB1 | 3.15 | 4.09 | BBMN68_1345 |
| Thioredoxin domain-containing protein | NS | 1.14 | BBMN68_991 | |
| Alkyl hydroperoxide reductase subunit C | ahpC2 | 1.38 | NS | BBMN68_1346 |
| Glutaredoxin | grxC2 (nrdH) | 2.68 | 2.69 | BBMN68_1397 |
| Glutaredoxin | grxC1 | NS | 1.05 | BBMN68_125 |
| Energy/intermediary metabolism | ||||
| Nitroreductase | nfnB1 | NS | 1.14 | BBMN68_86 |
| Nitroreductase | nfnB2 | NS | 2.22 | BBMN68_1435 |
| Class I pyridine nucleotide-disulfide oxidoreductase | ipd2 | 1.79 | 2.18 | BBMN68_1660 |
| Dihydroorotate dehydrogenase | pyrD2 | 1.26 | 2.17 | BBMN68_979 |
| Nucleic acid repair | ||||
| DNA helicase II/ATP-dependent DNA helicase PcrA | uvrD1 | 2.27 | 2.76 | BBMN68_138 |
| Excinuclease ABC subunit A | uvrA1 | NS | 1.08 | BBMN68_394 |
| DNA polymerase V | dinp1 | 1.14 | 2.08 | BBMN68_863 |
| ADP-ribose pyrophosphatase | 1.76 | 1.53 | BBMN68_240 | |
| DNA-repair protein RecN | recN | 1.07 | 1.20 | BBMN68_793 |
| Nucleoside triphosphate pyrophosphohydrolase | mutT3 | 1.04 | 1.11 | BBMN68_1517 |
| Ribonucleoside-triphosphate reductase | nrdG | 2.67 | 1.92 | BBMN68_1786 |
| Ribonucleoside-triphosphate reductase | nrdD | 1.61 | 1.11 | BBMN68_1785 |
| Protein involved in ribonucleotide reduction | nrdI | 2.06 | 1.63 | BBMN68_1398 |
| Ribonucleoside-diphosphate reductase alpha chain | nrdE | 3.47 | 3.01 | BBMN68_1399 |
| Ribonucleoside-diphosphate reductase beta chain | nrdF | 5.21 | 4.77 | BBMN68_1401 |
| Iron-responsive/iron-related (metal metabolism) | ||||
| Cysteine desulfurase | csdB(sufS) | NS | 1.78 | BBMN68_609 |
| Fe–S cluster assembly protein SufB | sufB2 | NS | 1.49 | BBMN68_612 |
| Fe–S cluster assembly protein SufD | sufB1 | 1.10 | 2.12 | BBMN68_611 |
| Iron complex transport system ATP-binding protein | modF | NS | 1.46 | BBMN68_569 |
| P-type ATPase | zntA1 | NS | 1.01 | BBMN68_1149 |
| Protein repair/chaperones | ||||
| Heat-shock molecular chaperone | ibpA | 2.91 | 2.77 | BBMN68_1305 |
| Molecular chaperone DnaJ | dnaJ1 | NS | 1.44 | BBMN68_410 |
| Co-chaperonin HSP10 | groES | NS | 1.94 | BBMN68_1589 |
| Chaperonin HSP60 | groEL | NS | 1.27 | BBMN68_44 |
| ATP-dependent Clp proteases; protease subunit ClpB | clpA2 | NS | 1.48 | BBMN68_1510 |
| Proteases | ||||
| ATP-dependent Clp proteases; protease subunit | clpP1 | NS | 1.43 | BBMN68_692 |
| ATP-dependent Clp proteases; protease subunit | clpP2 | NS | 1.41 | BBMN68_693 |
| Protease I | thiJ | 1.81 | 1.45 | BBMN68_377 |
| Putative endopeptidase | pepO | NS | 1.03 | BBMN68_1763 |
| Leader peptidase (prepilin peptidase)/N-methyltransferase | 2.08 | 2.46 | BBMN68_618 | |
| Glycolysis | ||||
| Probable phosphoglycerate mutase | phoE | 1.88 | 3.24 | BBMN68_1437 |
| 3-Bisphosphoglycerate-dependent phosphoglycerate mutase | gpmA | NS | 1.07 | BBMN68_1687 |
| Aldehyde dehydrogenase (NAD+) | putA1 | NS | 1.08 | BBMN68_872 |
| Enolase | eno | NS | 2.11 | BBMN68_771 |
| Valine, leucine and isoleucine biosynthesis | ||||
| 2-Isopropylmalate synthase | leuA | 1.35 | 1.13 | BBMN68_1222 |
| 3-Isopropylmalate dehydrogenase | leuB | 1.55 | 1.42 | BBMN68_984 |
| 3-Isopropylmalate/(R)-2-methylmalate dehydratase large subunit | leuC | 2.32 | 2.98 | BBMN68_1521 |
| 3-Isopropylmalate/(R)-2-methylmalate dehydratase small subunit | leuD | 1.42 | 1.78 | BBMN68_1522 |
| Ketol-acid reductoisomerase | ilvc1 | NS | 1.44 | BBMN68_1262 |
| Ketol-acid reductoisomerase | ilvc2 | 1.55 | 1.28 | BBMN68_1263 |
| Branched-chain amino acid aminotransferase | ilvE | 2.02 | 1.40 | BBMN68_592 |
| Branched-chain amino acid transport system substrate-binding protein | livK | 1.63 | 1.93 | BBMN68_1747 |
| Branched-chain amino acid transport system permease protein | livH | 1.46 | 1.74 | BBMN68_1748 |
| Branched-chain amino acid transport system permease protein | livM | 1.27 | 1.61 | BBMN68_1749 |
| Branched-chain amino acid transport system ATP-binding protein | livG | 1.72 | 1.76 | BBMN68_1750 |
| Branched-chain amino acid transport system ATP-binding protein | livF | 1.33 | 1.59 | BBMN68_1751 |
| Carbohydrate transport systems | ||||
| Solute-binding protein of ABC transporter system | −2.01 | −1.97 | BBMN68_1170 | |
| Sugar ABC transporter ATP-binding protein | mglA3 | NS | −1.10 | BBMN68_1727 |
| Putative multiple sugar transport system permease protein | xylH | −1.75 | −1.59 | BBMN68_1728 |
| MalE-type ABC sugar transport system periplasmic component | −2.03 | −1.98 | BBMN68_217 | |
| MalF-type ABC sugar transport systems permease component | −2.66 | −1.62 | BBMN68_218 | |
| MalG-type ABC sugar transport system permease component | −1.74 | −1.49 | BBMN68_219 | |
| Transmembrane transport protein | 2.06 | 2.07 | BBMN68_1264 | |
| Transmembrane transporter activity; MFS transporter (putative metabolite:H+ symporter) | 1.63 | 1.52 | BBMN68_157 | |
| Peptide transport | ||||
| Peptide/nickel transport system substrate-binding protein | ddpA1 | 1.16 | NS | BBMN68_236 |
| Peptide/nickel transport system permease protein | dppB1 | 1.29 | 1.66 | BBMN68_237 |
| Peptide/nickel transport system ATP-binding protein | appF1 | 1.05 | 1.06 | BBMN68_239 |
| Folate biosynthesis | ||||
| GTP cyclohydrolase I | folE | 1.36 | 1.93 | BBMN68_1717 |
| Dihydroneopterin aldolase/2-amino-4-hydroxy-6-hydroxymethyldihydro- | ||||
| pteridine diphosphokinase | folB | NS | 1.15 | BBMN68_1719 |
| Dihydropteroate synthase | folP | NS | 1.73 | BBMN68_1718 |
| Dihydrofolate synthase/folylpolyglutamate synthase | folC | NS | 1.49 | BBMN68_243 |
| Dihydrofolate reductase | folA | NS | 1.36 | BBMN68_1698 |
| Cell wall/membrane/envelope biogenesis | ||||
| Cyclopropane-fatty-acyl-phospholipid synthase | cfa | 1.44 | 2.27 | BBMN68_1705 |
| Bile salt hydrolase | cbaH | NS | 1.83 | BBMN68_536 |
| Hypothetical protein | −1.49 | −2.04 | BBMN68_1491 | |
| Rhamnosyltransferase | −1.86 | −1.93 | BBMN68_1492 | |
| Rhamnosyltransferase | NS | −1.21 | BBMN68_1493 | |
| ABC-2 type transport system permease protein | tagG | NS | −1.43 | BBMN68_1495 |
| ABC-2 type transport system ATP-binding protein | tagH | NS | −1.29 | BBMN68_1496 |
| S-layer protein | −1.46 | −1.40 | BBMN68_882 | |
| Signal transduction | ||||
| Two-component system, OmpR family, response regulator RegX3 | 1.62 | 1.14 | BBMN68_1079 | |
| Histidine kinase sensor of two-component system | 1.48 | 1.64 | BBMN68_1678 | |
| Response regulator of two-component system | NS | 1.02 | BBMN68_750 | |
| S-ribosylhomocysteine lyase | luxS | 1.16 | 1.75 | BBMN68_914 |
| Transcriptional factors | ||||
| Transcriptional regulator of heat shock | hrcA | NS | 2.26 | BBMN68_409 |
| LacI-type transcriptional repressor | 1.71 | 1.61 | BBMN68_223 | |
| SOS-response transcriptional repressor | lexA1 | 1.44 | 1.81 | BBMN68_195 |
| Leucine-responsive regulatory protein | irp | 1.50 | 1.39 | BBMN68_1361 |
| Atypical LysR-type transcriptional regulator | lysR | NS | 1.38 | BBMN68_843 |
| Putative transcriptional regulator | 2.44 | 2.51 | BBMN68_1661 | |
| Putative transcriptional regulator | 1.29 | NS | BBMN68_905 | |
| Hypothetical protein | ||||
| Hypothetical protein | 4.79 | 4.64 | BBMN68_1400 | |
| Hypothetical protein | 4.63 | 4.00 | BBMN68_582 | |
| Hypothetical protein | 2.87 | 3.49 | BBMN68_105 | |
| Hypothetical protein | 1.92 | 3.02 | BBMN68_248 | |
| Hypothetical protein | 1.70 | 2.71 | BBMN68_519 | |
| Hypothetical protein | 1.58 | 2.39 | BBMN68_520 | |
| Hypothetical protein | 1.92 | 2.20 | BBMN68_1662 | |
aLog2 ratio represents the ratio of mRNA transcript levels in oxygen-treated samples (30 min and 60 min) to untreated samples (Cont).
bOpen reading frame (ORF) ID is as annotated in KEGG (http://www.genome.jp/kegg/kegg2.html).
NS, not statistically significant.
Interestingly, two nitroreductase-homolog genes, nfnB1 (BBMN68_86) and nfnB2 (BBMN68_1435), were markedly induced after 60 min exposure of BBMN68 to oxygen (Table 2). NfnB2 shows 46.9% amino acid identity with NfrA1 from Bacillus subtilis37. In the latter, NfrA1 plays a dual role that leads to high concentrations of H2O2 based on its NADH oxidase activity, whereas it can also scavenge H2O2 and degrade NAD+ 37. No high homology of NfnB1 with identified proteins in well-studied bacteria has been found. Nitroreductase is involved in the defense against oxidative stress in Lactococcus lactis38 and Staphylococcus aureus39. Therefore, the two nitroreductases might protect B. longum BBMN68 from oxygen-induced oxidative stress, warranting further investigation.
In Lactobacillus plantarum, Mn2+ not only replaces superoxide dismutase in scavenging superoxide anions, but it can also scavenge H2O240. It has been reported that P-type ATPase might be involved in taking up Mn2+, which then scavenges superoxide anions in bifidobacteria41. In strain BBMN68, expression of the homologous protein-encoding gene zntA1 (BBMN68_1149) was upregulated 2.01-fold after 60 min of oxygen exposure (Table 2). In addition, BBMN68 grew faster in MRS broth supplemented with Mn2+ than in the non-supplemented MRS upon exposure to 3% oxygen (Fig. S1A), but it grew normally under anaerobic conditions (Fig. S1B). This result suggested that manganese can protect bifidobacteria from oxidative stress.
Oxygen induces a multiple stress response in BBMN68
Chaperones and proteases related to several stress conditions were induced in strain BBMN68 in response to oxygen (Fig. 3, Table 2). The transcription of groEL (BBMN68_44) and groES (BBMN68_1589) was upregulated after 60 min exposure to oxygen. The GroEL/GroES complex is required for proper protein folding and is frequently involved in responses to heat, low-pH and bile-salt stresses in bifidobacteria42–45. Genes encoding other chaperones, such as BBMN68_410 and BBMN68_1510 encoding DnaJ and ClpB genes, respectively, were upregulated in strain BBMN68 after 60 min exposure to oxygen (Table 2). ClpB cooperates with DnaK, DnaJ, and GrpE in suppressing protein aggregation; this is a universal phenomenon found in different organisms’ responses to various abiotic stress conditions46,47. Note that expression of the gene BBMN68_1305 encoding the small heat-shock protein (sHsp) IbpA was consecutively induced more than 6-fold after both 30 min and 60 min of oxygen exposure in BBMN68. The ibpA homolog BL0576 is the most rapidly and strongly induced gene in B. longum NCC2705’s response to oxidative stress41. This result suggested that IbpA is important in preventing protein aggregation and misfolding, representing an early and persistent response to oxidative stress in BBMN68. In addition, several genes encoding proteases and peptidases were upregulated in BBMN68 after 60 min exposure to oxygen, including clpP1 (BBMN68_692), clpP2 (BBMN68_693), thiJ (BBMN68_377), and pepO (BBMN68_1763) (Table 2). These proteases and peptidases play a major role in the degradation and turnover of damaged proteins.
Genes involved in the SOS response were also upregulated. The SOS response in bacteria is a global regulatory network for DNA-damage repair, governed by the repressor LexA and inducer RecA21. In BBMN68, lexA (BBMN68_195) expression was upregulated 2.72- and 3.50-fold after 30 and 60 min oxygen exposure, respectively (Table 2). Accordingly, several genes belonging to the LexA regulon were also upregulated upon exposure to oxygen (Table 2). Among them, the DNA-repair protein RecN encoded by BBMN68_793 was upregulated 2.09- and 2.30-fold in BBMN68 upon oxygen exposure for 30 and 60 min, respectively; this protein has also been shown to be regulated by the ferric-uptake regulator (Fur) and to play a role in oxidative-damage protection in Neisseria gonorrhoeae48. This result suggested that oxygen-induced DNA damage leads to activation of RecA–LexA, which subsequently protects BBMN68 from oxidative stress.
Effect of oxygen stress on carbohydrate, nucleotide, and amino acid metabolism
Most of the genes involved in carbohydrate transport and metabolism, belonging to COG category G, were downregulated in strain BBMN68 relative to controls, especially after 30 min exposure to oxygen (Fig. 3). An overall transcriptome map presents a clear picture of the proposed carbohydrate metabolism of BBMN68 grown under oxygen stress49 (Fig. S2). In general, the expression profiles of genes involved in the glycolysis and pentose phosphate pathways were not significantly modified. However, the expression of genes encoding three enzymes related to utilization of complex carbohydrate sources—enolase (BBMN68_771) and two phosphoglycerate mutases (BBMN68_1437, BBMN68_1687)—was upregulated in BBMN68 after 60 min of oxygen exposure (Table 2). Enolase overproduction in BBMN68’s response to oxygen was also confirmed in our previous proteomics study16; these three enzymes fuel the bifid shunt, although expression of genes encoding the key enzymes of that shunt—fructose-6-phosphate phosphoketolase (FPPK, BBMN68_708) and glyceraldehyde 3-phosphate dehydrogenase (Gap, BBMN68_254)—was not significantly induced. Many of the genes encoding proteins in oligosaccharide and disaccharide metabolism were downregulated relative to controls (Table S2), suggesting that polysaccharide utilization is repressed in BBMN68 in response to oxidative stress. On the other hand, the following transport systems genes were heavily downregulated: BBMN68_1170 encoding a solute-binding protein of the ABC transporter system and predicted to be a putative transporter for oligofructose50; BBMN68_1728 encoding a putative multiple sugar transport system permease protein and suggested to be involved in the transport of multiple sugars with fructose and mannose moieties50; BBMN68_217–219 encoding proteins involved in transporting mannose-containing oligosaccharides50 (Table 2). These results corresponded with the repressed polysaccharide and oligosaccharide utilization in BBMN68 under oxygen stress, which has also been detected in BBMN68 in response to acid and bile-salt stress51,52.
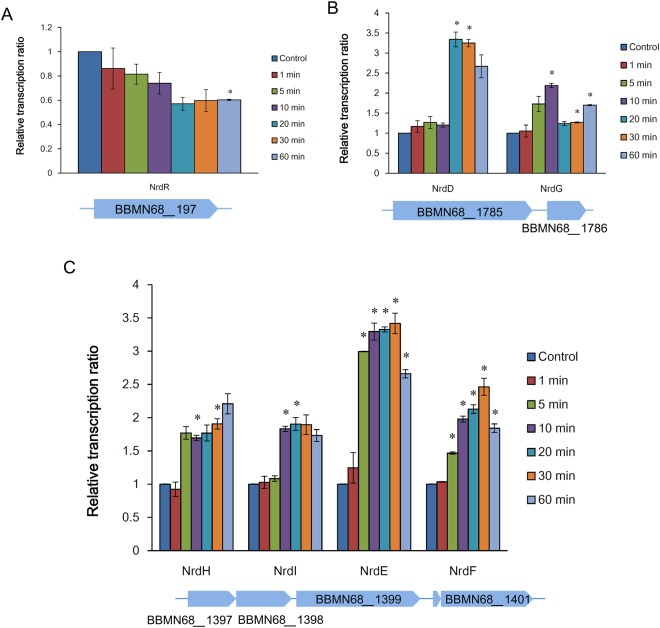
The expression of ribonucleotide reductase (RNR) gene clusters, including class III RNR nrdDG (BBMN68_1785/1786), and class Ib RNR nrdHIEF operon (BBMN68_1397/1398/1399/1401), was highly induced in BBMN68 in response to oxygen (Table 2). NrdDG is an oxygen-sensitive enzyme in anaerobes that is normally expressed under microaerophilic and anaerobic conditions53. While the class Ib RNR, which was the highest upregulated gene cluster (nrdHIEF operon) in this study, has been suggested to act primarily in response to oxidative stress54. nrdE upregulation in BBMN68 in response to oxygen stress has also been confirmed at the translational level14. However, nrdHIEF induction in B. animalis subsp. lactis BL-04 and B. longum NCC2705 in response to sublethal levels of H2O2 was only transitory17,18. We therefore analyzed the temporal expression of RNR cluster genes in BBMN68 upon exposure to oxygen by RT-qPCR. The result showed that nrdD was induced after 20 min and nrdG was induced after 10 min oxygen exposure (Fig. 4). In the nrdHIEF cluster, nrdH, nrdE, and nrdF were induced after 5 min, and nrdI was induced after 10 min oxygen exposure (Fig. 4). Accordingly, transcription of the putative transcriptional repressor NrdR-encoding gene BBMN68_197 decreased rapidly in BBMN68 upon exposure to oxygen (Fig. 4); putative NrdR-binding sites, as determined by Rodionov and Gelfand55, were located in the promoter region of BBMN68_1785 and BBMN68_1397, respectively (data not shown). Upregulation of RNRs supported deoxynucleoside diphosphate/deoxynucleoside triphosphate (dNDP/dNTP) biosynthesis, which could be used for turnover and scavenging of oxidatively damaged DNA in BBMN68.
Figure 4.
Time-course expression of ribonucleotide reductase gene clusters in B. longum BBMN68 response to 3% (v/v) oxygen stress detected by RT-qPCR. (A) Putative RNR regulator gene nrdR (BBMN68_197). (B) Class III RNRs nrdDG (BBMN68_1785/1786). (C) Class Ib RNRs nrdHIEF (BBMN68_1397/1398/1399/1401). Relative expression ratio was calculated as the ratio between signals observed in oxygen-treated samples (1 min, 5 min, 10 min, 20 min, 30 min, 60 min) and oxygen-untreated sample (Control). The mean values from three independent determinations ± SD are shown. Asterisks indicate a statistically significant difference (*P < 0.05).
Downregulation of pyrimidine-biosynthesis genes, including members of the pyr gene cluster (pyrB/I/C/F2–ubiB–pyrD1/E; BBMN68_534–528), was transiently observed in response to oxygen stress in BBMN68 (Table S2). Downregulation of pyrimidine biosynthesis is a common response to different stress conditions in bifidobacteria52,56. In contrast, genes involved in purine metabolism were upregulated, including BBMN68_909, BBMN68_1636, and BBMN68_591 (Table S2), leading to enhanced ATP and GTP production.
Genes belonging to COG category E (amino acid transport and metabolism) were strongly and persistently induced compared to controls (Fig. 3), indicating that the processes of amino acid and protein biosynthesis, transport and metabolism are strengthened upon BBMN68 exposure to oxygen-induced oxidative stress. However, the mRNA levels of most of the ribosomal protein-encoding genes were downregulated (Table S2). Ribosomal proteins are necessary for ribosome assembly and stability. It has been suggested that ribosomal protein synthesis is controlled primarily at the translational level, and that rRNA transcription is the rate-limiting step in ribosome synthesis in model organisms such as E. coli and B. subtilis57. It is speculated that the downregulation of ribosomal protein-encoding genes will have less influence on protein synthesis in BBMN68. A similar observation has been reported in B. longum in response to low pH and heat stress43,45, and in Lactobacillus rhamnosus in response to bile salt stress56. Nevertheless, most of the tRNA-encoding genes were transiently upregulated after 30 min but downregulated or unchanged after 60 min exposure to oxygen (except tRNA–Thr-encoding BBMN68_tRNA39, which was upregulated) (Table S2). This suggested that protein synthesis is strengthened at the early stage of oxidative stress in BBMN68, but is then suppressed at the later stage. Since the target of ROS are biomacromolecules, such as nucleic acids and proteins, their protection from ROS damage is essential for cell survival under oxidative stress58. From the observation that most tRNAs were first upregulated and then downregulated, together with the strong upregulation of chaperones and proteases, we hypothesized that B. longum BBMN68 reduces the global rates of protein synthesis, along with enhanced production of chaperones and Clp proteases to promote recycling of the misfolded and aggregated proteins under oxidative stress. Such a change in expression has also been observed in Bacteroides fragilis in response to oxygen exposure59, and in B. longum in response to high temperature45.
Notably, most genes encoding the biosynthesis of the branched-chain amino acids (BCAAs) L-Ile, L-Val, and L-Leu were significantly upregulated49 (Table 2, Fig. S3), including leuABCD (BBMN68_1222/984/1521/1522), ilvC1 (BBMN68_1262), and ilvE (BBMN68_592). Upregulation of leuA expression in BBMN68 in response to oxygen-induced stress had also been confirmed at the translational level16. Correspondingly, genes involved in BCAA transport—livKHMGF (BBMN68_1747−1751)—were also induced after both 30 and 60 min of oxygen exposure (Table 2). Transcription of BCAA synthesis-related genes has been found to be induced in bifidobacteria under conditions of low-pH and bile-salt stress42,43,51. Deamination of BCAAs has been postulated as a mechanism for maintaining internal cell pH43, but it has not been characterized in bifidobacteria’s oxidative stress response. The upregulation of BCAA biosynthesis might provide ATP for energy metabolism and hydrophobic amino acids for protein synthesis in BBMN68 in response to oxidative stress.
Expression of genes encoding Fe–S cluster-assembly proteins, including sufB (BBMN68_612), sufD (BBMN68_611), csdB (BBMN68_609, also known as sufS), was upregulated in BBMN68 exposed to oxygen for 60 min (Table 2). CsdB, an IscS/Nifs homolog, plays a main role in the assembly of Fe–S clusters by mobilizing the S atom of L-Cys through cysteine desulfurase activity60. The SufBCD complex acts as a scaffold which donates Fe–S clusters to SufA under oxidative stress and during iron starvation in E. coli61. However, gene BBMN68_269 encoding another Fe–S cluster assembly-related protein NifS, was not significantly induced, suggesting that only the suf system is induced by oxidative stress in BBMN68. Thus, to restore the necessary biochemical metabolism in response to oxidative stress, BBMN68 shows adaptable strengthening of Fe–S cluster-containing protein biosynthesis.
Oxidative stress accelerates folate biosynthesis in BBMN68
Genes involved in tetrahydrofolate (H4-folate) biosynthesis were upregulated in BBMN68 after 60 min oxygen exposure49 (Table 2, Fig. S4). H4-folate serves as a donor of 1-C units involved in the biosynthesis of purines, thymidine, glycine, methionine and pantothenate. In bacteria, H4-folate is also required for the synthesis of formylmethionyl tRNAfMet, which is essential for the initiation of protein synthesis62,63. The induction of folate biosynthesis may contribute to repairing the DNA and protein damage caused by oxidative stress in BBMN68. In addition, the aforementioned increase in GTP production from purine metabolism supports precursors for H4-folate synthesis.
BBMN68 alters cell-surface properties in response to oxidative stress
Oxygen exposure causes changes in fatty acids in the bifidobacteria cells and an extension of the lag phase of growth; the cells become elongated and develop a rough surface due to abnormal or incomplete cell division64. In this study, autoaggregation and hydrophobicity properties of BBMN68 cells exposed to oxygen were increased compared to untreated cells (Fig. 5), suggesting that BBMN68 cell-surface components were modified in response to oxidative stress. Remarkably, BBMN68_1705 encoding cyclopropane-fatty-acyl-phospholipid synthase, which catalyzes cyclopropane fatty acid biosynthesis, showed 2.71- and 4.81-fold upregulation in BBMN68 upon exposure to oxygen after 30 min and 60 min, respectively (Table 2). Cyclopropane fatty acid plays a role in the defense against environmental stresses via modification of the viscosity and permeability of cell membranes in lactic acid bacteria and bifidobacteria51,52,65–67. Increased cyclopropane fatty acid composition in cell membranes leads to a more hydrophobic cell surface, and the surface hydrophobicity of BBMN68 cells increased 70% to 100% upon exposure to oxygen (Fig. 5A). In addition, three adjacent operons (BBMN68_1487–BBMN68_1490, BBMN68_1493–BBMN68_1491, and BBMN68_1494–BBMN68_1496) encoding proteins involved in polysaccharide biosynthesis and transport were repressed in BBMN68 upon exposure to oxygen (Table 2). In particular, the transcription of two genes encoding rhamnosyltransferase was downregulated—BBMN68_1492 and BBMN68_1493 (Table 2). This revealed that BBMN68 reduces polysaccharide synthesis in response to oxidative stress, which might also contribute to improved hydrophobicity and autoaggregation of BBMN68 cells upon exposure to oxygen, because polysaccharides are likely to hinder cell aggregation and adhesion68,69. This, in turn, might reduce penetration of the surrounding dissolved oxygen into the cells70,71, thereby reducing the damage caused by the oxidative stress.
Figure 5.
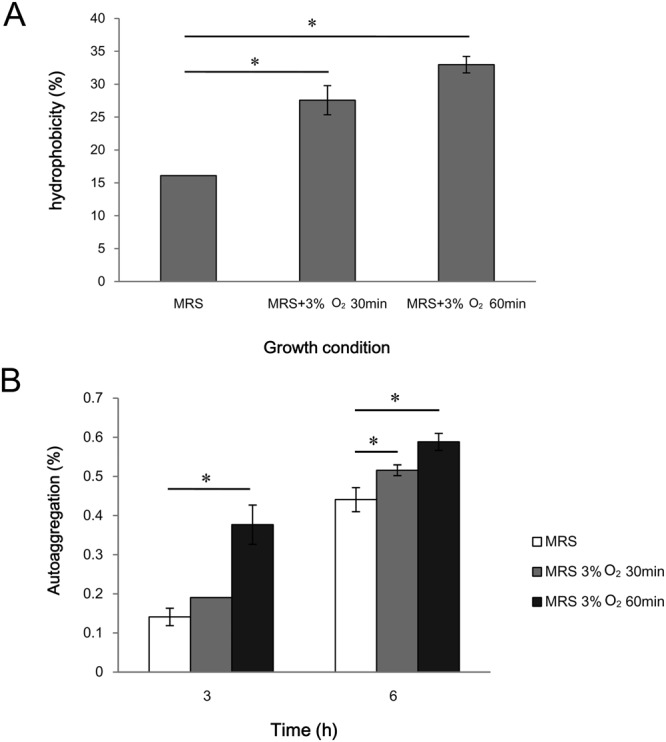
Hydrophobicity (A) and autoaggregation (B) properties of B. longum BBMN68 under different growth conditions. The mean values from three independent determinations ± SD are shown. Asterisks indicate a statistically significant difference (*P < 0.05).
Conclusion
In this study, we used RNA-Seq transcriptome profiling to investigate the mechanism governing the response to oxygen in the potentially probiotic B. longum strain BBMN68. Analysis of the pathways associated with the genes showing altered expression suggested that B. longum BBMN68 employs a complex global mechanism to cope with oxidative stress. First, the thioredoxin–thioredoxin reductase system, with thioredoxin-dependent pathways such as AhpC, provide a primary defense against ROS generated by aerobic metabolism. Moreover, several physiological processes were modulated for adaptation to the oxidative stress. To effectively cope with oxidative stress, B. longum BBMN68 enhanced BCAA, Fe–S, dNDP/dNTP and H4-folate production, toward protein and nucleotide biosynthesis and repair. In addition, B. longum BBMN68 increased cyclopropane-fatty-acyl-phospholipid synthase biosynthesis, while reduced cell-wall components and polysaccharide synthesis in response to oxidative stress. This could contribute to an increase in cell hydrophobicity and autoaggregation, protecting the cells from oxygen exposure. Taken together, our study provides the transcriptional landscape of B. longum BBMN68 grown under oxygen challenge and provides a wealth of clues for further detailed study.
Electronic supplementary material
Acknowledgements
This work was funded by the National Natural Science Foundation of China (no. 31071507), the National High Technology Research and Development Program (“863” Program, no. 2008AA10Z310), and the National Science and Technology Support Program, Ministry of Science and Technology of China (2011BAD09B03).
Author Contributions
S.C. designed the study; F.Z. and M.X. collected the samples; F.Z., R.Y., G.B.K., and X.S. performed the laboratory work; F.Z., B.Z., and S.C. analyzed the data; F.Z. wrote the manuscript; H.M., F.R., and S.C. reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-35286-7.
References
- 1.Ventura M, et al. Genomics of Actinobacteria: tracing the evolutionary history of an ancient phylum. Microbiol. Mol. Biol. Rev. 2007;71:495–548. doi: 10.1128/MMBR.00005-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JH, O’Sullivan DJ. Genomic insights into bifidobacteria. Microbiol. Mol. Biol. Rev. 2010;74:378–416. doi: 10.1128/MMBR.00004-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell DA, Ross RP, Fitzgerald GF, Stanton C. Metabolic activities and probiotic potential of bifidobacteria. Int. J. Food Microbiol. 2011;149:88–105. doi: 10.1016/j.ijfoodmicro.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Shah NP. Probiotic bacteria: selective enumeration and survival in dairy foods. J. Dairy Sci. 2000;83:894–907. doi: 10.3168/jds.S0022-0302(00)74953-8. [DOI] [PubMed] [Google Scholar]
- 5.Boylston TD, Vinderola CG, Ghoddusi HB, Reinheimer JA. Incorporation of bifidobacteria into cheeses: challenges and rewards. Int. Dairy J. 2004;14:375–387. doi: 10.1016/j.idairyj.2003.08.008. [DOI] [Google Scholar]
- 6.Talwalkar A, Kailasapathy K. Metabolic and biochemical responses of probiotic bacteria to oxygen. J. Dairy Sci. 2003;86:2537–2546. doi: 10.3168/jds.S0022-0302(03)73848-X. [DOI] [PubMed] [Google Scholar]
- 7.Simpson PJ, Stanton C, Fitzgerald GF, Ross RP. Intrinsic tolerance of Bifidobacterium species to heat and oxygen and survival following spray drying and storage. J. Appl. Microbiol. 2005;99:493–501. doi: 10.1111/j.1365-2672.2005.02648.x. [DOI] [PubMed] [Google Scholar]
- 8.Imlay JA. Cellular defenses against superoxide and hydrogen peroxide. Annu. Rev. Biochem. 2008;77:755–776. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brioukhanov AL, Netrusov AI. Catalase and superoxide dismutase: distribution, properties, and physiological role in cells of strict anaerobes. Biochemistry-Moscow. 2004;69:949–962. doi: 10.1023/B:BIRY.0000043537.04115.d9. [DOI] [PubMed] [Google Scholar]
- 10.Brioukhanov AL, Netrusov A. Aerotolerance of strictly anaerobic microorganisms and factors of defense against oxidative stress: A review. Appl. Biochem. Microbiol. 2007;43:567–582. doi: 10.1134/S0003683807060014. [DOI] [PubMed] [Google Scholar]
- 11.O’Callaghan A, van Sinderen D. Bifidobacteria and their role as members of the human gut microbiota. Front. Microbiol. 2016;7:925. doi: 10.3389/fmicb.2016.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashi K, et al. Purification and characterization of oxygen-inducible haem catalase from oxygen-tolerant Bifidobacterium asteroides. Microbiology. 2013;159:89–95. doi: 10.1099/mic.0.059741-0. [DOI] [PubMed] [Google Scholar]
- 13.Zuo FL, et al. Homologous overexpression of alkyl hydroperoxide reductase subunit C (ahpC) protects Bifidobacterium longum strain NCC2705 from oxidative stress. Res. Microbiol. 2014;165:581–589. doi: 10.1016/j.resmic.2014.05.040. [DOI] [PubMed] [Google Scholar]
- 14.Ruiz L, et al. Molecular clues to understand the aerotolerance phenotype of Bifidobacterium animalis subsp. lactis. Appl. Environ. Microbiol. 2012;78:644–650. doi: 10.1128/AEM.05455-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schell MA, et al. The genome sequence of Bifidobacterium longum reflects its adaptation to the human gastrointestinal tract. Proc. Natl. Acad. Sci. USA. 2002;99:14422–14427. doi: 10.1073/pnas.212527599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao M, et al. Oxidative stress-related responses of Bifidobacterium longum subsp. longum BBMN68 at the proteomic level after exposure to oxygen. Microbiology. 2011;157:1573–1588. doi: 10.1099/mic.0.044297-0. [DOI] [PubMed] [Google Scholar]
- 17.Oberg TS, Ward RE, Steele JL, Broadbent JR. Genetic and physiological responses of Bifidobacterium animalis subsp. lactis to hydrogen peroxide stress. J. Bacteriol. 2013;195:3743–3751. doi: 10.1128/JB.00279-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oberg TS, Ward RE, Steele JL, Broadbent JR. Transcriptome analysis of Bifidobacterium longum strains that show a differential response to hydrogen peroxide stress. J. Biotechnol. 2015;212:58–64. doi: 10.1016/j.jbiotec.2015.06.405. [DOI] [PubMed] [Google Scholar]
- 19.Zomer A, van Sinderen D. Intertwinement of stress response regulons in Bifidobacterium breve UCC2003. Gut Microbes. 2010;1:100–102. doi: 10.4161/gmic.1.2.11477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zomer A, et al. An interactive regulatory network controls stress response in Bifidobacterium breve UCC2003. J. Bacteriol. 2010;191:7039–7049. doi: 10.1128/JB.00897-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erill I, Campoy S, Barbé J. Aeons of distress: an evolutionary perspective on the bacterial SOS response. FEMS Microbiol. Rev. 2007;31:637–656. doi: 10.1111/j.1574-6976.2007.00082.x. [DOI] [PubMed] [Google Scholar]
- 22.Berger B, Moine D, Mansourian R, Arigoni F. HspR mutations are naturally selected in Bifidobacterium longum when successive heat shock treatments are applied. J. Bacteriol. 2010;192:256–263. doi: 10.1128/JB.01147-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimamura S, et al. Relationship between oxygen sensitivity and oxygen metabolism of Bifidobacterium species. J. Dairy Sci. 1992;75:3296–3306. doi: 10.3168/jds.S0022-0302(92)78105-3. [DOI] [PubMed] [Google Scholar]
- 24.Yang HY, et al. Oral administration of live Bifidobacterium substrains isolated from centenarians enhances intestinal function in mice. Curr. Microbiol. 2009;59:439–445. doi: 10.1007/s00284-009-9457-0. [DOI] [PubMed] [Google Scholar]
- 25.Yang HY, et al. Oral administration of live Bifidobacterium substrains isolated from healthy centenarians enhanced immune function in BALB/c mice. Nutr. Res. 2009;29:281–289. doi: 10.1016/j.nutres.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Yang J, et al. Bifidobacterium longum BBMN68-specific modulated dendritic cells alleviate allergic responses to bovine β-lactoglobulin in mice. J. Appl. Microbiol. 2015;119:1127–1137. doi: 10.1111/jam.12923. [DOI] [PubMed] [Google Scholar]
- 27.Hao YL, et al. Complete Genome Sequence of Bifidobacterium longum subsp. longum BBMN68, a New Strain from a Healthy Chinese Centenarian. J. Bacteriol. 2011;193:787–788. doi: 10.1128/JB.01213-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 29.Del Re B, Dgorbati B, Miglioli M, Palenzona D. Adhesion, autoaggregation and hydrophobicity of 13 strains of Bifidobacterium longum. Lett. Appl. Microbiol. 2000;31:438–442. doi: 10.1046/j.1365-2672.2000.00845.x. [DOI] [PubMed] [Google Scholar]
- 30.Pablo FP, Yessica M, Edgardo AD, Graciela LDA. Surface properties of bifidobacterial strains of human origin. Appl. Environ. Microbiol. 1998;64:21–26. doi: 10.1128/aem.64.1.21-26.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan WH, Li PL, Liu ZY. The correlation between surface hydrophobicity and adherence of Bifidobacterium strains from centenarians’ faeces. Anaerobe. 2006;12:148–152. doi: 10.1016/j.anaerobe.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Tatusov RL, Koonin EV, Lipman DJ. A genomic perspective on protein families. Science. 1997;278:631–637. doi: 10.1126/science.278.5338.631. [DOI] [PubMed] [Google Scholar]
- 33.Carmel-Harel O, Storz G. Roles of the glutathione-and thioredoxin-dependent reduction systems in the Escherichia coli and Saccharomyces cerevisiae responses to oxidative stress. Ann. Rev. Microbiol. 2000;54:439–461. doi: 10.1146/annurev.micro.54.1.439. [DOI] [PubMed] [Google Scholar]
- 34.Jordan A, Åslund F, Pontis E, Reichard P, Holmgren A. Characterization of Escherichia coli NrdH: a glutaredoxin-like protein with a thioredoxin-like activity profile. J. Biol. Chem. 1997;272:18044–18050. doi: 10.1074/jbc.272.29.18044. [DOI] [PubMed] [Google Scholar]
- 35.Zeller T, Klug G. Thioredoxins in bacteria: functions in oxidative stress response and regulation of thioredoxin genes. Naturwissenschaften. 2006;93:259–266. doi: 10.1007/s00114-006-0106-1. [DOI] [PubMed] [Google Scholar]
- 36.Zuo FL, et al. Combination of heterogeneous catalase and superoxide dismutase protects Bifidobacterium longum strain NCC2705 from oxidative stress. Appl. Microbiol. Biotechnol. 2014;98:7523–7534. doi: 10.1007/s00253-014-5851-z. [DOI] [PubMed] [Google Scholar]
- 37.Cortial S. NADH oxidase activity of Bacillus subtilis nitroreductase NfrA1: insight into its biological role. FEBS Lett. 2010;584:3916–3922. doi: 10.1016/j.febslet.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 38.Mérmod M, et al. Structure and function of CinD (YtjD) of Lactococcus lactis, a copper-induced nitroreductase involved in defense against oxidative stress. J. Bacteriol. 2010;192:4172–4180. doi: 10.1128/JB.00372-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Streker K, Freiberg C, Labischinski H, Hacker J, Ohlsen K. Staphylococcus aureus NfrA (SA0367) is a flavin mononucleotide-dependent NADPH oxidase involved in oxidative stress response. J. Bacteriol. 2005;187:2249–2256. doi: 10.1128/JB.187.7.2249-2256.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horsburgh MJ, Wharton SJ, Karavolos M, Foster SJ. Manganese: elemental defence for a life with oxygen. Trends Microbiol. 2002;10:496–501. doi: 10.1016/S0966-842X(02)02462-9. [DOI] [PubMed] [Google Scholar]
- 41.Klijn A, Mercenier A, Arigoni F. Lessons from the genomes of bifidobacteria. FEMS Microbiol. Rev. 2005;29:491–509. doi: 10.1016/j.fmrre.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 42.Sánchez B, et al. Proteomic analysis of global changes in protein expression during bile salt exposure of Bifidobacterium longum NCIMB 8809. J. Bacteriol. 2005;187:5799–5808. doi: 10.1128/JB.187.16.5799-5808.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sánchez B, et al. Low-pH adaptation and the acid tolerance response of Bifidobacterium longum biotype longum. Appl. Environ. Microbiol. 2007;73:6450–6459. doi: 10.1128/AEM.00886-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Savijoki K, et al. Effect of heat-shock and bile salts on protein synthesis of Bifidobacterium longum revealed by [35S]methionine labelling and two dimensional gel electrophoresis. FEMS Microbiol. Lett. 2005;248:207–215. doi: 10.1016/j.femsle.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 45.Rezzonico E, et al. Global transcriptome analysis of the heat shock response of Bifidobacterium longum. FEMS Microbiol. Lett. 2007;271:136–145. doi: 10.1111/j.1574-6968.2007.00704.x. [DOI] [PubMed] [Google Scholar]
- 46.Zolkiewski M. ClpB cooperates with DnaK, DnaJ, and GrpE in suppressing protein aggregation: A novel multi-chaperone system from Escherichia coli. J. Biol. Chem. 1999;274:28083–28086. doi: 10.1074/jbc.274.40.28083. [DOI] [PubMed] [Google Scholar]
- 47.Lund PA. Microbial molecular chaperones. Adv. Microb. Physiol. 2001;44:93–140. doi: 10.1016/S0065-2911(01)44012-4. [DOI] [PubMed] [Google Scholar]
- 48.Stohl EA, Criss AK, Seifert HS. The transcriptome response of Neisseria gonorrhoeae to hydrogen peroxide reveals genes with previously uncharacterized roles in oxidative damage protection. Mol. Microbiol. 2005;58:520–532. doi: 10.1111/j.1365-2958.2005.04839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanehisa M, Goto S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parche S, et al. Sugar transport systems of Bifidobacterium longum NCC2705. J. Mol. Microbiol. Biotechnol. 2007;12:9–19. doi: 10.1159/000096455. [DOI] [PubMed] [Google Scholar]
- 51.Jin JH, et al. Mechanism analysis of acid tolerance response of Bifidobacterium longum subsp. longum BBMN68 by gene expression profile using RNA-Sequencing. PLOS ONE. 2012;7:e50777. doi: 10.1371/journal.pone.0050777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.An HR, et al. Integrated transcriptomic and proteomic analysis of the bile stress response in a centenarian-originated probiotic Bifidobacterium longum BBMN68. Mol. Cell Proteomics. 2014;13:2558–2572. doi: 10.1074/mcp.M114.039156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Torrents E, et al. NrdR controls differential expression of the Escherichia coli ribonucleotide reductase genes. J. Bacteriol. 2007;189:5012–5021. doi: 10.1128/JB.00440-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Monje-Casas F, Jurado J, Prieto-Alamo MJ, Holmgren A, Pueyo C. Expression analysis of the nrdHIEF operon from Escherichia coli. Conditions that trigger the transcript level in vivo. J. Biol. Chem. 2001;276:18031–18037. doi: 10.1074/jbc.M011728200. [DOI] [PubMed] [Google Scholar]
- 55.Rodionov DA, Gelfand MS. Identification of a bacterial regulatory system for ribonucleotide reductases by phylogenetic profiling. Trends Genet. 2005;21:385–389. doi: 10.1016/j.tig.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 56.Koskenniemi K, et al. Proteomics and transcriptomics characterization of bile stress responsein probiotic Lactobacillus rhamnosus GG. Mol. Cell. Proteomics. 2011;10:M110.002741. doi: 10.1074/mcp.M110.002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paul BJ, Ross W, Gaal T, Gourse RL. rRNA transcription in Escherichia coli. Annu. Rev. Genet. 2004;38:749–770. doi: 10.1146/annurev.genet.38.072902.091347. [DOI] [PubMed] [Google Scholar]
- 58.Imlay JA. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat. Rev. Microbiol. 2013;11:443–454. doi: 10.1038/nrmicro3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sund CJ, et al. The Bacteroides fragilis transcriptome response to oxygen and H2O2: the role of OxyR and its effect on survival and virulence. Mol. Microbiol. 2008;67:129–142. doi: 10.1111/j.1365-2958.2007.06031.x. [DOI] [PubMed] [Google Scholar]
- 60.Kurihara T, Mihara H, Kato S, Yoshimura T, Esaki N. Assembly of iron-sulfur clusters mediated by cysteine desulfurases, IscS, CsdB and CSD, from Escherichia coli. BBA-Proteins Proteom. 2003;1647:303–309. doi: 10.1016/S1570-9639(03)00078-5. [DOI] [PubMed] [Google Scholar]
- 61.Py B, Moreau PL, Barras F. Fe-S clusters, fragile sentinels of the cell. Curr. Opin. Microbiol. 2011;14:218–223. doi: 10.1016/j.mib.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 62.Bermingham A. & Derrick, J. P. The folic acid biosynthesis pathway in bacteria: evaluation of potential for antibacterial drug discovery. BioEssays. 2002;24:637–648. doi: 10.1002/bies.10114. [DOI] [PubMed] [Google Scholar]
- 63.Levin I, Giladi M, Altman-Price N, Ortenberg R, Mevarech M. An alternative pathway for reduced folate biosynthesis in bacteria and halophilic archaea. Mol. Microbiol. 2004;54:1307–1318. doi: 10.1111/j.1365-2958.2004.04339.x. [DOI] [PubMed] [Google Scholar]
- 64.Ahn JB, Hwang HJ, Park JH. Physiological responses of oxygen-tolerant anaerobic Bifidobacterium longum under oxygen. J. Microbiol. Biotechnol. 2001;11:443–451. [Google Scholar]
- 65.Guillot A, Obis D, Mistou MY. Fatty acid membrane composition and activation of glycine-betaine transport in Lactococcus lactis subjected to osmotic stress. Int. J. Food Microbiol. 2000;55:47–51. doi: 10.1016/S0168-1605(00)00193-8. [DOI] [PubMed] [Google Scholar]
- 66.Grandvalet C, et al. Changes in membrane lipid composition in ethanol- and acid-adapted Oenococcus oeni cells: characterization of the cfa gene by heterologous complementation. Microbiology. 2008;154:2611–2619. doi: 10.1099/mic.0.2007/016238-0. [DOI] [PubMed] [Google Scholar]
- 67.Montanari C, Sado Kamdem SL, Serrazanetti DI, Etoa FX, Guerzoni ME. Synthesis of cyclopropane fatty acids in Lactobacillus helveticus and Lactobacillus sanfranciscensis and their cellular fatty acids changes following short term acid and cold stresses. Food Microbiol. 2010;27:493–502. doi: 10.1016/j.fm.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 68.Aires J, et al. Proteomic comparison of the cytosolic proteins of three Bifidobacterium longum human isolates and B. longum NCC2705. BMC Microbiol. 2010;10:29. doi: 10.1186/1471-2180-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Polak-Brecka M, Waśko A, Paduch R, Skrzypek T, Sroka-Bartnicka A. The effect of cell surface components on adhesion ability of Lactobacillus rhamnosus. Antonie van Leeuwenhoek. 2014;106:751–762. doi: 10.1007/s10482-014-0245-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sigalevich P, Meshorer E, Helman Y, Cohen Y. Transition from anaerobic to aerobic growth conditions for the sulfate-reducing bacterium Desulfovibrio oxyclinae results in flocculation. Appl. Environ. Microbiol. 2000;66:5005–5012. doi: 10.1128/AEM.66.11.5005-5012.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McLean JS, et al. Oxygen-dependent autoaggregation in Shewanella oneidensis MR-1. Environ. Microbiol. 2008;10:1861–1876. doi: 10.1111/j.1462-2920.2008.01608.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.