Abstract
We previously made a RNAi-based Cyp2b-knockdown (Cyp2b-KD) mouse to determine the in vivo role of the Cyp2b subfamily in xenobiotic detoxification. Further studies reported here indicate a role for Cyp2b in unsaturated fatty acid (UFA) metabolism and in turn obesity. Mice were treated i.p. with 100 μl corn oil as a carrier or the potent Cyp2b-inducer TCPOBOP (TC) dissolved in corn oil. Surprisingly, female Cyp2b-KD mice but not male mice showed increased liver lipid accumulation. Male Cyp2b-KD mice had higher serum triacylglycerols, cholesterol, VLDL, LDL, and HDL than wildtype (WT) mice; females had higher cholesterol, LDL, and HDL. Thus, Cyp2b-KD mice are unable to clear a high bolus dose of corn oil, potentially because the Cyp2b-KD mice were unable to metabolize the UFA in the corn oil. Therefore, WT and Cyp2b-KD mice were housed for 35 weeks and necropsies performed to test whether Cyp2b-KD mice develop age onset obesity. Cyp2b-KD mice exhibited a significant increase in body weight caused by an increase in white adipose tissue deposition relative to WT mice. Serum cholesterol, triacylglycerol, LDL, and VLDL were significantly greater in 35-week old Cyp2b-KD males compared to WT males; only serum triacylglycerol and LDL were higher in females. In conclusion, changes in Cyp2b expression led to perturbation in lipid metabolism and depuration in Cyp2b-KD mice. This suggests that Cyp2b is more than a detoxification enzyme, but also involved in the metabolism of UFA, as Cyp2b-KD mice have increased body weight, fat deposition and serum lipids.
Introduction
Cyp2b members participate in the metabolism of endogenous and exogenous compounds. Xenobiotic chemicals metabolized by Cyp2b isoforms include parathion, efavirenz, chlorpyrifos, phenobarbital, nonylphenol, some PCBs, and DDT (Foxenberg et al., 2007; Hodgson and Rose, 2007; Lee et al., 1998). Evidence suggests that several endogenous chemicals are also metabolized by CYP2B members including steroid hormones, prostaglandins, and fatty acids (Du et al., 2005; Finn et al., 2009; Waxman, 1988). For example, Cyp2b’s are potent arachidonic acid epoxygenases (El-Sherbeni et al., 2013; Keeney et al., 1998a). Cyp2b19 in mouse and CYP2B12 in rat are primarily found in keratinocytes and important in 14,15-epoxyeicosatrienoic acid formation, which is a key factor in epithelial cornification (Du et al., 2005; Keeney et al., 1998b).
Cyp2b’s are widely expressed. They are expressed in the kidney (Jarukamjorn et al., 2001), small intestine (Zhang et al., 2003), brain (Hersman and Bumpus, 2014; Johri et al., 2007), lungs (Hersman and Bumpus, 2014), heart (Isensee et al., 2007), testes, skeletal muscle (Finger et al., 2011), skin (Du et al., 2005; Keeney et al., 1998b), adipose (Yoshinari et al., 2004), and prostate (Kumagai et al., 2007). Five Cyp2b isoforms have been identified in mice (Cyp2b9, 2b10, 2b13, 2b19, and 2b23) compared to only one in humans (CYP2B6)(Kumar et al., 2017). However, only three Cyp2b isoforms are primarily expressed in the liver with Cyp2b9, Cyp2b10, and to a lesser extent Cyp2b13 being the major hepatic Cyp2b isoforms (Mota et al., 2010; Peng et al., 2012). Cyp2b19 is primarily expressed in skin (Du et al., 2005; Keeney et al., 1998b) and until recently, no study had established that Cyp2b23 is expressed and this recent study indicates very low expression of Cyp2b23 in the liver and only in mice younger than 21-days old (Peng et al., 2012).
The hepatic CYP also show sexual dimorphism as female mice express more Cyp2b9 and Cyp2b13 than males (Hernandez et al., 2009b; Wiwi et al., 2004). The sexually dimorphic expression of Cyp2b members is probably regulated by several transcription factors including hepatocyte nuclear factor 4α (HNF-4α) (Wiwi et al., 2004), Forkhead box A2 (Foxa2) (Hashita et al., 2008), and the constitutive androstane receptor (CAR) (Hernandez et al., 2009b; Mota et al., 2010). Of special interest are CAR (NR1I3) and Foxa2. CAR is a xenobiotic sensor that regulates the induction of Cyp2b10, and several other detoxification genes after exposure to a variety of chemicals, including phenobarbital or 1,4-bis[2-(3,5-dichloropyridyloxy)] benzene (TC) (Hernandez et al., 2009a; Honkakoski et al., 1998; Wei et al., 2000). Foxa2, which regulates Cyp2b9 expression, has been implicated in age-onset obesity and sporadic cases of early onset Type II diabetes (Bochkis et al., 2013). Foxa2 is activated by fasting, fatty acids, and bile acids and inhibited by insulin (Bochkis et al., 2008; Wolfrum et al., 2004). Fatty liver and diabetes activate both CAR and Foxa2 and increase drug metabolism due in part to increased Cyp2b9 and Cyp2b10 (Dong et al., 2009a; Wolfrum et al., 2004). Interestingly recent data showed that Cyp2b9 was the most highly induced gene in liver of diet-induced obese mice (Leung et al., 2016).
CAR much like Foxa2 is also a metabolic sensor. For example, TC-activation of CAR ameliorated diabetic activity and fatty liver disease in ob/ob mice, and double mutants (ob/ob mice that are also CAR-null) did not respond to TC-mediated amelioration of diabetes and fatty liver, indicating a role for CAR in inducing β-oxidation (Dong et al., 2009b). This provides a link between drug metabolism and energy metabolism. Additionally, the hepatic P450 oxidoreductase-null mouse (hepatic reductase null, POR-null, or HRN), which lacks hepatic CYP activity, shows profound changes in lipid homeostasis and liver size (Finn et al., 2009). This is associated with significantly elevated Cyp2b10, and linked to hepatic triacylglycerol accumulation and increased hepatic polyunsaturated fatty acids (PUFA) in the HRN mice, indicating a crucial role for CYP in unsaturated fatty acid metabolism. Furthermore, double HRN/CAR-null mice did not demonstrate Cyp2b10 induction, and linoleic acid, an unsaturated fatty acid, activated CAR in transactivation assays. The authors suggest that increases in Cyp2b10 could be an adaptive response against unsaturated fatty acid toxicity and indicated that their study is the first evidence that P450s, and particularly Cyp2b10, play a major role in controlling unsaturated fatty acid homoeostasis via CAR (Finn et al., 2009).
We previously constructed a Cyp2b-knockdown (Cyp2b-KD) mouse model using RNAi technology under the control of a lentiviral promoter, and demonstrated reduced expression of all three major hepatic Cyp2b isoforms (Damiri et al., 2012). The Cyp2b-KD mice developed normally and were fertile; however, we observed enlarged livers and a putative increase in abdominal fat deposition. Furthermore, our initial studies reported here indicate that the Cyp2b-KD mice do not clear corn oil from the liver as well as wildtype (WT) mice. Therefore, we examined serum parameters associated with perturbed lipid homeostasis compared to WT mice, and followed up by investigating whether Cyp2b-KD mice show increased lipid stores as they age. The endogenous role of the Cyp2b subfamily is not known (Wang and Tompkins, 2008; Yamada et al., 2006), and this study suggests a role for Cyp2b’s in fatty acid metabolism, and therefore they may act as anti-obesity CYP.
Materials and Methods
Mice treatment
All studies were carried out according to NIH guidelines for the humane use of research animals and pre-approved by Clemson University’s IACUC. Mice were provided water, and fed ad libitum with a typical chow diet (Envigo Harlan Tekland 2918) containing 3.1 kcal/g with 18% of its kcal from fat (61% PUFA/ 23% MUFA / 16% saturated fats), 58% carbohydrate, 24% protein, prior to and during treatments. Construction of Cyp2b-KD mice was described previously (Damiri et al., 2012), and the Cyp2b-KD mice were maintained on a FVB/NJ background, and bred with FVB/NJ (wild-type; WT) mice to maintain the genetic background. The shRNA construct, named Cyp2b-KD2 (GGATCCCAAGAACACTGAGGTGTACCCCTTGATATCCGGGGGTACACCTCAGTGTTCTTTTTTTTCCAACTCGAG; underlined regions recognize the Cyp2b genes), was designed to recognize all five murine Cyp2b subfamily members. The shRNA construct was cloned into the pRNAT-U6.2/Lenti plasmid, virus produced, and injected into fertilized single cell FVB embryos as described previously (Damiri et al., 2012). The zygotes were cultured overnight and transplanted into pseudopregnant CD-1 mice the next day. shRNA integration was detected by PCR genotyping (Cyp2b-KD2; Forward-5’- GAGGGCCTATTTCCCATGAT – 3’; Reverse-5’ – AGGCACAGTCGAGGCTGAT – 3’) for recognition of the Cyp2b-KD construct and/or the presence of the U.6/Lentivirus (U.6/Lenti; Forward-5’- TTATCGTTTCAGACCCACCTCCCAA – 3’; Reverse-5’ – TCCCATAAGGTCATGTACTGGGCA – 3’) (Damiri et al., 2012).
Untreated WT and Cyp2b-KD mice at 9-weeks or 35-weeks of age were weighed, blood was collected by heart puncture, and then mice were euthanized in separate experiments. In addition, WT and Cyp2b-KD mice (9-weeks old) were treated with 3 mg/kg TCPOBOP (TC) or received 100 μl corn oil i.p. as a vehicle control. TC-treated and corn oil control mice were weighed, blood collected, and then euthanized 24 hours after treatment. Animals were weighed prior to the necropsies. During the necropsies, livers were excised and weighed. A portion of the liver was placed in formalin for histology investigation via hemotoxylin and eosin (H&E) staining and Oil Red O evaluation of liver triacylglycerols. Another portion was snap for microsome or RNA isolation for Western blots or qPCR. Abdominal/renal/inguinal fat was also collected, pooled, and weighed.
Quantitative Real-time Polymerase Chain Reaction (qPCR):
Quantitative real-time PCR (qPCR) was performed as described previously by us and other groups (Damiri et al., 2012; Muller et al., 2002). First, Total RNA was extracted from liver tissue with Tri-Reagent according to the manufacturer’s instructions (Molecular Research Center (Cincinnati, OH) followed by DNase digestion to remove residual genomic DNA (Promega Corporation, Madison, WI). RNA concentrations were determined spectrophotometrically at 260/280 nm (Molecular Devices, Ramsey, MN) prior to cDNA preparation via reverse transcription with 200 units of Moloney murine leukemia virus, 10mM of a dNTP mixture, and 0.05 mg of random hexamers (Promega). RNA was stored at −80oC and cDNA was stored at −20oC. cDNA from liver samples was diluted 1:10 prior to qPCR. To generate a standard curve and determine the PCR efficiency of each reaction, a composite sample of cDNA from FVB (WT) and Cyp2b-KD mice, both treated and untreated mice, was made and dilutions from 1:1 to 1:10−6 were prepared. During qPCR, samples were denatured at 95°C for 10 s, lowered to the appropriate annealing temperature for 30 s, and extended at 72°C for 20 s. Amplifications of the samples and the standard curve were performed in triplicate using a 96-well iQ5 Real-Time PCR Detection System (Bio-Rad) with 0.25X SybrGreen (SA Biosciences, Frederick, MD) as the fluorescent double strand-intercalating agent to quantify changes in relative gene expression as described previously using our inverted Muller’s equation to determine relative quantities of each CYP (Muller et al., 2002; Roling et al., 2004). A minimum of forty cycles was run on all real-time samples to ensure a log based growth curve. 18S was used as the housekeeping gene as confirmed by taking the ratio of the quantification of the housekeeper compared to total RNA content (Bustin, 2002). Melting curve analysis was performed during qPCR runs on all of the primer sets. The primers and their annealing temperatures are presented in Table 1.
Table 1: PCR primer sequences used in qPCR.
| Gene | Forward primer | Reverse primer | Tm (°C) | Reference |
|---|---|---|---|---|
| Car | GGAGCGGCTGTGGAAATATTGCAT | TCCATCTTGTAGCAAAGAGGCCCA | 56.5 | This manuscript |
| Cpt1a | TTGATCAAGTGCCGGACGAGT | GTCCATCATGGCCAGCACAAAGTT | 55.5 | This manuscript |
| Cyp2a4 | AGCAGGCTACCTTCGACTGG | GCTGCTGAAGGCTATGCCAT | 63.6 | (Wiwi et al., 2004) |
| Cyp2b9 | CTGAGACCACAAGCGCCAC | CTTGACCATGAGCAGGACTCC | 64.3 | (Wiwi et al., 2004) |
| Cyp2b10 | CTGAATCCGCTCCTCCACACTC | TGAGCCAACCTTCAAGGAATAT | 61.4 | (Hernandez et al., 2006) |
| Cyp2b13 | GAACTGAGACTACCAGCACCACTCCT | TGAGCATGAGCAGGAAACCACT | 61.5 | (Wiwi et al., 2004) |
| Cyp2c29 | GGCCTCAAAGCCCTACTGTCA | AACGCCAAAACCTTTAATC | 53.6 | (Luo et al., 1998) |
| Cyp3a11 | CTTTCCTTCACCCTGCATTCC | CTCATCCTGCAGTTTTTTCTGGAT | 64.2 | (Wiwi et al., 2004) |
| Foxa2 | TCAACGACTGCTTTCTCAAGGTGC | TTCTCGAACATGTTGCCCGAGTCT | 57.8 | (Wiwi et al., 2004) |
| 18s rRNA | CGCCGCTAGAGGTGAAATTC | CCAGTCGGCATCGTTTATGG | 51 | (Hernandez et al., 2006) |
Tm: Annealing Temperature
Western Blots:
Microsomes were prepared from diced livers by dounce homogenization followed by differential centrifugation including high speed microsome separation from cytosol at 100,000XG for 45 minutes as described previously (Van der Hoeven and Coon, 1974). The Bio-Rad (Hercules, CA) protein assay based on the Bradford method was used to determine protein concentrations. Microsomes were stored at −80oC. Western blots were performed with 30 μg of hepatic microsomal protein as described previously using our rabbit-anti-mouse CYP2B antibody and a goat anti-rabbit IgG (Bio-Rad) alkaline-phosphatase coupled secondary antibody (Mota et al., 2010). β-actin (Sigma Aldrich, St. Louis, MO) was used as a housekeeper to ensure equal loading of samples. Bands were visualized via chemiluminescent detection (Bio-Rad) and quantified with the Chemi-Doc XRS HQ using Quantity One 4.6.5 software (Bio-Rad). Density was determined relative to β-actin (Kumar et al., 2017).
Histopathology and Oil Red O staining
Samples fixed in 10% formalin were processed and stained with hemotoxylin and eosin or Oil Red O at Colorado Histo-Prep for blind histopathological evaluation (Fort Collins, CO). Standard toxicology pathology criteria and nomenclature for mice was used to categorize microscopic tissue changes of H&E and Oil Red O stained samples (Banks, 1993; Percy and Barthold, 2001). Individual parameters (i.e. hepatocellular swelling, necrosis, hypertrophy, hyperplasia, inflammation, bile duct hyperplasia, mineralization, hepatocellular involvement) were scored 0–4 (0 = none, 1 = minimal, 2 = mild, 3 = moderate, 4 = severe) and then summed. Total scores for the hepatic histopathology lesions or Oil Red O staining in each mouse were ranked and significance determined by Kruskal–Wallis followed by Dunn’s post hoc test.
Serum Chemistry
Serum was prepared by centrifugation as described previously (Tuck et al., 2009) and analyzed at the Comparative Pathology Laboratory at Baylor College of Medicine (Houston, TX) for levels of non-fasting cholesterol, triacylglycerols, high density lipoporotein (HDL), low density lipoporotein (LDL), very low density lipoprotein (VLDL), phosphorus, albumin, and glucose using a Beckman-Coulter AU480 analyzer and the appropriate Beckman-Coulter biochemical kits according to the manufacturer’s instructions (n = 4–6).
Statistical Significance:
Data are presented as mean ± SEM. Statistical analysis was performed by ANOVA followed by Tukey’s multiple comparison test as the post-hoc test when comparing more than two groups with the exception of the histopathology data that was examined by Kruskal-Wallace followed by Dunn’s post-hoc test. Student’s t-tests were used when comparing two groups. Statistical analysis was performed using Graphpad Prism version 4.0 (La Jolla, CA USA). A p-value ≤ 0.05 was considered statistically significant.
Results
Corn oil treated Cyp2b-KD mice have higher hepatic lipid levels:
Corn oil is a commonly used carrier for chemical treatments as it dissolves lipid soluble chemicals in a non-toxic digestible form. We injected WT and Cyp2b-KD mice with corn oil or TC dissolved in corn oil i.p., and then euthanized the mice 24 hours later. Surprisingly, we observed a significant increase in hepatic lipids within the hepatocytes (steatosis) of female Cyp-KD mice (but not male Cyp2b-KD mice) as indicated by Oil Red O staining (Fig. 1). TC provided protection from steatosis in previous studies (Dong et al., 2009b), however, TC-treatment did not provide significant protection from corn oil treatment in Cyp2b-KD female mice (Fig. 1); but the number of mice with severe effects dropped.
Fig. 1: Lipid deposition in livers of Cyp2b-KD and WT mice treated with corn oil.
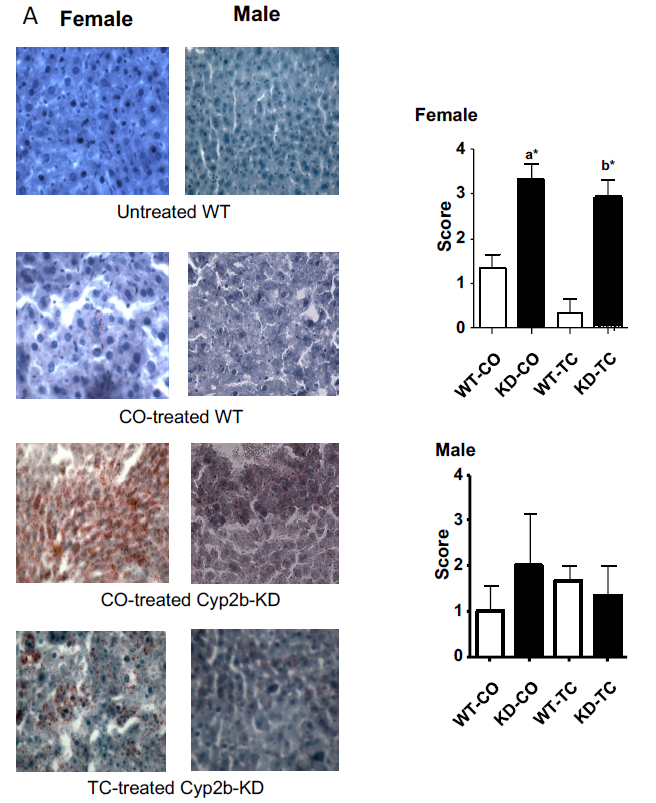
(A) Oil Red O staining of the hepatocytes of WT and Cyp2b-KD mice treated with corn oil or TC. (B) Average Oil Red O staining scores for female and male WT and Cyp2b-KD mice (n = 3). An [a] indicates a significant difference between WT and Cyp2b-KD mice treated with corn oil. A [b] indicates a significant difference between WT and Cyp2b-KD mice treated with TC. An asterisk indicates significant difference with a p < 0.05 as determined by Kruskal-Wallace followed by Dunn’s post-hoc test using the GraphPad Prism 4.0 software package. WT= wild-type, KD= Cyp2b-KD mice, M=male, F= female. CO = Corn oil, TC = TCOPBOP.
Corn Oil Increases Serum Lipids in Cyp2b-KD Mice:
Cyp2b-KD mice also displayed a significant increase in several serum lipid parameters, including total serum cholesterol, triacylglycerol, HDL, LDL, and VLDL compared to the corresponding WT controls (Fig. 2). Other than phosphorous, which increased 44% in corn oil treated Cyp2b-KD males and 42% in TC-treated Cyp2b-KD males compared to WT males, no other changes (glucose, albumin) were observed between WT and Cyp2b-KD mice following exposure to corn oil or TC (data not shown).
Fig. 2: Non-fasting serum cholesterol, triacylglycerols, and lipoprotein profiles in WT and Cyp2b-KD mice after corn oil and TC-treatments.
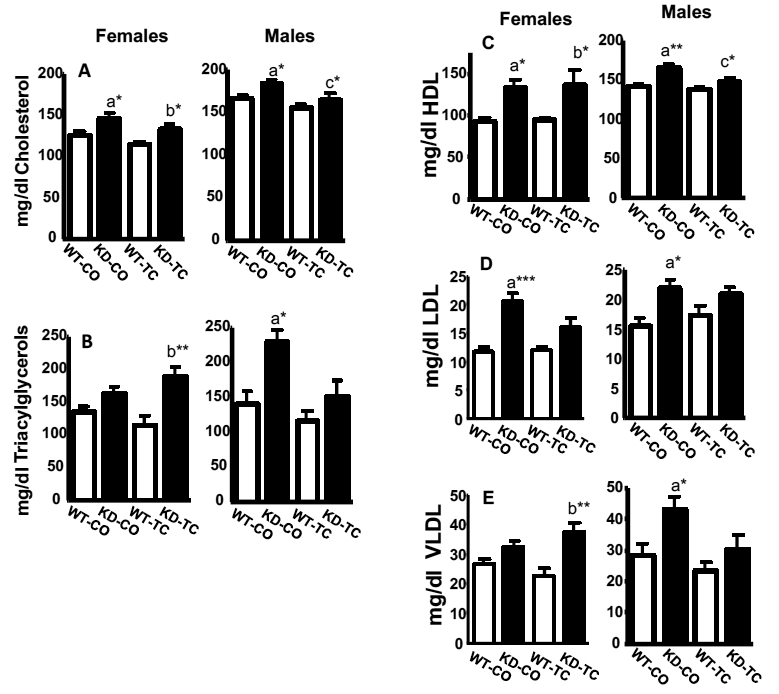
Measured (A) cholesterol, (B) triacylglycerols, (C) HDL, (D) LDL, and (E) VLDL concentrations in WT and Cyp2b-KD female and male mice following treatment with corn oil or TC. Significant differences between WT and Cyp2b-KD mice were assessed by ANOVA followed by Tukey’s multiple comparison tests using GraphPad Prism 4.0 (n = 4–6). An asterisk indicates a significant difference with a p < 0.05, two asterisks indicate a p < 0.01, and three asterisks indicate a p < 0.001. The letter [a] indicates a significant difference between WT and Cyp2b-KD mice treated with corn oil, the letter [b] indicates a significant difference between WT and Cyp2b-KD mice treated with TC, and the letter [c] indicates a significant difference between Cyp2b-KD mice treated with corn oil and Cyp2b-KD mice treated with TC. WT = wild-type, KD= Cyp2b-KD mice, M = male, F= female, CO = corn oil, TC= TCPOBOP.
Serum triacylglycerol and VLDL concentrations were significantly increased in male (53%), but not female Cyp2b-KD mice (21%)(Fig. 2), potentially because the lipids were stored in the livers of the females (Fig. 1). Females and males exhibited a significant increase in cholesterol (16.5%; 11.1%), HDL (42.6%; 17.3%), and LDL (48.7%; 42.1%)(Fig. 2).
The CAR activator and Cyp2b inducer, TC, reversed the effects of corn oil on cholesterol and HDL levels in male Cyp2b-KD mice. The effects of TC in female Cyp2b-KD mice were not as prominent as in male mice (Fig. 2). In addition, male TC-treated Cyp2b-KD mice did not show significant increases in triacylglycerols, LDL, and VLDL unlike the male corn oil treated Cyp2b-KD mice (Fig. 2). TC-treatment led to an increase in serum triacylglycerols (60%) and VLDL levels (67%) in TC-treated Cyp2b-KD female mice compared to TC-treated WT mice.
Changes in Cyp and metabolic transcription factor expression in Corn Oil and TC-treated WT and Cyp2b-KD Mice:
Previously we demonstrated that Cyp2b-KD mice showed repression of Cyp2b expression (Damiri et al., 2012); however, we did not investigate whether there was compensatory changes in other CYP regulated by CAR or if TC-treatment could overcome RNAi-mediated repression of Cyp2b expression. Surprisingly, corn oil-treated mice showed no significant changes in Cyp2b mRNA expression (Table 2–3), indicating that both corn oil and TC could overcome RNAi-mediated repression of Cyp2b RNA expression. Therefore, we examined Cyp2b protein expression by Western blotting, and found significant repression of Cyp2b protein levels (Fig. 3). The lack of RNA repression in Cyp2b-KD mice was not observed previously in untreated Cyp2b-KD mice where repression varied from 0 – 80% depending on the Cyp2b isoform and gender (Damiri et al., 2012). However, TC-treated mice previously showed no repression or slight induction of Cyp2b RNA expression along with repressed protein levels, indicating that the lack of Cyp protein was causing a compensatory transcriptional response and increased Cyp2b mRNA (Damiri et al., 2012). A similar response from corn oil suggests its components such as linoleic acid also activate CAR, which was shown previously (Finn et al., 2009). Studies have also shown that the monounsaturated fatty acid, olive oil can induce Cyp2b9 and Cyp2b13 (Guillen et al., 2009). Taken together, TC and unsaturated fatty acid components of corn oil are probably inducing Cyp2b expression; however, the shRNA is still able to cause enough destruction of the mRNA to reduce protein. In addition, there are no significant differences between WT and Cyp2b-KD female and male mice after corn oil or TC-treatment as to the expression of Car, Foxa2, Cpt1a, and a number of Cyp (Table 2–3).
Table 2: qPCR measured expression of Cyp and metabolic transcription factors in corn oil (CO)- and TC-treated, WT and Cyp2b-KD (KD) female mice.
| Females | Treated (8–12) weeks | |||
|---|---|---|---|---|
| WT-CO | KD-CO | WT -TC | KD-TC | |
| Cyp2b9 | 1.0 ± 0.05 | 2.23 ± 0.35a,** | 1.07 ± 0.02 | 0.13 ± 0.03d,*** |
| Cyp2b10 | 1.0 ± 0.03 | 2.14 ± 0.70 | 51.0 ± 8.39c,** | 135.0 ± 25.2d,*** |
| Cyp2b13 | 1.0 ± 0.28 | 2.31 ± 0.59 | 0.59 ± 0.08 | 1.74 ± 0.27 |
| Cyp2a4 | 1.0 ± 0.38 | 0.96 ± 0.22 | 1.54 ± 0.34 | 1.05 ± 0.41 |
| Cyp2c29 | 1.0 ± 0.10 | 4.63 ± 1.56 | 45.8 ± 9.93 | 126.0 ±32.9 |
| Cyp3a11 | 1.0 ± 0.22 | 1.74 ± 0.49 | 7.19 ± 2.08c,*** | 20.6 ± 1.83b,d,*** |
| Car | 1.0 ± 0.24 | 3.1 ± 1.03 | 0.46 ± 0.08 | 2.66 ± 0.55 |
| Foxa2 | 1.0 ± 0.40 | 4.2 ± 1.17 | 0.53 ± 0.23 | 3.0 ± 0.75 |
| Cpt1a | 1.0 ± 0.19 | 3.9 ± 0.99 | 0.41 ± 0.23 | 1.3 ± 0.28 |
Data are presented as mean ± SEM normalized to WT-CO treated mice. Significant changes in gene expression were determined by ANOVA followed by Tukey’s multiple comparison tests using the GraphPad Prism 4.0 software package (n = 5–6).
P value <0.05
< 0.01
< 0.001
WT-CO vs KD-CO
WT-TC vs KD-TC
WT-CO vs WT-TC
KD-CO vs KD-TC
Table 3: qPCR measured expression of Cyp and metabolic transcription factors in corn oil (CO)- and TC-treated, WT and Cyp2b-KD (KD) male mice.
| Males | Treated (8–12) weeks | |||
|---|---|---|---|---|
| WT-CO | KD-CO | WT -TC | KD-TC | |
| Cyp2b9 | 1.0 ± 0.92 | 19.0 ± 7.05 | 91.1 ± 29.8 | 13.8 ± 6.61 |
| Cyp2b10 | 1.0 ± 0.37 | 6.31 ± 2.33 | 514 ± 171a,* | 552 ± 138b,* |
| Cyp2b13 | 1.0 ± 0.98 | 3.37 ± 2.53 | 5.34 ± 4.52 | 0.136 ± 0.07 |
| Cyp2a4 | 1.0 ± 0.20 | 1.19 ± 0.42 | 1.5 ± 0.65 | 0.37± 0.11 |
| Cyp2c29 | 1.0 ± 0.54 | 0.043 ± 0.023 | 13.4 ± 5.1 | 21.7 ± 8.5 |
| Cyp3a11 | 1.0 ± 0.42 | 0.46 ± 0.15 | 2.9 ± 1.36 | 2.88 ± 0.45 |
| Car | 1.0 ± 0.47 | 1.74 ± 0.39 | 4.41 ± 1.74 | 1.34 ± 0.46 |
| Foxa2 | 1.0 ± 0.51 | 5.79 ± 2.2 | 9.67 ± 7.03 | 2.60 ± 0.77 |
| Cpt1a | 1.0 ± 0.34 | 1.33 ± 0.32 | 0.59 ± 0.16 | 1.84 ± 0.55 |
Data are presented as mean + SEM normalized to WT-CO treated mice. Significant changes in gene expression were determined by ANOVA followed by Tukey’s multiple comparison tests using the GraphPad Prizm 4.0 software package (n = 5–6).
P value <0.05
< 0.01
< 0.001
WT-CO vs WT-TC
KD-CO vs KD-TC
Fig. 3: Western blots from microsomes of female and male WT and Cyp2b-KD (KD) mice treated ip with either corn oil (CO) or TCPOBOP (TC).
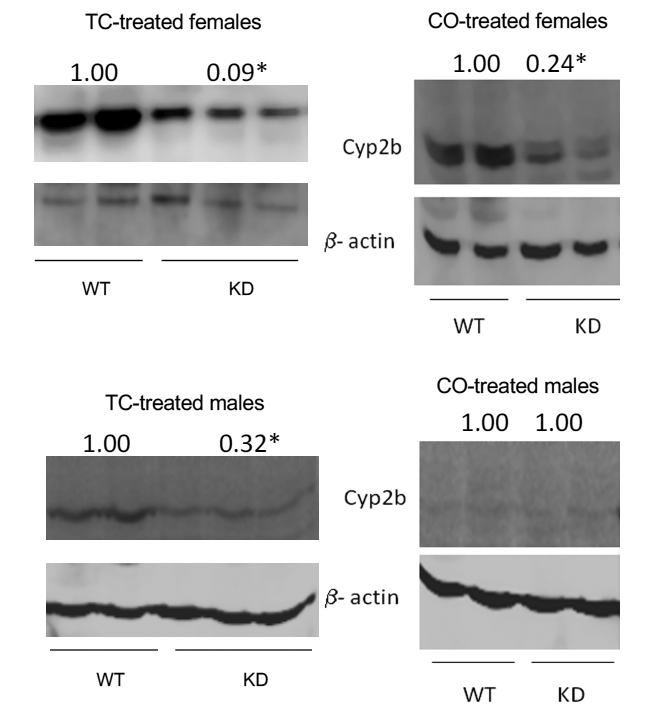
Results are expressed as relative mean and statistical differences were determined by Student’s t-tests using GraphPad Prism 4.0 with an asterisk indicating a p-value < 0.05.
Weight gain, Hepatic Histopathology, and Serum Lipids in 35-week old Cyp2b-KD mice
Because we observed what appeared to be a putative increase in adipose deposition in our initial studies (Damiri et al., 2012), and corn oil was not as quickly depurated from the liver of Cyp2b-KD mice (Fig. 1), we examined whether older Cyp2b-KD mice developed age-onset obesity, including weight gain, adipose deposition, and serum lipids in 35-week old mice. Adipose weight from the inguinal, abdominal, and renal regions of Cyp2b-KD mice was 2.12-fold greater in males and 2.88-fold greater in females than WT controls (Fig. 4A). In addition, the Cyp2b-KD mice weighed more with females weighing 10.9% more and male weighing 22.3% more than WT counterparts (Fig. 4B), indicating a change in body composition.
Fig. 4: Weight, serum, and hepatic differences between 35-week old WT and Cyp2b-KD mice.
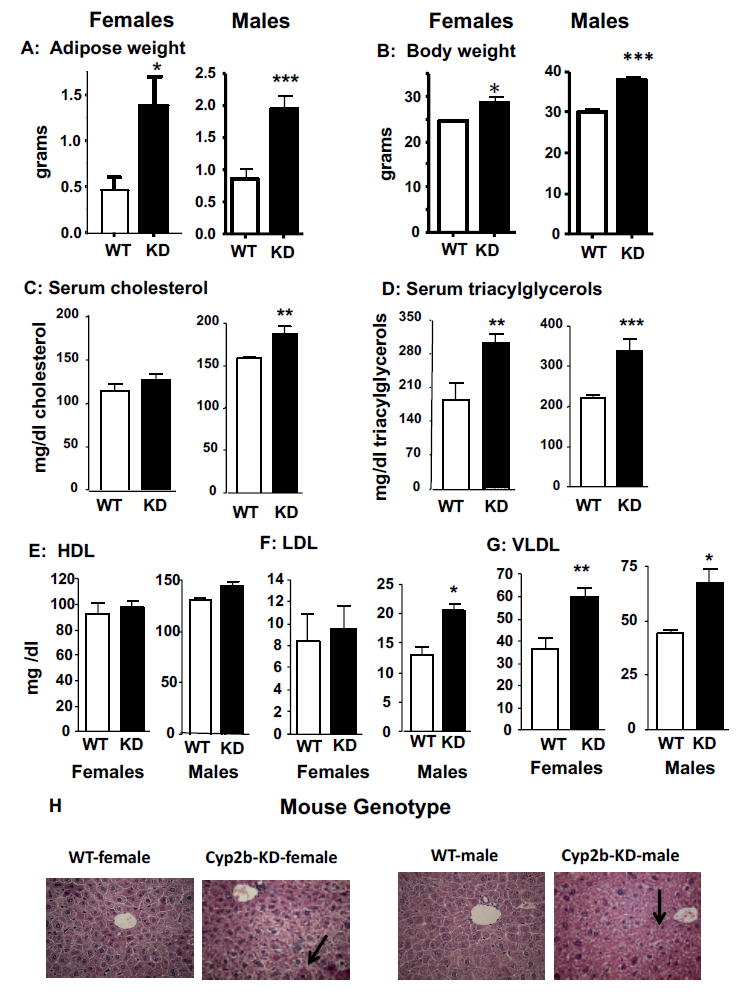
(A) Adipose weight, (B) body weight (C), serum cholesterol, (D) triacylglycerols, (E) HDL, (F) LDL, and (G) VLDL concentrations, and (H) histopathology observations in female and male WT mice compared to female and male Cyp2b-KD mice at 35-weeks of age show minor periportal hypertrophy in Cyp2b-KD mice. Significant differences between WT and Cyp2b-KD mice were assessed by Student’s t-test (n = 9–11). An asterisk indicates a significant difference with a p < 0.05, two asterisks indicate a p < 0.01, and three asterisks indicate a p < 0.001. WT = wild-type mice, KD = Cyp2b-KD mice, M = male, F= female.
To test whether Cyp2b repression caused significant perturbations in serum lipid concentrations in older mice; cholesterol, triacylglycerols, HDL, LDL, and VLDL were measured. The male 35-week old Cyp2b-KD mice exhibited a significant increase in serum cholesterol compared to WT mice (Fig. 4C). Both female and male Cyp2b-KD mice showed an increase in serum triacylglycerols (62%) compared to WT mice (Fig. 4D). Healthy HDL serum concentrations (Fig 4E) showed no change; however, serum LDL concentrations increased in males (Fig. 4F), and serum VLDL concentrations increased in both genders (Fig. 4G). Interestingly, Cyp2b-KD male mice also showed increased serum phosphorus similar to observed in corn oil treated Cyp2b-KD mice (26.6%). Overall, the age-related changes in serum lipids were more profound in Cyp2b-KD males than Cyp2b-KD females. Hypertriglyceremia is a key biomarker of metabolic disorder and the pre-diabetic condition. Therefore, we tested non-fasting blood sugar in both young and old mice. No significant changes in blood sugar or albumin were observed in the 35-week old Cyp2b-KD mice (data not shown).
There are few differences in the hepatic histopathology results between the WT and Cyp2b-KD mice. Hepatocytes from 35-week old Cyp2b-KD-female and male mice are more likely to show increased periportal hypertrophy as all of the Cyp2b-KD mice showed periportal hypertrophy and none of the WT mice showed periportal hypertrophy (Fig. 4H). However, the score was one (minimal) in each individual (Fig. 4H). In addition, Oil Red O staining results indicated no significant differences in fat accumulation in Cyp2b-KD mice compared to WT mice (data not shown).
Changes in Cyp and metabolic transcription factor expression in young and old mice:
We also examined Cyp2b expression for RNAi-mediated repression, and subsequent effects on other Cyp and metabolic transcription factors in 35-week old WT and Cyp2b-KD mice. To provide some perspective, young (9-week old) mice were also measured. As observed previously, Cyp2b expression is repressed in young Cyp2b-KD mice (Damiri et al., 2012)(Table 4–5); however, this repression was lost in the older mice regardless of sex (Table 4–5). Other than a similar drop in Cyp2a4 expression in Cyp2b-KD male mice, no other significant changes in gene expression were observed for Cyp or metabolic transcription factors in young or old mice.
Table 4: qPCR measured expression of Cyp and metabolic transcription factors in young (9-week old) and old (35-week old) WT and Cyp2b-KD (KD) female mice.
| Females | Untreated (8–12) weeks | Old 35-weeks | ||
|---|---|---|---|---|
| WT | KD | WT | KD | |
| Cyp2b9 | 1.0 ± 0.18 | 1.33 ± 0.24 | 1.0 ± 0.30 | 0.98 ± 0.18 |
| Cyp2b10 | 1.0 ± 0.29 | 0.33 ± 0.09* | 1.0 ± 0.24 | 0.61 ± 0.11 |
| Cyp2b13 | 1.0 ± 0.07 | 0.46 ± 0.09* | 1.0 ± 0.49 | 3.11 ± 1.12 |
| Cyp2a4 | 1.0 ± 0.03 | 0.81 ± 0.15 | 1.0 ± 0.53 | 1.10 ± 0.15 |
| Cyp2c29 | 1.0 ± 0.29 | 1.05 ± 0.34 | 1.0 ± 0.27 | 0.74 ± 0.12 |
| Cyp3a11 | 1.0 ± 0.29 | 1.03 ± 0.22 | 1.0 ± 0.79 | 1.17 ± 0.25 |
| Car | 1.0 ± 0.30 | 0.30 ± 0.30 | 1.0 ± 0.27 | 0.85 ± 0.09 |
| Foxa2 | 1.0 ± 0.21 | 0.42 ± 0.08 | 1.0 ± 0.36 | 1.34 ± 0.55 |
| Cpt1a | 1.0 ± 0.16 | 0.85 ± 0.23 | 1.0 ± 0.31 | 0.84 ± 0.15 |
Data are presented as mean ± SEM normalized to untreated mice at 9- or 35-weeks of age. Significant changes in gene expression were determined by Student’s t-tests using the GraphPad Prism 4.0 software package (n = 5–6).
P value <0.05
< 0.01
< 0.001
Table 5: qPCR measured expression of Cyp and metabolic transcription factors in young (9-week old) and old (35-week old) WT and Cyp2b-KD (KD) male mice.
| Males | Untreated (9) weeks | Old 35-weeks | ||
|---|---|---|---|---|
| WT | KD | WT | KD | |
| Cyp2b9 | 1.0 ± 0.28 | 0.29 ± 0.31** | 1.0 ± 0.43 | 8.76 ± 2.89 |
| Cyp2b10 | 1.0 ± 0.31 | 0.33 ± 0.08 | 1.0 ± 0.26 | 2.12 ± 0.99 |
| Cyp2b13 | 1.0 ± 0.30 | 0.21 ± 0.03*** | 1.0 ± 0.26 | 8.75 ± 5.43 |
| Cyp2a4 | 1.0 ± 0.19 | 0.48 ± 0.12* | 1.0 ± 0.53 | 0.20 ± 0.03 |
| Cyp2c29 | 1.0 ± 0.32 | 1.19 ± 0.41 | 1.0 ± 0.28 | 1.79 ± 0.5 |
| Cyp3a11 | 1.0 ± 0.18 | 1.43 ± 0.64 | 1.0 ± 0.11 | 1.49 ± 0.48 |
| Car | 1.0 ± 0.18 | 0.89 ± 0.24 | 1.0 ± 0.22 | 1.44 ± 0.54 |
| Foxa2 | 1.0 ± 0.23 | 0.60 ± 0.12 | 1.0 ± 0.23 | 0.83 ± 0.21 |
| Cpt1a | 1.0 ± 0.32 | 0.85 ± 0.29 | 1.0 ± 0.32 | 0.65± 0.41 |
Data are presented mean ± SEM normalized to untreated 9-week old mice. Significant changes in gene expression were determined by Student’s t-tests using the GraphPad Prism 4.0 software package (n = 5–6).
P value <0.05
< 0.01
< 0.001
Western blots corroborate that CYP2B expression is no longer significantly repressed in the Cyp2b-KD mice as they reached 35-weeks of age (Fig. 5), indicating either the RNAi was no longer repressing expression, or more likely compensatory changes occurred such as increased lipids that led to a loss of Cyp2b suppression. While not statistically significant, the latter appears plausible based on an 8-fold increase in Cyp2b9 and Cyp2b13 expression in the male Cyp2b-KD mice (Table 5). This also suggests that much of the age-onset obesity either occurred in mice at a younger age or the early effects of Cyp2b loss led to increased susceptibility to obesity as the mice aged.
Fig. 5: Western blots from microsomes of female and male WT and Cyp2b-KD mice after 35-weeks (old) of age.
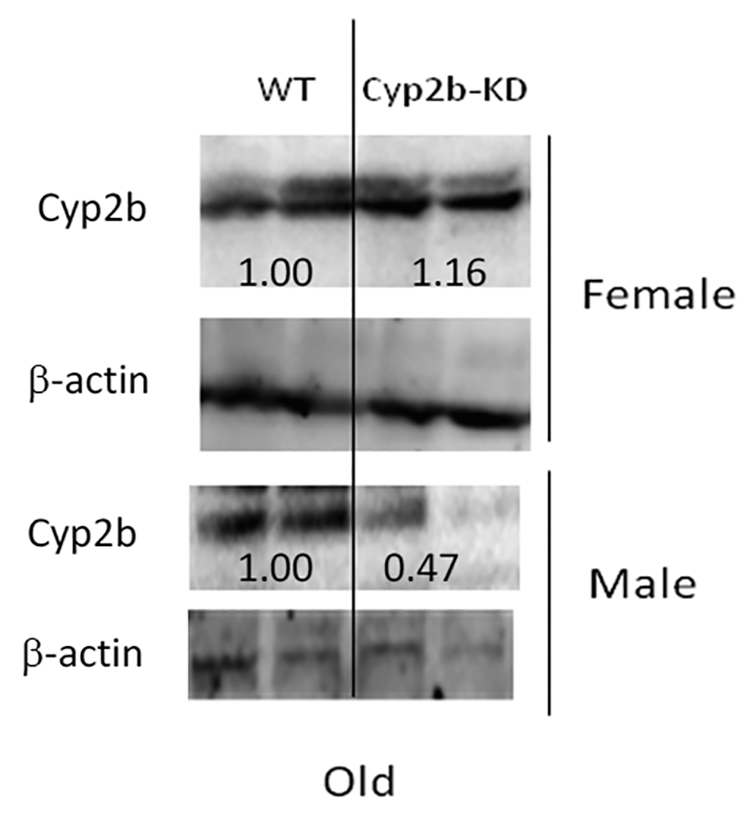
Results are expressed as relative mean. Student’s t-tests were performed using GraphPad Prism 4.0. No significant differences were detected.
DISCUSSION
Corn oil is primarily made of UFA, and contains approximately 55–57% linoleic acid (polyunsaturated), 1% linolenic acid, 30% oleic acid (monounsaturated) (85–88% total UFA), and 12–15% saturated fatty acids (US Department of Agriculture, 2007). In this manuscript, we demonstrate that Cyp2b-KD mice are age-dependent obese, show increased fat deposition, increased serum lipids, and a reduced ability to eliminate corn oil in comparison to WT mice. Female Cyp2b-KD mice treated with corn oil show greater hepatic Oil Red O staining indicative of higher lipid levels than WT mice injected with corn oil. TC-treatment was unable to ameliorate the increased liver lipids in females, which may in part be caused by lower Cyp2b induction in knockdown mice (Damiri et al., 2012).
Serum lipids are also increased by corn oil-treatment in Cyp2b-KD mice compared to their WT counterparts with TC-treatment providing little amelioration with more changes in males than females (Fig. 2). Slightly greater concentrations of triacylglycerols and VLDL were measured in the serum following TC-treatment in females. This suggests that TC is initiating elimination of lipids, most likely from the liver into the serum. However, in males TC-treatment ameliorates serum cholesterol and HDL. It is interesting that Cyp2b-KD females showed greater perturbations in lipids in the liver and males showed greater perturbations in the serum and this may be due to the gender predominant expression and induction of Cyp2b isoforms in female liver measured in multiple strains including FVB/NJ mice (Hashita et al., 2008; Hernandez et al., 2006; Hernandez et al., 2009b; Mota et al., 2011; Wiwi et al., 2004). Overall, the positive effects of TC-treatment on serum parameters in males were not reproduced in females (Fig. 2), indicating a sexually dimorphic difference in the mice possibly due to higher constitutive Cyp2b levels in the female mice. It is also possible that the greater serum effects measured in males may also have provided an environment more likely to show TC-mediated amelioration (Fig. 2).
A single high dose of corn oil was not cleared as well in Cyp2b-KD mice as it was WT mice. Interestingly, excess sunflower oil in the diet of hepatic POR-null mice (HRN) caused fatty liver presumably because of a lack of CYP activity and the inability to metabolize and eliminate fatty acids (Finn et al., 2009). Sunflower oil is 69% PUFA with linoleic acid the primary PUFA. Oleic acid (20%), and saturated fatty acids (11%) comprise the rest of the sunflower oil, similar to corn oil. Furthermore, mice treated with sunflower oil or linoleic acid show significant increases in Cyp2b10 presumably through linoleic acid activation of CAR (Finn et al., 2009). However, the POR-null mice treated with sunflower oil had lower serum cholesterol and triacylglycerols than WT mice treated with sunflower oil, presumably due to poor depuration from the liver in the mice lacking all CYP activity (Finn et al., 2009).
Cyp2b enzymes are highly regulated by the metabolic transcription factors CAR and Foxa2 (Hashita et al., 2008; Honkakoski et al., 1998; Wei et al., 2000), and their expression is also regulated by other transcription factors such as GR, STAT5b and HNF4α (Audet-Walsh and Anderson, 2009; Nakamura et al., 2007; Wiwi et al., 2004). It has been demonstrated that fatty liver and diabetes activate CAR and Foxa2 and increase drug metabolism due in part to increased Cyp2b9 and Cyp2b10 activity (Bochkis et al., 2013; Dong et al., 2009a; Hashita et al., 2008; Wolfrum et al., 2004). CAR activation also modulates hepatic metabolism and lowers hepatic triacylglycerols and serum glucose levels (Dong et al., 2009b), while protecting mice from diet induced obesity (Gao et al., 2009). However, others have shown that CAR activation by TC led to increased serum triacylglycerols in mice fed a high fat diet, potentially due to depuration of liver fatty acids (Maglich et al., 2009). Our studies show that the CAR activator TC partially reversed serum hyperlipidemia in male but not female Cyp2b-KD mice. The TC-induced reversal in male mice is probably due to the regulation of multiple genes, and Cyp2b’s appear to play a crucial role (Fig 1,2).
Corn oil and other vegetable oils are relatively common carriers for toxicology studies and 100 μl injections are common. However, this level of lipid in one dose is quite high. For example, 100 μl in a 25 g mouse is equivalent by weight to 300 ml in a 75 kg human; an unrealistic and incredibly heavy dose of fatty acids for one meal. There are almost certainly physiological and toxicological consequences to the high dose of unsaturated fatty acids as this amount is likely to activate CAR and induce CYP (Finn et al., 2009). This could confound some toxicology studies. However, within 24 hours, WT mice appeared to acclimate and clear the high amounts of unsaturated fat, while the Cyp2b-KD mice did not fully acclimate to the corn oil treatment and in turn showed greater Oil Red O staining in female livers and higher triacylglycerols, cholesterol, LDL, HDL, and VLDL in male serum with higher cholesterol, HDL, and LDL in female serum.
Cyp2b isoforms metabolize linoleic acid (Bylund et al., 1998) and arachidonic acid into epoxides (Maayah et al., 2014), and in turn produce epoxyeicosatrienoic acids that are critical in kerotinocyte development (Du et al., 2005; Ladd et al., 2003). Cyp2b induction in the liver during exposure to lipids or in POR-null mice (Finn et al., 2009; Wang et al., 2005) indicates other roles for Cyp2b enzymes in the metabolism of unsaturated fatty acids. In addition, it suggests that toxicants that inhibit Cyp2b metabolism may disrupt unsaturated fatty acid metabolism.
The increased serum lipids in Cyp2b-KD mice following corn oil treatment may manifest itself as increased lipid deposition and obesity as the mice age. Therefore, age-onset obesity was followed. 35-week old Cyp2b-KD mice showed increased white adipose tissue and weighed significantly more than WT mice (Fig. 4). Both female and male Cyp2b-KD mice showed much greater levels of white adipose tissue and both female and male Cyp2b-KD mice weighed more than their WT counterparts. However, male weight differences were much greater than female weight differences. Similar to treatments with corn oil, the male Cyp2b-KD mice showed more robust effects on serum lipids, as female Cyp2b-KD mice did not demonstrate higher serum cholesterol or LDL concentrations. Obesity, increased abdominal fat deposition, and increased serum triacylglycerols are all key symptoms of prediabetic conditions and associated with metabolic syndromes and indicate that the lack or inhibition of Cyp2b’s has metabolic consequences. Taken together, males with low Cyp2b levels appear to be more susceptible to age-onset obesity even though they express less Cyp2b enzymes (Hernandez et al., 2006; Kumar et al., 2017).
Foxa2 mutants also develop age-onset obesity in males (Bochkis et al., 2013) even though Foxa2 is a female predominant transcription factor that regulates the expression of Cyp2b9 and potentially Cyp2b13 (Bochkis et al., 2013; Hashita et al., 2008). The Foxa2 mutants show perturbed bile acid and growth hormone signaling most likely due to disruptions in the interactions of Foxa2 with Stat5b, FXR, or PXR (Bochkis et al., 2013; Nakamura et al., 2007). Interestingly, Cyp2b9 is also the most induced gene in the liver (Leung et al., 2016) and brown fat (McGregor et al., 2013) of diet-induced obese mice. Furthermore, db/db mice also show increased Car and Cyp2b10 expression (Yoshinari et al., 2006). Interestingly, the older Cyp2b-KD mice lost their RNAi-mediated Cyp2b repression and this may be lipid-mediated induction or compensation that is consistent with induction of Cyp2b members following exposure to corn oil (Finn et al., 2009), a high-fat diet (Leung et al., 2016; McGregor et al., 2013), or a compensatory mechanism due to changes in key metabolites (Hernandez et al., 2009b; Kumar et al., 2017; Mota et al., 2011). Taken together, Cyp2b9, Cyp2b10, and the transcription factors they are regulated by (Foxa2/CAR) are associated with obesity and age-onset obesity.
The endogenous role of the Cyp2b subfamily is poorly understood (Wang and Tompkins, 2008). We propose that hepatic Cyp2b’s are increased in response to unsaturated fatty acids (Finn et al., 2009) and are important in the regulation, signaling, or depuration of unsaturated fatty acids in addition to their role as detoxification CYP. Thus, they either directly metabolize and depurate unsaturated fats as suggested previously (Finn et al., 2009), or they produce a key signaling molecule from unsaturated fatty acids that regulate the use of lipids. Therefore, significant inhibition of Cyp2b activity could decrease lipid metabolism, increase fatty liver, and induce obesity by increasing fat deposition in individuals eating a high-fat diet. Cyp2b substrates that act as inhibitors or competitors such as atrazine, polychlorinated biphenyls, nonylphenol, diazinon, parathion, and chlorpyrifos may be obesogens (Acevedo et al., 2005; Hodgson and Rose, 2007). In fact, there is data implicating several potential Cyp2b inhibitors such as polychlorinated biphenyls, nonylphenol, bisphenol A, chlorpyrifos, diazinon, and parathion in increased fat deposition, pre-diabetic states, and obesity (Lassiter et al., 2008; Masuno et al., 2003; Slotkin, 2011; Wada et al., 2007; Wahlang et al., 2013). Furthermore, metabolic changes in in the livers of endosulfan-treated mice are consistent with diabetes and insulin resistance (Canlet et al., 2013).
Overall, our data indicate an influence of Cyp2b isoforms on the homeostasis of serum and hepatic lipids. Recent reports from other laboratories provide supporting data. For example, transcription factors that regulate Cyp2b such as CAR and Foxa2 are nutrient sensors involved in energy homeostasis and lipid metabolism (Bochkis et al., 2013; Finn et al., 2009; Gao et al., 2009; Wolfrum et al., 2004), and hepatic deletion of P450 oxidoreductase (POR; HRN mouse) led to a profound time and CAR-dependent induction of Cyp2b10 associated with fatty liver (Finn et al., 2009). In conclusion, our findings indicate that Cyp2b-KD mice are unable to either utilize or metabolize unsaturated fatty acids efficiently which leads to an accumulation of central fat and the potential for metabolic disorders.
Acknowledgments
Acknowledgments: Funding was provided by NIH R15ES017321.
Abbreviations:
- CAR:
constitutive androstane receptor
- cDNA:
complimentary DNA
- Cpt1a:
carnitine palmitoyltransferase 1a
- Cyp2b-KD:
Cyp2b-knockdown
- Cyp2b:
Cytochrome P450 2b
- DDT:
1,1,1-Trichloro-2,2-bis(4-chlorophenyl)ethane
- dNTP:
deoxynucleotide triphosphate
- Foxa2:
forkhead box a2; also known as HNF3B, hepatocyte nuclear factor 3 beta
- H&E:
hematoxylin and eosin
- HDL:
High-density lipoprotein
- IACUC:
Institutional Animal Care and Use Committee
- LDL:
Low-density lipoprotein
- i.p.:
intraperitoneal
- PCBs:
polychlorinated biphenyls
- PCR:
polymerase chain reaction
- PUFA:
polyunsaturated fatty acid
- qPCR:
Quantitative real-time PCR
- shRNA:
short hairpin RNA
- TCPOBOP; TC:
3,3′,5,5′-Tetrachloro-1,4-bis(pyridyloxy)benzene
- Tm:
Annealing temperature
- UFA:
Unsaturated fatty acid
- VLDL:
very low-density lipoprotein
- WT:
wildtype
REFERENCES:
- Acevedo R, Villanueva H, Parnell PG, Chapman LM, Gimenez T, Gray SL, Baldwin WS (2005) The contribution of hepatic steroid metabolism to serum estradiol and estriol concentrations in nonylphenol treated mmtvneu mice and its potential effects on breast cancer incidence and latency. Journal of Applied Toxicology, 25:339–353. [DOI] [PubMed] [Google Scholar]
- Audet-Walsh E, Anderson A (2009) Dexamethasone induction of murine cyp2b genes requires the glucocorticoid receptor. Drug Metabolism and Disposition, 37:580–588. [DOI] [PubMed] [Google Scholar]
- Banks WJ (1993) Applied veterinary histology. C V Mosby, 3rd edition, St. Louis. [Google Scholar]
- Bochkis IM, Rubins NE, White P, Furth EE, Friedman JR, Kaestner KH (2008) Hepatocyte-specific ablation of foxa2 alters bile acid homeostasis and results in er stress. Nature Medicine, 14:828–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochkis IM, Shin S, Kaestner KH (2013) Bile acid-induced inflammatory signaling in mice lacking foxa2 in the liver leads to activation of mtor and age-onset obesity. Molecular Metabolism, 2:447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin SA (2002) Quantification of mrna using real-time reverse transcription pcr (rt-pcr): Trends and problems. Journal of Molecular Endocrinology, 29:23–39. [DOI] [PubMed] [Google Scholar]
- Bylund J, Kunz T, Valmsen K, Oliw EH (1998) Cytochromes p450 with bisallylic hydroxylation activity on arachidonic and linoleic acids studied with human recombinant enzymes and with human and rat liver microsomes. Journal of Pharmacology and Experimental Therapeutics, 284:51–60. [PubMed] [Google Scholar]
- Canlet C, Tremblay-Franco M, Gautier R, Molina J, Métais B, Blas-Y EF, Gamet-Payrastre L (2013) Specific metabolic fingerprint of a dietary exposure to a very low dose of endosulfan. Journal of Toxicology, 2013:545802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiri B, Holle E, Yu X, Baldwin WS (2012) Lentiviral-mediated rnai knockdown yields a novel mouse model for studying cyp2b function. Toxicological Sciences, 125:368–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B, Qatanani M, Moore DD (2009a) Constitutive androstane receptor mediates the induction of drug metabolism in mouse models of type 1 diabetes. Hepatology, 50:622–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B, Saha PK, Huang W, Chen W, Abu-Elheiga LA, Wakil SJ, Stevens RD, Ilkayeva O, Newgard CB, Chan L, Moore DD (2009b) Activation of nuclear receptor car ameliorates diabetes and fatty liver disease. Proceedings of the National Academy of Sciences U S A, 106:18831–18836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, Yermalitsky V, Ladd PA, Capdevila JH, Mernaugh R, Keeney DS (2005) Evidence that cytochrome p450 cyp2b19 is the major source of epoxyeicosatrienoic acids in mouse skin. Archives of Biochemistry and Biophysics, 435:125–133. [DOI] [PubMed] [Google Scholar]
- El-Sherbeni AA, Aboutabl ME, Zordoky BNM, Anwar-Mohamed A, El-Kadi AOS (2013) Determination of the dominant arachidonic acid cytochrome p450 monooxygenases in rat heart, lung, kidney, and liver: Protein expression and metabolite kinetics. The American Association of Pharmaceutical Scientists Journal 15:112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger JH, Smith CM, Hayamizu TF, McCright IJ, Eppig JT, Kadin JA, Richardson JE, Ringwald M (2011) The mouse gene expression database (gxd): 2011 update. Nucleic Acids Research, 39(suppl 1):D835–D841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RD, Henderson CJ, Scott CL, Wolf CR (2009) Unsaturated fatty acid regulation of cytochrome p450 expression via a car-dependent pathway. Biochemical Journal, 417:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxenberg RJ, McGarrigle BP, Knaak JB, Kostyniak PJ, Olson JR (2007) Human hepatic cytochrome p450-specific metabolism of parathion and chlorpyrifos. Drug Metabolism and Disposition, 35:189–193. [DOI] [PubMed] [Google Scholar]
- Gao J, He J, Zhal Y, Wada T, Xie W (2009) The constitutive androstane receptor is an anti-obesity nuclear receptor that improves insulin sensitivity. Journal of Biological Chemistry, 284:25984–25992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillen N, Acín S, Surra JC, Arnal C, Godino J, García-Granados A, Muniesa P, Ruiz-Gutiérrez V, Osada J (2009) Apolipoprotein e determines the hepatic transcriptional profile of dietary maslinic acid in mice. Journal of Nutritional Biochemistry, 20:882–893. [DOI] [PubMed] [Google Scholar]
- Hashita T, Sakuma T, Akada M, Nakajima A, Yamahara H, Ito S, Takesako H, Nemoto N (2008) Forkhead box a2-mediated regulation of female-predominant expression of the mouse cyp2b9 gene. Drug Metabolism and Disposition, 36:1080–1087. [DOI] [PubMed] [Google Scholar]
- Hernandez JP, Chapman LM, Kretschmer XC, Baldwin WS (2006) Gender specific induction of cytochrome p450s in nonylphenol-treated FVB/NJ mice. Toxicology and Applied Pharmacology, 216:186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez JP, Mota LC, Baldwin WS (2009a) Activation of car and pxr by dietary, environmental and occupational chemicals alters drug metabolism, intermediary metabolism, and cell proliferation. Current Pharmacogenomics and Personalized Medicine, 7:81–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez JP, Mota LC, Huang W, Moore DD, Baldwin WS (2009b) Sexually dimorphic regulation and induction of p450s by the constitutive androstane receptor (car). Toxicology, 256:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersman EM, Bumpus NN (2014) A targeted proteomics approach for profiling murine cytochrome p450 expression. Journal of Pharmacology and Experimental Therapeutics, 349:221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson E, Rose RL (2007) The importance of cytochrome p450 2b6 in the human metabolism of environmental chemicals. Pharmacology and Therapeutics, 113:420–428. [DOI] [PubMed] [Google Scholar]
- Honkakoski P, Zelko I, Sueyoshi T, Negishi M (1998) The nuclear orphan-receptor car-retinoid x receptor heterodimer activates the phenobarbital-responsive module of the cyp2b gene. Molecular and Cell Biology, 18:5652–5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isensee J, Witt H, Pregla R, Hetzer R, Retitz-Zagrosek V, Ruiz-Noppinger P (2007) Sexually dimorphic gene expression in the heart of mice and men. Journal of Molecular Medicine, 86:61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarukamjorn K, Sakuma T, Yamamoto M, Ohara A, Nemoto N (2001) Sex-associated expression of mouse hepatic and renal cyp2b enzymes by glucocorticoid hormones. Biochemical Pharmacology, 62:161–169. [DOI] [PubMed] [Google Scholar]
- Johri A, Yadav S, Dhawan A, Parmar D (2007) Overexpression of cerebral and hepatic cytochrome p450 alters behavioral activity of rat offspring following prenatal exposure to lindane. Toxicology and Applied Pharmacology, 225:278–292. [DOI] [PubMed] [Google Scholar]
- Keeney DS, Skinner C, Travers JB, Capdevila JH, Nanney LB, King LE Jr., Water MR (1998a) Differentiating keratinocytes express a novel cytochrome p450 enzyme, cyp2b19, having arachidonate monoxygenase activity. Journal of Biological Chemistry, 273:32071–32079. [DOI] [PubMed] [Google Scholar]
- Keeney DS, Skinner S, Weii S, Friedberg T, Waterman MR (1998b) A keratinocyte-specific epoxygenase, cyp2b12, metabolizes arachidonic acid with unusual selectivity, producing a single major epoxyeicosatrienoic acid. Journal of Biological Chemistry, 273:9279–9284. [DOI] [PubMed] [Google Scholar]
- Kumagai J, Fumimura T, Takahashi S, Urano T, Ogushi T, Hori-Inoue K, Ouchi Y, Kitamura T, Muramatsu M, Blumberg B, Inoue S (2007) Cytochrome p450 2b6 is a growth-inhibitory and prognostic factor for prostate cancer. Prostate, 67:1029–1037. [DOI] [PubMed] [Google Scholar]
- Kumar R, Mota LC, Litoff EJ, Rooney JP, Boswell WT, Courter E, Henderson CM, Hernandez JP, Corton JC, Moore DD, Baldwin WS (2017) Compensatory changes in cyp expression in three different toxicology mouse models: Car-null, cyp3a-null, and cyp2b9/10/13-null mice. PLOS One, 12:e0174355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladd PA, Du L, Capdevila JH, Mernaugh R, Keeney DS (2003) Epoxyeicosatrienoic acids activate transglutaminases in situ and induce cornification of epidermal keratinocytes. Journal of Biological Chemistry, 278:35184–35192. [DOI] [PubMed] [Google Scholar]
- Lassiter TL, Ryde IT, Mackillop EA, Brown KK, Levin ED, Seidler FJ, Slotkin TA (2008) Exposure of neonatal rats to parathion elicits sex-selective reprogramming of metabolism and alters the response to a high-fat diet in adulthood. Environmental Health Perspectives, 2008:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Marquardt M, Lech JJ (1998) Metabolism of nonylphenol by rat and human microsomes. Toxicology Letters, 99:117–126. [DOI] [PubMed] [Google Scholar]
- Leung A, Trac C, Du J, Natarajan R, Schones DE (2016) Persistent chromatin modifications induced by a high fat diet. Journal of Biological Chemistry, 291:10446–10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G, Zeldin DC, Blaisdell JA, Hodgson E, Goldstein JA (1998) Cloning and expression of murine cyp2cs and their ability to metabolize arachidonic acid. Archives of Biochemistry and Biophysics, 357:45–57. [DOI] [PubMed] [Google Scholar]
- Maayah ZH, Elshenawy OH, Althurwi HN, Abdelhamid G, El-Kadi AO (2015) Human fetal ventricular cardiomyocyte, rl-14 cell line, is a promising model to study drug metabolizing enzymes and their associated arachidonic acid metabolites. Journal of Pharmacology and Toxicology Methods, 71:33–41 [DOI] [PubMed] [Google Scholar]
- Maglich JM, Lobe DC, Moore JT (2009) The nuclear receptor car (nr1i3) regulates serum triglyceride levels under conditions of metabolic stress. Journal of Lipid Research, 50:439–445. [DOI] [PubMed] [Google Scholar]
- Masuno H, Okamoto S, J I, Honda K, Shiosaka T, Kidani T, Sakayama K, Yamamoto H (2003) Effect of 4-nonylphenol on cell proliferation and adipocyte formation in cultures of fullly differentiated 3t3-l1 cells. Toxicological Sciences, 75:314–320. [DOI] [PubMed] [Google Scholar]
- McGregor RA, Kwon E-Y, Shin S-K, Jung UJ, Kim E, Park JHY, Yu R, Yun JW, Choi M-S (2013) Time-course microarrays reveal modulation of developmental, lipid metabolism and immune gene networks in intrascapular brown adipose tissue during the development of diet-induced obesity. International Journal of Obesity, 37:1524–1531. [DOI] [PubMed] [Google Scholar]
- Mota LC, Barfield C, Hernandez JP, Baldwin WS (2011) Nonylphenol-mediated cyp induction is pxr-dependent: The use of humanized mice and human hepatocytes suggests that hpxr is less sensitive than mouse pxr to nonylphenol treatment. Toxicology and Applied Pharmacology, 252:259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota LC, Hernandez JP, Baldwin WS (2010) Constitutive androstane receptor-null mice are sensitive to the toxic effects of parathion: Association with reduced cytochrome p450-mediated parathion metabolism. Drug Metab Dispos, 38:1582–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller PY, Janovjak H, Miserez AR, Dobbie Z (2002) Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques, 32:1372–1379. [PubMed] [Google Scholar]
- Nakamura K, Moore R, Negishi M, Sueyoshi T (2007) Nuclear pregnane x receptor cross-talk with foxa2 to mediate the drug-induced regulation of lipid metabolism in fasting mouse liver. Journal of Biological Chemistry, 282:9768–9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, Yoo B, Gunewardena SS, Lu H, Klaassen CD, Zhong X-B (2012) RNA sequencing reveals dynamic changes of mrna abundance of cytochromes p450 and their alternative transcripts during mouse liver development. Drug Metabolism and Disposition, 40:1198–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy DH, Barthold SW (2001). Pathology of laboratory rodents and rabbits. Iowa State University Press, Ames, IA. [Google Scholar]
- Roling JA, Bain LJ, Baldwin WS (2004) Differential gene expression in mummichogs (Fundulus heteroclitus) following treatment with pyrene: Comparison to a creosote contaminated site. Marine Environmental Research, 57:377–395. [DOI] [PubMed] [Google Scholar]
- Slotkin TA (2011) Does early-life exposure to organophosphate insecticides lead to prediabetes and obesity? Reproductive Toxicology, 31:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuck MK, Chan DW, Chia D, Godwin AK, Grizzle WE, Krueger KE, Rom W, Sanda M, Sorbara L, Stass S, Wang W, Brenner DE (2009) Standard operating procedures for serum and plasma collection: Early detection research network consensus statement. Journal of Proteome Research, 8:113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Agriculture, Agricultural Research Service, 2007. USDA nutrient database for standard reference, release 20. Retrieved November 15, 2011 fromvthe nutrient data laboratory home page: http://www.ars.usda.gov/ba/bhnrc/ndl.
- Van der Hoeven TA, Coon MJ (1974) Preparation and properties of partially purified cytochrome p450 and nadph-cytochrome p450 reductase from rabbit liver microsomes. Journal of Biological Chemistry, 249:6302–6310. [PubMed] [Google Scholar]
- Wada K, Sakamoto H, Nishikawa K, Sakuma S, Nakajima A, Fujimoto Y, Kamisaki Y (2007) Life style-related diseases of the digestive system: Endocrine disruptors stimulate lipid accumulation in target cells related to metabolic syndrome. Journal of Phamacological Sciences, 105:133–137. [DOI] [PubMed] [Google Scholar]
- Wahlang B, Falkner KC, Gregory B, Ansert D, Young D, Conklin DJ, Bhatnagar A, McClain CJ, Cave M (2013) Polychlorinated biphenyl 153 is a diet-dependent obesogen that worsens nonalcoholic fatty liver disease in male C57Bl6/J mice. Journal of Nutritional Biochemistry, 24:1587–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Tompkins LM (2008) Cyp2b6: New insights into a historically overlooked cytochrome p450 isozyme. Current Drug Metabolism, 9:598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, Chamberlain M, Vassieva O, Henderson CJ, Wolf CR (2005) Relationship between hepatic phenotype and changes in gene expression in cytochrome p450 reductase (por) null mice. Biochemical Journal, 388:857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman DJ (1988) Interactions of hepatic cytochromes p-450 with steroid hormones: Regioselectivity and stereoselectivity of steroid metabolism and hormonal regulation of rat p-450 enzyme expression. Biochemical Pharmcology, 37:71–84. [DOI] [PubMed] [Google Scholar]
- Wei P, Zhang J, Egan-Hafley M, Liang S, Moore DD (2000) The nuclear receptor car mediates specific xenobiotic induction of drug metabolism. Nature, 407:920–923. [DOI] [PubMed] [Google Scholar]
- Wiwi CA, Gupte M, Waxman DJ (2004) Sexually dimorphic p450 gene expression in liver-specific hepatocyte nuclear factor 4a-deficient mice. Molecular Endocrinology, 18:1975–1987. [DOI] [PubMed] [Google Scholar]
- Wolfrum C, Asilmaz E, Luca E, Friedman JM, Stoffel M (2004) Foxa2 regulates lipid metabolism and ketogenesis in the liver during fasting and in diabetes. Nature, 432:1027–1032. [DOI] [PubMed] [Google Scholar]
- Yamada H, Ishii Y, Yamamoto M, Oguri K (2006) Induction of the hepatic cytochrome p450 2b subfamily by xenobiotics: Research history, evolutionary aspect, relation to tumorigenesis, and mechanism. Current Drug Metabolism, 7:397–409. [DOI] [PubMed] [Google Scholar]
- Yoshinari K, Sato T, Okino N, Sugatani J, Miwa M (2004) Expression and induction of cytochromes p450 in rat white adipose tissue. Journal of Pharmacology and Experimental Therapeutics, 311:147–154. [DOI] [PubMed] [Google Scholar]
- Yoshinari K, Takagi S, Sugatani J, Miwa M (2006) Changes in the expression of cytochromes p450 and nuclear receptors in the liver of genetically diabetic db/db mice. Biological and Pharmaceutical Bulletin, 29:1634–1638. [DOI] [PubMed] [Google Scholar]
- Zhang QY, Dunbar D, Kaminsky LS (2003) Characterization of mouse small intestinal cytochrome p450 expression. Drug Metabolism and Disposition, 31:1346–1351. [DOI] [PubMed] [Google Scholar]


