Abstract
Background:
The chemokine CCL25, and its receptor CCR9, constitute a unique chemokine/receptor pair, which regulates trafficking of T lymphocytes to the small intestine under physiological conditions and is an attractive target for small bowel Crohn’s disease drug development. We have previously shown that CCL25/CCR9 interactions regulate the recovery from acute dextran sulfate sodium–induced colonic inflammation. In this study, we explored whether these interactions also regulate chronic colitis development in 2 independent murine models of experimental colitis.
Methods:
Histological flow cytometry and qPCR analyses were performed to evaluate the role of CL25 and CCR9 in chronic colonic inflammation induced by serial exposures to dextran sulfate sodium salts or by adoptive transfer of CD45RBhi CD4+ T cell into lymphopenic mice devoid of CCL25/CCR9 interactions.
Results:
Chronic dextran sulfate sodium exposure results in exacerbated colitis in mice deficient for either CCR9 or CCL25 when compared with wild-type control mice. Although CCR9-deficient T cells traffic to the colon and induce severe colitis similar to wild-type T cells in the CD45RB transfer model, naive wild-type T cells induce more severe disease in recipient animals devoid of CCL25 expression.
Conclusions:
CCL25/CCR9 interactions are required for modulating protection against large intestinal inflammation in 2 models of chronic colitis. These data may have implications for the potential effects of disrupting CCL25/CCR9 interactions in humans in the setting of intestinal disorders including inflammatory bowel disease.
Keywords: CCL25, CCR9, colitis, T cell, innate immune cell
Human inflammatory bowel diseases (IBD) can be broadly subcategorized into Crohn’s disease (CD) and ulcerative colitis, which affect the small and the large bowel, respectively.1,2 These are complex chronic diseases associated with intestinal inflammation of unknown etiology, but several factors have been implicated including genetic predisposition, microbial dysbiosis, and other environmental triggers, and aberrant adaptive and/or innate mucosal immune function. Studies in various colitis models have highlighted a critical role for the balance between effector and regulatory T-cell populations and cross talk with innate immune cells in maintaining mucosal homeostasis and preventing intestinal inflammation.3
Chemokines and their receptors play a major role in recruiting T cells in health and disease to sites of injury.4 In health, CD4+ and CD8+ effector/memory T-cell trafficking to the small intestine is mediated by CCR9- and α4β7-mediated cell surface interactions with CCL255–8 and MadCAM-1,9 respectively, whereas trafficking to the large intestine is mediated at least in part by α4β7/MadCAM-1 interactions.10–12 CCL25 is expressed constitutively by thymic and small intestinal epithelial cells in mice and in humans.13–15 Upregulation of CCL25 expression, along with concomitant increase in CCR9+ CD4+ T cells, are found in the inflamed small bowel of wild-type (WT) mice during inflammatory states16 or SAMP1/YitFC mice (which develop spontaneous enteritis) and in the small intestine of patients with CD,17,18 suggesting that such interactions mediate recruitment of T cells to inflamed sites, further contributing to intestinal inflammation. Indeed, these interactions are the target of an anti-CCR9 agent that is being evaluated in patients with CD with active inflammation.19
Although augmented T-cell effector responses are associated with colonic inflammation in the setting of IBD,20 the mechanisms underlying how T-cell migration to the large intestine is modulated during inflammation is largely unknown.10–12 We previously reported increased colonic expression of CCL25 on recovery of acute dextran sodium sulfate (DSS)–mediated colitis in mice.21 In addition, acute DSS administration in Ccr9−/− and Ccl25−/− mice led to exacerbated large intestinal inflammation.21 These data suggested that CCL25/CCR9 interactions may be important for modulating T-cell responses in the colon, during induction and recovery phases of acute colitis. In this study, we aimed to determine the role of CCL25/CCR9 interactions in the setting of chronic inflammation using 2 independent models. Our results show that conventional and regulatory T cells (Tregs) do not require CCR9 expression to traffic into and function in the inflamed colonic lamina propria (cLP). However, colitic mice devoid of CCL25/CCR9 interactions display exacerbated colitis in association with altered innate immune cell distribution.
MATERIALS AND METHODS
Animals
The generation of Ccr9−/− and Ccl25−/− mice has been described previously.8,22 Rag1−/− mice were purchased from The Jackson Laboratories (Bar Harbor, ME). Rag1−/−Ccl25−/− were generated by crossing Ccl25−/− onto Rag1−/− mice and kept at the homozygous state. All strains of mice including C57Bl/6 control mice were bred in our animal facility at Boston Children’s Hospital, born and held in the same room under specific pathogen-free conditions. To avoid variations of commensal bacteria as a confounding factor in our experiments, littermate controls were used. All animal experiments were approved by Boston Children’s Hospital Institutional Animal Care and Use Committee. Boston Children’s Hospital’s assurance number is A3303-01. Boston Children’s Hospital is accredited by AAALAC International. All efforts were made to minimize suffering of animals.
DSS-mediated Chronic Colitis
Sex- and age-matched WT and Ccr9−/− mice received 1.5% to 2% DSS (36–50 kDa; MP Biomedicals, LCC, Solon, OH) in the drinking water for 4 cycles consisting of 7-day DSS administration followed by 7-day water administration. The animal weight was recorded daily. For flow cytometry, histology, and mRNA analysis, mice were euthanized at indicated time points, with day 0 corresponding to the initiation of DSS treatment.
T cell Transfer-mediated Chronic Colitis
For CD45RBhi transfers and CD45RBhi and CD45RBlo cotransfers into Rag1−/− and Rag1−/−Ccl25−/− mice, spleens (SPLs) were harvested from WT donor mice. CD4+ T cells were enriched by negative selection using a depletion monoclonal antibody (mAb) cocktail (consisting of unconjugated anti-B220, anti-CD8α, anti-CD11b, anti-CD11c, anti-Gr-1, anti-Dx5, and anti-NK1.1 mAbs [BioXcell, West Lebanon, NH], followed by Rat anti-Mouse Igk chain conjugated magnetic beads [Miltenyi Biotec, Auburn, CA]). Enriched CD4+ T cells subsequently sorted by flow cytometry on a MoFlo Fluorescence Activated Cell Sorter (DakoCytomation, Carpinteria, CA) into 2 fractions: CD45RBhi CD4+ to induce colitis in immunocompromised mice and CD45RBlo CD4+ to prevent colitis induction when cotransferred with CD45RBhi CD4+ T cells. Postsort purity was typically >98%. Age-matched lymphopenic recipient Rag1−/− mice (lacking or not CCL25 expression) were injected with 4 × 105 CD45RBhi CD4+ T cells with or without 2 × 105 CD45RBlo CD4+ T cells. Mice were typically analyzed between week 8 and 10 after transfer.
Preparation of Cell Suspensions
SPLs and mesenteric lymph nodes (mLNs) were harvested in HBSS with Ca2+ and Mg2+ supplemented with 2% fetal calf serum and 10 mM HEPES. For CD4+ T cell investigation, SPL and mLN cell suspensions were prepared with frosted slides and filtered through nylon mesh after red blood cell lysis. For analyses of innate immune cell, SPL and mLN suspensions were obtained by collagenase VIII digestion (Sigma-Aldrich, St. Louis, MO) for 30 minutes at 37°C. Colonic lamina propria lymphocyte suspensions were obtained as previously described21: colons were flushed in phosphate-buffered saline 1× and opened longitudinally. To remove intraepithelial lymphocytes and epithelial cells, intestinal pieces were incubated in HBSS without Ca2+/Mg2+ supplemented with 10 mM EDTA, 10 mM HEPES, 0.5% fetal calf serum, and 1.5 mM DTET, for 2 × 20 minutes at 37°C. Intestinal pieces were digested in HBSS with Ca2+/Mg2+, 20% fetal calf serum, 100 U/mL collagenase VIII, and 5 μg/mL DNase (Sigma-Aldrich) for 60 to 90 minutes at 37°C. Lamina propria lymphocytes were purified over a 40% to 100% Percoll gradient (GE Healthcare Bio-Sciences Corp, Piscataway, NJ).
Flow Cytometry Analysis
Cell suspensions from SPL, mLN, and cLP were analyzed by flow cytometry. Conventional CD4+ T cells and Tregs were stained with anti-CD3ε and anti-CD4 mAbs (BD Biosciences, San Jose, CA) and intracytoplasmic anti-foxp3 mAbs (eBioscience, San Diego, CA). Neutrophils were stained with Gr-1-FITC, CD11b-PECy7, and Ly6G-AF647 mAbs (eBioscience). Plasmacytoid dendritic cells (pDCs) and conventional dendritic cells (cDCs) were stained with MHCII-FITC, CD11b-PECy7, PDCA-1-AF647, and CD11c-APCCy7 mAbs with a lineage-negative mAb cocktail containing B220-biotin, CD3ε-biotin, Ly6G-biotin (eBioscience) and PerCP-Cy5.5-conjugated streptavidin. The cDC subsets were stained with MHCII-FITC, CD11c-APCCy7, CD11b-PECy7, and CD103-PE mAbs with a lineage-negative mAb cocktail containing B220-biotin, CD3ε-biotin, Ly6G-biotin (eBioscience) and PerCP-Cy5.5-conjugated streptavidin (eBioscience). Monocytes and macrophages (MΦ) were stained with Gr-1-FITC, MHCII-PE, Ly6C-PerCP-Cy5.5, CD11b-PECy7, Ly6G-APC, and CD11c-APCCy7 mAbs (eBioscience). Blocking of FcγR binding was performed using mouse and rat serum (Jackson ImmunoResearch, West Grove, PA). Cells were analyzed on a FACS Canto II (BD Biosciences). Data were collected using FACS Diva software (BD Biosciences) and analyzed with FlowJo software (TreeStar, Ashland, OR).
Histology and IBD and Clinical Scoring
Intestinal samples of the distal colon were harvested, fixed in 10% formalin, and embedded in paraffin for hematoxylin and eosin (H&E) staining. Histological IBD scoring was performed blindly by a pathologist as follows: IBD scores corresponding to 0 = normal, 1 = mild, 2 = moderate, and 3 = severe were attributed to activity grade, changes of crypt architecture, basal lymphoplasmacytosis, expansion of lamina propria, and epithelial hyperplasia. Scores were graphed in a total range of 0–15.
mRNA Quantification
Total RNA was extracted with TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer’s guidelines. Quantification of total RNA was performed with a NanoDrop spectrophotometer (Thermo Scientific, Nanodrop Technologies, Wilmington, DE). For real-time RT-PCR, cDNA was synthesized with iScript Select cDNA Synthesis kit (Biorad, Hercules, CA). Amplification was performed with the CFX96 quantitative PCR System (Biorad) and iQ SYBR Green Supermix (Biorad) on 50 ng cDNA. Transcripts were quantified using HPRT mRNA for normalization. Primer sequences are available upon request.
Statistical Analysis
Weight loss, IBD scoring, and cellular distributions were compared using either Student’s t test or ANOVA. Differences with P < 0.05 were considered significant. Statistical analysis was performed using Prism (Graph Pad Software, La Jolla, CA).
RESULTS
DSS-mediated Chronic Colitis Is Exacerbated in Ccr9−/− Mice
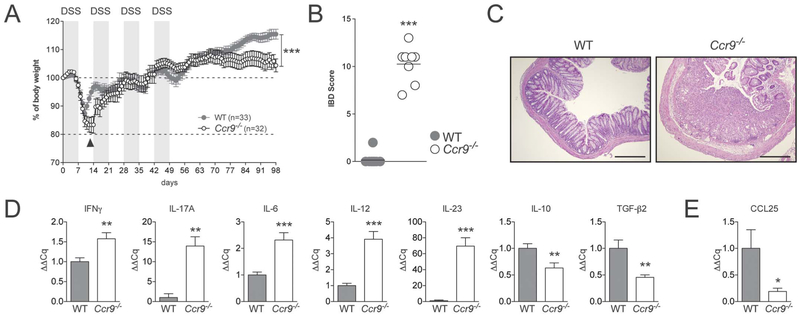
We previously reported that Ccr9−/− and Ccl25−/− mice are more susceptible to acute DSS colitis than WT controls.21 As human ulcerative colitis is associated with signs of chronic colonic inflammation, we sought to assess whether the increased susceptibility to acute inflammation associated with defective CCL25/CCR9 interactions would also translate into increased susceptibility to chronic inflammation. WT and Ccr9−/− mice were exposed to DSS in drinking water for 4 cycles and monitored daily (Fig. 1). As previously reported, Ccr9−/− mice showed delayed weight loss recovery after the first DSS exposure (Fig. 1A, arrowhead, and Wurbel et al21), but these mice recovered without any evidence of increased chronic inflammation after each of 3 additional DSS cycles. However, 3 to 4 weeks after the last DSS exposures, Ccr9−/− mice and Ccl25−/− mice stopped thriving and failed to gain weight (Fig. 1A and data not shown). In addition, analysis of H&E-stained colonic sections revealed that Ccr9−/− mice exhibited severe colonic inflammation, whereas WT mice had fully recovered without evidence of histological signs of colitis (Fig. 1B, C). As evidenced by qPCR RNA analyses from colonic tissue, the exacerbation of chronic colonic inflammation in CCR9−/− animals was associated with a mixed Th1/Th17 immune response (Fig. 1D). In contrast, regulatory cytokines (IL-10 and TGFb2) and CCL25 transcripts were down-regulated in tissues from Ccr9−/− mice when compared with WT mice (Fig. 1D, E). Taken together, these data indicate that CCL25/CCR9 interactions are required to modulate the severity of DSS-mediated chronic colonic inflammation.
FIGURE 1.
Ccr9−/− mice show signs of exacerbated DSS-mediated chronic colitis. A, WT and Ccr9−/− mice were subjected to 4 DSS cycles (consisting of 2% DSS administration for 7 d in drinking water followed by water for 7 d). Weight was monitored daily and weight loss was calculated as percent of initial body weight. Data represent the mean of 4 independent experiments. Arrowhead highlights differences between WT and Ccr9−/− mice after first DSS cycle. B, IBD scores of chronic DSS-treated WT and Ccr9−/− mice were determined blindly by a pathologist after H&E staining of distal colonic tissue sections. C, Representative H&E staining of colonic tissue sections harvested in WT (left) and Ccr9−/− mice (right) >100 days after initiation of DSS exposure (10× magnification). D, mRNA profiling of proinflammatory and anti-inflammatory cytokines in colonic tissues from DSS-treated WT and Ccr9−/− mice. The mRNA transcripts were quantified by qPCR and normalized to the housekeeping gene HPRT. Data represent 1 out of 4 experiments. E, CCL25 transcript qPCR analysis in colons harvested from WT and Ccr9−/− mice treated with DSS. Data represent an individual experiment out of 4. *P < 0.05; **P < 0.005; ***P < 0.0005.
CD4+ T cells Home to the Large Bowel and Induce Colitis Independent of CCR9
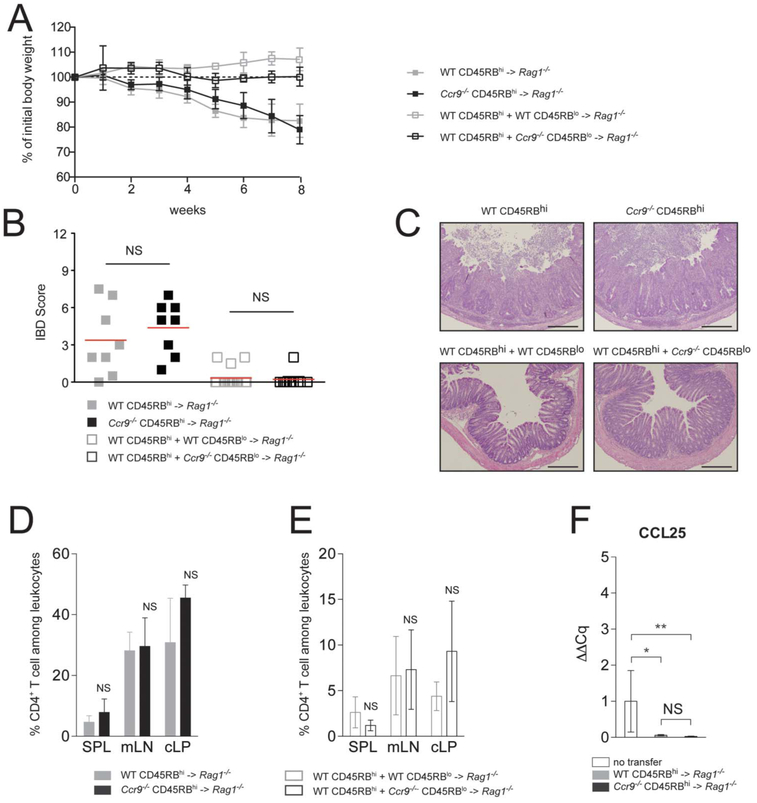
We next used the CD45RBhi transfer model to assess the role CCL25/CCR9 interactions in regulating a T cell–mediated chronic colitis model. In this model, colitis induction by naive CD45RBhi CD4+ cells into lymphopenic mice can be prevented by the cotransfer of CD45RBlo CD4+ T cells (which contain naturally occurring thymically derived FOXP3+ regulatory T cells, nTregs).3 To determine the role of CCR9 on colonic homing and effector T-cell colitic activity, we adoptively transferred naive WT or Ccr9−/− CD45RBhi CD4+ T cells into Rag1−/− recipient mice (Fig. 2). Rag1−/− recipient animals receiving either WT (gray squares) or Ccr9−/− (black squares) CD45RBhi CD4+ T cells developed comparable signs of inflammation (Fig. 2A–C). Both groups of mice gradually lost 10% to 20% of their initial body weight (Fig. 2A) and developed severe inflammation (Fig. 2B, C). The percentages of CD4+ T cells in SPL, mLN, and cLP after transfer of WT (gray bars) or Ccr9−/− (black bars) CD45RBhi CD4+ colitogenic T cells into Rag1−/− recipient mice were comparable (Fig. 2D, E). Inflammatory cytokine elevations were also similar in colitic colons independent of CCR9 expression in recipient mice after CD45RB transfer (see Fig., Supplemental Digital Content 1, http://links.lww.com/IBD/A470). CCL25 transcripts were markedly reduced in colitic mice upon transfer of either WT or Ccr9−/− CD45RBhi CD4+ T cells (Fig. 2F). As this chemokine is expressed by both thymic and intestinal epithelial cells,13 this finding may result from altered CCL25 epithelial expression in the setting of inflammation. To assess the role of CCR9 on nTreg activity, we adoptively cotransferred WT CD45RBhi naive CD4+ T cells with either WT or Ccr9−/− CD45RBlo’ CD4+ T cells. In cotransfer experiments, both WT and Ccr9−/− CD45RBlo prevented colitis development as depicted by absence of both weight loss (Fig. 2A) and evidence of intestinal inflammation (Fig. 2B, C), indicating normal trafficking and in vivo suppressive function of Tregs in the absence of CCR9 expression. Cotransfer of WT CD45RBhi CD4+ T cells with either WT CD45RBlo (open gray bars) or Ccr9−/− CD45RBlo (open black bars) CD4+ T cells resulted in similar distribution of CD4+ T cells in lymphoid tissues (Fig. 2E). The maintenance of Tregs was also not affected by CCR9 expression in cotransfer experiments (data not shown). In addition, T-cell suppression assays indicated that the suppressive function of CCR9-deficient Tregs in vitro was not impaired (data not shown). Taken together, these data suggest that in vivo trafficking and function of colitogenic T cells and Tregs to the large intestinal mucosa occur independent of CCR9 expression.
FIGURE 2.
CD45RBhi CD4+ T cells and CD45RBlo CD4+ T cell home to the large bowel independent of CCR9 expression. A, Weight loss monitoring of Rag1−/− recipient mice adoptively transferred with either WT CD45RBhi (filled gray squares) or Ccr9−/− CD45RBhi (filled black squares) CD4+ T cells and in Rag1−/− recipient mice cotransferred with WT CD45RBhi CD4+ T cells and either WT CD45RBlo (open gray squares) or Ccr9−/− CD45RBlo (open black squares) CD4+ T cells. Mice were monitored weekly and weight loss was reported and expressed as percentage of initial body weight (mean ± SD). Data represent 3 pooled experiments (n = 15). B, IBD scores of distal colons of paraffin-embedded sections of Rag1−/− mice are after transfer of either WT CD45RBhi CD4+ T cell transfer alone (filled gray squares) or with WT CD45RBlo CD4+ T cells (open gray squares), with Ccr9−/− CD45RBlo CD4+ T cells (open black squares), or Ccr9−/− CD45RBhi CD4+ T cell transfer alone (filled black squares) (mean ± SD). Data represent 3 pooled experiments. NS, not significant (P values). C, Representative H&E staining of colonic sections harvested in Rag1−/− mice transferred with either WT CD45RBhi or Ccr9−/− CD45RBhi CD4+T cells alone, or WT CD45RBhi T cells cotransferred with either WT CD45RBlo or Ccr9−/− CD45RBlo CD4+ T cells (10× magnification). D, Percentage of CD4+ T cells among leukocytes determined in SPL, mLN, and cLP from Rag1−/− mice transferred with either WT or Ccr9−/− CD45RBhi CD4+ T cells. E, Percentage of CD4+ T cells among leukocytes was determined in SPL, mLN, and cLP determined in Rag1−/− mice transferred with either WT CD45RBhi CD4+ T cells and WT CD45RBlo CD4+ T cells or WT CD45RBhi CD4+ T cells and Ccr9−/− CD45RBlo CD4+ T cells. Mean ± SD values of 3 pooled experiments are shown. F, A qPCR analysis of CCL25 transcript levels in Rag1−/− colitic host mice transferred with either WT or Ccr9−/− CD45RBhi CD4+ T cells after normalization to HPRT and to untransferred Rag1−/− mice (mean ± SD). Data represent of 1 out of 3 experiments. *P < 0.05; **P < 0.005.
CCL25 Deficiency Leads to Exacerbated T cell–mediated Chronic Colitis
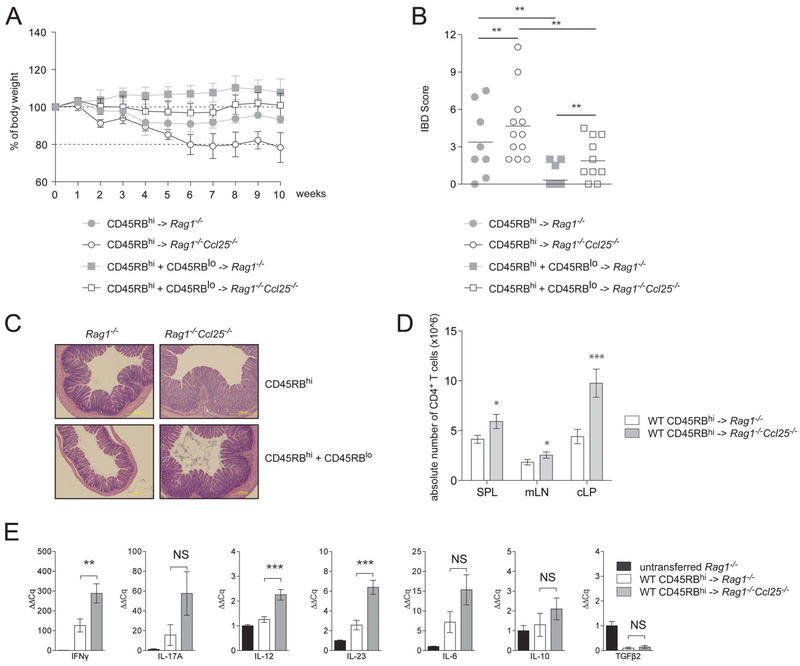
We next sought to assess the effect on chronic colitis development in animals that were devoid of the CCR9 ligand, CCL25. We adoptively transferred sorted WT CD45RBhi CD4+ T cells into either Rag1−/− or Rag1−/−Ccl25−/− mice (Fig. 3). WT CD45RBhi CD4+ T cell transfer into Rag1−/−Ccl25−/− recipient mice, when compared with Rag1−/− recipient mice, developed increased weight loss (Fig. 3A), splenomegaly, and exacerbated colonic inflammation (Fig. 3B, C and see Fig., Supplemental Digital Content 2, http://links.lww.com/IBD/A471). Moreover, the overall cellularity in SPL, mLN, and cLP was significantly higher in Rag1−/−Ccl25−/− mice when compared with Rag1−/− mice (data not shown). In addition, the overall number of CD4+ T cells was increased in the SPL, mLN, and cLP of Rag1−/−Ccl25−/− mice (Fig. 3D). Transfer of WT CD45RBhi CD4+ T cells into Rag1−/−Ccl25−/− recipient mice was also associated with increased proinflammatory cytokine transcript levels when compared with Rag1−/− recipient mice (Fig. 3E). In contrast to studies by Mizuno et al36 who reported small bowel inflammation in Rag2−/−Ccr9−/− recipient mice that received WT CD45RBhi CD4+ T cells, ileitis was not observed in Rag1−/−Ccl25−/− recipient mice after T cell transfer (see Fig., Supplemental Digital Content 2, http://links.lww.com/IBD/A471). Together, incorporating the results from the last section, these data suggest that while CCR9 expression on T cells does not modulate T cell–mediated colitis in immunodeficient hosts, expression of CCL25 in recipient mice regulates the severity of T cell–mediated colitis development.
FIGURE 3.
Rag1−/−Ccl25−/− mice display exacerbated T cell-mediated chronic colitis. A, Sex- and aged-matched Rag1−/− and Rag1−/−Ccl25−/− mice were adoptively transferred with WT CD45RBhi or CD45RBhi and CD45RBlo CD4+ T cells to respectively induce or protect from experimental T cell-mediated colitis. Mice were monitored weekly and weight loss was reported and expressed as the percentage of initial body weight (mean ± SD). Data representative of 5 pooled experiments. B, Comparison of IBD scores obtained by H&E histological examination of paraffin-embedded sections from distal colons. Rag1−/− recipient mice are depicted in filled gray circles (after CD45RBhi transfer) or filled gray squares (after CD45RBhi and CD45RBlo cotransfers), and Rag1−/−Ccl25−/− mice are depicted in open circles (after CD45RBhi transfer) or open squares (after CD45RBhi and CD45RBlo cotransfers). C, Representative H&E staining of Rag1−/− and Rag1−/−Ccl25−/− recipient mice after transfer of CD45RBhi CD4+ T cells or after cotransfer of CD45RBhi and CD45RBlo CD4+ T cells (10× magnification). D, Absolute CD4+ T-cell numbers among leukocytes quantified by flow cytometry in Rag1−/− and Rag1−/−Ccl25−/− SPL, mLN, and cLP after CD45RBhi CD4+ T cell transfer (mean ± SD). Data are representative of 5 pooled experiments. E, The qPCR mRNA profiling in Rag1−/− and Rag1−/−Ccl25−/− colons after transfer of WT CD45RBhi CD4+ T cells. Normalization was performed to HPRT and untransferred Rag1−/− control mice. Data represent of 1 of 5 experiments. *P < 0.05; **P < 0.005; ***P < 0.0005; NS, not significant.
Treg Development and Function Are Independent of CCL25/CCR9 Interactions
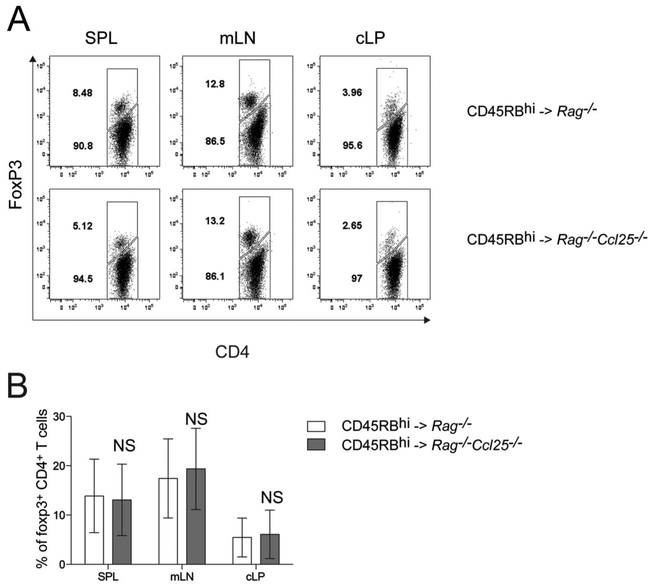
To assess the role of CCL25 expression on the function of WT Tregs in suppressing colitogenic T cells, WT CD45RBhi CD4+ T cells were cotransferred with WT CD45RBlo CD4+ T cells (containing nTregs) in either Rag1−/− or Rag1−/−Ccl25−/− recipient mice. Colitis development was similarly suppressed in Rag1−/− and Rag1−/−Ccl25−/− recipient mice indicating that WT nTreg function in vivo is independent of CCL25/CCR9 interactions (Fig. 3A–C). One potential explanation for the increased colitis observed in Rag1−/−Ccl25−/− recipient mice after naive CD4+ T cell transfer might be the aberrant generation of inducible Tregs (iTregs) from naive T cells in the setting of CCL25 deficiency. Therefore, to test whether generation of iTreg is deficient in Rag1−/−Ccl25−/− host mice after naive CD45RBhi CD4+ T cells, we analyzed the generation of CD4+ Foxp3+ T cells 8 weeks after transfer (Fig. 4). Generation of iTreg was similar between Rag1−/− (white bars) and Rag1−/−Ccl25−/− (gray bars) recipient mice (Fig. 4A, B). Collectively, these data indicate that the development and function of Tregs is independent of CCL25/CCR9 interactions.
FIGURE 4.
Generation of inducible Tregs (iTregs) is not impaired in the absence of CCL25. A, Data represent the percentage of CD4+ Foxp3-positive iTregs in SPL, mLN, and cLP of Rag1−/− and Rag1−/−Ccl25−/− mice 8 weeks after transfer of naive CD4+ T cells. Data show 1 representative experiment out of 5. B, Mean ± SD percentages of CD4+ T cells that are Foxp3+ pooled from 3 independent experiments. NS, not significant.
Increased Neutrophils in Colitic Mice Devoid of CCL25/CCR9 Interactions
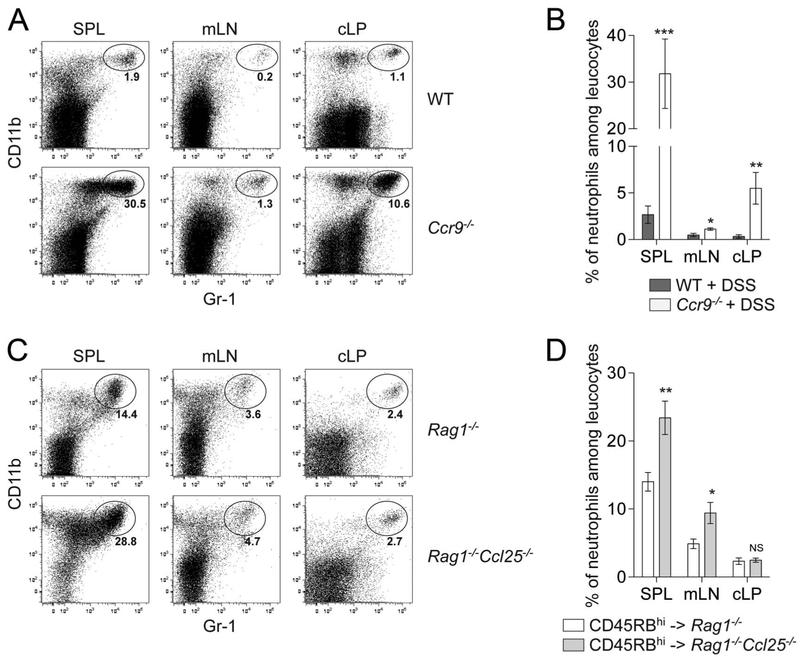
Neutrophil recruitment and activation are key steps in the intestinal innate immune response observed in IBD,23 and studies with animal models of colitis highlight the relationship between neutrophil infiltration and disease severity.24,25 We characterized neutrophil infiltration in chronic DSS-mediated colitis in Ccr9−/− mice and in Rag1−/−Ccl25−/− recipient mice upon naive CD4+ T cell transfer (Fig. 5). Flow cytometry analyses revealed increased Gr-1hi CD11bhi neutrophils in the SPL, mLN, and cLP of Ccr9−/− DSS-treated mice (Fig. 5A, B) and in the SPL and mLN, but not cLP, of Rag1−/−Ccl25−/− mice upon naive CD4+ T cell transfer (Fig. 5C, D). Together, these data demonstrate that enhanced neutrophil levels correlate with colitis severity seen in 2 models of colitis associated with defective CCL25/CCR9 interactions.
FIGURE 5.
Increased frequencies of circulating and intestinal granulocytes. Granulocytes defined by Gr-1hi (Ly6Ghi Ly6Clo) CD11bhi CD11− MHCII− expression were analyzed by flow cytometry in SPL, mLN, and cLP of WT versus Ccr9−/− mice upon chronic DSS-mediated colitis (A and B) and of Rag1−/− versus Rag1−/−Ccl25−/− mice adoptively transferred with WT CD45RBhi CD4+ T cells (C and D). A and C, Display representative flow cytometry dot plots of Gr-1hi CD11bhi granulocytes. B and D, Display granulocyte quantification in SPL, mLN, and cLP of mice developing chronic colitis (mean ± SD). Data representative of 5 pooled experiments. *P < 0.05; **P < 0.005; ***P < 0.0005; NS, not significant.
Altered Conventional Dendritic Cell Subset Distribution in Colitic Mice Devoid of CCL25/CCR9 Interactions
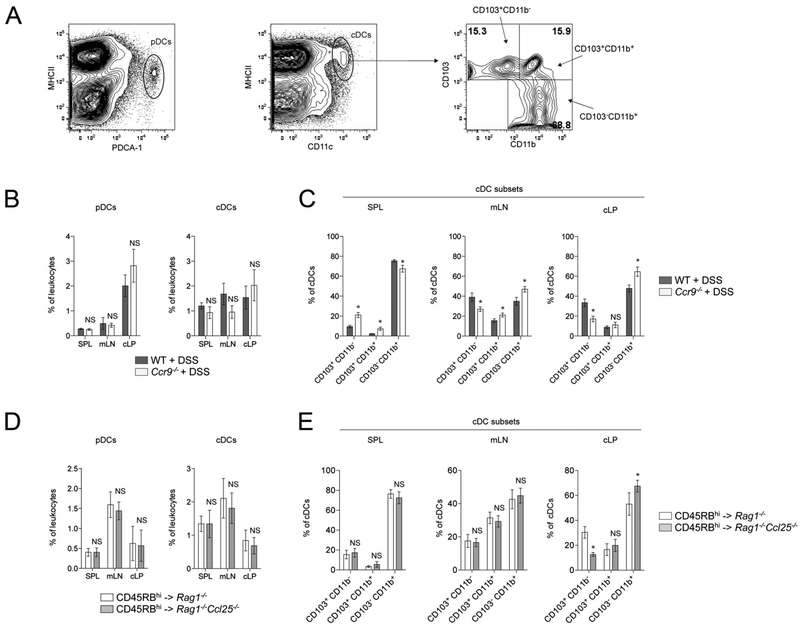
We next hypothesized that CCL25 CCR9 interactions may play a role in innate immune cell distribution upon colonic inflammation because CCL25/CCR9 interactions were not necessary in effector and regulatory functions of CD4+ T cells. We analyzed the distribution of dendritic cell (DC) populations in SPL, mLN, and cLP of Ccr9−/− DSS-treated mice and Rag1−/−Ccl25−/− mice upon naive CD4+ T cell transfer (Fig. 6). The percentage of pDC and cDC were comparable in all lymphoid organs isolated from DSS-treated Ccr9−/− mice and Rag1−/−Ccl25−/− recipient mice upon naive CD4+ T cell transfer (Fig. 6B, D). Recently, the characterization of conventional DC subsets in the intestinal LP has been intensely studied. Based on the differential expression of CD103 and CD11b markers, cDC subset display distinct functions (reviewed in Persson et al26). We analyzed cDC subsets and observed that CD103+CD11b− cDCs were decreased in cLP, whereas CD103−CD11b+ cDCs were increased in the inflamed colons of DSS-treated Ccr9−/− intestinal mucosa and Rag1−/−Ccl25−/− recipient mice receiving WT naive T cells (Fig. 6C, E). In addition, the overall percentage of CD103+ cDCs (CD11b+ and CD11b−), a DC subset that has been implicated in the generation and function of iTregs,26 was decreased in the inflamed cLP of both DSS-treated Ccr9−/− mice (28.2 ± 3.2% Ccr9−/− cLP versus 42.3 ± 4.3% WT cLP, P = 0.0120) and Rag1−/−Ccl25−/− recipient mice after WT T cell transfer (28.2 ± 2.6% Rag1−/−Ccl25−/− cLP versus 62.3 ± 3.7% Rag1−/− cLP, P = 0.0022). These data suggest that chronic colonic inflammation alters cDC distribution in mice devoid of CCL25/CCR9 interactions.
FIGURE 6.
Altered cDC subset distribution in colitic mice lacking CCL25/CCR9 interactions. A, Flow cytometry gating strategy to identify PDCA-1+ MHCIIlo pDCs (left), CD11chi MHCIIhi cDCs (middle), and cDC subsets (right). B, The pDC (left) and cDC (right) distribution analyses in SPL, mLN, and cLP of DSS-treated WT and Ccr9−/− mice. C, The cDC subsets distribution analyses in SPL, mLN, and cLP of DSS-treated WT and Ccr9−/− mice. D, The pDC (left) and cDC (right) distribution analyses in SPL, mLN, and cLP of Rag1−/− and Rag1−/−Ccl25−/− mice transferred with WT CD45RBhi CD4+ T cells. E, The cDC subsets distribution analyses in SPL, mLN, and cLP of Rag1−/− and Rag1−/−Ccl25−/− mice transferred with WT CD45RBhi CD4+ T cells (mean ± SD). Data represent 5 pooled experiments. *P < 0.05; NS, not significant.
Distribution of Proinflammatory and Anti-inflammatory Colonic Macrophages Is Independent of CCL25/CCR9 Interactions
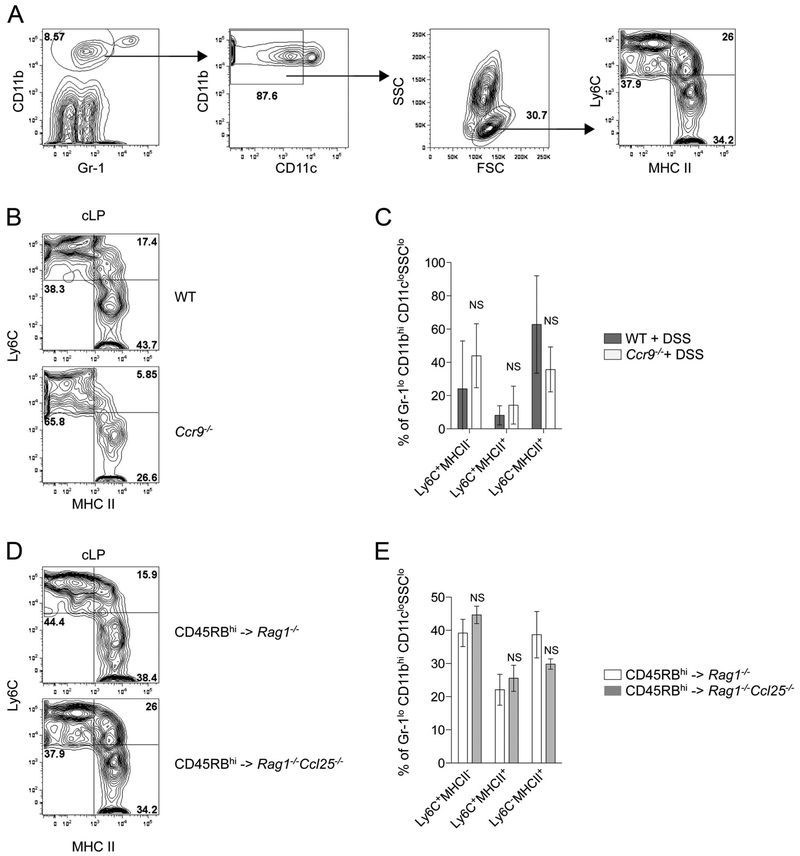
We have previously reported that acute DSS exposure leads to increased frequencies of intestinal inflammatory monocytes in Ccr9−/− mice.21 Here, we wanted to determine whether the frequencies of proinflammatory and anti-inflammatory MΦ were altered in mice lacking CCL25/CCR9 interactions in the setting of chronic colitis. We did not observe any statistically significant altered distribution in proinflammatory MΦ (Ly6ChiMHCIIhi) and anti-inflammatory MΦ (Ly6CloMHCIIhi) in the cLP of Ccr9−/− mice upon DSS treatment and Rag1−/−Ccl25−/− mice after naive T cell transfer (Fig. 7). This suggests that the distribution of proinflammatory and anti-inflammatory MΦ in the LP of colitic mice is independent of CCL25/CCR9 interactions.
FIGURE 7.
Distribution of proinflammatory and anti-inflammatory colonic macrophages is independent of CCL25/CCR9 interactions. A, Flow cytometry gating strategy used to investigate proinflammatory and anti-inflammatory macrophages. B and D, Representative dot plot of proinflammatory and anti-inflammatory macrophages in cLP of DSS-treated WT and Ccr9−/− mice (B) and Rag1−/− and Rag1−/−xCcl25−/− recipient mice transferred with WT CD45RBhi CD4+ T cells (D). Indicated percentages are relative to gated Gr-1loCD11bhiCD11cloSSClo leukocytes. C and E, Frequencies of proinflammatory and anti-inflammatory intestinal macrophages in DSS-treated WT and Ccr9−/− mice (C) and Rag1−/− and Rag1−/−xCcl25−/− mice transferred with WT CD45RBhi CD4+ T cells (E). Pooled data from 5 (B) and 3 experiments (D) (mean ± SD). Data represent 5 pooled experiments. NS, not significant.
DISCUSSION
T-cell homing to intestinal compartments requires expression of chemokine receptors on the surface of leukocytes and the attraction offered by chemokine receptor–specific ligands secreted predominantly by epithelial cells.10–12 The gut homing receptor CCR9 and its ligand CCL25 have been shown to play an important role in regulating the homing of lymphocytes in health and in disease.27 Moreover, because patients with CD have increased expression of CCL25 in the intestine and increased numbers of infiltrating CCR9+ T cells, blockade of this pathway has been postulated as a therapeutic target for CD.14,28 Although a phase 2 study using a CCR9 antagonist (Vercirnon) demonstrated clinical efficacy in inducing remission in moderate to severe CD,19 preliminary reports of a phase 3 study in patients with CD (including those with moderate to severe small bowel and/or colonic involvement) failed to show improvement in clinical response and clinical remission.29 Moreover, CCR9 antagonist administration showed dose-dependent increase in adverse reactions among all treatment groups.29 These data highlight that further investigation is required to determine whether blockade of CCL25/CCR9 interactions will be a safe and effective target for the treatment of CD.
CCR9 expression appears to be important for both effector and regulatory T-cell trafficking to the small bowel,17,30 but not to the large bowel.31 In our present study, we show that CCR9 expression on naive T effector cells is not required to mediate colitis when transferred into lymphopenic hosts. Moreover, such expression is also not required for generation and function of Tregs because CCR9-deficient Tregs are able to home to the cLP and suppress colitis. These data imply that CCR9 expression on T cells is not required for trafficking to the cLP or either effector/memory T-cell functions or regulatory T-cell functions. Recently, the G-coupled protein receptor 15 has been implicated in mediating T-cell trafficking (in particular Tregs) to the large bowel.32 The unique trafficking functions of CCR9 and G-coupled protein receptor 15 in T-cell migration suggest that these molecules may be the restrictive elements targeting leukocytes to specific intestinal compartments.
Our data suggest that CCL25/CCR9 interactions regulate innate immune cell trafficking/function. Both DSS-treated Ccr9−/− mice and Rag1−/−CCL25−/− recipient mice after naive WT T cell transfer are more susceptible to colitis than their respective controls. Since, as described above, CCL25/CCR9 interactions appear not to regulate T-cell colonic trafficking and function, exacerbation of colitis in these mice suggest that these interactions may influence the trafficking/function of other hematopoietic cells. Indeed, when compared with controls, Rag1−/−Ccl25−/− recipient mice transferred with naive CD4+ T cells and DSS-treated Ccr9−/− mice display increased numbers of neutrophils. Although neutrophils can have an anti-inflammatory role in certain IBD models,33 most studies have suggested that neutrophils play an important proinflammatory role in the pathogenesis of IBD and correlate with disease severity.23,25
The pDCs were not affected in DSS-treated Ccr9−/− mice and Rag1−/−CCL25−/− recipient mice after naive T cell transfer. CCR9-expressing pDCs have been reported to home to small intestinal tissues in steady state and in the setting of intestinal inflammation and can modulate immune responses in extraintestinal sites.34–36 Mizuno et al observed that Rag2−/− Ccr9−/− recipient mice develop ileitis, as well as colitis, when transferred with WT CD45RBhi CD4+ T cells.36 The authors attributed the ileitis to altered localization and function of regulatory pDCs within the small intestine of Rag2−/−Ccr9−/− recipient mice.36 Although in our studies Rag1−/−CCL25−/− recipient mice after T cell transfer developed colitis but not ileitis, we did not assess the effects of the absence of CCR9 expression directly on innate immune cells.
Although overall cDC numbers were unaffected, we demonstrated a decrease in CD103+CD11b− cDC and an increase in CD103−CD11b+ cDC subsets in colonic tissues of mice devoid of CCL25/CCR9 interactions in 2 independent chronic colitis models. In addition, we observed a decrease in the overall percentage of CD103+ cDCs (CD11b+ and CD11b−). An imbalance in cDC subsets has been reported in humans and mice with colonic inflammation37 and suggests that inflammatory conditions may lead to and/or result from an altered balance in tolerogenic/inflammatory DC subpopulations. CD103+CD11b− cDCs have been reported to play a crucial role in promoting tolerance to commensal bacteria and food antigens with enhanced capacity to generate iTregs from naive CD4+ T cells.26 The decrease in the CD103+ cDC subset seen in both chronic IBD models was accompanied by an increase in CD103−CD11b+ cDCs, a fraction reported to increase in the sensitivity of CD4+ T-cell responses to bacterial antigens.38 All in all, the exacerbated colitis observed in Rag1−/−Ccl25−/− mice upon transfer of naive T cells and in Ccr9−/− mice after chronic DSS exposure may result from altered cDC subset distribution, which is associated with reduced Treg function and augmented effecter T cell function.
Monocytes migrate into the intestinal LP in a CCR2-dependent manner and undergo a sequential differentiation process into proinflammatory and anti-inflammatory intestinal MΦ.39 Interestingly, Tamoutounour et al39 show that in the setting of a T cell–mediated colitis, MΦ differentiation is impaired and is associated with an increase in proinflammatory MΦ. The signals that regulate proinflammatory versus anti-inflammatory MΦ differentiation remain unknown, but our results show that this transition does not require CCL25/CCR9 interactions. Nonetheless, activated MΦ are known to respond to CCL25 chemotactic gradients,40 and CCL25 appears to drive CCR9+ MΦ differentiation in human inflammatory conditions.41 CCR9+ MΦ can display both proinflammatory and anti-inflammatory functions: for example, CCR9+ MΦ can drive acute murine liver inflammation and fibrosis in some conditions,42,43 whereas CCR9+ MΦ can also prevent peritoneal sepsis in association with reduction of inflammatory cytokines and accumulation of neutrophils.44 Therefore, these studies suggest that further investigation is required to understand how CCR9/CCL25 interactions regulate MΦ differentiation and/or function.
Together, we have demonstrated that murine T cells lacking CCR9 expression can induce large intestinal inflammation and that Treg trafficking and function are not affected by CCR9 deficiency. However, mice lacking CCR9 have exaggerated colonic inflammation in response to DSS and those devoid of CCR9/CCL25 interactions have enhanced colitogenicity after naive T cell transfer. In both the chronic colitis models resulting from aberrant CCR9/CCL25 interactions, enhanced neutrophil numbers correlate with the degree of intestinal inflammation. Although overall pDC and cDC numbers are not significantly different than in WT control mice, cDC proinflammatory and anti-inflammatory subsets are regulated by CCR9/CCL25 interactions. Finally, CCR9/CCL25-dependent innate immune cell lineage specificity and lineage-dependent functions will be aided significantly by targeted deletions of CCR9/CCL25 in innate immune cell and/or epithelial compartments.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Drs. Dror Shouval, Jeremy Goettel, and Amlan Biwas for critical review of the data and the article.
Supported by a Pilot Project Grant from the Harvard Digestive Diseases Center (P30 DK034854) and a Crohn’s and Colitis Foundation of America Career Development Award # 2821 (M.A.W.); by a NIH grant AI075037 (E.F.); S.B.S. is supported by NIH Grants HL59561, DK034854, and AI50950, the Helmsley Charitable Trust, and the Wolpow Family Chair in IBD Treatment and Research.
Footnotes
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380:1590–1605. [DOI] [PubMed] [Google Scholar]
- 2.Ordas I, Eckmann L, Talamini M, et al. Ulcerative colitis. Lancet. 2012; 380:1606–1619. [DOI] [PubMed] [Google Scholar]
- 3.Neurath MF. Animal models of inflammatory bowel diseases: illuminating the pathogenesis of colitis, ileitis and cancer. Dig Dis. 2012;30(suppl 1): 91–94. [DOI] [PubMed] [Google Scholar]
- 4.Islam SA, Luster AD. T cell homing to epithelial barriers in allergic disease. Nat Med. 2012;18:705–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Svensson M, Marsal J, Ericsson A, et al. CCL25 mediates the localization of recently activated CD8alphabeta(+) lymphocytes to the small-intestinal mucosa. J Clin Invest. 2002;110:1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansson-Lindbom B, Svensson M, Wurbel MA, et al. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J Exp Med. 2003; 198:963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stenstad H, Ericsson A, Johansson-Lindbom B, et al. Gut-associated lymphoid tissue-primed CD4+ T cells display CCR9-dependent and -independent homing to the small intestine. Blood. 2006;107:3447–3454. [DOI] [PubMed] [Google Scholar]
- 8.Wurbel MA, Malissen M, Guy-Grand D, et al. Impaired accumulation of antigen-specific CD8 lymphocytes in chemokine CCL25-deficient intestinal epithelium and lamina propria. J Immunol. 2007;178:7598–7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Briskin M, Winsor-Hines D, Shyjan A, et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol. 1997;151:97–110. [PMC free article] [PubMed] [Google Scholar]
- 10.Agace WW. T-cell recruitment to the intestinal mucosa. Trends Immunol. 2008;29:514–522. [DOI] [PubMed] [Google Scholar]
- 11.Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008;8: 685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sigmundsdottir H, Butcher EC. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nat Immunol. 2008;9:981–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wurbel MA, Philippe JM, Nguyen C, et al. The chemokine TECK is expressed by thymic and intestinal epithelial cells and attracts double- and single-positive thymocytes expressing the TECK receptor CCR9. Eur J Immunol. 2000;30:262–271. [DOI] [PubMed] [Google Scholar]
- 14.Papadakis KA, Prehn J, Nelson V, et al. The role of thymus-expressed chemokine and its receptor CCR9 on lymphocytes in the regional specialization of the mucosal immune system. J Immunol. 2000;165:5069–5076. [DOI] [PubMed] [Google Scholar]
- 15.Kunkel EJ, Campbell JJ, Haraldsen G, et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med. 2000;192:761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosoe N, Miura S, Watanabe C, et al. Demonstration of functional role of TECK/CCL25 in T lymphocyte-endothelium interaction in inflamed and uninflamed intestinal mucosa. Am J Physiol Gastrointest Liver Physiol. 2004;286:G458–G466. [DOI] [PubMed] [Google Scholar]
- 17.Rivera-Nieves J, Ho J, Bamias G, et al. Antibody blockade of CCL25/CCR9 ameliorates early but not late chronic murine ileitis. Gastroenterology. 2006; 131:1518–1529. [DOI] [PubMed] [Google Scholar]
- 18.Saruta M, Yu QT, Avanesyan A, et al. Phenotype and effector function of CC chemokine receptor 9-expressing lymphocytes in small intestinal Crohn’s disease. J Immunol. 2007;178:3293–3300. [DOI] [PubMed] [Google Scholar]
- 19.Keshav S, Vanasek T, Niv Y, et al. A randomized controlled trial of the efficacy and safety of CCX282-B, an orally-administered blocker of chemokine receptor CCR9, for patients with Crohn’s disease. PLoS One. 2013;8:e60094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. [DOI] [PubMed] [Google Scholar]
- 21.Wurbel MA, McIntire MG, Dwyer P, et al. CCL25/CCR9 interactions regulate large intestinal inflammation in a murine model of acute colitis. PLoS One. 2011;6:e16442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wurbel MA, Malissen M, Guy-Grand D, et al. Mice lacking the CCR9 CC-chemokine receptor show a mild impairment of early T- and B-cell development and a reduction in T-cell receptor gammadelta(+) gut intraepithelial lymphocytes. Blood. 2001;98:2626–2632. [DOI] [PubMed] [Google Scholar]
- 23.Kucharzik T, Walsh SV, Chen J, et al. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am J Pathol. 2001;159:2001–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erdman SE, Rao VP, Poutahidis T, et al. Nitric oxide and TNF-alpha trigger colonic inflammation and carcinogenesis in Helicobacter hepaticus-infected, Rag2-deficient mice. Proc Natl Acad Sci U S A. 2009;106:1027–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McBee ME, Zeng Y, Parry N, et al. Multivariate modeling identifies neutrophil- and Th17-related factors as differential serum biomarkers of chronic murine colitis. PLoS One. 2010;5:e13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Persson EK, Scott CL, Mowat AM, et al. Dendritic cell subsets in the intestinal lamina propria: ontogeny and function. Eur J Immunol. 2013;43:3098–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koenecke C, Forster R. CCR9 and inflammatory bowel disease. Expert Opin Ther Targets. 2009;13:297–306. [DOI] [PubMed] [Google Scholar]
- 28.Papadakis KA, Prehn J, Moreno ST, et al. CCR9-positive lymphocytes and thymus-expressed chemokine distinguish small bowel from colonic Crohn’s disease. Gastroenterology. 2001;121:246–254. [DOI] [PubMed] [Google Scholar]
- 29.ChemoCentryx. Press release. 2013. Available at: http://irchemocentryxcom/releasedetailcfm?ReleaseID—786941. Accessed August 23, 2013.
- 30.Wermers JD, McNamee EN, Wurbel MA, et al. The chemokine receptor CCR9 is required for the T-cell-mediated regulation of chronic ileitis in mice. Gastroenterology. 2011;140:1526–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karlsson F, Martinez NE, Gray L, et al. Therapeutic evaluation of ex vivo-generated versus natural regulatory T-cells in a mouse model of chronic gut inflammation. Inflamm Bowel Dis. 2013;19:2282–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim SV, Xiang WV, Kwak C, et al. GPR15-mediated homing controls immune homeostasis in the large intestine mucosa. Science. 2013;340: 1456–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuhl AA, Kakirman H, Janotta M, et al. Aggravation of different types of experimental colitis by depletion or adhesion blockade of neutrophils. Gastroenterology. 2007;133:1882–1892. [DOI] [PubMed] [Google Scholar]
- 34.Hadeiba H, Sato T, Habtezion A, et al. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nat Immunol. 2008;9:1253–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wendland M, Czeloth N, Mach N, et al. CCR9 is a homing receptor for plasmacytoid dendritic cells to the small intestine. Proc Natl Acad Sci U S A. 2007;104:6347–6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mizuno S, Kanai T, Mikami Y, et al. CCR9+ plasmacytoid dendritic cells in the small intestine suppress development of intestinal inflammation in mice. Immunol Lett. 2012;146:64–69. [DOI] [PubMed] [Google Scholar]
- 37.Strauch UG, Grunwald N, Obermeier F, et al. Loss of CD103+ intestinal dendritic cells during colonic inflammation. World J Gastroenterol. 2010; 16:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atif SM, Uematsu S, Akira S, et al. CD103−CD11b+ dendritic cells regulate the sensitivity of CD4 T-cell responses to bacterial flagellin. Mucosal Immunol. 2014;7:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamoutounour S, Henri S, Lelouard H, et al. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur J Immunol. 2012; 42:3150–3166. [DOI] [PubMed] [Google Scholar]
- 40.Vicari AP, Figueroa DJ, Hedrick JA, et al. TECK: a novel CC chemokine specifically expressed by thymic dendritic cells and potentially involved in T cell development. Immunity. 1997;7:291–301. [DOI] [PubMed] [Google Scholar]
- 41.Schmutz C, Cartwright A, Williams H, et al. Monocytes/macrophages express chemokine receptor CCR9 in rheumatoid arthritis and CCL25 stimulates their differentiation. Arthritis Res Ther. 2010;12:R161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chu PS, Nakamoto N, Ebinuma H, et al. C-C motif chemokine receptor 9 positive macrophages activate hepatic stellate cells and promote liver fibrosis in mice. Hepatology. 2013;58:337–350. [DOI] [PubMed] [Google Scholar]
- 43.Nakamoto N, Ebinuma H, Kanai T, et al. CCR9+ macrophages are required for acute liver inflammation in mouse models of hepatitis. Gastroenterology. 2012;142:366–376. [DOI] [PubMed] [Google Scholar]
- 44.Mizukami T, Kanai T, Mikami Y, et al. CCR9+ macrophages are required for eradication of peritoneal bacterial infections and prevention of polymicrobial sepsis. Immunol Lett. 2012;147:75–79. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.