Key Points
Question
Does inhaled inorganic nitrite improve exercise capacity in patients with heart failure with preserved ejection fraction (HFpEF)?
Findings
In this randomized clinical trial that included 105 patients with HFpEF, inhaled inorganic nitrite compared with placebo for 4 weeks resulted in an exercise capacity (measured as peak oxygen consumption) of 13.5 vs 13.7 mL/kg/min, a difference that was not statistically significant.
Meaning
Inhaled inorganic nitrite was not effective in improving exercise capacity in patients with HFpEF.
Abstract
Importance
There are few effective treatments for heart failure with preserved ejection fraction (HFpEF). Short-term administration of inorganic nitrite or nitrate preparations has been shown to enhance nitric oxide signaling, which may improve aerobic capacity in HFpEF.
Objective
To determine the effect of 4 weeks’ administration of inhaled, nebulized inorganic nitrite on exercise capacity in HFpEF.
Design, Setting, and Participants
Multicenter, double-blind, placebo-controlled, 2-treatment, crossover trial of 105 patients with HFpEF. Participants were enrolled from July 22, 2016, to September 12, 2017, at 17 US sites, with final date of follow-up of January 2, 2018.
Interventions
Inorganic nitrite or placebo administered via micronebulizer device. During each 6-week phase of the crossover study, participants received no study drug for 2 weeks (baseline/washout) followed by study drug (nitrite or placebo) at 46 mg 3 times a day for 1 week followed by 80 mg 3 times a day for 3 weeks.
Main Outcomes and Measures
The primary end point was peak oxygen consumption (mL/kg/min). Secondary end points included daily activity levels assessed by accelerometry, health status as assessed by the Kansas City Cardiomyopathy Questionnaire (score range, 0-100, with higher scores reflecting better quality of life), functional class, cardiac filling pressures assessed by echocardiography, N-terminal fragment of the prohormone brain natriuretic peptide levels, other exercise indices, adverse events, and tolerability. Outcomes were assessed after treatment for 4 weeks.
Results
Among 105 patients who were randomized (median age, 68 years; 56% women), 98 (93%) completed the trial. During the nitrite phase, there was no significant difference in mean peak oxygen consumption as compared with the placebo phase (13.5 vs 13.7 mL/kg/min; difference, −0.20 [95% CI, −0.56 to 0.16]; P = .27). There were no significant between–treatment phase differences in daily activity levels (5497 vs 5503 accelerometry units; difference, −15 [95% CI, −264 to 234]; P = .91), Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (62.6 vs 61.9; difference, 1.1 [95% CI, −1.4 to 3.5]; P = .39), functional class (2.5 vs 2.5; difference, 0.1 [95% CI, −0.1 to 0.2]; P = .43), echocardiographic E/e′ ratio (16.4 vs 16.6; difference, 0.1 [95% CI, −1.2 to 1.3]; P = .93), or N-terminal fragment of the prohormone brain natriuretic peptide levels (520 vs 533 pg/mL; difference, 11 [95% CI, −53 to 75]; P = .74). Worsening heart failure occurred in 3 participants (2.9%) during the nitrite phase and 8 (7.6%) during the placebo phase.
Conclusions and Relevance
Among patients with HFpEF, administration of inhaled inorganic nitrite for 4 weeks, compared with placebo, did not result in significant improvement in exercise capacity.
Trial Registration
ClinicalTrials.gov Identifier: NCT02742129
This randomized clinical trial compares the effect of 4 weeks’ administration of inhaled, nebulized inorganic nitrite vs placebo on exercise capacity in patients with heart failure with preserved ejection fraction (HFpEF).
Introduction
Approximately half of patients with heart failure have a preserved ejection fraction (HFpEF).1,2 There is no proven effective medical treatment for this syndrome. Multiple lines of evidence suggest that impairments in nitric oxide availability have a potentially important role in the pathophysiology of HFpEF.1,2 Therapies that enhance signaling in the nitric oxide pathway have been tested in HFpEF but have failed to demonstrate a clear benefit in randomized trials performed to date.3,4,5
The inorganic nitrate/nitrite pathway represents a different means of restoring nitric oxide signaling.6 Unlike the organic nitrates (eg, isosorbide mononitrate and dinitrate), inorganic nitrite is converted to nitric oxide in a 1-step reaction that is facilitated in the presence of hypoxia and acidosis, conditions that develop in the tissues and venous circulation during exercise, and there is no tolerance to nitrite. Because the cardiac, vascular, and skeletal muscle abnormalities that limit physical capacity and contribute to symptoms in patients with HFpEF characteristically develop during exercise,7 inorganic nitrate/nitrite may provide a way to better target nitric oxide delivery precisely at the time of greatest need: during exercise.6
Multiple acute and short-term, single-center studies have demonstrated improved cardiac hemodynamics and exercise capacity with inorganic nitrate/nitrite in patients with HFpEF,8,9,10,11,12,13 but to our knowledge, this approach has not been tested with longer-term use or in a multicenter trial. This trial tested the hypothesis that inhaled, nebulized inorganic nitrite would enhance peak exercise capacity in patients with HFpEF.
Methods
Study Oversight
This trial was sponsored by the National Heart, Lung, and Blood Institute. The trial protocol (Supplement 1) was approved by the protocol review committee of the institute’s Heart Failure Clinical Research Network and monitored by the network’s data and safety monitoring board. All patients provided written informed consent. The ethics committee at each participating site approved the trial design. Data collection, management, and analysis were performed at the network’s coordinating center at the Duke Clinical Research Institute. The study protocol (with protocol amendments) and statistical analysis plan are provided in Supplement 1 and Supplement 2, respectively.
Study Patients
Ambulatory patients with a diagnosis of HF were eligible if they were 40 years of age or older and had HF while they were receiving stable medical therapy. Patients were required to have an EF of 50% or more and objective evidence of HF, as shown by 1 or more of the following criteria: previous hospitalization for HF with radiographic evidence of pulmonary congestion; elevated left ventricular end-diastolic pressure or pulmonary capillary wedge pressure at rest (≥15 mm Hg) or with exercise (≥25 mm Hg); an elevated level of N-terminal fragment of the prohormone brain natriuretic peptide (NT-proBNP) (>400 pg/mL) or brain natriuretic peptide (BNP) (>200 pg/mL); or echocardiographic evidence of diastolic dysfunction (medial E/e′ ratio ≥15 or left atrial enlargement) together with chronic treatment with a loop diuretic.
All patients were required to report on a screening questionnaire that the primary reason for their inability to be active was dyspnea, fatigue, or chest pain (rather than orthopedic, neurologic, or lifestyle factors), and all patients were required to display objective exercise limitation, evidenced by reduced peak oxygen consumption (V̇o2) on cardiopulmonary exercise testing of less than 75% predicted,14 with a respiratory exchange ratio indicative of maximal effort (≥1.0).
Exclusion criteria included a systolic blood pressure of less than 115 mm Hg seated or less than 90 mm Hg while standing, a previous adverse reaction to or current use of organic nitrate or phosphodiesterase type 5 inhibitor therapy, or inability to tolerate the open-label run-in dose.
Race and ethnicity were included as data elements to satisfy the National Heart, Lung, and Blood Institute Policy for Inclusion of Women and Minorities in Clinical Research. Race and ethnicity determinations were made by the participants and collected as fixed categories. Cardiopulmonary exercise testing was performed by Core Laboratory–certified sites using equipment and calibration approaches that met American Thoracic Society standards. Cardiopulmonary exercise tests were performed using a 10-W/min incremental ramp protocol and breath-by-breath measures of oxygen uptake were uniformly analyzed by the Core Laboratory (Massachusetts General Hospital, Boston).
Study Design
The design of the trial, which was a multicenter, randomized, double-blind, placebo-controlled, crossover study, has been described.6 After enrollment, patients underwent baseline studies, including cardiopulmonary exercise testing and assessments of secondary end points, including quality-of-life scores, New York Heart Association functional class, and Core Laboratory assessment of NT-proBNP. Following qualifying exercise testing, eligible participants received an open-label, single-dose run-in of inhaled, nebulized sodium nitrite (80 mg) to assess tolerability, symptoms, and orthostatic vital signs. Patients developing hypotension (systolic blood pressure <90 mm Hg seated or standing), light-headedness, or any other intolerance were categorized as a run-in failure and were not randomized.
Randomization
A computer-generated, permuted-block randomization stratified according to study site was used to assign patients in a 1:1 ratio to 1 of 2 treatment groups (placebo first with crossover to nitrite or nitrite first with crossover to placebo), with block size of 2.
Interventions
The study drugs were identical in appearance and prepared as 80-mg ampules of inorganic sodium nitrite (AIR001 Inhalation Solution, Mast Therapeutics Inc, a subsidiary of Savara Pharmaceuticals Inc) and matching placebo.
Study drug was administered 3 times a day using the Philips I-neb Adaptive Aerosol Delivery nebulizer over 10 to 15 minutes for each dose.6 The nebulizer is a small, battery-powered, lightweight, portable drug delivery device. During each 6-week period, patients were instructed to take no study drug for the first 2 weeks (baseline phase during the first period and washout phase during the second period), followed by 46 mg 3 times daily for 1 week, and then 80 mg 3 times daily for 3 weeks, for a treatment duration of 4 weeks. The 80-mg dose using this nebulizer system produces plasma nitrite levels that are equivalent to the 90-mg dose used in prior acute studies.10,12 The study drug was administered at least 4 hours apart during the daytime hours. Patients were called weekly to assess any adverse effects and reinforce adherence with study procedures. Patients who had unacceptable adverse effects were allowed to return to the 46-mg dose. After the first period, patients returned to the study center to repeat end-point assessments, receive the crossover study drug, and exchange accelerometers. After the second period, patients returned to repeat end-point assessments and to return the accelerometers and any unused study medicine.
Outcome Measures
The prespecified primary end point was peak V̇o2, measured as the highest 30-second average during upright cycle ergometry, during the 4-week period in which patients were receiving inorganic nitrite as compared with placebo. A 6% increase in peak V̇o2 represents a minimal clinically important difference (0.6 mL/kg/min for a patient with baseline peak V̇o2 of 10.0 mL/kg/min).15 All patients received 1 directly observed dose of the study drug allocated for each period of the trial immediately preceding the exercise test.
Secondary end points included daily activity levels assessed by average accelerometry units, health-related quality-of-life scores on the self-administered Kansas City Cardiomyopathy Questionnaire (score range, 0-100, with higher scores indicating better quality of life; minimum clinically important change, 5 points16), echocardiographic indicators of cardiac filling pressures measured at trough drug levels (E/e′ ratio, estimated pulmonary artery systolic pressure, and left atrial volume index; lower scores indicate better health for all), ventilatory efficiency (VE/V̇co2, lower indicating better health), exercise time (higher indicating better health), and NT-proBNP levels (lower indicating better health).
Tertiary end points included plasma cyclic guanosine monophosphate (cGMP) and cystatin C levels, and were assessed prior to receipt of study drug at trough. Details on the methods for end point assessment are included the eAppendix in Supplement 3. At the end of the study, patients completed a questionnaire indicating in which period they felt better (first period, second period, or no preference). Adverse events were recorded throughout the study.
Statistical Analysis
For the 2 × 2 crossover design, enrollment of 90 patients would provide a power of 80% to detect a difference of 0.6 mL/kg/min in peak V̇o2, 100 patients would provide a power of 90% to detect an increase in averaged accelerometry units of approximately 2.5%, and 70 patients would have a power of 80% to detect a clinically significant difference of 5 points on the Kansas City Cardiomyopathy Questionnaire. The target enrollment was approximately 100 patients. For all end points, patients were analyzed according to the randomized group, regardless of treatment received (if any), using a linear mixed model with fixed-effect terms for the sequence, study period, treatment, and including the baseline value for the end point variable (when available). A random-effect term was included to account for the correlated measurements for each patient. A post hoc analysis was also performed including enrolling site as a random-effect term. The mixed-effects model is valid under the “missing at random” assumption, similar to the multiple imputation approach.
For the primary end point, a sensitivity analysis included an analysis restricted to patients who were taking the study drug at the time of the corresponding visit. Because there was no adjustment of significance threshold to account for type I error in the secondary and tertiary end points, those analyses should be interpreted as exploratory. For the primary end point, interaction between treatment effect and a number of prespecified baseline characteristics was assessed. A 2-sided P value of .05 was considered to indicate statistical significance. All analyses were conducted with the use of SAS version 9.4 (SAS Institute).
Results
Study Patients
From July 22, 2016, to September 12, 2017, a total of 105 patients with chronic HFpEF were enrolled at 17 sites in the United States, with 53 assigned to nitrite first and placebo second, and 52 assigned to placebo first and nitrite second (Figure 1). Qualifying criteria for HFpEF are provided in eTable 1 in Supplement 3. The median duration of HF prior to enrollment was 1.9 years. The mean age of patients was 68 years and 56% were women (Table 1). Most patients (75%) were obese, and 95% were either overweight or obese. Most patients were white and had New York Heart Association class II or III symptoms, severely depressed exercise capacity, and poor quality of life. Comorbidities were common including high prevalence of hypertension, coronary disease, atrial fibrillation, sleep apnea, and diabetes. Most patients were treated with multiple cardiovascular medications. Mean ejection fraction was 61%. Clinical characteristics of the study population were similar to other recent trials in HFpEF (eTable 2 in Supplement 3).
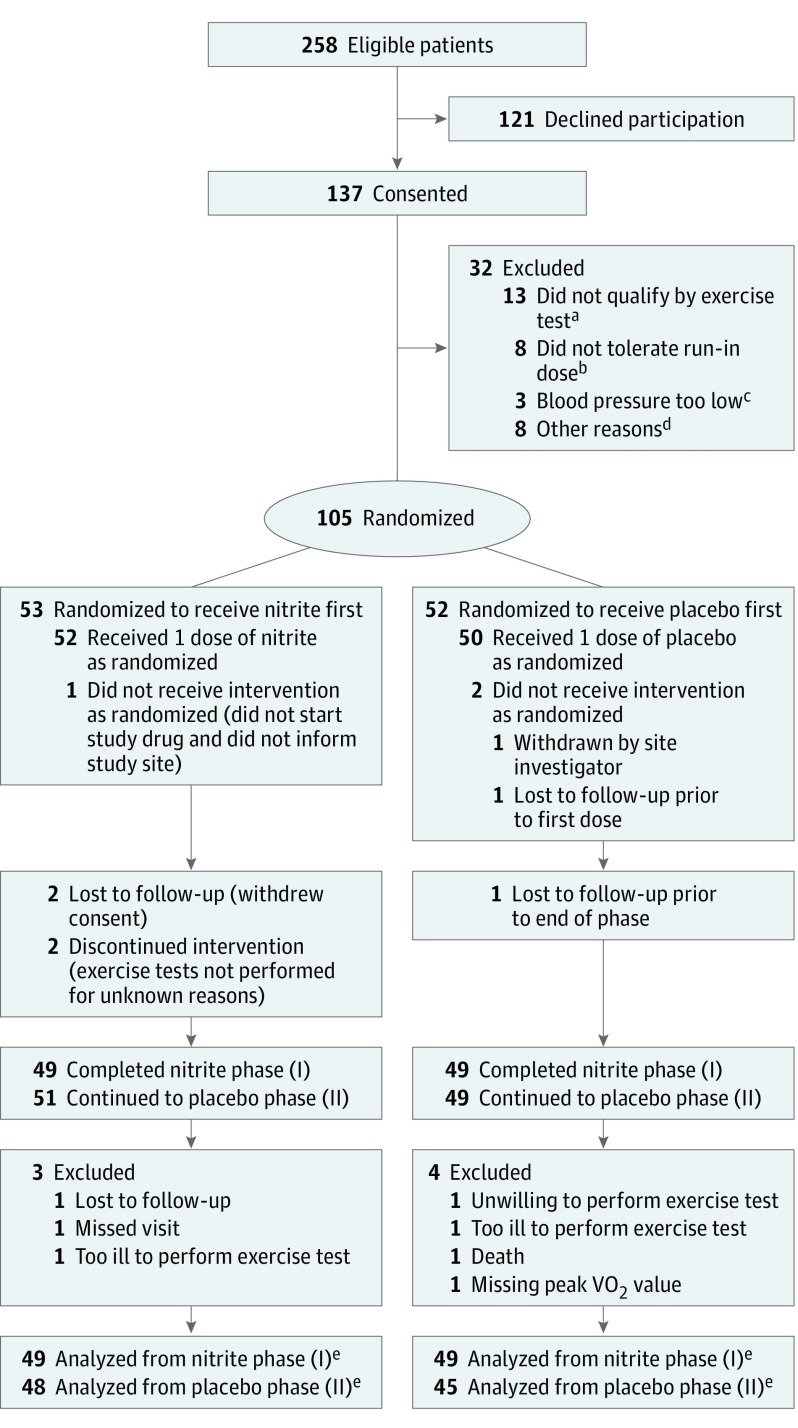
Figure 1. Flow of Participants Through the Study of Nitrite Delivery in Heart Failure With Preserved Ejection Fraction.
aA total of 12 patients did not reach an adequate peak respiratory exchange ratio (≥1.0) during cardiopulmonary exercise testing.
bOf the 8 patients failing the run-in, 2 were for low blood pressure, 2 were for anxiety and hyperventilation, and 4 were other symptomatic intolerances that developed following the run-in dose (abdominal discomfort, dizziness, nausea, cough, and shortness of breath).
cIn 3 patients, blood pressure levels were too low to administer the nitrite run-in dose.
dThe 8 remaining patients were excluded because 4 could not complete the necessary assessments in the specified time window, 2 withdrew consent, 1 reported after consent that heart failure was not their primary limitation, and 1 was found to have an ejection fraction below 50% after obtaining consent. No imputation for missing data was used because the primary analysis model uses all available data and estimates model parameters using maximum likelihood.
eThe study drug was permanently discontinued prior to the primary end-point assessment for 8 patients taking inorganic nitrite (2 unable or unwilling to administer treatment, 1 device malfunction, 1 symptom intolerance [dizziness, nausea, lassitude], 1 discontinued due to dyspepsia, 1 family event, 1 withdrew consent, and 1 death), and 5 taking placebo (2 unable or unwilling to administer treatment, 2 symptom intolerance [tremor and diaphoresis; hypotension], and 1 by investigator decision.
Table 1. Baseline Characteristics.
| Characteristic | No. (%)a | |
|---|---|---|
| Nitrite First (n = 53) | Placebo First (n = 52) | |
| Age, mean (SD), y | 68 (9) | 68 (12) |
| Sex | ||
| Female | 36 (68) | 23 (44) |
| Male | 17 (32) | 29 (56) |
| White race | 47 (89) | 45 (87) |
| BMI, mean (SD) | 35.6 (6.4) | 35.0 (7.0) |
| Functional measures | ||
| New York Heart Association classb | ||
| II: slight limitation | 25 (47) | 20 (38) |
| III: marked limitation with ordinary activity | 27 (51) | 32 (62) |
| Orthopneac | 22 (42) | 23 (45) |
| Peak oxygen consumption, mean (SD), mL/kg/min | 13.9 (3.1) | 13.8 (3.8) |
| Peak respiratory exchange ratio, mean (SD)d | 1.1 (0.1) | 1.1 (0.1) |
| Peak exercise work, mean (SD), W | 83 (30) | 83 (30) |
| Ventilatory efficiency, mean (SD)e | 32 (6) | 34 (8) |
| Overall score on KCCQ, mean (SD)f | 59 (17) | 52 (20) |
| Physical examinationc | ||
| Systolic blood pressure, mean (SD), mm Hg | 130 (16) | 131 (17) |
| Heart rate, mean (SD), bpm | 71 (12) | 71 (11) |
| Elevated jugular venous pressure | 17 (33) | 20 (39) |
| Edema | 26 (49) | 26 (50) |
| Medical historyc | ||
| Hospitalization for heart failure in previous year | 13 (25) | 10 (19) |
| Hypertension | 43 (81) | 42 (81) |
| Ischemic heart disease | 36 (68) | 37 (71) |
| History of atrial fibrillation | 24 (45) | 23 (45) |
| Diabetes | 20 (38) | 17 (33) |
| Chronic obstructive pulmonary disease | 4 (8) | 9 (17) |
| Hyperlipidemia | 38 (72) | 38 (73) |
| Sleep apnea | 31 (58) | 34 (65) |
| Anemia | 22 (42) | 23 (44) |
| Chronic kidney disease, stage ≥3 | 12 (23) | 19 (37) |
| Depression treated with prescription drug | 13 (25) | 10 (19) |
| Medications at enrollmentc | ||
| Loop diuretic | 41 (77) | 40 (77) |
| Thiazide diuretic | 6 (11) | 10 (19) |
| ACE inhibitor or ARB | 28 (53) | 28 (54) |
| β-Blocker | 32 (60) | 35 (67) |
| Aldosterone antagonist | 16 (30) | 17 (33) |
| Calcium channel blocker | 18 (34) | 14 (27) |
| Lipid-lowering agent | 35 (66) | 33 (63) |
| Antiplatelet agent | 26 (49) | 30 (58) |
| Anticoagulant agent | 22 (42) | 21 (40) |
| Laboratory or echocardiographic measures, mean (SD) | ||
| Creatinine, mg/dL | 1.1 (0.4) | 1.2 (0.4) |
| Cystatin C, mg/L | 1.3 (0.4) | 1.4 (0.5) |
| Hemoglobin, g/dL | 13.0 (1.5) | 13.1 (1.7) |
| NT-proBNP, pg/mL | 471 (624) | 528 (669) |
| Cyclic guanosine monophosphate, pmol/mL | 124 (40) | 122 (40) |
| Ejection fraction, %g | 61.4 (5.0) | 60.6 (6.7) |
Abbreviations: ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); bpm, beats per minute; KCCQ, Kansas City Cardiomyopathy Questionnaire; NT-proBNP, N-terminal fragment of the prohormone brain natriuretic peptide.
SI conversion factor: To convert creatinine to micromoles per liter, multiply by 88.4.
Values indicate mean and SD or number and percentage.
New York Heart Association functional class (ranges from I to IV, lower class representing better health) quantifies clinician-estimated severity of functional limitation. Class I indicates no limiting symptoms, class II reflects slight limitation, class III reflects marked symptoms develop with even ordinary activity, and class IV reflects patients who are symptomatic at rest or minimal activity.
Symptoms, medical history data, and examination findings determined by study investigators based on medical record review, patient interview, and physical examination at first study visit.
Respiratory exchange ratio is defined by the ratio of carbon dioxide produced relative to oxygen consumed during exercise. Values exceeding 1.0 provide objective evidence of maximal or near-maximal effort during exercise testing.
Ventilatory efficiency is a unitless index that is defined by minute ventilation (in L/min) relative to carbon dioxide production (in L/min) throughout exercise. Higher values represent worse efficiency.
The KCCQ (ranges from 0-100, higher scores representing better health) quantifies patient-reported quality of life in heart failure.
Reflects most recent historical result from echocardiography prior to randomization.
Primary End Point
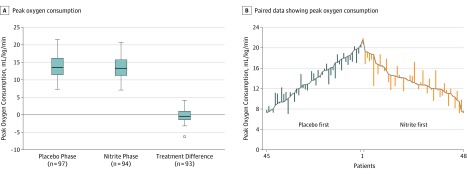
As compared with placebo, there was no significant effect of nitrite on the primary end point of peak VO2 achieved during cardiopulmonary exercise testing (13.5 vs 13.7 mL/kg/min), with a treatment effect of −0.20 mL/kg/min (95% CI, −0.56 to 0.16) (P = .27) (Table 2 and Figure 2). Post hoc analyses, including random-effect terms for both participant and enrolling site, were not meaningfully different. Similar treatment effects were observed on the basis of sensitivity analyses after restricting to patients who were taking the study drug at the time of the corresponding visit (13.5 vs 13.7 mL/kg/min; difference, −0.22 [95% CI, −0.58 to 0.14]; P = .24).
Table 2. Efficacy End Points.
| End Point | Mean (SD) | Adjusted Treatment Difference (95% CI)a | P Value | |
|---|---|---|---|---|
| Nitrite (n = 105) | Placebo (n = 105) | |||
| Primary End Point | ||||
| Peak V̇o2, mL/kg/min | 13.5 (3.3) | 13.7 (3.5) | −0.20 (−0.56 to 0.16) | .27 |
| Secondary End Points | ||||
| Activity assessed on accelerometry | ||||
| Daily arbitrary accelerometry units | 5497 (2922) | 5503 (3027) | −15 (−264 to 234) | .91 |
| Quality of life | ||||
| Overall score on the KCCQb | 61.8 (20.4) | 60.8 (18.5) | 1.3 (−1.3 to 3.8) | .32 |
| Clinical score on the KCCQb | 62.6 (20.1) | 61.9 (18.4) | 1.1 (−1.4 to 3.5) | .39 |
| NYHA classc | 2.5 (0.6) | 2.5 (0.5) | 0.1 (−0.1 to 0.2) | .43 |
| Echocardiography | ||||
| Medial E/e′ ratiod | 16.4 (9.8) | 16.6 (9.8) | 0.1 (−1.2 to 1.3) | .93 |
| PASP, mm Hg | 37.7 (9.4) | 37.4 (11.2) | 0.8 (−1.4 to 3.1) | .47 |
| Left atrial volume index, mL/m2 | 37.7 (18.4) | 38.6 (17.4) | −0.3 (−2.8 to 2.2) | .82 |
| NT-proBNP, pg/ml | 520 (671) | 533 (678) | 11 (−53 to 75) | .74 |
| Ventilatory efficiencye | 32.7 (6.6) | 33.0 (6.5) | −0.5 (−1.1 to 0.1) | .11 |
| V̇o2 at ventilatory threshold, mL/kg/minf | 7.8 (1.8) | 7.9 (1.7) | −0.1 (−0.4 to 0.2) | .44 |
| Exercise time, min | 10.8 (2.8) | 11.0 (3.0) | −0.2 (−0.5 to 0.2) | .30 |
| Tertiary End Points | ||||
| Plasma Cystatin-C, mg/L | 1.3 (0.5) | 1.4 (0.6) | −0.1 (−0.1 to −0.0) | .02 |
| Plasma cGMP, pmol/ml | 126 (41) | 125 (43) | 0.7 (−6.2 to 7.6) | .84 |
Abbreviations: cGMP, cyclic guanosine monophosphate; KCCQ, Kansas City Cardiomyopathy Questionnaire; NT-proBNP, N-terminal fragment of the prohormone brain natriuretic peptide; NYHA, New York Heart Association; PASP, pulmonary artery systolic pressure; V̇o2, oxygen consumption.
Adjusted treatment differences are adjusted for treatment sequence, period effect, a random effect for each participant, and baseline value (when available).
The KCCQ (ranges from 0-100, higher scores representing better health) quantifies patient-reported quality of life in heart failure.
NYHA functional class (ranges from I to IV, lower class representing better health).
Medial E/e′ ratio is a surrogate measure of left ventricular filling pressure, calculated by the ratio of transmitral inflow velocity to septal annular tissue velocity during early diastole. Lower values reflect decreased cardiac filling pressures.
Ventilatory efficiency is a unitless index that is defined by minute ventilation (in L/min) relative to carbon dioxide production (in L/min) throughout exercise. Higher values represent worse efficiency.
V̇o2 at the point where anaerobic metabolism increases during exercise, with higher values representing better outcomes.
Figure 2. Peak Oxygen Consumption Following Placebo and Nitrite Treatments .
A, Box plot showing peak oxygen consumption (V̇o2) measured following treatment in the placebo phase and nitrite phase, as well as the primary end point of the treatment difference between these phases (P = .27 for comparison of treatment difference after adjusting for treatment sequence, period effect, a random effect for each participant, and baseline peak V̇o2). The horizontal line in the box represents the mean. The edges of the boxes are the 25th and 75th quartile values. If there are no outliers, the ends of the whiskers are minimum and maximum values (left and middle). If there is an outlier (right; open circle), the ends of the whiskers are the highest or lowest values that are not outliers. Outliers are defined as values outside of Q1 − 1.5 × (Q3 − Q1) and Q3 + 1.5 × (Q3 − Q1). B, Each vertical bar represents paired data from an individual patient for peak V̇o2 values during the placebo period on (gray line) with bar extending to values during the nitrite period.
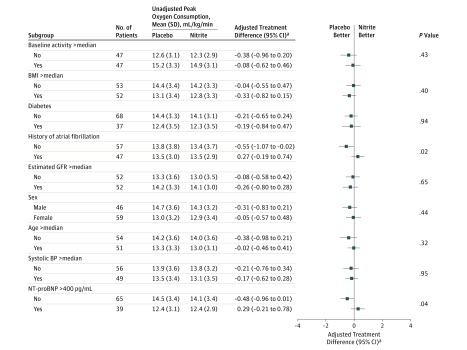
For the primary end point, there were statistically significant interactions between treatment effect and baseline NT-proBNP levels (interaction P = .04) and history of atrial fibrillation (interaction P = .02), where peak V̇o2 was lower during nitrite therapy in patients with NT-proBNP levels less than 400 pg/mL (14.1 vs 14.5 mL/kg/min; difference, −0.48 [95% CI, −0.96 to 0.01], P = .04) and in patients without a history of atrial fibrillation (13.4 vs 13.8 mL/kg/min; difference, −0.55 [95% CI, −1.07 to −0.02]; P = .02; Figure 3).
Figure 3. Treatment Differences in the Primary End Point According to Predefined Subgroups.
Error bars indicate 95% confidence intervals. P values for subgroup comparisons correspond to test for interaction. BMI indicates body mass index; BP, blood pressure; GFR, glomerular filtration rate; and NT-proBNP, N-terminal fragment of the prohormone brain natriuretic peptide.
aTreatment differences shown are after adjustment for treatment sequence, period effect, and baseline peak oxygen consumption values along with a random effect for each participant.
Secondary End Points
Treatment with nitrite, as compared with placebo, had no significant effect on daily activity levels (5497 vs 5503 accelerometry units per day; difference, −15 [95% CI, −264 to 234]; P = .91), the Clinical Summary Score (62.6 vs 61.9 points; difference, 1.1 [95% CI, −1.4 to 3.5]; P = .39), or overall summary score (61.8 vs 60.8 points; difference, 1.3 [95% CI, −1.3 to 3.8]; P = .32) on the Kansas City Cardiomyopathy Questionnaire (Table 2).
As compared with placebo, there was no significant effect of nitrite on New York Heart Association functional class (2.5 vs 2.5; difference, 0.1 [95% CI, −0.1 to 0.2]; P = .43), echocardiographic estimates of ventricular filling pressures measured prior to receiving study medication at each visit (E/e′ ratio: 16.4 vs 16.6, difference, 0.1 [95% CI, −1.2 to 1.3], P = .93; left atrial volume index: 37.7 vs 38.6 mL/m2, difference, −0.3 [95% CI, −2.8 to 2.2], P = .82; and pulmonary artery pressure: 37.3 vs 37.4 mm Hg, difference, 0.8 [95% CI, −1.4 to 3.1], P = .47), NT-proBNP levels (520 vs 533 pg/mL; difference, 11 [95% CI, −53 to 75]; P = .74), ventilatory efficiency during exercise testing (VE/V̇co2 slope: 32.7 vs 33.0; difference, −0.5 [95% CI, −1.1 to 0.1]; P = .11), or exercise time (10.8 vs 11.0 minutes; difference, −0.2 [95% CI, −0.5 to 0.2]; P = .30) (Table 2). A total of 95 patients were asked which period they preferred at the final visit: 45% of patients indicated that they felt better during receipt of nitrite, 34% reported feeling better during receipt of placebo, and 21% had no preference.
Adverse Events
The study drug was discontinued prior to end-point assessment in 8 patients receiving nitrite and 5 patients receiving placebo (Figure 1). There were 11 events of interest in participants while receiving placebo and 6 events of interest while receiving nitrite (Table 3). Worsening HF was observed in 8 patients during the placebo phase and 3 patients during the nitrite phase. There were 5 serious adverse events during the nitrite phase, including 1 death, and 4 serious adverse events during the placebo phase (Table 3).
Table 3. Adverse Events.
| Adverse Event | No. (%) | |
|---|---|---|
| Nitrite (n = 105) | Placebo (n = 105) | |
| Any adverse event of interest | 6 (5.7) | 11 (10.5) |
| Dysrhythmiaa | 1 (1.0) | 2 (1.9) |
| Worsening heart failureb | 3 (2.9) | 8 (7.6) |
| Acute coronary syndromec | 1 (1.0) | 1 (1.0) |
| Stroke or transient ischemic attackd | 0 | 0 |
| Worsening renal functione | 1 (1.0) | 1 (1.0) |
| Any serious adverse eventf | 5 (4.8) | 4 (3.8) |
| Death | 1 (1.0) | 0 |
New and clinically significant atrial or ventricular dysrhythmias.
Worsening signs or symptoms of heart failure requiring change in medical therapy such as need for intravenous diuretics.
Defined as unstable angina, non–ST-segment elevation myocardial infarction, and ST-segment elevation myocardial infarction.
Defined as cerebrovascular accidents (stroke) of any cause (hemorrhagic, ischemic, or embolic) and transient ischemic attack.
Defined as increase in serum creatinine of 0.3 mg/dL (to convert to micromoles per liter, multiply by 88.4).
There was a total of 6 serious adverse events in 5 participants during the nitrite phase (sudden death, Crohn disease flare, chest pain, diabetic ketoacidosis, hypoglycemia, and foreign body in gastrointestinal tract). There was a total of 6 serious adverse events in 4 participants during the placebo phase (2 pneumonia, sepsis, syncope, fall, and staphylococcal bacteremia).
Adherence and Nonprespecified End Points
Median adherence rates to study drug administration (determined by device readouts) during the nitrite and placebo phases were 86% and 84%, respectively. Systolic blood pressure was higher prior to receipt of study drug during the nitrite phase (130 vs 125 mm Hg; difference, 5 [95% CI, 2 to 9]; P = .001), while systolic pressure following study drug administration tended to be lower (121 vs 124 mm Hg; difference, −3 [95% CI, −7 to 1]; P = .10), such that systolic blood pressure was reduced by nitrite compared with placebo at rest (−9 vs −2 mm Hg; difference, −7 [95% CI, −11 to −4]; P < .0001; eTable 3 in Supplement 3). Nitrite also reduced resting systolic blood pressure during the open-label run-in dose (117 vs 130 mm Hg; difference, −12 [95% CI, −9 to −15]; P < .001; eTable 4 in Supplement 3). There was no effect of nitrite on exercise systolic blood pressure (154 vs 156 mm Hg; difference, −1 [95% CI, −6 to 3]; P = .59), but exercise diastolic (70 vs 72 mm Hg; difference, −3 [95% CI, −6 to −1]; P = .01) and mean (97 vs 101 mm Hg; difference, −3 [95% CI, −5 to 0]; P = .048) blood pressures were reduced (eTable 5 in Supplement 3). Compared with placebo, treatment with nitrite did not alter hours active per day (6.2 vs 6.5 hours; difference, −0.02 [95% CI, −0.20 to 0.17]; P = .84; eTable 6 in Supplement 3).
Discussion
In this study, inhaled sodium nitrite, a nitric oxide–providing therapy, did not improve peak aerobic capacity, daily activity levels, quality-of-life scores, or other indicators of clinical status in patients with chronic HFpEF. These results are in contrast to multiple earlier studies suggesting administration of inorganic nitrite or nitrate may have benefits in HFpEF.8,9,10,11,12,13,17
As is typical of HFpEF in the community, multimorbidity was common in the patients participating in this study, with high prevalence of hypertension, coronary disease, diabetes, and sleep apnea.18 Obesity, which is associated with a more severe phenotype of HFpEF,19 was present in 75% of patients, while 95% were either overweight or obese. Plasma natriuretic peptide levels were lower in this study than many prior trials, likely related to the high prevalence of obesity/overweight, and because elevated NT-proBNP levels were not required for eligibility. Despite this, the patients enrolled in this trial displayed objective evidence of severe exercise limitation, and all participants indicated that their functional capacity was most strongly limited by HF symptoms rather than pain, balance, motivation, or other issues.
Measures of both maximal functional capacity (peak V̇o2) and volume of daily activity (accelerometry) were examined to assess the efficacy of inorganic nitrite in patients with HFpEF. Peak V̇o2, which is the gold-standard indicator of functional capacity, is depressed in patients with HFpEF, and greater impairment is associated with adverse outcomes.20,21 Reduction in peak V̇o2 in HFpEF is related in part to high cardiac filling pressures that promote symptoms of dyspnea,21,22 as well as abnormalities peripheral to the heart, in the vasculature and skeletal muscle, which limit oxygen delivery and use in the tissues.23,24,25 Through its potential effects on the heart, blood vessels, and muscle, nitrite could improve each of these components and thus enhance peak aerobic capacity.6
In contrast to peak V̇o2, accelerometry may provide an assessment of the volume of daily activity that is quantitative, high density, and more applicable to activities of daily living. Accelerometry provides insight on the effect of medical interventions that may not be apparent from intermittent assessments and is complementary with other measures, such as peak exercise capacity.4,26 The lack of treatment effect on both measures, in concert with absent effects on quality of life, NT-proBNP levels, and other indicators, shows that inhaled nitrite as administered in this study did not improve clinical status in people with HFpEF.
The current results differ from those reported in single-center studies demonstrating improvements in hemodynamics, submaximal exercise endurance, and peak V̇o2 using therapies that target the inorganic nitrate/nitrite pathway.6,8,9,10,11,12,13,17 The reasons for the discrepant findings are not clear. While the half-life of inhaled nitrite is short (approximately 40 minutes),10 study medication was administered immediately prior to exercise testing in this study. There was no improvement in echocardiographic markers of cardiac filling pressures and no increase in plasma cGMP levels with nitrite, but these assessments were performed at trough rather than peak drug levels, and blood pressure was reduced, suggesting that a biological effect was achieved at the time of peak concentrations. The absence of benefit with inhaled nitrite may relate to its short plasma half-life or the relatively brief duration of the trial (4 weeks), which might not have allowed adequate exposure to observe a favorable effect on cardiovascular structure and function.
To our knowledge, this is the first multicenter trial testing an inhaled therapy for HF. The pulmonary alveolar–capillary interface represents one of the fastest and most efficient sites for absorption of systemically active drugs, eliminating problems related to first pass metabolism with oral administration. Despite these advantages, difficulties with proper use of the nebulizer device may have interfered with drug delivery, for example, with longer-than-expected treatment times. Favorable acute hemodynamic effects with nitrite have been observed with the Aerogen Solo-Idehaler nebulizer device,10,12 but the latter is only feasible for single-time use, rather than the nebulizer used in the current study that is suitable for chronic use, and this might have influenced the effectiveness of delivery. Clinical trials testing alternative, orally active formulations targeting the inorganic nitrate/nitrite pathway are currently under way in HFpEF (NCT02918552, NCT03015402, NCT02980068, NCT02840799, NCT03289481, and NCT02713126). Given the longer duration of action for these alternative formulations, the results of these trials could differ from the current study.
The only intervention that has been consistently shown to improve aerobic capacity in HFpEF is exercise training,27 and perhaps it is necessary in future trials to couple cardiovascular medicines (such as nitrite) with concomitant exercise training and/or weight loss to achieve meaningful improvements in functional capacity. Administration of inorganic nitrate via beetroot juice did not improve functional capacity in a recent pilot study, but the duration of exposure and dose administered may not have been sufficient.28 Another trial is currently under way testing whether more sustained use of higher-dose nitrite might improve the efficacy and tolerability of exercise training in HFpEF (NCT02713126).
Limitations
This study has several limitations. First, the hypothesis for this study was predicated on the assumption that improved hemodynamics would cause patients with HFpEF to become more active and able to reach a higher peak exercise capacity. However, patients with HFpEF and obesity who are habitually sedentary might not experience the effect of improved hemodynamics on symptoms. Second, the exploratory secondary end point of accelerometry measures volitional activity, which is dependent on psychosocial factors and motivation in addition to limitations related to cardiac insufficiency. As such, volume of activity might be less apt to improve with interventions targeted to cardiovascular abnormalities alone, without concomitant behavioral and psychosocial interventions. Third, plasma nitrite levels were not measured, which limits the ability to relate pharmacokinetics to treatment effects. Fourth, blood tests, including cGMP levels, were measured prior to receipt of study drug, when plasma nitrite levels would be at their trough. This may partly explain the lack of difference in cGMP with nitrite administration, and future studies might evaluate cGMP levels closer to the time of peak plasma nitrite levels.
Conclusions
Among patients with HFpEF, administration of inhaled inorganic nitrite for 4 weeks, compared with placebo, did not result in significant improvement in exercise capacity.
Trial Protocol
Statistical Analysis Plan
eTable 1. Qualifying Criteria for Enrollment
eTable 2. Patient Characteristics in Comparison to Other Contemporary Trials in HFpEF
eTable 3. Hemodynamic Effects of Nitrite vs Placebo
eTable 4. Effects of Open Label Nitrite on Blood Pressure During Run-in
eTable 5. Effects of Nitrite vs Placebo on Additional Exercise End Points
eTable 6. Effects of Nitrite vs Placebo on Hours Active per Day
eAppendix. Detailed Description of End Point Definitions and Methods
eReferences
Data Sharing Statement
References
- 1.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263-271. doi: 10.1016/j.jacc.2013.02.092 [DOI] [PubMed] [Google Scholar]
- 2.Redfield MM. Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2017;376(9):897. [DOI] [PubMed] [Google Scholar]
- 3.Redfield MM, Chen HH, Borlaug BA, et al. ; RELAX Trial . Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309(12):1268-1277. doi: 10.1001/jama.2013.2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Redfield MM, Anstrom KJ, Levine JA, et al. ; NHLBI Heart Failure Clinical Research Network . Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med. 2015;373(24):2314-2324. doi: 10.1056/NEJMoa1510774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pieske B, Maggioni AP, Lam CSP, et al. . Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES-PRESERVED) study. Eur Heart J. 2017;38(15):1119-1127. doi: 10.1093/eurheartj/ehw593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reddy YNV, Lewis GD, Shah SJ, et al. . INDIE-HFpEF (Inorganic Nitrite Delivery to Improve Exercise Capacity in Heart Failure With Preserved Ejection Fraction): rationale and design. Circ Heart Fail. 2017;10(5):e003862. doi: 10.1161/CIRCHEARTFAILURE.117.003862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3(5):588-595. doi: 10.1161/CIRCHEARTFAILURE.109.930701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borlaug BA, Koepp KE, Melenovsky V. Sodium nitrite improves exercise hemodynamics and ventricular performance in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2015;66(15):1672-1682. doi: 10.1016/j.jacc.2015.07.067 [DOI] [PubMed] [Google Scholar]
- 9.Zamani P, Rawat D, Shiva-Kumar P, et al. . Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation. 2015;131(4):371-380. doi: 10.1161/CIRCULATIONAHA.114.012957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borlaug BA, Melenovsky V, Koepp KE. Inhaled sodium nitrite improves rest and exercise hemodynamics in heart failure with preserved ejection fraction. Circ Res. 2016;119(7):880-886. doi: 10.1161/CIRCRESAHA.116.309184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eggebeen J, Kim-Shapiro DB, Haykowsky M, et al. . One week of daily dosing with beetroot juice improves submaximal endurance and blood pressure in older patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2016;4(6):428-437. doi: 10.1016/j.jchf.2015.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simon MA, Vanderpool RR, Nouraie M, et al. . Acute hemodynamic effects of inhaled sodium nitrite in pulmonary hypertension associated with heart failure with preserved ejection fraction. JCI Insight. 2016;1(18):e89620. doi: 10.1172/jci.insight.89620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zamani P, Tan V, Soto-Calderon H, et al. . Pharmacokinetics and pharmacodynamics of inorganic nitrate in heart failure with preserved ejection fraction. Circ Res. 2017;120(7):1151-1161. doi: 10.1161/CIRCRESAHA.116.309832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fletcher GF, Balady G, Froelicher VF, Hartley LH, Haskell WL, Pollock ML; Writing Group . Exercise standards: a statement for healthcare professionals from the American Heart Association. Circulation. 1995;91(2):580-615. doi: 10.1161/01.CIR.91.2.580 [DOI] [PubMed] [Google Scholar]
- 15.Swank AM, Horton J, Fleg JL, et al. ; HF-ACTION Investigators . Modest increase in peak VO2 is related to better clinical outcomes in chronic heart failure patients: results from heart failure and a controlled trial to investigate outcomes of exercise training. Circ Heart Fail. 2012;5(5):579-585. doi: 10.1161/CIRCHEARTFAILURE.111.965186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flynn KE, Piña IL, Whellan DJ, et al. ; HF-ACTION Investigators . Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1451-1459. doi: 10.1001/jama.2009.457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reddy YNV, Andersen MJ, Obokata M, et al. . Arterial stiffening with exercise in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2017;70(2):136-148. doi: 10.1016/j.jacc.2017.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mentz RJ, Kelly JP, von Lueder TG, et al. . Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2014;64(21):2281-2293. doi: 10.1016/j.jacc.2014.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136(1):6-19. doi: 10.1161/CIRCULATIONAHA.116.026807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guazzi M, Myers J, Arena R. Cardiopulmonary exercise testing in the clinical and prognostic assessment of diastolic heart failure. J Am Coll Cardiol. 2005;46(10):1883-1890. doi: 10.1016/j.jacc.2005.07.051 [DOI] [PubMed] [Google Scholar]
- 21.Reddy YNV, Olson TP, Obokata M, Melenovsky V, Borlaug BA. Hemodynamic correlates and diagnostic role of cardiopulmonary exercise testing in heart failure with preserved ejection fraction. JACC Heart Fail. 2018;6(8):665-675. doi: 10.1016/j.jchf.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Obokata M, Olson TP, Reddy YNV, Melenovsky V, Kane GC, Borlaug BA. Haemodynamics, dyspnoea, and pulmonary reserve in heart failure with preserved ejection fraction. Eur Heart J. 2018;39(30):2810-2821. doi: 10.1093/eurheartj/ehy268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Houstis NE, Eisman AS, Pappagianopoulos PP, et al. . Exercise intolerance in heart failure with preserved ejection fraction: diagnosing and ranking its causes using personalized O2 pathway analysis. Circulation. 2018;137(2):148-161. doi: 10.1161/CIRCULATIONAHA.117.029058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisman AS, Shah RV, Dhakal BP, et al. . Pulmonary capillary wedge pressure patterns during exercise predict exercise capacity and incident heart failure. Circ Heart Fail. 2018;11(5):e004750. doi: 10.1161/CIRCHEARTFAILURE.117.004750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss K, Schär M, Panjrath GS, et al. . Fatigability, exercise intolerance, and abnormal skeletal muscle energetics in heart failure. Circ Heart Fail. 2017;10(7):e004129. doi: 10.1161/CIRCHEARTFAILURE.117.004129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snipelisky D, Kelly J, Levine JA, et al. . Accelerometer-measured daily activity in heart failure with preserved ejection fraction: clinical correlates and association with standard heart failure severity indices. Circ Heart Fail. 2017;10(6):e003878. doi: 10.1161/CIRCHEARTFAILURE.117.003878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pandey A, Parashar A, Kumbhani D, et al. . Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail. 2015;8(1):33-40. doi: 10.1161/CIRCHEARTFAILURE.114.001615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaltout HA, Eggebeen J, Marsh AP, et al. . Effects of supervised exercise and dietary nitrate in older adults with controlled hypertension and/or heart failure with preserved ejection fraction. Nitric Oxide. 2017;69:78-90. doi: 10.1016/j.niox.2017.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eTable 1. Qualifying Criteria for Enrollment
eTable 2. Patient Characteristics in Comparison to Other Contemporary Trials in HFpEF
eTable 3. Hemodynamic Effects of Nitrite vs Placebo
eTable 4. Effects of Open Label Nitrite on Blood Pressure During Run-in
eTable 5. Effects of Nitrite vs Placebo on Additional Exercise End Points
eTable 6. Effects of Nitrite vs Placebo on Hours Active per Day
eAppendix. Detailed Description of End Point Definitions and Methods
eReferences
Data Sharing Statement