Abstract
The new diterpene methoxy-ent-8(14)-pimarenely-15-one (1) and three known metabolites: ent-8(14)-pimarene-15R,16-diol (2), stigmasterol (3) and β-sitosterol (4), were isolated from the roots of the mangrove plant Ceriops tagal. Their structures and relative stereochemistry were elucidated by means of extensive NMR, IR and MS analysis. Compounds 1, 2, 3 and 4 exhibited significant antifouling activities against cyprid larvae of the barnacle Balanus albicostatus Pilsbry, with EC50 values of 0.32 ± 0.01, 0.04 ± 0.00, 4.05 ± 0.15 and 18.47 ± 0.40 µg/cm2, respectively, whereas their toxicities towards cyprids were very low, with LC50 values all above 10 µg/cm2.
Keywords: Diterpenes, antifouling activity, barnacle, Ceriops tagal
Introduction
Marine fouling organisms often cause technical and economic problems by settling on artificial surfaces submerged in seawaters [1]. Although organotin compounds such as tributyltin (TBT) and tributyltin oxide (TBTO) have been widely used for controlling these fouling organisms, they were also found to be toxic to many non-target marine organisms [2]. The Marine Environment Protection Committee of the International Maritime Organization has proposed a ban on the application of TBT-based antifouling paints from January 1, 2003 and the ban of the presence of such paints on the surface of vessels from January 1, 2008 [3]. Currently, some booster biocides are used for antifouling paints, but they may also pollute the aquatic environments [4]. Therefore, effective and environmentally friendly antifoulants are urgently needed. An important source of such effective and environmentally friendly antifoulants is the antifouling active substances isolated from marine organisms. To date, a variety of natural products with antifouling activities have been isolated from lots of different marine organisms, including marine bacteria, algae, seagrass, sponge, coral, bryozoa, ascidian, etc. [5,6]. However, there is scant information on isolation of antifouling compounds from mangrove species, which is one kind of marine higher plants [7].
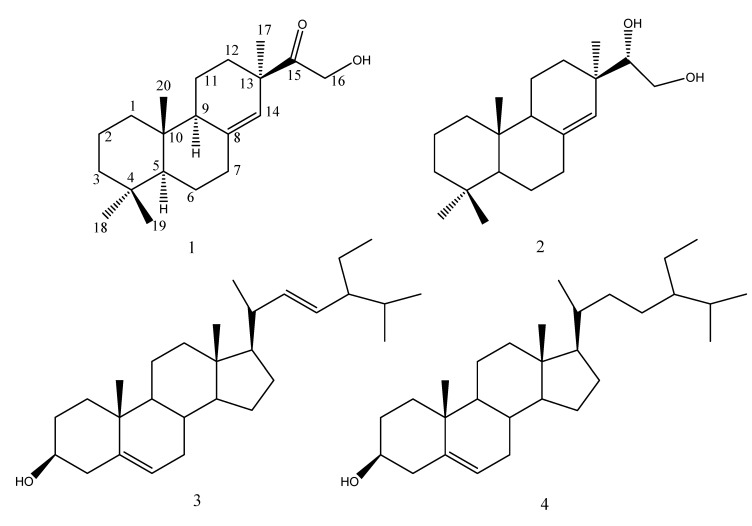
Marine organisms use the diverse mechanisms to protect their own surfaces from fouling [8]. Within the marine ecosystem, evolution has allowed for the development of certain antifouling properties. Marine organisms have both physical and chemical methods to protect themselves from the harmful process of biofouling [8,9]. The key chemical antifouling mechanism of marine organisms occurs via the production of secondary metabolites (also known as natural products) which deter foulers [1]. During our search for structurally interesting and antifouling natural products from the mangrove plants, we found that compounds from the roots of Ceriops tagal inhibited significantly larval settlement of the barnacle Balanus albicostatus, which is one important fouling organism in East Asian coastal waters. These antifouling active compounds included one new diterpene, methoxy-ent-8(14)-pimarenely-15-one (1) and three known metabolites, designated as ent-8(14)-pimarene-15R,16-diol (2), stigmasterol (3) and β-sitosterol (4) (Figure 1). The isolation, structural elucidation and antifouling activity of these compounds are described in this paper.
Figure 1.
Structure of the compounds.
Results and Discussion
One new diterpene, methoxy-ent-8(14)-pimarenely-15-one (1), and three known metabolites ent-8(14)-pimarene-15R,16-diol (2), stigmasterol (3) and β-sitosterol (4) were isolated from the roots of C. tagal. These compounds showed potent non-toxic antifouling activities against larval settlements of B. albicostatus.
Methoxy-ent-8(14)-pimarenely-15-one (1) was obtained as amorphous powder, [α]D25-2.37º (c ‑0.002, CHCl3). Its molecular composition of C20H32O2 was established by HRESIMS at m/z 305.2487 [M + 1]+ (calcd. 305.2481 for C20H33O2). The ESIMS of 1 showed quasimolecular ion peaks at m/z 305.4 [M + 1] +. The IR absorptions at 3433, 1735 cm-1 showed the presence of hydroxyl and carbonyl groups. Analysis of the 1H-NMR, 13C-NMR, DEPT and HMQC spectra revealed not only the presence of four methyls, eight methylenes (including one oxygenated methylene at δC 65.9, t), three methines and five quaternary carbon (including one carbonyl group at δC 214.9, s), but also the presence of one trisubstituted double bond (Table 1). Four singlet methyls (δC 22.4, q; 22.0, q; 33.6, q; 14.5, q) and one trisubstituted double bond (δC 143.3, s; 122.8, d), in addition to the optical rotation data of 1, suggested that a pimarane diterpenoid structure might be presented [10,11,12,13,14].
Table 1.
1H-NMR Data (500 MHz) and 13C- NMR Data (125 MHz) for Compound 1 (CDCl3).
| No. | δC | δH (J = Hz) |
|---|---|---|
| 1 | 38.8 t | 1.01 (m), 1.57 (m) |
| 2 | 18.8 t | 1.43 (m) |
| 3 | 42.0 t | 1.20 (m), 1.39 (m) |
| 4 | 33.3 s | |
| 5 | 54.6 d | 1.07 (m) |
| 6 | 22.4 t | 1.36 (dd, 4.5, 13), 1.64 (m) |
| 7 | 35.8 t | 2.11 (ddd, 5, 13.5, 18.5), 2.36 (m) |
| 8 | 143.3 s | |
| 9 | 51.0 d | 1.75 (m) |
| 10 | 38.5 s | |
| 11 | 20.1 t | 1.61 (m) |
| 12 | 32.8 t | 1.13 (m), 2.31 (m) |
| 13 | 46.8 s | |
| 14 | 122.8 d | 5.37 (s) |
| 15 | 214.9 s | |
| 16 | 65.9 t | 4.36 (s) |
| 17 | 22.4 q | 1.12 (s) |
| 18 | 33.6 q | 0.88 (s) |
| 19 | 22.0 q | 0.83 (s) |
| 20 | 14.5 q | 0.64 (s) |
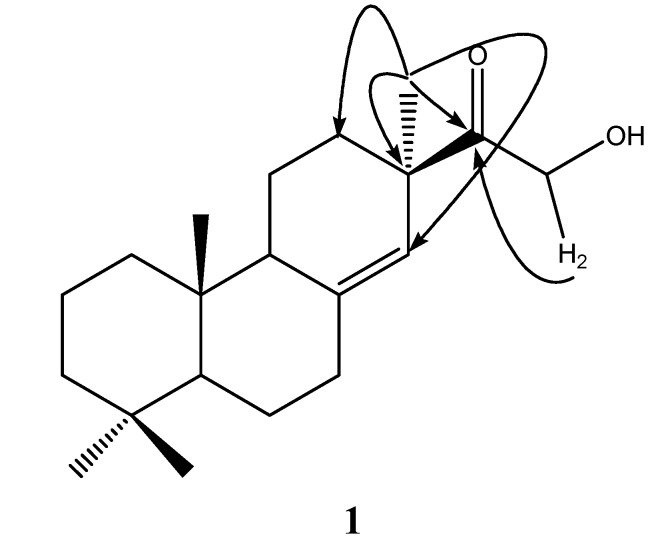
The planar structure of 1 was elucidated by the analysis of 2D-NMR data and by comparison with the NMR data of 2 (Table 1). The NMR spectra of 1 were similar to those of 2, except for the fact that the chemical shift of C-15 (δC 214.9, s) were shifted more upfield than that of 2 (δC 78.7, d), indicating that the oxygenated methine of 2 was replaced by carbonyl group. Furthermore, The HMBC correlations from Me-17 (δH 1.12, s) to C-15 (δC 214.9, s), C-14 (δC 122.8, d), C-13 (δC 46.8, s) and C-12 (δC 32.8, t), and from H-16 (δH 4.36, s) to C-15 (δC 214.9, s) indicated the connectivity between C-13/C-15 and C-15/C-16 (Figure 2). Thus, the gross structure of 1 was assigned as a new pimarane diterpenoid.
Figure 2.
Key HMBC correlations of Compound 1.
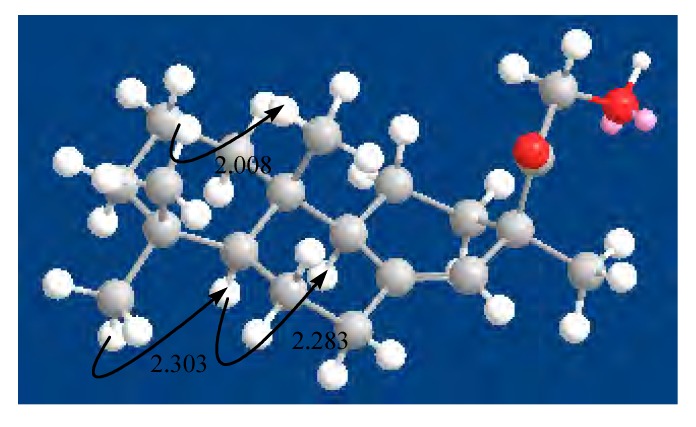
In addition, the comparison of its NMR data with that of compound 2 and the NOE cross-signals confirmed the relative stereochemistry of compound 1. Comparison of the NMR data of 1 with 2 showed that the Me-18 (33.6, q), Me-17 (22.4, q) was in an α-orientation, while Me-19 (22.0, q) was in a β-orientation. Then, the 3-D structure was obtained using CHEM 3D ULTRA V 9.0, with MM2 force-file calculations for energy minimization in the computer (Figure 3). NOE correlations between Me-19 and Me-20 (2.013 Ǻ) confirmed that Me-20 was in a β-orientation. Then, the cross-peaks of NOE correlations between Me-18 and H-5 (2.306 Ǻ) and between H-5 and H-9 (2.286 Ǻ) indicated that the H-5 and H-9 were on the β-side of 1.
Figure 3.
Key NOE correlations of compound 1 and corresponding interatomic distances (Ǻ).
The known compounds ent-8(14)-pimarene-15R,16-diol (2), stigmasterol (3) and β-sitosterol (4) were identified by comparison of their physical and spectral data with those in the literature [14,15,16].
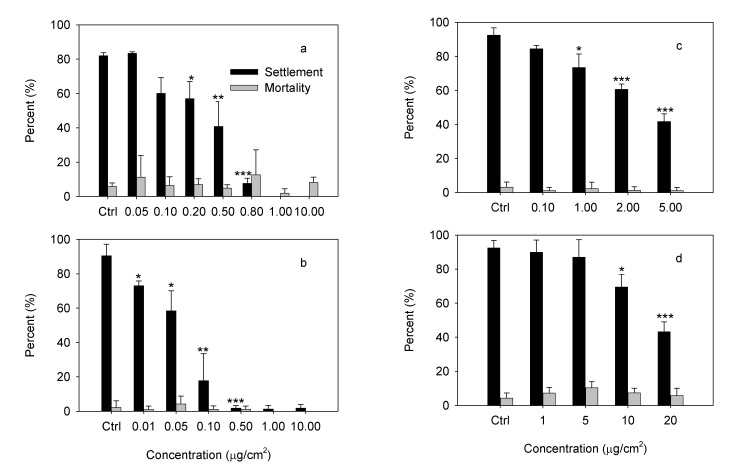
The antifouling activities of four compounds are shown in Figure 4. Compounds 1, 2, 3 and 4 exhibited significant antifouling activities against cyprid larvae of the barnacle B. albicostatus Pilsbry, with EC50 values of 0.32 ± 0.01, 0.04 ± 0.00, 4.05 ± 0.15 and 18.47 ± 0.40 µg/cm2, respectively. On the other hand, the LC50 values of four compounds were all above10 µg/cm2 (Figure 4), indicating that their toxicities to cyprids were quite low and they all inhibited cyprid settlement in a non-toxic way.
Figure 4.
Effects of Compounds 1-4 on settlement and survival of cyprid larvae in B. albicostatus. a) Methoxy-ent-8(14)-pimarenely-15-one (1); b) Ent-8(14)-pimarene-15R,16-diol (2); c) Stigmasterol (3); d) β-sitosterol (4). Data were analyzed using one-way ANOVA, where *P < 0.05; **P < 0.01 and ***P < 0.001 were significantly different from the control.
Pimarane diterpenoid had a stronger antifouling activity than phytosterin. Our structure-activity relationship analysis of pimarane diterpenoid suggested that the antifouling activity might be due to the number of free hydroxyl group in side chain of pimarane diterpenoid. Compound 2, with two free hydroxyl groups had the higher antifouling activity than compound 1 with only one free hydroxyl group. However, there was no great effect of the number of free hydroxyl group on the toxicity of pimarane diterpenoids. In addition, the structure-activity relationship analysis of phytosterin suggested that the presence of double bond in side chain of phytosterin might be important in expression of the antifouling activity in these compounds. Compound 3 had the stronger antifouling activity than compound 4 due to its double bond group. But, it seemed that the double bond in side chain of phytosterin did not influence the toxicity of phytosterins.
Experimental
General
1H-NMR spectra were measured with Varian UNITY PLUS 500 spectrometer (USA). 1H-, 13C- and 2D-NMR spectra were measured with Varian INOVA 600 spectrometer (USA). High-resolution ESI mass spectra data were measured with a Waters Q-TOF MicroTM spectrometer (USA) and were provided by Zhengzhou University, China. Low-resolution ESI mass spectra data were recorded on an AB 3200Q TRAP spectrometer (USA). IR spectra were measured using a Nicolet 380 FT-IR spectrophotometer (USA). The optical rotation data were obtained on a Rudolph Autopol IV polarimeter (USA).
Extraction and Isolation
The roots of C. tagal were collected from Hainan Province, China in July 2005. The air-dried and powdered material (4.1 kg) was extracted with 95% EtOH at room temperature. The extract was evaporated under vacuum to give a residue (715 g), and then the residue was partitioned between petroleum ether, ethyl acetate and H2O. The petroleum ether extract (11.5 g) was chromatographed on a silica gel column, eluted with a petroleum ether – ethyl acetate gradient to obtain 13 fractions (A-M). Fraction H (1.1 g) was chromatographed over silica gel and Sephadex LH-20 repeatedly (elution with 24:1 hexane - acetone) to yield compound 1 (23 mg). Compound 2 (200 mg) was recrystallized with acetone –MeOH (4:1) from fraction M (1.562 g). Fraction L (438 mg) was chromatographed over Sephadex LH-20 column eluting with CHCl3-MeOH (1:1) and the major fraction obtained was then purified by silica gel column chromatography eluting with petroleum ether- acetone (60:1) to yield compound 3 (11 mg). Fraction J (850 mg) was subjected to silica gel column chromatography eluting with petroleum ether- acetone (9:1) to yield compound 4 (646 mg).
Methoxy-ent-8(14)-pimarenely-15-one (1): C20H32O2; amorphous powder; IR νmax (KBr): 3433, 1735 cm-1; ESIMS, m/z 305.4 [M + 1] +, HRESIMS at m/z 305.2487 [M + 1]+ (calcd. 305.2481 for C20H33O2); 1H-NMR and 13C-NMR data for 1 are listed in Table 1.
Antifouling Assay
Antifouling efficacies of the four compounds towards C. tagal were evaluated by the settlement inhibition assay with cyprid larvae of B. albicostatus. Adult B. albicostatus were obtained from intertidal zone in Xiamen, China. In the laboratory, the nauplius I and II were released by subjecting the adult barnacles to aerial exposure for a period of 12 h, followed by immersion in filtered seawater (FSW 0.22 µm, salinity 30‰, and temperature 25°C). Naupilar larvae were collected using a pipette and placed into 8-liter glass beakers containing FSW. Naupilar larvae were reared at a density of 1 larvae mL-1 on a diet of the diatom Chaetoceros muelleri with a concentration of 2.0 × 105 cells·mL-1. The seawater and diet were renewed everyday. After 5~6 days, the cultures were monitored for the presence of cyprids and the cyprids were harvested within 24 h after molting.
Tested compounds were dissolved in CH2Cl2. Aliquots of the solution were added to the glass dishes with diameter of 6 cm and air-dried. Then, 30 cyprids and 10 mL FSW were placed into each glass dish. Cyprids were exposed to different concentrations of the compounds determined as shown in Figure 4. There were three replicates for the FSW control and each concentration. After 48 h incubation at 25°C, the numbers of larvae which settled, died or did not settle in each replicate were enumerated with the aid of a stereomicroscope.
Statistical analysis
Differences in the settlement or mortality percentages of experimental and control treatments were tested for significance by one-way ANOVA. Significance was set at the 5% level. The EC50 (the concentration that reduces the settlement rate by 50% relative to the control) and LC50 (the concentration that results in 50% mortality relative to the control) both with a 95% confidence interval were estimated by using the Spearman-Karber method [17,18].
Acknowledgements
This work was supported by grants from Scientific and Technological Projects of Fujian Province (2003Y036), the National Natural Science Foundation of China (40376026, 30671646, 30530150), by Program for New Century Excellent Talents in University (NCET-07-0725) and by Program for Innovative Research Team in Science and Technology in Fujian Province University. We thank Professor Sun Handong at Kunming Institute of Botany, the Chinese Academy of Sciences for assistance with NMR determination.
Footnotes
Sample Availability: Samples of the compounds are available from authors.
References
- 1.Richmond M. D., Seed R. A review of marine macrofouling communities with special reference to animal fouling. Biofouling. 1991;2:151–168. doi: 10.1080/08927019109378169. [DOI] [Google Scholar]
- 2.Ellis D. V. New dangerous chemicals in the environment: lessons from TBT. Mar. Pollut. Bull. 1991;22:8–10. doi: 10.1016/0025-326X(91)90437-W. [DOI] [Google Scholar]
- 3.Yebra D. M., Kiil S., Dam-Johansen K. Antifouling technology-past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004;50:75–104. doi: 10.1016/j.porgcoat.2003.06.001. [DOI] [Google Scholar]
- 4.Thomas K. V. The environmental fate and behavior of antifouling paint booster biocides: A review. Biofouling. 2001;17:73–86. doi: 10.1080/08927010109378466. [DOI] [Google Scholar]
- 5.Clare A. S. Marine natural product antifoulants: status and potential. Biofouling. 1996;9:211–229. doi: 10.1080/08927019609378304. [DOI] [Google Scholar]
- 6.Rittschof D. In: Natural product antifoulants and coatings development. McClintock J. B., Baker B. J., editors. Marine Chemical Ecology. CRC Press; Boca Raton: 2001. pp. 543–566. [Google Scholar]
- 7.Chambers L. D., Stokes K. R., Walsh F. C., Wood R. J. K. Modern approaches to marine antifouling coatings. Surf. Coat. Technol. 2006;201:3642–3652. doi: 10.1016/j.surfcoat.2006.08.129. [DOI] [Google Scholar]
- 8.Wahl M., Kröger K., Lenz M. Non-toxic protection against epibiosis. Biofouling. 1998;12:205–226. doi: 10.1080/08927019809378355. [DOI] [Google Scholar]
- 9.Wahl M., Marine epibiosis I. Fouling and antifouling: some basic aspects. Mar. Ecol. Prog. Ser. 1989;58:175–189. doi: 10.3354/meps058175. [DOI] [Google Scholar]
- 10.Lai A. R., Cambie R. C., Rutledge P. S., Woodgate P. D. Ent-Pimarane and ent-abietane diterpenes from Euphorbia fidjiana. Phytochemistry. 1990;29:2239–2246. doi: 10.1016/0031-9422(90)83045-3. [DOI] [Google Scholar]
- 11.Ma G. X., Wang T. S., Yin L., Pan Y. Two pimarane diterpenoids from Ephemerantha ionchophylla and their evaluation as modulators of the multidrug resistance phenotype. J. Nat. Prod. 1998;61:112–115. doi: 10.1021/np970065o. [DOI] [PubMed] [Google Scholar]
- 12.Luo X. D., Wu S. H., Ma Y. B., Wu D. G. Ent-pimarane derivatives from Dysoxylum hainanense. Phytochemistry. 2001;57:131–134. doi: 10.1016/S0031-9422(00)00482-9. [DOI] [PubMed] [Google Scholar]
- 13.Xiang Y., Zhang H., Fan C. Q., Yue J. M. Novel diterpenoids and diterpenoid glycosides from Siegesbeckia orientalis. J. Nat. Prod. 2004;67:1517–1521. doi: 10.1021/np0400407. [DOI] [PubMed] [Google Scholar]
- 14.Han L., Huang X. S., Sattler L., Dahse H. M., Fu H. Z., Grabley S., Lin W. H. Three new pimaren diterpenoids from mangrove plant, Bruguiera gymnorrhiza. Pharmazie. 2005;60:705–707. [PubMed] [Google Scholar]
- 15.Zhao Q. S., Tian J., Yue J. M., Chen S. N., Lin Z. W., Sun H. D. Diterpenoids from Isodon flavidus. Phytochemistry. 1998;48:1025–1029. doi: 10.1016/S0031-9422(97)00608-0. [DOI] [Google Scholar]
- 16.Wright J. L. C., Mcinnes A. G., Shimizu S., Smith D. G., Watter J. A. Identification of C-24 alkyl epimers of marine sterols by 13C nuclear magnetic resonance spectroscopy. Can. J. Chem. 1978;56:1898–1903. doi: 10.1139/v78-308. [DOI] [Google Scholar]
- 17.Hamilton M. A., Russo R. C., Thurston R. V. Trimmed Spearman–Karber method for estimating median lethal concentrations in toxicity bioassays. Environ. Sci. Technol. 1977;11:714–719. doi: 10.1021/es60130a004. [DOI] [Google Scholar]
- 18.Hamilton M. A., Russo R. C., Thurston R. V. Correction to: Hamilton, M. A.; Russo, R. C. and Thurston, R. V. Trimmed Spearman–Karber method for estimating median lethal concentrations in toxicity bioassays. Environ. Sci. Technol. 1978;12:417. doi: 10.1021/es60140a017. [DOI] [Google Scholar]