Abstract
Background
To report our experience utilizing a multidisciplinary clinic (MDC) at Indiana University (IU) since the publication of the International Germ Cell Cancer Collaborative Group (IGCCCG), and to compare our overall survival (OS) to that of the National Cancer Institute (NCI) Surveillance, Epidemiology, and End Results (SEER) Program.
Patients and methods
We conducted a retrospective analysis of all patients with metastatic germ-cell tumor (GCT) seen at IU from 1998 to 2014. A total of 1611 consecutive patients were identified, of whom 704 patients received an initial evaluation by our MDC (including medical oncology, pathology, urology and thoracic surgery) and started first-line chemotherapy at IU. These 704 patients were eligible for analysis. All patients in this cohort were treated with cisplatin–etoposide-based combination chemotherapy. We compared the progression-free survival (PFS) and OS of patients treated at IU with that of the published IGCCCG cohort. OS of the IU testis cancer primary cohort (n = 622) was further compared with the SEER data of 1283 patients labeled with ‘distant’ disease. The Kaplan–Meier method was used to estimate PFS and OS.
Results
With a median follow-up of 4.4 years, patients with good, intermediate, and poor risk disease by IGCCCG criteria treated at IU had 5-year PFS of 90%, 84%, and 54% and 5-year OS of 97%, 92%, and 73%, respectively. The 5-year PFS for all patients in the IU cohort was 79% [95% confidence interval (CI) 76% to 82%]. The 5-year OS for the IU cohort was 90% (95% CI 87% to 92%). IU testis cohort had 5-year OS 94% (95% CI 91% to 96%) versus 75% (95% CI 73% to 78%) for the SEER ‘distant’ cohort between 2000 and 2014, P-value <0.0001.
Conclusion
The MDC approach to GCT at high-volume cancer center associated with improved OS outcomes in this contemporary dataset. OS is significantly higher in the IU cohort compared with the IGCCCG and SEER ‘distant’ cohort.
Keywords: testicular cancer, germ-cell tumor, IGCCCG, multidisciplinary, SEER
Key Message
We investigated the role of multidisciplinary clinic in improving the outcomes of patients with metastatic germ-cell tumors at our high volume cancer center at Indiana University (IU) and observed a better survival compared with the historical IGCCCG and the SEER distant cohort.
Introduction
Germ-cell tumors (GCTs) are the most common cancer in men between 15 and 35 years of age, with an estimated 8720 cases diagnosed annually in the United States and 410 deaths [1]. First-line chemotherapy with bleomycin–etoposide–cisplatin (BEP) became the standard of care for patients with advanced GCT [2–5]. The International Germ Cell Cancer Collaborative Group (IGCCCG) in 1997 published a consensus statement classifying patients with metastatic GCT into good, intermediate, and poor risk disease [6]. Good risk GCT had a 5-year progression-free survival (PFS) of 88% and a 5-year overall survival (OS) of 91%. Intermediate risk GCT had a 5-year PFS of 75% and a 5-year OS of 79%. The poor risk category had a 5-year PFS of 41% and a 5-year OS of 48%.
The optimal management of GCTs is complex, with options including chemotherapy and surgery. At Indiana University Cancer Center (IU), we have established a multidisciplinary clinic (MDC) to evaluate newly diagnosed GCT patients and those needing additional consultation. The goals of this MDC are to provide state-of-the-art oncology care and to educate patients, their families, medical students, residents, and fellows in training. Our MDC integrate dedicated team including medical oncologists, pathologists, urologic and thoracic surgical oncologists, full-time coordinator (responsible for data acquisition, scheduling, and following up with patients and referring physicians) and oncology nurses. The team meets on a weekly basis in a multidisciplinary tumor board. Through this clinic, we can establish the accurate pathologic diagnosis, offer combination chemotherapy, surgical resection of residual tumor and enroll patients on clinical trials all in one visit (supplementary Figure S2, available at Annals of Oncology online).
Institutional experience, hospital and physician volume have been associated with improved outcomes of testicular cancer [7–10]. Recent outcome data from large datasets are missing, and the difference in results of patients treated in large volume centers and community centers is unknown. We, therefore, report survival outcomes in 704 consecutive patients with metastatic GCT treated at our MDC at IU since the publication of IGCCCG and compare the outcome to those of National Cancer Institute (NCI) Surveillance, Epidemiology, and End Results (SEER) Program.
Patients and methods
Patients
The IU Cancer Registry database was queried, and a retrospective review was carried out to compare the PFS and OS of patients treated at IU with that of IGCCCG [6]. This study used the secure Web-based, Title 21 Code of Federal Regulations Part 11—compliant Research Electronic Data Capture (REDCap) system for data input. Eligible patients who had metastatic GCT treated at IU after the establishment of IGCCCG between January 1998 and December 2014 were included. All patients were treated with standard cisplatin–etoposide-based combination chemotherapy consisting of at least three to four cycles of cisplatin and etoposide with or without bleomycin or ifosfamide [11, 12]. SEER Research Data (1973–2014) was also obtained to compare OS of the IU cohort the SEER distant cohort. The SEER distant cohort consisted of patients in the SEER database with testis cancer diagnosed between 2000 and 2014, who had an SEER historical stage of distant, and who had available survival data [13].
Statistical analysis
The end points of the study were the PFS and OS probabilities at 5 years. For the IU cohort, PFS started with the initiation of chemotherapy and ended with progression or death, whichever occurred first. OS started with the initiation of chemotherapy and ended with the death of a patient. Survival status was identified from medical charts or death certificates. Patients without an event were censored at the date of last follow-up. For the SEER distant cohort, OS started with the date of diagnosis and ended with the death of a patient. Patients alive at the date of last contact were censored. PFS and OS were calculated according to the Kaplan–Meier method and using the log-rank test. Analyses were completed compared using SAS software, version 9.4 and figures were created in R, version 3.3.2. Five-year PFS and OS were reported along with 95% confidence intervals (CIs) calculated using the log–log method.
Results
Patient and disease characteristics
For the IU cohort, 1611 consecutive patients with metastatic GCT were evaluated in the MDC at IU between 1998 and 2014. Of these, 704 patients started the initial chemotherapy at IU and were included in the primary outcome analysis (supplementary Figure S1, available at Annals of Oncology online). Median age at diagnosis was 29 (range 13–62). Median follow-up time was 4.4 years. The primary tumor site was testis in 622 (88.4%), retroperitoneum in 26 (3.7%), and mediastinum in 54 (7.7%). Eighty-five percent of patients had nonseminomatous GCT (NSGCT). Ninety-seven percent of patients were white, 1% were black, and the remaining 2% were a variety of races/ethnicities. Of note, we did not include LDH in our database. Elevations in LDH are highly nonspecific and may be found in a vast number of benign and malignant conditions [14]. Table 1 lists patients and disease characteristics at the time of initiation of first-line chemotherapy. Supplementary Figure S3, available at Annals of Oncology online, presents a map of the United States showing the zip codes of patients seen at our center.
Table 1.
Patient’s and disease’s characteristics at the beginning of first-line chemotherapy
| Characteristic | Overall (N = 704) | Gooda (N = 449; 63.8%) | Intermediatea (N = 74; 10.5%) | Poora (N = 181; 25.7%) |
|---|---|---|---|---|
| Median age (range) | 29.3 (13.1–61.5) | 30.54 (14.9–61.5) | 26.8 (16.0–49.6) | 26.9 (13.1–55.7) |
| Location of primary tumor | ||||
| Testis | 622 (88.4%) | 433 (96.4%) | 70 (94.6%) | 119 (65.8%) |
| Retroperitoneum | 26 (3.7%) | 9 (2.0%) | 3 (4.0%) | 14 (7.7%) |
| Mediastinum | 54 (7.7%) | 5 (1.1%) | 1 (1.4%) | 48 (26.5%) |
| Unknown | 2 (0.2%) | 2 (0.5%) | 0 | 0 |
| Tumor histology | ||||
| Seminoma | 106 (15.1%) | 99 (22.1%) | 7 (9.5%) | 0 |
| NSGCT | 598 (84.9%) | 350 (77.9%) | 67 (90.5%) | 181 (100%) |
| Predominant histology | ||||
| Embryonal | 257 (36.5%) | 205 (45.7%) | 22 (29.7%) | 30 (16.6%) |
| Choriocarcinoma | 48 (6.8%) | 1 (0.2%) | 5 (6.8%) | 42 (23.2%) |
| Yolk sac tumor | 69 (9.8%) | 16 (3.6%) | 9 (12.2%) | 44 (24.3%) |
| Teratoma | 65 (9.2%) | 29 (6.5%) | 7 (9.5%) | 29 (16.0%) |
| Mixed | 104 (14.8%) | 53 (11.8%) | 21 (28.4%) | 30 (16.6%) |
| Seminoma | 63 (9.0%) | 59 (13.1%) | 3 (4.0%) | 1 (0.6%) |
| Pure seminoma | 79 (11.2%) | 74 (16.5%) | 5 (6.8%) | 0 |
| Necrosis | 11 (1.6%) | 5 (1.1%) | 2 (2.7%) | 4 (2.2%) |
| IGCN (CIS) | 8 (1.1%) | 7 (1.6%) | 0 | 1 (0.6%) |
| Median serum AFP (ng/ml) (range) | 10.8 (0.2–280 000) | 5.9 (0.2–999) | 1323.6 (0.6–9653) | 270.3 (0.9–280 000) |
| Serum AFP | ||||
| <1000 | 578 (82.8%) | 444 (100%) | 29 (39.2%) | 105 (58.3%) |
| 1000–10 000 | 79 (11.3%) | 0 | 45 (60.8%) | 34 (18.9%) |
| ≥10 000 | 41 (5.9%) | 0 | 0 | 41 (22.8%) |
| Median serum HCG (mIU/ml) (range) | 21.7 (0–1 700 000) | 6.2 (0–4981.9) | 1334.5 (0–41 000) | 10838.0 (0.5–1 700 000) |
| Serum HCG | ||||
| <5000 | 571 (81.8%) | 444 (100%) | 44 (59.5%) | 83 (46.1%) |
| 5000–50 000 | 51 (7.3%) | 0 | 30 (40.5%) | 21 (11.7%) |
| ≥50 000 | 76 (10.9%) | 0 | 0 | 76 (42.2%) |
| Metastatic site(s) | ||||
| Retroperitoneum | 555 (78.8%) | 362 (80.6%) | 68 (91.9%) | 125 (69.1%) |
| Pulmonary | 270 (38.4%) | 96 (21.4%) | 41 (55.4%) | 133 (73.5%) |
| NPVM | 93 (13.2%) | 0 | 5 (6.8%) | 88 (48.6%) |
| Liver | 60 (8.5%) | 0 | 1 (1.4%) | 59 (32.6%) |
| Brainb | 34 (4.8%) | 0 | 3 (4.1%) | 31 (17.1%) |
| Boneb | 16 (2.3%) | 0 | 2 (2.7%) | 14 (7.7%) |
| Other | 12 (1.7%) | 0 | 1 (1.4%) | 11 (6.1%) |
| First-line chemotherapy | ||||
| BEPX3 | 384 (54.6%) | 371 (82.6%) | 7 (9.5%) | 6 (3.3%) |
| BEPX4 | 123 (17.5%) | 6 (1.3%) | 26 (35.1%) | 91 (50.3%) |
| BEPX3+EPX1 | 69 (9.8%) | 15 (3.3%) | 33 (44.6%) | 21 (11.6%) |
| EPX4 | 42 (6.0%) | 41 (9.1%) | 1 (1.3%) | 0 |
| VIPX4 | 50 (7.1%) | 0 | 2 (2.7%) | 48 (26.5%) |
| Other | 36 (5.1%) | 16 (3.6%) | 5 (6.8%) | 15 (8.3%) |
Risk per IGCCCG classification.
Brain/bone imaging was not mandatory.
NPVM, nonpulmonary visceral metastasis; IGCCCG, International Germ Cell Cancer Collaborative Group; AFP, alpha fetoprotein; HCG, human chorionic gonadotropin; IU, International unit; NSGCT, nonseminomatous germ-cell tumor; IGCN, intratubular GERM cell neoplasia.
For the SEER distant cohort, 1283 patients were identified from the SEER database with testis cancer diagnosed between 2000 and 2014. To be included in the cohort, patients must have had an SEER historical stage of distant and available survival data. Patients with a survival time of 0 (i.e. date of diagnosis and date of last contact are the same) were excluded. Median age at diagnosis was 32 (range 0–87). Eighty-seven percent of patients were white, 5% were black, and the remaining 8% were a variety of races/ethnicities. A 73.5% of patients had NSGCT.
Treatment administration
All 704 assessable patients in the IU cohort were treated with cisplatin–etoposide combination chemotherapy. Details regarding first-line treatment regimen stratified per IGCCCG risk classification are listed in Table 1. Overall, 82% of patients achieved a complete response and remained disease-free after first-line chemotherapy. A total of 250 patients (36%) underwent post-chemotherapy retroperitoneal lymph node dissection (PCRPLND), 129 (18%) thoracic surgery, 21 cervical lymph node dissection and 9 patients had a resection of brain metastasis. One hundred and fifty-three patients failed first-line chemotherapy, 118 received salvage chemotherapy including high-dose chemotherapy (HDCT) (n = 76), and 51 had salvage surgery. At last follow-up, 635 patients (90%) had no evidence of disease (NED), 65 patients (9%) had died, and 4 patients (1%) were alive with relapsed disease. Among patients who died, 52 patients were dead of disease progression, and 13 patients died of other causes including treatment-related toxicity, secondary malignancy, or surgical complications.
We also reviewed the GCT patients who came to IU for a second opinion. Nine hundred and seven patients sought a second opinion or were evaluated after receiving first-line therapy at an outside institution and were not included in the primary analysis. Four hundred and ninety-two (56%) underwent PCRPLND, 432 (51%) underwent salvage chemotherapy, and 172 (21%) underwent thoracic surgery as a result of the multidisciplinary evaluation.
Survival outcomes
With a median follow-up of 4.4 years, the estimated 5-year PFS was 79% (95% CI 76% to 82%) and the 5-year OS was 90% (95% CI 87% to 92%) for the IU cohort (Table 2). The 5-year PFS for good, Intermediate and poor risk were 90%, 84%, and 54%, respectively (Figure 1A), and the estimated 5-year OS was 97%, 92%, and 73% (Figure 1B), respectively. In sub-segment of patients with testis as the primary site at presentation (IU testis cohort n = 622), the 5-year OS was 94% (95% CI 91% to 96%). Patients with primary mediastinal nonseminomatous GCT (PMNSGCT) had an estimated 5-year PFS of 50% (95% CI 35% to 63%) and 5-year OS of 59% (95% CI 43% to 72%). Patients with brain metastasis at diagnosis had an estimated 5-year PFS of 15% (95% CI 5% to 28%) and 5-year OS of 46% (95% CI 28% to 63%).
Table 2.
Comparison of survival outcomes between the IGCCCG, IU and NCI SEER dataset
| Risk per IGCCCG criteria | 5-Year | Indiana University 1998–2014 (%) | IGCCCG 1975–1990 (%) | NCI SEER 2000–2013 (%) |
|---|---|---|---|---|
| Good risk | PFS | 90 | 88 | NA |
| OS | 97 | 91 | ||
| Intermediate risk | PFS | 84 | 75 | NA |
| OS | 92 | 79 | ||
| Poor risk | PFS | 54 | 41 | NA |
| OS | 73 | 48 | ||
| Testis cancer cohort | OS | 94 | NA | 75 |
IGCCCG, International Germ Cell Cancer Collaborative Group; PFS, progression-free survival; OS, overall survival, NCI SEER: National Cancer Institute Surveillance, Epidemiology, and End Results Program.
Figure 1.

Kaplan–Meier estimates of progression-free survival (A) and overall survival (B) according to IGCCCG risk stratification.
To demonstrate the impact of our MDC approach, we compared OS of patients in the IU testicular primary cohort with the SEER distant cohort. The 5-year OS for the SEER distant cohort was 75% (95% CI 73% to 78%) compared with 94% (95% CI 91% to 96%) for IU testis cohort (P-value <0.0001; Figure 2). The SEER database does not allow stratification according to the IGCCCG risk category; therefore comparisons of survival between groups are not possible.
Figure 2.
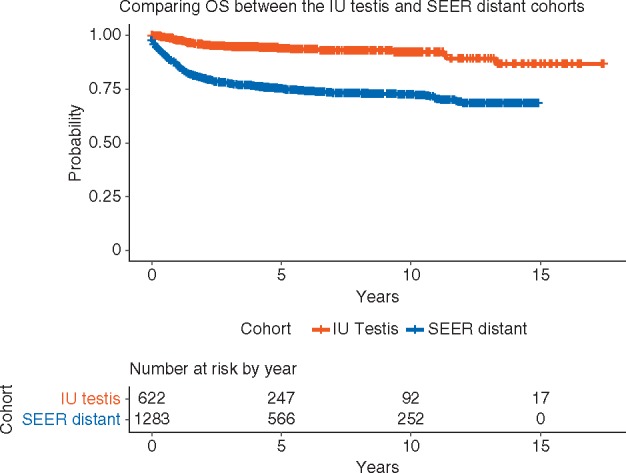
Kaplan–Meier estimates of overall survival of patients with newly diagnosed metastatic testicular GCT at the IU (1998–2014) and patients in NCI SEER (2000–2014).
Discussion
To our knowledge, this is the largest single-institution study evaluating survival outcomes of patients with metastatic GCT. Survival results of patients treated at IU appear superior to the results of the IGCCCG (Table 2) and the NCI SEER distant cohort (Figure 2). This observation is supported by a large multi-institutional initiative that provided outcome results from high-volume centers that were superior to the original IGCCCG [15]. These data were, however, not directly compared with community outcomes. Several factors may account for excellent survival outcomes seen at our center compared with the IGCCCG and SEER database. This could be attributed to the uniform utilization of cisplatin–etoposide-based combination chemotherapy, improvement in supportive care avoiding delays between cycles, expertise in post-chemotherapy surgical resection of residual disease and the experience resulting from a large volume of patients. Our dedicated multidisciplinary team of medical, urologic and thoracic oncologists, and pathologists have specific academic interest in GCT supported by strong research and clinical trials designed to refine treatment, improve supportive care, and patient education.
Surgical treatment is crucial for the management of metastatic GCT to improve survival and reduce complications [16, 17]. Appropriate patient selection and timing of surgery have lowered morbidity while improving oncologic outcomes at high volume centers [18, 19]. The marked improvement in OS in all-risk categories maybe driven by the development of successful salvage therapy options including salvage surgery, and the long-term experience in HDCT followed by autologous peripheral blood stem-cell transplant [20–25].
This analysis has limitations. This is a retrospective single institution study, and potential bias exists in our patient population. We did not have access to matched patient’s characteristics between the contemporary IU cohort and the historical IGCCCG cohort, and the community patients reported in SEER. Referral bias might have affected the results of this study. However, this study has a large sample size of consecutive patients with metastatic GCT treated at a tertiary care center with long follow-up. A large portion of patients enrolled in the study had poor risk disease 25.7% compared with 14% of patients from the IGCCCG [6]; hence survival outcomes for patients treated at other institutions or in the community might vary. Besides, a limitation of this study is that NCI SEER uses a staging system including local, regional, and distant metastases which are not typically used in GCT. The IGCCCG classification of good, intermediate, and poor risk is not included in the SEER database which makes further analysis not possible. That is why we compared all patients with metastatic disease as one group.
Despite substantial improvement in outcomes of patients with metastatic GCT treated in the modern era, many challenges remain. There is a clear disparity in health care outcomes among patients with testis cancer [26–28]. This could be related to patient’s factors such as under insurance, poor socioeconomic status, ethnicity, and a language barrier that delays diagnosis. Also, it could be attributed to the rare nature of this cancer and lack of experience in the community to establish an accurate diagnosis and deliver a treatment plan.
In conclusion, in this modern cohort of newly diagnosed metastatic GCT, there was an improvement in PFS and OS for good, intermediate, and poor-risk disease compared with IGCCCG. Furthermore, we demonstrated that a multidisciplinary team care approach is associated with improved survival outcomes compared with SEER distant cohort. Taken together, these data support reconstructing health delivery models to enhance value and improve clinical outcomes [9, 19].
Funding
Walther Cancer Foundation, Walther Scholars Program (grant number 0053.01 to CA); Slovak Research and Development Agency (contract number APVV-0016-11 and APVV-15-0086 grants to MC).
Disclosure
The authors have declared no conflicts of interest.
Supplementary Material
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017; 67: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Williams SD, Birch R, Einhorn LH. et al. Treatment of disseminated germ-cell tumors with cisplatin, bleomycin, and either vinblastine or etoposide. N Engl J Med 1987; 316(23): 1435–1440. [DOI] [PubMed] [Google Scholar]
- 3. de Wit R, Roberts JT, Wilkinson PM. et al. Equivalence of three or four cycles of bleomycin, etoposide, and cisplatin chemotherapy and of a 3- or 5-day schedule in good-prognosis germ cell cancer: a randomized study of the European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group and the Medical Research Council. J Clin Oncol 2001; 19(6): 1629–1640. [DOI] [PubMed] [Google Scholar]
- 4. Einhorn LH, Williams SD, Loehrer PJ. et al. Evaluation of optimal duration of chemotherapy in favorable-prognosis disseminated germ cell tumors: a Southeastern Cancer Study Group protocol. JCO 1989; 7: 387–391. [DOI] [PubMed] [Google Scholar]
- 5. Culine S, Kerbrat P, Kramar A. et al. Refining the optimal chemotherapy regimen for good-risk metastatic nonseminomatous germ-cell tumors: a randomized trial of the Genito-Urinary Group of the French Federation of Cancer Centers (GETUG T93BP). Ann Oncol 2007; 18(5): 917–924. [DOI] [PubMed] [Google Scholar]
- 6. International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol 1997; 15: 594–603. [DOI] [PubMed] [Google Scholar]
- 7. Collette L, Sylvester RJ, Stenning SP. et al. Impact of the treating institution on survival of patients with “poor-prognosis” metastatic nonseminoma. European Organization for Research and Treatment of Cancer Genito-Urinary Tract Cancer Collaborative Group and the Medical Research Council Testicular Cancer Working Party. J Natl Cancer Inst 1999; 91(10): 839–846. [DOI] [PubMed] [Google Scholar]
- 8. Nayan M, Jewett MA, Anson-Cartwright L. et al. The association between institution at orchiectomy and outcomes on active surveillance for clinical stage I germ cell tumours. CUAJ 2016; 10: 204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jeldres C, Pham KN, Daneshmand S. et al. Association of higher institutional volume with improved overall survival in clinical stage III testicular cancer: results from the National Cancer Data Base (1998–2011). JCO 2014; 32(4 Suppl): 4519–4519. [Google Scholar]
- 10. Adra N, Althouse SK, Liu H. et al. Prognostic factors in patients with poor-risk germ-cell tumors: a retrospective analysis of the Indiana University experience from 1990 to 2014. Ann Oncol 2016; 27(5): 875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blanke C, Loehrer PJ, Nichols CR, Einhorn LH.. A phase II trial of VP-16, ifosfamide, cisplatin, vinblastine, and bleomycin in advanced germ-cell tumors. Am J Clin Oncol 1996; 19(5): 487–491. [DOI] [PubMed] [Google Scholar]
- 12. Motzer RJ, Nichols CJ, Margolin KA. et al. Phase III randomized trial of conventional-dose chemotherapy with or without high-dose chemotherapy and autologous hematopoietic stem-cell rescue as first-line treatment for patients with poor-prognosis metastatic germ cell tumors. J Clin Oncol 2007; 25(3): 247–256. [DOI] [PubMed] [Google Scholar]
- 13.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) Research Data (1973–2014), National Cancer Institute, DCCPS, Surveillance Research Program, released April 2017, based on the November 2016 Submission.
- 14. Gilligan TD, Seidenfeld J, Basch EM. et al. American Society of Clinical Oncology Clinical Practice Guideline on uses of serum tumor markers in adult males with germ cell tumors. JCO 2010; 28: 3388–3404. [DOI] [PubMed] [Google Scholar]
- 15. Necchi A, Pond GR, Nicolai N. et al. A suggested prognostic reclassification of intermediate and poor-risk nonseminomatous germ cell tumors. Clin Genitourin Cancer 2017; 15(2): 306–312 e303. [DOI] [PubMed] [Google Scholar]
- 16. Cary C, Pedrosa JA, Jacob J. et al. Outcomes of postchemotherapy retroperitoneal lymph node dissection following high-dose chemotherapy with stem cell transplantation. Cancer 2015; 121(24): 4369–4375. [DOI] [PubMed] [Google Scholar]
- 17. Albers P, Siener R, Krege S. et al. Randomized phase III trial comparing retroperitoneal lymph node dissection with one course of bleomycin and etoposide plus cisplatin chemotherapy in the adjuvant treatment of clinical stage I nonseminomatous testicular germ cell tumors: AUO trial AH 01/94 by the German Testicular Cancer Study Group. JCO 2008; 26: 2966–2972. [DOI] [PubMed] [Google Scholar]
- 18. Cho JS, Kaimakliotis HZ, Cary C. et al. Modified retroperitoneal lymph node dissection for post-chemotherapy residual tumour: a long-term update. BJU Int 2017; 120(1): 104–108. [DOI] [PubMed] [Google Scholar]
- 19. Tandstad T, Kollmannsberger CK, Roth BJ. et al. Practice makes perfect: the rest of the story in testicular cancer as a model curable neoplasm. J Clin Oncol 2017; 35(31): 3525–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Einhorn LH, Williams SD, Chamness A. et al. High-dose chemotherapy and stem-cell rescue for metastatic germ-cell tumors. N Engl J Med 2007; 357(4): 340–348. [DOI] [PubMed] [Google Scholar]
- 21. Feldman DR, Sheinfeld J, Bajorin DF. et al. TI-CE high-dose chemotherapy for patients with previously treated germ cell tumors: results and prognostic factor analysis. J Clin Oncol 2010; 28(10): 1706–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kondagunta GV, Bacik J, Donadio A. et al. Combination of paclitaxel, ifosfamide, and cisplatin is an effective second-line therapy for patients with relapsed testicular germ cell tumors. J Clin Oncol 2005; 23(27): 6549–6555. [DOI] [PubMed] [Google Scholar]
- 23. Loehrer PJ Sr, Einhorn LH, Williams SD.. VP-16 plus ifosfamide plus cisplatin as salvage therapy in refractory germ cell cancer. J Clin Oncol 1986; 4(4): 528–536. [DOI] [PubMed] [Google Scholar]
- 24. Loehrer PJ Sr, Gonin R, Nichols CR. et al. Vinblastine plus ifosfamide plus cisplatin as initial salvage therapy in recurrent germ cell tumor. J Clin Oncol 1998; 16(7): 2500–2504. [DOI] [PubMed] [Google Scholar]
- 25. Murphy BR, Breeden ES, Donohue JP. et al. Surgical salvage of chemorefractory germ cell tumors. J Clin Oncol 1993; 11(2): 324–329. [DOI] [PubMed] [Google Scholar]
- 26. Macleod LC, Cannon S, Ko O. et al. Nationwide disparities in testicular cancer care delivery: racial, ethnic, and economic markers of patient vulnerability. JCO 2017; 35(6 Suppl): 421–421. [Google Scholar]
- 27. Sun M, Abdollah F, Liberman D. et al. Racial disparities and socioeconomic status in men diagnosed with testicular germ cell tumors: a survival analysis. Cancer 2011; 117(18): 4277–4285. [DOI] [PubMed] [Google Scholar]
- 28. Nichols CR, Jeldres C, Pham K. et al. Influence of social demographics and African-American race on outcomes in testicular cancer: analysis of 75,902 patients in the National Cancer database. JCO 2014; 32(4 Suppl): 391–391. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


