Abstract
Objectives:
To determine the interaction of age and habitual sleep duration in predicting cognitive performance in a large sample of participants aged 15 to 89 years.
Methods:
This study is a cross-sectional analysis of performance data gathered between January 2012 and September 2013. First-time players (N = 512823) of three internet cognitive training games measuring processing speed, working memory, visuospatial memory, and arithmetic participated in the study.
Results:
Performance was based on a measure of speed and accuracy for each game. The relationship between performance and self-reported habitual sleep duration was examined in the sample as a whole and across 10-year age groups starting at age 15 and ending at 75 and older. Performance peaked at 7 h of sleep duration for all three games in the sample as a whole, and the decrements in performance for sleep durations greater than 7 h were either comparable or greater in the youngest as compared to the oldest age groups.
Conclusions:
These findings challenge the hypothesis that deteriorating cognitive performance with long sleep duration is driven by medical comorbidities associated with aging. Further, these data are consistent with an optimal dose model of sleep and suggest that the model for the homeostatic recovery of cognitive function as a function of sleep duration should incorporate a curvilinear decline with longer duration sleep, indicating that there may be a cost to increased sleep. Replication and further research is essential for clarifying the sleep duration–cognition relationship in youth and adults of all ages.
Keywords: Sleep duration, cognitive performance, aging, adolescence.
Statement of Significance
While most studies are consistent in finding that short sleep durations are problematic, little research has focused on the effects of long sleep durations. The assumptions that long sleep durations are better for functioning and that associations detected between longer sleep durations and poor functioning found in some studies must be attributable to medical or other unmeasured confounders, are reflected in current recommendations regarding sleep duration. Recent clinical guidelines recommend that healthy adults obtain at least 7 hours of habitual sleep and that 9 or more hours may be appropriate in young adults. The current study focused on sleep duration and cognitive performance in a large internet-based sample of cognitive-training-game players aged 15–89. This study found a reliable peak in performance at 7 hours’ sleep duration in all 3 games examined in all but the oldest age categories, with deteriorations in performance beyond the peak equal or more steap in the youngest as compared to older age groups. These findings run counter to existing assumptions, especially with respect to younger individuals. Further research is necessary to further understand how sleep duration affects cognitive functioning across the lifespan.
INTRODUCTION
There is a growing interest in the role of sleep and sleep duration on cognitive functioning across the lifespan. With respect to younger age groups, research is motivated by evidence that chronic sleep deprivation is pervasive and associated with poorer school performance and increased risk-taking behavior.1–3 For example, two systematic reviews of research on sleep and functioning in school-aged children and/or adolescents provide convincing evidence that low sleep duration is associated with lower school performance and increases in risk-taking behavior, such as substance use.1,2 The threshold of sleep duration below which less sleep is associated with loss of performance has ranged across studies from 6 h,4 to approximately 7 h5,6; to as much as 8 h.7 Nonetheless, the sleep variables and the duration thresholds that define sleep duration as “low” vary across studies, making it difficult to compare findings and confidently conclude what sleep durations are problematic. In elderly populations, studies with fairly large sample sizes also indicate that inadequate or poor quality sleep contributes to cognitive dysfunction.8–10 Using validated measures of cognitive performance, such as the Delayed Word Recall Test (DWRT), digit span tasks, verbal fluency tests, and/or the Mini-Mental State Examination (MMSE), these studies demonstrated that scores were significantly lower in subjects reporting sleep durations of 3–4 h as compared to sleep durations of 7–8 h,8 ≤5 h as compared to 7 h,10 or <6 h as compared to 6–9 h.9 Xu and colleagues identified a significant trend in the sleep duration–cognitive performance relationship, demonstrating a dose–response relationship between sleep duration and improved performance from 3 to 7 h of sleep duration.8
Although there is sizeable evidence indicating that inadequate sleep contributes to cognitive deficits,1–7 less is known about the relationship of long sleep duration and cognitive performance across the lifespan. A growing body of research in older-age subjects indicates that habitual sleep duration in excess of 7 to 9 h may be associated with decreased cognitive performance.8,9,11–13 In one of the studies described above, Xu et al. identified an inverted U-shaped relationship between sleep duration and MMSE score which peaked at 7 h.8 While the change in score across sleep durations was small (mean MMSE exam scores ranged from 26.6 to 27.3 across sleep durations), the trend was highly statistically significant and controlled for a number of demographic and health factors that are often thought to contribute to both sleep duration and cognition. Despite this, the association between longer sleep duration and poorer functioning, including cognitive performance, has generally been attributed to unmeasured confounding factors such as medical comorbidities that independently degrade cognition, rather than an indication that excess sleep is independently detrimental to cognitive function.9 In contrast to research in older-age subjects, research on the topic of sleep duration and cognition in youth is focused primarily on the effects of sleep deprivation, and has generally concluded that children and adolescents would benefit from an increase in habitual sleep duration above 7 h for optimal functioning.3 The potential consequences of excess sleep have not been explored. Based on existing epidemiological research and a small body of experimental research on individuals aged 18–60 years, the most recent clinical guidelines recommend that healthy adults obtain at least 7 or more h of habitual sleep and that 9 or more h may be appropriate in young adults.14 It is generally recommended that adolescents sleep 8 h at the very least.3
To better understand the interaction of age and habitual sleep duration on predicting cognitive functioning, we analyzed data from three large samples of Lumosity brain game users on tasks of working memory, short-term visuospatial memory, and arithmetic, respectively. Although we expected age and education to be the strongest predictors of performance, as has been widely demonstrated in the literature,15–18 we hypothesized that (1) short sleep duration would be associated with worse task performance (in all age groups); (2) peak performance would occur at longer habitual sleep duration in younger subjects as compared to older subjects; and (3) performance would degrade more rapidly with sleep durations beyond the peak in older as compared to younger participants.
METHODS
We used data collected by the Lumosity brain game database between January 2012 and September 2013 for three games, Speed Match, Memory Matrix, and Raindrops, focused on working memory/processing speed, short-term visuospatial memory, and basic arithmetic, respectively. Lumosity is an online brain training program that consists of tasks invoking processing speed, memory, problem solving and other cognitive skills. First-time use of the platform does not require payment, but repeated use for the purposes of training requires a paid registration. Additional details on the Lumosity platform and tasks have been published elsewhere.19 Data were included for all players greater than or equal to 15 years of age at the time of play. All data were derived from a player’s first use of the game so as to eliminate practice effects. Only data for participants whose preferred language was English, and who used keyboards for desktops or laptops (as opposed to tablets, smartphones, and touchscreens), were included. Data for participants with scores of zero were excluded. Zero scores are clear outliers on each of the tasks and are likely to result from participants beginning a game but not responding to the questions. These excluded responses constituted 0.10% of the data for Speed Match, 0.01% for Memory Matrix, and 2.27% for Raindrops. After removing zero scores, we computed the standard deviation of the scores for each game (after log transformation to normalize the distributions) and further removed scores that were above or below 4 SD from the mean. These exceptionally long or short play durations are likely to result from participants starting a game but not actively playing. This resulted in an additional 1541 participants being dropped from Raindrops (0.66% of the sample) and 124 participants dropped from Speed Match (0.02% of sample). Play duration was not available for Memory Matrix. The total number of players in the analysis sample was 512823. Of these, 42.6% completed all three games, 44.5% completed two games, and 12.8% completed only one game.
To maximize the sample size for each game, data for each game were analyzed separately. Sample size for each game are as follows: Speed Match: N = 499273; Memory Matrix: N = 447665; and Raindrops: N = 231658.
Measures
Demographic Information
At time of registration on the Lumosity website, users entered basic demographic information, including date of birth, gender, and educational attainment. Age at time of each game play is calculated by the Lumosity software.
Sleep Duration
Information about habitual sleep duration was obtained at registration. Participants answered the question “How much sleep do you typically get each night?” by selecting a sleep duration in “number of hours” ranging from “less than 4 h,” then increasing integrally from 4 to 10 h, and ending with “more than 10 h” as the final option.
Speed Match
Speed Match is a simple test of working memory and processing speed and is a version of the 1-back test. Players are shown a variety of colored shapes in sequence and are required to indicate, using a keyboard stroke, whether each presented shape is the same as or different from the previously presented shape. Users are instructed to respond to as many trials as they can within the task’s duration, which is 45 s. n-Back tasks, among which the 1-back is the simplest, are used widely in clinical and research settings as a measure of working memory.20–23 Neuroimaging studies provide evidence for a consistent pattern of activation of frontal and parietal cortical regions by various versions of the n-back tasks,22 providing support that the 1-back task can effectively measure simple working memory despite slight variations in task stimuli and duration.
Memory Matrix
Memory Matrix is a measure of visuospatial working memory in which participants are briefly presented with a pattern of squares on a grid, which they must reproduce on an empty grid using keyboard strokes. Participants complete 12 grids, or trials, with the complexity increasing with each successful trial or decreasing with each failed trial according to a predefined algorithm. There is no time limit for completion of the task. This computer-based test is based on a validated measure of visual memory, the Visual Patterns Test (VPT) used in research and clinical settings.24,25 While the Lumosity version of the task is not the same as the official VPT, the task is designed according to rules that are considered important for visual working memory assessments, such as the use of patterns that cannot easily be coded verbally.25 Researchers studying visual working memory have frequently modified matrix-based visual working memory tasks based on specific study aims and study populations.24,26 They have the common feature of addressing the important question regarding how much can be remembered and are generally designed to be reliable and easy to use.24
Raindrops
Raindrops is a measure of calculations, in which participants are presented with a series of raindrops containing simple arithmetic problems at the top of their screen. Participants must enter numerical solutions using the keypad before each of the raindrops reaches the bottom of the screen. The concentration of raindrops on the screen and the complexity of the problems increase gradually over time (and therefore as a consequence of successful problem solving) according to a predefined algorithm, such that players must complete harder and harder problems at an increasingly fast pace to prevent game termination. The game ends once three raindrops have hit the ground. Raindrops is similar to other tasks used in neuroscience research on arithmetic.27,28 While it is not a formally validated measure of cognitive function, its straightforward design lends it face validity as a measure of speeded, simple mathematical problem solving.
Statistical Analysis
The game score for each task was used as a measure of performance. The Lumosity scoring algorithm awards increasing numbers of points for each correct response as performance level increases, as a means to enhance motivation. Consequently, the score distributions are approximately exponential, with extreme right-skew. In order to normalize the distributions and to linearize the scoring scales, all performance score data were natural-log transformed prior to analysis. Samples were categorized into 10-year age groups with 15- to 24-year olds at one extreme and >75-year olds at the other extreme to examine the relationship of performance to sleep duration across age categories. Analyses of performance by habitual sleep duration across age groups controlled for gender and education level. Because higher education contributed to performance, and because education is naturally confounded with age in a sample that includes young people who haven’t completed their education, we used the following method to adjust the log scores for education in each game: We noted that education levels were stable in age groups above 35, so we constructed regression models to predict performance scores from education level in the 35 and older participants only. We then used the regression coefficients to adjust log scores for education level for participants in all age groups. Thus scores for each participant in each age group, including the youngest group, were adjusted for estimated effects of education level derived from age groups above 35, in which age and education were not confounded.
We also noted a confound between age and gender, with women tending to be older (median age 34.1, interquartile range [IQR] 22.1–50.5) than men (median age 28.2, IQR 20.9–44.2). We therefore further adjusted the scores for gender by constructing regression models to predict education-adjusted scores from gender, age group, and gender by age group interactions, to allow for varying gender differences across age groups. We used the gender and gender by age group interaction parameters to adjust scores for gender within each age group. Education- and gender-adjusted log scores were then used for all subsequent analyses.
RESULTS
The demographic and sleep duration characteristics of the sample of participants playing at least one game are presented in Table 1. The sample was predominantly female (64%), and the mean age of the participant population was 35.8 (16.3) years. Because of the small size of the sample of participants between the ages of 85 and 89, these participants were combined with the 75- to 84-year age category for purposes of data analysis. Table 1 provides a profile of subsample size and sleep characteristics for the gender, age, and education level categories. The youngest age group comprised the largest age category, containing 35.8% of the sample and the oldest age group contained only 1% of sample, but nonetheless represents over 5000 participants. There was a broad range of education categories, with the median education being some college. The “other” education category represented only 4% of the sample and although little information is known about this sample’s educational attainment, the assumption that it represents individuals with less than a high school education is consistent with this group’s relatively low age- and gender-adjusted performance as compared to other groups. Age, gender, and education were very similarly distributed in the three games, with gender and education categories equal across games to the nearest 1 percentage point and mean age equal within 0.3 years.
Table 1.
Demographic and Sleep Characteristics of the Sample of Subjects With Scores on at Least One Game.
| n/% N = 512823 | Self-reported sleep duration M (SD) | |
|---|---|---|
| Gender | ||
| Male | 186577 (36.4) | 6.9 (1.4) |
| Female | 326245 (63.6) | 6.9 (1.4) |
| Age (years) | ||
| 15–24 | 183664 (35.8) | 7.2 (1.5) |
| 25–34 | 100988 (19.7) | 6.9 (1.4) |
| 35–44 | 71830 (14.0) | 6.7 (1.4) |
| 45–54 | 75819 (14.8) | 6.7 (1.3) |
| 55–64 | 54136 (10.6) | 6.8 (1.3) |
| 65–74 | 21263 (4.2) | 6.9 (1.3) |
| 75–89 | 5123 (1.0) | 7.0 (1.3) |
| Education level | ||
| Other | 20374 (4.0) | 7.0 (1.6) |
| Some HS | 35272 (6.9) | 6.9 (1.6) |
| HS diploma | 106430 (20.8) | 7.0 (1.5) |
| Some college | 125865 (24.5) | 6.8 (1.5) |
| Associate degree | 33118 (6.5) | 6.8 (1.4) |
| Bachelor’s degree | 101804 (19.9) | 6.9 (1.3) |
| Master’s degree | 46721 (9.1) | 7.0 (1.2) |
| PhD/Professional | 43239 (8.4) | 7.0 (1.3) |
Due to the large sample size, all pairwise comparisons of mean sleep duration for gender, age, and education level are statistically significant, even when effect sizes are negligible.
The mean subjective habitual sleep duration approximated 7 h of sleep for all demographic categories, with 15- to 24-year olds reporting a mean of 7.2 h and middle-aged individuals in the 35- to 44-year and 45- to 54-year categories demonstrating the shortest sleep duration (Table 1). A more detailed breakdown of the sample by age and sleep duration is provided in Supplementary Table 1.
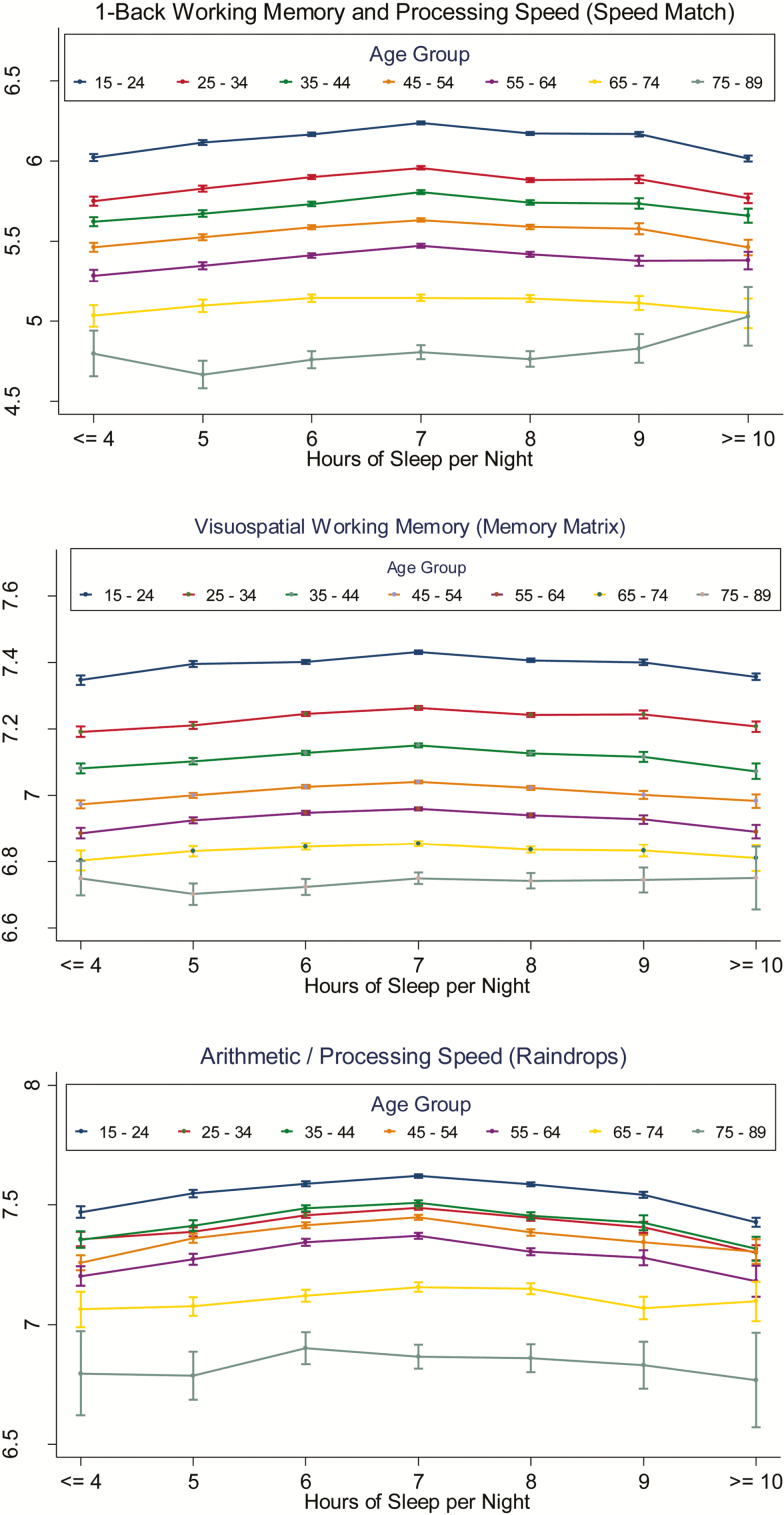
For the group as a whole, peak performance occurred at 7 h of sleep in all games, and the difference in performance at 7-h sleep duration was statistically significantly higher than performance at both 6-h and 8-h sleep duration. When the sample was broken down by 10-year age category, a similar pattern in the relationship between performance and sleep duration was found. Figure 1A–C depicts the relationship between task performance and sleep duration for all three brain games for all age categories. In addition to a predictable age-related decline in cognitive performance, these graphs reveal a consistent inverted u-shaped curve for the relationship between performance and sleep duration in younger and middle-aged age groups in all three games. Furthermore, peak performance occurred reliably at 7-h self-reported habitual sleep time for all three games for all age groups in which this inverted u-shaped relationship was present. This curve appears to flatten out in older age groups, such that the peak at 7 h is less discernible in 65- to 74-year olds and absent in 75- to 89-year olds.
Figure 1.
The three panels depict the relationship of mean-adjusted log score and self-reported habitual sleep duration by 10-year age category for working memory/processing speed (Speed Match; N = 499273), visuospatial working memory (Memory Matrix; N = 447665), and speeded arithmetic (Raindrops; N =231658). Due to small sample size in the 85- to 89-year-old category, this group was combined with the 75- to 84-year-old category. Error bars represent 95% confidence intervals.
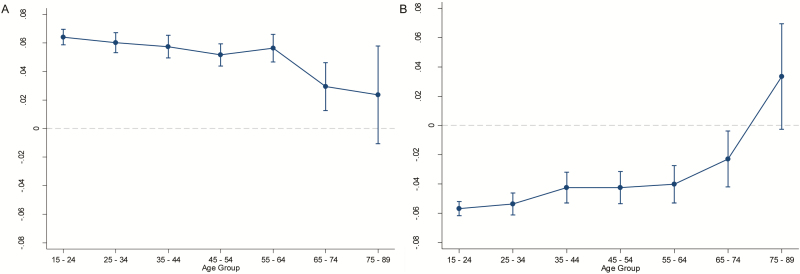
Given the consistency of the peak in performance at 7-h sleep duration in most age groups and the flattening of the curves in older age groups on visual inspection, we estimated linear slopes for change in performance for each hour change in sleep duration for both sides of the curve (i.e., between <4 and 7 h and between 7 h and >10 h), in order to determine whether rates of change in performance to and beyond the peak of 7-h sleep duration varied with age category in a statistically significant manner. Although performance in each age group is not perfectly linear with sleep duration on either side of the curve, the linear slope provides an approximate measure of steepness of the sleep–performance relationship that is more easily interpretable than a more complex nonlinear characterization. We modeled the flattening of the curves in terms of the sleep duration by age interaction in linear mixed models. Although results were subtle, we found evidence supporting a flattening of the slope with age on the left side of the curve (<4–7 h) as well as on the right side of the curve (7–10+ h). On the lower end of sleep durations (<4–7 h), analysis of linear trend for change in slope indicated less improvement in performance for each hour increase in habitual sleep duration up to 7 h in older age groups as compared to younger groups, for two of the three cognitive tasks. The flattening, with age, of the lower half of the curve observed in Figure 1 was statistically significant for Speed Match and Memory Matrix (p < .01) but not for Raindrops (p = .16). For sleep durations beyond 7 h, the flattening of the slope was significant only for Speed Match (p < .001). This indicates that there is a greater degradation in performance for each additional hour of sleep in younger people than in the oldest age groups for Speed Match, but not significantly in the other two tasks (p > .50). Figure 2A depicts the effect of each additional hour of sleep from <4 up to 7 h on performance in the Speed Match task. Figure 2B depicts the effect of each additional hour of sleep longer than 7 h. The slope coefficient in the oldest age group in Figure 2B appears positive, but the wide confidence interval overlaps with zero.
Figure 2.
Performance change per hour of increased sleep duration from <4 to 7 h (A) and for 7 h to 10+ h (B) for the working memory/processing speed task (Speed Match; N = 499273). In Panel A, more positive values indicate a greater gain in performance for each additional hour of sleep in the <4 to 7 h range. In Panel B, more negative values indicate a greater loss in performance for each additional hour of sleep in the 7–10+ h range. All age groups except the oldest demonstrated a statistically significant negative change in performance, while the oldest age group had a broad confidence interval that overlapped with a zero slope. Error bars represent 95% confidence intervals.
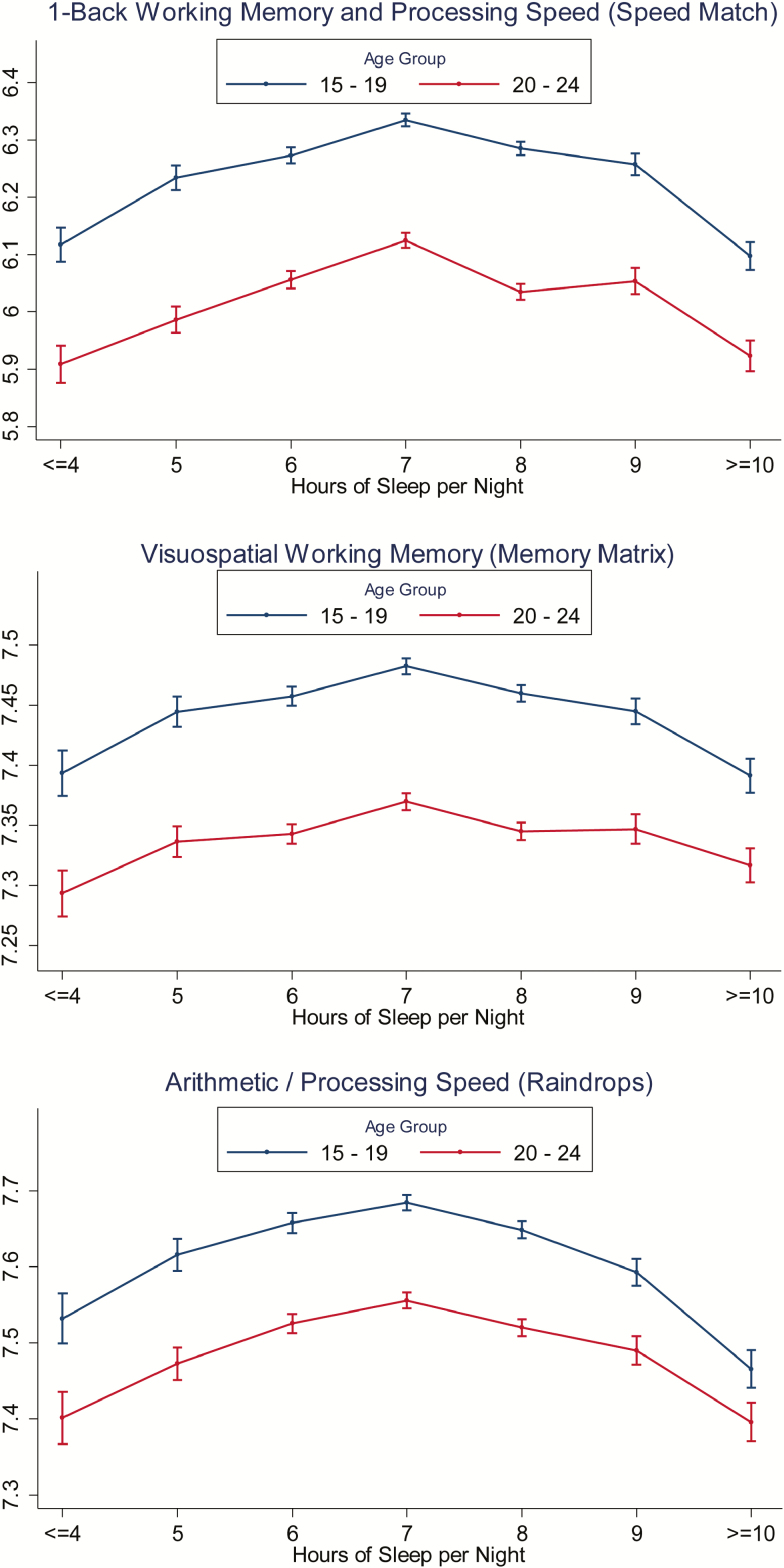
Given the unexpected finding that performance peaked at 7-h sleep duration, even in the youngest age group which was expected to require more sleep, we performed a post-hoc analysis to examine these relationships more closely in adolescents as compared to young adults, with the expectation that peak performance would be associated with a longer sleep duration at least in the adolescent age category. Figure 3 demonstrates that the difference in performance between 15- to 19-year olds and 20- to 24-year olds was comparable in magnitude to that found between 10-year age groups across the sample. With respect to the relationship with sleep, and contrary to expectations, 15- to 19-year olds also demonstrated peak performance at 7-h sleep duration, with a decline in performance beyond that peak comparable (Speed Match (p = .175)) or greater (Memory Matrix (p = .002) and Raindrops (p = .009)) than that seen in 20- to 24-year olds. For sleep durations between <4 and 7 h of sleep, the benefits of increasing number of hours of sleep were numerically, but not statistically, greater in the 15- to 19-year olds as compared to the 20- to 24-year olds (Speed Match (p = .866), Memory Matrix (p = .065), and Raindrops (p = .482)).
Figure 3.
The three panels depict the relationship of mean-adjusted log score and self-reported habitual sleep duration for 15- to 19-year olds (blue curve) and 20- to 24-year olds (red curve) for working memory/processing speed (Speed Match; N = 179031, including 96927 15- to 19-year olds and 82104 20- to 24-year olds), visuospatial working memory (Memory Matrix; N = 157618, including 84598 15- to 19-year olds and 73020 20- to 24-year olds), and speeded arithmetic (Raindrops; N = 82915, including 44092 15- to 19-year olds and 38823 20- to 24-year olds). Error bars represent 95% confidence intervals.
Analysis of gender-specific effects indicated a main effect for gender on game performance, with males performing modestly better than women on all three tasks, but no consistent age by gender by sleep interaction effects.
DISCUSSION
These present findings demonstrate a reliable peak in cognitive performance at 7 h of self-reported habitual sleep duration in the group as a whole and, when the sample is broken into age categories, in all except the oldest two age categories in all three cognitive tasks. While this effect is small, it is remarkably consistent across younger and middle-aged age groups and is reliably reproduced in different tasks.
For the group as a whole, these data support existing evidence that short sleep durations (most commonly defined in the literature as sleep durations falling below the 6- to 9-h range) are detrimental to cognitive functioning.8,9,29 While the detrimental effect of short sleep durations was not evident in the two oldest age groups, the smaller number of these older individuals at each sleep duration may mask any potential effects of sleep duration. Although various studies of sleep and cognitive functioning in older individuals do indicate worse performance at shorter habitual sleep duration,8,9 it is important to note that some studies did not find this effect, even when multiple potential confounding factors were taken into account.11–13 It is also important to note that most of the large-scale studies of sleep and performance on cognitive tasks have been conducted in middle-age and elderly subjects.30,31 Studies in youth tend to focus on the relationship of sleep duration and school performance, or other behaviors that may be indirect evidence of suboptimal cognitive functioning, such as risk-taking behaviors. Further research is therefore crucial for better understanding the effects of short sleep duration on various aspects of cognitive performance in all age groups.
Contrary to our hypothesis that peak performance would occur at higher sleep durations in younger as compared to older participants, these data clearly indicate that performance peaks at 7-h sleep duration for all age groups between 15 and 65 years. A peak is less discernible in the 65- to 75-year olds, and not at all evident in the 75- to 89-year olds. Several studies provide evidence that middle-aged and older individuals tend to over-report sleep duration as compared to objective measures of sleep,32–34 although factors that contribute to (e.g., age, gender, and cognitive status) or moderate such overestimations (e.g., objective sleep duration) differ across studies. Nonetheless, an overestimation would be consistent with evidence that total sleep time (measured objectively) decreases with age decreases with age35, and to a greater degree than we observed in our study. Given this possibility, it is plausible that the peaks of the sleep duration–performance curves (Figure 1) are artificially shifted to the right as individuals become older and that true peak performance occurs more solidly to the left of 7-h sleep duration. Such a shift would not, however, have a self-evident impact on the slope of the curve we saw, nor would it alter the unexpected shape of the sleep duration–performance curves in younger participants. There is limited research comparing subjective and objective sleep measures in adolescents,36,37 but we are not aware of literature suggesting that adolescents underestimate sleep time and that average sleep times and/or sleep times associated with peak performance are therefore biased toward being too low in our sample.
There is no research of which we are aware that indicates that teens or young adults might experience decrements in cognitive performance as a result of excess habitual sleep duration. The majority of previous research in youth has been focused on the detrimental consequences of sleep loss.1–3,30 In contrast, our findings are more consistent with an “optimal dose model” of sleep, as described by Marshall and colleagues, which proposes that a specified amount of sleep is required for optimal health and functioning and that more or less of it is detrimental.38 As was recently explained by a consensus panel of experts in sleep research and clinical sleep medicine, while a biological mechanism by which “excess” sleep could be detrimental has not been uncovered, this relationship would be consistent with many biological systems.14
The association we saw between long sleep durations and cognitive performance decline in young participants raises questions about the argument that the decline in performance associated with increasing habitual sleep duration is attributable to the confounding factors of increased age-related medical comorbidities. While several recent research studies of older adult samples have demonstrated associations between worsening cognitive performance and increasing sleep,8,9,11–13 the traditional argument has been that age-related medical comorbidities lead to both increased sleep need and worsening cognitive performance.9 If this were the case, one would expect the slope of the decline from peak performance at 7 h to longer sleep durations to be flatter in the younger age groups, in which medical comorbidities are presumably low, or at the very least lower than they are in the older age groups in our sample. Analyses of the linear slopes in the present dataset indicate the opposite; younger participants demonstrated a steeper decline in one task and indistinguishable decline in performance in the other tasks for each additional hour of sleep beyond 7 h.
Could there be other, unmeasured confounding factors that could affect our most surprising findings, which were that performance peaked at a mere 7-h sleep duration in the youngest groups and that performance declined reliably at longer sleep durations? It may be worth considering the possibility that intrinsic features of the cognitive task, such as emotional content, might moderate some of the relationships that we found. This would be different from subject-related features such as the age-related medical comorbidities that we have discussed previously. Adolescents are undergoing a period of profound social-emotional development, and more sleep may be required for emotional homeostasis during these years. Ample research has demonstrated that sleep is important for emotional processing and recalibration.39 School-related cognitive performance, and solid judgment in the context of teen driving and substance use, for example, which are the types of cognitive performance outcomes most often examined in large-scale research on sleep in youth, may be inextricably influenced by social-emotional pressures generated by peers, parents, and society. These may create moderating variables in the sleep–cognition relationship that may be absent in the context of internet-based activities (potentially devoid of such emotional loads). While this proposal is speculative, and while such a possibility does not undermine the findings described here with respect to emotionally neutral tasks, future research is essential to further understand this and other potential influences on the sleep duration and cognitive performance relationship in youth, and across the lifespan.
The current data may support the momentum to refocus research and public health attention to other problematic aspects of sleep in adolescents. For example, it may be the timing of sleep, which may be misaligned with the delayed chronotype that many adolescents temporarily develop, rather than the duration of sleep, that needs to be addressed in teens and young adults.3,40,41 In post-hoc analyses using a subset of scores for which time of game play was available, we found significant effects of time of play on performance for Speed Match and Raindrops (p values < .0001), but did not find time of play by sleep duration by age group effects on performance. Chronotype information was not available for this dataset. As much as possible, future analyses should take into account the timing of sleep, timing of task performance, and chronotype when examining sleep duration–cognition relationships.
The great strength of these findings is that they are derived from an unparalleled sample size and represent an impressive range of ages. This generates the statistical power to examine the relationship between cognitive performance and small changes in habitual sleep duration and identify a small, but precise, relationship between sleep duration and cognitive performance. The finding that peak performance consistently occurs at 7 h in all but the oldest two age groups raises intriguing questions about sleep homeostasis. Is 7 h of habitual sleep duration optimal for cognitive recovery and functioning? This peak at 7 h is consistent with recent studies demonstrating a similar curvilinear relationship between sleep duration and cognitive performance in older populations,8,19 but the consideration of its implications for sleep regulation has been limited by the assumption that confounding factors such as medical comorbidities must explain the decline in performance with increasing sleep duration. Future research may benefit from examining this more closely in young and middle-aged samples.
Because this is a cross-sectional study, a major question that this study cannot answer is what the 7-h sleep duration for peak performance implies for people at an individual level. Does this mean that all individuals would benefit by extending or reducing, in the case of long sleepers, their sleep duration to 7 h? This goes against the evidence that sleep need varies across individuals, and there is no one-size-fits-all for optimal sleep duration. On the other hand, it may be worth entertaining the possibility that a 7-h sleep need is a correlate, or marker, of optimal sleep regulatory health. That is, it is possible that individuals who need and achieve 7 h habitual sleep duration have the biological substrate to optimize the physiological recovery functions of sleep, including those related to cognitive functioning. Recent genetics research has identified genes that have independent effects on the timing and duration of sleep, but which may have coordinated roles with respect to achieving the physiological functions of sleep.42,43 There may be a genetic make-up, then, that promotes optimal cognitive functioning through independent and interacting effects on circadian rhythmicity, sleep duration, and the cognitive recovery functions of sleep and that is reflected in a 7-h sleep duration. Some researchers have also proposed that efficient sleep may reflect an all-around neural efficiency that may be reflected in cognitive performance.44 Future analyses using repeated measures of sleep and cognitive performance, with sophisticated approaches to controlling for practice effects, will be crucial in addressing the implications of these findings for understanding the relationship of acute and chronic sleep duration with cognitive function.
Despite the many strengths of this dataset, it is important to acknowledge several limitations. Due to protections to privacy for participants who engaged in brain games, factors such as health status could not be considered in the present analyses. Future research should take advantage of the large-scale recruitment potential afforded by the internet and include demographic, health, and lifestyle information to maximize the ability to control for a range of factors that impact cognitive performance. Nonetheless, it is unlikely that the decline in performance with increasing sleep duration is explained by health factors alone, as has been suggested, simply because this relationship is strong, if not stronger, in the younger age groups where health problems would be expected to be lower than in older age groups. Additionally, other reports that demonstrated a similar relationship between increasing sleep duration and worsening cognitive performance controlled for some of these factors (e.g., depression and Alzheimer’s disease) and still found significant results.8,13 It is possible, however, that older brain game users may be healthier in many respects than their non-internet using counterparts.45
An obvious limitation of the data is that information on habitual sleep duration is based on a one-item self-report measure. Although this is not ideal, single-question assessments of sleep duration have been used in most epidemiologic studies of sleep and health outcomes and more objective measures of sleep are prohibitive on a large scale.46–50 All the studies we cite examining the sleep duration–cognitive task performance relationship use single-question measures of sleep duration, although some studies explicitly incorporate nap time into the question regarding sleep duration,10,11 while others do not.8,9,12,13 Although naptime is a feature that may distinguish sleep patterns in the elderly people from that in other age groups, this difference does not explain the long sleep duration effects on cognitive performance in the above-mentioned studies on elderly participants. Hopefully, future studies will be able to parse the role of naps out further. Additionally, because teens and young adults may be particularly prone to social jetlag, which is marked by short weekday sleep durations accompanied with longer weekend “recovery” sleep, it is possible that the young participants’ self-reported sleep duration underestimates average sleep duration. Such an underestimate could artificially shift the curve to the left in younger age groups. When possible, future studies should consider whether self-reported sleep duration and task performance information was obtained on weekdays or weekends and/or ensure that estimates include all 7 days of the week.
With respect to the measures of cognitive performance, the Lumosity games are based on standardized cognitive tests (Speed Match and Memory Matrix) and basic arithmetic (Raindrops). They are also designed to be visually appealing and engaging to an internet audience. While it would be preferable to use tasks that have been validated against standardized measures of processing speed, working memory, visuospatial memory, and arithmetic skill, the scientific research demonstrates that n-back tests and grid-based visuospatial memory tasks are frequently modified across studies but that they generally function consistently in assessing the main domains of interest. Furthermore, the game scores vary as would be expected as a function of education and age, the largest predictors of cognitive performance, which increases our confidence in the reliability of our findings. While the scores are generated using an algorithm resulting in a nonlinear increase in score with higher performance, our findings were consistent with findings previously reported for a similar dataset using simpler outcomes such as number correct or memory thresholds.19 We chose the overall score as the outcome because of its consistency with previously reported findings and because it reflects a more global measure of performance encompassing both speed and accuracy of performance. Finally, because performance on all these tasks requires the ability to manipulate a computer keyboard, it is important to consider the degree to which performance reflects motor speed and coordination, all factors which are known to decline with age.51 Regardless, the impact of sleep on these functions would also be evidence of sleep’s effect on neural function, albeit of a different type. Additionally, it may be important to note that these tasks can be considered to be mild (Speed Match and Memory Matrix) to moderate (Raindrops) in complexity and that the sleep duration–performance relationships may have behaved differently with more complex tasks. It will be important for future research to determine whether task complexity moderates sleep duration–performance relationships and the impact of age on these relationships. Finally, while elderly individuals are increasingly savvy with the use of computers and the internet, it is possible that decreased skill and experience with computers, and computer- and internet-based tasks, resulted in an artificial downward shift in cognitive performance with increasing age. While very possible, this would not create a shift in peak cognitive performance relative to reported sleep duration.
In conclusion, our findings indicate a subtle but reliable inverted u-shaped relationship between self-reported habitual sleep duration and cognitive performance in a large sample of internet brain game users. These findings shift the focus of the role of sleep duration in predicting performance from older to younger age groups. Given the current concerns about sleep duration in younger people, further research is crucial to better understand sleep timing and duration effects in this age group. The internet may be a viable and effective strategy for studying this population. Further research is also crucial to examine mechanisms whereby increasing sleep may have negative effects on outcome.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at SLEEP online.
FUNDING
Work on this project was funded in part by grants to Dr. Richards from the U.S. Department of Veteran Affairs (1IK2CX000871-01A2) and the U.S. Department of Defense (W81XWH-16-1-0259).
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Neylan has consulted for Genentech, and received study medication from Actelion for a study funded by the Department of Defense, Glaxo-Smith Kline for a study funded by the Department of Veterans Affairs. All other authors have reported no financial conflicts of interest.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge Lumosity, Inc, including Daniel Sternberg, Director of Data Science, for the provision of the data to the investigators free of charge and with no restrictions on how the data were analyzed or interpreted.
REFERENCES
- 1. Shochat T, Cohen-Zion M, Tzischinsky O. Functional consequences of inadequate sleep in adolescents: a systematic review. Sleep Med Rev. 2014; 18(1): 75–87. [DOI] [PubMed] [Google Scholar]
- 2. Dewald JF, Meijer AM, Oort FJ, Kerkhof GA, Bogels SM. The influence of sleep quality, sleep duration and sleepiness on school performance in children and adolescents: a meta-analytic review. Sleep Med Rev. 2010; 14(3): 179–189. [DOI] [PubMed] [Google Scholar]
- 3. Owens J; Adolescent Sleep Working Group, Committee on Adolescence Insufficient sleep in adolescents and young adults: an update on causes and consequences. Pediatrics. 2014; 134(3):e921–e932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roberts RE, Roberts CR, Duong HT. Sleepless in adolescence: prospective data on sleep deprivation, health and functioning. J Adolesc. 2009; 32(5): 1045–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998; 69(41): 875–887. [PubMed] [Google Scholar]
- 6. Chung KF, Cheung MM. Sleep-wake patterns and sleep disturbance among Hong Kong Chinese adolescents. Sleep. 2008; 31(2): 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McKnight-Eily LR, Eaton DK, et al. Relationships between hours of sleep and health-risk behaviors in US adolescent students. Prev Med. 2011; 53(4-5): 271–273. [DOI] [PubMed] [Google Scholar]
- 8. Xu L, Jiang CQ, Lam TH, et al. Short or long sleep duration is associated with memory impairment in older Chinese: the Guangzhou Biobank Cohort Study. Sleep. 2011; 34(5): 575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gildner TE, Liebert MA, Kowal P, Chatterji S, Snodgrass JJ. Associations between sleep duration, sleep quality, and cognitive test performance among older adults from six middle income countries: results from the Study on Global Ageing and Adult Health (SAGE). J Clin Sleep Med. 2014; 10: 613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu L, Jiang CQ, Lam TH, et al. Sleep duration and memory in the elderly Chinese: longitudinal analysis of the Guangzhou Biobank Cohort Study. Sleep. 2014; 37(11): 1737–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Faubel R, Lopez-Garcia E, Guallar-Castillon P, Graciani A, Banegas JR, Rodriguez-Artalejo F. Usual sleep duration and cognitive function in older adults in Spain. J Sleep Res. 2009; 18(4): 427–435. [DOI] [PubMed] [Google Scholar]
- 12. Ramos AR, Dong C, Elkind MS, et al. Association between sleep duration and the mini-mental score: the Northern Manhattan study. J Clin Sleep Med. 2013; 9(7): 669–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schmutte T, Harris S, Levin R, Zweig R, Katz M, Lipton R. The relation between cognitive functioning and self-reported sleep complaints in nondemented older adults: results from the Bronx aging study. Behav Sleep Med. 2007; 5: 39–56. [DOI] [PubMed] [Google Scholar]
- 14. Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American academy of sleep medicine and sleep research society. Sleep. 2015; 38: 843–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 2002; 17(2): 299–320. [PubMed] [Google Scholar]
- 16. Barnes DE, Tager IB, Satariano WA, Yaffe K. The relationship between literacy and cognition in well-educated elders. J Gerontol A Biol Sci Med Sci. 2004; 59(4): 390–395. [DOI] [PubMed] [Google Scholar]
- 17. Heaton R, Ryan L, Grant I, Matthews CG. Demographic influences on neuropsychological test performance. In: Grant IAK, ed. Neuropsychological Assessment of Neuropsychiatric Disorders. New York: Oxford University Press; 1996: 141–163. [Google Scholar]
- 18. Salthouse TA. Selective review of cognitive aging. J Int Neuropsychol Soc. 2010; 16(5): 754–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sternberg DA, Ballard K, Hardy JL, Katz B, Doraiswamy PM, Scanlon M. The largest human cognitive performance dataset reveals insights into the effects of lifestyle factors and aging. Front Hum Neurosci. 2013; 7: 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wilhelm O, Hildebrandt A, Oberauer K. What is working memory capacity, and how can we measure it? Front Psychol. 2013; 4: 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kirchner WK. Age differences in short-term retention of rapidly changing information. J Exp Psychol. 1958; 55(4): 352–358. [DOI] [PubMed] [Google Scholar]
- 22. Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005; 25(1): 46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cansino S, Hernández-Ramos E, Estrada-Manilla C, et al. The decline of verbal and visuospatial working memory across the adult life span. Age (Dordr). 2013; 35: 2283–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilson JTL, Scott JH, Power KG. Developmental differences in the span of visual memory for pattern. Brit J Dev Psychol. 1987; 5: 249–255. [Google Scholar]
- 25. Della Sala S, Gray C, Baddeley A, Wilson L. The Visual Patterns Test: A New Test of Short-Term Visual Recall. Feltham, Suffolk: Thames Valley Test Company; 1997. [Google Scholar]
- 26. Ichikawa S. Measurement of visual memory span by means of the recall of dot-in-matrix patterns. Behav Res Methods Instrument. 1982; 14: 309–313. [Google Scholar]
- 27. Pauli P, McClelland JL, Seidenberg MS, Patterson KE. Extensive practice in mental arithmetic and practice transfer over a ten-month retention interval Math Cogn. 1998; 4: 21–46. [Google Scholar]
- 28. Delazer M, Domahs F, Bartha L, et al. Learning complex arithmetic—an fMRI study. Brain Res Cogn Brain Res. 2003; 18(1): 76–88. [DOI] [PubMed] [Google Scholar]
- 29. Tworoger SS, Lee S, Schernhammer ES, Grodstein F. The association of self-reported sleep duration, difficulty sleeping, and snoring with cognitive function in older women. Alzheimer Dis Assoc Disord. 2006; 20: 41–8. [DOI] [PubMed] [Google Scholar]
- 30. Benitez A, Gunstad J. Poor sleep quality diminishes cognitive functioning independent of depression and anxiety in healthy young adults. Clin Neuropsychol. 2012; 26(2): 214–23. [DOI] [PubMed] [Google Scholar]
- 31. Scullin MK, Bliwise DL. Sleep, cognition, and normal aging: integrating a half century of multidisciplinary research. Perspect Psychol Sci. 2015; 10(1): 97–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Silva GE, Goodwin JL, Sherrill DL, et al. Relationship between reported and measured sleep times: the sleep heart health study (SHHS). J Clin Sleep Med. 2007; 3: 622–630. [PMC free article] [PubMed] [Google Scholar]
- 33. Van Den Berg JF, Van Rooij FJ, Vos H, et al. Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. J Sleep Res. 2008; 17: 295–302. [DOI] [PubMed] [Google Scholar]
- 34. Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008; 19(6): 838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004; 27: 1255–1273. [DOI] [PubMed] [Google Scholar]
- 36. Wolfson AR, Carskadon MA, Acebo C, et al. Evidence for the validity of a sleep habits survey for adolescents. Sleep. 2003; 26(2): 213–216. [DOI] [PubMed] [Google Scholar]
- 37. Short MA, Gradisar M, Lack LC, Wright HR, Chatburn A. Estimating adolescent sleep patterns: parent reports versus adolescent self-report surveys, sleep diaries, and actigraphy. Nat Sci Sleep. 2013; 5:23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marshall NS, Glozier N, Grunstein RR. Is sleep duration related to obesity? A critical review of the epidemiological evidence. Sleep Med Rev. 2008; 12(4): 289–98. [DOI] [PubMed] [Google Scholar]
- 39. Goldstein AN, Walker MP. The role of sleep in emotional brain function. Annu Rev Clin Psychol. 2014; 10: 679–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carskadon MA, Acebo C, Jenni OG. Regulation of adolescent sleep: implications for behavior. Ann NY Acad Sci. 2004; 1021: 276–291. [DOI] [PubMed] [Google Scholar]
- 41. Colrain IM, Baker FC. Changes in sleep as a function of adolescent development. Neuropsychol Rev. 2011; 21(1): 5–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hallows WC, Ptáček LJ, Fu YH. Solving the mystery of human sleep schedules one mutation at a time. Crit Rev Biochem Mol Biol. 2013; 48: 465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kurien PA, Chong SY, Ptáček LJ, Fu YH. Sick and tired: how molecular regulators of human sleep schedules and duration impact immune function. Curr Opin Neurobiol. 2013; 23: 873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Geiger A, Achermann P, Jenni OG. Association between sleep duration and intelligence scores in healthy children. Dev Psychol. 2010; 46: 949–954. [DOI] [PubMed] [Google Scholar]
- 45. Xavier AJ, d’Orsi E, Wardle J, Demakakos P, Smith SG, von Wagner C. Internet use and cancer-preventive behaviors in older adults: findings from a longitudinal cohort study. Cancer Epidemiol Biomarkers Prev. 2013; 22: 2066–2074. [DOI] [PubMed] [Google Scholar]
- 46. Kurina LM, McClintock MK, Chen JH, Waite LJ, Thisted RA, Lauderdale DS. Sleep duration and all-cause mortality: a critical review of measurement and associations. Ann Epidemiol. 2013; 23(4): 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ford ES, Cunningham TJ, Croft JB. Trends in self-reported sleep duration among US adults from 1985 to 2012. Sleep. 2015; 38(5): 829–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Krueger PM, Friedman EM. Sleep duration in the United States: a cross-sectional population-based study. Am J Epidemiol. 2009; 169(9): 1052–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Maslowsky J, Ozer EJ. Developmental trends in sleep duration in adolescence and young adulthood: evidence from a national United States sample. J Adolesc Health. 2014; 54: 691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fang SC, Subramanian SV, Piccolo R, et al. Geographic variations in sleep duration: a multilevel analysis from the Boston Area Community Health (BACH) Survey. J Epidemiol Community Health. 2015; 69(1): 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve. 2002; 25(1): 17–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.