Abstract
Although implicated in cardiovascular disease, little is known about the fat surrounding the heart. In humans, epicardial fat is the visceral fat depot of the heart, which directly contacts the myocardium. This strategically placed fat depot is thought to produce bioactive molecules that could affect cardiac function. A major limitation in understanding the biology of epicardial fat is its restricted access in humans and its seeming absence in commonly-used experimental animal models. Although laboratory mice do not have epicardial fat per se, they do have a fat depot around the heart. In this study, we found that mouse pericardial fat has the molecular signature, small adipocyte size, and resistance to differentiation consistent with visceral fat. In addition, we show that mouse pericardial fat is regulated by pregnancy-associated plasma protein-A (PAPP-A), a key modulator of local insulin-like growth factor bioavailability. PAPP-A is highly expressed in mouse pericardial fat at levels equivalent to those in mesenteric visceral fat and 10-fold higher than in subcutaneous inguinal fat (P = 0.0003). Cultured pre-adipocytes isolated from pericardial fat show 2-fold increased PAPP-A secretion compared to preadipocytes isolated from inguinal fat. Furthermore, PAPP-A knock-out mice fed a high fat diet for 20 weeks have significantly reduced pericardial fat (by 60%; P < 0.0001) compared to wild-type littermates. There was no significant difference in inguinal fat between wild-type and PAPPA knock-out mice. These data characterize a new mouse model of visceral-like pericardial fat and lay a foundation for understanding its role in human heart disease.
Keywords: pericardial fat, visceral fat, PAPP-A, mice
INTRODUCTIION
Although implicated in cardiovascular disease and heart failure, very little is known about the fat surrounding the heart. In humans and non-human primates,[1, 2]. epicardial fat is the visceral fat depot of the heart [1–3]. It shares a common embryologic origin with other intra-abdominal visceral fat depots -- omental fat (attached to stomach and spleen) and mesenteric fat (located along intestines). Epicardial fat is situated between the myocardium and the inner layer of the pericardium. It directly contacts the myocardium with no intervening fascia, and shares the same microcirculation. Human epicardial fat is thought to have metabolic and secretory functions similar to other visceral fat depots, and it has been hypothesized that its generation of bioactive molecules could significantly affect cardiac function [3–5]. Epicardial fat is easily detected and measured by imaging. There are several clinical studies showing that epicardial thickness is a proxy measure of visceral adiposity [6, 7], and that it correlates with atherosclerosis, heart failure, atrial fibrillation, metabolic syndrome, insulin resistance, and fatty liver disease [3–5, 8–11]. However, correlations need to be validated by experimental evidence, and there are very few studies directly assessing human epicardial physiology and pathophysiology. This is in large part due to the limited ability to access epicardial adipose tissue, and is especially true of accessing epicardial tissue from heathy people. Thus, there is an urgent need for alternative models to study the role of cardiac adipose tissue. In particular, use of genetically-engineered animal models would significantly expand our molecular understanding of cardiac fat and its impact on the heart.
A major limitation in understanding the biology of epicardial fat, i.e., visceral fat directly abutting the myocardium, is its reported absence in commonly used experimental animal models -- laboratory mice and rats [12]. Although mice do not have epicardial fat per se (i.e., no direct contact with the myocardium except for attachment at the atrial-ventricular groove [13]), they do have a fat depot around the heart. We will refer to this as pericardial fat. This fat depot has not been characterized in any detail or functionally explored. Furthermore, mice have numerous pores in the pericardium allowing direct access of surrounding adipose tissue-derived products to the myocardium [14].
Pregnancy-associated plasma protein-A (PAPP-A) is a novel zinc metalloproteinase that regulates local insulin-like growth factor (IGF) action through cleavage of inhibitory IGF binding proteins, in particular IGFBP-4 [15]. PAPP-A is highly expressed in human and non-human primate pre-adipocytes from visceral fat [16, 17]. Using genome-wide expression profiling, PAPP-A was found to be one of the most distinctive genes expressed, with levels in preadipocytes from omental > mesenteric >> subcutaneous fat. We recently established that there is similar fat depot-specific expression of PAPP-A in mice [18]. PAPP-A mRNA expression was 7-fold higher in visceral (mesenteric) fat compared to subcutaneous (inguinal, subscapular) fat of mice. Thus, there was a preferential impact of PAPP-A deficiency in mice on a high fat diet to prevent increases in mesenteric adipocyte size and lipid accumulation, with no significant effect on subcutaneous fat depots. Although not analyzed in that study [18], we noted that PAPP-A knock-out (KO) mice on high fat diet had significantly decreased pericardial fat compared to wild-type (WT) mice. Is this fat surrounding the heart in mice visceral-like as with human epicardial fat? What is the role of PAPP-A? The present study was designed to address these important questions.
MATERIALS AND METHODS
Materials for cultures and assays:
Fetal bovine and calf serum, bovine insulin, IBMX, rosiglitizone, dexamethasone, Oil Red-O, crystal violet, methylene blue (MilliporeSigma St. Louis, MO); DMEM/F12, αMEM, penicillin, streptomycin, HBSS (Thermo Fisher Scientific, Waltham, MA); Collagenase type II (Worthington Biochemical Corporation, Lakewood, NJ); IGFII (R&D Systems, Minneapolis, MN); HEPES (Gibco, Waltham, MA); IGFBP-4 antibodies (Abcam, Cambridge, MA); Fluorescently-labeled secondary antibodies (LI-COR, Lincoln, NE); IgG2a (Bio X Cell, West Lebanon, NH). An ultrasensitive mouse PAPP-A ELISA and inhibitory PAPP-A monoclonal antibody (mAb-PA 1/41) were generous gifts of Ansh Laboratories (Webster, TX). Recombinant human IGFBP-4 and recombinant antibody that recognizes mouse PAPP-A in immunohistochemistry were kindly provided by Professor Claus Oxvig (Aarhus University, Denmark).
Mice.
Wild-type (WT) and PAPP-A KO littermates from matings of heterozygous mice on a mixed C57BL/6 and 129/SvE background were used in these studies. Genotyping was performed as previously described [18–20]. Mice were fed a high fat diet (HFD; 60% of calories from fat; Dyets, Inc, Bethlehem, PA) starting at four weeks postweaning and continuing for 20 weeks. Mice were housed five per cage (males and females in separate cages with a mix of WT and KO mice) in static autoclaved HEPA-ventilated micro-isolator cages measuring 446 square centimeters (27 × 16.5 × 15.5 cm). Cages were opened only within Class II biosafety cabinets. These cages were located in a standard pathogen-free facility. At the end of the experiments, the mice were weighed, and fat depots (mesenteric, pericardial, inguinal), heart, liver, and skeletal muscle were harvested, weighed, and processed for RNA, cell culture, immunohistochemistry or lipid content. All studies were reviewed and approved by the Institutional Animal Care and Use Committee of Mayo Clinic.
RNA isolation and real-time qPCR.
At harvest, fat depots were rapidly isolated, minced in Trizol (Life Technologies, Carlsbad, CA), snap frozen, and stored at −80°C. Once thawed on ice, samples were homogenized by passing fat tissue through a 21-gauge needle several times. Total RNA was then isolated, reversed transcribed with the SuperScript II First-Strand Synthesis System (Life Technologies), and mRNA expression was evaluated by quantitative real-time PCR using the iCycler iQ5 Detection System with iQ SYBR green PCR Master Mix (Bio-Rad, Hercules, CA). Amplification plots were analyzed with iQ5 Optimal System Software version 2.1 (Bio-Rad). Statistical significance of gene expression was determined by the Pfaffl method using (REST)2009 (Qiagen, Valencia, CA). The primer sequences for mouse genes differentially expressed in subcutaneous and visceral adipose tissue (HoxC9, Nrf2, PAPP-A), and the reference gene, TBP, are listed in Table 1.
Table 1.
Primer sets for real-time qPCR
| Forward | Reverse | |
|---|---|---|
| Mouse Hox C9 | cagcaagcacaaagaggaga | cgacggtccctggttaaatac |
| Mouse NrF2 | caacctgcgctgcaaacc | cattttctgaccagacacga |
| Mouse PAPP-A | gccgtgggagcaatatc | gatgccacactctgaccctat |
| Mouse TBP | ctcagttcacaggtggcagca | cagcacagagcaagcaactc |
Adipocyte size and lipid content.
Fresh samples of pericardial and inguinal fat were digested with collagenase in HEPES in a 37°C shaking water bath for 8–10 minutes or until fat globules were no longer present. Following centrifugation and methylene blue staining, size of the dispersed adipocytes was determined using a Nikon Labophot-2 with Coolpix camera and customized software, Analyze/Cell Counting and Application Processing [21]. Circularity, diameter, and area measurements were computed. Images were analyzed until ~ 300 cells were sized.
Fat, liver, heart and skeletal muscle were analyzed for percent lipid, as described [18].
Immunohistochemistry.
Formalin-fixed, paraffin-embedded adipose tissue was processed and immunostained for PAPP-A, using a previously described recombinant antibody that recognizes mouse PAPP-A [22], with the following modification: antigen retrieval in steamed citrate buffer pH 6.0 for 25 minutes was used. We found this to improve sensitivity, allowing reduction of the primary antibody concentration to 0.2 μg/ml. Color was visualized using ImmPACT NovaRed (Vector Laboratories, Burmingame, CA) per manufacturer’s instructions. Gill’s hematoxylin was used for counterstain.
Pre-adipocyte cell culture and differentiation.
Pre-adipocytes were isolated from pericardial and inguinal fat as previously described [23]. Tissue was minced and digested with collagenase in HBSS for 30 minutes at 37°C, vortexing every 10 minutes. The solution was filtered through 100 μm nylon cell strainer, and centrifuged at 1000 × g for 10 minutes at room temperature. Preadipocytes in the pellet were plated in αMEM with 10% heat-inactivated calf serum plus penicillin/streptomycin, and cultured in a humidified incubator set at 3% O2/5% CO2. Cells up to passage 5 were used in the experiments. For in vitro PAPP-A expression, 72-hour conditioned medium (1% calf serum) was collected, and cell number determined by hemocytometer. For in vitro differentiation, pre-adipocytes at 85–90% confluency were changed to DMEM/F12 containing 10% FBS, 1 μg/ml insulin, 250 nM dexamethasone, 0.5 mM IBMX and 2.5 μM rosiglitazone, and then transferred to an incubator with 20% O2/5% CO2. After 48 hours the medium was replaced with DMEM/F12 containing 10% FBS and 1 μg/ml insulin. Medium was changed every other day. At day 4, 6 and 8, cells were treated with Oil Red-O, to stain neutral lipids, which was eluted with isopropanol and absorbance read at 490–500 nm. Cells were then washed with phosphate-buffered saline, stained with crystal violet to determine viable cell number, eluted with 10% acetic acid, and absorbance read at 590 nm. Results are expressed as μg/ml Oil Red-O (from standard curve) per OD reading of crystal violet.
PAPP-A.
Ultrasensitive ELISA kits that measure mouse PAPP-A were used to determine levels of PAPP-A in pre-adipocyte conditioned media. For proteolysis assays, human IGFBP-4 precomplexed to IGF-II was incubated in cell-free conditioned medium without and with inhibitory PAPP-A monoclonal antibody (mAb-PA 1/41) for 4 hours at 37°C. IGF must be bound to IGFBP-4 for IGFBP-4 to be a substrate for PAPP-A proteolysis [24, 25]. mAb-PA 1/41 specifically inhibits PAPP-A-mediated IGFBP-4 proteolysis [26]. Western blotting was performed as previously described [26]. Briefly, samples were separated by 12% SDS-PAGE, blotted onto a PVDF membrane, blocked, and probed for IGFBP-4 fragments using specific N- and C-terminal antibodies. Fluorescently-labeled secondary antibodies were used for detection of intact and cleaved IGFBP-4, images were captured using a LI-COR Odyssey scanner, and intensities measured using ImageJ soft-ware.
Statistical analysis.
Results are expressed as mean ± SEM. We used paired t-tests to compare pericardial and inguinal samples from the same mouse, and unpaired t-test for two group comparisons. For multiple comparisons to a single control, we used ANOVA followed by Dunnett’s test. Significance was set at P < 0.05.
RESULTS
Characterization of mouse pericardial fat.
Visceral and subcutaneous fat cells in humans and mice can be defined by their distinct molecular signatures. To determine if mouse pericardial fat derives from a visceral-like lineage, we compared mRNA expression in pericardial fat to that of mesenteric fat (visceral) and inguinal fat (subcutaneous) from WT mice on chow diet. We chose genes identified as being preferentially expressed in subcutaneous or visceral fat [27]. Real-time qPCR analyses are presented in Figure 1.
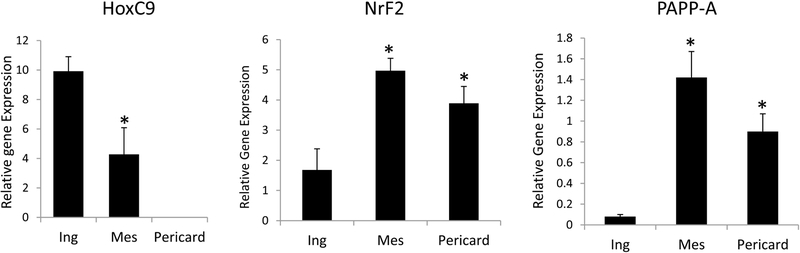
Figure 1.
Differential mRNA expression in inguinal (Ing), mesenteric (Mes), and pericardial (Pericard) fat depots from WT mice
HoxC9: subcutaneous > visceral fat
NrF2, PAPP-A: visceral > subcutaneous fat
Results are mean ± SEM of N = 8 male mice
*Significantly different from inguinal.
HoxC9 is known to be elevated in subcutaneous compared to visceral fat, and expression in mesenteric fat was less than 50% of the expression in inguinal fat (P = 0.014). Interestingly, there was no detectable HoxC9 expression in pericardial fat. NrF2 is known to be elevated in visceral compared to subcutaneous fat, and was found to be elevated 2- to 3-fold in pericardial and mesenteric fat compared to inguinal fat (P < 0.0001). Tchkonia et al. reported PAPP-A as being differentially expressed in human pre-adipocytes, with expression levels being markedly elevated in cells from omental and mesenteric fat compared to cells from subcutaneous fat [16]. We previously reported elevated PAPP-A expression in mouse mesenteric fat compared to inguinal fat depots [18]. As shown in Figure 1, PAPP-A expression was elevated ~10-fold in pericardial and mesenteric fat compared to inguinal fat (P = 0.0003).
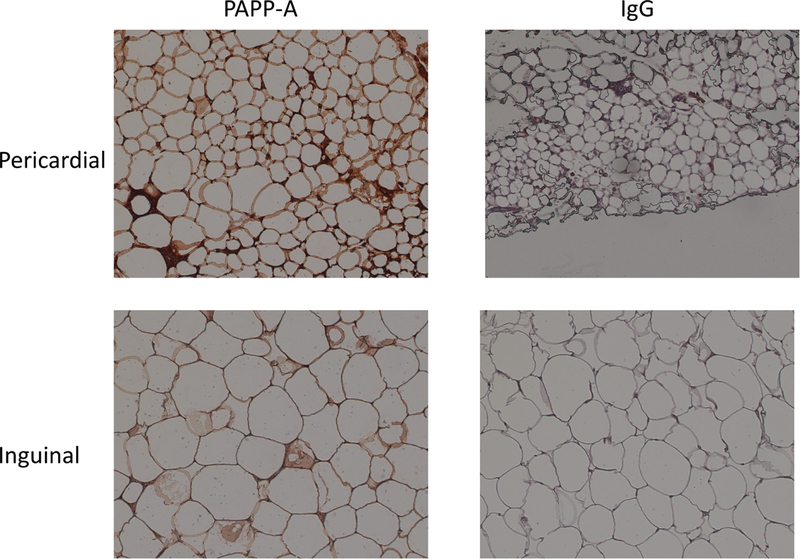
Adipocyte size was also consistent with visceral fat, mouse pericardial adipocytes being significantly smaller (2532 ± 24 μm2) than inguinal adipocytes (4924 ± 72 μm2) (P < 0.0001), and similar in size to mouse mesenteric adipocytes [18]. Human epicardial adipocytes are also smaller than subcutaneous adipocytes [28]. Immunohistochemistry using a monoclonal antibody that detects mouse PAPP-A [22] indicated intense staining associated with the stromal vascular portion of the pericardial fat compared to inguinal fat (Fig. 2). Adipocyte size differences between the depots are also apparent in this figure.
Figure 2.
Immunohistochemistry for mouse PAPP-A in inguinal and pericardial fat depots from WT mice
IgG: control for non-specific staining
Photographs were taken at 20X
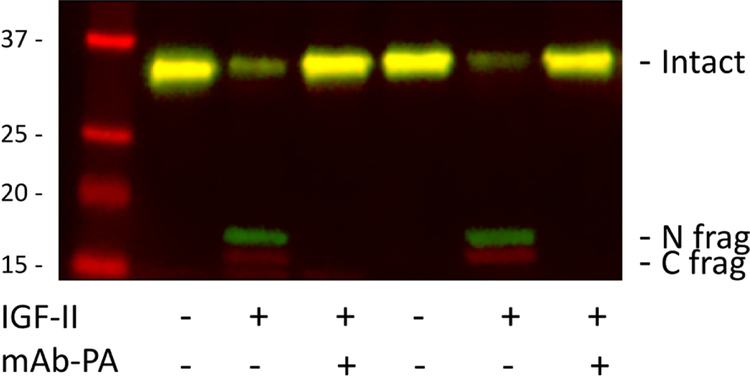
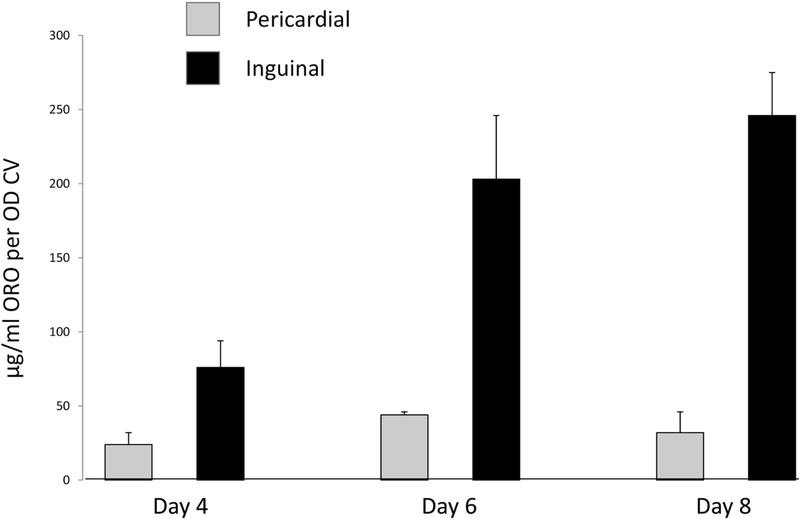
Pre-adipocytes were isolated from pericardial and inguinal fat from WT mice on chow diet, and established in culture. Pre-adipocytes isolated from pericardial fat secreted twice as much PAPP-A into 72-hour conditioned medium than pre-adipocytes from inguinal fat, 39 ± 7 and 20 ± 5 μg/ml per 105 cells, repectively. These results are similar to what was seen in human preadipocytes from visceral compared to subcutaneous fat [29]. This secreted PAPP-A was proteolytically active as evidenced by loss of intact and generation of IGFBP-4 fragments with the addition of IGF-II (Fig. 3). IGF needs to be bound to the substrate, IGFBP-4, for proteolysis by PAPP-A to proceed [24, 25]. Without the addition of IGF-II, there was no proteolysis. Pre-incubation with mAb-PA 1/41 before the addition of IGF-II completely inhibited IGFBP-4 proteolysis, indicating the specificity of the enzymatic reaction. Furthermore as shown in Figure 4, pericardial pre-adipocytes were markedly resistant to experimentally-induced differentiation compared to inguinal pre-adipocytes (P < 0.0001). This resistance to differentiation is a hallmark of visceral fat pre-adipocytes [30].
Figure 3.
PAPP-A-mediated IGFBP-4 proteolysis in pericardial pre-adipocyte conditioned medium
Seventy-two hour conditioned medium was incubated for 4 hours at 37°C with IGFBP-4 without (−) or with (+) pre-complexing to IGF-II, and without (−) or with (+) the addition of inhibitory mAb-PA 1/41. Samples were processed by Western blot. Conditioned medium from two separate pre-adipocyte isolations are shown.
Intact IGFBP-4 and N- and C-terminal fragments are indicated on the right.
Molecular weight markers are indicated on the left.
Figure 4.
Pre-adipocyte differentiation in vitro
Cultured pre-adipocytes isolated from pericardial and inguinal fat depots from WT mice were induced to differentiate as described in Materials and Methods. At day 4, 6 and 8, cells were stained with Oil Red-O for lipid droplets and with crystal violet for viable cells. Results (expressed as μg/ml Oil Red-O per OD crystal violet) are mean + SEM of three separate cell isolations from 1 male and 2 female mice.
Thus, the depot-specific expression data, small adipocyte size, and markedly reduced response to in vitro differentiation are consistent with a visceral lineage for pericardial fat cells. Furthermore, PAPP-A is highly expressed in pericardial adipose tissue and it is proteolytically active.
PAPP-A KO mice.
PAPP-A KO and WT littermates were started on a HFD (60% of calories from fat) starting at 7 weeks of age and continuing for 20 weeks. Tissue weights after 20 weeks HFD are presented in Table 2. Both male and female PAPP-A KO mice had a striking 50–60% reduction in pericardial fat weight compared to WT mice (males P = 0.0003; females P = 0.0002). There were no significant differences in tissue weight in the other fat depots, or in liver and quadriceps muscle. In females, the heart was slightly but significantly heavier in PAPP-A KO than in WT mice. When the data are expressed as pericardial weight to heart weight, in male PAPP-A KO mice it was 39 ± 4% and in female PAPP-A KO mice it was 46 ± 4% compared to WT (set as 100%). Using body weight as a covariate in the analysis, we found an R2 = 0.84 with P < 0.0001.
Table 2.
Tissue weights of PAPP-A KO mice
| % of WT | ||
|---|---|---|
| Males | Females | |
| Pericardial fat | 40 ± 6** | 51 ± 10** |
| Inguinal fat | 84 ± 6 | 101 ± 7 |
| Mesenteric fat | 87 ± 7 | 73 ± 7 |
| Liver | 106 ± 9 | 105 ± 7 |
| Heart | 106 ± 6 | 111 ± 2* |
| Skeletal muscle | 111 ± 5 | 109 ± 6 |
PAPP-A KO and WT litter-mates were fed a HFD for 20 weeks. Tissue weights were divided by body weight prior to the start of HFD to take into account the smaller size of PAPP-A KO mice compared to WT littermates [20] and females compared to males. These values were expressed as % of WT (set as 100%). Results are mean ± SEM of N = 7–10 mice.
P < 0.05;
P = 0.0002
Adipocyte size was reduced in pericardial fat from PAPP-A KO mice compared to WT mice on HFD (1953 ± 102 vs. 3363 ± 441 μm2; P = 0.009). However, adipocyte size in inguinal fat was not significantly different in the two groups (5216 ± 354 vs. 6435 ± 617 μm2; P = 0.092).
Percent lipid per gram tissue was also determined (Table 3). There were no significant differences between WT and PAPP-A KO mice in percent lipid in the fat depots, heart and skeletal muscle. However, there was significantly less lipid in the liver of male and female PAPP-A KO mice (males P = 0.005; females P = 0.027).
Table 3.
Tissue lipid of PAPP-A KO and WT mice
| % Lipid | ||||
|---|---|---|---|---|
| Males | Females | |||
| WT | KO | WT | KO | |
| Fat Depot | ||||
| – Pericardial | 72 ± 2.5 | 69 ± 4.2 | 77 ± 1.6 | 77 ± 2.9 |
| – Inguinal | 76 ± 0.9 | 79 ± 1.9 | 76 ± 2.2 | 75 ± 1.4 |
| – Mesenteric | 78 ± 2.4 | 79 ± 2.3 | 76 ± 2.1 | 75 ± 0.9 |
| Liver | 14 ± 1.5 | 8 ± 0.4* | 17 ± 1.7 | 12 ± 0.8* |
| Heart | 8 ± 0.8 | 9 ± 1.4 | 9 ± 0.6 | 9 ± 0.8 |
| Skeletal muscle | 15 ± 1.3 | 12 ± 1.2 | 15 ± 2.0 | 12 ± 1.4 |
PAPP-A KO and WT litter-mates were fed a HFD for 20 weeks. Results are mean ± SEM of N = 7–9 mice.
P < 0.05
We performed a small pilot study with the PAPP-A immunoneutralizing monoclonal antibody [26] to complement the genetic loss of PAPP-A expression with loss of PAPP-A proteolytic activity. Mice (female; N = 6 in each group) were fed HFD for five weeks and then administered mAb-PA 1/41 or isotype control antibody (IgG2a) once a week at 30 mg/kg body weight for 10 additional weeks while continuing on HFD. Mice receiving mAb-PA had a 25% reduction in pericardial fat depot weight compared to control mice (0.28 ± 0.027 and 0.38 ± 0.045 percent of starting weight), but likely due to the small numbers this difference did not reach statistical difference (P = 0.08). There was no difference between groups for inguinal fat depot weights (4.4 ± 0.36 and 4.1 ± 0.27) or heart (0.50 ± 0.014 and 0.55 ± 0.024).
DISCUSSION
In this study, we provide the following evidence that pericardial adipose tissue is a visceral fat depot in mice: (1) Genes associated with visceral fat were significantly elevated in pericardial (and mesenteric) fat compared to subcutaneous (inguinal) fat. Conversely, a gene known to be elevated in subcutaneous fat [27] was undetectable in pericardial fat. (2) Small adipocyte size in the pericardial fat depot is consistent with visceral fat [18, 28]. (3) In vitro differentiation of preadipocytes from pericardial fat was markedly impeded compared to pre-adipocytes from inguinal fat. This resistance to differentiation is seen in pre-adipocytes from human visceral fat [30]. (4) PAPP-A, which is over-expressed in visceral fat compared to subcutaneous fat in humans, non-human primates and mice [16–18, 29], was elevated in pericardial fat. It is of note that confusion in terminology used to define cardiac fat in humans, also appears to apply to mice and rats. Thus, studies of purported ‘epicardial’ fat in mice and rats seem to be referring to the fat surrounding the heart not attached to it, as indicated by the schematics and photos of the fat depot studied [31, 32]. Interestingly, the study of Chau et al. [31] indicated that fat surrounding the mouse heart had markers of visceral fat progenitors. Thus, mouse pericardial fat is a visceral fat depot that may be relevant to human epicardial fat.
In this study, we further show that PAPP-A plays a role in the regulation of pericardial adipose tissue. PAPP-A mRNA expression was significantly elevated in pericardial fat compared to inguinal fat. Pre-adipocytes from pericardial fat secreted two-fold more PAPP-A into the culture media than did inguinal pre-adipocytes. Secreted PAPP-A can cell-associate through proteoglycan binding, and PAPP-A in the conditioned medium may be only the “tip of the iceberg” [33, 34]. In conditioned media (and whole cell lysates, data not shown), PAPP-A was proteolytically active, cleaving IGFBP-4 into two fragments with markedly reduced affinity for IGFs. This is the primary mechanism by which PAPP-A enhances local IGF bioavailability for receptor activation [15]. PAPP-A is expressed primarily by pre-adipocytes in fat tissue.
Adipocytes and macrophages do not express PAPP-A ([34] and our unpublished results), but secretory products from pre-adipocytes, such as PAPP-A, and the byproducts of its proteolytic activity e.g., ‘free’ IGF-I, can have paracrine effects on adipocytes and other cells in the stromal vascular fraction of adipose tissue.
We have previously shown that PAPP-A expression is higher in visceral fat compared to subcutaneous fat in mice. Therefore, the impact of PAPP-A deficiency in mice on HFD was on visceral fat, with a prevention of increased adipocyte size and lipid accumulation in the mesenteric fat, but not the subcutaneous fat depot weight and adipocyte size was reduced in pericardial fat from compared to WT mice on HFD [18]. In this study we had similar findings, in that PAPP-A KO mice. These differences were not seen in the inguinal fat depot. Interestingly, the adipocyte size and frequency histograms indicated bimodal peaks in several of the inguinal fat depots from WT, but not PAPP-A KO, mice on HFD (Supplemental Fig.1). The smaller size peak in mice corresponded to the peak adipocyte size in mice on chow diet, suggesting recruitment of new adipocytes. High fat feeding can induce adipose tissue hyperplasia to meet the need for additional fat storage capacity [35, 36]. We did not see this in our previous study [18], but that may be explained by the higher fat content and the 20 week duration of the present study.
In humans, epicardial fat thickness is associated with heart dysfunctions and also fatty liver disease [8–11]. PAPP-A KO mice on HFD had significantly reduced liver lipid. It will be of interest to determine if PAPP-A KO mice on HFD have improved heart function.
Preliminary findings using an inhibitory monoclonal antibody against PAPP-A, suggest PAPP-A as a possible therapeutic target for visceral fat accumulation. We have shown that this antibody can inhibit atherosclerotic plaque progression in mice [37]. A proof-of-principle study using this antibody is warranted, but a small molecule inhibitor of PAPP-A would be preferable for long-term preventative strategies.
Further studies are necessary to delineate the mechanism of PAPP-A in visceral fat. Although PAPP-A is highly expressed in pre-adipocytes in mouse pericardial fat, its effects are likely to be paracrine, e.g., on adipocytes and other cells in the stromal vascular compartment of the fat depot and, hypothetically, the heart. Furthermore, once the cell targets are identified, it will need to be confirmed that the effects of PAPP-A are IGF-dependent. Elevated circulating levels of IGF-I appear to be cardio-protective, and decreases in circulating IGF-I can have detrimental effects on the structure and function of the heart [38, 39]. It is of note that PAPP-A acts locally to regulate IGF action, and PAPP-A KO mice have normal circulating levels of IGF-I [19].
Results of these studies are foundational for establishing this new mouse model and its value for future mechanistic studies of cardiac fat. Indeed, it may turn out to be important for general study of visceral fat in mice, since mesenteric fat, with its attachment to the intestine, is nearly impossible to isolate in a sterile manner for culture. Further studies are necessary to determine the mechanism of PAPP-A’s effect on pericardial fat and its potential impact on heart function.
Supplementary Material
Supplemental Fig. 1. Adipocyte size distribution and frequency
Examples of inguinal adipocyte histograms of male WT mice 20 weeks on (A) HFD and (B) chow diet. Bimodal distribution in adipocyte size was seen in ~ 15% of inguinal adipocyte histograms from WT mice on HFD and none from PAPP-A KO mice on HFD.
Highlights.
Mouse pericardial fat has the characteristics of visceral adipose tissue
PAPP-A expression was significantly elevated in pericardial fat compared to subcutaneous fat
Pre-adipocytes from pericardial fat secreted proteolytically active PAPP-A
PAPP-A KO mice on high fat diet had significantly reduced pericardial fat compared to wild-type mice
Acknowledgements –
The authors would like to thank Hanne Lucier for help in formatting this manuscript.
Funding –
This work was supported in part by the National Institute of Health (R01 AG28141 to CAC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Please send all reprint requests to the corresponding author.
Declarations of interest – none
Disclosure statement: Dr. Cheryl A. Conover, who is co-authoring this paper, also serves as editor of Growth Hormone and IGF Research. However, this has not influenced the handling of the paper, which has been subjected to the Journal’s usual procedures. Thus, the peer review process has been handled independently of Dr. Conover, who has been blinded to the review process.
REFERENCES:
- [1].Sacks HS, Fain JN, Human epicardial adipose tissue: a review, Am Heart J, 153 (2007) 907–917. [DOI] [PubMed] [Google Scholar]
- [2].Kaushik M, Reddy YM, Distinction of “fat around the heart”, J Am Coll Cardiol, 58 (2011) 1640; author reply 1640–1641. [DOI] [PubMed] [Google Scholar]
- [3].Iacobellis G, Local and systemic effects of the multifaceted epicardial adipose tissue depot, Nat Rev Endocrinol, 11 (2015) 363–371. [DOI] [PubMed] [Google Scholar]
- [4].Fitzgibbons TP, Czech MP, Epicardial and perivascular adipose tissues and their influence on cardiovascular disease: basic mechanisms and clinical associations, J Am Heart Assoc, 3 (2014) e000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Talman AH, Psaltis PJ, Cameron JD, Meredith IT, Seneviratne SK, Wong DT, Epicardial adipose tissue: far more than a fat depot, Cardiovasc Diagn Ther, 4 (2014) 416–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Iacobellis G, Assael F, Ribaudo MC, Zappaterreno A, Alessi G, Di Mario U, Leonetti F, Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction, Obes Res, 11 (2003) 304–310. [DOI] [PubMed] [Google Scholar]
- [7].Iacobellis G, Ribaudo MC, Assael F, Vecci E, Tiberti C, Zappaterreno A, Di Mario U, Leonetti F, Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk, J Clin Endo Metab, 88 (2003) 5163–5168. [DOI] [PubMed] [Google Scholar]
- [8].Iacobellis G, Lonn E, Lamy A, Singh N, Sharma AM, Epicardial fat thickness and coronary artery disease correlate independently of obesity, Int J Cardiol, 146 (2011) 452–454. [DOI] [PubMed] [Google Scholar]
- [9].Iacobellis G, Barbarini G, Letizia C, Barbaro G, Epicardial fat thickness and nonalcoholic fatty liver disease in obese subjects, Obesity (Silver Spring), 22 (2014) 332–336. [DOI] [PubMed] [Google Scholar]
- [10].Iwayama T, Nitobe J, Watanabe T, Ishino M, Tamura H, Nishiyama S, Takahashi H, Arimoto T, Shishido T, Miyashita T, Miyamoto T, Toyama S, Sadahiro M, Kubota I, Role of epicardial adipose tissue in coronary artery disease in non-obese patients, J Cardiol, 63 (2014) 344–349. [DOI] [PubMed] [Google Scholar]
- [11].Thanassoulis G, Massaro JM, O’Donnell CJ, Hoffmann U, Levy D, Ellinor PT, Wang TJ, Schnabel RB, Vasan RS, Fox CS, Benjamin EJ, Pericardial fat is associated with prevalent atrial fibrillation: the Framingham Heart Study, Circ Arrhythm Electrophysiol, 3 (2010) 345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Marchington JM, Mattacks CA, Pond CM, Adipose tissue in the mammalian heart and pericardium: structure, foetal development and biochemical properties, Comp Biochem Physiol B, 94 (1989) 225–232. [DOI] [PubMed] [Google Scholar]
- [13].Yamaguchi Y, Cavallero S, Patterson M, Shen H, Xu J, Kumar SR, Sucov HM, Adipogenesis and epicardial adipose tissue: a novel fate of the epicardium induced by mesenchymal transformation and PPARgamma activation, Proc Natl Acad Sci U S A, 112 (2015) 2070–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nakatani T, Shinohara H, Fukuo Y, Morisawa S, Matsuda T, Pericardium of rodents: pores connect the pericardial and pleural cavities, Anat Rec, 220 (1988) 132–137. [DOI] [PubMed] [Google Scholar]
- [15].Conover CA, Key questions and answers about pregnancy-associated plasma protein-A, Trends Endocrinol Metab, 23 (2012) 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tchkonia T, Lenburg M, Thomou T, Giorgadze N, Frampton G, Pirtskhalava T, Cartwright A, Cartwright M, Flanagan J, Karagiannides I, Gerry N, Forse RA, Tchoukalova Y, Jensen MD, Pothoulakis C, Kirkland JL, Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns, A J Physiol Endo Metab, 292 (2007) E298–307. [DOI] [PubMed] [Google Scholar]
- [17].Tchoukalova YD, Nathanielsz PW, Conover CA, Smith SR, Ravussin E, Regional variation in adipogenesis and IGF regulatory proteins in the fetal baboon, Biochem Biophys Res Comm, 380 (2009) 679–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Conover CA, Harstad SL, Tchkonia T, Kirkland JL, Preferential impact of pregnancy-associated plasma protein-A deficiency on visceral fat in mice on high-fat diet, Am J Physiol: Endo Metab, 305 (2013) E1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Conover CA, Bale LK, Mader JR, Mason MA, Keenan KP, Marler RJ, Longevity and age-related pathology of mice deficient in pregnancy-associated plasma protein-A, J Gerontol A Biol Sci Med Sci, 65 (2010) 590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Conover CA, Bale LK, Overgaard MT, Johnstone EW, Laursen UH, Fuchtbauer E-M, Oxvig C, van Deursen J, Metalloproteinase pregnancy-associated plasma protein A is a critical growth regulatory factor during fetal development, Development, 131 (2004) 1187–1194. [DOI] [PubMed] [Google Scholar]
- [21].Tchoukalova YD, Harteneck DA, Karwoski RA, Tarara J, Jensen MD, A quick, reliable, and automated method for fat cell sizing, J Lipid Res, 44 (2003) 1795–1801. [DOI] [PubMed] [Google Scholar]
- [22].Mikkelsen JH, Steffensen LB, Oxvig C, Development of a recombinant antibody towards PAPP-A for immunohistochemical use in multiple animal species, J Immunol Methods, 404 (2014) 33–40. [DOI] [PubMed] [Google Scholar]
- [23].Stout MB, Tchkonia T, Pirtskhalava T, Palmer AK, List EO, Berryman DE, Lubbers ER, Escande C, Spong A, Masternak MM, Oberg AL, LeBrasseur NK, Miller RA, Kopchick JJ, Bartke A, Kirkland JL, Growth hormone action predicts age-related white adipose tissue dysfunction and senescent cell burden in mice, Aging (Albany NY), 6 (2014) 575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Qin X, Byun D, Lau K-HW, Baylink DJ, Mohan S, Evidence that the interaction between insulin-like growth factor (IGF)-II and IGF binding protein (IGFBP)-4 is essential for the action of the IGF-II-dependent IGFBP-4 protease, Arch Biochem Biophys, 379 (2000) 209–216. [DOI] [PubMed] [Google Scholar]
- [25].Laursen LS, Overgaard MT, Soe R, Boldt HB, Sottrup-Jensen L, Giudice LC, Conover CA, Oxvig C, Pregnancy-associated plasma protein-A (PAPP-A) cleaves insulin-like growth factor binding protein (IGFBP)-5 independent of IGF: implications for the mechanism of IGFBP-4 proteolysis by PAPP-A, FEBS Lett, 504 (2001) 36–40. [DOI] [PubMed] [Google Scholar]
- [26].Mikkelsen JH, Resch ZT, Kalra B, Savjani G, Kumar A, Conover CA, Oxvig C, Indirect targeting of IGF receptor signaling in vivo by substrate-selective inhibition of PAPP-A proteolytic activity, Oncotarget, 5 (2014) 1014–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gesta S, Tseng YH, Kahn CR, Developmental origin of fat: tracking obesity to its source, Cell, 131 (2007) 242–256. [DOI] [PubMed] [Google Scholar]
- [28].Bambace C, Telesca M, Zoico E, Sepe A, Olioso D, Rossi A, Corzato F, Di Francesco V, Mazzucco A, Santini F, Zamboni M, Adiponectin gene expression and adipocyte diameter: a comparison between epicardial and subcutaneous adipose tissue in men, Cardiovasc Pathol, 20 (2011) e153–156. [DOI] [PubMed] [Google Scholar]
- [29].Davidge-Pitts C, Escande CJ, Conover CA, Preferential expression of PAPPA in human preadipocytes from omental fat, J Endocrinol, 222 (2014) 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tchkonia T, Thomou T, Zhu Y, Karagiannides I, Pothoulakis C, Jensen MD, Kirkland JL, Mechanisms and metabolic implications of regional differences among fat depots, Cell Metab, 17 (2013) 644–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chau YY, Bandiera R, Serrels A, Martinez-Estrada OM, Qing W, Lee M, Slight J, Thornburn A, Berry R, McHaffie S, Stimson RH, Walker BR, Chapuli RM, Schedl A, Hastie N, Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source, Nat Cell Biol, 16 (2014) 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bohm C, Benz V, Clemenz M, Sprang C, Hoft B, Kintscher U, Foryst-Ludwig A, Sexual dimorphism in obesity-mediated left ventricular hypertrophy, Am J Physiol Heart Circ Physiol, 305 (2013) H211–218. [DOI] [PubMed] [Google Scholar]
- [33].Laursen LS, Overgaard MT, Weyer K, Boldt HB, Ebbesen P, Christiansen M, Sottrup-Jensen L, Giudice LC, Oxvig C, Cell surface targeting of pregnancy-associated plasma protein A proteolytic activity. Reversible adhesion is mediated by two neighboring short consensus repeats, J Biol Chem, 277 (2002) 47225–47234. [DOI] [PubMed] [Google Scholar]
- [34].Conover CA, Harrington SC, Bale LK, Oxvig C, Surface association of pregnancy-associated plasma protein-A accounts for its colocalization with activated macrophages, Am J Physiol Heart Circ Physiol, 292 (2007) H994–H1000. [DOI] [PubMed] [Google Scholar]
- [35].Jo J, Gavrilova O, Pack S, Jou W, Mullen S, Sumner AE, Cushman SW, Periwal V, Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth, PLoS Comput Biol, 5 (2009) e1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gealekman O, Gurav K, Chouinard M, Straubhaar J, Thompson M, Malkani S, Hartigan C, Corvera S, Control of adipose tissue expandability in response to high fat diet by the insulin-like growth factor-binding protein-4, J Biol Chem, 289 (2014) 18327–18338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Conover CA, Bale LK, Oxvig C, Targeted inhibition of pregnancy-associated plasma protein-A activity reduces atherosclerotic plaque burden in mice, J Cardiovasc Transl Res, 9 (2016) 77–79. [DOI] [PubMed] [Google Scholar]
- [38].Scharin Tang M, Redfors B, Lindbom M, Svensson J, Ramunddal T, Ohlsson C, Shao Y, Omerovic E, Importance of circulating IGF-1 for normal cardiac morphology, function and post infarction remodeling, Growth Horm IGF Res, 22 (2012) 206–211. [DOI] [PubMed] [Google Scholar]
- [39].Prele CM, Reichelt ME, Mutsaers SE, Davies M, Delbridge LM, Headrick JP, Rosenthal N, Bogoyevitch MA, Grounds MD, Insulin-like growth factor-1 overexpression in cardiomyocytes diminishes ex vivo heart functional recovery after acute ischemia, Cardiovasc Pathol, 21 (2012) 17–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1. Adipocyte size distribution and frequency
Examples of inguinal adipocyte histograms of male WT mice 20 weeks on (A) HFD and (B) chow diet. Bimodal distribution in adipocyte size was seen in ~ 15% of inguinal adipocyte histograms from WT mice on HFD and none from PAPP-A KO mice on HFD.