Abstract
The selective incorporation of gem-difluoroalkyl groups into biologically active molecules has long been used as an efficient strategy for drug design and discovery. However, the catalytic C(sp3)-CF2 bond-forming cross-coupling reaction for selective incorporation of difluoromethylene group into diverse alkyl chains, especially more sterically demanding secondary and tertiary functionalized alkanes, still remains as a major challenge. Herein, we describe a cobalt-catalyzed difluoroalkylation of tertiary aryl ketones for facile synthesis of quaternary alkyl difluorides, which exhibited high efficiency, broad scope and mild conditions. The synthetic utility of this method is demonstrated by late-stage difluoroalkylation of donepezil, a well-known acetylcholinesterase inhibitor used to treat the Alzheimer’s disease. Preliminary mechanistic investigations indicate that a difluoroalkyl radical is involved in a Co(I)/Co(III) catalytic cycle. This cobalt-catalyzed fluoroalkylation thus offers insights into an efficient way for the synthesis of fluoroalkylated bioactive molecules for drug discovery.
The catalytic C(sp3)-CF2 bond formation is of high interest in drug design and discovery. Here, the authors report a cobalt-catalyzed difluoroalkylation of tertiary α-C-H bonds of aryl ketones for facile synthesis of quaternary alkyl difluorides and show the late-stage functionalization of marketed drugs.
Introduction
The wide use of fluorinated compounds in pharmaceuticals, agrochemicals, and advanced materials vigorously propelled the development of efficient methods for the incorporation of fluorine or fluorinated moieties into organic molecules1–3. In the past decades, transition-metal-catalyzed fluoroalkylation has emerged as an efficient and convenient alternative approach to direct fluorination for facile synthesis of fluorine-containing organic compounds4–11. Among all well-developed active catalysts, palladium-12-19 and copper-catalyzed20–29 or -mediated30–33 fluoroalkylation reactions have long proved to be the most useful and reliable tools for effective synthesis of fluoroalkylated molecules. Recently, the earth-abundant, inexpensive, and environmentally friendly first-row transition metals, including Ni, Fe, and Co, have attracted increasing attention on C–C bond-forming reactions due to their different and complementary catalytic reactivities to late and noble transition metals34–36. Whereas nickel37–44 and iron45–48 have already been demonstrated as efficient catalysts for such fluoroalkylation reactions, cobalt-catalyzed fluoroalkylation has been less studied and remained as a major challenge. To the best of our knowledge, the only two examples on cobalt-catalyzed49,50 fluoroalkylation were limited to aryl zinc and magnesium nucleophiles, whereas the cross-coupling of alkyl species for construction of fluorinated alkanes still remained as an issue left to be solved so far.
The selective incorporation of gem-difluoroalkyl groups into drug-like molecules has long been known as a powerful strategy for drug design and discovery, as their structural diversity and the ability to modulate the electronic properties of parent molecules51–54. To date, a number of difluoroalkylation reactions have been well-developed into the construction of C(sp2 or sp)-CF2R bonds, in which fluorine atoms were normally oriented at relatively unique benzyl, allyl, and propargyl positions12–20,23–28,46–48,55–57. In contrast, the catalytic C(sp3)-CF2 bond-forming reaction for selective incorporation of difluoromethylene group into the alkyl chain at any special position, is still scarce and remains as an unsolved problem58. The only example on transition-metal catalyzed cross-coupling between alkyl substrates and difluoroalkylating reagents was just reported by Zhang and coworkers59; however, the alkylzinc reagents in this transformation were still limited to primary alkyl compounds, probably due to the lack of reactivity with more sterically demanding secondary and tertiary alkylating reagents (Fig. 1a)60.
Fig. 1.
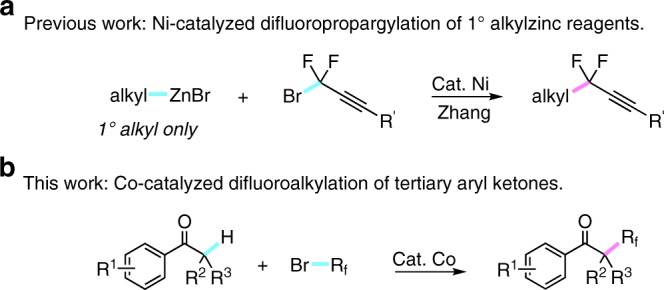
Transition-metal-catalyzed cross-coupling for facile synthesis of difluoroalkylated alkanes. a Ni-catalyzed difluoropropargylation of 1° alkylzinc reagents. b Co-catalyzed difluoroalkylation of tertiary aryl ketones
Different from palladium or nickel catalyzed alkylations, the decomposition of alkyl-cobalt intermediates via β-hydrogen elimination is really not a limitation34. Whereas cobalt catalysis has demonstrated especially efficient for coupling of alkyl halides, we conceived the unique catalytic characteristics of cobalt could enable the facile constuction of C(sp3)-CF2 bonds. Herein, we report a cobalt-catalyzed difluoroalkylation of tertiary aryl ketones with fluoroalkyl bromides (Fig. 1b). This approach has demonstrated high-catalytic reactivity and broad substrate scope, thus enabling the late-stage fluoroalkylation of biologically active molecules. This strategy offers a solution for facile synthesis of quaternary alkyl difluorides, and provides an efficient way for the synthesis of fluoroalkylated bioactive molecules for drug discovery.
Results
Optimization of the co-catalyzed cross-coupling
Our study commenced with 2-phenyl-3,4-dihydronaphthalen-1(2H)-one 1a as the pilot substrate and bromodifluoroacetate 2a as the coupling partner in the presence of catalytic amount of CoBr2 (10 mol%) and dppBz (10 mol%) in THF at −10 °C. Not surprisingly, only trace amount of the desired product 3a was observed when tBuOK was used as the base (Table 1, entry 1). To our delight, 30% yield of 3a was isolated when zinc metal (0.5 equiv) was added as the key reductant to generate the active cobalt catalyst (Table 1, entry 2)63. Further investigation indicated that manganese was not the suitable reductant (Table 1, entry 3), and reducing the amount of zinc resulted in a decline in yield (17%, Table 1, entry 4). To further improve the yield, a careful examination of base was carried out when 0.5 equiv of zinc was added. Whereas inorganic bases gave almost none of the product 3a, organic bases, including KHMDS and LiHMDS showed good reactivity, and LDA proved as the optimal choice with 90% yield (Table 1, entries 5–7). Different kinds of nitrogen, phosphine, and carbene ligands were next studied, but all gave obviously lower yields or even none of 3a (Supplementary Table 3). Additionally, careful screening of solvents and cobalt sources indicated THF and CoBr2 were still the best choices (Table 1, entries 8–12 and Supplementary Tables 2 and 6). The reaction temperature was also investigated, which demonstrated that lower temperature even to −30 °C still gave the same excellent yield, but increasing higher temperature (0 °C) resulted in a significant reduction in yield (Table 1, entries 13–14). Finally, control experiments confirmed that almost none of the desired product 3a was detected in the absence of cobalt catalyst (Table 1, entry 15).
Table 1.
Cobalt-catalyzed difluoroalkylation: optimization of conditionsa
|
dppBz = 1,2-bis(diphenylphosphino)benzene
aReaction conditions: 1a (0.2 mmol, 1.0 equiv), 2a (3.0 equiv), [Co] (10 mol%), dppBz (10 mol%), base (105 mol%), Zn (0.5 equiv), solvent (2.0 mL), −10 °C, 12 h, N2
bYields of the isolated products given
cDioxane was used as solvent
dT = −30 °C
eT = 0 °C
Scope of the co-catalyzed cross-coupling
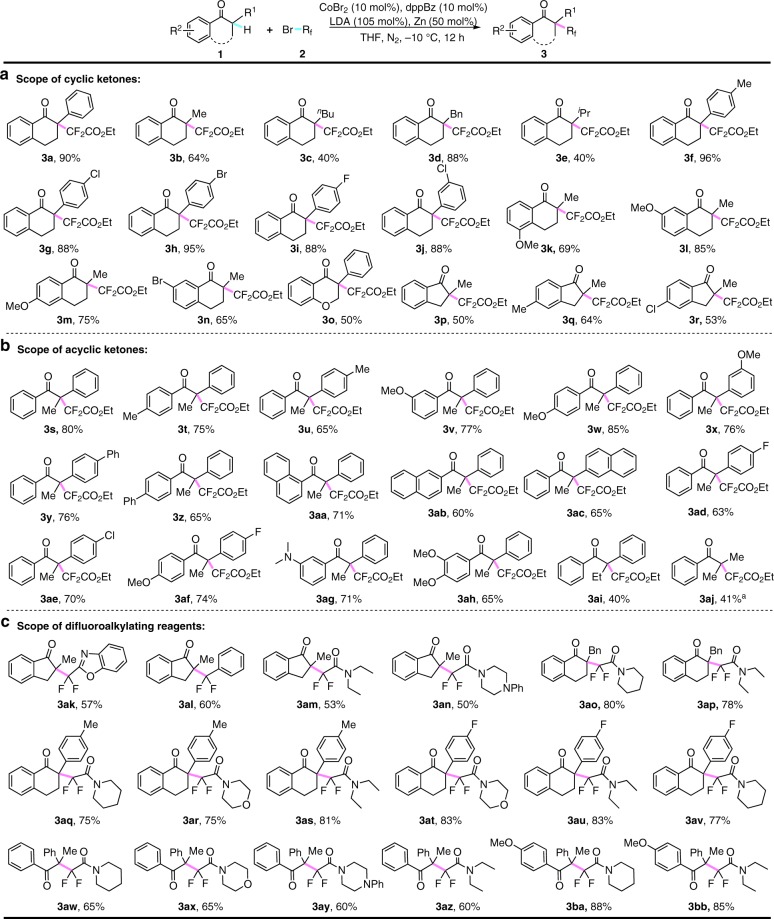
With these optimized condition in hand, various aryl ketone 1 were next tested in this catalytic fluoroalkylation system. As shown in Fig. 2, a variety of cyclic and acyclic aryl ketones were difluoroalkylated successfully, furnishing the desired products 3 with difluoroalkylated quaternary carbon centers in good to excellent yields. To our satisfaction, the examination of α-substituents (R1) of cyclic aryl ketones showed that not only a great number of substituted aryl groups, but also various alkyl groups like Me (3b), n-Bu (3c), Bn (3d), and i-Pr (3e), were smoothly difluoroalkylated with good yields. It should be noted that five- or six-membered carbon rings and even O-containing chromanone were well tolerated in this reaction (3a-3r). Indeed, cyclic and acyclic aryl ketones installed with both electron-donating groups, including Me (3f, 3q, 3t, 3u), MeO (3k-3m, 3v-3×, 3af, 3ah), Me2N (3ag), and electron-withdrawing groups, such as F (3i, 3ad, 3af), Cl (3g, 3j, 3r, 3ae), and Br (3h, 3n), on the phenyl rings were fluoroalkylated effectively to give the desired products with acceptable yields in our catalytic system. Remarkably, both cyclic and acyclic aryl ketones containing F (3i, 3ad, 3af), Cl (3g, 3j, 3r, 3ae), Br (3h, 3n) were also suitable coupling partners, which clearly demonstrated good functional group tolerance of our method and offered the synthetic potential for further elaboration by transition-metal-catalyzed coupling reactions. Gratifyingly, acyclic aryl ketones bearing two alkyl groups at the α-position (3aj) were well tolerated in our reaction. In addition, aryl ketones containing primary and secondary α-C-H bonds had also been investigated in this catalytic system. While no reaction was observed with PhCOCH3, PhCOCH2R (R=Me, Ph) afforded tetrasubstituted monofluoroalkenes 8 and 9 in 31% and 41% yield, respectively. In contrast, cyclic aryl ketone furnished a 3,3-difluorofuran-2-one derivative 10 accordingly (Supplementary Figure 186).
Fig. 2.
Cobalt-catalyzed difluoroalkylation of aryl ketones. a Scope of cyclic ketones. b Scope of acyclic ketones. c Scope of difluoroalkylating reagents. Conditions: 1 (0.2 mmol, 1.0 equiv), 2 (3.0 equiv), CoBr2 (10 mol%), dppBz (10 mol%), LDA (105 mol%), Zn (0.5 equiv), THF (2.0 mL), −10 °C, 12 h, under N2 atmosphere. aConditions: 20 mol% of CoBr2 and 20 mol% of dppBz were used. dppBz = 1,2-bis(diphenylphosphino)benzene
To further demonstrate the scope of fluoroalkylating reagents, various kinds of difluoroalkyl bromides were examined in this catalytic system. Not surprisingly, as the analogues with similar reactivity to difluoroacetate 2a, bromodifluoroacetamides were also compatible with this reaction. Different acetamides, including acyclic diethyl amine, cyclic piperidine, morpholine, and piperazine, could be well tolerated in this transformation with acceptable yields. Additionally, the difluoromethylated arene (3al) and heteroarene (3ak) could be smoothly coupled to the aryl ketones using this method. Furthermore, the scope of difluoroalkylating reagents had been demonstrated via a diversity of cross-coupling reactions between different kinds of cyclic or acyclic aryl ketones and difluoroalkyl bromides. It is known that the gem-difluoromethyl group (CF2) serves widely as a key motif to improve the biological activity of target drug molecules53,54, and this method thus demonstrates its application prospect to access diverse difluoroalkylated aryl ketones for drug screen.
Owing to the mild conditions and good functional group tolerance demonstrated in this catalytic system, the synthetic potential of this method was next elucidated via late-stage modification of biologically active molecules with structural diversity. Indeed, donepezil, a well-known acetylcholinesterase inhibitor used to treat the Alzheimer’s disease61, could be difluoroalkylated smoothly with good yields. As shown in Fig. 3, different functional groups, such as ester (5a), benzo[d]oxazole (5b), and arene (5c), in the fluoroalkylating reagents were all well compatible with this catalytic transformation. As an efficient and expedient tactic for the construction of fluorine-containing analogues, these late-stage fluoroalkylations of complex molecules could offer a useful strategy to modify the lead compounds in drug development.
Fig. 3.
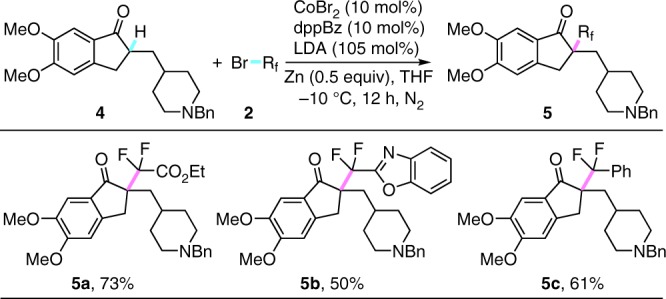
Late-stage difluoroalkylation of biologically active molecules. Conditions: 4 (0.2 mmol, 1.0 equiv), 2 (3.0 equiv), CoBr2 (10 mol%), dppBz (10 mol%), LDA (105 mol%), Zn (0.5 equiv), THF (2.0 mL), −10 °C, 12 h, under N2 atmosphere. dppBz = 1,2-bis(diphenylphosphino)benzene
Mechanistic studies
To gain some insights into this cobalt-catalyzed difluoroalkylation, a series of control experiments were next performed. Firstly, the subjection of β-piene into the reaction could afford the cycle-opening product 7 in 12% yield along with 35% yield of the desired difluoroalkylated product 3s (Fig. 4a). This result indicated that a difluoroalkyl radical was in situ generated in the catalytic cycle. Considering the key role of zinc metal as a reductant for generation of active cobalt species, to understand the catalytically active species in this transformation, Co(I)Cl(PPh3)3 had been synthesized and subjected into the reaction63. It was found that the addition of zinc metal or not into the standard catalytic conditions had no effect to this transformation (Fig. 4b), giving the desired product 3s in almost the same yield (70% and 71%). Whereas zinc metal showed indeed not a strong enough reductant to access Co(0) species62, these results clearly revealed that Co(I) was the productive catalyst. To further investigate the evidence of generation of the difluoroalkyl radical, β-piene 6 was tested as a radical clock with different cobalt catalysts. As shown in Fig. 4c, the subjection of 1 equiv of Co(II)Br2/dppBz and zinc were added together into the reaction affording the radical-scavenging product 7, whereas the omission of zinc furnished none of 7. Moreover, 1 equiv of premade Co(I)Cl(PPh3)3 could give almost the same result as the combination of Co(II) and zinc used in the reaction system (Fig. 4c). All these results implied that the difluoroalkyl radical was generated by single-electron oxidation of Co(I) with difluoroalkyl bromide 2a, and zinc served as an efficient reductant to reduce Co(II) to active Co(I) species62. The in-situ reductive process mentioned not only existed in our system, but also has been reported before63. Furthermore, sequential addition of a mixture of 1a (1 equiv)/LDA (1 equiv), and then fluoroalkylating reagent 2a (3 equiv) gave a comparable yield in 72%, but the reverse order of addition with the same reagents gave only 10% yield of the desired product 3s (Fig. 4d). These results indicated the transmetallation step should occur before the activation of fluoroalkyl bromide.
Fig. 4.
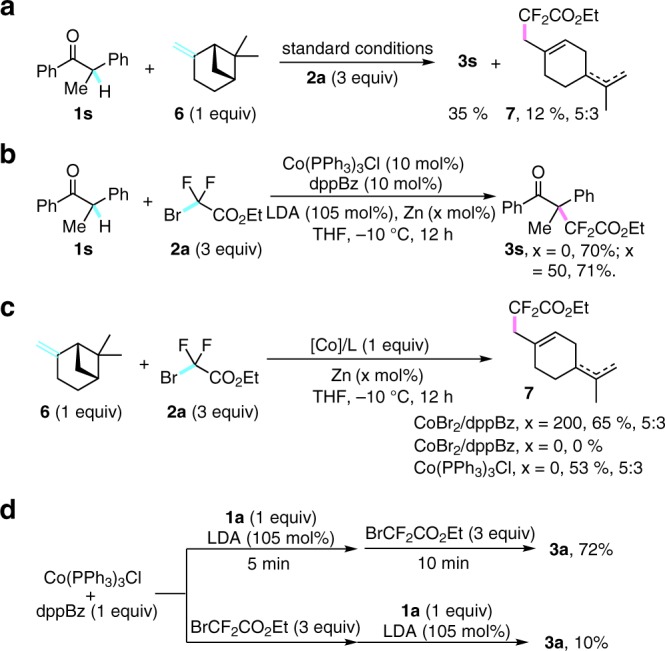
Mechanistic studies. a Radical trapping experiment with β-piene. b Control experiments using Cobalt(I) complex. c Studies on generation of the difluoroalkyl radical. d Determination of the sequence of transmetallation and activation of fluoroalkyl bromide
Based on all of these results and the previous reports64–66, a proposed mechanism of Co(I) initiated cross-coupling was described in Fig. 5. First of all, reduction of Co(II) by zinc metal afforded the catalytically active Co(I) species A to start the cycle66. Transmetallation between Co(I) A and enol anion B, which was in situ generated with the assistance of LDA, afforded the corresponding Co(I) complex C and D. A single electron oxidation of Co(I) C by fluoroalkylating reagent 2 generated the corresponding radical and Co(II) species, which was further transformed to Co(III) intermediate F after the following radical oxidation. At last, the reductive elimination of F furnished the final product 3 and regenerated Co(I) species to enter the next catalytic circle.
Fig. 5.
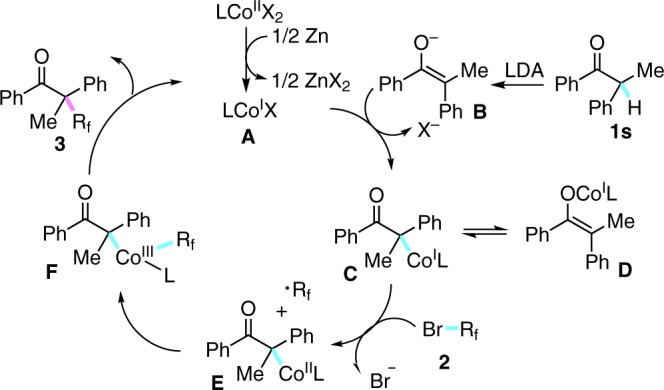
Proposed mechanism. The possible reaction pathway based on our studies and the previous literatures
Discussion
In summary, we have developed a difluoroalkylation of tertiary C–H bonds through cobalt-catalyzed cross-coupling between aryl ketones and fluoroalkyl bromides. Mechanistic investigations indicated this C–H fluoroalkylation proceeds via a Co(I)/Co(III) catalytic cycle involving an in situ generated difluoroalkyl radical. This method has demonstrated mild conditions, broad substrate scope, and thus enabled the late-stage difluoroalkylation of complex molecules. This strategy will offer a solution for facile synthesis of quaternary alkyl difluorides. Further application of this method to fluorine-containing modification of complex biologically active molecules is still underway in our laboratory.
Methods
General procedure for the cobalt-catalyzed cross-coupling
To a 50 mL of Schlenk tube was added aryl ketone 1 (1.0 equiv, 0.2 mmol), CoBr2 (10 mol %, 0.02 mmol) and dppBz (10 mol %, 0.02 mmol) under air, followed by Zn (0.5 equiv, 0.1 mmol). The mixture was evacuated and backfilled with N2 (three times). THF (2 mL) was added then followed by LDA (105 mol%, 0.21 mmol) subsequently. The Schlenk tube was then sealed with a Teflon lined cap and put into a cooled bath (−10 °C). After stirring for 5 min, bormdifluoroacetate 2a (3.0 equiv, 0.6 mmol) was added to the reaction mixture, and the Schlenk tube was then resealed with a Teflon lined cap and put back into the cooled bath (−10 °C). After stirring for another 12 h, the reaction mixture was diluted with ethyl acetate (5 mL). The solvent was removed under reduced pressure, and the residue was purified by flash column chromatography on silica gel to give the desired product.
Electronic supplementary material
Acknowledgements
We gratefully acknowledge the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB20000000), the National Basic Research Program of China (973 Program 2015CB856600), the National Science Foundation of China (21772187, 21522208) for financial support.
Author contributions
C.L. and Y.-X.C. designed and performed the experiments. R.W., Y.-N.W., and Q.L. helped to complete the experiments. X.-S.W. directed the project and wrote the manuscript. All authors interpreted the results on the manuscript.
Data availability
The authors declare that all the data supporting the findings of this research are available within the article and its supplementary information.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Chao Li, Yi-Xuan Cao.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-07525-y.
References
- 1.Müller K, Faeh C, Diederich F. Fluorine in pharmaceuticals: looking beyond intuition. Science. 2007;317:1881–1886. doi: 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]
- 2.Mikami K, Itoh Y, Yamanaka M. Fluorinated carbonyl and olefinic compounds: basic character and asymmetric catalytic reactions. Chem. Rev. 2004;104:1–16. doi: 10.1021/cr030685w. [DOI] [PubMed] [Google Scholar]
- 3.Uneyama K, Katagiri T, Amii H. α-trifluoromethylated carbanion synthons. Acc. Chem. Res. 2008;41:817–829. doi: 10.1021/ar7002573. [DOI] [PubMed] [Google Scholar]
- 4.Ma JA, Cahard D. Asymmetric fluorination, trifluoromethylation, and perfluoroalkylation reactions. Chem. Rev. 2004;104:6119–6146. doi: 10.1021/cr030143e. [DOI] [PubMed] [Google Scholar]
- 5.Zhang CP, Chen QY, Guo Y, Xiao JC, Gu YC. Progress in fluoroalkylation of organic compounds via sulfinatodehalogenation initiation system. Chem. Soc. Rev. 2012;41:4536–4559. doi: 10.1039/c2cs15352a. [DOI] [PubMed] [Google Scholar]
- 6.Liang T, Neumann CN, Ritter T. Introduction of fluorine and fluorine-containing functional groups. Angew. Chem. Int. Ed. 2013;52:8214–8264. doi: 10.1002/anie.201206566. [DOI] [PubMed] [Google Scholar]
- 7.Ni C, Hu M, Hu J. Good partnership between sulfur and fluorine: sulfur-based fluorination and fluoroalkylation reagents for organic synthesis. Chem. Rev. 2015;115:765–825. doi: 10.1021/cr5002386. [DOI] [PubMed] [Google Scholar]
- 8.Belhomme MC, Besset T, Poisson T, Pannecoucke X. Recent progress toward the introduction of functionalized difluoromethylated building blocks onto C(sp2) and C(sp) centers. Chem. Eur. J. 2015;21:12836–12865. doi: 10.1002/chem.201501475. [DOI] [PubMed] [Google Scholar]
- 9.Feng Z, Xiao YL, Zhang X. Transition-metal (Cu, Pd, Ni)-catalyzed difluoroalkylation via cross-coupling with difluoroalkyl halides. Acc. Chem. Res. 2018;51:2264–2278. doi: 10.1021/acs.accounts.8b00230. [DOI] [PubMed] [Google Scholar]
- 10.Nagib DA, Scott ME, MacMillan DWC. Enantioselective α-trifluoromethylation of aldehydes via photoredox organocatalysis. J. Am. Chem. Soc. 2009;131:10875–10877. doi: 10.1021/ja9053338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shibata N, Mizuta S, Kawai H. Recent advances in enantioselective trifluoromethylation reactions. Tetrahedron.: Asymmetry. 2008;19:2633–2644. doi: 10.1016/j.tetasy.2008.11.011. [DOI] [Google Scholar]
- 12.Cho EJ, et al. The palladium-catalyzed trifluoromethylation of aryl chlorides. Science. 2010;328:1679–1681. doi: 10.1126/science.1190524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo C, Wang RW, Qing FL. Palladium catalyzed direct α-arylation of α,α-difluoroketones with aryl bromides. J. Fluor. Chem. 2012;143:135–142. doi: 10.1016/j.jfluchem.2012.05.001. [DOI] [Google Scholar]
- 14.Feng Z, Min QQ, Xiao YL, Zhang B, Zhang X. Palladium-catalyzed difluoroalkylation of aryl boronic acids: a new method for the synthesis of aryldifluoromethylated phosphonates and carboxylic acid derivatives. Angew. Chem. Int. Ed. 2014;53:1669–1673. doi: 10.1002/anie.201309535. [DOI] [PubMed] [Google Scholar]
- 15.Min QQ, Yin Z, Feng Z, Guo WH, Zhang X. Highly selective gem-difluoroallylation of organoborons with bromodifluoromethylated alkenes catalyzed by palladium. J. Am. Chem. Soc. 2014;136:1230–1233. doi: 10.1021/ja4114825. [DOI] [PubMed] [Google Scholar]
- 16.Shao C, Shi G, Zhang Y, Pan S, Guan X. Palladium-catalyzed C-H ethoxycarbonyldifluoromethylation of electron-rich heteroarenes. Org. Lett. 2015;17:2652–2655. doi: 10.1021/acs.orglett.5b01024. [DOI] [PubMed] [Google Scholar]
- 17.Gu Y, Leng X, Sheng Q. Cooperative dual palladium/silver catalyst for direct difluoromethylation of aryl bromides and iodides. Nat. Commun. 2014;5:5405–5411. doi: 10.1038/ncomms6405. [DOI] [PubMed] [Google Scholar]
- 18.Feng Z, Min QQ, Fu XP, An L, Zhang X. Chlorodifluoromethane-triggered formation of difluoromethylated arenes catalysed by palladium. Nat. Chem. 2017;9:918–923. doi: 10.1038/nchem.2746. [DOI] [PubMed] [Google Scholar]
- 19.Aikawa K, Serizawa H, Ishii K, Mikami K. Palladium-catalyzed Negishi cross-coupling reaction of aryl halides with (difluoromethyl)zinc reagent. Org. Lett. 2016;18:3690–3693. doi: 10.1021/acs.orglett.6b01734. [DOI] [PubMed] [Google Scholar]
- 20.Oishi M, Kondo H, Amii H. Aromatic trifluoromethylation catalytic in copper. Chem. Commun. 2009;45:1909–1911. doi: 10.1039/b823249k. [DOI] [PubMed] [Google Scholar]
- 21.Feng Z, Chen F, Zhang X. Copper catalyzed cross-coupling of iodobenzoates with bromozinc-difluorophosphonate. Org. Lett. 2012;14:1938–1941. doi: 10.1021/ol3006425. [DOI] [PubMed] [Google Scholar]
- 22.Belhomme MC, Poisson T, Pannecoucke X. Copper-catalyzed direct C-2 difluoromethylation of furans and benzofurans: access to C-2 CF2H derivatives. J. Org. Chem. 2014;79:7205–7211. doi: 10.1021/jo5010907. [DOI] [PubMed] [Google Scholar]
- 23.Jiang X, Chu L, Qing FL. Copper-mediated oxidative difluoromethylenation of aryl boronic acids with α-silyldifluoromethylphosphonates: a new method for aryldifluorophosphonates. New J. Chem. 2013;37:1736–1741. doi: 10.1039/c3nj00044c. [DOI] [Google Scholar]
- 24.Serizawa H, Ishii K, Aikawa K, Mikami K. Copper-catalyzed difluoromethylation of aryl iodides with (difluoromethyl)zinc reagent. Org. Lett. 2016;18:3686–3689. doi: 10.1021/acs.orglett.6b01733. [DOI] [PubMed] [Google Scholar]
- 25.Gao X, Xiao YL, Wan X, Zhang X. Copper-catalyzed highly stereoselective trifluoromethylation and difluoroalkylation of secondary propargyl sulfonates. Angew. Chem. Int. Ed. 2018;57:3187–3191. doi: 10.1002/anie.201711463. [DOI] [PubMed] [Google Scholar]
- 26.Ivanova MV, Bayle A, Besset T, Poisson T, Pannecoucke X. Copper-mediated formation of aryl, heteroaryl, vinyl and alkynyl difluoromethylphosphonates: a general approach to fluorinated phosphate mimics. Angew. Chem. Int. Ed. 2015;54:13406–13410. doi: 10.1002/anie.201507130. [DOI] [PubMed] [Google Scholar]
- 27.Jiang X, Chu L, Qing FL. Copper-mediated oxidative cross-coupling reaction of terminal alkynes with α-silyldifluoromethylphosphonates: an efficient method for α,α-difluoropropargylphosphonates. Org. Lett. 2014;14:2870–2873. doi: 10.1021/ol301113v. [DOI] [PubMed] [Google Scholar]
- 28.Belhomme MC, Poisson T, Pannecoucke X. Copper catalyzed β-difluoroacetylation of dihydropyrans and glycals by means of direct C–H functionalization. Org. Lett. 2013;15:3428–3431. doi: 10.1021/ol401483j. [DOI] [PubMed] [Google Scholar]
- 29.Zhu J, Ni C, Gao B, Hu J. Copper(0)-mediated fluoroalkylation of iodobenzene with 2-bromo-1,1,2,2-tetrafluoroethyl compounds: investigation on the influence of R substituent on the reactivity of RCF2Cu species. J. Fluor. Chem. 2015;171:139–147. doi: 10.1016/j.jfluchem.2014.08.011. [DOI] [Google Scholar]
- 30.He Z, et al. Copper-mediated trifluoromethylation of propiolic acids: facile synthesis of α-trifluoromethyl ketones. Chem. Sci. 2013;4:3478–3483. doi: 10.1039/c3sc51613j. [DOI] [Google Scholar]
- 31.Qi Q, Shen Q, Lu L. Copper-mediated aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides at room temperature. J. Am. Chem. Soc. 2012;134:6548–6551. doi: 10.1021/ja301705z. [DOI] [PubMed] [Google Scholar]
- 32.Hu M, Ni C, Hu J. Copper-mediated trifluoromethylation of α-diazo esters with TMSCF3: the important role of water as a promoter. J. Am. Chem. Soc. 2012;134:15257–15260. doi: 10.1021/ja307058c. [DOI] [PubMed] [Google Scholar]
- 33.Moselage M, Li J, Ackermann L. Cobalt-catalyzed C–H activation. ACS Catal. 2016;6:498–525. doi: 10.1021/acscatal.5b02344. [DOI] [Google Scholar]
- 34.Cahiez G, Moyeux A. Cobalt-catalyzed cross-coupling reactions. Chem. Rev. 2010;110:1435–1462. doi: 10.1021/cr9000786. [DOI] [PubMed] [Google Scholar]
- 35.Su B, Cao ZC, Shi ZJ. Exploration of earth-abundant transition metals (Fe, Co, and Ni) as catalysts in unreactive chemical bond activations. Acc. Chem. Res. 2015;48:886–896. doi: 10.1021/ar500345f. [DOI] [PubMed] [Google Scholar]
- 36.Liang Y, Fu GC. Catalytic asymmetric synthesis of tertiary alkyl fluorides: Negishi cross-couplings of racemic α,α-dihaloketones. J. Am. Chem. Soc. 2016;136:5520–5524. doi: 10.1021/ja501815p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang X, Sakthivel S, Kulbitski K, Nisnevich G, Gandelman M. Efficient synthesis of secondary alkyl fluorides via suzuki cross-coupling reaction of 1-Halo-1-fluoroalkanes. J. Am. Chem. Soc. 2014;136:9548–9551. doi: 10.1021/ja504089y. [DOI] [PubMed] [Google Scholar]
- 38.Su YM, Feng GS, Wang ZY, Lan Q, Wang XS. Nickel-catalyzed monofluoromethylation of aryl boronic acids. Angew. Chem. Int. Ed. 2015;54:6003–6007. doi: 10.1002/anie.201412026. [DOI] [PubMed] [Google Scholar]
- 39.Schwaebe MK, McCarthy JR, Whitten JP. Nickel(0)-catalyzed coupling of vinylzirconiums to α-bromo-α,α-difluoro esters Convenient generation of a functionalized allyldifluoro moiety. Tetrahedron Lett. 2000;41:791–794. doi: 10.1016/S0040-4039(99)02212-1. [DOI] [Google Scholar]
- 40.Sheng J, Ni HQ, Liu G, Li Y, Wang XS. Combinatorial nickel-catalyzed monofluoroalkylation of aryl boronic acids with unactivated fluoroalkyl iodides. Org. Lett. 2017;19:4480–4483. doi: 10.1021/acs.orglett.7b02012. [DOI] [PubMed] [Google Scholar]
- 41.Sheng J, Ni HQ, Zhang HR, Wang YN, Wang XS. Nickel-catalyzed reductive cross-coupling of aryl halides with monofluoroalkyl halides for late-stage monofluoroalkylation. Angew. Chem. Int. Ed. 2018;57:7634–7639. doi: 10.1002/anie.201803228. [DOI] [PubMed] [Google Scholar]
- 42.Gu JW, Min QQ, Yu LC, Zhang X. Tandem difluoroalkylation-arylation of enamides catalyzed by nickel. Angew. Chem. Int. Ed. 2016;55:12270–12274. doi: 10.1002/anie.201606458. [DOI] [PubMed] [Google Scholar]
- 43.Xiao YL, Guo WH, He GZ, Pan Q, Zhang X. Nickel-catalyzed cross-coupling of functionalized difluoromethyl bromides and chlorides with aryl boronic acids: a general method for difluoroalkylated arenes. Angew. Chem. Int. Ed. 2014;53:9909–9913. doi: 10.1002/anie.201405653. [DOI] [PubMed] [Google Scholar]
- 44.Xu C, et al. Difluoromethylation of (hetero)aryl chlorides with chlorodifluoromethane catalyzed by nickel. Nat. Commun. 2018;9:1170–1179. doi: 10.1038/s41467-018-03532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.An L, Xiao YL, Zhang S, Zhang X. Bulky diamine ligand promotes cross-coupling of difluoroalkyl bromides by iron catalysis. Angew. Chem. Int. Ed. 2018;57:6921–6925. doi: 10.1002/anie.201802713. [DOI] [PubMed] [Google Scholar]
- 46.Lin X, Zheng F, Qing FL. Iron-catalyzed cross-coupling reactions between arylzinc reagents and alkyl halides bearing β-fluorines. Organometallics. 2012;31:1578–1582. doi: 10.1021/om2005904. [DOI] [Google Scholar]
- 47.Miao W, et al. Iron-catalyzed difluoromethylation of arylzincs with difluoromethyl 2-pyridyl sulfone. J. Am. Chem. Soc. 2018;140:880–883. doi: 10.1021/jacs.7b11976. [DOI] [PubMed] [Google Scholar]
- 48.Ohtsuka Y, Yamakawa T. Direct ethoxycarbonyldifluoromethylation of aromatic compounds using Fenton reagent. Tetrahedron. 2011;67:2323–2331. doi: 10.1016/j.tet.2011.01.049. [DOI] [Google Scholar]
- 49.Araki K, Inoue M. Cobalt-catalyzed cross-coupling reaction of arylzinc reagents with ethyl bromodifluoroacetate. Tetrahedron. 2013;69:3913–3918. doi: 10.1016/j.tet.2013.03.041. [DOI] [Google Scholar]
- 50.Ohtsuka Y, Yamakawa T. Cobalt/diamine-catalyzed 1,1-difluoroethylation and 2,2,2-trifluoroethylation of aryl Grignard reagents with corresponding fluoroalkyl halides. J. Fluor. Chem. 2016;185:96–102. doi: 10.1016/j.jfluchem.2016.03.007. [DOI] [Google Scholar]
- 51.Chou TS, et al. Stereospecific synthesis of 2-deoxy-2,2-difluororibonolactone and its use in the preparation of 2′-Deoxy-2′,2′-difluoro-β-D-ribofuranosyl pyrimidine nucleosides: the key role of selective crystallization. Synthesis. 1992;6:565–570. doi: 10.1055/s-1992-26167. [DOI] [Google Scholar]
- 52.Yue X, Qiu XL, Qing FL. Synthesis of 2′,3′-dideoxy-6′,6′-difluoro-3′-azanucleosides. J. Fluor. Chem. 2008;129:866–874. doi: 10.1016/j.jfluchem.2008.02.008. [DOI] [Google Scholar]
- 53.Nakayama K, et al. Synthesis and antifungal activity of rhodopeptin analogues. 2. Modification of the west amino acid moiety. Org. Lett. 2000;2:977–980. doi: 10.1021/ol005630k. [DOI] [PubMed] [Google Scholar]
- 54.Uoto K, et al. Synthesis and structure-activity relationships of novel 2’,2’-difluoro analogues of docetaxel. Chem. Pharm. Bull. 1997;45:1793–1804. doi: 10.1248/cpb.45.1793. [DOI] [PubMed] [Google Scholar]
- 55.Fujikawa K, Fujioka Y, Kobayashi A, Amii H. A new method for aromatic difluoromethylation: copper-catalyzed cross-coupling and decarboxylation sequence from aryl iodides. Org. Lett. 2011;13:5560–5563. doi: 10.1021/ol202289z. [DOI] [PubMed] [Google Scholar]
- 56.Ge S, Arlow SI, Mormino MG, Hartwig JF. Pd-catalyzed α-arylation of trimethylsilyl enolates of α,α-difluoroacetamides. J. Am. Chem. Soc. 2014;136:14401–14404. doi: 10.1021/ja508590k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu L, Vicic DA. Direct difluoromethylation of aryl halides via base metal catalysis at room temperature. J. Am. Chem. Soc. 2016;138:2536–2539. doi: 10.1021/jacs.6b00053. [DOI] [PubMed] [Google Scholar]
- 58.Aikawa K, Maruyama K, Nitta J, Hashimoto R, Mikami K. Siladifluoromethylation and difluoromethylation onto C(sp3), C(sp2), and C(sp) centers using ruppert–prakash reagent and fluoroform. Org. Lett. 2016;18:3354–3357. doi: 10.1021/acs.orglett.6b01476. [DOI] [PubMed] [Google Scholar]
- 59.An L, Xu C, Zhang X. Highly selective nickel-catalyzed gem-difluoropropargylation of unactivated alkylzinc reagents. Nat. Commun. 2017;8:1460–1468. doi: 10.1038/s41467-017-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cinderella AP, Vulovic B, Watson DA. Palladium-catalyzed cross-coupling of silyl electrophiles with alkylzinc halides: a Silyl-Negishi reaction. J. Am. Chem. Soc. 2017;139:7741–7744. doi: 10.1021/jacs.7b04364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo Z, et al. Synthesis and evaluation of multi-target-directed ligands against Alzheimer’s disease based on the fusion of donepezil and ebselen. J. Med. Chem. 2013;56:9089–9099. doi: 10.1021/jm401047q. [DOI] [PubMed] [Google Scholar]
- 62.Chen QA, Kim DK, Dong VM. Regioselective hydroacylation of 1,3-Dienes by cobalt catalysis. J. Am. Chem. Soc. 2014;136:3772–3775. doi: 10.1021/ja500268w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liebeskind LS, Baysdon SL, South MS, Iyer S, Leeds JP. The development of an organotransition metal synthesis of quinones. Tetrahedron. 1985;41:5839–5853. doi: 10.1016/S0040-4020(01)91423-1. [DOI] [Google Scholar]
- 64.Fillon H, Gosmini C, Périchon J. New chemical synthesis of functionalized arylzinc compounds from aromatic or thienyl bromides under mild conditions using a simple cobalt catalyst and zinc dust. J. Am. Chem. Soc. 2003;125:3867–3870. doi: 10.1021/ja0289494. [DOI] [PubMed] [Google Scholar]
- 65.Jin MY, Yoshikai N. Cobalt−Xantphos-catalyzed, LiCl-mediated preparation of arylzinc reagents from aryl iodides, bromides, and chlorides. J. Org. Chem. 2011;76:1972–1978. doi: 10.1021/jo102417x. [DOI] [PubMed] [Google Scholar]
- 66.Brennan MR, Kim D, Fout AR. A synthetic and mechanistic investigation into the cobalt(I) catalyzed amination of aryl halides. Chem. Sci. 2014;5:4831–4839. doi: 10.1039/C4SC01257G. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all the data supporting the findings of this research are available within the article and its supplementary information.