ABSTRACT
The present review highlights the idea that antioxidant supplementation can be optimized when tailored to the precise antioxidant status of each individual. A novel methodologic approach involving personalized nutrition, the mechanisms by which antioxidant status regulates human metabolism and performance, and similarities between antioxidants and other nutritional supplements are described. The usefulness of higher-level phenotypes for data-driven personalized treatments is also explained. We conclude that personally tailored antioxidant interventions based on specific antioxidant inadequacies or deficiencies could result in improved exercise performance accompanied by consistent alterations in redox profile.
Keywords: antioxidants, exercise, glutathione, oxidative stress, personalized nutrition, redox phenotyping, stratification, vitamin C
Introduction
Antioxidant supplements have been placed over the years at both “ends” of the exercise nutrition field and have been characterized from absolutely necessary to totally detrimental (1, 2). Approximately 35 y ago, when the first reports about reactive oxygen, nitrogen, and sulfur species (referred to as reactive species) production during exercise appeared, antioxidant supplements were considered essential in order to combat the “damaging” oxidative stress induced by exercise (1). However, during the past decade, antioxidant supplements have been considered a “villain” in the exercise nutrition field (2). In fact, many original studies and review articles have strongly supported the view that antioxidant supplementation should be discouraged during exercise training, because it leads to diminished molecular, biochemical, and physiologic adaptations (3, 4).
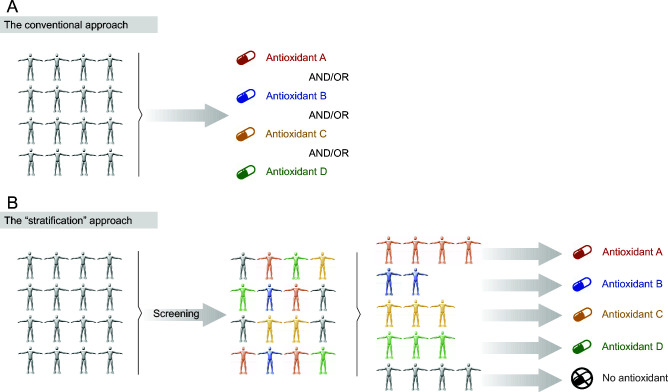
In a series of studies conducted by our group we found that large redox interindividual variability exists both at rest and in response to acute exercise (5–10). This variability was substantiated in both antioxidant (i.e., glutathione, vitamin C) and oxidative stress (i.e., F2-isoprostanes, protein carbonyls) biomarkers. We asserted that this redox heterogeneity among individuals might actually explain the equivocal findings with regard to the effectiveness of antioxidants as possible ergogenic aids. More specifically, antioxidant treatments have been typically applied to young, healthy individuals with normal concentrations of antioxidants or oxidative stress biomarkers (11–14). Therefore, the participants recruited may not have been derived from the appropriate cohort to experience any potential benefit from the antioxidant treatments. On the basis of this idea, we exploited an emerging practice in biomedical research, known as stratified purposive sampling, and tried to identify those individuals who would be more likely to benefit from the treatment. Figure 1 summarizes the central idea of our approach. In particular, contrary to the conventional strategy (Figure 1A), which is characterized by the indiscriminate use of antioxidants (either a single antioxidant or a combination of antioxidants) by all individuals, we herein propose a “stratified” approach (Figure 1B) based on the redox profile of the individuals. According to this profile, the antioxidant in deficiency should be administered in each participant individually. Built on this “data-driven” participant recruitment, we hereby argue that the effects of antioxidant supplements on exercise responses and adaptations depend on the baseline redox status of each individual, and consequently, the ergogenic effects of antioxidant supplements are evident only in individuals with low baseline concentrations of antioxidants or high baseline levels of oxidative stress.
FIGURE 1.
The conventional (A) and the novel “stratification” (B) approach with regard to antioxidant supplementation. The conventional approach is characterized by the indiscriminate administration of antioxidants irrespective of the redox profile of the individual. On the contrary, the stratified approach aims to identify potential antioxidant deficiencies in order to tailor the most suitable treatment (if needed).
In light of the above, the main objectives of the present article are as follows: 1) to highlight the necessity for more personalized approaches in exercise studies utilizing antioxidant supplements and 2) to present a novel methodologic strategy—namely, the stratification of individuals on the basis of their antioxidant profile (i.e., redox phenotyping) in order to identify the most “susceptible” individuals and to exclude those who are not in need of treatment or, even worse, those for whom an otherwise beneficial treatment will probably have a harmful effect. Secondary aims of the article are 1) to describe the mechanisms through which antioxidant supplements may exert their ergogenic potential in individuals with a disturbed redox profile and 2) to present possible applications in sports nutrition and to draw similarities to other nutritional supplements whose effectiveness has also been a matter of debate.
Current Status of Knowledge
Antioxidant supplements: panacea, deleterious, or neutral?
It is increasingly recognized that the reactive species produced during exercise are essential signaling molecules driving exercise adaptations, such as mitochondrial biogenesis, angiogenesis, and neurogenesis, leading to increased physical performance (3, 7, 15–18). Our knowledge on the role of reactive species in exercise responses and adaptations has been acquired through diverse in vitro, ex vivo, in situ, and in vivo experiments (3, 19–22). The vast majority of the relevant studies (and actually all in vivo studies) have utilized agents with purported antioxidant properties. According to the current consensus, antioxidant supplementation either does not affect exercise adaptations (11, 12) or blocks the beneficial effects of reactive species, leading to a more “reductive” state than the optimal state, resulting thereby in hampered exercise adaptations (23). Similar negative or neutral effects of antioxidant supplementation have been observed in the progression of cancer and diabetes and in increased mortality (24–26). As a result, currently, antioxidant supplements have a negative reputation in the nutrition field and biomedicine in general.
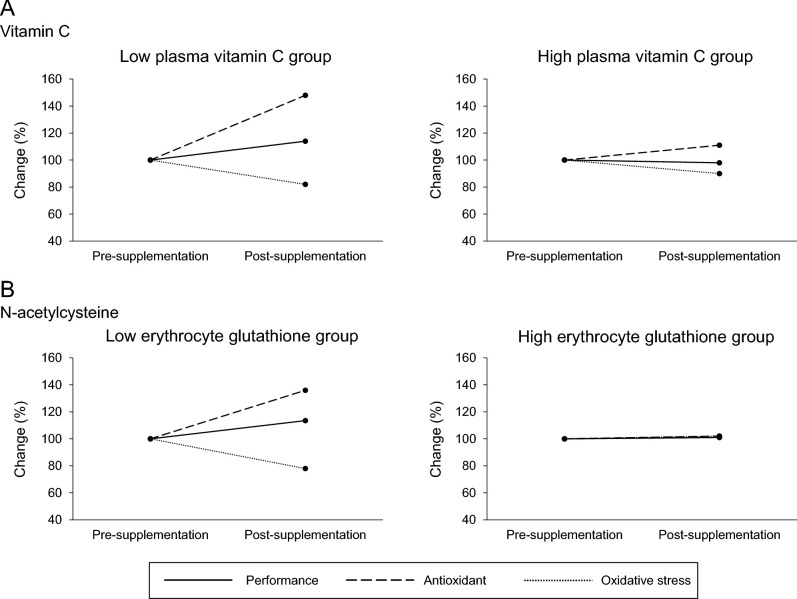
In our first study on the topic, we showed a large interindividual variability in redox (both antioxidant and oxidative stress) responses after acute exercise among 100 participants and argued that this heterogeneity was partially determined by the baseline values of biomarkers measured (5). In a subsequent study, we showed that this redox individuality, assessed through the exercise-induced changes in the levels of the reference oxidative stress biomarker (i.e., F2-isoprostanes), partially predicts aerobic and anaerobic trainability (7). On the basis of this documented redox variability, we hypothesized that baseline antioxidant concentrations could also affect exercise physiology and nutrition outcomes. In order to examine this hypothesis, we conducted a study in 100 individuals screened for plasma vitamin C concentrations, and subsequently, 2 groups (10 individuals/group) were formed: one with the highest (78 ± 11 μmol/L) and one with the lowest (35 ± 8 μmol/L) plasma vitamin C concentrations. After supplementing both groups with 1 g vitamin C/d for 1 mo, we observed that the “low” vitamin C group exhibited a marginally significant increase in maximal oxygen uptake (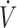 O2max) equal to 14% (P = 0.079), along with a concomitant decrease in baseline oxidative stress (i.e., −18% in urine F2-isoprostanes and −23% in plasma protein carbonyls) (8). In contrast, no changes were observed in the “high” vitamin C group after supplementation. By implementing the same methodologic strategy, we investigated the effect of N-acetylcysteine (NAC) supplementation (i.e., 1.2 g NAC/d for 1 mo) on 3 whole-body exercise tests evaluating aerobic and anaerobic capacity (i.e.,
O2max) equal to 14% (P = 0.079), along with a concomitant decrease in baseline oxidative stress (i.e., −18% in urine F2-isoprostanes and −23% in plasma protein carbonyls) (8). In contrast, no changes were observed in the “high” vitamin C group after supplementation. By implementing the same methodologic strategy, we investigated the effect of N-acetylcysteine (NAC) supplementation (i.e., 1.2 g NAC/d for 1 mo) on 3 whole-body exercise tests evaluating aerobic and anaerobic capacity (i.e., 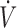 O2max, time trial, and Wingate tests) in groups of individuals with low (2.05 μmol/g hemoglobin), moderate (3.06 μmol/g hemoglobin), and high (3.96 μmol/g hemoglobin) resting erythrocyte reduced glutathione (GSH) levels (9). We found that NAC supplementation improved both aerobic and anaerobic capacity only in the “low” GSH group (i.e., +13.6% in
O2max, time trial, and Wingate tests) in groups of individuals with low (2.05 μmol/g hemoglobin), moderate (3.06 μmol/g hemoglobin), and high (3.96 μmol/g hemoglobin) resting erythrocyte reduced glutathione (GSH) levels (9). We found that NAC supplementation improved both aerobic and anaerobic capacity only in the “low” GSH group (i.e., +13.6% in 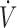 O2max, +15.4% in time trial, and +11.4% in Wingate; P < 0.05 for all tests), whereas an improvement in Wingate was also observed in the “moderate” GSH group (i.e., +10.4%; P < 0.05). Interestingly, an adverse effect was found in the “high” GSH group after NAC supplementation in time trial performance (i.e., −3.5%; P < 0.05) (9). Collectively, it can be reasonably assumed that the ergogenic effects of antioxidant supplements appear only when a relevant “deficiency” or inadequacy exists in the population under study. Otherwise, either neutral or even negative effects may be experienced (Figure 2). These findings could be interpreted by the hormesis concept, according to which there is an optimal range where reactive species exert their favorable effects on exercise performance (27). In addition, our findings are also in line with the very recent International Olympic Committee consensus statement on dietary supplements and performance, which underlined that some supplements exert their ergogenic effects by correcting nutrient deficiencies and reverse the associated impairment of health, training capacity, or performance (28).
O2max, +15.4% in time trial, and +11.4% in Wingate; P < 0.05 for all tests), whereas an improvement in Wingate was also observed in the “moderate” GSH group (i.e., +10.4%; P < 0.05). Interestingly, an adverse effect was found in the “high” GSH group after NAC supplementation in time trial performance (i.e., −3.5%; P < 0.05) (9). Collectively, it can be reasonably assumed that the ergogenic effects of antioxidant supplements appear only when a relevant “deficiency” or inadequacy exists in the population under study. Otherwise, either neutral or even negative effects may be experienced (Figure 2). These findings could be interpreted by the hormesis concept, according to which there is an optimal range where reactive species exert their favorable effects on exercise performance (27). In addition, our findings are also in line with the very recent International Olympic Committee consensus statement on dietary supplements and performance, which underlined that some supplements exert their ergogenic effects by correcting nutrient deficiencies and reverse the associated impairment of health, training capacity, or performance (28).
FIGURE 2.
The differential effects of antioxidant supplementation on physical performance (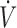 O2max), oxidative stress (F2-isoprostanes), and antioxidant concentrations [vitamin C (A) and glutathione (B)] according to baseline antioxidant concentration. The 100% level corresponds to the presupplementation values. Values are based on data from references 8 and 9.
O2max), oxidative stress (F2-isoprostanes), and antioxidant concentrations [vitamin C (A) and glutathione (B)] according to baseline antioxidant concentration. The 100% level corresponds to the presupplementation values. Values are based on data from references 8 and 9.
Researchers outside the exercise nutrition field have also asserted that the effectiveness of antioxidant supplements to decrease oxidative stress and promote health depends on the baseline redox status (29–34). For instance, Block et al. (29) showed that the beneficial effects of vitamin C or E supplementation on plasma F2-isoprostane concentrations are genuine, yet limited only to individuals with high baseline oxidative stress levels (29). On this basis, the authors proposed the concentration of 50 μg F2-isoprostanes/mL in plasma as a “cutoff point” for enrollment eligibility in studies using antioxidants. Similarly, Dow et al. (30) showed that a 6-wk antioxidant treatment reduced urine F2-isoprostane concentrations only in overweight adults with high baseline values (30). The importance of baseline oxidative stress on the effectiveness of antioxidants in translational medicine has also been highlighted in commentaries and reviews (31–34). Some authors have proposed that the “antioxidant paradox” (i.e., the lack of therapeutic effect of antioxidant supplements despite the fact that reactive species are involved in several human diseases) may be explained by the enrollment of individuals with normal levels of oxidative stress in clinical trials (35).
An important methodologic distinction should be made between these studies and ours. The vast majority (if not all) of the previous studies have focused on generic oxidative stress biomarkers for stratifying participants (e.g., low or high baseline F2-isoprostane concentrations) (36). We, instead, focused on specific antioxidant inadequacies (i.e., vitamin C and GSH) and attempted to reverse the specific aberrant nutritional status in each case by restoring the concentrations of the antioxidant in deficiency (i.e., supplementation with vitamin C and NAC, respectively). In other words, we followed a “targeted” supplementation strategy instead of administering antioxidants indiscriminately in order to combat a generically defined state of “oxidative stress.” Collectively, it seems that the effects of antioxidant supplementation (i.e., beneficial, detrimental, or neutral) on physiology and pathology largely depend on the baseline antioxidant profile of the individuals who receive the treatment, a fact that necessitates the implementation of more personalized experimental approaches in the exercise field.
Precision nutrition studies: a novel methodologic design
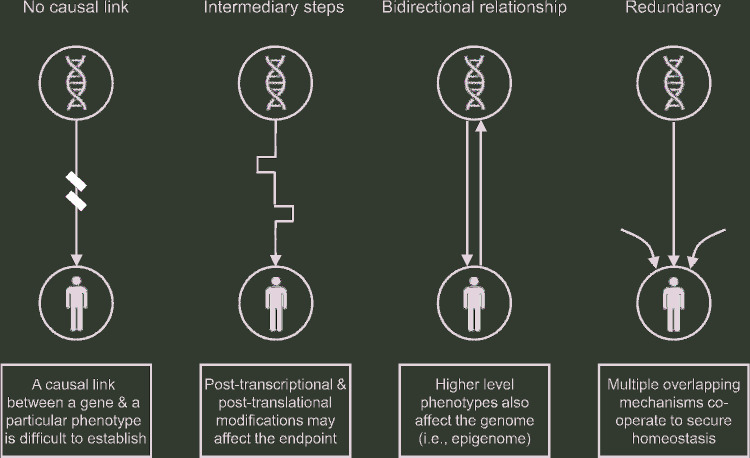
The idea to tailor the right nutrient to the right individual, at the right time point, every single time undoubtedly sounds like a fascinating prospect and is reasonably regarded as the next-generation approach in nutrition. The evaluation of variability in individuals’ responses to nutritional treatments is central to assessing potential benefits from personalized nutrition (37). Although this requirement is obvious, fulfilling it is more difficult than generally considered (38). Precision nutrition, as a facet of the general concept of precision (also known as personalized) medicine, has been predominantly built on studies that tried to identify how an individual's genetic makeup predisposes that individual to respond differently to the same nutritional intervention or supplement (i.e., nutrigenetics) (39). However, this genotype-to-phenotype-to-selection approach has been challenged, because, with very few exceptions [e.g., diagnosis of hypolactasia and phenylketonuria screening (40)], it failed to deliver its promise to revolutionize experimental and clinical treatments (41, 42). Some explanations for this failure are as follows: 1) the still unidentified causality chain between genetic makeup and nutritional effectiveness, 2) the nontransformable genetic report on nutritional properties due to the dynamic intermediary steps from genes to phenotype (e.g., post-transcriptional and post-translational modifications), and 3) the complex nature of high-level physiologic phenotypes, which manifest redundancy and cross-talk [i.e., multiple overlapping mechanisms cooperate at many levels in order to secure optimal function (43)]. Furthermore, the 2-way interaction (i.e., gene-diet interaction) seems to be what truly matters, instead of the conventional 1-way reductionist approach (i.e., from genes to diet) (42) (Figure 3).
FIGURE 3.
Four major limitations when designing genome-driven personalized nutritional treatments.
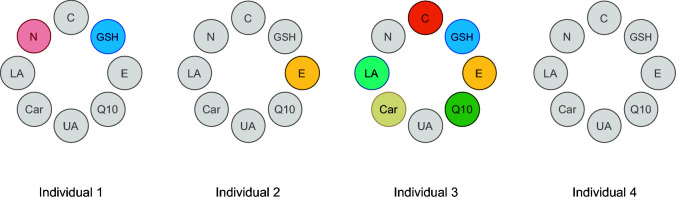
As a result, it is currently acknowledged that precision nutrition cannot be solely based on nutrigenetics (44, 45). More specifically, other factors that may determine individual responses to nutritional intakes should be taken into consideration, such as dietary and physical activity habits and the microbiome (40). It should also be noted that these higher-level phenotypes are biologically “closer” to the commonly evaluated nutritional endpoints compared with the genome and thus may be more realistic tools to design precision nutritional treatments. In this regard, and despite the fact that the genomic element is generally deemed the main driver of precision nutrition (possibly due to its more “attractive” nature), nutritional advice based on the assessment of the individual's diet or based on phenotypic markers (e.g., anthropometric measures, clinical physiologic or chemical variables, markers of nutritional status) still represents the 2 main pillars of precision nutrition (46). With regard to our studies, poor dietary habits [i.e., low vitamin C or sulfur-containing nutrient intake (47, 48)] could reasonably explain the deficiencies observed in the “low” antioxidant groups (plasma vitamin C and erythrocyte GSH, respectively), which were subsequently reversed by the targeted antioxidant supplementation. Nevertheless, a deviant genetic background cannot be ruled out, especially for the low GSH group (vitamin C cannot be synthesized in humans, thus low vitamin C concentrations are most likely attributed to low dietary intake). In fact, gene mutations or missing genes that produce the enzymes that synthesize the GSH tripeptide (i.e., glutamate-cysteine ligase, glutathione synthetase, and γ-glutamyl transpeptidase) or regulate its function (i.e., glutathione reductase, peroxidase, and S-transferase) may lead to GSH deficiency, necessitating exogenous administration or increased nutritional intake of GSH or its precursors (49, 50). It is important to note that our approach actually refers to tailored antioxidant treatments at a group level rather than at an individual level, which is in line with the widely adopted strategy to identify groups with a common phenotypic or metabolic profile and then to apply a targeted treatment. Analogous to the concept of metabolic phenotyping [or metabotyping (51)], which describes the categorization of individuals on the basis of metabolic or phenotypic characteristics into more homogeneous subgroups, we herein propose a redox (antioxidant) phenotyping. This idea can be extrapolated in the future for developing analytical tools that will offer the opportunity to rapidly screen individuals—for instance, via a capillary blood sample, for assessing their antioxidant profile to determine the antioxidant needed. Such an “idealized” tool and its potential utility (i.e., to tailor the most optimal nutritional redox treatment) is described in Figure 4. For instance, a tool developed on the basis of the idea of redox phenotyping would provide the opportunity to distinguish a person with low concentrations of GSH [e.g., glucose-6-phosphate dehydrogenase (G6PD) deficient or an individual with aberrant NAD(P)H redox metabolism; i.e., individual no. 1] from a vitamin E–deficient person (e.g., due to malnutrition; i.e., individual no. 2), or equally important, to identify individuals with a normal antioxidant status who do not need any exogenous antioxidant supplement (individual no. 4). Taking into account that oxidative stress is widely regarded as an underlying mechanism of several diseases and a factor aggravating a pre-existing pathology (52, 53), the administration of the right antioxidant in each case seems imperative. Of course, many mechanistic details should be taken into consideration when integrating reactive species into the pathophysiology of a disease (54); yet, a clinical point-of-care tool designed to identify specific antioxidant deficiencies in each patient individually would be useful for more successful antioxidant therapies.
FIGURE 4.
An “idealized” analytical tool to assess (e.g., via a capillary blood sample) an individual's systemic antioxidant profile in order to tailor the most optimal nutritional redox treatment. Individual 1 represents a person with low concentrations of GSH and NAD(P)H [e.g., due to dysregulated NAD(P)H redox metabolism]; individual 2 represents a vitamin E–deficient person (e.g., due to malnutrition); individual 3 represents a person with a highly disturbed antioxidant profile (e.g., due to severe illness); individual 4 represents an apparently healthy person with normal antioxidant status who does not need any exogenous antioxidant supplement. C, vitamin C; Car, carotene; E, vitamin E; GSH, reduced glutathione; LA, lipoic acid; N, NAD(P)H; Q10, coenzyme Q10; UA, uric acid.
With regard to exercise, to the best of our knowledge, we are unaware of any study that specifically addressed the issue of personalized responses to a specific diet or nutritional supplement. To fill this gap, the stratified purposive sampling of participants on the basis of a specific biological phenotype (considered critical for the objective of each study) seems a promising experimental design (55). The stratified purposive sampling is a nonprobability sampling methodologic technique, where the key goal is to identify and enroll “information-rich” subjects for an a priori–defined characteristic from a larger population. The stratification approach typically generates 2 (i.e., “above” and “below” a predefined threshold) or 3 (i.e., “low,” “moderate,” and “high”) strata of the biological trait (i.e., “classifier”) under study. Thus, the distinct groups formed are expected to capture a wide range of conditions (including the extremes and the average), which provides the opportunity to gain a comprehensive understanding of the topic under investigation using only part of the population. By implementing the aforementioned approach, we have shown that 2 different redox supplements, an antioxidant (i.e., vitamin C) and a precursor of an antioxidant (i.e., NAC as a cysteine donor for GSH synthesis), exert ergogenic effects only in individuals with low baseline antioxidant concentrations (8, 9). On the contrary, neutral or even detrimental effects may be seen in individuals with moderate or high baseline antioxidant concentrations. Our studies focused only on 2 well-investigated antioxidants and thus future studies are warranted that will “scan for” and “identify” additional, and potentially multiple and coexistent, antioxidant deficiencies that may necessitate targeted antioxidant treatments. On this basis, the stratification approach may also facilitate the creation of data sets summarizing the redox characteristics of the individuals who will probably not respond favorably to a nutritional treatment (i.e., identification of “nonresponders” or “low responders”).
The stratification approach is not without disadvantages. When trying to identify the most suitable subjects for enrollment in a study, the initial screening of the classifier trait requires a large pool of available participants. This becomes more demanding when the normal (optimal) values of the classifier trait is unspecified, because, despite the advances in analytical chemistry (56–58), there are no reference intervals for most redox biomarkers. This is largely due to the numerous available analytical techniques and assays used for the determination of the biomarkers, which yield noncomparable values (59, 60). Thus, the initial screening of the pool should delineate the range of variation in the classifier trait in order to form the distinct groups.
Another disadvantage of the stratification approach is the methodologic artifact known as “regression to the mean”: individuals with an extreme initial value in a specific trait are typically prone to exhibit a less extreme value (i.e., tend toward the mean) in a subsequent measurement (6, 61, 62). Hence, regression to the mean may become a major problem in studies in which participants are stratified on the basis of an extreme initial value, such as the antioxidant-insufficient individuals in our case. Several methodologic and statistical approaches have been proposed to bypass or reduce the impact of this artifact (61, 63, 64). In our studies, we addressed regression to the mean by performing a duplicate pretreatment measurement, using the second one as a more realistic baseline value of the groups (7, 9). The key idea of this approach is based on the fact that the impact of regression to the mean predominantly takes place between the first and the second measurement (65). Certainly, this duplicate pretreatment measurement increases the amount of work required. Yet, on the bright side, the stratification approach represents a novel methodologic strategy that allows to distinguish individualized responses to antioxidant and nutritional supplementation in general.
How does redox status affect physical performance?
In our studies, the “low” antioxidant group (i.e., the individuals with the lowest antioxidant concentrations) exhibited inferior physical performance at baseline compared with the “moderate” and “high” antioxidant groups (8, 9). More specifically, the “low” plasma vitamin C group (35 ± 8 μmol/L) had ∼21% lower 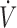 O2max compared with the “high” vitamin C group (78 ± 11 μmol/L) (8). Likewise, the “low” erythrocyte GSH group (2.05 μmol/g hemoglobin) exhibited ∼14% lower
O2max compared with the “high” vitamin C group (78 ± 11 μmol/L) (8). Likewise, the “low” erythrocyte GSH group (2.05 μmol/g hemoglobin) exhibited ∼14% lower 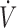 O2max compared with both “moderate” (3.06 μmol/g hemoglobin) and “high” (3.96 μmol/g hemoglobin) GSH groups, whereas the performance in the time trial was ∼17% lower than that in the “high” GSH group (9). However, all of these features almost disappeared after the 1-mo antioxidant (i.e., vitamin C or NAC) supplementation period.
O2max compared with both “moderate” (3.06 μmol/g hemoglobin) and “high” (3.96 μmol/g hemoglobin) GSH groups, whereas the performance in the time trial was ∼17% lower than that in the “high” GSH group (9). However, all of these features almost disappeared after the 1-mo antioxidant (i.e., vitamin C or NAC) supplementation period.
An interesting question that emerges from the aforementioned data is how an aberrant antioxidant status can affect physical performance. Antioxidant molecules do not work independently of one other; instead, they represent a part of a greater whole, which includes enzymatic and nonenzymatic mechanisms (66). That is, each antioxidant molecule interacts, supports, and is supported by other molecules. Thus, low concentrations of an antioxidant, due to poor dietary habits for example, may compromise the entire biochemical pathway that involves this antioxidant. Indeed, when we analyzed our redox data from the “low” GSH group, we noticed that many molecules in the GSH-dependent redox metabolism pathway were affected, such as the concentration of the reductive substrate NAD(P)H and the activity of many antioxidant enzymes (i.e., glutathione peroxidase and reductase, superoxide dismutase, and catalase) (9). This indicates that there is a disturbed flux in the whole antioxidant buffering machinery, which results in increased systemic oxidative stress levels (as shown by the increased resting values of urine F2-isoprostanes and plasma protein carbonyls). Interestingly, NAC supplementation not only increased GSH production but also upregulated the flux in the abovementioned redox pathway (9). Collectively, a nutritional antioxidant insufficiency may lead to a dysregulation of a whole redox metabolic network (9).
Two possible mechanisms whereby an aberrant redox homeostasis may affect physical performance are the redox control of cell signaling and the redox control of energy metabolism. With regard to cell signaling, mounting evidence suggests that antioxidant enzymes act as key “nodes” (e.g., sensors or transmitters) that fine-tune redox signaling triggered by reactive species (67, 68). In fact, antioxidant enzymes exhibit a high selectivity against reactive species featuring signaling properties, such as hydrogen peroxide (H2O2) and NO (19, 69), while at the same time they are kinetically favored (up to 107-fold higher rate constants) compared with other low-molecular-weight antioxidants (e.g., GSH, vitamins C and E) (70, 71). Although the precise biochemical cascades have yet to be defined and remain largely debatable, 2 proposed mechanisms have received the greatest attention with regard to how antioxidant enzymes control redox signaling and, more specifically, how they facilitate the selective reversible reaction between a precise reactive species (e.g., hydrogen peroxide) and the active cysteine residue of a target signaling protein [e.g., Kelch-like ECH-associated protein 1 (KEAP1)] (72, 73). The first mechanism is known as the “redox relay.” According to this mechanism, antioxidant enzymes “transfer” the oxidizing equivalents to the downstream effector protein. For instance, it has been shown that peroxiredoxin-2 acts as a redox receptor and transmitter (i.e., as a “relay”) for the transcription factor signal transducer and activator of transcription 3 (STAT3) signaling (74). The second mechanism is known as the “floodgate model.” According to this model, the reactive species (i.e., hydrogen peroxide) sequesters or inactivates the antioxidant enzyme (i.e., peroxiredoxin), thereby flooding the area, and subsequently oxidizes the cysteine residues in the target protein (75).
With regard to cell metabolism, a wide spectrum of redox-dependent post-translational modifications has been described that modulate key aspects of cellular metabolic fluxes, many of them tightly linked with exercise metabolism (76, 77). It is now well established that redox processes coordinate the regulation (i.e., activation or inactivation) of kinases and phosphatases that catalyze the (de)phosphorylation of proteins, thereby controlling the metabolic fate of cells. For instance, reactive species activate several receptor and nonreceptor tyrosine kinases and phosphatases, such as the protein kinase C, Rho-kinase, and mitogen-activated protein kinases, as well as the Src homology-2 domain-containing phosphatase 2, phosphatase and tensin homolog, 5′ AMP-activated protein kinase, c-Jun amino-terminal kinases, and extracellular signal–regulated kinases (78–84). Along with these enzymes, compartmentalized and reversible redox reactions also regulate, either directly (i.e., by oxidative modifications) or indirectly (e.g., via intermediate tyrosine kinases), the activity of fundamental and ubiquitous transcription factors and coactivators, such as peroxisome proliferator-activated receptor γ coactivator 1α (PGC1-α), nuclear factor (erythroid-derived 2)–like 2 (Nrf2), and NF-κB (67, 85). These transcription regulators have been implicated in diverse exercise-induced adaptations, such as mitochondrial biogenesis and angiogenesis (86–89). With regard to energy metabolism, several signaling pathways have been described to regulate glucose uptake during exercise, predominantly by controlling glucose transporter 4 (GLUT4) trafficking (90). Among the diverse upstream signals, reactive species have been shown to control, in part, this process. More specifically, mounting evidence exists about the key role of NO (the parent reactive nitrogen species) in the contraction-induced glucose uptake, whereas in vitro and ex vivo (although not in vivo) data support a role for reactive oxygen species in this process as well (91–93). Finally, it is now well known that the activity of many enzymes depends on their oxidation state. Taking into account that some of these enzymes, such as creatine kinase (94), play a pivotal role in energy utilization and recycling, a disturbed redox state has strong implications in energy production during exercise.
Taking into account that the inhibition of all of the aforementioned signaling pathways is as an important event as is their induction (19), the vital role of antioxidants (especially of enzymes) in fine-tuning these processes becomes clear (95). As a result, it is reasonable to argue that an aberrant flux in an antioxidant pathway (as was the case in our GSH-related pathway in the “low” GSH group) may result in deviant cell signaling and impaired energy metabolism, especially under conditions of increased stress (e.g., during exercise).
Similarities to other nutrition supplements
Analogous to our concept on the effectiveness of antioxidant supplementation (i.e., restricted to individuals with low baseline antioxidant concentrations), some authors have proposed a similar idea for other nutritional supplements as well. Two representative examples are vitamins E and D and their efficacy to reduce molecular and biochemical deregulation and reverse an adverse condition observed at the physiologic level.
Vitamin E (typically seen in the literature in the α-tocopherol form) is a well-described lipophilic antioxidant and regulator of cellular metabolism (96). It has been previously reported that the vast majority of adult individuals, sometimes up to 80–90% (97), do not consume sufficient amounts of dietary vitamin E and fail to meet the Estimated Average Requirements. As a result, vitamin E inadequacy or deficiency is a common phenomenon in humans and has been linked to a wide array of symptoms, such as anemia, increased risk of infection, impaired cognitive function, and developmental maladies (34). Mechanistic studies, mainly in animal models (e.g., rat and zebrafish), have shown that long-term low concentratons of vitamin E are associated with dysregulated energy metabolism and neurological function, suboptimal tissue lipid profile, aberrant mitochondrial function, and extensive tissue damage due to the prolonged lipid peroxidation (mostly of membrane PUFAs) (34, 98, 99). It is noteworthy that it has been shown that long-lasting impairment of redox homeostasis and cellular metabolism due to vitamin E deficiency may sometimes persist even after vitamin E remediation through diet or supplementation, whereas extreme situations of deficiency have been linked to embryonic death (100, 101). On the bright side, supplements providing several orders of magnitude greater vitamin E intakes increase steady-state vitamin E concentrations, reverse complex comorbidities, and lead to health benefits (34). However, the potential benefits of long-term and excessive intake of vitamin E beyond the optimal levels have been a matter of debate (102, 103).
Similar to vitamin E, vitamin D deficiency and inadequacy are widespread among both athletes and the general population (104, 105). Vitamin D has received great attention during the past decade mainly due to the resurgence of the comorbidities associated with vitamin D deficiency. In fact, low concentrations of vitamin D have been associated with impaired muscle function, disturbed immune function, poor bone health, and aberrant cardiovascular function (105, 106). Most of these data come from epidemiologic studies and the causal link is lacking. Yet, a recent study has shown that vitamin D is essentially implicated in skeletal muscle regeneration and that the maintenance of optimal vitamin D concentrations is critical for keeping the best regenerative capacity during recovery after eccentric exercise and this may facilitate the hypertrophic response (107). On the other hand, like the use of antioxidants, chronic high-dose vitamin D supplementation not only does not convey further beneficial effects, it may instead become detrimental for its targeted purposes (108). Collectively, maintaining optimal concentrations of vitamins D and E seems imperative for diverse biological phenotypes, although the provision of larger amounts is at best neutral (if not detrimental).
Conclusions
We have shown that personally tailored antioxidant interventions based on specific antioxidant inadequacies or deficiencies result in improved exercise performance accompanied by consistent alterations in the redox profile (8, 9). Despite any possible limitations, current empirical data offer hope of the promise of stratified nutrition on the basis of phenotype (and not necessarily genotype). This “targeted” redox framework may have important ramifications in the antioxidant supplementation strategies used by athletes (e.g., guarantee of optimal antioxidant concentrations during competition) and in other population cohorts as well (e.g., in a clinical setting). In a broader perspective, the use of higher level biological phenotypes phenotypes, which are biologically closer to the target endpoint, to predict or modulate the most suitable intervention [e.g., toward personalized nutrition (37)] is emerging as a promising strategy in research and everyday practice (109). As Halliwell (110) provocatively stated, “Perhaps, we should only test the effects of antioxidants on the most ‘rancid’ people, who may be those at greatest risk of disease.” We herein tried to refine this idea by suggesting that for each “rancid” individual under oxidative stress, the specific antioxidant deficiency or deficiencies have to be revealed and the appropriate antioxidant or antioxidants should be administered.
Acknowledgments
All authors read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: NVM, VP, AAT, AK, and MGN, no conflicts of interest.
Abbreviations used:
- GSH
reduced glutathione
- NAC
N-acetylcysteine
-
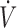 O2max
O2max maximal oxygen uptake
References
- 1. Packer L. Vitamin E, physical exercise and tissue damage in animals. Med Biol 1984;62(2):105–9. [PubMed] [Google Scholar]
- 2. Nikolaidis MG, Kyparos A, Spanou C, Paschalis V, Theodorou AA, Vrabas IS. Redox biology of exercise: an integrative and comparative consideration of some overlooked issues. J Exp Biol 2012;215(Pt 10):1615–25. [DOI] [PubMed] [Google Scholar]
- 3. Gomez-Cabrera MC, Salvador-Pascual A, Cabo H, Ferrando B, Viña J. Redox modulation of mitochondriogenesis in exercise. Does antioxidant supplementation blunt the benefits of exercise training? Free Radic Biol Med 2015;86:37–46. [DOI] [PubMed] [Google Scholar]
- 4. Merry TL, Ristow M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J Physiol 2016;594(18):5135–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Margaritelis NV, Kyparos A, Paschalis V, Theodorou AA, Panayiotou G, Zafeiridis A, Dipla K, Nikolaidis MG, Vrabas IS. Reductive stress after exercise: the issue of redox individuality. Redox Biol 2014;2:520–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Margaritelis NV, Theodorou AA, Paschalis V, Veskoukis AS, Dipla K, Zafeiridis A, Panayiotou G, Vrabas IS, Kyparos A, Nikolaidis MG. Experimental verification of regression to the mean in redox biology: differential responses to exercise. Free Radic Res 2016;50(11):1237–44. [DOI] [PubMed] [Google Scholar]
- 7. Margaritelis NV, Theodorou AA, Paschalis V, Veskoukis AS, Dipla K, Zafeiridis A, Panayiotou G, Vrabas IS, Kyparos A, Nikolaidis MG. Adaptations to endurance training depend on exercise-induced oxidative stress: exploiting redox inter-individual variability. Acta Physiol (Oxf) 2017(Epub ahead of print; doi: 10.1111/alpha.12898). [DOI] [PubMed] [Google Scholar]
- 8. Paschalis V, Theodorou AA, Kyparos A, Dipla K, Zafeiridis A, Panayiotou G, Vrabas IS, Nikolaidis MG. Low vitamin C values are linked with decreased physical performance and increased oxidative stress: reversal by vitamin C supplementation. Eur J Nutr 2016;55(1):45–53. [DOI] [PubMed] [Google Scholar]
- 9. Paschalis V, Theodorou AA, Margaritelis NV, Kyparos A, Nikolaidis MG. N-acetylcysteine supplementation increases exercise performance and reduces oxidative stress only in individuals with low levels of glutathione. Free Radic Biol Med 2018;115:288–97. [DOI] [PubMed] [Google Scholar]
- 10. Theodorou AA, Paschalis V, Kyparos A, Panayiotou G, Nikolaidis MG. Passive smoking reduces and vitamin C increases exercise-induced oxidative stress: does this make passive smoking an anti-oxidant and vitamin C a pro-oxidant stimulus? Biochem Biophys Res Commun 2014;454(1):131–6. [DOI] [PubMed] [Google Scholar]
- 11. Theodorou AA, Nikolaidis MG, Paschalis V, Koutsias S, Panayiotou G, Fatouros IG, Koutedakis Y, Jamurtas AZ. No effect of antioxidant supplementation on muscle performance and blood redox status adaptations to eccentric training. Am J Clin Nutr 2011;93(6):1373–83. [DOI] [PubMed] [Google Scholar]
- 12. Yfanti C, Akerström T, Nielsen S, Nielsen AR, Mounier R, Mortensen OH, Lykkesfeldt J, Rose AJ, Fischer CP, Pedersen BK. Antioxidant supplementation does not alter endurance training adaptation. Med Sci Sports Exerc 2010;42(7):1388–95. [DOI] [PubMed] [Google Scholar]
- 13. Petersen AC, McKenna MJ, Medved I, Murphy KT, Brown MJ, Della Gatta P, Cameron-Smith D. Infusion with the antioxidant N-acetylcysteine attenuates early adaptive responses to exercise in human skeletal muscle. Acta Physiol (Oxf) 2012;204(3):382–92. [DOI] [PubMed] [Google Scholar]
- 14. Michailidis Y, Karagounis LG, Terzis G, Jamurtas AZ, Spengos K, Tsoukas D, Chatzinikolaou A, Mandalidis D, Stefanetti RJ, Papassotiriou I et al. Thiol-based antioxidant supplementation alters human skeletal muscle signaling and attenuates its inflammatory response and recovery after intense eccentric exercise. Am J Clin Nutr 2013;98(1):233–45. [DOI] [PubMed] [Google Scholar]
- 15. Camera DM, Smiles WJ, Hawley JA. Exercise-induced skeletal muscle signaling pathways and human athletic performance. Free Radic Biol Med 2016;98:131–43. [DOI] [PubMed] [Google Scholar]
- 16. Mason SA, Morrison D, McConell GK, Wadley GD. Muscle redox signalling pathways in exercise: role of antioxidants. Free Radic Biol Med 2016;98:29–45. [DOI] [PubMed] [Google Scholar]
- 17. Morales-Alamo D, Calbet JA. AMPK signaling in skeletal muscle during exercise: role of reactive oxygen and nitrogen species. Free Radic Biol Med 2016;98:68–77. [DOI] [PubMed] [Google Scholar]
- 18. Radak Z, Suzuki K, Higuchi M, Balogh L, Boldogh I, Koltai E. Physical exercise, reactive oxygen species and neuroprotection. Free Radic Biol Med 2016;98:187–96. [DOI] [PubMed] [Google Scholar]
- 19. Margaritelis NV, Cobley JN, Paschalis V, Veskoukis AS, Theodorou AA, Kyparos A, Nikolaidis MG. Principles for integrating reactive species into in vivo biological processes: examples from exercise physiology. Cell Signal 2016;28(4):256–71. [DOI] [PubMed] [Google Scholar]
- 20. Katz A. Role of reactive oxygen species in regulation of glucose transport in skeletal muscle during exercise. J Physiol 2016;594(11):2787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Westerblad H, Allen DG. Emerging roles of ROS/RNS in muscle function and fatigue. Antioxid Redox Signal 2011;15(9):2487–99. [DOI] [PubMed] [Google Scholar]
- 22. Jackson MJ. Redox regulation of muscle adaptations to contractile activity and aging. J Appl Physiol (1985) 2015;119(3):163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Radak Z, Ishihara K, Tekus E, Varga C, Posa A, Balogh L, Boldogh I, Koltai E. Exercise, oxidants, and antioxidants change the shape of the bell-shaped hormesis curve. Redox Biol 2017;12:285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA 2007;297(8):842–57. [DOI] [PubMed] [Google Scholar]
- 25. Sayin VI, Ibrahim MX, Larsson E, Nilsson JA, Lindahl P, Bergo MO. Antioxidants accelerate lung cancer progression in mice. Sci Transl Med 2014;6(221):221ra15. [DOI] [PubMed] [Google Scholar]
- 26. Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Glynn RJ, Gaziano JM. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial. JAMA 2008;300(18):2123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reid MB. Invited review: redox modulation of skeletal muscle contraction: what we know and what we don't. J Appl Physiol (1985) 2001;90(2):724–31. [DOI] [PubMed] [Google Scholar]
- 28. Maughan RJ, Burke LM, Dvorak J, Larson-Meyer DE, Peeling P, Phillips SM, Rawson ES, Walsh NP, Garthe I, Geyer H et al. IOC consensus statement: dietary supplements and the high-performance athlete. Int J Sport Nutr Exerc Metab 2018;28(2):104–25. [DOI] [PubMed] [Google Scholar]
- 29. Block G, Jensen CD, Morrow JD, Holland N, Norkus EP, Milne GL, Hudes M, Dalvi TB, Crawford PB, Fung EB et al. The effect of vitamins C and E on biomarkers of oxidative stress depends on baseline level. Free Radic Biol Med 2008;45(4):377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dow CA, Wertheim BC, Patil BS, Thomson CA. Daily consumption of grapefruit for 6 weeks reduces urine F2-isoprostanes in overweight adults with high baseline values but has no effect on plasma high-sensitivity C-reactive protein or soluble vascular cellular adhesion molecule. J Nutr 2013;143(10):1586–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blumberg JB, Frei B. Why clinical trials of vitamin E and cardiovascular diseases may be fatally flawed. Commentary on “The relationship between dose of vitamin E and suppression of oxidative stress in humans”. Free Radic Biol Med 2007;43(10):1374–6. [DOI] [PubMed] [Google Scholar]
- 32. Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, Chen S, Corpe C, Dutta A, Dutta SK et al. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr 2003;22(1):18–35. [DOI] [PubMed] [Google Scholar]
- 33. Steinhubl SR. Why have antioxidants failed in clinical trials? Am J Cardiol 2008;101(10A):14D–9D. [DOI] [PubMed] [Google Scholar]
- 34. Traber MG. Vitamin E inadequacy in humans: causes and consequences. Adv Nutr 2014;5(5):503–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Halliwell B. The antioxidant paradox: less paradoxical now? Br J Clin Pharmacol 2013;75(3):637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Margaritelis NV, Cobley JN, Paschalis V, Veskoukis AS, Theodorou AA, Kyparos A, Nikolaidis MG. Going retro: oxidative stress biomarkers in modern redox biology. Free Radic Biol Med 2016;98:2–12. [DOI] [PubMed] [Google Scholar]
- 37. Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, Ben-Yacov O, Lador D, Avnit-Sagi T, Lotan-Pompan M et al. Personalized nutrition by prediction of glycemic responses. Cell 2015;163(5):1079–94. [DOI] [PubMed] [Google Scholar]
- 38. Wang DD, Hu FB. Precision nutrition for prevention and management of type 2 diabetes. Lancet Diabetes Endocrinol 2018;6(5):416–26. [DOI] [PubMed] [Google Scholar]
- 39. Corella D, Coltell O, Mattingley G, Sorlí JV, Ordovas JM. Utilizing nutritional genomics to tailor diets for the prevention of cardiovascular disease: a guide for upcoming studies and implementations. Expert Rev Mol Diagn 2017;17(5):495–513. [DOI] [PubMed] [Google Scholar]
- 40. de Toro-Martín J, Arsenault BJ, Després JP, Vohl MC. Precision nutrition: a review of personalized nutritional approaches for the prevention and management of metabolic syndrome. Nutrients 2017;9(8):E913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Joyner MJ. Has neo-Darwinism failed clinical medicine: does systems biology have to? Prog Biophys Mol Biol 2015;117(1):107–12. [DOI] [PubMed] [Google Scholar]
- 42. Pavlidis C, Patrinos GP, Katsila T. Nutrigenomics: a controversy. Appl Transl Genom 2015;4:50–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Joyner MJ, Pedersen BK. Ten questions about systems biology. J Physiol 2011;589(Pt 5):1017–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brennan L. Use of metabotyping for optimal nutrition. Curr Opin Biotechnol 2017;44:35–38. [DOI] [PubMed] [Google Scholar]
- 45. O'Donovan CB, Walsh MC, Gibney MJ, Gibney ER, Brennan L. Can metabotyping help deliver the promise of personalised nutrition? Proc Nutr Soc 2016;75(1):106–14. [DOI] [PubMed] [Google Scholar]
- 46. Gibney MJ, Walsh MC. The future direction of personalised nutrition: my diet, my phenotype, my genes. Proc Nutr Soc 2013;72(2):219–25. [DOI] [PubMed] [Google Scholar]
- 47. Hampl JS, Taylor CA, Johnston CS. Vitamin C deficiency and depletion in the United States: the Third National Health and Nutrition Examination Survey, 1988 to 1994. Am J Public Health 2004;94(5):870–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nimni ME, Han B, Cordoba F. Are we getting enough sulfur in our diet? Nutr Metab (Lond) 2007;4:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Njålsson R, Ristoff E, Carlsson K, Winkler A, Larsson A, Norgren S. Genotype, enzyme activity, glutathione level, and clinical phenotype in patients with glutathione synthetase deficiency. Hum Genet 2005;116(5):384–9. [DOI] [PubMed] [Google Scholar]
- 50. Shi ZZ, Habib GM, Rhead WJ, Gahl WA, He X, Sazer S, Lieberman MW. Mutations in the glutathione synthetase gene cause 5-oxoprolinuria. Nat Genet 1996;14(3):361–5. [DOI] [PubMed] [Google Scholar]
- 51. Riedl A, Gieger C, Hauner H, Daniel H, Linseisen J. Metabotyping and its application in targeted nutrition: an overview. Br J Nutr 2017;117(12):1631–44. [DOI] [PubMed] [Google Scholar]
- 52. Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem 2006;52(4):601–23. [DOI] [PubMed] [Google Scholar]
- 53. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007;39(1):44–84. [DOI] [PubMed] [Google Scholar]
- 54. Margaritelis NV. Antioxidants as therapeutics in the intensive care unit: have we ticked the redox boxes? Pharmacol Res 2016;111:126–32. [DOI] [PubMed] [Google Scholar]
- 55. Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health 2015;42(5):533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kalyanaraman B, Darley-Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ II, Ischiropoulos H. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med 2012;52(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tsikas D, Theodoridis G. Analytical tools and protocols in oxidative stress. J Chromatogr B Analyt Technol Biomed Life Sci 2016;1019:1–3. [DOI] [PubMed] [Google Scholar]
- 58. Winterbourn CC. The challenges of using fluorescent probes to detect and quantify specific reactive oxygen species in living cells. Biochim Biophys Acta 2014;1840(2):730–8. [DOI] [PubMed] [Google Scholar]
- 59. Cobley JN, Close GL, Bailey DM, Davison GW. Exercise redox biochemistry: conceptual, methodological and technical recommendations. Redox Biol 2017;12:540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nikolaidis MG, Margaritelis NV, Paschalis V, Theodorou AA, Kyparos A, Vrabas IS. Common questions and tentative answers on how to assess oxidative stress after antioxidant supplementation and exercise. In: Lamprecht M, editor. Antioxidants in sport nutrition. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. p. 221–46 [PubMed] [Google Scholar]
- 61. Atkinson G, Batterham AM. True and false interindividual differences in the physiological response to an intervention. Exp Physiol 2015;100(6):577–88. [DOI] [PubMed] [Google Scholar]
- 62. Shephard RJ. Regression to the mean: a threat to exercise science? Sports Med 2003;33(8):575–84. [DOI] [PubMed] [Google Scholar]
- 63. Tu YK, Gilthorpe MS. Revisiting the relation between change and initial value: a review and evaluation. Stat Med 2007;26(2):443–57. [DOI] [PubMed] [Google Scholar]
- 64. Yudkin PL, Stratton IM. How to deal with regression to the mean in intervention studies. Lancet 1996;347(8996):241–3. [DOI] [PubMed] [Google Scholar]
- 65. Barnett AG, van der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it. Int J Epidemiol 2005;34(1):215–20. [DOI] [PubMed] [Google Scholar]
- 66. Halliwell B, Gutteridge J. Free radicals in biology and medicine. New York: Oxford University Press; 2015. [Google Scholar]
- 67. Brigelius-Flohé R, Flohé L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxid Redox Signal 2011;15(8):2335–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Forman HJ, Ursini F, Maiorino M. An overview of mechanisms of redox signaling. J Mol Cell Cardiol 2014;73:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Thomas DD, Ridnour LA, Isenberg JS, Flores-Santana W, Switzer CH, Donzelli S, Hussain P, Vecoli C, Paolocci N, Ambs S et al. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic Biol Med 2008;45(1):18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cobley JN, McHardy H, Morton JP, Nikolaidis MG, Close GL. Influence of vitamin C and vitamin E on redox signaling: implications for exercise adaptations. Free Radic Biol Med 2015;84:65–76. [DOI] [PubMed] [Google Scholar]
- 71. Winterbourn CC. The biological chemistry of hydrogen peroxide. Methods Enzymol 2013;528:3–25. [DOI] [PubMed] [Google Scholar]
- 72. Reczek CR, Chandel NS. ROS-dependent signal transduction. Curr Opin Cell Biol 2015;33:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sies H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol 2017;11:613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sobotta MC, Liou W, Stöcker S, Talwar D, Oehler M, Ruppert T, Scharf AN, Dick TP. Peroxiredoxin-2 and STAT3 form a redox relay for H2O2 signaling. Nat Chem Biol 2015;11(1):64–70. [DOI] [PubMed] [Google Scholar]
- 75. Woo HA, Yim SH, Shin DH, Kang D, Yu DY, Rhee SG. Inactivation of peroxiredoxin I by phosphorylation allows localized H(2)O(2) accumulation for cell signaling. Cell 2010;140(4):517–28. [DOI] [PubMed] [Google Scholar]
- 76. Hawley JA, Hargreaves M, Joyner MJ, Zierath JR. Integrative biology of exercise. Cell 2014;159(4):738–49. [DOI] [PubMed] [Google Scholar]
- 77. Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 2013;17(2):162–84. [DOI] [PubMed] [Google Scholar]
- 78. Chiarugi P, Cirri P. Redox regulation of protein tyrosine phosphatases during receptor tyrosine kinase signal transduction. Trends Biochem Sci 2003;28(9):509–14. [DOI] [PubMed] [Google Scholar]
- 79. Corcoran A, Cotter TG. Redox regulation of protein kinases. FEBS J 2013;280(9):1944–65. [DOI] [PubMed] [Google Scholar]
- 80. Giannoni E, Taddei ML, Chiarugi P. Src redox regulation: again in the front line. Free Radic Biol Med 2010;49(4):516–27. [DOI] [PubMed] [Google Scholar]
- 81. Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002;298(5600):1911–2. [DOI] [PubMed] [Google Scholar]
- 82. Knock GA, Ward JP. Redox regulation of protein kinases as a modulator of vascular function. Antioxid Redox Signal 2011;15(6):1531–47. [DOI] [PubMed] [Google Scholar]
- 83. Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell 2002;9(2):387–99. [DOI] [PubMed] [Google Scholar]
- 84. Parker L, Shaw CS, Stepto NK, Levinger I. Exercise and glycemic control: focus on redox homeostasis and redox-sensitive protein signaling. Front Endocrinol (Lausanne) 2017;8:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Piantadosi CA, Suliman HB. Redox regulation of mitochondrial biogenesis. Free Radic Biol Med 2012;53(11):2043–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Done AJ, Traustadóttir T. Nrf2 mediates redox adaptations to exercise. Redox Biol 2016;10:191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kang C, O'Moore KM, Dickman JR, Ji LL. Exercise activation of muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha signaling is redox sensitive. Free Radic Biol Med 2009;47(10):1394–400. [DOI] [PubMed] [Google Scholar]
- 88. Narasimhan M, Rajasekaran NS. Exercise, Nrf2 and antioxidant signaling in cardiac aging. Front Physiol 2016;7:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Perry CGR, Hawley JA. Molecular basis of exercise-induced skeletal muscle mitochondrial biogenesis: historical advances, current knowledge, and future challenges. Cold Spring Harb Perspect Med 2017(Epub ahead of print; doi: 10.1101/cshperspect.a029686). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Richter EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev 2013;93(3):993–1017. [DOI] [PubMed] [Google Scholar]
- 91. Merry TL, McConell GK. Skeletal muscle glucose uptake during exercise: a focus on reactive oxygen species and nitric oxide signaling. IUBMB Life 2009;61(5):479–84. [DOI] [PubMed] [Google Scholar]
- 92. Katz A. Role of reactive oxygen species in regulation of glucose transport in skeletal muscle during exercise. J Physiol 2016;594(11):2787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pinheiro CH, Silveira LR, Nachbar RT, Vitzel KF, Curi R. Regulation of glycolysis and expression of glucose metabolism-related genes by reactive oxygen species in contracting skeletal muscle cells. Free Radic Biol Med 2010;48(7):953–60. [DOI] [PubMed] [Google Scholar]
- 94. Koufen P, Stark G. Free radical induced inactivation of creatine kinase: sites of interaction, protection, and recovery. Biochim Biophys Acta 2000;1501(1):44–50. [DOI] [PubMed] [Google Scholar]
- 95. Rhee SG, Kang SW, Jeong W, Chang TS, Yang KS, Woo HA. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol 2005;17(2):183–9. [DOI] [PubMed] [Google Scholar]
- 96. Azzi A. Many tocopherols, one vitamin E. Mol Aspects Med 2018;61:92–103. [DOI] [PubMed] [Google Scholar]
- 97. Maras JE, Bermudez OI, Qiao N, Bakun PJ, Boody-Alter EL, Tucker KL. Intake of alpha-tocopherol is limited among US adults. J Am Diet Assoc 2004;104(4):567–75. [DOI] [PubMed] [Google Scholar]
- 98. Brigelius-Flohé R, Kelly FJ, Salonen JT, Neuzil J, Zingg JM, Azzi A. The European perspective on vitamin E: current knowledge and future research. Am J Clin Nutr 2002;76(4):703–16. [DOI] [PubMed] [Google Scholar]
- 99. Galli F, Azzi A, Birringer M, Cook-Mills JM, Eggersdorfer M, Frank J, Cruciani G, Lorkowski S, Özer NK. Vitamin E: emerging aspects and new directions. Free Radic Biol Med 2017;102:16–36. [DOI] [PubMed] [Google Scholar]
- 100. McDougall M, Choi J, Kim HK, Bobe G, Stevens JF, Cadenas E, Tanguay R, Traber MG. Lethal dysregulation of energy metabolism during embryonic vitamin E deficiency. Free Radic Biol Med 2017;104:324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. McDougall M, Choi J, Truong L, Tanguay R, Traber MG. Vitamin E deficiency during embryogenesis in zebrafish causes lasting metabolic and cognitive impairments despite refeeding adequate diets. Free Radic Biol Med 2017;110:250–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Abner EL, Schmitt FA, Mendiondo MS, Marcum JL, Kryscio RJ. Vitamin E and all-cause mortality: a meta-analysis. Curr Aging Sci 2011;4(2):158–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Miller ER III, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 2005;142(1):37–46. [DOI] [PubMed] [Google Scholar]
- 104. Gill TK, Hill CL, Shanahan EM, Taylor AW, Appleton SL, Grant JF, Shi Z, Dal Grande E, Price K, Adams RJ. Vitamin D levels in an Australian population. BMC Public Health 2014;14:1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Owens DJ, Fraser WD, Close GL. Vitamin D and the athlete: emerging insights. Eur J Sport Sci 2015;15(1):73–84. [DOI] [PubMed] [Google Scholar]
- 106. Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol 2010;10(4):482–96. [DOI] [PubMed] [Google Scholar]
- 107. Owens DJ, Sharples AP, Polydorou I, Alwan N, Donovan T, Tang J, Fraser WD, Cooper RG, Morton JP, Stewart C et al. A systems-based investigation into vitamin D and skeletal muscle repair, regeneration, and hypertrophy. Am J Physiol Endocrinol Metab 2015;309(12):E1019–31. [DOI] [PubMed] [Google Scholar]
- 108. Owens DJ, Tang JC, Bradley WJ, Sparks AS, Fraser WD, Morton JP, Close GL. Efficacy of high-dose vitamin D supplements for elite athletes. Med Sci Sports Exerc 2017;49(2):349–56. [DOI] [PubMed] [Google Scholar]
- 109. Noble D, Jablonka E, Joyner MJ, Müller GB, Omholt SW. Evolution evolves: physiology returns to centre stage. J Physiol 2014;592(11):2237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Halliwell B. The antioxidant paradox. Lancet 2000;355(9210):1179–80. [DOI] [PubMed] [Google Scholar]