EXECUTIVE SUMMARY
Guidelines for the clinical management of patients with neurocysticercosis (NCC) were prepared by a panel of the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). The guidelines are intended for infectious disease specialists, neurologists, neurological surgeons, internists, pediatricians, and family practitioners.
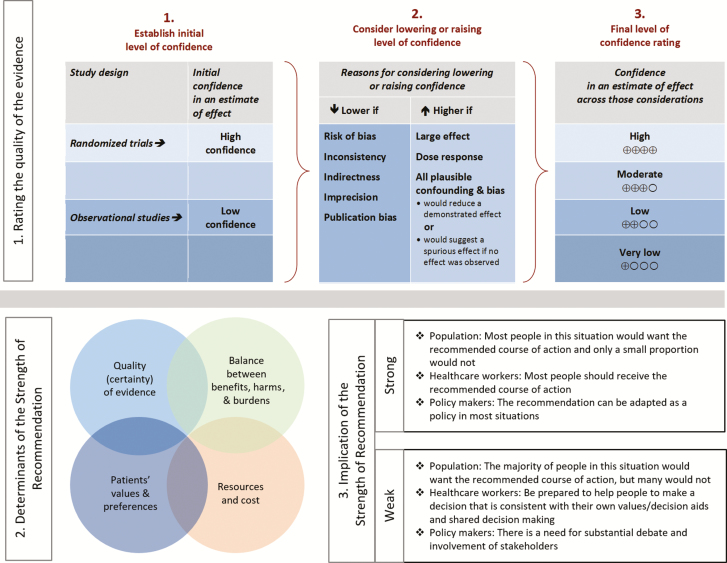
These guidelines present our approaches to the diagnosis and management of patients with the different forms of neurocysticercosis, including viable parenchymal neurocysticercosis, single enhancing lesions, calcified parenchymal neurocysticercosis, ventricular neurocysticercosis, and subarachnoid neurocysticercosis. Our recommendations are based on the best evidence available. Due to the complex variations in clinical manifestations and the limitations of the literature, many of the recommendations are based on observational studies, anecdotal data, or expert opinion rather than randomized clinical trials. The approaches we describe are intended to be both applicable and feasible in the United States and Canada (for simplicity, referred to here as North America). The recommendations may not apply for settings where resource constraints may limit their applicability. The executive summary below lists the recommendations for the diagnosis and clinical management of neurocysticercosis. A detailed description of the methods, background, and evidence summaries that support each of the recommendations can be found online in the full text of the guidelines. A criterion for grading evidence is presented in Figure 1 [1]. Note that diagnosis and management of patients with neurocysticercosis can be challenging even with expert guidelines. Due to this complexity, clinicians with little experience with this disease should have a low threshold for consultation with an expert in the disease.
Figure 1.
Grading of Recommendations, Assessment, Development and Evaluation (GRADE) framework for grading of evidence.
RECOMMENDATIONS FOR DIAGNOSIS AND BASELINE EVALUATION
I. How should NCC be diagnosed?
Recommendations
While there is a wide range of clinical manifestations of neurocysticercosis, the 2 most common clinical presentations are with seizures and increased intracranial pressure (not graded).
Initial evaluation should include careful history and physical examination, and neuroimaging studies (not graded).
We recommend serologic testing with enzyme-linked immunotransfer blot as a confirmatory test in patients with suspected neurocysticercosis (strong, moderate). Enzyme-linked immunosorbent assays using crude antigen should be avoided due to poor sensitivity and specificity (strong, moderate).
II. What imaging studies should be used to classify disease?
Recommendation
4. We recommend both brain magnetic resonance imaging (MRI) and a noncontrast computed tomography (CT) scan for classifying patients with newly diagnosed neurocysticercosis (strong, moderate). The classification is outlined in Table 1.
Table 1.
Classification of Neurocysticercosis Based on Location and Appearance of the Parasite and Surrounding Host Tissue on Neuroimaging
| Forma | Characteristic on Neuroimaging | Histopathology |
|---|---|---|
| Parenchymalb | ||
| Nonviable calcified | Nodular calcifications <20 mm in diameter (often 1–5 mm) with or without surrounding edema and/or contrast enhancement. | Calcified granuloma with or without surrounding inflammation and/or gliosis. |
| Single, small enhancing | Cystic or nodular enhancing lesion <2 cm in size. | Single parenchymal parasites in the process of degeneration with surrounding inflammation and variable opacification or absence of the cyst fluid. |
| Viable parenchymal | Vesicular lesions often with evidence of associated contrast enhancement and/or surrounding edema. The scolex is often visible on high-definition imaging. | Parasites with intact cyst wall, vesicular fluid and scolex, with variable amounts of inflammation surrounding the parasite sometimes invading the cyst wall. |
| Extraparenchymalc | ||
| Intraventricular | Cysticerci within the ventricles, obstructive hydrocephalus or loculated hydrocephalus with disproportionate dilatation of the ventricles in CT/MRI (suggestive of a cysticercus). | Viable cysticercus cyst within the ventricle and/or obstructive hydrocephalus. |
| Subarachnoid | Cysticerci in the Sylvian fissure, in the basilar cisterns, or interhemispheric spaces. Strokes or meningitis without discrete cysts. | Cysticerci in the subarachnoid space often with arachnoiditis, vasculitis. The cysticerci are often in clusters with proliferating membranes (racemose) and may lack a scolex. |
| Spinal | Cysticerci within the spinal subarachnoid space with or without evidence of inflammation/diffuse spinal arachnoiditis. Intramedullary cysticerci within the spinal cord. | Subarachnoid cysticerci often with associated arachnoiditis. Intramedullary cysticerci similar pathologically to parenchymal cysticerci. |
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging.
aPatients with >1 form are classified with the form found lower on the chart, with the exception that single enhancing lesions that are also viable are grouped with single enhancing lesions. Ocular cysticercosis is classified separately.
bRefers to cysticerci in the brain parenchyma. Small cysticerci in the gyri over the cerebral convexity behave clinically like parenchymal cysticerci and are grouped with parenchymal cysticerci. Rare forms of neurocysticercosis include multiple inflamed parenchymal cysticerci with diffuse cerebral edema, termed cysticercal encephalitis, large parenchymal cysticerci (>20 mm).
cRefers to cysticerci in the central nervous system outside of the brain parenchyma.
III. What additional tests should be performed prior to initiation of therapy?
Recommendations
5. We suggest screening for latent tuberculosis infection in patients likely to require prolonged corticosteroids (weak, low).
6. We suggest screening or empiric therapy for Strongyloides stercoralis in patients likely to require prolonged corticosteroids (weak, low).
7. We recommend that all patients with NCC undergo a funduscopic examination prior to initiation of anthelminthic therapy (strong, moderate).
8. We suggest that patients with NCC who probably acquired NCC in a nonendemic area have their household members screened for tapeworm carriage (weak, low). Remark: This is a public health issue and can often be addressed by the local health department.
IV. How should antiparasitic and anti-inflammatory therapy be monitored?
Recommendations
9. We recommend that patients treated with albendazole for >14 days be monitored for hepatotoxicity and leukopenia (strong, moderate).
10. No additional monitoring is needed for patients receiving combination therapy with albendazole and praziquantel beyond that recommended for albendazole monotherapy (strong, moderate).
RECOMMENDATIONS FOR THE TREATMENT OF VIABLE INTRAPARENCHYMAL NEUROCYSTICERCOSIS
V. What is the role of antiparasitic drugs in viable intraparenchymal neurocysticercosis (VPN)?
Recommendations
11. In patients with untreated hydrocephalus or diffuse cerebral edema, we recommend management of elevated intracranial pressure alone and not antiparasitic treatment (strong, moderate). Remarks: The management of patients with diffuse cerebral edema should be anti-inflammatory therapy such as corticosteroids, whereas hydrocephalus usually requires a surgical approach.
12. In the absence of elevated intracranial pressure, we recommend use of antiparasitic drugs in all patients with VPN (strong, moderate), (Table 2).
13. For patients with 1–2 viable parenchymal cysticerci, we recommend albendazole monotherapy for 10–14 days compared to either no antiparasitic therapy (strong, high) or combination antiparasitic therapy (weak, moderate). Remarks: The usual dose of albendazole is 15 mg/kg/day divided into 2 daily doses for 10–14 days with food. We recommend a maximum dose of 1200 mg/day.
14. We recommend albendazole (15 mg/kg/day) combined with praziquantel (50 mg/kg/day) for 10–14 days rather than albendazole monotherapy for patients with >2 viable parenchymal cysticerci (strong, moderate).
15. We suggest retreatment with antiparasitic therapy for parenchymal cystic lesions persisting for 6 months after the end of the initial course of therapy (weak, low).
Table 2.
Summary of Treatment Recommendations for Different Forms of Parenchymal Neurocysticercosis
| Form | Type of Therapy/Subgroup | Recommendation | Comment | Strength of Recommendation; Quality of Evidence |
|---|---|---|---|---|
| Viable parenchymal | Antiparasitic therapy | Antiparasitic drugs should be used in all patients with viable parenchymal NCC unless there is increased intracranial pressure. | The preponderance of studies demonstrated more rapid radiologic resolution in patients treated with antiparasitic drugs compared with placebo and decreased numbers of generalized seizuresa. | Strong; moderate |
| 1–2 viable cysts | Monotherapy with albendazole (15 mg/kg/d in 2 daily doses up to 1200 mg/d) with food for 10 d. | Combination therapy showed no additional benefit with 1 or 2 cysts and more complex pharmacologyb. | Strong; moderate | |
| >2 viable cysts | Albendazole (15 mg/kg/d in 2 daily doses up to 1200 mg/d) combined with praziquantel (15 mg/kg/d in 3 daily doses) for 10 d. | Both the pharmacokinetic study and a recent randomized trial demonstrated improved radiologic resolution with the combination compared to albendazole alone in those with >2 cysticercib. | Strong; moderate | |
| Anti-inflammatory therapy | Corticosteroids should be used whenever antiparasitic drugs are used. | Adjuvant use of corticosteroids is associated with fewer seizures during therapy Optimal doses have not been clearly definedc. | Strong; moderate | |
| Antiepileptic therapy | Antiepileptic drugs should be used in all patients with seizures. | Antiepileptic drugs appear to be effective in controlling seizures in patients with parenchymal NCC; consider tapering off after 2 years if meet criteria for withdrawal as in idiopathic epilepsyd. | Strong; moderate | |
| Single enhancing lesion due to neurocysticercosis | Antiparasitic therapy | Albendazole (15 mg/kg/d in 2 daily doses up to 800 mg/d) for 1–2 wk. | Albendazole shown to improve seizure outcome in meta-analysese. Different studies have employed a range of durations of treatment without clear advantages of longer duration. | Weak; high |
| Anti-inflammatory therapy | Corticosteroids should be given concomitantly with antiparasitic agentse. | Given the data on worsening symptoms with antiparasitic drugs, most authorities recommend use of corticosteroids in patients treated with antiparasitic drugsf. | Strong; moderate | |
| Antiepileptic therapy | Antiepileptic drugs should be used in all patients with seizuresg. | Antiepileptic drugs can be discontinued after resolution of cystic lesions if no risk factors for recurrenceg. Risk factors for recurrent seizures include (1) calcifications on follow-up CT, (2) breakthrough seizures, and (3) >2 seizures during the course of the disease. | Strong; moderate | |
| Calcified parenchymal neurocysticercosis with or without perilesional edema | Antiparasitic therapy | Antiparasitic treatment not recommended. | There are no viable cysts and thus no indication for antiparasitic therapy. | |
| Antiepileptic therapy | Treatment with antiepileptic drugsg. | Management guidelines are similar to that in other patients with seizures. | Strong; moderate | |
| Anti-inflammatory therapy | Corticosteroids should not be routinely used.h | There are a few case reports suggesting that when corticosteroids are stopped or lowered previously quiescent calcifications develop perilesional edema. | Strong; low | |
| Cysticercal encephalitis (with diffuse cerebral edema) | Avoid antiparasitic drugs, treat diffuse cerebral edema with corticosteroidsi. | Cerebral edema mediated by the host inflammatory response. Antiparasitic drugs are associated with worsening edema. | Strong; low |
Abbreviations: CT, computed tomography; NCC, neurocysticercosis.
aTwo well-designed randomized trials demonstrated more rapid radiologic responses and fewer generalized seizures in patients treated with albendazole compared to placebo.
bThe combination of praziquantel and albendazole was superior to albendazole alone in patients with >2 cysts, but was not better in those with 1 or 2 viable cysts.
cThe optimal anti-inflammatory regimen has not been clearly defined. A trial comparing 6 mg/day of dexamethasone for 10 days with 8 mg/day for 28 days followed by a taper noted fewer seizures in the higher-dose group. Other studies have used prednisone 1–1.5 mg/kg/day during therapy.
dThere are no clear data on optimal duration of antiepileptic drugs. Risk factors for recurrent seizures include calcifications on follow-up CT, breakthrough seizures, and >2 seizures during the course of the disease. In patients without any of these risk factors and no seizures in the prior 3 months, antiepileptic drugs can be safely withdrawn within a few weeks of the resolution of the enhancing lesion on high-resolution imaging studies.
eTwo meta-analyses of randomized controlled trials have concluded that albendazole improved the outcome in patients with single enhancing lesions due to neurocysticercosis.
fAlbendazole should be given along with anti-inflammatory drugs. The optimal dose and duration has not been defined, but doses have included dexamethasone 0.1 mg/kg/day for the duration of therapy or 1–2 mg/kg/day of prednisone or prednisolone have been used.
gManagement guidelines are similar to those in other patients with seizures. Many can be managed with a single drug. There are no data on relative efficacy of different antiepileptic drugs.
hA few case reports suggest that when corticosteroids are lowered or stopped, rebound perilesional edema can occur. Therefore, anti-inflammatory drugs should be used cautiously, if at all, in patients presenting with perilesional edema around a calcified lesion.
iAntiparasitic agents can worsen cerebral edema and should generally be avoided in patients with increased intracranial pressure from either diffuse cerebral edema (cysticercal encephalitis) or untreated hydrocephalus.
VI. What is the role of anti-inflammatory therapy in management of VPN?
Recommendation
16. We recommend adjunctive corticosteroid therapy begun prior to antiparasitic drugs rather than no adjunctive therapy in all patients treated with antiparasitic therapy (strong, moderate).
VII. What is the role of antiepileptic drugs in VPN?
Recommendations
17. We recommend antiepileptic drugs in all NCC patients with seizures (strong, low).
18. In patients with few seizures prior to antiparasitic therapy, resolution of the cystic lesion on imaging studies, and no seizures for 24 consecutive months, we suggest that tapering off and stopping antiepileptic drugs be considered (weak, moderate).
19. In the absence of controlled data, the choice of antiepileptic drugs should be guided by local availability, cost, drug interactions, and potential side effects (not graded)
VIII. What follow-up is recommended after initial antiparasitic therapy for patients with VPN?
Recommendation
20. We suggest that MRI be repeated at least every 6 months until resolution of the cystic component (strong, low).
RECOMMENDATION FOR THE TREATMENT OF DEGENERATING INTRAPARENCHYMAL NCC INCLUDING PATIENTS WITH SINGLE ENHANCING LESIONS DUE TO NCC
IX. What should be the initial approach to the patient with multiple enhancing lesions from NCC?
Recommendation
21. We recommend that patients with multiple enhancing lesions and seizures be initially treated with antiepileptic drugs, antiparasitic therapy, and corticosteroids as outlined in the section on viable parenchymal cysticerci (weak, moderate), (Table 2).
X. What is the role of antiepileptic medications in patients with single enhancing lesions (SELs) from cysticercosis with seizures?
Recommendations
22. We recommend antiepileptic drugs for all patients with SELs and seizures (strong, moderate).
23. In the absence of controlled data, the choice of antiepileptic drugs can be guided by local availability, cost, drug interactions, and potential side effects (not graded).
24. In patients who have been seizure free for 6 months, we suggest tapering off and stopping antiepileptic drugs after resolution of the lesion in patients with SELs without risk factors for recurrent seizures (weak, moderate). Remark: Risk factors for recurrent seizures include residual cystic lesions or calcifications on neuroimaging studies, breakthrough seizures, or >2 seizures.
XI. What is the role of antiparasitic drugs in patients with SELs?
Recommendation
25. We suggest albendazole therapy rather than no antiparasitic therapy for all patients with SELs (weak, moderate). Remarks: Albendazole (15 mg/kg/day in twice-daily doses up for 1–2 weeks) should be given with meals.
XII. What is the role of anti-inflammatory therapy in SELs?
Recommendation
26. We recommend that patients with SELs treated with antiparasitic drugs also be treated with corticosteroids initiated prior to antiparasitic therapy (strong, moderate).
XIII. How should patients with SELs be followed?
Recommendation
27 We suggest that MRI be repeated at least every 6 months until resolution of cystic lesions for patients with SELs (weak, low).
RECOMMENDATIONS FOR THE TREATMENT OF CALCIFIED PARENCHYMAL NEUROCYSTICERCOSIS
XIV. What should the initial approach be to patients with calcified lesions suggestive of calcified parenchymal neurocysticercosis (CPN)?
Recommendation
28 We suggest brain MRI in patients with seizures or hydrocephalus and only calcified parenchymal NCC on CT (weak, low).
XV. What is the role of antiparasitic drugs, antiepileptic drugs, and anti-inflammatory medications in the management of patients with CPN?
Recommendations
29. We recommend symptomatic therapy alone instead of antiparasitic drugs in patients with calcified parenchymal lesions (strong, moderate), (Table 2).
30. We suggest that corticosteroids not be routinely used in patients with isolated CPN and perilesional edema (weak, low).
XVI. Is there a role for surgical therapy in refractory cases?
Recommendation
31. In patients with refractory epilepsy and CPN, we suggest evaluation for surgical removal of seizure foci (weak, low).
RECOMMENDATIONS FOR THE TREATMENT OF INTRAVENTRICULAR NEUROCYSTICERCOSIS
XVII. How are extraparenchymal cysts best identified?
Recommendation
32 We recommend MRI with 3D volumetric sequencing to identify intraventricular and subarachnoid cysticerci in patients with hydrocephalus and suspected NCC (strong, moderate).
XVIII. What is the optimal approach to management of intraventricular neurocysticercosis (IVN) in the lateral and third ventricles?
Recommendation
33. When possible, we recommend removal of the cysticerci by minimally invasive neuroendoscopy over other surgical or medical approaches for cysticerci of the lateral and third ventricles (strong, moderate), (Table 3). Remark: Most experts recommend that antiparasitic drugs not be used preoperatively, as such treatment could result in disruption of parasite integrity and an inflammatory response that could prevent successful cyst removal.
Table 3.
Recommendations for Therapy of Extraparenchymal Neurocysticercosis
| Form | Recommended Therapy | Comment | Strength of Recommendation; Quality of Evidence |
|---|---|---|---|
| Intraventricular (lateral or third ventricle) |
Removal of the cysticerci by minimally invasive, neuroendoscopy when feasiblea,b. | Most cases with isolated nonadherent cysts in the lateral or third ventricle can be cured by neuroendoscopy and do not require subsequent antiparasitic drugs or shunt therapy if all cysticerci are removed. | Strong; low |
| Intraventricular (fourth ventricle) |
Either endoscopic or microsurgical cystectomy is suitable, depending on the experience of the surgeon. | Microsurgical resection is from a suboccipital approach. The endoscopic approach can be either from the conventional lateral-third ventricular-trans aqueductal route (technically demanding) or through the posterior approach. | Strong; low |
| Intraventricular—when surgical removal not feasible (eg, adherent cyst) | CSF diversion via a ventriculoperitoneal shuntc. | In cases of marked inflammation in the ventricles or degenerating cysticerci, the cyst may adhere to the ventricular wall, making removal hazardous. CSF diversion with medical therapy is the recommended approachc. | Weak; low |
| Adjuvant antiparasitic and anti-inflammatory therapyc. | Medical therapy should be limited to patients in whom surgery is contraindicated due to various reasons. A CSF diversion (shunt) should always be performed prior to chemotherapy if there is hydrocephalus, since there are reports of precipitation of hydrocephalus with antiparasitic therapy. | Strong; moderate | |
| Subarachnoidd | Surgical management of hydrocephalus | Initial management should focus on treatment of hydrocephalus. This often requires ventriculoperitoneal shunting. | Strong; low |
| Antiparasitic therapye | Subarachnoid cysts do not respond well to typical doses and durations of therapy. Options to improve responses include prolonged administration of albendazole (15 mg/kg/d for months) or combination therapy with albendazole (15 mg/kg/d plus praziquantel 50 mg/ kg/d). | Strong; low | |
| Anti-inflammatory therapy | Concomitant administration of corticosteroids with antiparasitic drugs is essential in the treatment of patients with subarachnoid neurocysticercosis. Inflammation is exacerbated as a result of antiparasitic treatmentf. | Strong; low |
Abbreviation: CSF, cerebrospinal fluid.
aThe endoscopic surgical approach often requires ventriculomegaly. Cyst rupture is the norm and not associated with adverse consequences. The microsurgical approach is also facilitated by presence of hydrocephalus.
bAlternative approaches include CSF diversion along with medical management, or craniotomy with microsurgical excision.
cShunts are initially efficacious acutely for hydrocephalus, but there is a very high rate of shunt malfunction in patients with neurocysticercosis. Shunt failure may be lower when combined with corticosteroids and antiparasitic treatment.
dSubarachnoid neurocysticercosis should be aggressively treated with antiparasitic and anti-inflammatory drugs. Hydrocephalus should be addressed before antiparasitic therapy.
eUntreated hydrocephalus is a contraindication to antiparasitic therapy and needs to be treated first. Some cases respond to anti-inflammatory treatment, but most cases require CSF diversion.
fGenerally, 1 mg/kg/day of prednisone or .2–.4 mg/kg/day of dexamethasone are administered 3–4 days before and during antiparasitic treatment. The dose is slowly decreased, depending on the intensity of the inflammatory response. Methotrexate or antibody to tumor necrosis factor can be used as steroid-sparing agents.
XIX. What is the optimal surgical approach to management of IVN in the fourth ventricle?
Recommendation
34. In cases in which surgical removal of fourth ventricular cysticerci is possible, we recommend surgical removal rather than medical therapy and/or shunt surgery (strong, moderate).
XX. What is the optimal approach to adherent IVN?
Recommendation
35. We suggest shunt surgery for hydrocephalus rather than cyst removal when surgical removal is technically difficult (weak, low). Remark: Attempted removal of inflamed or adherent ventricular cysticerci is associated with increased risk of complications.
XXI. Does medical therapy as an adjunct to procedures or as primary therapy have an impact on outcome in treating patients with IVN?
Recommendations
36. We recommend corticosteroids to decrease brain edema in the perioperative period (not graded).
37. We suggest antiparasitic drugs with corticosteroid therapy following shunt insertion to decrease subsequent shunt failure in patients in whom surgical removal of isolated intraventricular cysts is not possible (weak, low), but neither after successful removal of intraventricular cysts (weak, low). Remark: Note that intraventricular cysts may be accompanied by other lesions with indications for antiparasitic therapy.
RECOMMENDATIONS FOR SUBARACHNOID NEUROCYSTICERCOSIS
XXII. What is the role of medical therapy in subarachnoid neurocysticercosis (SAN) in the basilar cisterns or Sylvian fissures?
Recommendations
38. We recommend that patients with subarachnoid cysts be treated with antiparasitic drugs (strong, low), (Table 3).
39. We suggest that antiparasitic therapy be continued until there is radiologic resolution of viable cysticerci on MRI and resolution of other evidence of cysticerci (weak, low). Responses often require prolonged therapy, which can last for more than a year.
40. We recommend anti-inflammatory therapy (such as high-dose corticosteroids) for subarachnoid NCC initiated prior to antiparasitic drugs (strong, moderate).
41. We suggest that methotrexate be considered as a steroid-sparing agent in patients requiring prolonged courses of anti-inflammatory therapy (weak, low).
XXIII. What is the role of neurosurgery in SAN?
Recommendations
42. We recommend that patients with hydrocephalus from subarachnoid NCC be treated with shunt surgery in addition to medical therapy (strong, low).
43. We suggest that some patients may benefit from surgical debulking over shunt surgery alone (weak, low).
RECOMMENDATIONS FOR SPINAL NEUROCYSTICERCOSIS
XXIV. How is spinal neurocysticercosis (SN) best treated?
Recommendations
44. We recommend corticosteroid treatment for patients with SN with evidence of spinal cord dysfunction (eg, paraparesis or incontinence) or as adjunctive therapy along with antiparasitic therapy (strong, moderate).
45. We suggest that both medical (antiparasitic drugs plus anti-inflammatory drugs) and surgical approaches be considered for SN (weak, low), (Table 3). Practice statement: There are anecdotes of good responses of spinal neurocysticercosis to medical and/or surgical therapy. However, there are no good data supporting one approach over the other. We suggest that management of spinal NCC should be individualized based on symptoms, location of the cysticerci, degree of arachnoiditis, and surgical experience. Recommendations for antiparasitic drugs, reimaging, and follow-up of SAN should also be considered for subarachnoid SN.
RECOMMENDATIONS FOR MANAGEMENT OF OCULAR CYSTICERCOSIS
XXV. What is the optimal management of ocular cysticercosis?
Recommendation
46. We suggest that intraocular cysticerci be treated with surgical removal rather than with antiparasitic drugs (weak, low).
RECOMMENDATIONS FOR THE TREATMENT OF SPECIAL POPULATIONS
XXVI. Should children be managed differently than adults?
Recommendation
47. There is no evidence that management of NCC in children should be different than in adults with the same form of disease (strong, moderate). Dosing should be weight based.
XXVII. Should management be different in pregnant women?
Recommendation
48. We suggest that antihelminthic therapy be deferred until after pregnancy (weak, low). Remarks: Pregnant patients with elevated intracranial pressure need to be aggressively managed as they would be if not pregnant. Corticosteroids can be used in pregnancy when necessary. The use of antiepileptic drugs should take into account altered pharmacokinetics and potential teratogenicity. Phenobarbital and valproic acid are known to have high rates of teratogenicity. Antihelminthic drugs are rarely required emergently and their use can usually be deferred until after delivery. Methotrexate is teratogenic and should be avoided. Key principles are summarized in Table 4.
Table 4.
Clinical Pearls for Management of Neurocysticercosis
|
Abbreviations: CSF, cerebrospinal fluid; NCC, neurocysticercosis.
aSymptomatic therapy includes antiepileptic drugs for seizures, anti-inflammatory drug such as corticosteroids and methotrexate, and surgery for hydrocephalus.
bAntiparasitic therapy for subarachnoid NCC may include prolonged courses of albendazole, high-dose albendazole, or combinations of praziquantel and albendazole.
cSurgical therapy for subarachnoid NCC may include CSF diversion for hydrocephalus or minimally invasive surgical debulking.
dAdherent cysticerci should be managed with CSF diversion along with antiparasitic drugs. Open craniotomy is effective for fourth ventricular lesions and the choice of approaches should depend on local surgical expertise.
INTRODUCTION
In the first section, the panel summarizes background information relevant to the topic. In the second section, the panel poses questions regarding the diagnosis and treatment of neurocysticercosis (NCC), evaluates applicable clinical trial and observational data, and makes recommendations using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) framework [1]. The following 27 clinical questions were answered:
How should neurocysticercosis be diagnosed?
What imaging studies should be used to classify disease?
What additional tests should be performed prior to initiation of therapy?
How should antiparasitic and anti-inflammatory therapy be monitored?
What is the role of antiparasitic drugs in viable parenchymal neurocysticercosis?
What is the role of anti-inflammatory therapy in management of viable parenchymal neurocysticercosis?
What is the role of antiepileptic drugs in viable parenchymal neurocysticercosis?
What follow-up is recommended after initial antiparasitic therapy for patients with viable parenchymal neurocysticercosis?
What should be the initial approach to the patient with multiple enhancing lesions from neurocysticercosis?
What is the role of antiepileptic medications in patients with single enhancing lesions from cysticercosis with seizures?
What is the role of antiparasitic drugs in patients with single enhancing lesions?
What is the role of anti-inflammatory therapy in single enhancing lesions?
How should patients with single enhancing lesions be followed?
What should the initial approach be to patients with calcified lesions suggestive of calcified parenchymal neurocysticercosis?
What is the role of antiparasitic drugs, antiepileptic drugs, and anti-inflammatory medications in the management of patients with calcified parenchymal neurocysticercosis?
Is there a role for surgical therapy in refractory cases?
How are extraparenchymal cysts best identified?
What is the optimal approach to management of ventricular neurocysticercosis in the lateral and third ventricles?
What is the optimal surgical approach to management of ventricular neurocysticercosis in the fourth ventricles?
What is the optimal approach to adherent ventricular neurocysticercosis?
Does medical therapy as an adjunct to procedures or as primary therapy have an impact on outcome in treating patients with ventricular neurocysticercosis?
What is the role of medical therapy in subarachnoid neurocysticercosis in the basilar cisterns or Sylvian fissures?
What is the role of neurosurgery in subarachnoid neurocysticercosis?
How is spinal neurocysticercosis best treated?
What is the optimal management of ocular cysticercosis?
Should children be managed differently than adults?
Should management be different in pregnant women?
BACKGROUND
Neurocysticercosis, caused by the larval form of the cestode parasite Taenia solium, is a major cause of seizure and neurologic disease worldwide and is common among immigrant populations in the United States. Highly endemic regions include Latin America, sub-Saharan Africa, and parts of Asia [2, 3]. In endemic areas, it is linked to approximately 29% of cases of seizures [4]. Estimates suggest that there are >2000 cases per year in the United States, with hospital charges of nearly $100 million per year [5, 6]. Humans can be hosts to both the tapeworm form and larval forms of the parasite. Taeniasis (also termed taeniosis) refers to infestation of the human intestines with the tapeworm form. The tapeworm is acquired by ingestion of undercooked pork. The scolex evaginates and attaches to the intestinal wall, and segments termed proglottids form a long ribbon-like chain referred to as the strobili. The gravid proglottids and eggs are passed in stool. Humans can also develop cysticercosis after ingestion of ova. Cysticercosis refers to infection of the tissues with the larval cyst (or metacestode). Normally, pigs host the cysts, which are acquired by ingestion of ova or proglottids from human feces. However, humans can be infected by ingestion of ova and develop cysticercosis. Neurocysticercosis refers to cysticercosis involving the central nervous system, including the brain parenchyma, ventricles, basilar cisterns, sulci, gyri, spine, and retina.
The pathogenesis, natural history, clinical manifestations, and management vary with the location of the cysticerci [3]. For example, the main clinical manifestations vary between different forms of disease (Table 1). Parenchymal NCC typically presents with seizures or headache. Ventricular NCC most often presents with obstructive hydrocephalus. Subarachnoid NCC can present with communicating hydrocephalus, meningitis, stroke, or focal neurologic findings. Mixed forms are also common. Due to the complexity of diagnosis and management, the American Society for Tropical Medicine and Hygiene (ASTMH) and the Infectious Diseases Society of America (IDSA) agreed to jointly develop guidelines for the diagnosis and management of NCC.
METHODOLOGY
Panel Composition
The IDSA and the ASTMH convened experts in the diagnosis and management of NCC from the fields of tropical and infectious diseases, neurology, and neurosurgery, including experts coming from endemic areas as well as from North America. All panel members were selected on the basis of their clinical expertise in NCC as well as their expertise in the disciplines of infectious diseases, tropical medicine, neurology, and neurosurgery.
Evidence Review—The GRADE Method
Grading of Recommendations, Assessment, Development and Evaluation (GRADE) is a systematic approach to guideline development that has been described in detail elsewhere [1, 7]. The IDSA adopted GRADE in 2008. In the GRADE system, the guideline panel assigns each recommendation with separate ratings for the underlying quality of evidence supporting the recommendation and for the strength with which the recommendation is made (Figure 1). Data from randomized controlled trials begin as “high” quality, and data from observational studies begin as “low” quality. However, the panel may judge that specific features of the data warrant decreasing or increasing the quality of evidence rating, and GRADE provides guidance on how such factors should be weighed [7]. The strength assigned to a recommendation chiefly reflects the panel’s confidence that the benefits of following the recommendation are likely to outweigh potential harms. While the quality of evidence is an important factor in choosing recommendation strength, it is not prescriptive.
Process Overview
Panel members were each assigned to review the recent literature for at least 1 topic, evaluate the evidence, determine the strength of recommendations, and develop written evidence in support of these recommendations. The panel had several in-person meetings, and conducted most of its work though teleconferences and electronically based discussion during 2011–2017. Recommendations and grading of evidence were developed by the panel members based on GRADE criteria. All members of the panel participated in the preparation and/or review of the draft guidelines.
Conflicts of Interest
Members of the expert panel complied with the IDSA policy regarding conflicts of interest, which requires disclosure of any financial or other interest that might be construed as constituting an actual, potential, or apparent conflict. IDSA provided a conflicts of interest disclosure statement to panel members and asked them to identify ties to companies manufacturing or developing products that might be affected by promulgation of the guideline. Information was requested regarding employment, consultancies, stock ownership, honoraria, research funding, expert testimony, and membership on company advisory committees. Regular updates of information pertaining to conflicts of interest were requested from each panel member following scheduled teleconference meetings. The panel made decisions on a case-by-case basis as to whether an individual’s role should be limited as a result of a conflict. No limiting conflicts were identified. Complying with IDSA policy, the majority of panel members were free of conflicts and 1 of the chairs was free of all conflicts.
Review and Approval Process
The panel obtained feedback from 3 external peer reviewers. The final document was reviewed and approved by the entire panel. The contents of the guidelines and manuscript were reviewed and approved by the IDSA Standards and Practice Guidelines Committee (SPGC) and the boards of directors of the IDSA and ASTMH prior to dissemination.
Future Guideline Revisions
At annual intervals, the panel chairs will be asked for their input on the need to update the guideline based on an examination of the current literature. The SPGC of the IDSA will consider this input and determine the necessity and timing of an update. If warranted, the entire panel or a subset thereof will be convened to discuss potential changes.
BACKGROUND INFORMATION ON CYSTICERCOSIS
More than 2000 cases of NCC are diagnosed each year in the United States [5, 6]. Epidemiologic studies suggest that NCC is the cause of approximately 29% of seizures in endemic areas and about 2% of patients presenting with seizures presenting to US emergency rooms [2–5, 8–10]. The seizures can be focal, focal with generalization, or generalized. Thus, NCC should be considered in all patients with seizures potentially exposed to a tapeworm carrier. Increased intracranial pressure is also a common manifestation of NCC. Approximately 20% of cases present with increased intracranial pressure, mainly obstructive hydrocephalus [2–4, 8, 10, 11].
A wide range of additional neurologic symptoms may be the initial symptoms of NCC. Patients can present with headaches, including migraine headaches. Less common manifestations include spinal radiculopathies, cerebrovascular accidents (lacunar infarctions, thrombotic, and hemorrhagic strokes), visual changes, and mass lesions.
RECOMMENDATIONS FOR DIAGNOSIS AND BASELINE EVALUATION
I. How should NCC be diagnosed?
Recommendations
While there is a wide range of clinical manifestations of neurocysticercosis, the 2 most common clinical presentations are seizures and increased intracranial pressure (not graded).
Initial evaluation should include careful history and physical examinations, and neuroimaging studies (not graded).
We recommend serologic testing with enzyme-linked immunotransfer blot as a confirmatory test in patients with suspected neurocysticercosis (strong, moderate). Enzyme-linked immunosorbent assays (ELISAs) using crude antigen should be avoided due to poor sensitivity and specificity (strong, moderate).
Evidence Summary
All patients with suspected NCC should undergo a thorough history and physical examination with particular attention to exposure. Since there is typically a latent period of years (months to decades) between infection and onset of symptoms, exposure history should not be limited to recent periods. Because there is marked variability in exposure within given countries, this should not be limited to country of origin or residence, but should also include queries about access to safe water and improved sanitation throughout life, contact with tapeworm carriers, and contact with pork-raising areas (especially among family and neighbors), which may have occurred months to years prior to onset of symptoms. Because tapeworm carriers can infect themselves, patients should also be queried about consumption of undercooked pork or passage of tapeworm segments. A thorough history should include query about symptoms of diseases that might be confused with NCC (eg, fever, night sweats, and weight loss would suggest tuberculosis). Examination should pay careful attention to signs of diseases that could be confused with NCC (eg, regional adenopathy may suggest tuberculosis or a malignancy) [12].
All patients with suspected NCC should undergo neuroimaging [13, 14]. Computed tomography (CT) is generally more sensitive at detecting calcified lesions and magnetic resonance imaging (MRI) is more sensitive for detection of the scolex, edema, small parenchymal lesions, posterior fossa lesions, and involvement of the subarachnoid spaces and ventricles. Fluid attenuation inversion recovery (FLAIR) sequences are particularly helpful for identifying associated edema and the scolex [14, 15].
There are a number of causes of cystic lesions on radiographic studies that can have a similar appearance to NCC. These include a number of infections, particularly tuberculomas, brain abscesses, or occasionally parasitic lesions (Echinococcus granulosus, Paragonimus species). Tumors can also resemble NCC (including metastatic lesions, primary brain cancers or lymphoma, and histiocytosis). Often the parasite scolex is visible as an intracystic nodule, typically round to slightly elongated, 1–2 mm in diameter, isodense or slightly more dense than brain parenchyma on CT or T1 imaging.
If a scolex is definitively identified, the diagnosis is certain. However, there are a number of artifacts that can be confused with a scolex. Parenchymal cysticerci are rounded in shape. Most are between 5 and 20 mm in diameter, but they can be larger, especially if located in the gyri and fissures. Parenchymal lesions with cystic areas diameter >20 mm, with irregular borders, or accompanied by midline shift are more likely to have other causes [12]. Midline shift is usually limited to larger cysts. Symptoms and signs of systemic illnesses (evidence of a primary tumor, fevers, night sweats, weight loss, and adenopathy) also make NCC less likely.
The serologic antibody test of choice is the enzyme-linked immunotransfer blot (EITB) using parasite glycoproteins (available from the Centers for Disease Control and Prevention and some reference laboratories) performed on serum [16, 17]. ELISAs using crude antigens to detect antibody are associated with frequent false-positive and false-negative results and should generally be avoided. For example, Proaño-Narvaez and colleagues noted a sensitivity of 41% for ELISA compared to 86% for EITB [18, 19]. The sensitivity of EITB varies with the form of NCC and specimen. Testing of serum is generally more sensitive than cerebrospinal fluid (CSF) using the EITB assay [16]. In patients with multiple parenchymal, with ventricular, or with subarachnoid NCC, the sensitivity of serum EITB is close to 100% [16]. However, the sensitivity is poor in patients with a single parenchymal lesion or with only calcifications [20].
Assays for parasite antigen in CSF, serum, or even urine may also be used to confirm the diagnosis [21]. Antigen detection assays are not currently available commercially in the United States. They are also thought to be less sensitive than EITB. However, positive results correlate with the number of viable cysticerci. Parasite antigen is commonly detected in both serum and CSF in cases with multiple cysticerci such as subarachnoid NCC, and serial measurements may be helpful in follow-up of complex cases [22–24].
II. What imaging studies should be used to classify disease?
Recommendation
4. We recommend both a brain MRI and a noncontrast CT scan for classifying patients with newly diagnosed NCC (strong, moderate).
Evidence Summary
The pathogenesis, clinical manifestations, prognosis, and management vary depending on the location of the cysticerci and viability of the cysticercus and the associated host response (Tables 1–3) [3, 4, 8, 11, 25]. The mildest form of NCC is the patient with a single enhancing lesion (SEL). Lesions in these patients are often cystic. Patients also frequently present with 1 or more viable cysticerci, which are identified as cystic lesions on neuroimaging studies. A third group of patients presents with parenchymal calcifications. Many of these patients present with chronic epilepsy. A fourth group has 1 or more cysticerci in the ventricles. They often present with symptoms of obstructive hydrocephalus. Finally, a fifth group has cysticerci in the subarachnoid space and can present with a range of presentations associated with basilar arachnoiditis. Many patients have mixed forms. In this case, they should generally be managed based on the more severe manifestation, with parenchymal disease usually milder, and ventricular and subarachnoid disease carrying a worse prognosis.
Management depends on careful staging. NCC patients should be classified to determine if they have enhancing parenchymal lesions, viable parenchymal lesions, ventricular disease, subarachnoid NCC, isolated spinal disease, and/or ocular disease (Tables 2 and 3). Patients can have cysts in >1 of the above locations. For example, while most cases with NCC have isolated parenchymal lesions, patients with parenchymal lesions may also have additional lesions in parenchyma, subarachnoid space, or ventricles. If possible, MRI should be performed in all cases to look for additional cysticerci. Recent advances in imaging include MRI with 3D volumetric sequencing, such as fast imaging employing steady-state acquisition (FIESTA), 3-dimensional constructive interference in steady state (3D CISS), or balance fast field echo (BFFE). These sequences provide enhanced resolution in areas with high T2 signal such as CSF. In cysticercosis, they have enhanced sensitivity for detection of extra-axial cysticerci in the ventricles or subarachnoid spaces [14, 15, 26–28]. There is a strong association of basal subarachnoid NCC with asymptomatic involvement of the spine [29]. Thus, all patients with intracranial subarachnoid disease should also undergo MRI of the spine.
III. What additional tests should be performed prior to initiation of therapy?
Recommendations
5. We suggest screening for latent tuberculosis infection in patients likely to require prolonged corticosteroids (weak, low).
6. We suggest screening or empiric therapy for Strongyloides stercoralis in patients likely to require prolonged corticosteroids (weak, low).
7. We recommend that all patients with NCC undergo a funduscopic examination prior to initiation of anthelminthic therapy (strong, moderate).
8. We suggest that patients with NCC who probably acquired NCC in a nonendemic area have their household members screened for tapeworm carriage (weak, low). Remark: This is a public health issue and can often be addressed by the local health department.
Evidence Summary
Management of NCC often involves use of corticosteroids or other anti-inflammatory therapy. Depending on the anticipated dose and duration of therapy and prior exposures, the patients may be at increased risk for opportunistic infections including reactivation tuberculosis. Testing for latent infection with Mycobacterium tuberculosis is frequently performed in patients with NCC. It is recommended for all patients who will undergo prolonged treatment with corticosteroids [30, 31]. The course of steroids used for many patients with SELs from NCC is often not a clear indication for prophylactic therapy for latent tuberculosis. By contrast, when a month or more of therapy is anticipated, experts believe that patients should be screened for latent tuberculosis and considered for chemoprophylaxis, as would be the case for any subject from endemic areas.
Corticosteroids increase the risk for Strongyloides stercoralis hyperinfection. S. stercoralis is coendemic with T. solium in many areas. However, the prevalence is poorly defined in most populations. There is considerable controversy about how best to prevent Strongyloides hyperinfection in patients from Strongyloides-endemic areas, who will be treated with steroids. The rates of hyperinfection are low, and most patients with NCC are treated with albendazole along with steroids, which may successfully treat most patients. Strategies for prevention of strongyloidiasis hyperinfection range from testing all patients for larvae in stool, serologic testing in all patients, stool and/or serologic testing only in symptomatic patients, or empiric treatment with ivermectin. Stool studies require specialized testing for Strongyloides such as Baermann concentration method. Even when performed, the sensitivity is poor even when testing multiple specimens. Serologic tests for antibody are more sensitive, but have lower specificity. Because of difficulty in diagnosis, some authorities recommend empiric treatment with ivermectin rather than depending on imperfect testing.
Funduscopy is an important part of evaluation of patients with potential hydrocephalus or cerebral edema. By contrast, in parenchymal NCC with small number of cysts or granulomas, funduscopy is unlikely to detect papilledema. However, funduscopic examination should be performed to exclude intraocular cysticerci, which occurs in a small proportion of patients. Antiparasitic therapy may lead to blindness in some cases with unsuspected intraocular parasites. An indirect funduscopic examination may be more sensitive for detection of parasites. Ocular ultrasound examination is an alternative method to screen for ocular involvement.
Patients acquire infection from a tapeworm carrier (usually either the patient with NCC or a close contact). However, there is a prolonged incubation period between infection with NCC and onset of symptoms. Many of the tapeworm carriers who originally transmitted infection may have cleared the intestinal infection or may no longer live near the patient. Currently, stool microscopy is the only available diagnostic test for tapeworms. Stool examination for ova is often negative in tapeworm carriers. Even multiple examinations may not detect the tapeworm carrier. Even when ova are found, the morphology of the ova cannot distinguish T. solium from other Taenia species. Thus, the yield of microscopy for identification of tapeworm carriers is generally low even in cases with apparent transmission outside endemic areas. Nevertheless, among patients who apparently acquired infection in the United States, Sorvillo and colleagues documented tapeworms in close contacts of 22% of NCC cases [32]. Thus, most authorities would recommend screening for cases acquired outside endemic areas. Newer methods such as antigen detection in stool or detection of tapeworm stage–specific antibodies by immunoblot might improve the usefulness of screening, but these are currently only research techniques and not commercially available at present.
Tapeworm carriers pose a public health risk, especially if they are food handlers. There are also risks of transmission within the household and from mother to child. Thus, identification of a tapeworm carrier is an important public health issue and local public health authorities should be notified of cases of NCC (NCC or tapeworm carriage is reportable in many states and regions, but reporting is not mandated nationally). Public health authorities should be notified of cases and involved in investigation of tapeworm carriers.
IV. How should antiparasitic and anti-inflammatory therapy be monitored?
Recommendations
9. We recommend that patients treated with albendazole for >14 days be monitored for hepatotoxicity and leukopenia (strong, moderate).
10. No additional monitoring is needed for patients receiving combination therapy with albendazole and praziquantel beyond that recommended for albendazole monotherapy (strong, moderate).
Evidence Summary
Albendazole is generally poorly absorbed. Absorption can be improved by dosing it with food, especially with fatty meals. The main side effects of albendazole in patients treated with doses of 15 mg/kg/day (up to 1200 mg/day) or less for 28 days are due to the parasiticidal activity and treatment-induced inflammation, including headaches, seizures, and dizziness. Thus, there is a transient increase in the number of seizures after therapy. Hepatoxicity and leukopenia are known side effects of albendazole and are relative contraindications to its continued use. In studies of chronic therapy, mainly for echinococcosis, elevated liver enzymes were seen in up to 16% of cases, requiring drug discontinuation in 3.8% [33]. The elevated transaminases normalized in almost all cases when the drug is discontinued promptly. Leukopenia is also noted in up to 10% of cases receiving prolonged therapy, but only requires discontinuation in <1% of cases. Reversible alopecia may also occur in up to 10% of cases. Most patients tolerate continuous therapy without interruption. Higher doses (30 mg/kg/day) have been used in some case of subarachnoid cysticercosis, but there are limited data on safety [34]. Few adverse events were noted with duration of up to 4 weeks. Thus, prolonged or high-dose albendazole can be used when needed (eg, subarachnoid NCC or giant cysticerci).
Both liver enzymes and complete blood counts should be monitored during the first month in patients receiving albendazole alone or in combination with praziquantel. The consensus of the panel was that patients who will receive albendazole or albendazole plus praziquantel for >14 days should be monitored with complete blood counts and liver enzymes during the first month. The optimal frequency of monitoring is unknown, but our panel felt that monitoring laboratory test weekly is adequate. In those receiving prolonged duration of albendazole, liver enzymes should continue to be monitored with the frequency based on clinical indications and tolerance. In the presence of absolute neutropenia or elevation of transaminase >5 times the upper limits of normal, albendazole should be withheld until laboratory tests normalize and alternative approaches considered (eg, praziquantel or no anthelminthics). This is usually only an issue in prolonged courses of therapy such as those used for subarachnoid disease.
The adverse effects noted with praziquantel depend on the indication, dose, and duration of therapy [35]. Most adverse effects in patients with NCC are due to its cysticidal activity, including headaches, dizziness, and seizures. Initial dose-ranging studies of praziquantel did not note other significant adverse events with doses of to 50 mg/kg/day for up to 28 days. Doses of up to 100 mg/kg/day for up to 28 days have been used in NCC without additional adverse laboratory adverse events. However, >10% of those treated with praziquantel develop gastrointestinal side effects such as nausea, vomiting, or abdominal pain. Allergic reactions including urticaria and other rashes are also noted in a small proportion of cases. Thus, patients should be advised about gastrointestinal and allergic reactions.
In 2 trials of combination therapy using both albendazole and praziquantel in parenchymal NCC, there were no more or different adverse events with combination therapy than with albendazole alone [36, 37]. Just as in monotherapy, liver enzymes and complete blood counts should be monitored.
Antiparasitic drugs can worsen symptoms of NCC by inducing an inflammatory response. Evidence from large case series suggests fewer adverse events in patients treated with antiparasitic drugs and steroids compared to antiparasitic drugs alone. Based on this fact, most authorities recommend using corticosteroids whenever antiparasitic therapy is planned. The doses and duration vary with different forms of NCC (Tables 2 and 3).
Short courses of corticosteroids are usually well tolerated. However, the adverse events profile is well defined, including hyperglycemia and gastritis. Additional risks of prolonged therapy include opportunistic infections, osteopenia, Cushing syndrome, aseptic necrosis of joints, altered mood (eg, depression, psychosis), and skin changes. Thus, prolonged steroid therapy should be used with caution. Best practices are to monitor patients on chronic steroids for these adverse events. Patients on corticosteroids for >2 weeks should undergo monitoring for blood sugar. Many authorities place all subjects on an H2 blocker or proton pump inhibitor to prevent gastritis.
Methotrexate has been used as an alternative treatment, especially in those patients who cannot tolerate steroids, or as a steroid-sparing agent during prolonged therapy of subarachnoid NCC [38]. Initial dose of 7.5 mg weekly can be increased to a maximum of 20 mg weekly. The treatment is generally well tolerated. Hepatotoxicity including cirrhosis, pulmonary complications, and myelosuppression can complicate chronic therapy with methotrexate when used daily for malignancies, but this is rare at doses used for NCC. Other side effects may include gastrointestinal intolerance, stomatitis, macular rash, alopecia, central nervous system problems, and hematologic abnormalities, but are rare with the low doses used in NCC. Patients receiving chronic therapy should receive folate supplementation, but not on the day that methotrexate is given.
RECOMMENDATIONS FOR THE TREATMENT OF VIABLE INTRAPARENCHYMAL NEUROCYSTICERCOSIS
V. What is the role of antiparasitic drugs in viable intraparenchymal neurocysticercosis (VPN)?
Recommendations
11. In patients with untreated hydrocephalus or diffuse cerebral edema, we recommend management of elevated intracranial pressure alone and not antiparasitic treatment (strong, moderate). Remarks: The management of patients with diffuse cerebral edema should be anti-inflammatory therapy such as corticosteroids, whereas hydrocephalus usually requires a surgical approach.
12. In the absence of elevated intracranial pressure, we recommend use of antiparasitic drugs in all patients with VPN (strong, moderate).
13. For patients with 1–2 viable parenchymal cysticerci, we recommend albendazole monotherapy for 10–14 days compared to either no antiparasitic therapy (strong, high) or combination antiparasitic therapy (weak, low). Remarks: The usual dose of albendazole is 15 mg/kg/day divided into 2 daily doses for 10–14 days with food. We recommend a maximum dose of 1200 mg/day.
14. We recommend albendazole (15 mg/kg/day) combined with praziquantel (50 mg/kg/day) for 10–14 days rather than albendazole monotherapy for patients with >2 viable parenchymal cysticerci (strong, moderate).
15. We suggest retreatment with antiparasitic therapy for parenchymal cystic lesions persisting for 6 months after the end of the initial course of therapy (weak, low).
Evidence Summary
Viable cysts are usually defined based on the radiologic appearance of a cystic lesion with a fluid-filled center. On CT or MRI, the best correlate for a viable organism is a cystic lesion with a hypodense center on CT or T1 images or a hyperintense center on T2 images. By contrast, nonviable lesions lack the cystic component (eg, lesions with isodense centers or calcified lesions). The natural history of parenchymal NCC includes an asymptomatic period that typically lasts several years, followed by gradual degeneration over a period of at least a year. While the exact proportion is unknown, many patients with parenchymal NCC go on to develop calcified lesions, a risk factor for chronic epilepsy. A higher number of seizures at baseline, poor adherence to antiepileptic drugs (AEDs), and development of calcifications are risk factors for seizure recurrence.
Antiparasitic drugs can worsen cerebral edema and should generally be avoided in patients with increased intracranial pressure from either diffuse cerebral edema (cysticercal encephalitis) or untreated hydrocephalus [39]. In both cases, antiparasitic drugs can lead to fatal adverse events, such as herniation. Cases with cysticercal encephalitis already have an inflammatory response and management should focus on anti-inflammatory therapy such as corticosteroids. In cases of hydrocephalus, antiparasitic drugs can also lead to worsening. Thus, increased intracranial pressure should be addressed before initiating antiparasitic therapy.
The use of antiparasitic drugs in cystic NCC was first reported in 1979, yet the role of antiparasitic drugs in cystic lesions remains controversial. Two recent meta-analyses have analyzed data on antiparasitic drug in viable parenchymal cysticercosis [40, 41]. One of these meta-analyses [41] was limited by not separating analyses of cystic lesions from enhancing lesions. Most early studies either used historic controls or patients who refused to enroll in trials [40].These early studies suggested improved radiologic resolution and clinical prognosis in those treated with either praziquantel or albendazole. However, studies mainly from the United States also demonstrated a good prognosis in those not treated with antiparasitic drugs [42]. In the 1990s, several poor-quality, randomized trials were reported in which there was no clear benefit of antiparasitic drugs compared to placebo [43]. However, there were methodological concerns about these studies.
Despite the large numbers of studies reported on the subject, there are only 2 high-quality, placebo-controlled trials of antiparasitic drugs in viable NCC. Garcia and colleagues enrolled 120 patients in a placebo-controlled randomized trial of albendazole 800 mg/day plus dexamethasone 6 mg/day, both in divided doses for 10 days, compared to placebos for both [44]. Therapy was well tolerated. At 6 months of follow-up, 21 of 55 in the albendazole group compared to 8 of 54 in the placebo group demonstrated radiologic resolution on MRI (P = .007). Carpio and colleagues reported a second high-quality, placebo-controlled randomized trial of albendazole in cysticercosis [45]. Patients were randomized to albendazole 800 mg/day or placebo for 8 days. Both groups received prednisone 75 mg/day. Separate strata analyzed patients with only parenchymal disease and those with both extraparenchymal disease and for those with “active” cysts from those with “transitional” cysts. Among those with active parenchymal cysts, resolution was demonstrated at 6 months in 19 of 39 (49%) treated with albendazole compared with 8 of 27 (23%) of those treated with placebo (P = .021). In both studies, the rate of resolution was much lower in patients with multiple nonenhancing parenchymal cysticerci.
The effect of antiparasitic drugs on seizures in viable parenchymal NCC has been difficult to demonstrate. Garcia and colleagues noted no seizures between 2 and 30 months of follow-up in 32 of 57 (56%) patients in the albendazole group and 30 of 59 (51%) in the placebo group [44]. However, there were 46% fewer seizures in the albendazole and steroid group. The overall reduction in the number of seizures was not significant. However, there was a significant reduction in the numbers of generalized seizures (22 vs 68, P = .003). Similarly, Carpio et al noted a higher proportion of patients that were seizure-free at 12 months in those receiving albendazole, but the difference was not statistically significant (62% vs 52%, P = .274). However, subgroup analysis has also demonstrated a decrease in the number of recurrent focal seizures with generalization [46].
Both praziquantel and albendazole have cysticidal activity, but there are limited high-quality data on the relative efficacy of the 2 drugs in NCC. Treatment with praziquantel has generally been less effective than albendazole. A single randomized trial comparing 2-week courses of these agents did not demonstrate significant differences [45]. In open-label studies, radiologic response rates with albendazole (generally dosed at 15 mg/kg/day in 2 daily doses) have tended to be better than with praziquantel (generally used at doses of 50 mg/kg/day given in 3 daily doses for 14 days). However, praziquantel has more complex drug interactions, with extensive first-pass metabolism induced by drugs such as AEDs and corticosteroids, which are often coadministered with antiparasitic drugs. First-pass metabolism of praziquantel can be inhibited by cimetidine. Thus, higher drug levels occur with coadministration of cimetidine and praziquantel, but there are no controlled clinical data demonstrating the clinical impact of coadministration. A single report and expert opinion also support the use of high doses of praziquantel (eg, 100 mg/kg/day). The studies of albendazole demonstrating efficacy have used doses of 15 mg/kg/day divided into 2 daily doses. Drugs have been continued for 10–14 days. There are limited data on the safety of doses over 1200 mg/day. Thus, we suggest that doses be limited to 1200 mg/day.
Three trials have compared the combination of albendazole and praziquantel to albendazole alone for parenchymal NCC. One initial report noted a higher rate of lesion resolution in patients treated with the combination. However, that study was not randomized and did not mask assessments of differences between groups [47]. In a phase 1/2 study of albendazole plus placebo compared to albendazole plus praziquantel, Garcia and colleagues noted improved radiologic resolution in the combination treatment group (12/16 [75%] with the combination compared to 4/16 [25%] with albendazole alone) [37]. Similarly, Garcia and colleagues completed a randomized controlled trial of albendazole (15 mg/kg/day up to 800 mg/day), higher-dose albendazole (22.5 mg/kg/day), or combination therapy (albendazole 15 mg/kg/day plus praziquantel 50 mg/kg/day) each for 10 days [36]. Each group was treated with dexamethasone 0.1 mg/kg/day. Among those with 3 or more cysticerci, on MRI at 180 days of follow-up, resolution of all viable cysts was demonstrated in 13 of 19 (68%) in the combination group compared to 1 of 21 (5%) in the standard-dose albendazole group and 5 of 20 (25%) in the high-dose albendazole group (P < .0001) [36].
Persistence of cystic lesions after chemotherapy is associated with recurrent seizures [36, 48]. While there are no convincing data that retreatment is better than symptomatic therapy, most experts recommend retreatment of patients with persistent cystic lesions beyond 6–12 months of therapy. Options for retreatment include a second course of albendazole, switching to praziquantel, or using the combination of albendazole and praziquantel. Combination therapy may have superior parasiticidal activity as noted above.
VI. What is the role of anti-inflammatory therapy in management of VPN?
Recommendation
16. We recommend adjunctive corticosteroid therapy begun prior to antiparasitic drugs rather than no adjunctive therapy in all patients treated with antiparasitic therapy (strong, moderate).
Evidence Summary
Antiparasitic drugs can worsen symptoms of NCC by inducing an inflammatory response [44]. Anecdotal evidence suggests fewer adverse events in patients treated with antiparasitic drugs and steroids compared to antiparasitic drugs alone [49]. Based on this fact, most authorities recommend using corticosteroids whenever antiparasitic therapy is planned. The impact of corticosteroids on lesion resolution and on development of calcifications in VPN is unknown. Trials using corticosteroids along with albendazole have not demonstrated different proportions developing calcifications. A recent trial of enhanced dexamethasone (8 mg/day in 3 daily doses for 28 days with a 14-day taper compared to 6 mg/day [also 3 times daily] for 10 days, both receiving albendazole for 10 days at 15 mg/kg and AEDs) revealed a significant decrease in partial seizures over the first 21 days as well as over the first 180 days in the enhanced corticosteroid arm [50]. There was no significant difference in cyst resolution or other side effects between the arms. However, the study was underpowered due to slow enrollment. While the optimal dose of corticosteroids has not been defined, this study suggests that higher doses may be preferable when patients are treated for VPN.
VII. What is the role of antiepileptic drugs in VPN?
Recommendations
17. We recommend antiepileptic drugs in all NCC patients with seizures (strong, low).
18. In patients with few seizures prior to antiparasitic therapy, resolution of the cystic lesion on imaging studies, and no seizures for 24 consecutive months, we suggest that tapering off and stopping antiepileptic drugs be considered (weak, moderate).
19. In the absence of controlled data, the choice of antiepileptic drugs should be guided by local availability, cost, drug interactions, and potential side effects (not graded).
Evidence Summary
While there are no controlled trials, AEDs appear to be as effective at controlling seizures in patients with parenchymal NCC as they are in other seizure disorders. There is also indirect evidence of effectiveness. For example, poor adherence to AEDs is a major risk factor for seizure recurrence [44]. AEDs should be used in patients with viable parenchymal NCC and seizures [44].
There are no controlled data comparing efficacy of different AEDs in patients with viable parenchymal cysticercosis. Phenytoin, carbamazepine, and phenobarbital have been used in many cases. Besides efficacy, other considerations that need to be considered include drug interactions with antiparasitic agents and corticosteroids, which are especially problematic for phenobarbital. While any AED can be used, it may be better to avoid phenobarbital with antiparasitic therapy, due to high rates of drug interactions. Newer agents such as levetiracetam with fewer drug interactions may be preferable to older agents.
There are limited data on optimal duration of AEDs in patients with viable parenchymal cysticerci [51]. Anecdotal reports suggest that AEDs can eventually be effectively tapered and discontinued in patients with resolution of the parasite cysts. In prospective studies, seizure recurrences have been noted in those who discontinued AEDs after being seizure free for at least 1 year. Guidelines for management of idiopathic seizures suggest continuations of AEDs for at least 24 months. Risk factors for seizure recurrence include the number of seizures prior to treatment and development of calcified lesions [48, 52]. Recurrence of seizures is unusual in patients with CT resolution without the development of calcifications and no subsequent seizures for >12 months [48, 52].
VIII. What follow-up is recommended after initial antiparasitic therapy for patients with VPN?
Recommendation
20. We suggest that MRI be repeated at least every 6 months until resolution of the cystic component (strong, low).
Evidence Summary
Patients should be followed clinically for seizure recurrence and optimization of AEDs as recommended for other patients with seizures. The first clinical follow-up after initial diagnosis should be performed at 2–4 weeks to determine if the patient has developed any recurrent seizures or new or worsening symptoms/signs. New or worsening symptoms should prompt reimaging. Monitoring of the antiparasitic response should mainly involve serial imaging studies. At a minimum, an MRI should be performed at least every 6 months until resolution of the cystic lesion, since we suggest retreatment in those with persistent lesions. This recommendation of frequency of follow-up scans is largely based on expert opinion. However, data from controlled trials of antiparasitic therapy have demonstrated that most cystic lesions have resolved by 6 months of follow-up [36, 44, 45]. Some recommend earlier imaging to look for an initial response to therapy or with clinical worsening. A CT scan should be performed prior to consideration of stopping AEDs to determine if calcifications have developed.
RECOMMENDATION FOR THE TREATMENT OF DEGENERATING INTRAPARENCHYMAL NCC INCLUDING PATIENTS WITH SINGLE ENHANCING LESIONS DUE TO NCC
IX. What should be the initial approach to the patient with multiple enhancing lesions from NCC?
Recommendation
21. We recommend that patients with multiple enhancing lesions and seizures be initially treated with antiepileptic drugs, antiparasitic therapy, and corticosteroids as outlined in the section on viable parenchymal cysticerci (weak, moderate).
Evidence Summary
Patients with 1 or, perhaps, 2 enhancing lesions have a better prognosis than those with multiple cysts [53]. Clinical trials of therapy for patients with viable cysticerci often included patients with multiple enhancing cysticerci [44, 45]. Thus, recommendations for viable cysticerci can also apply to these patients. By contrast, the data on optional treatment choice for patients with SELs (also termed solitary cysticercus granuloma) are significantly different and have been studied separately.
X. What is the role of antiepileptic medications in patients with SELs from cysticercosis with seizures?
Recommendations
22. We recommend antiepileptic drugs for all patients with SELs and seizures (strong, moderate).
23. In the absence of controlled data, the choice of antiepileptic drugs can be guided by local availability, cost, drug interactions, and potential side effects (not graded).
24. In patients who have been seizure free for 6 months, we suggest tapering off and stopping antiepileptic drugs after resolution of the lesion in patients with SELs without risk factors for recurrent seizures (weak, moderate). Remark: Risk factors for recurrent seizures include residual cystic lesions or calcifications on neuroimaging studies, breakthrough seizures, or >2 seizures.
Evidence Summary
In a prospective cohort study of 185 patients [54], most did not develop recurrent seizures. A minority of patients (16.2%) developed a seizure 1 week or more after the initial seizure while on AEDs. With long-term follow-up of 24 to 125 months, 28 patients (15.1%) with SELs developed recurrence of seizures after withdrawal of AEDs.
Symptomatic therapy in the form of AEDs is recommended for all patients with SELs and seizures. Those who present with headache alone (about 7%) do not need AEDs [12]. The goals of therapy are to prevent subsequent seizures in those presenting with seizures. The impact of AEDs on the natural history of seizures is not known. As 16.2% developed seizures despite antiepileptic therapy, it is likely that this number would be higher if AEDs were not prescribed.
There is limited evidence for the superiority of a particular AED. We suggest that the choice of AEDs be guided by local availability, cost, drug interactions, and side effects. Monotherapy with phenytoin or carbamazepine controlled seizures in 86.5% of patients in a study involving 185 patients with SELs [54]. A single, open-label comparative trial of clobazam vs phenytoin in patients with SELs suggested that the former might be more effective. However, clobazam has not been widely used. In contrast, carbamazepine and phenytoin appear to be used most often, largely due to availability and cost considerations in T. solium–endemic regions [55]. Both are potent hepatic P450 enzyme inducers, and the antihelminthic drugs praziquantel and albendazole are metabolized by the hepatic P450 enzyme system. In pharmacokinetic studies, phenytoin and carbamazepine decrease the areas under the curve of praziquantel and, to a lesser extent, albendazole [44, 56, 57]. The clinical significance of the pharmacokinetic interaction in context of a solitary cysticercus granuloma is unknown. One potential way of circumventing this interaction is to administer a non-enzyme-inducing AED (eg, levetiracetam) at least for the period of time for which antihelminthic treatment is being administered. This treatment strategy, however, has not been tested in any controlled trial.
An important management issue resolves around the duration for which AED treatment is given. At least 3 trials compared short-term (6 months) with slightly longer-term (12–24 months) AED treatment [58–60]. The trials found no benefit in seizure control with longer duration AEDs in those people in whom the solitary cysticercus granuloma had completely resolved. The studies also revealed that the risk of recurrent seizures remained high in people in whom the lesion resolved but resulted in a calcific residue visible on CT scan. The increased risk of seizures in those who developed calcification appeared to be offset by using a longer duration of AED treatment (12–24 months) [55]. Hence, people with solitary cysticercus granuloma, in which the granuloma leaves behind a calcific residue, should receive a longer duration of AEDs. How long should AEDs be advocated in these circumstances and when AEDs can be safely discontinued remains unsettled, and AEDs should probably be managed according to guidelines for chronic epilepsy.
XI. What is the role of antiparasitic drugs in patients with SELs?
Recommendation
25. We suggest albendazole therapy rather than no antiparasitic therapy for all patients with SELs (weak, moderate). Remarks: Albendazole (15 mg/kg/day in twice-daily doses up for 1–2 weeks) should be given with meals.
Evidence Summary
The role of antiparasitic drugs in patients with SELs has been controversial. Initial treatment studies with praziquantel and, later, albendazole demonstrated that patients treated with antiparasitic drugs had few adverse events and a benign course [61, 62]. However, the same pattern was noted in those treated with only AEDs [54, 63]. In a meta-analysis, Otte and colleagues identified 10 randomized controlled trials of antiparasitic treatment of patients with 1–2 enhancing cysticerci, involving 765 subjects [5, 9–18]. There was considerable heterogeneity in the studies regarding duration of albendazole (3–28 days), frequency of follow-up, and radiologic response. A single study compared single-day treatment with praziquantel to placebo and demonstrated a higher rate of radiologic resolution at 3 months [64]. Most lesions in the placebo arm demonstrate resolution with symptomatic therapy alone within a year. However, the radiologic resolution tends to be more rapid in patients treated with albendazole (usually with corticosteroids). This meta-analysis concluded that albendazole treatment modestly accelerates resolution of lesions and seizure freedom in patients with SELs. Six trials reported radiologic resolution at 6 months, which was noted in 142 of 212 (67%) treated with albendazole vs 80 of 168 (48%) treated with placebo (P = .04) [58, 65–69]. However, there was considerable heterogeneity between trials. Seven studies examined the number of subjects that remain free of seizures, but the length of follow-up was variable. In the 5 studies reporting follow-up at 6 months, 180 of 199 (90%) were seizure-free in the albendazole arm compared to 137 of 165 (83%) treated with placebo [58, 66, 68–70]. A single study reported 12-month follow-up and reported no significant difference in those who were free of seizures [67]. Overall, there was no significant difference in the frequency of calcifications at the end of follow-up, a surrogate marker for chronic epilepsy.
A single trial compared treatment with albendazole alone to albendazole combined with praziquantel [71]. There were trends toward more rapid resolution and fewer residual calcifications in the combination arm, but the results were not statistically significant.
The duration of albendazole therapy for SELs in different studies has ranged from 3 to 30 days. In comparative studies, no significant differences were demonstrated comparing 7 and 28 days, 7 and 14 days, or 2 and 15 days (though there was a trend toward a better response in the latter) [72]. Based on those data, our panel suggests that therapy should be administered for 7–14 days.
XII. What is the role of anti-inflammatory therapy in SELs?
Recommendation
26. We recommend that patients with SELs treated with antiparasitic drugs also be treated with corticosteroids initiated prior to antiparasitic therapy (strong, moderate).
Evidence Summary
In the meta-analysis of Otte and colleagues, randomized studies of corticosteroids alone (ie, not concomitantly with antihelminthic drugs) in patients with SELs did not demonstrate a significant impact on lesion resolution, seizure recurrence, or development of calcifications [73]. However, that analysis misinterpreted data from 1 study [74]. The group assignments were different in the abstract (suggesting worse with steroids) than in the body of the article and conclusions (steroids were better) [74]. If that study were analyzed correctly, the results would have been statistically significant. A subsequent meta-analysis using network analysis noted that the combination of albendazole plus corticosteroids had the optimal effect on lesion resolution and recurrent seizures [75].
XIII. How should patients with SELs be followed?
Recommendation
27. We suggest that MRI be repeated at least every 6 months until resolution of cystic lesions for patients with SELs (weak, low).
Evidence Summary
There are limited data on optimal follow-up of patients with SELs due to NCC. Most controlled trials have followed the patients at 3–6 months after therapy. Data suggest that imaging studies often normalize and antiepileptic drugs can often be discontinued after 6 months of therapy. Based on this, follow-up at 6 months appears to be important.
RECOMMENDATIONS FOR THE TREATMENT OF CALCIFIED PARENCHYMAL NEUROCYSTICERCOSIS
XIV. What should the initial approach be to patients with calcified lesions suggestive of calcified parenchymal neurocysticercosis (CPN)?
Recommendation
28. We suggest brain MRI in patients with seizures or hydrocephalus and only calcified parenchymal NCC on CT (weak, low).
Evidence Summary
The extent of evaluation required for patients with CPN depends on symptoms. Asymptomatic subjects without evidence of viable cysts do not require additional evaluation. By contrast, patients with seizures should be studied with MRI to exclude coexisting viable cysticerci, especially in the posterior fossa. Patients with symptoms of increased intracranial pressure should be studied by MRI looking for subarachnoid or ventricular disease.
The calcifications are best visualized by noncontrast CT examination (most sensitive), but can also be identified by MRI using gradient echo techniques (generally less sensitive). Typical calcifications are usually small (eg, 1–4 mm), dense, and round but at times are large and irregular in shape. The number of calcifications varies from 1 to hundreds, but most patients have 1–2 calcifications. Calcified lesions may not be diagnostic but in endemic areas are highly suggestive of NCC. The scolex embedded within the calcification can be visualized by MRI gradient spin echo imaging in some cases. Perilesional edema and the absence of viable cysts are best visualized by MRI using FLAIR or T2 sequences. Calcifications appear as voids or black regions by MRI. At present, the management of patients with transient perilesional calcifications edema is symptomatic and similar to other patients with calcifications. Thus, repeated imaging is only recommended in the setting of new or worsening symptoms. Serologic tests including EITB are frequently negative in patients with only calcifications, and parasite antigen in the blood or CSF is not generally detected.
XV. What is the role of antiparasitic drugs, antiepileptic drugs, and anti-inflammatory medications in the management of patients with CPN?
Recommendations
29. We recommend symptomatic therapy alone instead of antiparasitic drugs in patients with calcified parenchymal lesions (strong, moderate).
30. We suggest that corticosteroids not be routinely used in patients with isolated CPN and perilesional edema (weak, low).
Evidence Summary
Calcified lesions do not contain viable parasites. Thus, there is no reason to use antiparasitic therapy. Prior to widespread use of CT scanning, calcifications were primarily identified and characterized by gross examination at autopsy as hard or chalky nodules in the brain [76]. Microscopic descriptions noted varying amounts of degenerated membranes, calcareous corpuscles, and areas of amorphous calcifications surrounded by a collagenous capsule with little or no surrounding or intralesional inflammation. This contrasts with viable organisms and exuberant inflammation that is commonly associated with degeneration cysts and led to the suggestion that calcified granulomas are “inactive” and play little or no part in the pathophysiology of disease [77]. However, some calcified granulomas are foci of seizure activity [78, 79].
There are limited data on cessation of AEDs in patients with calcified NCC. Existing guidelines for the treatment and prevention of epilepsy, including choice of agents and doses and cessation of antiseizure medications, should be followed. In a prospective study, at least 36% of patients developed recurrent seizures [80]. Risk factors for recurrences have included the number of prior seizures and seizure-free interval. Thus, those with few prior seizures and a long seizure-free period (eg, 2 years of good control) may be candidates for tapering off and discontinuing AEDs.
Approximately 30%–50% of the time, transient episodes of perilesional edema develop around calcified foci that are foci of seizure activation when symptoms first appear [81]. Perilesional edema, which is most intense immediately surrounding the calcification, is best visualized using the FLAIR MRI technique; marked enhancement is almost always present as well. In an urban population in Lima with a history of seizures and only calcifications, 50% with recurring seizures had perilesional edema. These data indicate this phenomenon is common and likely a major cause of seizures in endemic rural populations where the prevalence of calcifications is high [82]. The natural history is incompletely known, but repeated episodes are common, sometimes reoccurring multiple times over decades [80]. Perilesional edema regresses without treatment in 3–6 weeks.
It is likely that perilesional edema is due to an immune-mediated inflammatory response to antigen present in the calcified granuloma. This is supported by the persistence of microglial activation around calcifications during and following episodes despite the early resolution of edema [83]. Further evidence of an immune-regulated process is suggested by the apparent induction or reactivation of perilesional edema following abrupt corticosteroid withdrawal [84]. There appear to be functional and histopathological differences among calcified granulomas. A subset demonstrate enhancement, which is due to abnormal leakage of serum components by blood–brain barrier [85], mostly likely caused by persisting perivascular inflammation and gliosis. An inflammatory cause of perilesional edema episodes is further supported by the presence of exuberant capsular and intralesional inflammation in calcified granulomas surgically removed from 2 patients experiencing seizures and perilesional edema episodes [3, 86, 87]. Both the presence of a scolex within the calcification [88] and gliosis around a calcification are associated with increased seizure activity [89].
There are no specific proven measures for treatment or prevention of symptoms related to calcifications with or without associated perilesional edema. High-dose corticosteroids are commonly used to control edema in other brain conditions. As perilesional edema appears to be inflammatory, use of chronic immunosuppressive agents might be expected to be useful. Immunosuppressive drugs such as methotrexate have also been tried. For example, the administration of methotrexate appeared to prevent further incapacitating episodes of perilesional edema in 1 patient for about 8 years [38]. However, the use of corticosteroids and other immunosuppressive drugs to control symptoms related to perilesional edema episodes is clouded by the apparent initiation or exacerbation of perilesional edema episodes following abrupt corticosteroid withdrawal [84]. Thus, at present there is not sufficient evidence to determine whether the benefits of these drugs outweigh the risks.
XVI. Is there a role for surgical therapy in refractory cases?
Recommendation
31. In patients with refractory epilepsy and CPN, we suggest evaluation for surgical removal of seizure foci (weak, low).
Evidence Summary
Calcified NCC has been associated with refractory epilepsy, though this is uncommon. In some cases, the focus can be mapped to the calcification. In others, the calcification may be associated with hippocampal sclerosis and mesial temporal lobe epilepsy. There are case series of surgical resolution of epilepsy in calcified NCC when the seizure focus is removed [90, 91]. Thus, mapping of the seizure foci followed by surgical therapy should be considered in refractory cases.
RECOMMENDATIONS FOR THE TREATMENT OF INTRAVENTRICULAR NEUROCYSTICERCOSIS
XVII. How are extraparenchymal cysts best identified?
Recommendation
32. We recommend MRI with 3D volumetric sequencing to identify intraventricular and subarachnoid cysticerci in patients with hydrocephalus and suspected NCC (strong, moderate).
Evidence Summary
In most case series, 10%–20% of patients with NCC present with cysticerci in the ventricles [11, 92]. These cysticerci are typically asymptomatic for years prior to the development of symptoms. Most cases present clinically when the cysticerci obstruct CSF flow, causing hydrocephalus. The cysticerci can be found in any of the ventricles. Obstruction typically occurs when the cysticerci lodge in an area of narrowing at the outflow of the ventricle such as the foramen of Monro, entrance to the aqueduct of Sylvius, or foramenae of Luschka or Magendie. Older series noted most of the ventricular cysticerci in the fourth ventricle, but this may have reflected more severe disease when the cysticerci are in that location. In most cases, the cysticerci cause symptoms while still viable [93]. As viable cysticerci have thin walls with cyst fluid isodense with CSF, they may be difficult to detect on imaging studies. CT scanning usually only shows evidence of obstructive hydrocephalus and/or distortion of the shape of the involved ventricle. MRI and CT ventriculography are more sensitive. However, MRI with 3D volumetric sequencing (FIESTA, 3D CISS, or BFFE) [14, 26, 94, 95] is considered to be the best method of identifying intraventricular cysts. These patients may also have parenchymal cysticerci, which can be associated with parenchymal inflammation and seizures.
XVIII. What is the optimal approach to management of intraventricular neurocysticercosis (IVN) in the lateral and third ventricles?
Recommendation
33. When possible, we recommend removal of the cysticerci by minimally invasive neuroendoscopy over other surgical or medical approaches for cysticerci of the lateral and third ventricles (strong, moderate). Remark: Most experts recommend that antiparasitic drugs not be used preoperatively, since such treatment could result in disruption of parasite integrity and an inflammatory response that could prevent successful cyst removal.
Evidence Summary
Ventricular NCC presents with symptoms or signs of raised intracranial pressure. Symptoms may include headaches, nausea, or vomiting, altered mental status, visual changes, or dizziness. The onset varies and can be abrupt (due to acute obstruction), intermittent, or gradual. Cysticerci may form a ball valve in the foramina that may come and go with position changes. This is especially common for cysticerci in the lateral ventricles. Cysticerci in the fourth ventricle have been associated with acute obstructive hydrocephalus that can lead to drop attacks (Bruns syndrome) [96]. Ventricular disease is prone to a high case-fatality rate and requires careful management.
There are no high-quality data on optimal management of ventricular NCC. All patients with obstructive hydrocephalus should be treated by either cyst removal or CSF diversion. Prior to the 1990s, most patients were managed by surgical excision of the cysticerci from the ventricles [97, 98]. The cure rate was high, but this approach was associated with significant operative morbidity and some mortality, especially for cysticerci in the third ventricle. Furthermore, cases in which the cysticerci were adherent to ependyma or associated with significant periventricular inflammation (as defined by enhancement on imaging studies or marked CSF pleocytosis) could not be readily removed without high rates of surgical complications [98].
The development of minimally invasive surgical approaches via neuroendoscopy has been increasingly used in neurosurgery. Early reports noted high cure rates and minimal morbidity when lateral and third ventricular cysts were removed by neuroendoscopic approaches [11, 99–104]. A number of case series have now been reported with this approach and outcomes have been generally favorable. Initial concerns were raised about the consequences of rupture of the cysticerci, which has been a frequent occurrence [99]. To date, there is no convincing evidence of adverse outcomes with cyst rupture. Only retrospective data are available comparing management strategies. However, in case series, outcomes were better with endoscopic removal than open procedures [11, 103].
There are, however, contraindications to endoscopic cyst removal. Inflamed or degenerating cysticerci are frequently adherent to the ventricular walls and ependyma. As is the case for open removal, attempted removal of adherent cysts is associated with a high risk of hemorrhage and neurologic sequelae. Thus, inflamed cysticerci are a contraindication to neurosurgical removal regardless of whether they are approached by neuroendoscopy or microdissection. Unlike hydatid disease, cysticerci do not produce daughter cysts in the human host. However, antiparasitic drugs may induce cyst inflammation, making cyst removal more difficult. For those reasons, preoperative antiparasitic therapy should generally be avoided.
XIX. What is the optimal surgical approach to management of IVN in the fourth ventricle?
Recommendation
34. In cases in which surgical removal of fourth ventricular cysticerci is possible, we recommend surgical removal rather than medical therapy and/or shunt surgery (strong, moderate).
Evidence Summary
There are more limited data on endoscopic approaches to cysticerci in the fourth ventricle. There are reports of excision of fourth ventricular cysts via the lateral and third ventricles and aqueduct [99, 103–106]. Case series note excellent results. However, concerns remain regarding the safety of this procedure, as the approach requires removal through the aqueduct and should only be attempted by an experienced neuroendoscopist. Cysticerci in the fourth ventricle can also be approached through a suboccipital craniotomy with cyst excision by either microsurgical dissection or by neuroendoscopy. As is the case with any surgery, attempted removal of adherent cysticerci is associated with a high rate of complications.
XX. What is the optimal approach to adherent IVN?
Recommendation
35. We suggest shunt surgery for hydrocephalus rather than cyst removal when surgical removal is technically difficult (weak, low). Remark: Attempted removal of inflamed or adherent ventricular cysticerci is associated with increased risk of complications.
Evidence Summary
Medical treatment alone with antiparasitic drugs and corticosteroids has been proposed [107, 108]. However, most of the cases described were actually treated with a shunt procedure prior to medical therapy, and there are limited data on the safety and efficacy of medical therapy. Medical therapy alone has been associated with poor outcomes in some series [11].
In the 1990s, investigators reported management of IVN with CSF diversion alone (typically via a ventriculoperitoneal shunt). However, most cases were complicated by shunt failure [11, 93, 109]. In comparative studies, neurologic outcome with shunting alone was worse than with cyst removal, either endoscopically or by open procedures. Thus, shunting should be regarded as an alternative strategy when cyst removal is not possible (eg, with adherent or inflamed cysticerci). Three approaches were used to try to prevent shunt failure, including low-flow shunts, adjunctive corticosteroids, and antiparasitic drugs. There is no good evidence to support any of these approaches. Sotelo and colleagues reported using a low-flow shunt and noted lower rates of shunt failure [110]. However, subsequent studies have noted inconsistent control of hydrocephalus with these shunts, and they are not widely available [111].
XXI. Does medical therapy as an adjunct to procedures or as primary therapy have an impact on outcome in treating patients with IVN?
Recommendations
36. We recommend corticosteroids to decrease brain edema in the perioperative period (not graded).
37. We suggest antiparasitic drugs with corticosteroid therapy following shunt insertion to decrease subsequent shunt failure in patients in whom surgical removal of isolated intraventricular cysts is not possible (weak, low), but neither after successful removal of intraventricular cysts (weak, low). Remark: Note that intraventricular cysts may be accompanied by other lesions with indications for antiparasitic therapy.
Evidence Summary
There are no high-quality data on the use of corticosteroids in ventricular NCC. Corticosteroids are often administered as an adjunct prior to and during the surgery and in the postoperative period to reduce inflammation and brain edema. Although there are no good data on the efficacy for these indications in NCC, they are well established in other neurologic diseases. There is anecdotal experience using high-dose corticosteroids (eg, dexamethasone 8–24 mg/day in divided doses) to stabilize patients prior to definitive surgical therapy.
Treatment with shunts without antiparasitic drugs had a high rate of shunt failure (approximately 60%). Suastegui Roman and colleagues treated patients who had received ventriculoperitoneal shunts for IVN with prednisone 50 mg 3 days per week and were also noted to have few shunt failures [112]. However, these data have not been confirmed in other studies, and the risks of chronic corticosteroids are significant. Lower rates of shunt failure were noted in those who received antiparasitic drugs and corticosteroids [93, 107]. None of these studies included randomized controls. However, given the significant morbidity associated with shunt failure, we suggest that patients undergoing shunting also be treated with antiparasitic drugs and steroids.
Some authorities have recommended that antiparasitic drugs be used after successful removal of ventricular cysts [113]. This recommendation came from the time in which earlier-generation CT and MRI were used for imaging. Recent data have noted that when cysts are successfully removed by either endoscopy or open surgery, if imaging studies are negative, outcomes are excellent without further antiparasitic therapy (T. Nash, unpublished observations).
RECOMMENDATIONS FOR SUBARACHNOID NEUROCYSTICERCOSIS
XXII. What is the role of medical therapy in subarachnoid neurocysticercosis (SAN) in the basilar cisterns or Sylvian fissures?
Recommendations
38. We recommend that patients with subarachnoid cysts be treated with antiparasitic drugs (strong, low).
39. We suggest that antiparasitic therapy be continued until there is radiologic resolution of viable cysticerci on MRI and resolution of other evidence of cysticerci (weak, low). Responses often require prolonged therapy, which can last for more than a year.
40. We recommend anti-inflammatory therapy (such as high-dose corticosteroids) for subarachnoid NCC initiated prior to antiparasitic drugs (strong, moderate).
41. We suggest that methotrexate be considered as a steroid-sparing agent in patients requiring prolonged courses of anti-inflammatory therapy (weak, low).
Evidence Summary
Cysts of T. solium that lodge or develop within the subarachnoid spaces of the brain frequently cause serious disease with considerable morbidity (SAN). Structurally normal cysts commonly lodge in the subarachnoid spaces within fissures abutting on the surface of the brain of the convexities of cerebral hemispheres. These cases present with a benign clinical course similar to parenchymal cysts and should be managed similarly [3, 114]. When located in the basal cisterns of the subarachnoid space or Sylvian fissure, the manifestations can be severe. There is almost always accompanying host inflammatory responses to the parasite resulting in meningitis, edema, and scarring, which are responsible for complications (eg, communicating hydrocephalus, focal neurological symptoms, nerve entrapments, and cerebrovascular complications including lacunar infarctions, thrombotic strokes, and hemorrhages). Intracranial hypertension is a cause of morbidity and mortality [115, 116] in SAN. It is usually due to communicating hydrocephalus, which is a common complication usually requiring shunt placement [117–119].
Vascular complications may be the initial presentation, with strokes occurring in 4%–12% of patients [120, 121], but may occur at any time during the course of disease. Perivascular inflammation can result in endarteritis, inflammatory aneurysms, and thrombosis [120–124]. Most cases of cerebral infarction associated with NCC involve occlusive arteritis in small perforating vessels, resulting in lacunar infarcts [125, 126]. Large vessel strokes and hemorrhage occur, but are less common [127, 128].
Other manifestations of SAN depend upon the location and extent of infection. These include cognitive dysfunction, psychiatric disease, and Parkinson-like syndromes [129–132]. Papilledema, frequently observed in patients with hydrocephalus, may lead to optic nerve damage and atrophy resulting in visual loss or impairment [133]. Cranial nerves can become compromised, leading to isolated neurologic deficits [116, 134, 135].
There are no randomized or well-controlled studies on antiparasitic treatment in SAN. Prior to the availability of antiparasitic drugs, patients with SAN had a very poor prognosis. For example, Sotelo and colleagues documented a 50% mortality rate in patients treated with ventriculoperitoneal shunting alone [136]. Several early small series reported successful treatment with short courses of albendazole at doses used in parenchymal disease [107, 109, 137]. However, these reports have not been confirmed, with treatment failure and the requirement for multiple courses of therapy noted in most patients. A majority of experts have concluded that this form of NCC is poorly responsive to cysticidal agents at doses and durations developed for parenchymal disease. A study of 33 patients with giant cysts found that patients had a good response to repeated courses of albendazole and/or praziquantel [115]. Most cases failed a single course of therapy but eventually responded to repeated courses of albendazole or praziquantel. Interestingly, no deaths were reported in that series, in sharp contrast to historical controls not treated with antiparasitic drugs [136]. Thus, most experts have concluded that there are benefits of cysticidal therapy, but that more intensive therapy may be needed that is traditionally used for parenchymal disease.
Alternative treatment regimens have been tried in SAN. Some investigators have noted good outcomes with longer courses of treatment compared to parenchymal disease [116, 118]. High doses of albendazole (30 mg/kg/day) in short courses have demonstrated better cysticidal activity than traditional doses, but the safety of this approach is not well established [34, 138]. Based on the improved response of patients with multiple parenchymal cysticerci to the combination of albendazole (15 mg/kg/day) and concurrent praziquantel (50 mg/kg/day), some experts have begun to use this combination in patients with subarachnoid disease. However, at present none of these data have been published. Corticosteroids are universally used to prevent damaging effects of ongoing and cysticidal-induced host inflammation, but the manner of use is practitioner dependent. The duration and dosing of cysticidal drugs and steroids or other immunosuppressive drugs have not been studied. Our expert panel suggests that the duration of therapy should be individualized. Our practice is to continue treatment until there is resolution of cystic lesions on neuroimaging studies. In addition, steroids should be continued until resolution of other evidence of viable cysticerci including CSF abnormalities when present and resolution of antigen in CSF and/or serum when detected.
Inflammation plays a key role in the pathogenesis of SAN. Nearly all of the complications of subarachnoid NCC are the result of the inflammatory reaction to parasite antigens. Complications include communicating hydrocephalus, meningitis, and vasculitis. Thus, concomitant corticosteroid therapy is essential to avoid complications due to the ensuing inflammation in the subarachnoid space, especially after the administration of antiparasitic agents with a careful tapering schedule to avoid cerebrovascular complications and hydrocephalus [127, 139]. It is usually prudent to stabilize the patient clinically before initiating antiparasitic therapy, which includes administration of high-dose corticosteroids to suppress ongoing as well as antiparasitic drug–induced inflammation. Anti-inflammatory treatment is indicated for the duration of antiparasitic cysticidal therapy, which often is continued for months. Because high-dose corticosteroids are associated with serious complications, some authorities recommend using methotrexate as a steroid-sparing agent. Close follow-up of the patient is essential while corticosteroids are being tapered.
Corticosteroids are critical in the setting of intracranial hypertension to stabilize the patient. Doses are not well standardized, but may include dexamethasone, 0.2 mg/kg/day. Untreated hydrocephalus is a contraindication to antiparasitic therapy. Hydrocephalus usually requires shunt placement or cyst removal.
Endpoints for therapy have not been established. The MRI should be followed and examined for regression of the cystic lesions and improvement of enhancement. Enhancement may not completely disappear and the subarachnoid space may remain distorted, presumably due to scarring, presenting a challenge for the clinician deciding on a course of therapy. CSF analysis may be helpful if lumbar puncture can be performed safely. Cellularity and hypoglycorrhachia should normalize, but in patients with shunts the protein may remain elevated. Antigen detection of the secreted metacestode antigen using a monoclonal antibody–based test is a useful adjunctive tool for following SAN patients undergoing treatment [22–24, 114, 140–142]. Antigen detection assays are currently commercially available in Europe, but standardized assays are not commercially available in the United States. Normalization of CSF cellularity and glucose, absence of cystic lesions on MRI, and negative antigen assays should be an endpoint for ending therapy. Long-term follow-up is required. SAN is a slowly developing infection and successful therapy may require years of treatment.
XXIII. What is the role of neurosurgery in SAN?
Recommendations
42. We recommend that patients with hydrocephalus from subarachnoid NCC be treated with shunt surgery in addition to medical therapy (strong, low).
43. We suggest that some patients may benefit from surgical debulking over shunt surgery alone (weak, low).
Evidence Summary
Theoretically, surgical removal of parasite mass would be expected to shorten the course of treatment and decrease provoked inflammation. However, because there is a risk of surgery and bleeding due to adherent cysts, the role for debulking in SAN is controversial. Several groups have described endoscopic removal of cysts from SAN via a third ventriculostomy [103]. When successful, this approach may lead to faster resolution of the cysticerci and decreased antigen-stimulated inflammation. However, this approach is not always possible and attempts to remove adherent cysts in the basilar cisterns can be catastrophic [143].
RECOMMENDATIONS FOR SPINAL NEUROCYSTICERCOSIS
XXIV. How is spinal neurocysticercosis (SN) best treated?
Recommendations
44. We recommend corticosteroid treatment for patients with SN with evidence of spinal cord dysfunction (eg, paraparesis or incontinence) or as adjunctive therapy along with antiparasitic therapy (strong, moderate).
45. We suggest that both medical (antiparasitic drugs plus anti-inflammatory drugs) and surgical approaches be considered for SN (weak, low). Practice statement: There are anecdotes of good responses of spinal NCC to medical and/or surgical therapy. However, there are no good data supporting one approach over the other. We suggest that management of spinal NCC should be individualized based on symptoms, location of the cysticerci, degree of arachnoiditis, and surgical experience. Recommendations for antiparasitic drugs, reimaging, and follow-up of SAN should also be considered for subarachnoid SN.
Evidence Summary
Corticosteroids are recommended in all patients with evidence of cord dysfunction. Doses and duration should follow guidelines for management of cord dysfunction. In contrast to cases of spinal epidural abscess, cord compromise is frequently due to edema surrounding cysticerci and is not necessarily a surgical emergency. Similar to subarachnoid disease of the brain, cysticidal treatment can worsen inflammation and exacerbate neurologic symptoms. Corticosteroids should be used concomitantly with antiparasitic agents.
There are no good data on optimal management of spinal NCC. Intramedullary SN has traditionally been treated with laminectomy and subsequent myelotomy. There have been increasing numbers of reports of treatment with cysticidal drugs with complete recovery and disappearance of lesions on neuroimaging. A recent review suggested that the outcome of intramedullary cysticercosis may be better for medically treated patients than for surgically treated patients [144]. A trial with cysticidal drugs in suspected cases is warranted to confirm the diagnosis by documenting resolution of the lesion on follow-up imaging studies. If antiparasitic drugs are used, they should be given together with sufficient corticosteroids to reduce the risk of transient clinical deterioration secondary to worsening inflammation and edema.
For those patients with subarachnoid disease of the spine and concurrent basilar SAN, medical therapy should be undertaken as outlined in section XXII. Corticosteroids are critical in patients with symptomatic extramedullary spinal disease. As is the case for cerebral subarachnoid disease, doses are not well standardized, but may include dexamethasone 0.2 mg/kg/day with albendazole 15 mg/kg/day. The clinical picture along with the MRI of the spine should be followed and examined for regression of the cystic lesions and improvement of enhancement. Nerve clumping or displacement of the nerve roots may persist, presumably due to adhesive arachnoiditis and scarring [29]. CSF analysis can reveal cellularity and hypoglycorrhachia, but with successful medical therapy it should normalize. Endpoints for therapy have not been established, but as in SAN of the brain, close follow-up of the patient while tapering corticosteroids is important. Surgical treatment is indicated in the management of acute symptomatic disease to relieve mass effect, as well as in patients who have mass effect and experience severe and progressive neurological dysfunction despite corticosteroids. Cysts degenerating in the spine can elicit inflammation and scarring, making excision of these lesions technically difficult. Subarachnoid scarring may be severe enough to obstruct CSF flow necessitating duraplasty to reestablish CSF flow. Treatment regimens are not standardized; therefore, close follow-up of the patient is critical as corticosteroids are tapered. Surgical treatment with excision of the cysticerci after laminectomy has been the norm in cases of spinal cord or radicular compression. Surgical removal in the setting of severe arachnoiditis, where cysts have adhered to the sacral roots and adjacent dura, can be very difficult and in some cases complete removal of the cysts is impossible. Unless the surgeon is confident that the cysticerci have been completely removed, patients should be treated with antiparasitic drugs postoperatively and undergo follow-up with imaging and CSF analysis as outlined above for intracranial subarachnoid cysticercosis.
RECOMMENDATIONS FOR MANAGEMENT OF OCULAR CYSTICERCOSIS
XXV. What is the optimal management of ocular cysticercosis?
Recommendation
46. We suggest that intraocular cysticerci be treated with surgical removal rather than with antiparasitic drugs (weak, low).
Evidence Summary
Eye involvement in cysticercosis may include the extraocular muscles or subconjunctiva. Intraocular disease may involve the anterior chamber, vitreous, or subretinal location. Orbital cysticercosis, extraocular muscle cysticercosis, and subconjunctival cysticercosis are often amenable to medical therapy. Intraocular cysticercosis has traditionally been approached surgically [145, 146]. Untreated intraocular disease was thought to be a contraindication to cysticidal therapy due to the risk of inducing inflammation, which can lead to blindness. While there are anecdotal reports of successful medical therapy of ocular NCC [147, 148], there are not sufficient data on the safety of this approach. Thus, at the present time, intraocular cysticerci should be treated by surgical removal.
RECOMMENDATIONS FOR THE TREATMENT OF SPECIAL POPULATIONS
XXVI. Should children be managed differently than adults?
Recommendation
47. There is no evidence that management of NCC in children should be different than in adults with the same form of disease (strong, moderate). Dosing should be weight based.
Evidence Summary
The clinical spectrum of NCC is somewhat different in children than adults [54, 149]. Children are more likely to present with SELs and cysticercal encephalitis. Both albendazole and praziquantel have been used safely in millions of children >1 year of age. The pharmacokinetics of the drugs is similar to those seen in adults. Due to the incubation period between infection and onset of symptoms, it is rare for NCC to present in infants and there are limited data on drug safety, pharmacokinetics, or efficacy in this population.
XXVII. Should management be different in pregnant women?
Recommendation
48. We suggest that antihelminthic therapy be deferred until after pregnancy (weak, low). Remarks: Pregnant patients with elevated intracranial pressure need to be aggressively managed as they would be if not pregnant. Corticosteroids can be used in pregnancy when necessary. The use of antiepileptic drugs should take into account altered pharmacokinetics and potential teratogenicity. Phenobarbital and valproic acid are known to have high rates of teratogenicity. Antihelminthic drugs are rarely required emergently and their use can usually be deferred until after delivery. Methotrexate is teratogenic and should be avoided.
Evidence Summary
The management of NCC in pregnant women presents challenges, due to concerns about teratogenicity of the drugs. Patients who are symptomatic and have significant morbidity should receive optimal symptomatic therapy. Pregnant patients with elevated intracranial pressure need to be aggressively managed as they would be if not pregnant. The use of antiepileptic drugs should take into account altered pharmacokinetics and potential teratogenicity. Valproate is associated with a higher rate of teratogenicity. Otherwise, the choice of antiepileptic drugs should follow guidelines for the management of epilepsy in pregnancy.
By contrast, antihelminthic drugs are rarely required emergently and their use can usually be deferred until after delivery. Praziquantel is a category B agent with no clear associated teratogenicity in animal studies and limited data suggesting that it is safe in pregnancy [150]. Based on teratogenicity observed in animal models, albendazole is not considered safe in pregnancy [151]. However, limited data are emerging that treatment of pregnant women with albendazole for intestinal helminths actually improves birth outcomes without evidence of increased rates of birth defects [152]. In general, cysticidal therapy should be deferred until after delivery.
Corticosteroids can be used in pregnancy when necessary. Methotrexate is teratogenic and should be avoided.
Notes
Acknowledgments. The panel expresses its gratitude for thoughtful reviews of an earlier version by Oscar H. Del Brutto, Joe Zunt, Jose A. Serpa-Alvarez, Edward Ryan (Chair), and members of the ASTMH Guidelines Committee. The panel also thanks Dean Winslow, the liaison of the IDSA Standards and Practice Guidelines Committee (SPGC), and Genet Demisashi for their efforts guiding us through the process.
Financial support. Support for these guidelines was provided by the Infectious Diseases Society of America and the American Society of Tropical Medicine and Hygiene. This work was in part supported by intramural National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Potential conflicts of interest. The following list is a reflection of what has been reported to IDSA. To provide thorough transparency, IDSA requires full disclosure of all relationships, regardless of relevancy to the guideline topic. Evaluation of such relationships as potential conflicts of interest (COI) is determined by a review process that includes assessment by the SPGC Chair, the SPGC liaison to the development panel, the Board of Directors liaison to the SPGC, and, if necessary, the COI Task Force of the Board. This assessment of disclosed relationships for possible COI will be based on the relative weight of the financial relationship (ie, monetary amount) and the relevance of the relationship (ie, the degree to which an association might reasonably be interpreted by an independent observer as related to the topic or recommendation of consideration). The reader of these guidelines should be mindful of this when the list of disclosures is reviewed. For activities outside of the submitted work, A. C. W. has received royalties from UpToDate. For activities outside of the submitted work, A. H. has served as a member of a data and safety monitoring board for Neuropace. For activities outside of the submitted work, H. G. has received research grants from the National Institute of Neurological Disorders and Stroke and the Fogarty International Center of the National Institutes of Health. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Disclaimer. It is important to realize that guidelines cannot always account for individual variation among patients. They are not intended to supplant physician judgment with respect to particular patients or special clinical situations. IDSA considers adherence to the guidelines listed below to be voluntary, with the ultimate determination regarding their application to be made by the physician in the light of each patient’s individual circumstances. While IDSA makes every effort to present accurate and reliable information, the information provided in these guidelines is “as is” without any warranty of accuracy, reliability, or otherwise, either express or implied. Neither IDSA nor its officers, directors, members, employees, or agents will be liable for any loss, damage, or claim with respect to any liabilities, including direct, special, indirect, or consequential damages, incurred in connection with these guidelines or reliance on the information presented.
References
- 1. Guyatt GH, Oxman AD, Vist GE et al. ; GRADE Working Group GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coyle CM, Mahanty S, Zunt JR et al. . Neurocysticercosis: neglected but not forgotten. PLoS Negl Trop Dis 2012; 6:e1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garcia HH, Nash TE, Del Brutto OH. Clinical symptoms, diagnosis, and treatment of neurocysticercosis. Lancet Neurol 2014; 13:1202–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ndimubanzi PC, Carabin H, Budke CM et al. . A systematic review of the frequency of neurocyticercosis with a focus on people with epilepsy. PLoS Negl Trop Dis 2010; 4:e870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O’Neal SE, Flecker RH. Hospitalization frequency and charges for neurocysticercosis, United States, 2003–2012. Emerg Infect Dis 2015; 21:969–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O’Keefe KA, Eberhard ML, Shafir SC, Wilkins P, Ash LR, Sorvillo FJ. Cysticercosis-related hospitalizations in the United States, 1998–2011. Am J Trop Med Hyg 2015; 92:354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 2011; 64:380–2. [DOI] [PubMed] [Google Scholar]
- 8. Del Brutto OH. Neurocysticercosis. Neurohospitalist 2014; 4:205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ong S, Talan DA, Moran GJ et al. ; EMERGEncy ID NET Study Group Neurocysticercosis in radiographically imaged seizure patients in U.S. emergency departments. Emerg Infect Dis 2002; 8:608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Serpa JA, Graviss EA, Kass JS, White AC Jr. Neurocysticercosis in Houston, Texas: an update. Medicine (Baltimore) 2011; 90:81–6. [DOI] [PubMed] [Google Scholar]
- 11. Rangel-Castilla L, Serpa JA, Gopinath SP, Graviss EA, Diaz-Marchan P, White AC Jr. Contemporary neurosurgical approaches to neurocysticercosis. Am J Trop Med Hyg 2009; 80:373–8. [PubMed] [Google Scholar]
- 12. Rajshekhar V, Chandy MJ. Validation of diagnostic criteria for solitary cerebral cysticercus granuloma in patients presenting with seizures. Acta Neurol Scand 1997; 96:76–81. [DOI] [PubMed] [Google Scholar]
- 13. Del Brutto OH, Rajshekhar V, White AC Jr et al. . Proposed diagnostic criteria for neurocysticercosis. Neurology 2001; 57:177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lerner A, Shiroishi MS, Zee CS, Law M, Go JL. Imaging of neurocysticercosis. Neuroimaging Clin N Am 2012; 22:659–76. [DOI] [PubMed] [Google Scholar]
- 15. Hernández RD, Durán BB, Lujambio PS. Magnetic resonance imaging in neurocysticercosis. Top Magn Reson Imaging 2014; 23:191–8. [DOI] [PubMed] [Google Scholar]
- 16. Tsang VC, Brand JA, Boyer AE. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium). J Infect Dis 1989; 159:50–9. [DOI] [PubMed] [Google Scholar]
- 17. Del Brutto OH, Nash TE, White AC Jr, et al. Revised diagnostic criteria for neurocysticercosis. J Neurol Sci 2017; 372:202–10. [DOI] [PubMed] [Google Scholar]
- 18. Proaño-Narvaez JV, Meza-Lucas A, Mata-Ruiz O, García-Jerónimo RC, Correa D. Laboratory diagnosis of human neurocysticercosis: double-blind comparison of enzyme-linked immunosorbent assay and electroimmunotransfer blot assay. J Clin Microbiol 2002; 40:2115–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carod JF, Randrianarison M, Razafimahefa J et al. . Evaluation of the performance of 5 commercialized enzyme immunoassays for the detection of Taenia solium antibodies and for the diagnosis of neurocysticercosis. Diagn Microbiol Infect Dis 2012; 72:85–9. [DOI] [PubMed] [Google Scholar]
- 20. Wilson M, Bryan RT, Fried JA et al. . Clinical evaluation of the cysticercosis enzyme-linked immunoelectrotransfer blot in patients with neurocysticercosis. J Infect Dis 1991; 164:1007–9. [DOI] [PubMed] [Google Scholar]
- 21. Gabriël S, Blocher J, Dorny P et al. . Added value of antigen ELISA in the diagnosis of neurocysticercosis in resource poor settings. PLoS Negl Trop Dis 2012; 6:e1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nguekam, Zoli AP, Ongolo-Zogo P, Dorny P, Brandt J, Geerts S. Follow-up of neurocysticercosis patients after treatment using an antigen detection ELISA. Parasite 2003; 10:65–8. [DOI] [PubMed] [Google Scholar]
- 23. Fleury A, Hernández M, Avila M et al. . Detection of HP10 antigen in serum for diagnosis and follow-up of subarachnoidal and intraventricular human neurocysticercosis. J Neurol Neurosurg Psychiatry 2007; 78:970–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Garcia HH. Serological diagnosis and follow-up of severe neurocysticercosis using HP10 antigen detection. Nat Clin Pract Neurol 2007; 3:488–9. [DOI] [PubMed] [Google Scholar]
- 25. Nash TE, Garcia HH. Diagnosis and treatment of neurocysticercosis. Nat Rev Neurol 2011; 7:584–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Govindappa SS, Narayanan JP, Krishnamoorthy VM, Shastry CH, Balasubramaniam A, Krishna SS. Improved detection of intraventricular cysticercal cysts with the use of three-dimensional constructive interference in steady state MR sequences. AJNR Am J Neuroradiol 2000; 21:679–84. [PMC free article] [PubMed] [Google Scholar]
- 27. Hingwala D, Chatterjee S, Kesavadas C, Thomas B, Kapilamoorthy TR. Applications of 3D CISS sequence for problem solving in neuroimaging. Indian J Radiol Imaging 2011; 21:90–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carrillo Mezo R, Lara García J, Arroyo M, Fleury A. Relevance of 3D magnetic resonance imaging sequences in diagnosing basal subarachnoid neurocysticercosis. Acta Trop 2015; 152:60–5. [DOI] [PubMed] [Google Scholar]
- 29. Callacondo D, Garcia HH, Gonzales I, Escalante D, Nash TE; Cysticercosis Working Group in Peru High frequency of spinal involvement in patients with basal subarachnoid neurocysticercosis. Neurology 2012; 78:1394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lewinsohn DM, Leonard MK, LoBue PA et al. . Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis 2017; 64:111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Recomm Rep 2000; 49:1–51. [PubMed] [Google Scholar]
- 32. Sorvillo FJ, Waterman SH, Richards FO, Schantz PM. Cysticercosis surveillance: locally acquired and travel-related infections and detection of intestinal tapeworm carriers in Los Angeles County. Am J Trop Med Hyg 1992; 47:365–71. [DOI] [PubMed] [Google Scholar]
- 33. Albenza (albendazole). Product information. Philadelphia, PA: SmithKline Beecham; Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207844s000lbl.pdf. Accessed July 2016. [Google Scholar]
- 34. Göngora-Rivera F, Soto-Hernández JL, González Esquivel D et al. . Albendazole trial at 15 or 30 mg/kg/day for subarachnoid and intraventricular cysticercosis. Neurology 2006; 66:436–8. [DOI] [PubMed] [Google Scholar]
- 35. Biltricide (praziquantel). Product information. West Haven, CT: Bayer; Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/018714s012lbl.pdf. Accessed July 2017. [Google Scholar]
- 36. Garcia HH, Gonzales I, Lescano AG et al. ; Cysticercosis Working Group in Peru Efficacy of combined antiparasitic therapy with praziquantel and albendazole for neurocysticercosis: a double-blind, randomised controlled trial. Lancet Infect Dis 2014; 14:687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Garcia HH, Lescano AG, Gonzales I et al. ; Cysticercosis Working Group in Peru Cysticidal efficacy of combined treatment with praziquantel and albendazole for parenchymal brain cysticercosis. Clin Infect Dis 2016; 62:1375–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mitre E, Talaat KR, Sperling MR, Nash TE. Methotrexate as a corticosteroid-sparing agent in complicated neurocysticercosis. Clin Infect Dis 2007; 44:549–53. [DOI] [PubMed] [Google Scholar]
- 39. Del Brutto O, Sotelo J, Roman GC.. Neurocysticercosis: a clinical handbook. Exton, PA: Swets and Zeitlinger Publisher, 1998. [Google Scholar]
- 40. Del Brutto OH, Roos KL, Coffey CS, García HH. Meta-analysis: cysticidal drugs for neurocysticercosis: albendazole and praziquantel. Ann Intern Med 2006; 145:43–51. [DOI] [PubMed] [Google Scholar]
- 41. Baird RA, Wiebe S, Zunt JR, Halperin JJ, Gronseth G, Roos KL. Evidence-based guideline: treatment of parenchymal neurocysticercosis: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2013; 80:1424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mitchell WG, Crawford TO. Intraparenchymal cerebral cysticercosis in children: diagnosis and treatment. Pediatrics 1988; 82:76–82. [PubMed] [Google Scholar]
- 43. Carpio A, Santillán F, León P, Flores C, Hauser WA. Is the course of neurocysticercosis modified by treatment with antihelminthic agents?Arch Intern Med 1995; 155:1982–8. [PubMed] [Google Scholar]
- 44. Garcia HH, Pretell EJ, Gilman RH et al. ; Cysticercosis Working Group in Peru A trial of antiparasitic treatment to reduce the rate of seizures due to cerebral cysticercosis. N Engl J Med 2004; 350:249–58. [DOI] [PubMed] [Google Scholar]
- 45. Carpio A, Kelvin EA, Bagiella E et al. ; Ecuadorian Neurocysticercosis Group Effects of albendazole treatment on neurocysticercosis: a randomised controlled trial. J Neurol Neurosurg Psychiatry 2008; 79:1050–5. [DOI] [PubMed] [Google Scholar]
- 46. Romo ML, Wyka K, Carpio A et al. ; Ecuadorian Neurocysticercosis Group The effect of albendazole treatment on seizure outcomes in patients with symptomatic neurocysticercosis. Trans R Soc Trop Med Hyg 2015; 109:738–46. [DOI] [PubMed] [Google Scholar]
- 47. Guo DM, Xie SP, Jia JP. Therapeutic efficacy of praziquantel, albendazole and a combination of the two drugs in cysticercosis. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2003; 21:187–8. [PubMed] [Google Scholar]
- 48. Carpio A, Hauser WA. Prognosis for seizure recurrence in patients with newly diagnosed neurocysticercosis. Neurology 2002; 59:1730–4. [DOI] [PubMed] [Google Scholar]
- 49. deGhetaldi LD, Norman RM, Douville AW Jr. Cerebral cysticercosis treated biphasically with dexamethasone and praziquantel. Ann Intern Med 1983; 99:179–81. [DOI] [PubMed] [Google Scholar]
- 50. Garcia HH, Gonzales I, Lescano AG et al. ; Cysticercosis Working Group in Peru Enhanced steroid dosing reduces seizures during antiparasitic treatment for cysticercosis and early after. Epilepsia 2014; 55:1452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sharma M, Singh T, Mathew A. Antiepileptic drugs for seizure control in people with neurocysticercosis. Cochrane Database Syst Rev 2015; CD009027. [DOI] [PubMed] [Google Scholar]
- 52. Del Brutto OH. Prognostic factors for seizure recurrence after withdrawal of antiepileptic drugs in patients with neurocysticercosis. Neurology 1994; 44:1706–9. [DOI] [PubMed] [Google Scholar]
- 53. Singhi P, Suthar R, Deo B, Malhi P, Khandelwal NK. Long-term clinical and radiologic outcome in 500 children with parenchymal neurocysticercosis. Pediatr Infect Dis J 2017; 36:549–55. [DOI] [PubMed] [Google Scholar]
- 54. Rajshekhar V, Jeyaseelan L. Seizure outcome in patients with a solitary cerebral cysticercus granuloma. Neurology 2004; 62:2236–40. [DOI] [PubMed] [Google Scholar]
- 55. Singh G, Rajshekhar V, Murthy JM et al. . A diagnostic and therapeutic scheme for a solitary cysticercus granuloma. Neurology 2010; 75:2236–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bittencourt PR, Gracia CM, Martins R, Fernandes AG, Diekmann HW, Jung W. Phenytoin and carbamazepine decreased oral bioavailability of praziquantel. Neurology 1992; 42:492–6. [DOI] [PubMed] [Google Scholar]
- 57. Lanchote VL, Garcia FS, Dreossi SA, Takayanagui OM. Pharmacokinetic interaction between albendazole sulfoxide enantiomers and antiepileptic drugs in patients with neurocysticercosis. Ther Drug Monit 2002; 24:338–45. [DOI] [PubMed] [Google Scholar]
- 58. Thussu A, Arora A, Prabhakar S, Lal V, Sawhney IM. Acute symptomatic seizures due to single CT lesions: how long to treat with antiepileptic drugs?Neurol India 2002; 50:141–4. [PubMed] [Google Scholar]
- 59. Verma A, Misra S. Outcome of short-term antiepileptic treatment in patients with solitary cerebral cysticercus granuloma. Acta Neurol Scand 2006; 113:174–7. [DOI] [PubMed] [Google Scholar]
- 60. Gupta RK, Kumar R, Chawla S, Pradhan S. Demonstration of scolex within calcified cysticercus cyst: its possible role in the pathogenesis of perilesional edema. Epilepsia 2002; 43:1502–8. [DOI] [PubMed] [Google Scholar]
- 61. Thussu A, Chattopadhyay A, Sawhney IM, Khandelwal N. Albendazole therapy for single small enhancing CT lesions (SSECTL) in the brain in epilepsy. J Neurol Neurosurg Psychiatry 2008; 79:272–5. [DOI] [PubMed] [Google Scholar]
- 62. Rajshekhar V. Incidence and significance of adverse effects of albendazole therapy in patients with a persistent solitary cysticercus granuloma. Acta Neurol Scand 1998; 98:121–3. [DOI] [PubMed] [Google Scholar]
- 63. Rajshekhar V, Oommen A. Utility of the cysticercus immunoblot in a patient with an atypical solitary cerebral cysticercus granuloma. Neurol India 2001; 49:75–7. [PubMed] [Google Scholar]
- 64. Pretell EJ, Garcia HH, Custodio N et al. . Short regimen of praziquantel in the treatment of single brain enhancing lesions. Clin Neurol Neurosurg 2000; 102:215–8. [DOI] [PubMed] [Google Scholar]
- 65. Alarcón F, Tolosa E, Muñoz E. Focal limb dystonia in a patient with a cerebellar mass. Arch Neurol 2001; 58:1125–7. [DOI] [PubMed] [Google Scholar]
- 66. Chaurasia RN, Garg RK, Agarwall A et al. . Three day albendazole therapy in patients with a solitary cysticercus granuloma: a randomized double blind placebo controlled study. Southeast Asian J Trop Med Public Health 2010; 41:517–25. [PubMed] [Google Scholar]
- 67. de Souza A, Nalini A, Kovoor JM, Yeshraj G, Siddalingaiah HS, Thennarasu K. Perilesional gliosis around solitary cerebral parenchymal cysticerci and long-term seizure outcome: a prospective study using serial magnetization transfer imaging. Epilepsia 2011; 52:1918–27. [DOI] [PubMed] [Google Scholar]
- 68. Gogia S, Talukdar B, Choudhury V, Arora BS. Neurocysticercosis in children: clinical findings and response to albendazole therapy in a randomized, double-blind, placebo-controlled trial in newly diagnosed cases. Trans R Soc Trop Med Hyg 2003; 97:416–21. [DOI] [PubMed] [Google Scholar]
- 69. Singhi P, Jain V, Khandelwal N. Corticosteroids versus albendazole for treatment of single small enhancing computed tomographic lesions in children with neurocysticercosis. J Child Neurol 2004; 19:323–7. [DOI] [PubMed] [Google Scholar]
- 70. Kalra V, Dua T, Kumar V. Efficacy of albendazole and short-course dexamethasone treatment in children with 1 or 2 ring-enhancing lesions of neurocysticercosis: a randomized controlled trial. J Pediatr 2003; 143:111–4. [DOI] [PubMed] [Google Scholar]
- 71. Kaur S, Singhi P, Singhi S, Khandelwal N. Combination therapy with albendazole and praziquantel versus albendazole alone in children with seizures and single lesion neurocysticercosis: a randomized, placebo-controlled double blind trial. Pediatr Infect Dis J 2009; 28:403–6. [DOI] [PubMed] [Google Scholar]
- 72.Singh G, Murthy JM. Solitary cysticercus granuloma-treatment with albendazole: what is the optimal duration? Neurol India 2010; 58:507–8. [DOI] [PubMed] [Google Scholar]
- 73. Otte WM, Singla M, Sander JW, Singh G. Drug therapy for solitary cysticercus granuloma: a systematic review and meta-analysis. Neurology 2013; 80:152–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kishore D, Misra S. Short course of oral prednisolone on disappearance of lesion and seizure recurrence in patients of solitary cysticercal granuloma with single small enhancing CT lesion: an open label randomized prospective study. J Assoc Physicians India 2007; 55:419–24. [PubMed] [Google Scholar]
- 75. Zhao BC, Jiang HY, Ma WY et al. . Albendazole and corticosteroids for the treatment of solitary cysticercus granuloma: a network meta-analysis. PLoS Negl Trop Dis 2016; 10:e0004418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Henneberg R. Die tierischen parasiten des zentralnerven-systems. Handbuch Der Neurologie. M. Lewandowsky. Berlin: Verlag Von Julius Springer, 1912:69. [Google Scholar]
- 77. Sotelo J, Guerrero V, Rubio F. Neurocysticercosis: a new classification based on active and inactive forms. A study of 753 cases. Arch Intern Med 1985; 145:442–5. [PubMed] [Google Scholar]
- 78.Singh G, Chowdhary AK. Epilepsy surgery in context of neurocysticercosis. Ann Indian Acad Neurol 2014;17:S65–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cukiert A, Puglia P, Scapolan HB, Vilela MM, Marino Júnior R. Congruence of the topography of intracranial calcifications and epileptic foci. Arq Neuropsiquiatr 1994; 52:289–94. [DOI] [PubMed] [Google Scholar]
- 80. Nash TE, Pretell EJ, Lescano AG et al. ; Cysticercosis Working Group in Peru Perilesional brain oedema and seizure activity in patients with calcified neurocysticercosis: a prospective cohort and nested case-control study. Lancet Neurol 2008; 7:1099–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nash T. Edema surrounding calcified intracranial cysticerci: clinical manifestations, natural history, and treatment. Pathog Glob Health 2012; 106:275–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Moyano LM, O’Neal SE, Ayvar V et al. ; Cysticercosis Working Group in Peru High prevalence of asymptomatic neurocysticercosis in an endemic rural community in Peru. PLoS Negl Trop Dis 2016; 10:e0005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fujita M, Mahanty S, Zoghbi SS, et al. PET reveals inflammation around calcified Taenia solium granulomas with perilesional edema. PLoS One 2013; 8:e74052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mejia R, Nash TE. Corticosteroid withdrawal precipitates perilesional edema around calcified Taenia solium cysts. Am J Trop Med Hyg 2013; 89:919–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gupta RK, Awasthi R, Garg RK et al. . T1-weighted dynamic contrast-enhanced MR evaluation of different stages of neurocysticercosis and its relationship with serum MMP-9 expression. AJNR Am J Neuroradiol 2013; 34:997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ooi WW, Wijemanne S, Thomas CB, Quezado M, Brown CR, Nash TE. Short report: a calcified Taenia solium granuloma associated with recurrent perilesional edema causing refractory seizures: histopathological features. Am J Trop Med Hyg 2011; 85:460–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nash TE, Bartelt LA, Korpe PS, Lopes B, Houpt ER. Calcified neurocysticercus, perilesional edema, and histologic inflammation. Am J Trop Med Hyg 2014; 90:318–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gupta M, Agarwal P, Khwaja GA et al. . Randomized prospective study of outcome of short term antiepileptic treatment in small single enhancing CT lesion in brain. Neurol India 2002; 50:145–7. [PubMed] [Google Scholar]
- 89. Pradhan S, Kathuria MK, Gupta RK. Perilesional gliosis and seizure outcome: a study based on magnetization transfer magnetic resonance imaging in patients with neurocysticercosis. Ann Neurol 2000; 48:181–7. [PubMed] [Google Scholar]
- 90. Singh G, Chowdhary AK. Epilepsy surgery in context of neurocysticercosis. Ann Indian Acad Neurol 2014; 17:S65–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bianchin MM, Velasco TR, Coimbra ER et al. . Cognitive and surgical outcome in mesial temporal lobe epilepsy associated with hippocampal sclerosis plus neurocysticercosis: a cohort study. PLoS One 2013; 8:e60949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Salgado P, Rojas R, Sotelo J. Cysticercosis. Clinical classification based on imaging studies. Arch Intern Med 1997; 157:1991–7. [DOI] [PubMed] [Google Scholar]
- 93. Kelley R, Duong DH, Locke GE. Characteristics of ventricular shunt malfunctions among patients with neurocysticercosis. Neurosurgery 2002; 50:757–61; discussion 61–2. [DOI] [PubMed] [Google Scholar]
- 94. Neyaz Z, Patwari SS, Paliwal VK. Role of FIESTA and SWAN sequences in diagnosis of intraventricular neurocysticercosis. Neurol India 2012; 60:646–7. [DOI] [PubMed] [Google Scholar]
- 95. Mont’Alverne Filho FE, Machado Ldos R, Lucato LT, Leite CC. The role of 3D volumetric MR sequences in diagnosing intraventricular neurocysticercosis: preliminar results. Arq Neuropsiquiatr 2011; 69:74–8. [DOI] [PubMed] [Google Scholar]
- 96. Torres-Corzo J, Rodriguez-della Vecchia R, Rangel-Castilla L. Bruns syndrome caused by intraventricular neurocysticercosis treated using flexible endoscopy. J Neurosurg 2006; 104:746–8. [DOI] [PubMed] [Google Scholar]
- 97. Colli BO, Carlotti CG Jr, Assirati JA Jr, Machado HR, Valença M, Amato MC. Surgical treatment of cerebral cysticercosis: long-term results and prognostic factors. Neurosurg Focus 2002; 12:e3. [PubMed] [Google Scholar]
- 98. Colli BO, Martelli N, Assirati Júnior JA et al. . Cysticercosis of the central nervous system. I. Surgical treatment of cerebral cysticercosis: a 23 years experience in the Hospital das Clínicas of Ribeirão Preto Medical School. Arq Neuropsiquiatr 1994; 52:166–86. [DOI] [PubMed] [Google Scholar]
- 99. Husain M, Jha DK, Rastogi M, Husain N, Gupta RK. Neuro-endoscopic management of intraventricular neurocysticercosis (NCC). Acta Neurochir (Wien) 2007; 149:341–6. [DOI] [PubMed] [Google Scholar]
- 100. Psarros TG, Coimbra C. Endoscopic third ventriculostomy for patients with hydrocephalus and fourth ventricular cysticercosis: a review of five cases. Minim Invasive Neurosurg 2004; 47:346–9. [DOI] [PubMed] [Google Scholar]
- 101. Singh I, Haris M, Husain M, Husain N, Rastogi M, Gupta RK. Role of endoscopic third ventriculostomy in patients with communicating hydrocephalus: an evaluation by MR ventriculography. Neurosurg Rev 2008; 31:319–25. [DOI] [PubMed] [Google Scholar]
- 102. Jiménez-Vázquez OH, Nagore N. Endoscopic evidence of ventricular and cisternal inflammatory changes after intraoperative cysticercal rupture during endoscopic third-ventriculostomy removal. Br J Neurosurg 2013; 27:137–8. [DOI] [PubMed] [Google Scholar]
- 103. Proaño JV, Torres-Corzo J, Rodríguez-Della Vecchia R, Guizar-Sahagun G, Rangel-Castilla L. Intraventricular and subarachnoid basal cisterns neurocysticercosis: a comparative study between traditional treatment versus neuroendoscopic surgery. Childs Nerv Syst 2009; 25:1467–75. [DOI] [PubMed] [Google Scholar]
- 104. Goel RK, Ahmad FU, Vellimana AK et al. . Endoscopic management of intraventricular neurocysticercosis. J Clin Neurosci 2008; 15:1096–101. [DOI] [PubMed] [Google Scholar]
- 105. Bergsneider M. Endoscopic removal of cysticercal cysts within the fourth ventricle. Technical note. J Neurosurg 1999; 91:340–5. [DOI] [PubMed] [Google Scholar]
- 106. Suri A, Goel RK, Ahmad FU, Vellimana AK, Sharma BS, Mahapatra AK. Transventricular, transaqueductal scope-in-scope endoscopic excision of fourth ventricular neurocysticercosis: a series of 13 cases and a review. J Neurosurg Pediatr 2008; 1:35–9. [DOI] [PubMed] [Google Scholar]
- 107. Proaño JV, Madrazo I, García L, García-Torres E, Correa D. Albendazole and praziquantel treatment in neurocysticercosis of the fourth ventricle. J Neurosurg 1997; 87:29–33. [DOI] [PubMed] [Google Scholar]
- 108. Cuetter AC, Garcia-Bobadilla J, Guerra LG, Martinez FM, Kaim B. Neurocysticercosis: focus on intraventricular disease. Clin Infect Dis 1997; 24:157–64. [DOI] [PubMed] [Google Scholar]
- 109. Bandres JC, White AC Jr, Samo T, Murphy EC, Harris RL. Extraparenchymal neurocysticercosis: report of five cases and review of management. Clin Infect Dis 1992; 15:799–811. [DOI] [PubMed] [Google Scholar]
- 110. Sotelo J, Izurieta M, Arriada N. Treatment of hydrocephalus in adults by placement of an open ventricular shunt. J Neurosurg 2001; 94:873–9. [DOI] [PubMed] [Google Scholar]
- 111. Rajshekhar V. Surgical management of neurocysticercosis. Int J Surg 2010; 8:100–4. [DOI] [PubMed] [Google Scholar]
- 112. Suastegui Roman RA, Soto-Hernandez JL, Sotelo J. Effects of prednisone on ventriculoperitoneal shunt function in hydrocephalus secondary to cysticercosis: a preliminary study. J Neurosurg 1996; 84:629–33. [DOI] [PubMed] [Google Scholar]
- 113. Khade P, Lemos RS, Toussaint LG. What is the utility of postoperative antihelminthic therapy after resection for intraventricular neurocysticercosis?World Neurosurg 2013; 79:558–67. [DOI] [PubMed] [Google Scholar]
- 114. Garcia HH, Gonzalez AE, Gilman RH et al. ; Cysticercosis Working Group in Peru Circulating parasite antigen in patients with hydrocephalus secondary to neurocysticercosis. Am J Trop Med Hyg 2002; 66:427–30. [DOI] [PubMed] [Google Scholar]
- 115. Proaño JV, Madrazo I, Avelar F, López-Félix B, Díaz G, Grijalva I. Medical treatment for neurocysticercosis characterized by giant subarachnoid cysts. N Engl J Med 2001; 345:879–85. [DOI] [PubMed] [Google Scholar]
- 116. Fleury A, Carrillo-Mezo R, Flisser A, Sciutto E, Corona T. Subarachnoid basal neurocysticercosis: a focus on the most severe form of the disease. Expert Rev Anti Infect Ther 2011; 9:123–33. [DOI] [PubMed] [Google Scholar]
- 117. Sotelo J, Del Brutto OH. Therapy of neurocysticercosis. Childs Nerv Syst 1987; 3:208–11. [DOI] [PubMed] [Google Scholar]
- 118. Cárdenas G, Carrillo-Mezo R, Jung H, Sciutto E, Hernandez JL, Fleury A. Subarachnoidal neurocysticercosis non-responsive to cysticidal drugs: a case series. BMC Neurol 2010; 10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Oka Y, Fukui K, Shoda D et al. . Cerebral cysticercosis manifesting as hydrocephalus—case report. Neurol Med Chir (Tokyo) 1996; 36:654–8. [DOI] [PubMed] [Google Scholar]
- 120. Alarcón F, Vanormelingen K, Moncayo J, Viñán I. Cerebral cysticercosis as a risk factor for stroke in young and middle-aged people. Stroke 1992; 23:1563–5. [DOI] [PubMed] [Google Scholar]
- 121. Alarcón F, Hidalgo F, Moncayo J, Viñán I, Dueñas G. Cerebral cysticercosis and stroke. Stroke 1992; 23:224–8. [DOI] [PubMed] [Google Scholar]
- 122. Del Brutto OH, Lama J; Atahualpa Project Investigators The importance of neurocysticercosis in stroke in rural areas of a developing Latin American country. Am J Trop Med Hyg 2013; 89:374–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Barinagarrementeria F, Cantú C. Neurocysticercosis as a cause of stroke. Stroke 1992; 23:1180–1. [DOI] [PubMed] [Google Scholar]
- 124. Arauz A, Ruiz-Navarro F, Silos H et al. . Concurrent asymptomatic inflammatory aneurysm and ischemic stroke due to cysticercal arteritis. Clin Neurol Neurosurg 2013; 115:2540–2. [DOI] [PubMed] [Google Scholar]
- 125. Barinagarrementeria F, Cantú C. Frequency of cerebral arteritis in subarachnoid cysticercosis: an angiographic study. Stroke 1998; 29:123–5. [DOI] [PubMed] [Google Scholar]
- 126. Del Brutto OH. Cysticercosis and cerebrovascular disease: a review. J Neurol Neurosurg Psychiatry 1992; 55:252–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Levy AS, Lillehei KO, Rubinstein D, Stears JC. Subarachnoid neurocysticercosis with occlusion of the major intracranial arteries: case report. Neurosurgery 1995; 36:183–8; discussion 188. [DOI] [PubMed] [Google Scholar]
- 128. Viola GM, White AC Jr, Serpa JA. Hemorrhagic cerebrovascular events and neurocysticercosis: a case report and review of the literature. Am J Trop Med Hyg 2011; 84:402–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Forlenza OV, Filho AH, Nobrega JP et al. . Psychiatric manifestations of neurocysticercosis: a study of 38 patients from a neurology clinic in Brazil. J Neurol Neurosurg Psychiatry 1997; 62:612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Keane JR. Tremor as the result of shunt obstruction: four patients with cysticercosis and secondary parkinsonism: report of four cases. Neurosurgery 1995; 37:520–2. [DOI] [PubMed] [Google Scholar]
- 131. Sharma S, Modi M, Lal V, Prabhakar S, Bhardwaj A, Sehgal R. Reversible dementia as a presenting manifestation of racemose neurocysticercosis. Ann Indian Acad Neurol 2013; 16:88–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Wiwanitkit S, Wiwanitkit V. Racemose neurocysticercosis and reversible dementia. Ann Indian Acad Neurol 2013; 16:730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Chang GY, Keane JR. Visual loss in cysticercosis: analysis of 23 patients. Neurology 2001; 57:545–8. [DOI] [PubMed] [Google Scholar]
- 134. Ranjith MP, Divya R, Sahni A. Isolated third cranial nerve palsy: a rare presentation of neurocysticercosis. Ir J Med Sci 2011; 180:905–7. [DOI] [PubMed] [Google Scholar]
- 135. Kim JS, Jeong SM, Moon SY, Park SH. Third cranial nerve palsy from midbrain neurocysticercosis: repeated exacerbation on tapering corticosteroids. J Neuroophthalmol 2004; 24:217–20. [DOI] [PubMed] [Google Scholar]
- 136. Sotelo J, Marin C. Hydrocephalus secondary to cysticercotic arachnoiditis. A long-term follow-up review of 92 cases. J Neurosurg 1987; 66:686–9. [DOI] [PubMed] [Google Scholar]
- 137. Del Brutto OH. Albendazole therapy for subarachnoid cysticerci: clinical and neuroimaging analysis of 17 patients. J Neurol Neurosurg Psychiatry 1997; 62:659–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Márquez-Caraveo C, Góngora-Rivera F, Santos Zambrano J, Hernández R, Soto-Hernández JL. Pre-treatment with corticosteroids and a single cycle of high dose albendazole for subarachnoidal cysticercosis. J Neurol Neurosurg Psychiatry 2004; 75:938–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Bang OY, Heo JH, Choi SA, Kim DI. Large cerebral infarction during praziquantel therapy in neurocysticercosis. Stroke 1997; 28:211–3. [DOI] [PubMed] [Google Scholar]
- 140. Bobes RJ, Hernández M, Márquez C et al. . Subarachnoidal and intraventricular human neurocysticercosis: application of an antigen detection assay for the diagnosis and follow-up. Trop Med Int Health 2006; 11:943–50. [DOI] [PubMed] [Google Scholar]
- 141. Garcia HH, Harrison LJ, Parkhouse RM et al. . A specific antigen-detection ELISA for the diagnosis of human neurocysticercosis. The Cysticercosis Working Group in Peru. Trans R Soc Trop Med Hyg 1998; 92:411–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Garcia HH, Parkhouse RM, Gilman RH et al. ; Cysticercosis Working Group in Peru Serum antigen detection in the diagnosis, treatment, and follow-up of neurocysticercosis patients. Trans R Soc Trop Med Hyg 2000; 94:673–6. [DOI] [PubMed] [Google Scholar]
- 143. Jimenez-Vazquez OH, Nagore N. Cisternal neurocysticercosis. Br J Neurosurg 2008; 22:774–5. [DOI] [PubMed] [Google Scholar]
- 144. Del Brutto OH, Garcia HH. Intramedullary cysticercosis of the spinal cord: a review of patients evaluated with MRI. J Neurol Sci 2013; 331:114–7. [DOI] [PubMed] [Google Scholar]
- 145. Sharma T, Sinha S, Shah N et al. . Intraocular cysticercosis: clinical characteristics and visual outcome after vitreoretinal surgery. Ophthalmology 2003; 110:996–1004. [DOI] [PubMed] [Google Scholar]
- 146. Wender JD, Rathinam SR, Shaw RE, Cunningham ET Jr. Intraocular cysticercosis: case series and comprehensive review of the literature. Ocul Immunol Inflamm 2011; 19:240–5. [DOI] [PubMed] [Google Scholar]
- 147. Lim WK, Chee SP. Nonsurgical management of subretinal cysticercosis. Retina 2004; 24:469–71. [DOI] [PubMed] [Google Scholar]
- 148. Kaliaperumal S, Rao VA, Parija SC. Cysticercosis of the eye in South India—a case series. Indian J Med Microbiol 2005; 23:227–30. [PubMed] [Google Scholar]
- 149. Singhi P, Singhi S. Neurocysticercosis in children. Indian J Pediatr 2009; 76:537–45. [DOI] [PubMed] [Google Scholar]
- 150. Olveda RM, Acosta LP, Tallo V et al. . Efficacy and safety of praziquantel for the treatment of human schistosomiasis during pregnancy: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 2016; 16:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Delatour P, Parish RC, Gyurik RJ. Albendazole: a comparison of relay embryotoxicity with embryotoxicity of individual metabolites. Ann Rech Vet 1981; 12:159–67. [PubMed] [Google Scholar]
- 152. Salam RA, Haider BA, Humayun Q, Bhutta ZA. Effect of administration of antihelminthics for soil-transmitted helminths during pregnancy. Cochrane Database Syst Rev 2015; CD005547. [DOI] [PubMed] [Google Scholar]