Abstract
The synthesis of konjac glucomannan-graft-polyacrylamide (KGM-g-PAM) was carried out at 25°C by γ-irradiation under a N2 atmosphere. The effects of absorbed radiation dosage and monomer concentration on grafting yield and water absorbency were studied. The grafted copolymers were characterized using Fourier Transform Infrared (FTIR) spectroscopy, nuclear magnetic resonance (NMR), x-ray diffraction (XRD), thermogravimetric analysis (TGA) and gel permeation chromatography (GPC). The grafting yield was observed to increase with increasing absorbed dosage and monomer concentration. Compared with the original KGM, the grafted copolymers exhibited better thermal stability and water absorbency. The results suggest that γ-irradiation is convenient and efficient for inducing graft copolymerization of KGM and acrylamide (AM).
Keywords: Konjac glucomannan, polyacrylamide, graft copolymerization, γ-irradiation
Introduction
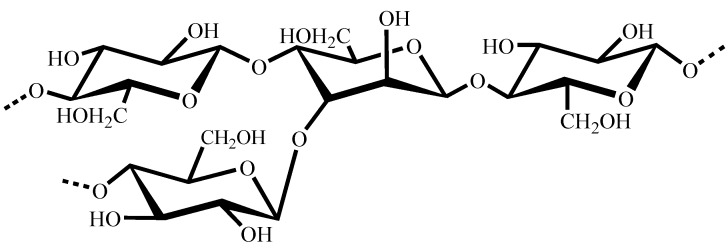
Konjac glucomannan (KGM) is a type of neutral heteropolysaccharide extracted from tubers of Amorphophallus konjac C. Koch. Chemically, KGM has β-(1→4) linked D-mannose and D-glucose units in a molar ratio of 1.6:1as the main chain, with branches joined through C-3 of the D-glucosyl and D-mannosyl residues and a low number of acetyl groups (approximately one acetyl group per 17 residues) at the C-6 position [1,2,3]. The chemical structure of KGM is shown in Figure 1. KGM has long been used as a health food in China and Japan. Due to its characteristics of low cost, high viscosity, excellent film-forming ability, good biocompatibility and biodegradability, as well as gel-forming properties, KGM and its derivatives have been used widely in various fields, such as food and food additives, and the pharmaceutical, biotechnology and fine chemical industries [4].
Figure 1.
The chemical structure of konjac glucomannan.
Graft copolymerization of natural polysaccharides such as chitosan, carrageenan and guar is becoming an important resource for developing advanced materials as it can improve the functional properties of natural polysaccharides [5,6,7]. The synthesis of grafted copolymers is usually conducted by the conventional redox grafting method or high-energy radiation including microwave, UV ray, γ-ray and electron beam [8,9,10]. Of these methods, 60Co γ-irradiation exhibits the most potential to synthesize the grafted copolymers, due to the higher energy emission, simpler preparation and lower cost [11]. γ-Irradiation is an ionic, non-thermal processing technology which continues to receive attention as a preservation and functional modification agent in polymer research and application [12]. With regard to its safety, a Joint FAO/IAEA/WHO Expert Committee on Food Irradiation (JECFI) declared in 1997 that food irradiated to any dosage appropriate to achieve the intended technological objective was both safe to consume and nutritionally adequate [13].
The synthesis of KGM with vinyl monomer for colon-specific drug delivery and as a superabsorbent by the conventional redox grafting method has been reported [14,15]. In our previous work we examined the effect of γ-irradiation on the properties of KGM and suggested that it was convenient to modify KGM [16]. However, little information on graft copolymerization of KGM and acrylamide (AM) by γ-irradiation is available. The objective of the present work was to investigate the feasibility of graft copolymerization of KGM and AM by γ-irradiation. The effects of absorbed dosage and monomer concentration on grafting yield and water absorbency and the properties of the grafted products were investigated.
Results and Discussion
Synthesis and properties
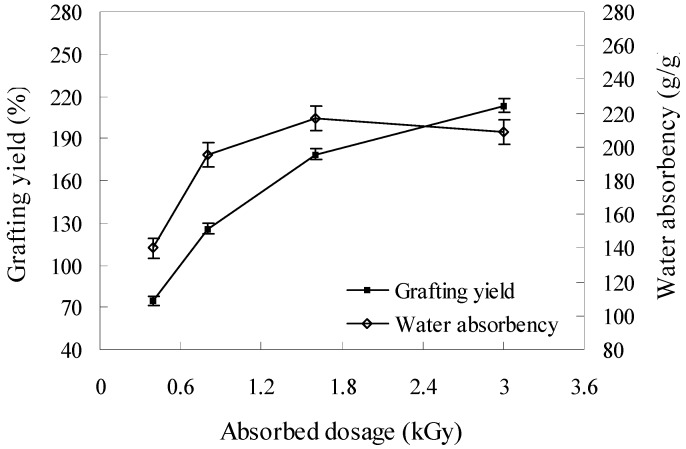
The synthesis of KGM-g-PAM was conducted by a simultaneous irradiation method, as described by Biswal et al. [7]. The effects of absorbed dosage and monomer concentration on grafting yield and water absorbency of the grafted copolymers were investigated. Figure 2 shows the effect of total absorbed dosage on the grafting yield and water absorbency of KGM-g-PAM. The grafting yield increased with irradiation dosage. A high grafting yield (>100%) could be achieved at a dosage above 0.8 kGy and it was 213.1% at a dosage of 3.2 kGy. However, the water absorbency of the grafted copolymer increased with increasing dosage at first and then decreased slightly when the dosage was higher than 1.6 kGy. The water absorbency was 217g/g at the dosage of 1.6 kGy. The increasing grafting yield with increasing dosage was mainly attributed to the increasing presence of radicals induced by higher irradiation dosage, as first described by Chapiro [17]. However, the results indicated that water absorbency of the grafted copolymer was not always positively associated with grafting yield. This might be explained by the Flory’s theory [18]: there is an appropriate crosslink density for the maximal water absorbency. The increased grafting yield might improve the crosslink density of the copolymers, which resulting in a difficulty to absorb water. Luo et al. [19] and Huacai et al. [9] also reported that higher irradiation dosage could decrease the water absorbency of graft copolymers.
Figure 2.
Effect of absorbed dosage on grafting yield and water absorbency of KGM-g-PAM (grafting condition: [KGM] = 0.6%,[AM] = 0.3 mol/L).
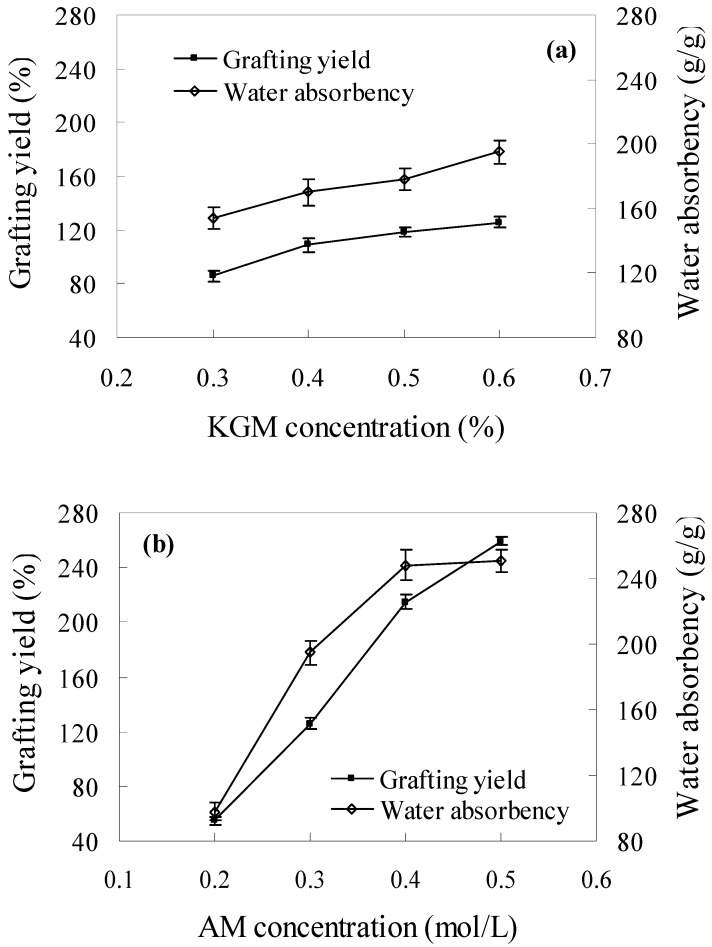
Figure 3 illustrates the effects of (a) KGM concentration and (b), AM concentration on grafting yield and water absorbency of the grafted copolymer. Increases in both KGM and AM contents could enhance grafting yield, but the concentration of AM played a more important role in the grafting yield than that of KGM. This may due to the inherent properties of KGM and AM upon irradiation exposure: whereas AM predominantly undergoes crosslinking [20], KGM, like other natural polymers such as carrageenan and alginates [21,22], undergoes degradation [16,23]. Moreover, the G value for radical formation of AM on γ-irradiation was reported to be much higher than that of natural polymers [24,25]. Therefore, the higher G value and predominant crosslinking nature of AM on irradiation both favored faster grafting with increase in monomer concentration. The water absorbency of grafted copolymer increased with increase of grafting yield at first and then decreased slightly.
Figure 3.
Effects of (a) KGM concentration; grating condition: dosage =0.8 kGy, [AM] = 0.3 mol/L) and (b), AM concentration; grafting condition: dosage = 0.8 kGy, [KGM] = 0.6%) on grafting yield and water absorbency of KGM-g-PAM.
Characterization
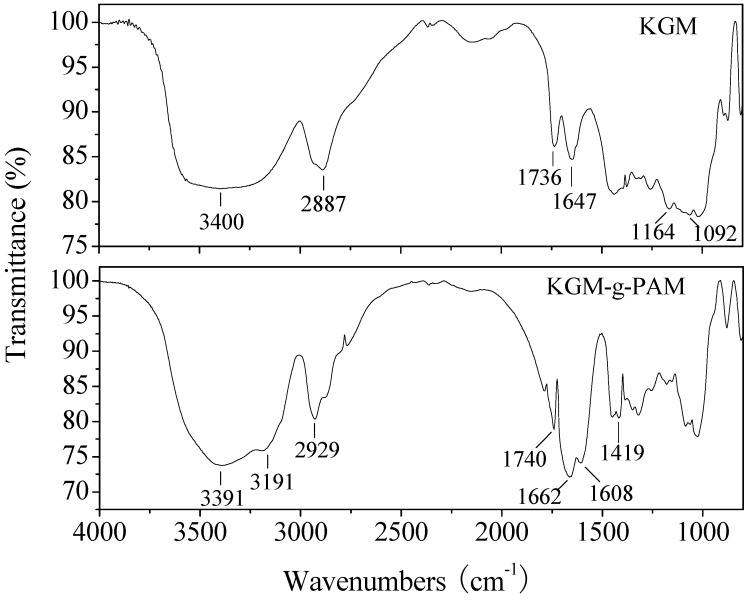
The characteristic FTIR absorption bands of the original KGM and grafted copolymer are presented in Figure 4. The main characteristic peaks of KGM were at 3400 cm-1 (O–H stretch), 2887 cm-1 (C–H stretch), 1736 cm-1 (C=O stretch), 1164 cm-1 (bridge O stretch), and 1092 cm-1 (C–O stretch).
Figure 4.
FTIR spectra of KGM and KGM-g-PAM.
The result was similar to the reports of Huacai et al. [9] and Yu et al. [26]. In the spectra of the grafted copolymer, in addition to the KGM characteristic peaks, peaks at 3139 and 1662 cm-1 indicated the N–H stretching and N–H bending of the amide bands, which are characteristic of the ‑CONH2 group present in the acrylamide. In addition, a peak at 1419 cm-1 was assigned to C–N stretching. These changes provided strong evidence supporting the grafting of acrylamide onto the KGM.
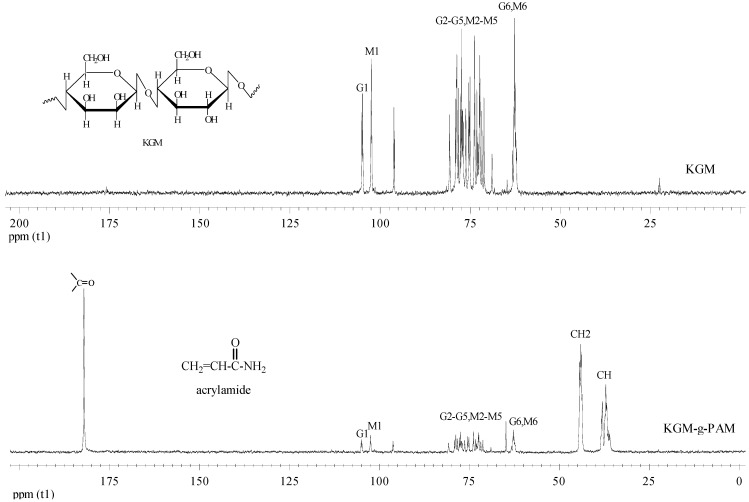
Figure 5 shows the 13C-NMR spectra of KGM and its grafted copolymers. According to the studies on the NMR spectra of KGM by Vieira and Gil [27] and Katsuraya et al. [28], the overlapping peaks in the 71−80 ppm region were assigned to the pyranose ring, including the presence of glucosyl (G) and mannosyl (M) units. The peak at about 63 ppm can be assigned as C6 of both G and M units, while the C1 of both G and M units was observed at 102−106 ppm. For KGM-g-PAM, the presence of a very intense peak at 182.1 ppm was due to the polyacrylamide carbonyl groups. The two peaks at about 37 ppm and 44 ppm were assigned as the methylene group and the carbon connected to carbonyl group of polyacrylamide, respectively [29]. The NMR spectra results thus further confirmed the FTIR results.
Figure 5.
13C-NMR spectra of KGM and KGM-g-PAM.
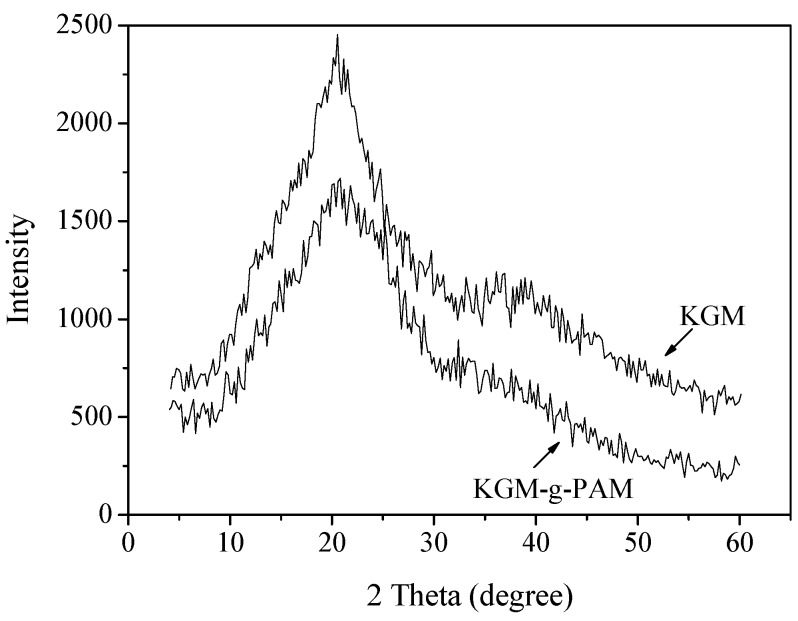
X-ray diffractograms of KGM and KGM-g-PAM are shown in Figure 6. The XRD pattern of KGM showed a weak but broad reflection falling at about 2θ = 20ο. For the grafted copolymer, the peak at 2θ = 20ο decreased significantly, indicating that the conjugation of KGM with PAM reduced the crystallinity of KGM. KGM was reported to have a hydrated crystal of low crystallinity [30] and PAM to be an amorphous material, which did not show any significant crystallinity peak [31]. The decrease of crystallinity might due to the intra- and intermolecular- hydrogen bonding interactions between KGM and PAM, which destroyed the original molecular orientation of KGM. Xiao et al. [32] also found that the graft copolymerization of KGM and PAM could lower the crystallinity of KGM, which suggested that KGM and PAM chains were mixed well at a molecular level.
Figure 6.
XRD patterns of KGM and KGM-g-PAM.
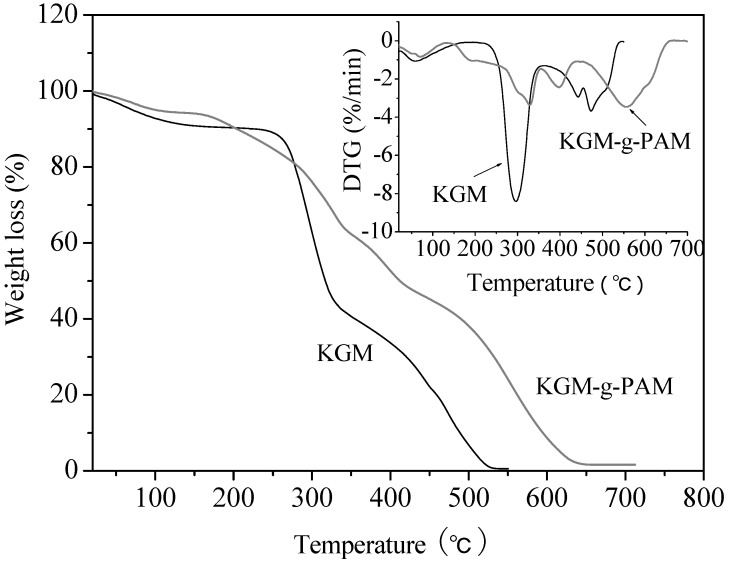
The thermogravimetric analysis (TGA) technique was employed to characterize the thermal properties of KGM and KGM-g-PAM; the results are shown in Figure 7. The decomposition of KGM started at about 250°C. The weight loss rate increased with increasing temperature up to 296°C and then decreased. Nearly 61% of KGM degraded at the first step and about 0.5% survived up to 550°C. However, the TGA profile of KGM-g-PAM was distinctly different from that of KGM.
Figure 7.
TG and DTG curves of KGM and KGM-g-PAM.
The grafted copolymers had a continuous weight loss with increasing temperature and began to degrade at about 180°C, with the maximum weight loss rate occurring at 328°C. Furthermore, the weight loss rate of the KGM-g-PAM was slower than that of KGM at above 276°C and about 1.6 % still existed at 700°C. The results indicated that the initial decomposition temperature (Ti) of KGM-g-PAM was lower than that of KGM, and the maximum decomposition temperature (Tm) and final decomposition temperature (Tf) were higher than that of KGM. The previous reports also revealed that the graft copolymerization of natural polysaccharides with vinyl monomer could enhance their thermal stability [7,9,29].
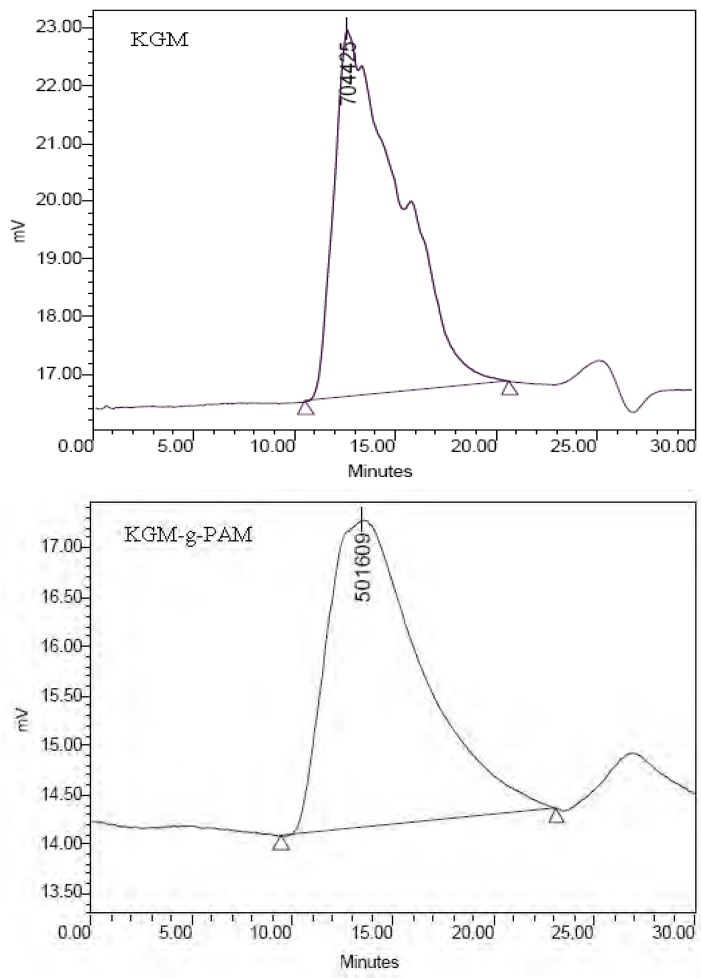
The molecular weights of KGM and its grafted copolymer were determined by GPC. The elution curves are presented in Figure 8. The peak molecular mass (Mp) of the grafted copolymer decreased significantly and the weight average molecular mass (Mw) and number average molecular mass (Mn) decreased slightly compared with that of KGM (Table 1). The elution curve of KGM-g-PAM was smoother than that of KGM, which could be a reflection of the decrease in polydispersity. The slight decrease of molecular weight might be attributed to the degradation of KGM expose to irradiation. The results were in agreement with some earlier reports [33,34].
Figure 8.
GPC elution curves of KGM and KGM-g-PAM.
Table 1.
The molecular weight and polydispersity of KGM and KGM-g-PAM
| Sample | Mw | Mn | Polydispersity (Mw/Mn) |
|---|---|---|---|
| KGM | 4.81×105 | 3.33×105 | 1.441 |
| KGM-g-PAM | 4.28×105 | 3.02×105 | 1.418 |
Conclusions
The synthesis and characterization of konjac glucomannan-graft-polyacrylamide via γ-irradiation was studied. The results indicated that γ-irradiation was convenient for inducing graft copolymerization of KGM and AM at low dosage and obtaining graft copolymers with high grafting yield (>100%). The grafting yield increased with increasing dosage and monomer concentration, while the water absorbency increased first and then decreased slightly with increasing dosage and monomer concentration. FTIR, NMR and XRD analyses showed that AM had been grafted onto KGM successfully. TGA results indicated that the grafted copolymer exhibited better thermal stability in comparison with original KGM. The molecular weight of grafted copolymer decreased slightly according to the results of GPC.
Experimental
Materials
KGM was obtained from Sanai Konjac Food Corp., Yibin, P.R. China. It was purified by washing several times with aqueous methanol. All the other regents were of analytical grade and used without further purification. Double-distilled water was used for these experiments.
Graft copolymerization
KGM (0.6 g) was dissolved in water (90 mL) in a glass tube and kept stirring for 12 h. AM solution (3 mol/L, 10 mL) was then added to the solution with stirring and stirring was continued for another 2 h. The solutions were deoxygenated by nitrogen bubbling and then irradiated for 20, 40, 80 and 150 min at a fixed dosage rate of 20 Gy/min at 25°C. The irradiated solution was placed overnight for a complete reaction and then precipitated in excess of ethanol. The precipitate was dried and washed several times with a 3:2 mixture of acetic acid-dimethylformamide to remove the unreacted monomer and homopolymer. Finally, it was washed with ethanol and then dried at 55°C under vacuum.
Determination of grafting yield and water absorbency
The grafting yield was determined gravimetrically using the following equation:
| Grafting yield (%) = [(Wg − Wo) / Wo] ×100 | (1) |
where Wg and Wo were the weight of KGM after grafting and the initial weight of KGM, respectively.
The dried sample (1.0 g) was dispersed in water (800 mL) and kept stirring for at least 12 h at 25°C to reach swelling equilibrium, then the residual water was removed by filtration through a 100-mesh stainless steel screen. Water absorbency was calculated using the following equation:
| Absorbency (g/g) = (W1 − W0) / W0 | (2) |
where W1 and W0 are the weight of the water-swollen and the dry sample, respectively.
Characterization
Fourier transformed infrared spectroscopy (FTIR) measurements were performed on a Vector33 FTIR spectrometer (Bruker, Germany). Samples were mixed with KBr and then scanned against a blank KBr pellet background. The 13C-NMR spectra were recorded on a Bruker Model Avance DRX-400 spectrometer at about 30°C. X-ray diffraction (XRD) patterns were obtained with a D/max-IIIA x-ray diffractometer at the scattering angle (2θ) of 4ο to 60ο and the scanning rate of 10ο/min. Thermogravimetric analysis (TGA) was conducted with a Netzsch TG 209 analyzer, the scan was carried out at a heating rate of 10.0 °C/min from 20 to 700 °C. The molecular weights and molecular weight distributions were determined by 515-gel permeation chromatography (GPC). Unless otherwise stated, the grafting yield of samples used for analysis, which were synthesized under the following condition: absorbed dosage = 0.8 kGy, [KGM] = 0.6%, [AM] = 0.3 mol/L, was 125.94%.
Acknowledgements
The authors are grateful for the financial support of the National Natural Science Foundation of China (Grant Nos. 30070533, 30471218 and 30371009).
Footnotes
Sample Availability: Available from the authors.
References
- 1.Smith F., Srivastava H.C. Constitution studies on the glucomannan of konjac flower. J. Am. Chem. Soc. 1959;81:1715–1718. doi: 10.1021/ja01516a048. [DOI] [Google Scholar]
- 2.Kato K., Matsuda K. Studies on the chemical structure of konjac mannan. Agr. Biol. Chem. 1969;33:1446–1453. doi: 10.1271/bbb1961.33.1446. [DOI] [Google Scholar]
- 3.Maeda M., Shimahara H., Sugiyama N. Detailed examination of the branched structure of konjac glucomannan. Agr. Biol. Chem. 1980;44:245–252. [Google Scholar]
- 4.Zhang Y.Q., Xie B.J., Gan X. Advance in the applications of konjac glucomannan and its derivatives. Carbohydr. Polym. 2005;60:27–31. doi: 10.1016/j.carbpol.2004.11.003. [DOI] [Google Scholar]
- 5.Francis S., Kumar M., Varsheny L. Radiation synthesis of superabsorbent poly(acrylic acid)-carrageenan hydrogels. Radiat. Phys. Chem. 2004;69:481–486. doi: 10.1016/j.radphyschem.2003.09.004. [DOI] [Google Scholar]
- 6.Wang J.P., Chen Y.Z., Ge X.W., Yu H.Q. Gamma radiation-induced grafting of a cationic monomer onto chitosan as a flocculant. Chemosphere. 2007;66:1752–1757. doi: 10.1016/j.chemosphere.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 7.Biswal J., Kumar V., Bhardwaj Y.K., Goel N.K., Dubey K.A., Chaudhari C.V., Sabharwal S. Radiation-induced grafting of acrylamide onto guar gum in aqueous medium: Synthesis and characterization of grafted polymer guar-g-acrylamide. Radiat. Phys. Chem. 2007;76:1624–1630. doi: 10.1016/j.radphyschem.2006.11.014. [DOI] [Google Scholar]
- 8.Pandey P.K., Srivastava A., Tripathy J., Behari K. Graft copolymerization of acrylic acid onto guar gum initiated by vanadium (V)–mercaptosuccinic acid redox pair. Carbohydr. Polym. 2006;65:414–420. doi: 10.1016/j.carbpol.2006.01.022. [DOI] [Google Scholar]
- 9.Huacai G., Wang P., Dengke L. Graft copolymerization of chitosan with acrylic acid under microwave irradiation and its water absorbency. Carbohydr. Polym. 2006;66:372–378. [Google Scholar]
- 10.Vahdat A., Bahrami H., Ansari N., Ziaie F. Radiation grafting of styrene onto polypropylene fibres by a 10 MeV electron beam. Radiat. Phys. Chem. 2007;76:787–793. doi: 10.1016/j.radphyschem.2006.05.009. [DOI] [Google Scholar]
- 11.Gupta B., Scherer G. Proton exchange membranes by radiation-induced graft copolymerization of monomers into Teflon-FEP films. Chimia. 1994;48:127–37. [Google Scholar]
- 12.Abu J.O., Duodu K.G., Minnaar A. Effect of γ-irradiation on some physicochemical and thermal properties of cowpea (Vigna unguiculata L. Walp) starch. Food. Chem. 2006;95:386–393. [Google Scholar]
- 13.Molins R.A, Motarjemi Y., Kaferstein F.K. Irradiation: a critical control point in ensuring the microbiological safety of raw foods. Food Control. 2001;12:347–356. [Google Scholar]
- 14.Chen L.G., Liu Z.L., Zhuo R.X. Synthesis and properties of degradable hydrogels of konjac glucomannan grafted acrylic acid for colon-specific drug delivery. Polymer. 2005;46:6274–6281. doi: 10.1016/j.polymer.2005.05.041. [DOI] [Google Scholar]
- 15.Wang F., Zou H.N., Zhou D.H., Wu G.H. Preparation and characterization of high water absorption resin based KGM-graft-AA. Nat. Prod. Res. Dev. (Chin). 2006;18:282–285. [Google Scholar]
- 16.Xu Z., Sun Y., Yang Y., Ding J., Pang J. Effect of γ-irradiation on some physiochemical properties of konjac glucomannan. Carbohydr. Polym. 2007;70:444–450. doi: 10.1016/j.carbpol.2007.05.011. [DOI] [Google Scholar]
- 17.Chapiro A. Preparation des copolymers greffes du polytetrafluoroethylene (Teflon) par viie radiochimique. J. Polym. Sci. 1959;34:481–501. [Google Scholar]
- 18.Flory P.J. Principles of Polymer Chemistry. Cornell University Press; Ithaca, NY: 1953. [Google Scholar]
- 19.Luo W., Zhang W., Chen P., Fang Y. Synthesis and properties of starch grafted poly[acrylamide-co-(acrylic acid)]/montmorillonite nanosuperabsorbent via γ-ray irradiation technique. J. Appl. Polym. Sci. 2005;96:1341–1346. doi: 10.1002/app.21447. [DOI] [Google Scholar]
- 20.Nishii M., Hayashi K. Solid-state polymerization. Annu. Rev. Mater. Sci. 1975;5:135–149. doi: 10.1146/annurev.ms.05.080175.001031. [DOI] [Google Scholar]
- 21.Nagasawa N., Mitomo H., Yoshii F., Kume T. Radiation-induced degradation of sodium alginate. Polym. Degrad. Stab. 2000;69:279–285. doi: 10.1016/S0141-3910(00)00070-7. [DOI] [Google Scholar]
- 22.Relleve L., Nagasawa N., Luan L.Q., Yagi T., Aranilla C., Abad L., Kume T., Yoshii F., dela Rosa A. Degradation of carrageenan by radiation. Polym. Degrad. Stab. 2005;87:403–410. doi: 10.1016/j.polymdegradstab.2004.09.003. [DOI] [Google Scholar]
- 23.Prawitwong P., Takigami S., Phillips G.O. Effect of γ-irradiation on molar mass and properties of konjac mannan. Food Hydrocolloid. 2007;21:1362–1367. doi: 10.1016/j.foodhyd.2006.10.015. [DOI] [Google Scholar]
- 24.Molotkov V.A., Kurlyankina V.I., Klenin S.I. Investigation of the kinetics of graft copolymerization of acrylamide with cellulose in the presence of trivalent cobalt salts. Polym. Sci. 1972;14:2890–2898. [Google Scholar]
- 25.Blundell D.J., Osborn B.N. The morphology of poly(aryl-ether-ether-ketone) Polymer. 1983;24:954–960. doi: 10.1016/0032-3861(83)90144-1. [DOI] [Google Scholar]
- 26.Yu H.Q., Huang Y.H., Ying H., Xiao C.B. Preparation and characterization of a quaternary ammonium derivative of konjac glucomannan. Carbohyd. Polym. 2007;69:29–40. doi: 10.1016/j.carbpol.2006.08.024. [DOI] [Google Scholar]
- 27.Vieria M.C., Gil A.M. A solid state NMR study of locust bean gum glatactomannan and Konjac glucomannan gels. Carbohyd. Polym. 2005;60:439–448. doi: 10.1016/j.carbpol.2005.02.013. [DOI] [Google Scholar]
- 28.Katsuraya K., Okuyama K., Hatanaka K., Oshima R., Satoc T., Matsuzaki K. Constitution of konjac glucomannan: chemical analysis and 13C NMR spectroscopy. Carbohydr. Polym. 2003;53:183–189. doi: 10.1016/S0144-8617(03)00039-0. [DOI] [Google Scholar]
- 29.Silva D.A., Paula R.C.M., Feitosa J.P.A. Graft copolymerization of acrylamide onto cashew gum. Eur. Polym. J. 2007;43:2620–2629. doi: 10.1016/j.eurpolymj.2007.03.041. [DOI] [Google Scholar]
- 30.Ogawa K., Yui T., Mizuno T. X-Ray-diffraction study of glucomannans and their acetates. Agr. Biol. Chem. 1991;55:2105–2111. doi: 10.1271/bbb1961.55.2105. [DOI] [Google Scholar]
- 31.Tripathy T., Pandey S.R., Karmakar N.C., Bhagat R.P., Singh R.P. Novel flocculating agent based on sodium alginate and acrylamide. Eur. Polym. J. 1999;35:2057–2072. doi: 10.1016/S0014-3057(98)00284-5. [DOI] [Google Scholar]
- 32.Xiao C.B., Lu Y.S., Zhang L.N. Preparation and physical properties of konjac glucomannan-polyacrylamide blend films. J. Appl. Polym. Sci. 2001;81:882–888. doi: 10.1002/app.1507. [DOI] [Google Scholar]
- 33.Garnett J.L., Jakiewicz S.V., Sangster D.F. Mechanistic aspects of the acid and salt effect in radiation grafting. Radiat. Phys. Chem. 1990;36:571–579. [Google Scholar]
- 34.Ducouret C., Betz N., Le Moel A. Study of the molecular weight distribution of polystyrene grafted by means of swift heavy ions. J. Chim. Phys. 1996;93:70–77. [Google Scholar]