Abstract
Peptic ulcer disease is a deep gastrointestinal erosion disorder that involves the entire mucosal thickness and can even penetrate the muscular mucosa. Numerous natural products have been evaluated as therapeutics for the treatment of a variety of diseases, including this one. These products usually derive from plant and animal sources that contain active constituents such as alkaloids, flavonoids, terpenoids, tannins and others. The alkaloids are natural nitrogen-containing secondary metabolites mostly derived from amino acids and found in about 20% of plants. There has been considerable pharmacological research into the antiulcer activity of these compounds. In this work we review the literature on alkaloids with antiulcer activity, which covers about sixty-one alkaloids, fifty-five of which have activity against this disease when induced in animals.
Keywords: Alkaloids; Antiulcer activity; Peptic ulcer, Review.
Introduction
For over a century, peptic ulcer disease has been one of the leading causes of gastrointestinal surgery, with high morbidity and mortality rates. The prevalence of gastrointestinal ulcers differs around the world: duodenal ulcers are dominant the Western populations and gastric ulcers are more frequent in Asia, especially in Japan. As the prevalence of this disease increases over time, one would expect peptic ulcers to continue to have a significant global impact in the basic health and economic systems and in patients’ life quality [1].
Peptic ulcers are a deep gastrointestinal erosion disorder that involves the entire mucosal thickness, penetrating the muscular mucosa [2]. For decades it was believed that gastrointestinal ulcerations were caused by the excessive secretion of gastric acid, but many patients presenting such ulcerations had normal acid secretion rates [3]. Then, researchers reported that peptic ulcers were been caused by an imbalance between the aggressive factors and a number of known defense mechanisms. Exogenous aggressive factors such as smoke, anti-inflammatory drugs, alcohol, stress, fatty foods and Helicobacter pylori infections triggered tissue necrosis through mucosal ischemia, free radical generation and cessation of nutrient delivery, hydrochloric acid together with pepsin, pancreatic enzymes and bile decreased the defense mechanisms of gastrointestinal mucosa such as the intercellular junctions, local blood flow, mucus/bicarbonate secretion and cellular growth [2,4,5].
In recent years, a large advance in chemical and pharmacological studies has contributed to the knowledge about new therapeutically active compounds obtained from the natural products [6]. These compounds can be used directly as leads for the development of new medicines or as pharmacological tools to discover new active compounds, so they can be life-saving or determine the quality of life in long-lasting diseases [7,8]. However, the incorrect use of the natural products offers dangers to society, so it is important to identify the active compounds, linking its structure with the biological activity and report the correct manner to use them with regards to dose, route of administration and frequency of use [9].
The natural active compounds classes or secondary metabolites as alkaloids, flavonoids, terpenoids, tannins and others have attracted researchers to investigate their chemical, toxicological and pharmacological features. The alkaloids represent a group of natural products that has had a major impact throughout history on the economic, medical, political and social affairs of humans. They are a diverse group of low molecular weight nitrogen-containing compounds derived mostly from amino acids [10]. These secondary metabolites are found in about 20 % of plant species and they classified as true alkaloids, which have nitrogen atoms in heterocyclic rings, protoalkaloids, which do not have the nitrogen atom(s) in heterocyclic rings and pseudoalkaloids, which don’t derive from amino acids but may have nitrogen atoms in heterocyclic rings [11].
Several alkaloids are being used in therapeutics and as pharmacological tools. A wide range of biological activities of alkaloids have been reported: emetic, anti-cholinergic, antitumor, diuretic, sympathomimetic, antiviral, antihypertensive, hypnoanalgesic, antidepressant, miorelaxant, antitussigen, antimicrobial and anti-inflammatory [11]. However, the alkaloids and other natural compounds have complex activities and it is necessary to analyze pharmacological activities in the general tissues, linking the structure with the activity presented. It is common to find pharmacological results where a single experimental model generalizes a biological answer, but these can’t be accepted because all the pathologies in question are also complex and it is necessary to investigate specific experimental models.
In the course of our continuing search for bioactive natural products from plants, we have recently published reviews on crude plant extracts and plant-derived compounds with the following potential activities and uses: as inhibitors of mammary, uterine cervical and ovarian neoplasia [12,13,14]; as inhibitors of HMG CoA reductase [15]; with central analgesic activity [16]; employed in prevention of osteoporosis [17]; for the treatment of Parkinson’s disease [18]; antileishmanial, hypoglycemic and anti-inflammatory activities [19,20,21,22]; as inhibitors of the acetylcholinesterase enzyme [23], inhibitors of the angiotensin-converting enzymes [24], with giardicidal and antileprotic activities [25,26] and plant extracts with antiulcer activity [27].
In this article, we have reviewed some reports about alkaloids with antiulcer activity in the specialized literature pubished up to December 2007. The search was carried out on data banks such as SciFinder Scholar, Periódicos CAPES, Pubmed and NAPRALERT (acronym for Natural Products ALERT - University of Illinois in Chicago, U.S.A.). The references were consulted for details of the experimental models used for testing the alkaloids against peptic ulcer, activities, route of administration, organism tested and subtypes of alkaloids studied.
Alkaloids studied in models that investigate the antiulcer activity
For this review we identified sixty-one alkaloids studied in models that evaluate the antiulcer activity and distributed among thirteen subtypes: imidazole, indole, isoquinoline, non-nitrogen heterocycle alkaloid, phenylalkylamide, piperidine, pyrazine, pyridine, pyrrolidine, pyrrolizidine, quinolizidine, steroid and tropane alkaloids. Fifty-five of these alkaloids have reported antiulcer activity (see Table 1).
Table 1.
Alkaloids with gastrointestinal antiulcer activity
| Substance | Experimental models/dose – Route of administration | Organism tested | Effect |
|---|---|---|---|
| Imidazole alkaloids | |||
| Allantoin | Phenylbutazone induced ulcer/6 g/kg – gastric intubation | Rat | Active [61] |
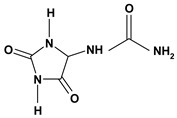
|
*/oral | Human adult | * [62] |
| Histamine, para-coumaroyl | */ 100 mg/kg – intragastric | Mouse | Equivocal [63] |
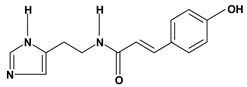
|
50% ethanol induced gastric ulcer/2.5; 10 and 20 mg/kg – oral and intraperitoneal | Mouse | Active [59] |
4-Methoxycantin-6-one 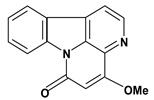
|
50% ethanol induced gastric ulcer/ 10 and 20 mg/kg – oral and intraperitoneal | Mouse | Active [59] |
Hirsuteine 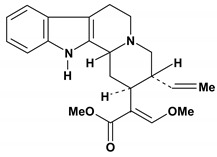
|
*/60 mg/kg – intraperitoneal | Mouse | Active [56] |
Hirsutine 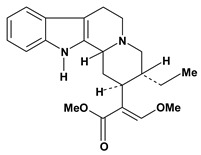
|
*/60 mg/kg – intraperitoneal | Mouse | Active [56] |
LSD-25 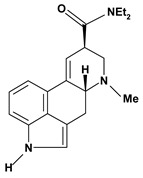
|
5-HT induced gastric ulcers/30mg/kg – intramuscular | Guinea pig | Active [64] |
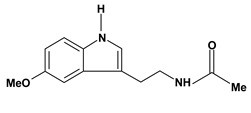
| Melatonin | */intragastric | Rat | Active [55] |
|
Ischemia-reperfusion induced ulcer/intragastric | Rat | Active [55] |
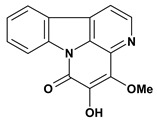
| Nigakinone | */* | Rat | Active [57] |
|
Pylorus-ligated induced ulcer/31.3 mg/kg - * | Rat | Active [57] |
Nigakinone, methyl 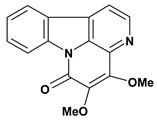
|
Pylorus-ligated induced ulcer/62.5 mg/kg - * | Rat | Active [58] |
| Reserpine | Stress induced ulcer/2.5 mg/kg –subcutaneous | Mouse | Inactive [46] |
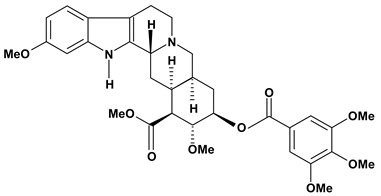
| |||
Rhynchophylline 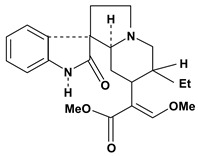
|
*/60 mg/kg – intraperitoneal | Mouse | Active [56] |
Rhynchophylline, iso 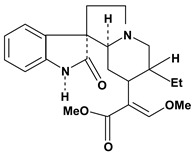
|
*/60 mg/kg – intraperitoneal | Mouse | Inactive [56] |
Tabersonine 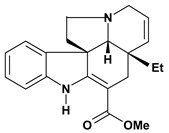
|
*/50 mg/kg – intragastric | Rat | Active [34] |
Vinpocetine 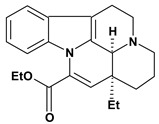
|
*/10 mg/kg – oral | Human adult | Active [65] |
Yohimbine, beta 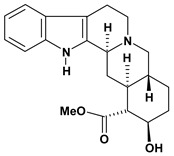
|
*/20 mg/kg – intraperitoneal | Mouse | Active [56] |
| Isoquinoline alkaloids | |||
| Berberine | Ethanol induced gastric ulcer/25 mg/kg – intragastric | Rat | Inactive [30] |
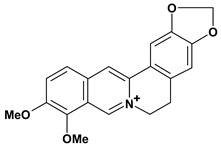
|
*/15 mg/kg – gastric intubation | Rat | Weak activity [31] |
| Pylorus-ligated induced ulcer/10 mg/kg – oral | Rat | Inactive [32] | |
Berberine, oxy 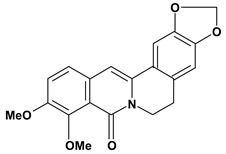
|
Ethanol induced gastric ulcer/50 mg/day - intragastric | Rat | Inactive [30] |
Cathinone 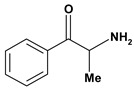
|
*/intragastric | Rat | Active [66] |
| Cathinone, (-) | Aspirin induced gasric ulcer/10 mg/kg - intragastric | Rat | Active [28] |
| Indomethacin induced gastric ulcer/10 mg/kg - intragastric | Rat | Active [28] | |
| Phenylbutazone induced gastric ulcer/10 mg/kg - intragastric | Rat | Active [28] | |
| Cathinone, (+) | Aspirin induced gasric ulcer/10 mg/kg - intragastric | Rat | Active [28] |
| Indomethacin induced gastric ulcer/10 mg/kg - intragastric | Rat | Active [28] | |
| Phenylbutazone induced gastric ulcer/10 mg/kg - intragastric | Rat | Active [28] | |
| Coptisine | Ethanol induced gastric ulcer/0.1 mg/kg - intragastric | Rat | Active [30] |
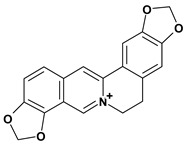
|
80% ethanol induced ulcer/0.1 mg/kg - intragastric | Rat | Active [35] |
| 80% ethanol induced ulcer/0.1 mg/kg - intragastric | Rat | Active [36] | |
| Coptisine, 8-oxo | Ethanol induced gastric ulcer/0.1 mg/kg - intragastric | Rat | Active [30] |
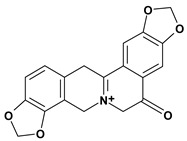
|
80% ethanol induced ulcers/0.1 mg/kg - intragastric | Rat | Active [35] |
Corydaline 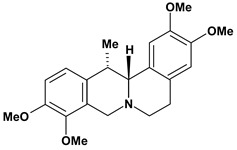
|
*/15 mg/kg - oral | Human adult | Active [67] |
| Corydaline, dehydro | */40 mg/kg - oral | Mouse | Active [68] |
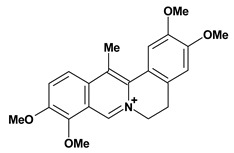
|
*/* | Bullfrog | Active [68] |
| Pylorus-ligated induced gastric ulcer/oral | Rat | Active [69] | |
| Stress induced gastric ulcer/oral | Rat | Active [69] | |
| Pylorus-ligated induced gastric ulcer/subcutaneous | Rat | Active [69] | |
| Stress induced gastric ulcer/subcutaneous | Rat | Active [69] | |
| Histamine induced ulcers of duodenum/oral | Guinea pig | Active [69] | |
| Histamine induced ulcers of duodenum/subcutaneous | Guinea pig | Inactive [69] | |
| Reserpine induced gastric ulcer/oral | Rat | Active [69] | |
| Reserpine induced gastric ulcer/subcutaneous | Rat | Inactive [69] | |
Corydamine 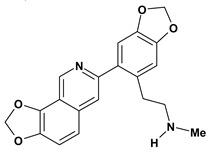
|
*/* | * | Active [70] |
Corydamine, tetrahydro 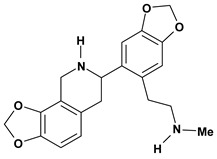
|
*/* | * | Active [71] |
| Ephedrine, nor- n-formyl (-) | Aspirin induced ulcers/5 mg/kg - intragastric | Rat | Active [28] |
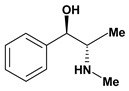 |
Indomethacin induced ulcers/10 mg/kg - intragastric | Rat | Active [28] |
Ephedrine, nor-
n-formyl (+) 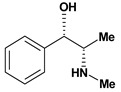
|
Aspirin induced ulcers/5 mg/kg - intragastric | Rat | Active [28] |
| Glaziovine | Histamine induced ulcers/5 mg/kg - intraperitoneal | Guinea pig | Active [72] |
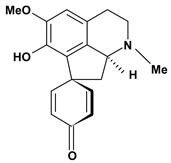
|
Reserpine induced ulcers/5 mg/kg - intraperitoneal | Rat | Active [72] |
| Serotonin induced ulcers/5 mg/kg - intraperitoneal | Rat | Active [72] | |
| Restraint stress induced ulcers/5 mg/kg - intraperitoneal | Rat | Active [72] | |
| Pylorus-ligated induced ulcers/5 mg/kg - intraperitoneal | Rat | Active [72] | |
| */10 mg/person - oral | Human adult | Active [73] | |
Morphine 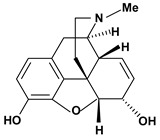
|
Reserpine induced ulcers/10 mg/kg - oral | Rat | Active [29] |
| Palmatine, 7-8-dihydro: 8-hydroxy | Ethanol induced gastric ulcers/50 mg/kg - intragastric | Rat | Active [33] |
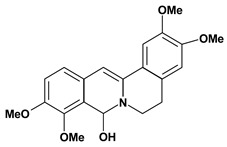
|
Pylorus-ligated induced ulcers/50 mg/kg - intragastric | Rat | Active [33] |
| Acetic acid induced ulcers/80 mg/kg - intragastric | Rat | Active [33] | |
| */100 mg/kg - intragastric | Rat | Active [34] | |
| Non- nitrogen heterocycle alkaloid | |||
| Chlorophyll | Pylorus-ligated induced ulcers/1 g/kg - intravenous | Rat | Active [74] |
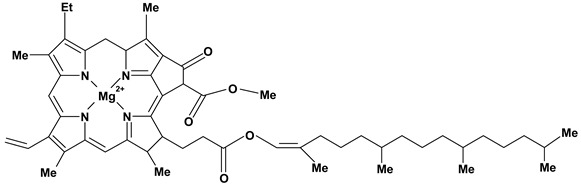 | |||
| Phenylalkylamide alkaloid | |||
| Capsaicin | Ethanol induced gastric ulcer/0.3 nanomol/kg - intragastric | Rat | Active [52] |
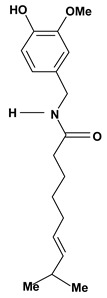 |
80% ethanol induced ulcer/2 mg/kg - intragastric | Rat | Active [75] |
| 80% ethanol induced ulcer/2 mg/kg - subcutaneous | Rat | Active [75] | |
| Pylorus-ligated induced ulcer/1 μg/kg - intragastric | Rat | Active [76] | |
| Aspirin induced ulcer/0.6 μg/kg - intragastric | Rat | Active [77] | |
| Ethanol induced ulcer/0.105 μg/kg - intragastric | Rat | Active [77] | |
| HCl induced ulcer/0.1 μg/kg - intragastric | Rat | Active [77] | |
| Acetic acid induced ulcer/10 mg/kg - intragastric | Rat | Active [78] | |
| */0.5 mg/kg - intragastric | Rat | Active [53] | |
| Piperidine alkaloid | |||
| Piperine | Aspirin induced ulcer/1,5 mg/kg - intravenous | Rabbit | Active [50] |
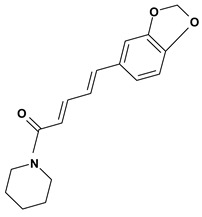
|
Restraint stress induced ulcer/25 mg/kg - intragastric | Rat | Active [51] |
| Indomethacin induced ulcer/25 mg/kg - intragastric | Rat | Active [51] | |
| HCl/ethanol induced gastric ulcer/25 mg/kg - intragastric | Rat | Active [51] | |
| Pylorus-ligated induced ulcer/25 mg/kg - intragastric | Rat | Active [51] | |
| Restraint stress induced ulcer/25 mg/kg - intragastric | Mouse | Active [51] | |
| Indomethacin induced ulcer/25 mg/kg - intragastric | Mouse | Active [51] | |
| HCl/ethanol induced gastric ulcer/25 mg/kg - intragastric | Mouse | Active [51] | |
| Pylorus-ligated induced ulcer/25 mg/kg – intragastric | Mouse | Active [51] | |
| Pyrazine alkaloid | |||
| Ligustrazine | Water-immersion stress induced ulcer/intragastric | Rat | Active [79] |
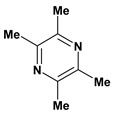
|
Restraint stress induced ulcer/intragastric | Rat | Active [79] |
| Pyridine alkaloids | |||
| Gentianine | Pylorus-ligated induced ulcers/100 mg/kg - oral | Rat | Weak activity [80] |
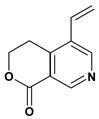
|
Water-immersion stress-induced ulcers/100 mg/kg - oral | Rat | Active [80] |
Mallorepine 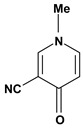
|
Stress induced gastric ulcer/300 mg/kg - subcutaneous | Mouse | Inactive [81] |
Nicotine 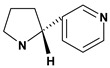
|
Aspirin induced ulcer/1mg/animal - intragastric | Rat | Active [44] |
| Pyrrolidine alkaloid | |||
Cuscohygrine 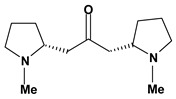
|
*/* | Rat | Active [82] |
| Pyrrolizidine alkaloids | |||
| Interregimine | Hypothermic restraint stress induced gastric ulcer/12.5 mg/kg of crude alkaloid extract - oral | Mouse | Active [60] |
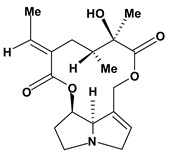
|
Ethanol induced gastric ulcer/12.5 mg/kg of crude alkaloid extract - oral | Rat | Active [60] |
| Cysteamine induced duodenal ulcer/12.5 mg/kg of crude alkaloid extract - oral | Rat | Active [60] | |
| Indomethacin induced gastric ulcer/12.5 mg/kg of crude alkaloid extract - oral | Mouse | Active [60] | |
Retrorsine 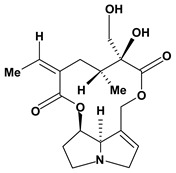
|
Hypothermic restraint stress induced gastric ulcer/12.5 mg/kg of crude alkaloid extract - oral | Mouse | Active [60] |
| Ethanol induced gastric ulcer/12.5 mg/kg of crude alkaloid extract - oral | Rat | Active [60] | |
| Cysteamine induced duodenal ulcer/12.5 mg/kg of crude alkaloid extract - oral | Rat | Active [60] | |
| Indomethacin induced gastric ulcer/12.5 mg/kg of crude alkaloid extract – oral | Mouse | Active [60] | |
Senecionine 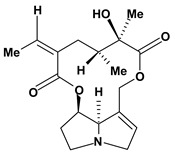
|
Hypothermic restraint stress induced gastric ulcer/12.5 mg/kg of crude alkaloid extract – oral | Mouse | Active [60] |
| Ethanol induced gastric ulcer/12.5 mg/kg of crude alkaloid extract - oral | Rat | Active [60] | |
| Cysteamine induced duodenal ulcer/12.5 mg/kg of crude alkaloid extract - oral | Rat | Active [60] | |
| Indomethacin induced gastric ulcer/12.5 mg/kg of crude alkaloid extract – oral | Mouse | Active [60] | |
Seneciphylline 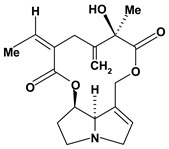
|
Hypothermic restraint stress induced gastric ulcer/12.5 mg/kg of crude alkaloid extract - oral | Mouse | Active [60] |
| Ethanol induced gastric ulcer/12.5 mg/kg of crude alkaloid extract - oral | Rat | Active [60] | |
| Cysteamine induced duodenal ulcer/12.5 mg/kg of crude alkaloid extract - oral | Rat | Active [60] | |
| Indomethacin induced gastric ulcer/12.5 mg/kg of crude alkaloid extract - oral | Mouse | Active [60] | |
Usaramine 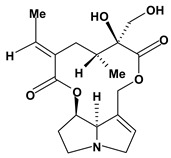
|
Hypothermic restraint stress induced gastric ulcer/12.5 mg/kg of crude alkaloid extract - oral | Mouse | Active [60] |
| Ethanol induced gastric ulcer/12.5 mg/kg of crude alkaloid extract - oral | Rat | Active [60] | |
| Cysteamine induced duodenal ulcer/12.5 mg/kg of crude alkaloid extract - oral | Rat | Active [60] | |
| Indomethacin induced gastric ulcer/12.5 mg/kg of crude alkaloid extract - oral | Mouse | Active [60] | |
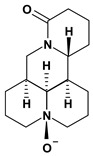
| Quinolizidine alkaloids | |||
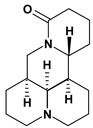
| Matrine | Water-immersion stress induced ulcers/25 mg/kg - gastric intubation | Mouse | Active [45] |
|
Restraint stress induced ulcers/25 mg/kg - gastric intubation | Mouse | Active [45] |
| Water-immersion stress induced ulcers/10 mg/kg - intraperitoneal | Mouse | Weak activity [45] | |
| Restraint stress induced ulcers/10 mg/kg - intraperitoneal | Mouse | Weak activity [45] | |
| Water-immersion stress induced ulcers/10 mg/kg - intravenous | Mouse | Active [45] | |
| Restraint stress induced ulcers/10 mg/kg – intravenous | Mouse | Active [45] | |
| Stress induced ulcer/50 mg/kg - gastric intubation | Mouse | Active [46] | |
| Stress induced ulcer/50 mg/kg -Intraperitoneal | Mouse | Active [46] | |
| Restraint stress induced ulcers/50 mg/kg - gastric intubation | Mouse | Active [47] | |
| Restraint stress induced ulcers/50 mg/kg - intraperitoneal | Mouse | Weak activity [47] | |
| Restraint stress induced ulcers/50 mg/kg – intravenous | Mouse | Active [47] | |
| Pylorus-ligated induced ulcers/50 mg/kg - intraduodenal | Rat | Inactive [45] | |
| Indomethacin induced ulcers/100 mg/kg - gastric intubation | Mouse | Inactive [45] | |
| Reserpine induced ulcers/ 50 mg/kg – gastric intubation | Mouse | Active [45] | |
| Reserpine induced ulcers/25 mg/kg - intravenous | Mouse | Active [45] | |
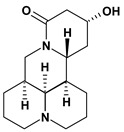
| Matrine, 13-alpha-hydroxy | Water-immersion stress induced ulcers/* - intragastric | Rat | Active [48] |
|
Indomethacin plus alcohol induced gastric ulcers/ * -intragastric | Rat | Active [48] |
| Matrine, oxy | Pylorus-ligated induced ulcers/* | * | Active [49] |
|
Indomethacin induced ulcers/* | * | Active [49] |
| Restraint stress induced ulcers/ * - intraduodenal | Rat | Active [49] | |
| Water-immersion stress induced ulcers/25mg/kg - gastric intubation | Mouse | Active [45] | |
| Restraint stress induced ulcers/25 mg/kg - gastric intubation | Mouse | Active [45] | |
| Water-immersion stress induced ulcers/10 mg/kg - intraperitoneal | Mouse | Active [45] | |
| Restraint stress induced ulcers/10 mg/kg – intraperitoneal | Mouse | Active [45] | |
| Water-immersion stress induced ulcers/10 mg/kg - intravenous | Mouse | Weak activity [45] | |
| Restraint stress induced ulcers/10 mg/kg - intravenous | Mouse | Weak activity [45] | |
| Stress induced ulcer/50 mg/kg - gastric intubation | Mouse | Active [46] | |
| Stress induced ulcer/50 mg/kg -intraperitoneal | Mouse | Active [46] | |
| Restraint stress induced ulcers/50 mg/kg - gastric intubation | Mouse | Active [47] | |
| Restraint stress induced ulcers/50 mg/kg - intraperitoneal | Mouse | Active [47] | |
| Restraint stress induced ulcers/50 mg/kg - intravenous | Mouse | Weak activity [47] | |
| Indomethacin induced ulcers/100 mg/kg - gastric intubation | Mouse | Active [45] | |
| Reserpine induced ulcers/50 mg/kg - gastric intubation | Mouse | Active [45] | |
| Reserpine induced ulcers/50 mg/kg - intravenous | Mouse | Inactive [45] | |
| Pylorus-ligated induced ulcers/* - gastric intubation | Rat | Active [84] | |
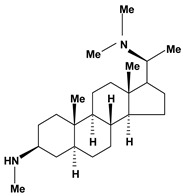
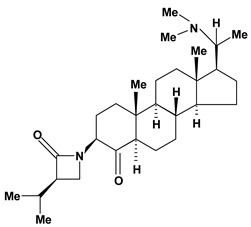
| Steroid alkaloids | |||
| Pachysamine A, epi | Water-immersion stress induced ulcers/50 mg/kg - intraperitoneal | Mouse | Active [54] |
|
Restraint stress induced ulcers/50 mg/kg – intraperitoneal | Mouse | Active [54] |
Pachysandrine A 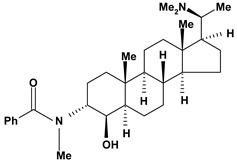
|
Water-immersion stress induced ulcers/50 mg/kg - intraperitoneal | Mouse | Active [54] |
| Pachystermine A | Restraint stress induced ulcers/50 mg/kg - intraperitoneal | Mouse | Active [54] |
| Water-immersion stress induced ulcers/50 mg/kg - intraperitoneal | Mouse | Active [54] | |
 | |||
| Spiropachysine | Water-immersion stress induced ulcers/50 mg/kg - intraperitoneal | Mouse | Active [54] |
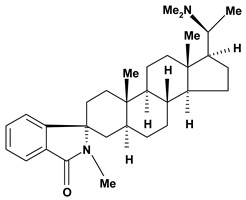
|
Restraint stress induced ulcers/50 mg/kg - intraperitoneal | Mouse | Active [54] |
| Tropane alkaloids | |||
| Anisodamine | Water-immersion stress induced ulcers/10 mg/kg – intraperitoneal | Rat | Active [41] |
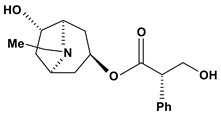
|
Reserpine induced ulcers/1.5 mg/kg - intraperitoneal | Rat | Active [42] |
| Cold stress induced ulcers/2.5 mg/kg - intraperitoneal | Rat | Active [39] | |
| Indomethacin induced ulcers/0.5 mg/kg - intraperitoneal | Rat | Active [39] | |
| Acetic acid induced ulcers/10 mg/kg - intraperitoneal | Rat | Active [39] | |
| Ethanol induced ulcers/25 mg/kg- intragastric | Rat | Active [43] | |
| Indomethacin induced ulcers/25 mg/kg - intragastric | Rat | Active [43] | |
| Restraint stress induced ulcers/25 mg/kg - intragastric | Rat | Active [43] | |
| Pylorus-ligated induced ulcers/25 mg/kg - intragastric | Rat | Active [43] | |
| Reserpine induced ulcers/1.5 mg/kg - intraperitoneal | Rat | Active [42] | |
| Anisodine | Cold stress induced ulcers/2 mg/kg - intraperitoneal | Rat | Active [39] |
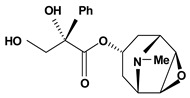
|
Indomethacin induced ulcers/8 mg/kg - intraperitoneal | Rat | Active [39] |
| Acetic acid induced ulcers/32 mg/kg - intraperitoneal | Rat | Active [39] | |
| Atropine | Pylorus-ligated induced ulcers/10 mg/kg - intragastric | Rat | Active [37] |
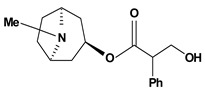
|
Pylorus-ligated induced ulcers/10 mg/kg - gastric intubation | Rat | Active [38] |
| Aspirin induced ulcers/10 mg/kg - gastric intubation | Rat | Active [38] | |
Cocaine 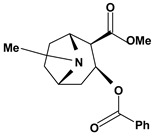
|
Reserpine induced ulcers/10 mg/kg - oral | Rat | Active [29] |
| Scopolamine | Cold stress induced ulcers/0.5 mg/kg - intraperitoneal | Rat | Active [39] |
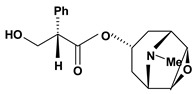
|
Indomethacin induced ulcers/1 mg/kg - intraperitoneal | Rat | Active [39] |
| Acetic acid induced ulcers/8 mg/kg – intraperitoneal | Rat | Active [39] | |
Scopolamine, methyl 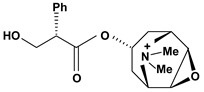
|
Propionitrile-induced duodenal ulcers/2.5 mg/kg - subcutaneous | Rat | Active [40] |
| Tropine, n-(4’-ethoxy-carbonyl-phenyl-amine-n’-acetyl) | */10 mg/kg - intragastric | Rat | Active [83] |
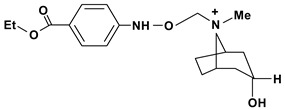
| |||
| Tropine, n-(4’-ethoxy-phenyl-amine-n’-acetyl) | */10 mg/kg - intragastric | Rat | Active [83] |
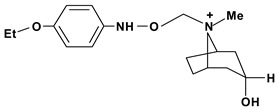
| |||
| Tropine, N-(4’-sulfamoyl-phenyl-amine-N’-acetyl) | */10 mg/kg - intragastric | Rat | Inactive [83] |
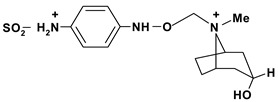
| |||
* = Data not provided
Among the different alkaloids showing potent pharmacological properties are the narcotic analgesic morphine, the antimicrobial berberine and the sympathomimetic ephedrine. These isoquinoline alkaloids occur mainly in the Papaveraceae, Berberidaceae and Ephedraceae families [10]. The compounds morphine and ephedrine also have confirmed antiulcer activity, inhibiting gastric lesions induced by reserpine, aspirin or indomethacin [28,29]. Berberine didn’t display this activity towards ethanol induced ulcers [30,31,32], but 7,8-dihydro-8-hydroxypalmatine, a new type of protoberberine alkaloid obtained from the bark of Enantia chlorantha, accelerated ulcer-healing and increased the gastric mucus production after the lesions have been caused by acetic acid, HCl/ethanol or absolute ethanol and it also has been investigated in the pylorus ligature model [33,34]. Other isoquinoline alkaloids isolated from Coptidis rhizome, coptisine and 8-oxocoptisine, showed protection of gastric mucosa similar to that offered by gastroprotective medicines such as cimetidine and sucralfate [30,35,36].
Tropane alkaloids are dicyclic compounds formed by condensation of a pyrrolidine precursor amino acid (ornithine) with three acetate-derived carbon atoms. Some of these alkaloids such as atropine and scopolamine constitute an important class of anticholinergic compounds derived from plants that occur in several Atropa and Datura species [10]. Clinically, they are used to block the muscarinic activity of acetylcholine showing antispasmodic and antisecretory effects in the treatment of spastic colitis, gastroenteritis and peptic ulcer. They also are useful pharmacological tools to discover new active principles with gastrointestinal tract actions [37,38,39,40]. Anisodamine and anisodine are analogs of atropine and they were evaluated as protectors of gastric mucosa against the damaging effects induced in rats by indomethacin, reserpine, stress, pylorus ligature, acetic acid or absolute ethanol. In this research, these compounds inhibited the lesions caused by aggressor agents and altered the gastric acid secretion through increase of luminal gastric output of basal bicarbonate and pH [39,41,42,43]. Another well known tropane alkaloid, cocaine, showed antiulcer activity against ulcers induced by reserpine in rats when it was used in the dose of 10 mg/kg by oral route of administration [29]. This substance is obtained of the Erythroxylum coca leaves and has multiple actions in the central and peripheral nervous system. It is a psychomotor stimulant with a strong abuse potential and has ability to dominate or decreasing behaviors such as eating and sleeping.
A pyridine alkaloid well known in society is nicotine, which is found in the Solanaceae family, mainly in the dried leaves of the tobacco plant Nicotiana tabacum Linné. This substance acts on the nicotinic receptors of acetylcholine in autonomic ganglia, adrenal medulla, neuromuscular junction and brain of mammals. The chronic use of nicotine may result in psychologic and physical dependence. However, this alkaloid protected the stomach from damage induced by aspirin by decreasing hemorrhages and increasing the pH gradient/gastric fluid volume [44].
Quinolizidine alkaloids such as matrine, 13-alpha-hydroxymatrine and oxy-matrine were isolated from Sophora flavescens (Fabaceae) and they were experimentally tested for inhibition of gastric ulcers induced by pylorus ligature, water immersion stress and indomethacin. These alkaloids decreased the acid secretion and inhibited the gastric motility [45,46,47,48,49].
A piperidine alkaloid is piperine, which has a pungent taste and was studied in connection with the gastric mucosa damage caused by stress, indomethacin, ethanol or pylorus ligature in rats or mice. This substance protected the stomach against ulceration by decreasing the volume of gastric juice, gastric acidity and pepsin-A activity in doses of 1.5 mg/kg and 25 mg/kg after intravenous and oral administrations, respectively [50,51]. Capsaicin is a phenylakylamide alkaloid and it also has a pungent taste. This substance provides a selective probe for mechanisms involving slowly conducting primary afferent neurons. In the stomach, capsaicin (0.3 nanolmol/kg - 10 mg/kg) stimulates these neurons and signalizes for protection inhibiting the acid secretion, stimulating the alkali/mucus secretions and mainly increasing the gastric mucosal blood flow which help in prevention and healing of ulcers against injury caused by aggressive agents. However, neurotoxic doses of capsaicin have augmented the susceptibility of gastric mucosa to injury caused by strong irritants [52,53].
Steroidal alkaloids have been found in the Apocynaceae, Buxaceae, Liliaceae and Solanaceae families. In this review, pachystermine A, pachysamine A, epipachysamine A, pachysandrine A and spiropachysine obtained from Pachysandra terminalis (Buxaceae), used in Ainu folk medicine for gastrointestinal diseases, were investigated for their preventive effects on gastric lesions induced by water-immersion stress in mice and the results this evaluation suggested that these alkaloids may contribute partly to the traditional use of the plants for the gastrointestinal complaints [54].
An important class of alkaloid is the indole constituted by melatonin, cantinone, hirsuteine, reserpine, lysergic acid (LSD), yohimbine and nigakinone. Melatonin has been found in algae and humans. This human hormone is secreted by pineal gland and gastrointestinal cells. Moreover, it has antiulcer activity because showed to protect the gastric mucosa from the damage caused by ischemia-reperfusion and absolute ethanol through of the attenuation of the gastric blood flow failed and scavenging of free radicals [55]. Reserpine was isolated of Rauvolfia serpentina (Apocynaceae) and didn’t show antiulcer effect in the dose of 2.5 mg/kg when the gastric ulcers were induced by stress in mice [46], while yohimbine, obtained of Pausinystalia yohimbe (Rubiaceae), was active in the reduction of gastrointestinal ulceration [56]. Other alkaloids such as hirsutine, hirsuteine, and rhynchophylline isolated from the domestic plant Uncaria rhynchophylla Miq. showed mild central depressive, anti-spasmodic and hypotensive effects in mice or rats. These substances also were effective in the dose of 60 mg/kg against gastric lesions, while isorhynchophylline didn´t have a preventive effect on the development of gastric erosions in mice [56]. Nigakinone and methylnigakinone are also indole alkaloids and they can be found in Picrasma quassioides, P. ailanthoides or Ailanthus altissima. These substances had antiulcer effects associated with decreases in gastric acid/pepsin secretions and protection of the mucous membrane [57,58]. Cantin-6-one and 4-methoxycantinone are alkaloids extracted from Simaroubaceae plants. These substances were found in Quassia amara, Simaba multiflora, S. polyphylla, S. feruginea and Eurycoma longifolia which are popularly indicated for gastrointestinal disorders, obesity, anti-inflammatory, stimulant of the intestinal motility and central nervous system activities. These compounds were effective against gastric lesions induced by ethanol and indomethacin [59].
The pyrrolizidine alkaloids integerrimine, retrorsine, senecionine, usaramine and seneciphylline were extracted from Senecio brasiliensis. These alkaloids demonstrate significant activity in acute and chronic gastric ulcers in the dose of 12.5 mg/kg. These alkaloids increased free mucus and prostaglandin in the mucosal gastric. Moreover, they showed a reduction of exfoliation of superficial cells, hemorrhages and blood cell infiltration that can be mediated by increase in gastrin secretion and mRNA expression of epidermal growth factors [60].
Conclusions
The studied alkaloids have shown gastroprotetive and antiulcer activities, nevertheless many of them as senecionine, cocaine, nicotine and LSD have several toxic effects on the body such as hepatotoxic, neurotoxic and carcinogenic properties, although some compounds such as morphine (hypnoanalgesic) and ephedrine (nasal decongestant) are more effective in other afflictions. Consequently many alkaloids are not viable for development as gastroprotective drugs or they would need modification of functional groups in their chemical structures for this propose. Nevertheless, they can be used as pharmacological tools in pathophysiological understanding of gastrointestinal diseases, particulalry peptic ulcers, and in the viability of new active compounds for synthesis of new medicines. In this context, it is necessary to support financially the multidisciplinary and interdisciplinary research, mostly those related to natural products enabling the discovery of new antiulcer or gastroprotective pharmaceuticals.
Acknowledgements
The authors thanks to College of Pharmacy, the University of Illinois at Chicago, U.S.A., for helping with the computer database NAPRALERT and CNPq/CAPES/RENORBIO/IMSEAR - Brazil for financial support.
Footnotes
Sample Availability: Not available.
References and Notes
- 1.Yuan Y., Padol I.T., Hunt R.H. Peptic ulcer disease today. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006;3:80–89. doi: 10.1038/ncpgasthep0393. [DOI] [PubMed] [Google Scholar]
- 2.Tarnawski A.S. Cellular and molecular mechanisms of gastrointestinal ulcer healing. Digest. Dis. Sci. 2005;50:24–33. doi: 10.1007/s10620-005-2803-6. [DOI] [PubMed] [Google Scholar]
- 3.Wallace J.L., Granger D.N. The cellular and molecular basis of gastric mucosal defense. FASEB J. 1996;10:731–740. doi: 10.1096/fasebj.10.7.8635690. [DOI] [PubMed] [Google Scholar]
- 4.Kaunitz J.D., Akiba Y. Gastroduodenal mucosal defense: role of endogenous mediators. Curr. Opin. Gastroenterol. 2004;20:526–532. doi: 10.1097/00001574-200411000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Bandyopadhyay D., Biswas K., Bhattacharyya M., Reiter R.J., Banerjee R.K. Gastric toxicity and mucosal ulceration induced by oxygen-derived reactive species: protection by melatonin. Curr. Mol. Med. 2001;1:501–513. doi: 10.2174/1566524013363483. [DOI] [PubMed] [Google Scholar]
- 6.Cechinel Filho V., Yunes R.A. Estratégias para obtenção de compostos farmacologicamente ativos a partir de plantas medicinais: conceitos sobre modificação estrutural para otimização da atividade. Quim. Nova. 1998;21:99–105. doi: 10.1590/S0100-40421998000100015. [DOI] [Google Scholar]
- 7.Kaplan M.A.C., Gottlieb O.R. Busca racional de princípios ativos em plantas. Interciência. 1990;15:26–29. [Google Scholar]
- 8.Elisabetsky E., Costa-Campos L. Medicinal plant genetic resources and international cooperation: the Brazilian perspective. J. Ethnopharmacol. 1996;51:111–119. doi: 10.1016/0378-8741(95)01353-9. [DOI] [PubMed] [Google Scholar]
- 9.Secco R.S. Produtos naturais: alternativa segura? Cienc. Cult. 1990;42:807–810. [Google Scholar]
- 10.Ziegler J., Facchini P.J. Alkaloid biosynthesis: metabolism and trafficking. Annu. Rev. Plant Biol. 2008;59:735–769. doi: 10.1146/annurev.arplant.59.032607.092730. [DOI] [PubMed] [Google Scholar]
- 11.Henriques A.T., Limberger R.P., Kerber V.A., Moreno P.R.H. In: Farmacognosia: da planta ao medicamento. 5 ed. Simões C. M. O., et al., editors. Editoras of the Universidades Federais de Santa Catarina and Rio Grande do Sul; Porto Alegre/Florianópolis, Brazil: 2004. pp. 765–792. Chapter 29. [Google Scholar]
- 12.Moura M.D., Torres A.R., Oliveira R.A.G., Diniz M.F.F.M., Barbosa-Filho J.M. Natural products as inhibitors of models of mammary neoplasia. Brit. J. Phytother. 2001;5:124–145. [Google Scholar]
- 13.Moura M.D., Silva J.S., Oliveira R.A.G., Diniz M.F.F.M., Barbosa-Filho J.M. Natural products reported as potential inhibitors of uterine cervical neoplasia. Acta Farm. Bonaerense. 2002;21:67–74. [Google Scholar]
- 14.Silva J.S., Moura M.D., Oliveira R.A.G., Diniz M.F.F.M., Barbosa-Filho J.M. Natural product inhibitors of ovarian neoplasia. Phytomedicine. 2003;10:221–232. doi: 10.1078/094471103321659988. [DOI] [PubMed] [Google Scholar]
- 15.Gonçalves M.C.R., Moura L.S.A., Rabelo L.A., Barbosa-Filho J.M., Cruz H.M.M., Cruz J. Natural products inhibitors of HMG CoA reductase. Rev. Bras. Farm. 2000;81:6371. [Google Scholar]
- 16.Almeida R.N., Navarro D.S., Barbosa-Filho J.M. Plants with central analgesic activity. Phytomedicine. 2001;8:310–322. doi: 10.1078/0944-7113-00050. [DOI] [PubMed] [Google Scholar]
- 17.Pereira J.V., Modesto-Filho J., Agra M.F., Barbosa-Filho J.M. Plant and plant-derived compounds employed in prevention of the osteoporosis. Acta Farm. Bonaer. 2002;21:223–234. [Google Scholar]
- 18.Morais L.C.S.L., Barbosa-Filho J.M., Almeida R.N. Plants and bioactive compounds for the treatment of Parkinson’s disease. Arq. Fitomed. 2003;1:127–132. [Google Scholar]
- 19.Rocha L.G., Almeida J.R.G.S., Macedo R.O., Barbosa-Filho J.M. A review of natural products with antileishmanial activity. Phytomedicine. 2005;12:514–535. doi: 10.1016/j.phymed.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Barbosa-Filho J.M., Vasconcelos T.H.C., Alencar A.A., Batista L.M., Oliveira R.A.G., Guedes D.N., Falcão H.S., Moura M.D., Diniz M.F.F.M., Modesto-Filho J. Plants and their active constituents from South, Central, and North America with hypoglycemic activity. Rev. Bras. Farmacogn. 2005;15:392–413. doi: 10.1590/S0102-695X2005000400021. [DOI] [Google Scholar]
- 21.Falcão H.S., Lima I.O., Santos V.L., Dantas H.F., Diniz M.F.F.M., Barbosa-Filho J.M., Batista L.M. Review of the plants with anti-inflammatory activity studied in Brazil. Rev. Bras. Farmacogn. 2005;15:381–391. doi: 10.1590/S0102-695X2005000400020. [DOI] [Google Scholar]
- 22.Barbosa-Filho J.M., Piuvezam M.R., Moura M.D., Silva M.S., Lima K.V.B., Cunha E.V.L., Fechine I.M., Takemura O.S. Anti-inflammatory activity of alkaloids: a twenty century review. Rev. Bras. Farmacogn. 2006a;16:109–139. doi: 10.1590/S0102-695X2006000100020. [DOI] [Google Scholar]
- 23.Barbosa-Filho J.M., Medeiros K.C.P., Diniz M.F.F.M., Batista L.M., Athayde-Filho P.F., Silva M.S., Cunha E.V.L., Almeida J.R.G.S., Quintans-Júnior L.J. Natural products inhibitors of the enzyme acetylcholinesterase. Rev. Bras. Farmacogn. 2006b;16:258–285. doi: 10.1590/S0102-695X2006000200021. [DOI] [Google Scholar]
- 24.Barbosa-Filho J.M., Martins V.K.M., Rabelo L.A., Moura M.D., Silva M.S., Cunha E.V.L., Souza M.F.V., Almeida R.N., Medeiros I.A. Natural products inhibitors of the angiotensin converting enzyme (ACE). A review between 1980-2000. Rev. Bras. Farmacogn. 2006c;16:421–446. doi: 10.1590/S0102-695X2006000300021. [DOI] [Google Scholar]
- 25.Amaral F.M.M., Ribeiro M.N.S., Barbosa-Filho J.M., Reis A.S., Nascimento F.R.F., Macedo R.O. Plants and chemical constituents with giardicidal activity. Rev. Bras. Farmacogn. 2006;16(Suppl.):696–720. [Google Scholar]
- 26.Barbosa-Filho J.M., Nascimento-Júnior F.A., Tomaz A.C.A., Athayde-Filho P.F., Silva M.S., Cunha E.V.L. Natural products with antileprotic activity. Rev. Bras. Farmacogn. 2007;17:141–148. doi: 10.1590/S0102-695X2007000100022. [DOI] [Google Scholar]
- 27.Falcão H.S., Mariath I.R., Diniz M.F.F.M., Batista L.M., Barbosa-Filho J.M. Plants of the American continent with antiulcer activity. Phytomedicine. 2008;15:132–146. doi: 10.1016/j.phymed.2007.07.057. [DOI] [PubMed] [Google Scholar]
- 28.Al-Shabanah O.A., Islam M.W., Al-Gharably N.M., Al-Harbi M.M. Effect of khatamines and their enantiomers on aspirin, indomethacin, phenylbutazone and reserpine induced gastric ulcers in rats. Res Commun. Subst. Abuse. 1993;14:81–94. [Google Scholar]
- 29.Sandor V., Cuparencu B. Analysis of the mechanism of the protective activity of some sympathomimetic amines in experimental ulcers. Pharmacology. 1977;15:208–217. doi: 10.1159/000136691. [DOI] [PubMed] [Google Scholar]
- 30.Hirano H., Tokuhira T., Yokoi T., Shingu T. Gastric mucous membrane-protective principles of Coptidis rhizoma. Nat. Med. 1997;51:516–518. [Google Scholar]
- 31.Yamahara J. Behavioral pharmacology of berberine-type alkaloids. (1). Central depressive action of Coptidis rhizoma and its constituents. Nippon Yakurigaku Zasshi. 1976;72:899–908. doi: 10.1254/fpj.72.899. [DOI] [PubMed] [Google Scholar]
- 32.Sabir M., Akhter M.H., Bhide N.K. Further studies on pharmacology of berberine. Indian J. Physiol. Pharmacol. 1978;22:9–23. [PubMed] [Google Scholar]
- 33.Tan P.V., Nyasse B., Enow-Orock G.E., Wafo P., Forcha E.A. Prophylactic and healing properties of a new anti-ulcer compound from Enantia chlorantha in rats. Phytomedicine. 2000;7:291–296. doi: 10.1016/S0944-7113(00)80046-X. [DOI] [PubMed] [Google Scholar]
- 34.Tan P.V., Nyasse B., Dimo T., Wafo P., Akahkuh B.T. Synergistic and potentiating effects of ranitidine and two new anti-ulcer compounds from Enantia chlorantha and Voacanga africana in experimental animal models. Pharmazie. 2002;57:409–412. [PubMed] [Google Scholar]
- 35.Hirano H., Tokuhira T., Yoshioka Y., Yokoi T., Shingu T. Analysis of gastric mucosus membrane-protective compounds in Coptidis rhizoma. Nat. Med. 2000;54:209–212. [Google Scholar]
- 36.Hirano H., Osawa E., Yamaoka Y., Yokoi T. Gastric-mucous membrane protection activity of coptisine derivatives. Biol. Pharm. Bull. 2001;24:1277–1281. doi: 10.1248/bpb.24.1277. [DOI] [PubMed] [Google Scholar]
- 37.Sakurai T., Sugawara H., Saito K.I., Kano Y. Effects of TDEYA from Atractylodes rhizome on experimental gastric ulcer. Phytother. Res. 1995;9:340–345. doi: 10.1002/ptr.2650090507. [DOI] [PubMed] [Google Scholar]
- 38.Kubo M., Nogami M., Nishimura M., Moriura T., Arichi S. Studies of crude drugs about its origin, process and quality. I. The preventive effects of chinese crude drug "zhu" on experimental stomach ulcer an its pharmacological evaluation (1) Yakugaku Zasshi. 1983;103:442–448. [PubMed] [Google Scholar]
- 39.Zhang J.F., Yan C.D., Zhang Y.Q., Gao W.Z., Zhai X.M. Effect of scopolia drugs on the gastric mucosal lesion in rats. Acta Pharmacol. Sin. 1990;25:90–94. [PubMed] [Google Scholar]
- 40.Robert A., Nezamis J. E., Lancaster C. Duodenal ulcers produced in rats by propionitrile. Factors inhibiting and aggravating such ulcers. Toxicol. Appl. Pharmacol. 1975;31:201–207. doi: 10.1016/0041-008X(75)90156-8. [DOI] [PubMed] [Google Scholar]
- 41.Wan Y.J. Effect of anisodamine on gastric ulcer induced by restraint water-immersion in rats. Clin. Gastroenterol. 1993;3:154–158. [Google Scholar]
- 42.Wang J.L. Effect of anisodamine on the reserpine-induced gastric mucosal lesion in rats. Zhongguo Bingli Shengli Zazhi. 2000;16:237–242. [Google Scholar]
- 43.Yong D.G., Geng B.Q., Gu G.G., Zhong F.M., Yu W.H. Anti-ulcer effect of anisodamine in rats. Acta Pharmacol. Sin. 1991;12:522–525. [PubMed] [Google Scholar]
- 44.Fallone C.A., Morris G.P. Topical nicotine protects rat gastric mucosa against ASA-induced damage. A role for mucosal fluid secretion in cytoprotection. Digest. Dis. Sci. 1995;40:936–942. doi: 10.1007/BF02064180. [DOI] [PubMed] [Google Scholar]
- 45.Yamazaki M., Arai A., Suzuki S., Takeuchi T. Protective effects of matrine and oxymatrine on stress ulcer in relation to their effects on the central nervous system. Yakugaku Zasshi. 1984;104:293–301. doi: 10.1248/yakushi1947.104.3_293. [DOI] [PubMed] [Google Scholar]
- 46.Yamazaki M., Shirota H. Application of experimental stress ulcer test in mice for the survey of neurotropic naturally occurring drug materials. Shoyakugaku Zasshi. 1981;35:96–102. [Google Scholar]
- 47.Yamazaki M., Arai A. Antiulcerogenic activity of oxymatrine. J. Pharmacobiodyn. 1985;8:s-51. [Google Scholar]
- 48.Zhu Z.P., Zhang M.F., Shen Y.Q. Antiulcer components of Sophora viciifolia alkaloids. Tianran Chanwu Yanjiu Yu Kaifa. 1993;5:26–29. [Google Scholar]
- 49.Yamazaki M. The pharmacological studies on matrine and oxymatrine. Yakugaku Zasshi. 2000;120:1025–1033. doi: 10.1248/yakushi1947.120.10_1025. [DOI] [PubMed] [Google Scholar]
- 50.Annamalai A.R., Manavalan R. Effects of Trikatu and its individual components and piperine on gastrointestinal tract: Trikatu - a bioavailable enhancer. Indian Drugs. 1990;27:595–604. [Google Scholar]
- 51.Bai Y.F., Xu H. Protective action of piperine against experimental gastric ulcer. Acta Pharmacol. Sin. 2000;21:357–359. [PubMed] [Google Scholar]
- 52.Tramontana M., Renzi D., Panerai C., Surrenti C., Nappi F., Abelli L., Evangelista S. Capsaicin-like effect of resiniferatoxin in the rat stomach. Neuropeptides. 1994;26:29–32. doi: 10.1016/0143-4179(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 53.Park J.S., Choi M.A., Kim B.S., Han I.S., Kurata T., Yu R. Capsaicin protects against ethanol-induced oxidative injury in the gastric mucosa of rats. Life Sci. 2000;67:3087–3093. doi: 10.1016/S0024-3205(00)00890-0. [DOI] [PubMed] [Google Scholar]
- 54.Watanabe H., Watanabe K., Shimadzu M., Kikuchi T., Liu Z. Anti-ulcer effect of steroidal alkaloids extracted from Pachysandra terminalis. Planta Med. 1986;52:56–58. doi: 10.1055/s-2007-969070. [DOI] [PubMed] [Google Scholar]
- 55.Konturek P.C., Konturek S.J., Majka J., Zembala M., Hahn E.G. Melatonin affords protection against gastric lesions induced by ischemia-reperfusion possibly due to its antioxidant and mucosal microcirculatory effects. Eur. J. Pharmacol. 1997;322:73–77. doi: 10.1016/S0014-2999(97)00051-4. [DOI] [PubMed] [Google Scholar]
- 56.Ozaki Y. Pharmacological studies of indole alkaloids obtained from domestic plants Uncaria rhynchophylla Miq. and Amsonia elliptica Roem. et Schult. Nippon Yakurigaku Zasshi. 1989;94:17–26. doi: 10.1254/fpj.94.17. [DOI] [PubMed] [Google Scholar]
- 57.Omoto T., Shinho Y., Nakajima K., Ishiwatari H., Ito H. Antiulcer alkaloids from Picrasma ailanthus. 02004790. Jpn Kokai Tokkyo Koho. 1990
- 58.Niiho Y., Mitsunaga K., Koike K., Ohmoto T. Studies on the gastric antiulcer components from the woods of Picrasma quassioides (Simaroubaceae) Nat. Med. 1994;48:116–121. [Google Scholar]
- 59.Noldin V.F., Martins D.T.O., Marcello C.M., Lima J.C.S., Monache F.D., Cechinel-Filho V. Phytochemical and antiulcerogenic properties of rhizomes from Simaba ferruginea St. Hill. (Simaroubaceae) Z. Naturforsch. C. 2005;60:701–706. doi: 10.1515/znc-2005-9-1007. [DOI] [PubMed] [Google Scholar]
- 60.Toma W., Trigo J.R., Bensuaski de Paula A.C., Souza Brito A.R.M. Preventive activity of pyrrolizidine alkaloids from Senecio brasiliensis (Asteraceae) on gastric and duodenal induced ulcer on mice and rats. J. Ethnopharmacol. 2004;95:345–351. doi: 10.1016/j.jep.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 61.Cicotti I. Pharmaceutical composition for treating gastro-duodenal ulcers. GB 2054373. 1981
- 62.Istudor V., Mantoiu M., Badescu I. Study on the preparation of an "instant" type product (Ulcovex) for gastric and duodenal ulcers. Part I. Chemical study of Calendulae flores product preparation of the "instant" type extract and determination of the control methodology. Farmacia (Bucharest) 1981;29:41–48. [Google Scholar]
- 63.Hikino H., Kiso Y., Ogata M., Konno C., Aisaka K., Kubota H., Hirose N., Ishihara T. Pharmacological actions of analogues of feruloylhistamine, an imidazole alkaloid of Ephedra roots. Planta Med. 1984;50:478–480. doi: 10.1055/s-2007-969777. [DOI] [PubMed] [Google Scholar]
- 64.Sinha M., Sikdar S., Dasgupta S.R. Study of the effects of curcumin on 5-HT induced gastric lesions in guinea pigs. Indian Med. Gaz. 1975;15:318. [Google Scholar]
- 65.Machova J., Nosalova V., Babulova A. Pharmaceuticals for stomach ulcer treatment. 274070. Czech Patent. 1992
- 66.Al-Shabanah O.A., Al-Gharably N.A., Islam M.W., Al-Harbi M.M. Effect of cathinone, an active constituent of khat (Catha edulis Forsk) and amphetamine on experimental gastric ulcer and secretions. Med. Sci. Res. 1992;20:817–819. [Google Scholar]
- 67.Iwasa J., Naruto S., Ikeda N., Sohji Y. Salt of dehydrocorydaline. CA 934547. Can. Pat. 1974
- 68.Watanabe K., Goto Y., Murakami M. Mode of action of dehydrocorydaline on gastric acid secretion in rats. Oyo Yakuri. 1974;8:1105. [Google Scholar]
- 69.Sohji Y., Kawashima K., Shimizu M. Effect of dehydrocorydaline chloride on experimental gastric and duodenal ulcers. Nippon Yakurigaku Zasshi. 1974;70:425–437. doi: 10.1254/fpj.70.425. [DOI] [PubMed] [Google Scholar]
- 70.Nishioka I., Takao N., Nonaka G., Iwasa K. Corydamine and tetrahydrocorydamine derivatives. 49125399. Jpn. Kokai Tokkyo Koho. 1974
- 71.Nishioka I., Takao N., Nonaka G., Iwasa K. Tetrahydrocorydamine. 49125398. Jpn. Kokai Tokkyo Koho. 1974
- 72.Chaumontet M., Capt M., Gold-Aubert P. Comparative study of two anti-ulcerogenic drugs-glaziovine and sulpiride. Arzneimittelforsch. 1978;28:2119–2121. [PubMed] [Google Scholar]
- 73.Galeone M., Cacioli D., Moise G., Gherardi G., Quadro G. Glaziovine as antiulcer: a double-blind short-term controlled clinical trial in comparison with cimetidine. Curr. Ther. Res. 1981;30:44–49. [Google Scholar]
- 74.Ishii Y., Fujii Y. Effects of several mild antiulcer agents on pylorus ligated rats (shay rats) Nippon Yakurigaku Zasshi. 1974;70:863. doi: 10.1254/fpj.70.863. [DOI] [PubMed] [Google Scholar]
- 75.Kang J.Y., Teng C.H., Wee A., Chen F.C. Effect of capsaicin and chilli on ethanol induced gastric mucosal injury in the rat. Gut. 1995;36:664–669. doi: 10.1136/gut.36.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abdel Salam O.M.E., Szolcsanyi J., Mozsik G. Capsaicin and its analogue resiniferatoxin inhibit gastric acid secretion in pylorus-ligated rats. Pharmacol. Res. 1995;31:341–345. doi: 10.1016/1043-6618(95)80087-5. [DOI] [PubMed] [Google Scholar]
- 77.Abdel Salam O.M.E., Mozsik G., Szolcsanyi J. Studies on the effect of intragastric capsaicin on gastric ulcer and on the prostacyclin-induced cytoprotection in rats. Pharmacol. Res. 1995;32:209–215. doi: 10.1016/s1043-6618(05)80024-6. [DOI] [PubMed] [Google Scholar]
- 78.Kang J.Y., Teng C.H., Chen F.C. Effect of capsaicin and cimetidine on the healing of acetic acid induced gastric ulceration in the rat. Gut. 1996;38:832–836. doi: 10.1136/gut.38.6.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wan J.L., Wang C., Cui S.Z. Effect of tetramethylpyrazine on gastric ulcer induced by restraint/water-immersion stress in rats. Chung Ts’ao Yao. 2000;31:115–117. [Google Scholar]
- 80.Yamahara J., Konoshima T., Sawada T., Fujimura H. Biologically active principles of crude drugs: pharmacological actions of Swertia japonica extracts, swertiamarin and gentianine. Yakugaku Zasshi. 1978;98:1446–1451. doi: 10.1248/yakushi1947.98.11_1446. [DOI] [PubMed] [Google Scholar]
- 81.Hikino H., Tamada M., Yen K.Y. Mallorepine, cyano-gamma-pyridone from Mallotus repandus. Planta Med. 1978;33:385–388. doi: 10.1055/s-0028-1097397. [DOI] [PubMed] [Google Scholar]
- 82.Sun Y.S., Ma Z.S., Fan Y.H., Wang W.L., Yin S.Y., Liu C.K., Chen W.Y., Liu Y.H., Deng S.J. Chemical reformation of cuscohygrine. K’o Hsueh T’ung Pao. 1975;20:427–428. [Google Scholar]
- 83.Gorecki P., Drozdzynska M., Kedzia B., Przybylska D. Synthesis and biological activity of tropine alkaloids quaternary derivatives I. Tropine derivatives. Herba Pol. 1983;29:135–149. [Google Scholar]
- 84.Arai A., Yamazaki M. Study of antisecretory effect of oxymatrine, a constituent of Sophora flavescens. J. Pharmacobiodyn. 1987;10:s-55. [Google Scholar]


