Abstract
Prior evidence suggests that vitamin D supplementation may reduce fall risk, but existing data are inconsistent and insufficient to guide policy. We designed a two-stage Bayesian response-adaptive dose-finding and seamless confirmatory randomized trial of vitamin D supplementation to prevent falls. Up to 1200 community-dwelling persons, aged ≥70 years, of predominantly white and African-American race, with serum 25(OH)D concentrations of 10–29 ng/mL and at elevated fall risk, will be randomized to one of four vitamin D3 (cholecalciferol) supplement doses: 200 (control), 1000, 2000, or 4000 IU/day and treated for up to 2 years. Stage 1 is designed to identify the best of the non-control doses for fall prevention. If a best dose is selected, Stage 2 will start seamlessly, with enrollees assigned to control or the best dose in Stage 1 continuing on that dose unchanged, enrollees assigned to the two non-control, non-best doses in Stage 1 switched to the best dose, and new enrollees randomly assigned 1:1 to control or the best dose. In Stage 2, we will compare the control dose group to the best dose group to potentially confirm the efficacy of that dose for fall prevention. The primary outcome measure in both stages is time to first fall or death, whichever comes first. Falls are ascertained from calendars, scheduled interviews, or interim self-reports. Secondary outcome measures include time to each component of the composite primary outcome and gait speed. Additional outcomes include the Short Physical Performance Battery score, physical activity level (assessed by accelerometry), and frailty score.
Keywords: Vitamin D, Falls, Aging, Randomized Trial, Adaptive Design
1. INTRODUCTION
The public health burden of falls in older persons is substantial. In the U.S., 2.8 million older adults are treated in emergency departments and over 800,000 are hospitalized for fall-related injuries each year.[1] Increasing age and female sex are associated with higher fall incidences and greater economic burden.[2] Direct medical costs of fatal and non-fatal falls for U.S. adults aged ≥65 years were estimated at $637.5 million and $31.3 billion, respectively, in 2015.[2] Identifying evidence-based interventions for fall prevention is paramount.
Vitamin D, a fat-soluble vitamin, is critical for the maintenance of bone mineral density.[3, 4] Optimal serum concentrations of 25-hydroxyvitamin D [25(OH)D], the storage form of vitamin D, may be potentially important for the prevention of falls in older adults through several proposed biologic mechanisms (Figure 1). Low concentrations of 25(OH)D have been associated with muscle weakness,[5] reduced muscle mass (sarcopenia),[6] low physical performance,[7] and frailty.[8] Trials of vitamin D interventions on muscle performance have been inconclusive, but suggest a possible benefit among those with 25(OH)D deficiency.[9]
Figure 1.
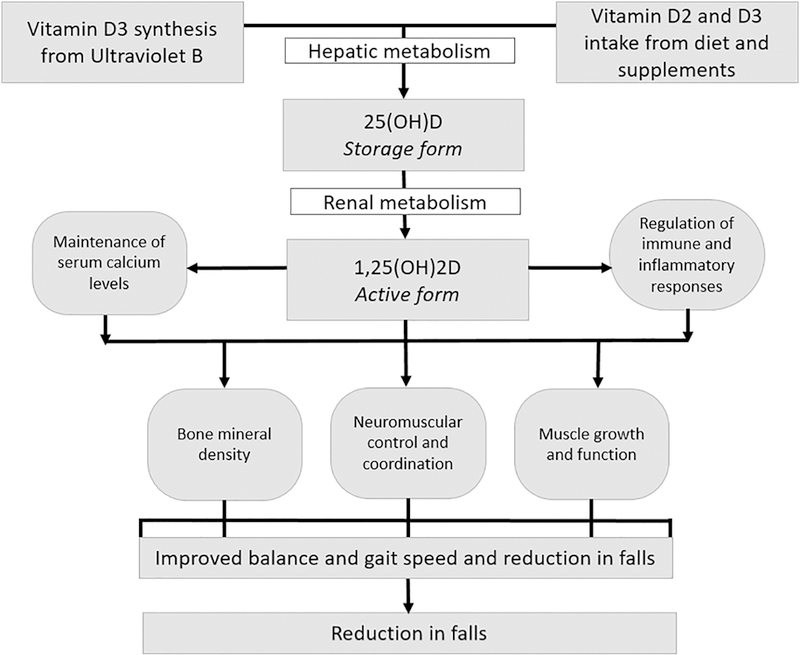
Proposed biologic mechanisms for effects of vitamin D on reduction in falls
Several professional organizations have advocated for vitamin D supplementation to prevent falls in at-risk groups (such as deficient individuals), including the American Geriatrics Society,[10] the Endocrine Society,[11] and the International Osteoporosis Foundation.[12] Meta-analyses of randomized trials conducted before 2010 suggested that vitamin D supplementation in the range of 800– 1000 IU/day could reduce fall risk by ~20%.[13, 14] However, some trials suggested benefit, while others had null results or documented an increased risk of falls. Two recent randomized trials reported that relatively high doses of vitamin D supplementation increased fall risk[15, 16]; in another trial testing 800 IU/day, vitamin D supplementation neither reduced fall rate nor improved physical function.[17] A systematic review and meta-analysis argued that there is little evidence that vitamin D prevents falls.[18] While a 2015 systematic review by the U.S. Preventive Services Task Force (USPSTF) acknowledged a possible benefit of vitamin D treatment for decreased fall risk,[19] a more recent 2018 USPSTF statement recommended against vitamin D supplementation in community-dwelling older adults for the purposes of fall prevention.[20]
This inconsistent evidence may be due to differences in study populations, vitamin D dosages, type of vitamin D supplement, co-administration with calcium supplements, baseline 25(OH)D concentrations, and methodological considerations (e.g., suboptimal ascertainment of falls).[21] Given uncertainty of the benefits of vitamin D supplementation for fall prevention in older adults based on existing trial evidence,[21] further randomized trials with fall as a primary outcome are needed.
The Study To Understand Fall Reduction and Vitamin D in You (STURDY) is a two-stage, Bayesian, response-adaptive dose-finding and seamless confirmatory randomized trial designed to find the best dose of vitamin D supplementation for fall prevention and, if a best dose is found, potentially confirm efficacy of that dose for fall prevention. It will also examine additional outcomes related to fall risk, mobility, and physical function, and will explore effects in pre-defined at-risk subgroups that may benefit more from supplementation, including those with baseline deficiency [25(OH)D concentrations of 10–19 ng/mL] and those with low physical function on enrollment.
2. MATERIALS AND METHODS
2.1. Overview of Study Design
STURDY is a double-masked, two-stage, Bayesian, response-adaptive dose- finding and seamless confirmatory randomized trial designed to select the best dose of vitamin D supplementation from three candidate doses and, if a best dose is selected, potentially confirm the efficacy of that dose for fall prevention. STURDY is recruiting up to 1200 older adults with 25(OH)D concentration in the range 10–29 ng/mL and who are at elevated risk for falling. Recruitment occurs in two communities in Maryland (located in Baltimore and Washington Counties, at approximately 39 degrees latitude), which provides the opportunity to enroll a large sample of whites and African-Americans, including both men and women. Participants will be randomized to one of four vitamin D3 (cholecalciferol) supplement doses: 200 (control), 1000, 2000, or 4000 IU/day and followed for 2 years or until the trial ends, whichever occurs first (minimum follow-up of 6 months). Stage 1 is designed to select the best dose for fall prevention from the three non-control doses. If a best dose is found, Stage 2 will start seamlessly, with enrollees assigned in Stage 1 to control or the best dose continuing on that dose unchanged; those enrollees who were assigned in Stage 1 to the two non-control, non-best doses will be switched to the best dose, and new enrollees will be randomly assigned 1:1 to control or the best dose. We will compare the control dose group to the best dose group, using the experience of each participant while on the best dose. The primary outcome measure in both stages is time to first fall or death, whichever comes first. Falls are ascertained from calendars, scheduled interviews, or interim self-reports. Secondary outcomes include each component of the composite primary outcome and gait speed. Additional outcomes include rates of different types of falls (e.g., injurious falls, falls with fractures, indoor falls, etc.), physical function, physical activity (from accelerometry), frailty, and achieved 25(OH)D concentration. STURDY is registered on ClinicalTrials.gov as NCT02166333. The first screening visit was held in June 2015, and randomization began in October 2015.
2.2. Funding Announcement, Sponsors, and Study Oversight:
The primary funder of STURDY is the National Institutes of Health (NIH)/National Institute on Aging (NIA). The Office of Dietary Supplements (ODS) also funds the trial. Application for funding of this trial was made possible through a request for application announcement (RFA) (RFA-AG-14–001) that outlined the need for a randomized trial to assess the efficacy (and dose-response) of vitamin D supplementation for fall prevention among older adults across a range of doses and baseline serum 25(OH)D concentrations.
Recommendations in the RFA included 1) a secondary outcome on gait speed, 2) an adaptive design strategy that included a dose-finding phase followed by an efficacy phase for the selected dose, 3) use of Liquid Chromatography Mass Spectrometry (LC-MS) to measure serum 25(OH)D concentrations following the specifications of the Vitamin D Standardization Program, and 4) the amount of vitamin D intake in the comparison group should meet the recommended dietary allowance (RDA) per the Institute of Medicine (IOM) guidelines.[22]
A Data and Safety Monitoring Board (DSMB) appointed by the sponsors and an Institutional Review Board (IRB) at Johns Hopkins University approved the trial protocol prior to implementation. The DSMB monitors the conduct of the trial, making recommendations to the NIA regarding trial continuation, participant safety, and trial performance.
2.3. Participant Eligibility
The inclusion and exclusion criteria for STURDY are listed in Table 1. Briefly, participants are eligible for the study if they 1) are aged 70 years or older; 2) fell in the past year with injury, fell at least twice in the past year regardless of injury, are afraid of falling due to balance or walking problems, have difficulty maintaining their balance, or use an assistive device when walking; and 3) have a serum 25(OH)D concentration ≥10 ng/mL (25 nmol/L) and <30 ng/mL (75 nmol/L), determined by a non-fasting blood draw. Main exclusion criteria include 1) use of vitamin D supplements >1000 IU/day, 2) use of calcitriol-analogues, 3) use of calcium supplements >1200 mg/day, 4) history of kidney stone in past 2 years or 2 or more lifetime kidney stones, 5) Mini-Mental State Examination (MMSE) score <24 (to help assure competency to comply with protocol requirements), and 6) hypercalcemia (serum calcium concentration ≥11 mg/dL or >10.5 mg/dL confirmed on a repeat check) or hypocalcemia (serum calcium concentration <8.5 mg/dL), as determined at screening.
Table 1:
STURDY Eligibility Criteria.
| Inclusion Criteria |
|---|
| ▪ Age 70 and older |
| ▪ Non-institutionalized |
| ▪ High risk for falling, defined by a ‘yes’ response to at least one of the following: |
| ▪ Have you fallen and hurt yourself in the past year? |
| ▪ Have you fallen 2 or more times in the past year? |
| ▪ Are you afraid that you might fall because of balance or walking |
| problems? |
| ▪ Do you have difficulty maintaining your balance when bathing, dressing, or |
| getting in and out of a chair? |
| ▪ Do you use a cane, walker, or other device when walking inside or outside |
| your home? |
| ▪ Serum vitamin D [25(OH)D] concentration ≥10 and <30 ng/ml (≥25 and <75 |
| nmol/L) |
| ▪ Able to provide informed consent |
| ▪ Able to walk (with or without assistive device) |
| ▪ Willing to accept randomization to each vitamin D dose |
| ▪ One of the following: |
| ▪ No vitamin D supplementation at screening |
| ▪ Average daily vitamin D supplementation judged by study staff as being |
| consistent with the goal of ≤1000 IU/day at screening and willing to |
| continue the dose unchanged throughout the trial |
| ▪ One of the following: |
| ▪ No calcium supplementation at screening |
| ▪ Average daily calcium supplementation judged by study staff as being |
| consistent with the goal of ≤1200 mg/day at screening and willing to |
| continue the dose unchanged throughout the trial |
|
Exclusion Criteria |
| ▪ Cognitive impairment, defined as Mini-Mental State Exam (MMSE) score <24 |
| ▪ Hypercalcemia, serum Ca2+ >10.5 mg/dl (confirmed) |
| ▪ Hypocalcemia, serum Ca2+ <8.5 mg/dl |
| ▪ Kidney, ureteral, or bladder stones made of calcium compounds (≥2 in lifetime, or 1 |
| in the last 2 years); in the absence of information on type of stone, stones will be |
| assumed to be made of calcium compounds |
| ▪ Planning to move out of area within 2 years, where plans would prevent |
| compliance with the study protocol |
| ▪ Disease or condition expected to cause death or to prevent compliance with the |
| study protocol in the next 2 years |
| ▪ Participation in another trial of vitamin D or falls, or any trial that might affect the |
| risk of falls |
| ▪ Lactose allergy (lactose intolerance is okay) |
| ▪ Use of any form of oral or injected calcitriol (brand names: Rocaltrol®, Calcijex®, |
| and Zemplar®; generic names: calcitriol, paricalcitol, doxycalcitriol, 22- |
| oxacalcitriol) |
Participants taking ≤1000 IU/day of vitamin D and ≤1200 mg/day of calcium supplements are eligible for enrollment. Participants are asked to maintain their dosage of personal vitamin D and calcium supplementation throughout the trial. Individuals who initially were excluded due to personal use of supplemental vitamin D and calcium that exceeded eligibility doses, but are willing to reduce their supplemental doses to those within the eligibility range, are allowed to re-screen after a wash-out period.
Of note, for the first year of STURDY’s recruitment, the eligible serum vitamin D [25(OH)D] range was 10–25 ng/mL, but this was found to be too restrictive in terms of recruitment, as many older adults on supplementation had concentrations between 25– 30 ng/mL. After approval from the DSMB and the IRB, in July 2016, this range was expanded to 10–29 ng/mL, which remains in line with the Endocrine Society’s definition of insufficient 25(OH)D,[11] as well as the eligibility criteria originally proposed in our grant application.
2.4. Participant Recruitment
STURDY implements multiple recruitment strategies including 1) mass mailing of brochures to older persons, 2) print stories and advertisements in local newspapers, 3) advertisements at local venues frequented by older adults (e.g., placemats at diners, brochures at the symphony), 4) screening events at senior health fairs, senior centers, and assisted living communities, 5) distribution of brochures and flyers in public locations and health care facilities, 6) messages sent via patient electronic medical record portals (i.e., secure emails sent to patients of Johns Hopkins Medical Institutions age >70 who live within specific zip codes near the STURDY field centers via Epic MyChart), and 7) web-based advertisements targeting demographics, locations, and user interest (e.g., banner ads on Facebook and Google AdWords) directing participants to the study website (http://www.sturdystudy.org). The website contains information about STURDY and a secure form to sign up for a prescreening phone call. The website URL was also included in all brochures and mailings. All recruitment strategies were approved by the IRB at Johns Hopkins University.
2.5. Study Data Collection and Contact Schedule
Eligibility, baseline, and follow-up data are collected by phone contacts, mailings, and in-person visits per the schedule shown in Table 2. In a Pre-Screen Contact, a brief questionnaire is administered by phone or in person to identify potentially eligible participants. During the Screening Visit, participants undergo more detailed questions and testing (MMSE assessment and blood draw for measurement of 25(OH)D and calcium concentrations) to determine eligibility.
Table 2:
STURDY Data Collection Schedule.
| Months from randomization | Pre-RZ | RZ | Year 1 | Year 2 | |||||||||
| −2+ | -2 | −1 | 0 | 1 | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 24 | |
| [C]linic, [T]elephone, or [E]ither |
E | C | C | C | T | C | T | T | C | T | T | T | C |
| Visit | PS | SV+ | BV+ | RZ | F01 | F03 | F06 | F09 | F12 | F15 | F18 | F21 | F24 |
| Consent | O | W | W | O | • | • | • | • | • | • | • | • | • |
| Prescreen questionnaire | X | • | • | • | • | • | • | • | • | • | • | • | • |
| Registration (demographics, eligibility questions) |
• | X | • | • | • | • | • | • | • | • | • | • | • |
| Blood draw* | • | X | X | • | • | X | • | • | X | • | • | • | X |
| Urine collection (stored) | • | • | X | • | • | X | • | • | X | • | • | • | X |
| Medical history, incl. adverse events |
• | X | X | X | X | X | X | X | X | X | X | X | X |
| Cognitive testing$ | • | X | • | • | • | X | • | • | X | • | • | • | X |
| Physical measurements# | • | • | X | • | • | X | • | • | X | • | • | • | X |
| Short Physical Performance Battery (SPPB) |
• | • | X | • | • | X | • | • | X | • | • | • | X |
| Timed Up and Go (TUG) Test |
• | • | X | • | • | X | • | • | X | • | • | • | X |
| Accelerometry | • | • | X | • | • | X | • | • | X | • | • | • | X |
| 6-minute walk | • | • | X | • | • | X | • | • | X | • | • | • | X |
| Grip strength | • | • | X | • | • | X | • | • | X | • | • | • | X |
| Physical function questionnaire+ |
• | • | X | • | • | X | • | • | X | • | • | • | X |
| Physical activity questionnaire+ |
• | • | X | • | • | X | • | • | X | • | • | • | X |
| Vitamin D and calcium food frequency questionnaire+ |
• | • | X | • | • | • | • | • | • | • | • | • | • |
| SF-12 health survey+ | • | • | X | • | • | X | • | • | • | • | • | • | • |
| Adherence reminders | • | • | X | X | X | X | X | X | X | X | X | X | X |
| Fall calendar | • | • | RI^ | X | X | X | X | X | X | X | X | X | X |
| Pill distribution/return | • | • | RI^ | X | • | M | M | M | X | M | M | M | X |
Abbreviations: RZ=Randomization Visit; PS=Pre-screening; SV=Screening Visit; BV=Baseline Visit; Fxx=Follow-up visit; O=oral; W=written; RI=run-in; M=by mail;
physical assessments and questionnaires can be completed at either SV or BV, as long as they are completed prior to RZ
real-time 25(OH)D and calcium; stored blood
MMSE at screening visit; Mini-Cog® at follow-up visits
height (baseline only), weight, blood pressure, including orthostatic blood pressure, and heart rate
at the end of BV, the participant will be given placebo pills and instructed to take one daily and complete the pill/fall calendar for a period of about 10 days, which the participant will return at RZ
At the Baseline Visit, questionnaires measuring quality of life, dietary intake of vitamin D and calcium, medical history, and physical function are completed and physical performance is assessed. After the baseline visit, participants are asked to complete a run-in period of approximately 10 days, taking placebo pills and completing a fall and study pill calendar, to demonstrate their ability to adhere to study protocols.
During the Randomization Visit, which generally takes place within 90 days of the screening visit, treatment is randomly assigned using a web-based system that maintains masking of participants, study staff, and investigative teams. Each eligible and consenting participant is given a randomly assigned bottle of study pills and instructions on taking pills, completing the fall and study pill calendar, and reporting falls and safety issues.
Routinely scheduled Telephone Contacts to maintain rapport, promote adherence, and obtain interim history information occur at 1, 6, 9, 15, 18, and 21 months post-randomization. During these calls participants are queried about changes to personal supplements, incidence of falls, and number of study pills taken in the past 7 days.
Follow-Up Visits, conducted in person at the field centers, take place at 3, 12, and 24 months after randomization, and include interim history, adherence to study pills, physical activity and function questionnaires, physical performance assessments, blood draws for testing and banking, urine collection for banking, and the same questions posed during the telephone contacts regarding incidence of falls,
Participants are asked to wear an accelerometer for 7 days at the baseline and 3-, 12- and 24-month follow-up visits.
Written consent for screening is obtained at the screening visit, and separate written consent for the trial is obtained at the baseline visit; consent is confirmed orally at the randomization visit.
2.6. Intervention and Treatment Group Assignment
STURDY is a dose-finding and confirmatory trial testing the efficacy of vitamin D3 (cholecalciferol) supplementation for the primary outcome across a broad range of doses which are thought to be safe, non-toxic, and potentially beneficial. The four doses studied are 200 (control dose), 1000, 2000, and 4000 IU/day; Table 3 provides the rationale for each dose. Briefly, the control dose (200 IU/day) was selected to meet the minimal RDA for participants in conjunction with their dietary and personal supplemental sources; 1000 IU/day is close to the dose used in the trials that documented a benefit of supplemental vitamin D on falls (800 IU/day)[23, 24] and muscle strength (1000 IU/day)[25]; 2000 IU/day is a commonly used dose that is being tested in the ongoing NIH-funded VITamin D and OmegA-3 (VITAL) trial;[26] and 4000 IU/day is the maximal tolerable daily allowance set by the IOM.[22] All four doses are also well below the levels might lead to vitamin D toxicity,[27] especially among these individuals who are pre-selected for vitamin D insufficiency/deficiency.
Table 3:
STURDY Trial Intervention: Four Cholecalciferol Doses and Rationale.
| Dose | Rationale |
|---|---|
| 200 IU/day |
This supplement dose, combined with mean estimated dietary intake and mean estimated intake from supplements, was estimated to provide > 800 IU/d, the recommended dietary intake from the IOM for adults ages 70 and older and the level recommended by the RFA for this trial. |
| 200 IU/day + 525 IU/day + 200 IU/day ≈ 925 IU/day mean dietary avg. background lowest dose (> RDA) intake supplementation (75% taking vitamin D x avg. dose of 700 IU/day) Mean dietary intake was estimated from NHANES 2005–2006 data, and the average dose was assumed as the average of the most common multivitamin dose (400 IU/day) and the maximum allowable supplement dose (1000 IU/day). |
|
| 1000 IU/day |
This is close to the dose used in the trials which documented a benefit of supplemental vitamin D on falls (800 IU/day)[23, 24] and muscle strength (1000 IU/day).[25] |
| 2000 IU/day |
This is the dose used in the ongoing VITAL trial.[26] |
| 4000 IU/day |
This corresponds to the Upper Limit for total vitamin D intake set by the IOM and is well below the ‘maintenance tolerable upper limit’ of 10,000 IU/day , recommended by the Endocrine Society Clinical Practice Guideline.[11] This dose is also well below the concentrations that might lead to vitamin D toxicity, among these individuals pre- selected for vitamin D insufficiency. |
The placebo pills (used during run-in only) and the vitamin D3 pills (200, 1000, 2000, and 4000 IU) are manufactured by Continental Vitamin Company (Vernon, CA), and are all identical in appearance to ensure masking. The pills are small tablets and can be swallowed or consumed sublingually, thereby facilitating pill taking and adherence. Three independent outside laboratories tested samples from the initial order of study pills to confirm dosing and adherence to Food and Drug Administration (FDA) overage standardization for supplements (slight overage is typical in industry and allows for some degradation during storage); one laboratory was selected for all subsequent testing. Samples from orders are tested upon receipt and periodically thereafter to confirm dosage and stability. Each study pill bottle contains 100 pills and is received from the manufacturer labeled with a unique 5-digit bottle identification number.
After confirmation of eligibility via a web-based application that compares the participant’s keyed data to the protocol eligibility criteria, a participant’s random treatment assignment is generated centrally by a web-based application (see Methods section 2.13 for details regarding generation of the treatment assignment) and the participant is issued his/her first bottle of study pills. The treatment assignment consists of a dose group assignment (i.e., 200, 1000, 2000, or 4000 IU) and a 5-digit number identifying the bottle to be given to the participant. Dose group assignments are masked to the participants and the personnel of the field centers, but not to a restricted set of personnel at the Data Coordinating Center (DCC).
Each randomized participant receives the numbered bottle of study pills in person at the randomization visit and is instructed to take one pill per day. At approximately 95-day intervals from randomization, another web-based application is used to issue the participant a replacement pill bottle consistent with their dose group assignment and also identified by a unique 5-digit number. Issue of replacement bottles continues until completion of the 24-month visit, when follow-up and study pill administration ends for the participant.
2.7. Primary Outcome
The primary outcome measure for STURDY is the time to first fall, death, censoring due to completion of 24 months of follow-up, or early withdrawal from follow- up, whichever comes first. We chose to use the composite outcome of first fall or death, rather than fall alone, so that an outcome would be defined for a participant who dies during study follow-up prior to falling. In this trial, a fall is defined as any fall, slip, or trip in which the participant loses his or her balance and lands on the floor or ground or at a lower level (i.e., the World Health Organization definition[28]). Falls are ascertained by participant self-report, obtained via multiple modalities. Starting with the baseline visit and continuing through 24 months or the end of the study, whichever comes first, participants are asked to keep a fall calendar, which is considered the gold standard for falls ascertainment.[29, 30] The calendar is similar to traditional calendars with monthly pages. Participants are instructed to mark daily whether they fell or not, with instructions to notify the field center after any fall (after seeking medical attention, if needed). Instructions are given to mail back the completed calendar at the end of each month using a postage-paid envelope provided by STURDY. If a calendar is not received as expected, an interviewer calls the participant to remind the participant to mail the calendar. During this ad hoc telephone contact and at every scheduled follow-up visit (telephone contacts and in person visits, see Table 2), the interview includes a question about whether the participant has experienced any falls that have not already been reported to study staff.
Each reported fall is further documented by completion of a detailed interview of the participant regarding the time, location, circumstances of the fall, whether the fall resulted in medical attention (such as emergency department visit or hospitalization), and whether it resulted in injuries (such as cut, bruise, sprain, dislocation, fracture) and which body parts were injured.
Vital status, the second component of the composite primary outcome, is ascertained through regular contacts with participants via mail, phone and in person visits. Proxy informants, who are usually close family members, have been identified for each participant, and these proxies may be contacted if the status of the participant is uncertain or to obtain information about the death of a participant, including the date of death.
2.8. Secondary Outcome
Gait speed, a secondary outcome of STURDY, is collected as part of the Short Physical Performance Battery (SPPB), a well-established tool for assessing lower extremity physical performance, at baseline and the 3-, 12-, and 24-month visits.[31] The SPPB includes timed tests for usual gait speed, balance, and the ability to rise from a chair. A usual-paced 4-meter walk is timed to assess gait speed. For balance, the participants are asked to maintain their feet in side-by-side, semi-tandem, and tandem positions for 10 seconds each. Finally, participants are asked to stand up and sit down with their arms crossed against their chests five times as quickly as possible. Each test is scored from 0 to 4 using cut points from a large population-based study.[31] The final SPPB score is calculated as the sum of the three scores and ranges from 0 to 12, with higher score reflecting better physical performance. Each of the components is a secondary outcome of the trial.
2.9. Other Measurements
Physical measurements.
Height is obtained at the baseline visit using a stadiometer. Weight is obtained at baseline and each in-person follow-up visit using a calibrated scale. Blood pressure is assessed in triplicate 5 minutes after sitting and 1 minute after standing by trained certified observers using the Omron 907 device which records blood pressure via an oscillometric technique. Each assessment is separated by a 30 second pause.[32]
Grip strength:
Muscle strength is assessed via six (three for each hand) grip compressions of a hydraulic hand-held dynamometer, measured by kilograms squeezed at baseline and at the 3-, 12-, and 24-month visits.
Frailty phenotype.
STURDY is collecting data on the 5 components of the frailty phenotype based on the Fried criteria[33] using standard procedures at the baseline and 3-, 12-, and 24-month visits. These components include 1) low gait speed, 2) low grip strength, 3) low physical activity, 4) feelings of fatigue or exhaustion, and 5) unintentional weight loss. Frailty is defined as the presence of any 3 of these 5 components, and pre-frail as the presence of 1 or 2 components.
6-minute walk.
Cardiorespiratory fitness and endurance walking ability are assessed using the 6-minute walk test at baseline and 3, 12, and 24 months. The test is performed on a 10-meter course in a corridor marked at both ends. Participants are instructed to walk back and forth, “covering as much ground as possible in 6 minutes.” Total distance is recorded at the end of 6 minutes. Standard phrases of encouragement are read to the participant after each minute, and the participant is allowed to rest, while standing, as needed.[34]
Timed Up and Go (TUG) test.
The TUG test is often used in clinical settings to identify older adults who are at high risk for falling.[35] Participants are instructed to sit in a standard armchair, stand up from the chair, walk at their normal pace to a line 3 meters away, turn, walk back to the chair at their normal pace, and sit down again. Timing begins at the instruction “go” and ends when the buttocks touch the chair again. The TUG is obtained at the baseline and 3-, 12-, and 24-month visits.
Accelerometry.
Free-living physical activity is assessed at baseline and 3, 12, and 24 months using the Actigraph Link activity monitor (Actigraph, LLC, Pensacola, FL) positioned on the non-dominant wrist. Accelerometry counts are measured at a sampling frequency of 80 hertz for 7 days in the free-living environment. Participants are asked to wear the monitor at all times during those 7 days to assess quantity, patterns, and trends of daily physical activity and sleep. Data are downloaded in one-minute epochs using commercial software (Actilife®) to derive activity counts per minute, and raw acceleration data are stored for future analyses.
Physical function.
Structured interview questions are used to collect information on whether participants have difficulty or need assistance from others to perform instrumental (e.g., shopping, preparing meals, housework, managing medications, transportation) and basic (e.g., using the toilet, bathing, dressing, eating) activities of daily living; the interview is administered at the baseline and 3-, 12-, and 24-month visits.
Vitamin D and calcium intake and medications used.
At the baseline visit, each participant completes a vitamin D and calcium food frequency questionnaire to determine dietary intake. Participants are asked to bring all medications, including supplements, to each in-person visit. Classes of medications taken are recorded. Supplements containing vitamin D or calcium are recorded, along with amount taken and frequency, so that average daily intake from personal supplementation can be estimated. Vitamin D is also obtained from sunlight exposure, but no validated questionnaire exists to assesses sunlight exposure, so exposure to sunlight is not being measured in this study.[36]
Cognitive testing.
The Mini-Mental State Exam (MMSE)[37] is a commonly used screening tool for assessing general cognitive functioning and screening for possible dementia. Scores range from 0 to 30, with lower scores indicating greater cognitive impairment. The MMSE is administered at the screening visit, and screenees who score below 24 are excluded from further participation to help assure that participants will be able to complete protocol requirements such as the fall calendar. The Mini-Cog®[38] is administered at the 3-, 12-, and 24-month visits. The Mini-Cog® is a 2-item assessment for cognitive impairment (outcome is positive or negative for cognitive impairment) and serves as an alert to study staff that a participant may have difficulty completing protocol requirements.
SF-12.
Health-related quality of life and general self-rated health status (excellent, very good, good, fair, poor), based on a 4-week recall, are assessed using the 12-item short form (SF-12)[39] from the Medical Outcomes Study 36-Item Health Survey at baseline and 3 months. The SF-12 is widely used in clinical and epidemiological research and provides summary measures of physical and mental health functioning.
2.10. Adherence.
Self-reported adherence is obtained via two modalities. Each day on the fall and study pill calendar includes a box for checking that the study pill was taken that day. Additionally, during each regularly scheduled telephone and in person visit, the participant is asked how many days in the past week they took the study pill.
2.11. Blood Assays.
Non-fasting blood samples are collected at the screening, baseline, and in- person follow-up visits through standard venipuncture technique. The screening visit sample is assessed for study-eligible serum vitamin D (total 25(OH)D) and calcium levels by the University of Maryland School of Medicine’s Clinical Core Research Lab (Baltimore, MD). Serum 25(OH)D and serum calcium levels are also checked by the same laboratory for safety at 3, 12, and 24 months post-randomization. Provided that the participant has given consent for biospecimen banking, other samples obtained at the baseline and 3-, 12-, and 24-month visits are centrifuged for plasma and serum components and then aliquoted; whole blood samples are also aliquoted. Buffy coat is stored from the baseline visit. Blood is also collected in a single PAXgeneTM RNA tube (Qiagen) at the baseline and 3 months follow-up visits. The banked samples are stored at −70 degrees Celsius at both field centers, using customary secure procedures.
Serum concentrations of 25(OH)D2, 25(OH)D3, and C-3 epimers are measured using LC-MS calibrated to meet guidelines set for the National Institute of Standards and Technology.[40] Measurements in the University of Maryland Clinical Core Research Laboratory are certified by the Center for Disease Control (CDC) Vitamin D Standardization-Certification Program. The method used is high performance liquid chromatography coupled to tandem mass spectrometry, which is considered the “gold standard” for the 25(OH)D assay. According to the CDC Vitamin D Standardization- Certification Program, the total coefficients of variation (CVs) for 25(OH)D3 and 25(OH)D2 were 6.0% at a total level of 4 ng/mL, 4.4% for a level of 10 ng/mL and 4.2% at concentrations of 20 ng/mL or greater; bias is <1.5%. A lower limit of detection for both analytes is 2 ng/mL and the limit of quantitation is 4 ng/mL. Concentrations of 25(OH)D3 and 25(OH)D2 are summed to determine total 25(OH)D concentrations. Serum calcium is quantified with the Vitros system from Ortho Clinical Diagnostics (Raritan, NJ); these measurements have a total CV of 2.0%.
Potential future assays, supported by ancillary studies, can be measured at a later date from stored samples; these could include additional blood chemistries (e.g., renal function), related biomarkers (e.g., parathyroid hormone, FGF-23, inflammatory markers), and/or genetic markers.
2.12. Safety
Cholecalciferol supplements up to doses of 10,000 IU/day have been shown to be safe.[27] Side effects of vitamin D are rare but could include gastro-intestinal symptoms (nausea, constipation, or diarrhea), hypercalcemia, or kidney stones. To minimize these, we excluded participants with a confirmed baseline serum calcium level of ≥10.6 mg/dL, or with a history of kidney, bladder, or ureteral stones made of calcium compounds (≥2 in lifetime, or ≥1 in the last 2 years).
Measurements of serum calcium and vitamin D on treatment are obtained at each in-person follow-up visit. To maintain masking, study investigators are not told the participant’s 25(OH)D concentration unless toxicity is identified (level ≥150 ng/mL), at which point the study pill will be discontinued, or if extreme deficiency is detected (level <10 ng/mL), at which point the participant would be referred to his/her healthcare provider. The study pill will also be discontinued if hypercalcemia (confirmed serum calcium ≥11 mg/dL) is detected at any time during follow-up or if a kidney stone develops. Participants with confirmed calcium concentration of 10.6–10.9 mg/dL during the study will be encouraged to speak with their health care providers about discontinuing any personal calcium supplements they might be taking. The DSMB monitors incidence of adverse events in participants.
2.13. Statistical Methods
2.13.1. Adaptive Design
STURDY is a two-stage, Bayesian, response-adaptive, dose-finding and seamless confirmatory randomized trial of vitamin D supplementation for the prevention of falls. When Stage 1 starts, participants are randomly assigned to vitamin D3 (cholecalciferol) at a dose of 200 IU/day (control dose) with assignment probability of 0.50 or to a dose of 1000, 2000, or 4000 IU/day, each with assignment probability of 0.1667 and with randomizations assigned in a permuted block scheme and separate schedules for each field site. This ‘burn-in’ period of fixed treatment assignment probabilities continues to the time when the 100th participant randomized to a non- control group has completed 6 months of follow-up; it allows the outcome event rates to stabilize prior to application of any adaptive randomization probability changes.[41]
The response-adaptation process begins at the end of the burn-in period. In this process, assignment probabilities for the non-control groups are adjusted using the accumulated primary outcome data for the participants in the non-control groups; the assignment probability for the control group remains 0.50 throughout both stages of the trial. The adjustments occur at intervals defined by follow-up on particular participants (e.g., end of burn-in when the 100th participant randomized to a non-control group has achieved 6 months of follow-up; when the 200th participant randomized to a non-control group has achieved 6 months of follow-up; etc.) until Stage 2 begins or until 1200 participants have been randomized in Stage 1.
At each adjustment time, candidate new assignment probabilities are calculated using Bayes’ Theorem, given the pre-specified prior distribution of time to the composite primary outcome (fall or death), the cumulative number of primary outcome events observed in each of the non-control groups, and the total at-risk time observed in each of the non-control groups. Based on a synthesis of the previous literature, [13–15, 23, 24, 42–46] we expect approximately 20% of participants to have a primary outcome event in 6 months; for planning purposes, we assumed mortality in our study population would be low over the 2 years of follow-up. Following suggestions in Berry et al.[41], we assumed inverse gamma distributions with shape parameter α=2[47] for the mean times to primary outcome for both the posterior and conjugate prior distributions. [48]
The posterior distribution for mean time to primary outcome is calculated for each of the three non-control doses (i.e., 1000, 2000, and 4000 IU/day) via a Monte Carlo Markov Chain algorithm with 200,000 simulations using the observed data up to that point and pre-defined random number seeds for each dose. This number of simulations provides precision to the third decimal place in the estimate of the posterior predicted probabilities. Then, for the ith simulation, we define the best dose among the three doses as the dose with the longest time to first fall or death using the posterior distribution for each dose.[41, 47]
Because the probability of being assigned to the control dose is always 0.50, the probability of assignment to each remaining dose (1000, 2000, or 4000 IU/day) is set to the predictive probability of that dose being the best, multiplied by 0.50.
Following the advice of Berry et al.[41], we specified decision rules to be applied at each probability adjustment time. If the Bayesian probability that the 1000, 2000, or 4000 IU/day dose is the best dose is less than 0.025, then accrual to that dose group will be suspended. Participants already enrolled in the suspended group continue at that dose and any new falls or deaths experienced by them are recorded. The suspended dose will return to active status if, on a subsequent adjustment, the Bayesian probability that the dose is best is 0.025 or greater. If the Bayesian probability that the 1000, 2000, or 4000 IU/day dose is the best is greater than 0.95, then Stage 1 will end with that dose declared the best dose and Stage 2 will start.
During Stage 1, the proposed new assignment probabilities are reviewed and approved by the DSMB prior to implementation; the process and decisions are masked to all but a few DCC staff. Once the proposed new assignment probabilities are reviewed and approved by the DSMB, they are implemented by generating treatment assignments without a blocking scheme or stratification by site, and they remain in effect until the next pre-specified adjustment time.
The cycle of randomization with periodic adjustments to the assignment probabilities, as described above, continues until a best dose is identified. We expect to find a best dose and terminate Stage 1 using fewer than 1200 participants, and therefore, expect to proceed to Stage 2. However, if no best dose is found (no dose has Bayesian probability that the dose is best > 0.95), then Stage 1 will continue to the planned study end (recruitment of 1200 participants with at least 6 months of follow-up), subject to DSMB review.
If a best dose is found, Stage 2 will begin seamlessly. Participants from Stage 1 assigned to the control (200 IU/day) and best dose groups will remain at their assigned doses, and Stage 1 participants in the other two dose groups will be switched to the best dose at their next pill distribution. Participants enrolled during Stage 2 will be randomized to either the control group or the best dose group in a 1:1 ratio, without a blocking scheme or stratification by site. The transition from Stage 1 to Stage 2 will be seamless; field center staff and other non-DCC staff are masked to if and when the switch from Stage 1 to Stage 2 occurs. Because each pill bottle is uniquely identified by a 5-digit number, the DCC can manage the web-based study pill bottle distribution application such that a bottle of the dosage appropriate to the current stage of the trial is issued to a participant, while maintaining masking of which dosages are active in the trial. In both Stages 1 and 2, all participants take study pills and are followed for two years or until the end of the study, whichever comes first.
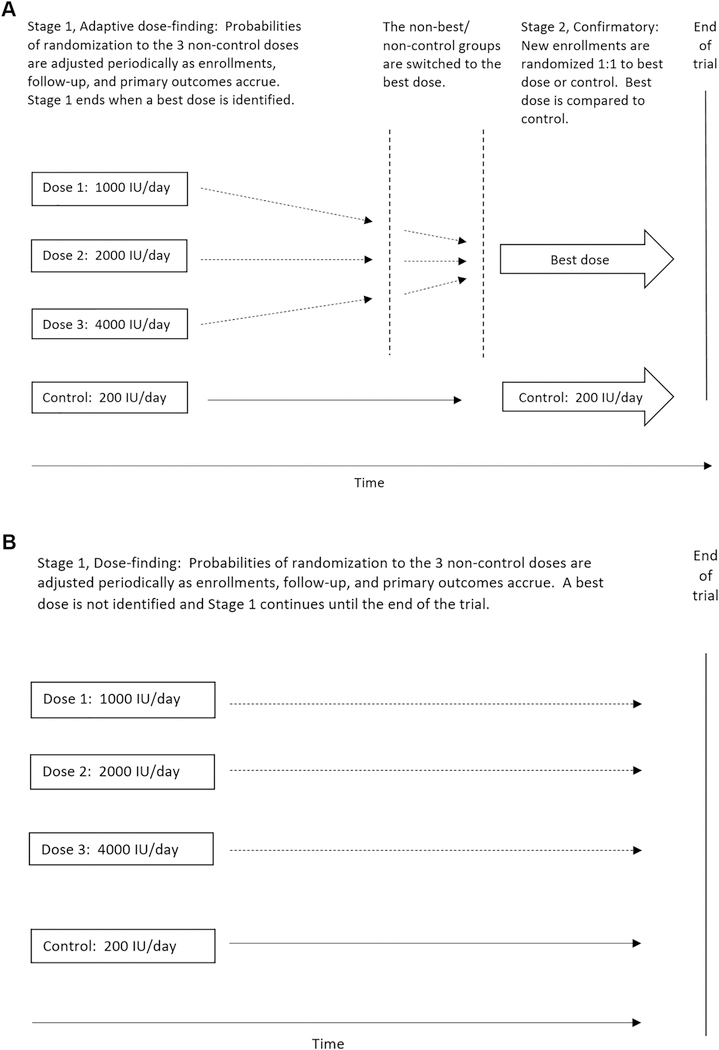
Figure 2 shows two possible scenarios for a Bayesian response-adaptive randomizations to dose groups. In Scenario A, a best dose is found during Stage 1, and that dose is compared to the control dose in Stage 2. In Scenario B, a best dose is not found (Stage 1 continues until the end of the trial), and Stage 2 is not initiated.
Figure 2.
Bayesian response-adaptive design: Schematics of two possible scenarios of response-adaptive randomizations to dose groups.
Panel A: Scenario 1. A best dose is found during Stage 1 and that dose is compared to the control dose in Stage 2.
Panel B: Scenario 2. Stage 1 dose-finding continues to the end of the trial with no best dose identified.
2.13.2. Analytic Plan for Stage 2 and Study Results Reporting
At the start of Stage 2, we will perform the first of three formal interim analyses for efficacy; two additional analyses will occur during Stage 2 at equally spaced calendar time intervals. The primary efficacy analysis is a comparison of the time to first fall or death for the control (200 IU/day) group versus the combined three >200 IU/day groups while on the best dose using the logrank test. Participants will be analyzed in either the control or best dose group using all available time at risk while on the control or best dose. The proportionality of the hazards will be examined using standard diagnostic plots and inspection of the Schoenfeld residuals[49] as implemented in SAS PROC PHREG. Events and follow-up time while on the control or best dose will be included in this primary analysis; for participants switched to the best dose from either non-best dose, the clock for occurrence of a first fall or death will reset to the time from the switch to the best dose.
We will examine the consistency of treatment effects for the primary outcome in subgroups of participants; 12 subgrouping factors, all measured at baseline, were specified in the protocol: race (African-American vs. non-African-American), 25(OH)D concentrations (10–19 vs. 20–29 ng/mL), physical function (SPPB total score <10 vs. ≥10), use of vitamin D supplements (none vs. any), total baseline vitamin D intake (<800 IU/day vs. ≥800 IU/day), gender, age (<80 years vs. ≥80 years), body mass index (BMI; <18.5 vs. ≥18.5 to <25 vs. ≥25 to <30 vs. ≥30), medications classes (any antihypertensive [including diuretics, ACE inhibitors, beta blockers or calcium channel blockers] vs. no antihypertensive and any diabetic drugs [insulin or oral anti-diabetic drugs] vs. no diabetic drugs), frailty status (frail vs. pre-frail vs. robust), and prior fall status (any vs. no prior fall). We will use the model described above augmented with treatment by subgroup interaction terms. Results of subgroup analyses will be interpreted cautiously, even for pre-specified subgroups, given the potential for false discovery. For the primary outcome, we will present both nominal p-values for tests for subgroups (interaction terms) and Bonferroni-corrected p-values for any subgroups not pre-specified.
We will also explore differences between the control group and the best dose group in our secondary outcomes (first fall, death, gait speed) and the additional outcomes that are described in section 2.9, as well as differences between the control group and pooled dose groups (control dose group versus pooled 1000, 2000 and 4000 IU/day groups). Sensitivity analyses may include “as treated” analyses, such as analysis by total vitamin D dose (combined study dose and personal supplementation dose) and/or analysis by achieved level of serum 25(OH)D. Unless otherwise specified, tests will be two-sided and P<0.05 will be considered statistically significant.
2.13.3. Sample Size and Power
Two goals of the trial design were to have high power in Stage 1 to detect a dose-response if it exists and to have at least 80% power in Stage 2 when comparing the control group versus the best dose group. We used the Compass 2.0® software (Cytel, Cambridge, MA) and public domain software ARandDesktop (Version 4.1.1, Houston, TX; 2015) to simulate trial scenarios with dose-response patterns of several types (flat, decreasing, increasing, U-shaped); assumptions for each scenario included accrual of 25 participants per month over 48 months, allocation ratio of 3:1:1:1 across the 4 doses, 6-month event rate in the 200 IU group=0.20, four parameter logistic dose- response curves with a uniform prior distribution, 2-sided Type 1 error=0.05, the Cochran-Armitage trend test for dose-response, and the following two rules: 1) Early success rule, stop for success if the Bayesian probability for any dose satisfies: P(6- month fall rate<0.15)=0.80; and 2) Early futility rule, stop for futility if the Bayesian probability for all doses satisfies: P(6-month fall rate>0.20)=0.80. Each scenario was simulated 200 times. The sample size needed for Stage 1 was variable but averaged N=400. With N=600, the trial has high statistical power (92%) for detecting a dose- response trend, if it exists, in 6-month fall proportions across 4 dose levels with sample sizes of 300, 100, 100, and 100, with equally-spaced scores for the doses, proportions equal to 0.20, 0.15, 0.11 and 0.08; Power Analysis and Sample Size System (PASS) 11 software (NCSS, LLC. Kaysville, UT) was used for the calculation.
The primary outcome variable for Stage 2 is time to first fall or death over 2 years, where time is measured from the date of randomization for the control group and date that the best dose was started (randomization or date switched to best dose) for the best dose group; the experience of the control group (from both Stage 1 and Stage 2) will be compared to the experience of the best dose group (i.e., Stage 1 and Stage 2 experience of Stage 1 enrollees randomized to the best dose group, plus Stage 2 experience of Stage 2 enrollees randomized to the best dose group, plus the Stage 2 experience of Stage 1 enrollees who are switched to the best dose group at the start of Stage 2). A 2-sided logrank test with 1200 subjects (600 in the control group vs. 600 in the best dose group) has 98% power to detect a difference between the control and best dose groups in time to first fall or death, assuming Type 1 error=0.05, hazard ratio=0.80, 10% dropouts, and 20% crossovers of each type. If Stage 1 ends with 200 participants randomized to the best dose group and limiting the analysis to the control group (N=600) and participants in the best dose group followed through both stages (N=200), the trial has 82% power to detect a difference between the control and best dose groups in time to first fall or death. PASS 11 software (NCSS, LLC. Kaysville, UT) was used for the calculations.
3. DISCUSSION
3.1. Inconclusive Prior Evidence and Need for a New Trial
Falls are a common problem in older adults,[1, 2] and there is a strong need for public health interventions that can prevent falls in this growing population. Suboptimal 25(OH)D concentrations are also common among older adults. Greater than 60% of U.S. adults aged ≥65 years have 25(OH)D concentrations <30 ng/mL (75 nmol/L).[50] The potential role of vitamin D for muscle strength and fall prevention has garnered attention. A 2009 meta-analysis based on 8 randomized trials and 2,426 older adults determined that supplemental vitamin D in the dose range of 700–1000 IU/day could reduce the risk of falls by 19%, but that falls were not notably reduced unless achieved blood concentrations reached at least 24 ng/mL (60 nmol/L).[13] An older (2015) systematic review by the USPSTF also found a possible decreased risk of falls with vitamin D supplementation.[19]
However, more recent trial data have challenged the purported benefit of vitamin D on fall prevention. In one randomized trial of older women, a very large single dose of vitamin D3 (500,000 IU) paradoxically increased fall risk,[15] but the non-physiologic dosing hindered interpretation and relevance of this study. Similarly, another small randomized trial of men and women aged ≥70 (N=200) found that participants randomized to higher monthly doses of D3 (60,000 IU/month or 24,000 IU/month) plus 300 μg of calcifediol actually had increased rate of falls compared to those randomized to lower D3 dosing (24,000 IU/month without calcifediol) despite higher achieved 25(OH)D concentration, and there was with no benefit on lower extremity function either.[16] In a recent clinical trial of approximately 400 older women, vitamin D supplementation at 800 IU/day did not reduce fall rate nor improve physical function conferred by exercise training.[17] In the context of inconsistent evidence, some experts have argued that there is little justification for prescribing vitamin D supplements to prevent falls,[18] despite guidelines in place that recommend supplementation to older adults for fall prevention.[10–12, 19] Indeed, the most recent (2018) statement of the USPSTF did not recommend vitamin D supplementation for the purposes of fall prevention among community-dwelling adults aged ≥65 years.[20]
In the midst of this controversy, the IOM Committee responded that the verdict on vitamin D and falls was inconclusive and more trial data are needed.[51] They cited that there were still relatively few studies of vitamin D and fall prevention, that many of the prior studies were “repurposed” for fall outcomes, and that there were discrepancies regarding ascertainment of the fall outcome across studies (i.e., fall rate per individual vs. simply total number of falls). It is possible that vitamin D supplementation might reduce the number of falls among individuals who fall often, but not reduce the total number of falls occurring in a cohort as noted in the OSTPRE trial.[52] Furthermore, even if vitamin D supplementation is helpful, there is great uncertainty concerning the optimal dose and the healthy level of 25(OH)D. In line with this, a recent 2018 systematic review of over 30 randomized trials of vitamin D supplements for fall prevention found substantial heterogeneity across trial designs and that most trials did not incorporate key design features.[21] Thus, rather than concluding that vitamin D supplementation is ineffective for fall prevention, it was felt that the existing trial evidence is insufficient to guide clinical recommendations.[21]
STURDY will address these gaps in knowledge by evaluating the benefit of vitamin D for fall prevention across a broad range of doses and using an adaptive design, potentially identifying the best dose for fall prevention, and exploring differential treatment effects among subgroups defined by race, physical function, and baseline and achieved 25(OH)D concentrations. STURDY will construct dose-response models for the relationships between falls (and physical function) and a) assigned vitamin D treatment dose; b) achieved serum 25(OH)D concentrations; and c) total vitamin D intake including dietary and supplemental sources. Such analyses will inform the ongoing debate on whether to treat patients by providing standard vitamin D doses vs. titrating doses to achieve specific target 25(OH)D concentrations, e.g., ≥30 ng/mL. Additionally, STURDY will be positioned to explore important mechanisms by which vitamin D may influence falls including physical function, muscle strength, and physical activity, vis-à-vis accelerometry. Finally, STURDY will establish a biorepository and other infrastructure to facilitate future ancillary studies for further research on vitamin D, falls, and physical functioning.
3.2. Design Considerations and Limitations
3.2.1. Adaptive design
STURDY is implementing a two-stage design that could substantially enhance trial efficiency by including a response-adaptive dose-selection phase followed by a seamless confirmatory efficacy phase for the selected dose (if a best dose is found). However, there is the potential for an extended dose-finding stage (uncertainty in length of Stage 1), in which a best dose may never emerge. Other outcomes are relevant to the question of benefit of vitamin D and will be reported, but these outcomes do not enter into the calculation of assignment probabilities during the adaptive dose-selection process.
3.2.2. Population
STURDY is well-positioned and on target to recruit a large number of African- American participants due to the fact that one of the two clinical field centers is located within a predominantly African-American neighborhood and the record of the investigators in recruiting minority study participants. Despite the well-recognized phenomenon that African-Americans have substantially lower 25(OH)D concentrations,[53] there is a striking dearth of vitamin D trials with clinically relevant outcomes in this subgroup which might be especially responsive to vitamin D supplementation. In fact, few of the trials that tested the effects of vitamin D on falls enrolled any African-Americans. However, other important minority groups (i.e., Hispanic, Asian, etc.) may not be well-represented from our recruitment locations. Because our enrolled participants either had a prior fall or are at elevated risk for falls, our results may not be generalizable to the broader population of older adults at lower risk of falling. All participants are recruited from one geographic region (Maryland, approximately 39°N) and may not be reflective of vitamin D status of individuals living at higher or lower latitudes.
3.2.3. Baseline Vitamin D Status
STURDY is enrolling only individuals who have either insufficient (20–29 ng/mL) or moderately deficient (10–19 ng/mL) 25(OH)D concentrations, as prior studies have not found clear evidence of additional benefits of vitamin D supplementation among those who already have adequate 25(OH)D concentrations.[54] However, there is ongoing controversy about what concentrations of 25(OH)D are considered “adequate”.[55] Vitamin D deficiency has historically been defined as 25(OH)D concentrations <20 ng/mL (50 nmol/L), with ≥20–29 ng/mL considered insufficient, and ≥30 ng/mL considered optimal — cut-points endorsed by the Endocrine Society.[11] However in their 2010 document, the IOM Committee concluded that 25(OH)D concentrations of ≥20 ng/mL should be adequate for bone health for the vast majority of Americans.[22] Other experts have disagreed with the IOM cut-points for adequacy.[56] Given the uncertainty of the potential for effect modification of vitamin D supplementation on fall prevention by baseline 25(OH)D concentration, STURDY is including individuals with either ‘deficient’ or ‘insufficient’ concentration (10–29 ng/mL).
While intermediate 25(OH)D concentrations of 20–30 ng/mL are debated as being adequate or insufficient, most experts consider those with concentrations of 10–20 ng/mL as clearly deficient, and we gave this latter group careful consideration when designing our study to ensure safety. First, we excluded all participants with severe deficiency [25(OH)D<10 ng/mL] and are referring them to seek evaluation with their healthcare providers for potential treatment of that deficiency. Second, our trial has no placebo, and all individuals are randomly assigned to one of four vitamin D treatments, with the lowest dose (control) being 200 IU/day. We allow participants to continue to take their own supplemental vitamin D up to 1000 IU/day and anticipate that between dietary sources and personal supplement use, even participants assigned to the lowest treatment group should be able to reach their RDA of vitamin D, which is 800 IU/day for adults aged ≥70 years.[22]
STURDY is recruiting participants all-year long, and seasonality will likely have an impact on baseline and achieved 25(OH)D concentrations; this will be addressed in exploratory analyses.[57, 58] Measurement of 25(OH)D concentrations, using the gold standard LC-MS assay, at baseline and each follow-up visit, is a strength of the study and will facilitate analyses examining outcomes by on-treatment concentrations achieved.
3.2.4. Accelerometry
STURDY is incorporating novel assessments of physical activity using accelerometry that will address important ancillary questions, including whether vitamin D supplementation increases physical activity levels, and possible subsequent effect(s) on falls. STURDY’s battery of physical function and physical activity measurements will allow assessment of the extent to which the observed dose-response relationship is mediated by changes in these factors. This may elucidate the causal mechanisms through which supplemental vitamin D affects the risk of falls.
4. CONCLUSIONS
In summary, STURDY is an innovative, adaptive, dose-selection randomized trial investigating vitamin D supplementation for fall prevention in older adults, and selection of the best dose for this purpose. In this era of uncertainty with conflicting prior studies and remarkably disparate guideline recommendations, STURDY is uniquely positioned to expand the existing knowledge base and inform our understanding of the implications of vitamin D for fall prevention, particularly among underrepresented groups such as African-Americans. We anticipate the trial results from STURDY will be informative in shaping future guidelines in this field. Irrespective of trial results, our study will have important clinical and public health implications – identifying an effective dose of vitamin D for fall prevention or documenting futility for supplementation beyond the current RDA recommendations.
Acknowledgements:
The authors thank the participants and the field center staff for their invaluable contributions to the STURDY trial.
Funding: This work was funded by the National Institute on Aging (NIA AG047837) and the Office of Dietary Supplements (ODS). Dr. Juraschek was supported by a National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (NIH/NIDDK) training grant T32DK007732-20 and National Heart, Lung, and Blood (NIH/NHLBI) K23HL135273-01. Dr. Plante was funded by a Health Research Services Administration (HRSA) Institutional National Research Service award T32HP10025B0 and an NIH/NHLBI training grant 2T32HL007180-41A1. Dr. Schrack was supported by K01AG048765. Dr. Michos was supported by the Blumenthal Scholars Fund in Preventive Cardiology.
Footnotes
This paper is subject to the NIH Public Access Policy.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors do not report any disclosures related to the topic of the submitted work. Dr. Schrack acts as a consultant for EMD Serono. Dr. Christenson is a consultant for Roche Diagnostics, Siemens Healthcare Diagnostics, Beckman Coulter, Quidel, PixCell and Becton Dickinson Medical Technology. No other authors report any disclosures.
REFERENCES
- [1].Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Important Facts about Falls https://www.cdc.gov/homeandrecreationalsafety/falls/adultfalls.html (Accessed November 27, 2017).
- [2].Burns ER, Stevens JA, Lee R, The direct costs of fatal and non-fatal falls among older adults - United States, J Safety Res 58 (2016) 99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Holick MF, Vitamin D deficiency, N Engl J Med 357(3) (2007) 266–81. [DOI] [PubMed] [Google Scholar]
- [4].Hannan MT, Litman HJ, Araujo AB, McLennan CE, McLean RR, McKinlay JB, Chen TC, Holick MF, Serum 25-hydroxyvitamin D and bone mineral density in a racially and ethnically diverse group of men, J Clin Endocrinol Metab 93(1) (2008) 40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dawson-Hughes B, Serum 25-hydroxyvitamin D and muscle atrophy in the elderly, Proc Nutr Soc 71(1) (2012) 46–9. [DOI] [PubMed] [Google Scholar]
- [6].Visser M, Deeg DJ, Lips P, A. Longitudinal Aging Study, Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam, J Clin Endocrinol Metab 88(12) (2003) 5766–72. [DOI] [PubMed] [Google Scholar]
- [7].Toffanello ED, Perissinotto E, Sergi G, Zambon S, Musacchio E, Maggi S, Coin A, Sartori L, Corti MC, Baggio G, Crepaldi G, Manzato E, Vitamin D and physical performance in elderly subjects: the Pro.V.A study, PLoS One 7(4) (2012) e34950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhou J, Huang P, Liu P, Hao Q, Chen S, Dong B, Wang J, Association of vitamin D deficiency and frailty: A systematic review and meta-analysis, Maturitas 94 (2016) 70–76. [DOI] [PubMed] [Google Scholar]
- [9].Stockton KA, Mengersen K, Paratz JD, Kandiah D, Bennell KL, Effect of vitamin D supplementation on muscle strength: a systematic review and meta-analysis, Osteoporos Int 22(3) (2011) 859–71. [DOI] [PubMed] [Google Scholar]
- [10].American Geriatrics Society Workgroup on Vitamin D Supplementation For Older Adults, Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for Prevention of Falls and Their Consequences, J Am Geriatr Soc 62(1) (2014) 147–52. [DOI] [PubMed] [Google Scholar]
- [11].Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM, Endocrine S, Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline, J Clin Endocrinol Metab 96(7) (2011) 1911–30. [DOI] [PubMed] [Google Scholar]
- [12].Dawson-Hughes B, Mithal A, Bonjour JP, Boonen S, Burckhardt P, Fuleihan GE, Josse RG, Lips P, Morales-Torres J, Yoshimura N, IOF position statement: vitamin D recommendations for older adults, Osteoporos Int 21(7) (2010) 1151–4. [DOI] [PubMed] [Google Scholar]
- [13].Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, Wong JB, Egli A, Kiel DP, Henschkowski J, Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials, BMJ 339 (2009) b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kalyani RR, Stein B, Valiyil R, Manno R, Maynard JW, Crews DC, Vitamin D treatment for the prevention of falls in older adults: systematic review and meta- analysis, Journal of the American Geriatrics Society 58(7) (2010) 1299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, Nicholson GC, Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial, Jama 303(18) (2010) 1815–22. [DOI] [PubMed] [Google Scholar]
- [16].Bischoff-Ferrari HA, Dawson-Hughes B, Orav EJ, Staehelin HB, Meyer OW, Theiler R, Dick W, Willett WC, Egli A, Monthly High-Dose Vitamin D Treatment for the Prevention of Functional Decline: A Randomized Clinical Trial, JAMA Intern Med 176(2) (2016) 175–83. [DOI] [PubMed] [Google Scholar]
- [17].Uusi-Rasi K, Patil R, Karinkanta S, Kannus P, Tokola K, Lamberg-Allardt C, Sievanen H, Exercise and vitamin D in fall prevention among older women: a randomized clinical trial, JAMA Intern Med 175(5) (2015) 703–11. [DOI] [PubMed] [Google Scholar]
- [18].Bolland MJ, Grey A, Gamble GD, Reid IR, Vitamin D supplementation and falls: a trial sequential meta-analysis, Lancet Diabetes Endocrinol 2(7) (2014) 573–80. [DOI] [PubMed] [Google Scholar]
- [19].LeBlanc ES, Zakher B, Daeges M, Pappas M, Chou R, Screening for vitamin D deficiency: a systematic review for the U.S. Preventive Services Task Force, Ann Intern Med 162(2) (2015) 109–22. [DOI] [PubMed] [Google Scholar]
- [20].U.S. Preventive Services Task Force, Grossman DC, Curry SJ, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni CA, Epling JW Jr., Kemper AR, Krist AH, Kubik M, Landefeld S, Mangione CM, Pignone M, Silverstein M, Simon MA, Tseng CW, Interventions to Prevent Falls in Community-Dwelling Older Adults: US Preventive Services Task Force Recommendation Statement, Jama 319(16) (2018) 1696–1704. [DOI] [PubMed] [Google Scholar]
- [21].Tang O, Juraschek SP, Appel LJ, Design Features of Randomized Clinical Trials of Vitamin D and Falls: A Systematic Review, Nutrients 10(8) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA, The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know, J Clin Endocrinol Metab 96(1) (2011) 53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pfeifer M, Begerow B, Minne HW, Suppan K, Fahrleitner-Pammer A, Dobnig H, Effects of a long-term vitamin D and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals, Osteoporos Int 20(2) (2009) 315–22. [DOI] [PubMed] [Google Scholar]
- [24].Broe KE, Chen TC, Weinberg J, Bischoff-Ferrari HA, Holick MF, Kiel DP, A higher dose of vitamin d reduces the risk of falls in nursing home residents: a randomized, multiple-dose study, Journal of the American Geriatrics Society 55(2) (2007) 234–9. [DOI] [PubMed] [Google Scholar]
- [25].Zhu K, Austin N, Devine A, Bruce D, Prince RL, A randomized controlled trial of the effects of vitamin D on muscle strength and mobility in older women with vitamin D insufficiency, J. Am. Geriatr. Soc 58(11) (2010) 2063–8. [DOI] [PubMed] [Google Scholar]
- [26].Manson JE, Bassuk SS, Lee IM, Cook NR, Albert MA, Gordon D, Zaharris E, Macfadyen JG, Danielson E, Lin J, Zhang SM, Buring JE, The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease, Contemp Clin Trials 33(1) (2012) 159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hathcock JN, Shao A, Vieth R, Heaney R, Risk assessment for vitamin D, Am J Clin Nutr 85(1) (2007) 6–18. [DOI] [PubMed] [Google Scholar]
- [28].World Health Organization, WHO global report on falls prevention in older age World Health Organization, WHO Press, Geneva, 2008. [Google Scholar]
- [29].Gillespie LD, Robertson MC, Gillespie WJ, Sherrington C, Gates S, Clemson LM, Lamb SE, Interventions for preventing falls in older people living in the community, Cochrane Database Syst Rev 9 (2012) CD007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lamb SE, Jorstad-Stein EC, Hauer K, Becker C, E. Prevention of Falls Network, G. Outcomes Consensus, Development of a common outcome data set for fall injury prevention trials: the Prevention of Falls Network Europe consensus, J. Am. Geriatr. Soc 53(9) (2005) 1618–22. [DOI] [PubMed] [Google Scholar]
- [31].Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB, A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission, J Gerontol 49(2) (1994) M85–94. [DOI] [PubMed] [Google Scholar]
- [32].White WB, Anwar YA, Evaluation of the overall efficacy of the Omron office digital blood pressure HEM-907 monitor in adults, Blood Press. Monit 6(2) (2001) 107– 10. [DOI] [PubMed] [Google Scholar]
- [33].Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, Cardiovascular G Health Study Collaborative Research, Frailty in older adults: evidence for a phenotype, J Gerontol A Biol Sci Med Sci 56(3) (2001) M146–56. [DOI] [PubMed] [Google Scholar]
- [34].ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories, ATS statement: guidelines for the six-minute walk test, Am J Respir Crit Care Med 166(1) (2002) 111–7. [DOI] [PubMed] [Google Scholar]
- [35].Mathias S, Nayak US, Isaacs B, Balance in elderly patients: the “get-up and go” test, Arch Phys Med Rehabil 67(6) (1986) 387–9. [PubMed] [Google Scholar]
- [36].McCarty CA, Sunlight exposure assessment: can we accurately assess vitamin D exposure from sunlight questionnaires?, Am. J. Clin. Nutr 87(4) (2008) 1097S–101S. [DOI] [PubMed] [Google Scholar]
- [37].Folstein MF, Folstein SE, McHugh PR, “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician, J. Psychiatr. Res 12(3) (1975) 189–98. [DOI] [PubMed] [Google Scholar]
- [38].Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A, The mini-cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly, Int. J. Geriatr. Psychiatry 15(11) (2000) 1021–7. [DOI] [PubMed] [Google Scholar]
- [39].Ware J Jr., Kosinski M, Keller SD, A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity, Med Care 34(3) (1996) 220–33. [DOI] [PubMed] [Google Scholar]
- [40].Phinney KW, Bedner M, Tai SS, Vamathevan VV, Sander LC, Sharpless KE, Wise SA, Yen JH, Schleicher RL, Chaudhary-Webb M, Pfeiffer CM, Betz JM, Coates PM, Picciano MF, Development and certification of a standard reference material for vitamin D metabolites in human serum, Anal Chem 84(2) (2012) 956–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Berry SM, Carlin BP, Lee JJ, Muller P, Bayesian Adaptive Methods for Clinical Trials, Taylor and Francis Group, LLC, Boca Raton, FL, 2011. [Google Scholar]
- [42].Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, Staehelin HB, Bazemore MG, Zee RY, Wong JB, Effect of Vitamin D on falls: a meta-analysis, Jama 291(16) (2004) 1999–2006. [DOI] [PubMed] [Google Scholar]
- [43].Bischoff-Ferrari HA, Orav EJ, Dawson-Hughes B, Effect of cholecalciferol plus calcium on falling in ambulatory older men and women: a 3-year randomized controlled trial, Archives of internal medicine 166(4) (2006) 424–30. [DOI] [PubMed] [Google Scholar]
- [44].Dukas L, Bischoff HA, Lindpaintner LS, Schacht E, Birkner-Binder D, Damm TN, Thalmann B, Stahelin HB, Alfacalcidol reduces the number of fallers in a community-dwelling elderly population with a minimum calcium intake of more than 500 mg daily, Journal of the American Geriatrics Society 52(2) (2004) 230–6. [DOI] [PubMed] [Google Scholar]
- [45].Bischoff HA, Stahelin HB, Dick W, Akos R, Knecht M, Salis C, Nebiker M, Theiler R, Pfeifer M, Begerow B, Lew RA, Conzelmann M, Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial, J Bone Miner Res 18(2) (2003) 343–51. [DOI] [PubMed] [Google Scholar]
- [46].Flicker L, MacInnis RJ, Stein MS, Scherer SC, Mead KE, Nowson CA, Thomas J, Lowndes C, Hopper JL, Wark JD, Should older people in residential care receive vitamin D to prevent falls? Results of a randomized trial, Journal of the American Geriatrics Society 53(11) (2005) 1881–8. [DOI] [PubMed] [Google Scholar]
- [47].Cook JD, Numerical evaluation of gamma inequalities, Technical report UTMDABTR-001–06, 2006.
- [48].Klein JP, Moeschberger ML, Survival analysis: techniques for censored and truncated data, Springer, New York, NY, 1997. [Google Scholar]
- [49].Schoenfeld D, Partial residuals for the proportional hazards regression model, Biometrika 69(1) (1982) 239–241. [Google Scholar]
- [50].Ginde AA, Scragg R, Schwartz RS, Camargo CA Jr., Prospective study of serum 25-hydroxyvitamin D level, cardiovascular disease mortality, and all-cause mortality in older U.S. adults, J Am Geriatr Soc 57(9) (2009) 1595–603. [DOI] [PubMed] [Google Scholar]
- [51].Rosen CJ, Taylor CL, Vitamin D supplementation and fall risk, Lancet Diabetes Endocrinol 2(7) (2014) 532–4. [DOI] [PubMed] [Google Scholar]
- [52].Karkkainen MK, Tuppurainen M, Salovaara K, Sandini L, Rikkonen T, Sirola J, Honkanen R, Arokoski J, Alhava E, Kroger H, Does daily vitamin D 800 IU and calcium 1000 mg supplementation decrease the risk of falling in ambulatory women aged 65–71 years? A 3-year randomized population-based trial (OSTPRE-FPS), Maturitas 65(4) (2010) 359–65. [DOI] [PubMed] [Google Scholar]
- [53].Harris SS, Vitamin D and African Americans, J Nutr 136(4) (2006) 1126–9. [DOI] [PubMed] [Google Scholar]
- [54].Rizzoli R, Boonen S, Brandi ML, Bruyere O, Cooper C, Kanis JA, Kaufman JM, Ringe JD, Weryha G, Reginster JY, Vitamin D supplementation in elderly or postmenopausal women: a 2013 update of the 2008 recommendations from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO), Curr Med Res Opin 29(4) (2013) 305–13. [DOI] [PubMed] [Google Scholar]
- [55].Rosen CJ, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo- Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Manson JE, Mayne A ST.Ross C, Shapses SA, Taylor CL, IOM committee members respond to Endocrine Society vitamin D guideline, J Clin Endocrinol Metab 97(4) (2012) 1146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Heaney RP, Holick MF, Why the IOM recommendations for vitamin D are deficient, J Bone Miner Res 26(3) (2011) 455–7. [DOI] [PubMed] [Google Scholar]
- [57].Shoben AB, Kestenbaum B, Levin G, Hoofnagle AN, Psaty BM, Siscovick DS, de Boer IH, Seasonal variation in 25-hydroxyvitamin D concentrations in the cardiovascular health study, Am J Epidemiol 174(12) (2011) 1363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wang Y, Jacobs EJ, McCullough ML, Rodriguez C, Thun MJ, Calle EE, Flanders WD, Comparing methods for accounting for seasonal variability in a biomarker when only a single sample is available: insights from simulations based on serum 25- hydroxyvitamin d, Am J Epidemiol 170(1) (2009) 88–94. [DOI] [PubMed] [Google Scholar]