Abstract
Scopolamine is an alkaloid widely used in medicine for its anticholinergic activity. The aim of this review is to show that metabolic engineering techniques constitute a suitable tool to improve the production of tropane alkaloids, focusing in particular on scopolamine. We present an overview of results obtained by various research groups, including our own, who have studied the overexpression of genes involved in the biosynthesis of scopolamine in different plant species that produce tropane alkaloids. Experiments carried out to improve production in hairy root cultures will also be described, as well as those attempting to biotransform hyoscyamine into scopolamine in roots and transgenic tobacco cells.
Keywords: Scopolamine, hyoscyamine, hairy roots, putrescine N-methyltransferase, hyoscyamine-6-β-hydroxylase
Introduction
In developing countries, plants are the main source of medicines: according to the World Health Organization, as much as 80% of the world’s population in developing countries relies on traditional medicine for its primary health care, and this is mainly based on plant remedies. The use of herbal medicine in developed countries is also growing; for example, it has been reported that 25% of the UK population regularly takes herbal medicines [1]. Bioactive compounds currently extracted from plants, besides being used as important therapeutic and remedy products, also serve as food additives, pigments, dyes, insecticides, cosmetics and perfumes. However, the fast rate at which the habitats for medicinal plants are disappearing, together with geopolitical problems, has resulted in certain plant-derived compounds becoming increasingly difficult to obtain.
Advances in biotechnology, particularly methods for culturing plant cells and organs, and for micropropagation of medicinal plants will provide new means for the commercial processing of even rare plants and the chemicals they provide. Metabolic engineering involves the targeted and purposeful alteration of metabolic pathways found in an organism to achieve better understanding and use of cellular pathways for chemical transformation, energy transduction, and supramolecular assembly [2]. This technique applied to plants will permit endogenous biochemical pathways to be manipulated, resulting in the generation of transgenic crops in which the range, scope, or nature of a plant’s existing natural products are modified to provide beneficial commercial, agronomic and/or post-harvest processing characteristics [3].
Over the last decades, plant cell cultures have been intensively investigated as a possible tool for the production of commercial plant secondary metabolites, including fine chemicals such as pharmaceuticals, agrochemicals, flavors, insecticides, fragrances and cosmetics [4]. In spite of the efforts in the field of in vitro production of phytochemicals, few industrial processes have been developed, involving only a limited number of secondary products, such as shikonin, berberine, ginsenosides and paclitaxel [5]. As in many cases production is too low for commercialization, metabolic engineering can provide various strategies to improve productivity, such as:
Increasing the number of producing cells
Increasing the carbon flux through a biosynthetic pathway by overexpression of genes codifying for rate-limiting enzymes or blocking the mechanism of feedback inhibition and competitive pathways
Decreasing catabolism
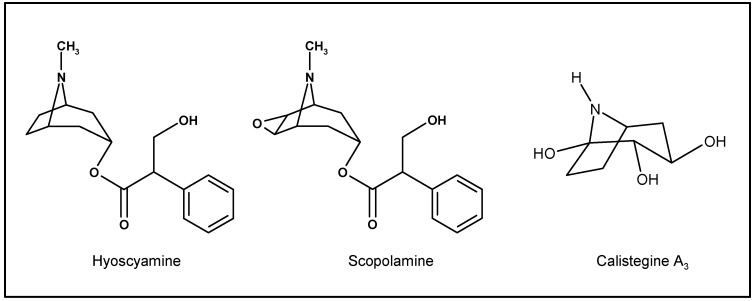
Many of the isolated pure compounds with biological activity are alkaloids, a diverse group of nitrogen-containing chemical ring structure compounds, with alkali-like chemical reactivity and pharmacological activity. Although the pharmacological effects of alkaloids have been studied, the biosynthetic pathways of these compounds are still obscure. Among the most famous are the tropane alkaloids, such as (-)-hyoscyamine, its racemate atropine, and scopolamine (hyoscine), which have an 8-azabicyclo[3.2.1]octane esterified nucleus (Figure 1). These alkaloids are commonly found in plants of different families: Solanaceae, Erythroxylaceae, Convolvulaceae, Proteaceae, Euphorbiaceae, Rhizophoraceae and Cruciferae [6]. Related to the tropane alkaloids, a new group of nortropane alkaloids, the calystegines, was discovered only 15 years ago. Calystegines bear three to five hydroxyl groups in various positions, making them water-soluble (Figure 1), and they share metabolic steps and enzymes of the formation of tropane alkaloids.
Figure 1.
Chemical structures of the anticholinergic alkaloids hyoscyamine, scopolamine and calystegine A3.
The tropane alkaloids, scopolamine and its precursor hyoscyamine, which are found mainly in the Duboisia, Datura, Hyoscyamus, Atropa and Scopolia species (see Table 1), together with their semisynthetic derivatives, are used as parasympatholytics that competitively antagonize acetylcholine. Anticholinergics are generally used as mydriatics, and to control the secretion of saliva and gastric acidity, slow gut motility and prevent vomiting. The roots are the principal site of alkaloid biosynthesis but leaves can also accumulate significant quantities of these compounds. Hyoscyamine is normally the more abundant alkaloid in plants, with scopolamine produced in greater quantities only in Duboisia spp and Datura metel [7]. The main source of raw material for tropane alkaloid production worldwide is Duboisia leaves, which contain 2-4% of total alkaloids, with more than 60% of scopolamine and 30% of hyoscyamine. Conventional cultivation of some varieties that can accumulate up to 6% of scopolamine has been established in Australia, Ecuador and Brazil, producing 1 t/ha of this alkaloid.
Table 1.
Distribution of hyoscyamine and/or scopolamine in the Solanaceae family [6].
| Solanoidae | Daturae | Datura | D. stramonium, D. ferox, D. quercifolia, D. pruinosa, D. leichahhardtii, D. inoxia, D. discolor, D. metel, D. wrightii |
| Brugmansia | B. aurea, B. sanguinea, B. arborea, B. xcandida, B. xdolichocarpa, B. xinsignis, B. versicolor, B. vulcanicola | ||
| Solandrae | Solandra | S. longifolia, S. grandifolia, S. guttata, S. hartvegii, S. hirsute, S. macranthe | |
| Solaneae | Atropa | A. belladonna | |
| Latua | L. pubiflora | ||
| Acristus | A. arborea | ||
| Mandragora | M. autumnale, M. vernalis | ||
| Salpichroa | S. organiflora | ||
| Hyoscyameae | Scopolia | S. carniolica, S. parviflora | |
| Hyoscyamus | H. muticus, H. niger, H. albus, H. aureus | ||
| Physochlaina | P. physaloides, P. orientalis | ||
| Przewalskia | P. tangutica | ||
| Cestroideae | Anthocercidae | Duboisia | D. hopwoodii, D. leichhardtii, D. myoporoides, D. arenitensis, D. hybrid |
| Anthotroche | A. myoporoides, A. pannosa, A. walcottii | ||
| Anthocercis | A. littorea, A. viscose, A. fasciculote, A. ilicitolia, A. genistoides | ||
| Cyphanthera | C. anthocercidea, C. albicans | ||
| Symonanthus | S. aromaticus | ||
| Grammosolen | G. dixonii |
Scopolamine is the most valuable tropane alkaloid, preferred for its higher physiological activity and fewer side effects. The world demand for this alkaloid is estimated to be about 10 times greater than for hyoscyamine and its racemic form atropine. Accordingly, there has been a long-standing interest in boosting the scopolamine content of producing plants and their in vitro cultures. In the case of biotechnological production of scopolamine, increasing the number of cells able to produce this compound is essential because tropane alkaloids are mostly synthesized in young cells and translocated to the aerial parts of the plants [8], so biotechnological processes based on undifferentiated systems such as calli or cell cultures are not productive [9,10]. Thus the attempts to produce scopolamine in biotechnological systems are based mainly on hairy root cultures.
Biosynthesis of scopolamine
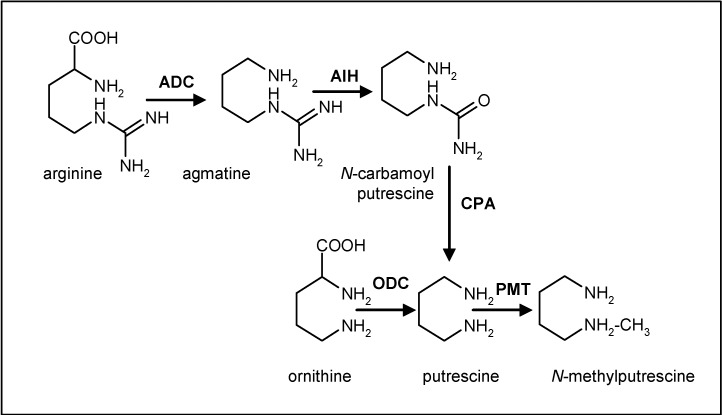
At present, the metabolic pathway for scopolamine biosynthesis is still not fully understood and only a few of the enzymes involved have been isolated and corresponding genes cloned, while practically nothing is known about how the synthesis is regulated [11]. The tropane ring present in the molecule of scopolamine derives from putrescine via an N-methylpyrrolinium salt. Walton et al. [12] demonstrated that two amino acids, ornithine and arginine, are involved in the biosynthesis of putrescine by alternative pathways (Figure 2). The decarboxylation of ornithine yields putrescine directly, whereas arginine has to be transformed into agmatine to produce putrescine (see Figure 2).
Figure 2.
Biosynthesis of N-methylputrescine from arginine and ornithine. ADC: Arginine decarboxylase. AIH: Agmatine iminohydrolase. CPA: N-carmaboylputrescine amidohydrolase. ODC: Ornithine decarboxylase. PMT: putrescine N-methyltransferase.
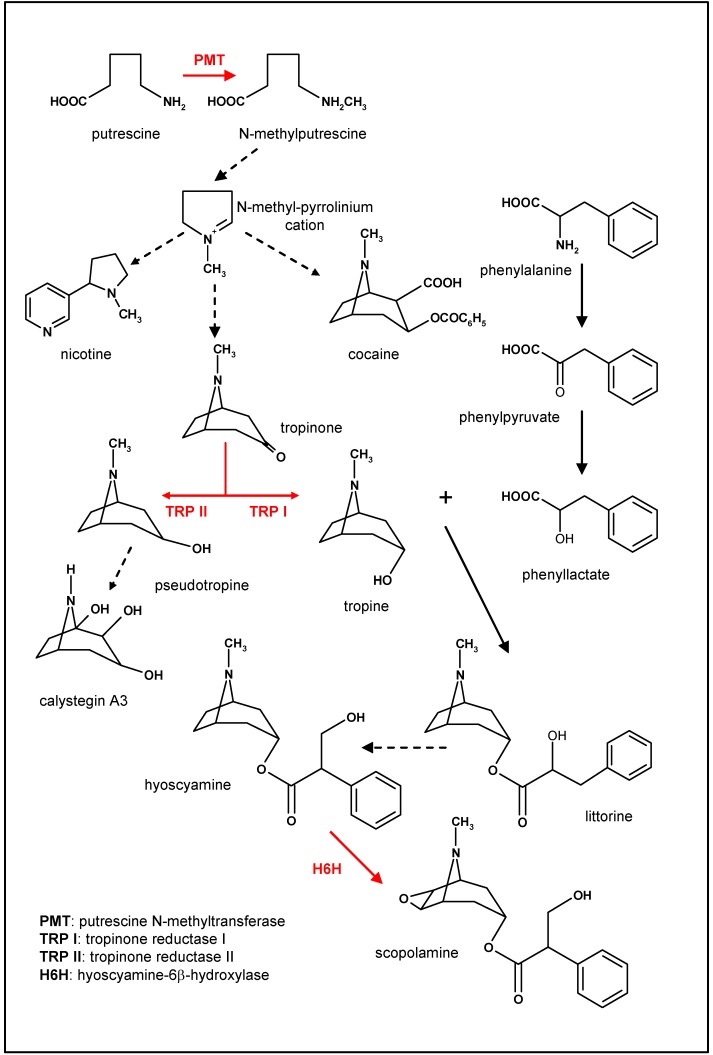
The next step is the S-adenosyl-methionine (SAM)-dependent methylation of putrescine catalysed by putrescine N-methyltransferase (PMT; EC2.1.1.53), forming N-methylputrescine. This enzyme removes putrescine from the polyamine pool and drives the methylated compound exclusively toward alkaloid production (Figure 3). Feeding experiments [13,14,15] have demonstrated that this is a flux limiting step. The oxidative transamination of N-methylputrescine yields an aminoaldehyde that is primed for cyclisation to an N-methylpyrrolinium intermediate [16].
Figure 3.
Overview of the most important steps in the scopolamine biosynthetic pathway. The enzymes overexpressed in scopolamine-producing hairy root cultures are in red.
The next steps in the biosynthesis require the condensation of an appropriate acetate-derived intermediate with N-methylpyrrolinium. Hygrine, an alkaloid that is formally the product of an acetone condensation with N-methylpyrrolidine, was for many years considered to be an intermediate in the biosynthesis of scopolamine, but recent studies suggest that hygrine is not involved in tropane ring biosynthesis [16]. Two tropinone reductases responsible for tropane alcohol formation have been isolated and characterized from root cultures of several species (see [17] and references therein). All the plant species studied so far possess two strictly stereospecific tropinone reductase activities, one (TRI) producing tropine and the other (TRII) producing pseudotropine (see Figure 3). Pseudotropine, as a product of tropinone reduction, undergoes a rapid conversion to calystegines, while tropine is esterified to yield hyoscyamine, the precursor of scopolamine [19].
The biosynthesis of tropic acid, the ester moiety of scopolamine, has been a topic of interest for many years [19,20,21,17]. Isotopic labelling studies in transformed root cultures of Datura stramonium have demonstrated that littorine [(R)-phenyllactoyl tropine] is the direct biosynthetic precursor of hyoscyamine by an intramolecular rearrangement [22] (Figure 3). Thus, the tropate ester moiety of hyoscyamine is derived by isomerisation from the (R)-phenyllactate ester of littorine. However, the results obtained by Patterson and O’Hagan [23] suggest that the rearrangement of littorine into hyoscyamine does not occur with a vicinal interchange process as previously thought, and thus the mechanism of isomerisation remains to be determined. Recently, a multifunctional cytochrome P450 capable of littorine rearrangement via a hyoscyamine aldehyde has been identified from Hyoscyamus niger [24].
Scopolamine, the 6,7-epoxide of hyoscyamine, is formed by direct oxidation of hyoscyamine without the intermediacy of a double bond. Hyoscyamine 6β-hydroxylase (H6H; EC 1.14.11.11) is an oxoglutarate-dependent dioxygenase that mediates a two-step reaction to generate the epoxide. The first step involves a hydroxylation to lead 6β-hydroxyhyoscyamine and the same enzyme mediates the epoxide ring closure to generate scopolamine [17].
Hairy root cultures as a source of scopolamine
The hairy root system based on inoculation with Agrobacterium rhizogenes has become popular in the two last decades as a method of producing secondary metabolites synthesized in plant roots [25,26]. Unorganized plant tissue cultures are frequently unable to produce secondary metabolites at the same levels as the intact plant. This is also the case of scopolamine production in undifferentiated in vitro cultures of Solanaceae, probably due to the specific location of some of the key enzymes involved in this biosynthetic pathway [27]. Suzuki et al. [28] have demonstrated that the expression of the pmt gene was pericycle-specific, and it has also been shown that H6H is localized in the root pericycle [29,30]. In addition, Nakajima and Hashimoto [31] have observed that TR proteins accumulate in the lateral roots of Hyoscyamus niger. Another possible reason for the low production of scopolamine in undifferentiated in vitro cultures could be that the auxin added to the callus and cell culture media for normal growth inhibits the activity of some of the key enzymes involved in scopolamine biosynthesis, such as PMT [32].
The hairy root phenotype is characterized by fast hormone-independent growth, lack of geotropism, lateral branching and genetic stability. The secondary metabolites produced by hairy roots arising from the infection of plant material by A. rhizogenes are the same as those usually synthesized in intact parent roots, with similar or higher yields [33]. This feature, together with genetic stability and generally rapid growth in simple media lacking phytohormones, makes them especially suitable for biochemical studies not easily undertaken with root cultures of an intact plant.
During the infection process A. rhizogenes transfers a part of the DNA (transferred DNA, T-DNA) located in the root-inducing plasmid Ri to plant cells and the genes contained in this segment are expressed in the same way as the endogenous genes of the plant cells [34]. Some A. rhizogenes, such as strain A4, have the T-DNA divided in two sections, the TR-DNA and TL-DNA, each of which can be incorporated separately into the plant genome. Two sets of pRi genes are involved in the root induction process: the aux genes located in the TR region of the pRi T-DNA and the rol (root loci) genes of the TL region [35]. The ags genes responsible for opine biosynthesis in the transformed tissues are also located in the TR region [36]. Opines are synthesized by plant transformed cells and are only used by Agrobacterium as a source of nitrogen and carbon.
Due to the similarities of the A. rhizogenes and A. tumefaciens infection processes, and because both microorganisms are very closely related, it has been suggested that the most important A. rhizogenes oncogenes encode proteins involved in the regulation of plant hormone metabolism. Aux genes provide transformed cells with an additional source of auxin [37,38], but they do not seem essential for developing hairy root disease [39]. However, rol genes have functions that are most likely other than that of producing mere alterations in plant hormone concentrations [40].
Several authors have investigated the effect of TR and TL regions of A. rhizogenes on growth and morphology of transformed roots and plants, but until now there have been few studies on the direct effects of oncogenes on secondary metabolism. As has been previously reported, a correlation exists between the expression of the rolC gene and tropane alkaloids [41,42,43], Catharanthus roseus alkaloids [44], and ginsenoside production [45]. No correlation between rolA and rolB expression and secondary metabolism was found in any of these studies. Moyano et al. [46] showed that the inoculation of leaf sections of tobacco, Duboisia hybrid and Datura metel plants with the A4 strain of A. rhizogenes induced transformed roots with the capacity to produce putrescine-derived alkaloids such as nicotine, hyoscyamine and scopolamine. In general, the obtained hairy roots presented two morphologies: typical hairy roots with a high capacity to produce alkaloids, and callus-like roots with faster growth and lower alkaloid production. The aux1 gene of A. rhizogenes located in the TR-DNA of A. rhizogenes was detected in all roots showing callus-like morphology. However, this gene was only detected in 25-60% of the established root cultures showing typical hairy morphology. These results demonstrate a significant role of aux genes in the morphology of transformed roots and the importance of typical hairy root morphology in the production of scopolamine. The studies with Panax ginseng hairy roots also support the effects of the genes located in the TR-DNA on root morphology and secondary metabolism [47].
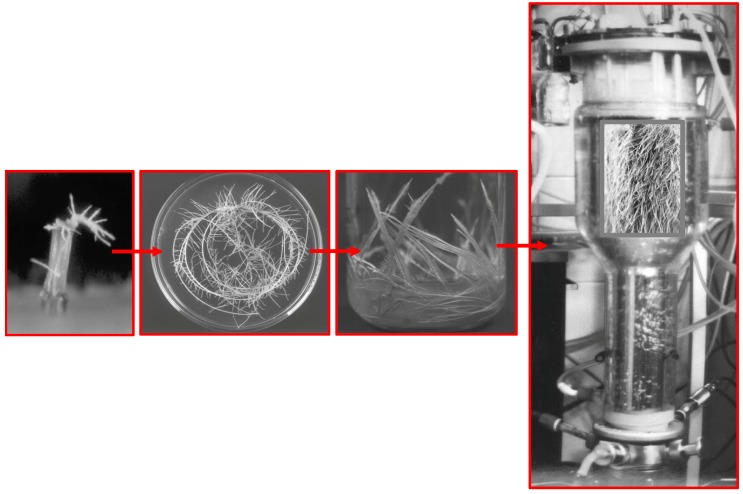
The hairy roots are normally induced on aseptic, wounded parts of plants by inoculating them with A. rhizogenes. In scopolamine-producing Solanaceae plants, roots usually emerge at the inoculation sites after 1-4 weeks (Figure 4), but in the case of other plant species such as Taxus it can be more than 4 months before the roots emerge [48]. Root tips are cultured separately in a hormone-free medium, the most commonly used being MS (Murashige-Skoog, [49]), Gamborg’s B5 [50] or SH [51]. The next step for establishing hairy root cultures is to select and characterize the root clones according to their capacity for growth and production of the desired compounds. Sevón et al. [33] reported the productivity of scopolamine in more than 15 species, amounts ranging from 0.2 to 32 mg/g DW. Sometimes these productions were achieved after a laborious process to optimize the growth conditions, such as the selection of the more productive clones, and optimization of the production conditions by testing different ionic concentrations as well as the carbon source and pH of the medium.
Figure 4.
The main steps for the establishment and culture of hairy roots from Datura metel.
The treatment with biotic or abiotic elicitors, such as methyl jasmonate, chitosan, salicylic acid or silver nitrate, can also improve the production of tropane alkaloids [11], and references therein, but in some cases the effect of the elicitor is due to an enhancement of cell permeability, which may increase the formation of secondary products by inhibiting operative feedback mechanisms or intracellular degradation of the products [52]. In this context, Cusidó et al. [53] have reported that treatment with the permeabilizing agent Tween-20 increases the production of scopolamine in hairy root cultures of D. metel.
The main constraint for the commercial exploitation of hairy root cultures is scaling up to an industrial level, since it has become clear that standard bioreactors are not suitable vessels to achieve this. The uneven distribution of biomass in the vessel does not permit the growth of interconnected tissues, which results in cell necrosis. The growth behavior of the roots also hampers the inoculation, harvesting and sampling procedures. Furthermore, in order to protect the root integrity, the design of mechanical stirred bioreactors should be modified by including stainless or nylon meshes to protect against mechanical shear [54].
Srivastava et al. [55] have recently summarized the attempts to adapt bioreactor design to hairy root cultures; stirred tank, airlift, bubble columns, connective flow, turbine blade, rotating drum, as well as different gas phase reactors have all been used successfully. In the case of tropane alkaloids, different types of bioreactors are used for scopolamine production (Figure 5). Wilson [56] describes the only large droplet bioreactor system with a volume of 500 L designed for hairy root cultures of Datura stramonium. On a smaller scale, modified airlift and stirred tanks have been used for scopolamine production in hairy root cultures of D. metel [53], connective flow reactors for H. muticus [57] and Atropa belladonna [58] and more recently a bubble column bioreactor has been employed for root cultures of Scopolia parviflora [59]. One such advance is the development of disposable wave bioreactor systems, whose working principle is based on wave-induced agitation, which significantly reduces stress levels. This type of bioreactor has been successfully used for H. muticus and Panax ginseng hairy root cultures [60].
Figure 5.
Detail of a hairy root culture of Duboisia hybrid showing the anchorage of the roots in the stainless mesh added to protect the culture in the stirred system.
Overexpression of the pmt gene to improve scopolamine production
It is known that tropane alkaloids are derived from putrescine via N-methylputrescine, and that putrescine can also be metabolized into polyamines such as spermidine and spermine. As previously mentioned, the formation of N-methylputrescine is catalyzed by putrescine N-methyltransferase, which is the first committed step in the biosynthesis of these alkaloids (Figure 2). This suggests that scopolamine production by plant cell cultures can be improved by overexpressing the pmt gene. This reaction is common to both tropane and tobacco alkaloids. The cDNA of pmt has been cloned from tobacco and Nicotiana sylvestris and the enzyme PMT has been purified from tobacco plant roots, where its activity has been measured [61]. Root cultures of Datura stramonium, [12,62,63], Hyoscyamus albus [64,65] and H. niger [66] contain PMT with similar properties to the tobacco enzyme and the expression of the gene has been found in the root pericycle of Atropa belladonna [28] as well as in the endodermis, xylem and outer cortex cells of Nicotiana sylvestris [67]. In tobacco, PMT is stress-responsive and inducible by methyl jasmonate [67,68,69,70] but, in contrast to the tobacco PMT promoter, in Atropa belladonna no jasmonate-responsive element has been identified in the promoter region [28], and correspondingly, hyoscyamine is not enhanced by elicitation in root cultures of A. belladonna [18].
The key role of PMT in tropane alkaloid biosynthesis converts this enzyme into a prime target for metabolic engineering, and there have been many attempts to increase the scopolamine production by over-expressing the pmt gene. In most cases, the plant material has been transformed with a heterologous gene, such as the pmt gene from tobacco, under the control of the CaMV35-S promoter, with the advantages of no feedback inhibition by downstream products, and a higher affinity for the substrate [71]. PMT-overexpressing plants of A. belladonna and N. sylvestris have already been produced by Sato et al. [72]. These authors report an opposite effect of the transgene expression, since no changes were observed in the Atropa alkaloid content, while the nicotine content in N. sylvestris leaves increased significantly.
In order to increase the bioproduction of hyoscyamine and scopolamine alkaloids, we have been working with two scopolamine-rich phenotypes, D. metel and a hybrid of Duboisia developed by Boehringer Ingelheim as an industrial source of scopolamine (personal communication), as well as a scopolamine-poor plant, H. muticus. In order to overexpress the tobacco pmt gene, we developed a binary system, consisting of a disarmed Agrobacterium tumefaciens C58C1, which contained the plasmid pRiA4 together with the plasmid pBMI. The latter plasmid carried the pmt gene, under the control of the promoter CaMV-35S, and the nptII gene. The results of the agroinfection showed that the Agrobacterium used in the three plant species had a high virulence. In all cases we obtained root lines with a high capacity for growth, similar to the root lines obtained after infection with a wild strain of A. rhizogenes, but the metabolic behavior changed considerably compared with the plant species. In the Duboisia hybrid the levels of N-methylputrescine (the direct product of the reaction catalyzed by PMT) of the engineered roots increased up to four-fold compared with wild type hairy roots, but the tropane alkaloids hyoscyamine and scopolamine did not increase significantly [73].
The results obtained from engineered roots of H. muticus and D. metel were also very different. As Moyano et al. [74] reports, in both species the overexpression of the pmt gene from tobacco under the control of the CaMV-35S promoter increased the hyoscyamine content, but while the production of scopolamine improved significantly in Datura, in Hyoscyamus the tropane alkaloid level remained similar to that of wild type hairy roots. In the engineered roots of Duboisia, alkaloid production was closely related to the presence of the 35S-pmt transgene, showing that ectopic expression of tobacco pmt increased the biosynthetic flux towards the tropane alkaloids and, consequently, the tropane alkaloid contents of transformed roots were significantly enhanced. In A. belladonna, it has been shown that regulation of the plant's endogenous pmt is pericycle specific [28]. It is evident that the transcriptional control by the 35S promoter of the transgenic pmt gene in the Datura root lines is not cell type specific. Furthermore, it may also indicate that the transgene allows the bypassing of the endogenous control of the metabolic flux to the alkaloids, which would take place as the first committed enzymatic step in their biosynthesis. Similar results have been obtained in H. muticus roots by Biondi et al. [75]. On the contrary, as already mentioned, in Duboisia and A. belladonna overexpression of the pmt gene only increased the accumulation of the direct metabolite N-methylputrescine [32,73], while the effect on the alkaloid level was marginal. These results, unlike those obtained by Moyano et al. [74] for H. muticus and D. metel, show that the operative regulatory pathways in tropane alkaloid plant species vary.
More recently, the tobacco pmt gene has been overexpressed in scopolamine-producing H. niger [76]. The engineered lines showed a significant increase in PMT activity and the contents of N-methylputrescine also increased more than five-fold, although the level of tropane alkaloids remained constant. It has also been demonstrated that the exposure of the roots to the elicitor methyl jasmonate increases the levels of polyamines as well as tropane alkaloids, and suggests that the C-flux for tropane alkaloid production could be restricted in later steps of the biosynthetic pathway.
Overexpression of tropinone reductases
Another branching point in scopolamine biosynthesis is at the tropinone reductase level (see Figure 2). Atropa, Datura, Hyoscyamus and Duboisia contain additional tropane alkaloids, calystegines, which are characterized by the loss of a methyl group on the nitrogen bridge and by the presence of three to five hydroxyl groups on the tropane heterocycle [17]. Two separate tropinone reductases have been isolated and characterized from root cultures of several species, D. stramonium [77,78,79], Hyoscyamus niger [29,80] and A. belladonna [81] and the corresponding cDNAs were cloned [83,84]. The activity of the tropine-forming enzyme TRI (EC 1.1.1.206) could be flux-limiting for the biosynthesis of hyoscyamine and scopolamine, whereas TRII (EC 1.1.1.236) catalyzes the formation of pseudotropine and consequently drives the C-metabolic flux from tropane alkaloids to calystegines [84].
Overexpression of both tropinone reductases TRI and TRII have been achieved in A. belladonna hairy roots [17]. Engineered root lines with strong overexpression of the trI or trII gene from D. stramonium under the control of the CaMV 35S-promotor showed more enzyme activity of the respective reductase and a higher level of the enzyme products, tropine and pseudotropine. Strong expression of the trI gene was accompanied by a significant enhancement of the contents of hyoscyamine and scopolamine. On the contrary, calystegine levels were lower than in the control roots. These results show the effectiveness of the system for increasing scopolamine production in hairy roots and suggest that the tropane alkaloid and calystegine pathways compete for the C-flux. On the contrary, overexpression of the pseudotropine-forming reductase increased the accumulation of calystegines while the hyoscyamine content of the engineered roots remained constant [17].
Overexpression of the h6h gene to increase the epoxidation of hyoscyamine into scopolamine
As mentioned above, the last step in the scopolamine biosynthetic pathway is the epoxidation of hyoscyamine catalyzed by hyoscyamine 6-β-hydroxylase (Figure 2). H6H, therefore, is a promising target enzyme that, if overexpressed in hyoscyamine-accumulating tissues, would result in increased scopolamine levels in transgenic plants or roots. In this way, several unattractive hyoscyamine-rich but scopolamine-poor plants, such as Hyoscyamus spp. could be converted into an industrial source of scopolamine [71].
The first example of how pharmaceutically important plants can be successfully altered by metabolic engineering was provided by Yun et al. [85]. Their research focused on Atropa belladonna, which is a hyoscyamine-rich phenotype. The h6h gene from H. niger was overexpressed in the target plant and the alkaloid pattern in a single primary transformed plant and its progenies showed elevated scopolamine contents, resulting in near to complete conversion of hyoscyamine to scopolamine in the mature plants. Working with hairy roots, Hashimoto et al. [86] showed that overexpression of the h6h transgene led to a higher production of scopolamine than hyoscyamine, the reverse of alkaloid production in wild-type roots. Similar results were achieved with H. muticus hairy roots overexpressing the same gene, in which the best transgenic clone had a 100-fold increase of scopolamine, while the hyoscyamine content remained unaltered [7]. These results indicate that overexpressing the h6h gene can be a way of increasing scopolamine production in hairy root cultures of those species that produce low levels of this alkaloid. However, the yields of scopolamine obtained so far are still too low for commercialization.
The previous experiments were mainly carried out with phenotypes that were poor in scopolamine and accumulated hyoscyamine as the principal alkaloid. In the case of a scopolamine-rich phenotype, such as the Duboisia hybrid (D. myoporoides x D. leichhardtii), overexpression of the h6h gene from H. niger under the control of the CaMV 35-S promoter significantly increased the scopolamine contents of the engineered root lines at the end of the culture period, with notable differences in alkaloid production observed among the different root lines established. The total alkaloid content (measured as hyoscyamine + scopolamine) was also higher in the root lines overexpressing the h6h gene compared with the typical hairy roots [87]. The results thus showed that overexpression of the 35S-h6h gene not only enhanced the capacity of root lines to convert hyoscyamine to scopolamine but also the total alkaloid production of these roots.
In all the cases commented above, significant quantities of hyoscyamine remain in the root tissues without biotransformation into scopolamine. In contrast, engineered hairy root cultures of another scopolamine-poor genotype, Atropa baetica, overexpressing the 35S-h6h transgene, showed an altered alkaloid profile in which hyoscyamine, the main alkaloid in the plant, was entirely converted into scopolamine. In the best h6h clone, scopolamine accumulation increased 9-fold compared to plants, some of which was released into the liquid medium. Only negligible amounts of hyoscyamine were detected [88].
Recently, Zhang et al. [89] reported the simultaneous introduction and overexpression of genes encoding the rate-limiting upstream enzyme PMT and the downstream enzyme H6H of scopolamine biosynthesis in hairy root cultures of H. niger. Transgenic root lines expressing both pmt and h6h genes produced significantly higher levels of scopolamine compared with the wild-type roots. The best line produced 411 mg/L scopolamine, the highest scopolamine content achieved so far by a genetically engineered plant. This study on simultaneously engineering pmt and h6h genes in scopolamine-producing plant species has resulted in a significant enhancement of scopolamine accumulation in cultured hairy root lines. Overexpression of multiple biosynthetic genes or transcription factors that control the expression of genes in pathways targeted by bioengineering is a promising strategy to alter the accumulation of certain secondary metabolites.
Biotransformation of hyoscyamine into scopolamine in transgenic tobacco hairy roots
Metabolic engineering can also provide techniques to transfer a whole metabolic pathway, or some of its steps, from one plant species to another. Rocha et al. [90] have simultaneously introduced two genes involved in tropane-alkaloid biosynthesis, TR I and H6H, in Nicotiana tabacum, expressed under the control of the CaMV 35S promoter. Detached leaves from the transgenic plants were fed with hyoscyamine and the expected H6H reaction product was generated. In addition, in most cases leaves of the transgenic plants showed higher nicotine contents than control plants, suggesting changes in the activity of the enzymes in the nicotine biosynthetic pathway.
We recently obtained three tobacco hairy root lines carrying the 35S-h6h gene from Hyoscyamus niger. The transformation was performed using a binary vector system based on Agrobacterium rhizogenes, as previously described by Palazón et al., [87], and the production of scopolamine in hairy roots was clearly correlated with the 35S-h6h transcript expression. The engineered Nicotiana tabacum hairy roots were studied for bioconversion after feeding the cultures with exogenous hyoscyamine. Engineered roots carrying the 35S-h6h transgene showed an efficient uptake of hyoscyamine (average of 95%) from the culture medium and also a higher rate of bioconversion of hyoscyamine to scopolamine (10-45%). Another important trait of this bioprocess was the remarkably high secretion of scopolamine from the roots, with up to 85% of the total scopolamine being released to the culture medium [91]. This contrasted with the normal metabolic behavior of tropane alkaloid-producing hairy roots in which the scopolamine remains accumulated in the root tissues [53].
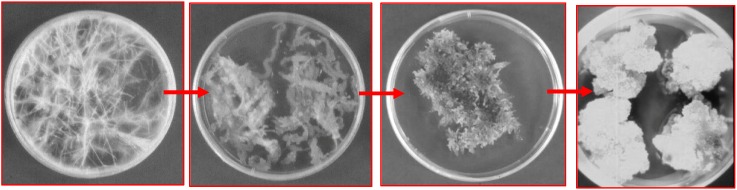
As mentioned previously, one of the most important obstacles for the industrial production of pharmaceuticals in biotechnological systems based on hairy root cultures is the scale-up to bioreactors. With the aim of scaling-up the biotransformation of hyoscyamine into scopolamine we obtained cell cultures derived from hairy roots overexpressing the h6h gene from H. niger. The hairy root cultures, obtained as described by Häkkinen et al. [91], were treated with indolacetic acid (11.5 μM) and kinetin (1 μM) to dedifferentiate the root tissues and obtain friable calli (Figure 7). After 3-5 subcultures in hormone-supplemented MS media the friable calli were transferred to the same liquid medium in order to obtain a fine cell suspension. The cell cultures were fed with hyoscyamine and 4 weeks later the amount of scopolamine produced was quantified by HPLC. The transgenic cell suspension cultures, like the hairy roots they derived from, showed a considerable capacity for the bioconversion of hyoscyamine into scopolamine (16%), and released it to the culture medium [92]. Although the scale-up from shake-flask to bioreactor culture usually results in reduced productivities, the transgenic cells grown in a 5-L turbine stirred tank reactor in a batch mode significantly increased the scopolamine accumulation. The total content (cell-associated+extracellular) of scopolamine was 35.5 mg/L, which was 1.6 times higher than that obtained in small-scale cultures. In this case almost 18% of the hyoscyamine added to the medium was transformed into scopolamine, which represented an increase of 65% with respect to the same alkaloid obtained by bioconversion in shake flasks.
Figure 7.
Dedifferentiation of tobacco hairy roots by subculturing in hormone-supplemented MS medium.
Future Challenges
Metabolic engineering, either alone or in combination with traditional cultivation techniques, provides the means to develop novel sources of plants with quantitatively and qualitatively improved pharmacological properties. However, the regulation towards the desired medicinal products requires a complete knowledge of the steps of the biosynthetic pathway and the respective cloned genes. Another problem is the difficulty of predicting the results of overexpressing a single or reduced number of genes, due to the frequent presence of multiple rate-limiting steps. Unfortunately, as expressed by the title of Humphrey and O’Hagan’s paper [16], “Tropane alkaloid biosynthesis. A century old problem unresolved”, several aspects of scopolamine biosynthesis remain unknown and we are also far from understanding the mechanism of their regulation. It is therefore necessary to consider not only the genes coding for enzymes involved in individual steps of the pathway, but also the homeotic genes controlling the transcription of numerous genes involved in the regulation of the whole pathway and interconnecting cellular pathways [11]
Oksman-Caldentey and Inzé [93] have described a novel gene discovery platform based on functional genomics. When comparing transcriptomic and metabolomic profiling, it is possible to obtain a large number of genes whose expression correlates with the accumulation of secondary metabolites. In this context, the elicitation of tobacco cell cultures with methyl jasmonate has permitted the identification of more than 600 tags modulated by the elicitor and probably related with the tobacco alkaloid pathway [94]. Similar studies carried out with plant cell cultures of Catharanthus roseus, a plant species that produces more than 120 alkaloids, have established a correlation within the transcriptomic profile of 417 tags and the metabolic accumulation of 178 alkaloids [95]. The genomic approach applied to scopolamine biosynthesis could constitute a potent tool to elucidate this unresolved pathway in its entirety with the aim of increasing the biotechnological production of this alkaloid. The transfer of part of a metabolic pathway from one plant species to another could also be an excellent system to obtain novel phytochemical compounds with new or improved biological activities.
Acknowledgements
Work in the authors’ laboratory was financially supported by the Spanish MEC, project BIO2005-05583. The stay of Dr. Hernandez-Vazquez was funded by a postdoctoral grant of CONACYT (REF: 75317). The stay of Mr. M.H. Mirjalili was funded by a grant from the Iranian Ministry of Sciences, Research and Technology.
References
- 1.Canter P. H., Thomas H., Ernst E. Brinding medicinal plants into cultivation: opportunities and challenges for biotechnology. Trends Biotechnol. 2005;23:180–185. doi: 10.1016/j.tibtech.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Lessard P. Metabolic engineering, the concept coalesces. Nat. Biotechnol. 1996;14:1654–1655. doi: 10.1038/nbt1296-1654. [DOI] [PubMed] [Google Scholar]
- 3.Kinney A. J. Manipulating flux through plant metabolic pathways. Curr. Opin. Plant Biol. 1998;1:173–178. doi: 10.1016/S1369-5266(98)80021-6. [DOI] [PubMed] [Google Scholar]
- 4.Whitmer S., Van der Heijden R., Verpoorte R. In: Plant Biotechnology and Transgenic Plants. Oksman-Caldentey K. -M., Barz W.H., editors. Marcel & Dekker; New York-Basel: 2002. pp. 373–405. [Google Scholar]
- 5.Ramachandra S. R., Ravishankar G. A. Plant cell cultures: Chemical factories of secondary metabolites. Biotechnol. Adv. 2002;20:1001–153. doi: 10.1016/s0734-9750(02)00007-1. [DOI] [PubMed] [Google Scholar]
- 6.Griffing W. J., Lin G. D. Chemotaxonomy and geographical distribution of tropane alkaloids. Phytochemistry. 2000;53:623–637. doi: 10.1016/S0031-9422(99)00475-6. [DOI] [PubMed] [Google Scholar]
- 7.Jouhikainen K., Lindgren L., Jokelainen T., Hiltunen R., Teeri T.H., Oksman-Caldentey K. M. Enhancement of scopolamine production in Hyoscyamus muticus L. hairy root cultures by genetic engineering. Planta. 1999;208:545–551. doi: 10.1007/s004250050592. [DOI] [Google Scholar]
- 8.Hashimoto T., Yamada Y. Alkaloid biogenesis: molecular aspects. Annu. Rev. Plant Phys. 1994;45:257–285. doi: 10.1146/annurev.pp.45.060194.001353. [DOI] [Google Scholar]
- 9.Oksman-Caldentey K. M., Strauss A. Somaclonal variation of scopolamine content in protoplast-derived cell culture clones of Hyoscyamus muticus. Planta Med. 1986;52:6–12. doi: 10.1055/s-2007-969053. [DOI] [Google Scholar]
- 10.Palazón J., Altabella T., Cusidó R. M., Ribó M., Piñol M. T. Growth and tropane alkaloid production in Agrobacterium transformed roots and derived callus of Datura. Biol. Plantar. 1995;37:161–168. doi: 10.1007/BF02913204. [DOI] [Google Scholar]
- 11.Arroo R., Woolley J., Oksman-Caldentey K. M. In: Biotechnology in Agriculture and Forestry. Pua E. C., Davey M. R., editors. Springer; Berlin-Heidelberg-New York: 2007. pp. 189–204. Vol. 61, Transgenic Crops VI,. Pua, E.C.; Davey, M.R., Eds.; Springer: Berlin Heidelberg New York, 2007. [Google Scholar]
- 12.Walton N. J., Robins R. J., Peerless A. C. J. Enzymes of N-methylputrescine biosynthesis in relation to hyoscyamine formation in transformed root cultures of Datura stramonium and Atropa belladonna. Planta. 1990;182:136–141. doi: 10.1007/BF00239995. [DOI] [PubMed] [Google Scholar]
- 13.Yamada Y., Hashimoto T., Endo T., Yukimune Y., Cono J., Hamaguchi N., Dräger B. In: Secondary Products from Plant Tissue Culture. Charlwood R. V., Rhodes M. J. C., editors. Clarendon Press; Oxford: 1990. pp. 227–242. [Google Scholar]
- 14.Robins R. J., Parr A. J., Bent E. G. Studies on the biosynthesis of tropane alkaloids in Datura stramonium L. transformed root cultures. Planta. 1991;183:385–390. doi: 10.1007/BF00201061. [DOI] [PubMed] [Google Scholar]
- 15.Ansarin M., Woolly J. G. The biosynthesis of tropic acid. Part 6. Enantioselective, intact incorporation of (R)-(+)-3-phenyllactic acid into the tropic acid ester alkaloids of Datura. J. Chem. Soc. Perkin Trans. I. 1995:487–490. doi: 10.1039/p19950000487. [DOI] [Google Scholar]
- 16.Humphrey A. J., O’Hagan D. Tropane alkaloid biosynthesis. A century old problem unresolved. Nat. Prod. Rep. 2001;18:494–502. doi: 10.1039/b001713m. [DOI] [PubMed] [Google Scholar]
- 17.Richter U., Rothe G., Fabian A. K., Rahfeld B., Dräger B. Overexpression of tropinone reductases alters alkaloid composition in Atropa belladonna root cultures. J. Exp. Bot. 2005;56:645–652. doi: 10.1093/jxb/eri067. [DOI] [PubMed] [Google Scholar]
- 18.Rothe G., Garske U., Draeger B. Calystegines in root cultures of Atropa belladonna respond to sucrose, not to elicitation. Plant Sci. 2001;160:1043–1053. doi: 10.1016/S0168-9452(01)00355-7. [DOI] [PubMed] [Google Scholar]
- 19.Leete E., Kowano N., Newmark R. A. Use of Carbon-13 Nuclear Magnetic Resonance to establish that the biosynthesis of tropic acid involves an intramolecular rearrangement of phenylalanine. J. Am. Chem. Soc. 1975;97:6826–6830. doi: 10.1021/ja00856a038. [DOI] [PubMed] [Google Scholar]
- 20.Ansarin M., Woolly J. G. The rearrangement of phenyllactate in the biosynthesis of tropic acid. Phytochemistry. 1994;35:935–939. doi: 10.1016/S0031-9422(00)90642-3. [DOI] [Google Scholar]
- 21.Chesters N. C. J. E., O’Hagan D., Robins R. J. The biosynthesis of tropic acid: The (R)-D-Phenyllactic moiety is processed by the mutase involved in hyoscyamine biosynthesis in Datura stramonium. J. Chem. Soc. Chem. Comm. 1995;2:127–129. [Google Scholar]
- 22.Robins R. J., Chesters N. C. G. E., O’Hagan D., Parr A., Walton N. J., Woolley J. G. The biosynthesis of hyoscyamine: the process by which littorine rearranges to hyoscyamine. J. Chem. Soc. Perkin Trans. I. 1995:481–485. [Google Scholar]
- 23.Patterson S., O’Hagan D. Biosynthetic studies on the tropane alkaloid hyoscyamine in Datura stramonium; hyoscyamine is stable to in vivo oxidation and is not derived from littorine via a vicinal interchange process. Phytochemistry. 2002;61:323–329. doi: 10.1016/S0031-9422(02)00200-5. [DOI] [PubMed] [Google Scholar]
- 24.Li R., Reed D. W., Liu E., Nowak J., Pelcher L. E., Page J. E., Covello P. S. Functional genomic analysis of alkaloid biosynthesis in Hyoscyamus niger reveals a cytochrome P450 involved in littorine rearrangement. Chem. Biol. 2006;13:513–520. doi: 10.1016/j.chembiol.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Toivonen L. Utilization of hairy root cultures for production of secondary metabolites. Biotechnol. Prog. 1993;9:12–20. doi: 10.1021/bp00019a002. [DOI] [Google Scholar]
- 26.Palazón J., Piñol M. T., Cusido R. M., Morales C., Bonfill M. Application of transformed root technology to the production of bioactive metabolites. Recent Res. Dev. Plant Phys. 1997;1:125–143. [Google Scholar]
- 27.Palazón J., Moyano E., Bonfill M., Cusidó R. M., Piñol M. T. In: Floriculture, Ornamental and Plant Biotechnology: Advances and Topical Issues. Teixeira da Silva J. A., editor. Vol. II. Global Science Books, Ltd; London, UK: 2006. pp. 209–221. [Google Scholar]
- 28.Suzuki K., Yamada Y., Hashimoto T. Expression of Atropa belladonna putrescine N-methyltransferase gene in root pericycle. Plant Cell Physiol. 1999;40:289–297. doi: 10.1093/oxfordjournals.pcp.a029540. [DOI] [PubMed] [Google Scholar]
- 29.Hashimoto T., Hayashi A., Amano Y., Cono J., Iwanari H., Usuda S., Yamada Y. Hyoscyamine 6β-hydroxilase, an enzyme envolved in tropane alkaloid biosynthesis, is localized at the pericycle of the roots. J. Biol. Chem. 1992;266:4648–4653. [PubMed] [Google Scholar]
- 30.Kanegae T., Kajiya H., Amano Y., Hashimoto T., Yamada Y. Species-dependent expression of the hyoscyamine 6β-hydroxilase gene in the pericycle. Plant Physiol. 1994;105:483–490. doi: 10.1104/pp.105.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakajima K., Hashimoto T. Two tropinone reductases, that catalyze opposite stereospecific reductions in tropane alkaloid biosynthesis, are localized in plants root with different cell-specific patterns. Plant Cell Physiol. 1999;40:1099–1107. doi: 10.1093/oxfordjournals.pcp.a029494. [DOI] [PubMed] [Google Scholar]
- 32.Rothe G., Hachiya A., Yamada Y., Hashimoto T., Dräger B. Alkaloids in plants and roots cultures of Atropa belladona overexpressing putrescine N-methyltransferase. J. Exp. Bot. 2003;54:2065–2070. doi: 10.1093/jxb/erg227. [DOI] [PubMed] [Google Scholar]
- 33.Sevón N., Oksman-Caldentey K. M. Agrobacterium rhizogenes-mediates transformation: Root cultures as a source of alkaloids. Planta Med. 2002;68:859–868. doi: 10.1055/s-2002-34924. [DOI] [PubMed] [Google Scholar]
- 34.White F. F., Nester E. W. Hairy root: plasmid encodes virulence in Agrobacterium rhizogenes. J. Bacteriol. 1980;141:1134–1141. doi: 10.1128/jb.141.3.1134-1141.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jouanin L. Restriction map of an agropine-type Ri-plasmid and its homologies with Ti-plasmids. Plasmid. 1984;12:91–102. doi: 10.1016/0147-619X(84)90055-6. [DOI] [PubMed] [Google Scholar]
- 36.Binns A. N., Tomashow J. V. Cell biology of Agrobacterium infection and transformation of plants. Annu. Rev. Microbiol. 1988;42:575–606. doi: 10.1146/annurev.mi.42.100188.003043. [DOI] [Google Scholar]
- 37.Morris R. O. Genes specifying auxin and cytokinin biosynthesis in phytopathogens. Annu. Rev. Plant Physiol. 1986;37:509–538. doi: 10.1146/annurev.pp.37.060186.002453. [DOI] [Google Scholar]
- 38.Chriqui D., Guivarch A., Dewitte W., Prinsen E., Van Onkelen H. Rol genes and root initiation and development. Plant Soil. 1996;187:47–55. doi: 10.1007/BF00011656. [DOI] [Google Scholar]
- 39.Palazón J., Cusidó R. M., Roig C., Piñol M. T. Effect of rol genes from Agrobacterium rhizogenes TL-DNA on nicotine production in tobacco root cultures. Plant Physiol. Biochem. 1997;35:155–162. [Google Scholar]
- 40.Nilsson O., Olsson O. Getting to the root: the role of the Agrobacterium rhizogenes rol genes in the formation of hairy roots. Physiol. Plant. 1997;100:463–473. doi: 10.1111/j.1399-3054.1997.tb03050.x. [DOI] [Google Scholar]
- 41.Piñol M. T., Palazón J., Cusidó R., Serrano M. Effects of Ri T-DNA from Agrobacterium rhizogenes on growth and hyoscyamine production in Datura stramonium root cultures. Bot. Acta. 1996;109:133–138. doi: 10.1111/j.1438-8677.1996.tb00553.x. [DOI] [Google Scholar]
- 42.Bonhomme V., Laurain-Mattar D., Fliniaux M. A. Effects of the rol C gene on hairy root: Induction development and tropane alkaloid production by Atropa belladonna. J. Nat. Prod. 2000;63:1249–1252. doi: 10.1021/np990614l. [DOI] [PubMed] [Google Scholar]
- 43.Bonhomme V., Laurain-Mattar D., Lacoux J., Fliniaux M. A., Jacquin-Dubreil A. Tropane alkaloid production by hairy roots of Atropa belladonna obtained after transformation with Agrobacterium rhizogenes 15834 and Agrobacterium tumefaciens containing rol A, B, C genes only. J. Biotechnol. 2000;81:151–158. doi: 10.1016/S0168-1656(00)00287-X. [DOI] [PubMed] [Google Scholar]
- 44.Palazón J., Cusidó R. M., Gonzalo J., Bonill M., Morales C., Piñol M. T. Relation between the amount of rol C gene product and indole alkaloid accumulation in Catharanthus roseus transformed root cultures. J. Plant Physiol. 1998;153:712–718. [Google Scholar]
- 45.Bulgakov V. P., Khodakovskaya M. V., Labetskaya N. V., Chernoded G. K., Zhuravlev Y. N. The impact of plant rol C oncogene on ginsenoside production by ginseng hairy root cultures. Phytochemistry. 1998;49:1929–1934. doi: 10.1016/S0031-9422(98)00351-3. [DOI] [Google Scholar]
- 46.Moyano E., Fornalé S., Palazón J., Cusidó R. M., Bonfill M., Morales C., Piñol M. T. Effect of Agrobacterium rhizogenes T-DNA on alkaloid production in Solanaceae plants. Phytochemistry. 1999;52:1287–1292. doi: 10.1016/S0031-9422(99)00421-5. [DOI] [Google Scholar]
- 47.Mallol A., Cusidó R. M., Palazón J., Bonfill M., Morales C., Piñol M. T. Ginsenoside production in different phenotypes of Panax ginseng transformed roots. Phytochemistry. 2001;57:365–371. doi: 10.1016/S0031-9422(01)00062-0. [DOI] [PubMed] [Google Scholar]
- 48.Furmanova M., Syklowska-Baranek K. Hairy root cultures of Taxus x media var. Hicksii Rehd. as a new source of paclitaxel and 10-deacetylbaccatin III. Biotechnol. Lett. 2000;22:606–616. [Google Scholar]
- 49.Murashige T., Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 50.Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968;50:151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- 51.Schenk R. V., Hildebrandt A. C. Medium techniques for induction and growth of monocotyledonous and dycotyledonous plant cell cultures. Can. J. Bot. 1972;50:199–204. doi: 10.1139/b72-026. [DOI] [Google Scholar]
- 52.Sevón N., Oksman-Caldentey K. M. Agrobacterium rhizogenes-mediated transformation: Root cultures as a source of alkaloids. Planta Med. 2002;68:859–868. doi: 10.1055/s-2002-34924. [DOI] [PubMed] [Google Scholar]
- 53.Cusidó R.M., Palazón J., Piñol M. T., Bonfill M., Morales C. Datura metel: In vitro production of tropane alkaloids. Planta Med. 1999;65:144–148. doi: 10.1055/s-1999-13976. [DOI] [PubMed] [Google Scholar]
- 54.Eibl R., Eibl D. In: Plant Biotechnology and Transgenic Plant. Oksman-Caldentey K.M., Barz W. H., editors. Marcel & Dekker; New York: 2002. pp. 163–200. [Google Scholar]
- 55.Srivastava S., Srivastava A. K. Hairy root culture for mass-production of high-value secondary metabolites. Crit. Rev. Biotechnol. 2007;27:29–43. doi: 10.1080/07388550601173918. [DOI] [PubMed] [Google Scholar]
- 56.Wilson P. D. G. In: Hairy roots: Culture and applications. Doran P. M., editor. Harwood Academic Publishers; Amsterdam: 1997. pp. 179–190. [Google Scholar]
- 57.Carvalho E. B., Curtis W. R. Characterization of fluid-flow resistance in root cultures with a connective flow tubular bioreactor. Biotechnol. Bioeng. 1998;60:375–384. doi: 10.1002/(SICI)1097-0290(19981105)60:3<375::AID-BIT15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 58.Williams G. R., Doran P. M. Hairy root culture in a liquid-dispersed bioreactor: characterization of spatial heterogeneity. Biotechnol. Progr. 2000;16:391–401. doi: 10.1021/bp0000306. [DOI] [PubMed] [Google Scholar]
- 59.Min J. Y., Jung H. Y., Kang S. M., Kim Y. D., Kang Y. M., Park D. J., Prasad D. T., Choi M. S. Production of tropane alkaloids by small-scale bubble column bioreactor cultures of Scopolia parviflora adventitious roots. Bioresour. Technol. 2007;98:1748–1753. doi: 10.1016/j.biortech.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 60.Eibl R., Eibl D. In: Plant Tissue Culture Engineering. Focus on Biotechnology. Gupta S. D., Ibaraki Y., editors. vol 6. Springer; Berlin-Heidelberg-New York: 2006. pp. 203–227. [Google Scholar]
- 61.Dräger B. Chemistry and biology of calystegines. Nat. Prod. Rep. 2004;21:211–223. doi: 10.1039/b300289f. [DOI] [PubMed] [Google Scholar]
- 62.Feth F., Arfmann H. A., Wray V., Wagner K. G. Determination of putrescine N-methyltransferase by high performance liquid chromatography. Phytochemistry. 1985;24:921–923. doi: 10.1016/S0031-9422(00)83153-2. [DOI] [Google Scholar]
- 63.Walton N. J., Peerless A. C. J., Robins R. J., Rhodes M. J. C., Boswell H. D., Robins D. J. Purification and properties of putrescine N-methyltransferase from transformed roots of Datura stramonium L. Planta. 1994;193:9–15. [Google Scholar]
- 64.Hashimoto T., Yukimune Y., Yamada Y. Putrescine and putrescine N-methyltransferase in the biosynthesis of tropane alkaloids in cultured roots of Hyoscyamus albus. Planta. 1989;178:123–130. doi: 10.1007/BF00392535. [DOI] [PubMed] [Google Scholar]
- 65.Hashimoto T., Yukimune Y., Yamada Y. Putrescine and putrescine N-methyltransferase in the biosynthesis of tropane alkaloids in cultured roots of Hyoscyamus albus. Planta. 1989;178:131–137. doi: 10.1007/BF00392536. [DOI] [PubMed] [Google Scholar]
- 66.Hibi N., Fujita T., Hatano M., Hashimoto T., Yamada Y. Putrescine N-methyltransferase in cultured roots of Hyoscyamus albus: n-butylamine as a potent inhibitor of the transferase both in vitro and in vivo. Plant Physiol. 1992;100:820–825. doi: 10.1104/pp.100.2.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shoji T., Yamada Y., Hashimoto T. Jasmonate induction of putrescine N-methyltransferase genes in the root of Nicotiana sylvestris. Plant Cell Physiol. 2000;41:831–839. doi: 10.1093/pcp/pcd001. [DOI] [PubMed] [Google Scholar]
- 68.Baldwin I. T., Zhang Z., Diab N., Ohnmeiss T. E., McCloud E. S., Lynds G. Y., Schmelz E. A. Quantification, correlations and manipulations of wound-induced changes in jasmonic acid and nicotine in Nicotiana sylvestris. Planta. 1997;201:397–404. doi: 10.1007/s004250050082. [DOI] [Google Scholar]
- 69.Shoji T., Nakajima K., Hashimoto T. Ethylene suppresses jasmonate-induced gene expression in nicotine biosynthesis. Plant Cell Physiol. 2000;41:1072–1076. doi: 10.1093/pcp/pcd027. [DOI] [PubMed] [Google Scholar]
- 70.Imanishi S., Katsuhito K., Nakakita M., Kojima H., Matsubayashi Y., Hashimoto T., Sakagami Y., Yamada Y., Nakamura K. Differential induction by methyl jasmonate of genes encoding ornithine decarboxylase and other enzymes involved in nicotine biosynthesis in tobacco cell cultures. Plant Mol. Biol. 1998;38:1101–1111. doi: 10.1023/A:1006058700949. [DOI] [PubMed] [Google Scholar]
- 71.Zhang L., Kai G. Y., Lu B. B., Zhang H. M., Tang K. X., Jiang J. H., Chen W. S. Metabolic engineering of tropane alkaloid biosynthesis in plants. J. Integr. Plant Biol. 2005;47:136–143. doi: 10.1111/j.1744-7909.2005.00024.x. [DOI] [Google Scholar]
- 72.Sato F., Hashimoto T., Hachiya A., Tamura K., Choi K., Morishige T., Fujimoto H., Yamada Y. Metabolic engineering of plant alkaloid biosynthesis. Proc. Natl. Acad. Sci. USA. 2001;98:367–372. doi: 10.1073/pnas.98.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moyano E., Fornalé S., Palazón J., Cusidó R. M., Bagni N., Piñol M. T. Alkaloid production in Duboisia hybrid hairy root cultures overexpressing the pmt gene. Phytochemistry. 2002;59:697–702. doi: 10.1016/S0031-9422(02)00044-4. [DOI] [PubMed] [Google Scholar]
- 74.Moyano E., Jouhikainen K, Tammela P., Palazón J, Cusido R. M., Piñol M. T., Teeri T. H., Oksman-Caldentey K. M. Effect of pmt gene overexpression on tropane alkaloid production in transformed root cultures of Datura metel and Hyoscyamus muticus. J. Exp. Bot. 2003;54:203–211. doi: 10.1093/jxb/erg014. [DOI] [PubMed] [Google Scholar]
- 75.Biondi S., Scaramagli S., Oksman-Caldentey K. M. Secondary metabolism in roots and callus cultures of Hyoscyamus muticus L.: The relationship between morphological organization and the response to methyl jasmonate. Plant Sci. 2002;163:563–569. doi: 10.1016/S0168-9452(02)00161-9. [DOI] [Google Scholar]
- 76.Zhang L., Yang B., Lu B., Kai G., Wang Z., Xia Y., Ding R., Zhang H., Sun X., Chen W., Tang K. Tropane alkaloids production in transgenic Hyoscyamus niger hairy root cultures over-expressing putrescine N-methyltransferase is methyl jasmonate-dependent. Planta. 2007;225:887–896. doi: 10.1007/s00425-006-0402-1. [DOI] [PubMed] [Google Scholar]
- 77.Nakajima K, Hashimoto T., Yamada Y. Two tropinone reductases with different stereospecificities are short-chain dehydrogenases evolved from a common ancestor. P. Nat. Acad. Sci. USA. 1993;90:9591–9595. doi: 10.1073/pnas.90.20.9591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nakajima K., Yamashita A., Akama H., Nakatsu T., Kato H., Hashimoto T., Oda J., Yamada Y. Crystal structures of two tropinone reductases: different reactions stereospecificities in the same protein fold. Proc. Natl. Acad. Sci. USA. 1998;95:4876–4881. doi: 10.1073/pnas.95.9.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Portsteffen A., Draeger B., Nahrstedt A. The reduction of tropinone in Datura stramonium root cultures by two specific reductases. Phytochemistry. 1994;37:391–400. doi: 10.1016/0031-9422(94)85066-6. [DOI] [PubMed] [Google Scholar]
- 80.Draeger B., Hashimoto T., Yamada Y. Purification and characterization of pseudotropine forming tropinone reductase from Hyoscyamus niger root cultures. Agric. Biol. Chem. 1988;52:2663–2667. doi: 10.1271/bbb1961.52.2663. [DOI] [Google Scholar]
- 81.Draeger B., Schaal A. Tropinone reduction in Atropa belladonna root cultures. Phytochemistry. 1994;35:1441–1447. doi: 10.1016/S0031-9422(00)86871-5. [DOI] [Google Scholar]
- 82.Nakajima K., Hashimoto T., Yamada Y. cDNA encoding tropinone reductase-II from Hyoscyamus niger. Plant Physiol. 1993;103:1465–1466. doi: 10.1104/pp.103.4.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nakajima K., Hashimoto T. Two tropinone reductases that catalyze opposite stereospecific reductions in tropane alkaloid biosynthesis are localized in plant root with different cell-specific patterns. Plant Cell Physiol. 1999;40:1099–1107. doi: 10.1093/oxfordjournals.pcp.a029494. [DOI] [PubMed] [Google Scholar]
- 84.Dräger B. Tropinone reductases, enzymes at the branch point of tropane alkaloid metabolism. Phytochemistry. 2006;67:327–337. doi: 10.1016/j.phytochem.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 85.Yun D. J., Hashimoto T., Yamada Y. Metabolic engineering of medicinal plants: Transgenic Atropa belladonna with an improved alkaloid composition. Proc. Natl. Acad. Sci. USA. 1992;89:11799–11803. doi: 10.1073/pnas.89.24.11799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hashimoto T., Yun D. J., Yamada Y. Production of tropane alkaloids in genetically engineered root cultures. Phytochemistry. 1993;32:713–718. doi: 10.1016/S0031-9422(00)95159-8. [DOI] [Google Scholar]
- 87.Palazón J., Moyano E., Cusido R.M., Bonfill M., Oksman-Caldentey K. M., Piñol M. T. Alkaloid production in Duboisia hybrid hairy roots and plants overexpresing the h6h gene. Plant Sci. 2003;165:1289–1295. doi: 10.1016/S0168-9452(03)00340-6. [DOI] [Google Scholar]
- 88.Zarate R., Jaber-Vazdekis N., Medina B., Ravelo A. G. Tailoring tropane alkaloid accumulation in transgenic hairy roots of Atropa baetica by over-expressing the gene encoding hyoscyamine 6β-hydroxylase. Biotechnol. Lett. 2006;28:1271–1277. doi: 10.1007/s10529-006-9085-8. [DOI] [PubMed] [Google Scholar]
- 89.Zhang L., Ding R., Chai Y., Bonfill M., Moyano E., Oksman-Caldentey K. M., Xu T., Pi Y., Wang Z., Zhang H., Kai G., Liao Z., Sun X., Tang K. Engineering tropane biosynthetic pathway in Hyoscyamus niger hairy root cultures. Proc. Natl. Acad. Sci. USA. 2004;101:6786–6791. doi: 10.1073/pnas.0401391101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rocha P., Stenzel O., Parr A., Walton N., Christou P., Dräger B., Leech M. J. Functional expression of tropinone reductase I (trI) and hyoscyamine-6b-hydroxylase (h6h) from Hyoscyamus niger in Nicotiana tabacum. Plant Sci. 2002;162:905–913. doi: 10.1016/S0168-9452(02)00033-X. [DOI] [Google Scholar]
- 91.Häkkinen S. T., Moyano E., Cusidó R. M., Palazón J., Piñol M. T., Oksman-Caldentey K. M. Enhanced secretion of tropane alkaloids in Nicotiana tabacum hairy roots expressing heterologous hyoscyamine-6ß-hydroxylase. J. Exp. Bot. 2005;56:2611–2618. doi: 10.1093/jxb/eri253. [DOI] [PubMed] [Google Scholar]
- 92.Moyano E, Palazón J., Bonfill M., Osuna L., Cusido R. M., Oksman-Caldentey K. M., Piñol M. T. Biotransformation of hyoscyamine into scopolamine in transgenic tobacco cell cultures. J. Plant Physiol. 2007;164:521–524. doi: 10.1016/j.jplph.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 93.Oksman-Caldentey K. M., Inzé D. Plant cell factories in the post genomic era: new ways to produce designer secondary metabolites. Trends Plant Sci. 2004;9:433–440. doi: 10.1016/j.tplants.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 94.Goossens A., Häkkinen T., Laakso I., Seppänen-Laakso T., Biondi S., De Sutter V., Lammertyn F., Nuutila M., Söderlund H., Zabeau M., Inzé D., Oksman-Caldentey K. M. A functional genomics approach toward the understanding of secondary metabolism in plant cells. Proc. Natl. Acad. Sci. USA. 2003;100:8595–8600. doi: 10.1073/pnas.1032967100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rischer H., Orešič M., Seppänen-Laakso T., Katajamaa M., Lammertyn F., Ardiles-Diaz W., Van Montagu M. C. E., Inzé D., Oksman-Caldentey K. M., Goossens A. Gene-to-metabolite networks for terpenoid indole alkaloid biosynthesis in Catharanthus roseus cells. Proc. Natl. Acad. Sci. USA. 2006;103:5614–5619. doi: 10.1073/pnas.0601027103. [DOI] [PMC free article] [PubMed] [Google Scholar]