Abstract
A new phenolic amide 8, together with the nine known phenolic compounds 1-7, 9 and 10 were isolated from the MeOH extract of the roots of Paris verticillata. The structure of the new compound 8 was determined to be 1-N-feruloylaminobutyl-4-ρ-hydroxybenzamide by spectroscopic methods. The isolated compounds were tested for cytotoxicity against four human tumor cell lines using the SRB assay.
Keywords: Paris verticillata, Liliaceae, cytotoxicity, 1-N-feruloyl-aminobutyl-4-ρ-hydroxybenzamide
Introduction
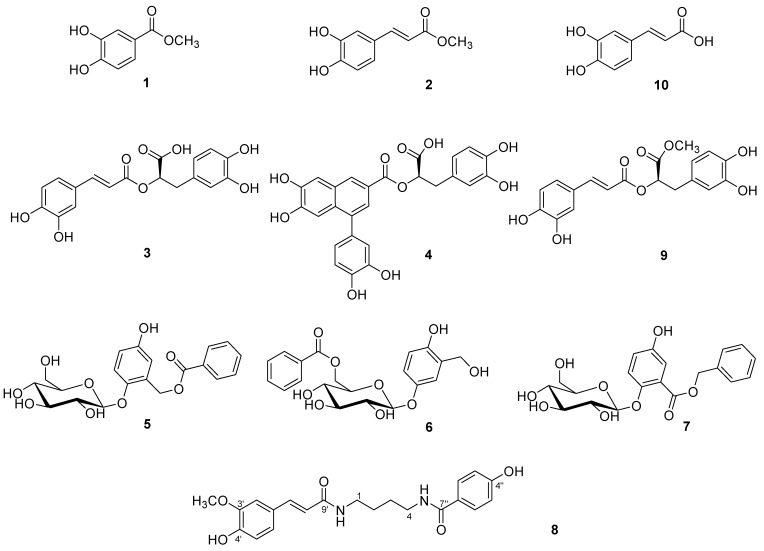
The perennial herb Paris veriticillata BIEB. (Liliaceae) is found in Korean valleys and has been used in Korean folk medicine as a treatment for asthma and chronic bronchitis [1]. Steroids and flavonol glycosides have been isolated from P. verticillata [2]. In continuation of our search for biologically active compounds from Korean medicinal plant sources, we have investigated the constituents of the roots of P. verticillata. Repeated column chromatographic separation of the MeOH extract of this plant led to the isolation of a new phenolic amide 8, as well as nine known phenolic constituents (1 - 7, 9 and 10, Figure 1). The known compounds are reported from this plant source for the first time. This paper deals with the isolation of the phenolic constituents, their cytotoxicity and the structure determination of the new phenolic amide.
Figure 1.
The structures of the isolated compounds 1-10 from P. verticillata.
Results and Discussion
The known compounds (Figure 1) were identified as methyl 3,4-dihydroxybenzoate (1) [3], methyl caffeoate (2) [4], rosmarinic acid (3) [5], globoidnan A (4) [6], salireposide (5) [7], xylosmacin (6) [8], trichocarpin (7) [9], methyl rosmarinate (9) [5] and caffeic acid (10) [10] by comparing their spectroscopic data with those reported in the literature.
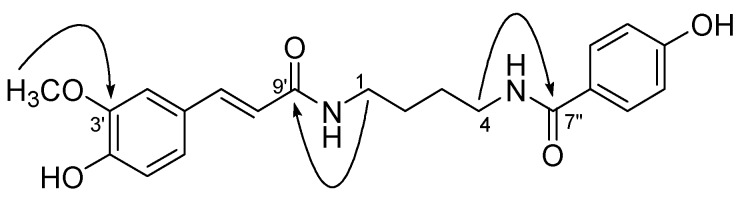
Compound 8 was obtained as a yellowish gum. Its molecular formula C21H25O5N2 was determined by HR FAB-MS (m/z) 385.1782 [M+H]+, (calcd. 385.1758). The IR spectrum showed a hydroxyl group at 3334 cm-1 and an amide group at 1575 cm-1. The 1H-NMR spectrum showed two benzene rings [δ 7.70 (2H, d, J = 8.5 Hz, H-2″, 6″) and 7.13 (1H, d, J = 2.0 Hz, H-2′), 7.03 (1H, dd, J = 8.0, 2.0 Hz, H-6′), 6.82 (2H, d, J = 8.5 Hz, H-3″, 5″) and 6.80 (1H, d, J = 8.0 Hz, H-5′)] and one double bond with trans coupling [δ 7.44 (1H, d, J = 15.5 Hz, H-7′) and 6.43 (1H, d, J = 15.5 Hz, H-8′)]. The 13C-NMR spectrum exhibited 21 carbon signals, including two amide signals at δ 169.0 (C-7″), and 168.1 (C-9′), fourteen olefinic carbon signals at δ 160.8 (C-4″), 148.7 (C-4′), 148.1 (C-3′), 140.8 (C-7′), 129.0 (2C-2″, 6″), 127.1 (C-1′), 125.4 (C-1″), 122.0 (C-6′), 117.6 (C-8′), 115.3 (C-5′), 114.9 (2C-3″, 5″) and 110.5 (C-2′), four aliphatic carbon signals at δ 39.3 (C-4), 39.1 (C-1), 26.9 (C-3) and 26.8 (C-2), and one methoxy signal at δ 55.2 (OMe). The correlation of δ 3.35 (2H, t, J = 7.0 Hz, H-1) with 168.1 (C-9′), δ 3.40 (2H, t, J = 7.0 Hz, H-4) with 169.0 (C-7″), and δ 3.89 (OMe) with 148.1 (C-3′) in the HMBC spectrum of 8 confirmed the connectivities of ρ-hydroxybenzamide and feruloyl amide group with the n-butyl chain (Figure 2). Thus, the structure of new compound 8 was determined to be 1-N-feruloyl-aminobutyl-4-ρ-hydroxybenzamide.
Figure 2.
Key HMBC correlations of 8 (1H →13C)
Biological activity
The isolated compounds were tested for cytotoxicity against four human tumor cell lines using the sulforhodamin B (SRB) assay. Compounds 1, 2, 4 and 9 showed mild cytotoxicity against only SK-OV-3 (ovary malignant ascites), with ED50 values of 10.14, 9.13, 12.27 and 7.22 μM, respectively. The other compounds exhibited little activity (ED50 > 30 μM) against the tested cancer cell lines.
Experimental
General
NMR spectra were recorded on a Varian Unity Inova 500Nb spectrometer. MS spectra were recorded on a JEOL JMS-700 mass spectrometer. Preparative HPLC used a Knauer K1001 instrument equipped with a refractive index detector, a UV detector and an Econosil silica 5 μm column (length: 250 mm, I.D 22 mm, Alltech) and an Econosil C18 5 μm column (length: 250 mm, I.D 4.6 mm, Alltech). For open column chromatography, silica gel (Merck, 70-230), ODS (Cosmosil 140 C18) and Sephadex LH-20 (Pharmacia) were used. Low pressure liquid chromatography was carried out over Merck LiChroprep Lobar-A Si 60 or Merck LiChroprep Lobar-A RP-C18 columns with an FMI QSY pump (Isco).
Plant material
The roots of P. verticillata (1.5 kg) were collected at Mt. O-Dae, Gang Won province, S. Korea in October 2002. A voucher specimen (SKKU-2002-04) was deposited at the College of Pharmacy, Sungkyunkwan University, Korea.
Extraction and Isolation
The dried and chopped root of P. verticillata (1.5 kg) was extracted three times with MeOH at room temperature. The MeOH extract (180 g) was suspended in distilled water (1.6 L) and successively partitioned with n-hexane, CH2Cl2, EtOAc and n-butanol, followed by evaporation, to yield 5.2, 3.5, 4.4 and 20 g fractions, respectively. The EtOAc fraction (4.4 g) was chromatographed over a silica gel column using a gradient solvent system (CHCl3-MeOH = 5:1 → 0:1) to give five subfractions (E1- E5). The E1 fraction (0.2 g) was chromatographed over silica gel (CHCl3-MeOH = 10:1), Sephadex LH-20 filtration (MeOH) and purified using HPLC (RP C-18 resin, 30% MeOH) to yield 1 (7 mg) and 2 (15 mg). The E2 fraction (1.4 g) was chromatographed over RP C-18 resin (50% MeOH), silica gel (CHCl3-MeOH = 7:1) and purified using HPLC (RP C-18 resin, 45% MeOH) to yield 3 (15 mg), 4 (4 mg), 5 (5 mg), 6 (6 mg) and 7 (5 mg). The E3 fraction (1.2 g) was chromatographed over silica gel (CHCl3-MeOH = 10:1), LPLC (RP C-18 resin, 40% MeOH) and purified using HPLC (silica gel, hexane-CHCl3-MeOH = 3:5:1 and 2:5:1) to yield 8 (4 mg), 9 (25 mg) and 10 (34 mg).
Spectroscopic data for 1-N-feruloyl-aminobutyl-4-ρ-hydroxybenzamide (8): Yellowish gum; UV (MeOH) λmax nm (log ε): 317 (3.446) and 247 (3.631); IR νmax: 3334, 2936, 1575 and 1128 cm-1; HR FAB-MS (m/z) 385.1782 [M+H]+, (C21H25O5N2 calcd. 385.1758); 1H-NMR (CD3OD, 500 MHz): δ 7.70 (2H, d, J = 8.5 Hz, H-2″, 6″), 7.44 (1H, d, J = 15.5 Hz, H-7′), 7.13 (1H, d, J = 2.0 Hz, H-2′), 7.03 (1H, dd, J = 8.0, 2.0 Hz, H-6′), 6.82 (2H, d, J = 8.5 Hz, H-3″, 5″), 6.80 (1H, d, J = 8.0 Hz, H-5′), 6.43 (1H, d, J = 15.5 Hz, H-8′), 3.89 (3H, s, OMe), 3.40 (2H, t, J = 7.0 Hz, H-4), 3.35 (2H, t, J = 7.0 Hz, H-1), 1.68 (4H, m, H-2, 3); 13C-NMR (CD3OD, 125 MHz): δ 169.0 (C-7″), 168.1 (C-9′), 160.8 (C-4″), 148.7 (C-4′), 148.1 (C-3′), 140.8 (C-7′), 129.0 (2C-2″, 6″), 127.1 (C-1′), 125.4 (C-1″), 122.0 (C-6′), 117.6 (C-8′), 115.3 (C-5′), 114.9 (2C-3″, 5″), 110.5 (C-2′), 55.2 (OMe), 39.3 (C-4), 39.1 (C-1), 26.9 (C-3), 26.8 (C-2).
Test for in vitro cytotoxicity.
A sulforhodamin B bioassay (SRB) was used to determine the cytotoxicity of each compound against four cultured human cancer cell lines [11]. The assays were performed at the Korea Research Institute of Chemical Technology. The cell lines used were A549 (non small cell lung carcinoma), SK-OV-3 (ovary malignant ascites), SK-MEL-2 (skin melanoma) and HCT (colon adenocarcinoma).
Acknowledgements
The authors would like to thank Drs. Eun Jung Bang, Sang Gu Kim and Jung Ju Seo at the Korea Basic Science Institute for the measurements of NMR and MS spectra.
Footnotes
Sample Availability: Milligram quantities of compounds 2, 3, 9 and 10 are available from the authors.
References
- 1.Ahn D. K. Illustrated book of Korean medicinal herbs. Kyo-Hak Publishing Co.; Seoul: 1998. [Google Scholar]
- 2.Nakano K., Murakami K., Nohara T., Tomimatsu T., Kawasaki T. The constituents of Paris verticillata M.v. Bieb. Chem. Pharm. Bull. 1981;29:1445–1451. doi: 10.1248/cpb.29.1445. [DOI] [Google Scholar]
- 3.Miyazawa M., Oshima T., Koshio K. Tyrosinase inhibitor from black rice bran. J. Agric. Food Chem. 2003;51:6953–6956. doi: 10.1021/jf030388s. [DOI] [PubMed] [Google Scholar]
- 4.Martic S., Brennan J. D., Brook M. A., Ackloo S., Nagy N. Towards the development of a covalenty tethered MALDI system- a study of allyl-modified MALDI matrixed. Can. J. Chem. 2007;85:66–76. doi: 10.1139/v06-185. [DOI] [Google Scholar]
- 5.Woo E. R., Piao M. S. Antioxidative constituents from Lycopus lucidus. Arch. Pharm. Res. 2004;27:173–176. doi: 10.1007/BF02980102. [DOI] [PubMed] [Google Scholar]
- 6.Ovenden S. P. B., Yu J., Wan S. S., Sberna G., Tait R. M., Rhodes D., Cox S., Coates J., Walsh N. G., Meurer-Grimes B. M. Globoidnan A: a lignan from Eucalyptus globoides inhibits HIV integrase. Phytochemistry. 2004;65:3255–3259. doi: 10.1016/j.phytochem.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Ahmad V. U., Abbasi M. A., Hussain H., Akhtar M. N., Farooq U., Fatima N., Choudhary M. I. Phenolic glycosides from Symplocos racemosa: natural inhibitors of phosphodiesterase I. Phytochemisty. 2003;63:217–220. doi: 10.1016/S0031-9422(03)00075-X. [DOI] [PubMed] [Google Scholar]
- 8.Cordell G. A., Chang P. T. O., Fong H. H. S., Farnsworth N. R. Xylosmacin, a new phenolic glucoside ester from Xylosma velutina (Flacourtiaceae) Lloydia. 1977;40:340–343. [PubMed] [Google Scholar]
- 9.Dommisse R. A., Van Hoof L., Vlietinck A. J. Structural analysis of phenolic glucosides from Salicaceae by NMR spectroscopy. Phytochemistry. 1986;25:1201–1204. doi: 10.1016/S0031-9422(00)81580-0. [DOI] [Google Scholar]
- 10.Saito N., Tatsuzawa F., Yazaki Y., Shigihara A., Honda T. 7-Polyacylated delphinidin 3,7-diglucosides from the blue flowers of Leschenaultia cv. Violet Lena. Phytochemistry. 2007;68:673–679. doi: 10.1016/j.phytochem.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Skehan P., Storeng R., Schudiero D., Monks A., McMahon J., Vistica D., Warren J. T., Bokesch H., Kenney S., Boyd M. R. New colorimetric assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]