Abstract
Aging, an irreversible biological process, serves as an independent risk factor for chronic disease including cancer, pulmonary, neurodegenerative and cardiovascular diseases. In particular, high morbidity and mortality has been associated with cardiovascular aging although effective clinical therapeutic remedy is suboptimal for the ever-rising aging population. Recent evidence suggests a unique role for aberrant aggregate clearance and protein quality control machinery - the process of autophagy in shortened lifespan, compromised healthspan, onset and development of aging-associated cardiovascular diseases. Autophagy degrades and removes long-lived or damaged cellular organelles and proteins, the functions of which decline with advanced aging. Induction of autophagy using rapamycin, resveratrol, nicotinamide derivatives, metformin, urolithin A and spermidine delays aging, prolongs lifespan and improves cardiovascular function in aging. Given the ever-rising human lifespan and aging population as well as the prevalence of cardiovascular disease provoked with increased age, it is pertinent to understand the contribution and underlying mechanisms for autophagy and organelle-selective autophagy (e.g., mitophagy) in the regulation of lifespan, healthspan and cardiovascular aging. Here we will dissect the mechanism of action for autophagy failure in aging and discuss the potential rationale of targeting autophagy using pharmacological agents as new avenues in the combat of biological and cardiovascular aging.
Keywords: Biology of aging, lifespan, autophagy, mitophagy, cardiovascular
Biology of aging and cardiovascular sequelae of aging
Human life expectancy has gradually extended over the past several decades, contributing to a fast-growing elderly population and a high prevalence of aging-related diseases in particularly cardiovascular diseases [1, 2]. Aging is a complicated biological process, leading to progressive deterioration of cardiovascular structure and function mainly manifested as cardiac and vascular remodeling, dampened cardiac reserve (see Glossary) and function, endothelial defect and loss of vascular compliance [2-5]. Epidemiological evidence suggests that aging often serve as an independent risk factor for heart disease yet most of the current therapeutic strategies focus on management and prevention of the comorbidity while ignoring the nature of aging process [2]. With aging, the heart transits from a compensatory adaptive to a decompensatory maladaptive state, ultimately leading to decreased myocardial contractile capacity including increased left ventricular (LV) wall thickness and chamber dimension, altered diastolic filling pattern such as prolonged diastole, and impaired cardiac pump function [4, 6]. Cardiovascular aging is also featured by overt apoptosis, dampened endogenous autonomic (e.g., adrenergic) responsiveness (which stimulates cardiac contractile function), calcification and fibrosis, all of which contribute to deteriorated cardiac geometry and function [2, 3, 5, 7]. Several pathogenic factors are postulated for cardiovascular aging including oxidative stress, mitochondrial injury, genetic and epigenetic modifications, telomere shortening and metabolic dysregulation [2, 4, 8]. Among these theories, the ‘free radical theory of aging’ considers buildup of oxygen-derived free radical species as the main drive force for biological aging [2, 3]. This theory receives validation of lifespan extension with antioxidants such as vitamins, superoxide dismutase, catalase and metallothionein [8]. Accumulation of the pro-oxidant advanced glycation end-products (AGEs) lays the foundation for the ‘glycation theory of aging’ [3]. Given the critical role of mitochondrial energy supply in organismal homeostasis, the ‘mitochondrial decline theory’ favors a decline in mitochondrial capacity and function (ATP production) in the aging process [2, 3]. In addition to their role in energy supply, mitochondria also function as the main sites for reactive oxygen species (ROS) production courtesy of mitochondrial respiration [3], coinciding with the aforementioned “free radical theory”. Mitochondrial defect in aging may result from loss of mitochondrial integrity following mitochondrial permeation transition pore (mPTP) opening [3, 9]. In addition, telomere shortening has been reported to govern cell replication and senescence with defective telomere possibly serving as a marker for cardiac aging [2]. Furthermore, increased adiposity and metabolic derangement cause premature aging and cardiac aging possibly through inflammation and mitochondrial injury [10, 11]. It is thus imperative to identify the mechanisms of action underscoring deteriorated cardiac reserve and function with aging, with an ultimate goal to offer effective measures to halt or manage the course of biological and cardiac aging.
Recent findings has suggested a role for autophagy, a highly conservative process governing the clearance of long-lived or damaged organelles or cellular components, in the regulation of longevity and cardiovascular function in aging [12-15]. While previous reviews had focused on the cell-autonomous nature of autophagy in aging, relevant drug therapies targeting autophagy is less clear in longevity and cardiovascular aging. Through examining the relationships among autophagy, longevity and cardiovascular aging, this review aims to explain how autophagy dysregulation shapes cardiovascular in particular cardiac aging. We will discuss how these basic concepts can be translated to pharmacological therapy of lifespan and healthspan.
Autophagy and autophagy regulation in lifespan and aging
Autophagy denotes a cellular process for degradation and recycling of long-lived or damaged organelles and proteins, including microautophagy - the invagination of lysosomal membranes, chaperone-mediated autophagy (CMA) – shipping of soluble proteins to lysosomes through chaperones and lysosomal membrane receptors, and macroautophagy (or autophagy) – engulfing cargos via double-membrane autophagosomes prior to fusion with lysosomes [12, 16-18]. Autophagy is initiated by the class III phosphatidylinositol-3 kinase (PI-3K) and Beclin-1. Autophagosomes undergoes elongation, microtubule light chain-3 (LC3) recruitment, proteolytic cleavage of LC3 (lipidation) and formation of autophagolysosomes (autophagosome fusion with lysosomes). Autophagy may be divided into selective and non-selective forms depending on the nature of cargo contents (such as mitophagy for selective tagging and degradation of long-lived or injured mitochondrial organelles). Two types of mitophagy are present namely the PTEN-induced putative kinase 1 (Pink1)/Parkin and the mitophagy receptors [BCL2 19 kD Protein-Interacting Protein 3 (BNIP3) and FUN14 domain containing 1 (FundC1)] [11]. As shown in Fig. 1, autophagy process is stimulated by AMP-dependent protein kinase (AMPK) and is suppressed by Akt and mechanistic target of rapamycin (mTOR) [16, 17]. The serine/threonine kinase mTOR is sensitive to changes in amino acids, fatty acids, growth factors such as insulin and insulin-like growth factor 1 (IGF-1), and regulates cell growth and metabolism [19]. Autophagy participates in a wide variety of physiological and pathophysiological processes such as growth and development, metabolism, inflammation, neurodegenerative diseases, cancer, metabolic and cardiovascular diseases. Autophagy may be both adaptive and maladaptive through promoting cell survival or conversely, cell death with excessive autophagy (i.e., autosis) [20]. Dysregulated autophagy is evident in cardiovascular aging as well as other forms of cardiovascular pathologies including heart failure, myocardial hypertrophy, ischemic heart disease, hypertension, stroke, diabetes and alcoholic complications [11, 15, 18, 20].
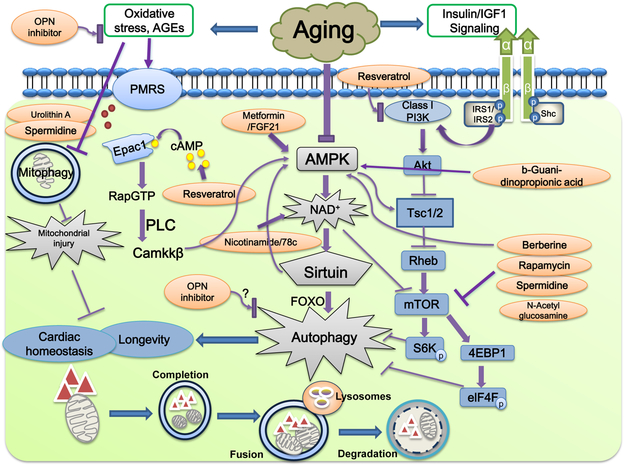
Fig. 1:
Schematic diagram depicting dysregulation of autophagy in advanced aging and how various pharmacological interventions may intervene the autophagy signaling process. Aging is commonly associated with impaired autophagy and mitophagy. Two main regulatory signaling machineries involved in dysregulated autophagy are suppressed AMPK activation and elevated class I PI-3K/Akt signaling, resulting in overactivated mTOR signaling, autophagy failure and changes in longevity and cardiac homeostasis. Improved lifespan and cardiac function in aging may be achieved through activation of AMPK directly or NAD+-dependent sirtuins indirectly. Sirtuins in turn promote longevity through FOXO-dependent induction of stress response and autophagy. Some pharmacological anti-agents such as resveratrol may benefit longevity and cardiac aging through autophagy-independent mechanism such as cAMP accumulation, leading to phospholipase C (PLC)-mediated activation of CamKKβ. Rapamycin, spermidine and N-acetyl glucosamine are considered autophagy inducers to directly act on mTOR. Free radical accumulation and oxidative stress such as AGEs turn on multiple oxido-reductase enzymes in aging commonly known as plasma membrane redox system (PMRS) that regulates cellular redox homeostasis and mitochondrial integrity. Bottom panel displays the essential steps in autophagy including nucleation and elongation, completion, fusion between autophagosomes and lysosomes as well as lysosomal degradation. Abbreviations PMRS: plasma membrane redox system, IGF-1: insulin like growth factor-1, FOXO: Forkhead O transcriptional factor; IRS: Insulin receptor substrate; EPac1: exchange protein directly activated by cAMP; OPN: osteopontin; CamKKβ: Ca2+/calmodulin-dependent protein kinase kinase β; mTOR: mammalian target of Rapamycin, PI3K: Phosphatidylinositol 3-kinase.
Ample of evidence has suggested a role for autophagy in the survival and longevity through removal and recycling of long-lived or injured cellular components or proteins [18, 20, 21]. Autophagy declines with aging while pharmacological and genetic approaches for lifespan extension are closely related with autophagy induction [8, 12, 16]. Table 1 summarizes the effect of genetically-modified autophagy gene and autophagy regulators on lifespan and cardiovascular aging in rodents. Similar findings were also noted in lower species providing the genetic link of Atg autophagy genes with longevity (reviewed in [21]). In general, suppression of autophagy induces cell death, accelerates aging and shortens lifespan [21]. This is in line with the notion that autophagy failure or mutation in autophagy genes promotes premature aging, cardiac aging and neurodegenerative diseases (including Parkinson disease, spastic paraplegia and ataxia), in association with buildup of damaged intracellular components, disturbed cellular homeostasis [3, 8, 16, 21, 22]. This is supported by the apparent cardiovascular anomalies in loss-of-function autophagy models [3, 8, 16]. In lower species, muscle and cardiac aging are characterized by progressive accumulation of protein aggregates associated with impaired function in Drosophila. Forkhead Box O transcriptional factor (FOXO) overexpression and increased activity of its target Thor/4E-BP preserve muscle function and extend lifespan via autophagy induction [23, 24]. Atg8b, one autophagy-associated gene, was downregulated with age in Drosophila heart. Age-dependent ectopic fat accumulation (EFA) in non-adipose tissues contributes to metabolic diseases such as obesity in aging. Chaperone dHsc4-assisted autophagy inhibits aging-dependent EFA and extends lifespan in Drosophila by maintaining the proteostasis of lipid droplets [25].
Table 1:
Lifespan and cardiac aging in genetically engineered murine models of autophagy
| Genotype | Target organ | model | Lifespan and cardiovascular aging | Reference |
|---|---|---|---|---|
| Atg5−/− neonatal mice | Global | starvation | Normal at birth but die within 1 day of delivery, survival time of starved Atg5-deficient neonates (12 h) < wild-type mice (21 h) | [80] |
| Atg5 transgenic mice | Global | aging | anti-aging phenotypes, including leanness, increased insulin sensitivity and improved motor function | [81] |
| Transgenic HSP27 mice | Cardiac specific | aging | Attenuates aging-induced impairment of cardiac function | [82] |
| Akt1+/−mice | Global | aging | Reduced ribosomal biogenesis, mitochondrial DNA content and oxidative stress | [83] |
| Akt2 −/− mice | Global | aging | prolongs life span and improves myocardial contractile function with adaptive cardiac remodeling | [15] |
| hypomorphic (mTOR(Δ/Δ)) alleles mice | Global | aging | increases lifespan, exhibits a marked functional preservation in various organs | [84] |
| heterozygous mice lacking global/hepatic Rictor, global Rictor deletion mice | Liver, global | aging | decreases male, but not female, lifespan, independent of the role of hepatic mTORC2 in promoting glucose tolerance | [85] |
| S6K1−/−mice | Global | aging and high fat diet | protects against obesity due to enhanced beta-oxidation, sensitive to insulin owing to loss of a negative feedback loop from S6K1 to insulin receptor substrate 1 (IRS1), which blunts S307 and S636/S639 phosphorylation | [86] |
| a Phe121Ala mutation in beclin 1 (BecnlF121A/F121A) to interrupt interaction with BCL2 | Global | aging | Increases lifespan, diminishes age-related renal and cardiac pathological changes and spontaneous tumorigenesis via Disruption of the beclin 1-BCL2 autophagy regulatory complex | [14] |
| TSC1 transgenic mice | Global | aging | lifespan extension in female, but not male | [87] |
Several explanations can be considered for dysregulated autophagy in aging, among which mTOR serves as the main driving force for autophagy inhibition, aging and lifespan control [8, 19]. A number of lifespan regulatory molecules including Sirtuins, AMPK, insulin/IGF-1, and FOXO transcription factors all converge at mTOR [8, 19]. Inhibition of the TOR pathway extends lifespan in yeast, C. elegans, Drosophila and rodents [19, 26]. Extension of lifespan with caloric-restriction is mediated through mTOR suppression, supporting the role of mTORC as a bona-fide target for lifespan extension. Activation of mTOR with tuber sclerosis complex 1 (TSC1) conditional deletion in young mice presented aging phenotypes [19]. Intermittent, life-long administration of the mTOR inhibitor rapamycin extends lifespan and suppresses aging-induced weight gain [27]. These findings favor the rationale of targeting mTOR in lifespan control. Given that calorie restriction is not practical in the elderly, due to malnutrition and immunodeficiency, the use of mTOR inhibitors as a ‘gerosuppressants’ to manage senescence and related comorbidities has received much attention [17]. Nonetheless, it may be argued that lifespan extension with mTOR inhibition may be due to alternate mechanism independent of autophagy such as the inhibition of protein synthesis. Deletion of S6K1, the downstream protein synthesis effector of mTOR, effectively prolongs lifespan and retards aging complications. Rapamycin was unable to offer lifespan extension with S6K overexpression [12]. More evidence revealed that mTORC1 phosphorylates the serine/threonine kinase unc-51-like kinase 1 (ULK1 or Atg1), an initiator of autophagy, while rapamycin upregulates Atg1 and promotes autophagy, even with surplus of nutrients [8].
Findings from our lab indicated that activation of the mTOR upstream signal Akt accentuated cardiac aging (both geometry and function) through dampened autophagy (as depicted in Fig. 1). Our data revealed that induction of autophagy rescued aging-induced cardiac anomalies [28]. Evaluation of autophagy regulatory machineries revealed suppressed tumor suppressor phosphatase and tensin homolog deleted from chromosome 10 (PTEN), AMPK and ACC in conjunction with elevated PI-3K/Akt/mTOR signaling. Along the same line, our recent report indicated that obliteration of Akt2 extended lifespan and rescued against cardiac aging through restored Foxo1-mediated autophagy and mitophagy [15]. This is consistent with the earlier notion that PI-3K inhibition stimulated autophagy, suppressed senescence and preserved contractile function in murine hearts [29]. In addition, data from our group also suggested a role for the innate pro-inflammatory mediator toll-like receptor 4 (TLR4) in declined autophagy and cardiac remodeling and contractile dysfunction in aging, via a histone deacetylase (HDAC1)-nuclear receptor corepressor 1 (NCoR1)-dependent mechanism [13]. These findings support a unique role for dysregulated autophagy in cardiac aging [15, 16].
Intervention of cardiac aging by targeting autophagy
Although caloric restriction appears to be the most robust way for autophagy induction and lifespan extension through improved mitochondrial respiration and retarding aging comorbidities [2], it may not be ideal for the elderly due to immunodeficiency and malnutrition. A number of anti-aging interventions became available to improve healthspan and/or lifespan, and display beneficial effects against cardiac aging [2, 30]. As shown in Fig. 1, several natural compounds may function as caloric-restriction mimetics including rapamycin, and the AMPK/Sirt1 activators resveratrol and metformin as well as polyphenol products to improve the healthspan in aging [2, 31]. Given the essential role of autophagy dysregulation in cardiac aging [8, 16], we will update the contemporary understanding of rapamycin, resveratrol, metformin, nicotinamide and NAD+ precursors, and other compounds in the management of aging and cardiac anomalies related to autophagy regulation (Table 2). Non-pharmacological approaches such as caloric restriction and recent epigenetic intervention will also be discussed.
Table 2:
List of anti-aging drugs with cardiovascular benefits involving autophagy induction
| Drug | Species | Lifespan & cardiovascular response | Possible mechanisms | Reference |
|---|---|---|---|---|
| Rapamycin | Rodents, human (Hutchinson-Gilford progeria syndrome) | Prolongs lifespan, reverses age-related oxidative stress, cardiac and vascular dysfunction, reverses cellular phenotype of fibroblasts from children | Activates AMPK, inhibits mTOR, S6 kinase and ULK1 phosphorylation, induces autophagy, clears progerin through autophagy, promotes mitochondrial biogenesis. | [26, 88-90] |
| Resveratrol | Rodents, human, Yeast, C. elegans, Drosophila | Prolongs lifespan, improves cardiac and vascular function as well as mitochondrial number but not glucose metabolism in aging | Activates AMPK and Sirtuins, mimics calorie restriction, affects acetylproteome, promotes lipolysis and attenuates lipogenesis | [91-93] |
| Metformin | Rodents, human aortic endothelial cells, C. elegans, Drosophila | Prolongs lifespan, improves Physiological and metabolic parameters in aging (glucose tolerance, exercise capacity and cardiac function), delays endothelial senescence via mitochondrial biogenesis/function | Activation of AMPK, H3K79 methylation, inhibits mTOR, reduces hyperglycemia and hyperinsulinemia and alleviates insulin resistance | [94, 95] |
| Nicotinamide derivatives, specific CD38 inhibitor 78c | Rodents, human | Improves healthspan but not lifespan, reverses age-related NAD+ decline and improves cardiac function in natural and accelerate aging | Stimulates autophagy and mitophagy, increases NAD+ levels, activation of Sirtuins, AMPK and poly (ADP ribose) polymerases (PARPs) | [59, 96] |
| Spermidine | Rodents, human, yeast nematodes and flies | Prolongs lifespan, improves healthspan, reduces cardiac hypertrophy and remodeling, preserves diastolic function in aging, reduces blood pressure and incidence of cardiovascular disease and cancer mortality | Induces autophagy, Mitophagy and mitochondrial respiration, inhibits histone acetyltransferases, inflammation, oxidative stress, affects glutathione metabolism, lipid metabolism | [60, 61, 63, 93] |
| OPN inhibitor Agelastatin A | Rodents | Rescues cardiac aging and induces a selective fibroblast senescence | Modulates fibroblast senescence by osteopontin (OPN) production. | [10] |
| Fibroblast growth factor 21 (FGF21) | Rodents, C. elegans | Improves healthspan and extends lifespan; alleviates age-related metabolic disorders, including atherosclerosis, obesity, type 2 diabetes | Activates AMPK, Autophagy and anti-inflammation | [65] |
| Urolithin A | Rodents and C. elegans | Prolongs lifespan, improves healthspan, exercise capacity and mitochondrial function | Stimulates mitophagy, prevents accumulation of dysfunctional mitochondria | [67] |
| N-Acetyl-glucosamine | C. elegans | Extends mean lifespan | Enhances autophagy, ER-Associated protein degradation, and proteasomal activity | [68] |
| Curcumin | Human | Improves vascular endothelial function in healthy middle-aged and older adults. | Increased nitric oxide (NO) bioavailability and reduced oxidative stress | [71] |
| b-Guani-dinopropionic acid | Drosophila melanogaster | Prolongs lifespan | Activates autophagy by AMPK-Atg1 signaling pathway | [69] |
| Kallistatin | Rodents, C. elegans | Extends lifespan, reduces vascular senescence and aging. | MicroRNA-34a-Sirt1-dependent | [72, 73] |
| Melatonin | Cardiac progenitor cells | Antagonizes premature senescence of cardiac progenitor cells | H19/miR-675/USP10-depndent pathway | [70] |
Rapamycin and mTOR inhibitors
Rapamycin is a US Food and Drug Administration (FDA)-approved mTOR inhibitor and autophagy inducer [19]. mTOR presents as mTORC1 and mTORC2 with distinct structures and activities. mTORC1, composed of mTOR, Raptor, mammalian lethal with SEC13 protein 8 (mLST8), proline-rich Akt substrate of 40 kDa (PRAS40), is rapamycin-sensitive and essential for autophagy regulation. mTORC2, on the other hand, consists of Rictor, mSin1, mLST8 and is rapamycin-insensitive [19]. Rapamycin and its analogs (termed rapalogs such as RAD001) are used in clinical settings such as suppression of organ rejection after kidney transplantation, occlusion of cardiac stents, and certain cancers. Inhibition of mTOR extends lifespan in various organisms including C. elegans, Drosophila and mice [19, 32]. The National Institute on Aging (NIA) Intervention Testing Program found extension of lifespan after rapamycin treatment beginning at 9 or 18 months of age in mice [26]. Rapamycin benefits cardiac aging and age-related diseases such as Alzheimer’s disease [33, 34]. Short-term rapamycin treatment (10 weeks) improves diastolic function and attenuates LV hypertrophy in aged mice [35]. Along the same line, late-life administration of rapamycin reverses aging-related cardiac dysfunction [36]. The beneficial effect of rapamycin on cardiac aging is likely due to proteomic and metabolic remodeling as it promotes mitochondrial content, inhibits inflammation and reverses aging-associated metabolic switch from fatty acid oxidation to glycolysis [7, 36]. Rapamycin is capable of re-inducing proliferation by lifting cell cycle inhibition, therefore allowing transition from “an irreversible arrest into a reversible state” without forcing cells to proliferate in the event of cell cycle arrest [37]. Interestingly, similar findings were noted in young mice following a 3-month rapamycin treatment [38], denoting an age-independent effects for rapamycin. More evidence suggested that rapamycin is capable of altering the balance between nuclear- and mitochondrial-encoded oxidative phosphorylation, with the mito-nuclear imbalance serving as a conserved mechanism for mitochondrial unfolded protein response (UPRmt) and longevity [39]. Although inhibition of mTOR is beneficial for cardiac aging, complete removal of mTORC1 may lead to cardiomyopathy, impaired hypertrophic response and accelerated heart failure [40]. Adaptive hypertrophic response is common and deemed adaptive in response to pressure overload or hemodynamic stress in aging.
Resveratrol and Sirtuin activators
The class III histone deacetylases, the NAD+-dependent Sirtuins, are appealed as “the fountain of youth” with a pivotal role in longevity and aging anomalies. Resveratrol, the first natural flavonoid found in red wine, mulberries, peanuts, and rhubarb, exerts cardiovascular and antiaging benefits through Sirtuin activation in a variety of pathological conditions [41, 42]. Resveratrol produces its beneficial effect on cardiac aging via Sirt1/PI3K/Akt-mediated Foxo3 phosphorylation, mitochondrial preservation and inhibition of cAMP phosphodiesterase, resulting in improved cardiac function [43]. Sirt3 deficiency abrogated the protective effect of resveratrol against cardiac hypertrophy [44]. Furthermore, administration of Longevinex, a commercialized resveratrol formulation, improves cardiac performance and longevity through induction of autophagy, upregulation of Sirt1 and Sirt3 and nuclear translocation of FOXOs in the heart [45]. More evidence has suggested that resveratrol and Sirtuins confer longevity and cardiac benefits through protein hypoacetylation in autophagy control [45]. It is noteworthy that resveratrol inhibits the Akt-insulin signaling promoting Class I PI3K by competing with ATP for the catalytic site, which may promote longevity independent of Sirtuins [46].
Metformin, Berberine and AMPK activators
Metformin is an anti-diabetic drug capable of stimulating AMPK via direct phosphorylation of Ser633 and Ser1177 [47]. Metformin extends lifespan and improves healthspan in C. elegans and mice via mitohormetic regulation of ROS production, AMPK-mediated induction of autophagy and elevation of NAD+ levels [47, 48]. Data from our lab suggested that short-term treatment of metformin improved cardiac aging phenotype in mice [49]. Findings from the United Kingdom Prospective Diabetes Study revealed that metformin lowers the 10-year mortality in myocardial infarction, stroke, or all-causes compared with sulfonylurea, insulin, or dietary control [50, 51]. The natural occurring compound Berberine lowers serum LDL cholesterol and benefits healthspan through AMPK activation [52]. Berberine enhances autophagy through inhibition of mTOR to limit cardiac remodeling, collagen deposition, apoptosis and fibrosis, resulting in improved cardiac function [53]. In patients with chronic congestive heart failure, berberine improves cardiac function and decreases mortality of the patients during long-term follow-up [54]. More evidence revealed that Berberine retards H2O2-induced senescence, in association with restored autophagic flux and NAD+ levels in senescent cells [55]. Berberine also alleviates postoperative cognitive defects via suppression of neuroinflammation in aging [56]. In addition to AMPK and autophagy induction, other signaling machineries have also been implicated in berberine-elicited anti-aging benefits such as Sirt1, PGC-1α and ATP production [57].
Nicotinamide and NAD+ precursors
NAD+ levels decrease with aging due to age-related CD38 upregulation or downregulation of nicotinamide phosphoribosyl transferase (Nampt), a key enzyme for NAD+ synthesis [16]. NAD+ depletion is found in neurodegenerative diseases, and cardiovascular aging, which could be rescued by pharmacological intervention to bolster cellular NAD+ levels [58]. Chronic intake of the NAD+ precursor nicotinamide or the specific CD38 inhibitor 78c improves healthspan but not lifespan. Nicotinamide may accelerate autophagy degradation of mitochondria (mitophagy) and improve glucose homeostasis in association with less hepatic steatosis and inflammation, increased glycogen deposition and clearance through glycolytic pathways [59]. Sadoshima and colleagues indicated that NAD+ precursors including nicotinamide mononucleotide and nicotinamide riboside or upregulation of Nampt may suppress cardiac aging through activation of Sirtuins, including Sirt1 and Sirt3, en route to protein deacetylation and activation of autophagy [16]. Targeted NAD metabolome analysis revealed decreased levels of nicotinamide mononucleotide salvage in nicotinamide mononucleotide-treated mice, an effect offset by overexpression of de novo NAD biosynthetic enzymes. Although nicotinamide mononucleotide may not drastically enhance NAD+ levels, it may promote acetylation of Sirtuin targets and alleviate cardiac aging in the absence of survival effects [59].
Spermidine
Spermidine is a polyamine participating in an array of biological events including autophagy induction, DNA stability, transcription, translation and apoptosis. Supplementation of spermidine, an autophagy-inducing agent, protects against neurodegeneration and cognitive decline, memory loss and motor impairment in aging. Eisenberg and colleagues recently reported that spermidine improved lifespan and health span through autophagy activation [60]. Using proteomics and metabolomics analyses, these investigators identified proteins and metabolites up- or down- regulated by spermidine in aging rat hearts. The molecules identified were mainly associated with immunity, blood coagulation, lipid metabolism, and glutathione metabolism [61]. To this end, spermidine may offer protective effect against aging hearts and display therapeutic promises in cardiac aging. Further study confirmed that supplementation of spermidine-rich plant extract is safe and well-tolerated in the elderly and mice, making it practical for longer-term intervention of spermidine in humans [62]. At this point, dietary polyamine (spermidine in particular)-offered cardiovascular endpoints seems to be attributed to mitochondrial homeostasis, anti-inflammation, and delay of stem cell senescence [63].
Osteopontin (OPN) Inhibitor
Biological aging is often tied with increased adiposity [11, 17] although the precise interplay remains elusive. Recent work from Derumeaux and colleagues suggested that visceral adipose tissue (VAT) triggers OPN expression and drives interstitial fibrosis in aging hearts. Given that cardiac aging is commonly associated with elevated adiposity and extracellular matrix including OPN (a marker for cardiac anomalies including calcification), these investigators tested the effect of surgical removal of VAT and a small-molecule OPN inhibitor Agelastatin A on cardiac aging. Interestingly, VAT removal significantly reduced circulating levels of OPN and TGFβ, restored cardiac function and alleviated myocardial fibrosis in aging hearts, the effects of which were mirrored by OPN deficiency. VAT removal and OPN deficiency promoted senescence of cardiac fibroblasts and thus limited their activation. Agelastatin A reversed aging-related cardiac fibrosis and dysfunction [10]. These findings supported the nature of VAT as the main source of OPN in aging to compromise cardiac structure and function through profibrotic secretome. Although a role for autophagy has not yet been identified here, OPN is well known to promote autophagy [64] thus raising a possible beneficial effect of autophagy inhibition in aging. Interventions targeting OPN such as VAT removal and OPN inhibition may be promising therapeutic targets for cardiac aging possibly through induced fibroblast senescence [10].
Fibroblast growth factor 21 (FGF21)
FGF21 belongs to a hormone-like FGF family and serves as a longevity factor governing energy expenditure, stress responses, glucose and lipid metabolism. In ER stress, dysfunctional mitochondria and autophagy, FGF21 regulates stress and metabolism through somatotropic and hypothalamic-pituitary-adrenal (HPA) axis. It was demonstrated that FGF21 alleviates aging pathologies in metabolism such as type 2 diabetes, obesity, atherosclerosis and cardiovascular diseases. Overexpressing FGF21 prolonged lifespan in mice although FGF21 resistance may develop under metabolic and stress-related disorders to compromise healthy aging [65]. Several mechanisms have been postulated for FGF21-induced healthy aging in integrated stress response including AMPK activation, autophagy induction and anti-inflammation. Likewise, FGF21 resistance, may jeopardize human healthspan and accelerate the aging process through disturbed autophagy, similar to insulin resistance [65].
Urolithin A and mitophagy regulators
Aging is commonly associated with mitochondrial dysfunction and compromised mitophagy [66]. However, effective measures to enhance mitochondrial function in particular mitophagy are still lacking due to toxicity and non-specificity. Urolithin A, a natural compound that induces mitophagy, was found to extend lifespan and promote health aging likely through mitophagy and clearance of injured mitochondria. Rye and colleagues revealed that urolithin A improved various activity (e.g., mobility, pharyngeal pumping and exercise capacity) and maintained mitochondrial respiration in aging in C. elegans and rodents [67]. Urolithin A and other food consumption components including asnicotinamide riboside and tomatidine, may protect against age-related loss in muscle function (sarcopenia) [67]. These findings highlight the health benefits of urolithin A and mitophagy enhancers in preservation of mitochondrial and cardiac function in aging.
Other anti-aging drugs with autophagy regulatory potential
There are a number of additional anti-aging drugs with promises in autophagy regulation. As shown in Table 2, N-acetyl-glucosamine and b-Guanidinopropionic acid may prolong lifespan through facilitated autophagy [68, 69]. In addition, natural compounds curcumin and melatonin have also exhibited beneficial responses against cardiovascular aging [70, 71] although direct evidence is still lacking for a permissive role of autophagy herein. Kallistatin is an endogenous protein although recent evidence suggested some promises for Kallistatin in lifespan extension and protection against senescence through inhibition of Akt-Bcl2, upregulation of Sirt1 and inhibition of oxidative stress and inflammation [72, 73].
Caloric restriction and lifestyle modification
In addition to pharmacological interventions, lifestyle modification such as caloric restriction also slows aging, extends lifespan and counters age-related diseases in various species [17, 74]. Epidemiological, clinical and experimental studies have all confirmed the benefits of caloric restriction in extended lifespan and healthspan, and benefited cardiac aging through autophagy induction [74]. Suppression of mTOR and induced autophagy were noted in calorically restricted hearts, indicating contribution of mTOR-dependent autophagy in caloric restriction-elicited benefit in cardiac aging [75]. Moreover, autophagy improves neuroendocrine and metabolic profiles of adiposity, glucose and lipid metabolism in aging [16, 17]. Pharmacological autophagy induction reduces serum leptin levels whereas genetic removal of leptin triggers autophagy. Decreased autophagy in aging may also be attributed to lipid accumulation and metabolic derangement with age [76]. Autophagy controls the formation of lipid droplets in the heart, with autophagy failure prompting accumulation of triglycerides and lipid droplets [76].
Epigenetic intervention
Aging is associated with altered epigenetic profiles of both DNA and histones such as DNA methylation and histone acetylation and methylation, while longevity intervention may attenuate age-associated chromatin decline [77]. Several epigenetic-targeting drugs have received FDA approval and many others are under clinical trials (although most for cancer therapy). A number of bioactive phytochemicals such as metformin and caloric restriction have been shown to possess epigenetic modulatory activities in aging (Table 2) [77]. These epigenetic modulators delay aging and minimize the risk of cancer. For example, chromatin alterations are indicated in the pathophysiology of age-related disease. The “designated epigenetic modulators” in cardiac aging are still unknown and little information is available for the role of autophagy regulation herein. It is thus intriguing to find out how much overlap exists between the current list of cardiovascular aging interventions and the epigenetic phenotypes these drugs may influence.
Conclusion and future perspectives
Evidence has suggested that autophagy is essential to longevity and healthspan, and altered autophagy contributes to cardiac aging and the transition of healthy state of organism into a pre-senescent state [12, 21, 78]. Although autophagy inducers have been indicated to benefit lifespan and aging cardiovascular pathologies [12], controversy still exists in term of the protective versus deleterious effect of autophagy induction in aging complications [79] (see Outstanding Questions). Much challenges remain for the field of aging. Multiple organismal models are used to dissect the correlation between aging and autophagy, although it is unclear with regards to the model specificity for autophagy in aging. Although autophagy is permissive to longevity, it remains unclear whether cardiac aging develops as a consequence of autophagy decline in aging or vice versa. Besides the therapeutic options discussed here in this review, more viable anti-aging regimen are available based on the activation of telomerase, NO modulation, antioxidants, PARP inhibition, senolytic therapeutics, plasma membrane redox system (PMRS) activators, and stem cell therapies [2]. Involvement of autophagy regulation is less clear for these measures. In-depth understanding of the mechanism behind autophagy dysregulation in aging and aging-related cardiovascular anomalies is vital for the development of appropriate therapeutic strategies to halt aging and cardiac complications in aging.
Outstanding Questions:
Given the heavy use of multiple organismal model in aging and autophagy field, do changes in autophagy vary across model/species in aging?
Do changes in autophagy exhibit organ and tissue specificity, even in the same organism?
Besides mTOR, what else could contribute to aging-associated decline in autophagy?
Do pharmacological activation and inhibition of autophagy both offer benefits against aging?
Are autophagy different in aging based on the form of autophagy (selective or non-selective) ?
Are changes in autophagy results of metabolic derangement in aging or vice versa (failure to clear excess lipids in aging)?
Highlights.
Clinical and experimental data depicted a tie between age-related cardiovascular diseases and autophagy failure.
Autophagy deficiency shortens lifespan and compromises cardiovascular function in aging.
Autophagy induction delays aging, prolongs lifespan and, improves cardiac aging.
Acknowledgements:
The authors received support in part from the National Institute of Aging (R03 AG21324), NSFC81522004, 91749128, 81770261.
Glossary:
- Autosis:
a form of cell death triggered by autophagy mediated by Na+-K+-ATPase pump.
- Advanced glycation end-products (AGEs):
a subset of glycated and oxidized proteins and lipids after continued contact of reducing sugars or short-chain aldehydes with amino group.
- Cardiac reserve:
the ability of heart to expel a larger quantity of blood beyond the basal level.
- Diastole:
relaxation and dilation of the heart chambers.
- Mitophagy:
the specific autophagic elimination of mitochondria.
- Mitochondrial permeation transition pore (mPTP):
protein formed in the inner membrane of the mitochondria under pathological conditions, the opening of which leads to cell death.
- NAD precursors:
molecules with NAD producing ability, including nicotinamide and vitamin B3 derivatives such as nicotinamide riboside and nicotinamide mononucleotide.
- Selective autophagy:
autophagy process involving recognition and targeting of specific cargo, such as damaged organelles, misfolded proteins, or invading pathogens for lysosomal destruction.
- Sirtuins:
NAD-dependent deacetylases governing metabolism, healthspan, and aging.
Footnotes
Conflict of Interest: Both authors had nothing to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- 1.Lutz W et al. (2008) The coming acceleration of global population ageing. Nature 451 (7179), 716–9. [DOI] [PubMed] [Google Scholar]
- 2.Alfaras I et al. (2016) Pharmacological Strategies to Retard Cardiovascular Aging. Circ Res 118 (10), 1626–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Picca A et al. (2018) Mitochondrial quality control mechanisms as molecular targets in cardiac ageing. Nat Rev Cardiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Obas V and Vasan RS (2018) The aging heart. Clin Sci (Lond) 132 (13), 1367–1382. [DOI] [PubMed] [Google Scholar]
- 5.Buford TW (2016) Hypertension and aging. Ageing Res Rev 26, 96–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liguori I et al. (2018) Oxidative stress, aging, and diseases. Clin Interv Aging 13, 757–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiao YA and Rabinovitch PS (2015) The Aging Heart. Cold Spring Harb Perspect Med 5 (9), a025148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nair S and Ren J (2012) Autophagy and cardiovascular aging: lesson learned from rapamycin. Cell Cycle 11 (11), 2092–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hafner AV et al. (2010) Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging (Albany NY) 2 (12), 914–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sawaki D et al. (2018) Visceral Adipose Tissue Drives Cardiac Aging Through Modulation of Fibroblast Senescence by Osteopontin Production. Circulation. [DOI] [PubMed] [Google Scholar]
- 11.Ren J et al. (2018) Metabolic Stress, Autophagy, and Cardiovascular Aging: from Pathophysiology to Therapeutics. Trends Endocrinol Metab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura S and Yoshimori T (2018) Autophagy and Longevity. Mol Cells 41 (1), 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S et al. (2018) Ablation of toll-like receptor 4 attenuates aging-induced myocardial remodeling and contractile dysfunction through NCoRI-HDAC1-mediated regulation of autophagy. J Mol Cell Cardiol 119, 40–50. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez AF et al. (2018) Disruption of the beclin 1-BCL2 autophagy regulatory complex promotes longevity in mice. Nature 558 (7708), 136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren J et al. (2017) Akt2 ablation prolongs life span and improves myocardial contractile function with adaptive cardiac remodeling: role of Sirt1-mediated autophagy regulation. Aging Cell 16 (5), 976–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shirakabe A et al. (2016) Aging and Autophagy in the Heart. Circ Res 118 (10), 1563–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y et al. (2018) Targeting autophagy in obesity: from pathophysiology to management. Nat Rev Endocrinol 14 (6), 356–376. [DOI] [PubMed] [Google Scholar]
- 18.Delbridge LMD et al. (2017) Myocardial stress and autophagy: mechanisms and potential therapies. Nat Rev Cardiol 14 (7), 412–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kennedy BK and Lamming DW (2016) The Mechanistic Target of Rapamycin: The Grand ConducTOR of Metabolism and Aging. Cell Metab 23 (6), 990–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sciarretta S et al. (2018) The Role of Autophagy in the Heart. Annu Rev Physiol 80, 1–26. [DOI] [PubMed] [Google Scholar]
- 21.Hansen M et al. (2018) Autophagy as a promoter of longevity: insights from model organisms. Nat Rev Mol Cell Biol 19 (9), 579–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bravo-San Pedro JM et al. (2017) Autophagy and Mitophagy in Cardiovascular Disease. Circ Res 120 (11), 1812–1824. [DOI] [PubMed] [Google Scholar]
- 23.Blice-Baum AC et al. (2017) Modest overexpression of FOXO maintains cardiac proteostasis and ameliorates age-associated functional decline. Aging Cell 16 (1), 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demontis F and Perrimon N (2010) FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell 143 (5), 813–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan Y et al. (2017) HDAC6 Suppresses Age-Dependent Ectopic Fat Accumulation by Maintaining the Proteostasis of PLIN2 in Drosophila. Dev Cell 43 (1), 99–111 e5. [DOI] [PubMed] [Google Scholar]
- 26.Harrison DE et al. (2009) Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460 (7253), 392–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anisimov VN et al. (2011) Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle 10 (24), 4230–6. [DOI] [PubMed] [Google Scholar]
- 28.Hua Y et al. (2011) Chronic Akt activation accentuates aging-induced cardiac hypertrophy and myocardial contractile dysfunction: role of autophagy. Basic Res Cardiol 106 (6), 1173–91. [DOI] [PubMed] [Google Scholar]
- 29.Inuzuka Y et al. (2009) Suppression of phosphoinositide 3-kinase prevents cardiac aging in mice. Circulation 120 (17), 1695–703. [DOI] [PubMed] [Google Scholar]
- 30.Saraswat K and Rizvi SI (2017) Novel strategies for anti-aging drug discovery. Expert Opin Drug Discov 12 (9), 955–966. [DOI] [PubMed] [Google Scholar]
- 31.de Cabo R and Navas P (2016) Spermidine to the rescue for an aging heart. Nat Med 22 (12), 1389–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weichhart T (2018) mTOR as Regulator of Lifespan, Aging, and Cellular Senescence: A Mini-Review. Gerontology 64 (2), 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leslie M (2013) Biomedicine. A putative antiaging drug takes a step from mice to men. Science 342 (6160), 789. [DOI] [PubMed] [Google Scholar]
- 34.Van Skike CE et al. (2018) Inhibition of mTOR protects the blood-brain barrier in models of Alzheimer's disease and vascular cognitive impairment. Am J Physiol Heart Circ Physiol 314 (4), H693–H703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai DF et al. (2014) Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell 13 (3), 529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flynn JM et al. (2013) Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell 12 (5), 851–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demidenko ZN et al. (2009) Rapamycin decelerates cellular senescence. Cell Cycle 8 (12), 1888–95. [DOI] [PubMed] [Google Scholar]
- 38.Neff F et al. (2013) Rapamycin extends murine lifespan but has limited effects on aging. J Clin Invest 123 (8), 3272–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Houtkooper RH et al. (2013) Mitonuclear protein imbalance as a conserved longevity mechanism. Nature 497 (7450), 451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang D et al. (2010) MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J Clin Invest 120 (8), 2805–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arafa MH et al. (2014) Protective effect of resveratrol against doxorubicin-induced cardiac toxicity and fibrosis in male experimental rats. J Physiol Biochem 70 (3), 701–11. [DOI] [PubMed] [Google Scholar]
- 42.Lin CH et al. (2014) Resveratrol enhanced FOXO3 phosphorylation via synergetic activation of SIRT1 and PI3K/Akt signaling to improve the effects of exercise in elderly rat hearts. Age (Dordr) 36 (5), 9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y et al. (2014) Mitochondrial aldehyde dehydrogenase 2 accentuates aging-induced cardiac remodeling and contractile dysfunction: role of AMPK, Sirt1, and mitochondrial function. Free Radic Biol Med 71, 208–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen T et al. (2015) Activation of SIRT3 by resveratrol ameliorates cardiac fibrosis and improves cardiac function via the TGF-beta/Smad3 pathway. Am J Physiol Heart Circ Physiol 308 (5), H424–34. [DOI] [PubMed] [Google Scholar]
- 45.Mukherjee S et al. (2010) Effects of Longevinex (modified resveratrol) on cardioprotection and its mechanisms of action. Can J Physiol Pharmacol 88 (11), 1017–25. [DOI] [PubMed] [Google Scholar]
- 46.Frojdo S et al. (2007) Resveratrol is a class IA phosphoinositide 3-kinase inhibitor. Biochem J 406 (3), 511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Haes W et al. (2014) Metformin promotes lifespan through mitohormesis via the peroxiredoxin PRDX-2. Proc Natl Acad Sci U S A 111 (24), E2501–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin-Montalvo A et al. (2013) Metformin improves healthspan and lifespan in mice. Nat Commun 4, 2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turdi S et al. (2010) AMP-activated protein kinase deficiency exacerbates aging-induced myocardial contractile dysfunction. Aging Cell 9 (4), 592–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tzoulaki I et al. (2009) Risk of cardiovascular disease and all cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK general practice research database. BMJ 339, b4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schramm TK et al. (2011) Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: a nationwide study. Eur Heart J 32 (15), 1900–8. [DOI] [PubMed] [Google Scholar]
- 52.Lee YS et al. (2006) Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes 55 (8), 2256–64. [DOI] [PubMed] [Google Scholar]
- 53.Li MH et al. (2014) Berberine improves pressure overload-induced cardiac hypertrophy and dysfunction through enhanced autophagy. Eur J Pharmacol 728, 67–76. [DOI] [PubMed] [Google Scholar]
- 54.Zeng XH et al. (2003) Efficacy and safety of berberine for congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 92 (2), 173–6. [DOI] [PubMed] [Google Scholar]
- 55.Han X et al. (2016) AMPK activation protects cells from oxidative stress-induced senescence via autophagic flux restoration and intracellular NAD(+) elevation. Aging Cell 15 (3), 416–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Z et al. (2016) Berberine alleviates postoperative cognitive dysfunction by suppressing neuroinflammation in aged mice. Int Immunopharmacol 38, 426–33. [DOI] [PubMed] [Google Scholar]
- 57.Yu Y et al. (2018) Berberine Improves Cognitive Deficiency and Muscular Dysfunction via Activation of the AMPK/SIRT1/PGC-1a Pathway in Skeletal Muscle from Naturally Aging Rats. J Nutr Health Aging 22 (6), 710–717. [DOI] [PubMed] [Google Scholar]
- 58.Fang EF et al. (2017) NAD(+) in Aging: Molecular Mechanisms and Translational Implications. Trends Mol Med 23 (10), 899–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mitchell SJ et al. (2018) Nicotinamide Improves Aspects of Healthspan, but Not Lifespan, in Mice. Cell Metab 27 (3), 667–676 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eisenberg T et al. (2016) Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med 22 (12), 1428–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang H et al. (2017) Spermine and spermidine reversed age-related cardiac deterioration in rats. Oncotarget 8 (39), 64793–64808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwarz C et al. (2018) Safety and tolerability of spermidine supplementation in mice and older adults with subjective cognitive decline. Aging (Albany NY) 10 (1), 19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Madeo F et al. (2018) Spermidine in health and disease. Science 359 (6374). [DOI] [PubMed] [Google Scholar]
- 64.Zheng YH et al. (2012) Osteopontin stimulates autophagy via integrin/CD44 and p38 MAPK signaling pathways in vascular smooth muscle cells. J Cell Physiol 227 (1), 127–35. [DOI] [PubMed] [Google Scholar]
- 65.Salminen A et al. (2017) Regulation of longevity by FGF21: Interaction between energy metabolism and stress responses. Ageing Res Rev 37, 79–93. [DOI] [PubMed] [Google Scholar]
- 66.Rubinsztein DC et al. (2011) Autophagy and aging. Cell 146 (5), 682–95. [DOI] [PubMed] [Google Scholar]
- 67.Ryu D et al. (2016) Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat Med 22 (8), 879–88. [DOI] [PubMed] [Google Scholar]
- 68.Denzel MS et al. (2014) Hexosamine pathway metabolites enhance protein quality control and prolong life. Cell 156 (6), 1167–1178. [DOI] [PubMed] [Google Scholar]
- 69.Yang S et al. (2015) beta-Guanidinopropionic acid extends the lifespan of Drosophila melanogaster via an AMP-activated protein kinase-dependent increase in autophagy. Aging Cell 14 (6), 1024–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cai B et al. (2016) Long noncoding RNA H19 mediates melatonin inhibition of premature senescence of c-kit(+) cardiac progenitor cells by promoting miR-675. J Pineal Res 61 (1), 82–95. [DOI] [PubMed] [Google Scholar]
- 71.Santos-Parker JR et al. (2017) Curcumin supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging (Albany NY) 9 (1), 187–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chao J et al. (2017) Role of Kallistatin Treatment in Aging and Cancer by Modulating miR-34a and miR-21 Expression. Oxid Med Cell Longev 2017, 5025610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guo Y et al. (2017) Kallistatin reduces vascular senescence and aging by regulating microRNA-34a-SIRT1 pathway. Aging Cell 16 (4), 837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seals DR et al. (2018) Strategies for Optimal Cardiovascular Aging. Am J Physiol Heart Circ Physiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shinmura K et al. (2011) Impact of long-term caloric restriction on cardiac senescence: caloric restriction ameliorates cardiac diastolic dysfunction associated with aging. J Mol Cell Cardiol 50 (1), 117–27. [DOI] [PubMed] [Google Scholar]
- 76.Lapierre LR et al. (2011) Autophagy and lipid metabolism coordinately modulate life span in germline-less C. elegans. Curr Biol 21 (18), 1507–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Field AE and Adams PD (2017) Targeting chromatin aging - The epigenetic impact of longevity-associated interventions. Exp Gerontol 94, 29–33. [DOI] [PubMed] [Google Scholar]
- 78.Cuervo AM et al. (2005) Autophagy and aging: the importance of maintaining "clean" cells. Autophagy 1 (3), 131–40. [DOI] [PubMed] [Google Scholar]
- 79.Schafer MJ et al. (2017) Cellular senescence: Implications for metabolic disease. Mol Cell Endocrinol 455, 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kuma A et al. (2004) The role of autophagy during the early neonatal starvation period. Nature 432 (7020), 1032–6. [DOI] [PubMed] [Google Scholar]
- 81.Pyo JO et al. (2013) Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nat Commun 4, 2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin S et al. (2016) HSP27 Alleviates Cardiac Aging in Mice via a Mechanism Involving Antioxidation and Mitophagy Activation. Oxid Med Cell Longev 2016, 2586706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nojima A et al. (2013) Haploinsufficiency of akt1 prolongs the lifespan of mice. PLoS One 8 (7), e69178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu JJ et al. (2013) Increased mammalian lifespan and a segmental and tissue-specific slowing of aging after genetic reduction of mTOR expression. Cell Rep 4 (5), 913–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lamming DW et al. (2014) Depletion of Rictor, an essential protein component of mTORC2, decreases male lifespan. Aging Cell 13 (5), 911–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Um SH et al. (2004) Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 431 (7005), 200–5. [DOI] [PubMed] [Google Scholar]
- 87.Zhang HM et al. (2017) Moderate lifelong overexpression of tuberous sclerosis complex 1 (TSC1) improves health and survival in mice. Sci Rep 7 (1), 834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lesniewski LA et al. (2017) Dietary rapamycin supplementation reverses age-related vascular dysfunction and oxidative stress, while modulating nutrient-sensing, cell cycle, and senescence pathways. Aging Cell 16 (1), 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chiao YA et al. (2016) Rapamycin transiently induces mitochondrial remodeling to reprogram energy metabolism in old hearts. Aging (Albany NY) 8 (2), 314–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Graziotto JJ et al. (2012) Rapamycin activates autophagy in Hutchinson-Gilford progeria syndrome: implications for normal aging and age-dependent neurodegenerative disorders. Autophagy 8 (1), 147–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pollack RM et al. (2017) Resveratrol Improves Vascular Function and Mitochondrial Number but Not Glucose Metabolism in Older Adults. J Gerontol A Biol Sci Med Sci 72 (12), 1703–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chung JH et al. (2012) Resveratrol as a calorie restriction mimetic: therapeutic implications. Trends Cell Biol 22 (10), 546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morselli E et al. (2009) Autophagy mediates pharmacological lifespan extension by spermidine and resveratrol. Aging (Albany NY) 1 (12), 961–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Karnewar S et al. (2018) Metformin regulates mitochondrial biogenesis and senescence through AMPK mediated H3K79 methylation: Relevance in age-associated vascular dysfunction. Biochim Biophys Acta 1864 (4 Pt A), 1115–1128. [DOI] [PubMed] [Google Scholar]
- 95.Anisimov VN (2013) Metformin: do we finally have an anti-aging drug? Cell Cycle 12 (22), 3483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tarrago MG et al. (2018) A Potent and Specific CD38 Inhibitor Ameliorates Age-Related Metabolic Dysfunction by Reversing Tissue NAD(+) Decline. Cell Metab 27 (5), 1081–1095 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]