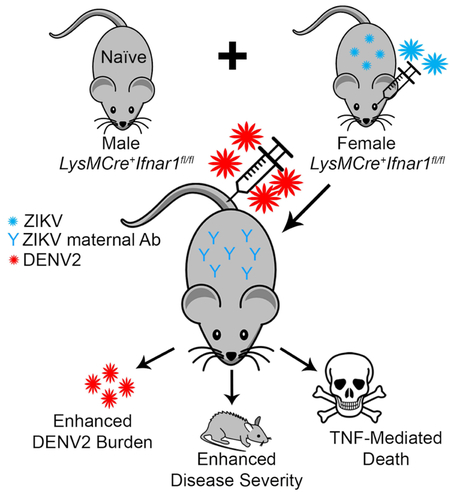
Summary
Antibody-dependent enhancement can exacerbate Dengue virus (DENV) infection due to cross-reactive antibodies from an initial DENV infection facilitating replication of a second DENV. Zika virus (ZIKV) emerged in DENV endemic areas, thus raising questions about whether existing immunity could impact these related flaviviruses. We show that mice born with circulating maternal Abs against ZIKV develop severe disease upon DENV infection. Compared to pups of naïve mothers, those born to ZIKV-immune mice lacking type I interferon receptor in myeloid cells (LysMCre+Ifnar1fl/fl) exhibit heightened disease and viremia upon DENV infection. Passive transfer of IgG isolated from mice born to ZIKV-immune mothers resulted in increased viremia in naïve recipient mice. Treatment with Abs blocking inflammatory cytokine TNF linked to DENV disease or Abs blocking DENV entry improved survival of DENV-infected mice born to ZIKV-immune mothers. Thus, the maternal Ab response to ZIKV infection or vaccination might predispose to severe dengue disease in infants.
Keywords: Zika, Dengue, antibody dependent enhancement (ADE), maternal antibody, mouse model, viral pathogenesis
Graphical Abstract
eTOC Blurb (50 or less)
The emergence of Zika virus (ZIKV) in dengue virus (DENV) endemic regions raises questions regarding the impact of ZIKV immunity on DENV disease severity. Fowler and Tang et. al. developed a mouse model to demonstrate ZIKV maternal antibody-mediated enhancement of DENV infection and pathogenesis.
Introduction
Since the emergence of Zika virus (ZIKV) in Dengue virus (DENV) endemic areas, a question that remains unanswered is how ZIKV and DENV immunity reciprocally impact each other in the context of sequential infections. DENV is the leading mosquito-transmitted viral infection globally (Guzman et al., 2010) with an estimated 390 million infections per year, and results in clinical symptoms ranging from inapparent to life-threatening (Bhatt et al., 2013). DENV disease creates a substantial burden on public health resources with more than 3 billion people at risk for infection worldwide (Shepard et al., 2016). DENV is a flavivirus and circulates as four different serotypes (DENV1-4) that vary by 25 to 40% at the amino acid level. Although primary DENV infection usually manifests as a self-limiting febrile illness, secondary infections with a heterotypic serotype can result in dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS), also referred to as severe dengue, which is associated with vascular leakage, hemodynamic shock, and death. One model for the pathogenesis of severe dengue involves the phenomenon of antibody (Ab)-dependent enhancement (ADE), where circulating cross-reactive Abs from the first DENV infection bind to the second DENV and facilitate its entry and replication in Fcγ receptor-expressing cells (Halstead, 2007).
ZIKV, the causal agent of Congenital Zika Syndrome (Organization, 2016), is genetically and antigenically similar to DENV with ~56% amino acid identity (Chang et al., 2017), and cross-reactivity between the two viruses at the Ab epitope level has been documented extensively (Bardina et al., 2017; Charles and Christofferson, 2016; Dejnirattisai et al., 2016; Kawiecki and Christofferson, 2016; Priyamvada et al., 2016; Stettler et al., 2016; Swanstrom et al., 2016). Indeed, studies have begun to evaluate the impact of the cross-reactive Ab response in protection against or pathogenesis of ZIKV and DENV infections. Although some cross-reactive monoclonal Abs generated against DENV protect against ZIKV (Barba-Spaeth et al., 2016; Fernandez et al., 2017b), others generated against ZIKV can enhance DENV infection (Stettler et al., 2016. In the context of polyclonal Ab responses, prior ZIKV infection resulted in increased peak DENV viremia in macaques (George et al., 2017) and DENV-immune plasma enhanced ZIKV infection and disease severity in Stat2−/− mice (Bardina et al., 2017. These studies suggest that ADE can occur in different ZIKV and DENV infection scenarios.
In humans, maternal Abs from DENV-immune mothers can provide protection, enhancement, or no effect when passively transferred to an infant (Chau et al., 2009; Elong Ngono and Shresta, 2018; Halstead et al., 2002; Simmons et al., 2007). As levels of maternal Abs in infants fall to sub-neutralizing levels, there is an increased risk of developing severe dengue (Halstead et al., 2002). As the geographic range of ZIKV expands, it will become possible for mothers to be exposed to ZIKV and their infants to be infected with DENV. It currently remains unknown if ZIKV Abs can result in enhancement or protection when transferred passively to infants. Here, we develop a model of severe dengue in mice born to ZIKV-immune mothers using established LysMCre+Ifnar1fl/fl mouse models of ZIKV and DENV infection (Elong Ngono et al., 2017; Pinto et al., 2015; Tang et al., 2016). Our results demonstrate that maternally acquired ZIKV Abs can enhance DENV infection and disease severity in young mice.
Results
Decreased survival of pups born to ZIKV-immune mothers after DENV2 infection.
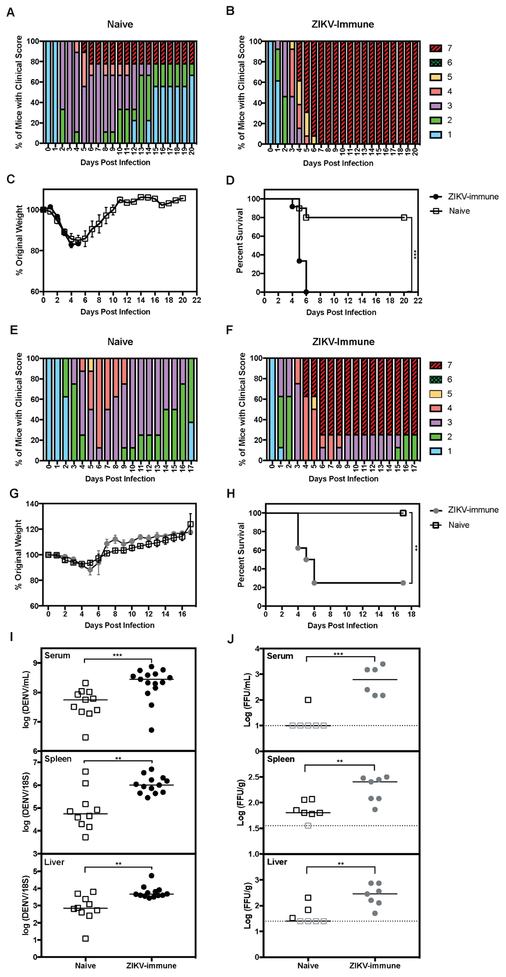
To investigate whether maternal Abs from ZIKV-immune mothers conferred protection or promoted severe dengue disease in young mice, 4- to 5-week old LysMCre+Ifnar1fl/fl pups born to ZIKV-immune or naïve mothers were inoculated with DENV2-S221 and monitored for weight loss, clinical signs, and survival. Pups born to ZIKV-immune mothers had similar immune cell numbers and frequency in the spleen as those born to naïve mothers (Fig. S1). Animals born to long-term (8-12 months) ZIKV-immune mothers and challenged with DENV2 had increased clinical scores compared to naïve LysMCre+Ifnar1fl/fl mice, although both groups exhibited similar weight loss (Fig. 1A-C). Most of the mice in the naïve control group recovered from DENV infection, whereas 100% mice born to long-term (8-12 months) ZIKV-immune mothers died by day 6 post-infection (p.i.) (Fig. 1A-B and D). To determine whether short-term ZIKV infection period in mothers also affected the outcome of DENV infection in their pups, we assessed clinical score, weight loss, and survival in pups born to short-term (2 months) ZIKV-immune mothers (Fig. 1E-H). Both naïve and ZIKV-immune groups exhibited similar weight loss after DENV2 challenge (Fig. 1G), yet mice born to ZIKV-immune mothers had increased clinical scores and decreased survival compared to those born to naïve mothers (Fig. 1E-F and 1H). These results demonstrate that, at 4- to 5-weeks of age, pups born to ZIKV-immune but not naïve mice develop lethal disease upon challenge with DENV2.
Figure 1. Mice born to ZIKV-immune mothers have increased dengue disease severity and DENV burden.
Four- to 5-week-old LysMCre+Ifnar1fl/fl mice born to mothers previously infected with ZIKV strain SD001 (106 FFU via retro-orbital route) or to naïve mothers were challenged with DENV2 strain S221 (106 FFU via tail vein). (A) Clinical scores of infected mice (n=11) from naïve mothers (n=2). (B) Clinical scores of infected mice (n=15) from ZIKV-immune mothers infected for 8-12 months (n=3). (C-D) Weight loss and survival data of infected mice from naïve (open squares) versus ZIKV-immune mothers infected for 8-12 months (black circles). (E) Clinical scores of infected mice (n=8) from naïve mothers (n=2) (F) Clinical scores of infected mice (n=8) from ZIKV-immune mothers infected for 2 months (n=2). (G-H) Weight loss and survival data of infected mice from naïve (open squares) versus ZIKV-immune mothers infected for 2 months (grey circles). To determine DENV viral burden, mice were euthanized at 3 days p.i. and serum, spleens, and livers were harvested. (I) The levels of DENV RNA from each tissue were measured via qRT-PCR. Viral RNA levels in the serum, spleen, and liver of DENV2-infected pups (open squares, n=11) from naïve mothers (n=2) were compared to pups (black circles, n=13) from ZIKV-immune mothers infected for 6-13 months (n=4). (J) The levels of infectious DENV2 were measured via focus forming assay in the serum, spleen, and liver of DENV2-infected pups (open squares, n=7) from naïve mothers (n=2) and pups (grey circles, n=7) from ZIKV-immune mothers infected for 2 months (n=2). Data were pooled from two independent experiments and are expressed as mean ± standard error of mean (C and G). Unpaired Student’s t test of groups for each day. (D and H) log-rank test (*** P ≤ 0.001, ** P < 0.01). (I and J) Data were pooled from 2 independent experiments. Mann-Whitney test (*** P< 0.001, ** P < 0.01).
Increased DENV2 burden in pups born to ZIKV-immune mice.
To determine if enhanced disease severity in pups born to long-term (6-13 months) ZIKV-immune mothers was associated with increased levels of DENV2 infection, viral RNA levels were compared in pups born to ZIKV-immune versus naïve mothers at day 3 p.i. DENV2 RNA levels were increased significantly in the serum (5-fold, *** P < 0.001), spleen (13-fold, ** P < 0.01), and liver (8-fold, ** P < 0.01) in pups born to ZIKV-immune mothers relative to naïve pups (Fig. 1I). Thus, severe dengue disease manifestations correlated with increased DENV2 tissue burden in mice born to ZIKV-immune mothers.
To examine if enhanced disease severity in mice born to short-term ZIKV-infected mothers was due to increased DENV infection, levels of infectious DENV were measured in pups born to short-term (2 months) ZIKV-immune or naïve mothers at day 3 p.i. Infectious DENV levels were higher in the serum (25-fold, *** P < 0.001), spleen (3-fold, ** P < 0.01), and liver (9-fold, ** P < 0.01) in pups born to ZIKV-immune than to naïve mothers (Fig. 1J), confirming the correlation between severe dengue disease and increased DENV2 burden in mice born to ZIKV-immune mothers.
Improved clinical phenotypes and decreased ZIKV burden in pups born to ZIKV-immune compared to naïve or DENV-immune mothers.
As we observed a negative impact of maternal ZIKV immunity in pups upon challenge with DENV, we next examined the reciprocal conditions by testing whether maternal ZIKV or DENV immunity influenced the outcome of subsequent challenge of pups with ZIKV. When pups born to ZIKV-immune mothers were challenged with ZIKV, they had significantly less infectious ZIKV in the serum, spleen, liver, brain, and eyes compared to pups born to naïve or DENV2-immune mothers (Fig. S2A). Similar levels of infectious ZIKV were detected in pups born to DENV2-immune and naïve mothers, with the exception of the liver where maternal DENV immunity had a protective effect (Fig. S2A). Consistent with these data, pups born to ZIKV-immune mothers and challenged with ZIKV had better clinical scores and less weight loss than those born to naïve mice (Fig. S2B-C, F). In contrast, pups born to DENV2-immune mothers and challenged with ZIKV had similar clinical scores to those born to naïve mice, but had a slightly different weight change (Fig. S2D-E, G). These data suggest that ZIKV maternal immunity protects against ZIKV challenge in infancy whereas DENV maternal immunity has a more neutral effect. As expected (Ng et al., 2014), pups born to DENV2-immune mothers and then challenged with DENV2 exhibited better clinical scores and no weight loss compared to pups born to naïve mothers (Fig. S3A-B), indicating that maternal DENV immunity protects against homologous DENV challenge. Thus, at least under the conditions tested, the enhanced pathogenesis observed in pups from ZIKV-immune mothers that are challenged with DENV2 is unique and does not occur when pups born to ZIKV-immune or DENV2-immune mothers are challenged with ZIKV.
Maternally acquired ZIKV Abs bind but do not neutralize DENV2.
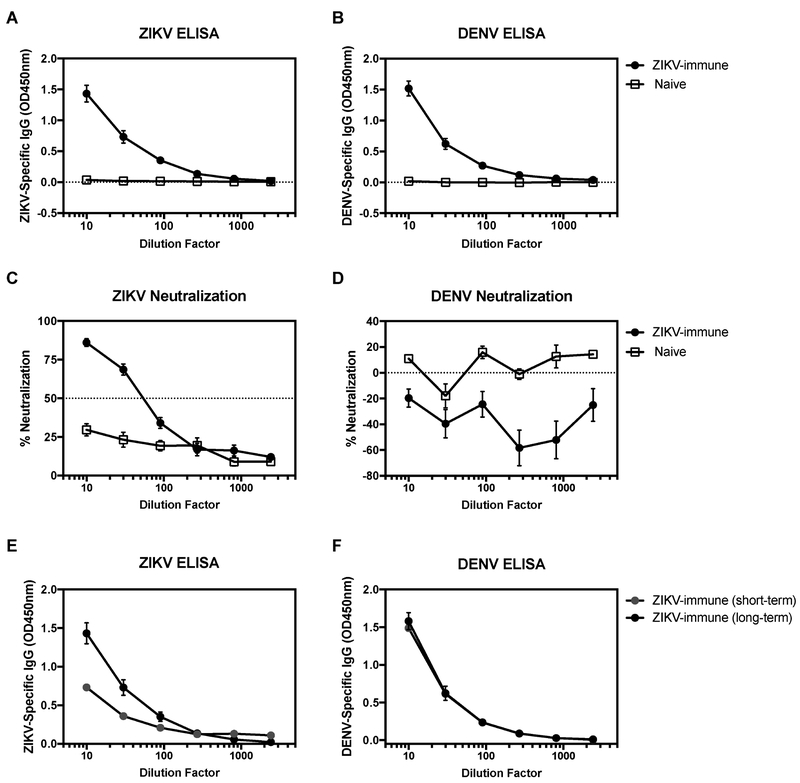
To begin defining the mechanism of maternal ZIKV Ab-mediated DENV pathogenesis, we tested maternal ZIKV Abs from the serum of 4- to 5-week-old pups from ZIKV-immune mothers for their capacity to bind and neutralize DENV2. Sera of pups born to ZIKV-immune but not na’ive mothers contained ZIKV- and DENV2-reactive Abs (Fig. 2A-B). However, these sera neutralized ZIKV but not DENV2 infection in U937-DC-SIGN cells and even appeared to enhance DENV2 infection (Fig. 2C-D). Levels of ZIKV-binding Abs in pups born to short-term (2 months) mothers was lower than the levels observed in pups born to long-term ZIKV-immune mothers, whereas DENV2-binding Ab levels were similar between the two groups of mice (Fig. 2E-F). Thus, maternal Abs in 4- to 5-week-old pups born to ZIKV-immune mothers cross-react with but do not cross-neutralize DENV2 in vitro.
Figure 2. Sera from mice born to ZIKV-immune mothers neutralize ZIKV but not DENV2 infection.
Serum was collected from 4- to 5-week-old LysMCre+Ifnar1fl/fl mice born to mothers previously infected with ZIKV strain SD001 (106 FFU via retro-orbital route) or naïve mothers. Serum of pups (open squares, n=10) from naïve mothers (n=2) were compared to pups (black circles, n=13) from ZIKV-immune mothers infected for 6-13 months (n=4). (A) anti-ZIKV IgG and (B) anti-DENV IgG were detected via ELISA. Neutralization capacity was assessed against (C) ZIKV SD001 and (D) DENV S221 via U937-DC-SIGN cells and a flow cytometry-based assay (Wen et al., 2017). Dotted line indicates limit of detection for ELISA and 50% or 0% neutralization line for the neutralization assay. Serum of pups (black circles, n=13) from ZIKV-immune mothers infected for 6-13 months (n=4) were compared to serum of pups (grey circles, n=10) from ZIKV-immune mothers infected for 2 months (n=3). (E) anti-ZIKV IgG and (F) anti-DENV IgG were detected via ELISA. Data are pooled from 3 independent experiments and are expressed as mean +/− standard error of mean.
To understand the lack of enhancement of ZIKV pathogenesis in pups with maternally acquired DENV2 Abs, we analyzed the binding and neutralization capacity of sera from 4- to 5-week old pups born to DENV2-immune mothers. Serum samples from mice born to DENV2-immune mothers bound to DENV2, but not ZIKV (Fig. S3C-D). Additionally, these sera could neutralize DENV2 (Fig. S3E). Thus, the absence of enhanced ZIKV pathogenesis in pups born to DENV2-immune mothers is likely due to a lack of ZIKV-binding by maternally-acquired DENV2 Abs.
Increased viral burden in DENV2-infected mice with passively transferred IgG from pups born to ZIKV-immune mothers.
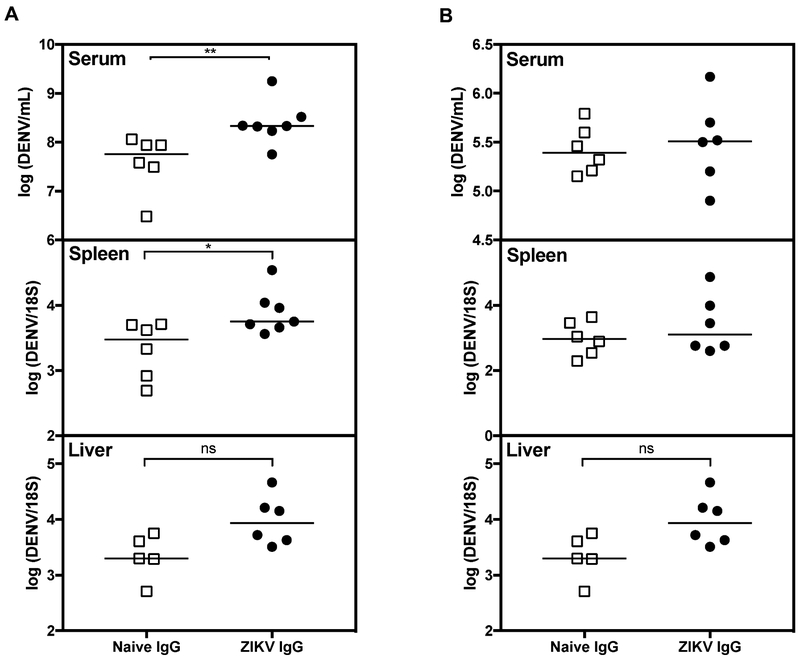
To confirm that maternal ZIKV Abs were responsible for enhanced DENV2 infection, serum IgG was isolated from 4- to 5-week old pups born to ZIKV-immune or naive mothers and then passively transferred into age-matched naïve 4- to 5-week old LysMCre+Ifnar1fl/fl recipient mice immediately prior to infection. Two different preparations of IgG that were isolated from pooled sera collected from pups born to ZIKV-immune or naïve mothers showed binding to both ZIKV and DENV but no neutralizing activity against DENV (Fig S4A-D). Mice that received 145 μg (amount that was determined from IgG isolated from a single mouse) of IgG from pups born to ZIKV-immune mothers had higher DENV2 RNA levels in the serum (7-fold, ** P < 0.01) and spleen (4-fold, * P < 0.05) than mice receiving IgG from pups born to naïve mothers at day 3 after challenge (Fig. 3A). These results imply that maternally acquired IgG obtained from ZIKV-immune mothers contributes to the enhanced DENV2 infection and pathogenesis phenotypes observed after DENV2 challenge of pups at 4- to 5-weeks of age. As expected, when 10-fold less IgG was transferred to naïve 4- to 5-week old recipient mice, no difference in DENV2 RNA levels in tissues was observed between mice that received IgG from ZIKV-immune versus naïve mothers (Fig 3B), revealing the Ab concentration-dependent nature of the DENV2 enhancement phenotype.
Figure 3. Passive transfer of IgG isolated from mice born to ZIKV-immune mothers into naïve mice increases viral burden upon DENV2 challenge.
IgG was isolated from serum of 36 pups born to ZIKV-immune mothers infected for 6-12 months (n=8) or from 32 age-matched naïve mice from naïve LysMCre+Ifnar1fl/fl mothers (n=7). (A) 145 μg or (B) 14.5 μg of purified IgG isolated from pups born to ZIKV-immune mothers (ZIKV IgG) or naïve mothers (naïve IgG) was passively transferred into naïve 4- to 5-week old LysMCre+Ifnar1fl/fl recipient mice, followed by challenge of the passively transferred recipient mice with 2 × 105 FFU of DENV2 strain S221 via tail vein injection. Tissues were harvested 3 days p.i. and DENV2 RNA levels in the (A and B) serum, and spleen, and liver were quantified by qRT-PCR. n=6-7 ZIKV IgG recipient mice and n=6 naïve IgG recipient mice. Mann-Whitney test (** P < 0.01, * P < 0.05).
TNF levels are increased in mice with passively transferred ZIKV IgG upon DENV2 challenge.
We next determined if TNF levels were increased in mice that received IgG from pups born to ZIKV-immune pups relative to mice that received IgG from naïve pups. Human studies have shown that patients with severe dengue have higher levels of several pro-inflammatory cytokines, including TNF, compared to individuals with mild dengue (Green et al., 1999; Hober et al., 1993; Kittigul et al., 2000; Wang et al., 2007), and in a small observational study, individuals on anti-TNF Ab therapy did not develop DHF/DSS (Deligny et al., 2014). Consistent with several studies demonstrating that the lethal ADE-mediated dengue disease in mice is TNF-dependent (Ng et al., 2014; Phanthanawiboon et al., 2016; Shresta et al., 2006; Watanabe et al., 2015; Zellweger et al., 2010), TNF levels were increased in ZIKV IgG recipient mice compared to naïve IgG recipient mice (Fig. S4E), implying that ZIKV IgG treatment potentiates TNF induction.
TNF blockade decreases DENV2-induced lethality in mice born to ZIKV-immune mothers.
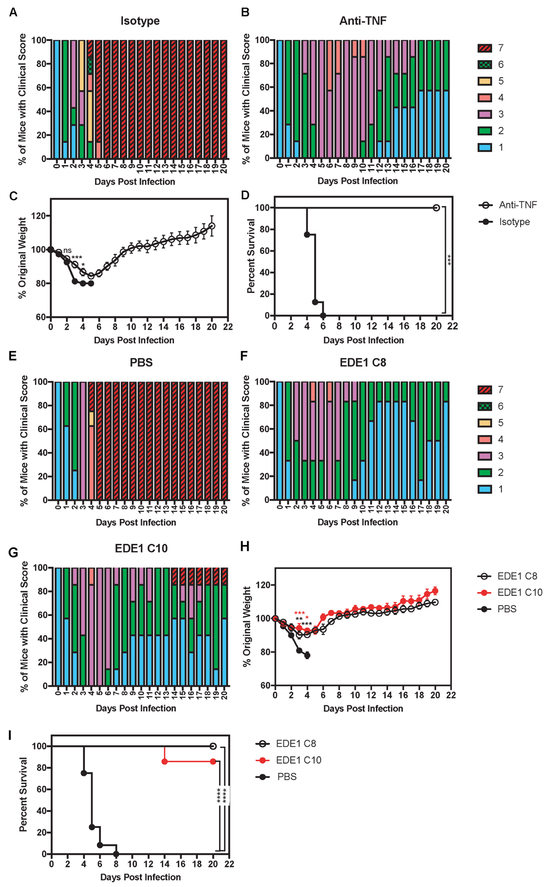
To assess the significance of TNF induction in our mouse model, we determined whether DENV2-induced lethal disease in pups born to ZIKV-immune mice was mediated by TNF by performing blocking experiments with a neutralizing anti-TNF Ab. Mice born to ZIKV-immune mothers were inoculated with DENV2 and administered 100 μg of an anti-TNF or an isotype control Ab on days 1, 2, and 3 p.i. The anti-TNF Ab-treated group exhibited improved clinical scores, weight gain, and increased survival compared to isotype control Ab-treated mice (Fig. 4A-D). This result is consistent with the hypothesis that DENV2-infected pups born to ZIKV-immune mothers have increased disease severity through ADE and over-exuberant production of pro-inflammatory cytokines.
Figure 4. Administration of anti-TNF Ab or EDE1 C8 and C10 Abs, which recognize EDE1 epitopes, prevents lethal dengue disease in mice born to ZIKV-immune mothers.
Four- to 5-week-old LysMCre+Ifnar1fl/fl mice born to mothers infected with ZIKV strain SD001 (1×106 FFU via retro-orbital route) for 7-9 months (n=3) were treated via an intraperitoneal injection with 100 μg of isotype control Ab (clone HPRN) or anti-TNF Ab (clone XT3.11) on days 1, 2, and 3 following inoculation with 106 FFU of DENV2 strain S221 via tail vein injection. (A) Clinical scores of isotype control Ab-treated mice (n=7), (B) Clinical scores of anti-TNF Ab-treated mice (n=8), and (C) Weight loss and (D) Survival rates of isotype control Ab-treated mice (black circles) and anti-TNF Ab-treated mice (open circles). Administration of EDE1 C8 or EDE1 C10 Abs was performed in 4- to 5-week-old LysMCre+Ifnar1fl/fl mice born to mothers infected with ZIKV strain SD001 (1×106 FFU via retro-orbital route) for 12-13 months (n=4). These pups were injected via an intraperitoneal route with PBS, EDE1 C8 Ab (100 μg), or EDE1 C10 Ab (100 μg) on days 1, 2, and 3 following challenge with DENV2 strain S221 (106 FFU via tail vein). (E) Clinical scores of PBS control mice (n=9), (F) Clinical scores of EDE1 C8 Ab-administered mice (n=10), (G) Clinical scores of EDE1 C10 Ab-administered mice (n=8), and (H) Weight loss and (I) Survival rates of PBS-treated (black circles), EDE1 C8-treated (open circles), and EDE1 C10-treated (red circles) mice. Data are pooled from two independent experiments and are expressed as mean ± standard error of mean. (C and H) Unpaired Student’s t test of groups for each day. (D and I) log-rank test * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001.
Monoclonal Ab treatment decreases DENV2-induced lethality in mice born to ZIKV-immune mothers.
The original studies demonstrating ADE-mediated lethal dengue disease in mice showed that neutralizing DENV mAbs that blocked fusion could abrogate ADE (Balsitis et al., 2010; Zellweger et al., 2010). Given this data, we assessed whether the DENV2-induced lethal disease in pups born to ZIKV-immune mothers could also be prevented via treatment with neutralizing mAbs. We tested two human EDE1 mAbs (C8 and C10) that cross-neutralize different DENV serotypes and ZIKV (Dejnirattisai et al., 2015; Fernandez et al., 2017a; Swanstrom et al., 2016) for their ability to reduce disease severity in DENV2-infected pups born to ZIKV-immune LysMCre+Ifnar1fl/fl mothers. Administration of 100 μg of EDE1 C8 or EDE1 C10 Ab on days 1, 2, and 3 p.i. decreased dengue disease compared to treatment with PBS alone, based on clinical scores (Fig. 4E-G), weight loss (Fig. 4H), and survival (Fig. 4I). Thus, administration of EDE1 mAbs can prevent severe dengue disease in mice born to ZIKV-immune mothers.
Discussion
Currently, little is known about how prior ZIKV immunity affects DENV pathogenesis and infection in vivo. One study showed that previous ZIKV exposure increased peak DENV viremia in macaques (George et al., 2017), and another study reported enhanced DENV pathogenesis in mice administered a ZIKV mAb (Stettler et al., 2016). Consistent with these findings, our data show increased viral burden, worsened clinical signs, and decreased survival in mice born to ZIKV-immune mothers relative to those born to naïve mothers. Our study demonstrates maternal Ab-mediated infant DHF/DSS in the context of preexisting anti-flavivirus immune sera other than anti-DENV immune sera (Martinez Gomez et al., 2016; Ng et al., 2014). In comparison, maternal DENV Abs exerted mainly neutral effects against ZIKV in our mouse model, in agreement with the observation that prior DENV immunity (1-to 3-year-long) did not influence subsequent ZIKV infection in macaques (McCracken et al., 2017; Pantoja et al., 2017) and consistent with reports that the anti-DENV Ab response in humans becomes less cross-neutralizing against ZIKV over time (Collins et al., 2017; Montoya et al., 2018). However, our result contrasts with a published study demonstrating increased ZIKV infection and pathogenesis in Stat2−/− mice that were passively transferred with DENV-immune human plasma (Bardina et al., 2017). The disparity in results may be related to the magnitude and quality of Ab responses (e.g., binding, neutralization, isotype, specificity, and avidity) and perhaps the use of LysMCre+Ifnar1fl/fl versus Stat2−/− mice.
By modeling a potential epidemiologic scenario in which infants born to ZIKV-experienced women are infected with DENV, our study has revealed a pathogenic potential of ZIKV maternal Abs during DENV infection. Mouse versus human differences related to the quantity and potentially quality of maternal IgG transferred into infants may affect the timing or duration of enhanced DENV pathogenesis in human infants versus mice. In our mouse model, 4- to 5-week old pups born to flavivirus-naïve mothers with either short-term (2 months) or long-term (>6 months) exposure to ZIKV exhibited increased dengue disease severity. In humans, the magnitude and quality of the Ab response to ZIKV may vary depending on the length of exposure (acute versus early convalescence versus late convalescence) and flavivirus-naïve versus immune status of mothers (Collins et al., 2017; Montoya et al., 2018; Yu et al., 2017), thereby impacting the window of both potentially protective and pathogenic periods in infants.
In summary, our findings have implications for understanding DENV infections in countries with co-circulation of ZIKV and DENV or women with prior ZIKV immunity through natural infection or possibly, vaccination. Mounting evidence supports a key role for ADE in pathogenesis of DENV infection in children and adults with secondary heterotypic DENV infection and infants born to DENV-immune women (Balsitis et al., 2010; Halstead, 2007; Katzelnick et al., 2017; Ng et al., 2014; Zellweger et al., 2010). Our results suggest that if women are infected with ZIKV or potentially immunized with a ZIKV vaccine that elicits a cross-reactive Ab response, their infants might have an increased risk of developing DHF/DSS. A ZIKV vaccine with fusion loop mutations in the E protein has been reported to reduce cross-reactive Ab responses and minimize enhancement of DENV infection and pathogenesis in mice (Richner et al., 2017), suggesting one potential avenue for designing ZIKV vaccines that avoid ZIKV maternal Ab-mediated severe DENV disease in infants. Thus, the emergence of ZIKV immunity in DENV-endemic regions may create an added risk for ADE-mediated severe dengue disease. Our mouse model may be useful for testing the effects of ZIKV and DENV (including different serotypes) on infant DENV and ZIKV infections and for evaluating the effects of ZIKV and DENV vaccine candidates that are designed for deployment in DENV-endemic regions. As T, B, and most dendritic cell responses are normal in LysMCre+Ifnar1fl/fl mice, this mouse model may also be useful for investigating mechanisms of DENV pathogenesis and immunity.
STAR Methods
CONTACT FOR REAGENT AND RESCOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Sujan Shresta (sujan@lji.org).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Viruses.
SD001 is a ZIKV clinical isolate obtained from an adult female traveler infected in Caracas, Venezuela in 2016 (Carlin et al., submitted). Infectious virus was isolated from a filtered urine sample from patient SD001 and propagated in C6/36 Aedes albopictus cells (ATCC, cat. # ATCC: CRL 1660). Mouse-adapted DENV2 strain S221 was propagated in C6/36 cells. ZIKV-SD001 and DENV2-S221 were titrated by focus-forming assay using baby hamster kidney (BHK-21) (ATCC, cat. #ATCC: CCL 10) cells as described in the virus quantification section.
Cell lines.
C6/36 mosquito cells were propagated in Leibovitz's L-15 (Thermo Fisher Scientific, cat. #11415064) supplemented with 10% fetal bovine serum (FBS) (Thermo Fisher Scientific, cat. #16000044), 1% penicillin/streptomycin (Thermo Fisher Scientific cat. #15140-122), and 1% HEPES (Thermo Fisher Scientific, cat. #15630080) at 28°C. BHK-21 cells were grown in MEM α (Fisher, cat. #12-561-072) supplemented with 10% FBS, 1% penicillin/streptomycin, and 1% HEPES at 37°3 in a 5% CO2 atmosphere. U937-DC-SIGN cells were propagated in RPMI 1640 (Thermo Fisher Scientific, cat. #11875093) supplemented with 10% FBS, 1% penicillin/streptomycin, and 1% HEPES at 37°3 in a 5% CO 2 atmosphere.
Mice.
LysMCre+Ifnar1fl/fl C57BL/6 mice lack type I interferon (IFN) receptors in a subset of myeloid cells (Clausen et al., 1999; Diamond et al., 2011) and were characterized previously as a model for DENV (Pinto et al., 2015; Zust et al., 2014) and ZIKV (Elong Ngono et al., 2017; Tang et al., 2016) infection. Female mice were inoculated via retro-orbital route with 106 focus-forming units (FFU) of ZIKV-SD001 diluted in 10% FBS/PBS (100 μL total volume) or with 5 × 105 FFU of DENV2-S221 via tail vein injection. At four weeks post-ZIKV or DENV infection, females were bred with naïve 6- to 8-week old male LysMCre+Ifnar1fl/fl mice. Male and female pups born to ZIKV- or DENV-infected LysMCre+Ifnar1fl/fl females were challenged at 4- to 5-weeks of age. Analyses were not performed on whether or not the sex of the mouse influenced the overall outcome as the outcome did not differ based on the sex of the mouse. Four- to 5-week-old age-matched pups (both males and females) born to naïve LysMCre+Ifnar1fl/fl mothers were used as controls. The mothers’ ZIKV or DENV infection lengths are stated in each figure legend. All experiments were performed following the La Jolla Institute Animal Care and Use Committee-approved animal protocol #AP 00001029. Mice were housed at a maximum of 5 per cage with the same sex of mouse. Water, food, and housing with bedding and enrichment were autoclaved prior to being utilized. Cages were changed every 2 weeks under a laminar flow hood. Proper personal protective equipment was worn when in the vivarium and handling the mice. All mice were bred and maintained under specific pathogen free (SPF) conditions, and infected mice were housed in a BSL2 SPF room.
METHOD DETAILS
Disease scoring.
Pups born to ZIKV-immune or naïve mothers at 4- to 5-weeks of age were inoculated with 1 × 106 FFU of DENV2 S221 diluted in 10% FBS/PBS (200 μL total volume) per mouse via tail vein injection. Mice were monitored daily for weight and clinical scores from day 0 to day 20 p.i. Clinical scores ranged from 1 – 7: 1, heathy mice with a smooth coat and bright, alert eyes; 2, mice are slightly ruffled around the head and neck, but active and alert; 3, mice have a ruffled coat throughout the body, but still active and alert; 4, mice have a very ruffled coat and slightly closed eyes, they walk slowly, and they have mild lethargy; 5, mice have a very ruffled coat and closed inset eyes, slow to no movement but will return to the upright position if put on the side; 6, mice have a very ruffled coat and closed inset eyes, are moribund, they have no movement or uncontrollable spastic movements, will not return to upright position if put on its side, and completely unaware or in noticeable distress and require humane euthanasia; 7, mice are deceased. Mice were humanely euthanized if weight loss was greater than or equal to 20% of their body mass or if their clinical score was a 6.
Flow cytometric analysis of immune cell populations.
Spleens were harvested from pups born to ZIKV-immune or naïve mothers at 4- to 5-weeks of age after being humanely euthanized with CO2. Splenocytes were plated into 96-well round-bottom plates at 1 × 106 cells/well in RPMI 1640 medium supplemented with 10% FBS, 1% penicillin/streptomycin, and 1% HEPES. Splenocytes were washed with PBS and stained with anti-CD3 PerCP-Cy5.5 (Tonbo Biosciences, cat. #65-0031-U100), anti-CD4 APC eflour780 (Thermo Fisher Scientific, cat. #47-0041-82), and anti-CD8 BV510 (BioLegend, cat. #100751) or with anti-CD19 PE (Thermo Fisher Scientific, cat. #12-0193-83), anti-CD138 PerCP-Cy5.5 (BioLegend, cat. #142510), and anti-mouse IgD FITC (BD Biosciences, cat. #553439). Cells were incubated with these Abs (each at 1:200 dilution) for 30 minutes, followed by washing for 3 times with FACs buffer. The cells were then fixed and permeabilized with Cytofix/Cytoperm (BD Biosciences, cat. #554714), washed and resuspended in FACs buffer.
Infectious virus quantification.
Four- to 5-week old LysMCre+Ifnar1fl/fl mice born to ZIKV-immune or naïve mothers were inoculated with 2 × 105 FFU DENV2 S221 diluted in 10% FBS/PBS via tail vein injection (200 μL total volume) or with 1 × 105 FFU ZIKV in 10% FBS/PBS retro-orbitally (100 μL total volume). Mice were humanely euthanized with CO2 3 days p.i. Blood was obtained via cardiac puncture, centrifuged (16,363 × g for 15 min at 4°C), and serum was stored at −80°C. Mice were perfused with PBS. Spleen and livers were harvested and put in pre-weighed tubes containing complete MEM α containing a metal bead and stored at −80°C. Viral titers were measured using a BHK-21 cell-based focus forming assay (FFA). BHK-21 cells were plated at 2 × 105 cells per well in a 24-well plate and incubated overnight in complete MEM α medium supplemented with 10% FBS, 1% penicillin/streptomycin, and 1% HEPES at 37°C in a 5% CO2 atmosphere. Cells were infected with serial dilutions of virus for 1.5 hours with gentle shaking every 15 min. The medium was then aspirated and replaced with fresh MEM α supplemented with 1% carboxymethyl cellulose (Sigma, cat. #419273), 10% FBS, 1% penicillin/streptomycin, and 1% HEPES. Cells were then cultured for 3 days. At 3 days p.i., cells were fixed with 4% formalin (Fisher Scientific, cat. #SF98-4) for 30 min, washed 3 times with PBS, permeabilized with 1% Triton X-100 (Sigma, cat. #X100-100ML) for 30 min, washed 3 times with PBS, and blocked by 10% FBS in PBS for 30 min. ZIKV or DENV was detected by incubation of cells with 4G2, a pan-flavivirus E protein-specific monoclonal Ab (ATCC, cat. # D1-4G2-4-15 (ATCC HB-112)) (1 μg/mL in 1% FBS/PBS) for 1.5 h at room temperature or overnight at 4°C. Cells were washed 3 times with PBS and incubated for 1.5 h with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Jackson ImmunoResearch, cat. #115-035-072) (diluted 1:1000 in 1% bovine serum albumin (BSA)/PBS), followed by washing 3 times with PBS. Finally, foci were detected by incubation with KPL True Blue substrate (SeraCare, cat. #5510-0030) for 20 min and rinsed in diH2O.
Viral RNA quantification
Four- to 5-week old LysMCre+Ifnar1fl/fl mice born to ZIKV-immune or naïve mothers were inoculated with 2 × 105 FFU DENV2 S221 diluted in 10% FBS/PBS (200 μL total volume) via tail vein injection. Mice were humanely euthanized 3 days p.i. Blood was obtained via cardiac puncture, centrifuged (16,363 × g for 15 min at 4°C), and serum was stored at −20°C. Mice were perfused with PBS, and their spleens and livers were harvested and stored in RNAlater (Thermo Fisher Scientific, cat. #AM7021) at 4°C. Tissues were homogenized prior to RNA extraction. RNA was isolated via Qiagen QIAmp viral mini kit (Qiagen, cat. #52904) and qRT-PCR was performed as previously described (Prestwood et al., 2008) using the QuantaBio qScript one-step qRT-PCR kit (VWR, cat. #101414-172) and probes and primers described in the Key Resources Table. PCR mixtures were pre-incubated at 50°C for 2 min, then 95°C for 10 min followed by 40 cycles of two-step incubations at 95°C for 15 s and 60°C for 1 min for DENV2. DENV2 samples were compared to a standard curve of 103 – 107 copies of DENV2 RNA. 18S samples were diluted and compared to a standard of 18S rRNA that was derived from a mouse spleen and diluted to 102 – 104 18S RNA. 18S RNA was ran for 10 minutes at 48°C, 5 minutes at 98°C, and 39 cycles of 15 seconds at 95°C and 1 minute at 60°C.
KEY RESCOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| 4G2 | ATCC | Cat. #HB-112 RRID: CVCL_J890 |
| PerCP/Cy5.5 anti-mouse CD3e (clone: 145-2C11) | TONBO Biosciences | Cat. #65-0031-U100 RRID: AB_394599 |
| Brilliant Violet 510 anti-mouse CD8a (clone: 53-6.7) | BioLegend | Cat. #100751 RRID: AB_2561389 |
| CD4 Monoclonal Antibody APC-eFluor 780 (clone: GK1.5), eBioscience | Thermo Fisher Scientific | Cat. #47-0041-82 RRID: AB_11218896 |
| CD19 Monoclonal Antibody PE (eBio1D3 (1D3)), eBioscience | Thermo Fisher Scientific | Cat. #12-0193-83 RRID: AB_657660 |
| PerCp/Cy5.5 anti-mouse CD138 (Syndecan-1) (clone: 281-2) | BioLegend | Cat. #142510 RRID: AB_2561601 |
| BD Pharmingen FITC rat anti-mouse IgD (clone: 11-26c.2a) | BD Biosciences | Cat. #553439 RRID: AB_394859 |
| EDE1 C8 | Ralph Baric (Swanstrom, Plante et al., 2016) | N/A |
| EDE1 C10 | Ralph Baric (Swanstrom, Plante et al., 2016) | N/A |
| In vivo MAb rat IgG1 isotype control, anti-horseradish peroxidase (clone: HPRN) | BioXCell | Cat. #BE0088 RRID: AB_1107775 |
| BD Pharmingen PE-labeled anti-human CD209 (clone: DCN46) | BD Biosciences | Cat. #551265 RRID: AB_394123 |
| In vivo Mab anti-mouse TNFα (clone: XT3.11) | BioXCell | Cat. #BE0058 RRID: AB_1107764 |
| Peroxidase conjugated affini-pure goat anti-mouse IgG F(ab’)2 fragment | Jackson ImmunoResearch | Cat. #115-035-072 RRID: AB_2338507 |
| Peroxidase conjugated affini-pure goat anti-mouse IgG Fcγ | Jackson ImmunoResearch | Cat. #115-035-008 RRID: AB_2313585 |
| Bacterial and Virus Strains | ||
| ZIKV (SD001) | Carlin et al., Submitted | N/A |
| DENV2 (S221) | Yauch, Zellweger et al., 2009 | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Cytofix/Cytoperm | BD Biosciences | Cat. #554714 |
| Qiamp Viral Mini Kit | Qiagen | Cat. #52904 |
| RNAlater | Thermo Fisher Scientific | Cat. #AM7021 |
| QuantaBio qScript one-step qRT-PCR kit | VWR | Cat. #101414-172 |
| eBioscience TMB solution | Thermo Fisher Scientific | Cat. #00-4201-56 |
| Experimental Models: Cell Lines | ||
| Aedes albopicticus: C6/36 | ATCC | Cat. #ATCC: CRL-1660 RRID: CVCL_Z230 |
| Baby Hamster Kidney (BHK)-21 | ATCC | Cat. #ATCC: CCL-10 RRID: CVCL_1915 |
| U937-DC SIGN cells | ATCC | Cat. #CRL-3253 RRID: CVCL_2Z95 |
| Experimental Models: Organisms/Strains | ||
| Mouse: LysMCre+Ifnar1fl/fl C57BL/6 | Michael S. Diamond (Clausen, Burkhardt et al., 1999) | N/A |
| Oligonucleotides | ||
| DENV2 forward primer: CATATTGACGCTGGGAAAGA | Prestwood, Prigozhin et al., 2008 | N/A |
| DENV2 reverse primer: AGAACCTGTTGATTCAAC | Prestwood, Prigozhin et al., 2008 | N/A |
| 18S forward primer: CGGCTACCACATCCAAGGAA | Prestwood, Prigozhin et al., 2008 | N/A |
| 18S reverse primer: GCTGGAATTACCGCGGCT | Prestwood, Prigozhin et al., 2008 | N/A |
| DENV2 probe – [Fam]-TGCTGGCCTC – [TamraQ] | Eurofins | N/A |
| 18S probe – [Fam] – CTGTCTGGCA – [TamraQ] | Eurofins | N/A |
| Software and Algorithms | ||
| FlowJo version 10 | FlowJo | https://www.flowjo.com/ |
| Graphpad Prism 7 | Graphpad Prism Software | https://www.graphpad.com/ |
| Other | ||
| Pierce FITC antibody labelling kit | Thermo Fisher Scientific | Cat. #53027 |
| Nab Protein G Spin Kit | Thermo Fisher Scientific | Cat. #89979 |
| Slide-A-Lyzer | Thermo Fisher Scientific | Cat. #66212 |
| Mouse TNF-α Quantikine ELISA kit | R&D Systems | Cat. #MTA00B |
| ZIKV E protein Suriname strain | Native Antigen Company | Cat. #ZIKVSU-ENV |
| KPL True Blue | SeraCare | Cat. #5510-0030 |
ZIKV and DENV ELISA.
Serum samples from mice born to ZIKV- or DENV-immune mothers were tested for ZIKV- and DENV-binding antibodies using a direct ELISA. To detect DENV antibodies, sucrose purified DENV2 S221 virions were used at a concentration of 1 × 106 FFU/well as the coating antigen and UV-inactivated. DENV2 was diluted in 50 μL coating buffer (0.1M NaHCO3 in PBS) per well and incubated at 4°C overnight. Wells were washed 3 times with ELISA washing buffer (0.05% Tween20 in PBS) and then blocked with 5% casein in PBS for an hour at room temperature. Serum was first diluted 1:10 in 10% FBS/PBS and then 1:3 for subsequent dilutions and was incubated in wells for 1.5 hours at room temperature. Wells were washed 3 times with ELISA washing buffer. Wells were then incubated with peroxidase conjugated Affini-Pure Goat anti-mouse IgG Fcγ (Jackson ImmunoResearch, cat. #115-035-008) diluted 1:5000 in 1% BSA/PBS at room temperature. Wells were washed 3 times with ELISA washing buffer. 100 μL of TMB substrate solution was added until blue color change, and reaction was stopped with 50 μL of 2N sulfuric acid (Sigma, cat. #339741). To detect ZIKV Abs, ZIKV E protein (Suriname strain, Native Antigen Company, #ZIKVSU-ENV) was adsorbed to 96-well plates at a concentration of 1 μg/mL in coating buffer (0.1M NaHCO3 in PBS) overnight at 4 °C and the remaining steps were the same as the DENV ELISA.
Neutralization assays.
Serum samples from naïve pups born to ZIKV- or DENV-immune mothers were used in a standard flow cytometry-based neutralization assay using U937-DC-SIGN cells (Wen et al., 2017). Mouse serum was inactivated at 56°C for 30 minutes. Sera were diluted at 1:10 and then at 1:3 for subsequent dilutions up to 1:7290 and then incubated with 105 FFU of DENV2 S221 or with 6 × 104 FFU ZIKV-SD001 in RPMI 1640 supplemented with 1% penicillin/streptomycin and 1% HEPES in a 96-well round bottom plate. Virus and sera were incubated together at 37°C with 5% CO2 for 1 hour. U937-DC-SIGN cells were then seeded into each well (105 cells per well in a 96-well round bottom plate), followed by addition of the virus/serum mixture to cells. Plates were incubated at 37°C with 5% CO2 for 2 hours, rocking every 15 minutes. Plates were then centrifuged at 300 × g for 5 minutes and media was replaced with RPMI 1640 (supplemented with 10% FBS, 1% penicillin/streptomycin, and 1% HEPES). Sixteen hours later, cells were stained with PE-labeled anti-human CD209 diluted 1:100 (BD Pharmingen, cat. #551265), fixed and permeabilized with Cytofix/Cytoperm (BD Biosciences, cat. #554714), and then stained with FITC-labeled anti-flavivirus E protein antibody (clone 4G2) diluted 1:100. 4G2 was conjugated to Pierce FITC using an antibody labelling kit (Thermo Fisher Scientific, cat. #53027).
TNF blockade.
At days 1, 2, and 3 post challenge with DENV, pups born to ZIKV-immune mothers (n=3 mothers infected for 7-9 months) were treated via intraperitoneal injection with 100 μg of anti-TNF (BioXcell, clone XT3.11, Rat IgG1, cat. #BE0058) or an isotype control (BioXcell, clone HRPN, Rat IgG1, cat. #BE0088) Ab that was diluted in PBS (200 μL total volume per mouse). Mice were clinically scored, weighed, and monitored for survival, on a daily basis, until day 20 p.i.
Passive transfer of IgG.
Serum IgG was purified from 4- to 5-week old mice that were born to naïve or ZIKV-immune mothers. For the first IgG preparation, serum was isolated from 23 4- to 5-week old mice from 3 different ZIKV-immune mothers and 20 naïve mice from 2 different mothers. For the second IgG preparation, serum was isolated from 13 4- to 5-week old mice from 2 different ZIKV-immune mothers and 9 naïve mice from 2 different mothers. IgG was isolated from the pooled serum using the NAb protein G spin columns, 1 mL (Thermo Fisher Scientific, cat. #89957). Buffer was exchanged with PBS by the Slide-a-lyzer dialysis cassettes, 2K molecular weight cutoff, 12 mL (Thermo Fisher Scientific, cat. #66212) per manufacturer’s directions. The isolated IgGs were injected via intraperitoneal route into 4- to 5-week old naïve LysMCre+Ifnar1fl/fl mice 30 min prior to inoculation with 2 × 105 FFU of DENV2 S221 via the tail vein. Three days p.i., mice were humanely euthanized. Blood was drawn, serum was isolated, and spleens and livers were harvested.
TNF ELISA.
Serum samples from mice that received IgG was assessed for TNF levels using a R&D Systems quantikine ELISA kit (R&D Systems, cat. #MTA00B). 52 μL of the serum from each recipient mouse that received 145 μg of IgG was assessed to determine levels of TNF using the kit protocol.
Monoclonal Ab treatment.
At days 1, 2, and 3 post challenge with DENV2, pups born to ZIKV-immune mothers (n=4 mothers infected for 12-13 months) were treated via intraperitoneal injection with 100 μg of monoclonal Ab (C8 or C10) recognizing the DENV E-dimer epitope (EDE) (Dejnirattisai et al., 2015) diluted in PBS to a total of 200 μL total volume per mouse. Both EDE1-C8 and EDE1-C10 Abs were produced recombinantly (Swanstrom et al., 2016). Mice were clinically scored, weighed, and monitored for survival on a daily basis for 20 days p.i.
QUANTIFICATION AND STATISTICAL ANALYSIS
Flow cytometric analysis of immune cell populations.
Following Ab staining, splenocytes were resuspended in FACs buffer and analyzed on the LSRII flow cytometer. Data were analyzed using FlowJo software X 10.0.7 (Tree Star).
Infectious virus quantification.
Infectious viral foci were detected by True Blue™ peroxidase substrate reaction and counted manually. Viral titers were expressed as log FFU/g tissue or log FFU/mL serum.
Viral RNA quantification.
DENV2 RNA was quantified using the CFX96 Touch™ real-time PCR detection system (Bio-Rad CFX Manager 3.1) and normalized to volume for serum and to 18S RNA levels for spleens and livers.
ZIKV and DENV ELISA.
ZIKV and DENV specific IgG were detected by TMB reaction. Signals were quantified using a Spectramax M2E at 450 nm.
Neutralization assays.
After Ab staining, infected U937-DC-SIGN cells were resuspended in FACS buffer and analyzed on the LSRII flow cytometer. Data were analyzed using FlowJo software X 10.0.7 (Tree Star). Percent inhibition was calculated by taking the value of % infection of the control with no serum - % infection of sample and dividing all by the value of % infection of the control with no serum.
Statistical analysis.
n demonstrates the number of mice used per experiment. For Fig. 1A-D: 11 male and female pups were used from 2 naïve mothers and 15 male and female pups were used from 3 ZIKV-immune mothers that were infected for 8-12 months; Fig. 1E-H: 8 male and female mice were used from 2 naïve mothers and 8 male and female mice from 2 ZIKV-immune mothers infected for 2 months; Fig. 1I: 11 male and female pups from 2 naïve mothers and 13 male and female pups from 4 ZIKV-immune mothers infected for 6-13 months; Fig. 1J: 7 female and male pups from 2 naïve mothers and 7 male and female pups from 2 ZIKV-immune mothers infected for 2 months. For Fig. 2A-D: serum was obtained from 10 male and female pups born to 2 naïve mothers and 13 male and female pups born to 4 ZIKV-immune mothers infected for 6-13 months; Fig. 2E-F: serum from 10 male and female mice from 2 ZIKV-immune mothers infected for 2 months compared to serum obtained in Fig. 2A-D. For Fig. 3: IgG was isolated from 32 male and female pups born to 7 naïve mothers and 36 male and female pups born to 8 ZIKV-immune mothers infected for 6-12 months and 13 male and female mice from 3 naïve mothers were used as recipient mice. For Fig. 4A-D: 15 male and female mice from 3 ZIKV-immune mothers infected for 7-9 months; Fig. 4E-I 27 male and female mice from 4 ZIKV-immune mothers infected for 12-13 months. n values can also be found in figure legends.
Data were analyzed using Graphpad Prism, version 7 (Graphpad Software, Inc.). P values were obtained by using Mann Whitney test, unpaired Student’s t test, one-way ANOVA or by using the log-rank test for survival curves. Goodness of fit tests were performed for data analyzed by unpaired Student’s t tests to determine normal distribution of data as expected. Statistical details are described in corresponding figure legends along with P values.
Supplementary Material
Highlights (85 or less).
Maternal ZIKV antibodies increase dengue disease severity in DENV-challenged pups.
Maternal ZIKV antibodies increase dengue viral burden in DENV-challenged pups.
Maternal ZIKV antibodies cross-react but do not neutralize DENV2.
Acknowledgements
This research was funded by NIH grants (AI116813 and NS100477 to S.S., AI104972 and HD091218 to M.S.D., and AI106695 and AI125198 to RSB) and by the NIH/La Jolla Institute for Allergy and Immunology Training grant T32AI125179 to A.F.
Footnotes
Declaration of Interests
M.S.D. is a consultant for Inbios, and is on the Scientific Advisory Board of Moderna. R.S.B. has consulted with Takeda and Sanofi Pasteur. All other authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balsitis SJ, Williams KL, Lachica R, Flores D, Kyle JL, Mehlhop E, Johnson S, Diamond MS, Beatty PR, and Harris E (2010). Lethal antibody enhancement of dengue disease in mice is prevented by Fc modification. PLoS pathogens 6, e1000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barba-Spaeth G, Dejnirattisai W, Rouvinski A, Vaney MC, Medits I, Sharma A, Simon-Loriere E, Sakuntabhai A, Cao-Lormeau VM, Haouz A, et al. (2016). Structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature 536, 48–53. [DOI] [PubMed] [Google Scholar]
- Bardina SV, Bunduc P, Tripathi S, Duehr J, Frere JJ, Brown JA, Nachbagauer R, Foster GA, Krysztof D, Tortorella D, et al. (2017). Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science (New York, NY) 356, 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, et al. (2013). The global distribution and burden of dengue. Nature 496, 504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HH, Huber RG, Bond PJ, Grad YH, Camerini D, Maurer-Stroh S, and Lipsitch M (2017). Systematic analysis of protein identity between Zika virus and other arthropod-borne viruses. Bulletin of the World Health Organization 95, 517–525i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles AS, and Christofferson RC (2016). Utility of a Dengue-Derived Monoclonal Antibody to Enhance Zika Infection In Vitro. PLoS currents 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau TN, Hieu NT, Anders KL, Wolbers M, Lien le B, Hieu LT, Hien TT, Hung NT, Farrar J, Whitehead S, et al. (2009). Dengue virus infections and maternal antibody decay in a prospective birth cohort study of Vietnamese infants. The Journal of infectious diseases 200, 1893–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen BE, Burkhardt C, Reith W, Renkawitz R, and Forster I (1999). Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic research 8, 265–277. [DOI] [PubMed] [Google Scholar]
- Collins MH, McGowan E, Jadi R, Young E, Lopez CA, Baric RS, Lazear HM, and de Silva AM (2017). Lack of Durable Cross-Neutralizing Antibodies Against Zika Virus from Dengue Virus Infection. Emerging infectious diseases 23, 773–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejnirattisai W, Supasa P, Wongwiwat W, Rouvinski A, Barba-Spaeth G, Duangchinda T, Sakuntabhai A, Cao-Lormeau VM, Malasit P, Rey FA, et al. (2016). Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nature immunology 17, 1102–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejnirattisai W, Wongwiwat W, Supasa S, Zhang X, Dai X, Rouvinski A, Jumnainsong A, Edwards C, Quyen NTH, Duangchinda T, et al. (2015). A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nature immunology 16, 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deligny C, de Bandt M, Dehlinger V, Numeric P, Cabie A, Lombard F, Polomat K, JeanBaptiste G, and Arfi S (2014). Dengue fever in patients under biologics. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology 61, 442–443. [DOI] [PubMed] [Google Scholar]
- Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM, Kalinke U, et al. (2011). Type I interferon is selectively required by dendritic cells for immune rejection of tumors. The Journal of experimental medicine 208, 1989–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick GW (1952). Zika virus. II. Pathogenicity and physical properties. Transactions of the Royal Society of Tropical Medicine and Hygiene 46, 521–534. [DOI] [PubMed] [Google Scholar]
- Dick GW, Kitchen SF, and Haddow AJ (1952). Zika virus. I. Isolations and serological specificity. Transactions of the Royal Society of Tropical Medicine and Hygiene 46, 509–520. [DOI] [PubMed] [Google Scholar]
- Elong Ngono A, and Shresta S (2018). Immune Response to Dengue and Zika. Annual review of immunology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elong Ngono A, Vizcarra EA, Tang WW, Sheets N, Joo Y, Kim K, Gorman MJ, Diamond MS, and Shresta S (2017). Mapping and Role of the CD8+ T Cell Response During Primary Zika Virus Infection in Mice. Cell host & microbe 21, 35–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez E, Dejnirattisai W, Cao B, Scheaffer SM, Supasa P, Wongwiwat W, Esakky P, Drury A, Mongkolsapaya J, Moley KH, et al. (2017a). Human antibodies to the dengue virus E-dimer epitope have therapeutic activity against Zika virus infection. Nature immunology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez E, Dejnirattisai W, Cao B, Scheaffer SM, Supasa P, Wongwiwat W, Esakky P, Drury A, Mongkolsapaya J, Moley KH, et al. (2017b). Human antibodies to the dengue virus E-dimer epitope have therapeutic activity against Zika virus infection. Nature immunology 18, 1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J, Valiant WG, Mattapallil MJ, Walker M, Huang YS, Vanlandingham DL, Misamore J, Greenhouse J, Weiss DE, Verthelyi D, et al. (2017). Prior Exposure to Zika Virus Significantly Enhances Peak Dengue-2 Viremia in Rhesus Macaques. Scientific reports 7, 10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S, Vaughn DW, Kalayanarooj S, Nimmannitya S, Suntayakorn S, Nisalak A, Lew R, Innis BL, Kurane I, Rothman AL, et al. (1999). Early immune activation in acute dengue illness is related to development of plasma leakage and disease severity. The Journal of infectious diseases 179, 755–762. [DOI] [PubMed] [Google Scholar]
- Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, Hunsperger E, Kroeger A, Margolis HS, Martinez E, et al. (2010). Dengue: a continuing global threat. Nature reviews Microbiology 8, S7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead SB (2007). Dengue. Lancet (London, England) 370, 1644–1652. [DOI] [PubMed] [Google Scholar]
- Halstead SB, Lan NT, Myint TT, Shwe TN, Nisalak A, Kalyanarooj S, Nimmannitya S, Soegijanto S, Vaughn DW, and Endy TP (2002). Dengue hemorrhagic fever in infants: research opportunities ignored. Emerging infectious diseases 8, 1474–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hober D, Poli L, Roblin B, Gestas P, Chungue E, Granic G, Imbert P, Pecarere JL, Vergez-Pascal R, Wattre P, et al. (1993). Serum levels of tumor necrosis factor-alpha (TNF-alpha), interleukin-6 (IL-6), and interleukin-1 beta (IL-1 beta) in dengue-infected patients. The American journal of tropical medicine and hygiene 48, 324–331. [DOI] [PubMed] [Google Scholar]
- Katzelnick LC, Gresh L, Halloran ME, Mercado JC, Kuan G, Gordon A, Balmaseda A, and Harris E (2017). Antibody-dependent enhancement of severe dengue disease in humans. Science (New York, NY) 358, 929–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawiecki AB, and Christofferson RC (2016). Zika Virus-Induced Antibody Response Enhances Dengue Virus Serotype 2 Replication In Vitro. The Journal of infectious diseases 214, 1357–1360. [DOI] [PubMed] [Google Scholar]
- Kittigul L, Temprom W, Sujirarat D, and Kittigul C (2000). Determination of tumor necrosis factor-alpha levels in dengue virus infected patients by sensitive biotin-streptavidin enzyme-linked immunosorbent assay. Journal of virological methods 90, 51–57. [DOI] [PubMed] [Google Scholar]
- Martinez Gomez JM, Ong LC, Lam JH, Binte Aman SA, Libau EA, Lee PX, St John AL, and Alonso S (2016). Maternal Antibody-Mediated Disease Enhancement in Type I Interferon-Deficient Mice Leads to Lethal Disease Associated with Liver Damage. PLoS neglected tropical diseases 10, e0004536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken MK, Gromowski GD, Friberg HL, Lin X, Abbink P, De La Barrera R, Eckles KH, Garver LS, Boyd M, Jetton D, et al. (2017). Impact of prior flavivirus immunity on Zika virus infection in rhesus macaques. PLoS Pathog 13, e1006487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya M, Collins M, Dejnirattisai W, Katzelnick LC, Puerta-Guardo H, Jadi R, Schildhauer S, Supasa P, Vasanawathana S, Malasit P, et al. (2018). Longitudinal Analysis of Antibody Cross-Neutralization Following Zika and Dengue Virus Infection in Asia and the Americas. The Journal of infectious diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng JK, Zhang SL, Tan HC, Yan B, Martinez JM, Tan WY, Lam JH, Tan GK, Ooi EE, and Alonso S (2014). First experimental in vivo model of enhanced dengue disease severity through maternally acquired heterotypic dengue antibodies. PLoS pathogens 10, e1004031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization, W.H. (2016). Zika virus, microcephaly and Guillain-Barré syndrome. (Geneva, Switzerland: World Health Organization). [Google Scholar]
- Pantoja P, Perez-Guzman EX, Rodriguez IV, White LJ, Gonzalez O, Serrano C, Giavedoni L, Hodara V, Cruz L, Arana T, et al. (2017). Zika virus pathogenesis in rhesus macaques is unaffected by pre-existing immunity to dengue virus. Nature communications 8, 15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phanthanawiboon S, Limkittikul K, Sakai Y, Takakura N, Saijo M, and Kurosu T (2016). Acute Systemic Infection with Dengue Virus Leads to Vascular Leakage and Death through Tumor Necrosis Factor-alpha and Tie2/Angiopoietin Signaling in Mice Lacking Type I and II Interferon Receptors. PloS one 11, e0148564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto AK, Brien JD, Lam CY, Johnson S, Chiang C, Hiscott J, Sarathy VV, Barrett AD, Shresta S, and Diamond MS (2015). Defining New Therapeutics Using a More Immunocompetent Mouse Model of Antibody-Enhanced Dengue Virus Infection. mBio 6, e01316–01315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwood TR, Prigozhin DM, Sharar KL, Zellweger RM, and Shresta S (2008). A mouse-passaged dengue virus strain with reduced affinity for heparan sulfate causes severe disease in mice by establishing increased systemic viral loads. Journal of virology 82, 8411–8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyamvada L, Quicke KM, Hudson WH, Onlamoon N, Sewatanon J, Edupuganti S, Pattanapanyasat K, Chokephaibulkit K, Mulligan MJ, Wilson PC, et al. (2016). Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proceedings of the National Academy of Sciences of the United States of America 113, 7852–7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richner JM, Himansu S, Dowd KA, Butler SL, Salazar V, Fox JM, Julander JG, Tang WW, Shresta S, Pierson TC, et al. (2017). Modified mRNA Vaccines Protect against Zika Virus Infection. Cell 168, 1114–1125 e1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard DS, Undurraga EA, Halasa YA, and Stanaway JD (2016). The global economic burden of dengue: a systematic analysis. The Lancet Infectious diseases 16, 935–941. [DOI] [PubMed] [Google Scholar]
- Shresta S, Sharar KL, Prigozhin DM, Beatty PR, and Harris E (2006). Murine model for dengue virus-induced lethal disease with increased vascular permeability. Journal of virology 80, 10208–10217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons CP, Chau TN, Thuy TT, Tuan NM, Hoang DM, Thien NT, Lien le B, Quy NT, Hieu NT, Hien TT, et al. (2007). Maternal antibody and viral factors in the pathogenesis of dengue virus in infants. The Journal of infectious diseases 196, 416–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, Vanzetta F, Minola A, Jaconi S, Mele F, et al. (2016). Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science (New York, NY) 353, 823–826. [DOI] [PubMed] [Google Scholar]
- Swanstrom JA, Plante JA, Plante KS, Young EF, McGowan E, Gallichotte EN, Widman DG, Heise MT, de Silva AM, and Baric RS (2016). Dengue Virus Envelope Dimer Epitope Monoclonal Antibodies Isolated from Dengue Patients Are Protective against Zika Virus. mBio 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WW, Young MP, Mamidi A, Regla-Nava JA, Kim K, and Shresta S (2016). A Mouse Model of Zika Virus Sexual Transmission and Vaginal Viral Replication. Cell reports 17, 3091–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Chen RF, Liu JW, Yu HR, Kuo HC, and Yang KD (2007). Implications of dynamic changes among tumor necrosis factor-alpha (TNF-alpha), membrane TNF receptor, and soluble TNF receptor levels in regard to the severity of dengue infection. The American journal of tropical medicine and hygiene 77, 297–302. [PubMed] [Google Scholar]
- Watanabe S, Chan KW, Wang J, Rivino L, Lok SM, and Vasudevan SG (2015). Dengue Virus Infection with Highly Neutralizing Levels of Cross-Reactive Antibodies Causes Acute Lethal Small Intestinal Pathology without a High Level of Viremia in Mice. Journal of virology 89, 5847–5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J, Tang WW, Sheets N, Ellison J, Sette A, Kim K, and Shresta S (2017). Identification of Zika virus epitopes reveals immunodominant and protective roles for dengue virus cross-reactive CD8+ T cells. Nature microbiology 2, 17036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Wang R, Gao F, Li M, Liu J, Wang J, Hong W, Zhao L, Wen Y, Yin C, et al. (2017). Delineating antibody recognition against Zika virus during natural infection. JCI insight 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellweger RM, Prestwood TR, and Shresta S (2010). Enhanced infection of liver sinusoidal endothelial cells in a mouse model of antibody-induced severe dengue disease. Cell host & microbe 7, 128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zust R, Toh YX, Valdes I, Cerny D, Heinrich J, Hermida L, Marcos E, Guillen G, Kalinke U, Shi PY, et al. (2014). Type I interferon signals in macrophages and dendritic cells control dengue virus infection: implications for a new mouse model to test dengue vaccines. J Virol 88, 7276–7285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.