Abstract
Objective
Precision medicine allows healthcare interventions to be tailored to groups of patients based on their disease susceptibility, diagnostic or prognostic information, or treatment response. We analysed what developments are expected in precision medicine over the next decade and considered the implications for health technology assessment (HTA) agencies.
Methods
We performed a pragmatic literature search to account for the large size and wide scope of the precision medicine literature. We refined and enriched these results with a series of expert interviews up to 1 h in length, including representatives from HTA agencies, research councils and researchers designed to cover a wide spectrum of precision medicine applications and research.
Results
We identified 31 relevant papers and interviewed 13 experts. We found that three types of precision medicine are expected to emerge in clinical practice: complex algorithms, digital health applications and ‘omics’-based tests. These are expected to impact upon each stage of the HTA process, from scoping and modelling through to decision-making and review. The complex and uncertain treatment pathways associated with patient stratification and fast-paced technological innovation are central to these effects.
Discussion
Innovation in precision medicine promises substantial benefits but will change the way in which some health services are delivered and evaluated. The shelf life of guidance may decrease, structural uncertainty may increase and new equity considerations will emerge. As biomarker discovery accelerates and artificial intelligence-based technologies emerge, refinements to the methods and processes of evidence assessments will help to adapt and maintain the objective of investing in healthcare that is value for money.
Electronic supplementary material
The online version of this article (10.1007/s40273-018-0686-6) contains supplementary material, which is available to authorized users.
Key Points for Decision Makers
| Three types of precision medicine technologies are likely to become more widespread in clinical practice over the next decade: ‘omics’-based biomarkers; complex artificial intelligence-based algorithms; and digital health applications |
| These innovations will require health technology assessment and guideline-producing agencies to adapt their methods and processes |
| The fast pace of discovery technological innovation, along with the potentially complex and uncertain treatment pathways patients will be presented with, are at the centre of the new challenges |
Introduction
Recent technological developments have allowed healthcare to increasingly be tailored toward specific patients and subgroups—a medical model referred to as precision medicine [1]. Broadly, this process involves tailoring aspects of the patient pathway (i.e. advice, referral or treatment) based on their disease risk, prognosis or likely treatment response—a process that can yield additional benefits to patients and the wider healthcare system. The ‘precision’ is informed by tools that incorporate genetic, environmental and lifestyle information, and ranges from risk equations [2] to genetic testing [3].
Technological progress in precision medicine is expected to continue, spearheaded by programmes such as the Precision Medicine Initiative [4] and the 100,000 Genomes Project [5]. This innovation will likely change the way that healthcare services are organised and delivered: the creation of new molecular testing infrastructure and the development of ‘learning’ health information systems that analyse molecular and health record data to inform future prevention, detection or treatment strategies are two cited possibilities [6]. This will have consequences for the generation of clinical and economic evidence, meaning that healthcare decision makers, including health technology assessment (HTA) agencies and guideline producers, should consider how their methods and processes will accommodate these new technologies and services.
HTA agencies’ experience of precision medicine has primarily been with diagnostic and companion diagnostic tests, the latter referring to those that identify biomarkers correlated with treatment response such as the HER2 receptor protein for breast cancer pharmacotherapies [3]. Several countries have accommodated the additional complexities of evaluating these tests through new procedures, such as the Diagnostic Assessment Programme at the National Institute for Health and Care Excellence (NICE) in England or the Health Technology Assessment Access Point in Australia [7, 8]. However, additional procedures may be required for other emerging precision medicine technologies.
The objective of this study is to describe the possible landscape of precision medicine over the next decade, alongside the potential implications for HTA. Our analysis is the first to draw together the significant but disparate body of literature on the economics of precision medicine, present the potential issues arising at each stage of the decision-making process, and anticipate future challenges by consulting with experts in a range of relevant fields.
Methods
Our approach consisted of two components: a review of literature on the methodological and empirical challenges of precision medicine with respect to economic evaluation for HTA and a series of interviews with experts in fields related to precision medicine and/or healthcare decision-making. From these we determined the types of precision medicine technologies and services that are expected to emerge in the next decade and the challenges that these and existing technologies create for HTA.
Literature Review
We conducted searches to identify literature focusing on methodological considerations relating to guideline development, decision-making and economic evaluation of precision medicine technologies, medicines and healthcare.
We searched MEDLINE and MEDLINE In-Process, prioritising retrieving relevant records at the expense of sensitivity. This pragmatic approach was taken because of the large size and wide scope of precision medicine literature. The search strategy was not intended to be exhaustive and instead aimed to retrieve those studies most likely to be relevant to the research question, while maintaining manageable numbers of records. A number of pragmatic decisions were made to limit record volume, including using only highly relevant search terms, restricting search terms to the title field, searching for English-language publications only and excluding publication types unlikely to yield study reports (e.g. news items). Rather than use pre-determined cut-off dates for inclusion, a flexible approach was adopted to provide an additional lever to limit record volume. This was anticipated to be from between 2007 and 2012 up to the date the search was conducted (May 2017). We used supplementary search techniques to identify grey literature and unpublished research, with further articles identified through citation searches of included studies and author searches on a preliminary list of 11 expert interviewees. The full search strategy is described in Electronic Supplementary Material Appendix A.
Articles were included if they (i) presented or assessed of methodological challenges relevant to economic evaluations of precision medicine; or (ii) discussed the implications of new or emerging precision medicine technologies. The number of topics considered relevant to economic evaluation was broad, and included guideline development, trial design, comparative effectiveness and health equity.
Data were extracted from included papers by two reviewers (KE and AP). Consistency of approach was tested by comparing results from an initial single paper and discussing discrepancies. Findings were tentatively organised into 11 pre-identified topic areas (provided in ESM Appendix B), with new topics added where appropriate.
Expert Interviews
A list of experts was compiled based on prior familiarity to the authors and preliminary literature searches. Each met one of three criteria: (i) research outputs relating to precision medicine technologies and their evaluation; (ii) experience with decision-making in HTA; and (iii) membership of institutes and organisations involving the use or evaluation of precision medicines. A total of 20 experts were contacted, all of whom were based in the UK.
Semi-structured qualitative interviews [9] lasting between 30 and 60 min were conducted in person or via telephone by JL-K and AP. A standardised document describing the key areas of inquiry was distributed to experts prior to interviews (see Electronic Supplementary Material Appendix B). Interviewees were not required to contribute to every topic and were encouraged to raise additional relevant issues.
A pattern-coding approach was taken with the qualitative data, in which the contemporaneous notes taken during each interview were organised into the initial list of 11 topics, with new topics added where appropriate. Pattern coding was undertaken by one researcher (AP) and validated by a second (JL-K), with disagreements resolved deliberatively. These data were then compiled across all interviews using Microsoft Excel® (Microsoft Corp., Redmond, WA, USA). The combined findings from the review and interviews were independently assessed and discussed by three reviewers (JL-K, KE and AP).
Findings
Literature Search and Expert Interview Results
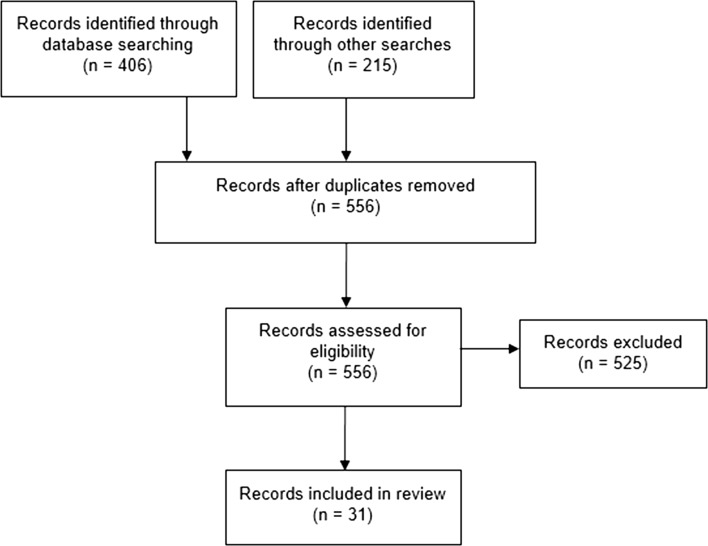
A total of 549 records were identified from the searches, with an additional seven identified by the authors (Fig. 1). In order to restrict the number of records to within our practical limit of 300, articles published before 2011 were not considered for screening. It was assumed that any methodological issues identified in earlier literature would have either been resolved or reiterated in later articles. Screening on the basis of title, abstract and, where necessary, full texts left 31 included papers.
Fig. 1.
Record flow diagram for pragmatic literature review. Note: Of the 525 records excluded for eligibility reasons, 382 were based on abstract and full-text review, with the remaining 143 removed due to being published prior to 2011
A total of 13 (65%) experts consented to be interviewed. Four represented the scientific affairs, technology appraisal, clinical guidelines and diagnostics assessment programmes at NICE, and expressed their personal views, rather than NICE policy. Other interviewees included four senior health economists, two researchers in digital health, a representative of the Medical Research Council (MRC), a specialist from the Precision Medicine Catapult institute, and professors of health informatics and primary care sciences. The interviews had an average length of approximately 50 min.
Defining Precision Medicine
A preliminary consideration for this study was to define the types of technologies and services that precision medicine encompasses. Ten papers from the review provided a definition for precision medicine, as did each of the consulted experts, resulting in a wide range of interpretations [10–12]. Most agreed that precision medicine encompasses more than just pharmacogenetic and pharmacogenomic tests, and the term is now used interchangeably with stratified medicine. It is also replacing the term personalised medicine, as it also covers technologies that offer unique treatment pathways for individual patients [10, 11].
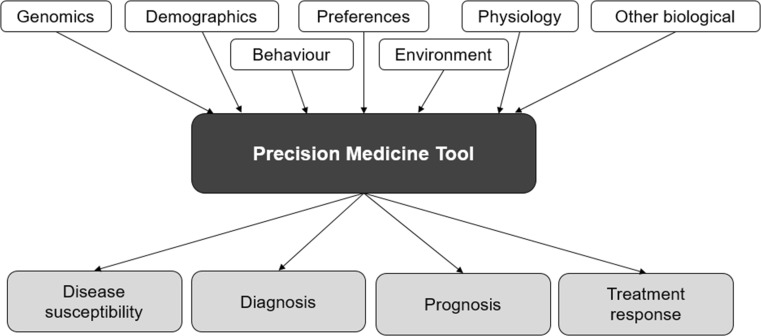
For the purposes of this study, we consider that a tool falls under the precision medicine ‘umbrella’ if it can be used to stratify patients to a specific treatment pathway or therapy, based on specific characteristics of the individual. These characteristics vary by tool but go beyond demographic or socioeconomic factors, and include genomic (or other ‘omic’) information, behavioural traits (including preferences), and environmental and physiological characteristics. Furthermore, tools will usually provide information on disease risk, diagnosis, prognosis or treatment response. This definition is summarised in Fig. 2.
Fig. 2.
Defining precision medicine
Interviewees stressed an additional and important distinction between prognostic and predictive tests. Prognostic tests indicate the likelihood that an individual patient will have a particular disease course or natural history. For example, the Decipher® (GenomeDx Biosciences Laboratory in San Diego, CA, USA) prostate cancer test, which calculates the probability of metastasis [13]. Predictive tests provide an estimate of the expected disease response to specific treatments, such as tests identifying the human epidermal growth factor receptor (HER2) gene to determine treatment allocation for patients with breast cancer. This distinction has direct implications for HTA: a senior health economist highlighted a recent instance in NICE Diagnostics Guidance in which the committee’s discussions focused on whether the technology could be considered predictive as well as prognostic, since this had an impact on the cost effectiveness of the test [14].
Technological Developments
Three major types of precision medicine technology likely to emerge over the next decade were identified: complex algorithms, digital health applications (‘health apps’) and ‘omics’-based tests. These are summarised in the following sections and, alongside existing precision medicine tools, in Table 1.
Table 1.
Types of precision medicine technologies
| Type of technology or service | Relevance to precision medicine | Estimated timescales for use |
|---|---|---|
| Tests for prognostic biomarkers Example: Decipher® tests [13]—indicate risk of disease progression after prostate cancer diagnosis |
Biomarkers indicate disease course and inform the patient treatment pathway | Genomic biomarkers are already in use. Rapid discovery of proteomic and metabolomic biomarkers is expected in the next 5 years |
| Tests for disease susceptibility biomarkers Example: Tests for BRCA1 gene—indicates risk of breast and ovarian cancer [26] |
Biomarkers indicate risk of developing a particular condition and inform the patient treatment pathway | |
| Tests for predictive biomarkers Example: HER2 protein tests—predicts response to breast cancer treatment [3] |
Biomarkers predict treatment response and inform therapy choice | An increasing number are being evaluated by HTA agencies—a review found NICE had evaluated seven by 2014 [8] Expected to expand rapidly in next 5 years |
| Diagnostic services Including genetic, genomic and molecular testing services but also other types of diagnostic support for clinicians, e.g. Computerised Decision Support [27] |
Services inform diagnoses and the patient treatment pathway | Some of these services are already in use |
| Complex algorithms Example: Sapientia [18]—combines genomic sequencing with clinical phenotyping to inform treatment decisions |
Clinical, genomic, behavioural (and more) data are utilised by these algorithms to inform diagnosis, recommendations for patient treatment pathways and therapy choices | Several are being developed and trialled—expected to be in clinical practice within the next decade Expected to be AI-based as the field progresses (e.g. AI Biocomputing [28]) |
| Digital health applications Example: MyHeart Counts [29]—records and analyses data on activity, risk factors and haematology, providing suggestions on improving heart health |
Apps draw on clinical and behavioural data and aim to influence patient behaviour, healthcare use and/or choice of treatment | Apps are already available but numbers are expected to increase dramatically in next decade |
| Risk prediction tools Example: QRISK [30]—static algorithm that determines risk for cardiovascular disease and informs statin prescribing |
Patient histories and characteristics (e.g. BMI, co-morbidities) are used to calculate disease risk, informing the patient treatment pathway | Currently available for a wide range of clinical areas |
| Patient decision aids Example: MAGIC [31]—produces dynamic decision aids that update based on published guidelines |
Instruments support patients in making decisions tailored to their preferences | Currently available for a wide range of clinical areas |
AI artificial intelligence, BMI body mass index, HTA health technology assessment, NICE National Institute for Health and Care Excellence
Complex Algorithms
The experts anticipated increased use of algorithms that use artificial intelligence (AI) to aid clinical decision-making over the next decade [15]. These algorithms require large datasets (‘knowledge bases’) that include a large number of variables, such as genetic information, sociodemographic characteristics and electronic health records. Using this information, the algorithms provide clinicians and patients with predictions on expected prognosis and optimal treatment choices using patient-level characteristics. Algorithms update regularly as new information is added to the knowledge base, an approach termed ‘evolutionary testing’. The first approaches of this type for clinical use are already being established [16–20]. AI-based technologies will also be combined with advances in imaging to develop algorithms that incorporate scan results into knowledge bases to offer more accurate information [21].
Health Apps
Health apps include a wide range of tools that provide disease management advice, receive and process patient-inputted data, and record physical activity and physiological data such as heart rate. A subset of apps will likely fall under precision medicine, with the most advanced also utilising AI-based technology as described in Sect. 3.3.1. Numbers of health apps are expected to increase significantly over the next decade. Digital health experts predicted that principal developments in this area would involve apps that analyse social or lifestyle determinants of health such as socioeconomic status or physical activity in order to stratify patients, including apps linked to activity monitoring devices (or wearable technologies). In November 2017 NICE published briefings on mobile technology health apps that were developed by the NICE medical technologies evaluation programme as a proof-of-concept activity, known as ‘Health App Briefings’. One of the first to be published concerned Sleepio, an app shown in placebo-controlled clinical trials to improve sleep through a virtual course of cognitive behavioural therapy [22].
‘Omics’-Based Biomarkers
Many current precision medicine tools use genetic and genomic information to estimate disease prognosis and predict treatment response [23]. A senior health economist predicted the use of other ‘omics’-based biomarkers, such as proteomics, metabolomics and lipidomics would become more common and partially replace genomics over the next decade.1
‘Omics’-based testing is expected to increase in complexity and scope, with single tests informing treatment pathway, therapy choice or disease risk for multiple diseases simultaneously [24]. This was described by one expert as “multi-parametric testing”. Whole-genome sequencing is at the broadest end of this scale and could feasibly provide information on risks and treatment decisions for hundreds of diseases [25].
Issues for Health Technology Assessment
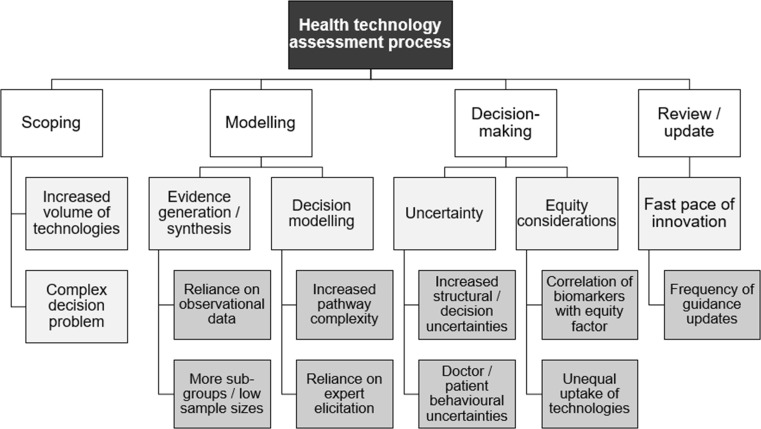
Precision medicine interventions will pose challenges at each stage of the HTA process, from scoping through to review (Fig. 3).
Fig. 3.
Challenges for health technology assessment agencies raised by precision medicine. Note: The first-tier categories (scoping to review) relate to the four principal stages of a typical health technology assessment appraisal, such as that used by National Institute for Health and Care Excellence (NICE) in England for traditional pharmaceutical technologies [32]
Scoping
The nature of the decision problem presented to HTA agencies and guideline developers will become more difficult to define when dealing with some precision medicine technologies and services. The emergence of multi-parametric tests, for instance, is expected to increase the number of relevant interventions, comparators and populations encompassed by a single assessment by providing information on multiple diseases simultaneously. The number of care pathways under consideration will also increase because tests may (i) not have a defined place in the care pathway and could potentially be used at a range of timepoints; and (ii) be used in combination with other tests [33–37]. Evaluating all of the relevant pathways, populations and comparators could be practically and computationally infeasible, and will likely necessitate increased use of expert opinion [11, 33–35, 38, 39]. One expert noted that these issues are particularly relevant for whole-genome sequencing, which can be performed at any point during an individual’s lifetime, inform care pathways for a wide range of diseases, and be analysed using many different methods [37].
The fast pace of innovation in precision medicine may also mean that assessment bodies face higher volumes of evaluations. Mixed views on how to address this emerged from expert interviews. A NICE analyst stated that scoping workshops, in which clinicians and other consultees determine which technologies should be evaluated, may be sufficient for technology appraisal. With respect to health apps, researchers agreed that new systems would need to be put in place to manage the burden of assessment. This could involve (i) a preliminary self-assessment phase; (ii) appraising classes of (rather than individual) apps; or (iii) setting priority areas using clinician input. Each present their own difficulties: classes would need to contain apps that are relatively homogenous, whilst any priority-setting process would require a clear and transparent decision-making framework.
Experts highlighted that adaptive AI-based algorithms would present a unique challenge in terms of regulation and evaluation. As more data are processed and the algorithm becomes more effective over time, evaluators would need to decide how frequently and exactly when to assess safety and clinical and cost effectiveness [40]. Interviewees also highlighted that technical validation of complex algorithms could be a challenge [41].
Modelling
Measuring Value
A number of studies stated that the value placed on knowing diagnostic test results may need to be included in economic evaluations of precision medicine [35, 39, 42–50]. This could be positive if such knowledge benefits patients and their families: directly in the case of hereditary conditions [12], or indirectly through enhanced autonomy or changes in lifestyle and screening behaviours [50]. Conversely, unintentional harms may also occur, for example due to psychological stress for patients and families.
Experts highlighted that the health-related quality of life instruments typically used in economic evaluations are unlikely to capture this value of knowing and that decision-makers may instead consider these factors through deliberation, taking into account the patient perspective, when making recommendations. Three studies [12, 35, 44] suggested that discrete choice experiments could be used to value patient preferences for increased knowledge, over and above any specific quality-adjusted life-year (QALY) gains deriving from subsequent treatment decisions. Quantifying these benefits separately (or in monetary terms) would be consistent with a welfarist framework but not the extra-welfarist one adopted by some agencies such as NICE [51]. Furthermore, incorporating these additional aspects of value on the benefits side of the cost-effectiveness equation also requires that they be incorporated when accounting for opportunity costs [52]. Incorporating non-health benefits into the evaluative framework of HTA would therefore require knowledge of (i) the extent to which society is willing to trade-off health and non-health benefits and (ii) what type of services might be displaced in order to fund a new intervention, and their associated non-health benefits.
Evidence Evaluation and Synthesis
Precision medicine presents numerous challenges for evidence evaluation. The stratification of patients to increasingly small subgroups will reduce sample sizes [10, 44, 53] and result in only certain subgroups (i.e. ones with specific biomarkers) being included in individual trials. Obtaining head-to-head estimates of comparative effectiveness for treatments and subgroups will become more difficult and will result in evidence networks being incomplete in cases where no common comparator links together the available trials. One study and several of the interviewees concluded that expert opinion will be needed more regularly to fill gaps in the evidence [12], along with suitably robust methods for eliciting these judgements [46]. Interviewees also noted that new trial designs are being developed that may be more compatible with precision medicine, including basket, umbrella and adaptive trials [54–56]. These designs, which are yet to contribute to any value dossiers submitted to HTA agencies, allow for trials to be adapted in terms of inclusion criteria and treatment response.
Nevertheless, the need to analyse multiple subgroups and more complex treatment pathways in decision models for precision medicine interventions is likely to necessitate additional sources of evidence [12] in terms of both cost and clinical data [33–36, 38, 39, 53]. An absence of relevant data recently resulted in the discontinuation of a diagnostic service delivery guideline being developed by NICE [57]. Regulatory efforts are being made to encourage the generation of clinical evidence, including the introduction of the In Vitro Diagnostic Device Regulations (IDVR) in 2017 by the European Commission [58]. However, as the new clinical evidence requirements for approval of the IDVR will not apply until 2022, evidence paucity is likely to be an issue in Europe in the medium term.
There was consensus that use of observational data for assessing precision medicine interventions will increase over the next decade [11, 33, 36, 39, 44], including registry data, cohort studies and electronic health records [16, 59, 60]. Experts noted that advanced statistical methods (and accompanying technical guidance) would be required to identify causality while controlling for the risks of selection bias and confounding in observational data.
Decision–Analytic Modelling
Multiple studies predicted that the complexity of clinical pathways in precision medicine could render traditional Markov-type model structures insufficient for capturing long-term costs and benefits [10, 12, 35, 42, 43, 61]. For example, multi-parametric testing may lead to secondary findings unrelated to the original test, as well as spill-over effects on family members and future generations [48]. A number of studies concluded that more research is needed to establish best practice guidelines for modelling precision medicines [12, 33, 43, 60], while others suggested approaches that could handle complex structures more adequately, such as microsimulation and discrete event simulations [12, 35, 62].
Decision-Making
Uncertainty
The stratification of a patient population may result in smaller sample sizes being recruited to trials for precision medicine interventions. Combined with more complex and variable treatment pathways, this could increase levels of uncertainty associated with cost-effectiveness estimates presented to decision makers.
Higher standard errors for estimates of treatment effect were raised as a concern [11, 35, 36, 42, 43, 46, 60, 61]. Several experts believed, however, that this concern is overstated. First, treatment effect variation between patients should be lower when therapies are targeted towards responders, thereby reducing standard errors. Second, any reduction in sample sizes could be compensated for in time through the use of large, linked observational datasets [16]. Value of information analysis, a technique for quantifying the value of reducing decision uncertainty, was also identified as key technique that could be beneficial to decision-making [12, 33, 44, 60, 63, 64]. Along with more typical factors such as patient population size, the key determinants of value of information in precision medicine will include the sensitivity and specificity of tests and predictions, and the intervention context (i.e. if it is used in combination with other tests).
Another source of uncertainty will be the unit costs, for example of ‘omics’-based tests, which vary by laboratory [36]. Such tests may also yield continuous results, meaning that thresholds must be set to determine the outcome of testing [11]. Thresholds will impact on the cost effectiveness of tests and, therefore, it was argued that determination of thresholds should go beyond analysis of receiver operating characteristic curves [12].
Complex clinical pathways will generate substantial uncertainties over model structure in economic evaluations of precision medicine interventions. Many experts and studies highlighted this as a critical aspect of decision modelling that would need to be addressed [11, 33, 35, 36, 39, 43]. Whilst it was agreed that the current approach of extensive sensitivity and scenario analyses should continue, interviewees expressed a desire for coherent frameworks for analysing and quantifying structural uncertainties. Approaches highlighted in the literature included multi-parameter evidence synthesis, although this approach may also be impeded by sparse data [65]. Value of information-type approaches can help to quantify the extent of this uncertainty and the value of reducing it, through techniques such as expert elicitation [66].
An additional consideration is uncertainty around the behaviour of clinicians and patients. Decisions made by these individuals, for example whether to follow the treatment pathway indicated by the result of a diagnostic test, could influence how clinically effective the intervention is and, thus, impact cost effectiveness [12, 33, 34, 38, 39, 43, 44]. In terms of clinician behaviour, low compliance to genotype-specific dosing recommendations has been observed [33]. Steep learning curves for some stratification tools have also been suggested as a cause of variability [67]. On the patient side, adherence remains an important yet under-researched determinant of effectiveness [36]. The development and application of evidence-based computerised decision support and patient decision aids could be a way to tackle these challenges.
Equity and Equality
When generating guidance that recommends different courses of treatment for different groups of patients, HTA agencies and other public bodies should aim to ensure that principles of non-discrimination and equality of opportunity are advanced [68, 69]. The main challenge lies in the specific instances where there are small numbers of patients in rare biomarker-stratified groups, for whom there is greater uncertainty around treatment effects [70]. An equality issue arises when the biomarkers used for stratification are correlated with factors such as ethnicity [36, 53]. In the NICE appraisal of sofosbuvir for treating chronic hepatitis C [71], low levels of evidence were available for some genotypes that were more common in minority ethnic patients. In this instance, a ‘pragmatic’ approach was explicitly taken on the grounds of equity, high unmet need and the lack of treatment options; evidence was extrapolated from genotypes where the treatment’s effectiveness was well-supported and the therapy was recommended for the rarer genotypes.
Stratifying patients to different treatment pathways based on measures of physiological dysregulation (such as blood pressure or cortisol level) may also introduce equity concerns. A significant, negative association between these measures and socioeconomic status has been established in the literature [72]; differential treatment recommendations may therefore result in individuals from low socioeconomic groups having a lower probability of receiving the most effective treatments. Concerns were also raised with respect to the differential uptake of some precision medicine interventions that require patient engagement. This is particularly true in digital health, where experts reported that use of health apps was much more common in younger age groups and those with higher social and educational status. If traditional (i.e. general practitioner-delivered) services were to be withdrawn in favour of digital-only access, the benefits of precision medicine may be unevenly distributed.
Review/Update
Experts working for HTA agencies noted that the rate of discovery of biomarkers means that the specificity and sensitivity of companion diagnostic tests is expected to steadily improve. Similarly, health apps and AI-based algorithms are regularly updated and upgraded, meaning that certain treatment pathways might become more cost effective over time. Although beneficial, this could reduce the ‘shelf life’ of guidance issued by HTA agencies and necessitate more frequent reviews and updates [35]. NICE have already begun addressing this issue with innovations to fast-track some evaluations [73] and increase the capacity of the technology appraisals programme [74]. Similar combined approaches to streamlining processes and increasing capacity will help the HTA community keep guidance up-to-date and useful while keeping the overall cost of HTA manageable.
Discussion
This study aimed to take a forward-looking view of precision medicine, considering what challenges are likely to be faced by HTA and guideline producing agencies as precision medicine technologies and services become more prevalent (see Electronic Supplementary Material Appendix C for a brief demonstrative case study).
We identified three key areas of precision medicine that are expected to expand in the next decade: complex algorithms, health apps and ‘omics’-based tests. The potential benefits to patients from these technologies are substantial, particularly as the costs of ‘omics’ testing are likely to decrease and manufacturers will be able to develop targeted therapies with greater efficiency. Complex algorithms and health apps will utilise AI and large, linked datasets to adapt all aspects of healthcare to patient subgroups and individuals in order to improve health outcomes. Additional technologies that were not discussed by experts or in the literature, such as the genome editing technique clustered regularly interspaced short palindromic repeats (CRISPR) [75], are also likely to fall under the umbrella of precision medicine as their application in healthcare is developed.
These new technologies will inevitably present challenges to decision makers. Researchers and clinicians should remain aware that it will not always be beneficial or ethical to use biomarker information to inform treatment decisions. Examples are already emerging of instances where a seemingly informative biomarker has not added predictive power to risk equations [76].
Early consideration of the evidence required by decision makers can improve evidence collection and analysis for precision medicine technologies and services in very early stages of development [77]. Innovative approaches for evidence generation to facilitate this are currently being developed: new trial designs [55] and robust statistical methods for analysing observational data [78] will help fill evidence gaps and improve trial recruitment numbers. Additionally, increasing use of health apps can improve the quality, frequency and accuracy of data collection.
Clear and transparent processes and principles will also be necessary to ensure equitable decision-making, particularly in cases where biomarkers are correlated with factors such as ethnicity and sociodemographic status. As with interventions such as vaccination and cancer screening [79, 80], unequal uptake of some precision medicines is also an area of concern that policy makers may want to consider and design strategies to counteract, such as targeting programmes.
Furthermore, it is not yet clear if any European agencies will be responsible for evaluating the safety and clinical and cost effectiveness of health apps and AI-based technologies. Addressing this regulatory and assessment vacuum is necessary to promote the uptake of safe and effective products. Evaluating these types of tools not only requires a new technical expertise within HTA agencies, but perhaps even a different system altogether given (i) the pace of innovation and (ii) the regularity with which apps and algorithms are updated, which can alter their effectiveness and cost effectiveness. Historical examples of assessments of these technologies by HTA agencies are sparse; developing a better understanding of the most appropriate approach for robustly evaluating AI-based technology should therefore be a valuable area of further research.
Resolving some of the issues presented in this paper, such as scoping increasingly complex treatment pathways, may require a thorough and balanced evaluation of the strengths and potential shortcomings of normative choices within an HTA framework. Any departure from current established frameworks will require considerable deliberation and co-operation between a wide range of stakeholders from across the health system. An appropriate solution will be dependent upon on the (i) decision-making context within which the HTA agency exists; (ii) stated objectives of the health system as a whole; (iii) practicality of the assessment; and (iv) relevance of the framework to the technology type [81].
A number of European organisations, such as International Consortium for Personalised Medicine (ICPerMed), European Network for Health Technology Assessment (EUnetHTA) and Horizon 2020, have identified the health economic evaluation of precision medicines as an important area of research [82–84]. The conclusions of these initiatives, which are at this point undetermined, can help to seek to address some of the other methodological issues we have highlighted, such as evidence generation and synthesis.
Strengths and Limitations
Whilst other studies have analysed the potential consequences of precision medicine on HTA processes [38, 60], their focus has been restricted to diagnostic and companion diagnostic tests. The more expansive definition of precision medicine adopted in this review, which includes technologies substantively different to diagnostics, therefore highlights a number of novel issues on the horizon for HTA agencies that will be realised in an evolving regulatory landscape.
However, our findings are limited by several factors. First, our qualitative interviews were conducted with UK-based experts only. Although the wider scope of the literature review also helps to relax the UK-centricity of the findings, future research should look at implications in other settings. This need aligns with the research objectives of the cross-country initiatives noted earlier.
Second, for practical reasons we made several pragmatic decisions when conducting the literature review. This may have resulted in relevant articles being excluded from our analysis, resulting in overlooked insights and issues.
Our review also primarily focused on the implications of precision medicine technologies on HTA rather than identifying those expected to come into practice in the decade. We were therefore more reliant on expert opinion for this aspect of the results.
Conclusion
Precision medicine interventions are likely to proliferate over the next decade and will change the way services are delivered and evaluated. It is possible to speculate that such changes will be driven firstly by the complexity and uncertainty around delivering therapies that use biomarker data and, secondly, by the innovative, evolutionary nature of AI-based technologies. Healthcare systems around the world will need to consider adjusting their evaluative methods and processes to accommodate these changes in such a way that they can continue to robustly assess the value for money of new treatments and services.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The findings and conclusions of this work are those of the authors and not necessarily those of NICE. The authors are grateful to the individuals who took part in the interviews for this research.
Author contributions
JL-K planned the analysis, conducted expert interviews and drafted the manuscript. AP conducted expert interviews and the literature review and drafted the manuscript. JCR-P and RL developed the original research idea, critically appraised the analysis and drafted the manuscript. KE conducted the literature review. AM and AC critically appraised the analysis and drafted the manuscript. HW conducted the literature searches. MT planned and project-managed the analysis and drafted the manuscript.
Funding
This project was funded by NICE.
Compliance with Ethical Standards
Conflict of interest
RL and JCR-P are employed by NICE and AM is a member of a NICE Technology Appraisal Committee. JL-K, AP, KE, AC, HW and MT have no potential conflicts of interest that are directly relevant to the content of this article.
Footnotes
These refer to fields of study that can identify biomarkers using proteins, metabolites and cellular lipids. These can be used instead of or in combination with genetic and genomic information.
The original version of this article was revised: In the original publication, the author name Juan Carlos Rejon-Parilla was published incorrectly. The correct name should be Juan Carlos Rejon-Parrilla.
Change history
10/26/2018
The article The Future of Precision Medicine: Potential Impacts for Health Technology Assessment written by James Love‑Koh, Alison Peel Juan, Carlos Rejon‑Parrilla, KateAnastasia Chalkidou, Hannah Wood, Matthew Taylor was originally published electronically on the publisher’s internet portal (currently Springer Link) on [13th July, 2018] with incorrect spelling of the co-author “Juan Carlos Rejon-Parilla”. The correct spelling is “Juan Carlos Rejon-Parrilla”.
Change history
10/26/2018
The article The Future of Precision Medicine: Potential Impacts for Health Technology Assessment written by James Love?Koh, Alison Peel Juan, Carlos Rejon?Parrilla, KateAnastasia Chalkidou, Hannah Wood, Matthew Taylor was originally published electronically on the publisher?s internet portal (currently Springer Link) on [13th July, 2018] with incorrect spelling of the co-author ?Juan?Carlos?Rejon-Parilla?. The correct spelling is ?Juan Carlos Rejon-Parrilla?.
References
- 1.Pearson ER. Personalized medicine in diabetes: the role of ‘omics’ and biomarkers. Diabet Med. 2016;33(6):712–717. doi: 10.1111/dme.13075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahmood SS, Levy D, Vasan RS, Wang TJ. The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective. Lancet. 2014;383(9921):999–1008. doi: 10.1016/s0140-6736(13)61752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rakha EA, Pinder SE, Bartlett JMS, Ibrahim M, Starczynski J, Carder PJ, et al. Updated UK recommendations for HER2 assessment in breast cancer. J Clin Pathol. 2015;68(2):93–99. doi: 10.1136/jclinpath-2014-202571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashley EA. The precision medicine initiative: a new national effort. JAMA. 2015;313(21):2119–2120. doi: 10.1001/jama.2015.3595. [DOI] [PubMed] [Google Scholar]
- 5.England NHS. 100,000 Genomes Project: paving the way to personalised medicine. London: NHS England; 2016. [Google Scholar]
- 6.Dzau Victor J., Ginsburg Geoffrey S. Realizing the Full Potential of Precision Medicine in Health and Health Care. JAMA. 2016;316(16):1659. doi: 10.1001/jama.2016.14117. [DOI] [PubMed] [Google Scholar]
- 7.Australian Government Department of Health. Applying through the HTA access point: a guide for potential applicants. 2011. http://www.health.gov.au/internet/hta/publishing.nsf/content/guide-1. Accessed 21 May 2018.
- 8.Byron SK, Crabb N, George E, Marlow M, Newland A. The health technology assessment of companion diagnostics: experience of NICE. Clin Cancer Res. 2014;20(6):1469–1476. doi: 10.1158/1078-0432.CCR-13-1955. [DOI] [PubMed] [Google Scholar]
- 9.Gill P, Stewart K, Treasure E, Chadwick B. Methods of data collection in qualitative research: interviews and focus groups. Br Dent J. 2008;204:291–295. doi: 10.1038/bdj.2008.192. [DOI] [PubMed] [Google Scholar]
- 10.Ijzerman MJ, Manca A, Keizer J, Ramsey SD. Implementation of comparative effectiveness research in personalized medicine applications in oncology: current and future perspectives. Comp Eff Res. 2015;5:65–72. doi: 10.2147/CER.S92212. [DOI] [Google Scholar]
- 11.Garattini L, Curto A, Freemantle N. Personalized medicine and economic evaluation in oncology: all theory and no practice? Expert Rev Pharmacoecon Outcomes Res. 2015;15(5):733–738. doi: 10.1586/14737167.2015.1078239. [DOI] [PubMed] [Google Scholar]
- 12.Rogowski W, Payne K, Schnell-Inderst P, Manca A, Rochau U, Jahn B, et al. Concepts of ‘personalization’ in personalized medicine: implications for economic evaluation. Pharmacoeconomics. 2015;33(1):49–59. doi: 10.1007/s40273-014-0211-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Decipher Test. 2017. http://deciphertest.com/. Accessed 21 July 2017.
- 14.National Institute for Health and Care Excellence . Gene expression profiling and expanded immunohistochemistry tests for guiding adjuvant chemotherapy decisions in early breast cancer management: MammaPrint, Oncotype DX, IHC4 and Mammostrat. London: NICE; 2013. [Google Scholar]
- 15.Alemayehu D, Berger ML. Big Data: transforming drug development and health policy decision making. Health Serv Outcomes Res Methodol. 2016;16(3):92–102. doi: 10.1007/s10742-016-0144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Guzauskas GF, Gu C, Wang BCM, Furnback WE, Xie G, et al. Precision health economics and outcomes research to support precision medicine: big data meets patient heterogeneity on the road to value. J Pers Med. 2016;6(4):20. doi: 10.3390/jpm6040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foundation Medicine. https://www.foundationmedicine.com/. Accessed 22 Jun 2017.
- 18.Congenica. https://www.congenica.com/. Accessed 22 Jun 2017.
- 19.deCODE genetics. https://www.decode.com/. Accessed 22 Jun 2017.
- 20.Illumina. https://www.illumina.com/. Accessed 22 Jun 2017.
- 21.Jha S, Topol EJ. Adapting to artificial intelligence: radiologists and pathologists as information specialists. JAMA. 2016;316(22):2353–2354. doi: 10.1001/jama.2016.17438. [DOI] [PubMed] [Google Scholar]
- 22.National Institute for Health and Care Excellence. Health app: Sleepio for adults with poor sleep (MIB129). 2017. https://www.nice.org.uk/advice/mib129. Accessed 12 Feb 2018.
- 23.Slade I, Riddell D, Turnbull C, Hanson H, Rahman N. Development of cancer genetic services in the UK: a national consultation. Genome Med. 2015;7(1):18. doi: 10.1186/s13073-015-0128-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthews H, Hanison J, Nirmalan N. “Omics”-informed drug and biomarker discovery: opportunities, challenges and future perspectives. Proteomes. 2016 doi: 10.3390/proteomes4030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Payne K, Eden M, Davison N, Bakker E. Toward health technology assessment of whole-genome sequencing diagnostic tests: challenges and solutions. Pers Med. 2017;14(3):235–247. doi: 10.2217/pme-2016-0089. [DOI] [PubMed] [Google Scholar]
- 26.Desai S, Jena AB. Do celebrity endorsements matter? Observational study of BRCA gene testing and mastectomy rates after Angelina Jolie’s New York Times editorial. BMJ. 2016;355:i6357. doi: 10.1136/bmj.i6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Institute for Health and Care Excellence. Computerised decision support: supporting hypertension management at the point-of-care. 2009. https://www.nice.org.uk/sharedlearning/computerised-decision-support-supporting-hypertension-management-at-the-point-of-care. Accessed 11 Aug 2017.
- 28.ATEM NT. ATEM NT: portfolio. http://www.atem-nt.com/#portfolio. Accessed 11 Aug 2017.
- 29.Stanford Medicine. MyHeart Counts. 2017. https://med.stanford.edu/myheartcounts.html. Accessed 11 Aug 2017.
- 30.ClinRisk. Welcome to the QRISK®2-2017 cardiovascular disease risk calculator. 2017. https://www.qrisk.org/2017/. Accessed 22 Jun 2017.
- 31.Magicproject.org. Decision aids: discussion tool for clinicians and patients. http://magicproject.org/magicapp/decision-aids/. Accessed 12 Feb 2018.
- 32.National Institute for Health and Care Excellence. STA timeline. 2018. https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/nice-technology-appraisal-guidance/process/sta-timeline. Accessed 12 Feb 2018.
- 33.Shabaruddin FH, Fleeman ND, Payne K. Economic evaluations of personalized medicine: existing challenges and current developments. Pharmgenom Pers Med. 2015;8:115–126. doi: 10.2147/PGPM.S35063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Institute of Health Economics. Summary report of session A1: research challenge – health economics methodology. Canada; 2012. https://www.ihe.ca/download/ihe_mf_personalized_medicine_final_report.pdf. Accessed 29 June 2018.
- 35.Wordsworth S, Buchanan J, Towse A. Health economic perspectives of genomics. In: Kumar D, Chadwick R, editors. Genomics and society: ethical, legal, cultural and socioeconomic implications. London: Academic Press; 2015. pp. 83–122. [Google Scholar]
- 36.Fugel H-J, Nuijten M, Postma M, Redekop K. Economic evaluation in stratified medicine: methodological issues and challenges. Front Pharmacol. 2016;7:113. doi: 10.3389/fphar.2016.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dewey FE, Grove ME, Pan C, Goldstein BA, Bernstein JA, Chaib H, et al. Clinical interpretation and implications of whole-genome sequencing. JAMA. 2014;311(10):1035–1045. doi: 10.1001/jama.2014.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Husereau D, Marshall DA, Levy AR, Peacock S, Hoch JS. Health technology assessment and personalized medicine: are economic evaluation guidelines sufficient to support decision making? Int J Technol Assess Health Care. 2014;30(2):179–187. doi: 10.1017/S0266462314000142. [DOI] [PubMed] [Google Scholar]
- 39.Annemans L, Redekop K, Payne K. Current methodological issues in the economic assessment of personalized medicine. Value Health. 2013;16(6 Suppl):S20–S26. doi: 10.1016/j.jval.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 40.U.S. Food & Drug Administration. Medical devices: digital health criteria. 2017. https://www.fda.gov/MedicalDevices/DigitalHealth/ucm575766.htm. Accessed 12 Feb 2018.
- 41.Hsu J. FDA assembles team to oversee AI revolution in health. 2017. https://spectrum.ieee.org/the-human-os/biomedical/devices/fda-assembles-team-to-oversee-ai-revolution-in-health. Accessed 12 Feb 2018.
- 42.Doble B. Budget impact and cost-effectiveness: can we afford precision medicine in oncology? Scand J Clin Lab Invest Suppl. 2016;245:S6–S11. doi: 10.1080/00365513.2016.1206437. [DOI] [PubMed] [Google Scholar]
- 43.Buchanan J, Wordsworth S, Schuh A. Issues surrounding the health economic evaluation of genomic technologies. Pharmacogenomics. 2013;14(15):1833–1847. doi: 10.2217/pgs.13.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goddard KAB, Knaus WA, Whitlock E, Lyman GH, Feigelson HS, Schully SD, et al. Building the evidence base for decision making in cancer genomic medicine using comparative effectiveness research. Genet Med. 2012;14(7):633–642. doi: 10.1038/gim.2012.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garrison L, Mestre-Ferrandiz J, Zamora B. The value of knowing and knowing the value: improving the health technology assessment of complementary diagnostics. White paper. London: Office of Health Economics, EPEMED; 2016. [Google Scholar]
- 46.Academy of Medical Sciences . Health economics for stratified medicine. Summary of a workshop held on 5 October 2016 by the Academy of Medical Sciences and the UK Pharmacogenetics and Stratified Medicine Network. London: Academy of Medical Sciences; 2016. p. 2016. [Google Scholar]
- 47.Towse A, Garrison LP., Jr Economic incentives for evidence generation: promoting an efficient path to personalized medicine. Value Health. 2013;16(6 Suppl):S39–S43. doi: 10.1016/j.jval.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Phillips KA, Douglas MP, Trosman JR, Marshall DA. “What goes around comes around”: lessons learned from economic evaluations of personalized medicine applied to digital medicine. Value Health. 2017;20(1):47–53. doi: 10.1016/j.jval.2016.08.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garrison LP, Kamal-Bahl S, Towse A. Toward a broader concept of value: identifying and defining elements for an expanded cost-effectiveness analysis. Value Health. 2017;20(2):213–216. doi: 10.1016/j.jval.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 50.Foster MW, Mulvihill JJ, Sharp RR. Evaluating the utility of personal genomic information. Genet Med. 2009;11(8):570–574. doi: 10.1097/GIM.0b013e3181a2743e. [DOI] [PubMed] [Google Scholar]
- 51.Brouwer WBF, Culyer AJ, van Exel NJA, Rutten FFH. Welfarism vs. extra-welfarism. J Health Econ. 2008;27(2):325–338. doi: 10.1016/j.jhealeco.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 52.Vallejo-Torres L, García-Lorenzo B, Castilla I, Valcárcel-Nazco C, García-Pérez L, Linertová R, et al. On the estimation of the cost-effectiveness threshold: why, what, how? Value Health. 2016;19(5):558–566. doi: 10.1016/j.jval.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 53.Lewis JRR, Lipworth WL, Kerridge IH, Day RO. The economic evaluation of personalised oncology medicines: ethical challenges. Med J Aust. 2013;199(7):471–473. doi: 10.5694/mja13.10046. [DOI] [PubMed] [Google Scholar]
- 54.Cunanan KM, Iasonos A, Shen R, Begg CB, Gönen M. An efficient basket trial design. Stat Med. 2017;36(10):1568–1579. doi: 10.1002/sim.7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Biomarker-guided trial designs (BiGTeD): an online tool to help develop personalised medicine. 2017. http://www.bigted.org/. Accessed 21 Jul 2017.
- 56.Kaplan R. The FOCUS4 design for biomarker stratified trials. Chin Clin Oncol. 2015;4(3):35. doi: 10.3978/j.issn.2304-3865.2015.02.03. [DOI] [PubMed] [Google Scholar]
- 57.National Institute for Health and Care Excellence. Diagnostic services. London: NICE; 2017. https://www.nice.org.uk/guidance/indevelopment/gid-cgwave0773. Accessed 5 July 2018.
- 58.Medicines and Healthcare Products Regulatory Agency. An introductory guide to the medical device regulation (MDR) and the in vitro diagnostic medical device regulation (IVDR). 2017. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/640404/MDR_IVDR_guidance_Print_13.pdf. Accessed 5 July 2018.
- 59.Becla L, Lunshof JE, Gurwitz D, In Schulte, den Baumen T, Westerhoff HV, Lange BMH, et al. Health technology assessment in the era of personalized health care. Int J Technol Assess Health Care. 2011;27(2):118–126. doi: 10.1017/S026646231100002X. [DOI] [PubMed] [Google Scholar]
- 60.Faulkner E, Annemans L, Garrison L, Helfand M, Holtorf A-P, Hornberger J, et al. Challenges in the development and reimbursement of personalized medicine-payer and manufacturer perspectives and implications for health economics and outcomes research: a report of the ISPOR personalized medicine special interest group. Value Health. 2012;15(8):1162–1171. doi: 10.1016/j.jval.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 61.Degeling K, Koffijberg H, Ijzerman MJ. A systematic review and checklist presenting the main challenges for health economic modeling in personalized medicine: towards implementing patient-level models. Expert Rev Pharmacoecon Outcomes Res. 2017;17(1):17–25. doi: 10.1080/14737167.2017.1273110. [DOI] [PubMed] [Google Scholar]
- 62.Hoogendoorn M, Feenstra TL, Asukai Y, Briggs AH, Borg S, Dal Negro RW, et al. Patient heterogeneity in health economic decision models for chronic obstructive pulmonary disease: are current models suitable to evaluate personalized medicine? Value Health. 2016;19(6):800–810. doi: 10.1016/j.jval.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 63.Espinoza MA, Manca A, Claxton K, Sculpher MJ. The value of heterogeneity for cost-effectiveness subgroup analysis: conceptual framework and application. Med Decis Mak. 2014;34(8):951–964. doi: 10.1177/0272989X14538705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Espinoza MA, Manca A, Claxton K, Sculpher M. Social value and individual choice. Health Econ. 2017 doi: 10.1002/hec.3559. [DOI] [PubMed] [Google Scholar]
- 65.Epstein D, Mochon LG, Espin J, Soares MO. Use of multiparameter evidence synthesis to assess the appropriateness of data and structure in decision models. Med Decis Mak. 2013;33(5):715–730. doi: 10.1177/0272989x13480130. [DOI] [PubMed] [Google Scholar]
- 66.Bojke L, Claxton K, Sculpher M, Palmer S. Characterizing structural uncertainty in decision analytic models: a review and application of methods. Value Health. 2009;12(5):739–749. doi: 10.1111/j.1524-4733.2008.00502.x. [DOI] [PubMed] [Google Scholar]
- 67.Skirton H, Lewis C, Kent A, Coviello DA. Genetic education and the challenge of genomic medicine: development of core competences to support preparation of health professionals in Europe. Eur J Hum Genet. 2010;18(9):972–977. doi: 10.1038/ejhg.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.National Institute for Health and Care Excellence . NICE’s equality objectives and equality programme 2016–2020. London: NICE; 2016. [Google Scholar]
- 69.Equality Act 2010. https://www.legislation.gov.uk/ukpga/2010/15. Accessed 12 Dec 2017.
- 70.National Institute for Health and Care Excellence . Evaluation consultation document—Eliglustat for treating type 1 Gaucher disease. London: NICE; 2017. [Google Scholar]
- 71.National Institute for Health and Care Excellence. Sofosbuvir for treating chronic hepatitis C. 2015. https://www.nice.org.uk/guidance/ta330. Accessed 16 Jun 2017.
- 72.Hawkley LC, Lavelle LA, Berntson GG, Cacioppo JT. Mediators of the relationship between socioeconomic status and allostatic load in the Chicago Health, Aging, and Social Relations Study (CHASRS) Psychophysiology. 2011;48(8):1134–1145. doi: 10.1111/j.1469-8986.2011.01185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.National Institute for Health and Care Excellence . Fast track appraisal: addendum to the guide to the processes of technology appraisal. London: NICE; 2017. [PubMed] [Google Scholar]
- 74.National Institute for Health and Care Excellence . Proposals for increasing capacity within NICE’s technology appraisal programme. London: NICE; 2017. [Google Scholar]
- 75.Mahmoudian-sani M-R, Farnoosh G, Mahdavinezhad A, Saidijam M. CRISPR genome editing and its medical applications. Biotechnol Biotechnol Equip. 2017 doi: 10.1080/13102818.2017.1406823. [DOI] [Google Scholar]
- 76.Noble D, Mathur R, Dent T, Meads C, Greenhalgh T. Risk models and scores for type 2 diabetes: systematic review. BMJ. 2011;343:d7163. doi: 10.1136/bmj.d7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gavan SP, Thompson AJ, Payne K. The economic case for precision medicine. Expert Rev Precis Med Drug Dev. 2018;3(1):1–9. doi: 10.1080/23808993.2018.1421858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Medical Research Council. Observational data in healthcare decision making. 2018. https://mrc.ukri.org/funding/how-we-fund-research/highlight-notices/observational-data-in-healthcare-decision-making/. Accessed 22 Jun 2018.
- 79.Crocker-Buque T, Edelstein M, Mounier-Jack S. Interventions to reduce inequalities in vaccine uptake in children and adolescents aged < 19 years: a systematic review. J Epidemiol Commun Health. 2017;71(1):87–97. doi: 10.1136/jech-2016-207572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hutt P, Gilmour S. Tackling inequalities in general practice: an inquiry into the quality of general practice in England. London: The King’s Fund; 2010. [Google Scholar]
- 81.Cowles E, Marsden G, Cole A, Devlin N. A review of NICE methods and processes across health technology assessment programmes: why the differences and what is the impact? Appl Health Econ Health Policy. 2017;15(4):469–477. doi: 10.1007/s40258-017-0309-y. [DOI] [PubMed] [Google Scholar]
- 82.International Consortium for Personalised Medicine. Action plan: actionable research and support activities. Cologne: Deutsches Zentrum für Luft- und Raumfahrt e. V. (DLR)/DLR Project Management Agency, Department Health; 2017.
- 83.Horgan D. From here to 2025: personalised medicine and healthcare for an immediate future. J Cancer Policy. 2018;16:6–21. doi: 10.1016/j.jcpo.2017.12.008. [DOI] [Google Scholar]
- 84.European Commission. Horizon 2020: health, demographic change and wellbeing. https://ec.europa.eu/programmes/horizon2020/en/h2020-section/health-demographic-change-and-wellbeing. Accessed 21 May 2018.
- 85.US Food & Drug Administration. FDA approves first cancer treatment for any solid tumor with a specific genetic feature. 2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm560167.htm. Accessed 26 Jun 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.