Abstract
Sleep–wake disturbances following traumatic brain injury (TBI) are increasingly recognized as a serious consequence following injury and as a barrier to recovery. Injury-induced sleep–wake disturbances can persist for years, often impairing quality of life. Recently, there has been a nearly exponential increase in the number of primary research articles published on the pathophysiology and mechanisms underlying sleep–wake disturbances after TBI, both in animal models and in humans, including in the pediatric population. In this review, we summarize over 200 articles on the topic, most of which were identified objectively using reproducible online search terms in PubMed. Although these studies differ in terms of methodology and detailed outcomes; overall, recent research describes a common phenotype of excessive daytime sleepiness, nighttime sleep fragmentation, insomnia, and electroencephalography spectral changes after TBI. Given the heterogeneity of the human disease phenotype, rigorous translation of animal models to the human condition is critical to our understanding of the mechanisms and of the temporal course of sleep–wake disturbances after injury. Arguably, this is most effectively accomplished when animal and human studies are performed by the same or collaborating research programs. Given the number of symptoms associated with TBI that are intimately related to, or directly stem from sleep dysfunction, sleep–wake disorders represent an important area in which mechanistic-based therapies may substantially impact recovery after TBI.
Keywords: TBI, sleep, EEG, animal models, pediatric, orexin, glutamate, BCAA
INTRODUCTION
Traumatic brain injury (TBI), defined as an alteration in brain function or other brain pathology caused by an external force, is a common injury and results in 2.5 million emergency room visits annually.1,2 Even in its mildest form (generally known as “concussion”), individuals with TBI can suffer from persistent sequelae that prevent the return to normal physical, cognitive, and emotional functioning—all of which are important components of overall recovery. Sleep–wake disturbances are among the most prevalent and persistent symptoms following TBI.3–5 Sleep–wake disturbances have been reported in ~30–70% of individuals with mild, moderate, or severe TBI up to 3 years postinjury.6–22 Furthermore, disrupted sleep contributes to several other complications, including memory and cognitive complaints, chronic pain, and psychological distress.23–28
Although sleep–wake disturbances after TBI have long been recognized in humans, the underlying neurologic mechanism(s) have yet to be clearly established. Only recently have studies utilized animal models of TBI that are capable of providing mechanistic insight into these sleep–wake disturbances. Thus, this review begins with a brief introduction to the epidemiology of sleep–wake disturbances in humans after TBI. We follow with a summary of the clinically relevant sleep–wake disturbances observed in humans after TBI and then present a working overview of the pathophysiology of these sleep–wake disturbances. This human clinical and pathophysiological background is then followed by a synthesis of the current literature on sleep–wake disturbances in animal models of TBI. Finally, we discuss the relationship between sleep and recovery from TBI as well as treatment options for sleep–wake disturbances after TBI. For additional in-depth discussion of relevant research concerning sleep–wake disturbances in humans after TBI, we refer the reader to previous reviews on the topic.29–41
This review presents a scoping overview of relevant literature to date, as an alternative to a strictly systematic approach. The advanced search function in PubMed was used to identify 243 publications with “TBI” and “Sleep” in the title and/or abstract as potentially relevant literature to include in this review. Studies from this literature search were excluded (n = 95 in total) on the basis of being unrelated (n = 41), or only peripherally related to sleep–wake disturbances following TBI (e.g., studies with a primary focus of neuropsychiatric and behavioral conditions (n = 18), surgical approaches and treatments (n = 10), cognitive function/memory (n = 6), headache (n = 4), rehabilitation (n = 4), epilepsy/seizure management (n = 3), nutrition and metabolic function (n = 3), posttraumatic stress disorder (n = 2), relationship with spinal cord injury (n = 2), and editorials/commentaries (n = 2). In addition, literature outside this search was included where appropriate, usually identified from references from primary literature identified by the initial PubMed search. Of the 243 identified publications on this topic, 181 of them were published between 2010 and the present time, indicating a nearly exponential rise in the number of manuscripts published on this emerging and important topic.
EPIDEMIOLOGY
TBI is typically classified using the Glasgow Coma Scale (GCS)42 as mild (GCS 13–15), moderate (GCS 9–12), and severe (GCS ≤8). While widely used since the 1970s, this scale has well-documented limitations for classifying TBI severity.43,44 Clinically, we know that individuals who have abnormal computed tomography scans with intracranial hemorrhage are at risk of hemorrhage expansion and/or neurological deterioration regardless of admission GCS score.45 Based on the World Health Organization criteria published in 2004, individuals with head trauma are classified as “mild TBI” if these lesions are nonoperable and GCS ≥13, although some authors and clinicians would consider these individuals to have a “moderate” injury that warrants a higher level of clinical monitoring in the hospital and often in an intensive care setting.46,47 Mild TBI, on the other hand, has been used interchangeably with term “concussion” to imply an innocuous and self-limited injury, although we now understand that these injuries too can lead to prolonged symptoms and sequelae.48
Studies indicate that 30–70% of TBI survivors across the entire injury spectrum experience disordered sleep after injury,6–22 a finding unique to traumatic cerebral injury, as traumatic spinal cord injuries do not cause similar sleep–wake disturbances.49 Numerous studies have demonstrated that the severity of the inciting head injury does not predict the degree of sleep–wake disturbances;6 individuals with traumatic brain insults of all severities are at risk. Reported rates vary widely due to methodological variances in many studies, including sample bias (i.e., samples referred to sleep laboratories), varied assessment methods (i.e., subjective self-report versus objective polysomnography or actigraphy), and poor recognition or underreporting by patients and clinicians. In a population-based study of 346 patients with mild TBI (concussion) in New Zealand, 40% of patients experienced sleep difficulties 1 year after injury, more than 3 times the incidence seen in the general population.50 Female gender, poor preinjury sleep quality, and symptoms of poor sleep/cognition within 2 weeks after injury were predictive of persistent sleep disturbance. In patients with moderate–severe TBI evaluated during acute rehabilitation, 66% had sleep–wake disturbances (evaluated using the Delirium Rating Scale-Revised-98) at 1-month postinjury.51 The severity of sleep–wake disturbances in this population predicted hospital length of stay and the duration of posttraumatic amnesia, even after controlling for injury severity.51 Baumann et al. studied 65 patients who required hospital admission with their first-ever moderate–severe TBI and found that more than 70% had sleep–wake disturbances in the first 6 months after TBI.6 In 51 of those patients who were available for follow-up 3 years later, less than 20% felt that their sleep symptoms had improved.7 Similarly, Imbach and Büchele et al. reported sleep–wake disturbances persisting for 18 months postinjury.52 A recent meta-analysis of 21 studies with 1706 participants across the spectrum of TBI severity showed a 50% prevalence of sleep–wake disturbances in TBI survivors, a number much higher than that seen in the general population.9 Taken together, these data suggest that sleep–wake disturbances are common, can persist, and impact recovery regardless of TBI severity.
SLEEP–WAKE DISTURBANCES IN HUMANS AFTER TBI
TBI is strongly associated with several clinically recognized sleep–wake disturbances. The most common sleep–wake disturbances are insomnia and hypersomnia/pleiosomnia, followed by sleep-related breathing disorder, circadian rhythm disorder, and parasomnia/movement disorders. Due to symptomatic overlap and high prevalence, we also summarize reports of fatigue after TBI.
Traditional methods of assessing for sleep–wake disturbances in individuals with TBI include polysomnography (PSG) and multiple sleep latency/maintenance of wakefulness testing. PSG and multiple sleep latency/maintenance of wakefulness testing are based upon scalp electroencephalogram (EEG) and therefore, beyond their use in clinical sleep staging, may have limited capabilities to capture functional neurobiological changes, especially in the subcortical regions that potentially contribute to sleep–wake disturbances. However, as we describe subsequently, promising quantitative EEG (qEEG) approaches may reveal additional insights into brain function beyond conventional sleep staging. There may also be a future role for novel neuroimaging techniques such as magnetic resonance spectroscopy for neurotransmitter content (e.g., glutamate and gamma-aminobutyric acid; GABA) in the cortex that might elucidate mechanisms underlying sleep–wake disturbances in TBI.53
Insomnia
Insomnia is characterized by difficulty in initiating and/or maintaining sleep. Symptoms include difficulty falling asleep, sleep fragmentation, and/or early morning awakenings, resulting in associated daytime sensations of fatigue, sleepiness, and mood/performance deficits.54 Insomnia in the context of TBI55 is reported in 30–60% of individuals following TBI of all severities,56 although insomnia may be more common following mild injuries.8,50 Repetitive traumatic head injuries may increase the risk of developing insomnia. Among military personal, rates of self-reported insomnia were 6% in those with no history of TBI, 20% after a single TBI, and 50% in those who had experienced multiple TBIs.57 Because insomnia is generally diagnosed using structured interviews and/or questionnaires, some caution is advised in interpreting these studies, as individuals have a tendency to either over- or underreport symptoms compared to findings from objective PSG studies.58
Excessive Daytime Sleepiness
Excessive daytime sleepiness (EDS) is characterized by daily or near-daily episodes of irrepressible need to sleep or unintentional lapses into sleep at potentially inappropriate times. In contrast to fatigue, which more commonly manifests during physical exertion, EDS is characterized by sleepiness during sedentary activities. While insomnia tends to be overreported by patients after head injury,58 EDS may actually be underreported. Imbach et al. found that 57% of TBI survivors had objective evidence of EDS as determined by multiple sleep latency testing, considerably higher than the prevalence of EDS in a control population without brain injury (<20%).59 Survivors, but not controls, markedly underestimated their sleepiness when subjective self-reports were employed.59 Similarly, in a rehabilitation setting, 50% of individuals with moderate to severe TBI were found to have objective evidence of EDS, but these difficulties were not detected on self-report questionnaires.60
Pleiosomnia
Following TBI, individuals may exhibit pleiosomnia, that is, an increased need for sleep.61 In fact, actigraphy monitoring 6 months after TBI showed that individuals with TBI required 1–2 hours more sleep/24 hours over a 2-week monitoring period compared to healthy controls.59 In this cohort, pleiosomnia positively correlated with TBI severity and was exacerbated in individuals who had intracranial hemorrhage. In a prospective electrophysiological study of 65 patients studied 6 months after TBI, 22% reported that they needed two more hours of sleep in a 24-hour period than prior to their injury.6 In both of these studies, similar to EDS symptoms, patients with TBI also underestimated pleiosomnia on self-report questionnaires compared to objective measures.6,59
Sleep-Related Breathing Disorders
Obstructive sleep apnea and central sleep apnea are reported more frequently in individuals after TBI than in the general population. Several small studies have reported a prevalence of 25–35% of obstructive sleep apnea following TBI of any severity, which is higher than most general population studies.5,62–64
Circadian Rhythm Sleep Disturbances
Circadian rhythm sleep disturbances, most commonly identified by delayed sleep phase syndrome and irregular sleep–wake pattern, may be easily overlooked following TBI. Complaints of difficulty falling asleep, staying asleep, and difficulty awakening at standard wake times may be misconstrued as insomnia rather than circadian rhythm sleep disturbances. For example, in a previous study by Ayalon et al. 15 (36%) of 42 patients with mild TBI who had insomnia, in fact, had a circadian rhythm sleep disturbance (either delayed sleep phase syndrome or irregular sleep–wake pattern).65 This distinction is important, as treatment approaches differ depending on whether the patient has insomnia versus a circadian rhythm sleep disturbance. In an observational study of 23 patients with TBI roughly 1-year postinjury by Shekleton et al., evening salivary melatonin production was reduced and was associated with decreased sleep efficiency, increase wake after sleep onset, and higher rates of anxiety and depression.66 Circadian rhythm sleep disturbances may be especially prominent in the acute phase after TBI. Duclos et al. studied 16 patients with moderate–severe TBI in the first 10 days after injury and found severe fragmentation of the rest–activity cycle using actigraphy recordings.3 Further studies are needed, as the confounding effects of acute hospitalization, pain, and anxiety complicate interpretation of these results.
Abnormal Movements and Behaviors During Sleep
Abnormal movements and behaviors during sleep have also been reported. In a study of adolescents with a history of chronic mild head injury, parasomnias were reported by 42% of head injured patients (compared to 19% of controls). Adolescents reported increased rates of sleep enuresis (involuntary urination; 21% versus 0%) and sleep bruxism (teeth grinding; 42% versus 6%) in head-injured subjects compared to noninjured control subjects.67 In this population, there was also an increased fear of falling asleep, fearful awakenings, and frightening dreams, which were not seen in control subjects. In a similar adolescent cohort followed after mild head injury (0.5–6 years from injury), there were more complaints of poor sleep and bruxism, although there were no group differences in total sleep time, bedtimes, or wakeup times.68
In 60 adult patients with chronic TBI (3 months–2 years from injury), 25% presented with parasomnia as their primary sleep complaint, the most frequent of which was REM sleep behavior disorder (RBD).17 While RBD has not been traditionally associated with TBI in clinical practice, the literature on this association is scant and has not been systematically examined. As RBD is widely considered to be a harbinger of synucleinopathy-related disorders such as Parkinson’s disease, and TBI increases lifetime risk of Parkinson’s disease, there may be a pathophysiological link between TBI and RBD, although this is still yet to be defined.
There is also emerging evidence that trauma exposure (e.g., TBI and/or post-traumatic stress disorder) is associated with a greater risk of dream enactment and disruptive nocturnal behaviors along with increased electromyographic (EMG) tone during both REM and NREM sleep, especially among the military population.69 While trauma-associated sleep disorder shares a number of features with RBD, it may encompass a separate nosologically defined parasomnia. It is not known whether trauma-associated sleep disorder carries an increased risk of neurodegeneration, although a recent report may indicate that the isolated finding of REM sleep without atonia carries an increased risk of synuclein-associated neurodegeneration.70
Fatigue
General physical and mental fatigue during the waking hours has long been recognized as a common and debilitating complication following TBI,71–73 that undoubtedly contributes to subjective measures of sleepiness, yet appears to be independent of pain, depression, and disrupted sleep.74,75 Not surprisingly, fatigue has been shown to impair quality of life.76 Symptoms of fatigue have been shown to extend at least 2 years postinjury.13,77,78 Although the cause remains unknown, neuroendocrine dysfunction, specifically deficiencies in basal cortisol levels and in the growth hormone response to glucagon stimulation, may contribute to symptoms of fatigue in patients with TBI.79
Sleep–Wake Disturbances in Children After TBI
Pediatric, children, and adolescent survivors of TBI also experience sleep–wake disorders postinjury reported either independently or from parental/caregiver observation.80–83 In an early study, Kaufman et al. reported an increase in subjective and objective markers of impaired sleep in adolescents (~13 years of age at the time of the study) ~3 years postinjury.67 These data were confirmed in a later, larger study (n = 98) of similarly aged adolescents in which 28% exhibited sleep–wake disturbances 6 months to 6 years postinjury.68 Three additional studies also reported continued impairment in sleep in adolescents (ages ~8–15 years) up to 1 year84,85 or 5 years86 postinjury.
Milroy et al. were unable to recapitulate this finding in a group of children who experienced head injury at an earlier age (~7 years old).87 Similarly, sleep–wake disturbances were largely absent in children who experienced a mild TBI early in life (~5 years of age) by 12 and 18 months postinjury.88 This raises the possibility that the age at which head injury is experienced may be an important factor in determining the long-term prognosis of sleep–wake disturbances after TBI. Indeed, Hooper et al. reported that, although sleep–wake disturbances were common acutely (1–4 months post-injury), they were absent by 10 months postinjury.81 This study spanned infancy to 18 years of age (average age = 8.7 years) in a large sample (n = 681), which may be broad enough to statistically obscure the presence of a developmental/aging effect. However, data from another study with a similarly large sample size (n = 729) and broad age range (2–17 years of age) showed persistent sleep–wake disturbances at 3, 12, and 24 months postinjury based on a single parent report question.89
These available studies highlight the unsolved questions on the nature of sleep–wake disturbances in the acute, subacute, and chronic periods after pediatric TBI. Larger and better designed pediatric observational trials are needed to understand the influence of age on sleep–wake disturbances across the recovery continuum. Perhaps even more intriguing, though, are how these disruptions impact cognitive and psychological development in the developing brain. Emerging evidence from animal models has implicated the role of sleep in synaptic plasticity,90,91 toxin clearance,92 and memory consolidation.93 Clarifying these roles for sleep in humans, both in the healthy and in the injured states, will be important to better appreciate how therapies targeting sleep–wake disturbances might impact long-term recovery.
Quantitative EEG Findings in Sleep After TBI
Diagnosis of these sleep–wake disturbances are heavily reliant on subjective symptoms and observational analysis performed as part of a comprehensive sleep evaluation. To determine if there are common objective electrophysiological changes that lead to disordered sleep after TBI, several studies have performed qEEG analyses obtained during PSG (Table 1). The overarching rationale of in-depth qEEG analyses stems from the fact that gross examination of sleep stages from in-lab PSG may be highly variable between subjects, and, therefore, changes in sleep staging after TBI may be difficult to discern. Accordingly, more detailed examination of specific EEG frequency bands (i.e., alpha, theta, beta, and delta power spectral analyses), coherence and cross-frequency coupling across channels, and other such measures may increase the ability to illuminate changes in brain activity after TBI.
Table 1.
Summary of Relevant Literature Reporting qEEG Data in TBI Patients.
| Publication | Ref | Sample size and sex | Mean time post injury | Injury Severity | Control | Polysomnogram data | Quantitative EEG data |
|---|---|---|---|---|---|---|---|
| Parsons et al, 1997, J Neurotrauma | 97 | 8 (2) | 72 hours, 6 weeks, and 12 weeks | Mild | None | · No differences | · Increase in delta, theta, and alpha-1 power at 72 hours post-injury that decreased across the 12 week time point |
| Williams et al, 2008, Clin Neurophysiol | 239 | 9 (3) | 27.8 months | Mild | Healthy non-TBI | · Decreased sleep efficiency and REM latency | · No differences |
| Gosselin et al, 2009, Sleep Medicine | 96 | 10 (7) | 6.2 months | Mild | Healthy non-TBI | · No differences | · No differences in NREM or REM sleep qEEG data,· Increased delta and reduced alpha power in the waking qEEG |
| Rao et al, 2011, J Neuropsy Clin Neurosci | 94 | 7 (1) | ≤1 week | Mild | Healthy non-TBI | · Increased sleep latency and lower sleep efficiency | · Decreased delta power, but increased alpha and beta power, during NREM sleep |
| Khoury et al, 2013, J Neurotrauma | 95 | 24 (9) | 48.7 days | Mild | Healthy non-TBI | · Increased sleep latency and lower sleep efficiency | · Decreased delta power, but increased alpha and beta power, during NREM sleep |
| Arbour et al, 2015, Sleep Medicine | 240 | 34 (11) | 10.5 weeks | Mild | Healthy non-TBI | · No differences | · Increased beta power, with no differences in absolute delta, theta, alpha, or sigma power |
| Cote et al, 2015, AIMS Neuroscience | 99 | 20 (11) | 6.7 years | 6, Mild; 8, Mod; 6, Severe | Healthy non-TBI | · Increased sleep latency | · Decreased density of spontaneous K-complexes and fewer evoked K-complexes |
| Imbach and Büchele et al, 2016 Neurology | 52 | 31 (11) | 18 months | 21, Mild; 2, Mod; 8, Severe | Healthy non-TBI | · Increased sleep time · Decreased sleep latency | · Increase in NREM sleep and higher delta power |
| Modarres et al, 2016, Neurobiol Sleep Circ Rhythm | 100 | 8 (0) | 58.4 months | Mild | Healthy non-TBI | · Decreased sleep latency · Decreased % REM sleep | · Increased slow waves during wakefulness, and decreased coherence across channels |
Value in parentheses next to sample size denotes number of female subjects.
Abbreviations: qEEG, quantitative electroencephalography; EMG, electromyography; NREM, non-rapid eye movement; REM, rapid eye movement; TBI, traumatic brain injury.
In the acute stage following mild TBI, patients may show longer sleep latency and lower sleep efficiency, along with lower delta power, but higher alpha and beta power, during non-REM (NREM) sleep.94,95 In the subacute to chronic stages following TBI, others have reported an increase in NREM sleep and higher delta power compared to matched controls.52 Concussed athletes reported worse sleep quality and greater sleep–wake disturbances and daytime dysfunction.96 However, there were no differences in PSG-derived variables or in qEEG analysis of NREM or REM sleep. In the waking qEEG, concussed athletes (compared to non-concussed athletes) exhibited increased delta and reduced alpha activities, potentially coinciding with self-reported daytime dysfunction. Similarly, in a small study of 8 patients with acute and subacute TBI, no differences in PSG-derived variables were observed, but qEEG analysis showed an increase in delta, theta, and alpha-1 power at 72 hours postinjury that progressively decreased across the 12-week time point.97
However, these studies have not been consistent. Several studies have found no or decreased delta power in patients with mild TBI who similarly reported poor quality sleep.77,78,81–83 The discrepancies may be because most qEEG studies to date have utilized power spectral analyses of frequency bands averaged over long time periods. Many human studies are plagued by small sample sizes, and any meaningful differences may be washed out by averaging spectra over long time durations (e.g., the entire night of sleep), when sleep quality may be very different at specific time points during the night. More sophisticated analyses that take into account phasic sleep events and examine qEEG in smaller temporal windows (e.g., cross-frequency coupling or coherence) may help to better identify signal from noise.98 In addition, examining more temporally precise features of sleep such as spindles, K-complexes, and local slow waves may be of greater utility. Cote et al. examined 20 subjects with chronic TBI (mean time since injury: 6.7 years) compared to controls and found more spindles during slow wave sleep, and a lower density of spontaneous K-complexes/fewer evoked K-complexes in response to stimulus presentation in subjects with TBI compared to controls.98,99 Modarres et al. quantified individual slow waves during the waking EEG and calculated the coherence of these slow waves across multiple channels and found that subjects with TBI had more slow waves during wakefulness and decreased coherence of waves across channels.100 Notably, in the Modarres study, striking parallels were seen in their highly controlled animal model of TBI as in their human subjects with TBI, indicating a shared neurophysiology of TBI between species.
The results of the Modarres et al.100 study highlight the value of employing qEEG analyses in human and animal models of TBI in parallel, using similar methodology, calculations, time spans, and other controllable parameters. Indeed, part of the challenge of unifying disparate qEEG findings across studies stems from heterogeneity in the acuity and severity of, duration elapsed since, and age at the time of injury. For this reason, studies that can combine animal and human data using similar qEEG approaches will increase the specificity and help refine the relevant biology to TBI that is fundamentally conserved between species. In this way, qEEG may eventually provide us with a reliable and quantitative biomarker for sleep–wake disturbances after TBI.
PATHOPHYSIOLOGY OF SLEEP–WAKE DISTURBANCES IN HUMANS AFTER TBI
The functional neuroanatomy and neuropharmacology of both normal and disordered sleep are active areas of investigation in which rapid progress is being made. Generalized electrographic, autonomic, and behavioral activation during waking emerges from specific arousal systems located in the brain stem, posterior and lateral hypothalamus, and basal forebrain. These networks utilize a variety of neurotransmitter/neuropeptide systems, including histamine, serotonin, norepinephrine, acetylcholine, dopamine, glutamate, and orexin/hypocretin (ORX). Each of these neuronal networks has specific roles in cognition, behavior, sensory processing, and/or autonomic control during waking and contributes to overall arousal. These arousal systems impact cortical activity indirectly through projections through the thalamus, lateral hypothalamus, and basal forebrain or via direct projections to the cortex. Differences in the pattern and intensity of neuronal activity across sleep–waking states among these various systems likely account for the varied manifestations of sleep–wake disorders after brain injury.
The underlying neuropathological and neurophysiological changes contributing to sleep–wake disturbances that occur after TBI are not well understood. Most individuals who suffer mild TBI do not exhibit abnormal radiographic findings and direct pathological examination is rarely possible. In patients with more severe traumatic injuries, case reports have identified structural brain injuries thought to contribute to secondary sleep–wake disturbances after TBI, including the suprachiasmatic nucleus and optic chiasm101 and the hypothalamus, amygdala, and brain stem.102 However, the vast majority of cases do not have an obvious structural abnormality contributing to sleep–wake dysfunction.103 Some have hypothesized that brain stem injury may contribute to arousal dysfunction after TBI,104,105 but a recent postmortem examination of 8 patients with TBI showed only mild changes in the number of brain stem neurons in patients with TBI when compared to controls.106
Hypothalamic Regulation of Sleep–Wake Disturbances After TBI
Given the lack of a structural correlate with sleep–wake disturbances after TBI and clinical features mimicking narcolepsy (including EDS and nighttime sleep fragmentation), several groups have now looked at the role of the ORX system in sleep–wake disturbances after TBI. ORX is a neuropeptide expressed by neurons in the perifornical lateral hypothalamus that have extensive projections to other hypothalamic nuclei, the limbic system, thalamus, cortex, and spinal cord, exciting several monoaminergic and cholinergic wake-promoting systems.107 Circadian oscillations in ORX within cerebrospinal fluid (CSF) correlate with arousal cycles,108,109 and exogenous administration of ORX promotes wakefulness.110,111 Interestingly, the phenotypes of human and canine narcolepsy and associated neurochemical imbalances, notably with respect to ORX expression deficiency, are strikingly similar.112 Using positional cloning, narcoleptic Doberman pinschers have been identified to be deficient in ORX neurotransmission, due to an ORX receptor gene mutation.113 In contrast, although the cause of human narcolepsy remains unknown, human narcoleptic patients have been shown to be deficient in ORX, as determined by ORX immunoreactivity from CSF samples.114 ORX knockout mice results in a condition of fragmented wakefulness similar to human narcolepsy.115 Additionally, ORX receptor antagonism116 promotes sleep in mice, rats, rabbits, dogs, monkeys, and humans.117
In humans, Baumann et al. measured CSF ORX levels of 44 patients in the first several days after moderate–severe TBI and showed CSF ORX levels were decreased in 95% of patients.6 Although CSF ORX levels recovered in the majority of patients 6 months after injury, low CSF ORX levels were associated with sleep–wake disturbances, including narcolepsy and a narcolepsy-like syndrome, in 3 of 4 patients in whom low CSF ORX levels persisted at 6 months. In a related study, autopsy examination of brains from 4 patients who died after severe TBI showed a reduction in ORX neurons in the hypothalamus compared to matched controls.118 Of note, autopsy examination of patients with narcolepsy also show a reduction in ORX neurons.119 Other injuries to the hypothalamus may also influence sleep following TBI. In a study of 12 TBI patients and 16 matched controls, 7 TBI patients had neuropathological abnormalities of the hypothalamus, particularly loss of wake-promoting histaminergic neurons (41%), whereas ORX neurons were decreased by 21%.120 Thus, there may be multiple mechanisms by which hypothalamic injury contributes to sleep–wake disorders after TBI.
Brain Stem and Pineal Dysregulation of Sleep After TBI
Recent work from Valko et al. examined the midbrain and pons in 8 patients on autopsy from fatal TBI.106 Severe TBI was associated with a 17% loss of serotonergic dorsal raphe nuclei neurons and a 29% loss of noradrenergic locus coeruleus neurons, while other arousal promoting neurons appeared less injured (e.g., median raphe nuclei, pedunculopontine, and laterodorsal tegmental nuclei).
Melatonin is produced by the pineal gland and regulates the sleep–wake cycle following a circadian pattern of release into the bloodstream. Although melatonin release may be disrupted following TBI,121 melatonin supplementation did not improve sleep yet does produce improvements in daytime alertness.122 Exogenous melatonin can be used to induce phase shifts in the sleep–wake cycle. In a study of 23 patients with chronic TBI (>6 months since injury), there was a decrease in evening melatonin production when compared to age- and sex-matched controls.66 Seifman et al. studied patients with acute TBI as well as other critically ill patients admitted to the hospital and found that both populations had decreased levels of serum melatonin compared to healthy controls.123 Recent work from Grima et al. extend these findings by demonstrating that patients with TBI produced 42% less salivary melatonin as well as a shift toward a later onset of melatonin synthesis.124
Glutamate Dysfunction as a Contributor to Sleep–Wake Disturbances After TBI
The excitatory neurotransmitter glutamate regulates the sleep–wake cycle across species. Homer proteins, which bind to metabotropic glutamate receptors and alter Ca2+ sensitivity, are involved in sleep–wake processes in both Drosophila and mice.125 Genetic deletion of the homer 1a protein in Drosophila (which is upregulated during sleep) results in fragmented sleep and the failure to sustain long bouts of sleep, despite increased sleep pressure.125 In contrast, genetic deletion of the homer 1a protein in mice (which is upregulated during wakefulness) produces fragmented sleep and failure to sustain long bouts of wakefulness.125 Simultaneous real-time measurements of sleep and glutamate concentration in the prefrontal cortex of healthy mice demonstrate that the concentration of extracellular glutamate increases during wakefulness, decreases during periods of extended sleep, and spikes during REM sleep.126 These data reveal a close temporal link between sleep/wake state and extracellular glutamate concentrations. Glutamate dysfunction, specifically an increase in glutamate signaling, has been described in rat cortex within the first hour after TBI induced via controlled cortical impact (CCI)127,128 and in humans 24 hours after TBI.129 Additionally, increases in cortical glutamate network activity can be attributed to impaired GABAergic control in a murine TBI model.53 Thus, it follows that TBI-induced changes in cortical glutamate could be one potential mechanism underlying sleep–wake dysfunction. We recently reported decreased glutamate within the presynaptic terminals synapsing onto ORX-positive cells in the hypothalamus after TBI, suggesting decreased excitatory inputs onto this critical wake-promoting system.130
In summary, sleepiness after TBI is likely the result of multiple factors, including direct damage to some arousal-promoting neurons, injury/inflammation in the cortex, thalamus and hypothalamus, diffuse axonal injury, and alterations in cerebral energy metabolism. It is possible that intervention in this early setting on these or other signaling pathways may help to ameliorate later sleep disturbance, but these approaches require further study.
ANIMAL MODELS OF SLEEP–WAKE DISTURBANCES AFTER TBI
Animal models of TBI recapitulate several sleep–wake disturbances seen in humans after TBI, including insomnia, EDS, and pleiosomnia. However, there are no well-defined animal models of TBI that accurately model sleep-related breathing disorder, circadian rhythm disorder, or abnormal movements during sleep. The majority of work using animal models of TBI have utilized rats and mice, although several have used larger animals such as swine or nonhuman primates. Experimentally inducing TBI is accomplished by subjecting the brain, with or without craniotomy, to an external mechanical force. In this section, we briefly summarize the most common approaches used to study sleep–wake disturbances in animal models of TBI, which include fluid percussion injury (FPI; midline and lateral), CCI injury, and the weight drop injury model (Table 2). We refer the reader to comprehensive reviews on the general topics of animal models of TBI,131,132 the translational and strategies of animal models of TBI,133 and neuroinflammation associated with TBI.134
Table 2.
Summary of Animal Models Used in Work Assessing sleep–wake Disturbances After TBI.
| Model | Type of injury | Strengths | Weaknesses | Species |
|---|---|---|---|---|
| FPI | · Mixed global/focal insult | · Very common model used | · Requires craniotomy | · Mouse, 100,164,169–171 Rat 165 |
| · Midline or lateral | · Highly reproducible · Mild regarded as the most translation model of TBI | · Does not replicate clinical TBI in terms of skull fracture · Limited mechanical control over neurological insult | ||
| CCI | · Predominantly focal | · Single or repetitive insults | · Requires craniotomy | · Mouse, 155,167,168,173 Piglet 172 |
| · Highly reproducible | ||||
| · Severity of neurological insult easily controlled | ||||
| Weight Drop | · Mixed global/focal insult | · Representative of human conditions· Does not require craniotomy | · Not highly reproducible· High mortality without post-injury 100% O2 ventilation | · Mouse, 157 Rat 158,159 |
Abbreviations: FPI, fluid percussion injury; CCI, controlled cortical impact injury; TBI, traumatic brain injury.
Fluid Percussion Injury
FPI is induced by a pendulum swinging and striking a piston of a fluid reservoir to generate a pressure pulse (i.e., percussion) to the exposed and intact dura.135–137 The percussion produces a brief displacement and deformation of brain tissue, with the severity of the injury determined by the strength of the pressure pulse. The two most common injury locations are centrally around the midline138 or laterally between bregma and lambda over the parietal bone.139 Although FPI does not replicate clinical TBI with associated skull fracture, it does replicate intracranial hemorrhage, brain swelling, and progressive gray matter damage. Historically, lateral FPI is one of the most common TBI animal models,137 despite limited mechanical control over the neurological insult (i.e., the height of the pendulum is the only modifiable parameter). The advantages of FPI over other rodent models of TBI is the high reproducibility and wide acceptance across dozens of laboratories worldwide, resulting in hundreds of publications over the past 3 decades that describe in detail the brain histological changes (e.g., markers of apoptosis and gliosis), electrophysiological changes, and behavioral deficits resulting from FPI.137 Furthermore, neurological testing for righting reflex time immediately after brain injury is highly predictive of histological severity of injury.140,141 Recent work has advanced this technique by utilizing a computer-controlled pneumatic instrument with precisely controlled impact pressure and dwell time, resulting in improved reproducibility.142
Mild FPI is widely regarded as the most translational model for nonpenetrating concussive injury,143,144 as the percussion injury (e.g., injected under the skull surface atop the intact dura) results in a mixed global/focal insult spanning both hemispheres.137 FPI also affects subcortical structures including hippocampus, prefrontal cortex, and amygdala electrophysiology, even if there is little structural damage to these distant sites.145–149 Although severe TBI induced by FPI has been performed in pigs150 and rats,151,152 researchers have not yet studied sleep–wake disturbances after severe FPI.
Controlled Cortical Impact Injury
The CCI injury model uses a pneumatic or electromagnetic impact device to drive a rigid impactor onto the exposed dura to create a cortical lesion.153 CCI causes a more focal insult compared to the mixed focal/global insult from FPI. The major advantage of CCI over other models of TBI is the extent to which mechanical factors (e.g., time, velocity, depth of impact) can be controlled. Furthermore, the severity of injury can be manipulated by adjusting the impact velocity, with increasing velocities leading to increasing TBI severity.154 However, the generalizability of CCI injury to human concussion is somewhat limited by the nature of the focal, cortically penetrating lesion. CCI is a useful model for mild, moderate, and severe focal TBI. Similar to FPI, the injury is not limited to cortical damage and can also affect subcortical structures.155
Weight Drop Injury
Weight drop injury can be either closed head or performed through an open craniotomy. Only closed head weight drop models been studied with regard to sleep–wake disturbances, and thus, most studies closely resemble the Shohami156 (mixed focal diffuse) model. Sleep–wake phenotypes from these models are summarized in Table 3. Sabir et al.157 utilized a closed head weight drop model in mice similar to what has been previously described,156 in which a 329-g rod is dropped from a 1-cm height that induces a mild TBI. Büchele et al.158 and Morawska et al.159 utilized a weight drop model in rats that was slightly modified from the classic Marmarou160,161 approach by allowing the weight to fall from a steep angle (rather than vertically). The weight drop model benefits from not requiring a craniotomy to be performed and, therefore, more closely resembles the human condition. Although this approach is easily implemented and well characterized, the severity of inciting injury is not highly reproducible yet can span the spectrum of mild to severe TBI (potentially with skull fracture). The weight drop model, particularly in more severe models with skull fracture, often requires ventilating the animal postinjury with 100% oxygen. The potential effects of administering supplemental oxygen postinjury are unknown, but considering the known increase in oxidative stress with supplemental oxygen,162,163 this is a notable methodological difference compared to FPI and CCI.
Table 3.
Summary of Relevant Literature Examining Sleep in an Animal Model of TBI.
| Publication | Ref | Species | TBI model | Injury severity | Control | Time between injury and sleep recording | Measure of sleep | Sleep or qEEG phenotype |
|---|---|---|---|---|---|---|---|---|
| Willie JT and Lim MM et al, 2012, J Neurotrauma | 155 | Mice | CCI | Moderately severe | Sham surgery | 0–3 days | EEG and EMG | · Increased sleep and shorter wake bouts · Increased sleep–wake fragmentation |
| Lim MM et al, 2013, Sci Trans Med | 164 | Mice | Lateral FPI | Mild | Sham surgery | 8–13 days | EEG and EMG | · Increased sleep · Increased sleep–wake fragmentation |
| Skopin MD and Kabadi SV et al, 2014, J Neurotrauma | 165 | Rats | Lateral FPI | Moderate | Sham surgery | 6, 19, and 29 days | EEG and EMG | · Increased sleep–wake fragmentation |
| Rowe RK et al, 2014, PLoS ONE | 169 | Mice | Midline FPI | Mild and moderate | Sham surgery | 0–7 days continuous | Piezoelectric cage system | · Increased sleep for first 6 hours post injury |
| Rowe RK et al, 2014, SLEEP | 170 | Mice | Midline FPI | Moderate | Sham surgery | 0–7 days continuous | Piezoelectric cage system | · Increase in sleep only during first hour of dark phase (~10 hours post injury) |
| Rowe RK and Harrison JL et al, 2014, Brain Inj | 171 | Mice | Midline FPI | Moderate | Sham surgery | 0–7 days continuous | Piezoelectric cage system | · Increased sleep for first week post injury with no change between weeks 2–5 |
| Petraglia AL et al, 2014, J Neurotrauma | 173 | Mice | Single and repetitive closed head CCI | Mild (single and repetitive) | Non- surgery control | 1 month | EEG and EMG | · Decreased sleep · Increased sleep–wake fragmentation · Decreased NREM |
| Hazra A et al, 2014, J Neuroscience Res | 168 | Mice | CCI | Mild | Sham surgery | 28 days | CageScan Software | · Increased sleep–wake fragmentation · Increased latency to reach peak sleep |
| Sabir M and Gaudreault PO et al, 2015, Brain Behavior Imm | 157 | Mice | Closed head weight drop | Mild | Sham surgery | 0–2 days | EEG and EMG | · Shorter bouts of wakefulness – state instability · Spectral changes - delta power |
| Büchele F and Morawska MM et al, 2016, J Neurotrauma | 158 | Rats | Closed head weight drop | Not specified | Sham surgery | 1, 7, and 28 days | EEG and EMG | · No change in the proportion of time spent in wakefulness, NREM, and REM |
| Thomasy HE et al, 2016, Neurobiol Sleep Circ Rhythm | 167 | Mice | CCI | Mild and moderate | Sham surgery | 7 and 15 days | EEG | · Increased sleep |
| Morawska MM and Büchele F et al, 2016, J Neuroscience | 159 | Rats | Closed head weight drop | Not specified | Sham surgery | 5 days | EEG and EMG | · Increased in sleep · Increase in sleep–wake fragmentation · Spectral changes - delta power |
| Olson E et al, 2016, J Neurotrauma | 172 | Piglets | CCI | Not specified | Non- surgery control | 4 days | Actigraphy | · Increased lethargy |
| Modarres et al, 2016, Neurobiol Sleep Circ Rhythm | 100 | Mice | Lateral FPI | Mild | Sham surgery | 8–13 days | EEG and EMG | · Increased slow waves during wakefulness · Spectral changes – theta:alpha ratio |
All mice were C57BL/6 and all rats were Sprague-Dawley. All studies used male animals with the exception of Hazra et al where the sex was not clearly identified, and Olson et al where piglets were all female.
Abbreviations: CCI, controlled cortical impact injury; FPI, fluid percussion injury; EEG, electroencephalography; EMG, electromyography; NREM, non-rapid eye movement; REM, rapid eye movement.
Comparison to Sleep–Wake Disturbances in Humans After TBI
Researchers have only recently begun to explore sleep physiology in these animal models of TBI (Table 3). One of the earliest reports of sleep–wake abnormalities in an animal model of CCI-induced moderate TBI demonstrated a trend for brain injured mice to show less wakefulness during the dark phase (when mice are typically more awake) and increased sleep fragmentation.155 Hypothalamic ORX levels, examined via in vivo microdialysis, were reduced in injured mice compared to uninjured control animals. Indeed, hypothalamic ORX levels in uninjured animals followed a normal diurnal fluctuation according to sleep–wake state and activity. These data suggest that TBI-related impairment in ORX neurotransmission may underlie the presence of sleep–wake disturbances after TBI.
More recent work using rodent models164,165 of lateral FPI-induced mild TBI have not only reproduced the phenotype of increased sleep–wake fragmentation and decreased levels of overall activity observed after moderate TBI but also support the hypothesis that TBI-related impairment in ORX neuron activation, at least in part, drives sleep–wake disturbances after TBI. In the mouse model of lateral FPI-induced mild TBI, increased sleep–wake fragmentation coincides with impaired ORX neuron activation, examined via cFOS activation, while restoration of ORX neuron activation restores normal sleep–wake state patterns.164 Restoration of ORX neuron activation was accomplished via dietary supplementation with branched chain amino acids (BCAAs), which are essential amino acids required for de novo cerebral glutamate and GABA synthesis that also ameliorate TBI-related cognitive impairment in mice.166 We recently reported decreased glutamate within the presynaptic terminals synapsing onto ORX-positive cells in the hypothalamus after TBI, suggesting decreased excitatory inputs onto this critical wake-promoting system.130 Accordingly, BCAA supplementation, via increasing glutamate synthesis, is likely restoring normal cortical excitability and thereby ORX neuron activation. Furthermore, in a mouse model of CCI-induced TBI there is both a reduction in the overall number of ORX positive cells in the perifornical region of the lateral hypothalamus as well as a reduction in the number of cholinergic neurons in the basal forebrain corresponding increased TBI-related NREM sleep time.167
Also, possibly consistent with a TBI-induced change in the ORX system, Hazra et al. reported increased awakenings from sleep and shorter bouts of sleep during the light phase at 28 days post-CCI induced TBI in mice.168 Astrocytosis, a histological marker of brain injury, occurred immediately in the cortex but was delayed in subcortical structures (e.g., the thalamus). Localized thalamic microglial activation also increased over time, suggesting a distinct temporal and spatial neurodegenerative response to TBI that may parallel the changes in sleep over the temporal course of TBI recovery.
More recent work using a midline FPI model of TBI in mice shows a similar increase in sleep-like activity acutely post-TBI. First, Rowe et al. reported an increase in sleep (>50%) during the first 6 hours post-TBI that was accompanied by increases in proinflammatory cytokines (i.e., IL-1β) and IBA-1 positive microglia.169 Subsequently, the same group investigated the effect of disrupting sleep for 6 hours following TBI.170 Sleep disruption acutely after injury increased sleep during the first hour of the dark period (~10 hours post-TBI). Mice that sustained TBI but were not subjected to sleep disruption did not exhibit an increase in sleep. Later, Rowe and Harrison et al. investigated the effect of TBI on chronic sleep disturbance (out to 5 weeks post-TBI).171 Total sleep, sleep during the dark cycle, and sleep bout length were increased in mice that sustained TBI in the first week after injury but did not extend to the more chronic period. These findings have also been supported in large animal models of TBI. In a CCI piglet model, alterations in daytime and nighttime activity levels corresponded to an increase in lethargy.172 Notably, these studies quantitated sleep-like activity using either actigraphy, or specialized noninvasive piezoelectric recording chambers and an algorithm based on respiration signals, rather than EEG/EMG recordings.
Several studies have utilized a closed head model of TBI such that no craniotomy is required to induce the injury. Petraglia et al. subjected mice to either a single impact or repetitive impacts in an attempt to mimic chronic traumatic encephalopathy.173 These authors reported a decrease in sleep 1 month postinjury, specifically manifested by less time spent in NREM sleep. No difference was observed in the total time spent in REM sleep; however, injured animals exhibited an increase in EMG activity during REM sleep, indicative of REM sleep disturbance. In contrast, Sabir et al. observed shorter bouts of wakefulness (indicating state instability) after a similar closed head model of TBI during the first 24 hours postinjury.157 However, more recent work found no differences in the proportion of time spent in wakefulness, NREM, and REM sleep during the light period between injured and control groups.158
Taken together, the majority of studies suggest a hypersomnolence phenotype in animal models of TBI that resembles reports of EDS and insomnia (e.g., manifesting as sleep fragmentation) in humans. Nevertheless, subtle differences in this finding are clearly present in the literature. Regardless of the experimental model of TBI and time course, 5 studies reported an acute increase in sleep,155,157,159,169,171 3 studies report a chronic increase in sleep,164,165,167 and 5 studies report an increase in sleep fragmentation.155,159,164,168,173 However, 2 studies did not demonstrate sleep–wake changes after TBI,158,170 and 1 study reported a decrease in total sleep time at a chronic time point after TBI.173 Several possibilities may explain these discrepancies. Methodological differences in the type and severity of injury (CCI versus lateral FPI versus midline FPI, mild versus moderate), the time point at which sleep is examined (acute versus subacute versus chronic), and the method of determining sleep (e.g., EEG versus activity-based surrogate) likely all contribute to these inconsistencies. Variation between individual animals and heterogeneity of response in this inherently heterogeneous disease also likely play a role. Notably, only 4 studies have examined animals pre- and postinjury, allowing for repeated measures testing and addressing the issue of individual heterogeneity in response to TBI.155,159,167,169 Furthermore, subtle differences may be expected based on the species, strain, sex, and potentially the age of the animals. Indeed, there is an increased appreciation for the importance of utilizing young-adult animals that are sufficiently mature to avoid potential contamination by early systemic maturation on a studies primary outcome variables.174 For example, although 8-week-old mice are frequently considered to be “adult,” recent work reported a marked change in respiratory mechanics by 6 months of age (when body weight stabilizes175) which was hypothesized to be attributable to continued systemic maturation.176 Other factors, such as inflammatory and cellular responses in the brain to injury, are strongly affected by age.177–179 Thus, it remains possible that the physiological response to TBI differs based on the animal’s age.
SLEEP AS AN EXACERBATING FACTOR AND PREDICTOR OF TBI RECOVERY
It is generally accepted that sleep is a critical neurophysiological process that is necessary for cognitive and behavioral functioning180; however, a clear consensus regarding the precise physiological function of sleep remains inconclusive.181 Impairments in cognition and physical functioning following sleep deprivation have been well documented.182–184 Mild disruption of even a single 24 hour sleep–wake cycle is associated with behavioral changes and emotional lability185 that resolve when normal sleep patterns resume.180 Given the impact of sleep disruption on the healthy brain and the pathophysiological mechanisms implicated in the studies described earlier, there has been much interest and speculation over the role that sleep disruption might impact recovery after TBI. Improved understanding of sleep disruption in TBI recovery is of particular interest, as there are numerous treatment options available.
Relationship of Sleep and Recovery Across the TBI Spectrum in Humans
Sleep–wake disturbances have long been recognized following moderate–severe head injury based on observations in the acute care and rehabilitation settings. More recently, the impact of sleep–wake disturbances in those with mild TBI is being increasingly recognized and appreciated for its impact on quality of life and recovery after mild TBI.
Following mild TBI in humans, there is frequently a period of hypersomnolence, which can last for a week or more.61,186 The role of this in TBI recovery and how interference during this time might impact healing is not well understood. Patients in whom sleep–wake disturbances persist beyond this acute period tend to have worse outcomes when followed longitudinally. In sports-related concussion, athletes who reported sleep–wake disturbances exhibited higher symptom burden on postconcussive questionnaires that persisted during clinical follow-up.187
It is long recognized that sleep and mental well-being are interrelated and mutually affect each other. Thus, sleep disruption after TBI may simply be a reflection of underlying psychiatric comorbidities such as depression and anxiety.188 However, several studies suggest that sleep–wake disturbances impact clinical outcome independent of these associated conditions. Workers with chronic mild TBI who reported sleep–wake disturbances/insomnia more than 6 months after injury were more likely to report marked/extreme global disability, even after controlling for depression, anxiety, and pain.189,190 In 374 patients who sustained mild TBI, 71% of patients reported sleep–wake disturbances at baseline assessment; at 1 year, 50% had persistent sleep disturbance.188 When analyzed in a multivariable model, sleep disruption was a predictor of poor functional outcome at 1 year while a measure of psychological distress was not. These data suggest that sleep–wake disturbances may represent a therapeutic target following mild TBI to promote recovery from other postconcussive symptoms.
In more severe forms of TBI, sleep disturbance following TBI has been best studied in the rehabilitation setting. Holcomb et al.191 prospectively examined the relationship between sleep disruption and cognitive recovery in 106 patients admitted to an acute rehabilitation center following TBI. They found that persistent sleep disruption was associated with poorer cognitive recovery over a 3-week period of admission. Importantly, this was not influenced by injury severity. Persistence of sleep–wake disturbances is associated with a longer length of stay in the acute rehabilitation setting51 while restoration of sleep is associated with recovery from the posttraumatic confusion state192 and return of memory.193 In the first 3 months following moderate to severe head injury, sleep–wake disturbances actually predicted the development of neuropsychological sequelae, including depression, anxiety, and apathy, at 6 and 12 months after injury.194 Whether treatment of sleep–wake disturbances could impact these long-term outcomes remains to be studied.
Much less is known about how sleep disruption in the acute setting might predict or impact long-term outcome after TBI. In the acute phase immediately after injury, Duclos et al. used actigraphy to monitor patients hospitalized after TBI.3 Rest episodes were highly fragmented in the acute period following moderate–severe TBI, correlating with poor sleep–wake cycles. Consolidation of rest and activity phases, which corresponds with restoration of sleep–wake cycles, was associated with a shorter length of acute hospitalization and lower disability rating scale scores at hospital discharge. Patients with a more rapid return to consolidated rest–activity patterns also exhibited more rapid resolution of posttraumatic amnesia. Others have examined sleep EEG to predict emergence from coma in disorders of consciousness (e.g., in some cases resulting from severe brain injury) and found that the amount of spindles and REM sleep are good prognostic indicators.195
We recently examined sleep characteristics in severely injured patients (n = 65) who underwent continuous EEG monitoring for a mean time of 51 hours during their acute hospitalization in the neurological intensive care unit for severe TBI.196 We found that objective evidence of sleep was present in 30% of patients with severe TBI and was associated with an improved outcome at hospital discharge. Importantly, the presence or absence of sleep characteristics was not predicted by injury severity as assessed by GCS score or Rotterdam neuroimaging score on admission. While much more work needs to be done, these studies raise the important possibilities that (1) sleep characteristics in the acute period following brain injury may provide prognostic information for patients and families and (2) optimization of sleep in the early period after moderate-to-severe TBI may impact brain recovery.196
Relationship of Sleep and Recovery Across the TBI Spectrum in Animals
Similar to humans, most reports of animal models of TBI indicate that they exhibit a period of hypersomnolence following TBI, although, just as in humans, the role this plays in recovery is not well understood. Morawska and Büchele et al. demonstrated in a rat model of TBI that slow oscillatory activity in the delta frequency range is key to facilitating cognitive improvement following TBI.159 In this study, both sleep induction with the drug gamma-Hydroxybutyric acid (GHB), and sleep restriction via gentle handling, prevented the development of cognitive impairment that was observed in animals that received TBI alone. Although these results may at first seem contradictory, the authors attributed these findings to an increase in delta power (slow wave sleep) in both experimental groups. Indeed, GHB increases oscillatory activity in the delta frequency.197,198 Similarly, sleep restriction results in more frequent and larger slow waves during subsequent sleep recovery periods in humans199 and rats.200 These data support previous work by the same group utilizing GHB to accelerate recovery after ischemia/brain damage, in light of their findings that sleep disruption exacerbated histological injury and delayed recovery after stroke.201 GHB administration in mice after focal cerebral ischemia accelerated stroke recovery as determined by increased body weight and motor grip strength, compared to control animals.202 Similar findings have been reported in rat models of cerebral ischemia, where GHB administration improved sensorimotor activities and memory.203,204 Previous work showing a neuroprotective effect from sleep deprivation on TBI in rats may be partially explained by the increase in delta power.205 Similarly, sleep deprivation prior to induction of focal cerebral ischemia in the rat206 or TBI207 is neuroprotective or at least does not increase neuronal susceptibility to injury.208 Interestingly, there is also evidence that acute sleep deprivation induces neurogenesis in the hippocampus209,210 and increases the expression of neurotrophins in the cortex.211
The mechanism underlying the delta power-associated improvements in neurocognition could be relevant to the recently described brain glymphatic system, which shows enhanced clearance of toxins and waste products from cerebral interstitium during sleep.212 This phenomenon may be relevant to the development of neurodegenerative disorders such as Alzheimer’s disease and chronic traumatic encephalopathy resulting from TBI.213,214 Interestingly, a recent study by Plog et al. show biomarkers of TBI are transported from the injured brain, to the blood, via the glymphatic system.92 To date, sleep modulation of the brain glymphatic system has only clearly been demonstrated in rodents, therefore much still remains to be determined with regard to glymphatics and TBI in humans.
TREATMENT OPTIONS FOR SLEEP–WAKE DISTURBANCES AFTER TBI
Given the paucity of empiric treatment options to facilitate TBI recovery, the prevalence of sleep disturbance following TBI, and the association of poor clinical outcomes with disturbed sleep, it is reasonable to consider therapies to optimize sleep early in the TBI recovery process. While current treatment options vary for individual patients according to the specific sleep disorder or dominant symptomatology, emerging data from preclinical models suggest that there are unique mechanisms to sleep–wake disturbances after TBI that may be amenable to therapy to improve clinical outcomes.
Symptom-Directed Therapy
Current therapies for sleep–wake disturbances after TBI are extrapolated from therapies used for non-TBI sleep–wake disturbances. Therefore, the lowest dose of medications for the shortest duration possible should be used whenever feasible, particularly in those with cognitive impairment who may be more sensitive to medication-related side effects.215 Any underlying condition, such as obstructive sleep apnea, restless legs syndrome, depression, thyroid dysfunction, and anemia, should be evaluated and treated.
In a study of 70 military personnel with TBI, initiation of behavioral therapy, medications, and continuous positive airway pressure (CPAP), as applicable based on individual patient characteristics, led to an improvement in measures of depression and PTSD in those who responded to treatment.216 Similar results were seen in a small, community-based study in patients with chronic TBI (1–22 years after injury).217
Cognitive–behavioral therapy for insomnia (CBT-I) is quite efficacious in general populations with insomnia.218,219 However, the efficacy of CBT-I after TBI has only been examined in small studies.186,187 In one such study, insomnia symptoms persisted after CBT-I, but depression and anxiety improved.186 In a study of 11 patients with TBI, CBT-I was effective in 73% of patients, resulting in a reduction in total wake time of >50%.220 This effect persisted over 3 months of follow-up and was accompanied by an improvement in related symptoms such as fatigue. Acupuncture was associated with an improvement in perception of sleep and cognition in a small sample of patients with TBI having insomnia, though it did not increase sleep duration.221 Blue light therapy has been shown to be helpful in a small study of patients with TBI.222 Additionally, moderate intensity aerobic exercise has been shown to be effective in improving sleep quality, cognitive function, and neurobehavioral function in patients with TBI.223,224
Both benzodiazepine and nonbenzodiazepine medications can be efficacious for treatment of insomnia after TBI,225 although these medications have not been studied in comparative trials in this patient population. It should be noted that benzodiazepines have been reported to suppress slow wave sleep, the stage of sleep which has recently been hypothesized to be important for recovery after TBI.35,159 Further research on the important role of slow wave sleep and TBI will inform the potential need to change our current clinical management of sleep–wake disturbances in patients with TBI. ORX receptor antagonists are the newest agents to become available for the treatment of insomnia. Three randomized controlled trials have shown that these agents are well tolerated and efficacious in the treatment of insomnia.226–228 Other pharmacologic treatment options for EDS include agents that, rather than optimize sleep, promote wakefulness (modafinil and armodafinil) and stimulants (methylphenidate). Four randomized trials investigating the use of modafinil and armodafinil in patients with TBI229–232 showed improvement in some, but not all, measures of subjective sleepiness. Finally, a pilot study employing a melatonin receptor agonist, ramelteon, that targets melatonin receptors 1 and 2 located in the suprachiasmatic nucleus of the hypothalamus, has shown promise in treating insomnia in patients with TBI.233 For review of additional pharmacotherapeutic options for sleep–wake disorders after TBI and other neurologic sequelae, we refer the reader to a previous review on the subject.234
Emerging Data on Novel Therapies for Sleep–Wake Disturbances After TBI
Emerging data from preclinical models suggests that there may be other options for the treatment of sleep after TBI. For example, we recently established a mouse model of mild TBI with sleep–wake disturbances that exhibited improved sleep through dietary supplementation of BCAAs, which are essential amino acids required for de novo glutamate, and subsequently GABA, synthesis in brain.164 Mice administered BCAA therapy ad libitum in the drinking water showed improvements in TBI-induced sleep–wake disturbances, maintenance of wakefulness, and ORX neuron activation. More recent work from our group demonstrated that BCAA supplementation is required for at least 5 continuous days of administration in order to successfully treat cognitive impairment after TBI.235 Interestingly, withdrawal of the BCAA therapy caused mice to revert back to the injured phenotype, suggesting that BCAA needs to be on board for efficacy. This time course and dependency is consistent with the purported mechanism of TBI causing decreased substrate required for glutamatergic synaptic neurotransmission. Indeed, recent data from our laboratory showed that mice with TBI on BCAA supplementation showed a restoration of glutamate within presynaptic terminals synapsing onto ORX-positive cells in the hypothalamus, compared to mice with TBI not on therapy.130 These data provide evidence that BCAA therapy may prove beneficial in enhancing the cognitive recovery of human subjects after mild TBI. In two small studies on patients with severe TBI, subjects given intravenous BCAA supplementation for 15 days showed improvement in the modified Rankin Scale score at hospital discharge, although sleep was not examined as an outcome in these studies.236,237 A clinical trial is currently underway to examine the efficacy of BCAA therapy after sports-related concussion in cognition and sleep as measured by actigraphy (www.clinicaltrials.gov, NCT01860404).
Another potential therapy may be to enhance sleep directly using agents that promote sleep and/or delta power during sleep, such as sodium oxybate or GHB.238 As discussed earlier (see Section 7.2), the cognitive impairment observed in rats after TBI can be attenuated by sleep modulation using GHB, through increasing delta power, and reducing posttraumatic amyloid precursor protein accumulation in the cortex and hippocampus.159 While early in the pipeline, these potential therapies are promising, mechanistic-based alternatives to current symptomatic treatment of sleep–wake disturbances after TBI.
SUMMARY
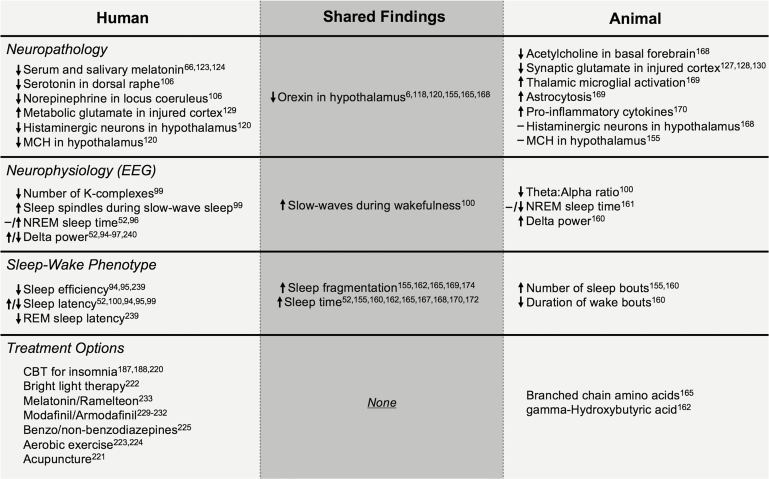
There are few disorders as heterogeneous and complex as TBI. The collective work described herein highlights the importance and value of clinicians and basic scientists working closely together to improve our understanding of this complex human condition. A summary figure that synthesizes what is known about the pathology, physiology, sleep–wake phenotype, and treatment options from both the human and the animal literature is shown in Figure 1. In this review of the existing literature, we showcase the ability of preclinical animal models to recapitulate many aspects of human sleep–wake disturbances, including hypersomnolence, sleep fragmentation, increased slow wave activity, and decreased orexin function in the hypothalamus. Animal studies have offered important insights into the pathophysiological mechanisms of TBI sleep–wake disturbances that had remained elusive from human studies plagued by injury heterogeneity and methodological variability. While this research is still early, it is clear that there is a dearth of treatments in use as a result of bench to bedside translation of findings from animal models. These models have led to the identification several sleep-targeted therapies with potential to improve recovery after TBI. Further work using quantitative metrics, both biochemical (ORX, other biomarkers) and electrophysiological (qEEG), will help to refine the diagnosis, prognosis, and treatment of sleep–wake disturbances after TBI. Finally, an implication of this review is that longitudinal/prospective studies with high-risk populations (in conjunction with parallel pre-post injury studies in animal models) are needed, to advance our understanding of the role of different aspects of sleep in TBI and recovery.
Figure 1.
Summary table showing the findings from animal and human studies with regard to several levels of analysis: neuropathology, neurophysiology, sleep–wake phenotype, and treatment options. Findings that are shared between animal and human studies are depicted in the center “Shared Findings” column. An absence of any Shared Findings in the Treatment category suggests that there is opportunity to move potential treatments identified in animal studies into the human condition. Abbreviations: EEG, electroencephalography; NREM, nonrapid eye movement sleep; REM, rapid eye movement; MCH, melanin-concentrating hormone; CBT, cognitive–behavioral therapy.
AUTHORS’ NOTE
Danielle K. Sandsmark and Jonathan E. Elliott equally contributed to authorship.
DISCLOSURE STATEMENT
None disclosed.
Acknowledgements
This material is the result of work supported with resources and the use of facilities at the VA Portland Health Care System, the VA Career Development Award #IK2 BX002712, Oregon Medical Research Foundation, and Portland VA Research Foundation (MML); N.I.H. T32 AT002688 (JEE). The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
References
- 1. Centers for Disease and Control. Injury Prevention and Control: Traumatic Brain Injury and Concussion.; 2016. http://www.cdc.gov/traumaticbraininjury/get_the_facts.html.
- 2. Katz DI, Cohen SI, Alexander MP. Mild traumatic brain injury . Handb Clin Neurol. 2015; 127: 131–156. [DOI] [PubMed] [Google Scholar]
- 3. Duclos C, Dumont M, Wiseman-Hakes C, et al. Sleep and wake disturbances following traumatic brain injury. Pathol Biol (Paris). 2014; 62(5): 252–261. [DOI] [PubMed] [Google Scholar]
- 4. Orff HJ, Ayalon L, Drummond SP. Traumatic brain injury and sleep disturbance: a review of current research. J Head Trauma Rehabil. 2009; 24(3): 155–165. [DOI] [PubMed] [Google Scholar]
- 5. Castriotta RJ, Wilde MC, Lai JM, Atanasov S, Masel BE, Kuna ST. Prevalence and consequences of sleep disorders in traumatic brain injury. J Clin Sleep Med. 2007; 3(4): 349–356. [PMC free article] [PubMed] [Google Scholar]
- 6. Baumann CR, Werth E, Stocker R, Ludwig S, Bassetti CL. Sleep-wake disturbances 6 months after traumatic brain injury: a prospective study. Brain. 2007; 130(Pt 7): 1873–1883. [DOI] [PubMed] [Google Scholar]
- 7. Kempf J, Werth E, Kaiser PR, Bassetti CL, Baumann CR. Sleep-wake disturbances 3 years after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2010; 81(12): 1402–1405. [DOI] [PubMed] [Google Scholar]
- 8. Ouellet MC, Savard J, Morin CM. Insomnia following traumatic brain injury: a review. Neurorehabil Neural Repair. 2004; 18(4): 187–198. [DOI] [PubMed] [Google Scholar]
- 9. Mathias JL, Alvaro PK. Prevalence of sleep disturbances, disorders, and problems following traumatic brain injury: a meta-analysis. Sleep Med. 2012; 13(7): 898–905. [DOI] [PubMed] [Google Scholar]
- 10. Ponsford JL, Parcell DL, Sinclair KL, Roper M, Rajaratnam SM. Changes in sleep patterns following traumatic brain injury: a controlled study. Neurorehabil Neural Repair. 2013; 27(7): 613–621. [DOI] [PubMed] [Google Scholar]
- 11. Parcell DL, Ponsford JL, Redman JR, Rajaratnam SM. Poor sleep quality and changes in objectively recorded sleep after traumatic brain injury: a preliminary study. Arch Phys Med Rehabil. 2008; 89(5): 843–850. [DOI] [PubMed] [Google Scholar]
- 12. Parcell DL, Ponsford JL, Rajaratnam SM, Redman JR. Self-reported changes to nighttime sleep after traumatic brain injury. Arch Phys Med Rehabil. 2006; 87(2): 278–285. [DOI] [PubMed] [Google Scholar]
- 13. Cantor JB, Bushnik T, Cicerone K, et al. Insomnia, fatigue, and sleepiness in the first 2 years after traumatic brain injury: an NIDRR TBI model system module study. J Head Trauma Rehabil. 2012; 27(6): E1–14. [DOI] [PubMed] [Google Scholar]
- 14. Lu W, Cantor JB, Gordon W, et al. The relationship between self-reported sleep disturbance and polysomnography in individuals with traumatic brain injury. Arch Phys Med Rehabil. 2015;95(10):e5. [DOI] [PubMed] [Google Scholar]
- 15. Makley MJ, English JB, Drubach DA, Kreuz AJ, Celnik PA, Tarwater PM. Prevalence of sleep disturbance in closed head injury patients in a rehabilitation unit. Neurorehabil Neural Repair. 2008; 22(4): 341–347. [DOI] [PubMed] [Google Scholar]
- 16. Rao V, Spiro J, Vaishnavi S, et al. Prevalence and types of sleep disturbances acutely after traumatic brain injury. Brain Inj. 2008; 22(5): 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Verma A, Anand V, Verma NP. Sleep disorders in chronic traumatic brain injury. J Clin Sleep Med. 2007; 3(4): 357–362. [PMC free article] [PubMed] [Google Scholar]
- 18. Mani A, Dastgheib SA, Chanor A, Khalili H, Ahmadzadeh L, Ahmadi J. Sleep Quality among Patients with Mild Traumatic Brain Injury: A Cross-Sectional Study. Bull Emerg Trauma. 2015; 3(3): 93–96. [PMC free article] [PubMed] [Google Scholar]
- 19. King NS, Crawford S, Wenden FJ, Moss NE, Wade DT. The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol. 1995; 242(9): 587–592. [DOI] [PubMed] [Google Scholar]
- 20. Chaput G, Giguère JF, Chauny JM, Denis R, Lavigne G. Relationship among subjective sleep complaints, headaches, and mood alterations following a mild traumatic brain injury. Sleep Med. 2009; 10(7): 713–716. [DOI] [PubMed] [Google Scholar]
- 21. Haboubi NH, Long J, Koshy M, Ward AB. Short-term sequelae of minor head injury (6 years experience of minor head injury clinic). Disabil Rehabil. 2001; 23(14): 635–638. [DOI] [PubMed] [Google Scholar]
- 22. Gardani M, Morfiri E, Thomson A, O’Neill B, McMillan TM. Evaluation of Sleep Disorders in Patients with Severe Traumatic Brain Injury during Rehabilitation. Arch Phys Med Rehabil. 2015;96(9):1691–1697e3. [DOI] [PubMed] [Google Scholar]
- 23. Boakye PA, Olechowski C, Rashiq S, et al. A Critical Review of Neurobiological Factors Involved in the Interactions Between Chronic Pain, Depression, and Sleep Disruption. Clin J Pain. 2016; 32(4): 327–336. [DOI] [PubMed] [Google Scholar]
- 24. Theadom A, Rowland V, Levack W, Starkey N, Wilkinson-Meyers L, McPherson K; TBI Experiences Group Exploring the experience of sleep and fatigue in male and female adults over the 2 years following traumatic brain injury: a qualitative descriptive study. BMJ Open. 2016; 6(4): e010453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beetar JT, Guilmette TJ, Sparadeo FR. Sleep and pain complaints in symptomatic traumatic brain injury and neurologic populations. Arch Phys Med Rehabil. 1996; 77(12): 1298–1302. [DOI] [PubMed] [Google Scholar]
- 26. Mahmood O, Rapport LJ, Hanks RA, Fichtenberg NL. Neuropsychological performance and sleep disturbance following traumatic brain injury. J Head Trauma Rehabil. 2004; 19(5): 378–390. [DOI] [PubMed] [Google Scholar]
- 27. Lavigne G, Khoury S, Chauny JM, Desautels A. Pain and sleep in post-concussion/mild traumatic brain injury. Pain. 2015; 156Suppl 1: S75–S85. [DOI] [PubMed] [Google Scholar]
- 28. Martindale SL, Morissette SB, Rowland JA, Dolan SL. Sleep quality affects cognitive functioning in returning combat veterans beyond combat exposure, PTSD, and mild TBI history. Neuropsychology. 2017; 31(1): 93–104. [DOI] [PubMed] [Google Scholar]
- 29. Singh K, Morse A, Tkachenko N, Kothare SV. Sleep Disorders Associated With Traumatic Brain Injury. Pediatr Neurol. 2016;60:1–4. [DOI] [PubMed] [Google Scholar]
- 30. Castriotta RJ, Lai JM. Sleep disorders associated with traumatic brain injury. Arch Phys Med Rehabil. 2001; 82(10): 1403–1406. [DOI] [PubMed] [Google Scholar]
- 31. Grima N, Ponsford J, Rajaratnam SM, Mansfield D, Pase MP. Sleep Disturbances in Traumatic Brain Injury: A Meta-Analysis. J Clin Sleep Med. 2016; 12(3): 419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lucke-Wold BP, Smith KE, Nguyen L, et al. Sleep disruption and the sequelae associated with traumatic brain injury. Neurosci Biobehav Rev. 2015; 55: 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mazwi NL, Fusco H, Zafonte R.Sleep in Traumatic Brain Injury. Vol 128 1st ed Elsevier Ltd.; 2015. [DOI] [PubMed] [Google Scholar]
- 34. Ponsford JL, Ziino C, Parcell DL, et al. Fatigue and sleep disturbance following traumatic brain injury–their nature, causes, and potential treatments. J Head Trauma Rehabil. 2012; 27(3): 224–233. [DOI] [PubMed] [Google Scholar]
- 35. Vermaelen J, Greiffenstein P, deBoisblanc BP. Sleep in traumatic brain injury. Crit Care Clin. 2015; 31(3): 551–561. [DOI] [PubMed] [Google Scholar]
- 36. Viola-Saltzman M, Musleh C. Traumatic brain injury-induced sleep disorders. Neuropsychiatr Dis Treat. 2016; 12: 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Viola-Saltzman M, Watson NF. Traumatic brain injury and sleep disorders. Neurol Clin. 2012; 30(4): 1299–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ouellet MC, Beaulieu-Bonneau S, Morin CM. Sleep-wake disturbances after traumatic brain injury. Lancet Neurol. 2015; 14(7): 746–757. [DOI] [PubMed] [Google Scholar]
- 39. Wickwire EM, Williams SG, Roth T, et al. Sleep, Sleep Disorders, and Mild Traumatic Brain Injury. What We Know and What We Need to Know: Findings from a National Working Group. Neurotherapeutics. 2016; 13(2): 403–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gilbert KS, Kark SM, Gehrman P, Bogdanova Y. Sleep disturbances, TBI and PTSD: Implications for treatment and recovery. Clin Psychol Rev. 2015; 40: 195–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rao V, Rollings P. Sleep Disturbances Following Traumatic Brain Injury. Curr Treat Options Neurol. 2002; 4(1): 77–87. [DOI] [PubMed] [Google Scholar]
- 42. Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974; 2(7872): 81–84. [DOI] [PubMed] [Google Scholar]
- 43. Ruff RM, Jurica P. In search of a unified definition for mild traumatic brain injury. Brain Inj. 1999; 13(12): 943–952. [DOI] [PubMed] [Google Scholar]
- 44. Roozenbeek B, Maas AI, Menon DK. Changing patterns in the epidemiology of traumatic brain injury. Nat Rev Neurol. 2013; 9(4): 231–236. [DOI] [PubMed] [Google Scholar]
- 45. Stein SC, Ross SE. Moderate head injury: a guide to initial management. J Neurosurg. 1992; 77(4): 562–564. [DOI] [PubMed] [Google Scholar]
- 46. Fabbri A, Servadei F, Marchesini G, Stein SC, Vandelli A. Early predictors of unfavourable outcome in subjects with moderate head injury in the emergency department. J Neurol Neurosurg Psychiatry. 2008; 79(5): 567–573. [DOI] [PubMed] [Google Scholar]
- 47. Compagnone C, d’Avella D, Servadei F, et al. Patients with moderate head injury: a prospective multicenter study of 315 patients. Neurosurgery. 2009; 64(4): 690–6; discussion 696. [DOI] [PubMed] [Google Scholar]
- 48. Zetterberg H, Smith DH, Blennow K. Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nat Rev Neurol. 2013; 9(4): 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wiseman-Hakes C, Duclos C, Blais H, et al. Sleep in the Acute Phase of Severe Traumatic Brain Injury: A Snapshot of Polysomnography. Neurorehabil Neural Repair. 2016; 30(8): 713–721. [DOI] [PubMed] [Google Scholar]
- 50. Theadom A, Cropley M, Parmar P, et al. ; BIONIC Research Group. Sleep difficulties one year following mild traumatic brain injury in a population-based study. Sleep Med. 2015; 16(8): 926–932. [DOI] [PubMed] [Google Scholar]
- 51. Nakase-Richardson R, Sherer M, Barnett SD, et al. Prospective evaluation of the nature, course, and impact of acute sleep abnormality after traumatic brain injury. Arch Phys Med Rehabil. 2013; 94(5): 875–882. [DOI] [PubMed] [Google Scholar]
- 52. Imbach LL, Büchele F, Valko PO, et al. Sleep-wake disorders persist 18 months after traumatic brain injury but remain underrecognized. Neurology. 2016; 86(21): 1945–1949. [DOI] [PubMed] [Google Scholar]
- 53. Cantu D, Walker K, Andresen L, et al. Traumatic Brain Injury Increases Cortical Glutamate Network Activity by Compromising GABAergic Control. Cereb Cortex. 2015; 25(8): 2306–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. American Acadamy of Sleep Medicine, ed. International Classification of Sleep Disorders. 3rd ed Darian, IL: American Acadamy of Sleep Medicine; 2014. [Google Scholar]
- 55. Zeitzer JM, Friedman L, O’Hara R. Insomnia in the context of traumatic brain injury. J Rehabil Res Dev. 2009; 46(6): 827–836. [DOI] [PubMed] [Google Scholar]
- 56. Ouellet MC, Beaulieu-Bonneau S, Morin CM. Insomnia in patients with traumatic brain injury: frequency, characteristics, and risk factors. J Head Trauma Rehabil. 2006; 21(3): 199–212. [DOI] [PubMed] [Google Scholar]
- 57. Bryan CJ. Repetitive traumatic brain injury (or concussion) increases severity of sleep disturbance among deployed military personnel. Sleep. 2013; 36(6): 941–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ouellet MC, Morin CM. Subjective and objective measures of insomnia in the context of traumatic brain injury: a preliminary study. Sleep Med. 2006; 7(6): 486–497. [DOI] [PubMed] [Google Scholar]
- 59. Imbach LL, Valko PO, Li T, et al. Increased sleep need and daytime sleepiness 6 months after traumatic brain injury: a prospective controlled clinical trial. Brain. 2016; 138(Pt 3): 726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Masel BE, Scheibel RS, Kimbark T, Kuna ST. Excessive daytime sleepiness in adults with brain injuries. Arch Phys Med Rehabil. 2001; 82(11): 1526–1532. [DOI] [PubMed] [Google Scholar]
- 61. Sommerauer M, Valko PO, Werth E, Baumann CR. Excessive sleep need following traumatic brain injury: a case-control study of 36 patients. J Sleep Res. 2013; 22(6): 634–639. [DOI] [PubMed] [Google Scholar]
- 62. Webster JB, Bell KR, Hussey JD, Natale TK, Lakshminarayan S. Sleep apnea in adults with traumatic brain injury: a preliminary investigation. Arch Phys Med Rehabil. 2001; 82(3): 316–321. [DOI] [PubMed] [Google Scholar]
- 63. Guilleminault C, Yuen KM, Gulevich MG, Karadeniz D, Leger D, Philip P. Hypersomnia after head-neck trauma: a medicolegal dilemma. Neurology. 2000; 54(3): 653–659. [DOI] [PubMed] [Google Scholar]
- 64. Collen J, Orr N, Lettieri CJ, Carter K, Holley AB. Sleep disturbances among soldiers with combat-related traumatic brain injury. Chest. 2012; 142(3): 622–630. [DOI] [PubMed] [Google Scholar]
- 65. Ayalon L, Borodkin K, Dishon L, Kanety H, Dagan Y. Circadian rhythm sleep disorders following mild traumatic brain injury. Neurology. 2007; 68(14): 1136–1140. [DOI] [PubMed] [Google Scholar]
- 66. Shekleton JA, Parcell DL, Redman JR, Phipps-Nelson J, Ponsford JL, Rajaratnam SM. Sleep disturbance and melatonin levels following traumatic brain injury. Neurology. 2010; 74(21): 1732–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kaufman Y, Tzischinsky O, Epstein R, Etzioni A, Lavie P, Pillar G. Long-term sleep disturbances in adolescents after minor head injury. Pediatr Neurol. 2001; 24(2): 129–134. [DOI] [PubMed] [Google Scholar]
- 68. Pillar G, Averbooch E, Katz N, Peled N, Kaufman Y, Shahar E. Prevalence and risk of sleep disturbances in adolescents after minor head injury. Pediatr Neurol. 2003; 29(2): 131–135. [DOI] [PubMed] [Google Scholar]
- 69. Mysliwiec V, O’Reilly B, Polchinski J, Kwon HP, Germain A, Roth BJ. Trauma associated sleep disorder: a proposed parasomnia encompassing disruptive nocturnal behaviors, nightmares, and REM without atonia in trauma survivors. J Clin Sleep Med. 2014; 10(10): 1143–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Stefani A, Gabelia D, Högl B, et al. Long-Term Follow-up Investigation of Isolated Rapid Eye Movement Sleep Without Atonia Without Rapid Eye Movement Sleep Behavior Disorder: A Pilot Study. J Clin Sleep Med. 2015; 11(11): 1273–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Borgaro SR, Baker J, Wethe JV, Prigatano GP, Kwasnica C. Subjective reports of fatigue during early recovery from traumatic brain injury. J Head Trauma Rehabil. 2005; 20(5): 416–425. [DOI] [PubMed] [Google Scholar]
- 72. Beaulieu-Bonneau S, Morin CM. Sleepiness and fatigue following traumatic brain injury. Sleep Med. 2012; 13(6): 598–605. [DOI] [PubMed] [Google Scholar]
- 73. Schiehser DM, Delano-Wood L, Jak AJ, et al. Predictors of cognitive and physical fatigue in post-acute mild-moderate traumatic brain injury. Neuropsychol Rehabil. 2016. [DOI] [PubMed] [Google Scholar]
- 74. Lezak MD. Subtle sequelae of brain damage. Perplexity, distractibility, and fatigue. Am J Phys Med. 1978; 57(1): 9–15. [PubMed] [Google Scholar]
- 75. Arciniegas DB, Topkoff J, Silver JM. Neuropsychiatric Aspects of Traumatic Brain Injury. Curr Treat Options Neurol. 2000; 2(2): 169–186. [DOI] [PubMed] [Google Scholar]
- 76. Cantor JB, Ashman T, Gordon W, et al. Fatigue after traumatic brain injury and its impact on participation and quality of life. J Head Trauma Rehabil. 2008; 23(1): 41–51. [DOI] [PubMed] [Google Scholar]
- 77. Bushnik T, Englander J, Wright J. Patterns of fatigue and its correlates over the first 2 years after traumatic brain injury. J Head Trauma Rehabil. 2008; 23(1): 25–32. [DOI] [PubMed] [Google Scholar]
- 78. Bushnik T, Englander J, Wright J. The experience of fatigue in the first 2 years after moderate-to-severe traumatic brain injury: a preliminary report. J Head Trauma Rehabil. 2008; 23(1): 17–24. [DOI] [PubMed] [Google Scholar]
- 79. Englander J, Bushnik T, Oggins J, Katznelson L. Fatigue after traumatic brain injury: Association with neuroendocrine, sleep, depression and other factors. Brain Inj. 2010; 24(12): 1379–1388. [DOI] [PubMed] [Google Scholar]
- 80. Beebe DW, Krivitzky L, Wells CT, Wade SL, Taylor HG, Yeates KO. Brief report: parental report of sleep behaviors following moderate or severe pediatric traumatic brain injury. J Pediatr Psychol. 2007; 32(7): 845–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hooper SR, Alexander J, Moore D, et al. Caregiver reports of common symptoms in children following a traumatic brain injury. NeuroRehabilitation. 2004; 19(3): 175–189. [PubMed] [Google Scholar]
- 82. Stores G, Stores R. Sleep disorders in children with traumatic brain injury: a case of serious neglect. Dev Med Child Neurol. 2013; 55(9): 797–805. [DOI] [PubMed] [Google Scholar]
- 83. Blinman TA, Houseknecht E, Snyder C, Wiebe DJ, Nance ML. Postconcussive symptoms in hospitalized pediatric patients after mild traumatic brain injury. J Pediatr Surg. 2009; 44(6): 1223–1228. [DOI] [PubMed] [Google Scholar]
- 84. Theadom A, Starkey N, Jones K, et al. Sleep difficulties and their impact on recovery following mild traumatic brain injury in children. Brain Inj. 2016; 30(10): 1243–1248. [DOI] [PubMed] [Google Scholar]
- 85. Tham SW, Fales J, Palermo TM. Subjective and Objective Assessment of Sleep in Adolescents with Mild Traumatic Brain Injury. J Neurotrauma. 2015;852:1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sumpter RE, Dorris L, Kelly T, McMillan TM. Pediatric sleep difficulties after moderate-severe traumatic brain injury. J Int Neuropsychol Soc. 2013; 19(7): 829–834. [DOI] [PubMed] [Google Scholar]
- 87. Milroy G, Dorris L, McMillan TM. Sleep disturbances following mild traumatic brain injury in childhood. J Pediatr Psychol. 2008; 33(3): 242–247. [DOI] [PubMed] [Google Scholar]
- 88. Shay N, Yeates KO, Walz NC, et al. Sleep problems and their relationship to cognitive and behavioral outcomes in young children with traumatic brain injury. J Neurotrauma. 2014; 31(14): 1305–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tham SW, Palermo TM, Vavilala MS, et al. The longitudinal course, risk factors, and impact of sleep disturbances in children with traumatic brain injury. J Neurotrauma. 2012; 29(1): 154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Frank MG, Cantera R. Sleep, clocks, and synaptic plasticity. Trends Neurosci. 2014; 37(9): 491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Maret S, Faraguna U, Nelson AB, Cirelli C, Tononi G. Sleep and waking modulate spine turnover in the adolescent mouse cortex. Nat Neurosci. 2011; 14(11): 1418–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Plog BA, Dashnaw ML, Hitomi E, et al. Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J Neurosci. 2015; 35(2): 518–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tononi G, Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014; 81(1): 12–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Rao V, Bergey A, Hill H, Efron D, McCann U. Sleep disturbance after mild traumatic brain injury: indicator of injury? J Neuropsychiatry Clin Neurosci. 2011; 23(2): 201–205. [DOI] [PubMed] [Google Scholar]
- 95. Khoury S, Chouchou F, Amzica F, et al. Rapid EEG activity during sleep dominates in mild traumatic brain injury patients with acute pain. J Neurotrauma. 2013; 30(8): 633–641. [DOI] [PubMed] [Google Scholar]
- 96. Gosselin N, Lassonde M, Petit D, et al. Sleep following sport-related concussions. Sleep Med. 2009; 10(1): 35–46. [DOI] [PubMed] [Google Scholar]
- 97. Parsons LC, Crosby LJ, Perlis M, Britt T, Jones P. Longitudinal sleep EEG power spectral analysis studies in adolescents with minor head injury. J Neurotrauma. 1997; 14(8): 549–559. [DOI] [PubMed] [Google Scholar]
- 98. Jones CE, Lim MM. Phasic Sleep Events Shape Cognitive Function after Traumatic Brain Injury: Implications for the Study of Sleep in Neurodevelopmental Disorders. AIMS Neurosci. 2016;3(2):232–236. [Google Scholar]
- 99. Cote KA, Milner CE, Speth TA. Altered Sleep Mechanisms following Traumatic Brain Injury and Relation to Waking Function. AIMS Neurosci. 2015;2(4):203–228. [Google Scholar]
- 100. Modarres M, Kuzma N, Kretzmer T, Pack AI, Lim MM. EEG slow waves in traumatic brain injury: Convergent findings in mouse and man. Neurobiol Sleep Circadian Rhythm. 2016. [PMC free article] [PubMed] [Google Scholar]
- 101. DelRosso LM, Hoque R, James S, Gonzalez-Toledo E, Chesson AL.Jr. Sleep-wake pattern following gunshot suprachiasmatic damage. J Clin Sleep Med. 2014; 10(4): 443–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Yassin W, Sugihara G, Oishi N, et al. Hypothalamic-amygdalar-brainstem volume reduction in a patient with narcolepsy secondary to diffuse axonal injury. J Clin Sleep Med. 2015; 11(5): 581–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hou L, Han X, Sheng P, et al. Risk factors associated with sleep disturbance following traumatic brain injury: clinical findings and questionnaire based study. PLoS One. 2013; 8(10): 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Crompton MR. Brainstem lesions due to closed head injury. Lancet. 1971; 1(7701): 669–673. [DOI] [PubMed] [Google Scholar]
- 105. Parvizi J, Damasio AR. Neuroanatomical correlates of brainstem coma. Brain. 2003; 126(Pt 7): 1524–1536. [DOI] [PubMed] [Google Scholar]
- 106. Valko PO, Gavrilov YV, Yamamoto M, et al. Damage to Arousal-Promoting Brainstem Neurons with Traumatic Brain Injury. Sleep. 2016; 39(6): 1249–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Peyron C, Tighe DK, van den Pol AN, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998; 18(23): 9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Gotter AL, Winrow CJ, Brunner J, et al. The duration of sleep promoting efficacy by dual orexin receptor antagonists is dependent upon receptor occupancy threshold. BMC Neurosci. 2013; 14(1): 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zeitzer JM, Buckmaster CL, Parker KJ, Hauck CM, Lyons DM, Mignot E. Circadian and homeostatic regulation of hypocretin in a primate model: implications for the consolidation of wakefulness. J Neurosci. 2003; 23(8): 3555–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Hagan JJ, Leslie RA, Patel S, et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A. 1999; 96(19): 10911–10916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Piper DC, Upton N, Smith MI, Hunter AJ. The novel brain neuropeptide, orexin-A, modulates the sleep-wake cycle of rats. Eur J Neurosci. 2000; 12(2): 726–730. [DOI] [PubMed] [Google Scholar]
- 112. Nishino S, Mignot E. Pharmacological aspects of human and canine narcolepsy. Prog Neurobiol. 1997; 52(1): 27–78. [DOI] [PubMed] [Google Scholar]
- 113. Lin L, Faraco J, Li R, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999; 98(3): 365–376. [DOI] [PubMed] [Google Scholar]
- 114. Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000; 355(9197): 39–40. [DOI] [PubMed] [Google Scholar]
- 115. Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999; 98(4): 437–451. [DOI] [PubMed] [Google Scholar]
- 116. Christopher JA. Orexin receptor antagonists. Pharm Pat Anal. 2012; 1(3): 329–346. [DOI] [PubMed] [Google Scholar]
- 117. Gotter AL, Forman MS, Harrell CM, et al. Orexin 2 Receptor Antagonism is Sufficient to Promote NREM and REM Sleep from Mouse to Man. Sci Rep. 2016; 6: 27147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Baumann CR, Bassetti CL, Valko PO, et al. Loss of hypocretin (orexin) neurons with traumatic brain injury. Ann Neurol. 2009; 66(4): 555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000; 6(9): 991–997. [DOI] [PubMed] [Google Scholar]
- 120. Valko PO, Gavrilov YV, Yamamoto M, et al. Damage to histaminergic tuberomammillary neurons and other hypothalamic neurons with traumatic brain injury. Ann Neurol. 2015; 77(1): 177–182. [DOI] [PubMed] [Google Scholar]
- 121. Naseem M, Parvez S. Role of melatonin in traumatic brain injury and spinal cord injury. ScientificWorldJournal. 2014; 2014: 586270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kemp S, Biswas R, Neumann V, Coughlan A. The value of melatonin for sleep disorders occurring post-head injury: a pilot RCT. Brain Inj. 2004; 18(9): 911–919. [DOI] [PubMed] [Google Scholar]
- 123. Seifman MA, Gomes K, Nguyen PN, et al. Measurement of serum melatonin in intensive care unit patients: changes in traumatic brain injury, trauma, and medical conditions. Front Neurol. 2014; 5: 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Grima NA, Ponsford JL, St Hilaire MA, Mansfield D, Rajaratnam SM. Circadian Melatonin Rhythm Following Traumatic Brain Injury. Neurorehabil Neural Repair. 2016; 30(10): 972–977. [DOI] [PubMed] [Google Scholar]
- 125. Naidoo N, Ferber M, Galante RJ, et al. Role of Homer proteins in the maintenance of sleep-wake states. PLoS One. 2012; 7(4): e35174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Naylor E, Aillon DV, Gabbert S, et al. Simultaneous real-time measurement of EEG/EMG and L-glutamate in mice: A biosensor study of neuronal activity during sleep. J Electroanal Chem (Lausanne). 2011; 656(1-2): 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Nilsson P, Ronne-Engström E, Flink R, Ungerstedt U, Carlson H, Hillered L. Epileptic seizure activity in the acute phase following cortical impact trauma in rat. Brain Res. 1994; 637(1-2): 227–232. [DOI] [PubMed] [Google Scholar]
- 128. Folkersma H, Foster Dingley JC, van Berckel BN, et al. Increased cerebral ®-[(11)C]PK11195 uptake and glutamate release in a rat model of traumatic brain injury: a longitudinal pilot study. J Neuroinflammation. 2011; 8(1): 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Chamoun R, Suki D, Gopinath SP, Goodman JC, Robertson C. Role of extracellular glutamate measured by cerebral microdialysis in severe traumatic brain injury. J Neurosurg. 2010; 113(3): 564–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. De Luche SE, Churchill MJ, Cocking DL, Moore C, Meshul CK, Lim MM. Glutamatergic input to orexin neurons is decreased after mild traumatic brain injury and restored by dietary therapy. SLEEP (abstact). 2015:p.55. [Google Scholar]
- 131. Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci. 2014; 14(2): 128–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Albert-Weissenberger C, Sirén AL. Experimental traumatic brain injury. Exp Transl Stroke Med. 2010; 2(1): 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Shultz SR, McDonald SJ, Haar CV, et al. The potential for animal models to provide insight into mild traumatic brain injury: translational challenges and strategies. Neurosci Biobehav Rev. 2016. [DOI] [PubMed] [Google Scholar]
- 134. Chiu CC, Liao YE, Yang LY, et al. Neuroinflammation in animal models of traumatic brain injury. J Neurosci Methods. 2016; 272: 38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Dixon CE, Lyeth BG, Povlishock JT, et al. A fluid percussion model of experimental brain injury in the rat. J Neurosurg. 1987; 67(1): 110–119. [DOI] [PubMed] [Google Scholar]
- 136. Lyeth BG. Historical Review of the Fluid-Percussion TBI Model. Front Neurol. 2016; 7: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Thompson HJ, Lifshitz J, Marklund N, et al. Lateral fluid percussion brain injury: a 15-year review and evaluation. J Neurotrauma. 2005; 22(1): 42–75. [DOI] [PubMed] [Google Scholar]
- 138. McIntosh TK, Noble L, Andrews B, Faden AI. Traumatic brain injury in the rat: characterization of a midline fluid-percussion model. Cent Nerv Syst Trauma. 1987; 4(2): 119–134. [DOI] [PubMed] [Google Scholar]
- 139. McIntosh TK, Vink R, Noble L, et al. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience. 1989; 28(1): 233–244. [DOI] [PubMed] [Google Scholar]
- 140. Carbonell WS, Maris DO, McCall T, Grady MS. Adaptation of the fluid percussion injury model to the mouse. J Neurotrauma. 1998; 15(3): 217–229. [DOI] [PubMed] [Google Scholar]
- 141. Morehead M, Bartus RT, Dean RL, et al. Histopathologic consequences of moderate concussion in an animal model: correlations with duration of unconsciousness. J Neurotrauma. 1994; 11(6): 657–667. [DOI] [PubMed] [Google Scholar]
- 142. Kabadi SV, Hilton GD, Stoica BA, Zapple DN, Faden AI. Fluid-percussion-induced traumatic brain injury model in rats. Nat Protoc. 2010; 5(9): 1552–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Rowe RK, Griffiths DR, Lifshitz J.Midline (Central) Fluid Percussion Model of Traumatic Brain Injury. Vol 1462 (Kobeissy F, ed.). Springer Science+Business Media; 2016. [DOI] [PubMed] [Google Scholar]
- 144. Lifshitz J, Rowe RK, Griffiths DR, et al. Clinical relevance of midline fluid percussion brain injury: Acute deficits, chronic morbidities and the utility of biomarkers. Brain Inj. 2016; 30(11): 1293–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Smith CJ, Xiong G, Elkind JA, Putnam B, Cohen AS. Brain Injury Impairs Working Memory and Prefrontal Circuit Function. Front Neurol. 2015; 6: 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Smith CJ, Johnson BN, Elkind JA, See JM, Xiong G, Cohen AS. Investigations on alterations of hippocampal circuit function following mild traumatic brain injury. J Vis Exp. 2012;(69):e4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Palmer CP, Metheny HE, Elkind JA, Cohen AS. Diminished amygdala activation and behavioral threat response following traumatic brain injury. Exp Neurol. 2016; 277: 215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Paterno R, Metheny H, Xiong G, Elkind J, Cohen AS. Mild Traumatic Brain Injury Decreases Broadband Power in Area CA1. J Neurotrauma. 2016; 33(17): 1645–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Beamer M, Tummala SR, Gullotti D, et al. Primary blast injury causes cognitive impairments and hippocampal circuit alterations. Exp Neurol. 2016; 283(Pt A): 16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Fritz HG, Walter B, Holzmayr M, Brodhun M, Patt S, Bauer R. A pig model with secondary increase of intracranial pressure after severe traumatic brain injury and temporary blood loss. J Neurotrauma. 2005; 22(7): 807–821. [DOI] [PubMed] [Google Scholar]
- 151. Balança B, Bapteste L, Lieutaud T, et al. Neuronal loss as evidenced by automated quantification of neuronal density following moderate and severe traumatic brain injury in rats. J Neurosci Res. 2016; 94(1): 39–49. [DOI] [PubMed] [Google Scholar]
- 152. Pierce JE, Smith DH, Trojanowski JQ, McIntosh TK. Enduring cognitive, neurobehavioral and histopathological changes persist for up to one year following severe experimental brain injury in rats. Neuroscience. 1998; 87(2): 359–369. [DOI] [PubMed] [Google Scholar]
- 153. Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991; 39(3): 253–262. [DOI] [PubMed] [Google Scholar]
- 154. Goodman JC, Cherian L, Bryan RM, Jr, Robertson CS. Lateral cortical impact injury in rats: pathologic effects of varying cortical compression and impact velocity. J Neurotrauma. 1994; 11(5): 587–597. [DOI] [PubMed] [Google Scholar]
- 155. Willie JT, Lim MM, Bennett RE, Azarion AA, Schwetye KE, Brody DL. Controlled cortical impact traumatic brain injury acutely disrupts wakefulness and extracellular orexin dynamics as determined by intracerebral microdialysis in mice. J Neurotrauma. 2012; 29(10): 1908–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Flierl MA, Stahel PF, Beauchamp KM, Morgan SJ, Smith WR, Shohami E. Mouse closed head injury model induced by a weight-drop device. Nat Protoc. 2009; 4(9): 1328–1337. [DOI] [PubMed] [Google Scholar]
- 157. Sabir M, Gaudreault PO, Freyburger M, et al. Impact of traumatic brain injury on sleep structure, electrocorticographic activity and transcriptome in mice. Brain Behav Immun. 2015; 47: 118–130. [DOI] [PubMed] [Google Scholar]
- 158. Büchele F, Morawska MM, Schreglmann SR, et al. Novel Rat Model of Weight Drop-Induced Closed Diffuse Traumatic Brain Injury Compatible with Electrophysiological Recordings of Vigilance States. J Neurotrauma. 2016; 33(13): 1171–1180. [DOI] [PubMed] [Google Scholar]
- 159. Morawska MM, Büchele F, Moreira CG, Imbach LL, Noain D, Baumann CR. Sleep Modulation Alleviates Axonal Damage and Cognitive Decline after Rodent Traumatic Brain Injury. J Neurosci. 2016; 36(12): 3422–3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Foda MA, Marmarou A. A new model of diffuse brain injury in rats. Part II: Morphological characterization. J Neurosurg. 1994; 80(2): 301–313. [DOI] [PubMed] [Google Scholar]
- 161. Marmarou A, Foda MA, van den Brink W, Campbell J, Kita H, Demetriadou K. A new model of diffuse brain injury in rats. Part I: Pathophysiology and biomechanics. J Neurosurg. 1994; 80(2): 291–300. [DOI] [PubMed] [Google Scholar]
- 162. Mulkey DK, Henderson RA, 3rd , Olson JE, Putnam RW, Dean JB. Oxygen measurements in brain stem slices exposed to normobaric hyperoxia and hyperbaric oxygen. J Appl Physiol (1985). 2001; 90(5): 1887–1899. [DOI] [PubMed] [Google Scholar]
- 163. Dean JB, Mulkey DK, Henderson RA, 3rd , Potter SJ, Putnam RW. Hyperoxia, reactive oxygen species, and hyperventilation: oxygen sensitivity of brain stem neurons. J Appl Physiol (1985). 2004; 96(2): 784–791. [DOI] [PubMed] [Google Scholar]
- 164. Lim MM, Elkind J, Xiong G, et al. Dietary therapy mitigates persistent wake deficits caused by mild traumatic brain injury. Sci Transl Med. 2013; 5(215): 215ra173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Skopin MD, Kabadi SV, Viechweg SS, Mong JA, Faden AI. Chronic decrease in wakefulness and disruption of sleep-wake behavior after experimental traumatic brain injury. J Neurotrauma. 2015; 32(5): 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Cole JT, Mitala CM, Kundu S, et al. Dietary branched chain amino acids ameliorate injury-induced cognitive impairment. Proc Natl Acad Sci. 2010;107(5):2373–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Thomasy HE, Febinger HY, Ringgold KM, Gemma C, Opp MR. Hypocretinergic and cholinergic contributions to sleep-wake disturbances in a mouse model of traumatic brain injury. Neurobiol Sleep Circadian Rhythm. 2016:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Hazra A, Macolino C, Elliott MB, Chin J. Delayed thalamic astrocytosis and disrupted sleep-wake patterns in a preclinical model of traumatic brain injury. J Neurosci Res. 2014; 92(11): 1434–1445. [DOI] [PubMed] [Google Scholar]
- 169. Rowe RK, Striz M, Bachstetter AD, et al. Diffuse brain injury induces acute post-traumatic sleep. PLoS One. 2014; 9(1): e82507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Rowe RK, Harrison JL, O’Hara BF, Lifshitz J. Recovery of neurological function despite immediate sleep disruption following diffuse brain injury in the mouse: clinical relevance to medically untreated concussion. Sleep. 2014; 37(4): 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Rowe RK, Harrison JL, O’Hara BF, Lifshitz J. Diffuse brain injury does not affect chronic sleep patterns in the mouse. Brain Inj. 2014; 28(4): 504–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Olson E, Badder C, Sullivan S, Smith C, Propert K, Margulies SS. Alterations in Daytime and Nighttime Activity in Piglets after Focal and Diffuse Brain Injury. J Neurotrauma. 2016; 33(8): 734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Petraglia AL, Plog BA, Dayawansa S, et al. The spectrum of neurobehavioral sequelae after repetitive mild traumatic brain injury: a novel mouse model of chronic traumatic encephalopathy. J Neurotrauma. 2014; 31(13): 1211–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Miller RA, Nadon NL. Principles of animal use for gerontological research. J Gerontol A Biol Sci Med Sci. 2000; 55(3): B117–B123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Berryman DE, List EO, Palmer AJ, et al. Two-year body composition analyses of long-lived GHR null mice. J Gerontol A Biol Sci Med Sci. 2010; 65(1): 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Elliott JE, Mantilla CB, Pabelick CM, Roden AC, Sieck GC. Aging-related changes in respiratory system mechanics and morphometry in mice. Am J Physiol Lung Cell Mol Physiol. 2016; 311(1): L167–L176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Kumar A, Stoica BA, Sabirzhanov B, Burns MP, Faden AI, Loane DJ. Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiol Aging. 2013; 34(5): 1397–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. McPherson CA, Aoyama M, Harry GJ. Interleukin (IL)-1 and IL-6 regulation of neural progenitor cell proliferation with hippocampal injury: differential regulatory pathways in the subgranular zone (SGZ) of the adolescent and mature mouse brain. Brain Behav Immun. 2011; 25(5): 850–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Timaru-Kast R, Luh C, Gotthardt P, et al. Influence of age on brain edema formation, secondary brain damage and inflammatory response after brain trauma in mice. PLoS One. 2012; 7(8): e43829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Zielinski MR, McKenna JT, McCarley RW. Functions and Mechanisms of Sleep. AIMS Neurosci. 2016;3(1):–67–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Krueger JM, Frank MG, Wisor JP, Roy S. Sleep function: Toward elucidating an enigma. Sleep Med Rev. 2016; 28: 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007; 3(5): 519–528. [PMC free article] [PubMed] [Google Scholar]
- 183. Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005; 25(1): 117–129. [DOI] [PubMed] [Google Scholar]
- 184. Dinges DF. Stress, fatigue, and behavioral energy. Nutr Rev. 2001; 59(1): 30–32. [DOI] [PubMed] [Google Scholar]
- 185. Van Someren EJ, Riemersma-Van Der Lek RF. Live to the rhythm, slave to the rhythm. Sleep Med Rev. 2007; 11(6): 465–484. [DOI] [PubMed] [Google Scholar]
- 186. Billiard M, Podesta C. Recurrent hypersomnia following traumatic brain injury. Sleep Med. 2013; 14(5): 462–465. [DOI] [PubMed] [Google Scholar]
- 187. Kostyun RO, Milewski MD, Hafeez I. Sleep disturbance and neurocognitive function during the recovery from a sport-related concussion in adolescents. Am J Sports Med. 2015; 43(3): 633–640. [DOI] [PubMed] [Google Scholar]
- 188. Chan LG, Feinstein A. Persistent Sleep Disturbances Independently Predict Poorer Functional and Social Outcomes 1 Year After Mild Traumatic Brain Injury. J Head Trauma Rehabil. 2015; 30(6): E67–E75. [DOI] [PubMed] [Google Scholar]
- 189. Mollayeva T, Mollayeva S, Shapiro CM, Cassidy JD, Colantonio A. Insomnia in workers with delayed recovery from mild traumatic brain injury. Sleep Med. 2016; 19: 153–161. [DOI] [PubMed] [Google Scholar]
- 190. Mollayeva T, Pratt B, Mollayeva S, Shapiro CM, Cassidy JD, Colantonio A. The relationship between insomnia and disability in workers with mild traumatic brain injury/concussion: Insomnia and disability in chronic mild traumatic brain injury. Sleep Med. 2016; 20: 157–166. [DOI] [PubMed] [Google Scholar]
- 191. Holcomb EM, Towns S, Kamper JE, et al. The Relationship Between Sleep-Wake Cycle Disturbance and Trajectory of Cognitive Recovery During Acute Traumatic Brain Injury. J Head Trauma Rehabil. 2016; 31(2): 108–116. [DOI] [PubMed] [Google Scholar]
- 192. Makley MJ, Johnson-Greene L, Tarwater PM, et al. Return of memory and sleep efficiency following moderate to severe closed head injury. Neurorehabil Neural Repair. 2009; 23(4): 320–326. [DOI] [PubMed] [Google Scholar]
- 193. Sherer M, Yablon SA, Nakase-Richardson R. Patterns of recovery of posttraumatic confusional state in neurorehabilitation admissions after traumatic brain injury. Arch Phys Med Rehabil. 2009; 90(10): 1749–1754. [DOI] [PubMed] [Google Scholar]
- 194. Rao V, McCann U, Han D, Bergey A, Smith MT. Does acute TBI-related sleep disturbance predict subsequent neuropsychiatric disturbances? Brain Inj. 2014; 28(1): 20–26. [DOI] [PubMed] [Google Scholar]
- 195. Cologan V, Drouot X, Parapatics S, et al. Sleep in the unresponsive wakefulness syndrome and minimally conscious state. J Neurotrauma. 2013; 30(5): 339–346. [DOI] [PubMed] [Google Scholar]
- 196. Sandsmark DK, Kumar MA, Woodward CS, Schmitt SE, Park S, Lim MM. Sleep Features on Continuous Electroencephalography Predict Rehabilitation Outcomes After Severe Traumatic Brain Injury. J Head Trauma Rehabil. 2016; 31(2): 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Entholzner E, Mielke L, Pichlmeier R, Weber F, Schneck H. [EEG changes during sedation with gamma-hydroxybutyric acid]. Anaesthesist. 1995; 44(5): 345–350. [DOI] [PubMed] [Google Scholar]
- 198. Meerlo P, Westerveld P, Turek FW, Koehl M. Effects of gamma-hydroxybutyrate (GHB) on vigilance states and EEG in mice. Sleep. 2004; 27(5): 899–904. [DOI] [PubMed] [Google Scholar]
- 199. Bellesi M, Pfister-Genskow M, Maret S, Keles S, Tononi G, Cirelli C. Effects of sleep and wake on oligodendrocytes and their precursors. J Neurosci. 2013; 33(36): 14288–14300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Borbély AA, Tobler I, Hanagasioglu M. Effect of sleep deprivation on sleep and EEG power spectra in the rat. Behav Brain Res. 1984; 14(3): 171–182. [DOI] [PubMed] [Google Scholar]
- 201. Gao B, Cam E, Jaeger H, Zunzunegui C, Sarnthein J, Bassetti CL. Sleep disruption aggravates focal cerebral ischemia in the rat. Sleep. 2010; 33(7): 879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Gao B, Kilic E, Baumann CR, Hermann DM, Bassetti CL. Gamma-hydroxybutyrate accelerates functional recovery after focal cerebral ischemia. Cerebrovasc Dis. 2008; 26(4): 413–419. [DOI] [PubMed] [Google Scholar]
- 203. Vergoni AV, Ottani A, Botticelli AR, et al. Neuroprotective effect of gamma-hydroxybutyrate in transient global cerebral ischemia in the rat. Eur J Pharmacol. 2000; 397(1): 75–84. [DOI] [PubMed] [Google Scholar]
- 204. Ottani A, Saltini S, Bartiromo M, et al. Effect of gamma-hydroxybutyrate in two rat models of focal cerebral damage. Brain Res. 2003; 986(1-2): 181–190. [DOI] [PubMed] [Google Scholar]
- 205. Martinez-Vargas M, Estrada Rojo F, Tabla-Ramon E, et al. Sleep deprivation has a neuroprotective role in a traumatic brain injury of the rat. Neurosci Lett. 2012; 529(2): 118–122. [DOI] [PubMed] [Google Scholar]
- 206. Cam E, Gao B, Imbach L, Hodor A, Bassetti CL. Sleep deprivation before stroke is neuroprotective: a pre-ischemic conditioning related to sleep rebound. Exp Neurol. 2013; 247: 673–679. [DOI] [PubMed] [Google Scholar]
- 207. Riechers RG, Shuster JL, Bryan KJ, Burant CJ, Ball SL. Prior housing conditions and sleep loss may affect recovery from brain injury in rats: a pilot study. J Rehabil Res Dev. 2013; 50(4): 455–462. [DOI] [PubMed] [Google Scholar]
- 208. Caron AM, Stephenson R. Sleep deprivation does not affect neuronal susceptibility to mild traumatic brain injury in the rat. Nat Sci Sleep. 2015; 7: 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Junek A, Rusak B, Semba K. Short-term sleep deprivation may alter the dynamics of hippocampal cell proliferation in adult rats. Neuroscience. 2010; 170(4): 1140–1152. [DOI] [PubMed] [Google Scholar]
- 210. Grassi Zucconi G, Cipriani S, Balgkouranidou I, Scattoni R. “One night” sleep deprivation stimulates hippocampal neurogenesis. Brain Res Bull. 2006; 69(4): 375–381. [DOI] [PubMed] [Google Scholar]
- 211. Brandt JA, Churchill L, Guan Z, Fang J, Chen L, Krueger JM. Sleep deprivation but not a whisker trim increases nerve growth factor within barrel cortical neurons. Brain Res. 2001; 898(1): 105–112. [DOI] [PubMed] [Google Scholar]
- 212. Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013; 342(6156): 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Kang JE, Lim MM, Bateman RJ, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009; 326(5955): 1005–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Iliff JJ, Chen MJ, Plog BA, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci. 2014; 34(49): 16180–16193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Larson EB, Zollman FS. The effect of sleep medications on cognitive recovery from traumatic brain injury. J Head Trauma Rehabil. 2010; 25(1): 61–67. [DOI] [PubMed] [Google Scholar]
- 216. Barr T, Livingston W, Guardado P, Baxter T, Mysliwiec V, Gill J. Chapter 8 Military Personnel With Traumatic Brain Injuries and Insomnia Have Reductions in PTSD and Improved Perceived Health Following Sleep Restoration: A Relationship Moderated by Inflammation. Annu Rev Nurs Res. 2015; 33: 249–266. [DOI] [PubMed] [Google Scholar]
- 217. Wiseman-Hakes C, Murray B, Moineddin R, et al. Evaluating the impact of treatment for sleep/wake disorders on recovery of cognition and communication in adults with chronic TBI. Brain Inj. 2013; 27(12): 1364–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive Behavioral Therapy for Chronic Insomnia: A Systematic Review and Meta-analysis. Ann Intern Med. 2015; 163(3): 191–204. [DOI] [PubMed] [Google Scholar]
- 219. Vitiello MV. Cognitive-behavioural therapy for insomnia: effective, long-lasting and safe. Evid Based Ment Health. 2016; 19(1): e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Ouellet MC, Morin CM. Efficacy of cognitive-behavioral therapy for insomnia associated with traumatic brain injury. Arch Phys Med Rehabil. 2007; 88(12): 1581–1592. [DOI] [PubMed] [Google Scholar]
- 221. Zollman FS, Larson EB, Wasek-Throm LK, Cyborski CM, Bode RK. Acupuncture for treatment of insomnia in patients with traumatic brain injury: a pilot intervention study. J Head Trauma Rehabil. 2012; 27(2): 135–142. [DOI] [PubMed] [Google Scholar]
- 222. Sinclair KL, Ponsford JL, Taffe J, Lockley SW, Rajaratnam SM. Randomized controlled trial of light therapy for fatigue following traumatic brain injury. Neurorehabil Neural Repair. 2014; 28(4): 303–313. [DOI] [PubMed] [Google Scholar]
- 223. Damiano DL, Zampieri C, Ge J, Acevedo A, Dsurney J. Effects of a rapid-resisted elliptical training program on motor, cognitive and neurobehavioral functioning in adults with chronic traumatic brain injury. Exp Brain Res. 2016; 234(8): 2245–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Chin LM, Keyser RE, Dsurney J, Chan L. Improved cognitive performance following aerobic exercise training in people with traumatic brain injury. Arch Phys Med Rehabil. 2015; 96(4): 754–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Li Pi Shan R, Ashworth N. Comparison of Lorazepam and with Stroke and Brain Injury: A Randomized. Crossover, Double-Blinded Trial. Am J Phys Med Rehabil. 2004;83(June):421–427. [DOI] [PubMed] [Google Scholar]
- 226. Michelson D, Snyder E, Paradis E, et al. Safety and efficacy of suvorexant during 1-year treatment of insomnia with subsequent abrupt treatment discontinuation: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2014; 13(5): 461–471. [DOI] [PubMed] [Google Scholar]
- 227. Herring WJ, Snyder E, Budd K, et al. Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant. Neurology. 2012; 79(23): 2265–2274. [DOI] [PubMed] [Google Scholar]
- 228. Sun H, Kennedy WP, Wilbraham D, et al. Effects of suvorexant, an orexin receptor antagonist, on sleep parameters as measured by polysomnography in healthy men. Sleep. 2013; 36(2): 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Kaiser PR, Valko PO, Werth E, et al. Modafinil ameliorates excessive daytime sleepiness after traumatic brain injury. Neurology. 2010; 75(20): 1780–1785. [DOI] [PubMed] [Google Scholar]
- 230. Menn SJ, Yang R, Lankford A. Armodafinil for the treatment of excessive sleepiness associated with mild or moderate closed traumatic brain injury: a 12-week, randomized, double-blind study followed by a 12-month open-label extension. J Clin Sleep Med. 2014; 10(11): 1181–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Jha A, Weintraub A, Allshouse A, et al. A randomized trial of modafinil for the treatment of fatigue and excessive daytime sleepiness in individuals with chronic traumatic brain injury. J Head Trauma Rehabil. 2008; 23(1): 52–63. [DOI] [PubMed] [Google Scholar]
- 232. Sheng P, Hou L, Wang X, et al. Efficacy of modafinil on fatigue and excessive daytime sleepiness associated with neurological disorders: a systematic review and meta-analysis. PLoS One. 2013; 8(12): e81802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Lequerica A, Jasey N, Portelli Tremont JN, Chiaravalloti ND. Pilot Study on the Effect of Ramelteon on Sleep Disturbance After Traumatic Brain Injury: Preliminary Evidence From a Clinical Trial. Arch Phys Med Rehabil. 2015; 96(10): 1802–1809. [DOI] [PubMed] [Google Scholar]
- 234. Bhatnagar S, Iaccarino MA, Zafonte R. Pharmacotherapy in rehabilitation of post-acute traumatic brain injury. Brain Res. 2016; 1640(Pt A): 164–179. [DOI] [PubMed] [Google Scholar]
- 235. Elkind JA, Lim MM, Johnson BN, et al. Efficacy, dosage, and duration of action of branched chain amino Acid therapy for traumatic brain injury. Front Neurol. 2015; 6: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Aquilani R, Iadarola P, Contardi A, et al. Branched-chain amino acids enhance the cognitive recovery of patients with severe traumatic brain injury. Arch Phys Med Rehabil. 2005; 86(9): 1729–1735. [DOI] [PubMed] [Google Scholar]
- 237. Aquilani R, Boselli M, Boschi F, et al. Branched-chain amino acids may improve recovery from a vegetative or minimally conscious state in patients with traumatic brain injury: a pilot study. Arch Phys Med Rehabil. 2008; 89(9): 1642–1647. [DOI] [PubMed] [Google Scholar]
- 238. Cauter E Van, Plat L, Scharf MB, et al. Simultaneous Stimulation of Slow-wave Sleep and Growth Hormone Secretion by Gamma-hydroxybutyrate in Normal Young Men. J Clin Invest. 1997;100(3):745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Williams BR, Lazic SE, Ogilvie RD. Polysomnographic and quantitative EEG analysis of subjects with long-term insomnia complaints associated with mild traumatic brain injury. Clin Neurophysiol. 2008; 119(2): 429–438. [DOI] [PubMed] [Google Scholar]
- 240. Arbour C, Khoury S, Lavigne GJ, et al. Are NREM sleep characteristics associated to subjective sleep complaints after mild traumatic brain injury? Sleep Med. 2015; 16(4): 534–539. [DOI] [PubMed] [Google Scholar]