Abstract
Background
Siamese fighting fish Betta splendens are notorious for their aggressiveness and accordingly have been widely used to study aggression. However, the lack of a reference genome has, to date, limited the understanding of the genetic basis of aggression in this species. Here, we present the first reference genome assembly of the Siamese fighting fish.
Findings
Frist, we sequenced and de novo assembled a 465.24-Mb genome for the B. splendens variety Giant, with a weighted average (N50) scaffold size of 949.03 Kb and an N50 contig size of 19.01 Kb, covering 99.93% of the estimated genome size. To obtain a chromosome-level genome assembly, we constructed one Hi-C library and sequenced 75.24 Gb reads using the BGISEQ-500 platform. We anchored approximately 93% of the scaffold sequences into 21 chromosomes and evaluated the quality of our assembly using the high-contact frequency heat map and Benchmarking Universal Single-Copy Orthologs. We also performed comparative chromosome analyses between Oryzias latipes and B. splendens, revealing a chromosome conservation evolution in B. splendens. We predicted 23,981 genes assisted by RNA-sequencing data generated from brain, liver, muscle, and heart tissues of Giant and annotated 15% repetitive sequences in the genome. Additionally, we resequenced five other B. splendens varieties and detected ∼3.4 M single-nucleotide variations and 27,305 insertions and deletions.
Conclusions
We provide the first chromosome-level genome for the Siamese fighting fish. The genome will lay a valuable foundation for future research on aggression in B. splendens.
Keywords: Betta splendens, fish genome, aggression, Hi-C, chromosomal genome assembly, resequencing
Data Description
Males of the Siamese fighting fish Betta splendens (NCBI:txid158456) are notorious for their aggressiveness. In nature, males establish and vigorously defend territories where they construct a bubble nest to hold fertilized eggs. In laboratory settings, males will readily attack an opponent, their mirror image, physical models of conspecifics, and video images of other males. Accordingly, the species has been widely used to study the neurobiological mechanisms of aggression. However, to date, the lack of a reference genome has limited studies on the genetic basis of aggression in B. splendens. The species is also one of the most relevant for the ornamental fish trade as it is easy to keep and reproduce in captivity. In addition, throughout its long domestication period, many varieties have been selected for their exuberant fins and colors, size, or aggressive behavior. Here, we sequenced the genome of B. splendens to provide the genomic foundation for future research on aggression and development of genomic tools.
Sampling and sequencing
We purchased five varieties of adult male Siamese fighting fish, including Giant, Half-moon, Half-moon plakat, Fighter, and Elephant Ear from Hong Kong supplier TC Northern Betta for DNA and RNA extraction [1, 2] (Supplementary Fig. S1). We constructed and sequenced six DNA libraries for the B. splendens variety Giant, including three short insert size libraries and three mate-pair libraries (Supplementary Table S1), and five RNA-sequencing (RNA-seq) libraries (Supplementary Table S2) using the HiSeq 2000 sequencing platform. One Hi-C library for Giant was also constructed and sequenced using the BGISEQ-500 sequencing platform, yielding 75.24 Gb of reads. Additionally, we sequenced four short insert size DNA libraries for the other four B. splendens varieties.
Genome assembly
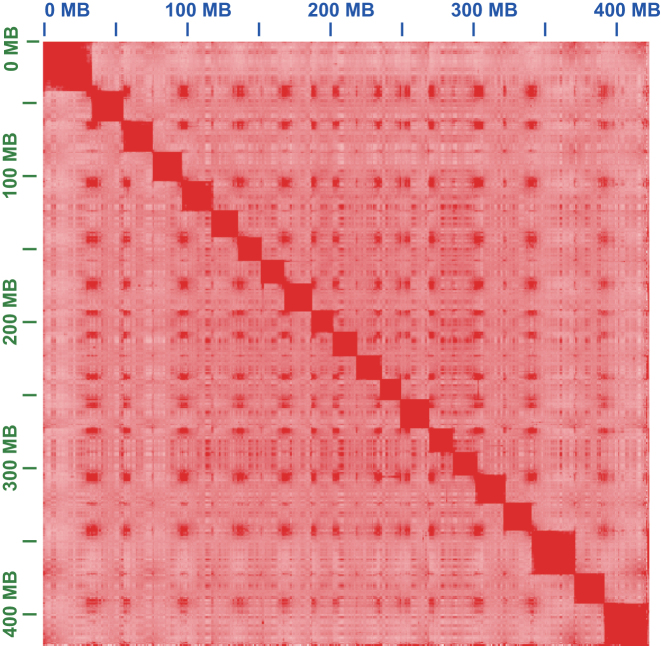
We obtained 52.34 Gb of clean reads using SOAPnuke, version 1.5.3 (SOAPnuke, RRID:SCR_015025) [3], with strict parameters, including removal of low-quality reads, adapter contamination, and Polymerase chain reaction (PCR) duplicates. Then, we performed the de novo assembly of the Giant reads using SOAPdenovo2, version 2.04 (SOAPdenovo2, RRID:SCR_014986) [4], assembler. For the genome assembly, the short insert size libraries were used to construct the contig sequences and the mate-paired libraries were used to link the scaffolds. We filled the gaps within the scaffolds using GapCloser, version 1.12 (GapCloser, RRID:SCR_015026). We obtained a genome assembly with a size of 465.24 Mb, with an N50 scaffold size of 949.03 Kb and an N50 contig size of 19.01 Kb (Table 1), covering 99.93% of the estimated genome size of 465.55 Mb using kmer, version 1.0, analysis (Supplementary Table S3 and Fig. S2). To construct the reference genome at the chromosome level, we used a MBOI endonuclease to cut the DNA and constructed a Hi-C library based on a previous protocol [5]. We sequenced 75.24 Gb of data using the BGISEQ-500 sequencing platform and obtained 34.5 Gb valid reads (∼45.8%) that could be used to anchor the scaffolds into chromosomes after quality control using the HiC-Pro, version 2.8.0, pipeline [6, 7] (Supplementary Figs. S3–S7). Last, we constructed 21 chromosomes that occupied 95.3% of the genome (Fig. 1, Table 1, Supplementary Table S4) using Juicer [8], version 1.5, and 3D-dna, version 170123, pipeline [9] based on the draft genome assembly. To evaluate the quality of the assembly, we found 95.4% of Benchmarking Universal Single-Copy Orthologs (BUSCO), version 3.0.1 (BUSCO, RRID:SCR_015008), genes that could be completely covered by our genome (Table 2) and approximately 98% of the transcripts assembled from RNA-seq data that could be aligned against the genome with more than 90% coverage (Supplementary Table S5).
Table 1:
Statistics of the assembly using SOAPdenovo and Hi-C data
| Type | Scaffold original | Contig original | Scaffold (Hi-C) | Contig (Hi-C) |
|---|---|---|---|---|
| Total number | 92,886 | 138,929 | 91,819 | 139,323 |
| Total length (bp) | 465,240,853 | 421,527,246 | 465,132,837 | 421,527,246 |
| Average length (bp) | 5,008.73 | 3,034.12 | 5,066 | 3,026 |
| N50 (bp) | 949,032 | 19,014 | 19,754,490 | 18,890 |
| N90 (bp) | 59,769 | 3,504 | 13,781,534 | 3,470 |
Figure 1:
Hi-C interaction heat map for B. Splendens reference genome showing interactions between the 21 chromosomes.
Table 2:
Evaluation results of the genome and gene set using BUSCO
| Genome | Genes | |||
|---|---|---|---|---|
| Number | Percentage (%) | Number | Percentage (%) | |
| Complete | 4,375 | 95.4 | 4,128 | 90.1 |
| Single-copy complete | 4,232 | 92.3 | 3,937 | 85.9 |
| Duplicated complete | 142 | 3.1 | 191 | 4.2 |
| Fragmented | 128 | 2.8 | 338 | 7.4 |
| Missing | 82 | 1.8 | 118 | 2.5 |
| Total | 4,584 | - | 4,584 | - |
Genome annotation
We annotated the repetitive sequences by combining de novo and homolog-based approaches [10]. First, we used LTR-FINDER, version 1.06 (LTR_Finder, RRID:SCR_015247) [11], and RepeatModeler, version 1.0.8 (RepeatModeler, RRID:SCR_015027), to construct a repetitive sequence library. Then, we used RepeatMasker, version 3.3.0 (RepeatMasker, RRID:SCR_012954) [12], to classify these repeat sequences. We also detected repetitive sequences using RepeatMasker and ProteinMasker, version 3.3.0, based on the Repbase library [13]. We identified 15.12% transposable elements in the genome (Supplementary Table S6).
For the protein-coding prediction, we combined the following approaches: gene model prediction using AUGUSTUS, version 3.0.3 (Augustus, RRID:SCR_008417) [14], and GENSCAN, version 1.0 (GENSCAN, RRID:SCR_012902) [15]; gene prediction using GeneWise, version 2.2.0 (GeneWise, RRID:SCR_015054) [16], based on the alignment results of protein sequences of other published species against our assembly; and five RNA-seq libraries were used to assist in predicting the gene structure with Cufflinks, version 2.2.1 (Cufflinks, RRID:SCR_014597) [17]. Last, we integrated all of this evidence into a nonredundancy gene set using GLEAN [18], version 1.0. The final gene set contained 23,981 genes (Supplementary Table S7), which is close to the number for Oryzias latipes [19] ( 24,674) and slightly less than that for Danio rerio [20] ( 26,046). We identified 90% of the 4,128 BUSCO gene models to be complete in the actinopterygii gene set (Table 2).
Comparative genomic analysis
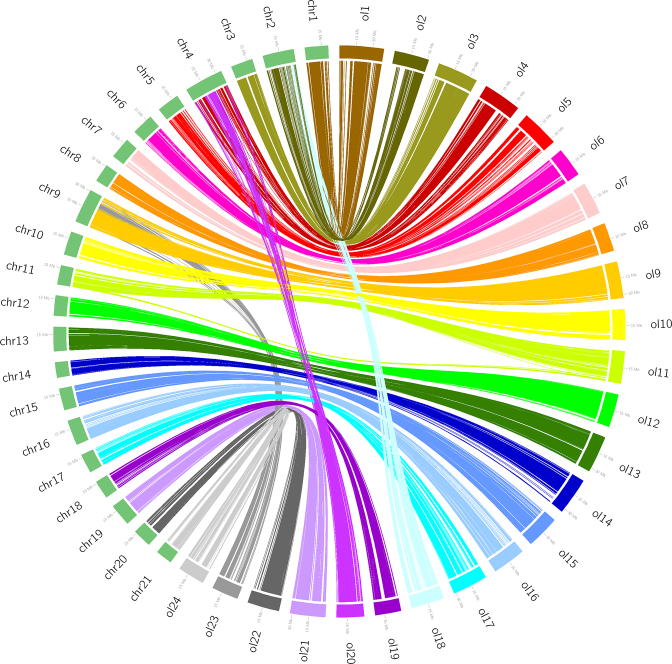
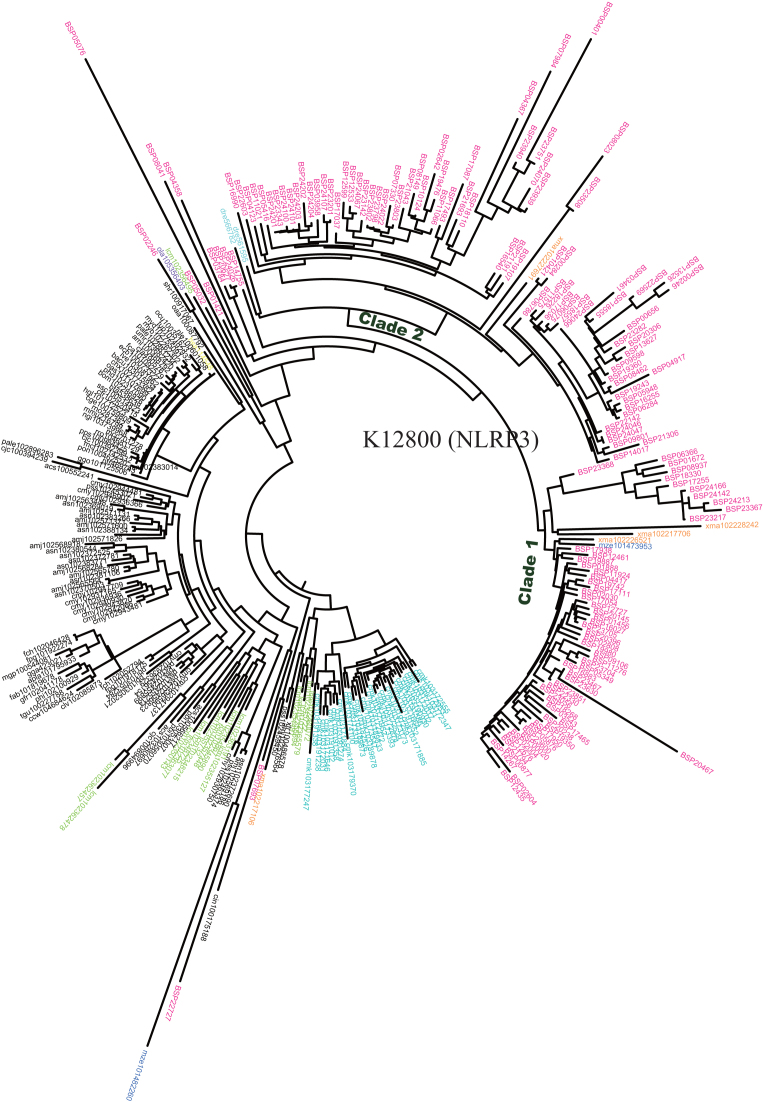
We compared the fighting fish genome with other species using Lastz, version 1.02.00, both at the whole-genome and gene levels. All of the 21 chromosomes assembled for the fighting fish could be matched to chromosomes of O. latipes with a mean coverage ratio of 75.3%. From these, 18 chromosomes had a single hit to one chromosome of O. latipes, and 3 chromosomes (1, 19, and 21) had a hit to two chromosomes of O. latipes (Fig. 2, Supplementary Table S8), indicating conservative evolution for most of chromosomes, as well as several chromosome reshuffling events between these two species. Furthermore, from the gene set level, KO (Kyoto Encyclopedia of Genes and Genomes [KEGG] Orthology) terms of animals from 109 species were counted and compared with the fighting fish gene set using the KEGG database [21], version 79. There were five KO terms notably expanded in fighting fish compared with all other animals, including 147 NACHT, LRR, and PYD domains-containing protein 3 (NLRP3, K12800), 86 tripartite motif-containing protein 47 (TRIM47, K12023), 43 chloride channel 7 (CLCN7, K05016), 29 arginine vasopressin receptor 2 (AVPR2, K04228), and 17 maltase-glucoamylase (MGAM, K12047) (Fig. 3). NLRP3 has two prominent expansions, one corresponding to clade 1, containing 56 genes, and one corresponding to clade 2, containing 79 genes, whereas other fish species in these two clades have less than three gene copies (Fig. 4). NLRP3 encodes a pyrin-like protein containing a pyrin domain, a nucleotide-binding site domain, and a leucine-rich repeat motif, and plays a role in the regulation of inflammation, the immune response, and apoptosis [22].
Figure 2:
Collinear relationship between B. splendens and O. latipes. Green represents the chromosomes of B. splendens and the other multicolor represent the chromosomes of O. latipes.
Figure 3:

Five gene families with prominent expansion in B. splendens when compared with 109 other species including NLRP3 (K12800), TRIM47 (K12023), CLCN7 (K05016), AVPR2 (K04228), and MGAM (K12047) using the KEGG database.
Figure 4:
The gene phylogenetic tree of NLRP3 gene family (KO: K12800) using the genes of B. splendens and other species. Clade 1 and clade 2 show two prominent expansion subfamilies of B. splendens.
Resequencing
Through mirror-image stimulation testing, we found that the different varieties of Siamese fighting fish have varying levels of aggressiveness. Male B. splendens were tested under a standardized mirror-elicited aggression paradigm as this elicits aggression levels similar to those of a real conspecific. One fighting fish was placed into the testing tank (30 × 19 × 23 cm) and left undisturbed for 30 minutes for acclimation. Then, the swimming behavior was recorded by taking a 5-minute video with a side digital camera; the swimming track was recorded using the Viewpoint ZebraLab Tracking System for 5 minutes. This represented the control state. After that, a mirror of similar size with a side wall was placed into the tank to induce aggression of the fish by its own mirror image. Aggression was observed through the following behaviors: opecular flare, fin spreading, 90° turn, and mirror hit. As expected, the mirror image elicited a high frequency of aggressive displays. Fish spent most of the time close to the mirror side and increased overall swimming distance compared to controls. Among all tested varieties, Giant had the highest frequency of aggressive displays and Half-moon had the lowest (Supplementary Fig. S8).
To evaluate the genetic diversity among the four varieties of B. splendens, we called the single-nucleotide variations (SNVs) and insertions and deletions (indels) based on the read alignment result using the Giant assembly as a reference. We obtained 70.25 Gb of clean reads filtered from 79.18 Gb of raw reads (Supplementary Table S9). We used BWA, version0.6.2 (BWA, RRID:SCR_010910) [23], to align all the resequencing data to the reference genome and the UnifiedGenotyper in Genome Analysis Toolkit, version2.8.1 (GATK, RRID:SCR_001876) [24], to call variations. In total, we detected approximately 3.4 M SNVs and 27,305 indels, which will provide a rich source of genomic data for use in future research and applications.
Availability of supporting data
Data is deposited in NCBI under the BioProject accession number PRJNA416843. Supporting data are also available via the GigaScience database GigaDB [25].
Additional files
Supplementary Figure S1: Five different varieties of adult male Siamese fighting fish.
Supplementary Figure S2: Distribution of the 17-mer analysis for the five Siamese fighting fish varieties.
Supplementary Figure S3: Quality control of Hi-C read alignment against genome sequence. Statistics for the type of separated pair-end read alignment. The aligned read ratio shown in the left bar including full read and trimmed read mapping.
Supplementary Figure S4: Quality control of read pairing. Considering the alignment type and read pairing, all paired reads include uniquely aligned pairs (Reported pairs), unmapped pairs (Unmapped pairs) and others (Not Reported pairs). The right bar shows the “Not Reported pairs”, which including low quality alignment, singleton and multiple hits.
Supplementary Figure S5: Statistic of read pair filtering. When assigned to restriction fragments, the aligned pairing reads can be divided to valid and invalid pairs. A valid paired-read involves two different restriction fragments and can be divided into four types according the direction of reads (the middle bar). F means Forward and R means Reverse. Invalid pairs content is shown in the right bar.
Supplementary Figure S6: Fraction of duplicated reads. The left bar shows the ratio of duplication for the valid read pairs. For all the non-duplicated reads, the percentage of cis and trans contacts are shown (right bar).
Supplementary Figure S7: Distribution of the fragment size. According to the distance between alignment site and the end of restriction fragment, a fragment size can be calculated.
Supplementary Figure S8: Evaluation of aggressive behavior in different varieties of Betta splendens. Number of opecular flare, mirror hit, 90° turning and fin spreading were counted manually from the 5-min video recording the swimming of normal and activated fish of different varieties. Data was expressed as mean ± S.D. of 6 replicates (n = 6).
Supplementary Table S1: Summary of sequencing data generated in this study.
Supplementary Table S2: Summary of the RNA-Seq data generated using the HiSeq 2000 sequencing platform.
Supplementary Table S3: 17-mer analysis information for the five Siamese fighting fish varieties.
Supplementary Table S4: Length of the 21 chromosomes constructed for the Siamese fighting fish Giant variety, given in descending order.
Supplementary Table S5: Assessment of the gene region coverage of assembly using RNA-seq data.
Supplementary Table S6: Statistics for the transposable element (TE) sequences present in the Siamese fighting fish genome.
Supplementary Table S7: Statistics of predicted gene models.
Supplementary Table S8: Summary of the alignment between Betta splendens and Oryzias latipes.
Supplementary Table S9: Summary of the resequencing data generated in this study.
Abbreviations
indel: insertions and deletions; KO: Kyoto Encyclopedia of Genes and Genomes Orthology; RNA-seq: RNA sequencing; SNV: single-nucleotide variation.
Author contributions
G.F., S.L., and J.C. conceived the project. G.F., X.L., and J.C. supervised the research. H.Z., C.S., and X.Y. conceived and designed the experiments. K.M., X.L., and B.Y. performed genome assembly and gene annotation. H.L., Z.R., Q.L., and Q.X. prepared the fighting fish sample. Jiahao. W., W.C., X.X., and L.S. performed sequencing. A.R., M.G., Jing. C., H.Y., and J.W. performed comparative genomic analysis. G.F., S.C., Y.W., and D.G. revised the paper. K.M. and X.Y. performed data accession.
Supplementary Material
3/13/2018 Reviewed
6/14/2018 Reviewed
3/16/2018 Reviewed
ACKNOWLEDGEMENTS
We thank the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA16010100), the Macau Science and Technology Development Fund for financial support (project 011/2014/A1), and Shenzhen Municipal Government of China (JCYJ20151015162041454 to W.C. and JCYJ20150529150505656 to X.L.).
References
- 1. Ma K. DNA extraction for the Betta splendens genome. Protocols.io. 2018. http://dx.doi.org/10.17504/protocols.io.qvedw3e. [Google Scholar]
- 2. Ma K. RNA extraction for the Betta splendens genome. Protocols.io. 2018. http://dx.doi.org/10.17504/protocols.io.qvfdw3n. [Google Scholar]
- 3. Chen Y, Chen Y, Shi C et al. . SOAPnuke: a MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. GigaScience. 2018;7:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Luo R, Liu B, Xie Y et al. . SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. GigaScience. 2012;1:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Durand NC, Shamim MS, Machol I et al. . Juicer provides a one-click system for analyzing loop-resolution Hi-C experiments. Cell Syst. 2016;3:95–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Servant N, Varoquaux N, Lajoie BR et al. . HiC-Pro: an optimized and flexible pipeline for Hi-C data processing. Genome Biol. 2015;16:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu X. The pipeline of Hi-C assembly. Protocols.io. 2018. http://dx.doi.org/10.17504/protocols.io.qradv2e. [Google Scholar]
- 8. Belton JM, McCord RP, Gibcus JH et al. . Hi-C: a comprehensive technique to capture the conformation of genomes. Methods. 2012;58:268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dudchenko O, Batra SS, Omer AD, et al. . De novo assembly of the Aedes aegyptigenome using Hi-C yields chromosome-length scaffolds. Science. 2017;356:92–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu X. An analytical pipeline of assembly and annotation of the Betta splendens genome. Protocols.io. 2018. http://dx.doi.org/10.17504/protocols.io.qq9dvz6. [Google Scholar]
- 11. Xu Z, Wang H. LTR_FINDER: an efficient tool for the prediction of full-length LTR retrotransposons. Nucleic Acids Res. 2007;35:W265–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tarailo-Graovac M, Chen N. Using RepeatMasker to identify repetitive elements in genomic sequences. Curr Protoc Bioinformatics. 2009, 4: 10, 10–14. [DOI] [PubMed] [Google Scholar]
- 13. Jurka J, Kapitonov VV, Pavlicek A, et al. . Repbase Update, a database of eukaryotic repetitive elements. Cytogenet Genome Res. 2005;110:462–7. [DOI] [PubMed] [Google Scholar]
- 14. Stanke M, Keller O, Gunduz I, et al. . AUGUSTUS: ab initio prediction of alternative transcripts. Nucleic Acids Res. 2006;34:W435–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burge C, Karlin S. Prediction of complete gene structures in human genomic DNA. J Mol Biol. 1997;268:78–94. [DOI] [PubMed] [Google Scholar]
- 16. Birney E. GeneWise and Genomewise. Genome Res. 2004;14:988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trapnell C, Roberts A, Goff L, et al. . Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7:562–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Elsik CG, Mackey AJ, Reese JT et al. . Creating a honey bee consensus gene set. Genome Biol. 2007;8:R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kasahara M, Naruse K, Sasaki S et al. . The medaka draft genome and insights into vertebrate genome evolution. Nature. 2007;447:714–9. [DOI] [PubMed] [Google Scholar]
- 20. Howe K, Clark MD, Torroja CF et al. . The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kanehisa M. The KEGG database. Novartis Found Symp. 2002;247:91–103. [PubMed] [Google Scholar]
- 22. Hirota SA, Ng J, Lueng A et al. . NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflamm Bowel Dis. 2011;17:1359–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McKenna A, Hanna M, Banks E et al. . The genome analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fan G, Chan J, Ma K et al. . Supporting data for “Chromosome-level reference genome of the Siamese fighting fish Betta splendens, a model species for the study of aggression.”. GigaScience Database. 2018. http://dx.doi.org/10.5524/100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
3/13/2018 Reviewed
6/14/2018 Reviewed
3/16/2018 Reviewed