ABSTRACT
Background
Many dietary indexes exist for chronic disease prevention, but the optimal dietary pattern for colorectal cancer prevention is unknown.
Objective
We sought to determine associations between adherence to various dietary indexes and incident colorectal cancer in 2 prospective cohort studies.
Design
We followed 78,012 women in the Nurses’ Health Study and 46,695 men in the Health Professionals Follow-up Study from 1986 and 1988, respectively, until 2012. We created dietary index scores for the Dietary Approaches to Stop Hypertension (DASH) diet, Alternative Mediterranean Diet (AMED), and Alternative Healthy Eating Index-2010 (AHEI-2010) and used Cox regression to estimate HRs and 95% CIs for risk of colorectal cancer (CRC) and by anatomic subsite. We also conducted latency analyses to examine associations between diet and CRC risk during different windows of exposure. We conducted analyses in men and women separately, and subsequently pooled these results in a random-effects meta-analysis.
Results
We documented 2690 colorectal cancer cases. Pooled multivariable HRs for colorectal cancer risk comparing the highest to lowest quintile of diet scores were 0.89 (95% CI: 0.74, 1.08; P-trend = 0.10) for DASH, 0.89 (95% CI: 0.73, 1.10; P-trend = 0.31) for AMED, and 0.95 (95% CI: 0.83, 1.09; P-trend = 0.56) for AHEI-2010 (P-heterogeneity ≥ 0.07 for all). In sex-specific analyses, we observed stronger associations in men for all dietary indexes (DASH: multivariable HR = 0.81, 95% CI: 0.66, 0.98; P-trend = 0.003; AMED: multivariable HR = 0.80, 95% CI: 0.65, 0.98; P-trend = 0.02; AHEI-2010: multivariable HR = 0.88, 95% CI: 0.72, 1.07; P-trend = 0.04) than in women (multivariable HRs range from 0.98 to 1.01).
Conclusions
Adherence to the DASH, AMED, and AHEI-2010 diets was inversely associated with colorectal cancer risk in men. These diets were not associated with colorectal cancer risk in women. This observational study was registered at http://www.clinicaltrials.gov as NCT03364582.
Keywords: colorectal cancer, dietary index, cohort studies, DASH diet, Mediterranean diet, Alternative Healthy Eating Index
INTRODUCTION
Associations between various foods and nutrients and colorectal cancer (CRC) incidence have been observed in many epidemiologic studies, with strong evidence of a harmful role of red and processed meats and alcohol, and of a protective role of whole grains, dairy products, dietary fiber, calcium, and folate (1). Fewer studies have reported associations for recommendation-based dietary indexes (2), which simultaneously account for synergistic relations between dietary components and represent combinations of dietary components according to established recommendations (3). While several cohort studies have reported associations between adherence to dietary indexes and CRC risk (4–11), it is unclear which pattern is optimal for CRC prevention. Moreover, studies on dietary index adherence and CRC incidence have generally not accounted for the long induction period between dietary intake and CRC diagnosis, despite evidence that diet in the distant past may be most relevant for CRC risk (12, 13).
The Dietary Approaches to Stop Hypertension (DASH) diet, Alternative Mediterranean Diet (AMED), and Alternative Healthy Eating Index-2010 (AHEI-2010) have all been inversely associated with overall mortality (14–16), cardiovascular disease risk (16, 17), and diabetes risk (18, 19). Although none of these diets were specifically developed for CRC prevention, they all contain components of diets that have been linked with CRC risk. However, evidence for their associations with CRC risk within the same population is limited.
We therefore conducted a study in 2 prospective cohorts of men and women to examine associations between adherence to the DASH, AMED, and AHEI-2010 dietary indexes and CRC incidence. We previously reported inverse associations between adherence to the DASH and AMED indexes and CRC risk in a study involving 2464 incident CRC cases through 2006 (20). In the present analysis, we extend our analyses of the DASH and AMED indexes through 2012, and additionally report on the AHEI-2010 index, providing 2690 incident cases of CRC, facilitating our ability to examine anatomical subsites and conduct latency analyses to explore the association between CRC incidence and dietary index adherence in the distant past.
METHODS
Study population
This study was conducted within the Nurses’ Health Study (NHS) and Health Professionals Follow-up Study (HPFS). The NHS is a cohort of 121,701 female nurses aged 30–55 y living in the United States at the time of initiation in 1976. The HPFS is a cohort of 51,529 male health professionals aged 40–75 y at the time of initiation in 1986. Both cohorts are ongoing, with updated data on medical, lifestyle, and other health-related information collected from participants via questionnaire every 2 y since baseline. Follow-up is >90% in both cohorts.
We excluded individuals who had a history of cancer (except nonmelanoma skin cancer) or ulcerative colitis, as well as those who were missing >70 items on the baseline 131-item food-frequency questionnaire (FFQ). We also excluded individuals with implausible energy intake (<500 or >3500 kcal/d for women and <800 or >4200 kcal/d for men), since these individuals may have filled out their questionnaires improperly. After these exclusions, there were 78,012 women and 46,695 men in the final analysis (Supplemental Figure 1). The study protocol was approved by the Institutional Review Boards at Brigham and Women's Hospital and Harvard TH Chan School of Public Health.
Dietary assessment
Dietary data were collected via self-administered, semiquantitative FFQs in 1984 in the NHS, 1986 in both cohorts, and every 4 y thereafter. We used only the expanded FFQ because it better estimates the intake of certain dietary index constituents than previously administered shorter FFQs (21, 22). These FFQs provided standard portion sizes for each item, and asked participants to record their frequency of intake, with 9 possible responses ranging from “never or less than once per month” to “six or more times per day.” Average daily nutrient intake was calculated by multiplying the frequency of intake by the nutrient content of each food and then summing nutrient values across all foods. Estimated intake of foods and nutrients by these FFQ has been validated previously against intake via multiple weeks of diet records (21–23), with correlations for dietary components ranging from 0.26 (cruciferous vegetables) to 0.78 (liquor).
Computation of DASH (24), AHEI-2010 (25), and AMED scores (26) in these studies has been described previously in detail. DASH diet scores consist of 8 components; for 5 components (fruits, vegetables, whole grains, nuts and legumes, and low-fat dairy), participants in the lowest quintile of intake are given 1 point, and an additional point is awarded for each increasing quintile. For 3 components (red and processed meats, sugar-sweetened beverages, and sodium), participants in the highest quintile of intake are given 1 point, and an additional point is awarded for each decreasing quintile. The component scores are summed for a total DASH score ranging from 8 to 40. AMED scores consist of 9 components. For 7 of these components (fruits, vegetables, legumes, nuts, whole grains, fish, and MUFA-to-SFA ratio), intake above the median is given 1 point; for red and processed meats, 1 point is awarded to those with intake below the median; and for alcohol, 1 point is awarded for moderate intake. The component scores are summed for a total AMED score ranging from 0 to 9 points. AHEI-2010 scores consist of 11 items, with predefined criteria for complete adherence and nonadherence for each. Higher intake is rewarded for 6 components (fruits, vegetables, whole grains, nuts and legumes, PUFAs, and omega-3 fatty acids), lower intake is rewarded for 4 components (red and processed meats, sugar-sweetened beverages, trans fatty acids, and sodium), and moderate intake is rewarded for alcohol (0.5–1.5 drinks/d for women, 0.5–2 drinks/d for men). Each component receives a score from 0 (complete nonadherence) to 10 (complete adherence), with partial adherence scores ranging between 0 and 10 directly proportional to intake. Component scores are summed for a total AHEI-2010 score ranging from 0 to 110. A comparison of dietary components included in each index is provided in Supplemental Table 1.
Ascertainment of CRC
Participants self-reported incident CRC between baseline and 2012 on biennial questionnaires, and a study physician blinded to exposure reviewed records to confirm cases and extract information on anatomic location. Diagnosis of CRC in participants who died from CRC but had not reported a diagnosis on a questionnaire was confirmed through various sources, including next of kin, the National Death Index, death certificates, and medical records. For the present study, CRC was the primary outcome, and 4 specific anatomic locations (colon cancer, proximal colon cancer, distal colon cancer, and rectal cancer) were the secondary outcomes.
Statistical analysis
Person-time was calculated for each participant from age (in months) 2 y after the date of the 1984 questionnaire in the NHS and 2 y after the date of the 1986 questionnaire in the HPFS until age at death, CRC diagnosis, loss to follow-up, or end of follow-up (1 June 2012 for the NHS and 1 January 2012 for the HPFS). We calculated the cumulative average of all dietary scores from FFQs completed prior to CRC diagnosis, loss to follow-up, death, or the year 2012 in order to represent long-term intake and reduce random within-person variation in diet (27), and lagged these exposures by 2 y, since changes in diet could result from symptoms of undiagnosed CRC (for example, in the NHS, the 1984 FFQ was used for follow-up time between 1986 and 1988; average dietary scores from the 1984 and 1986 FFQs were used for follow-up time between 1988 and 1990).
We used Cox regression (28) to estimate HRs and 95% CIs for associations between quintiles of the index scores and risk of CRC endpoints (total CRC, colon cancer, proximal colon cancer, distal colon cancer, and rectal cancer). For all analyses, we used age as the time scale and stratified the baseline hazard by calendar year. In multivariable analyses, we additionally adjusted for various dietary and lifestyle factors.The following covariates were included in the models: total energy intake (kcal/d, quintiles), alcohol intake (g/d, quintiles), physical activity (MET-hours/wk, quintiles), NSAID use [≥2 NSAIDs/wk vs. <2 NSAIDs/wk (ref)], family history of CRC [yes vs. no (ref)], previous CRC screening via colonoscopy or sigmoidoscopy [yes vs. no (ref)], history of polyps [yes vs. no (ref)], smoking [never smoker (ref), 0 -<10, 10 -<20, 20 -<30, 30 -<40, 40 -<50, ≥50 pack-years], multivitamin use [regular use vs. nonuse (ref)], supplemental calcium intake [none (ref), >0 -200, >200 -400, >400 -600, >600 mg/d], and young adult BMI [in kg/m2; <25 (ref), 25 -<27.5, 27.5 -<30, ≥30]). If exposure or covariate data were missing for a cycle, we carried forward nonmissing exposure and covariate data from the previous data cycle. We tested the proportional hazards assumption by evaluating the P-value of an interaction term between each exposure variable and age in multivariable models for CRC risk, and did not find violations for any exposure (P > 0.05 for all). We calculated a test of trend by modeling the index scores continuously, and additionally examined whether the association between the continuous scores and the CRC risk were linear by examining nonparametric regression curves with restricted cubic splines (29, 30). The model with linear and cubic spline terms, selected using a stepwise regression procedure, was compared with a model with only a linear term using the likelihood ratio test. We pooled data from the NHS and HPFS in a random-effects meta-analysis to obtain summary HRs and 95% CIs.
To determine whether the association between the dietary indexes and CRC risk differed according to anatomic location, we ran Cox proportional hazards models with a data augmentation method and performed a test of heterogeneity-comparing models that assume different associations for different CRC subtypes with a model that assumes a common association (31). We tested for heterogeneous associations for proximal colon, distal colon, and rectal cancers using the maximum likelihood ratio test.
We explored potential effect modification by regular nonsteroidal anti-inflammatory drug use (regular use compared with no regular use), family history of CRC (yes compared with no), obesity status (obese compared with not obese), smoking (ever compared with never smokers), regular multivitamin use (yes compared with no), physical activity (above compared with below median physical activity), young adult overweight [BMI (kg/m2) <25 compared with ≥25 at age 18 y in the NHS and age 21 y in the HPFS], history of CRC screening (yes compared with no), supplemental calcium intake (<50 compared with ≥50 mg/d), and, in women, oral contraceptive use (ever compared with never use) and postmenopausal hormone use (ever compared with never use) by running regression models with an interaction term between continuous dietary pattern scores and each potential effect modifier separately.
To evaluate associations with different windows of dietary intake, we conducted latency analyses, whereby we created different regression models based on dietary data collected at distinct time points. We analyzed simple updated intake, where index scores at each follow-up interval were constructed solely on the most recent FFQ, as well as with different latencies (0–4, 4–8, 8–12, and 12–16 y), where the index scores analyzed at each follow-up interval were constructed from lagged FFQ data (12). For example, in the 4- to 8-y lagged analyses, index scores created from the 1990 FFQ were related to CRC diagnoses between 1994 and 1998, while in the 8- to 12-y lagged analyses, the 1990 FFQ diet was related to diagnoses between 1998 and 2002.
In sensitivity analyses, we adjusted for BMI and diabetes, since these variables may be both confounders and mediators of associations between diet and CRC risk. We also conducted analyses where we removed alcohol from the AHEI-2010 and AMED indexes, since alcohol is a risk factor for CRC (32) and is given favorable points in these indexes. Lastly, we conducted analyses after removing history of diagnosed polyps (yes compared with no) from the model, since these could be potential intermediate precursor lesions.
All analyses were conducted using SAS version 9.4 for UNIX (Cary, NC). We calculated 2-sided 95% CIs for all statistical tests.
RESULTS
We documented 1528 incident CRC cases in the NHS over 26 y of follow-up and 1,834,968 total person-years, and 1162 incident CRC cases in the HPFS over 24 y of follow-up and 946,582 total person-years. For each of the DASH, AMED, and AHEI-2010 scores in women (NHS, Table 1) and men (HPFS, Table 2), individuals in the highest quintile of dietary index scores (most adherent) were more likely to engage in healthy behaviors (particularly physical activity) and less likely to engage in unhealthy behaviors (particularly smoking) than individuals in the lowest quintile. All 3 dietary patterns were strongly correlated, with pairwise Spearman correlation coefficients ranging from 0.62 to 0.74.
TABLE 1.
Age-standardized baseline characteristics and dietary intake in lowest and highest quintiles of DASH, AMED, and AHEI-2010 scores among women in the NHS1
| DASH (range: 8–40) | AMED (range: 0–9) | AHEI-2010 (range: 0–110) | ||||
|---|---|---|---|---|---|---|
| Q1 (n = 15,771) | Q5 (n = 13,809) | Q1 (n = 18,592) | Q5 (n = 16,213) | Q1 (n = 15,634) | Q5 (n = 15,544) | |
| Score | 15.9 ± 2.0 | 29.9 ± 1.8 | 1.5 ± 0.6 | 6.6 ± 0.7 | 33.4± 3.9 | 63.6 ± 5.8 |
| Baseline characteristics | ||||||
| Age, y | 52.8 ± 7.1 | 53.0 ± 7.2 | 52.8 ± 7.2 | 52.9 ± 7.1 | 52.8 ± 7.2 | 53.0 ± 7.1 |
| BMI, kg/m2 | 24.6 ± 4.7 | 24.3 ± 4.0 | 24.6 ± 4.5 | 24.3 ± 4.0 | 24.8 ± 4.8 | 24.0 ± 3.8 |
| Physical activity, MET-hours/wk | 9.4 ± 15.1 | 20.7 ± 28.7 | 10.6 ± 17.1 | 18.8 ± 24.8 | 9.8 ± 14.8 | 19.8 ± 26.3 |
| Current smokers, % | 34 | 12 | 28 | 15 | 28 | 15 |
| Past smokers, % | 28 | 41 | 30 | 40 | 27 | 44 |
| NSAID use, % | 43 | 41 | 42 | 42 | 44 | 40 |
| Multivitamin use, % | 28 | 31 | 28 | 31 | 27 | 32 |
| Family history of CRC, % | 8 | 8 | 8 | 8 | 8 | 8 |
| Screened for CRC, % | 3 | 4 | 3 | 3 | 3 | 4 |
| Postmenopausal, % | 57 | 57 | 56 | 57 | 57 | 56 |
| Postmenopausal hormone use, % | 28 | 33 | 29 | 33 | 29 | 34 |
| Baseline dietary intake | ||||||
| Energy, kcal/d | 1749 ± 530 | 1796 ± 491 | 1537 ± 471 | 1997 ± 531 | 1926 ± 497 | 1588 ± 505 |
| Alcohol (drinks/d), % | ||||||
| 0 | 51 | 53 | 53 | 52 | 52 | 52 |
| >0–1 | 37 | 35 | 36 | 36 | 36 | 36 |
| >1–2 | 7 | 7 | 6 | 7 | 6 | 7 |
| >2 | 5 | 5 | 5 | 5 | 5 | 5 |
| Fruits, servings/d | 1.2 ± 0.9 | 3.3 ± 1.5 | 1.3 ± 0.9 | 3.2 ± 1.5 | 1.7 ± 1.1 | 2.6 ± 1.6 |
| Vegetables, servings/d | 2.2 ± 1.1 | 4.8 ± 2.2 | 2.1 ± 1.0 | 4.9 ± 2.1 | 2.5 ± 1.2 | 4.3 ± 2.3 |
| Whole grains, servings/d | 0.5 ± 0.6 | 2.0 ± 1.3 | 0.6 ± 0.7 | 1.9 ± 1.3 | 0.8 ± 0.9 | 1.5 ± 1.3 |
| Nuts, servings/d | 0.5 ± 0.5 | 0.8 ± 0.4 | 0.3 ± 0.5 | 0.9 ± 0.3 | 0.5 ± 0.5 | 0.7 ± 0.4 |
| Legumes, servings/d | 0.3 ± 0.5 | 0.7 ± 0.5 | 0.2± 0.4 | 0.8 ± 0.4 | 0.4 ± 0.5 | 0.6 ± 0.5 |
| Low-fat dairy, servings/d | 0.3 ± 0.6 | 1.5 ± 1.0 | 0.7 ± 0.9 | 1.1 ± 1.0 | 0.8 ± 1.0 | 1.0 ± 0.9 |
| Fish, servings/d | 0.3 ± 0.5 | 0.7 ± 0.5 | 0.1 ± 0.4 | 0.9 ± 0.4 | 0.2 ± 0.4 | 0.7 ± 0.4 |
| PUFAs, % energy | 6.5 ± 1.7 | 6.4 ± 1.8 | 6.1 ± 1.7 | 6.8 ± 1.7 | 6.1 ± 1.5 | 6.9 ± 2.0 |
| ω-3 fatty acids, mg/d | 135 ± 112 | 278 ± 217 | 109 ± 88 | 304 ± 209 | 105 ± 81 | 296 ± 209 |
| MUFA:SFA ratio | 0.5 ± 0.5 | 0.5 ± 0.5 | 0.3 ± 0.5 | 0.7 ± 0.4 | 0.5 ± 0.5 | 0.6 ± 0.5 |
| Red and processed meat, servings/d | 1.0 ± 0.6 | 0.5 ± 0.3 | 0.8 ± 0.5 | 0.7 ± 0.5 | 1.1 ± 0.5 | 0.4 ± 0.3 |
| Sugar-sweetened beverages, drinks/d | 0.6 ± 0.9 | 0.1 ± 0.3 | 0.4 ± 0.7 | 0.2 ± 0.5 | 0.6 ± 0.8 | 0.1 ± 0.3 |
| Sodium, mg/d | 1923 ± 675 | 2031 ± 672 | 1672 ± 586 | 2274 ± 692 | 2145 ± 655 | 1767 ± 651 |
| Transfatty acids, % energy | 2.2 ± 0.6 | 1.5 ± 0.5 | 2.0 ± 0.6 | 1.7 ± 0.6 | 2.2 ± 0.6 | 1.5 ± 0.5 |
| Dietary fiber, 2 g/d | 12.2 ± 2.8 | 21.7 ± 4.9 | 13.1 ± 3.5 | 20.2 ± 4.9 | 13.0 ± 3.1 | 20.8 ± 5.4 |
| Folate, 2 μg/d | 279 ± 184 | 503 ± 252 | 321 ± 222 | 459 ± 233 | 316 ± 186 | 475 ± 278 |
| Calcium, 2 mg/d | 683 ± 344 | 1117 ± 452 | 828 ± 436 | 956 ± 415 | 772 ± 356 | 1030 ± 503 |
1Values are means ± SDs. AMED, Alternative Mediterranean Diet; AHEI-2010, Alternative Healthy Eating Index-2010; CRC, colorectal cancer; DASH, Dietary Approaches to Stop Hypertension; MET, metabolic equivalent of task; NHS, Nurses' Health Study; NSAID, nonsteroidal anti-inflammatory drug; Q, quintile.
2Nutrients were energy-adjusted.
TABLE 2.
Age-standardized baseline characteristics and dietary intake in lowest and highest quintiles of DASH, AMED, and AHEI-2010 scores among men in the HPFS1
| DASH (range: 8–40) | AMED (range: 0–9) | AHEI-2010 (range: 0–110) | ||||
|---|---|---|---|---|---|---|
| Q1 (n = 9399) | Q5 (n = 9721) | Q1 (n = 8726) | Q5 (n = 6818) | Q1 (n = 9327) | Q5 (n = 9291) | |
| Score | 16.7 ± 2.1 | 31.3 ± 2.2 | 1.5 ± 0.6 | 7.4 ± 0.6 | 37.0 ± 4.7 | 69.4 ± 5.5 |
| Baseline characteristics | ||||||
| Age, y | 56.1 ± 9.7 | 56.2 ± 9.7 | 56.1 ± 9.7 | 56.2 ± 9.7 | 56.1 ± 9.7 | 56.2 ± 9.7 |
| BMI, kg/m2 | 25.9 ± 3.5 | 25.0 ± 3.2 | 25.9 ± 3.3 | 25.0 ± 3.3 | 25.9 ± 3.5 | 25.1 ± 3.3 |
| Physical activity, MET-hours/wk | 14.9 ± 22.6 | 29.2 ± 36.5 | 15.3 ± 24.6 | 29.0 ± 33.9 | 15.3 ± 24.5 | 28.4 ± 36.8 |
| Current smokers, % | 18 | 4 | 16 | 4 | 16 | 5 |
| Past smokers, % | 40 | 41 | 40 | 43 | 38 | 45 |
| NSAID use, % | 33 | 36 | 33 | 37 | 35 | 36 |
| Multivitamin use, % | 53 | 68 | 55 | 68 | 55 | 68 |
| Family history of CRC, % | 8 | 9 | 7 | 10 | 8 | 9 |
| Screened for CRC, % | 31 | 43 | 33 | 42 | 31 | 43 |
| Baseline dietary intake2 | ||||||
| Energy, kcal/d | 1978 ± 605 | 2041 ± 583 | 1756 ± 549 | 2256 ± 608 | 2117 ± 605 | 1892 ± 587 |
| Alcohol (drinks/d), % | ||||||
| 0 | 23 | 26 | 28 | 17 | 35 | 15 |
| >0–1 | 45 | 50 | 47 | 47 | 35 | 57 |
| >1–2 | 14 | 14 | 7 | 28 | 6 | 23 |
| >2 | 18 | 9 | 19 | 8 | 24 | 6 |
| Fruits, servings/d | 0.8 ± 0.7 | 2.7 ± 1.6 | 0.8 ± 0.7 | 2.6 ± 1.6 | 0.9 ± 0.8 | 2.5 ± 1.6 |
| Vegetables, servings/d | 2.3 ± 1.1 | 4.6 ± 2.2 | 2.0 ± 0.9 | 4.9 ± 2.1 | 2.4 ± 1.2 | 4.4 ± 2.2 |
| Whole grains, servings/d | 0.6 ± 0.8 | 2.4 ± 1.7 | 0.7 ± 0.8 | 2.3 ± 1.6 | 1.0 ± 1.1 | 1.9 ± 1.6 |
| Nuts, servings/d | 0.5 ± 0.5 | 0.7 ± 0.5 | 0.3 ± 0.4 | 0.9 ± 0.3 | 0.5 ± 0.5 | 0.7 ± 0.5 |
| Legumes, servings/d | 0.3 ± 0.5 | 0.7 ± 0.5 | 0.2 ± 0.4 | 0.9 ± 0.4 | 0.4 ± 0.5 | 0.6 ± 0.5 |
| Low-fat dairy, servings/d | 0.4 ± 0.7 | 1.4 ± 1.2 | 0.8 ± 1.1 | 1.1 ± 1.0 | 0.9 ± 1.2 | 1.0 ± 1.0 |
| Fish, servings/d | 0.3 ± 0.5 | 0.6 ± 0.5 | 0.1 ± 0.3 | 0.9 ± 0.3 | 0.2 ± 0.4 | 0.7 ± 0.5 |
| PUFAs, % energy | 5.7 ± 1.5 | 5.8 ± 1.7 | 5.3 ± 1.4 | 6.1 ± 1.6 | 5.2 ± 1.3 | 6.3 ± 1.9 |
| ω-3 fatty acids, mg/d | 214 ± 205 | 412 ± 335 | 157 ± 133 | 504 ± 341 | 162 ± 174 | 438 ± 317 |
| MUFA:SFA ratio | 0.4 ± 0.5 | 0.6 ± 0.5 | 0.2 ± 0.4 | 0.9 ± 0.4 | 0.4 ± 0.5 | 0.7 ± 0.5 |
| Red and processed meat, servings/d | 1.4 ± 0.8 | 0.5 ± 0.5 | 1.2 ± 0.7 | 0.7 ± 0.6 | 1.5 ± 0.8 | 0.5 ± 0.4 |
| Sugar-sweetened beverages, drinks/d | 0.7 ± 0.8 | 0.1 ± 0.3 | 0.5 ± 0.7 | 0.3 ± 0.5 | 0.6 ± 0.9 | 0.1 ± 0.3 |
| Sodium, mg/d | 3491 ± 1484 | 2921 ± 1277 | 2864 ± 1276 | 3504 ± 1491 | 3664 ± 1484 | 2712 ± 1220 |
| Trans fatty acids, % energy | 1.5 ± 0.5 | 0.9 ± 0.4 | 1.5 ± 0.5 | 1.0 ± 0.4 | 1.6 ± 0.5 | 0.9 ± 0.4 |
| Fiber, g/d | 15.1 ± 3.7 | 28.2 ± 7.5 | 15.5 ± 4.4 | 27.1 ± 6.9 | 15.8 ± 4.2 | 27.3 ± 7.7 |
| Folate, μg/d | 361 ± 215 | 607 ± 303 | 390 ± 251 | 585 ± 284 | 396 ± 225 | 581 ± 322 |
| Calcium, mg/d | 712 ± 335 | 1086 ± 460 | 874 ± 441 | 924 ± 397 | 834 ± 399 | 968 ± 463 |
1Values are means ± SDs. AMED, Alternative Mediterranean Diet; AHEI-2010, Alternative Healthy Eating Index-2010; CRC, colorectal cancer; DASH, Dietary Approaches to Stop Hypertension; HPFS, Health Professionals Follow-up Study; MET, metabolic equivalent of task; NSAID, nonsteroidal anti-inflammatory drug; Q, quintile.
2Nutrients were energy-adjusted.
We present results stratified by sex for all of the analyses we conducted, based on previous literature suggesting that there are differences in these associations between men and women (33), in addition to pooled results.
When comparing those in the highest quintile with those in the lowest quintile of diet scores, we did not find any statistically significant associations for CRC risk with greater adherence to the DASH (HR = 0.89; 95% CI: 0.74, 1.08; P-trend = 0.10) (Table 3), AMED (HR = 0.89; 95% CI: 0.73, 1.10; P-trend = 0.31) (Table 4), or AHEI-2010 diets (HR = 0.95; 95% CI: 0.83, 1.09; P-trend = 0.56) (Table 5) when pooling data from the NHS and HPFS (P-heterogeneity ≥ 0.07 for all). However, associations were statistically significantly inverse in men for the DASH (HR = 0.81; 95% CI: 0.66, 0.98; P-trend = 0.003), AMED (HR = 0.80; 95% CI: 0.65, 0.98; P-trend = 0.02), and AHEI-2010 (with somewhat weaker results, HR = 0.88; 95% CI: 0.72, 1.07; P-trend = 0.04) diets, but were null in women (HRs range from 0.98 to 1.01 across all dietary patterns).
TABLE 3.
Associations between quintiles of the DASH diet and risk of CRC outcomes in the NHS and HPFS1
| Q1 | Q2 | Q3 | Q4 | Q5 | P-trend2 | P-nonlinearity3 | P-heterogeneity4 | |
|---|---|---|---|---|---|---|---|---|
| Total CRC | ||||||||
| Men | ||||||||
| Cases, n | 237 | 248 | 227 | 219 | 231 | |||
| Age-adjusted | 1.00 (ref) | 0.94 (0.78, 1.12) | 0.76 (0.63, 0.92) | 0.68 (0.56, 0.82) | 0.68 (0.56, 0.82) | <0.0001 | ||
| MV-adjusted5 | 1.00 (ref) | 1.00 (0.83, 1.20) | 0.83 (0.69, 1.01) | 0.77 (0.63, 0.93) | 0.81 (0.66, 0.98) | 0.003 | 0.15 | |
| Women | ||||||||
| Cases, n | 287 | 299 | 318 | 298 | 326 | |||
| Age-adjusted | 1.00 (ref) | 0.89 (0.75, 1.05) | 0.86 (0.73, 1.00) | 0.80 (0.67, 0.94) | 0.81 (0.69, 0.95) | 0.001 | ||
| MV-adjusted6 | 1.00 (ref) | 0.95 (0.80, 1.12) | 0.95 (0.81, 1.12) | 0.92 (0.77, 1.09) | 0.98 (0.82, 1.17) | 0.46 | 0.08 | |
| Pooled | ||||||||
| Cases, n | 524 | 547 | 545 | 517 | 557 | |||
| MV-adjusted7 | 1.00 (ref) | 0.97 (0.86, 1.10) | 0.90 (0.79, 1.02) | 0.85 (0.71, 1.01) | 0.89 (0.74, 1.08) | 0.10 | 0.10 | |
| Total colon cancer | ||||||||
| Men | ||||||||
| Cases, n | 187 | 193 | 186 | 170 | 185 | |||
| Age-adjusted | 1.00 (ref) | 0.93 (0.76, 1.14) | 0.78 (0.64, 0.96) | 0.65 (0.53, 0.81) | 0.68 (0.55, 0.84) | <0.0001 | ||
| MV-adjusted5 | 1.00 (ref) | 0.99 (0.80, 1.21) | 0.86 (0.69, 1.06) | 0.74 (0.60, 0.92) | 0.81 (0.65, 1.01) | 0.006 | ||
| Women | ||||||||
| Cases, n | 222 | 231 | 246 | 238 | 267 | |||
| Age-adjusted | 1.00 (ref) | 0.87 (0.72, 1.05) | 0.84 (0.69, 1.00) | 0.80 (0.66, 0.96) | 0.83 (0.69, 0.99) | 0.009 | ||
| MV-adjusted6 | 1.00 (ref) | 0.94 (0.78, 1.13) | 0.94 (0.78, 1.14) | 0.94 (0.77, 1.14) | 1.02 (0.84, 1.24) | 0.82 | 0.04 | |
| Pooled | ||||||||
| Cases, n | 409 | 424 | 432 | 408 | 452 | |||
| MV-adjusted7 | 1.00 (ref) | 0.96 (0.84, 1.10) | 0.90 (0.79, 1.04) | 0.84 (0.67, 1.05) | 0.92 (0.74, 1.15) | 0.24 | 0.07 | |
| Proximal colon cancer | ||||||||
| Men | ||||||||
| Cases, n | 67 | 88 | 72 | 78 | 91 | |||
| Age-adjusted | 1.00 (ref) | 1.18 (0.86, 1.63) | 0.83 (0.59, 1.16) | 0.83 (0.59, 1.16) | 0.91 (0.66, 1.26) | 0.12 | ||
| MV-adjusted5 | 1.00 (ref) | 1.22 (0.88, 1.69) | 0.88 (0.63, 1.24) | 0.90 (0.64, 1.26) | 1.02 (0.73, 1.43) | 0.45 | ||
| Women | ||||||||
| Cases, n | 135 | 149 | 150 | 140 | 181 | |||
| Age-adjusted | 1.00 (ref) | 0.90 (0.71, 1.14) | 0.82 (0.65, 1.03) | 0.75 (0.59, 0.96) | 0.89 (0.71, 1.12) | 0.08 | ||
| MV-adjusted6 | 1.00 (ref) | 0.97 (0.77, 1.23) | 0.91 (0.72, 1.16) | 0.87 (0.68, 1.11) | 1.08 (0.84, 1.37) | 0.88 | 0.10 | |
| Pooled | ||||||||
| Cases, n | 202 | 237 | 222 | 218 | 272 | |||
| MV-adjusted7 | 1.00 (ref) | 1.06 (0.85, 1.31) | 0.90 (0.74, 1.10) | 0.88 (0.72, 1.07) | 1.06 (0.86, 1.29) | 0.55 | 0.63 | |
| Distal colon cancer | ||||||||
| Men | ||||||||
| Cases, n | 75 | 73 | 78 | 55 | 54 | |||
| Age-adjusted | 1.00 (ref) | 0.87 (0.62, 1.20) | 0.82 (0.59, 1.13) | 0.53 (0.37, 0.76) | 0.51 (0.35, 0.72) | <0.0001 | ||
| MV-adjusted5 | 1.00 (ref) | 0.93 (0.67, 1.30) | 0.90 (0.65, 1.26) | 0.62 (0.43, 0.89) | 0.62 (0.43, 0.91) | 0.006 | 0.03 | |
| Women | ||||||||
| Cases, n | 81 | 77 | 91 | 90 | 78 | |||
| Age-adjusted | 1.00 (ref) | 0.82 (0.60, 1.13) | 0.88 (0.65, 1.19) | 0.86 (0.63, 1.17) | 0.70 (0.51, 0.96) | 0.03 | ||
| MV-adjusted6 | 1.00 (ref) | 0.89 (0.65, 1.22) | 1.01 (0.74, 1.38) | 1.04 (0.76, 1.43) | 0.89 (0.63, 1.24) | 0.65 | ||
| Pooled | ||||||||
| Cases, n | 156 | 150 | 169 | 145 | 132 | |||
| MV-adjusted7 | 1.00 (ref) | 0.91 (0.72, 1.14) | 0.96 (0.76, 1.20) | 0.81 (0.48, 1.35) | 0.75 (0.53, 1.06) | 0.16 | 0.10 | |
| Rectal cancer | ||||||||
| Men | ||||||||
| Cases, n | 50 | 55 | 41 | 49 | 46 | |||
| Age-adjusted | 1.00 (ref) | 0.98 (0.66, 1.44) | 0.68 (0.45, 1.03) | 0.78 (0.52, 1.16) | 0.65 (0.43, 0.98) | 0.03 | ||
| MV-adjusted5 | 1.00 (ref) | 1.05 (0.71, 1.56) | 0.75 (0.49, 1.15) | 0.89 (0.59, 1.35) | 0.79 (0.51, 1.22) | 0.28 | ||
| Women | ||||||||
| Cases, n | 65 | 68 | 72 | 60 | 59 | |||
| Age-adjusted | 1.00 (ref) | 0.95 (0.68, 1.34) | 0.93 (0.66, 1.30) | 0.78 (0.55, 1.12) | 0.73 (0.51, 1.05) | 0.05 | ||
| MV-adjusted6 | 1.00 (ref) | 0.98 (0.70, 1.39) | 0.98 (0.70, 1.39) | 0.85 (0.59, 1.23) | 0.82 (0.56, 1.21) | 0.24 | ||
| Pooled | ||||||||
| Cases, n | 115 | 123 | 113 | 109 | 105 | |||
| MV-adjusted7 | 1.00 (ref) | 1.01 (0.78, 1.31) | 0.88 (0.68, 1.16) | 0.87 (0.66, 1.15) | 0.81 (0.60, 1.08) | 0.11 | 0.96 | |
1Values are HRs (95% CIs) unless otherwise indicated. CRC, colorectal cancer; DASH, Dietary Approaches to Stop Hypertension; HPFS, Health Professionals Follow-up Study; MET, metabolic equivalent of task; MV, multivariable; NHS, Nurses' Health Study; NSAID, nonsteroidal anti-inflammatory drug; Q, quintile.
2 P-value for the continuous DASH score determined using the Wald test.
3 P-value for the likelihood ratio test comparing the model with cubic splines to the model without splines. If missing, no spline variables were selected from the stepwise procedure, and the relation between the dietary index and the CRC endpoint is assumed to be linear.
4 P-value for between-studies heterogeneity for continuous DASH score determined using the Q statistic.
5Adjusted for total energy intake (kcal/d, quintiles), alcohol intake (g/d, quintiles), physical activity (MET-hours/wk, quintiles), NSAID use [≥2 NSAIDs/wk vs. <2 NSAIDs/wk (ref)], family history of CRC [yes vs. no (ref)], previous CRC screening via colonoscopy or sigmoidoscopy [yes vs. no (ref)], history of polyps [yes vs. no (ref)], smoking [never smoker (ref), 0–<10, 10–<20, 20–<30, 30–<40, 40–<50, ≥50 pack-years], multivitamin use [regular use vs. nonuse (ref)], supplemental calcium intake [none (ref), >0–200, >200–400, >400–600, >600 mg/d], and young adult BMI [in kg/m2; <25 (ref), 25–<27.5, 27.5–<30, ≥30].
6Adjusted for the same multivariable models as in men + menopausal status [postmenopausal vs. not (ref)] and postmenopausal hormone use [never use (ref), past use, current use].
7Pooled HRs and 95% CIs were estimated by pooling the cohort-specific HRs using a random effects model.
TABLE 4.
Associations between quintiles of AMED scores and risk of CRC outcomes in the NHS and HPFS1
| Q1 | Q2 | Q3 | Q4 | Q5 | P-trend2 | P-nonlinearity3 | P-heterogeneity4 | |
|---|---|---|---|---|---|---|---|---|
| Total CRC | ||||||||
| Men | ||||||||
| Cases, n | 225 | 215 | 260 | 252 | 210 | |||
| Age-adjusted | 1.00 (ref) | 0.91 (0.76, 1.10) | 0.93 (0.78, 1.12) | 0.83 (0.69, 1.00) | 0.73 (0.60, 0.89) | 0.0002 | ||
| MV-adjusted5 | 1.00 (ref) | 0.94 (0.78, 1.14) | 0.98 (0.81, 1.17) | 0.89 (0.74, 1.08) | 0.80 (0.65, 0.98) | 0.02 | ||
| Women | ||||||||
| Cases, n | 294 | 313 | 301 | 303 | 317 | |||
| Age-adjusted | 1.00 (ref) | 0.86 (0.74, 1.01) | 0.78 (0.66, 0.91) | 0.79 (0.67, 0.93) | 0.84 (0.71, 0.99) | 0.03 | ||
| MV-adjusted6 | 1.00 (ref) | 0.91 (0.77, 1.07) | 0.85 (0.71, 1.00) | 0.89 (0.75, 1.05) | 0.99 (0.83, 1.18) | 0.98 | 0.05 | |
| Pooled | ||||||||
| Cases, n | 519 | 528 | 561 | 555 | 527 | |||
| MV-adjusted7 | 1.00 (ref) | 0.92 (0.82, 1.04) | 0.90 (0.78, 1.04) | 0.89 (0.78, 1.01) | 0.89 (0.73, 1.10) | 0.31 | 0.10 | |
| Total colon cancer | ||||||||
| Men | ||||||||
| Cases, n | 175 | 160 | 209 | 203 | 174 | |||
| Age-adjusted | 1.00 (ref) | 0.86 (0.69, 1.07) | 0.96 (0.78, 1.18) | 0.85 (0.69, 1.05) | 0.78 (0.63, 0.96) | 0.01 | ||
| MV-adjusted5 | 1.00 (ref) | 0.88 (0.71, 1.10) | 1.00 (0.81, 1.23) | 0.92 (0.74, 1.14) | 0.85 (0.67, 1.06) | 0.21 | ||
| Women | ||||||||
| Cases, n | 224 | 247 | 234 | 249 | 250 | |||
| Age-adjusted | 1.00 (ref) | 0.89 (0.74, 1.06) | 0.78 (0.65, 0.94) | 0.84 (0.70, 1.01) | 0.86 (0.71, 1.03) | 0.13 | ||
| MV-adjusted6 | 1.00 (ref) | 0.94 (0.78, 1.13) | 0.85 (0.70, 1.03) | 0.96 (0.79, 1.16) | 1.02 (0.83, 1.25) | 0.60 | 0.12 | |
| Pooled | ||||||||
| Cases, n | 399 | 407 | 443 | 452 | 424 | |||
| MV-adjusted7 | 1.00 (ref) | 0.92 (0.80, 1.05) | 0.92 (0.78, 1.08) | 0.94 (0.81, 1.08) | 0.94 (0.78, 1.12) | 0.59 | 0.21 | |
| Proximal colon cancer | ||||||||
| Men | ||||||||
| Cases, n | 71 | 68 | 88 | 91 | 78 | |||
| Age-adjusted | 1.00 (ref) | 0.89 (0.64, 1.25) | 1.00 (0.73, 1.37) | 0.94 (0.68, 1.29) | 0.85 (0.61, 1.17) | 0.46 | ||
| MV-adjusted5 | 1.00 (ref) | 0.89 (0.63, 1.24) | 0.98 (0.71, 1.35) | 0.94 (0.68, 1.30) | 0.85 (0.60, 1.20) | 0.56 | ||
| Women | ||||||||
| Cases, n | 140 | 170 | 145 | 157 | 143 | |||
| Age-adjusted | 1.00 (ref) | 0.97 (0.77, 1.21) | 0.76 (0.60, 0.97) | 0.83 (0.66, 1.04) | 0.77 (0.61, 0.98) | 0.04 | ||
| MV-adjusted6 | 1.00 (ref) | 1.00 (0.80, 1.25) | 0.80 (0.63, 1.02) | 0.88 (0.69, 1.13) | 0.85 (0.65, 1.10) | 0.36 | ||
| Pooled | ||||||||
| Cases, n | 211 | 238 | 233 | 248 | 221 | |||
| MV-adjusted7 | 1.00 (ref) | 0.96 (0.80, 1.16) | 0.86 (0.71, 1.04) | 0.94 (0.78, 1.15) | 0.85 (0.69, 1.05) | 0.28 | 0.89 | |
| Distal colon cancer | ||||||||
| Men | ||||||||
| Cases, n | 66 | 63 | 74 | 75 | 57 | |||
| Age-adjusted | 1.00 (ref) | 0.92 (0.65, 1.31) | 0.90 (0.65, 1.27) | 0.81 (0.58, 1.13) | 0.67 (0.47, 0.96) | 0.01 | ||
| MV-adjusted5 | 1.00 (ref) | 0.95 (0.67, 1.35) | 0.98 (0.69, 1.38) | 0.90 (0.63, 1.27) | 0.76 (0.52, 1.12) | 0.15 | ||
| Women | ||||||||
| Cases, n | 76 | 69 | 86 | 87 | 99 | |||
| Age-adjusted | 1.00 (ref) | 0.74 (0.53, 1.02) | 0.85 (0.63, 1.17) | 0.91 (0.67, 1.24) | 1.01 (0.74, 1.36) | 0.85 | ||
| MV-adjusted6 | 1.00 (ref) | 0.80 (0.58, 1.12) | 1.00 (0.72, 1.38) | 1.13 (0.81, 1.57) | 1.35 (0.96, 1.90) | 0.06 | ||
| Pooled | ||||||||
| Cases, n | 142 | 132 | 160 | 162 | 156 | |||
| MV-adjusted7 | 1.00 (ref) | 0.87 (0.68, 1.11) | 0.99 (0.78, 1.25) | 1.01 (0.80, 1.29) | 1.02 (0.58, 1.79) | 0.89 | 0.02 | |
| Rectal cancer | ||||||||
| Men | ||||||||
| Cases, n | 50 | 55 | 51 | 49 | 36 | |||
| Age-adjusted | 1.00 (ref) | 1.11 (0.75, 1.64) | 0.84 (0.56, 1.24) | 0.75 (0.50, 1.11) | 0.58 (0.37, 0.89) | 0.0007 | ||
| MV-adjusted5 | 1.00 (ref) | 1.13 (0.76, 1.67) | 0.87 (0.58, 1.30) | 0.79 (0.52, 1.20) | 0.62 (0.39, 0.99) | 0.008 | ||
| Women | ||||||||
| Cases, n | 70 | 66 | 67 | 54 | 67 | |||
| Age-adjusted | 1.00 (ref) | 0.79 (0.56, 1.11) | 0.77 (0.55, 1.08) | 0.62 (0.43, 0.88) | 0.80 (0.57, 1.13) | 0.08 | ||
| MV-adjusted6 | 1.00 (ref) | 0.81 (0.58, 1.14) | 0.82 (0.58, 1.16) | 0.66 (0.45, 0.97) | 0.88 (0.60, 1.29) | 0.28 | ||
| Pooled | ||||||||
| Cases, n | 120 | 121 | 118 | 103 | 103 | |||
| MV-adjusted7 | 1.00 (ref) | 0.94 (0.68, 1.30) | 0.84 (0.64, 1.09) | 0.72 (0.54, 0.95) | 0.76 (0.54, 1.07) | 0.02 | 0.25 | |
1Values are HRs (95% CIs) unless otherwise indicated. AMED, Alternative Mediterranean Diet; CRC, colorectal cancer; HPFS, Health Professionals Follow-up Study; MET, metabolic equivalent of task; MV, multivariable; NHS, Nurses' Health Study; NSAID, nonsteroidal anti-inflammatory drug; Q, quintile.
2 P-value for the continuous AMED score determined using the Wald test.
3 P-value for the likelihood ratio test comparing the model with cubic splines to the model without splines. If missing, no spline variables were selected from the stepwise procedure, and the relation between the dietary index and the CRC endpoint is assumed to be linear.
4 P-value for between-studies heterogeneity for continuous AMED score determined using the Q statistic.
5Adjusted for total energy intake (kcal/d, quintiles), alcohol intake (g/d, quintiles), physical activity (MET-hours/wk, quintiles), NSAID use [≥2 NSAIDs/wk vs. <2 NSAIDs/wk (ref)], family history of CRC [yes vs. no (ref)], previous CRC screening via colonoscopy or sigmoidoscopy [yes vs. no (ref)], history of polyps [yes vs. no (ref)], smoking [never smoker (ref), 0–<10, 10–<20, 20–<30, 30–<40, 40–<50, ≥50 pack-years], multivitamin use [regular use vs. nonuse (ref)], supplemental calcium intake [none (ref), >0–200, >200–400, >400–600, >600 mg/d], and young adult BMI [in kg/m2; <25 (ref), 25–<27.5, 27.5–<30, ≥30].
6Adjusted for the same multivariable models as in men + menopausal status [postmenopausal vs. not (ref)] and postmenopausal hormone use [never use (ref), past use, current use].
7Pooled HRs and 95% CIs were estimated by pooling the cohort-specific HRs using a random effects model.
TABLE 5.
Associations between quintiles of AHEI-2010 scores and risk of CRC outcomes in the NHS and HPFS1
| Q1 | Q2 | Q3 | Q4 | Q5 | P-trend2 | P-nonlinearity3 | P-heterogeneity4 | |
|---|---|---|---|---|---|---|---|---|
| Total CRC | ||||||||
| Men | ||||||||
| Cases, n | 228 | 251 | 231 | 220 | 232 | |||
| Age-adjusted | 1.00 (ref) | 0.99 (0.82, 1.18) | 0.87 (0.72, 1.04) | 0.79 (0.65, 0.95) | 0.77 (0.64, 0.92) | 0.0003 | ||
| MV-adjusted5 | 1.00 (ref) | 1.05 (0.88, 1.26) | 0.94 (0.78, 1.14) | 0.88 (0.72, 1.06) | 0.88 (0.72, 1.07) | 0.04 | ||
| Women | ||||||||
| Cases, n | 325 | 317 | 303 | 327 | 256 | |||
| Age-adjusted | 1.00 (ref) | 0.95 (0.81, 1.11) | 0.91 (0.78, 1.07) | 1.01 (0.86, 1.18) | 0.85 (0.72, 1.00) | 0.08 | ||
| MV-adjusted6 | 1.00 (ref) | 1.00 (0.85, 1.17) | 1.00 (0.85, 1.17) | 1.14 (0.97, 1.34) | 1.01 (0.85, 1.21) | 0.59 | ||
| Pooled | ||||||||
| Cases, n | 553 | 568 | 534 | 547 | 488 | |||
| MV-adjusted7 | 1.00 (ref) | 1.02 (0.91, 1.15) | 0.98 (0.86, 1.10) | 1.01 (0.78, 1.30) | 0.95 (0.83, 1.09) | 0.56 | 0.07 | |
| Total colon cancer | ||||||||
| Men | ||||||||
| Cases, n | 174 | 195 | 183 | 183 | 186 | |||
| Age-adjusted | 1.00 (ref) | 1.00 (0.81, 1.23) | 0.89 (0.72, 1.10) | 0.86 (0.69, 1.06) | 0.80 (0.65, 0.99) | 0.003 | ||
| MV-adjusted5 | 1.00 (ref) | 1.07 (0.86, 1.31) | 0.98 (0.79, 1.21) | 0.96 (0.77, 1.19) | 0.92 (0.74, 1.15) | 0.13 | ||
| Women | ||||||||
| Cases, n | 256 | 241 | 245 | 257 | 205 | |||
| Age-adjusted | 1.00 (ref) | 0.91 (0.76, 1.08) | 0.93 (0.78, 1.11) | 0.99 (0.83, 1.18) | 0.85 (0.70, 1.02) | 0.16 | ||
| MV-adjusted6 | 1.00 (ref) | 0.96 (0.80, 1.15) | 1.03 (0.86, 1.23) | 1.14 (0.95, 1.37) | 1.03 (0.85, 1.26) | 0.40 | ||
| Pooled | ||||||||
| Cases, n | 430 | 436 | 428 | 440 | 391 | |||
| MV-adjusted7 | 1.00 (ref) | 1.00 (0.88, 1.15) | 1.01 (0.88, 1.16) | 1.06 (0.89, 1.25) | 0.98 (0.85, 1.14) | 0.78 | 0.10 | |
| Proximal colon cancer | ||||||||
| Men | ||||||||
| Cases, n | 69 | 87 | 71 | 86 | 83 | |||
| Age-adjusted | 1.00 (ref) | 1.09 (0.79, 1.50) | 0.87 (0.62, 1.21) | 0.99 (0.72, 1.36) | 0.88 (0.64, 1.22) | 0.20 | ||
| MV-adjusted5 | 1.00 (ref) | 1.15 (0.83, 1.59) | 0.94 (0.67, 1.32) | 1.08 (0.78, 1.51) | 1.00 (0.71, 1.40) | 0.61 | ||
| Women | ||||||||
| Cases, n | 164 | 155 | 146 | 157 | 133 | |||
| Age-adjusted | 1.00 (ref) | 0.91 (0.73, 1.13) | 0.85 (0.68, 1.07) | 0.94 (0.76, 1.18) | 0.86 (0.68, 1.08) | 0.10 | ||
| MV-adjusted6 | 1.00 (ref) | 0.95 (0.76, 1.19) | 0.93 (0.74, 1.17) | 1.05 (0.84, 1.32) | 0.99 (0.77, 1.26) | 0.70 | ||
| Pooled | ||||||||
| Cases, n | 233 | 242 | 217 | 243 | 216 | |||
| MV-adjusted7 | 1.00 (ref) | 1.01 (0.84, 1.21) | 0.93 (0.77, 1.13) | 1.06 (0.88, 1.28) | 0.99 (0.81, 1.21) | 0.54 | 0.88 | |
| Distal colon cancer | ||||||||
| Men | ||||||||
| Cases, n | 66 | 74 | 70 | 60 | 65 | |||
| Age-adjusted | 1.00 (ref) | 1.02 (0.73, 1.43) | 0.90 (0.64, 1.27) | 0.75 (0.53, 1.07) | 0.75 (0.53, 1.07) | 0.02 | ||
| MV-adjusted5 | 1.00 (ref) | 1.11 (0.79, 1.56) | 1.01 (0.71, 1.43) | 0.87 (0.60, 1.25) | 0.90 (0.62, 1.30) | 0.23 | ||
| Women | ||||||||
| Cases, n | 88 | 81 | 89 | 94 | 65 | |||
| Age-adjusted | 1.00 (ref) | 0.89 (0.65, 1.20) | 1.01 (0.75, 1.35) | 1.05 (0.78, 1.41) | 0.78 (0.56, 1.08) | 0.62 | ||
| MV-adjusted6 | 1.00 (ref) | 0.96 (0.70, 1.30) | 1.15 (0.85, 1.56) | 1.27 (0.93, 1.72) | 1.03 (0.73, 1.45) | 0.17 | ||
| Pooled | ||||||||
| Cases, n | 154 | 155 | 159 | 154 | 130 | |||
| MV-adjusted7 | 1.00 (ref) | 1.02 (0.82, 1.29) | 1.09 (0.86, 1.36) | 1.06 (0.74, 1.53) | 0.97 (0.75, 1.24) | 0.95 | 0.07 | |
| Rectal cancer | ||||||||
| Men | ||||||||
| Cases, n | 54 | 56 | 48 | 37 | 46 | |||
| Age-adjusted | 1.00 (ref) | 0.96 (0.65, 1.39) | 0.79 (0.53, 1.16) | 0.58 (0.38, 0.88) | 0.67 (0.45, 0.99) | 0.03 | ||
| MV-adjusted5 | 1.00 (ref) | 1.01 (0.69, 1.48) | 0.83 (0.55, 1.23) | 0.63 (0.41, 0.98) | 0.73 (0.47, 1.11) | 0.11 | ||
| Women | ||||||||
| Cases, n | 69 | 76 | 58 | 70 | 51 | |||
| Age-adjusted | 1.00 (ref) | 1.11 (0.80, 1.53) | 0.85 (0.60, 1.21) | 1.07 (0.76, 1.50) | 0.84 (0.58, 1.21) | 0.27 | ||
| MV-adjusted6 | 1.00 (ref) | 1.13 (0.81, 1.58) | 0.89 (0.62, 1.28) | 1.16 (0.82, 1.64) | 0.93 (0.63, 1.38) | 0.65 | 0.03 | |
| Pooled | ||||||||
| Cases, n | 123 | 132 | 106 | 107 | 97 | |||
| MV-adjusted7 | 1.00 (ref) | 1.08 (0.84, 1.39) | 0.86 (0.66, 1.13) | 0.87 (0.48, 1.57) | 0.83 (0.62, 1.11) | 0.15 | 0.41 | |
1Values are HRs (95% CIs) unless otherwise indicated. AHEI-2010, Alternative Healthy Eating Index-2010; CRC, colorectal cancer; HPFS, Health Professionals Follow-up Study; MET, metabolic equivalent of task; MV, multivariable; NHS, Nurses' Health Study; NSAID, nonsteroidal anti-inflammatory drug; Q, quintile.
2 P-value for the continuous AHEI-2010 score determined using the Wald test.
3 P-value for likelihood ratio test comparing the model with cubic splines to the model without splines. If missing, no spline variables were selected from the stepwise procedure, and the relation between the dietary index and the CRC endpoint is assumed to be linear.
4 P-value for between-studies heterogeneity for continuous AHEI-2010 score determined using the Q statistic.
5Adjusted for total energy intake (kcal/d, quintiles), alcohol intake (g/d, quintiles), physical activity (MET-hours/wk, quintiles), NSAID use [≥2 NSAIDs/wk vs. <2 NSAIDs/wk (ref)], family history of CRC [yes vs. no (ref)], previous CRC screening via colonoscopy or sigmoidoscopy [yes vs. no (ref)], history of polyps [yes vs. no (ref)], smoking [never smoker (ref), 0–<10, 10–<20, 20–<30, 30–<40, 40–<50, ≥50 pack-years], multivitamin use [regular use vs. nonuse (ref)], supplemental calcium intake [none (ref), >0–200, >200–400, >400–600, >600 mg/d], and young adult BMI [in kg/m2; <25 (ref), 25–<27.5, 27.5–<30, ≥30].
6Adjusted for the same multivariable models as in men + menopausal status [postmenopausal vs. not (ref)] and postmenopausal hormone use [never use (ref), past use, current use].
7Pooled HRs and 95% CIs were estimated by pooling the cohort-specific HRs using a random effects model.
When examining specific anatomic subsites, we observed a strong inverse association between the DASH diet and distal colon cancer risk (HR = 0.62; 95% CI: 0.43, 0.91 for highest compared with lowest DASH quintile; P-trend = 0.006) in men (Table 3), as well as an inverse trend in the association between AMED adherence and rectal cancer (HR = 0.76; 95% CI: 0.54, 1.07 for highest compared with lowest quintile of AMED; P-trend = 0.02) in the pooled analyses of men and women (Table 4). We did not find any statistically significant associations for any dietary index and any anatomic subsite in women.
There was suggestive heterogeneity in the associations by anatomic location in men for the AMED diet (P-heterogeneity = 0.08), but not for the DASH or AHEI-2010 diets (P-heterogeneity = 0.12 and 0.61, respectively). Pairwise tests comparing each subsite to every other for the AMED diet in men revealed a statistically significantly stronger inverse association for rectal compared with proximal colon cancer (P-heterogeneity = 0.03). We did not find evidence of heterogeneity in associations by anatomic location for any dietary index in women (P-heterogeneity = 0.68, 0.27, and 0.83 for continuous DASH, AMED, and AHEI-2010 scores, respectively).
In general, associations were not materially altered when BMI and diabetes were added into the regression models, or when we removed history of polyps from all models. When we removed alcohol from the AMED and AHEI-2010 indexes and adjusted for it instead, we found generally similar results (Supplemental Table 2). We did not find any statistically significant interactions between any potential effect modifiers and dietary pattern scores with CRC risk (Supplemental Table 3).
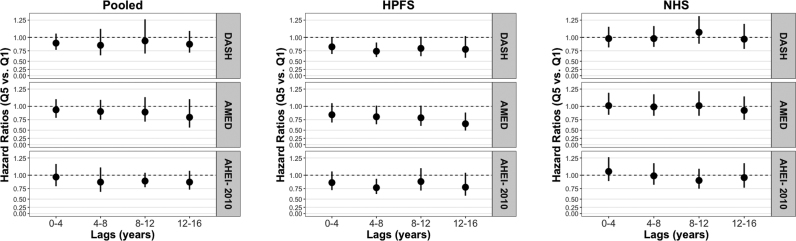
In latency analyses, we did not observe any modification by time for any dietary index and CRC risk when pooling men and women together, but we did observe some possible latent associations in men for the AMED diet (Figure 1). Specifically, we observed multivariable statistically nonsignificant HRs of 0.85 (95% CI: 0.75, 1.12), 0.81 (95% CI: 0.65, 1.01), and 0.79 (95% CI: 0.61, 1.01) for lags of 0–4, 4–8, and 8–12 y, respectively, and a statistically significant HR of 0.66 (95% CI: 0.49, 0.89) for a lag of 12–16 y when comparing those in the highest quintile with those in the lowest quintile of dietary scores. When examining the AMED diet and specific CRC subsites, we observed apparent modification by time specifically for proximal colon cancer risk [statistically nonsignificant multivariable HRs of 1.06 (95% CI: 0.74, 1.50), 0.96 (95% CI: 0.66, 1.38), and 0.81 (95% CI: 0.54, 1.23) for lags of 0–4, 4–8, and 8–12 y, respectively, and a statistically significant HR of 0.59 (95% CI: 0.36, 0.99) for a lag of 12–16 y], but not for distal colon or rectal cancer risk. Furthermore, we observed modification by time for the DASH diet and distal colon cancer risk specifically in men [statistically nonsignificant multivariable-adjusted HRs of 0.78 (95% CI: 0.54, 1.13) and 0.71 (95% CI: 0.48, 1.03) for lags of 0–4 and 4–8 y, respectively, and statistically significant HRs of 0.59 (95% CI: 0.37, 0.95), and 0.31 (95% CI: 0.17, 0.58) for lags of 8–12 and 12–16 y, respectively]. We did not observe any modification by time for any dietary index and any CRC endpoint in women.
FIGURE 1.
Multivariable HRs and 95% CIs for highest compared with lowest quintile of DASH, AMED, and AHEI-2010 scores and CRC risk by latency period in the NHS and HPFS separately and pooled. The circles and vertical lines correspond to multivariable HRs and 95% CIs, respectively, for those in the fifth quintile of the dietary index of interest compared with those in the first quintile of that dietary index with the given lag (n = 78,012 women in the NHS and n = 46,695 men in the HPFS). The HRs and 95% CIs were obtained from Cox regression analyses. The Pooled panel shows multivariable HRs and 95% CIs when men and women were pooled in a meta-analysis using a random effects model. All models were adjusted for total energy intake (kcal/d, quintiles), alcohol intake (g/d, quintiles), physical activity (MET-hours/wk, quintiles), NSAID use [≥2 NSAIDs/wk vs. <2 NSAIDs/wk (ref)], family history of CRC [yes vs. no (ref)], previous CRC screening via colonoscopy or sigmoidoscopy [yes vs. no (ref)], history of polyps [yes vs. no (ref)], smoking [never smoker (ref), 0–<10, 10–<20, 20–<30, 30–<40, 40–<50, ≥50 pack-years], multivitamin use [regular use vs. nonuse (ref)], supplemental calcium intake [none (ref), >0–200, >200–400, >400–600, >600 mg/d], and young adult BMI [in kg/m2; <25 (ref), 25–<27.5, 27.5–<30, ≥30]; in women, we additionally adjusted for menopausal status [postmenopausal vs. not (ref)] and postmenopausal hormone use [never use (ref), past use, current use]. AMED, Alternative Mediterranean Diet; AHEI-2010, Alternative Healthy Eating Index-2010; CRC, colorectal cancer; DASH, Dietary Approaches to Stop Hypertension; HPFS, Health Professionals Follow-Up Study; MET, metabolic task equivalent; NHS, Nurses' Health Study; NSAID, nonsteroidal anti-inflammatory drug.
DISCUSSION
In this analysis of 2 prospective cohorts, we found that the DASH, AMED, and AHEI-2010 diets were associated with a lower risk of CRC in men. When examining specific anatomic subsites in men, the DASH diet was associated with a lower risk of distal colon cancer, while the AMED diet was associated with a lower risk of rectal cancer. We did not observe any statistically significant associations for DASH, AMED, or AHEI-2010 scores and any CRC endpoints in women.
The inverse associations we observed for the DASH and AMED diets in men were similar to those in a previous study in the HPFS, although our results for the AMED diet were slightly stronger (20). However, the previous study also found inverse associations between the DASH and AMED diets and CRC risk in women in the NHS (20). Differences between our original report and this study may be because the present study had longer follow-ups for both cohorts and we added a 2-y lag to all analyses, unlike the initial study. Additionally, for the NHS, the previous study used the 1980 FFQ as the baseline dietary assessment, whereas we used the 1984 FFQ as the baseline assessment. This was necessary because 1984 was the first year that an expanded FFQ was administered, which allowed us to accurately calculate components of the AHEI-2010 (25). Inverse associations between the DASH and Mediterranean diets and CRC risk in men have been observed in previous cohort studies (5–8, 10), and one study additionally observed an inverse association for the AHEI-2010 diet (7). Previous prospective studies in women have also observed inverse associations between the DASH diet and CRC risk, with mixed results for Mediterranean diets and generally null results for the AHEI-2010 diet (4–10). Additionally, for previous analyses of the dietary indexes and CRC risk, most studies that included both sexes found stronger results in men (33).
The differing role of diet on CRC risk at specific anatomic subsites is not well understood. Stronger associations for dietary patterns have been observed for risk of distal colon cancer than proximal colon cancer in previous studies (6–8, 34, 35) as well as in the current study for the DASH diet. Proposed explanations for this include differences in the proximal and distal colon related to microbial communities (36), biochemical reactions during digestion (37), and molecular carcinogenic processes (34, 38, 39). Previous studies of the AMED diet have observed stronger results for rectal cancer risk than for other anatomic subsites (7–10), which we also observed in men. However, the mechanism behind this association remains unclear.
The DASH, AMED, and AHEI-2010 all consist of several dietary constituents that are independently inversely associated with CRC risk. All diets are low in red and processed meat, which is associated with increased CRC risk (40). This association may be driven by the formation of N-nitroso compounds (owing to high levels of heme iron) (41, 42), and heterocyclic amines and polycyclic aromatic hydrocarbons (owing to cooking meat at high temperatures) (43, 44). All diets are also rich in fiber, which is provided by whole grains, fruits, vegetables, nuts, and legumes. Evidence of a role of fiber on CRC risk is mixed (45, 46), although the World Cancer Research Fund/American Institute for Cancer Research has identified fiber as probably decreasing CRC risk (1). Potential mechanisms for these associations include production of short-chain fatty acids, reduction of fecal transit time, and improvements in insulin resistance (47, 48). The DASH diet specifically is rich in low-fat dairy, which is inversely associated with CRC risk (49), especially distal colon cancer risk (13, 50). Dairy is rich in calcium and vitamin D (49, 51, 52), which may reduce cellular proliferation and promote differentiation and cell apoptosis (53–55). Weaker associations observed for the AHEI-2010 in men may be because the AHEI-2010 considers intakes of PUFAs, ω-3 fatty acids, and trans fatty acids separately, all of which have either shown no association with CRC risk (56) or have been slightly positively associated with risk (57–59).
Mechanisms behind the differing associations we found between men and women are unclear, but may be partially explained by the effect of adiposity on CRC risk. Specifically, dietary index adherence may be associated with CRC risk through increased adiposity and weight gain, which are stronger risk factors for CRC in men than women (60, 61), although studies of early life adiposity suggest equally strong or stronger associations for women than for men (62–64). Moreover, weak associations between adult obesity and CRC risk in women may be because of the competing effects of metabolic abnormalities (increase risk) and increased estrogen production (decreases risk) (65). However, we did not find evidence of effect modification by adult obesity, young adult BMI, postmenopausal hormone use, or oral contraceptive use.
Latency analyses showed stronger associations for the AMED diet and CRC risk, as well as for the DASH diet and distal colon cancer risk, specifically in the increasingly distant past in men. Since CRC is a slow-growing disease, with a natural development of 10–15 y (66), it is possible that adhering to a healthy diet may interfere with the development of the early phases of colorectal carcinogenesis in men. Such latent associations have been observed for some specific dietary factors and CRC risk previously (12, 13), but not for dietary patterns. The present study supports the possible importance of diet in the early stages of colonic carcinogenesis in men.
This study's strengths include its prospective nature, low attrition, and long follow-up with multiple dietary assessments, allowing for continually updating diets and conducting latency analyses. Detailed collection of dietary, lifestyle, and medical information over several decades allowed us to adjust for all widely recognized confounders of these associations. However, our study has several limitations as well. Diet is measured with error, which could lead to biased results. However, we used FFQs that have been validated for measuring food and nutrient intake, as well as dietary patterns (21–23). Because we expect measurement error of diet to be nondifferential with respect to CRC risk, we anticipate our results to be biased toward the null, suggesting possibly stronger associations than our results imply. Second, both the NHS and the HPFS consist mainly of older, white health professionals, reducing the generalizability of our results. The relative homogeneity of these populations may have led to reduced variability in dietary intake, and it is possible that stronger associations would be observed in a population with a more heterogeneous diet. Lastly, we did not have information on diet in childhood or adolescence, which may be critical for CRC development. Although other studies have demonstrated a role of childhood diet in CRC development (67–69), this has not yet been studied using dietary indexes.
In summary, this study supports an inverse association between adherence to the DASH, AMED, and AHEI-2010 diets and CRC risk in men. Although we did not observe inverse associations between any dietary index and CRC risk in women, adherence to these diets is recommended for prevention of obesity, heart disease, diabetes, and other chronic diseases in men and women (14–19). More detailed studies of differences in dietary index adherence and CRC risk by sex are warranted, as are studies of early life adherence to dietary indexes and CRC risk.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, and WY. The authors assume full responsibility for analyses and interpretation of these data.
The authors' responsibilities are as follows—JP and FKT: designed the analysis; JP: conducted the analysis, interpreted the data, and wrote the manuscript; SAS-W, TTF, BR, ATC, FBH, ELG, and FKT: assisted in interpreting the data and edited the manuscript. JP and FKT: had responsibility for final content; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Notes
This work was supported by National Cancer Institute grant # K99CA207736 to FKT. The HPFS and NHS cohorts are supported by NIH grants UM1CA167552 (HPFS), P01 CA55075 (HPFS), UM1CA186107 (NHS), and P01 CA87969 (NHS).
Supplemental Tables 1–3 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used:
- AMED
Alternative Mediterranean Diet
- AHEI-2010
Alternative Health Eating Index-2010
- CRC
colorectal cancer
- DASH
Dietary Approaches to Stop Hypertension
- FFQ
food-frequency questionnaire
- HPFS
Health Professionals Follow-up Study
- NHS
Nurses’ Health Study
REFERENCES
- 1. World Cancer Research Fund International/American Institute for Cancer Research. Continuous Update Project Report: Diet, Nutrition, Physical Activity and Colorectal Cancer. 2017. [Google Scholar]
- 2. Potter J, Brown L, Williams RL, Byles J, Collins CE. Diet quality and cancer outcomes in adults: a systematic review of epidemiological studies. Int J Mol Sci. 2016;17(7): 1052. doi: 10.3390/ijms17071052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3–9. [DOI] [PubMed] [Google Scholar]
- 4. Vargas AJ, Neuhouser ML, George SM, Thomson CA, Ho GY, Rohan TE, Kato I, Nassir R, Hou L, Manson JE. Diet quality and colorectal cancer risk in the Women's Health Initiative Observational Study. Am J Epidemiol. 2016;184(1):23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bamia C, Lagiou P, Buckland G, Grioni S, Agnoli C, Taylor AJ, Dahm CC, Overvad K, Olsen A, Tjonneland A et al.. Mediterranean diet and colorectal cancer risk: results from a European cohort. Eur J Epidemiol. 2013;28(4):317–28. [DOI] [PubMed] [Google Scholar]
- 6. Miller PE, Cross AJ, Subar AF, Krebs-Smith SM, Park Y, Powell-Wiley T, Hollenbeck A, Reedy J. Comparison of 4 established DASH diet indexes: examining associations of index scores and colorectal cancer. Am J Clin Nutr. 2013;98(3):794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Park SY, Boushey CJ, Wilkens LR, Haiman CA, Le Marchand L. High-quality diets associate with reduced risk of colorectal cancer: analyses of diet quality indexes in the multiethnic cohort. Gastroenterology. 2017;153(2):386–94..e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reedy J, Mitrou PN, Krebs-Smith SM, Wirfalt E, Flood A, Kipnis V, Leitzmann M, Mouw T, Hollenbeck A, Schatzkin A et al.. Index-based dietary patterns and risk of colorectal cancer: the NIH-AARP Diet and Health Study. Am J Epidemiol. 2008;168(1):38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jones P, Cade JE, Evans CEL, Hancock N, Greenwood DC. The Mediterranean diet and risk of colorectal cancer in the UK Women's Cohort Study. Int J Epidemiol. 2017;46(6):1786–96. [DOI] [PubMed] [Google Scholar]
- 10. Agnoli C, Grioni S, Sieri S, Palli D, Masala G, Sacerdote C, Vineis P, Tumino R, Giurdanella MC, Pala V et al.. Italian Mediterranean Index and risk of colorectal cancer in the Italian section of the EPIC cohort. Int J Cancer. 2013;132(6):1404–11. [DOI] [PubMed] [Google Scholar]
- 11. Kyro C, Skeie G, Loft S, Overvad K, Christensen J, Tjonneland A, Olsen A. Adherence to a healthy Nordic food index is associated with a lower incidence of colorectal cancer in women: the Diet, Cancer and Health cohort study. Br J Nutr. 2013;109(5):920–7. [DOI] [PubMed] [Google Scholar]
- 12. Lee JE, Willett WC, Fuchs CS, Smith-Warner SA, Wu K, Ma J, Giovannucci E. Folate intake and risk of colorectal cancer and adenoma: modification by time. Am J Clin Nutr. 2011;93(4):817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang X, Keum N, Wu K, Smith-Warner SA, Ogino S, Chan AT, Fuchs CS, Giovannucci EL. Calcium intake and colorectal cancer risk: results from the Nurses' Health Study and Health Professionals Follow-up Study. Int J Cancer. 2016;139(10):2232–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sotos-Prieto M, Bhupathiraju SN, Mattei J, Fung TT, Li Y, Pan A, Willett WC, Rimm EB, Hu FB. Association of changes in diet quality with total and cause-specific mortality. N Engl J Med. 2017;377(2):143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. George SM, Ballard-Barbash R, Manson JE, Reedy J, Shikany JM, Subar AF, Tinker LF, Vitolins M, Neuhouser ML. Comparing indices of diet quality with chronic disease mortality risk in postmenopausal women in the Women's Health Initiative Observational Study: evidence to inform national dietary guidance. Am J Epidemiol. 2014;180(6):616–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reedy J, Krebs-Smith SM, Miller PE, Liese AD, Kahle LL, Park Y, Subar AF. Higher diet quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. J Nutr. 2014;144(6):881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bai G, Zhang J, Zhao C, Wang Y, Qi Y, Zhang B. Adherence to a healthy lifestyle and a DASH-style diet and risk of hypertension in Chinese individuals. Hypertens Res. 2017;40(2):196–202. [DOI] [PubMed] [Google Scholar]
- 18. Jacobs S, Harmon BE, Boushey CJ, Morimoto Y, Wilkens LR, Le Marchand L, Kroger J, Schulze MB, Kolonel LN, Maskarinec G. A priori-defined diet quality indexes and risk of type 2 diabetes: the Multiethnic Cohort. Diabetologia. 2015;58(1):98–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Koning L, Chiuve SE, Fung TT, Willett WC, Rimm EB, Hu FB. Diet-quality scores and the risk of type 2 diabetes in men. Diabetes Care. 2011;34(5):1150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fung TT, Hu FB, Wu K, Chiuve SE, Fuchs CS, Giovannucci E. The Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets and colorectal cancer. Am J Clin Nutr. 2010;92(6):1429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–26. [DOI] [PubMed] [Google Scholar]
- 22. Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. [DOI] [PubMed] [Google Scholar]
- 23. Hu FB, Rimm E, Smith-Warner SA, Feskanich D, Stampfer MJ, Ascherio A, Sampson L, Willett WC. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr. 1999;69(2):243–9. [DOI] [PubMed] [Google Scholar]
- 24. Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168(7):713–20. [DOI] [PubMed] [Google Scholar]
- 25. Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fung TT, Hu FB, McCullough ML, Newby PK, Willett WC, Holmes MD. Diet quality is associated with the risk of estrogen receptor-negative breast cancer in postmenopausal women. J Nutr. 2006;136(2):466–72. [DOI] [PubMed] [Google Scholar]
- 27. Willett W. Nutritional Epidemiology. 3 ed Oxford: Oxford University Press; 2013. [Google Scholar]
- 28. Cox DR. Regression models and life-tables. J R Stat Soc Series B Stat Methodol. 1972;34(2):187–220. [Google Scholar]
- 29. Smith PL. Splines as a useful and conveinent statistical tool. The American Statistician. 1979;33(2):57–62. [Google Scholar]
- 30. Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–61. [DOI] [PubMed] [Google Scholar]
- 31. Wang M, Spiegelman D, Kuchiba A, Lochhead P, Kim S, Chan AT, Poole EM, Tamimi R, Tworoger SS, Giovannucci E et al.. Statistical methods for studying disease subtype heterogeneity. Stat Med. 2016;35(5):782–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. World Cancer Research Fund / American Institute for Cancer Research. Continuous Update Project Report. Food, Nutrition, Physical Activity, and the Prevention of Colorectal Cancer. 2011. [Google Scholar]
- 33. Tabung FK, Brown LS, Fung TT. Dietary patterns and colorectal cancer risk: a review of 17 years of evidence (2000–2016). Curr Colorectal Cancer Rep. 2017;13(6):440–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mehta RS, Song M, Nishihara R, Drew DA, Wu K, Qian ZR, Fung TT, Hamada T, Masugi Y, da Silva A et al.. Dietary patterns and risk of colorectal cancer: analysis by tumor location and molecular subtypes. Gastroenterology. 2017;152(8):1944–53..e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shin S, Saito E, Sawada N, Ishihara J, Takachi R, Nanri A, Shimazu T, Yamaji T, Iwasaki M, Sasazuki S et al.. Dietary patterns and colorectal cancer risk in middle-aged adults: a large population-based prospective cohort study. Clin Nutr. 2017;37(3):1019–1026. doi: 10.1016/j.clnu.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 36. Gao Z, Guo B, Gao R, Zhu Q, Qin H. Microbiota disbiosis is associated with colorectal cancer. Front Microbiol. 2015;6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Macfarlane GT, Gibson GR, Cummings JH. Comparison of fermentation reactions in different regions of the human colon. J Appl Bacteriol. 1992;72(1):57–64. [DOI] [PubMed] [Google Scholar]
- 38. Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology. 2010;138(6):2088–100. [DOI] [PubMed] [Google Scholar]
- 39. Christie M, Jorissen RN, Mouradov D, Sakthianandeswaren A, Li S, Day F, Tsui C, Lipton L, Desai J, Jones IT et al.. Different APC genotypes in proximal and distal sporadic colorectal cancers suggest distinct WNT/beta-catenin signalling thresholds for tumourigenesis. Oncogene. 2013;32(39):4675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chan DS, Lau R, Aune D, Vieira R, Greenwood DC, Kampman E, Norat T. Red and processed meat and colorectal cancer incidence: meta-analysis of prospective studies. PLoS One. 2011;6(6):e20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bastide NM, Chenni F, Audebert M, Santarelli RL, Tache S, Naud N, Baradat M, Jouanin I, Surya R, Hobbs DA et al.. A central role for heme iron in colon carcinogenesis associated with red meat intake. Cancer Res. 2015;75(5):870–9. [DOI] [PubMed] [Google Scholar]
- 42. Kuhnle GG, Story GW, Reda T, Mani AR, Moore KP, Lunn JC, Bingham SA. Diet-induced endogenous formation of nitroso compounds in the GI tract. Free Radic Biol Med. 2007;43(7):1040–7. [DOI] [PubMed] [Google Scholar]
- 43. Le NT, Michels FA, Song M, Zhang X, Bernstein AM, Giovannucci EL, Fuchs CS, Ogino S, Chan AT, Sinha R et al.. A prospective analysis of meat mutagens and colorectal cancer in the Nurses' Health Study and Health Professionals Follow-up Study. Environ Health Perspect. 2016;124(10):1529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Butler LM, Sinha R, Millikan RC, Martin CF, Newman B, Gammon MD, Ammerman AS, Sandler RS. Heterocyclic amines, meat intake, and association with colon cancer in a population-based study. Am J Epidemiol. 2003;157(5):434–45. [DOI] [PubMed] [Google Scholar]
- 45. Park Y, Hunter DJ, Spiegelman D, Bergkvist L, Berrino F, van den Brandt PA, Buring JE, Colditz GA, Freudenheim JL, Fuchs CS et al.. Dietary fiber intake and risk of colorectal cancer: a pooled analysis of prospective cohort studies. JAMA. 2005;294(22):2849–57. [DOI] [PubMed] [Google Scholar]
- 46. Aune D, Chan DS, Lau R, Vieira R, Greenwood DC, Kampman E, Norat T. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2011;343:d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bingham SA. Mechanisms and experimental and epidemiological evidence relating dietary fibre (non-starch polysaccharides) and starch to protection against large bowel cancer. Proc Nutr Soc. 1990;49(2):153–71. [DOI] [PubMed] [Google Scholar]
- 48. Slavin JL. Mechanisms for the impact of whole grain foods on cancer risk. J Am Coll Nutr. 2000;19(3 Suppl):300s–7s. [DOI] [PubMed] [Google Scholar]
- 49. Cho E, Smith-Warner SA, Spiegelman D, Beeson WL, van den Brandt PA, Colditz GA, Folsom AR, Fraser GE, Freudenheim JL, Giovannucci E et al.. Dairy foods, calcium, and colorectal cancer: a pooled analysis of 10 cohort studies. J Natl Cancer Inst. 2004;96(13):1015–22. [DOI] [PubMed] [Google Scholar]
- 50. Larsson SC, Bergkvist L, Rutegard J, Giovannucci E, Wolk A. Calcium and dairy food intakes are inversely associated with colorectal cancer risk in the Cohort of Swedish Men. Am J Clin Nutr. 2006;83(3):667–73.; quiz 728–9. [DOI] [PubMed] [Google Scholar]
- 51. Keum N, Aune D, Greenwood DC, Ju W, Giovannucci EL. Calcium intake and colorectal cancer risk: dose-response meta-analysis of prospective observational studies. Int J Cancer. 2014;135(8):1940–8. [DOI] [PubMed] [Google Scholar]
- 52. Lee JE, Li H, Chan AT, Hollis BW, Lee IM, Stampfer MJ, Wu K, Giovannucci E, Ma J. Circulating levels of vitamin D and colon and rectal cancer: the Physicians' Health Study and a meta-analysis of prospective studies. Cancer Prev Res (Phila). 2011;4(5):735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lipkin M, Newmark H. Effect of added dietary calcium on colonic epithelial-cell proliferation in subjects at high risk for familial colonic cancer. N Engl J Med. 1985;313(22):1381–4. [DOI] [PubMed] [Google Scholar]
- 54. Lamprecht SA, Lipkin M. Cellular mechanisms of calcium and vitamin D in the inhibition of colorectal carcinogenesis. Ann N Y Acad Sci. 2001;952:73–87. [DOI] [PubMed] [Google Scholar]
- 55. Lamprecht SA, Lipkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat Rev Cancer. 2003;3(8):601–14. [DOI] [PubMed] [Google Scholar]
- 56. Limburg PJ, Liu-Mares W, Vierkant RA, Wang AH, Harnack L, Flood AP, Sellers TA, Cerhan JR. Prospective evaluation of trans-fatty acid intake and colorectal cancer risk in the Iowa Women's Health Study. Int J Cancer. 2008;123(11):2717–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dahm CC, Keogh RH, Lentjes MA, Spencer EA, Key TJ, Greenwood DC, Cade JE, Burley VJ, Shipley MJ, Brunner EJ et al.. Intake of dietary fats and colorectal cancer risk: prospective findings from the UK Dietary Cohort Consortium. Cancer Epidemiol. 2010;34(5):562–7. [DOI] [PubMed] [Google Scholar]
- 58. Murff HJ, Shu XO, Li H, Dai Q, Kallianpur A, Yang G, Cai H, Wen W, Gao YT, Zheng W. A prospective study of dietary polyunsaturated fatty acids and colorectal cancer risk in Chinese women. Cancer Epidemiol Biomarkers Prev. 2009;18(8):2283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen GC, Qin LQ, Lu DB, Han TM, Zheng Y, Xu GZ, Wang XH. N-3 polyunsaturated fatty acids intake and risk of colorectal cancer: meta-analysis of prospective studies. Cancer Causes Control. 2015;26(1):133–41. [DOI] [PubMed] [Google Scholar]
- 60. Keum N, Greenwood DC, Lee DH, Kim R, Aune D, Ju W, Hu FB, Giovannucci EL. Adult weight gain and adiposity-related cancers: a dose-response meta-analysis of prospective observational studies. J Natl Cancer Inst. 2015;107(2). doi: 10.1093/jnci/djv088. [DOI] [PubMed] [Google Scholar]
- 61. Ma Y, Yang Y, Wang F, Zhang P, Shi C, Zou Y, Qin H. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS One. 2013;8(1):e53916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang X, Wu K, Giovannucci EL, Ma J, Colditz GA, Fuchs CS, Willett WC, Stampfer MJ, Nimptsch K, Ogino S et al.. Early life body fatness and risk of colorectal cancer in U.S. women and men—results from two large cohort studies. Cancer Epidemiol Biomarkers Prev. 2015;24(4):690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jensen BW, Gamborg M, Gogenur I, Renehan AG, Sorensen TIA, Baker JL. Childhood body mass index and height in relation to site-specific risks of colorectal cancers in adult life. Eur J Epidemiol. 2017;32(12):1097–106. [DOI] [PubMed] [Google Scholar]
- 64. Russo A, Franceschi S, La Vecchia C, Dal Maso L, Montella M, Conti E, Giacosa A, Falcini F, Negri E. Body size and colorectal-cancer risk. Int J Cancer. 1998;78(2):161–5. [DOI] [PubMed] [Google Scholar]
- 65. Kim H, Giovannucci EL. Sex differences in the association of obesity and colorectal cancer risk. Cancer Causes Control. 2017;28(1):1–4. [DOI] [PubMed] [Google Scholar]
- 66. Winawer SJ. Natural history of colorectal cancer. Am J Med. 1999;106(1a):3S–6S.; discussion 50S–1S. [DOI] [PubMed] [Google Scholar]
- 67. Hughes LA, van den Brandt PA, Goldbohm RA, de Goeij AF, de Bruine AP, van Engeland M, Weijenberg MP. Childhood and adolescent energy restriction and subsequent colorectal cancer risk: results from the Netherlands Cohort Study. Int J Epidemiol. 2010;39(5):1333–44. [DOI] [PubMed] [Google Scholar]
- 68. van der Pols JC, Bain C, Gunnell D, Smith GD, Frobisher C, Martin RM. Childhood dairy intake and adult cancer risk: 65-y follow-up of the Boyd Orr cohort. Am J Clin Nutr. 2007;86(6):1722–9. [DOI] [PubMed] [Google Scholar]
- 69. Nimptsch K, Bernstein AM, Giovannucci E, Fuchs CS, Willett WC, Wu K. Dietary intakes of red meat, poultry, and fish during high school and risk of colorectal adenomas in women. Am J Epidemiol. 2013;178(2):172–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.