Abstract
In this study we investigated the potential roles of nanoparticles (< 100 nm) and submicron (100-1000 nm) particles in the formation of microparticles (> 1000 nm) in protein formulations under some pharmaceutically-relevant stress conditions. Exposure of intravenous immunoglobulin (IVIG) solutions to the interface-associated stresses of freeze-thawing or agitation resulted in relatively large increases in microparticle concentrations, which depended directly on the levels of preexisting nano- and submicron particles. Thus, agglomeration of nanoparticles and submicron particles appears to play a role in microparticle formation under these stresses. In contrast, increases in microparticle concentrations during quiescent incubation at elevated temperatures were independent of the initial nano- and submicron particle concentrations in solution.
INTRODUCTION
Subvisible particle levels in therapeutic protein products are critical quality attributes, potentially affecting patient health and safety. Importantly, even when present at relatively low levels (e.g., < 0.1% of total protein mass) these particles may cause immunogenic responses and/or infusion related hypersensitivity reactions in patients. For example, a recent article authored by FDA researchers reported 49 cases of anaphylaxis including 7 fatalities among patients receiving peginesatide, a commercial pegylated erythropoietin peptide mimetic. The product was found to comply with USP <788> requirements for particle content as measured by the traditional light obscuration method. However, using more sensitive nanoparticle tracking analysis (NTA) and micro flow imaging (MFI) techniques, higher concentrations of particles in the nano- and micron size range were observed in the immunogenic marketed formulation compared to those found in the non-immunogenic product used in the clinical studies.1 In further support of the role of subvisible particles in immunogenicity, numerous in vivo and a few in vitro studies have shown that small amounts of these particles can activate various pathways of the innate and adaptive immune system.2-8
Additionally, analysis of subvisible particles provides a sensitive tool to monitor and characterize aggregation in protein products. For example, stresses incurred during one cycle of freeze thawing or during filling operations for monoclonal antibodies (mAb) did not result in the formation of soluble aggregates that could be detected by size-exclusion chromatography (SEC) analysis, but thousands of microparticles per mL were detected by particle counting methods.9,10 Recently, several industry scientists have discussed case studies showing that monitoring subvisible particles enabled them to devise particle control strategies and make better decisions in support of formulation and process development, and ensure higher product quality.11
Subvisible particles are ubiquitous in protein solutions.12 Freeze thawing, oxidation, elevated temperatures, light exposure, agitation and mechanical shock – stresses to which the protein product may be exposed to during production, purification, storage, transport and delivery to patients – can cause formation of subvisible particles.9,13-17 However, detailed mechanisms by which subvisible particles form are unknown. Some studies have investigated agitation-induced microparticle formation and concluded that the mechanisms are protein-dependent with no clear relationship between levels of soluble oligomers and microparticle formation,15,16 but these studies did not examine the potential contribution of nanoparticles and submicron particles to the formation of microparticles. In another study, however, Bai; et al. observed a direct relation between initial particle concentrations in the size range of 100 to 1000 nm in solution and subsequent microparticle formation during isothermal incubation of interferon-β-1a.14
With that background, the goal of the current study was to investigate the potential roles of nanoparticles and submicron particles in the formation of microparticles in protein formulations subjected to the stresses of elevated temperature, freeze-thawing or agitation. Prior to the application of these stresses, we manipulated the concentrations of nano- and submicron particles in IVIG solutions using ultracentrifugation or freeze-thawing. Subsequent stress-induced particle formation was monitored using nanoparticle tracking analysis (NTA) and microflow imaging (MFI).
MATERIALS AND METHODS
Materials
Gammagard® (IVIG), 100 mg/mL in 200 mM glycine, pH 4.2 (Lot# LE12N107AB, Expiry: May 2016) manufactured by Baxter Healthcare Corporation (Westlake Village CA.) was used as the model protein in this study. Glycine, sodium phosphate, sodium chloride and polysorbate 20 were purchased from Fisher Scientific (Fair Lawn, NJ).
Methods
Effects of pre-existing particles on particle formation during freeze-thawing
In this experiment, the goal was to examine the effects of pre-existing particles on particle formation during freeze-thawing. IVIG from the manufacturer was diluted to 1 mg/ml in the formulation buffer (200 mM glycine, pH 4.2). To prepare a sample without pre-existing particles, 25 mL aliquots of the protein formulation were centrifuged (Beckman optima LE-80K ultracentrifuge) at 112,000g for 3 hours, and 20 mL of the supernatant was removed. IVIG samples (non-centrifuged and centrifuged) were frozen by immersion in liquid nitrogen and thawed in a water bath at room temperature. Submicron particle and micron sized particle concentrations in freeze-thawed solutions were characterized using NTA and MFI, respectively. For each determination of submicron and micron sized particle concentrations, three independent replicates were used.
Effects of freeze-thawing-induced particles on particle formation during agitation
In this experiment, the goal was to test the hypothesis that particles generated during freeze-thawing might influence particle formation during subsequent agitation. IVIG from the manufacturer was diluted to 1 mg/ml in the formulation buffer. A 25 mL aliquot of 1 mg/mL IVIG solution was agitated end-over-end at 40 RPM for 48 hours in a 50 mL conical centrifuge tube. This IVIG solution was then centrifuged for 3 hours at 112,000g, and 20 mL of the supernatant decanted. These steps served the dual purposes of preferentially removing components from the polyclonal IVIG that had a high tendency to aggregate, and creating an initial solution with minimal pre-existing particles. This initial solution was next subjected to either freeze thawing (procedure mentioned above) followed by agitation for 8 hours at 40 RPM in 50 mL polypropylene tubes, or to 8 hours of agitation alone. After agitation, submicron particle and micron sized particle concentrations were determined using NTA and MFI, respectively, for three independent replicates.
Effects of pre-existing micron and submicron sized particles on stress-induced particle formation
The goal of this study was to determine whether the size range of pre-existing particles influenced subsequent particle generation during agitation stress. We prepared samples that contained various levels of nanoparticles and submicron particles by fractionating a stock solution containing high levels of particles. This stock solution was prepared by end-over-end agitation of a 1 mg/ml IVIG formulation for 48 hours. Particles were selectively removed from the aliquots of this stock solution by preparative centrifugation. Samples were depleted of micron sized particles, leaving submicron particles and nanoparticles in solution, by centrifugation for 45 minutes at 2350g (30 mL in 35 mL Nalgene® tubes) using a Sorvall RC6plus centrifuge. Samples depleted of micron sized particles and a fraction of submicron particles were prepared by centrifugation for 45 minutes at 30,000g (30 mL in 35 mL Nalgene® tubes) using a Sorvall RC6plus centrifuge. Finally, samples (20 mL) depleted of both micron and submicron particles were prepared by centrifugation (Beckman optima LE-80K ultracentrifuge) for 3 hours at 112,000g in 25 mL polycarbonate tubes from Beckman. After centrifugation, aliquots from each of the three sample types were used in three experiments: 1) samples were incubated quiescently at 25° and 50° C for five days in 20 mL glass vials; 2) samples were freeze-thawed as described above; and 3) samples were agitated end-over-end at room temperature for 8 hours. For each experiment, nano- and microparticle concentrations were determined using NTA and MFI, respectively, for three independent replicates.
Particle characterization
Particles greater than 1000 nm in size were characterized using a Protein Simple (Ottawa, ON, Canada) 4200 MFI system with a 100 μm flow cell. Sample volumes of 0.5 mL were analyzed, of which 0.1 mL was used as purge volume, 0.05 mL was used for background optimization and 0.35 mL was used for particle data acquisition.
Nanoparticles and submicron particles (60-1000 nm) were characterized using a Nanosight (Amesbury, United Kingdom) LM 20 system with the LM 12B sample chamber or the NS 300 system with a LM 14 viewing unit equipped with a 405 nm laser light source. Samples were introduced into the instrument using a silicone oil-free 1 mL plastic syringe (National Scientific Company, TN). Videos were recorded for 60 seconds and subsequently analyzed using NTA 2.3 Build 127 software, using a screen gain of 1 and a detection threshold of 10.
RESULTS
Effects of pre-existing particles on particle formation during freeze-thawing
Ultracentrifugation of the IVIG samples effectively removed both submicron and micron sized particles from the solution (Fig. 1). Thus, by comparing centrifuged and uncentrifuged samples we could determine the effect of pre-existing particles on additional particle formation during freeze-thawing. The levels of microparticles formed by freeze-thawing were almost 4-fold higher in the samples that contained pre-existing particles than those observed for the centrifuged, particle-depleted samples (Fig. 1a). In contrast, the increases in submicron particle levels caused by freeze-thawing were not significantly dependent on the presence of pre-existing particles (Fig. 1b).
Figure 1.
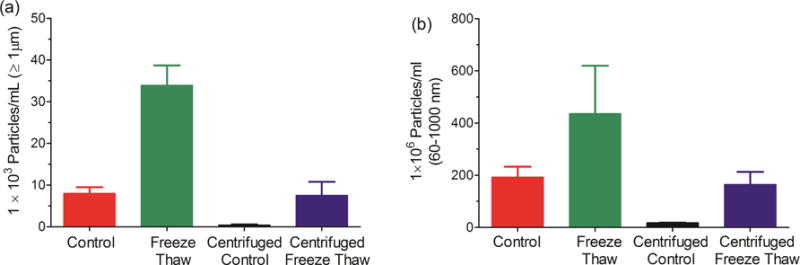
Total particle concentration following freeze-thawing of non-ultracentrifuged and ultracentrifuged IVIG samples. (a) Particles ≥ 1 μm; and (b) particles between 60-1000 nm. Error bars represent standard deviation from three independent replicates.
Effects of freeze-thawing-induced particles on particle formation during agitation
Microparticle formation during 8 hours of agitation was more rapid in samples that contained particles created by prior freeze-thawing (Fig. 2a). In contrast, the rate of submicron particle formation was independent of the presence of pre-existing particles (Fig. 2b).
Figure 2.
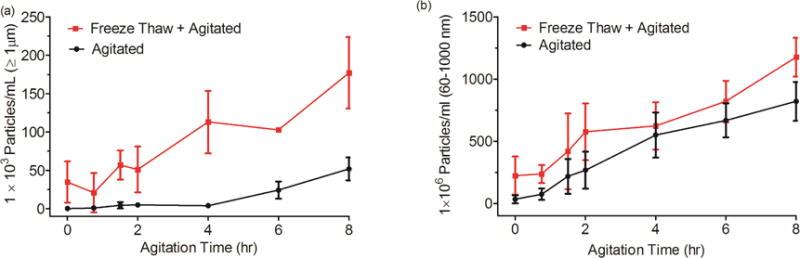
Total particle concentration for IVIG sample agitated for eight hours either with or without prior freeze thawing. (a) Particles ≥ 1 μm; and (b) particles between 60-1000 nm. Error bars represent standard deviation from three independent replicates.
Effects of pre-existing submicron and micron sized particles on stress-induced particle formation
To monitor further the role of pre-existing particles in stress-induced particle formation, samples with varying levels of particles were obtained by applying different centrifugation protocols to a 1 mg/ml IVIG formulation that had first been agitated for 48 hours. Agitation produced high concentrations of particles that exceeded the upper detection limit of the NanoSight instrument (ca. 109 particles/mL18) (Table.1). Centrifugation at 2350g removed most of these microparticles, but left a high concentration of submicron particles in solution. Centrifugation at 30,000g removed microparticles and an intermediate level of submicron particles. Centrifugation at 112,000g removed microparticles and most submicron particles. These IVIG solutions were then incubated quiescently at 25° or 50° C, subjected to one cycle of freeze thawing or subjected to 8 hours of agitation.
Table 1.
Particle concentration in IVIG solution after 48 hours of agitation and subsequent centrifugation
| Particles 60-1000 nm (108/mL) | Particles ≥ 1 μm (#/mL) | |
|---|---|---|
| Agitation for 48 hours | n/aa | 918,000 ± 260,000 |
| Centrifugation 45 minutes, 2350 g | 10.04 ± 7.0 | 1,900 ± 2000 |
| Centrifugation 45 minutes, 30000 g | 0.47 ± 0.14 | 1,300 ± 1,400 |
| Centrifugation 3 hours, 112,000 g | 0.07 ± 1.27 | 140 ± 80 |
Measurement error represents standard deviation from measurement of three independent samples
Samples above the limit of detection of the instrument (ca 109 particles/mL)
IVIG incubation at 25° C or 50° C in the presence of varying levels of submicron particles
The samples described above that had been subjected to different centrifugation speeds to yield different levels of submicron and micron sized particles were incubated quiescently at 25°C and 50° C. During incubation at 25° C, there were only slight increases in microparticle concentrations in the IVIG solution containing the most submicron particles, and the samples that had been subjected to more exhaustive centrifugation showed no change in microparticle levels over five days (Fig. 3a). During incubation at 50° C, microparticle levels increased more rapidly. However, for all three samples there were no discernable effects of the initial levels of submicron particles (Figure 3b). Interestingly, there were no significant changes in the submicron particle concentrations either in any of the IVIG samples during 5 days of incubation at either 25° C or at 50° C. (Table 2).
Figure 3.
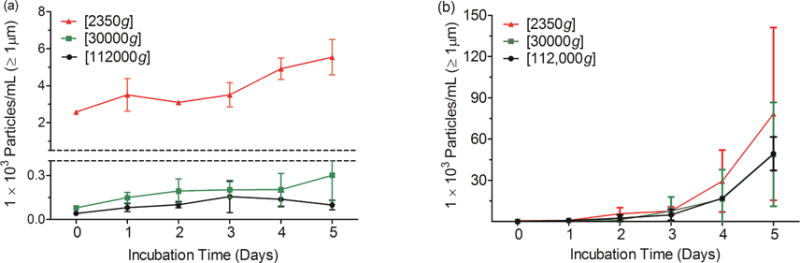
Total microparticle concentration during quiescent incubation of IVIG solutions previously processed by centrifugation at various g forces. (a) Incubation at 25° C (b) incubation at 50° C. Error bars represent standard deviation from three independent replicates.
Table 2.
Concentrations for particles of sizes between 60-1000 nm (108/mL) in IVIG solution centrifuged at various g forces and incubated at either 25° C or at 50° C
| 45 minutes @2,350g | 45 minutes @30,000g | 3 hours @112,000g | |
|---|---|---|---|
| Incubation at 25° C | |||
| Day 0 | 0.64 | 0.29 | |
| Day 1 | 0.32 ± 0.15 | 1.96 ± 1.15 | |
| Day 2 | n/aa | 0.29 ± 0.27 | 1.93 ± 1.13 |
| Day 3 | 0.42 ± 0.19 | 0.63 ± 0.22 | |
| Day 4 | 0.55 ±0.10 | 1.33 ± 0.62 | |
| Day 5 | 1.76 ± 2.10 | 1.18 ±0.41 | |
| Incubation at 50° C | |||
| Day 0 | 4.1 | 1.28 | |
| Day 1 | 4.49 ± 0.27 | 0.71 ± 0.26 | |
| Day 2 | n/aa | 4.19 ± 1.59 | 1.02 ± 0.68 |
| Day 3 | 2.35 ± 2.28 | 0.37 ± 0.14 | |
| Day 4 | 1.90 ± 0.24 | 0.08 ± 0.06 | |
| Day 5 | 1.80 ± 0.82 | 0.76 ± 0.48 |
Measurement error represents standard deviation from measurement of three independent samples
Particle levels above the upper limit of quantitation of the instrument (ca 109 particles/mL)
Freeze thawing or agitating IVIG solution with varying levels of nanoparticles
The solutions of IVIG processed by the different centrifugation procedures were also subjected to a single freeze-thawing cycle, which resulted in increases in microparticle levels that were based on the concentration of pre-existing submicron particles in the solution (Fig. 4a and Table 1). Freeze-thawing of the IVIG samples that had been subjected to centrifugation at 30,000g or 112,000g resulted in similar increases in the levels of submicron particles. There were no discernable changes in submicron particle levels during freeze-thawing of the sample processed at 2350g.
Figure 4.
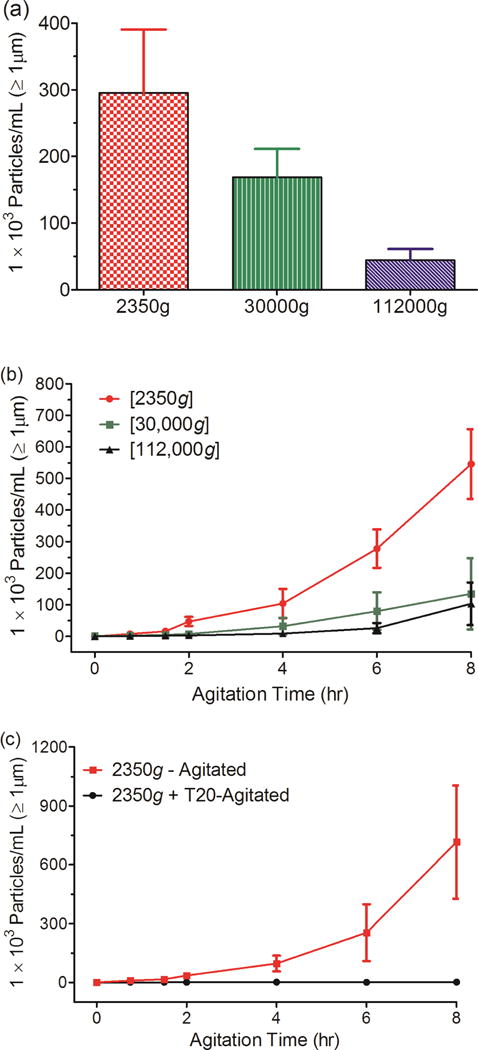
Total microparticle concentrations in IVIG solutions previously centrifuged at various g-forces and then exposed to (a) one cycle of freeze-thawing; (b) 8 hours of agitation (c) 8 hours of agitation in the presence (black circles) and absence (red squares) of polysorbate 20 (T20). Error bars represent standard deviation from three independent replicates.
The IVIG solutions processed by the different centrifugation procedures were also subjected to stress by agitation (Fig. 4b). In the solution that had been centrifuged at 2350g, which was depleted of microparticles but contained high levels of submicron particles, microparticle concentrations increased sharply during 8 hours of agitation. In contrast, in the IVIG solutions centrifuged at 30,000g or 112,000g, which were depleted in microparticles and also had reduced levels of submicron particles, fewer microparticles were formed.
Submicron particle concentrations increased during eight hours of agitation in IVIG samples that had been centrifuged at 30,000g or 112,000g (Table 3). In the sample that had been centrifuged at 2350g, submicron particle levels were near the upper detection limit for the NTA instrument even prior to agitation, and remained high during agitation.
Table 3.
Concentration of nanoparticles of sizes between 60-1000 nm (108/mL) in IVIG solution centrifuged at various g forces and exposed to either freeze thawing, agitation or agitation in the presence of polysorbate 20
| 45 minutes @2,350g | 45 minutes @30,000g | 3 hours @112,000g | |
|---|---|---|---|
| Control | 25.43 ± 1.56 | 0.47 ± 0.007 | 0.23 ± 0.20 |
| Freeze-Thawing | 19.90 ± 0.569 | 2.85 ± 2.68 | 1.50 ± 0.595 |
| Agitation | |||
| 0 hr | 10.04 ± 7.0 | 0.47 ± 0.14 | 0.07 ± 1.27 |
| 0.75 hr | 8.92 ± 2.82 | 2.89 ± 1.03 | 0.80 ± 0.45 |
| 1.5 hr | 8.73 ± 1.33 | 4.87 ± 1.09 | 2.85 ± 0.65 |
| 2 hr | 8.55 ± 2.02 | 5.66 ± 0.57 | 3.01 ± 0.85 |
| 4 hr | 6.85 ± 2.31 | 7.08 ± 0.71 | 5.82 ± 1.64 |
| 6 hr | 8.01 ± 1.54 | 7.48 ± 1.54 | 6.76 ± 1.49 |
| 8 hr | 8.13 ± 3.39 | 7.48 ± 2.07 | 6.03 ± 1.43 |
| Agitation with Tween 20 | n/aa | Not determinedb | Not determinedb |
Measurement error represents standard deviation from measurement of three independent samples
Particle levels above the limit of quantitation of the instrument (ca 109 particles/mL).
Samples not tested for this treatment
Nonionic surfactants are often used to reduce agitation-induced aggregation of therapeutic proteins. To test for such an effect in our system, the IVIG solution that had been centrifuged at 2350g was agitated with and without polysorbate 20. The presence of polysorbate 20 inhibited agitation-induced increase in microparticle concentrations (Fig. 4c). Submicron particle counts for these samples could not be obtained because the levels in all samples exceeded the upper detection limit for the NTA instrument.
DISCUSSION
Protein particle formation in therapeutic products can occur during all steps of manufacturing, shipping, storage and delivery to patients.14,15,17 In order to develop effective strategies to control particle levels – as required by regulatory authorities19 – it is critical to employ the appropriate analytical methods and to understand key factors that contribute to particle formation. In the current study with the therapeutic protein IVIG, we found that the presence of pre-existing nanoparticles and submicron particles fostered more rapid formation of microparticles during agitation and freeze-thawing. In contrast, in IVIG solutions depleted of these particles, much lower levels of microparticles were formed during these stresses. But we also observed that these stresses may induce formation of new smaller sized particles, and these particles presumably would subsequently contribute to the formation of microparticles. It is likely that nanoparticles and submicron particles agglomerate to form microparticles, as was shown previously for a monoclonal antibody formulation.20
Often protein particle formation is associated with freeze-thawing and agitation, to which products are exposed routinely.21,22 In the current study, it was observed that microparticle formation rates were insensitive to the initial concentrations of nanoparticles and submicron particles during quiescent incubation at elevated temperature (50° C). However, these particles promoted microparticle formation in IVIG solutions during freeze-thawing and agitation.
During freeze-thawing, multiple stresses occur that may influence protein particle formation and agglomeration.23 Ice formation during freezing leads to greatly increased concentrations of solutes in the remaining liquid phase, potentially increasing both particle production and agglomeration rates.23 Also, ice crystals present a relatively large interfacial surface to which protein molecules and nanoparticles might adsorb, potentially promoting particle formation and particle agglomeration.
During agitation, adsorption of protein molecules at the air-water interface contributes to protein particle formation.21,24-27 Consistent with this mechanism is the observation in the current and many earlier studies that inclusion of a non-ionic surfactant in the formulation can substantially inhibit agitation-induced protein aggregation and particle formation.28-31 In the absence of surfactant, protein molecules that adsorb onto the air-water interface may unfold and associate to form a gel or film.21,24,25 Protein particles in the bulk solution at the start of an agitation stress may also adsorb onto the interface and contribute to the formation of the layer of gelled protein.32,33 Mechanical stresses and rupture of the interfacial layer can lead to more particles in the bulk.21,24 During agitation, compression and expansion of the air-water interface and bursting of air bubbles can mechanically rupture the interfacial gel.34,35 Furthermore, agitation may increase the rate at which nanoparticles collide and agglomerate to form larger sized submicron and micron sized particles. In our experiments, IVIG samples depleted of nanoparticles prior to agitation did not form detectable levels of microparticles for a substantial period of time. A possible explanation for this observation is that agitation first generated nanoparticles at air-water interfaces, and subsequently the rates of submicron and micron sized particle formation increased as nanoparticle levels became more significant.
Overall the results from the current study and other recent studies on subvisible particle formation have important practical implications for therapeutic protein product development and quality assurance.9-11,14-16 For example, consider a fill-finish operation for a therapeutic protein formulation that occurs after frozen bulk drug substance is thawed. It is important to realize that the manner in which the frozen drug substance is prepared and handled, as well as the thawing regime, and the solution handling and formulation steps, can greatly affect the levels of micro- and nanoparticles/aggregates present in the protein solution.26 Of course, sterile filtration (an operation typically conducted just prior to filling) with a 0.2 μm filter will remove most particles, but particles smaller than 200 nm may still be present, and new particles might form due to the filtration operation itself.36 During the filling operation, nanoparticles can be shed from the pump materials and/or tubing used in peristaltic pumps, and protein molecules can readily adsorb to these particles.10,37 Protein molecules also adsorb to wetted surfaces of the pumping systems and can slough off as submicron and micron sized particles.38,39 Furthermore, particles (e.g., glass particles) may be contributed by the container itself. These initial particles in the product container, even at levels below USP limits, may agglomerate over time during shipping, storage and administration, potentially reaching unacceptable levels and/or resulting in visible particles.
Particle formation rates for a given protein product depend on numerous factors including: the protein’s physicochemical properties, the solution composition, the specific stresses to which the protein is exposed, and whether foreign submicron sized and/or nanometer sized particles are being shed into the protein solution.10,36,40 Therefore, it is essential for each step in a product’s life history that robust particle analytical methods, such as NTA and MFI, are employed routinely to investigate particle formation. As part of a quality-by-design study, relevant process and product parameters should be varied and appropriate analytical methods for micron and submicron sized particles must be used. Otherwise, the causes and control for particle formation will not be properly understood.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kotarek JSC, De Paoli SH, Simak J, Lin T, Gao Y, Ovanesov M, Liang Y, Scott D, Brown J, Bai Y, Metcalfe DD, Marszal E, Ragheb JA. Subvisible Particle Content, Formulation, and Dose of an Erythropoietin Peptide Mimetic Product Are Associated With Severe Adverse Postmarketing Events. Journal of Pharmaceutical Sciences. 5(3):1023–1027. doi: 10.1016/S0022-3549(15)00180-X. [DOI] [PubMed] [Google Scholar]
- 2.Joubert MK, Hokom M, Eakin C, Zhou L, Deshpande M, Baker MP, Goletz TJ, Kerwin BA, Chirmule N, Narhi LO, Jawa V. Highly aggregated antibody therapeutics can enhance the in vitro innate and late-stage T-cell immune responses. The Journal of biological chemistry. 2012;287(30):25266–25279. doi: 10.1074/jbc.M111.330902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmadi M, Bryson CJ, Cloake EA, Welch K, Filipe V, Romeijn S, Hawe A, Jiskoot W, Baker MP, Fogg MH. Small amounts of sub-visible aggregates enhance the immunogenic potential of monoclonal antibody therapeutics. Pharmaceutical research. 2015;32(4):1383–1394. doi: 10.1007/s11095-014-1541-x. [DOI] [PubMed] [Google Scholar]
- 4.Rombach-Riegraf V, Karle AC, Wolf B, Sordé L, Koepke S, Gottlieb S, Krieg J, Djidja M-C, Baban A, Spindeldreher S, Koulov AV, Kiessling A. Aggregation of Human Recombinant Monoclonal Antibodies Influences the Capacity of Dendritic Cells to Stimulate Adaptive T-Cell Responses In Vitro. PLoS ONE. 2014;9(1):e86322. doi: 10.1371/journal.pone.0086322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christie M, Torres RM, Kedl RM, Randolph TW, Carpenter JF. Recombinant Murine Growth Hormone Particles are More Immunogenic with Intravenous than Subcutaneous Administration. Journal of Pharmaceutical Sciences. 2014;103(1):128–139. doi: 10.1002/jps.23794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pisal DS, Kosloski MP, Middaugh CR, Bankert RB, Balu-Iyer SV. Native-like aggregates of factor VIII are immunogenic in von Willebrand factor deficient and hemophilia a mice. Journal of Pharmaceutical Sciences. 2012;101(6):2055–2065. doi: 10.1002/jps.23091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fradkin AH, Carpenter JF, Randolph TW. Immunogenicity of aggregates of recombinant human growth hormone in mouse models. Journal of Pharmaceutical Sciences. 2009;98(9):3247–3264. doi: 10.1002/jps.21834. [DOI] [PubMed] [Google Scholar]
- 8.Shomali MTS, Freitag AJ, Engert J, Winter G, Siedler M, KaymakCalan Z, Carpenter JF, Randolph TW. Dose levels in particulate containing formulations impact anti-drug antibody responses to murine monoclonal antibody in mice. Journal of Pharmaceutical Sciences. 2015;104:1610–1621. doi: 10.1002/jps.24413. [DOI] [PubMed] [Google Scholar]
- 9.Barnard JG, Singh S, Randolph TW, Carpenter JF. Subvisible particle counting provides a sensitive method of detecting and quantifying aggregation of monoclonal antibody caused by freeze-thawing: insights into the roles of particles in the protein aggregation pathway. Journal of Pharmaceutical Sciences. 2011;100(2):492–503. doi: 10.1002/jps.22305. [DOI] [PubMed] [Google Scholar]
- 10.Tyagi AK, Randolph TW, Dong A, Maloney KM, Hitscherich C, Jr, Carpenter JF. IgG particle formation during filling pump operation: a case study of heterogeneous nucleation on stainless steel nanoparticles. Journal of Pharmaceutical Sciences. 2009;98(1):94–104. doi: 10.1002/jps.21419. [DOI] [PubMed] [Google Scholar]
- 11.Corvari V, Narhi LO, Spitznagel TM, Afonina N, Cao S, Cash P, Cecchini I, DeFelippis MR, Garidel P, Herre A, Koulov AV, Lubiniecki T, Mahler HC, Mangiagalli P, Nesta D, Perez-Ramirez B, Polozova A, Rossi M, Schmidt R, Simler R, Singh S, Weiskopf A, Wuchner K. Subvisible (2-100 mum) particle analysis during biotherapeutic drug product development: Part 2, experience with the application of subvisible particle analysis. Biologicals: journal of the International Association of Biological Standardization. 2015;43(6):457–473. doi: 10.1016/j.biologicals.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Singh SK, Afonina N, Awwad M, Bechtold-Peters K, Blue JT, Chou D, Cromwell M, Krause HJ, Mahler HC, Meyer BK, Narhi L, Nesta DP, Spitznagel T. An industry perspective on the monitoring of subvisible particles as a quality attribute for protein therapeutics. Journal of Pharmaceutical Sciences. 2010;99(8):3302–3321. doi: 10.1002/jps.22097. [DOI] [PubMed] [Google Scholar]
- 13.Hawe A, Wiggenhorn M, van de Weert M, Garbe JHO, Mahler H-C, Jiskoot W. Forced degradation of therapeutic proteins. Journal of Pharmaceutical Sciences. 2012;101(3):895–913. doi: 10.1002/jps.22812. [DOI] [PubMed] [Google Scholar]
- 14.Bai S, Murugesan Y, Vlasic M, Karpes LB, Brader ML. Effects of submicron particles on formation of micron-sized particles during long-term storage of an interferon-beta-1a solution. Journal of Pharmaceutical Sciences. 2013;102(2):347–351. doi: 10.1002/jps.23414. [DOI] [PubMed] [Google Scholar]
- 15.Simler BR, Hui G, Dahl JE, Perez-Ramirez B. Mechanistic complexity of subvisible particle formation: links to protein aggregation are highly specific. Journal of Pharmaceutical Sciences. 2012;101(11):4140–4154. doi: 10.1002/jps.23299. [DOI] [PubMed] [Google Scholar]
- 16.Jayaraman M, Buck PM, Alphonse Ignatius A, King KR, Wang W. Agitation-induced aggregation and subvisible particulate formation in model proteins. European Journal of Pharmaceutics and Biopharmaceutics. 2014;87(2):299–309. doi: 10.1016/j.ejpb.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Kumru OS, Liu J, Ji JA, Cheng W, Wang YJ, Wang T, Joshi SB, Middaugh CR, Volkin DB. Compatibility, physical stability, and characterization of an IgG4 monoclonal antibody after dilution into different intravenous administration bags. Journal of Pharmaceutical Sciences. 2012;101(10):3636–3650. doi: 10.1002/jps.23224. [DOI] [PubMed] [Google Scholar]
- 18.Gross JSS, Karow AR, Bakowsky U, Garidel P. Nanoparticle tracking analysis of particle size and concentration detection in suspensions of polymer and protein samples: Influence of experimental and data evaluation parameters. European Journal of Pharmaceutics and Biopharmaceutics. 2016;104:30–41. doi: 10.1016/j.ejpb.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg AS, Verthelyi D, Cherney BW. Managing uncertainty: A perspective on risk pertaining to product quality attributes as they bear on immunogenicity of therapeutic proteins. Journal of Pharmaceutical Sciences. 2012;101(10):3560–3567. doi: 10.1002/jps.23244. [DOI] [PubMed] [Google Scholar]
- 20.Mehta S, Carpenter JF, Randolph TW. Colloidal Instability Fosters Agglomeration of Subvisible Particles Created by Rupture of Gels of a Monoclonal Antibody Formed at Silicone Oil-Water Interfaces. Journal of Pharmaceutical Sciences. 2016;105(8):2238–2348. doi: 10.1016/j.xphs.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Rudiuk S, Cohen-Tannoudji L, Huille S, Tribet C. Importance of the dynamics of adsorption and of a transient interfacial stress on the formation of aggregates of IgG antibodies. Soft Matter. 2012;8(9):2651. [Google Scholar]
- 22.Mehta SB, Lewus R, Bee JS, Randolph TW, Carpenter JF. Gelation of a monoclonal antibody at the silicone oil-water interface and subsequent rupture of the interfacial gel results in aggregation and particle formation. J Pharm Sci. 2015;104(4):1282–1290. doi: 10.1002/jps.24358. [DOI] [PubMed] [Google Scholar]
- 23.Bhatnagar BS, Bogner RH, Pikal MJ. Protein stability during freezing: separation of stresses and mechanisms of protein stabilization. Pharmaceutical development and technology. 2007;12(5):505–523. doi: 10.1080/10837450701481157. [DOI] [PubMed] [Google Scholar]
- 24.Shieh IC, Patel AR. Predicting the Agitation-Induced Aggregation of Monoclonal Antibodies Using Surface Tensiometry. Molecular pharmaceutics. 2015;12(9):3184–3193. doi: 10.1021/acs.molpharmaceut.5b00089. [DOI] [PubMed] [Google Scholar]
- 25.Mahler HCFS, Randolph TW, Carpenter JF. Protein Aggregation and Particle Formation: Effects of Formulation, Interfaces, and Drug Poduct Manufacturing Operations. In: Wang WR CJ, editor. Aggregation of Therapeutic Proteins. Wiley and Sons; 2010. pp. 301–311. [Google Scholar]
- 26.Rathore N. Current perspectives on stability of protein drug products during formulation, fill and finish operations. Biotechnology Progress. 2008;24:504–514. doi: 10.1021/bp070462h. [DOI] [PubMed] [Google Scholar]
- 27.Ghazvini S, Kalonia C, Volkin DB, Dhar P. Evaluating the Role of the Air-Solution Interface on the Mechanism of Subvisible Particle Formation Caused by Mechanical Agitation for an IgG1 mAb. Journal of Pharmaceutical Sciences. 2016;105(5):1643–1656. doi: 10.1016/j.xphs.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 28.Joshi O, Chu L, McGuire J, Wang DQ. Adsorption and function of recombinant Factor VIII at the air-water interface in the presence of Tween 80. J Pharm Sci. 2009;98(9):3099–3107. doi: 10.1002/jps.21569. [DOI] [PubMed] [Google Scholar]
- 29.Kerwin BA. Polysorbates 20 and 80 used in the formulation of protein biotherapeutics: structure and degradation pathways. Journal of Pharmaceutical Sciences. 2008;97(8):2924–2935. doi: 10.1002/jps.21190. [DOI] [PubMed] [Google Scholar]
- 30.Kreilgaard L. Effect of Tween 20 on Freeze-thawing and Agitation-induced aggregation of recombinant human factor XIII. Journal of Pharmaceutical Sciences. 1998;87(12):1597–1603. doi: 10.1021/js980126i. [DOI] [PubMed] [Google Scholar]
- 31.Chou DK, Krishnamurthy R, Randolph TW, Carpenter JF, Manning MC. Effects of Tween 20 and Tween 80 on the stability of Albutropin during agitation. Journal of Pharmaceutical Sciences. 2005;94(6):1368–1381. doi: 10.1002/jps.20365. [DOI] [PubMed] [Google Scholar]
- 32.Cheng H-L, Velankar SS. Film climbing of particle-laden interfaces. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2008;315(1–3):275–284. [Google Scholar]
- 33.Binks BP, Clint JH, Fletcher PD, Lees TJ, Taylor P. Particle film growth driven by foam bubble coalescence. Chemical communications. 2006;(33):3531–3533. doi: 10.1039/b606308j. [DOI] [PubMed] [Google Scholar]
- 34.Bee JS, Schwartz DK, Trabelsi S, Freund E, Stevenson JL, Carpenter JF, Randolph TW. Production of particles of therapeutic proteins at the air–water interface during compression/dilation cycles. Soft Matter. 2012;8(40):10329. [Google Scholar]
- 35.Randolph TW, Schiltz E, Sederstrom D, Steinmann D, Mozziconacci O, Schöneich C, Freund E, Ricci MS, Carpenter JF, Lengsfeld CS. Do Not Drop: Mechanical Shock in Vials Causes Cavitation, Protein Aggregation, and Particle Formation. Journal of Pharmaceutical Sciences. 2015;104(2):602–611. doi: 10.1002/jps.24259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu L, Randolph TW, Carpenter JF. Particles shed from syringe filters and their effects on agitation-induced protein aggregation. Journal of Pharmaceutical Sciences. 2012;101(8):2952–2959. doi: 10.1002/jps.23225. [DOI] [PubMed] [Google Scholar]
- 37.Saller VMJ, Grauschopf U, Bechtold-Peters K, Mahler HC, Friess W. Particle Shedding from Peristaltic Pump Tubing in Biopharmaceutical Drug Product Manufacturing. Journal of Pharmaceutical Sciences. 2015;104(4):1440–1450. doi: 10.1002/jps.24357. [DOI] [PubMed] [Google Scholar]
- 38.Tzannis ST, H WJM, Wood PA, Przybycien TM. Irreversible inactivation of interleukin 2 in a pump based delivery environment. Proc Natl Acad USA Applied Biological Sciences. 1996;93:5460–5465. doi: 10.1073/pnas.93.11.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pardeshi NN, Q W, Dahl K, Caplan L, Carpenter JF. Microparticles and Nanoparticles Delivered in Intravenous Saline and in an Intravenous Solution of a Therapeutic Antibody Product. Journal of Pharmaceutical Sciences. 2017;106(2):511–520. doi: 10.1016/j.xphs.2016.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bee JS, Chiu D, Sawicki S, Stevenson JL, Chatterjee K, Freund E, Carpenter JF, Randolph TW. Monoclonal antibody interactions with micro- and nanoparticles: Adsorption, aggregation, and accelerated stress studies. Journal of Pharmaceutical Sciences. 2009;98(9):3218–3238. doi: 10.1002/jps.21768. [DOI] [PMC free article] [PubMed] [Google Scholar]


