Abstract
Here we present the feasibility of NMR Signal Amplification by Reversible Exchange (SABRE) using radio-frequency irradiation at low magnetic field (0.05 T) in the regime, where the chemical shifts of free and catalyst bound species are similar. In SABRE, the 15N-containing substrate and parahydrogen perform simultaneous chemical exchange on an Iridium hexacoordinate complex. Shaped Spin-Lock Induced Crossing (SLIC) radio-frequency pulse sequence followed by a delay is applied at QUASi-Resonance (QUASR) condition of 15N spins of 15N-enriched substrate. As a result of this pulse sequence application, 15N z-magnetization is created from the spin-order of parahydrogen derived hyperpolarized hydrides. The repetition of the pulse-sequence block consisting of shaped radio-frequency pulse and the delay leads to the build-up of 15N magnetization. The modulation of this effect by the irradiation frequency, pulse duration and amplitude, delay duration, and the number of pumping cycles was demonstrated. Pyridine-15N, acetonitrile-15N, metronidazole-15N2-13C2 substrates were studied representing three classes of compounds (five- and six-membered heterocycles and nitrile) showing the wide applicability of the technique. Metronidazole-15N2-13C2 is an FDA-approved antibiotic that can be injected in large quantities promising non-invasive and accurate hypoxia sensing. The 15N hyperpolarization levels attained with QUASR-SABRE on metronidazole-15N2-13C2 were more than two-fold greater than with SABRE-SHEATH (SABRE in SHield Enables Alignment Transfer to Heteronuclei) demonstrating that QUASR-SABRE can deliver significantly more efficient means of SABRE hyperpolarization.
Table of Contents Graphic
Conventional NMR relies on equilibrium thermal nuclear spin polarization P dictated by the Boltzmann distribution among Zeeman energy levels in dependence of the applied static magnetic field B0. Although P can be boosted significantly by applying stronger magnetic field (because P∝B0), P is typically on the order of 10−5 to 10−6 for a conventional high-field NMR spectrometer (ca. 9.4 T) or MRI scanner (ca. 3 T) at room temperature, i.e. when the high temperature approximation holds. Hyperpolarization techniques increase P to the order of unity increasing NMR sensitivity increase by 4–5 orders of magnitude.1–3
Several hyperpolarization techniques exist.1–3 Signal Amplification by Reversible Exchange (SABRE) is one of more recent techniques pioneered by Duckett and co-workers in 2009.4–7 SABRE relies on simultaneous chemical exchange of parahydrogen (p-H2) and to-be-hyperpolarized substrate (Figure 1a).8 When the transient complex is formed, the parahydrogen symmetry is broken,9 and the network of spin-spin couplings can enable transfer polarization from parahydrogen-derived hydrides to the nuclear spins of the substrate.4–7 Two major groups of approaches have been developed for SABRE polarization transfer: the first group employs a matching static magnetic field Bevo,4, 10–13 and the second group applies radio-frequency (RF) pulse sequences,14 to approach Level Anti-Crossings (LAC)15–16 and induce polarization transfer. Both approaches have merit depending on the application. For biomedical applications, which represent the main driver for development of hyperpolarization technology,2 the key is to achieve high degrees of polarization with the long lifetimes in a suitable biomolecular motif.17 So far, approaches relying on static magnetic fields such as SABRE-SHEATH (SABRE in SHield Enables Alignment Transfer to Heteronuclei)18–20 have been the most efficient for preparation of long-lived 15N hyperpolarized spin states with exponential decay constant of more than 20 minutes21 and P15N exceeding 30%.22–23
Figure 1.
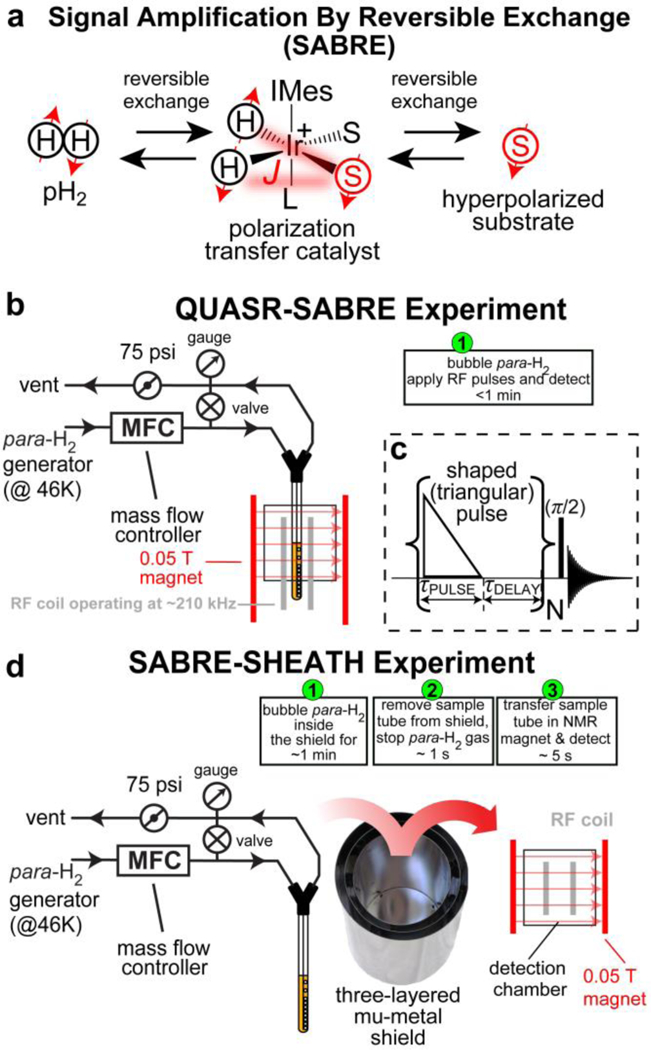
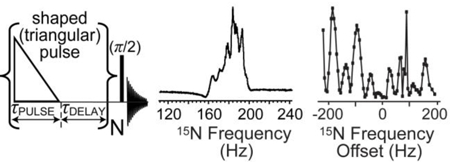
a) The diagram of molecular exchange with p-H2 in SABRE hyperpolarization. b) the experimental setup for QUASR-SABRE, c) the RF pulse sequence for QUASR-SABRE, d) corresponding experimental setup for SABRE-SHEATH experiment.
One likely explanation as to why RF-based methods are lagging behind in the context of SABRE is the reliance on conventional high-field NMR spectrometers with small coils that only encompass a small fraction of the liquid sample in a 5 mm NMR tube,14 which is continuously bubbled with p-H2.24 These factors result in major RF-inhomogeneities. In contrast, a previous approach used in hydrogenative Parahydrogen Induced Polarization (PHIP9, 25–26), employs low-field (ca. 5–50 mT) magnets and RF excitation coils,27–32 which encompass the entire sample volume, sometimes in excess of 50 mL.33–35 Moreover, the hardware behind such low-field devices is significantly less complex and less costly compared to that of the high-field NMR spectrometers.17, 31–32, 35–36 Here it is demonstrated that these advantageous features can also be translated to SABRE.
RF-based polarization transfer such as Low-Irradiation Generation of High Tesla (LIGHT)-SABRE employs RF irradiation of the catalyst-bound substrate (Figure 1A), which typically has a chemical shift difference of 30–50 ppm with respect to the free substrate.14, 37 Irradiation of the catalyst bound species allows for polarization transfer from p-H2-derived hydrides.14 When the complex dissociates, the 15N nuclear spin polarization is preserved in the free substrate, because it is not affected by the frequency-selective RF pulses.14 Achieving this frequency selective irradiation at high magnetic fields is trivial due to large chemical shift dispersion. For example, 50 ppm difference equals to ~2 kHz at 9.4 T. However, this difference vanishes at low magnetic fields: for example, 50 ppm difference equals 10 Hz at 0.05 T (the magnetic field employed in this Letter), and selective RF-excitation becomes challenging. We demonstrate that this challenge can be overcome through the use of quasi-resonance (QUASR) spin-lock induced crossing (SLIC)38 irradiation to polarize 15N spins from p-H2-derived hydrides.
The QUASR-SABRE experiment (Figure 1B) is performed at 0.05 T magnetic field using previously described p-H2 bubbling setup in a medium-walled 5-mm NMR tube.24, 39–40 During p-H2 bubbling a triangular-shaped pulse is applied for duration τPULSE followed by a delay period τDELAY. The process is repeated, and the net z-magnetization increases during this “rf-pumping” process. The resulting magnetization can be conveniently assessed by applying a broad-band excitation (π/2) RF pulse, Figure 1c. We compare the performance of this QUASR-SABRE approach with SABRE-SHEATH approach (Figure 1d), which has been employed previously to obtain record-high 15N polarization in excess of 30%.22–23
The previously described SABRE-SHEATH setup,39–40 15N RF coil and 0.05 T magnet have been employed here.41 Samples of three substrates and IrIMes catalyst precursor in perdeuterated methanol were prepared as follows: pyridine-15N/catalyst, ~20 mM/~1 mM, acetonitrile-15N/catalyst, ~40 mM/~1–2 mM, metronidazole-15N2-13C2/catalyst, ~20 mM/~1 mM. All 15N-enriched compounds were purchased from Isotec. The RF pulse sequences was coded and applied on Kea-2 NMR spectrometer (Magritek, New Zeeland) using Tomco RF amplifier. The employed Kea-2 spectrometer was operated in the signal averaging mode, where the signal is averaged during multi-scan acquisitions version being added. As a result, the signal integral value from multi-scan spectrum is similar to that acquired using 1-scan acquisition, e.g. the integral values of spectra shown in corresponding displays in Figures 2–4 can be compared directly without any scaling even though the spectra were recorded using different numbers of scans. A Three-layered mu-metal magnetic shield was employed (Magnetic Shield Corp., Bensenville, IL, P/N ZG-206). All data was acquired employing 75–80% parahydrogen prepared using home-built parahydrogen generator based on Sunpower cryo-chiller at a flow rate of 150 standard cubic centimeters per minute (sccm). For the SABRE experiments, the following conditions were used: 75 psi back-pressure and 70 sccm para-H2 flow rate (Figure 1b and Figure 1d). The flow rate was maintained by via a mass flow controller (MFC, Sierra Instruments, Monterey, CA, P/N C100L-DD-OV1-SV1-PV2-V1-S0-C0).
Figure 2.
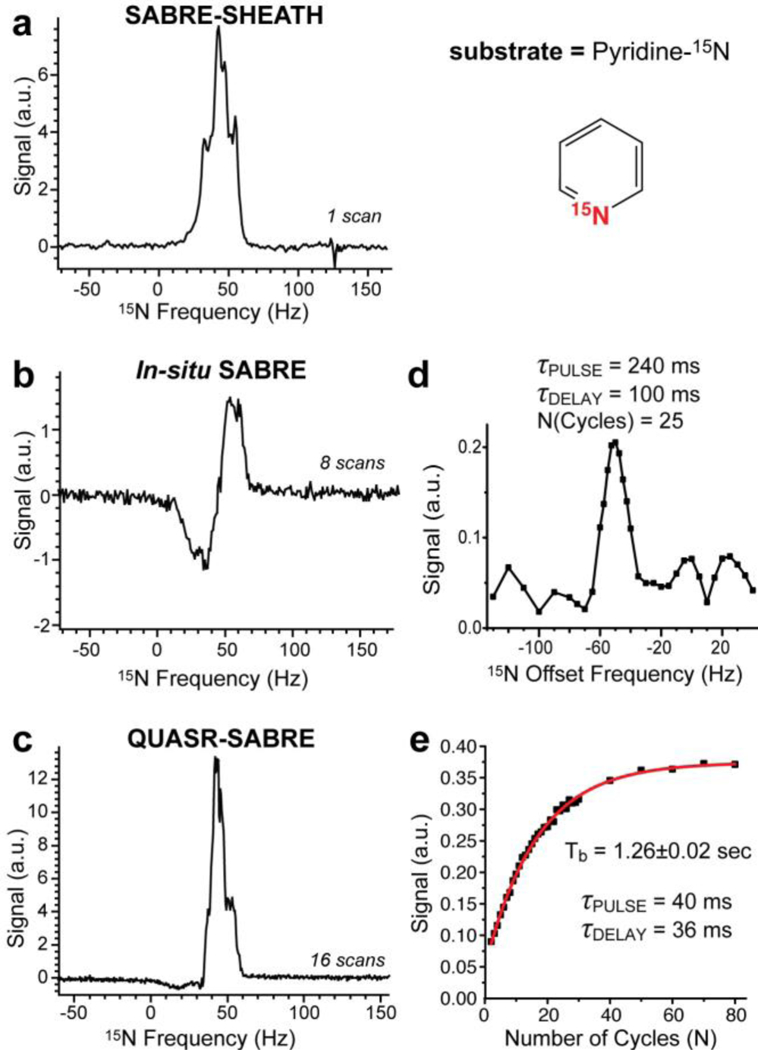
Pyridine-15N data. The other experimental conditions were as follows: 20 mM pyridine-15N, 1 mM catalyst in CD3OD. Note the width of the signal in displays b and d. a) the 15N spectrum obtained after performing SABRE-SHEATH; b) The 15N spectrum recorded using 90-degree excitation pulse when bubbling p-H2 in situ of the 0.05 T magnet; c) the 15N spectrum obtained after performing QUASR-SABRE; d) 15N QUASR-SABRE signal dependence on the applied radio frequency offset from the actual resonance condition; e) the build-up of 15N QUASR-SABRE signal as a function of the number of pumping cycles. Note the individual spectra employed for figures in displays d and e were auto-phased, and the data is presented in the magnitude mode.
Figure 4.
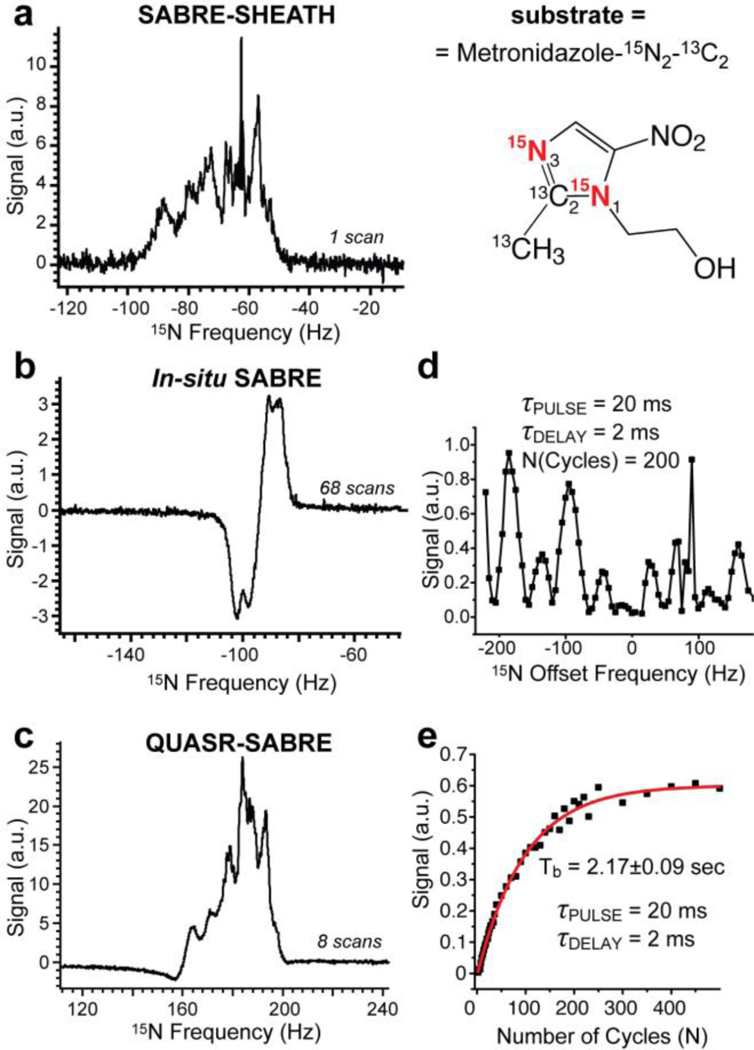
Metronidazole-15N2-13C2 data. The other experimental conditions were as follows: ~20 mM metronidazole-15N2-13C2, 1 or 2 mM catalyst in CD3OD. Note the width of the signal in displays a and c is nearly the same as opposed to pyridine-15N case. a) the 15N spectrum obtained after performing SABRE-SHEATH; b) The 15N spectrum recorded using 90-degree excitation pulse when bubbling p-H2 in situ of the 0.05 T magnet; c) the 15N spectrum obtained after performing QUASR-SABRE; d) 15N QUASR-SABRE signal dependence on the frequency of the applied RF shaped pulse; e) the build-up of 15N QUASR-SABRE signal as a function of the number of pumping cycles N. Note the individual spectra employed for figures in displays d and e were auto-phased, and the data is presented in the magnitude mode.
Low-field detection of hyperpolarized (HP) compounds offers sufficient detection sensitivity in the context of NMR detection of HP states.42 However, detection of thermal polarization is challenging due to low P even at high concentrations (see Supporting Information (SI) for details). As a result, the quantification of 15N enhancements (ε15N) and polarization (P15N) relied on signal to noise measurements (see SI for details) in order to determine the minimum values achieved.
15N NMR spectra with high SNR were obtained for all three studied molecules: pyridine-15N, acetonitrile-15N, and metronidazole-15N2-13C2 using SABRE-SHEATH (Figure 2a, Figure 3a, and Figure 4a respectively) and QUASR-SABRE (Figure 2c, Figure 3c, and Figure 4c respectively). We note that while p-H2 bubbling through the sample placed in 0.05 T leads to 15N signal even without RF pulses (Figure 2b, Figure 3b and Figure 4b respectively), this 15N signal is distinctly anti-phase,14, 41, 43 and has significantly lower intensity compared to those obtained using SABRE-SHEATH and QUASR-SABRE protocols.
Figure 3.
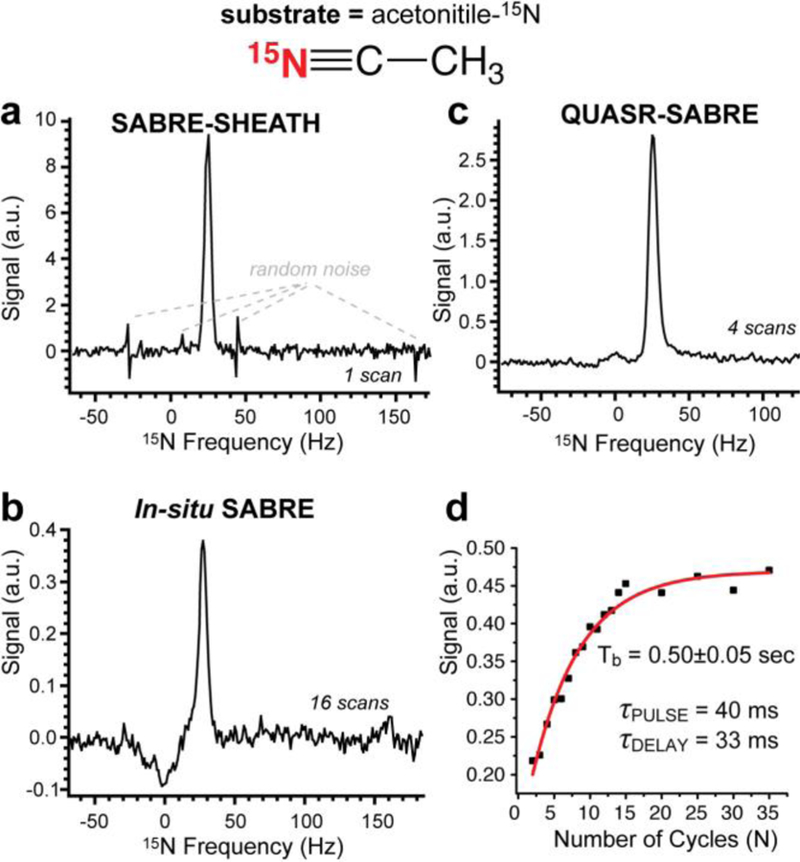
acetonitrile-15N data. The other experimental conditions were as follows: 40 mM acetonitrile-15N, 1 or 2 mM catalyst in CD3OD. Note the width of the signal in displays a and c is nearly the same as opposed to pyridine-15N case. a) the 15N spectrum obtained after performing SABRE-SHEATH; b) The 15N spectrum recorded using 90-degree excitation pulse when bubbling p-H2 in situ of the 0.05 T magnet; c) the 15N spectrum obtained after performing QUASR-SABRE; d) the build-up of 15N QUASR-SABRE signal as a function of the number of pumping cycles; Note the individual spectra employed for figures in display d were auto-phased, and the data is presented in the magnitude mode.
The key distinct feature of QUASR-SABRE phenomenon is strong RF offset frequency dependence: Figure 2d and Figure 4d demonstrate the dependence of the 15N QUASR-SABRE signal for pyridine-15N and metronidazole-15N2-13C2 respectively. Note that very small signal is obtained at the resonance frequency. The maximum intensity of 15N QUASR-SABRE was compared to that of SABRE-SHEATH and is reported here as the ratio of 15N QUASR-SABRE and SABRE-SHEATH signal, η. We find η of ~1.0 for pyridine-15N, η of ~0.44 for acetonitrile-15N, and η of (at least) ~2.4 for metronidazole-15N2-13C2. The relatively low η value for acetonitrile-15N is in part explained by the fact that frequency optimization was not performed, and the data was recorded using the frequency offset parameter optimized for pyridine-15N, which unfortunately did not provide a fair comparison. Based on the range of on the frequency optimization data for pyridine-15N (Figure 2e) and for metronidazole-15N2-13C2 (Figure 4d), we estimate that this optimization may potentially yield an improvement of up ten-fold. We note that η value for metronidazole-15N2-13C2 assumes that both 15N sites are hyperpolarized via SABRE-SHEATH and QUASR-SABRE protocols. While SABRE-SHEATH indeed yields hyperpolarization of both 15N sites due to spin-relay of polarization at very low magnetic fields (the Earth’s field (ca. 50 μT) and below), QUASR-SABRE may yield hyperpolarization of only one (N3) site, Figure 4. If that is the case, then η would be effectively doubled to 4.8, because only one 15N site contributes to the QUASR-SABRE signal (Figure 4c) versus two 15N sites to the SABRE-SHEATH NMR signal (Figure 4a). We note that the Full Width at the Half-Height (FWHH) was approximately the same for SABRE-SHEATH and QUASR-SABRE NMR spectra for acetonitrile-15N and metronidazole-15N2-13C2: Figure 3 and Figure 4 respectively, whereas QUASR-SABRE spectrum FWHH was approximately half of that for SABRE-SHEATH spectrum for pyridine-15N (Figure 2c and Figure 2a respectively). The latter observation may in part explain pyridine-15N η value, which is significantly lower than that of metronidazole-15N2-13C2.
All three molecules studies exhibited a clear and strong dependence of 15N QUASR-SABRE signal on the duration of the pulse (τPULSE) and the duration of the delay (τDELAY): Figure S2a, Figure S3a and Figure S3b, Figure S4a and Figure S2b, Figure S3c, Figure S4b respectively. This strong dependence is likely due to the dynamics and kinetics of the substrate and p-H2 exchange on the catalyst. When the τPULSE and τDELAY are in sync with chemistry of exchange, the maximum QUASR-SABRE signal may be achieved. However, we note that QUASR-SABRE signal has very complex dependence on τPULSE and τDELAY. For example, the τPULSE-curves for acetonictrile-15N are vastly different at τDELAY of 2 ms (Figure S3a) and τDELAY of 33 ms (Figure S3b). Future theoretical work is certainly warranted to study this complex behavior of QUASR-SABRE effect, which is outside the scope of this pioneering phenomenological report.
The second key feature of QUASR-SABRE effect allows for continuous “RF-pumping” of 15N z-magnetization: Figure 2e, Figure 3d, and Figure 4e for the three studied compounds respectively. This fitting of the exponential dependence of the build-up processed yielded Tb of 0.5±0.05 s for acetonitrile-15N, Tb of 1.26±0.02 s for pyridine-15N, and Tb of 2.17±0.09 s for metronidazole-15N2-13C2. The Tb values correlate well with η values with R2 of 0.93, Figure 5, suggesting that the build-up rate may be affecting the efficiency of QUASR-SABRE polarization process. A likely explanation of this observation in the contribution of the polarization destruction processes to Tb: when the destruction due to RF pulses is significant, Tb is reduced resulting in lower 15N signals in a manner analogous to that of batch-mode Spin Exchange Optical Pumping (SEOP).44–47 Further experimental and theoretical studies are certainly warranted in the future to maximize the efficiency of QUASR-SABRE approach described here.
Figure 5.
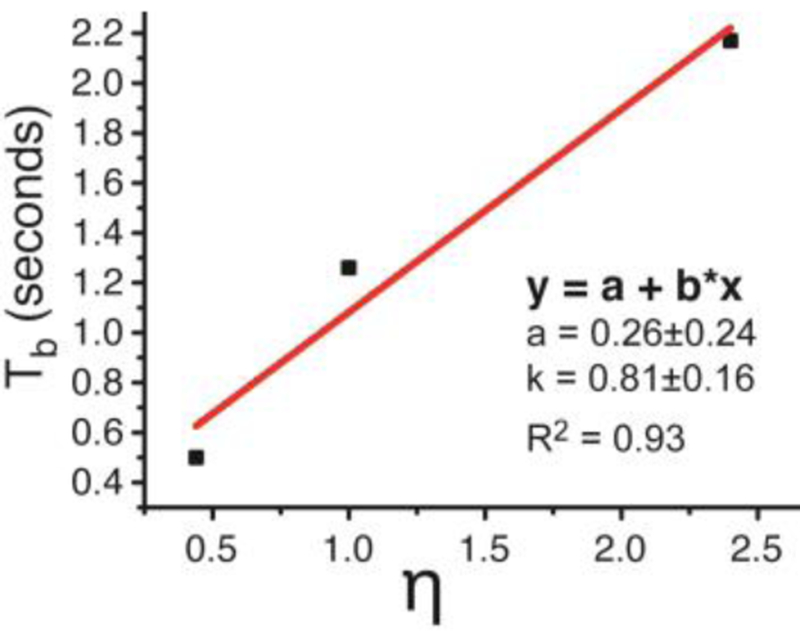
Correlation plot of Tb and η for the three studied compounds.
All experiments were performed at room temperature (ca. 298 K). We note that the rf pumping of QUASR-SABRE process needs to be effectively matched to the chemical exchange dynamics in order to maximize the polarization transfer efficiency to yield the highest P15N value. Therefore, it is expected that the optimal values of τPULSE and τDELAY (Figure S2, Figure S3, and Figure S4) would exhibit temperature dependence, because temperature modulates the rates of substrate and p-H2 exchange. Moreover, it is entirely possible that optimum temperature (i.e. yielding the highest value of P15N) may be significantly different from room temperature and may be different from optimal temperature of the SABRE-SHEATH process (note that the optimal SABRE-SHEATH temperature for pyridine-15N and for metronidazole-15N2-13C2 corresponds to approximately room temperature, i.e. SABRE-SHEATH experiments were optimized with respect to temperature). As a result, some additional improvement in the maximum value of P15N may be potentially expected for QUASR-SABRE process.
We have also investigated the dependence of the QUASR-SABRE signal on the amplitude of SLIC power amplitude. Figure S4c exhibits a plateau (with a range of approximately 6 decibels) with relatively steep slopes on both sides. This trend is expected, because LAC conditions are usually created in a relatively narrow power range.15, 48
Optimization of P15N was not the goal of this report. Moreover, due to lack of direct 15N signal reference (due to insufficient thermal equilibrium signal), we can only report the low limit of ε15N and P15N values. Metronidazole-15N2-13C2 QUASR-SABRE estimates were ε15N ~9*105 and P15N ~1.5% (these values are doubled if only N3 site is hyperpolarized). Metronidazole-15N2-13C2 SABRE-SHEATH estimates were ε15N ~3.7*105 and P15N ~0.6%. Note these lower-limit estimates are in good agreement with P15N~1.5% reported for SABRE-SHEATH under similar conditions using detection provided by high-resolution 9.4 T NMR spectrometer.39–40 Pyridine-15N lower-limit estimates were ε15N ~ 6.6*105 and P15N ~ 1.1% for both QUASR-SABRE and SABRE-SHEATH—in line with previous SABRE-SHEATH studies.18–19 Acetonitrile-15N lower-limit estimates were ε15N ~ 5.3*104 and P15N ~ 0.09% for QUASR-SABRE and ε15N ~ 1.2*105 and P15N ~ 0.2% for SABRE-SHEATH respectively. See SI for details.
With regards to the limitations of the QUASR-SABRE method, it remains to be seen if QUASR-SABRE is capable of hyperpolarization of long-range spin sites in the substrate compounds. Moreover, future systematic experimental and theoretical studies are certainly needed to further optimize the efficiency of QUASR-SABRE technique. For example, more advanced shaped forms (e.g. sine, exponential, trapezoid, etc.) and strategies (adiabatic pulses) can be envisioned.
In summary, radio-frequency based polarization transfer approach has been presented for polarization of 15N sites. At least in some compounds, this method appears to be more efficient than the SABRE-SHEATH approach, which has already been shown to yield more than 30% 15N polarization.23 This is remarkable because in all previous demonstrations RF-SABRE approaches yielded significantly less polarization than static field matching / field cycling approaches. We hope that QUASR-SABRE may ultimately yield 15N polarization of the order of unity for a wide range of biomolecules. The employed pulse-sequence is a shaped variant of SLIC pulse sequence,38 which has the benefit of using low power levels. The applicability of this technique has been explored for three different types of compounds (six- and five-membered N-heterocycles and acetonitrile) including the antibiotic metronidazole. Metronidazole is an antibiotic that can be administered in large doses,49 and contains the nitroimidazole moiety, which has been exploited in a wide range of molecular contrast agents for hypoxia sensing using position emission tomography (PET).50–55 Therefore, metronidazole is a promising candidate as molecular probe for hypoxia imaging using HP MRI.40
Supplementary Material
Acknowledgments
Funding Sources
This work was supported by NSF under Grants CHE-1058727, CHE-1363008, CHE-1416268, and CHE-1836308. Research reported in this publication was also supported by the National Institute of Biomedical Imaging and Bioengineering of the NIH under R21EB025313 and 1R21EB020323, by National Cancer Institute under 1R21CA220137, and by DOD CDMRP under BRP W81XWH-12–1-0159/BC112431 and under W81XWH-15–1-0271.
Footnotes
ASSOCIATED CONTENT
Supporting Information. Computation of 15N signal enhancement and polarization values, photograph of the shaped RF pulse detected by the oscilloscope, Supporting Figures of the QUASR-SABRE effect, PDF.
This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Goodson BM; Whiting N; Coffey AM; Nikolaou P; Shi F; Gust BM; Gemeinhardt ME; Shchepin RV; Skinner JG; Birchall JR, et al. Hyperpolarization Methods for MRS. Emagres 2015, 4, 797–810. [Google Scholar]
- 2.Nikolaou P; Goodson BM; Chekmenev EY NMR Hyperpolarization Techniques for Biomedicine. Chem. Eur. J 2015, 21, 3156–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kovtunov KV; Pokochueva E; Salnikov OG; Cousin S; Kurzbach D; Vuichoud B; Jannin S; Chekmenev EY; Goodson BM; Barskiy DA, et al. Hyperpolarized NMR: D‐DNP, PHIP, and SABRE. Chem. Asian J 2018, 13, 1857–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams RW; Aguilar JA; Atkinson KD; Cowley MJ; Elliott PIP; Duckett SB; Green GGR; Khazal IG; Lopez-Serrano J; Williamson DC Reversible Interactions with Para-Hydrogen Enhance NMR Sensitivity by Polarization Transfer. Science 2009, 323, 1708–1711. [DOI] [PubMed] [Google Scholar]
- 5.Adams RW; Duckett SB; Green RA; Williamson DC; Green GGR A Theoretical Basis for Spontaneous Polarization Transfer in Non-Hydrogenative Parahydrogen-Induced Polarization. J. Chem. Phys 2009, 131, 194505. [DOI] [PubMed] [Google Scholar]
- 6.Atkinson KD; Cowley MJ; Duckett SB; Elliott PIP; Green GGR; López-Serrano J; Khazal IG; Whitwood AC Para-Hydrogen Induced Polarization without Incorporation of Para-Hydrogen into the Analyte. Inorg. Chem 2009, 48, 663–670. [DOI] [PubMed] [Google Scholar]
- 7.Atkinson KD; Cowley MJ; Elliott PIP; Duckett SB; Green GGR; Lopez-Serrano J; Whitwood AC Spontaneous Transfer of Parahydrogen Derived Spin Order to Pyridine at Low Magnetic Field. J. Am. Chem. Soc 2009, 131, 13362–13368. [DOI] [PubMed] [Google Scholar]
- 8.Rayner PJ; Duckett SB Signal Amplification by Reversible Exchange (SABRE): From Discovery to Diagnosis. Angew. Chem. Int. Ed 2018, 57, 6742–6753. [DOI] [PubMed] [Google Scholar]
- 9.Bowers CR; Weitekamp DP Transformation of Symmetrization Order to Nuclear-Spin Magnetization by Chemical-Reaction and Nuclear-Magnetic-Resonance. Phys. Rev. Lett 1986, 57, 2645–2648. [DOI] [PubMed] [Google Scholar]
- 10.Shchepin RV; Truong ML; Theis T; Coffey AM; Shi F; Waddell KW; Warren WS; Goodson BM; Chekmenev EY Hyperpolarization of “Neat” Liquids by NMR Signal Amplification by Reversible Exchange. J. Phys. Chem. Lett 2015, 6, 1961–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green RA; Adams RW; Duckett SB; Mewis RE; Williamson DC; Green GGR The Theory and Practice of Hyperpolarization in Magnetic Resonance Using Parahydrogen. Prog. Nucl. Mag. Res. Spectrosc 2012, 67, 1–48. [DOI] [PubMed] [Google Scholar]
- 12.Hovener JB; Schwaderlapp N; Lickert T; Duckett SB; Mewis RE; Highton LAR; Kenny SM; Green GGR; Leibfritz D; Korvink JG, et al. A Hyperpolarized Equilibrium for Magnetic Resonance. Nat. Commun 2013, 4, 2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barskiy DA; Kovtunov KV; Koptyug IV; He P; Groome KA; Best QA; Shi F; Goodson BM; Shchepin RV; Truong ML, et al. In Situ and Ex Situ Low-Field NMR Spectroscopy and MRI Endowed by SABRE Hyperpolarization. ChemPhysChem 2014, 15, 4100–4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Theis T; Truong M; Coffey AM; Chekmenev EY; Warren WS LIGHT-SABRE Enables Efficient in-Magnet Catalytic Hyperpolarization. J. Magn. Reson 2014, 248, 23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pravdivtsev AN; Yurkovskaya AV; Zimmermann H; Vieth H-M; Ivanov KL Transfer of SABRE-Derived Hyperpolarization to Spin-1/2 Heteronuclei. RSC Adv 2015, 5, 63615–63623. [Google Scholar]
- 16.Ivanov KL; Pravdivtsev AN; Yurkovskaya AV; Vieth H-M; Kaptein R The Role of Level Anti-Crossings in Nuclear Spin Hyperpolarization. Prog. Nucl. Mag. Res. Spectrosc 2014, 81, 1–36. [DOI] [PubMed] [Google Scholar]
- 17.Hövener J-B; Pravdivtsev AN; Kidd B; Bowers CR; Glöggler S; Kovtunov KV; Plaumann M; Katz-Brull R; Buckenmaier K; Jerschow A, et al. Parahydrogen-Based Hyperpolarization for Biomedicine. Angew. Chem. Int. Ed 2018, 57, 11140–11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Theis T; Truong ML; Coffey AM; Shchepin RV; Waddell KW; Shi F; Goodson BM; Warren WS; Chekmenev EY Microtesla SABRE Enables 10% Nitrogen-15 Nuclear Spin Polarization. J. Am. Chem. Soc 2015, 137, 1404–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Truong ML; Theis T; Coffey AM; Shchepin RV; Waddell KW; Shi F; Goodson BM; Warren WS; Chekmenev EY 15N Hyperpolarization by Reversible Exchange Using SABRE-SHEATH. J. Phys. Chem. C 2015, 119, 8786–8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colell JFP; Logan AWJ; Zhou Z; Shchepin RV; Barskiy DA; Ortiz GX; Wang Q; Malcolmson SJ; Chekmenev EY; Warren WS, et al. Generalizing, Extending, and Maximizing Nitrogen-15 Hyperpolarization Induced by Parahydrogen in Reversible Exchange. J. Phys. Chem. C 2017, 121, 6626–6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theis T; Ortiz GX; Logan AWJ; Claytor KE; Feng Y; Huhn WP; Blum V; Malcolmson SJ; Chekmenev EY; Wang Q, et al. Direct and Cost-Efficient Hyperpolarization of Long-Lived Nuclear Spin States on Universal 15N2-Diazirine Molecular Tags. Sci. Adv 2016, 2, e1501438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barskiy DA; Shchepin RV; Coffey AM; Theis T; Warren WS; Goodson BM; Chekmenev EY Over 20% 15N Hyperpolarization in under One Minute for Metronidazole, an Antibiotic and Hypoxia Probe. J. Am. Chem. Soc 2016, 138, 8080–8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kidd BE; Gesiorski JL; Gemeinhardt ME; Shchepin RV; Kovtunov KV; Koptyug IV; Chekmenev EY; Goodson BM Facile Removal of Homogeneous SABRE Catalysts for Purifying Hyperpolarized Metronidazole, a Potential Hypoxia Sensor. J. Phys. Chem. C 2018, 122, 16848–16852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Truong ML; Shi F; He P; Yuan B; Plunkett KN; Coffey AM; Shchepin RV; Barskiy DA; Kovtunov KV; Koptyug IV, et al. Irreversible Catalyst Activation Enables Hyperpolarization and Water Solubility for NMR Signal Amplification by Reversible Exchange. J. Phys. Chem. B 2014, 18 13882–13889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bowers CR; Weitekamp DP Para-Hydrogen and Synthesis Allow Dramatically Enhanced Nuclear Alignment. J. Am. Chem. Soc. 1987, 109, 5541–5542. [Google Scholar]
- 26.Eisenschmid TC; Kirss RU; Deutsch PP; Hommeltoft SI; Eisenberg R; Bargon J; Lawler RG; Balch AL Para Hydrogen Induced Polarization in Hydrogenation Reactions. J. Am. Chem. Soc 1987, 109, 8089–8091. [Google Scholar]
- 27.Waddell KW; Coffey AM; Chekmenev EY In Situ Detection of Phip at 48 mT: Demonstration Using a Centrally Controlled Polarizer. J. Am. Chem. Soc 2011, 133, 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coffey AM; Shchepin RV; Wilkens K; Waddell KW; Chekmenev EY A Large Volume Double Channel 1H-X RF Probe for Hyperpolarized Magnetic Resonance at 0.0475 Tesla. J. Magn. Reson 2012, 220, 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovtunov KV; Truong ML; Barskiy DA; Koptyug IV; Coffey AM; Waddell KW; Chekmenev EY Long-Lived Spin States for Low-Field Hyperpolarized Gas MRI. Chem. Eur. J 2014, 20, 14629–14632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kovtunov KV; Truong ML; Barskiy DA; Salnikov OG; Bukhtiyarov VI; Coffey AM; Waddell KW; Koptyug IV; Chekmenev EY Propane-D6 Heterogeneously Hyperpolarized by Parahydrogen. J. Phys. Chem. C 2014, 118, 28234–28243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coffey AM; Shchepin RV; Truong ML; Wilkens K; Pham W; Chekmenev EY Open-Source Automated Parahydrogen Hyperpolarizer for Molecular Imaging Using 13C Metabolic Contrast Agents. Anal. Chem 2016, 88, 8279–8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coffey AM; Shchepin RV; Feng B; Colon RD; Wilkens K; Waddell KW; Chekmenev EY A Pulse Programmable Parahydrogen Polarizer Using a Tunable Electromagnet and Dual Channel NMR Spectrometer. J. Magn. Reson 2017, 284, 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hövener J-B; Chekmenev EY; Harris KC; Perman W; Robertson L; Ross BD; Bhattacharya P Pasadena Hyperpolarization of 13C Biomolecules: Equipment Design and Installation. Magn. Reson. Mater. Phy 2009, 22, 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hövener J-B; Chekmenev EY; Harris KC; Perman W; Tran T; Ross BD; Bhattacharya P Quality Assurance of PASADENA Hyperpolarization for 13C Biomolecules. Magn. Reson. Mater. Phy 2009, 22, 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kadlecek S; Vahdat V; Nakayama T; Ng D; Emami K; Rizi R A Simple and Low-Cost Device for Generating Hyperpolarized Contrast Agents Using Parahydrogen. NMR Biomed 2011, 24, 933–942. [DOI] [PubMed] [Google Scholar]
- 36.Borowiak R; Schwaderlapp N; Huethe F; Lickert T; Fischer E; Bär S; Hennig J; Elverfeldt D; Hövener J-B A Battery-Driven, Low-Field NMR Unit for Thermally and Hyperpolarized Samples. Magn. Reson. Mater. Phy 2013, 26, 491–499. [DOI] [PubMed] [Google Scholar]
- 37.Pravdivtsev AN; Yurkovskaya AV; Zimmermann H; Vieth H-M; Ivanov KL Enhancing NMR of Insensitive Nuclei by Transfer of SABRE Spin Hyperpolarization. Chem. Phys. Lett 2016, 661, 77–82. [Google Scholar]
- 38.DeVience SJ; Walsworth RL; Rosen MS Preparation of Nuclear Spin Singlet States Using Spin-Lock Induced Crossing. Phys. Rev. Lett 2013, 111, 5. [DOI] [PubMed] [Google Scholar]
- 39.Shchepin RV; Jaigirdar L; Theis T; Warren WS; Goodson BM; Chekmenev EY Spin Relays Enable Efficient Long-Range Heteronuclear Signal Amplification by Reversible Exchange. J. Phys. Chem. C 2017, 121, 28425–28434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shchepin RV; Jaigirdar L; Chekmenev EY Spin-Lattice Relaxation of Hyperpolarized Metronidazole in Signal Amplification by Reversible Exchange in Micro-Tesla Fields. J. Phys. Chem. C 2018, 122, 4984–4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shchepin RV; Barskiy DA; Coffey AM; Feldman MA; Kovtunova LM; Bukhtiyarov VI; Kovtunov KV; Goodson BM; Koptyug IV; Chekmenev EY Robust Imidazole-15N2 Synthesis for High-Resolution Low-Field (0.05 T) 15N hyperpolarized NMR Spectroscopy. ChemistrySelect 2017, 2, 4478–4483. [Google Scholar]
- 42.Coffey AM; Truong ML; Chekmenev EY Low-Field MRI Can Be More Sensitive Than High-Field MRI. J. Magn. Reson 2013, 237, 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kovtunov KV; Kidd BE; Salnikov OG; Bales LB; Gemeinhardt ME; Gesiorski J; Shchepin RV; Chekmenev EY; Goodson BM; Koptyug IV Imaging of Biomolecular NMR Signals Amplified by Reversible Exchange with Parahydrogen inside an MRI Scanner. J. Phys. Chem. C 2017, 121, 25994–25999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walker TG Fundamentals of Spin-Exchange Optical Pumping. Journal of Physics: Conference Series 2011, 294, 012001. [Google Scholar]
- 45.Nikolaou P; Coffey AM; Ranta K; Walkup LL; Gust B; Barlow MJ; Rosen MS; Goodson BM; Chekmenev EY Multi-Dimensional Mapping of Spin-Exchange Optical Pumping in Clinical-Scale Batch-Mode 129Xe Hyperpolarizers. J. Phys. Chem. B 2014, 118, 4809–4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nikolaou P; Coffey AM; Walkup LL; Gust BM; Whiting N; Newton H; Barcus S; Muradyan I; Dabaghyan M; Moroz GD, et al. Near-Unity Nuclear Polarization with an ‘Open-Source’ 129Xe Hyperpolarizer for NMR and MRI. Proc. Natl. Acad. Sci. U. S. A 2013, 110, 14150–14155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barskiy DA; Coffey AM; Nikolaou P; Mikhaylov DM; Goodson BM; Branca RT; Lu GJ; Shapiro MG; Telkki V-V; Zhivonitko VV, et al. NMR Hyperpolarization Techniques of Gases. Chem. Eur. J 2017, 23, 725–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barskiy DA; Salnikov OG; Romanov AS; Feldman MA; Coffey AM; Kovtunov KV; Koptyug IV; Chekmenev EY NMR Spin-Lock Induced Crossing (SLIC) Dispersion and Long-Lived Spin States of Gaseous Propane at Low Magnetic Field (0.05 T). J. Magn. Reson 2017, 276, 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Erickson SH; Oppenheim GL; Smith GH Metronidazole in Breast Milk. Obstet Gynecol 1981, 57, 48–50. [PubMed] [Google Scholar]
- 50.Kizaka-Kondoh S; Konse-Nagasawa H Significance of Nitroimidazole Compounds and Hypoxia-Inducible Factor-1 for Imaging Tumor Hypoxia. Cancer Sci 2009, 100, 1366–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Procissi D; Claus F; Burgman P; Koziorowski J; Chapman JD; Thakur SB; Matei C; Ling CC; Koutcher JA In Vivo 19F Magnetic Resonance Spectroscopy and Chemical Shift Imaging of Tri-Fluoro-Nitroimidazole as a Potential Hypoxia Reporter in Solid Tumors. Clin. Cancer Res 2007, 13, 3738–3747. [DOI] [PubMed] [Google Scholar]
- 52.Komar G; Seppänen M; Eskola O; Lindholm P; Grönroos TJ; Forsback S; Sipilä H; Evans SM; Solin O; Minn H 18F-EF5: A New PET Tracer for Imaging Hypoxia in Head and Neck Cancer. J. Nucl. Med 2008, 49, 1944–1951. [DOI] [PubMed] [Google Scholar]
- 53.Hendrickson K; Phillips M; Smith W; Peterson L; Krohn K; Rajendran J Hypoxia Imaging with [F-18] FMISO-PET in Head and Neck Cancer: Potential for Guiding Intensity Modulated Radiation Therapy in Overcoming Hypoxia-Induced Treatment Resistance. Radiother. Oncol 2011, 101, 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Masaki Y; Shimizu Y; Yoshioka T; Tanaka Y; Nishijima K.-i.; Zhao S; Higashino K; Sakamoto S; Numata Y; Yamaguchi Y, et al. The Accumulation Mechanism of the Hypoxia Imaging Probe “FMISO” by Imaging Mass Spectrometry: Possible Involvement of Low-Molecular Metabolites. Sci. Rep 2015, 5, 16802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwartz J; Grkovski M; Rimner A; Schöder H; Zanzonico PB; Carlin SD; Staton KD; Humm JL; Nehmeh SA Pharmacokinetic Analysis of Dynamic 18F-Fluoromisonidazole PET Data in Non–Small Cell Lung Cancer. J. Nucl. Med 2017, 58, 911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


