Abstract
Obesity, insulin resistance, and diabetes are strongly linked to the accumulation of excessive lipids in the liver parenchyma, a condition known as nonalcoholic fatty liver disease (NAFLD). Given its association with obesity and related metabolic diseases, it is not surprising that the prevalence of NAFLD has dramatically increased in the past few decades. NAFLD has become the most common liver disease in many areas of the world. The term, NAFLD, encompasses a spectrum of disorders that ranges from simple steatosis to steatosis with inflammatory lesions (nonalcoholic steatohepatitis [NASH]). Although simple steatosis might be relatively benign, epidemiologic studies have linked NASH to greatly increased risk of developing cirrhosis and hepatocellular carcinoma. Yet despite this, there are no approved treatments for the disease, and it remains a significant unmet medical need. This Perspective will review some of the relevant literature on the topic and examine approved and experimental NASH therapeutic concepts that target intermediary metabolism, insulin resistance, and diabetes to treat this emerging public health problem.
Nonalcoholic Fatty Liver Disease
Hepatic steatosis in the absence of significant alcohol intake or other identifiable cause is known as nonalcoholic fatty liver disease (NAFLD). Only recently recognized as a distinct disease entity, the term NAFLD encompasses a spectrum of severity (Fig. 1). Sporadic and zonal steatosis is the most common manifestation of the disease, which may not progress or lead to liver pathology. However, some patients develop inflammation with fibrotic lesions, termed nonalcoholic steatohepatitis (NASH). The progression of NAFLD to NASH is likely multifactorial, but most models invoke insulin resistance, accumulation of toxic lipid species, and some form of inflammation or reactive species in the liver. It can also not be discounted that alcohol consumption could be factor even in NAFLD, as most people consume alcohol in addition to caloric excess.
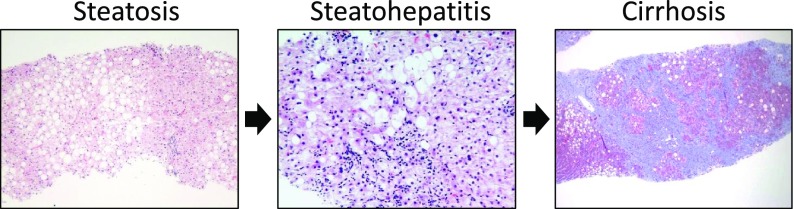
Figure 1.
The histologic spectrum of NAFLD. Left: Steatosis without inflammation, hepatocyte ballooning, or fibrosis. Middle: Steatohepatitis with hepatocyte ballooning, Mallory-Denk bodies, lobular inflammation, and perisinusoidal fibrosis. Right: Cirrhosis with residual steatosis. Pictures were kindly provided by Dr. Elizabeth Brunt (Washington University School of Medicine in St. Louis).
NASH is associated with a markedly increased risk of developing cirrhosis and hepatocellular carcinoma as well as other diseases not directly associated with liver damage, including increased risk of cardiovascular disease (1). Indeed, morbidity and all-cause mortality are increased in people with NASH compared with the general population (1). Powerful genome-wide association studies have demonstrated a clear genetic basis for susceptibility to NAFLD that may explain much of the difference in the incidence of NAFLD in various racial groups (2). However, the development and progression of this disease is closely associated with obesity and with the obesity epidemic, and NAFLD has now become the most common cause of liver disease in the U.S. Estimates of the worldwide prevalence of NAFLD range up to 35% in adults (3). With new and very effective treatments for hepatitis C virus, liver failure precipitated by NASH may already be the leading cause of liver transplantation in the U.S. Despite this, there are still no approved pharmacologic treatments for NAFLD/NASH, and this remains a major untreated public health issue.
The Role of Liver Cell Types in the Etiology of NASH
The liver is made up of several cell types that each play pivotal roles in the pathophysiology of the liver. Hepatocytes are the parenchymal cells of the liver and make up the majority of the liver by mass (4). A number of types of immune cells are found in liver, including resident macrophages of the liver (Kupffer cells), infiltrating macrophages, T cells, and neutrophils. Stellate cells are fibroblast-like cells that, when activated, translocate to sites of inflammatory lesions and secrete the extracellular matrix that leads to the development of fibrosis that occurs in response to many forms of liver injury, including NASH. Therapeutics that directly prevent or reverse stellate cell activation (antifibrotics) also have excellent promise for treating NASH and are under development but will not be discussed given the metabolic focus of this review. It is clear that the various types of cells in the liver have elaborate networks of intercellular communication. For instance, hepatocytes play an important role in activating stellate cells from a quiescent state to the matrix-secreting myofibroblasts by release of several proinflammatory cytokines, chemokines, mitochondrial DNA, lipids, or microRNAs. Several examples indicate that alleviating metabolic stress in hepatocytes can lead to diminished activation of stellate cells by reducing the release of these activating factors.
Hepatocytes are responsible for the major physiologic and metabolic functions of the liver (4). To fuel these metabolic and nonmetabolic functions, hepatocytes take up and use large amounts of lipids as free fatty acids and lipoprotein remnants. Hepatocytes are the main cells in the liver for triglyceride storage and the cells in which lipid accumulates in NAFLD. In obesity, the uptake and synthesis of lipids exceed to the flux through utilization or secretion pathways, leading to a net increase in intrahepatic lipid. Hepatic and extrahepatic factors have been implicated in the development of NAFLD in obesity and will be discussed.
Interrelationships Between NAFLD and Insulin Resistance or Diabetes
A number of studies conducted in humans and experimental animal models have linked hepatic steatosis and insulin resistance. Although the cause and effect relationship between lipid accumulation and insulin resistance is still open to debate (5), several groups have suggested that the accumulation of specific lipid species in liver leads to hepatic insulin resistance. Most studies suggest that the main storage lipid, triglyceride, is relatively inert, but other lipid intermediates have been mechanistically connected to impairments in insulin signaling. For example, diacylglycerol, the penultimate intermediate in the triglyceride synthesis pathways, and ceramide, a complex membrane lipid, have been shown to inhibit insulin signaling at the insulin receptor or proximal mediators (6,7). It is also possible that the block in signaling occurs further downstream in the insulin signaling cascades by mechanisms that remain to be determined (8).
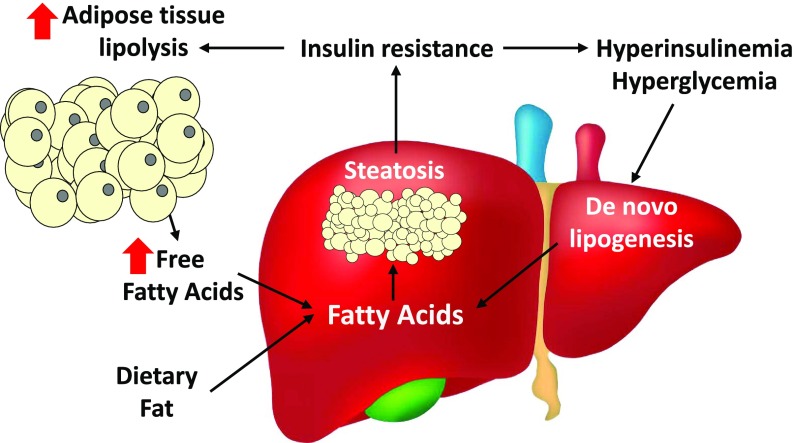
There is also excellent evidence that insulin resistance is also a driver of the progression of NAFLD (Fig. 2). Diabetes is highly predictive of the progression of simple steatosis to the more injurious NASH (9). Impairments in insulin signaling in hepatocytes could lead to perturbations in intermediary metabolism that contribute to lipid accumulation in these cells. For instance, obesity and NAFLD are associated with increased rates of hepatocyte de novo lipogenesis (10), which may be relatively low in normal human liver but is a significant contributor in obesity-related NAFLD. Hyperinsulinemia caused by peripheral insulin resistance can stimulate hepatic de novo lipogenesis through arms of the insulin signaling pathway that seem to be intact (selective insulin resistance). Insulin resistance in adipose tissue can dysregulate adipose tissue lipolysis to increase flux of fatty acids from adipocytes to the liver (11) (Fig. 2). In addition, diminished mitochondrial fat oxidation may also play a role in the accumulation of fat, though there is also evidence that mitochondrial oxidative metabolism is actually increased (12) and that the mitochondrial impairments may be a later manifestation of the progression of NASH (13). Impairments in the process of autophagy in hepatocytes and other types of cells may also contribute to the pathogenesis of NASH (14), including the failure to break down stored fat via lipophagy and accumulation of damaged mitochondria due to impaired mitophagy. Regardless of the cause and effect relationship between insulin resistance and NAFLD, therapeutics that improve both hepatic steatosis and other metabolic derangements will almost certainly have a beneficial effect on overall cardiometabolic risk.
Figure 2.
Interactions between insulin resistance and NAFLD. Insulin resistance can promote NAFLD by leading to increased release of free fatty acids by adipose tissue and hyperglycemia/hyperinsulinemia, which promotes hepatic de novo lipogenesis. The contribution of dietary fat also cannot be discounted. Hepatic steatosis is also associated with developing/exacerbating systemic insulin resistance by direct and indirect mechanisms.
Development of NASH Therapeutics
Exercise interventions and weight loss due to caloric restriction are known to be quite effective at reducing liver fat and improving NASH end points. However, these interventions have proven to be difficult to maintain long term as weight regain recidivism is very high and exercise regimens are unsustainable for many individuals. Thus, a number of therapeutic approaches have been tried in experimental models and clinical trials.
One of the challenges in developing NASH therapeutics are well-known issues with mouse models of the disease. Mice readily develop hepatic steatosis, but producing bridging fibrosis, cirrhosis, and hepatocellular carcinoma requires extensive manipulation and/or experiments with a very long duration. The translational value of these manipulations may be questionable. Mice also have a variety of differences in lipid metabolism, including higher reliance on hepatic de novo lipogenesis and differences in lipoprotein metabolism. This may give false confidence in agents targeting these pathways or obscure untoward effects, such as raising LDLs. Dietary models include the methionine and choline–deficient (MCD) diet, high-fat diets of varying compositions, fructose-containing diets, and high-cholesterol diets (15). The MCD diet results in significant steatohepatitis, but mice fed a MCD diet tend to lose weight rather than becoming obese. Most recently, diets containing high levels of fat and fructose in combination with added cholesterol have become popular and have been shown to induce signs of NASH (16). However, although these fat/fructose/cholesterol-containing diets can reliably produce findings consistent with NASH, the amounts of these nutrients are much higher than physiologic conditions in humans (17). Frequently used genetic models include ob/ob mice, db/db mice, and genetically engineered mice often superimposed with dietary or toxin administration (17), which all have their caveats. It is likely that other species, such as rats, hamsters, or pigs, may have more translational value, but these models are less genetically tractable compared with mice for mechanistic insights. Thus, preclinical results with NASH therapeutics must be viewed with caution until validated in clinical trials.
A number of end points, including liver fat content and inflammation, circulating levels of transaminase enzymes, and other metabolic parameters, are often measured as outcomes in preclinical and clinical studies. However, regulatory agencies will require that a successful phase III trial for NASH will either 1) improve histologic NAFLD activity scoring (NAS), which assesses steatosis, lobular inflammation, and hepatocyte ballooning, without exacerbating fibrosis or 2) reduce fibrosis scoring by at least one stage without exacerbating NAS. Results discussed below should be viewed in this context. To begin, current clinically used antidiabetes therapies will be outlined, but metformin, the first-line therapeutic, has largely been ineffective at treating NASH and will not be discussed. The final section will focus on other metabolic targets currently in clinical development. The present work will not be discussing novel therapeutics attempting to suppress fibrogenesis or target inflammation, which also show much promise. Also, given the wide variety of agents in development, it will not be possible to capture all of the therapeutic targets and I have focused on therapies already in clinical trials.
Clinically Used Antidiabetes Therapies for Treating NASH
Glucagon-Like Peptide 1 Modulators
Glucagon-like peptide 1 (GLP1) is an endogenous hormone produced by the intestine and some areas of the brain that regulates blood glucose levels by enhancing the secretion of insulin by β-cells and metabolic effects on a variety of tissues (18). GLP1 mediates its effects through a G-protein–coupled receptor that is expressed in many types of cells, including hepatocytes. The half-life of GLP1 in the plasma is very short due to the actions of a protease (dipeptidyl peptidase 4 [DPP4]) that cleaves GLP1 to inactivate it. To harness the potential beneficial effects of GLP1 activation, therapeutic efforts have focused on GLP1 receptor agonists that are not subject to proteolytic inactivation (e.g., exenatide, liraglutide, and semaglutide) and on inhibitors of DPP4 (the gliptins) to prolong the half-life of the endogenous hormone.
Preclinical studies have shown that GLP1 receptor agonists reduce hepatic steatosis and markers of liver injury in rodents, often in concert with effects on reducing body weight (19,20). It is believed that at least some of the effects of these agonists are due to direct effects on hepatocytes, which express the GLP1 receptor (20). Currently, exenatide and liraglitude are both in later-stage clinical trials for NASH. Previous work in people using liraglitude has suggested that it is effective at resolving NASH in a notable percentage of subjects (21). Liraglitude did not improve fibrosis in this 48-week study but met the criteria for NASH resolution without exacerbating fibrosis. Given the beneficial effects of these injected drugs on a variety of metabolic end points while being well tolerated, it is very possible that GLP1 receptor agonism is a viable therapeutic option for treating NASH.
Insulin Sensitizers
The thiazolidinediones (TZDs) pioglitazone and rosiglitazone are insulin-sensitizing agents that act as agonists for a nuclear receptor, the peroxisome proliferator–activated receptor γ (PPARγ) (22). PPARγ is most highly expressed in adipose tissue, where it serves an essential role in the regulation of adipocyte differentiation, adipogenesis, and lipid metabolism (23) as well as suppressing inflammation (24). PPARγ activation may suppress hepatic steatosis by promoting fat storage in adipocytes and decreasing adipose tissue lipolysis, thereby decreasing the concentration of fatty acids presented to the liver. TZDs also stimulate release of adipokines, including adiponectin, which enhance hepatic fatty acid oxidation. In rodent models, there is some evidence that strong activation of PPARγ in liver can actually exacerbate hepatic steatosis (25). However, this has not been seen in humans, and this may be a rodent-specific effect. The effects of TZDs in various tissues and how this impacts their beneficial effects on NASH have not been carefully teased apart.
Of the TZDs in clinical use, rosiglitazone is the most potent PPARγ ligand (22). In many rodent models, rosiglitazone almost universally reduces inflammation, fibrosis, and hepatic stellate cell activation (26). In humans, rosiglitazone treatment reduced plasma transaminase levels but failed to produce improvements in histologic features, such as fibrosis, hepatocyte ballooning, and inflammation (27). Moreover, concerns regarding weight gain and cardiovascular side effects have severely curtailed use of this drug as an insulin sensitizer.
Pioglitazone is a less potent PPARγ agonist compared with rosiglitazone (22) but clinically is very effective and is associated with fewer side effects (28), though weight gain is often seen in response to the drug. The role of pioglitazone in NAFLD has been evaluated in rodent studies using a variety of high-fat diets and has been shown to decrease hepatic triglyceride content and prevent (29) or reverse (30) hepatic fibrosis, including direct effects on stellate cell activation. There have been numerous clinical trials with pioglitazone over the years. A recent meta-analysis using randomized, placebo-controlled trials concluded that pioglitazone improved hepatic fibrosis, whereas rosiglitazone did not (31). The observation that the less potent PPARγ agonist pioglitazone may be superior for suppressing fibrosis perhaps suggests PPARγ-independent effects. As pioglitazone can be used at higher doses compared with rosiglitazone, it could engage alternate targets, such as the mitochondrial pyruvate carrier discussed later.
Sodium–Glucose Cotransporter 2 Inhibitors
The sodium–glucose cotransporter 2 (SGLT2) is a solute carrier that is highly expressed in kidney. SGLT2 inhibitors have been used since 2013 to lower blood glucose in patients with diabetes by preventing the kidneys from reabsorbing glucose, causing it to be released in urine. SGLT2 inhibitors currently in use include canagliflozin, dapagliflozin, ertugliflozin, empagliflozin, and ipragliflozin. SGLT2 is also expressed in other organs, including the heart, and other recent work has suggested that these inhibitors may also have beneficial effects on cardiovascular outcomes and risk (32) and, often, weight loss. Thus far, the SGLT2 inhibitors have been relatively well tolerated, though there may be an increased risk of amputation, acute kidney failure, and ketoacidosis in patients with diabetes taking them (33).
There has been recent work conducted in mice and other model organisms that suggests SGLT2 inhibitors hold promise for treating NASH. In a mouse model of NASH and hepatocellular carcinoma, canagliflozin reduced hepatic steatosis, fibrosis, and tumor incidence (34). Another SGLT2 inhibitor, ipragliflozin, also prevented liver steatosis and fibrosis in the MCD diet model (35). A 1-year study demonstrated that canagliflozin reduced transaminitis in patients with diabetes, but histologic analysis or other measures of hepatic steatosis and injury was not evaluated (36). Indeed, it does not appear that this has been evaluated in patients to date. However, empagliflozin is currently in a clinical trial that will determine whether this drug successfully attenuates NASH.
Agents in Clinical Development That Target Metabolism
Inhibitors of De Novo Fatty Acid Synthesis
As described above, hepatic de novo lipogenesis is activated in obesity and may be an important contributor to the development of NAFLD and hepatic insulin resistance. Several inhibitors of enzymes involved in the conversion of carbohydrates to new lipids have entered preclinical development with the idea of targeting this pathway.
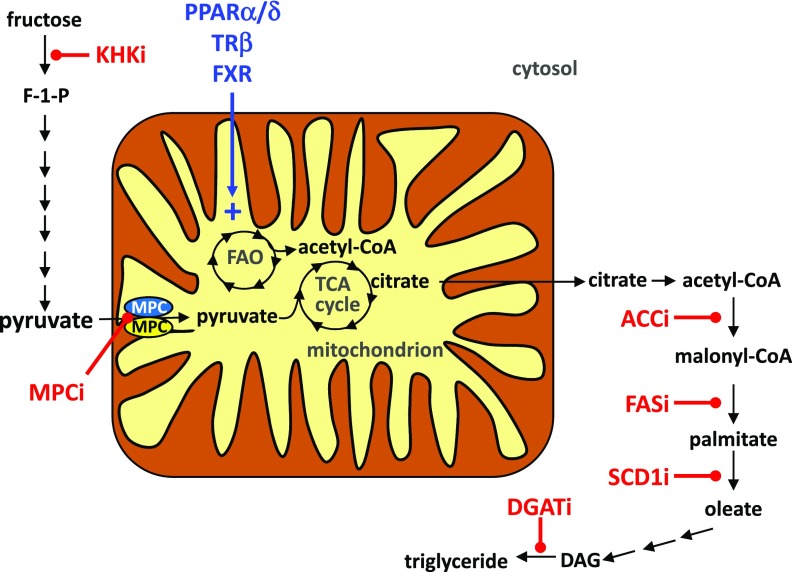
Ketohexokinase (KHK) catalyzes the conversion of fructose to fructose-1-phosphate, which shunts this dietary sugar into the pathway of de novo lipogenesis (Fig. 3). Recent work has suggested that mice lacking this enzyme are protected from a variety of metabolic abnormalities in the context of fructose feeding (37). Phase I clinical trials have been completed to evaluate the effects of ketohexokinase inhibitors (KHKi) on NAFLD end points, and results are pending.
Figure 3.
Investigational drugs for treating NASH. The proposed sites of action of various experimental NASH therapeutics are shown. Agonists for the PPARs, TRβ, and FXR nuclear receptors likely work by stimulating mitochondrial fatty acid oxidation. MPC inhibitors (MPCi) work by attenuating flux of pyruvate into mitochondrial pathways and likely stimulating fat oxidation. Inhibitors of various steps in de novo lipogenesis are also shown and include KHKi, ACCi, FASi, SCD1i, and DGATi. DAG, diacylglycerol; F-1-P, fructose-1-phosphate; FAO, fatty acid oxidation; TCA, tricarboxylic acid.
Acetyl-CoA carboxylase enzymes (ACC1 and ACC2) convert acetyl-CoA to malonyl-CoA (Fig. 3), which is a committed step in de novo lipogenesis. Malonyl-CoA is also an important signaling intermediate that is an allosteric inhibitor of fatty acid oxidation. Inhibition of ACC (ACCi) therefore has the potential to inhibit lipogenesis and to stimulate mitochondrial fatty acid oxidation. Knockdown of ACC1 and ACC2 showed potential at reducing hepatic steatosis (38), and small-molecule inhibitors of ACCs attenuated steatosis and reduced inflammation and fibrosis in rodent models (39). However, liver-specific ACC1 and ACC2 knockout mice actually exhibit worse hepatic steatosis due to impaired mitochondrial fat oxidation (40). Several inhibitors of ACC are in various stages of clinical development. Recently, it was reported that in clinical trials with an inhibitor and in liver-specific ACC1/2 double knockout mice, targeting this enzyme is very potent at reducing de novo lipogenesis but raises the plasma triglyceride concentration significantly (41). This is believed to occur due to a reduction in polyunsaturated fatty acid synthesis, leading to activation of SREBP1, which stimulates VLDL secretion. The consistency in elevation in blood triglyceride content with chemical inhibition in humans and the knockout mouse model could suggest a class side effect.
Fatty acid synthase (FAS) contains multiple enzymatic active sites sufficient to catalyze the elongation and reductive reactions necessary to use malonyl-CoA generated by ACC to synthesize palmitate, a saturated fatty acid containing 16 carbons (Fig. 3). Studies with a liver-directed FAS inhibitor reduced hepatic steatosis (42), and clinical trials with a potent inhibitor of FAS (FASi) are also ongoing.
Steroyl-CoA Desaturase 1 Inhibitors
After de novo synthesis or dietary absorption, saturated fatty acids can be converted to unsaturated fatty acids by steroyl-CoA desaturase (SCD) enzymes (Fig. 3). Because of the distinct biophysical properties and signaling effects of these different lipids, these enzymes could have a very profound effect on liver physiology and metabolism. Obesity and hepatic steatosis are well known to robustly induce the expression of SCD1 (43), and rodents lacking SCD1 in liver specifically are protected from developing hepatic steatosis (44). However, the effects of deleting SCD1 seem to be tissue specific, and some data suggest that SCD1 deletion may exacerbate liver injury in NASH (45). Aramchol is a chemical inhibitor of SCD1 in clinical testing for treatment of NASH. Earlier trials showed that Aramchol reduced liver fat content in people (46) and in mice fed the MCD diet and reduced hepatic lipid accumulation and fibrosis (47). Data from chemical and genetic SCD1 inhibition have suggested that attenuating its activity may direct fatty acids toward an oxidative fate (43). Moreover, there is some evidence that the monounsaturated fatty acids produced by SCD1 in liver can have signaling effects on other tissues (48), suggesting multifactorial effects on systemic metabolism.
Diacylglycerol Acyltransferase Inhibitors
As their name indicates, diacylglycerol acyltransferase (DGAT) enzymes catalyze the addition of a fatty acid to diacylglycerol to form triglyceride (Fig. 3). In animal models, the data suggest that preventing the esterification of fatty acids promotes their oxidation in β-oxidation pathways. Two isoforms of DGAT enzymes have been characterized (DGAT1 and DGAT2) with a distinct pattern of expression in various metabolic tissues (49). Pharmacologic or genetic inhibition of DGAT1 in mouse liver significantly improved hepatic steatosis, fibrosis, and insulin sensitivity (50,51). Human mutations in DGAT1 have been linked to severe congenital diarrhea syndrome due to malabsorption (52), potentially suggesting that DGAT inhibitors (DGATi) may elicit diarrheal side effects if active in intestine. Interestingly, knockdown of DGAT2 reduced hepatic steatosis (53) but actually exacerbated liver injury with the MCD diet (54). Currently, two DGAT targeting agents are in clinical development including a small-molecule DGAT1i and an antisense oligonucleotide to knock down the expression of DGAT2 in liver. The injectable antisense oligonucleotide may be beneficial in that it may spare intestinal DGAT activity and avoid diarrheal side effects, but questions remain regarding whether liver injury with DGAT2 inhibition will translate to humans.
Mitochondrial Pyruvate Carrier Inhibitors
Studies conducted in a variety of animal models have suggested that activating mitochondrial oxidative metabolism in liver could be an effective target for NAFLD by stimulating fat catabolism and promoting the disposal of excess fat. One compound targeting mitochondrial metabolism directly that has reached clinical trials for NASH surprisingly targets mitochondrial pyruvate metabolism. Pyruvate is produced in the cytosol but must enter the mitochondrial matrix by the mitochondrial pyruvate carrier (MPC) (55) to be further metabolized (Fig. 3). TZDs, including pioglitazone and rosiglitazone, interact with and inhibit the MPC at physiologic concentrations (56). MSDC-0602, a novel TZD with a very low affinity to bind or activate PPARγ (57), also binds the MPC (58) and has potent insulin-sensitizing effects in mouse models of obesity and diabetes (57).
How does inhibiting the MPC complex to suppress pyruvate flux into mitochondrial pathways elicit insulin sensitization and beneficial effects on NASH end points? As mitochondrial pyruvate metabolism is required for gluconeogenesis from pyruvate, MPC inhibition reduces hepatic glucose production. Genetic deletion of the MPC complex in mouse liver prevents diabetes in db/db or high-fat–diet fed models (59,60). It is possible that altered redox status via NAD+ generation by pyruvate being converted to lactate and/or stimulation of fatty acid oxidation as an energy source provides a mechanistic explanation for the effects. In addition, there is evidence that inhibition of the MPC specifically in hepatocytes promotes intercellular communication to suppress activation of stellate cells (61). Targeting MPC in hepatocytes prevented or reversed signs of NASH in a dietary model of liver injury and fibrosis (61) in association with reduced hepatic oxidative damage (61). MSDC-0602 is currently in a 52-week phase II clinical trial to evaluate its efficacy for treating NASH.
Thyroid Hormone Receptor β Agonists
The thyroid hormone mediates its effects by engaging the thyroid hormone receptor (TR)α and TRβ receptors to control myriad essential cellular and organismal functions, including important roles in stimulating oxidative metabolism. Whereas the side effects of thyroid hormone administration are too risky to warrant use as a treatment for metabolic disease, selective engagement of the TRβ isoform in liver has emerged as a potential treatment for NASH. MGL-3196 is a liver-directed TRβ agonist (62) that likely enhances fatty acid catabolism to promote fat burning and alleviate hepatic steatosis and dyslipidemia (Fig. 3). Though a peer-reviewed report has not yet emerged, a recent clinical trial with MGL-3196 was reported to be well tolerated and result in lower liver fat and plasma transaminase levels while improving fibrosis and NAS in a 36-week study. MGL-3196 also significantly reduced plasma LDL cholesterol and triglyceride concentrations (63). Longer-term clinical studies will define whether these effects and tolerability are sustained and whether efficacy is improved by longer drug exposure.
Farnesoid X Receptor Agonists
Originally considered a nuclear receptor for farnesyl, farnesoid X receptor (FXR) is now known to be a receptor for bile acids and their derivatives that is highly expressed in intestine and liver (64). Activation of the receptor suppresses the expression of several enzymes involved in cholesterol and bile acid synthesis. This constitutes a negative feedback loop to maintain physiologic levels of these lipids. FXR activation also stimulates the production of FGF19, an atypical hormone regulator of metabolism and bile acid homeostasis that has been linked to improvements in NASH (65).
In preclinical studies, a variety of FXR agonists have been shown to attenuate hepatic steatosis, reduce inflammation, and prevent fibrosis (66–68). Obeticholic acid (6α-ethyl-chenodeoxycholic acid) is a semisynthetic bile acid analog that is a very potent FXR agonist that has reached phase III clinical trials. Phase II clinical trials showed excellent effects on hepatic inflammation and fibrosis in patients with NASH (69), and this compound has received U.S. Food and Drug Administration approval for treating primary biliary cholangitis. Concerns around effects on elevating plasma LDL cholesterol concentrations and potential dosing issues remain for obeticholic acid, yet the effects on NASH end points hold excellent promise. It remains to be determined whether other FXR agonists in development share the signature of plasma lipid alterations.
PPAR Dual Agonists
The PPAR family of nuclear receptor transcription factors is composed of PPARα, PPARβ/δ, and PPARγ. In addition to the TZD PPARγ agonists, small-molecule modulators that serve as dual or pan-agonists for PPAR family members have also been explored as treatments for NASH. PPARα is most highly expressed in the liver and regulates fatty acid uptake, β-oxidation, ketogenesis, bile acid synthesis, and triglyceride turnover (70). The PPARδ isoform is most highly expressed in muscle, but also in liver and other tissues, where it is involved in regulating mitochondrial metabolism and fatty acid β-oxidation (71) (Fig. 3). Because PPARs have relatively large ligand binding pockets, it has been possible to design agonists that activate multiple isoforms. For example, IVA337 is a PPARα/δ/γ pan-agonist that has shown promise as an insulin-sensitizing and antifibrotic agent in preclinical models (72), leading to ongoing clinical trials.
A PPARα/δ dual agonist (GFT505, also known as elafibranor) has been examined for efficacy in treating NASH. Using various rodent models of NASH, treatment with GFT505 demonstrated improvement in histologic measures of NASH, decreased hepatic triglyceride content, and diminished expression of inflammatory cytokine and fibrosis markers (73). These effects on NASH end points were mediated independently of PPARα as PPARα knockout mice still responded to the drug. However, GFT505 treatment did not improve plasma triglyceride or free fatty acid levels in the knockouts, suggesting both PPARα-dependent and -independent effects (73). In humans, GFT505 caused a significant reduction in ALT compared with placebo control subjects (74) but did not resolve steatosis, ballooning, or inflammation scoring (75). However, subgroup analysis indicated that there may be an effect on fibrosis in patients with a higher baseline fibrosis.
Saroglitazar is a PPARα/γ dual agonist that predominantly activates PPARα (76). Saroglitazar has insulin-sensitizing and lipid-lowering effects in mice and reduces adipose tissue inflammation (77). In a choline-deficient diet mouse model, saroglitazar reduced steatosis, transaminitis, hepatocyte ballooning, inflammation, and fibrosis (78). On the basis of these promising preclinical findings, clinical trials have been initiated. Previous PPARα/γ dual agonists developed to target cardiometabolic disease have stalled in late-stage clinical trials due to issues with toxicity. It is possible that higher relative affinity of saroglitazar for PPARα will mitigate these issues.
Conclusions
Although NAFLD, like other obesity-related metabolic diseases, is highly treatable by exercise or weight loss due to lifestyle interventions or bariatric surgery, there is a need to develop therapeutic approaches to treat this widespread disease of the liver. Improving insulin sensitivity and intermediary metabolism treats the root causes of hepatic steatosis and NASH and is highly promising as a therapeutic avenue. The key will be to identify ideal targets and compounds with minimal side effects as improvements in glycemia with metformin are not sufficient to impact NASH end points and patients receiving these drugs will likely be on them for a lifetime. Current approaches in clinical development include targeting systemic insulin sensitivity and lipogenic enzymes and stimulating fatty acid oxidation by a variety of mechanisms. Several ongoing late-stage clinical trials hold promise that an effective treatment for NASH that also may have beneficial effects on systemic metabolism and cardiometabolic risk will be found in the near future. Finally, given the intrinsic heterogeneity of NASH caused by the well-recognized genetic linkages and diversity of diets human societies consume, it is possible that a personalized medicine approach will be needed to tailor the right metabolic therapy to optimize treatment regimens.
Article Information
Funding. Work in the laboratory of B.N.F. is supported by National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK104735).
Duality of Interest. B.N.F. is a shareholder and member of the scientific advisory board for Cirius Therapeutics. No other potential conflicts of interest relevant to this article were reported.
References
- 1.Ekstedt M, Franzén LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006;44:865–873 [DOI] [PubMed] [Google Scholar]
- 2.Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2008;40:1461–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 2016;64:1577–1586 [DOI] [PubMed] [Google Scholar]
- 4.Trefts E, Gannon M, Wasserman DH. The liver. Curr Biol 2017;27:R1147–R1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farese RV Jr, Zechner R, Newgard CB, Walther TC. The problem of establishing relationships between hepatic steatosis and hepatic insulin resistance. Cell Metab 2012;15:570–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samuel VT, Shulman GI. Nonalcoholic fatty liver disease as a nexus of metabolic and hepatic diseases. Cell Metab 2018;27:22–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaurasia B, Summers SA. Ceramides - lipotoxic inducers of metabolic disorders. Trends Endocrinol Metab 2015;26:538–550 [DOI] [PubMed] [Google Scholar]
- 8.Czech MP. Insulin action and resistance in obesity and type 2 diabetes. Nat Med 2017;23:804–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005;129:113–121 [DOI] [PubMed] [Google Scholar]
- 10.Lambert JE, Ramos-Roman MA, Browning JD, Parks EJ. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 2014;146:726–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jelenik T, Kaul K, Séquaris G, et al. Mechanisms of insulin resistance in primary and secondary nonalcoholic fatty liver. Diabetes 2017;66:2241–2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satapati S, Kucejova B, Duarte JA, et al. Mitochondrial metabolism mediates oxidative stress and inflammation in fatty liver [published correction appears in J Clin Invest 2016;126:1605]. J Clin Invest 2015;125:4447–4462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koliaki C, Szendroedi J, Kaul K, et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab 2015;21:739–746 [DOI] [PubMed] [Google Scholar]
- 14.Lavallard VJ, Gual P. Autophagy and non-alcoholic fatty liver disease. BioMed Res Int 2014;2014:120179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.London RM, George J. Pathogenesis of NASH: animal models. Clin Liver Dis 2007;11:55–74, viii [DOI] [PubMed] [Google Scholar]
- 16.Tetri LH, Basaranoglu M, Brunt EM, Yerian LM, Neuschwander-Tetri BA. Severe NAFLD with hepatic necroinflammatory changes in mice fed trans fats and a high-fructose corn syrup equivalent. Am J Physiol Gastrointest Liver Physiol 2008;295:G987–G995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maher JJ. Modeling fatty liver disease in animals: Is there an optimal approach, and is the effort worthwhile? Hepatology 2016;64:1398–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab 2018;27:740–756 [DOI] [PubMed] [Google Scholar]
- 19.Trevaskis JL, Griffin PS, Wittmer C, et al. Glucagon-like peptide-1 receptor agonism improves metabolic, biochemical, and histopathological indices of nonalcoholic steatohepatitis in mice. Am J Physiol Gastrointest Liver Physiol 2012;302:G762–G772 [DOI] [PubMed] [Google Scholar]
- 20.Gupta NA, Mells J, Dunham RM, et al. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology 2010;51:1584–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armstrong MJ, Gaunt P, Aithal GP, et al.; LEAN Trial Team . Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016;387:679–690 [DOI] [PubMed] [Google Scholar]
- 22.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J Biol Chem 1995;270:12953–12956 [DOI] [PubMed] [Google Scholar]
- 23.Zhu Y, Alvares K, Huang Q, Rao MS, Reddy JK. Cloning of a new member of the peroxisome proliferator-activated receptor gene family from mouse liver. J Biol Chem 1993;268:26817–26820 [PubMed] [Google Scholar]
- 24.Esposito K, Ciotola M, Merante D, Giugliano D. Rosiglitazone cools down inflammation in the metabolic syndrome. Arterioscler Thromb Vasc Biol 2006;26:1413–1414 [DOI] [PubMed] [Google Scholar]
- 25.Gavrilova O, Haluzik M, Matsusue K, et al. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem 2003;278:34268–34276 [DOI] [PubMed] [Google Scholar]
- 26.Yu J, Zhang S, Chu ES, et al. Peroxisome proliferator-activated receptors gamma reverses hepatic nutritional fibrosis in mice and suppresses activation of hepatic stellate cells in vitro. Int J Biochem Cell Biol 2010;42:948–957 [DOI] [PubMed] [Google Scholar]
- 27.Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology 2003;38:1008–1017 [DOI] [PubMed] [Google Scholar]
- 28.de Vries CS, Russell-Jones DL. Rosiglitazone or pioglitazone in type 2 diabetes? BMJ 2009;339:b3076. [DOI] [PubMed] [Google Scholar]
- 29.Kawaguchi K, Sakaida I, Tsuchiya M, Omori K, Takami T, Okita K. Pioglitazone prevents hepatic steatosis, fibrosis, and enzyme-altered lesions in rat liver cirrhosis induced by a choline-deficient L-amino acid-defined diet. Biochem Biophys Res Commun 2004;315:187–195 [DOI] [PubMed] [Google Scholar]
- 30.Uto H, Nakanishi C, Ido A, et al. The peroxisome proliferator-activated receptor-gamma agonist, pioglitazone, inhibits fat accumulation and fibrosis in the livers of rats fed a choline-deficient, l-amino acid-defined diet. Hepatol Res 2005;32:235–242 [DOI] [PubMed]
- 31.Boettcher E, Csako G, Pucino F, Wesley R, Loomba R. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2012;35:66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zinman B, Wanner C, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 33.Rådholm K, Wu JH, Wong MG, et al. Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular disease, death and safety outcomes in type 2 diabetes - A systematic review. Diabetes Res Clin Pract 2018;140:118–128 [DOI] [PubMed] [Google Scholar]
- 34.Shiba K, Tsuchiya K, Komiya C, et al. Canagliflozin, an SGLT2 inhibitor, attenuates the development of hepatocellular carcinoma in a mouse model of human NASH. Sci Rep 2018;8:2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayashizaki-Someya Y, Kurosaki E, Takasu T, et al. Ipragliflozin, an SGLT2 inhibitor, exhibits a prophylactic effect on hepatic steatosis and fibrosis induced by choline-deficient l-amino acid-defined diet in rats. Eur J Pharmacol 2015;754:19–24 [DOI] [PubMed] [Google Scholar]
- 36.Lavalle-González FJ, Januszewicz A, Davidson J, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia 2013;56:2582–2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller CO, Yang X, Lu K, et al. Ketohexokinase knockout mice, a model for essential fructosuria, exhibit altered fructose metabolism and are protected from diet-induced metabolic defects. Am J Physiol Endocrinol Metab 2018;315:E386–E393 [DOI] [PubMed] [Google Scholar]
- 38.Savage DB, Choi CS, Samuel VT, et al. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J Clin Invest 2006;116:817–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harriman G, Greenwood J, Bhat S, et al. Acetyl-CoA carboxylase inhibition by ND-630 reduces hepatic steatosis, improves insulin sensitivity, and modulates dyslipidemia in rats. Proc Natl Acad Sci U S A 2016;113:E1796–E1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chow JD, Lawrence RT, Healy ME, et al. Genetic inhibition of hepatic acetyl-CoA carboxylase activity increases liver fat and alters global protein acetylation. Mol Metab 2014;3:419–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim CW, Addy C, Kusunoki J, et al. Acetyl CoA carboxylase inhibition reduces hepatic steatosis but elevates plasma triglycerides in mice and humans: a bedside to bench investigation. Cell Metab 2017;26:576. [DOI] [PubMed] [Google Scholar]
- 42.Wu M, Singh SB, Wang J, et al. Antidiabetic and antisteatotic effects of the selective fatty acid synthase (FAS) inhibitor platensimycin in mouse models of diabetes. Proc Natl Acad Sci USA 2011;108:5378–5383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen P, Miyazaki M, Socci ND, et al. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science 2002;297:240–243 [DOI] [PubMed] [Google Scholar]
- 44.Gutiérrez-Juárez R, Pocai A, Mulas C, et al. Critical role of stearoyl-CoA desaturase-1 (SCD1) in the onset of diet-induced hepatic insulin resistance. J Clin Invest 2006;116:1686–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu X, Burhans MS, Flowers MT, Ntambi JM. Hepatic oleate regulates liver stress response partially through PGC-1α during high-carbohydrate feeding. J Hepatol 2016;65:103–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Safadi R, Konikoff FM, Mahamid M, et al.; FLORA Group. The fatty acid-bile acid conjugate Aramchol reduces liver fat content in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2014;12:2085–2091.e1 [DOI] [PubMed]
- 47.Iruarrizaga-Lejarreta M, Varela-Rey M, Fernández-Ramos D, et al. Role of Aramchol in steatohepatitis and fibrosis in mice. Hepatol Commun 2017;1:911–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.ALJohani AM, Syed DN, Ntambi JM. Insights into stearoyl-coa desaturase-1 regulation of systemic metabolism. Trends Endocrinol Metab 2017;28:831–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yen CL, Stone SJ, Koliwad S, Harris C, Farese RV Jr. Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res 2008;49:2283–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamaguchi K, Yang L, McCall S, et al. Diacylglycerol acyltranferase 1 anti-sense oligonucleotides reduce hepatic fibrosis in mice with nonalcoholic steatohepatitis. Hepatology 2008;47:625–635 [DOI] [PubMed] [Google Scholar]
- 51.Yamamoto T, Yamaguchi H, Miki H, et al. Coenzyme A: diacylglycerol acyltransferase 1 inhibitor ameliorates obesity, liver steatosis, and lipid metabolism abnormality in KKAy mice fed high-fat or high-carbohydrate diets. Eur J Pharmacol 2010;640:243–249 [DOI] [PubMed] [Google Scholar]
- 52.Haas JT, Winter HS, Lim E, et al. DGAT1 mutation is linked to a congenital diarrheal disorder. J Clin Invest 2012;122:4680–4684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu XX, Murray SF, Pandey SK, et al. Antisense oligonucleotide reduction of DGAT2 expression improves hepatic steatosis and hyperlipidemia in obese mice. Hepatology 2005;42:362–371 [DOI] [PubMed] [Google Scholar]
- 54.Yamaguchi K, Yang L, McCall S, et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology 2007;45:1366–1374 [DOI] [PubMed] [Google Scholar]
- 55.Bricker DK, Taylor EB, Schell JC, et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science 2012;337:96–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Divakaruni AS, Wiley SE, Rogers GW, et al. Thiazolidinediones are acute, specific inhibitors of the mitochondrial pyruvate carrier. Proc Natl Acad Sci U S A 2013;110:5422–5427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Z, Vigueira PA, Chambers KT, et al. Insulin resistance and metabolic derangements in obese mice are ameliorated by a novel peroxisome proliferator-activated receptor γ-sparing thiazolidinedione. J Biol Chem 2012;287:23537–23548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Colca JR, McDonald WG, Cavey GS, et al. Identification of a mitochondrial target of thiazolidinedione insulin sensitizers (mTOT)--relationship to newly identified mitochondrial pyruvate carrier proteins. PLoS One 2013;8:e61551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McCommis KS, Chen Z, Fu X, et al. Loss of mitochondrial pyruvate carrier 2 in the liver leads to defects in gluconeogenesis and compensation via pyruvate-alanine cycling. Cell Metab 2015;22:682–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gray LR, Sultana MR, Rauckhorst AJ, et al. Hepatic mitochondrial pyruvate carrier 1 is required for efficient regulation of gluconeogenesis and whole-body glucose homeostasis. Cell Metab 2015;22:669–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McCommis KS, Hodges WT, Brunt EM, et al. Targeting the mitochondrial pyruvate carrier attenuates fibrosis in a mouse model of nonalcoholic steatohepatitis. Hepatology 2017;65:1543–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kelly MJ, Pietranico-Cole S, Larigan JD, et al. Discovery of 2-[3,5-dichloro-4-(5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yloxy)phenyl]-3,5-dioxo-2,3,4,5-tetrahydro[1,2,4]triazine-6-carbonitrile (MGL-3196), a highly selective thyroid hormone receptor β agonist in clinical trials for the treatment of dyslipidemia. J Med Chem 2014;57:3912–3923 [DOI] [PubMed] [Google Scholar]
- 63.Taub R, Chiang E, Chabot-Blanchet M, et al. Lipid lowering in healthy volunteers treated with multiple doses of MGL-3196, a liver-targeted thyroid hormone receptor-β agonist. Atherosclerosis 2013;230:373–380 [DOI] [PubMed] [Google Scholar]
- 64.Han CY. Update on FXR biology: promising therapeutic target? Int J Mol Sci 2018;19:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Holt JA, Luo G, Billin AN, et al. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev 2003;17:1581–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tully DC, Rucker PV, Chianelli D, et al. Discovery of tropifexor (ljn452), a highly potent non-bile acid FXR agonist for the treatment of cholestatic liver diseases and nonalcoholic steatohepatitis (NASH). J Med Chem 2017;60:9960–9973 [DOI] [PubMed] [Google Scholar]
- 67.Ma Y, Huang Y, Yan L, Gao M, Liu D. Synthetic FXR agonist GW4064 prevents diet-induced hepatic steatosis and insulin resistance. Pharm Res 2013;30:1447–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu X, Xue R, Ji L, et al. Activation of farnesoid X receptor (FXR) protects against fructose-induced liver steatosis via inflammatory inhibition and ADRP reduction. Biochem Biophys Res Commun 2014;450:117–123 [DOI] [PubMed] [Google Scholar]
- 69.Mudaliar S, Henry RR, Sanyal AJ, et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 2013;145:574–582.e1 [DOI] [PubMed]
- 70.Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev 1999;20:649–688 [DOI] [PubMed] [Google Scholar]
- 71.Tanaka T, Yamamoto J, Iwasaki S, et al. Activation of peroxisome proliferator-activated receptor delta induces fatty acid beta-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc Natl Acad Sci U S A 2003;100:15924–15929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wettstein G, Luccarini JM, Poekes L, et al. The new-generation pan-peroxisome proliferator-activated receptor agonist IVA337 protects the liver from metabolic disorders and fibrosis. Hepatol Commun 2017;1:524–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Staels B, Rubenstrunk A, Noel B, et al. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology 2013;58:1941–1952 [DOI] [PubMed] [Google Scholar]
- 74.Cariou B, Hanf R, Lambert-Porcheron S, et al. Dual peroxisome proliferator-activated receptor α/δ agonist GFT505 improves hepatic and peripheral insulin sensitivity in abdominally obese subjects. Diabetes Care 2013;36:2923–2930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ratziu V, Harrison SA, Francque S, et al.; GOLDEN-505 Investigator Study Group. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-alpha and -delta, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology 2016;150:1147–1159.e5 [DOI] [PubMed]
- 76.Jani RH, Kansagra K, Jain MR, Patel H. Pharmacokinetics, safety, and tolerability of saroglitazar (ZYH1), a predominantly PPARα agonist with moderate PPARγ agonist activity in healthy human subjects. Clin Drug Investig 2013;33:809–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jain MR, Giri SR, Trivedi C, et al. Saroglitazar, a novel PPARα/γ agonist with predominant PPARα activity, shows lipid-lowering and insulin-sensitizing effects in preclinical models. Pharmacol Res Perspect 2015;3:e00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jain MR, Giri SR, Bhoi B, et al. Dual PPARα/γ agonist saroglitazar improves liver histopathology and biochemistry in experimental NASH models. Liver Int 2018;38:1084–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]