Abstract
In recent years, vegetables gain consumer attraction due to their reputation of being healthy in combination with low energy density. However, since fresh produce is often eaten raw, it may also be a source for foodborne illness. The presence of antibiotic-resistant bacteria might pose a particular risk to the consumer. Therefore, this review aims to present the current state of knowledge concerning the exposure of humans to antibiotic-resistant bacteria via food of plant origin for quantitative risk assessment purposes. The review provides a critical overview of available information on hazard identification and characterization, exposure assessment, and risk prevention with special respect to potential sources of contamination and infection chains. Several comprehensive studies are accessible regarding major antimicrobial-resistant foodborne pathogens (e.g., Salmonella spp., Listeria spp., Bacillus cereus, Campylobacter spp., Escherichia coli) and other bacteria (e.g., further Enterobacteriaceae, Pseudomonas spp., Gram-positive cocci). These studies revealed vegetables to be a potential—although rare—vector for extended-spectrum beta-lactamase-producing Enterobacteriaceae, mcr1-positive E. coli, colistin- and carbapenem-resistant Pseudomonas aeruginosa, linezolid-resistant enterococci and staphylococci, and vancomycin-resistant enterococci. Even if this provides first clues for assessing the risk related to vegetable-borne antimicrobial-resistant bacteria, the literature research reveals important knowledge gaps affecting almost every part of risk assessment and management. Especially, the need for (comparable) quantitative data as well as data on possible contamination sources other than irrigation water, organic fertilizer, and soil becomes obvious. Most crucially, dose–response studies would be needed to convert a theoretical “risk” (e.g., related to antimicrobial-resistant commensals and opportunistic pathogens) into a quantitative risk estimate.
Keywords: : ESBL, antimicrobial resistance, antibiotic resistance, foodborne pathogens, vegetables, risk assessment
Introduction
Vegetables are essential for healthy nutrition and obesity reduction in Western civilizations, due to a high nutrient density, correlated with low energy density (Darmon et al., 2005). They are defined as “a plant, root, seed, or pod that is used as food, especially in dishes that are not sweet” in the Cambridge dictionary. In 2003, WHO and FAO started an initiative to promote fruit and vegetable consumption for health worldwide, with a recommended minimum intake of 400 g fruits and vegetables per day (www.who.int).
However, vegetables are also implicated in foodborne outbreaks due to various—most often unknown—sources of contamination, the most recent familiar case in Europe being the enterohemorrhagic Escherichia coli (EHEC) outbreak of 2011, with 4321 reported cases, of which at least 50 had been fatal (Robert-Koch-Institute, Germany, cited from Buchholz et al., 2011). Besides other unusual features, the outbreak strain was characterized as an extended-spectrum beta-lactamase (ESBL) producer due to the presence of blaCTX-M15 genes (Mellmann et al., 2011). The press reported extensively on antimicrobial resistance (AMR), but not on the fact that this resistance was meaningless for therapy, due to contraindication of antibiosis in EHEC infections (see Hazard Identification and Characterization section).
According to the FAO definition, quantitative risk assessment is based on four steps: hazard identification, hazard characterization, exposure assessment, and risk characterization (www.fao.org). In this definition, hazard identification means to identify impacts on human health and the circumstances under which the danger is present, whereas hazard characterization aims to quantitatively or qualitatively evaluate the adverse effects on human health (e.g., using dose–response relationship). Exposure assessment provides the likely degree of consumption or intake of the hazardous agent, and the risk characterization tries to offer an estimate of the likely adverse effect in the target population by integrating the first three steps.
There have been well-recognized approaches for risk assessment related to general microbial hazards imposed by vegetable consumption (Hamilton et al., 2006; Franz et al., 2010; Danyluk and Schaffner, 2011; Pang et al., 2017). However, no specific quantitative risk assessment has been published for hazards related to antimicrobial-resistant bacteria (ARB) in vegetables, although AMR is seen as an obligate “risk amplifier” in public awareness. Steps to assess specific risks due to AMR would involve all steps of quantitative risk assessment. This starts from hazard identification (i.e., is a known adverse health effect aggravated by AMR features of the causative agent? Does an adverse health effect arise from spread of antibiotic resistance features in commensals, which otherwise would not be considered as microbial hazards?) but also includes hazard characterization as well as exposure assessment.
This literature survey aims to initiate a discussion on quantitative risk assessment related to the spread of antibiotic resistance in the vegetable food chain. For this purpose, we tried to provide an overview over current screening data starting from 2007. To allow risk prevention, sources of contamination were highlighted as well. Finally, important knowledge gaps were identified, which should be addressed in the future to facilitate quantitative risk assessment within a reasonable term.
Literature Search and Exclusion Criteria
Our PubMed search combined the keywords “antimicrobial resistance” or “antibiotic resistance” with “vegetables” or “fresh produce” in May 2018. We aimed to provide a systematic overview on recent screening data for antibiotic-resistant bacteria isolated from vegetables, so we covered the period from January 2007 to April 2018 and found 169 studies, after excluding double hits. The search result comprised 93 studies that reported screening data for antibiotic resistance in vegetable-borne isolates (Tables 1–7). We excluded 22 obvious mishits (one study dealing with the inhibition of bladder cancer cell proliferation by mustard oil, for example) and 13 studies that did not investigate vegetable-borne isolates (instead, these studies reported on, e.g., reptiles). Eight studies were excluded since they did not attribute resistant isolates to their source (these studies presented resistance rates from different sources, e.g., meat and vegetables, as one common value). We excluded opinion reports, reviews (unless they comprised relevant screening data), and publications that described modeling or method implementation or whole genome sequencing data of single isolates (in total 14 studies). We also excluded three studies in a language other than English (Polish, Ukrainian, and Chinese). One study that would not have met the exclusion criteria was excluded afterward since it presented mainly resistance data for Escherichia coli and vancomycin, a substance that is not effective in Enterobacteriaceae. All other studies presenting intrinsic resistance data (e.g., erythromycin in Salmonella spp.) were kept since significant results for acquired resistance were presented as well.
Table 1.
Studies on Major Antimicrobial-Resistant Foodborne Pathogens (Salmonella enterica subsp. enterica, Listeria spp., Bacillus cereus, Campylobacter spp.) in Vegetables
| Species/serovar | No. of isolates from vegetables | Specification of vegetables | Tested antimicrobials (method) | Region | Source |
|---|---|---|---|---|---|
| Salmonella Cubana, 50 others | 14, 138 | Cantaloupes, celery, cilantro, green onions, hot peppers, lettuce, parsley, spinach, alfalfa sprouts, and tomatoes | NARMS gram negative panel amoxicillin, ampicillin, cefoxitin,cephalothin, chloramphenicol,kanamycin, nalidixic acid,streptomycin, sulfamethoxazole, sulfisoxazole,tetracycline, trimethoprim (microdilution, sensititre) | United States | Reddy et al. (2016) |
| Salmonella Weltevreden, Salmonella Agona, others | 33, 24, 77 | Indigenous vegetables | Ampicillin, cefotaxime, ceftriaxone, chloramphenicol, erythromycin, gentamicin, nalidixic acid, streptomycin, tetracycline, trimethoprim–sulfamethoxazole (disk diffusion) | Malaysia | Yoke-Kqueen et al. (2008) |
| S. enterica subsp. enterica—serovar not specified | 26 | Tomato, spinach, carrot, radish, cantaloupe, and cucumber | Amikacin, amoxicillin–clavulanic acid, ampicillin, aztreonam, azithromycin, cefixime, cefoxitin, chloramphenicol, ciprofloxacin, colistin, co-trimoxazole, doxycycline, erythromycin, gatifloxacin, gentamicin, imipenem, kanamycin, meropenem, moxifloxacin, nalidixic acid, netilmicin, norfloxacin, ofloxacin, piperacillin, polymyxin B, sparfloxacin, streptomycin, tetracycline, tobramycin (disk diffusion) | India | Verma et al. (2018) |
| B. cereus | 110 | Fermented soybean products | Ampicillin, cefepime, cefotetan, ciprofloxacin, chloramphenicol, clindamycin, erythromycin, gentamicin, imipenem, oxacillin, penicillin, rifampin, sulfamethoxazole, tetracycline, trimethoprim, vancomycin (disk diffusion) | Korea | Kim et al. (2015) |
| B. cereus | 39 | Sunsik (a Korean ready-to-eat food prepared from grains, fruits, and vegetables) | Ampicillin, cefepime, cefoxitin, chloramphenicol, gentamicin, imipenem, oxacillin, penicillin, sulfamethoxazole, tetracycline, vancomycin (disk diffusion) | Korea | Lee et al. (2012) |
| B. cereus | 87 | Fermented soybean products | Ampicillin, cefepime, cefotetan, ciprofloxacin, chloramphenicol, clindamycin, erythromycin, gentamicin, imipenem, oxacillin, penicillin, rifampin, sulfamethoxazole/trimethoprim, tetracycline, vancomycin (disk diffusion) | Korea | Yim et al. (2015) |
| Listeria monocytogenes, Listeria grayi, Listeria welshimeri, Listeria ivanovii | 30, 42, 46, 28 | Ready-to-eat salads | Amikacin, ampicillin, cefotaxime, cephalothin, chloramphenicol, clindamycin, erythromycin, gentamicin, neomycin, oxacillin, penicillin, streptomycin, tetracycline, vancomycina (disk diffusion) | Turkey | Gurler et al. (2015) |
| L. monocytogenes | 144 | Fresh and frozen vegetables | Ampicillin, amoxicillin, chloramphenicol, ciprofloxacin, erythromycin, gentamicin, minocycline, norfloxacin, rifampicin, sulfamethoxazole, tetracycline, trimethoprim, vancomycin (microdilution, E-test) | Poland | Korsak et al. (2012) |
| Campylobacter jejuni | 33 | Salad style vegetables (ulam) | Ampicillin, amikacin, ciprofloxacin, enrofloxacin, erythromycin, gentamicin, norfloxacin, penicillin G, tetracycline, vancomycin (disk diffusion) | Malaysia | Khalid et al. (2015) |
| Each study <25 | n.d. | n.d. | n.d. | Singh et al. (2007); Miranda et al. (2009); Guchi and Ashenafi (2010); Yan et al. (2010); Raufu et al. (2013); Taban et al. (2013); Yu et al. (2014); Chen et al. (2015); Gurler et al. (2015); Mąka et al. (2015); Lee et al. (2016); Niyomdecha et al. (2016); de Vasconcelos Byrne et al. (2016); Liu and Kilonzo-Nthenge (2017) |
Font normal: resistance tested but not found; underlined: resistance in one single isolate; bold face: resistance in two or more isolates. Resistance defined as in the original study.
In L. ivanovii and L. welshimeri.
Table 2.
Studies on Antimicrobial-Resistant Escherichia coli in Vegetables
| No. of isolates from vegetables | Specification of vegetables | Tested antimicrobials (method) | Region | Source |
|---|---|---|---|---|
| 70 | Leafy green vegetables | Amoxicillin–clavulanic acid, ampicillin, cefoxitin, ceftazidime, ceftriaxone, co-trimoxazole, chloramphenicol, ciprofloxacin, gentamicin, nalidixic acid, streptomycin, tetracycline (disk diffusion) | South Africa | Jongman and Korsten (2016) |
| 208 | Leek, radish, basil, parsley, spinach, and lettuce, commercial and traditional whole salad | Ampicillin, cephalothin, chloramphenicol, ciprofloxacin, enrofloxacin, gentamicin, imipenem, lincomycin, nitrofurantoin, streptomycin, sulfamethoxazole, tetracycline, trimethoprim (disk diffusion) | Iran | Shakerian et al. (2016) |
| Approximately 230 (STEC) | Leafy vegetables and other vegetables from private produce farms | mcr1, mcr2 (PCR) | California | Mavrici et al. (2017) |
| 60 | Salad vegetables, cucumber, parsley, tomato, mint, and lettuce, salad (restaurant) | Ampicillin, cephalothin, colistin, co-trimoxazole, gentamicin, streptomycin, sulphatriad, tetracycline(disk diffusion) | Lebanon | Faour-Klingbeil et al. (2016) |
| 72 | Lettuce | Amikacin, amoxicillin–clavulanic acid, ampicillin, cefotaxime, cephalothin, chloramphenicol, ciprofloxacin, gentamicin, kanamycin, nalidixic acid, streptomycin, sulfonamide, tetracycline, trimethoprim (disk diffusion) | Belgium | Holvoet et al. (2013) |
| 82 (Diarrheagenic pathotypes) | Cactus salad (nopalitos) | Amikacin, amoxicillin–clavulanic acid, ampicillin, ceftriaxone, chloramphenicol, ciprofloxacin, colistin, co-trimoxazole, erythromycin, gentamicin, kanamycin, nalidixic acid, neomycin, streptomycin, tetracycline (disk diffusion) | Mexico | Gómez-Aldapa et al. (2016) |
| 78 (from 13 samples) | Packaged ready-to-eat salad | Amoxicillin–clavulanic acid, ciprofloxacin, chloramphenicol, co-trimoxazole, gentamicin, kanamycin, nalidixic acid, streptomycin, tetracycline (disk diffusion, E-test) | 78 (from 13 samples) | Campos et al. (2013) |
| 73 | Tomato, spinach, carrot, radish, cantaloupe, and cucumber | Amikacin, amoxicillin–clavulanic acid, ampicillin, aztreonam, azithromycin, cefixime, cefoxitin, chloramphenicol, ciprofloxacin, colistin, co-trimoxazole, doxycycline, erythromycin, gatifloxacin, gentamicin, imipenem, kanamycin, meropenem, moxifloxacin, nalidixic acid, netilmicin, norfloxacin, ofloxacin, piperacillin, polymyxin B, sparfloxacin, streptomycin, tetracycline, tobramycin (disk diffusion) | India | Verma et al. (2018) |
| 239 | Collard, cucumber, lettuce, tomato, and spinach | Amoxicillin–clavulanic acid, ampicillin, piperacillin, piperacillin–tazobactam, cefepime, ceftazidime, cefotaxime, imipenem, aztreonam, gentamicin, streptomycin, nalidixic acid, ciprofloxacin, tetracycline, chloramphenicol, co-trimoxazole (disk diffusion) | Portugal | Araújo et al. (2017) |
| 29 | Spinach, cabbage | Amikacin, amoxicillin–clavulanic acid, ampicillin, aztreonam, cefepime, cefotaxime, cefoxitin, cefquinome, ceftazidime, ceftriaxone, chloramphenicol, ciprofloxacin, enrofloxacin, florfenicol, gentamicin, kanamycin, nalidixic acid, neomycin, streptomycin, tetracycline, trimethoprim–sulfamethoxazole | South Africa | Du Plessis et al. (2017) |
| Each study <25 | n.d. | n.d. | n.d. | Hassan et al. (2011); Skočková et al. (2013); Kim and Woo (2014); Rasheed et al. (2014); Jones-Dias et al. (2016); Zurfuh et al. (2016); Lima et al. (2017); Randall et al. (2017); Wang et al. (2017) |
Font normal: resistance tested but not found; underlined: resistance in one single isolate; bold face: resistance in two or more isolates. Resistance defined as in the original study.
STEC, Shiga-toxin producing E. coli.
Table 3.
Studies on Antimicrobial-Resistant Pseudomonads in Vegetables
| Species | No. of isolates from vegetables | Specification of vegetables | Tested antimicrobials (method) | Region | Source |
|---|---|---|---|---|---|
| Pseudomonas aeruginosa, Pseudomonas putida | 295, 106 | Fruit, root, bulbous vegetables, salads, and cereals | Amikacin, apramycin, cefepime, ceftazidime, ciprofloxacin, colistin, doxycycline (only in P. putida), enrofloxacin, gentamicin, imipenem, neomycin, netilmicin, piperacillin+tazobactam, streptomycin, tobramycin, (microdilution) | Germany | Schwaiger et al. (2011a) |
| P. aeruginosa | 88 | Lettuce, white cabbage, red cabbage, carrots, sweet pepper, cucumber, and tomatoes, mixed | Ampicillin, aztreonam, ceftazidime, chloramphenicol, ciprofloxacin, gentamicin, imipenem, sulfamethoxazole–trimethoprim, tetracycline (disk diffusion) | Jamaica | Allydice-Francis and Brown (2012) |
| Pseudomonas spp. | 35 | Leaf lettuces, tomatoes, and carrots | Amikacin, cefepime, cefotaxime, ceftazidime, ciprofloxacin, imipenem, gentamicin (disk diffusion) | Portugal | Jones-Dias et al. (2016) |
| Each study <25 | n.d. | n.d. | n.d. | Bezanson et al. (2008); Hassan et al. (2011); Estepa et al. (2015) |
Font normal: resistance tested but not found; underlined: resistance in one single isolate; bold face: resistance in two or more isolates. Resistance defined as in the original study.
Table 4.
Studies on Other Antimicrobial-Resistant Gram Negatives in Vegetables
| Genus/species | No. of isolates from vegetables | Specification of vegetables | Tested antimicrobials (method) | Region | Source |
|---|---|---|---|---|---|
| Acinetobacter spp. | 89 | Leaf lettuces, tomatoes, and carrots | Amikacin, cefepime, cefotaxime, ceftazidime, ciprofloxacin, gentamicin, imipenem, tetracycline (disk diffusion) | Portugal | Jones-Dias et al. (2016) |
| Cronobacter spp. | 34 | Carrot, cucumber, leafy greens, sprouts, and others | Ampicillin, amoxicillin+-clavulanic acid, apramycin, aztreonam, cefotaxime, chloramphenicol, ciprofloxacin, colistin, enrofloxacin, gentamicin, kanamycin, meropenem, nalidixic acid, neomycin, streptomycin, sulfonamides, co-trimoxazole, tetracycline, trimethoprim (disk diffusion) | Czech Republic | Vojkovska et al. (2016) |
| Enterobacter cloacae, Enterobacter gergoviae, Pantoea agglomerans | 172, 92, 96 | Fruit, root, bulbous vegetables, salads, and cereals | Amikacin, amoxicillin+clavulanate (not tested in E. cloacae), ampicillin (E. gergoviae only), apramycin, cefaclor (not tested in E. cloacae), cefepime, ceftazidime, ciprofloxacin, colistin,adoxycycline, enrofloxacin, imipenem, mezlocillin, neomycin, netilmicin, piperacillin, piperacillin+tazobactam, gentamicin, streptomycin, co-trimoxazole, tobramycin (microdilution) | Germany | Schwaiger et al. (2011a) |
| Helicobacter pylori | 52 | Leek, radish, basil, parsley, spinach, lettuce, cabbage, carrot, scallion, chive, fenugreek, coriander, pepper, turnip, beet, garlic, maize, broccoli, and cucumber, commercial and traditional salad samples | Amoxicillin, ampicillin, cefsulodin, clarithromycin, erythromycin, furazolidone, levofloxacin, metronidazole, rifampin, spiramycin, streptomycin, tetracycline, trimethoprim (disk diffusion) | Iran | Yahaghi et al. (2014) |
| Enterobacteriaceae, Helicobacter pylori, Coliforms, Acinetobacter baumannii, Sphingobacterium multivorum, Cronobacter spp. | Each study <25 or studies, which mix/sum up resistance data of different genera | n.d. | n.d. | n.d. | Bezanson et al. (2008); Hassan et al. (2011); Mokhtari et al. (2012); Campos et al. (2013); Hemmatinezhad et al. (2016); Jones-Dias et al. (2016); Mesbah Zekar et al. (2017); Fei et al. (2018) |
Font normal: resistance tested but not found; underlined: resistance in one single isolate; bold face: resistance in two or more isolates. Resistance defined as in the original study.
R > 8 mg/L.
Table 5.
Studies on Antimicrobial-Resistant Gram-Positive Cocci in Vegetables
| Species | No. of isolates from vegetables | Specification of vegetables | Tested antimicrobials (method) | Region | Source |
|---|---|---|---|---|---|
| Staphylococcus aureus | 53 | Lettuce, perilla leaf, and sprouts | Amoxicillin–clavulanic acid, cephalothin, chloramphenicol, ciprofloxacin, clindamycin, erythromycin, gentamicin, imipenem, linezolid, nitrofurantoin, oxacillin, penicillin, quinupristin/dalfopristin, telithromycin, tetracycline, trimethoprim/sulfamethoxazole, vancomycin (disk diffusion) | Korea | Hong et al. (2015) |
| Staphylococcus saprophyticus, Staphylococcus succinus, Staphylococcus xylosus | 49, 14, 18 | Soybean products: meju and doenjang | Ampicillin, chloramphenicol, erythromycin, gentamicin, lincomycin, penicillin G, tetracycline, trimethoprim (disk diffusion) | Korea | Jeong et al. (2016) |
| Enterococcus faecalis | 100 | Fruit, root, bulbous vegetables, salads, and cereals | Amoxicillin–clavulanic acid, ampicillin, mezlocillin, imipenem, gentamicin high level, streptomycin high level, chloramphenicol, florfenicol, ciprofloxacin, enrofloxacin, moxifloxacin, vancomycin, teicoplanin, erythromycin, tylosin, linezolid, doxycycline, nitrofurantoin, rifampicin | Germany | Schwaiger et al. (2011a) |
| Enterococcus faecium, E. faecalis | 26, 9 | Herbs, spinach, and leafy greens | Ampicillin, bacitracin, chloramphenicol, ciprofloxacin, erythromycin, gentamicin, kanamycin, linezolid, nitrofurantoin, penicillin, quinupristin–dalfopristin,a streptomycin high level, teicoplanin, tetracycline, vancomycin (disk diffusion) | Canada | Allen et al. (2013) |
| Enterococcus casseliflavus, E. faecalis, E. faecium, Enterococcus hirae, Enterococcus gallinarum, Enterococcus spp. | 40, 20, 18, 9, 5, 16 | Ready-to-eat salads (split or mixed leaves, carrot, and corn) | Amoxicillin, chloramphenicol, ciprofloxacin, erythromycin, gentamicin, minocycline, nitrofurantoin, quinupristin–dalfopristin,b streptomycin, teicoplanin, tetracycline, vancomycin | Portugal | Campos et al. (2013) |
| E. faecium, E. hirae, E. faecalis, E. casseliflavus | 34, 23, 4, 4 | Vegetables, soil, and irrigation water | Ampicillin, chloramphenicol, ciprofloxacin, erythromycin, gentamicin high level, kanamycin high level, streptomycin high level, teicoplanin, tetracycline, trimethoprim–sulfamethoxazole, vancomycinc (disk diffusion) | Tunisia | Ben Said et al. (2016) |
| Each study <25 | n.d. | n.d. | n.d. | Abriouel et al. (2008); Gomes et al. (2008); Hassan et al. (2011); Burgos et al. (2014); Lavilla Lerma et al. (2014); Pesavento et al. (2014); Rodriguez-Palacios et al. (2014); Xu et al. (2014); Hao et al. (2015) |
Font normal: resistance tested, but not found; underlined: resistance in one single isolate; bold face: resistance in two or more isolates. Resistance defined as in the original study.
E. faecalis; bE. casseliflavus, E. faecalis; cE. casseliflavus.
Table 6.
Comparative Prevalence (%) of Antimicrobial-Resistant Escherichia coli in Meat and Vegetables (NonSelective Approach; Calculated from Percent Positive Samples and Percent Resistant Isolates)
| Antibiotic | Retail chicken (n = 250) | Retail pork (n = 250) | Vegetables (farm+retail, n = 1001) |
|---|---|---|---|
| Amoxicillin–clavulanate | 13.65a | 2.37b | 1.10b |
| Ampicillin | 34.58a | 5.90b | 1.70c |
| Piperacillin | 23.33a | 4.34b | 0.40c |
| Cefaclor | 9.30a | 2.76b | 1.90b |
| Cefoxitin | 2.40 | 0.78 | 1.30 |
| Cefuroxime | 1.95 | 0.39 | 0.80 |
| Imipenem | 1.65a | 0.00b | 0.00b |
| Gentamicin | 1.13a | 0.39a,b | 0.10b |
| Streptomycin | 23.63a | 7.12b | 0.30c |
| Tobramycin | 0.00 | 0.39 | 0.00 |
| Chloramphenicol | 3.60a | 1.17a,b | 0.50b |
| Florfenicol | 0.00 | 0.39 | 0.60 |
| Ciprofloxacin | 3.98a | 0.39b | 0.00b |
| Enrofloxacin | 4.50a | 0.39b | 0.00b |
| Colistin | 0.00 | 0.39 | 0.30 |
| Doxycycline | 30.53a | 9.46b | 0.50c |
| Sulfamethoxazole—trimethoprim | 31.35a | 5.51b | 0.20c |
Different superscripts in a row: values differ significantly in a chi-squared test (Fisher's exact test, if expected values are below 5 per cell).
Table 7.
Prevalence of Vegetable-Borne Bacteria with Particularly Relevant Antimicrobial Resistance (Examples)
| Microorganism | Antimicrobial resistance | Prevalence of resistance: No. of resistant isolates/total No. of isolates (%) | Vegetable source | Region | References |
|---|---|---|---|---|---|
| Salmonella enterica subsp. enterica (serovar Albany, Brunei, Kralingen) | Third-generation cephalosporin | 4/134 (3.0) | Indigenous vegetable food | Malaysia | Yoke-Kqueen et al. (2008) |
| Bacillus cereus | Vancomycin | 1/87 (1.2) | Fermented soybeans | Korea | Yim et al. (2015) |
| 5/35 (14.3) | Sunsik | Korea | Lee et al. (2012) | ||
| Escherichia coli | ESBL genes | 3/56 (5.4) | Lettuce | Portugal | Araújo et al. (2017) |
| One from 32 salad samples | Salad | Spain | Egea et al. (2011) | ||
| ESBL genes and phenotypes | Zero from 400 fruit or vegetable samples | Fruit and vegetables | United Kingdom | Randall et al. (2017) | |
| Carbapenem | 12/180 (6.6) | Leek, radish, basil, spinach, lettuce, traditional salads, commercial salads | Iran | Shakerian et al. (2016) | |
| mcr1 | 1/? | Lettuce | Portugal | Jones-Dias et al. (2016) | |
| 0/230 | Vegetables | United States | Mavrici et al. (2017) | ||
| 2/60 (3.3) from 42 samples | Imported vegetables | Switzerland | Zurfuh et al. (2016) | ||
| Enterobacteriaceae | ESBL phenotype | 44/97 | Various vegetables and fruits | Algeria | Mesbah Zekar et al. (2017) |
| Enterobacteriaceae (E. coli, Klebsiella pneumoniae, Enterobacter cloacae, Enterobacter aerogenes, Cronobacter sakazakii) | ESBL phenotype+ESBL genes | 25.4% of vegetable samples | Imported vegetables | Dominican Republic, India, Thailand, Vietnam (imported to Switzerland) | Zurfluh et al. (2015) |
| Enterobacteriaceae (K. pneumonia, Serratia marcescens) | ESBL phenotype+ESBL genes | 3/138 (2.2) from 9 samples | Iceberg lettuce | United States | Bhutani et al. (2015) |
| Raoultella terrigena | ESBL genes | 2/2 | Salads | Portugal | Campos et al. (2013) |
| Pseudomonas spp. | Colistin,a imipenem, meropenema | 8/401 (2.0), 26/401 (6.5), 1/401 (0.2) from 1001 samples | Fruit, root, bulbous vegetables, salads, and cereals | Germany | Schwaiger et al. (2011a) |
| Staphylococcus aureus (MSSA) | Linezolid | 1/53 | Leafy vegetables | Korea | Hong et al. (2015) |
| Enterococcus faecalis+faecium | Vancomycin | 3/160 (1.9) from 540 samples | Vegetables+environment (not specified) | Korea | Kim et al. (2017a) |
| E. faecalis+faecium | Linezolid | 1/100 (1.0), 1/59 (1.7) from 1001 samples | Fruit, root, bulbous vegetables, salads and cereals | Germany | Schwaiger et al. (2011a) |
R > 8; CLSI breakpoint: R ≥ 8, for colistin recently adjusted to R ≥ 4.
?, unknown number; CLSI, Clinical and Laboratory Standards Institute; ESBL, extended-spectrum beta-lactamase; MSSA, methicillin-sensitive Staphylococcus aureus.
All 13 experimental studies returned by the keywords were included in the text (e.g., experimental application of manure or biosolids to soil), as were two studies that reported metagenomic screening data.
Besides our systematic approach, we used targeted searches with individual keywords to add details (e.g., on vegetable consumption).
Hazard Identification and Characterization
A recent outbreak due to vegetables was related to a multiresistant strain of EHEC O104:H4, which produced ESBL enzymes (Buchholz et al., 2011). Did this fact add adverse effects to the outbreak? In general, antibiotics are not the first-line agents for treatment of diarrhea (Guerrant et al., 2001). On the contrary, for EHEC, as compiled by Goldwater and Bettelheim (2012), antibiotics—and especially beta-lactams—are even contraindicated due to the fact that cell-wall-impaired dead bacteria release more toxin (Tarr et al., 2005; Smith et al., 2012). Even subinhibitory concentrations are thought to increase toxin production and/or toxin release (Grif et al., 1998). Thus, in the absence of treatment indication, AMR could not lead to treatment failure. However, for other bacterial infections such as systemic listeriosis, antibiotic therapy is the treatment of choice (Safdar and Armstrong, 2003), and clinical resistance against first-line antibiotics is likely to impose additional health hazards. Centers for Disease Control and Prevention report recent cases of listeriosis in the United States due to vegetable consumption, which led to several deaths (www.cdc.gov); it is unknown whether these deaths were related to treatment failure or other reasons such as delayed diagnosis.
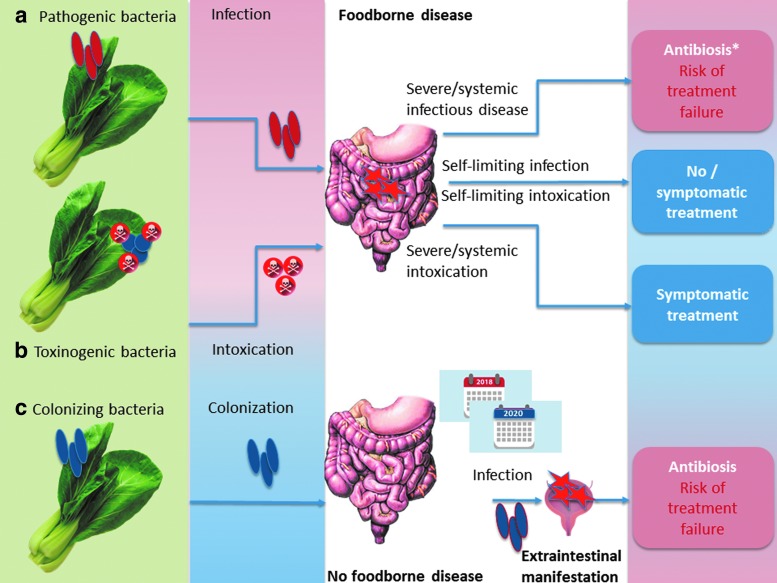
Providing comprehensive hazard identification of all foodborne pathogens associated with vegetables goes beyond the scope of this review. Thus, three more general situations will be exemplarily illustrated and connected to AMR: (1) primary foodborne infectious disease due to obligatory or opportunistic pathogens, (2) foodborne microbial intoxication, (3) foodborne colonization, maybe followed by opportunistic disease after a considerable time-shift (Fig. 1a–c).
FIG. 1.
Impact of antimicrobial resistance in different situations following vegetable-borne (a) infection, (b) intoxication, or (c) colonization (schematic illustration). *not for EHEC infection
Primary foodborne infectious disease is seen after infection with pathogenic serovars of, for example, Salmonella enterica subsp. enterica, Campylobacter spp., Listeria monocytogenes, Bacillus cereus, and E. coli (e.g., EHEC, Shiga-toxin producing E. coli [STEC]) (Butler et al., 2015). In case of systemic infection, as already mentioned, antimicrobial treatment is often indicated (except for EHEC), and AMR to clinically relevant antimicrobial drugs will lower the number of therapeutic options (Fig. 1a). In contrast, foodborne Staphylococcus aureus exclusively causes food poisoning due to the presence of preformed heat-stable toxins independent of viable bacteria (Kadariya et al., 2014). Thus, this kind of food poisoning is self-limiting, and antibiotic treatment is never indicated (Fig. 1b). However, S. aureus might cause opportunistic infections of wounds like cuts (van de Sande-Bruinsma et al., 2015), which might occur during food preparation and might call for topical treatment. Only a limited number of B. cereus lineages (Ehling-Schulz et al., 2005) are able to cause mere food poisoning due to the presence of cereulide (see Rosenquist et al., 2005, for example). Instead, in most cases, B. cereus needs to infect the organism to cause gastrointestinal disease (due to synthesis of Nhe and other enterotoxins in the intestine; EFSA, 2016). This is similar to the situation seen in Clostridium perfringens (Uzal et al., 2014). Antibiotic treatment of B. cereus infections is recommended in severe disease, whereas it is unreasonable in self-limited food poisoning (Spiliopoulou et al., 2014). C. perfringens might contribute to antibiotic-associated diarrhea (Borriello et al., 1984; Kim et al., 2017b); treatment of choice is discontinued primary antibiosis; only severe cases might be treated with glycopeptides or metronidazole (Bergogne-Bérézin, 2000).
A particular situation is found for organisms that are able to colonize the human gastrointestinal tract (Fig. 1c), such as E. coli or enterococci. These bacteria are useful indicators for AMR (Franklin et al., 2001; Schwaiger et al., 2011b) and were included in a recent EFSA recommendation to provide harmonized European monitoring of AMR (EFSA, 2012), as authorized by Directive 2003/99/EC. In addition, E. coli might produce ESBL enzymes, and this ESBL production (or other resistance phenotypes) might complicate the treatment of opportunistic infections. However, it is impossible to relate such colonization to a particular foodborne source, such as it is done for foodborne outbreaks: the onset of opportunistic infectious disease (e.g., urinary tract infection due to ESBL E. coli) might sporadically happen years after ingestion of the contaminated food. Thus, according to Depoorter et al. (2012), the human health risk posed by a given exposure to foodborne ESBL E. coli cannot be estimated yet.
In general, the situation described in Figure 1a–c applies for all foods, not only vegetables. However, the risk to encounter infection or colonization is elevated in vegetables, due to a high share of raw consumption.
Opportunistic pathogens (and apathogenic commensals) might further serve as a vehicle for the transfer of antimicrobial resistance genes (ARGs) to pathogens. This might happen in the intestine, but also before consumption as observed on lettuce with a resistance gene of clinical relevance in humans (blaSHV18, Jung and Matthews, 2016). DNA is partly degraded by heat, so raw consumed food is also more likely to pass high concentrations of ARGs into the human intestine.
In terms of hazard identification, additional hazard due to AMR is identified in systemic foodborne infection and in opportunistic colonization, but not in self-limiting diarrhea and intoxication.
In terms of hazard characterization, dose–response relationships are the most critical point for quantitative risk assessment. In case of obligate pathogens that would be antibiotically treated (Fig. 1a), the dose–response relationship is that of foodborne disease, so data are available for many pathogens. In contrast, dose–response data are completely missing for stable intestinal colonization or opportunistic extraintestinal disease. For colonization, hints are provided from studies using oronasal infection to colonize animals: Schoeni and Doyle (1994) isolated E. coli O157:H7 from cecal tissue of single chickens 3 months after experimental inoculation with as low as 2.6 × 101 colony-forming unit (cfu).
Exposure Assessment
Produce and human consumption of vegetables
Community members of the EU produced vegetables (including melons and strawberries) on a total area of 224,126,000 ha in 2016 (http://ec.europa.eu). In Germany, more than 8000 farms produced 3,672,660 tons of vegetables for human consumption (www.destatis.de); roots, salads, and other vegetables that are commonly consumed raw constituted approximately one-third of this produce. According to EFSA data (https://dwh.efsa.europa.eu) based on 60 studies (1997–2015) from 25 countries, the mean daily consumption of vegetables and vegetable products in adults ranged from 62 g per capita in Sweden to 382 g in Romania, with excessive consumption observed in individuals (99th percentile = 1047 g); these figures do not include plant products such as grains or legumes.
Prevalence of ARB in vegetables
A PubMed search combining the keywords “antimicrobial resistance” or “antibiotic resistance” with “vegetables” or “fresh produce” covered the period between January 2007 and April 2018. After applying the exclusion criteria as described above, 93 hits were condensed in Tables 1–7.
The studies included bacteria of more than 20 different genera (Tables 1–7), mainly E. coli, S. enterica subsp. enterica, Listeria spp., enterococci, pseudomonads, and B. cereus. To provide basic information from the studies, resistances are marked as present or absent in Tables 1–5 (irrespective of whether they are acquired or intrinsic). Additionally, prevalences are included in Tables 6 and 7. In general, comparisons between studies should be avoided since different choices of breakpoints introduce significant bias when comparing resistance data according to the European EUCAST organization with the U.S. Clinical and Laboratory Standards Institute (CLSI). Moreover, many studies lack the information of whether they used appropriate reference strains in antimicrobial susceptibility testing.
For Salmonella, studies are hard to compare due to a high diversity in serotypes. A huge number of vegetables (more than 100,000 samples) were investigated in the United States from 2002 to 2012, resulting in isolation of 152 Salmonella strains, of which as few as 10 had detectable AMRs (Reddy et al., 2016; Table 1). However, CLSI warned that Salmonella might be falsely reported as susceptible to several antibiotics (CLSI M100-S22E, table 2A in the CLSI document) due to differences between in vitro and in vivo susceptibility. Yoke-Kqueen et al. (2008) reported 56.7% of 134 isolates with a multiple antimicrobial resistance (MAR) index of more than 0.2. However, erythromycin was included in the MAR. Corrected for this fact, 44.3% of isolates from Malaysian vegetables were resistant to two or more antibiotics (mainly tetracycline+streptomycin—substances that are not the first- or second-line drugs in human therapy of salmonellosis). Relevant multiresistance (>5 resistances, including third-generation cephalosporins) was found in Salmonella Albany, Salmonella Brunei, and Salmonella Kralingen (Yoke-Kqueen et al., 2008).
All identified studies dealing with AMR in vegetable-borne B. cereus (n isolates = 39–110, Table 1) referred to Korean fermented or traditional food (Lee et al., 2012; Kim et al., 2015; Yim et al., 2015), thus statements are restrained to a limited variety of food and regions. In addition, resistance was assessed only by means of disk diffusion, and the source and validity of breakpoints is unclear, since these studies did not refer to CLSI or other acknowledged standards, but to the secondary literature. Apart from substances to which B. cereus should be considered as intrinsically resistant (e.g., ampicillin, oxacillin, penicillin, cefepime, rifampin), resistance rates were moderate, with complete susceptibility to gentamicin, imipenem, ciprofloxacin, tetracycline, and vancomycin in one study (Kim et al., 2015). However, resistance to vancomycin—a first-line therapeutic in (extraintestinal) B. cereus infections—was reported in 1/87 isolates from fermented soybeans (Yim et al., 2015) and 5/39 isolates from Sunsik (Lee et al., 2012), a ready-to-eat food made from grains, fruits, and vegetables.
Significant data for L. monocytogenes (n = 144, Table 1) from fresh and frozen vegetables were reported from a Polish study using E-test with CLSI-breakpoints for listeria and staphylococci (Korsak et al., 2012): only one vegetable-borne isolate had detectable AMR at all (to tetracyclines). In Turkish ready-to-eat salad, listerial AMR was most pronounced to erythromycin and cephalothin (Gurler et al., 2015). However, cephalosporin resistance is considered as an intrinsic feature of L. monocytogenes (Collins et al., 2012), and erythromycin is not considered as a treatment of choice (Morvan et al., 2010).
Campylobacter jejuni (n = 33, Table 1) from Malaysian “salad style vegetables” had moderate resistance rates except for intrinsic resistances and erythromycin resistance (60.6%; Khalid et al., 2015). The latter fact might be remarkable since macrolides have for long been used as a therapy of choice (Blaser et al., 1979; Engberg et al., 2001). However, the study is generally limited by the fact that breakpoints were outdated (taken from a document from 2003, when the CLSI was still named NCCLS). In any case, fluoroquinolones—a first-line therapy of today—were highly effective in this study.
Resistance data for E. coli (60–239 isolates, Table 2) differed significantly between studies; this might be related to the very different locations (South Africa, India, United States, South America, Europe, Lebanon, Iran), the different nature of samples, and the different choice of antibiotics and breakpoints.
The majority of studies reported pronounced resistance to streptomycin and tetracyclines—a finding of limited significance, given the fact that clinical breakpoints cut within natural populations. However, studies also reported significant resistance to amoxicillin combined with clavulanic acid (>15%) (e.g., Araújo et al., 2017) or resistance to carbapenems (Shakerian et al., 2016) and third- or fourth-generation cephalosporin as well as presence of blaTEM1-genes (Araújo et al., 2017). Remarkably, Gómez-Aldapa et al. (2016) reported 100% resistance to amikacin and colistin in diarrheagenic E. coli pathotypes from Mexican cactus salads. The study referred to CLSI; however, CLSI does not provide a breakpoint for colistin in E. coli. In addition, colistin resistance was assessed by disk diffusion, a practice that is discouraged nowadays (www.eucast.org).
For pathogenic E. coli, one study assessed the prevalence of plasmid-borne colistin resistance genes mcr1/mcr2 in STEC E. coli from the United States; not a single positive isolate was found in 1000 strains from different sources, including about 230 isolates from vegetables (Mavrici et al., 2017).
Due to their environmental sources, pseudomonads are frequently found in vegetables. Pseudomonads are not considered as major foodborne pathogens; however, six studies were identified, which reported resistance data of up to 401 Pseudomonas isolates from, mostly Pseudomonas aeruginosa (Table 3). Using microdilution and DIN58940-breakpoints where available, one study identified significantly higher resistance rates to aminoglycosides in Pseudomonas isolates from fruit vegetables compared with root vegetables or salads (Schwaiger et al., 2011a). In this study, resistance also included antibiotics used in clinics, such as gentamicin, tobramycin, amikacin, ciprofloxacin, colistin, piperacillin, ceftazidime, imipenem, or meropenem; however, such resistances were rare (Schwaiger et al., 2011a). Using CLSI breakpoints and disk diffusion, most of these resistances were also observed in Jamaican vegetable isolates, apart from resistance to carbapenems (Allydice-Francis and Brown, 2012).
Cronobacter spp. acts as a foodborne pathogen in immunocompromised patients (Healy et al., 2010) and will then be antibiotically treated, for example, with ampicillin plus gentamicin or chloramphenicol (Lai, 2001). However, vegetable isolates were characterized by a high rate of pansusceptible strains and lacked resistance against clinically relevant antimicrobials (Vojkovska et al., 2016; Table 4). For Enterobacter spp. (n = 264), several AMRs were significantly lowered at retail, compared with farm level, pointing toward a need for sampling close to consumption for particular questions of risk assessment (Schwaiger et al., 2011a; Table 4). Once more, resistance depended on the vegetable group: resistance to colistin was significantly more frequent in Enterobacter cloacae from fruit vegetables than from roots (Schwaiger et al., 2011a).
None of the studies on Gram-positive cocci (Table 5) reported staphylococci to be resistant to vancomycin, a critically important antibiotic especially for infection caused by methicillin-resistant S. aureus (MRSA). Korean leafy vegetables were partly contaminated with multiresistant MRSA strains (Hong et al., 2015). One methicillin-susceptible isolate was resistant to linezolid, a first-line drug for the treatment of MRSA-infected wounds (Gurusamy et al., 2013). Single linezolid-resistant strains of Enterococcus faecalis (1/100) and Enterococcus faecium were isolated from German vegetables (Schwaiger et al., 2011a). One linezolid-resistant E. faecium was detected in Canadian vegetables as well (Allen et al., 2013). Detectable resistance in E. faecalis isolates (n = 20) from Portuguese ready-to-eat salad was restrained to tetracyclines and erythromycin due to the presence of tet(M), tet(L), and erm(B) (Campos et al., 2013). From Tunisia, Ben Said et al. (2016) reported additional resistance to high level concentrations of aminoglycosides and chloramphenicol. By multilocus sequence typing, Leavis et al. (2006) identified vegetable-borne ARB of a so-called high-risk enterococcal clonal complex. Importantly, vancomycin–teicoplanin-resistant enterococci were isolated from fresh produce or its environment in Korea (Kim et al., 2017a).
Human exposure to vegetable-borne ARB
Assessing the exposure of humans to vegetable-borne ARB is not the same as assessing the prevalence of AMR in vegetable-borne bacteria: Considerable exposure to ARB might arise in a situation when bacteria are rarely found, but frequently resistant, or vice versa in the situation when AMR is moderate, but the prevalence of bacterial contamination is very high. For Salmonella or Listeria spp., both prevalence of bacteria and prevalence of AMR within vegetable-borne bacteria are low (Reddy et al., 2016). For other bacteria such as pseudomonads, prevalence of bacteria is high, but the prevalence of clinically significant resistance is low.
Vice versa, the prevalence of E. coli or E. faecalis is much lower in vegetables than in food of animal origin, but the prevalence of AMR is remarkably high for some antibiotics and, although rare, resistances to critically important isolates are present as well. For E. coli, we had the chance to comparatively calculate the prevalence of ARB (prevalence of bacteria × prevalence of resistance) in pork, poultry, and vegetables from two studies (Schwaiger et al., 2011a, 2012 and unpublished data) using identical methods; pork and poultry were included for the sake of comparison. The studies resembled each other in identical (nonselective) bacterial isolation, identical susceptibility testing (microdilution, DIN 58940-81), and identical source of breakpoints. Results are shown in Table 6. In total, the prevalence of E. coli was lowest in vegetables (3.4% compared with 75% in chicken and 25% in pork). However, for several antibiotics, the prevalence of resistant E. coli was quite comparable between pork and vegetables. This was because a much lower prevalence of E. coli in vegetables was accompanied by a high prevalence of resistance: up to 55% of the vegetable-borne E. coli were resistant to beta-lactams.
For quantitative risk assessment, it would be crucial to know not just the prevalence but also the concentration of ARB in vegetables since risk characterization has to consider dose-related data. However, this information is more than rare. Some of the studies listed in Tables 1–7 quantified bacteria (e.g., Hassan et al., 2011; Campos et al., 2013; Holvoet et al., 2013; Araújo et al., 2017; Du Plessis et al., 2017) but did not specifically refer to ARB. To assess these data, selective quantification for resistant bacteria would be needed, which is performed even rarer. Relevant data are provided by Ruimy et al. (2010), who reported densities of resistant bacteria as high as 104 cfu/g of product for Gram negatives grown in the presence of tetracycline, chloramphenicol, and nalidixic acid. Yang et al. (2016) quantified antibiotic-resistant endophytic bacteria in experimentally grown pak choi and found concentrations of up to 9 × 102 per g.
Risk Characterization
Risk characterization integrates the informations from hazard identification, hazard characterization, and exposure assessment into a quantitative estimate of risk (probability of adverse outcome or number of cases). Hazard identification related to resistant bacteria has to account for the fact whether this resistance is of therapeutic interest. This review revealed that clinically relevant antibiotics are underrepresented in current studies so that many pathogen-AMR combinations listed above do not imply any therapeutic consequence (hazard). However, ESBL production in Enterobacteriaceae or linezolid resistance in enterococci and staphylococci is surely of clinical interest.
Risk characterization would also include hazard characterization, which means dose–response relationships. Infectious doses vary in the individual host and situation; for many environmental bacteria, such infectious doses have not been defined at all. In addition, the amount of opportunistic pathogens or commensals, which is necessary to produce stable colonization in the human intestine, is unknown for most bacteria, and dose–response studies are absent. For foodborne outbreaks in general, case numbers are also used as a substitute in hazard characterization if dose–response data are missing. However, no specific case numbers are available for infectious disease due to vegetable-borne ARB.
In the absence of known colonization doses or known frequencies of colonization events, at least qualitative data might help to further characterize the risk. Thus, attempts were made to identify genetically related antimicrobially resistant organisms in food and human intestines or human clinical samples in a distinct region and period (e.g., Abriouel et al., 2008; Hannah et al., 2009; Burgos et al., 2014), but without showing significant association. Several studies tried to relate the presence of ARB in human feces to consumption behavior and found that vegetarians are at higher risk to carry ARB (Elder et al., 1993; Sannes et al., 2008). However, Sannes et al. (2008) also showed that this might be linked to a confounder related to the lifestyle of vegetarians: traveling abroad.
Exposure assessment identified vegetables as a relevant vehicle in terms of a high consumption frequency and in terms of frequent bacterial contamination in a ready-to-eat state. However, the exposure to clinically relevant pathogens or to commensals conferring clinically relevant resistance is low, compared with other food like meat.
Ultimately, taking together the individual steps of risk assessment, we have to state that the absence of dose–response data and the absence of published estimates on treatment failure due to AMR in foodborne infections do not allow quantitative risk characterization for vegetable-borne ARB yet.
Risk Prevention: Sources of Contamination
While sprout seeds from Egypt were considered as the most probable outbreak source for the multinational EHEC outbreak in 2011, the primary source of sprout contamination remained unknown. This is seen in most outbreaks (not only) related to vegetables, thus indirect clues are important for assessing infection chains. In rare cases, molecular typing of bacterial isolates from possible sources and contaminated vegetables is available to strengthen these indirect clues.
Irrigation water as a source of ARB
Worldwide, the FAO indicates a need for irrigation water as high as 1500 km3/year; of this irrigation water, 7.38% are used for vegetables and roots (www.fao.org, data from 2012). Groundwater constitutes 38.9% of this irrigation water, the rest is taken from sources such as surface water or used water, which are more easily prone to microbial contamination than groundwater. Microbial concentrations in irrigation water are generally much lower than they are in organic fertilizers; however, filter effects of soil might concentrate microorganisms at soil surfaces, and irrigation is more continuously applied to vegetables than is organic fertilizer. Blaak et al. (2015) found that Dutch surface water contains 2.2 × 102 multidrug-resistant E. coli (median) and up to 6 × 102 ESBL E. coli per microliter.
Several studies found that irrigation water and vegetables shared the same clones, as confirmed by repetitive-element polymerase chain reaction (rep-PCR: BOX-, REP-, and ERIC-PCR) (Jongman and Korsten, 2016; Araújo et al., 2017). Njage and Buys (2015) identified irrigation water as a possible pathway of transmission for ESBL E. coli.
Soil and organic fertilizer as sources of ARB
Vegetables produced in or close to soil—such as carrots and leaf vegetables—are at special risk for contamination with soil-borne bacteria, either belonging to natural soil microbiota or introduced into soil by manure fertilization. While the application of manure in ready-to-eat crops is discouraged, for example, by the U.K. Food Standards Agency (www.food.gov.uk); it is not generally prohibited by European law. In Germany, however, the use of liquid manure for top dressing is interdicted by national law; any vegetables must not be planted earlier than 12 weeks after fields had been fertilized with liquid manure.
Several studies focused on the prevalence of ARB or AMR genes in vegetables at harvest and the soil from which these vegetables were harvested (Marti et al., 2013, 2014; Wang et al., 2015; He et al., 2016; Lau et al., 2017; Tien et al., 2017); these studies found temporary—if any—impact of manuring compared with natural soil, which has a natural base level of ARG due to the presence of antimicrobial-producing microorganisms (Nesme and Simonet, 2015). Ruimy et al. (2010) showed that resistance scores were significantly higher in vegetables grown in or close to soil compared with fruits and vegetables grown above the soil. Rahube et al. (2014, 2016) reported that human biosolids are a relevant—but also temporary—source of ARG on vegetables at harvest, and Duan et al. (2017) found that ARG contents in soil and lettuce decreased after experimental application of biochar. Zhu et al. (2017) reported eightfold higher absolute copy numbers or ARG in manure-fertilized Chinese organic lettuce compared with conventionally produced lettuce; this difference was mainly due to different microbial communities in both types of samples. One recent study searched for ARGs in the viral DNA fraction (bacteriophages) from vegetables and soil and found that concentrations were highest for lettuce and soil, pointing toward soil as a relevant source of ARGs (Larrañaga et al., 2018).
Antibiotic residues in manure or soil might be more critical than is presence of manure-borne ARB, due to higher persistence. Antibiotic residues were able to select antimicrobially resistant endophytic bacteria, which are not removed by washing or peeling (Zhang et al., 2017; Esteban-Cuesta et al., 2018).
Direct contamination by humans
To our knowledge, systematic studies on human contamination of vegetables with ARB during production or processing are missing, as is systematic source tracking by molecular microbiological methods.
In the 2011 EHEC outbreak, it was assumed that, in a regional suboutbreak, vegetable dishes were contaminated by an infected caterer (www.rki.de). Genotyping raised suspicion that (multiresistant) S. aureus from Korean leafy vegetables were of human origin (Hong et al., 2015); the presence of new class 3-integrons in a pathogenic strain of Klebsiella pneumoniae was related to clinical sources by Jones-Dias et al. (2016). As an additional hint, we found that fruit vegetables were generally more likely to be contaminated with ARB than other vegetables; touching by consumers is discussed as a possible explanation, although not proven yet (Schwaiger et al., 2011a). A similar hypothesis was presented by Mesbah Zekar et al. (2017). Weak food hygiene standards on Nigerian farm were shown to correlate with higher bacterial loads in cut lettuce (Oyinlola et al., 2017), but these standards included only some parameters of personal hygiene besides a couple of nonhuman hygiene factors, such as surface water irrigation and manure application.
Besides the mentioned routes of contamination, other plausible ways of contamination include a broad variety of sources, either for the primary products (e.g., Salmonella contamination by reptiles and amphibians, Gorski et al., 2013) or during transport, processing, and distribution. Macrorestriction plus pulsed-field gel electrophoresis suggested that two different kinds of vegetables from two different countries were contaminated with a clonal E. coli strain due to packaging and distribution at the same factory. The role of processing is further illustrated by cut salads, which are generally considered as a risk food (www.fda.gov): here, processing is not only a possible source of cross-contamination but also a factor increasing water activity (thus decreasing microbial stability).
Outlook: Transfer of ARB to Humans by Food of Plant Origin—Current Lacks and Future Needs for Risk Assessment
A considerable share of studies used nonvalidated methods, reported intrinsic resistance, dealt with nonpathogenic bacteria, or provided data on AMRs/ARGs of low clinical relevance. Such limitations should be proactively addressed, if present. Commensal bacteria might be useful indicator organisms and serve as donors for AMR genes in the gut; in addition, the label pathogenic/nonpathogenic is conferred more carefully than in former since pathogenesis is more and more treated as a nonstatic interaction between strain-specific virulence and susceptibility of individual hosts. However, this fact gives even more emphasis to a need for prudent study design, overcoming the actual strategy of “count and collect” in favor of more functional approaches leading to dose–response data.
Important lacks of knowledge affect almost every part of risk assessment and management, starting from identifying sources of contamination and ending up to the black box of intestinal and infectious processes long after consumption. With regard to sources of contamination, search for plant-derived ARB should include processing and distribution steps since contamination close to consumption is more likely to result in human exposure, and human contamination might be more likely to introduce strains with AMR against human therapeutics. Further research should thus focus on clinically relevant antibiotics, should provide (or reject) evidence of transmission at clonal level, and should clarify and quantify the involvement of commensals in ARG transfer to pathogens as well as in extraintestinal opportunistic infections after transmission by food. Future studies addressing these gaps would be more than welcome.
Disclosure Statement
No competing financial interests exist.
References
- Abriouel H, Omar NB, Molinos AC, López RL, Grande MJ, Martínez-Viedma P, Ortega E, Cañamero MM, Galvez A. Comparative analysis of genetic diversity and incidence of virulence factors and antibiotic resistance among enterococcal populations from raw fruit and vegetable foods, water and soil, and clinical samples. Int J Food Microbiol 2008;123:38–49 [DOI] [PubMed] [Google Scholar]
- Allen KJ, Kovacevic J, Cancarevic A, Wood J, Xu J, Gill B, Allen JK, Mesak LR. Microbiological survey of imported produce available at retail across Canada. Int J Food Microbiol 2013;162:135–142 [DOI] [PubMed] [Google Scholar]
- Allydice-Francis K, Brown PD. Diversity of antimicrobial resistance and virulence determinants in Pseudomonas aeruginosa associated with fresh vegetables. Int J Microbiol 2012;2012:426241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo S A T.Silva I, Tacão M, Patinha C, Alves A, Henriques I. Characterization of antibiotic resistant and pathogenic Escherichia coli in irrigation water and vegetables in household farms. Int J Food Microbiol 2017;257:192–200 [DOI] [PubMed] [Google Scholar]
- Ben Said L, Klibi N, Dziri R, Borgo F, Boudabous A, Ben Slama K, Torres C. Prevalence, antimicrobial resistance and genetic lineages of Enterococcus spp. from vegetable food, soil and irrigation water in farm environments in Tunisia. J Sci Food Agric 2016;96:1627–1633 [DOI] [PubMed] [Google Scholar]
- Bergogne-Bérézin E. Treatment and prevention of antibiotic associated diarrhea. Int J Antimicrob Agents 2000;16:521–526 [DOI] [PubMed] [Google Scholar]
- Bezanson GS, MacInnis R, Potter G, Hughes T. Presence and potential for horizontal transfer of antibiotic resistance in oxidase-positive bacteria populating raw salad vegetables. Int J Food Microbiol 2008;127:37–42 [DOI] [PubMed] [Google Scholar]
- Bhutani N, Muraleedharan C, Talreja D, Rana SW, Walia S, Kumar A, Walia SK. Occurrence of multidrug resistant extended spectrum beta-lactamase-producing bacteria on iceberg lettuce retailed for human consumption. Biomed Res Int 2015;2015:547547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaak H, Lynch G, Italiaander R, Hamidjaja RA, Schets FM, de Roda Husman AM. Multidrug-resistant and extended spectrum beta-lactamase-producing Escherichia coli in Dutch surface water and wastewater. PLoS One 2015;10:e0127752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser MJ, Berkowitz ID, LaForce FM, Cravens J, Reller LB, Wang WL. Campylobacter enteritis: Clinical and epidemiologic features. Ann Intern Med 1979;91:179. [DOI] [PubMed] [Google Scholar]
- Borriello SP, Larson HE, Welch AR, Barclay F, Stringer MF, Bartholomew BA. Enterotoxigenic Clostridium perfringens: A possible cause of antibiotic-associated diarrhoea. Lancet 1984;1:305–307 [DOI] [PubMed] [Google Scholar]
- Buchholz U, Bernard H, Werber D, Böhmer MM, Remschmidt C, Wilking H, Deleré Y, an der Heiden M, Adlhoch C, Dreesman J, Ehlers J, Ethelberg S, Faber M, Frank C, Fricke G, Greiner M, Höhle M, Ivarsson S, Jark U, Kirchner M, Koch J, Krause G, Luber P, Rosner B, Stark K, Kühne M. German outbreak of Escherichia coli O104:H4 associated with sprouts. N Engl J Med 2011;365:1763–1770 [DOI] [PubMed] [Google Scholar]
- Burgos MJG, Aguayo MCL, Pulido RP, Gálvez A, López RL. Multilocus sequence typing and antimicrobial resistance in Enterococcus faecium isolates from fresh produce. Antonie Van Leeuwenhoek 2014;105:413–421 [DOI] [PubMed] [Google Scholar]
- Butler AJ, Thomas MK, Pintar KDM. Expert elicitation as a means to attribute 28 enteric pathogens to foodborne, waterborne, animal contact, and person-to-person transmission routes in Canada. Foodborne Pathog Dis 2015;12:335–344 [DOI] [PubMed] [Google Scholar]
- Campos J, Mourão J, Pestana N, Peixe L, Novais C, Antunes P. Microbiological quality of ready-to-eat salads: An underestimated vehicle of bacteria and clinically relevant antibiotic resistance genes. Int J Food Microbiol 2013;166:464–470 [DOI] [PubMed] [Google Scholar]
- [CLSI] Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement. CLSI document M100-S22. Wayne, PA: Clinical and Laboratory Standards Institute, 2012 [Google Scholar]
- Chen M, Wu Q, Zhang J, Wu S, Guo W. Prevalence, enumeration, and pheno- and genotypic characteristics of Listeria monocytogenes isolated from raw foods in South China. Front Microbiol 2015;6:1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins B, Guinane CM, Cotter PD, Hill C, Ross RP. Assessing the contributions of the LiaS histidine kinase to the innate resistance of Listeria monocytogenes to nisin, cephalosporins, and disinfectants. Appl Environ Microbiol 2012;78:2923–2929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danyluk MD, Schaffner DW. Quantitative assessment of the microbial risk of leafy greens from farm to consumption: Preliminary framework, data, and risk estimates. J Food Prot 2011;74:700–708 [DOI] [PubMed] [Google Scholar]
- Darmon N, Darmon M, Maillot M, Drewnowski A. A nutrient density standard for vegetables and fruits: Nutrients per calorie and nutrients per unit cost. J Am Diet Assoc 2005;105:1881–1887 [DOI] [PubMed] [Google Scholar]
- Depoorter P, Persoons D, Uyttendaele M, Butaye P, de Zutter L, Dierick K, Herman L, Imberechts H, van Huffel X, Dewulf J. Assessment of human exposure to 3rd generation cephalosporin resistant E. coli (CREC) through consumption of broiler meat in Belgium. Int J Food Microbiol 2012;159:30–38 [DOI] [PubMed] [Google Scholar]
- Du Plessis EM, Govender S, Pillay B, Korsten L. Exploratory study into the microbiological quality of spinach and cabbage purchased from street vendors and retailers in Johannesburg, South Africa. J Food Prot 2017;80:1726–1733 [DOI] [PubMed] [Google Scholar]
- Duan M, Li H, Gu J, Tuo X, Sun W, Qian X, Wang X. Effects of biochar on reducing the abundance of oxytetracycline, antibiotic resistance genes, and human pathogenic bacteria in soil and lettuce. Environ Pollut 2017;224:787–795 [DOI] [PubMed] [Google Scholar]
- EFSA. Technical specifications on the harmonised monitoring and reporting of antimicrobial resistance in Salmonella, Campylobacter and indicator Escherichia coli and Enterococcus spp. bacteria transmitted through food. EFSA J 2012;10:311 [Google Scholar]
- EFSA. Risks for public health related to the presence of Bacillus cereus and other Bacillus spp. including Bacillus thuringiensis in foodstuffs. EFSA J 2016;14:122 [Google Scholar]
- Egea P, López-Cerero L, Navarro MD, Rodríguez-Baño J, Pascual A. Assessment of the presence of extended-spectrum beta-lactamase-producing Escherichia coli in eggshells and ready-to-eat products. Eur J Clin Microbiol Infect Dis 2011;30:1045–1047 [DOI] [PubMed] [Google Scholar]
- Ehling-Schulz M, Svensson B, Guinebretiere M-H, Lindbäck T, Andersson M, Schulz A, Fricker M, Christiansson A, Granum PE, Märtlbauer E, Nguyen-The C, Salkinoja-Salonen M, Scherer S. Emetic toxin formation of Bacillus cereus is restricted to a single evolutionary lineage of closely related strains. Microbiology 2005;151(Pt 1):183–197 [DOI] [PubMed] [Google Scholar]
- Elder HA, Roy I, Lehman S, Phillips RL, Kass EH. Human studies to measure the effect of antibiotic residues. Vet Hum Toxicol 1993;35(Suppl 1):31–36 [PubMed] [Google Scholar]
- Engberg J, Aarestrup FM, Taylor DE, Gerner-Smidt P, Nachamkin I. Quinolone and macrolide resistance in Campylobacter jejuni and C. coli: Resistance mechanisms and trends in human isolates. Emerg Infect Dis 2001;7:24–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban-Cuesta I, Drees N, Ulrich S, Stauch P, Sperner B, Schwaiger K, Gareis M, Gottschalk C. Endogenous microbial contamination of melons (Cucumis melo) from international trade: An underestimated risk for the consumer? J Sci Food Agric 2018;98:5074–5081 [DOI] [PubMed] [Google Scholar]
- Estepa V, Rojo-Bezares B, Torres C, Sáenz Y. Genetic lineages and antimicrobial resistance in Pseudomonas spp. isolates recovered from food samples. Foodborne Pathog Dis 2015;12:486–491 [DOI] [PubMed] [Google Scholar]
- Faour-Klingbeil D, Kuri V, Fadlallah S, Matar GM. Prevalence of antimicrobial-resistant Escherichia coli from raw vegetables in Lebanon. J Infect Dev Ctries 2016;10:354–362 [DOI] [PubMed] [Google Scholar]
- Fei P, Jiang Y, Gong S, Li R, Jiang Y, Yuan X, Wang Z, Kang H, Ali MA. Occurrence, genotyping, and antibiotic susceptibility of Cronobacter spp. in drinking water and food samples from Northeast China. J Food Prot 2018:456–460 [DOI] [PubMed] [Google Scholar]
- Franklin A, Acar J, Anthony F, Gupta R, Nicholls T, Tamura Y, Thompson S, Threlfall EJ, Vose D, van Vuuren M, White DG, Wegener HC, Costarrica ML. Antimicrobial resistance: Harmonisation of national antimicrobial resistance monitoring and surveillance programmes in animals and in animal-derived food. Rev Sci Tech 2001;20:859–870 [DOI] [PubMed] [Google Scholar]
- Franz E, Tromp SO, Rijgersberg H, Van Der Fels-Klerx HJ. Quantitative microbial risk assessment for Escherichia coli O157:H7, Salmonella, and Listeria monocytogenes in leafy green vegetables consumed at salad bars. J Food Prot 2010;73:274–285 [DOI] [PubMed] [Google Scholar]
- Goldwater PN, Bettelheim KA. Treatment of enterohemorrhagic Escherichia coli (EHEC) infection and hemolytic uremic syndrome (HUS). BMC Med 2012;10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes BC, Esteves CT, Palazzo ICV, Darini ALC, Felis GE, Sechi LA, Franco BDGM, de Martinis ECP. Prevalence and characterization of Enterococcus spp. isolated from Brazilian foods. Food Microbiol 2008;25:668–675 [DOI] [PubMed] [Google Scholar]
- Gómez-Aldapa CA, Cerna-Cortes JF, Rangel-Vargas E, Torres-Vitela MR, Villarruel-López A, Gutiérrez-Alcántara EJ, Castro-Rosas J. Presence of multidrug-resistant Shiga toxin–producing Escherichia coli, enteropathogenic E. coli and enterotoxigenic E. coli, on raw nopalitos (Opuntia ficus-indica L.) and in nopalitos salads from local retail markets in Mexico. Foodborne Pathog Dis 2016;13:269–274 [DOI] [PubMed] [Google Scholar]
- Gorski L, Jay-Russell MT, Liang AS, Walker S, Bengson Y, Govoni J, Mandrell RE. Diversity of pulsed-field gel electrophoresis pulsotypes, serovars, and antibiotic resistance among Salmonella isolates from wild amphibians and reptiles in the California Central Coast. Foodborne Pathog Dis 2013;10:540–548 [DOI] [PubMed] [Google Scholar]
- Grif K, Dierich MP, Karch H, Allerberger F. Strain-specific differences in the amount of Shiga toxin released from enterohemorrhagic Escherichia coli O157 following exposure to subinhibitory concentrations of antimicrobial agents. Eur J Clin Microbiol Infect Dis 1998;17:761–766 [DOI] [PubMed] [Google Scholar]
- Guchi B, Ashenafi M. Microbial load, prevalence and antibiograms of Salmonella and Shigella in lettuce and green peppers. Ethiop J Health Sci 2010;20:41–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant RL, van Gilder T, Steiner TS, Thielman NM, Slutsker L, Tauxe RV, Hennessy T, Griffin PM, DuPont H, Sack RB, Tarr P, Neill M, Nachamkin I, Reller LB, Osterholm MT, Bennish ML, Pickering LK. Practice guidelines for the management of infectious diarrhea. Clin Infect Dis 2001;32:331–351 [DOI] [PubMed] [Google Scholar]
- Gurler Z, Pamuk S, Yildirim Y, Ertas N. The microbiological quality of ready-to-eat salads in Turkey: A focus on Salmonella spp. and Listeria monocytogenes. Int J Food Microbiol 2015;196:79–83 [DOI] [PubMed] [Google Scholar]
- Gurusamy KS, Koti R, Toon CD, Wilson P, Davidson BR. Antibiotic therapy for the treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections in surgical wounds. Cochrane Database Syst Rev 2013;(8):CD009726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AJ, Stagnitti F, Premier R, Boland A-M, Hale G. Quantitative microbial risk assessment models for consumption of raw vegetables irrigated with reclaimed water. Appl Environ Microbiol 2006;72:3284–3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah EL, Johnson JR, Angulo F, Haddadin B, Williamson J, Samore MH. Molecular analysis of antimicrobial-susceptible and -resistant Escherichia coli from retail meats and human stool and clinical specimens in a rural community setting. Foodborne Pathog Dis 2009;6:285–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao D, Xing X, Li G, Wang X, Zhang M, Zhang W, Xia X, Meng J. Prevalence, toxin gene profiles, and antimicrobial resistance of Staphylococcus aureus isolated from quick-frozen dumplings. J Food Prot 2015;78:218–223 [DOI] [PubMed] [Google Scholar]
- Hassan SA, Altalhi AD, Gherbawy YA, El-Deeb BA. Bacterial load of fresh vegetables and their resistance to the currently used antibiotics in Saudi Arabia. Foodborne Pathog Dis 2011;8:1011–1018 [DOI] [PubMed] [Google Scholar]
- He LY, Ying GG, Liu YS, Su HC, Chen J, Liu SS, Zhao JL. Discharge of swine wastes risks water quality and food safety: Antibiotics and antibiotic resistance genes from swine sources to the receiving environments. Environ Int 2016;92–93:210–219 [DOI] [PubMed] [Google Scholar]
- Healy B, Cooney S, O'Brien S, Iversen C, Whyte P, Nally J, Callanan JJ, Fanning S. Cronobacter (Enterobacter sakazakii): An opportunistic foodborne pathogen. Foodborne Pathog Dis 2010;7:339–350 [DOI] [PubMed] [Google Scholar]
- Hemmatinezhad B, Momtaz H, Rahimi E. VacA, cagA, iceA and oipA genotypes status and antimicrobial resistance properties of Helicobacter pylori isolated from various types of ready to eat foods. Ann Clin Microbiol Antimicrob 2016;15:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holvoet K, Sampers I, Callens B, Dewulf J, Uyttendaele M. Moderate prevalence of antimicrobial resistance in Escherichia coli isolates from lettuce, irrigation water, and soil. Appl Environ Microbiol 2013;79:6677–6683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J, Kim Y, Kim J, Heu S, Kim S-R, Kim K-P, Roh E. Genetic diversity and antibiotic resistance patterns of Staphylococcus aureus isolated from leaf vegetables in Korea. J Food Sci 2015;80:M1526–M1531 [DOI] [PubMed] [Google Scholar]
- Jeong D-W, Lee B, Her J-Y, Lee K-G, Lee J-H. Safety and technological characterization of coagulase-negative staphylococci isolates from traditional Korean fermented soybean foods for starter development. Int J Food Microbiol 2016;236:9–16 [DOI] [PubMed] [Google Scholar]
- Jones-Dias D, Manageiro V, Ferreira E, Barreiro P, Vieira L, Moura IB, Caniça M. Architecture of class 1, 2, and 3 integrons from gram negative bacteria recovered among fruits and vegetables. Front Microbiol 2016;7:1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongman M, Korsten L. Genetic diversity and antibiotic resistance of Escherichia coli isolates from different leafy green production systems. J Food Prot 2016;79:1846–1853 [DOI] [PubMed] [Google Scholar]
- Jung Y, Matthews KR. Potential transfer of extended spectrum β-lactamase encoding gene, blashv18 gene, between Klebsiella pneumoniae in raw foods. Food Microbiol 2016;60:39–48 [DOI] [PubMed] [Google Scholar]
- Kadariya J, Smith TC, Thapaliya D. Staphylococcus aureus and staphylococcal food-borne disease: An ongoing challenge in public health. Biomed Res Int 2014;2014:827965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid MI, Tang JYH, Baharuddin NH, Rahman NS, Rahimi NF, Radu S. Prevalence, antibiogram, and cdt genes of toxigenic Campylobacter jejuni in salad style vegetables (ulam) at farms and retail outlets in Terengganu. J Food Prot 2015;78:65–71 [DOI] [PubMed] [Google Scholar]
- Kim C-W, Cho S-H, Kang S-H, Park Y-B, Yoon M-H, Lee J-B, No W-S, Kim J-B. Prevalence, genetic diversity, and antibiotic resistance of Bacillus cereus isolated from Korean fermented soybean products. J Food Sci 2015;80:M123–M128 [DOI] [PubMed] [Google Scholar]
- Kim M-C, Cha M-H, Ryu J-G, Woo G-J. Characterization of vancomycin-resistant Enterococcus faecalis and Enterococcus faecium isolated from fresh produces and human fecal samples. Foodborne Pathog Dis 2017a;14:195–201 [DOI] [PubMed] [Google Scholar]
- Kim S, Woo G-J. Prevalence and characterization of antimicrobial-resistant Escherichia coli isolated from conventional and organic vegetables. Foodborne Pathog Dis 2014;11:815–821 [DOI] [PubMed] [Google Scholar]
- Kim YJ, Kim SH, Ahn J, Cho S, Kim D, Kim K, Lee H, Son H, Lee HJ, Yong D, Choi JY, Kim HR, Shin JH. Prevalence of Clostridium perfringens toxin in patients suspected of having antibiotic-associated diarrhea. Anaerobe 2017b;48:34–36 [DOI] [PubMed] [Google Scholar]
- Korsak D, Borek A, Daniluk S, Grabowska A, Pappelbaum K. Antimicrobial susceptibilities of Listeria monocytogenes strains isolated from food and food processing environment in Poland. Int J Food Microbiol 2012;158:203–208 [DOI] [PubMed] [Google Scholar]
- Lai KK. Enterobacter sakazakii infections among neonates, infants, children, and adults. Case reports and a review of the literature. Medicine 2001;80:113–122 [DOI] [PubMed] [Google Scholar]
- Larrañaga O, Brown-Jaque M, Quirós P, Gómez-Gómez C, Blanch AR, Rodríguez-Rubio L, Muniesa M. Phage particles harboring antibiotic resistance genes in fresh-cut vegetables and agricultural soil. Environ Int 2018;115:133–141 [DOI] [PubMed] [Google Scholar]
- Lau CH-F, Li B, Zhang T, Tien Y-C, Scott A, Murray R, Sabourin L, Lapen DR, Duenk P, Topp E. Impact of pre-application treatment on municipal sludge composition, soil dynamics of antibiotic resistance genes, and abundance of antibiotic-resistance genes on vegetables at harvest. Sci Total Environ 2017;587–588:214–222 [DOI] [PubMed] [Google Scholar]
- Lavilla Lerma L, Benomar N, Valenzuela AS, Casado Muñoz Mdel C, Gálvez A, Abriouel H. Role of EfrAB efflux pump in biocide tolerance and antibiotic resistance of Enterococcus faecalis and Enterococcus faecium isolated from traditional fermented foods and the effect of EDTA as EfrAB inhibitor. Food Microbiol 2014;44:249–257 [DOI] [PubMed] [Google Scholar]
- Leavis HL, Bonten MJM, Willems RJL. Identification of high-risk enterococcal clonal complexes: Global dispersion and antibiotic resistance. Curr Opin Microbiol 2006;9:454–460 [DOI] [PubMed] [Google Scholar]
- Lee N, Sun JM, Kwon KY, Kim HJ, Koo M, Chun HS. Genetic diversity, antimicrobial resistance, and toxigenic profiles of Bacillus cereus strains isolated from Sunsik. J Food Prot 2012;75:225–230 [DOI] [PubMed] [Google Scholar]
- Lee W-J, Kim H-B, Kim K-S. Isolation and characterization of spore-forming bacilli (SFB) from Shepherd's Purse (Capsella bursa-pastoris). J Food Sci 2016;81:M684–M691 [DOI] [PubMed] [Google Scholar]
- Lima CM, Souza IEGL, Dos Santos Alves T, Leite CC, Evangelista-Barreto NS, de Castro Almeida RC. Antimicrobial resistance in diarrheagenic Escherichia coli from ready-to-eat foods. J Food Sci Technol 2017;54:3612–3619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Kilonzo-Nthenge A. Prevalence of multidrug-resistant bacteria from U.S.-grown and imported fresh produce retailed in chain supermarkets and ethnic stores of Davidson County, Tennessee. J Food Prot 2017;80:506–514 [DOI] [PubMed] [Google Scholar]
- Mąka Ł, Maćkiw E, Ścieżyńska H, Popowska M. Occurrence and antimicrobial resistance of Salmonella spp. isolated from food other than meat in Poland. Ann Agric Environ Med 2015;22:403–408 [DOI] [PubMed] [Google Scholar]
- Marti R, Scott A, Tien Y-C, Murray R, Sabourin L, Zhang Y, Topp E. Impact of manure fertilization on the abundance of antibiotic-resistant bacteria and frequency of detection of antibiotic resistance genes in soil and on vegetables at harvest. Appl Environ Microbiol 2013;79:5701–5709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti R, Tien YC, Murray R, Scott A, Sabourin L, Topp E. Safely coupling livestock and crop production systems: How rapidly do antibiotic resistance genes dissipate in soil following a commercial application of swine or dairy manure? Appl Environ Microbiol 2014;80:3258–3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrici D, Yambao JC, Lee BG, Quiñones B, He X. Screening for the presence of mcr-1/mcr-2 genes in Shiga toxin–producing Escherichia coli recovered from a major produce-production region in California. PLoS One 2017;12:e0187827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellmann A, Harmsen D, Cummings CA, Zentz EB, Leopold SR, Rico A, Prior K, Szczepanowski R, Ji Y, Zhang W, McLaughlin SF, Henkhaus JK, Leopold B, Bielaszewska M, Prager R, Brzoska PM, Moore RL, Guenther S, Rothberg JM, Karch H. Prospective genomic characterization of the German enterohemorrhagic Escherichia coli O104:H4 outbreak by rapid next generation sequencing technology. PLoS One 2011;6:e22751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesbah Zekar F, Granier SA, Marault M, Yaici L, Gassilloud B, Manceau C, Touati A, Millemann Y. From farms to markets: Gram-negative bacteria resistant to third-generation cephalosporins in fruits and vegetables in a region of North Africa. Front Microbiol 2017;8:1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda JM, Mondragón AC, Martinez B, Guarddon M, Rodriguez JA. Prevalence and antimicrobial resistance patterns of Salmonella from different raw foods in Mexico. J Food Prot 2009;72:966–971 [DOI] [PubMed] [Google Scholar]
- Mokhtari W, Nsaibia S, Majouri D, Ben Hassen A, Gharbi A, Aouni M. Detection and characterization of Shigella species isolated from food and human stool samples in Nabeul, Tunisia, by molecular methods and culture techniques. J Appl Microbiol 2012;113:209–222 [DOI] [PubMed] [Google Scholar]
- Morvan A, Moubareck C, Leclercq A, Hervé-Bazin M, Bremont S, Lecuit M, Courvalin P, Le Monnier A. Antimicrobial resistance of Listeria monocytogenes strains isolated from humans in France. Antimicrob Agents Chemother 2010;54:2728–2731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesme J, Simonet P. The soil resistome: A critical review on antibiotic resistance origins, ecology and dissemination potential in telluric bacteria. Environ Microbiol 2015;17:913–930 [DOI] [PubMed] [Google Scholar]
- Niyomdecha N, Mungkornkaew N, Samosornsuk W. Serotypes and antimicrobial resistance of Salmonella enterica isolated from pork, chicken meat and lettuce, Bangkok and Central Thailand. Southeast Asian J Trop Med Public Health 2016;47:31–39 [PubMed] [Google Scholar]
- Njage PMK, Buys EM. Pathogenic and commensal Escherichia coli from irrigation water show potential in transmission of extended spectrum and AmpC β-lactamases determinants to isolates from lettuce. Microb Biotechnol 2015;8:462–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyinlola LA, Obadina AO, Omemu AM, Oyewole OB. Prevention of microbial hazard on fresh-cut lettuce through adoption of food safety and hygienic practices by lettuce farmers. Food Sci Nutr 2017;5:67–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang H, Lambertini E, Buchanan RL, Schaffner DW, Pradhan AK. Quantitative microbial risk assessment for Escherichia coli O157:H7 in fresh-cut lettuce. J Food Prot 2017;80:302–311 [DOI] [PubMed] [Google Scholar]
- Pesavento G, Calonico C, Ducci B, Magnanini A, Lo Nostro A. Prevalence and antibiotic resistance of Enterococcus spp. isolated from retail cheese, ready-to-eat salads, ham, and raw meat. Food Microbiol 2014;41:1–7 [DOI] [PubMed] [Google Scholar]
- Rahube TO, Marti R, Scott A, Tien YC, Murray R, Sabourin L, Duenk P, Lapen DR, Topp E. Persistence of antibiotic resistance and plasmid-associated genes in soil following application of sewage sludge and abundance on vegetables at harvest. Can J Microbiol 2016;62:600–607 [DOI] [PubMed] [Google Scholar]
- Rahube TO, Marti R, Scott A, Tien YC, Murray R, Sabourin L, Zhang Y, Duenk P, Lapen DR, Topp E. Impact of fertilizing with raw or anaerobically digested sewage sludge on the abundance of antibiotic-resistant coliforms, antibiotic resistance genes, and pathogenic bacteria in soil and on vegetables at harvest. Appl Environ Microbiol 2014;80:6898–6907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall LP, Lodge MP, Elviss NC, Lemma FL, Hopkins KL, Teale CJ, Woodford N. Evaluation of meat, fruit and vegetables from retail stores in five United Kingdom regions as sources of extended-spectrum beta-lactamase (ESBL)-producing and carbapenem-resistant Escherichia coli. Int J Food Microbiol 2017;241:283–290 [DOI] [PubMed] [Google Scholar]
- Rasheed MU, Thajuddin N, Ahamed P, Teklemariam Z, Jamil K. Antimicrobial drug resistance in strains of Escherichia coli isolated from food sources. Rev Inst Med Trop Sao Paulo 2014;56:341–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raufu I, Bortolaia V, Svendsen CA, Ameh JA, Ambali AG, Aarestrup FM, Hendriksen RS. The first attempt of an active integrated laboratory-based Salmonella surveillance programme in the north-eastern region of Nigeria. J Appl Microbiol 2013;115:1059–1067 [DOI] [PubMed] [Google Scholar]
- Reddy SP, Wang H, Adams JK, Feng PCH. Prevalence and characteristics of Salmonella serotypes isolated from fresh produce marketed in the United States. J Food Prot 2016;79:6–16 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Palacios A, Ilic S, LeJeune JT. Clostridium difficile with moxifloxacin/clindamycin resistance in vegetables in Ohio, USA, and prevalence meta-analysis. J Pathog 2014;2014:158601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenquist H, Smidt L, Andersen SR, Jensen GB, Wilcks A. Occurrence and significance of Bacillus cereus and Bacillus thuringiensis in ready-to-eat food. FEMS Microbiol Lett 2005;250:129–136 [DOI] [PubMed] [Google Scholar]
- Ruimy R, Brisabois A, Bernede C, Skurnik D, Barnat S, Arlet G, Momcilovic S, Elbaz S, Moury F, Vibet M-A, Courvalin P, Guillemot D, Andremont A. Organic and conventional fruits and vegetables contain equivalent counts of gram-negative bacteria expressing resistance to antibacterial agents. Environ Microbiol 2010;12:608–615 [DOI] [PubMed] [Google Scholar]
- Safdar A, Armstrong D. Antimicrobial activities against 84 Listeria monocytogenes isolates from patients with systemic listeriosis at a comprehensive cancer center (1955–1997). J Clin Microbiol 2003;41:483–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sannes MR, Belongia EA, Kieke B, Smith K, Kieke A, Vandermause M, Bender J, Clabots C, Winokur P, Johnson JR. Predictors of antimicrobial-resistant Escherichia coli in the feces of vegetarians and newly hospitalized adults in Minnesota and Wisconsin. J Infect Dis 2008;197:430–434 [DOI] [PubMed] [Google Scholar]
- Schoeni JL, Doyle MP. Variable colonization of chickens perorally inoculated with Escherichia coli O157:H7 and subsequent contamination of eggs. Appl Environ Microbiol 1994;60:2958–2962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaiger K, Helmke K, Hölzel CS, Bauer J. Antibiotic resistance in bacteria isolated from vegetables with regards to the marketing stage (farm vs. supermarket). Int J Food Microbiol 2011a;148:191–196 [DOI] [PubMed] [Google Scholar]
- Schwaiger K, Hölzel C, Bauer J. Detection of the macrolide-efflux protein A gene mef(A) in Enterococcus faecalis. Microb Drug Resist 2011b;17:429–432 [DOI] [PubMed] [Google Scholar]
- Schwaiger K, Huther S, Hölzel C, Kämpf P, Bauer J. Prevalence of antibiotic-resistant Enterobacteriaceae isolated from chicken and pork meat purchased at the slaughterhouse and at retail in Bavaria, Germany. Int J Food Microbiol 2012;154:206–211 [DOI] [PubMed] [Google Scholar]
- Shakerian A, Rahimi E, Emad P. Vegetables and restaurant salads as a reservoir for Shiga toxigenic Escherichia coli: Distribution of virulence factors, O-serogroups, and antibiotic resistance properties. J Food Prot 2016;79:1154–1160 [DOI] [PubMed] [Google Scholar]
- Singh BR, Singh P, Agrawal S, Teotia U, Verma A, Sharma S, Chandra M, Babu N, Kant Agarwal R. Prevalence of multidrug resistant Salmonella in coriander, mint, carrot, and radish in Bareilly and Kanpur, northern India. Foodborne Pathog Dis 2007;4:233–240 [DOI] [PubMed] [Google Scholar]
- Skočková A, Karpíšková R, Koláčková I, Cupáková Š. Characteristics of Escherichia coli from raw vegetables at a retail market in the Czech Republic. Int J Food Microbiol 2013;167:196–201 [DOI] [PubMed] [Google Scholar]
- Smith KE, Wilker PR, Reiter PL, Hedican EB, Bender JB, Hedberg CW. Antibiotic treatment of Escherichia coli O157 infection and the risk of hemolytic uremic syndrome, Minnesota. Pediatr Infect Dis J 2012;31:37–41 [DOI] [PubMed] [Google Scholar]
- Spiliopoulou A, Papachristou E, Foka A, Kolonitsiou F, Anastassiou ED, Goumenos DS, Spiliopoulou I. Relapsing Bacillus cereus peritonitis in a patient treated with continuous ambulatory peritoneal dialysis. JMM Case Rep 2014;1:e003400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taban BM, Aytac SA, Akkoc N, Akcelik M. Characterization of antibiotic resistance in Salmonella enterica isolates determined from ready-to-eat (RTE) salad vegetables. Braz J Microbiol 2013;44:385–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr PI, Gordon CA, Chandler WL. Shiga-toxin–producing Escherichia coli and haemolytic uraemic syndrome. Lancet 2005;365:1073–1086 [DOI] [PubMed] [Google Scholar]
- Tien Y-C, Li B, Zhang T, Scott A, Murray R, Sabourin L, Marti R, Topp E. Impact of dairy manure pre-application treatment on manure composition, soil dynamics of antibiotic resistance genes, and abundance of antibiotic-resistance genes on vegetables at harvest. Sci Total Environ 2017;581–582:32–39 [DOI] [PubMed] [Google Scholar]
- Uzal FA, Freedman JC, Shrestha A, Theoret JR, Garcia J, Awad MM, Adams V, Moore RJ, Rood JI, McClane BA. Towards an understanding of the role of Clostridium perfringens toxins in human and animal disease. Future Microbiol 2014;9:361–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de S.ande-Bruinsma N, Leverstein van Hall MA, Janssen M, Nagtzaam N, Leenders S, Greeff SC de, Schneeberger PM. Impact of livestock-associated MRSA in a hospital setting. Antimicrob Resist Infect Control 2015;4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vasconcelos Byrne V, Hofer E, Vallim DC, de Castro Almeida RC. Occurrence and antimicrobial resistance patterns of Listeria monocytogenes isolated from vegetables. Braz J Microbiol 2016;47:438–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma P, Saharan VV, Nimesh S, Singh AP. Phenotypic and virulence traits of Escherichia coli and Salmonella strains isolated from vegetables and fruits from India. J Appl Microbiol 2018;125:270–281 [DOI] [PubMed] [Google Scholar]
- Vojkovska H, Karpiskova R, Orieskova M, Drahovska H. Characterization of Cronobacter spp. isolated from food of plant origin and environmental samples collected from farms and from supermarkets in the Czech Republic. Int J Food Microbiol 2016;217:130–136 [DOI] [PubMed] [Google Scholar]
- Wang F-H, Qiao M, Chen Z, Su J-Q, Zhu Y-G. Antibiotic resistance genes in manure-amended soil and vegetables at harvest. J Hazard Mater 2015;299:215–221 [DOI] [PubMed] [Google Scholar]
- Wang L, Nakamura H, Kage-Nakadai E, Hara-Kudo Y, Nishikawa Y. Prevalence, antimicrobial resistance and multiple-locus variable-number tandem-repeat analysis profiles of diarrheagenic Escherichia coli isolated from different retail foods. Int J Food Microbiol 2017;249:44–52 [DOI] [PubMed] [Google Scholar]
- Xu J, Shi C, Song M, Xu X, Yang P, Paoli G, Shi X. Phenotypic and genotypic antimicrobial resistance traits of foodborne Staphylococcus aureus isolates from Shanghai. J Food Sci 2014;79:M635–M642 [DOI] [PubMed] [Google Scholar]
- Yahaghi E, Khamesipour F, Mashayekhi F, Safarpoor Dehkordi F, Sakhaei MH, Masoudimanesh M, Khameneie MK. Helicobacter pylori in vegetables and salads: Genotyping and antimicrobial resistance properties. Biomed Res Int 2014;2014:757941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Neogi SB, Mo Z, Guan W, Shen Z, Zhang S, Li L, Yamasaki S, Shi L, Zhong N. Prevalence and characterization of antimicrobial resistance of foodborne Listeria monocytogenes isolates in Hebei province of Northern China, 2005–2007. Int J Food Microbiol 2010;144:310–316 [DOI] [PubMed] [Google Scholar]
- Yang Q, Zhang H, Guo Y, Tian T. Influence of chicken manure fertilization on antibiotic-resistant bacteria in soil and the endophytic bacteria of Pakchoi. Int J Environ Res Public Health 2016;13:662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim J-H, Kim K-Y, Chon J-W, Kim D-H, Kim H-S, Choi D-S, Choi I-S, Seo K-H. Incidence, antibiotic susceptibility, and toxin profiles of Bacillus cereus sensu lato isolated from Korean fermented soybean products. J Food Sci 2015;80:M1266–M1270 [DOI] [PubMed] [Google Scholar]
- Yoke-Kqueen C, Learn-Han L, Noorzaleha AS, Son R, Sabrina S, Jiun-Horng S, Chai-Hoon K. Characterization of multiple-antimicrobial-resistant Salmonella enterica subsp. enterica isolated from indigenous vegetables and poultry in Malaysia. Lett Appl Microbiol 2008;46:318–324 [DOI] [PubMed] [Google Scholar]
- Yu T, Jiang X, Zhou Q, Wu J, Wu Z. Antimicrobial resistance, class 1 integrons, and horizontal transfer in Salmonella isolated from retail food in Henan, China. J Infect Dev Ctries 2014;8:705–711 [DOI] [PubMed] [Google Scholar]
- Zhang H, Li X, Yang Q, Sun L, Yang X, Zhou M, Deng R, Bi L. Plant growth, antibiotic uptake, and prevalence of antibiotic resistance in an endophytic system of Pakchoi under antibiotic exposure. Int J Environ Res Public Health 2017;14:1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B, Chen Q, Chen S, Zhu YG. Does organically produced lettuce harbor higher abundance of antibiotic resistance genes than conventionally produced? Environ Int 2017;98:152–159 [DOI] [PubMed] [Google Scholar]
- Zurfluh K, Nüesch-Inderbinen M, Morach M, Zihler Berner A, Hächler H, Stephan R. Extended-spectrum-β-lactamase-producing Enterobacteriaceae isolated from vegetables imported from the Dominican Republic, India, Thailand, and Vietnam. Appl Environ Microbiol 2015;81:3115–3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurfuh K, Poirel L, Nordmann P, Nüesch-Inderbinen M, Hächler H, Stephan R. Occurrence of the plasmid-borne mcr-1 colistin resistance gene in extended-spectrum-β-lactamase-producing Enterobacteriaceae in river water and imported vegetable samples in Switzerland. Antimicrob Agents Chemother 2016;60:2594–2595 [DOI] [PMC free article] [PubMed] [Google Scholar]