Abstract
Renal expression of klotho is reduced in hypertension. Experiments were performed to examine whether exogenous klotho protein supplementation ameliorates pressure natriuresis in early phase of hypertension, using stroke-prone spontaneously hypertensive rats (sp-SHR). The interactions between klotho protein and renal renin-Ang (angiotensin) system were examined with immunoprecipitation and cell culture methods. Uninephrectomy was performed in sp-SHRs to induce nephrosclerosis, and they were treated with exogenous klotho protein or vehicle. Exogenous klotho protein supplementation to sp-SHR decreased blood pressure, renal Ang II levels, AGT (angiotensinogen) expression, HIF (hypoxia-inducible factor)-1α abundance, and medullary fibronectin levels, with increased renal klotho expression and serum and urine klotho levels. Klotho supplementation also reduced kidney weight, renal phosphorylated Akt, and mTOR (mammalian target of rapamycin) abundance. Furthermore, klotho supplementation restored renal autoregulation of glomerular filtration rate and enhanced pressure-induced natriuresis in sp-SHR. Klotho protein bound to AT1R (Ang II type- 1 receptor) and decreased the presence of AT1R on HK-2 (human proximal tubular) cells, attenuating inositol triphosphate generation. Klotho protein suppressed Ang II-induced increments of AGT expression in HK-2 cells. Collectively, the present data demonstrate that klotho binds with the AT1R to suppress Ang signal transduction, participating in inactivating renal renin-Ang system. Our results also suggest that exogenous klotho supplementation represses Akt-mTOR signaling to reduce renal hypertrophy and restore the autoregulatory ability of glomerular filtration rate in uninephrectomized sp- SHRs. Finally, the present findings implicate that klotho supplementation inhibits HIF-1α pathway and medullary fibrosis, contributing to enhancements of pressure natriuresis and reduction in blood pressure.
Keywords: angiotensinogen, fibronectin, klotho protein, natriuresis, rats
Klotho is a type-1 membrane protein expressed mainly in the kidneys.1 Peptidases of a disintegrin and metalloproteinase family cleave m-klotho (membrane-bound klotho) to release its extracellular domain, which then diffuses into the renal interstitium. Secreted klotho reaches various regions within the kidneys, such as the glomeruli and medulla, and then enters systemic circulation.2,3 Thus, renal interstitial concentration of klotho could exceed serum klotho level. The kidneys are the main source of circulating klotho, which functions as an antiaging protei0n.4 Secreted klotho binds to Wnt, thus inhibiting Wnt signaling, which promotes aging and activates the renin-Ang (angiotensin) system (RAS).5,6 Both renal and circulating klotho levels are decreased in hypertensive nephrosclerosis.7 Klotho interacts with many membrane proteins, such as transient receptor potential channel 1, transient receptor potential vanilloid 5, type2 sodium-phosphate co-transporter, and receptors of fibroblast growth factor 23, IGF (insulin-like growth factor), parathyroid hormone, and TGFß (transforming growth factor β).1,4,8,9
Renal medulla plays an important role in the pathogenesis of hypertension. Pharmacological papillary necrosis prevents pressure-induced natriuresis,10 thus supporting that medullary circulation determines blood pressure levels.11 Hyperfiltration occurs in juxtamedullary nephrons but not in cortical nephrons of uninephrectomized spontaneously hypertensive rats (SHRs), suggesting that deep nephrons are exposed to excessive albumin excretion during hypertension.12,13 However, glomerular blood flow is almost perfectly autoregulated in juxtamedullary nephrons of normotensive rats.14 In SHRs, pathological lesions, such as fibrinoid necrosis, sclerosis, and tubulointerstitial changes are initially confined to the juxta- medullary zones, sparing the outer cortex. Normal kidneys possess resident fibroblasts in the medulla but almost no fibroblasts in the cortex. Ang II induces profibrotic changes in medullary fibroblasts by activating HIF (hypoxia-inducible factor)-1α.15 These findings indicate that the renal medulla is selectively damaged in the initial phase of hypertension.
The RAS contributes to the development and maintenance of high blood pressure. Ang II induces glomerular arteriolar constriction and enhances both proximal tubular reabsorption and tubuloglomerular feedback.16 We previously showed that the renal RAS is involved in the pathogenesis of hypertension in SHRs.17 Although Ang II maintains the autoregulation of glomerular filtration rate (GFR), it attenuates pressure natriuresis.16 There is general agreement that the renal RAS is regulated independently of the systemic RAS.18 Activation of the renal RAS during Ang II-infused hypertension involves increased renal AGT (angiotensinogen) expression, which is triggered, at least partly, by AT1R (Ang II type-1 receptor) internalization.19 In the present study, we examined the potential interaction between klotho and the AT1R and determined whether exogenous supplementation of rh-klotho (recombinant human klotho protein, 516 amino acids) ameliorated renal RAS, medullary fibrosis, and pressure natriuresis in early phase of hypertension.20
Methods
The authors declare that ah supporting data are available within the article and in the online-only Data Supplement.
In Vitro Cell-Free Analysis of Klotho Binding
As detailed previously,8 antibodies against the human AT1R (10 μg; Abcam) were incubated with protein G-Sepharose beads (25% v/v; GE Health Care Life Sciences) in 0.1 mL binding buffer (Krebs- Ringer-Hepes buffer with 1% BSA) for 2 hours at room temperature. After washing the beads with the binding buffer, full-length human AT1R (1 μΜ; Novus Biologicals) was added to the beads suspended in 0.1 mL binding buffer, and the beads were incubated further at 4°C for 15 hours. Next, the beads were washed, treated with rh-klotho (10−10–10−8 M in 0.1 mL binding buffer), and incubated further at 4°C for 15 hours. The solution of rh-klotho was made freshly with HEPES buffer on the day of each in vitro experiment. The samples were centrifuged, and the beads were washed 3× with the binding buffer and 3× with the Krebs-Ringer-Hepes buffer lacking BSA. Proteins bound to the beads were detected by Western blotting with a monoclonal anti-klotho antibody (KM2076; provided by Hyowa- Hakko Kirin, Co, Ltd.). Similar experiments were performed using β-glucuronidase (Abcam) or kidney homogenates containing endogenous AT1R and m-klotho. Klotho shows β-glucuronidase activity and similar size to β-glucuronidase.1
In additional experiments, Sepharose beads coated with the AT1R were incubated in 0.2 mL binding buffer containing fixed amount of 125I-Ang II (10−9 M) and increasing doses of rh-klotho (10−10–10−8 M) for 1 hour at room temperature.8 Next, the beads were washed 3× with the binding buffer and 3× with the Krebs-Ringer-Hepes buffer lacking BSA and were subjected to a scintillation counter. Nonspecific binding was determined by replacing rh-klotho with unlabeled Ang II (Sigma-Aldrich) in an excess of 100-fold 125I-Ang II.
Cell Culture
Human proximal tubular (HK-2) cells were purchased from American Type Culture Collection (CRL-2190) and were cultured in keratino- cyte serum-free medium supplemented with bovine pituitary extract and human recombinant epidermal growth factor until confluency.21 The cells were then cultured in keratinocyte serum-free medium lacking both bovine pituitary extract and epidermal growth factor for 1 day before performing acute experiments. Ang II (1–10 nmol/L) was added to the culture medium in the presence or absence of rh- klotho (10−9 M). This dose of rh-klotho showed considerable binding to AT1Rs. Inositol triphosphate (IP3) levels in the culture medium were measured at 5, 10, and 20 minutes after adding Ang II by using an ELISA kit (Antibodies-Online). Because variations in intracellular IP3 levels are relatively large among cultured sheets, the medium was used as a sample.8
Separate experime(PeproTech, Inc, Rocky Hill, NJnts were performed to examine the potential effects of klotho on AT1R internalization. As previously described,22 HK-2 cells were stimulated with Ang II (100 nmol/L) in the presence or absence of rh-klotho (10−10–10−8 M) for 5 to 20 minutes at 37°C. Cells were treated with rh-klotho or vehicle for 30 minutes before Ang II challenge. Surface-bound ligands were removed by treatment with a gentle acid wash (50 mmol/L sodium citrate, 0.2 mmol/L sodium phosphate, 90 mmol/L NaCl, and 0.1% BSA [pH 5.0]) for 10 minutes at 4°C. Radioligand binding assay was performed at 4°C for 5 hours to quantify receptors remaining on cell surface. Internalized receptors were expressed as the percentage loss of cell surface binding compared with that in cells exposed to neither Ang II nor rh-klotho.
In additional studies, we assessed the effects of klotho protein on the induction of AGT and AT1R expression by Ang II in HK-2 cells.21 First, rh-klotho (10−9 M) or vehicle was added to the media. After 30 minutes, Ang II (3 nM) was added. Cells were collected from the sheets under basal condition, 1 and 24 hours later from Ang II exposure to evaluate AGT and AT1R expressions.
In Vivo Animal Study
Six-week-old male stroke-prone SHRs were purchased from Charles River Labs (Yokohama, Japan). All experimental protocols were approved by the ethical committee for animal research of Keio University (permit No. 14 001-[0]).
At 7 weeks of age, the rats were anesthetized by intraperitone- ally administering pentobarbital sodium (50 mg/kg), and left unine-phrectomy was performed to induce nephrosclerosis for relatively short period.20,23 As described in Table S1 in the online-only Data Supplement, renal expression of klotho was reduced by hypertension and uninephrectomy. At 8 weeks of age, the rats were divided into 2 groups (n=12 per group)1: rats treated with a vehicle (control group)2 and rats treated with rh-klotho (PeproTech, Inc, Rocky Hill, NJ; klotho group). Rh-klotho ameliorates calcium and phosphate abnormalities in klotho-deficient animals, indicating that it is physiologically active.8 Rh-klotho was dissolved in 1 mL saline with 0.1% BSA and administered daily through a subcutaneous injection (10 μg/kg per day). A vehicle control group received the same amount of saline containing 0.1% BSA. Systolic blood pressure was measured every 2 weeks by using tail-cuff method, and urine was collected every 2 weeks to measure albumin, creatinine, klotho, and PGF2α (prostaglandin F2a) levels. At 12 weeks of age, 12 rats (6 rats from each group) were anesthetized, and their femoral artery was cannulated to obtain blood samples for various analyses, including measurement of Ang II, FGF (fibroblast growth factor) 23, and klotho levels. In addition, the kidneys were removed, weighed, and cut in half. One half of the kidneys was frozen quickly in liquid nitrogen for the analyses, and the other half was fixed in 4% formalin solution for pathology.6 The rats were then euthanized by administering an overdose of the anesthetic. Blood samples were centrifuged at 4°C for 10 minutes. Serum, plasma, urine, and tissues were deep-frozen until their use.3
For the other 12 rats, 2 days before acute experiments, the chow containing LiCl (5 mmol/Kg) was given to obtain measurable concentrations of lithium, and its clearance as an index of end-proximal tubular flow without acute diuresis.24 On the day of acute experiment, rats were anesthetized with intraperitoneal Inactin (100 mg/kg; Byk Gulden, Konstanz, Germany). To directly monitor renal perfusion pressure (RPP), femoral and carotid arteries were cannulated.17,25 Mean blood pressure was used as RPP. Jugular vein was also can- nulated (PE50) to infuse saline containing 1% BSA, 7.5% Inutest (Laevosan-Gesellschaft, Linz/Donau Austria). The ureter was can- nulated (PE10) with a midline abdominal incision to collect urine in a preweighed tube. Adjustable clamps were placed on the aorta above and below the renal artery to control RPP. Rats were allowed to breathe air enriched with 100% oxygen (aimed at FiO2 of 40%), which markedly improves the stability of arterial blood pressure. After completion of surgery, 1 hour of equilibration was allowed. Before initiating pressure natriuresis study, basal blood pressure was checked. The upper clamp was tightened to decrease RPP approximately by 20 mm Hg. After 30 minutes of equilibration, 2 separate 20-minute clearance studies were performed. The upper clamp was loosened to return RPP to the baseline. After 30 minutes of equilibration, clearance studies were performed in 2 separate 20-minute periods. The lower clamp was tightened to increase RPP by about 20 mm Hg. After 30 minutes of equilibration, 2 20-minute clearance studies were performed. Inulin clearance was considered as GFR. Because GFR altered with the variations of RPP in control group, fractional excretion of sodium was used as the index for natriuresis. All clearance studies were performed in 12-week-old uninephrecto- mized stroke-prone SHRs.
Please refer online-only Data Supplement for detailed in vivo study, reverse transcription polymerase chain reaction, Western blotting, and pathology.
Data are expressed as mean±SEM. Statistical analysis was performed using Student t test, linear regression analysis, or ANOVA followed by Newman-Keuls test. P value of <0.05 was considered statistically significant.
Results
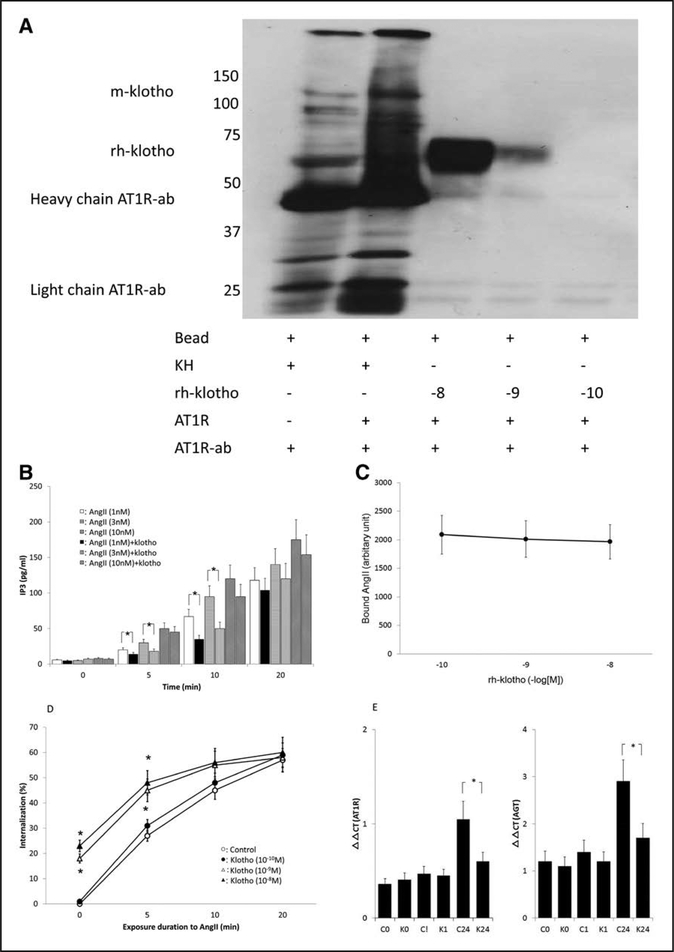
The present study was, to our knowledge, the first to show that klotho interacted with the AT1R (Figure 1A). Our results exhibited that ≥10−9 M rh-klotho was bound to beads coated with the human AT1R. Moreover, m-klotho was detected when these beads were treated with kidney homogenates, which should contain both the AT1R and m-klotho. In contrast, β-glucuronidase did not show substantial binding to AT1R (Figure S1). Multiple-way ANOVA for repeated measurements revealed that Ang II induced dose- and time-dependent elevations of IP3 in HK-2 cells19 and that klotho protein suppressed Ang II-induced IP3 elevations (P<0.005 for each). At 5 and 10 minutes, Ang II (1 and 3 nM) elicited smaller increments of IP3 in the presence of klotho protein (Figure 1B). However, our results showed that increasing doses of rh- klotho did not inhibit the amounts of Ang II bound on ATIRs (Figure 1C). Ang II elicited time-dependent increases in AT1R internalization in HK-2 cells, and klotho protein altered Ang II-induced AT1R internalization (P<0.001 for each). At 10−9 M and higher, rh-klotho also enhanced the amounts of AT1Rs lost following to Ang II stimulation for 5 minutes (Figure 1D). In addition, klotho protein lowered AT1Rs on HK-2 cells by itself. Thus, Ang II could internalize smaller amounts of tubular AT1Rs in the presence of klotho protein. As depicted in Figure 1E, ANOVA showed that Ang II caused time-dependent increments of AGT and AT1R expressions in HK-2 cells, and klotho protein attenuated them (P<0.005 for each). Although rh-klotho (10−9M) failed to alter AGT and AT1R expression by an hour of Ang II exposure, rh-klotho attenuated the expression of AT1R and AGT induced by the treatment with Ang II for a day in HK-2 cells.
Figure 1.
Summary of in vitro studies. A, Binding of the human AT1R (Ang II [angiotensin II] type-1 receptor) to rh-klotho (recombinant human klotho). This binding is evident at high concentrations of rh-klotho, which composed of KL-1 gene (n=5). Kidney homogenate (KH), AT1R-ab (antibody against the AT1R), m-klotho (membrane-bound klotho), −8: 10−8 M, −9: 10−9 M, −10: 10−10 M. B, Ang II stimulation increased inositol triphosphate (IP3) levels (n=6 for each). Vertical axis showed the amount of IP3. Ang II (1 and 3 nM) caused smaller increments of IP3 in the presence of klotho at 5 and 10 min. Open, horizontal line, and right oblique line bars indicate 1, 3, 10 nmol/L of Ang II exposure without klotho treatment. Closed, vertical line, and left oblique line bars depict 1, 3, 10 nmol/L of Ang II with klotho treatment, respectively. *Significant difference between control and klotho-treated groups. C, Treatment with rh- klotho did not alter the binding of Ang II to the AT1R (n=4 for each). D, Ang II-induced time- dependent internalization of the AT1R (n=6 for each). Vertical axis showed the magnitude of AT1R internalization. Klotho enhanced the amounts of AT1Rs lost following to Ang II stimulation for 5 min. Open and closed circles indicate control and 10−10 M rh-klotho-treated groups, respectively; open and closed triangles depict 10−9 and 10−8 M rh-klotho-treated groups, respectively. *Significant difference compared with the control group. E, Ang II elicited time-dependent inductions of AT1R and AGT (angiotensinogen; n=6 for each). Target gene expression was compared by relative changes in comparative threshold (ΔΔΟ) using Gapdh as reference. *Significant difference between control and klotho-treated groups. C0 indicates the values from control group at time 0; C1, the values from control group at 1 h; C24: the values from control group at 24 h; K0, the values from klotho-treated group at time 0; K1, the values from klotho-treated group at 1 h; and K24, the values from klotho-treated group at 24 h.
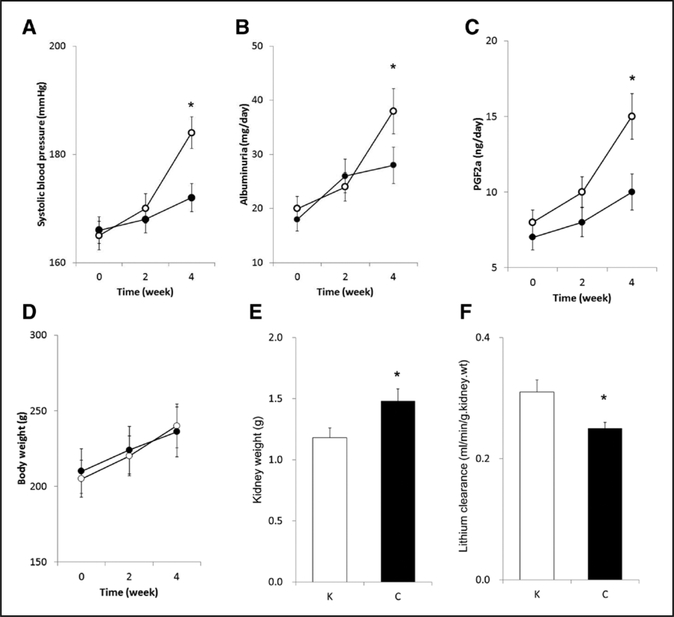
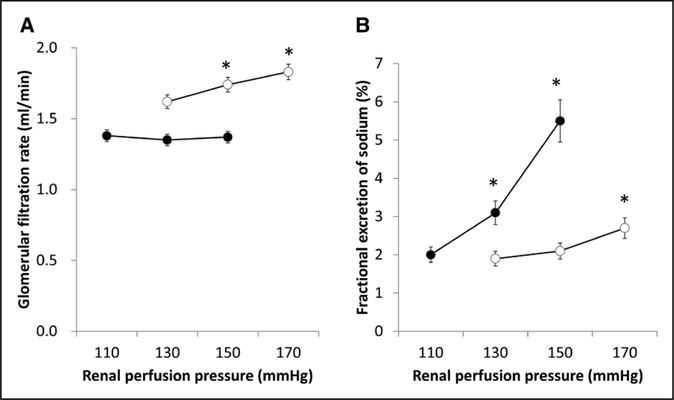
Although systolic blood pressure was elevated with time, daily supplementation of klotho protein significantly attenuated systolic blood pressure at 4 experimental weeks (Figure 2A). In addition, daily klotho protein supplementation declined time-dependent increase in albumin excretion (Figure 2B). Similarly, exogenous klotho protein supplementation decreased time-dependent increases in 8-epi-PGF2αexcretion by experimental week 4 (Figure 2C). Although exogenous klotho protein supplementation did not affect the temporal increase in body weight without considerable changes in appetite, drinking, and activity (Figure 2D), it decreased kidney weight by experimental week 4 (Figure 2E). Our data constitute new demonstration that klotho protein supplementation restored autoregulatory ability (Figure 3A) in association with considerable increments of lithium clearance at experimental week 4 (Figure 2F). Compared with control group (0.42±0.09), GFR autoregulatory index was attenuated in klotho group (0.02±0.02, P<0.01). Of note, at the baseline mean blood pressure levels, whole kidney GFR in klotho-treated animals (1.35±0.05 mL/min at 128±2 mm Hg) was significantly less than the controls (1.76±0.06 mL/min at 147±3 mm Hg, P<0.01). Furthermore, exogenous klotho protein supplementation markedly sharpened pressure natri- uresis responses in uninephrectomized stroke-prone SHRs (Figure 3B). Compared with control group (0.024±0.009 %/ mm Hg), the slope for fractional excretion of sodium against RPP was accentuated by klotho supplementation (0.074±0.014 %/mm Hg, P<0.05).
Figure 2.
Influences of klotho protein supplementation on systolic blood pressure (A), albuminuria (B), 8-epi-PGF2α (prostaglandin F2a) excretion (C), body weight (D), kidney weight (E), and lithium clearance at basal blood pressure (F). Open and closed circles indicate control (C) and klotho (K)-treated groups (F: n=6 for each; the other parameters: n=12 for each), respectively. *Significant difference between the 2 groups
Figure 3.
Impacts of exogenous klotho protein supplementation on autoregulation of glomerular filtration rate (A), pressure-induced natriuresis (B). Open and closed circles indicate control and klotho-treated groups (n=6 for each). *Significant difference from the values at the lowest renal perfusion pressure within the group.
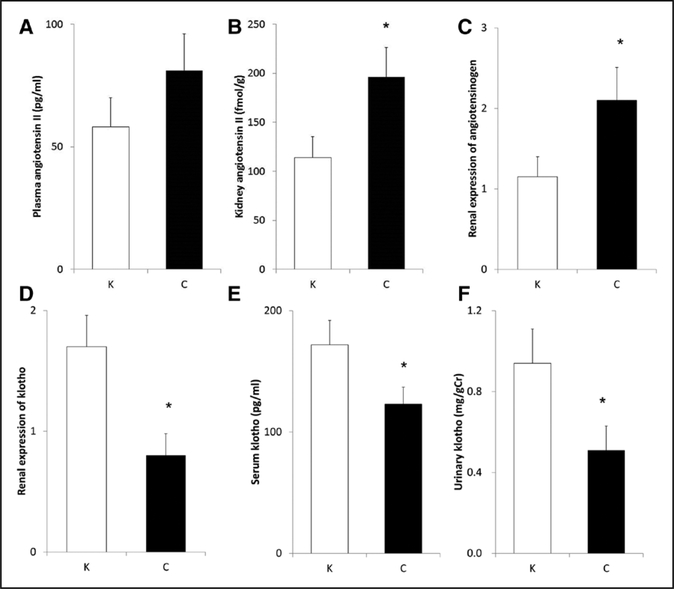
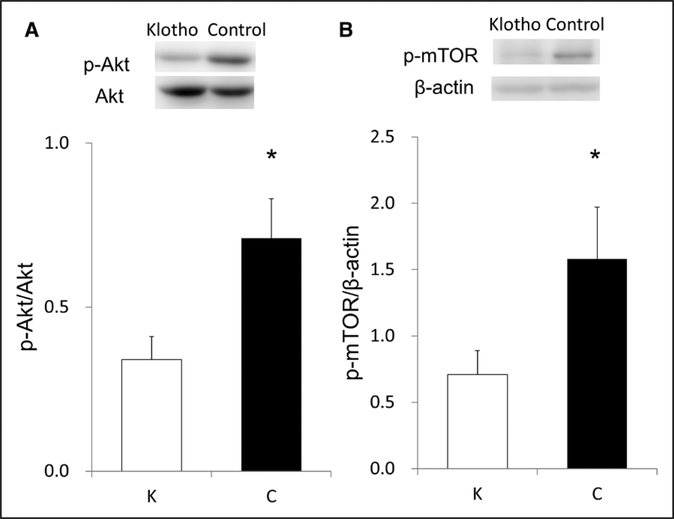
Although exogenous supplementation of klotho protein did not significantly reduce plasma Ang II concentrations (Figure 4A), it considerably decreased renal Ang II levels (Figure 4B) and renal AGT expression (Figure 4C) in uni- nephrectomized stroke-prone SHRs. Klotho protein supplementation substantially increased renal klotho expression (Figure 4D) and elevated both serum klotho levels and urinary klotho excretion (Figure 4E and 4F) to the levels similar to normotensive control rats.6 Of note, klotho supplementation failed to alter serum calcium, phosphate, and FGF23 levels in this model (Table S2). Early phase of kidney disease model may relate to small effects of klotho supplementation on mineral metabolism. In contrast, klotho protein supplementation significantly decreased phosphorylated Akt (Figure 5A) and mTOR (mammalian target of rapamycin; Figure 5B) abundance.
Figure 4.
Effects of exogenous klotho supplementation on plasma Ang II (angiotensin II) concentration (A), renal Ang II concentration (B), renal AGT (angiotensinogen) expression (C), renal klotho expression (D), serum klotho concentration (E), urine klotho levels (F). *Significant difference between the 2 groups.C indicates control group; and K, klotho group (n=6 for each).
Figure 5.
Influences of klotho protein supplementation on renal p-Akt (phosphorylated Akt) abundance (A) and renal p-mTOR (phosphorylated mTOR, mammalian target of rapamycin) abundance (B). *Significant difference between the 2 groups. C indicates control group; and K, klotho group (n=6 for each).
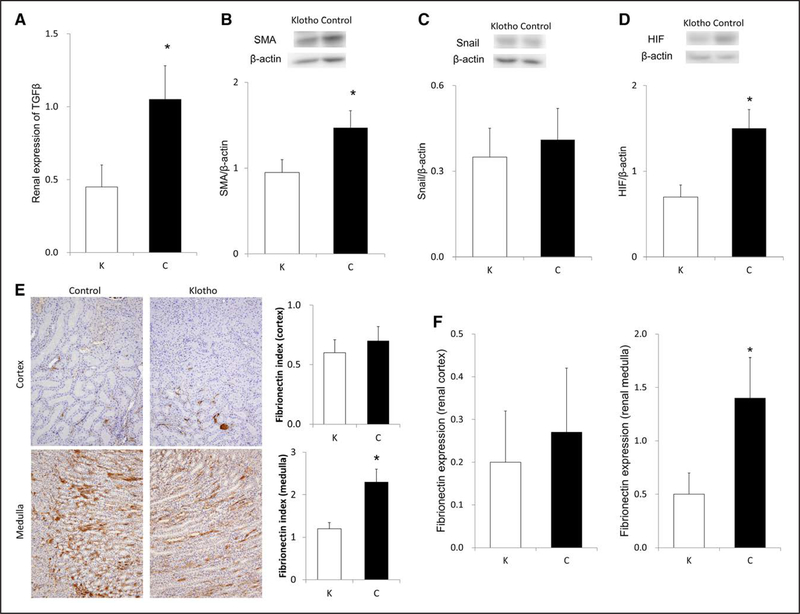
Because hypertension initially damages the juxtamedullary nephrons13 and because HIF-1α induces profibrotic changes in medullary fibroblasts,15 we assessed medullary fibrosis by performing immunohistochemical analysis. Klotho protein supplementation decreased TGFß expression (Figure 6A). Moreover, klotho protein supplementation marginally ameliorated α-SMA (α smooth muscle actin) (Figure 6B) but not Snail abundance (Figure 6C). Exogenous klotho supplementation considerably ameliorated renal abundance of HIF-1α (Figure 6D). Furthermore, klotho protein supplementation decreased fibronectin staining of renal medulla but not cortex (Figure 6E). Similarly, fibronectin expression was reduced by klotho protein supplementation in renal medulla (Figure 6F) but not cortex (Figure 6F). Moreover, the results of fibrosis indexes using Masson trichrome staining manifested similar trends to those of immunohistochemical analyses on fibronec- tin (Figure S2).
Figure 6.
Impacts of exogenous klotho protein supplementation on renal TGFß (transforming growth factor β) expression (A); renal α-SMA (α smooth muscle actln; B), Snail (C), and HIF (hypoxia-inducible factor)-1α abundance (D). Fibronectin staining in the renal cortex (E, upper) and medulla (E, lower). Klotho supplementation declined fibronectin index in the renal medulla (E, right). Fibronectin is stained in brown. Fibronectin index was calculated similarly to fibrosis index, by measuring the percentage of area stained in brown. Klotho also reduced fibronectin expression in the medulla (F, right). E: n=12 for each; the other parameters: n=6 for each. *Significant difference between the 2 groups. C indicates control group; and K, klotho group.
Discussion
Hypertensive nephrosclerosis is a common chronic kidney disease that requires renal replacement therapy. Although antihypertensive therapy is the main strategy for delaying the progression of nephrosclerosis, it may not completely prevent the progression to end-stage renal failure. Established hypertensive nephrosclerosis is characterized by tubulointerstitial fibrosis, arteriosclerosis, arteriolosclerosis, and glomerular sclerosis. Hypertension initially damages the juxtamedullary nephrons,12,13 and resultant medullary pathology further increases blood pressure.10,11 Thus, high blood pressure and renal medullary lesions form a vicious cycle, which could be disrupted by employing therapeutic strategies focused on medullary fibrosis.
The present data constitute new demonstrations that klotho interacts with AT1Rs to diminish their localization on the cell surface. Two domains of the carboxyl terminus and 1 domain of the third intracellular loop are important for the internalization of activated AT1R.22 AT1R stimulation by Ang II results in the translocation of β-arrestin to the AT1R and subsequently elicits the internalization of the receptor.26–28 Binding of klotho to the AT1R may elicit conformational changes in these regions, thus enhancing the internalization of the receptor to reduce surface density. In addition to AT1R stimulation, the internalized Ang II induces AGT expression.19 These in vitro data are consistent with the in vivo results that klotho protein supplementation attenuated renal AGT expression and elevations of blood pressure. The present findings also showed that exogenous klotho supplementation inhibited Akt phosphorylation. We recently showed that GSK3ß (glycogen synthase kinase 3β) inhibition increased AGT expression in the kidneys.6 GSK3ß is activated by the Akt pathway to control glucose metabolism. Collectively, the present findings suggested that klotho- induced modulations of renal RAS involve multiple pathways, AT1R internalization, as well as Akt phosphorylation and oxidative stress in hypertensive kidneys.
The present study indicated that klotho protein supplementation decreased TGFß expression and α-SMA abundance in hypertensive kidneys. However, exogenous klotho supplementation did not alter Snail abundance. Hypertensive kidneys show weak TGFß and Snail levels compared with kidneys with adriamycin nephropathy.6 Albuminuria strongly induces TGFß expression in tubular cells of the nephron.29 However, albumin excretion in uninephrectomized SHRs was much lower than that in rats with adriamycin nephropathy. Ang II induces TGFß expression in renal tubular cells, thus inducing epithelial-mesenchymal transition in an autocrine manner.30 Klotho inhibits TGFß signaling.9 These may account for the discrepant results. We previously showed that the contribution of epithelial-mesenchymal transition in renal fibrosis differs among various underlying kidney diseases.31 In addition, cortical fibrosis was not apparent in the present study. Together, these results extended the above-mentioned findings31 and suggest that the contribution of epithelial-mesenchymal transition to renal fibrosis is relatively small in the early phase of hypertension.
Renal fibrosis is the common final pathway that leads to end-stage renal failure.31 To our knowledge, the present study showed for the first time that klotho protein supplementation ameliorated PGF2α excretion and HIF-1α abundance in hypertensive kidneys. Ang II stabilizes HIF-1α, which promotes both fibrosis and cell growth.32 The excretion of PGF2α represents an index of oxidative stress.6,7 Thus, these findings suggested that klotho-induced decrease in renal Ang II levels and oxidative stress contributed to the decrements of HIF-1α abundance. Wang et al15 showed that Ang II induced profibrotic changes by activating HIF-1α in medullary fibroblasts. In the present study, we found that exogenous klotho supplementation inhibited medullary fibronectin expression and sharpened pressure-induced natriuresis responses. Our data extended those of Bank et al13 who showed that tubulointerstitial changes were first confined to the juxtamedul- lary zones except the outer cortex in SHRs and suggest that klotho-induced alterations of medullary pathology underlie the augmentation of pressure natriuresis. Taken together, the present study indicated that klotho protein supplementation attenuated renal medullary fibrosis in hypertensive rats and suggested that klotho could crosstalk with the renal RAS to suppress medullary fibrosis, contributing to enhancing pressure-induced natriuresis in hypertensive kidneys.
Compensatory renal hypertrophy is characterized by proximal tubular growth and elongation, which diminish the delivery to macula densa and then tubuloglomerular feedback.33,34 The present results that exogenous klotho supplementation decreased phosphorylated Akt levels in hypertensive kidneys are consistent with the notion that klotho interacts with IGF receptor to inhibit its signaling.4 In addition, our study is the first to show that klotho protein supplementation inhibited mTOR phosphorylation in the kidneys. Chen et al35 showed that class III PI3K (phosphoinositide 3-kinase)-mTOR signaling mediates compensatory renal hypertrophy. Furthermore, kidney weights of rats receiving exogenous klotho protein were lower than those of the controls, supporting that klotho suppressed compensatory renal hypertrophy. This was supported by physiological significance that lithium clearance was increased by klotho protein supplementation. These observations indicate that klotho reduced proximal tubular reabsorption and suggest that klotho-induced increase in distal delivey is involved in controlling tubuloglo- merular feedback and GFR autoregulation. Of course,14 we would not exclude the possibility that klotho-induced reductions of blood pressure could ameliorate renal arteriolopathy (Figure S3). Consequently, the present findings are consistent with those of Kumar et al36 and suggested that klotho-induced inhibition of the Akt-mTOR pathway decreased compensatory renal hypertrophy. However, mTOR signaling is important for normal growth, implicating that klotho supplementation may not suit for children with renal diseases, but for adult patients with diabetic nephropathy37 and polycystic kidney disease.38
Our study has several limitations. First, although our data did not prove endogenous klotho loss as a mechanism of hypertension, klotho gene delivery to 12-week-old SHR prevented the progression of hypertension.39·40 The present study focused on early phase of hypertension, using klotho protein supplementation that may ethically be more acceptable than gene transfer. It remained to be determined whether klotho improved the phenotypes or attenuated the development of disease-related phenotypes. Second, genetically modified ATIRs were not used. Thus, precise structural basis for klotho interactions remained to be elucidated. Third, klotho decreased IP3 levels after Ang II stimulation. However, we did not examine the effects of klotho supplementation on AT1R desensitization.28 Fourth, exogenous klotho supplementation enhanced endogenous klotho expression. Marginal reductions in FGF23 might have induced natriuresis,41 possibly contributing to the present results. Finally, healthy control arm and experimental group under AT1R blockade were not included in the present study. Because the present study only assessed the relative benefit of exogenous klotho treatment, cautions should be exercised before generalizing the results.
Perspectives
The present study was the first to show that klotho interacted with the AT1R to modulate its internalization and to inactivate the renal RAS. Furthermore, our results indicated that klotho suppressed medullary fibrosis through the HIF-1α pathway, enhancing pressure natriuresis. Our results also suggested that klotho supplementation decreased the Akt-mTOR signaling to ameliorate renal hypertrophy and GFR autoregulation. These results provide translational basis to evaluate the utility of exogenous klotho protein supplementation for treating patients with hypertensive nephrosclerosis.
Supplementary Material
Novelty and Significance
What Is New?
Klotho interacts with the AT1R (Ang II [angiotensin II] type 1 receptor) to inactivate the renal renin-Ang system.
Klotho suppresses medullary fibrosis through the HIF (hypoxia-inducible factor)-1α pathway in early hypertension.
Klotho supplementation decreases Akt-mTOR (mammalian target of rapamycin) signaling.
What Is Relevant?
Exogenous klotho protein supplementation would be useful for hypertensive kidney diseases
Renal renin-Ang system contributes at least partly to the pathogenesis of medullary fibrosis and high blood pressure.
Klotho supplementation could ameliorate renal hypertrophy.
Summary
Klotho suppresses renal renin-Ang system at least partly by interacting with the AT1Rs. Klotho supplementation suppresses Akt-mTOR signaling, declining renal hypertrophy and improving glomerular filtration rate autoregulation. Medullary fibrosis in early hypertension involves HIF-1α pathway, attenuating pressure natriuresis and elevating blood pressure.
Acknowledgments
We thank Maho Yamashita, Hiroko Sano, Yumi Sakane, Mami Fukuoka, and Maiko Sato for providing technical assistance.
Sources of Funding
This study is supported by grants from the Japanese Ministry of Education, Culture, Sports, Science and Technology (Japan Society for the Promotion of Science KAKENHI 16K09625) and the Japan Kidney Foundation (JKFB15–57).
Footnotes
Disclosures
None.
Contributor Information
Tsuneo Takenaka, Department of Medicine, International University of Health and Welfare, Minato, Tokyo, Japan.
Tsutomu Inoue, Department of Nephrology, Saitama Medical University, Iruma, Japan.
Takashi Miyazaki, Department of Nephrology, Saitama Medical University, Iruma, Japan.
Hiroyuki Kobori, Department of Medicine, International University of Health and Welfare, Minato, Tokyo, Japan.
Akira Nishiyama, Department of Pharmacology, Kagawa University, Kita, Japan.
Naohito Ishii, Department of Clinical Chemistry, Kitasato University, Sagamihara, Kanagawa, Japan.
Matsuhiko Hayashi, Department of Blood Purification Center, Keio University, Shinjuku, Tokyo, Japan.
Hiromichi Suzuki, Department of Nephrology, Saitama Medical University, Iruma, Japan.
References
- 1.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285 [DOI] [PubMed] [Google Scholar]
- 2.Takenaka T, Watanabe Y, Inoue T, Miyazaki T, Suzuki H. Fibroblast growth factor 23 enhances renal klotho abundance. Pflugers Arch. 2013;465:935–943. doi: 10.1007/s00424-013-1226-z [DOI] [PubMed] [Google Scholar]
- 3.Takenaka T, Inoue T, Miyazaki T, Nishiyama A, Ishii N, Hayashi M, Suzuki H. Antialbuminuric actions of calcilytics in the remnant kidney. Am J Physiol Renal Physiol. 2015;309:F216–F226. doi: 10.1152/ajprenal.00003.2015 [DOI] [PubMed] [Google Scholar]
- 4.Kurosu H, Yamamoto M, Clark JD, et al. Suppression of aging in mice by the hormone klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. doi: 10.1126/science.1143578 [DOI] [PubMed] [Google Scholar]
- 6.Takenaka T, Inoue T, Miyazaki T, Kobori H, Nishiyama A, Ishii N, Hayashi M, Suzuki H. Klotho suppresses the renin-angiotensin system in adriamycin nephropathy. Nephrol Dial Transplant. 2017;32:791–800. doi: 10.1093/ndt/gfw340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takenaka T, Inoue T, Ohno Y, Miyazaki T, Nishiyama A, Ishii N, Suzuki H. Calcitriol supplementation improves endothelium-dependent vasodilation in rat hypertensive renal injury. Kidney Blood Press Res. 2014;39:17–27. doi: 10.1159/000355773 [DOI] [PubMed] [Google Scholar]
- 8.Takenaka T, Inoue T, Miyazaki T, Hayashi M, Suzuki H. Xeno-klotho inhibits parathyroid hormone signaling. J Bone Miner Res. 2016;31:455–462. doi: 10.1002/jbmr.2691 [DOI] [PubMed] [Google Scholar]
- 9.Doi S, Zou Y, Togao O, Pastor JV, John GB, Wang L, Shiizaki K, Gotschall R, Schiavi S, Yorioka N, Takahashi M, Boothman DA, Kuro-o M. Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem. 2011;286:8655–8665. doi: 10.1074/jbc.M110.174037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen PS, Caldwell RM, Hsu CH. Role of renal papillae in the regulation of sodium excretion during acute elevation of renal perfusion pressure in the rat. Hypertension. 1984;6(6 pt 1):893–898. [DOI] [PubMed] [Google Scholar]
- 11.Cowley AW, Roman RJ, Fenoy FJ, Mattson DL. Effect of renal medullary circulation on arterial pressure. J Hypertens Suppl. 1992;10:S187–S193. [PubMed] [Google Scholar]
- 12.Feld LG, Van Liew JB, Brentjens JR, Boylan JW. Renal lesions and proteinuria in the spontaneously hypertensive rat made normotensive by treatment. Kidney Int. 1981;20:606–614. [DOI] [PubMed] [Google Scholar]
- 13.Bank N, Alterman L, Aynedjian HS. Selective deep nephron hyperfiltration in uninephrectomized spontaneously hypertensive rats. Kidney Int. 1983;24:185–191. [DOI] [PubMed] [Google Scholar]
- 14.Takenaka T, Harrison-Bernard LM, Inscho EW, Carmines PK, Navar LG. Autoregulation of afferent arteriolar blood flow in juxtamed- ullary nephrons. Am J Physiol. 1994;267(5 pt 2):F879–F887. doi: 10.1152/ajprenal.1994.267.5.F879 [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Tang L, Zhu Q,Yi F, Zhang F, Li PL, Li N. Hypoxia-inducible factor- 1α contributes to the profibrotic action of angiotensin II in renal medullary interstitial cells. Kidney Int. 2011;79:300–310. doi: 10.1038/ki.2010.326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navar LG, Inscho EW, Majid SA, Imig JD, Harrison-Bernard LM, Mitchell KD. Paracrine regulation of the renal microcirculation. Physiol Rev. 1996;76:425–536. doi: 10.1152/physrev.1996.76.2.425 [DOI] [PubMed] [Google Scholar]
- 17.Takenaka T, Suzuki H, Furukawa T, Ogata Y, Saruta T. Role of intrarenal renin-angiotensin system on pressure-natriuresis in spontaneously hypertensive rats. Clin Exp Hypertens A. 1990;12:1377–1394. [DOI] [PubMed] [Google Scholar]
- 18.Yang T, Xu C. Physiology and pathophysiology of the intrarenal renin- angiotensin system: an update. J Am Soc Nephrol. 2017;28:1040–1049. doi: 10.1681/ASN.2016070734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhuo JL, Kobori H, Li XC, Satou R, Katsurada A, Navar LG. Augmentation of angiotensinogen expression in the proximal tubule by intracellular angiotensin II via AT1a/MAPK/NF-KB signaling pathways. Am J Physiol Renal Physiol. 2016;310:F1103–F1112. doi: 10.1152/ajprenal.00350.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mashimo T, Nabika T, Kitada K, Serikawa T, Matsumoto C, Ikeda K, Nara Y, Masuda J, Yamori Y, Ohno Y, Saruta T. Mapping of four simple sequence repeat (SSR) markers on rat chromosome 4. Hypertens Res. 2000;23:47–50. [DOI] [PubMed] [Google Scholar]
- 21.Ryan MJ, Johnson G, Kirk J, Fuerstenberg SM, Zager RA, Torok-Storb B. HK-2: an immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int. 1994;45:48–57. [DOI] [PubMed] [Google Scholar]
- 22.Guo DF, Sun YL, Hamet P, Inagami T. The angiotensin II type 1 receptor and receptor-associated proteins. Cell Res. 2001;11:165–180. doi: 10.1038/sj.cr.7290083 [DOI] [PubMed] [Google Scholar]
- 23.Huang C, Davis G, Johns EJ. Effect of nitrendipine on autoregulation of perfusion in the cortex and papilla of kidneys from Wistar and stroke prone spontaneously hypertensive rats. Br J Pharmacol. 1994;111:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takenaka T, Inoue T, Ohno Y, Miyazaki T, Nishiyama A, Ishii N, Suzuki H. Elucidating mechanisms underlying altered renal autoregulation in diabetes. Am J Physiol Regul Integr Comp Physiol. 2012;303:R495–R504. doi: 10.1152/ajpregu.00217.2012 [DOI] [PubMed] [Google Scholar]
- 25.Takenaka T, Suzuki H, Sakamaki Y, Itaya Y, Saruta T. Contribution of prostaglandins to pressure natriuresis in Dahl salt-sensitive rats. Am J Hypertens. 1991;4:489–493. [DOI] [PubMed] [Google Scholar]
- 26.Feng YH, Ding Y, Ren S, Zhou L, Xu C, Karnik SS. Unconventional homologous internalization of the angiotensin II type-1 receptor induced by G-protein-independent signals. Hypertension. 2005;46:419–425. doi: 10.1161/01.HYP.0000172621.68061.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turu G, Szidonya L, Gáborik Z, Buday L, Spät A, Clark AJ, Hunyady L. Differential beta-arrestin binding of AT1 and AT2 angiotensin receptors. FEBS Lett. 2006;580:41–45. doi: 10.1016/j.febslet.2005.11.044 [DOI] [PubMed] [Google Scholar]
- 28.Oliveira L, Costa-Neto CM, Nakaie CR, Schreier S, Shimuta SI, Paiva AC. The angiotensin II AT1 receptor structure-activity correlations in the light of rhodopsin structure. Physiol Rev. 2007;87:565–592. doi: 10.1152/physrev.00040.2005 [DOI] [PubMed] [Google Scholar]
- 29.Okada H, Moriwaki K, Kalluri R, Takenaka T, Imai H, Ban S, Takahama M, Suzuki H. Osteopontin expressed by renal tubular epithelium mediates interstitial monocyte infiltration in rats. Am J Physiol. 2000;278:F110–F1121. [DOI] [PubMed] [Google Scholar]
- 30.Wolf G, Mueller E, Stahl RA, Ziyadeh FN. Angiotensin II-induced hypertrophy of cultured murine proximal tubular cells is mediated by endogenous transforming growth factor-beta. J Clin Invest. 1993;92:1366–1372. doi: 10.1172/JCI116710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inoue T, Umezawa A, Takenaka T, Suzuki H, Okada H. The contribution of epithelial-mesenchymal transition to renal fibrosis differs among kidney disease models. Kidney Int. 2015;87:233–238. doi: 10.1038/ki.2014.235 [DOI] [PubMed] [Google Scholar]
- 32.Patten DA, Lafleur VN, Robitaille GA, Chan DA, Giaccia AJ, Richard DE. Hypoxia-inducible factor-1 activation in nonhypoxic conditions: the essential role of mitochondrial-derived reactive oxygen species. Mol Biol Cell. 2010;21:3247–3257. doi: 10.1091/mbc.E10-01-0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pollock CA, Bostrom TE, Dyne M, Györy AZ, Field MJ. Tubular sodium handling and tubuloglomerular feedback in compensatory renal hypertrophy. Pflugers Arch. 1992;420:159–166. [DOI] [PubMed] [Google Scholar]
- 34.Capasso G, Mollica F, Saviano C, De Santo NG. Tubule effects of glomerular hyperfiltration: an integrated view. Semin Nephrol. 1995;15:419–425. [PubMed] [Google Scholar]
- 35.Chen JK, Nagai K, Chen J, Plieth D, Hino M, Xu J, Sha F, Ikizler TA, Quarles CC, Threadgill DW, Neilson EG, Harris RC. Phosphatidylinositol 3-kinase signaling determines kidney size. J Clin Invest. 2015;125:2429–2444. doi: 10.1172/JCI78945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar V, Wollner C, Kurth T, Bukowy JD, Cowley AW Jr. Inhibition of mammalian target of rapamycin complex 1 attenuates salt-induced hypertension and kidney injury in Dahl salt-sensitive rats. Hypertension. 2017;70:813–821. doi: 10.1161/HYPERTENSIONAHA.117.09456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagai K, Matsubara T, Mima A, Sumi E, Kanamori H, Iehara N, Fukatsu A, Yanagita M, Nakano T, Ishimoto Y, Kita T, Doi T, Arai H. Gas6 induces Akt/mTOR-mediated mesangial hypertrophy in diabetic nephropathy. Kidney Int. 2005;68:552–561. doi: 10.1111/j.1523-1755.2005.00433.x [DOI] [PubMed] [Google Scholar]
- 38.Fischer DC, Jacoby U, Pape L, Ward CJ, Kuwertz-Broeking E, Renken C, Nizze H, Querfeld U, Rudolph B, Mueller-Wiefel DE, Bergmann C, Haffner D. Activation of the AKT/mTOR pathway in autosomal recessive polycystic kidney disease (ARPKD). Nephrol Dial Transplant. 2009;24:1819–1827. doi: 10.1093/ndt/gfn744 [DOI] [PubMed] [Google Scholar]
- 39.Aizawa H, Saito Y, Nakamura T, et al. Downregulation of the Klotho gene in the kidney under sustained circulatory stress in rats. Biochem Biophys Res Commun. 1998;249:865–871. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Sun Z. Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension. 2009;54:810–817. doi: 10.1161/HYPERTENSIONAHA.109.134320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andrukhova O, Slavic S, Smorodchenko A, Zeitz U, Shalhoub V, Lanske B, Pohl EE, Erben RG. FGF23 regulates renal sodium handling and blood pressure. EMBO Mol Med. 2014;6:744–759. doi: 10.1002/emmm.201303716 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.