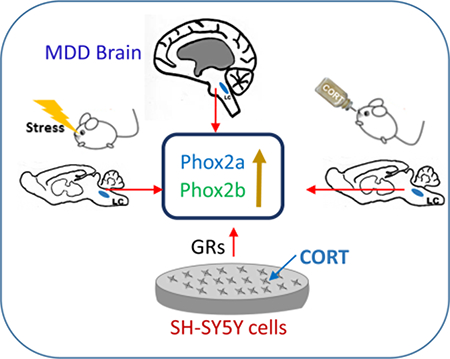
Abstract
Phox2a and Phox2b are two homeodomain transcription factors playing a pivotal role in the development of noradrenergic neurons during the embryonic period. However, their expression and function in adulthood remain to be elucidated. Using human postmortem brain tissues, rat stress models and cultured cells, this study aimed to examine the alteration of Phox2a and Phox2b expression. The results show that Phox2a and Phox2b are normally expressed in the human locus coeruleus (LC) in adulthood. Furthermore, the levels of Phox2a protein and mRNA and protein levels of Phox2b were significantly elevated in the LC of brain donors that suffered from the major depressive disorder, as compared to age-matched and psychiatrically normal control donors. Fischer 344 rats subjected to chronic social defeat showed higher mRNA and protein levels of Phox2a and Phox2b in the LC, as compared to non-stressed control rats. In rats chronically administered oral corticosterone, mRNA and protein levels of Phox2b, but not Phox2a, in the LC were significantly increased. In addition, the corticosterone-induced increase of Phox2b protein was reversed by simultaneous treatment with either mifepristone or spironolactone. Exposing SH-SY5Y cells to corticosterone significantly increased expression of Phox2a and Phox2b, which was blocked by corticosteroid receptor antagonists. Taken together, these experiments reveal that Phox2 genes are expressed throughout the lifetime in the LC of humans and Fischer 344 rats. Alterations in their expression may play a role in major depressive disorder and possibly other stress-related disorders through their modulatory effects on the noradrenergic phenotype.
Keywords: Phox2 genes, major depressive disorder, stress, corticosterone, postmortem, locus coeruleus
Graphical Abstract
Introduction
Phox2a and Phox2b are two closely related homeodomain transcription factors with relatively similar expression patterns, and play a pivotal role in the specification and differentiation of noradrenergic neurons during the embryonic period (Morin et al. 1997, Hirsch et al. 1998, Pattyn et al. 2000). A large body of experiments demonstrated that the silencing of the Phox2a gene leads to the agenesis of the noradrenergic locus coeruleus (LC) in mice (Morin et al. 1997). Inactivation of Phox2b disturbs noradrenergic differentiation in both central and peripheral nervous systems (Pattyn et al. 1999, Pattyn et al. 2000). These important roles of the Phox2a and Phox2b on noradrenergic neurons are further revealed by their involvement in the transcriptional control of noradrenergic phenotypes such as expression of tyrosine hydroxylase (TH) and dopamine β-hydroxylase (DBH). In fact, Phox2a−/− or Phox2b−/− embryos fail to express DBH (Morin et al. 1997, Pattyn et al. 1999), similar to that observed in DBH knock-out animals (Thomas et al. 1995, Pattyn et al. 2000). Forced expression of either Phox2a or Phox2b can induce TH in vitro (Lo et al. 1999, Stanke et al. 1999) and in vivo (Guo et al. 1999, VogelHopker & Rohrer 2002). These data establish Phox2 genes as determinants for development of the noradrenergic neuronal phenotype during embryogenesis.
Several studies demonstrate that Phox2a and Phox2b expression continues beyond embryonic development (Tiveron et al. 1996, Kang et al. 2007, Card et al. 2010). Functional investigations reveal that Phox2a and Phox2b are essential regulators of noradrenergic marker gene expressions past early development (Howard 2005). Their expression continues to be required for the control of adaptive homeostatic functions of mature neurons that contribute to brainstem networks (Pattyn et al. 2000, Tiveron et al. 2003). Our previous in vivo study showed that a significant reduction in mRNAs of DBH in the LC and adrenal glands of aging rats was paralleled with a decline in mRNA levels of Phox2a and Phox2b (Zhu et al. 2005), supporting the importance of Phox2 genes to the expression of the noradrenergic phenotype well beyond birth. Furthermore, in vitro investigations demonstrate a direct transcriptional activation of TH and DBH by Phox2 genes (Kim et al. 1998, Yang et al. 1998b, Yang et al. 1998a, Seo et al. 2002, Fan et al. 2009). Together, these investigations strongly implicate a regulatory effect of Phox2a and Phox2b on the expression of noradrenergic phenotypes into adulthood.
The noradrenergic system plays a critical role in attention, learning and memory, emotion, sleep/wakefulness and central responses to stress (Berridge & Waterhouse 2003, Sara 2009). A disruption of this system is believed to have a close relationship with major depressive disorder (MDD) (Ressler & Nemeroff 1999, Chandley & Ordway 2012). Pathologically, MDD can exhibit a pronounced and sustained central hyper-noradrenergic function as indicated by increased TH protein in the LC of postmortem brains from donors who died by suicide (Ordway et al. 1994, Gos et al. 2008) and/or donors that suffered from MDD at the time of death (Zhu et al. 1999), as compared to matched control donors. Consistent with these findings, elevated norepinephrine concentrations in the cerebrospinal fluid paralleled by an increase of plasma cortisol has been observed in depressed patients (Wong et al. 2000, Gold et al. 2005). Chronic stress is a major factor contributing to elevated risk of developing MDD (Mundt et al. 2000, Mann & Currier 2010). Stress greatly activates the LC (Robbins & Everitt 1995), resulting in elevated norepinephrine release (Pavcovich et al. 1990). Animal studies have shown that stress significantly increases TH expression and TH protein in the LC and other noradrenergic nuclei (Nankova et al. 1996, Wang et al. 1998, Makino et al. 2002). Given the critical role of Phox2 genes on the noradrenergic phenotype, it is reasonable to expect that Phox2a and Phox2b may regulate noradrenergic adaptations in response to stress and in MDD. There is one report of chronic stress-induced alterations of Phox2b expression in the mouse adrenal medulla (Santana et al. 2015), but how the Phox2 genes in the CNS are regulated by stress or in stress-related disorders is unknown.
Based on the important role of Phox2 genes on the expressional and functional regulation of the noradrenergic phenotype, we hypothesize that the expression of phox2a and Phox2b in the central noradrenergic system is possibly altered under depressive and stress conditions. Phox2 gene expression changes may mechanistically underlie increases in markers of the noradrenergic phenotypes in the LC of depressive patients and under stressful condition (Melia et al. 1992, Nankova et al. 1996, Zhu et al. 1999, Fan et al. 2014). Therefore, the present study was designed to investigate whether the expression of Phox2a and Phox2b is altered in MDD in human, or by exposure to chronic stress in rodents. mRNA and protein levels of Phox2a and Phox2b were assessed in the LC of postmortem brains from MDD donors, and in brains of rats subjected to chronic social defeat (CSD) or treated with corticosterone (CORT). Furthermore, an in vitro study was performed to examine the expression of these genes after exposing cells to CORT. The results from the present experiments indicate that Phox2 genes may play an important role in MDD and in adaptation to stress through modulation of the noradrenergic phenotype.
Experimental procedures
Human tissue collection and section:
The methods for obtaining human brains tissues are exactly as described in the previous report (Zhu et al. 1999). Briefly, human brain tissues were collected from the Cuyahoga County Coroner’s Office, Cleveland, Ohio, in accordance with an approved Institutional Review Board protocol for human studies. Information on the lifetime and current (within the last month) psychiatric status of all brain donors was obtained in structured clinical interviews with the next-of-kin by a trained interviewer for DSM-IV Axis I Disorders modified for third-person reporting (First et al. 1996). Brain tissues were collected from 12 donors diagnosed as having MDD at the time of death and from 12 matched control donors that had no Axis I diagnosis at the time of death or historically. From over 50 donors with MDD available from the brain collection, donors with MDD chosen for study were those that had the least number of potentially confounding factors (psychotherapeutic drugs in toxicology, low RNA quality, low tissue pH, comorbidities) and that could be matched for age and postmortem interval to available control donors that also had the least number of potentially confounding factors. The age of subjects ranged from 18–77 years with an average of 45 ± 5 years for control group (all males) and 48 ± 5 years for MDD group (all males). A summary of subject information as a result of psychiatry autopsy including a toxicology screen from all of the subjects is outlined in Table 1. All demographic data (e.g. ages) is not provided in the table to protect donor identities. Psychiatrically normal control and MDD donors were matched as closely as possible for age, postmortem interval (PMI), brain tissue pH and smoking history.
Table 1.
Subject demographics
| Subject Code | pH | RINa | PMIb | Smoker | Toxicology | Axis I diagnosis | Suicide | Tissue | Cause of death |
|---|---|---|---|---|---|---|---|---|---|
| Normal Control Donors | |||||||||
| KS 59 | 6.95 | 6.8 | 19.0 | No | Ethanol (0.03%) | None | No | LC | Heart disease |
| KS 31 | 6.79 | 7.6 | 6.0 | No,hxd | Lidocaine | None | No | LC | Heart disease |
| KS 63 | 6.40 | 6.4 | 31.5 | unknown | Midazolam | None | No | LC | Btonchial asthma |
| KS 65 | 5.80 | 6.4 | 27 | No | NDDc | None | No | LC | Accident |
| KS 82 | 6.50 | 6.5 | 26 | No | Morphine | None | No | LC | Paritonitis |
| KS 70 | 7 | 7.4 | 22.3 | No | NDD | None | No | LC | Distended aorta |
| KS 76 | 6.70 | 7.4 | 23.5 | No | Butalbital | None | No | LC | Trauma |
| KS 78 | 6.60 | 7.9 | 16.0 | unknown | NDD | None | No | LC | Cong. hearte |
| KS 74 | 6.30 | 7.3 | 16.4 | No | Diltiazem | None | No | LC | Trauma |
| KS 85 | 6.60 | 7 | 24.9 | No | Diltiazem | None | No | LC | Trauma |
| KS 23 | 6.78 | 7.7 | 21 | Yes | NDD | None | No | LC | Heart disease |
| KS 72 | 7.10 | 8.5 | 19.9 | Yes | Doxylamine | None | No | LC | Subarachnoid hemorrhage |
| MEAN | 6.63 | 7.24 | 21.15 | ||||||
| SEM | 0.10 | 0.18 | 1.89 | ||||||
| MDD Donors | |||||||||
| KS 56 | 6.47 | 6.9 | 31 | No | Ethanol | MDD | No | LC | Gunshot |
| KS 32 | 6.32 | 6.8 | 20 | Yes | Ethanol | MDD | No | LC | Gunshot |
| KS 66 | 6.68 | 6.7 | 17 | No | NDD | MDD | No | LC | Asphyxiation |
| KS 55 | 6.42 | 7.4 | 11.0 | No | NDD | MDD | No | LC | Heart disease |
| DD | 6.48 | 5.8 | 18 | No | CO | MDD | No | LC | CO poisoning |
| KS 71 | 6.60 | 7.5 | 5.3 | No | Citalopram, Venlafaxine | MDD | No | LC | Hanging |
| KS 77 | 6.70 | 8.7 | 18.8 | No | NDD | MDD | No | LC | Gunshot |
| KS 79 | 6.90 | 7.6 | 12.9 | Yes | NDD | MDD | No | LC | Gunshot |
| KS 75 | 6.70 | 6.7 | 19.6 | No | CO, Diazepam, Temazepam | MDD | No | LC | CO poisoning |
| KS 81 | 7 | 8.5 | 10.1 | No | Ethanol | MDD | No | LC | Hanging |
| KS 24 | 6.85 | 7.25 | 26 | Yes | Ethanol | MDD | No | LC | Gunshot |
| KS 73 | 6.50 | 9.2 | 11 | Yes | NDD | MDD | LC | Heart disease | |
| MEAN | 6.64 | 7.42 | 16.73 | ||||||
| SEM | 0.06 | 0.28 | 2.08 | ||||||
RNA integrity number generated by the Agilent Bioanalyzer 2100®
Postmortem interval
No drugs detectable
History
Congestive heart failure
Human brains were frozen (unfixed) and blocking was the same as previously reported (Zhu et al. 1999). The floor of the fourth ventricle and the pons were its dorsal and ventral surfaces, respectively. The rostral surface was formed by a transverse cut immediately caudal to the inferior colliculus (at the frenulum). Tissue lateral to the superior cerebellar peduncles was trimmed away. Particular care was taken in the freezing process to maintain gross morphology. For example, the block of pontine tissue was dissected to form a flat surface on the ventral pontine surface of the LC tissue block. This surface was placed on a hard piece of cardboard, which was then lowered for 10 sec into 2-methylbutane cooled on dry ice to −50 °C for quick freezing. Tissue blocks were then placed on powdered dry ice for 10 min and then stored in an ultracold freezer (−80°C). When sectioning, tissue blocks containing the LC were paired (MDD donor and age-matched control donor) and the caudal surfaces of each pair were co-mounted to the specimen chuck of a cryostat microtome (Leica, Cryocut 1800, Deerfield, IL). In this way, a single MDD-control pair was sectioned simultaneously and paired sections were mounted on the same microscope slide to be processed concurrently throughout the experiment. This pairing procedure reduced the influence of factors that could artificially contribute to differences between MDD and normal control donors. Frozen tissue blocks containing the LC were cut transversely at the same anatomical level in all donors, at approximately the middle along the rostro-caudal axis the LC (approximately 5 mm caudal from the frenulum). Tissue sections (20 μm) were cut at −16°C and thaw-mounted onto gelatin-coated microscope slides, and stored at −80°C until assay.
Animals:
Male Fischer 344 rats weighing 200–250g at the beginning of the experiment, Long-Evans retired male breeders, and ovariectomized female rats were purchased from Harlan Laboratories Inc. (Indianapolis, IN, USA). All animal procedures were approved by the Animal Care and Use Committee of East Tennessee State University (approval reference number: P130701), and complied with the NIH Guide for the Care and Use of Laboratory Animals. Rats were maintained on a 12-h light/dark cycle (lights on at 07:00 h) with ad-libitum access to food and tap water except as specifically described below. They were housed in a group cage (up to 3 rats per cage except for breeders and ovariectomized female rats). After an acclimation period of 5 days, rats were randomly assigned to experimental groups, in which animals were identified and recorded using a unique code during experiments of CSD and CORT ingestion, biochemical measurements and analysis. ARRIVE guidelines were taken into consideration in experimental reporting. This study was not pre-registered.
Chronic social defeat (CSD) paradigm:
This protocol has been reported previously (Chen et al. 2012). Briefly, each pair of Long-Evans rats (larger retired male breeders and sterile female rats, residents) was housed together in individual cages for 7 days. On the 8th day, the female was removed and an adult male Fischer 344 rat (intruder) was placed into the cage for 2 min. After being attacked and defeated, as shown by a supine and submissive posture, the “intruder” was rescued into a small protective cage within the resident’s cage, which precluded further physical contact, but allowing visual, auditory, and olfactory contact with the resident. The “intruder” was left in the cage of the “resident” for 1.5 h in the small enclosure. Some male Fischer 344 rats (controls) were transferred to the resident home cage when the residents had been removed. Therefore, these control rats were never physically attacked and defeated by the residents. The resident-intruder exposure was repeated 4 times in the first and fourth weeks, and 2 times in the second and third weeks. After the last session of CSD paradigm, rats were immediately sacrificed by rapid decapitation without anesthesia for biochemical measurements.
Oral administration with CORT and drug treatment:
This procedure is essentially as described previously (Fan et al. 2014). Briefly, male Fischer 344 rats were administered an oral solution containing CORT (Sigma, St. Louis, MO; 100 μg/ml solution in ad lib drinking bottles) at 9:00 am of each day for 21 days, which was freshly prepared daily. This dose of CORT, which is approximately 30 mg/kg/day (Gourley et al. 2008), results in an increased plasma CORT level approaching that observed during stress (Karatsoreos et al. 2010, Donner et al. 2012). Control rats were given the vehicle alone (a 2.4% ethanol solution used for preparation of CORT solution), which neither activates the hypothalamic-pituitary-adrenal axis, nor causes other biochemical alterations including organ weight (Magarinos et al. 1998, Nacher et al. 2004a, Nacher et al. 2004b, Gourley & Taylor 2009). A separate set of rats were injected with glucocorticoid receptor antagonist mifepristone (10 mg/kg, daily, s.c.; Sigma-Aldrich, St. Louis, MO, USA) or mineralocorticoid receptor antagonist spironolactone (15 mg/kg, daily, s.c.; Sigma-Aldrich, St. Louis, MO, USA); either alone or in combination at a similar time (around 9:00 am). In addition, a separate set of rats was injected daily with either mifepristone or spironolactone without CORT ingestion. The selection of doses of these antagonists was based on previous reports (Ratka et al. 1989, Ni et al. 1995, Haller et al. 1998, Macunluoglu et al. 2008) and our preliminary pilot data (Fan et al. 2014). Rats in the untreated control and CORT alone groups were injected with vehicle. After oral administration of CORT and related compounds for 21 days, animals were sacrificed on the 22nd day for biochemical measurements.
In situ hybridization to measure Phox2 mRNAs.
The in situ hybridization method has been described previously (Zhu et al. 2002). After rats were sacrificed, brains were removed and rapidly frozen in 2-methyl-butane on dry-ice, then stored at −80°C until sectioning. Sections (16 μm, not in pairs) were cut through the pontine LC region on a cryostat, and were mounted on SuperFrost Plus slides (Fisher Scientific; Pittsburg, PA), and stored at −80°C until assay. When hybridization was performed, slide-mounted tissue sections were fixed with 4% (w/v) paraformaldehyde followed by acetylation (with acetic anhydride) and washing (with an alcohol solution). [35S]-labeled cRNA probes were transcribed in vitro from cDNAs of Phox2a and Phox2b of human (for postmortem) or rats in pGEM-3Zf vectors with T3 RNA polymerase. The probe sizes for rat Phox2a and Phox2b were 0.85 and 0.95 kb, respectively, and for human Phox2a and Phox2b were 1.4 and 1.6 kb, respectively. The sequences of all these probes are located in the N-terminal of the genes. Pre-hybridized sections were incubated with radiolabeled probes followed by extensive washing, and then apposed to Biomax autoradiographic film (Kodak; Rochester, NY). The film exposure time is dependent upon the activities of radioprobes ranging from 24 to 48 hours, which was determined by the signal in the radioactivity meter measured on the slides after post-hybridization. For higher-resolution studies, sections were also dipped in Kodak NTB2 emulsion. The specificity of cRNA probes was tested using three criteria. First, sense probes synthesized from each cDNA were used to perform in situ hybridization in parallel with antisense probes. There were no specific signals on these slides. Second, antisense probes were used on control slides from the cerebellum and cortex and no hybridization signals were detected. Third, antisense probes were hybridized to slides that were treated with RNase A (20 μg/mL) and no hybridization signal was detected.
The same quantification method was performed for both rat and human. For analysis of in situ hybridization results, 3 sections from each rat and bilateral LC regions from each section were quantitated. Optical densities of developed autoradiograms were quantified using an image analysis system (MCID M2; Imaging Research Inc., Ontario, Canada). Relative abundance of Phox2+ silver grains are represented in optical density units, estimated by calibrating the image analysis system with an optical density stepwedge (Eastman Kodak Co., Rochester, New York). The compact cell body region of the LC was drawn on the image and optical densities within this drawing were recorded. Optical densities of Phox2 gene expressions were measured in a range where there was a linear relationship between Phox2a and Phox2b mRNA amounts and optical density of the film, confirmed with a set of calibrated recombinant Phox2 standards spotted onto Immobilin-P membrane. The optical density of background, i.e., representing areas of the membrane that were hybridized, but where no tissue was transferred, were very low (averaging 0.06 optical density unit).
Immunocytochemical staining:
The immunocytochemical staining procedures are the same as previously reported (Zhu et al. 2007). Briefly, human brain sections were pre-incubated in 5% bovine serum in phosphate buffer saline (PBS) supplemented with 0.2% Triton-X 100 for 1 h, followed by incubation in primary antibodies (1:200 dilution, monoclonal AB against Phox2a, RRID: AB_547013 and polyclonal AB against Phox2b, RRID:AB_10889846, both from Novus Biologicals, Littleton, CO, USA) overnight. On the following day, binding of Phox2a and Phox2b antibodies was detected with a biotinylated secondary antibody using the ABC kit (Vector Laboratories, Burlingame, CA) based on the manufacturer’s instructions. 3, 3’Diaminobenzidine tetrahydrochloride (DAB) was used as the substrate. Staining for Phox2immunoreactivity (ir) was then visualized and semi-quantitatively analyzed using ImageJ software (Rasband, US National Institutes of Health, Bethesda, http://rsbweb.nih.gov/ij, 2010). Briefly, images were acquired in an Olympus BX41 microscope (Tokyo, Japan) equipped with an Olympus U-TVO digital camera connected to a computer with MagnaFire image software (Goleta, CA). Three digital microscopic images under higher power (40x objective lens) were randomly captured of the cell body region of the LC. The image size of the analysis field was ~75 μm2. These grey-scale images were thresholded in ImageJ with a fixed level over the background, and this threshold was used for all images. The threshold values for Phox2-ir were obtained by manually sampling the signal intensity in each image, which was visually compared with the original grey-scale images to ensure that the tool effectively resolved all labeled cells. Thereafter, the intensity of Phox2-ir in the area was semi-quantitatively obtained using the measurement tool of ImageJ and the target (above threshold) area was expressed as a percentage of the sampled area. Two sections from each animal were examined. The average of the measurements (percent areas) obtained in 12 MDD donors from each group was a reflection of staining intensity for the LC.
Detection of protein levels:
Protein levels of Phox2a and Phox2b from rat LC and cultured cells were determined by western blotting as reported previously (Fan et al. 2011). Briefly, these samples were prepared by homogenization, centrifugation and measurement of protein concentrations. Then, equal amounts of samples (20 μg of protein per lane) were loaded on 10% SDS-polyacrylamide gels for electrophoresis. Electro-blotting was performed to transfer proteins in the gels to polyvinylidene diflouride membranes, which were incubated with primary antibodies against rat Phox2a (1:500 dilution; rabbit polyclonal; Abcam, Cambridge, MA, USA; RRID: AB_130121) or Phox2b (1:500 dilution; rabbit polyclonal; Abcam, Cambridge, MA, USA; RRID: AB_183741) overnight and further with secondary antibodies (horseradish peroxidase-conjugated anti-rabbit IgG, 1:3000; Amersham Biosciences, Little Chalfont, UK). Immunoreactive bands were visualized and detected by G:Box Imaging (Fyederick, MD, USA), or exposed on films and scanned by a Quantity One imaging devices (Bio-Rad, Hercules, CA). Band densities were then quantified by imaging software (Molecular Dynamics IQ solutions, Molecular Dynamics, Inc., Sunnyvale, CA). OD values of Phox2a and Phox2b signals were normalized with β-actin immunoreactivities, determined on the same blot. Normalized values were then averaged for all gels from separate animals (N numbers) of replicated experiments, and used to calculate the relative changes on the corresponding gel, presented as means ± SEM as described in 2.10.
To validate the antibodies, before the experiment, the antibody specificity was tested by using blocking peptides and results showed there was no band at the known molecular weight for the target after addition of the blocking peptide. Also, a linear standard curve was created from optical densities (ODs) of bands with a dilution series of total proteins prepared from cells. OD values of Phox2a or Phox2b were compared with those of the standard curve to ensure that detection was in the linear range of measurement. Thereafter, quantitated values were normalized with β-actin immunoreactivities determined on the same blot to assess equal protein loading. The value normalization is the same as described in above section.
Cell culture and drug exposure:
The human neuroblastoma cell line SH-SY5Y (from ATCC, Cat#: CRL-2266, RRID: CVCL_0019) was maintained in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% heat-inactivated fetal bovine serum (FBS), penicillin (100 U/ml) and streptomycin (100 μg/ml), at 37°C in humidified air containing 5% CO2 as described previously (Wang 2013). FBS was replaced by dialyzed FBS (Gibco-Invitrogen, Carlsbad, CA), when cells were treated with CORT. Drug exposures were started at day 3, when each subculture had become confluent. CORT (Sigma, St. Louis, MO), dissolved in 40 μl DMSO and then further diluted with saline, was added to 6-well plates in fresh medium that was changed daily. The same amount of vehicle was added into the drug-free medium for cells in the control group. Based on our previous study (Sun et al. 2010) and a preliminary experiment of this study, cells were exposed to different concentration of CORT for 3 days. Then cells were harvested after washing twice with fresh, ice-cold PBS and immediately lysed to obtain total proteins. In addition, SH-SY5Y cells were exposed to CORT plus either mifepristone (5 μM) or spironolactone (5 μM, both from Sigma-Aldrich, St. Louis, MO, USA) for 3 days. The selection of these concentrations of receptor antagonists was based on the literature, in which 1–10 μM of mifepristone or spironolactone was reported to fully block corticosteroid-induced biological effects in vitro (Xiao et al. 2000, Son et al. 2001, Golde et al. 2003, Pickering et al. 2003).
RNA isolation and quantitative real-time polymerase chain reaction (qPCR) analysis for mRNA of Phox2a and Phox2b.
The method is the similar as reported previously (Zha et al. 2011). Briefly, total RNA was extracted using RNAzol reagent (Molecular Research Center, Inc., Carlsbad, CA) from harvested cells after SH-SY5Y cells were exposed to 100 nM CORT for 3 days. Then cDNAs were converted using the superscript III First-Strand Synthesis Kit (Applied Biosystems/Life technologies, Forster City, CA) according to the manufacturer’s protocol. Real-time PCR was conducted using the SYBR green Platinum Quantitative PCR supermix (Invitrogen, Carlsbad, CA). The primers for q-PCR were as follows: human Phox2a: forward 5’-CAT TTA CAC GCG TGA GGA GCT GGC-3’ and reverse 5’- TCC TGT TTG CGG AAC TTG GCC C-3’. Human Phox2b: forward 5’-CAC CCT CAG GGA CCA CCA GA-3’ and reverse 5’-TTC TCG TTG AGG CCG CCG T-3’. Human β-actin (ACTB): forward 5’-TGT GCC CAT CTA CGA GGG GTA TGC-3’ and reverse 5’-GGT ACA TGG TGG TGC CGC CAG ACA-3’. A standard curve was generated by analysis of the serial dilutions of β-actin oligo fragment solutions (102–107 copies/μl). For each sample, the copy number of Phox2a, Phox2b and β-actin was extrapolated from their respective standard curves. The value of Phox2a and Phox2b gene expression was normalized with the β-actin copy number and expressed in arbitrary units. Reproducibility of results was determined by triplicate measurements of each cDNA aliquot, each using separate sets of samples.
Statistics:
The sample size of rats used in CSD and CORT administration were estimated by power analysis. It was calculated that to achieve 95% power (p<0.05), a sample size of 7–8 animals per group was required. All experimental data are presented in the text and graphs as the mean ± SEM. Data were analyzed by a paired t-test (for human postmortem study), unpaired t-test (animal and cell culture studies), or one way analysis of variance (ANOVA, SigmaStat, Systat Software Inc., Richmond, VA) when multiple treatment groups were compared. A post-hoc Student-Newman-Keuls test was performed for planned comparisons of multiple groups. Pearson correlations related to human tissues were computed for potential relationships between age or other possible modifiers [brain pH, postmortem interval (PMI), RNA quality assessed by RNA integrity number (RIN)] and dependent variables (levels of Phox2a and Phox2b mRNAs and proteins).
Results
Expression of Phox2a and Phox2b in the LC of MDD.
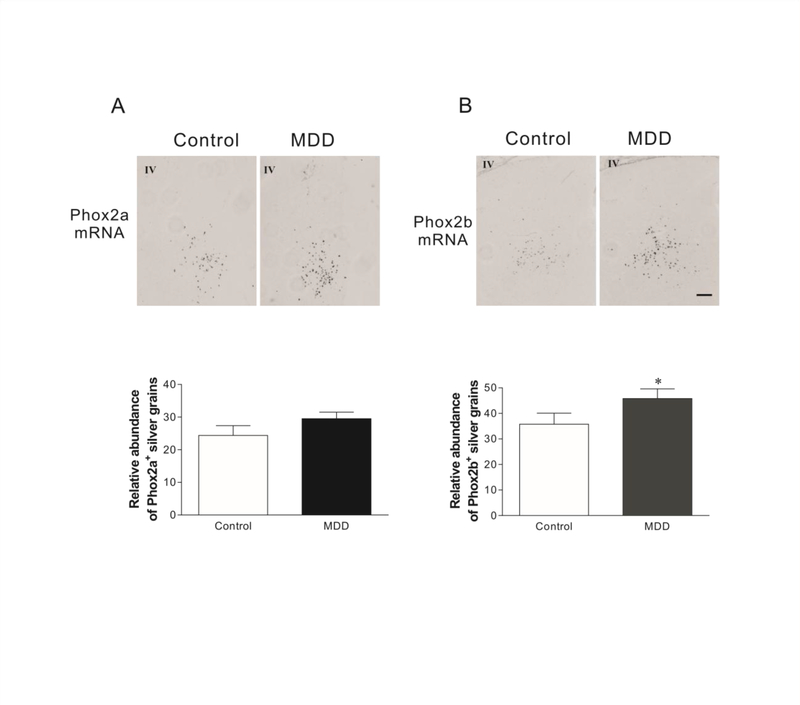
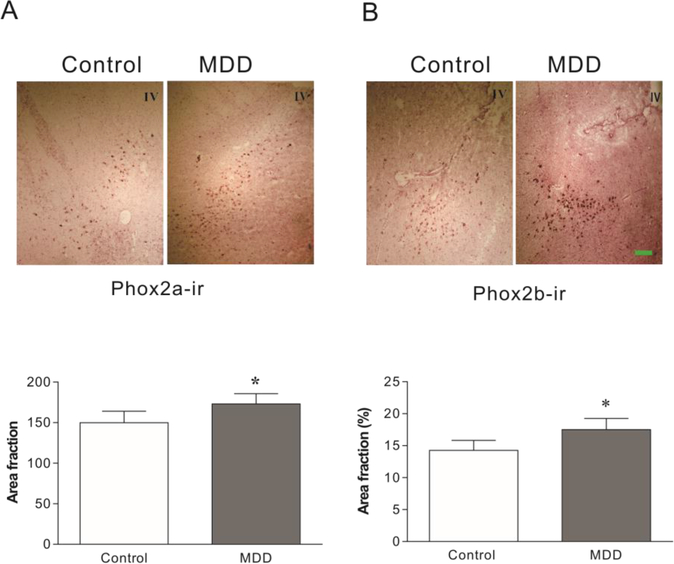
In situ hybridization was performed to examine mRNA levels of Phox2a and Phox2b in the LC of brain donors that had MDD and matched control donors that were psychiatrically normal (n=12/group for both groups). Grain densities of Phox2b mRNAs in MDD donors were 28% greater (Fig. 1B, t=3.16, p= 0.019) than that of the control donors. Although Phox2a expression levels were modestly increased, this was not statistically significant (Fig. 1A). Using brain tissues cut from same MDD/control pairs used for in situ hybridization, Phox2a and Phox2b protein levels were measured immunocytochemically. In MDD donors, levels of Phox2a-ir and Phox2b-ir in the LC region were elevated by 16% (Fig. 2A, t=2.37, p=0.035) and 23% (Fig. 2B, t=2.98, p=0.021), respectively, as compared to control donors. Pearson correlation analyses showed that except for a modest correlation between levels of Phox2b-ir and age (R=0.434; p=0.034), there were no other significant correlations between brain pH, PMI, RIN, or age and levels of Phox2 mRNAs or proteins (Table 2). A One-way ANCOVA analysis was conducted to determine whether age impacted the group effect (MDD vs control) on Phox2b-ir. Age did not alter the interpretation of the results; the ANCOVA with age as the covariate chowed a significant difference between the MDD and control groups for Phox2b-ir (F1, 23=12.66, p=0.002).
Figure 1:
The expression of Phox2a (A) and Phox2b (B) mRNAs in the LC of MDD donors and age-matched and psychiatrically normal control donors (N=12 for both). Upper panel: Representative images of Phox2a and Phox2b mRNA in the LC of brains detected by in situ hybridization. Lower panel: Quantitative analyses of Phox2a and Phox2b mRNA hybridizations for control and MDD donors. IV: Fourth ventricle. Scale bar: 250 μm for all images.
Figure 2:
Phox2a and Phox2b immunoreactivity (ir) in the LC of MDD donors and age-matched psychiatrically normal control donors (N=12 for both). Upper panel: Representative images of Phox2a- and Phox2b-ir in the LC of brains detected by immunocytochemical staining. Lower panel: semi-quantitative analyses of Phox2a-ir and Phox2b-ir in tissue sections. * p<0.05, compared to the control. IV: Fourth ventricle. Scale bar: 250 μm for all images.
Table 2.
Pearson correlations for potential confounding variables
| mRNA | Protein | ||||
|---|---|---|---|---|---|
| Phox2a | Phox2b | Phox2a | Phox2b | ||
| pH | Pearson Correlation | −0.249 | −0.286 | −0.017 | 0.242 |
| Sig. (2-tailed) | 0.241 | 0.176 | 0.938 | 0.255 | |
| N | 24 | 24 | 24 | 24 | |
| PMI | Pearson Correlation | −0.047 | −0.366 | 0.105 | −0.187 |
| Sig. (2-tailed) | 0.826 | 0.078 | 0.624 | 0.382 | |
| N | 24 | 24 | 24 | 24 | |
| RIN | Pearson Correlation | 0.154 | −0.076 | −0.035 | 0.193 |
| Sig. (2-tailed) | 0.471 | 0.725 | 0.87 | 0.367 | |
| N | 24 | 24 | 24 | 24 | |
| Age | Pearson Correlation | 0.263 | −0.035 | 0.001 | 0.434 |
| Sig. (2-tailed) | 0.213 | 0.869 | 0.998 | 0.034 | |
| N | 24 | 24 | 24 | 24 | |
Expression of Phox2a and Phox2b in the LC of rats subjected to CSD.
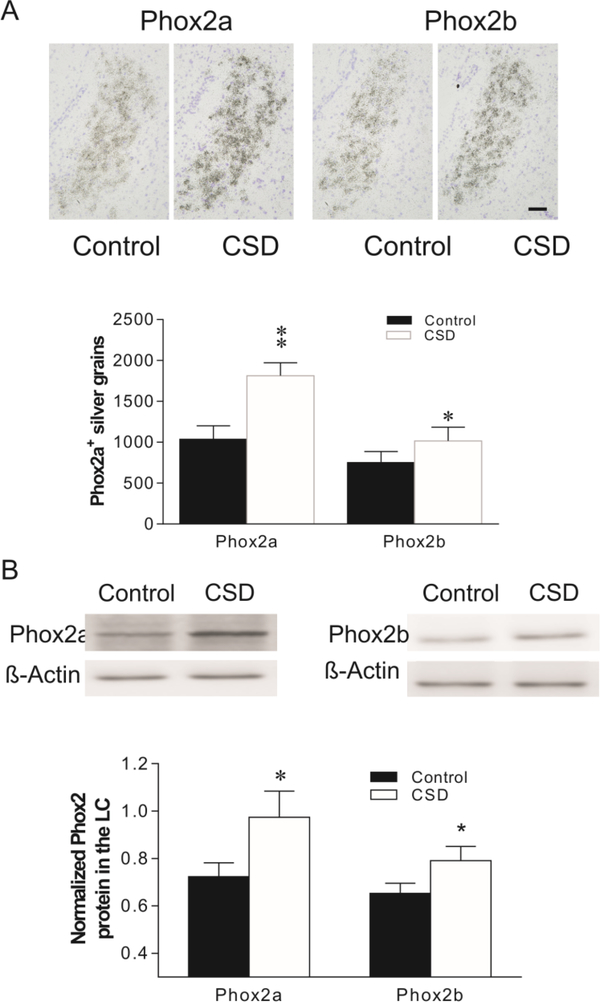
Ex vivo in situ hybridization was performed to examine effects of CSD on the mRNA levels of Phox2a and Phox2b in the LC of rats. As show in Fig. 3A, exposing to CSD significantly increased Phox2a expression by 76% (t=4.56, p<0.01), and Phox2b expression by 34% (t=3.07, p<0.05), compared to unstressed control rats. Western blotting was performed to determine levels of Phox2a-ir and Phox2b-ir in the LC from the CSD and control groups. Exposing to CSD significantly increased levels of Phox2a-ir by 34% (t=3.29, p<0.05) and Phox2b-ir by 21% (t=2.99, p<0.05), as compared to unstressed control rats (Fig. 3B).
Figure 3:
Effects of CSD on mRNAs (A) and protein (B) levels of Phox2a and Phox2b in the rat LC. Upper panel in A: Phox2a/2b mRNA in LC of rat brains detected by in situ hybridization (N=7/group). Lower panel in A: Quantitative analyses of mRNA in slides. Upper panel in B: Autoradiographs obtained by western blotting of Phox2a/2b in LC of rat brains (N=8/group). Lower panel in B: Quantitative analyses of band densities. Values of Phox2 bands were normalized to those of β-actin probed on the same blot. * p<0.05, ** p<0.01, compared to the control group. Scale bar: 50 μm for all images.
Expression levels of Phox2a and Phox2b in the LC of rats treated with oral CORT.
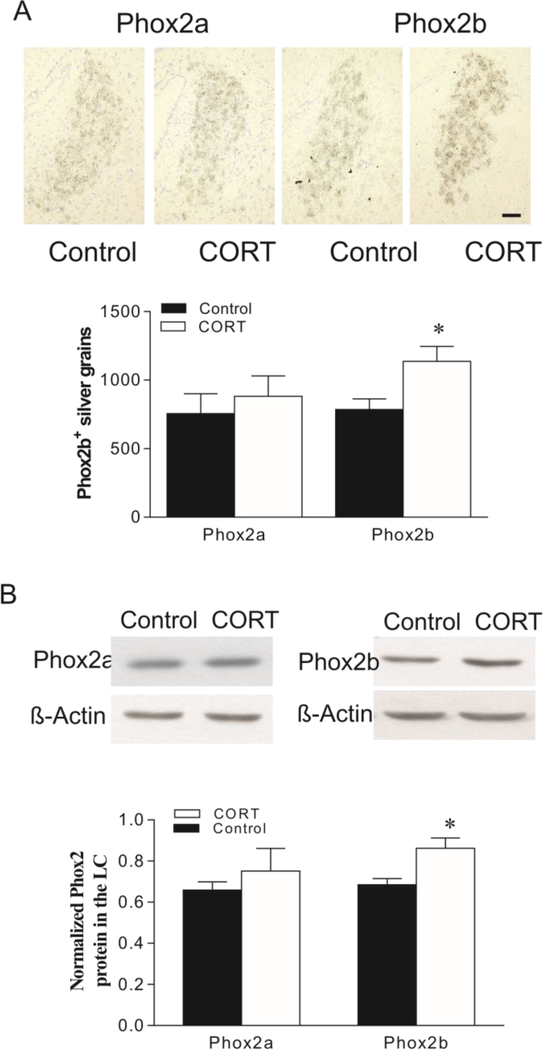
To further evaluate stress-related changes in the expression of Phox2a and Phox2b, as well as their proteins in the LC, rats were orally administered CORT (~30 mg/kg/day) via drinking water for 3 weeks. Although CORT treatment resulted in a significant increase in Phox2b expression (by 66%, t=4.01, p<0.01), it failed to significantly change Phox2a mRNA levels in the LC, as compared to the control group (Fig. 4A). Western blotting of Phox proteins revealed results parallel to in situ hybridization. Treatment with CORT enhanced levels of Phox2b-ir in the LC by about 26% (t=3.02, p<0.05). However, the same treatment did not significantly influence Phox2a-ir levels in the LC (Fig. 4B).
Figure 4:
Effects of oral administration of CORT on mRNAs (A) and protein (B) levels of Phox2a and Phox2b in the rat LC. Upper panel in A: Phox2a/2b mRNA in LC of rat brains detected by in situ hybridization (N=7/group). Lower panel in A: Quantitative analyses of mRNA in slides. Upper panel in B: Autoradiographs obtained by western blotting of Phox2a/2b in LC of rat brains (N=8/group). Lower panel in B: Quantitative analyses of band densities. Values of Phox2 bands were normalized to those of β-actin probed on the same blot. * p<0.05, compared to the control. Scale bar: 50 μm for all images.
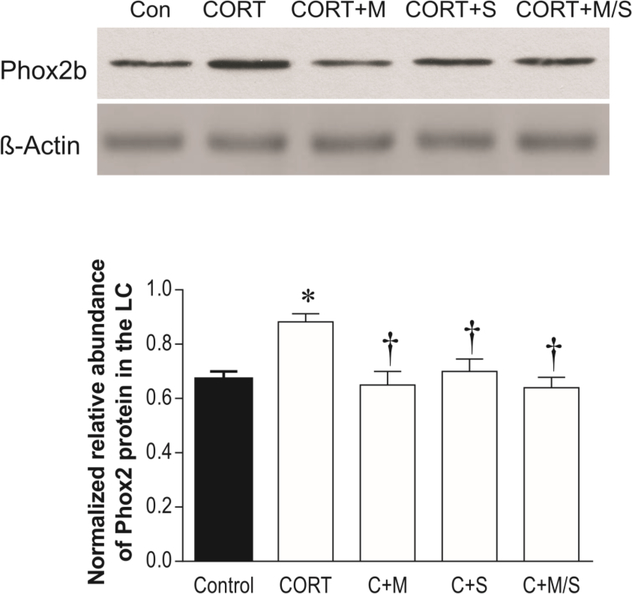
To examine whether the altered Phox2b protein levels caused by administration of CORT were related to corticosteroid receptors, mifepristone or spironolactone, alone or in combination, were administered to rats together with oral administration of CORT for the same period. Western blotting analysis exhibited a significant influence of these treatments on Phox2b-ir levels (F3.34 = 4.18, p<0.05, Fig. 5). Simultaneous treatment with either mifepristone or spironolactone prevented the CORT-induced increase in Phox2b-ir levels in the LC. However, there were no additional effects when both corticosteroid receptor antagonists were administered together (Fig. 5). In addition, treatment with either mifepristone or spironolactone alone (without oral administration of CORT) did not significantly affect Phox2b-ir levels (data not shown).
Figure 5:
Effects of CORT administration, and CORT administration plus treatment with corticosteroid receptor antagonists (N=7/group) on Phox2b immunoreactivities in the LC. Upper panel: Phox2b proteins in LC tissues of rats detected by western blotting. Lower panel: Quantitative analyses of band densities. * p<0.05, compared to the control; † p<0.05, compared to the CORT group. Con: control; CORT+M: CORT plus treatment with mifepristone; CORT+S: CORT plus treatment with spironolactone; C+M/S: CORT plus treatments with both mifepristone and spironolactone.
Phox2a and Phox2b in the SH-SY5Y cells exposed to CORT.
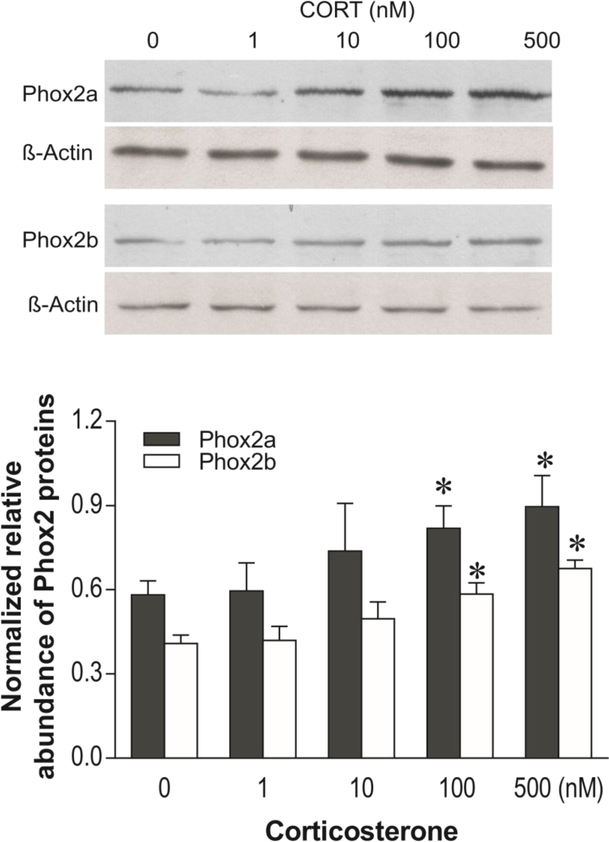
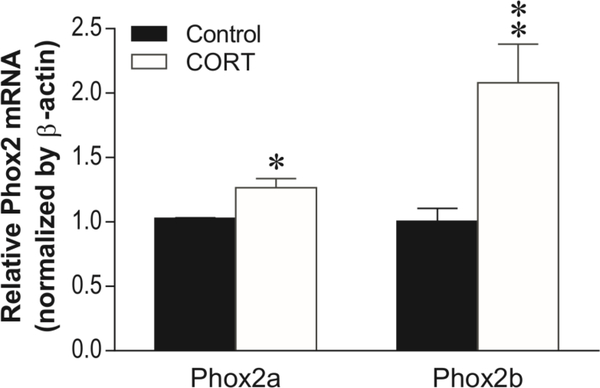
To verify whether CORT affects the expression of Phox2a and Phox2b in vitro, SHSY5Y cells were exposed to different concentrations of CORT for 3 days. The selection of this cell line is based on characteristics that closely mimic noradrenergic neurons with respect to display of the noradrenergic phenotype and to catecholamine metabolism (Balasooriya & Wimalasena 2007, Xicoy et al. 2017). Western blotting analysis showed that CORT significantly increased levels of Phox2a-ir and Phox2b-ir in a concentration-dependent manner (F4,19 = 4.67, p<0.05 for Phox2a; F4,19 = 4.32, p<0.05 for Phox2b, Fig. 6). Post-hoc tests showed exposure of cells to 100 and 500 nM of CORT significantly elevated Phox2a-ir by 35% and 46%, and Phox2b-ir by 34% and 52%, respectively (all p<0.05). Based on the observation of the concentration-response to CORT in western blotting, mRNA levels of Phox2a and Phox2b were examined after cells were exposed to 100 nM CORT for 3 days. Real time PCR analysis demonstrated that mRNA levels of both Phox2a (increased by 23%, p<0.05) and Phox2b (increased by 93%, p<0.001) were significantly enhanced by treatment with CORT (Fig. 7).
Figure 6:
Effects of exposing SH-SY5Y cells to different concentrations of CORT for 3 days on immunoreactivities of Phox2a and Phox2b (N=5/groups). Upper panel: Phox2a and Phox2b proteins detected by western blotting. Lower panel: Quantitative analyses of band densities. * p<0.05, compared to the 0 group (vehicle).
Figure 7:
Effects of exposing to CORT on mRNA levels of Phox2a and Phox2b. SH-SY5Y cells were exposed to 100 nM CORT for 3 days. Harvested cells were prepared for real-time PCR with β-actin as the internal control. The graphic data represent means ± SEM obtained from 7 separate experiments (N=7). *p < 0.05, ** p < 0.01; compared to the group treated with vehicle (control).
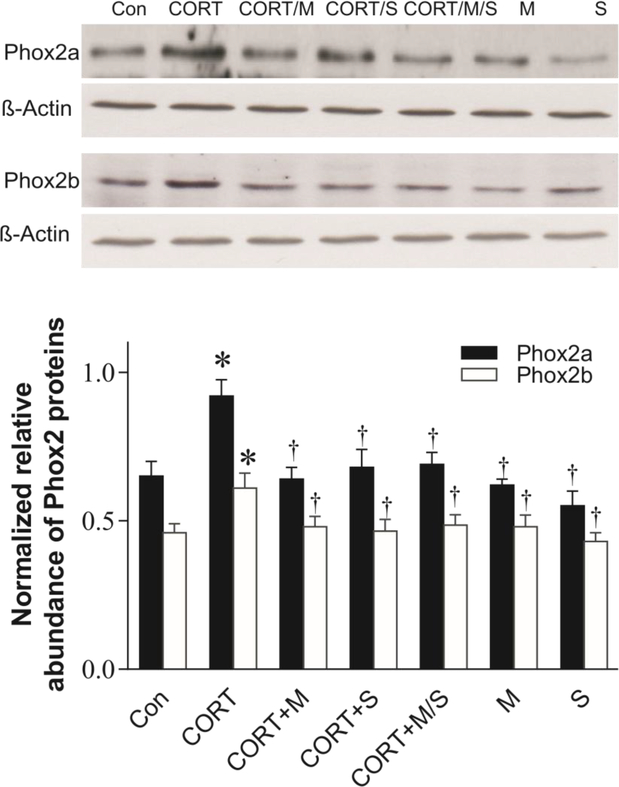
Further, SH-SY5Y cells were simultaneously exposed to 100 nM CORT alone, and 100 nM CORT plus either 5 μM mifepristone, 5 μM spironolactone, or both, as well as 5 μM mifepristone and 5 μM spironolactone without CORT for 3 days. Western blotting analysis showed that these treatments significantly affected levels of Phox2-ir and Phox2b-ir (F6,27 = 3.89, p<0.05 for Phox2a; F6,27 = 4.05, p<0.05). Further analysis revealed that while neither mifepristone or spironolactone alone did significantly affected levels of both Phox2a-ir and Phox2b-ir, these corticosteroid receptor antagonists significantly blocked CORT-induced increases of Phox2 protein levels (both p<0.05, Fig. 8). There is no additional effect when cells were exposed to CORT in combination with both mifepristone and spironolactone.
Figure 8:
Effects of CORT receptor antagonists on CORT-induced increases in Phox2b immunoreactivities. SH-SY5Y cells were simultaneously exposed to 100 nM CORT, CORT plus either mifepristone, spironolactone or both, as well as mifepristone, spironolactone alone for 3 days (N=5/group). Upper panel: Phox2b proteins detected by western blotting. Lower panel: Quantitative analyses of band densities. * p<0.05, compared to the control (Con), † p<0.05, compared to the CORT ingestion group. M: treated with mifepristone alone; S: treated with spironolactone; for other abbreviations see legend of Fig. 6.
Discussion
The most striking finding of this study is that there were elevated protein levels of Phox2a and Phox2b, as well as elevated mRNA levels of Phox2b in the LC of MDD donors , possibly contributing to the psychopathology of MDD. As the development of MDD is closely related to psychological stress exposure (Mundt et al. 2000, Mann & Currier 2010), the expression of these genes were also examined in the rats either exposed to CSD or treated with the stress hormone CORT. The results show that either CSD exposure or treatment with CORT similarly increased Phox2b mRNA and Phox2b protein levels in the LC, while CSD also increased Phox2a protein levels in the LC. Furthermore, exposure of SH-SY5Y cells to CORT upregulated the expression of Phox2a and Phox2b. Mifepristone and spironolactone reversed CORT-induced upregulation of Phox2b proteins in vivo and in vitro, indicating that corticosteroid receptors mediate CORT-induced changes in Phox2b proteins. Together, these experiments reveal the presence of Phox2a and Phox2b in the mature human brain and a potential role of these transcription factors in the pathology of MDD and stress. Considering that these transcription factors exert transcriptional control of the noradrenergic phenotypes, TH and DBH, the present studies may be linked to increased expression of TH in MDD (Zhu et al. 1999) and of TH and DBH in rodent stress models (Melia et al. 1992, Nankova et al. 1996, Fan et al. 2014).
Previous studies have revealed an apparent divergence in the distribution of Phox2a and Phox2b in animal brains. Phox2a is expressed in DBH-positive neurons such as A1, A2, A5 and the LC (A6) in postnatal mice (P12) (Tiveron et al 1996). However, the expression of Phox2b in the LC is transient and disappears around E11.5 rather than continuing up to birth like Phox2a (Pattyn et al 2000). Further, while a robust and stable expression of Phox2a can be found in all brainstem noradrenaline neurons of adult Sprague-Dawley rats (Card et al. 2010), there is no expression of Phox2b in the LC of the same strain of rats (Kang et al. 2007). Such divergent expression of Phox2a and Phox2b may indicate differences in their functions, and leads to the notion that expression of Phox2b is not necessary for the maintenance of the noradrenergic phenotype in the adult brainstem (Card et al. 2010). However, our previous study (Fan et al. 2011) and the current work showed that Phox2b is expressed in the LC of adult Fischer 344 rats. More importantly, gene expression and proteins of Phox2a and Phox2b are found in the LC of humans at ages ranging from 18–77 years (Table 1). These findings indicate that like Phox2a, Phox2b is expressed in the LC of adult humans and Fischer 344 rats, and may continue to play a significant role in the maintenance or modulation of noradrenergic transmission in normal and disease states.
Phox2a and Phox2b protein, as well as Phox2b gene expression, were significantly increased in the LC of MDD brain donors, compared to age-matched and psychiatrically normal control donors. This is the first report of the expression of Phox2a and Phox2b in the LC of humans that had MDD. It can be speculated that these genes may be involved in the neuronal control of adaptive homeostatic function in adulthood. Given psychiatric diseases are polygenetic disorders, aberrant gene expressions could be one of their pathophysiologic characteristics. Since transcription factors are involved in the orchestration of gene expression programs, abnormalities in their expression might be expected in MDD. Studies have shown that altered expression of certain transcription factors is associated with MDD. For example, CREB and phosphorylated CREB were found to be reduced in the temporal cortex of MDD patients (Yamada et al. 2003), resulting in an increased expression of pentraxin 3 (Shelton et al. 2004), transcription of the gene of which is inhibited by CREB (Sulser 2002). In addition, abnormal transcriptions caused by transcription factors LBP-1c (Schahab et al. 2006), Foxp2 (Li et al. 2013) and REST (Otsuki et al. 2010) have been associated with MDD. On the other hand, genetic disruption of Phox2 genes has been reported in other human diseases. For example, Phox2 gene mutations are associated with some human diseases such as the congenital fibrosis of the extraocular muscles syndrome type 2 (CFEOM2) (Nakano et al. 2001, Yazdani et al. 2003), congenital central hypoventilation syndrome (CCHS) (Amiel et al. 2003, Sasaki et al. 2003), and Hirschsprung disease (HSCR) (Carter et al. 2012, Fernandez et al. 2013). Considering the close relationship of Phox2a and Phox2b with the noradrenergic phenotype, an increased expression of Phox2a and Phox2b in the LC of MDD may suggest that these transcription factors could be a molecular marker for the pathophysiology of MDD. Further exploration of their roles in MDD might provide additional information for their genetic linkage with MDD.
Stress is a major risk factor for the development of depression, and stress induces release of CORT (in rodents) or cortisol (in humans) (Chrousos & Gold 1992). Hence, it is noteworthy that relatively similar enhancements of Phox2a and Phox2b were observed in the LC in MDD donors and Fischer 344 rats exposed to chronic stress, although CORT treatments only resulted in an increase of Phox2b in rats. It is conceivable that the molecular mechanisms responsible for elevated Phox2a and Phox2b in MDD and chronically stressed rats are similar. At least for Phox2b, the mechanism may involve CORT in rats, or cortisol in humans. Interestingly, several stress paradigms including single and repeated immobilization elicited expression levels of c-Fos, ΔFosB, Fra-2 and CREB in the LC (Hebert et al. 2005, Goebel et al. 2009, McDevitt et al. 2009, Imbe & Kimura 2016), as well as c-Fos, Fra-2, Egr1 in other brain regions such as the amygdala, as well as in the adrenal medulla (Sabban et al. 2004, Porter & Hayward 2011). Also of note is that Phox2b expression levels were significantly increased in adrenal medulla of stressed mice after 7 days of unpredictable chronic stress (Santana et al. 2015). Therefore, the results of the present study are consistent with these previous investigations.
One issue raised by the present study is physiological relevance of the upregulated Phox2a and Phox2b in the LC of MDD and stressed animals. Given Phox2a and Phox2b are essential for development of the noradrenergic system in the embryonic period, their upregulation may be related to their effects on the noradrenergic phenotype in adulthood too. Postmortem studies demonstrated biological abnormalities of the LC, including altered TH protein levels in subjects who suffered from MDD (Zhu et al. 1999) or committed suicide (Ordway et al. 1994, Xiang et al. 2004). Animal studies demonstrate that chronic stress significantly increases TH protein in the LC (Melia & Duman 1991, Smith et al. 1991, Brady 1994, Watanabe et al. 1995, McDougall et al. 2005). Studies from our and other laboratories report a marked elevation of DBH expression in the LC of stressed animals (Serova et al. 1999, Fan et al. 2013). In addition, stress-induced DBH upregulation was found in the stellate ganglia (Gavrilovic et al. 2009), adrenal medulla (Nankova et al. 1999, Spasojevic et al. 2010) and sympathetic ganglia (Kvetnansky et al. 2004), where Phox2a and Phox2b are normally expressed (Brunet & Pattyn 2002). It seems likely that induction of Phox2a and Phox2b expression may be a precursor to stress-induced in increases in LC activity and TH expression. Therefore, an increased expression of Phox2a and Phox2b would account for the upregulated expression of TH and DBH in the LC under depressive and stressful conditions. Although we do not have ample direct evidence to support this explanation currently, our previous study showed that forced expression of Phox2a and Phox2b in the LC favors the increased expression of the noradrenergic phenotype in adult rats (Fan et al. 2011). More studies are necessary to clarify this important issue.
In this study, compared to CSD paradigm, chronic administration of CORT to rats did not significantly upregulate Phox2a or Phox2a protein expression. This unchanged Phox2a expression might not be ascribed to CORT administration, as the in vitro study showed mRNA and protein levels of Phox2a and Phox2b were upregulated by exposure of cells to CORT. The possible reason for the discrepancy may be related to physiological or pharmacokinetic differences between the stress paradigm and oral CORT administration. This is similar to the observation in our previous study that while CSD upregulated DBH protein levels in all major terminal regions including the frontal cortex (Fan et al. 2013), but there was no significant alteration of DBH protein levels in the frontal cortex after CORT ingestion (Fan et al. 2014). Investigations from other laboratories also demonstrate discrepant results between stress paradigms and CORT treatments. For example, chronic stress significantly reduced expression of brain-derived neurotrophic factor (BDNF) in the frontal cortex (Roceri et al. 2004, Mao et al. 2010, Li et al. 2012), whereas chronic administration of CORT had no significant effect on BDNF mRNA or BDNF protein levels in the same brain region (Jacobsen & Mork 2006). The discrepancy in Phox2a expression effects between CSD paradigm and CORT ingestion may also be related to the fact that stress can activate multiple hormones and signaling pathways and therefore produce more complex effects than exposure to CORT alone.
The present study demonstrated intriguing findings regarding the alterations of Phox2 expression in MDD and induced by stress in rats. However, it is noteworthy that Phox2a mRNA levels in the LC from MDD donors, and mRNA and protein levels of Phox2a in the LC of rats exposed to CORT did not exhibit the significant changes as seen for Phox2b. One potential limitation for such complete correspondence between Phox2a and Phox2b may be a result of small sample sizes that precluded the demonstration of statistical significance. Small sample sizes reduced the power of the study and increased the margin of error. It will therefore be necessary to replicate our finding, especially for the human tissues, with a larger sample size.
In conclusion, the present study demonstrated that transcription factors Phox2a and Phox2b are expressed in the LC of adult humans and Fischer 344 rats, and the expression of these transcription factors appear to be plastic. In vitro experiments demonstrated that the expression of both Phox2a and Phox2b are upregulated after exposure of cells to CORT. Furthermore, CORT-induced upregulation of Phox2b is mediated by corticosteroid receptors, as verified by in vivo and in vitro experiments using corticosteroid receptor antagonists. Taken as a whole, the results of this study strongly suggest that Phox2a and Phox2b may play a regulatory role in the mature brain. Their altered expression in MDD and following chronic stress suggest that these transcription factors may contribute to neuronal pathology associated with these conditions.
Highlights.
Protein levels of Phox2a, mRNA and protein levels of Phox2b were elevated in the locus coeruleus from brain donors of MDD
CSD significantly increased Phox2a and Phox2b expression in the rat locus coeruleus
Corticosterone administration increased Phox2b protein levels in the rat locus coeruleus
Corticosterone increased Phox2a and Phox2b expression in the SK-SY5Y cells
Corticosterone-induced increase in Phox2 expression may be mediated through corticosteroid receptors
Acknowledgments:
This research was supported by the Postmortem Brain Core of COBRE P30 GM103328 (CAS), and a C06 grant (NIH RR030651) (GAO). We also gratefully acknowledge the support of the Cuyahoga County Medical Examiner’s Office, Cleveland, OH, in assisting with the collection of brain tissue. The authors have no conflicts of interest.
Abbreviations:
- CORT:
corticosterone
- CSD:
chronic social defeat
- DBH:
dopamine βhydroxylase
- ir:
immunoreactivity
- LC:
locus coeruleus
- MDD:
major depressive disorders
- PBS:
phosphate-buffer saline
- q-PCR:
quantitative real-time polymerase chain reaction
- TH:
tyrosine hydroxylase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Amiel J, Laudier B, Attie-Bitach T et al. (2003) Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet, 33, 459–461. [DOI] [PubMed] [Google Scholar]
- Balasooriya I and Wimalasena K (2007) Are SH-SY5Y and MN9D cell lines truly dopaminergic? Proceedings of the 3rd Annual GRASP Symposium, 25–26. [Google Scholar]
- Berridge CW and Waterhouse BD (2003) The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev, 42, 33–84. [DOI] [PubMed] [Google Scholar]
- Brady LS (1994) Stress, antidepressant drugs, and the locus coeruleus. Brain Res Bull, 35, 545–556. [DOI] [PubMed] [Google Scholar]
- Brunet JF and Pattyn A (2002) Phox2 genes - from patterning to connectivity. Curr Opin Genet Dev, 12, 435–440. [DOI] [PubMed] [Google Scholar]
- Card JP, Lois J and Sved AF (2010) Distribution and phenotype of Phox2a-containing neurons in the adult sprague-dawley rat. J Comp Neurol, 518, 2202–2220. [DOI] [PubMed] [Google Scholar]
- Carter TC, Kay DM, Browne ML et al. (2012) Hirschsprung’s disease and variants in genes that regulate enteric neural crest cell proliferation, migration and differentiation. Journal of human genetics, 57, 485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandley MJ and Ordway GA (2012) Noradrenergic Dysfunction in Depression and Suicide In: The Neurobiological Basis of Suicide, (Dwivedi Y ed.). Boca Raton (FL). [PubMed] [Google Scholar]
- Chen P, Fan Y, Li Y, Sun Z, Bissette G and Zhu MY (2012) Chronic social defeat up regulates expression of norepinephrine transporter in rat brains. Neurochem Int, 60, 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP and Gold PW (1992) The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. Jama, 267, 1244–1252. [PubMed] [Google Scholar]
- Donner NC, Montoya CD, Lukkes JL and Lowry CA (2012) Chronic non-invasive corticosterone administration abolishes the diurnal pattern of tph2 expression. Psychoneuroendocrinology, 37, 645–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Chen P, Li Y, Cui K, Noel DM, Cummins ED, Peterson DJ, Brown RW and Zhu MY (2014) Corticosterone administration up-regulated expression of norepinephrine transporter and dopamine beta-hydroxylase in rat locus coeruleus and its terminal regions. J Neurochem, 128, 445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Chen P, Li Y and Zhu MY (2013) Effects of chronic social defeat on expression of dopamine beta-hydroxylase in rat brains. Synapse, 67, 300–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Huang J, Duffourc M, Kao RL, Ordway GA, Huang R and Zhu MY (2011) Transcription factor Phox2 upregulates expression of norepinephrine transporter and dopamine beta-hydroxylase in adult rat brains. Neuroscience, 192, 37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Huang J, Kieran N and Zhu MY (2009) Effects of transcription factors Phox2 on expression of norepinephrine transporter and dopamine beta-hydroxylase in SK-NBE(2)C cells. J Neurochem, 110, 1502–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez RM, Mathieu Y, Luzon-Toro B, Nunez-Torres R, Gonzalez-Meneses A, Antinolo G, Amiel J and Borrego S (2013) Contributions of PHOX2B in the pathogenesis of Hirschsprung disease. PloS one, 8, e54043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilovic L, Spasojevic N and Dronjak S (2009) Psychosocial stress-related changes in gene expression of norepinephrine biosynthetic enzymes in stellate ganglia of adult rats. Autonomic neuroscience : basic & clinical, 150, 144–146. [DOI] [PubMed] [Google Scholar]
- Goebel M, Stengel A, Wang L and Tache Y (2009) Restraint stress activates nesfatin-1immunoreactive brain nuclei in rats. Brain Res, 1300, 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PW, Wong ML, Goldstein DS et al. (2005) Cardiac implications of increased arterial entry and reversible 24-h central and peripheral norepinephrine levels in melancholia. Proc Natl Acad Sci U S A, 102, 8303–8308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde S, Coles A, Lindquist JA and Compston A (2003) Decreased iNOS synthesis mediates dexamethasone-induced protection of neurons from inflammatory injury in vitro. Eur J Neurosci, 18, 2527–2537. [DOI] [PubMed] [Google Scholar]
- Gos T, Krell D, Bielau H, Brisch R, Trubner K, Steiner J, Bernstein HG, Jankowski Z and Bogerts B (2008) Tyrosine hydroxylase immunoreactivity in the locus coeruleus is elevated in violent suicidal depressive patients. European archives of psychiatry and clinical neuroscience, 258, 513–520. [DOI] [PubMed] [Google Scholar]
- Gourley SL, Kiraly DD, Howell JL, Olausson P and Taylor JR (2008) Acute hippocampal brain-derived neurotrophic factor restores motivational and forced swim performance after corticosterone. Biol Psychiatry, 64, 884–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL and Taylor JR (2009) Recapitulation and reversal of a persistent depression-like syndrome in rodents. Current protocols in neuroscience / editorial board, Jacqueline N. Crawley ... [et al. ], Chapter 9, Unit 9 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Brush J, Teraoka H, Goddard A, Wilson SW, Mullins MC and Rosenthal A (1999) Development of noradrenergic neurons in the zebrafish hindbrain requires BMP, FGF8, and the homeodomain protein soulless/Phox2a. Neuron, 24, 555–566. [DOI] [PubMed] [Google Scholar]
- Haller J, Millar S and Kruk MR (1998) Mineralocorticoid receptor blockade inhibits aggressive behaviour in male rats. Stress, 2, 201–207. [DOI] [PubMed] [Google Scholar]
- Hebert MA, Serova LI and Sabban EL (2005) Single and repeated immobilization stress differentially trigger induction and phosphorylation of several transcription factors and mitogen-activated protein kinases in the rat locus coeruleus. J Neurochem, 95, 484–498. [DOI] [PubMed] [Google Scholar]
- Hirsch MR, Tiveron MC, Guillemot F, Brunet JF and Goridis C (1998) Control of noradrenergic differentiation and Phox2a expression by MASH1 in the central and peripheral nervous system. Development, 125, 599–608. [DOI] [PubMed] [Google Scholar]
- Howard MJ (2005) Mechanisms and perspectives on differentiation of autonomic neurons. Dev Biol, 277, 271–286. [DOI] [PubMed] [Google Scholar]
- Imbe H and Kimura A (2016) Repeated forced swim stress affects the expression of pCREB and DeltaFosB and the acetylation of histone H3 in the rostral ventromedial medulla and locus coeruleus. Brain Res Bull, 127, 11–22. [DOI] [PubMed] [Google Scholar]
- Jacobsen JP and Mork A (2006) Chronic corticosterone decreases brain-derived neurotrophic factor (BDNF) mRNA and protein in the hippocampus, but not in the frontal cortex, of the rat. Brain Res, 1110, 221–225. [DOI] [PubMed] [Google Scholar]
- Kang BJ, Chang DA, Mackay DD, West GH, Moreira TS, Takakura AC, Gwilt JM, Guyenet PG and Stornetta RL (2007) Central nervous system distribution of the transcription factor Phox2b in the adult rat. J Comp Neurol, 503, 627–641. [DOI] [PubMed] [Google Scholar]
- Karatsoreos IN, Bhagat SM, Bowles NP, Weil ZM, Pfaff DW and McEwen BS (2010) Endocrine and physiological changes in response to chronic corticosterone: a potential model of the metabolic syndrome in mouse. Endocrinology, 151, 2117–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Seo H, Yang C, Brunet JF and Kim KS (1998) Noradrenergic-specific transcription of the dopamine beta-hydroxylase gene requires synergy of multiple cisacting elements including at least two Phox2a-binding sites. J Neurosci, 18, 8247–8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvetnansky R, Micutkova L, Rychkova N, Kubovcakova L, Mravec B, Filipenko M, Sabban EL and Krizanova O (2004) Quantitative evaluation of catecholamine enzymes gene expression in adrenal medulla and sympathetic Ganglia of stressed rats. Ann N Y Acad Sci, 1018, 356–369. [DOI] [PubMed] [Google Scholar]
- Li LF, Lu J, Li XM, Xu CL, Deng JM, Qu R and Ma SP (2012) Antidepressantlike effect of magnolol on BDNF up-regulation and serotonergic system activity in unpredictable chronic mild stress treated rats. Phytotherapy research : PTR, 26, 11891194. [DOI] [PubMed] [Google Scholar]
- Li T, Zeng Z, Zhao Q et al. (2013) FoxP2 is significantly associated with schizophrenia and major depression in the Chinese Han population. World J Biol Psychiatry, 14, 146–150. [DOI] [PubMed] [Google Scholar]
- Lo L, Morin X, Brunet JF and Anderson DJ (1999) Specification of neurotransmitter identity by Phox2 proteins in neural crest stem cells. Neuron, 22, 693–705. [DOI] [PubMed] [Google Scholar]
- Macunluoglu B, Arikan H, Atakan A, Tuglular S, Ulfer G, Cakalagaoglu F, Ozener C and Akoglu E (2008) Effects of spironolactone in an experimental model of chronic cyclosporine nephrotoxicity. Transplantation proceedings, 40, 273–278. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, Orchinik M and McEwen BS (1998) Morphological changes in the hippocampal CA3 region induced by non-invasive glucocorticoid administration: a paradox. Brain Res, 809, 314–318. [DOI] [PubMed] [Google Scholar]
- Makino S, Smith MA and Gold PW (2002) Regulatory role of glucocorticoids and glucocorticoid receptor mRNA levels on tyrosine hydroxylase gene expression in the locus coeruleus during repeated immobilization stress. Brain Res, 943, 216–223. [DOI] [PubMed] [Google Scholar]
- Mann JJ and Currier DM (2010) Stress, genetics and epigenetic effects on the neurobiology of suicidal behavior and depression. European psychiatry : the journal of the Association of European Psychiatrists, 25, 268–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao QQ, Huang Z, Zhong XM, Feng CR, Pan AJ, Li ZY, Ip SP and Che CT (2010) Effects of SYJN, a Chinese herbal formula, on chronic unpredictable stressinduced changes in behavior and brain BDNF in rats. J Ethnopharmacol, 128, 336–341. [DOI] [PubMed] [Google Scholar]
- McDevitt RA, Szot P, Baratta MV, Bland ST, White SS, Maier SF and Neumaier JF (2009) Stress-induced activity in the locus coeruleus is not sensitive to stressor controllability. Brain Res, 1285, 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall SJ, Widdop RE and Lawrence AJ (2005) Differential gene expression in WKY and SHR brain following acute and chronic air-puff stress. Brain Res Mol Brain Res, 133, 329–336. [DOI] [PubMed] [Google Scholar]
- Melia KR and Duman RS (1991) Involvement of corticotropin-releasing factor in chronic stress regulation of the brain noradrenergic system. Proc Natl Acad Sci U S A, 88, 83828386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melia KR, Nestler EJ and Duman RS (1992) Chronic imipramine treatment normalizes levels of tyrosine hydroxylase in the locus coeruleus of chronically stressed rats. Psychopharmacology (Berl), 108, 23–26. [DOI] [PubMed] [Google Scholar]
- Morin X, Cremer H, Hirsch MR, Kapur RP, Goridis C and Brunet JF (1997) Defects in sensory and autonomic ganglia and absence of locus coeruleus in mice deficient for the homeobox gene Phox2a. Neuron, 18, 411–423. [DOI] [PubMed] [Google Scholar]
- Mundt C, Reck C, Backenstrass M, Kronmuller K and Fiedler P (2000) Reconfirming the role of life events for the timing of depressive episodes. A two-year prospective followup study. J Affect Disord, 59, 23–30. [DOI] [PubMed] [Google Scholar]
- Nacher J, Gomez-Climent MA and McEwen B (2004a) Chronic non-invasive glucocorticoid administration decreases polysialylated neural cell adhesion molecule expression in the adult rat dentate gyrus. Neurosci Lett, 370, 40–44. [DOI] [PubMed] [Google Scholar]
- Nacher J, Pham K, Gil-Fernandez V and McEwen BS (2004b) Chronic restraint stress and chronic corticosterone treatment modulate differentially the expression of molecules related to structural plasticity in the adult rat piriform cortex. Neuroscience, 126, 503509. [DOI] [PubMed] [Google Scholar]
- Nakano M, Yamada K, Fain J et al. (2001) Homozygous mutations in ARIX(PHOX2A) result in congenital fibrosis of the extraocular muscles type 2. Nat Genet, 29, 315–320. [DOI] [PubMed] [Google Scholar]
- Nankova B, Kvetnansky R, Hiremagalur B, Sabban B, Rusnak M and Sabban EL (1996) Immobilization stress elevates gene expression for catecholamine biosynthetic enzymes and some neuropeptides in rat sympathetic ganglia: effects of adrenocorticotropin and glucocorticoids. Endocrinology, 137, 5597–5604. [DOI] [PubMed] [Google Scholar]
- Nankova BB, Tank AW and Sabban EL (1999) Transient or sustained transcriptional activation of the genes encoding rat adrenomedullary catecholamine biosynthetic enzymes by different durations of immobilization stress. Neuroscience, 94, 803–808. [DOI] [PubMed] [Google Scholar]
- Ni H, Mune T, Morita H, Daidoh H, Hanafusa J, Shibata T, Yamakita N and Yasuda K (1995) Inhibition of aldosterone turn-off phenomenon following chronic adrenocorticotropin treatment with in vivo administration of antiglucocorticoid and antioxidants in rats. Eur J Endocrinol, 133, 578–584. [DOI] [PubMed] [Google Scholar]
- Ordway GA, Smith KS and Haycock JW (1994) Elevated tyrosine hydroxylase in the locus coeruleus of suicide victims. J Neurochem, 62, 680–685. [DOI] [PubMed] [Google Scholar]
- Otsuki K, Uchida S, Wakabayashi Y, Matsubara T, Hobara T, Funato H and Watanabe Y (2010) Aberrant REST-mediated transcriptional regulation in major depressive disorder. J Psychiatr Res, 44, 378–384. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Goridis C and Brunet JF (2000) Specification of the central noradrenergic phenotype by the homeobox gene Phox2b. Mol Cell Neurosci, 15, 235–243. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Morin X, Cremer H, Goridis C and Brunet JF (1999) The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature, 399, 366–370. [DOI] [PubMed] [Google Scholar]
- Pavcovich LA, Cancela LM, Volosin M, Molina VA and Ramirez OA (1990) Chronic stress-induced changes in locus coeruleus neuronal activity. Brain Res Bull, 24, 293–296. [DOI] [PubMed] [Google Scholar]
- Pickering W, Cheng MK, Brown J, Butler H, Walls J and Bevington A (2003) Stimulation of protein degradation by low pH in L6G8C5 skeletal muscle cells is independent of apoptosis but dependent on differentiation state. Nephrol Dial Transplant, 18, 1466–1474. [DOI] [PubMed] [Google Scholar]
- Porter K and Hayward LF (2011) Stress-induced changes in c-Fos and corticotropin releasing hormone immunoreactivity in the amygdala of the spontaneously hypertensive rat. Behav Brain Res, 216, 543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratka A, Sutanto W, Bloemers M and de Kloet ER (1989) On the role of brain mineralocorticoid (type I) and glucocorticoid (type II) receptors in neuroendocrine regulation. Neuroendocrinology, 50, 117–123. [DOI] [PubMed] [Google Scholar]
- Ressler KJ and Nemeroff CB (1999) Role of norepinephrine in the pathophysiology and treatment of mood disorders. Biol Psychiatry, 46, 1219–1233. [DOI] [PubMed] [Google Scholar]
- Robbins T and Everitt B (1995) Central norepinephrine neurons and behavior In: Neuropsychopharmacology: the fourth generation of progress, (Bloom Fand Kupfer D eds.). Raven Press, New York. [Google Scholar]
- Roceri M, Cirulli F, Pessina C, Peretto P, Racagni G and Riva MA (2004) Postnatal repeated maternal deprivation produces age-dependent changes of brain-derived neurotrophic factor expression in selected rat brain regions. Biol Psychiatry, 55, 708–714. [DOI] [PubMed] [Google Scholar]
- Sabban EL, Hebert MA, Liu X, Nankova B and Serova L (2004) Differential effects of stress on gene transcription factors in catecholaminergic systems. Ann N Y Acad Sci, 1032, 130–140. [DOI] [PubMed] [Google Scholar]
- Santana MM, Rosmaninho-Salgado J, Cortez V, Pereira FC, Kaster MP, Aveleira CA, Ferreira M, Alvaro AR and Cavadas C (2015) Impaired adrenal medullary function in a mouse model of depression induced by unpredictable chronic stress. Eur Neuropsychopharmacol, 25, 1753–1766. [DOI] [PubMed] [Google Scholar]
- Sara SJ (2009) The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci, 10, 211–223. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Kanai M, Kijima K et al. (2003) Molecular analysis of congenital central hypoventilation syndrome. Hum Genet, 114, 22–26. [DOI] [PubMed] [Google Scholar]
- Schahab S, Heun R, Schmitz S, Maier W and Kolsch H (2006) Association of polymorphism in the transcription factor LBP-1c/CP2/LSF gene with Alzheimer’s disease and major depression. Dement Geriatr Cogn Disord, 22, 95–98. [DOI] [PubMed] [Google Scholar]
- Seo H, Hong SJ, Guo S, Kim HS, Kim CH, Hwang DY, Isacson O, Rosenthal A and Kim KS (2002) A direct role of the homeodomain proteins Phox2a/2b in noradrenaline neurotransmitter identity determination. J Neurochem, 80, 905–916. [DOI] [PubMed] [Google Scholar]
- Serova LI, Nankova BB, Feng Z, Hong JS, Hutt M and Sabban EL (1999) Heightened transcription for enzymes involved in norepinephrine biosynthesis in the rat locus coeruleus by immobilization stress. Biol Psychiatry, 45, 853–862. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Liang S, Liang P, Chakrabarti A, Manier DH and Sulser F (2004) Differential expression of pentraxin 3 in fibroblasts from patients with major depression. Neuropsychopharmacology, 29, 126–132. [DOI] [PubMed] [Google Scholar]
- Smith MA, Brady LS, Glowa J, Gold PW and Herkenham M (1991) Effects of stress and adrenalectomy on tyrosine hydroxylase mRNA levels in the locus ceruleus by in situ hybridization. Brain Res, 544, 26–32. [DOI] [PubMed] [Google Scholar]
- Son GH, Geum D, Jung H and Kim K (2001) Glucocorticoid inhibits growth factorinduced differentiation of hippocampal progenitor HiB5 cells. J Neurochem, 79, 10131021. [DOI] [PubMed] [Google Scholar]
- Spasojevic N, Gavrilovic L and Dronjak S (2010) Effects of repeated maprotiline and fluoxetine treatment on gene expression of catecholamine synthesizing enzymes in adrenal medulla of unstressed and stressed rats. Autonomic & autacoid pharmacology, 30, 213–217. [DOI] [PubMed] [Google Scholar]
- Stanke M, Junghans D, Geissen M, Goridis C, Ernsberger U and Rohrer H (1999) The Phox2 homeodomain proteins are sufficient to promote the development of sympathetic neurons. Development, 126, 4087–4094. [DOI] [PubMed] [Google Scholar]
- Sulser F (2002) The role of CREB and other transcription factors in the pharmacotherapy and etiology of depression. Ann Med, 34, 348–356. [DOI] [PubMed] [Google Scholar]
- Sun Z, Fan Y, Zha Q and Zhu MY (2010) Corticosterone up-regulates expression and function of norepinephrine transporter in SK-N-BE(2)C cells. J Neurochem, 113, 105116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SA, Matsumoto AM and Palmiter RD (1995) Noradrenaline is essential for mouse fetal development. Nature, 374, 643–646. [DOI] [PubMed] [Google Scholar]
- Tiveron MC, Hirsch MR and Brunet JF (1996) The expression pattern of the transcription factor Phox2 delineates synaptic pathways of the autonomic nervous system. J Neurosci, 16, 7649–7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiveron MC, Pattyn A, Hirsch MR and Brunet JF (2003) Role of Phox2b and Mash1 in the generation of the vestibular efferent nucleus. Dev Biol, 260, 46–57. [DOI] [PubMed] [Google Scholar]
- Vogel-Hopker A and Rohrer H (2002) The specification of noradrenergic locus coeruleus (LC) neurones depends on bone morphogenetic proteins (BMPs). Development, 129, 983–991. [DOI] [PubMed] [Google Scholar]
- Wang P, Kitayama I and Nomura J (1998) Tyrosine hydroxylase gene expression in the locus coeruleus of depression-model rats and rats exposed to short-and long-term forced walking stress. Life Sci, 62, 2083–2092. [DOI] [PubMed] [Google Scholar]
- Wang Y, Musich PR, Serrano MA, Zou Y, Zhu MY (2013) Neurotoxin DSP4 induces persistent DNA damage in neuroblastoma cells. . In: 15th Annual Midwest DNA Repair Symposium. Kentucky. [Google Scholar]
- Watanabe Y, McKittrick CR, Blanchard DC, Blanchard RJ, McEwen BS and Sakai RR (1995) Effects of chronic social stress on tyrosine hydroxylase mRNA and protein levels. Brain Res Mol Brain Res, 32, 176–180. [DOI] [PubMed] [Google Scholar]
- Wong ML, Kling MA, Munson PJ et al. (2000) Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proc Natl Acad Sci U S A, 97, 325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang L, Szebeni K, Stockmeier C, Roth BL, Overholser JC, Jurjus G and Ordway G (2004) Fos-related antigen-2 (Fra-2) gene expression in the locus coeruleus is elevated in major depression In: Society for Neuroscience, pp. Abstract 799.711. Washington, DC. [Google Scholar]
- Xiao F, Puddefoot JR and Vinson GP (2000) Aldosterone mediates angiotensin IIstimulated rat vascular smooth muscle cell proliferation. J Endocrinol, 165, 533–536. [DOI] [PubMed] [Google Scholar]
- Xicoy H, Wieringa B and Martens GJ (2017) The SH-SY5Y cell line in Parkinson’s disease research: a systematic review. Molecular neurodegeneration, 12, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Yamamoto M, Ozawa H, Riederer P and Saito T (2003) Reduced phosphorylation of cyclic AMP-responsive element binding protein in the postmortem orbitofrontal cortex of patients with major depressive disorder. J Neural Transm (Vienna), 110, 671–680. [DOI] [PubMed] [Google Scholar]
- Yang C, Kim HS, Seo H, Kim CH, Brunet JF and Kim KS (1998a) Paired-like homeodomain proteins, Phox2a and Phox2b, are responsible for noradrenergic cellspecific transcription of the dopamine beta-hydroxylase gene. J Neurochem, 71, 18131826. [DOI] [PubMed] [Google Scholar]
- Yang C, Kim HS, Seo H and Kim KS (1998b) Identification and characterization of potential cis-regulatory elements governing transcriptional activation of the rat tyrosine hydroxylase gene. J Neurochem, 71, 1358–1368. [DOI] [PubMed] [Google Scholar]
- Yazdani A, Chung DC, Abbaszadegan MR, Al-Khayer K, Chan WM, Yazdani M, Ghodsi K, Engle EC and Traboulsi EI (2003) A novel PHOX2A/ARIX mutation in an Iranian family with congenital fibrosis of extraocular muscles type 2 (CFEOM2). Am J Ophthalmol, 136, 861–865. [DOI] [PubMed] [Google Scholar]
- Zha Q, Wang Y, Fan Y and Zhu MY (2011) Dexamethasone-induced up-regulation of the human norepinephrine transporter involves the glucocorticoid receptor and increased binding of C/EBP-beta to the proximal promoter of norepinephrine transporter. J Neurochem, 119, 654–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MY, Kim CH, Hwang DY, Baldessarini RJ and Kim KS (2002) Effects of desipramine treatment on norepinephrine transporter gene expression in the cultured SKN-BE(2)M17 cells and rat brain tissue. J Neurochem, 82, 146–153. [DOI] [PubMed] [Google Scholar]
- Zhu MY, Klimek V, Dilley GE, Haycock JW, Stockmeier C, Overholser JC, Meltzer HY and Ordway GA (1999) Elevated levels of tyrosine hydroxylase in the locus coeruleus in major depression. Biol Psychiatry, 46, 1275–1286. [DOI] [PubMed] [Google Scholar]
- Zhu MY, Wang WP, Huang J and Regunathan S (2007) Chronic treatment with glucocorticoids alters rat hippocampal and prefrontal cortical morphology in parallel with endogenous agmatine and arginine decarboxylase levels. J Neurochem, 103, 1811–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MY, Wang WP, Iyo AH, Ordway GA and Kim KS (2005) Age-associated changes in mRNA levels of Phox2, norepinephrine transporter and dopamine betahydroxylase in the locus coeruleus and adrenal glands of rats. J Neurochem, 94, 828–838. [DOI] [PMC free article] [PubMed] [Google Scholar]