Abstract
BACKGROUND:
Gastrointestinal bleeding (GIB) is a common complication seen in patients supported with left ventricular assist devices (LVADs) and is related to increased inflammation and angiogenesis. Omega-3 is an unsaturated fatty acid that possesses anti-inflammatory and antiangiogenic properties. This study aims to assess the prophylactic efficacy of treatment with omega-3 on the incidence of GIB in LVAD patients.
METHODS AND RESULTS:
Among consecutive 166 LVAD patients enrolled in this analysis, 30 patients (49 years old and 26 male) received 4 mg/d of omega-3 therapy for 310±87 days and 136 patients in the control group (58 years old and 98 male) were observed for 302±102 days. One-year GIB-free rate was significantly higher in the omega-3 group as compared with the control group (97% versus 73%; P=0.02). Omega-3 therapy was associated with the occurrence of GIB in both the univariate (hazard ratio, 0.12; 95% CI, 0.02–0.91; P=0.040) and multivariate Cox proportional hazard ratio analyses (hazard ratio, 0.13; 95% CI, 0.02–0.98; P=0.047). The frequency of GIB was significantly lower in the omega-3 group (0.08±0.42 versus 0.37±0.93 events/y; P=0.01), accompanied by significantly lower blood product transfusion and shorter days in the hospital. The frequency of GIB remained lower among the omega-3 group after matching for patient background characteristics (96% versus 73%, P=0.028).
CONCLUSIONS:
LVAD patients treated with omega-3 had a significant increase in freedom from GIB. A randomized controlled study is warranted to evaluate the use of omega-3 in LVAD patients.
Keywords: fish oil, freedom, incidence, inflammation, therapeutics
Continuous-flow left ventricular assist devices (LVADs) have changed the clinical course of advanced heart failure (HF) patients, with significant improvement in both longevity and quality of life.1 However, patients supported with LVAD are prone to a high burden of adverse events requiring frequent readmissions. Hemocompatibility-related adverse events, composed of both bleeding and thrombotic adverse events (gastrointestinal bleeding [GIB], neurological events, pump thrombosis, and other thromboembolic events) are the leading causes for readmission, with GIB being the most common complication.2
Therapies to address GIB include temporary discontinuation of anticoagulant and antiplatelet medications, intravenous administration of proton pump inhibitors, octreotide therapy, and invasive endoscopic cauterization.3 In rare cases, administration of vitamin K, fresh frozen plasma, cryoprecipitate, or concentrated vWF (von Willebrand factors) is given for refractory bleeding episodes, but these agents may increase the risk of pump thrombosis. To date, a definitive strategy to prevent GIB has not been identified.4
Evidence is mounting that the development of arteriovenous malformations (AVMs) is a key factor in the development of GIB in LVAD patients,4 in addition to previously identified mechanisms, including LVAD-induced acquired von Willebrand disease and continuous anticoagulant and antiplatelet therapies.5 Recently, our team demonstrated that thrombin-induced angiopoietin-2 expression and higher level of TNF (tumor necrotic factor)-α in LVAD patients lead to increased angiogenesis, which may contribute to AVM formation.6,7
Omega-3, an unsaturated fatty acid, is known to have inhibitory effects on angiogenesis and inflammation and thus may potentially have a role in the prevention of GIB in these patients.8,9 In this study, we investigated for the first time the prophylactic effect of omega-3 therapy on reduction of GIB in LVAD patients.
METHODS
Patient Selection
The data, analytic methods, and study materials will be made available on the corresponding author to any researchers for purposes of reproducing the results or replicating the procedure. All patients implanted with LVAD in our center between April 2014 and May 2017 were included in this retrospective analysis. Patients were divided into 2 groups based on treatment with omega-3. Omega-3 therapy was administered at a dose of 4 g daily at discretion of the treating cardiologists. Patients treated with omega-3 were followed for 1 year from the time of omega-3 initiation. All other patients were followed for 1 year from the index admission discharge day and served as a control group.
All patients received guideline-directed medical therapy, including antiplatelet therapy, oral proton pump inhibitor, and warfarin with a target international normalized ratio (INR) between 2.0 and 3.0 for HeartWare LVAD and between 2.0 and 2.5 for HeartMate II LVAD, unless recurrent GIB occurred. The institutional review board of the University of Chicago approved this study. All subjects gave informed consent for this study.
Definition of GIB
GIB was defined according to the Interagency Registry for Mechanically Assisted Circulatory Support as any clinically suspected or documented bleeding from the GI tract as indicated by a new drop in hemoglobin and the appearance of melena, hematochezia, hematemesis, or guaiac-positive stool. The validity of the definition of GIB was confirmed by 2 independent researchers (Dr Imamura and P.M.).
Management of GIB
GIB was managed according to our institutional protocol, irrespective of administration of omega-3, by the attending physicians. Patients admitted to the hospital with GIB had both anticoagulants and antiplatelet agents discontinued. All patients received proton pump inhibitor therapy by intravenous infusion.10 Red blood cells were transfused to achieve a minimum hemoglobin of 7.0 g/dL. Vitamin K or fresh frozen plasma was administered when ongoing severe bleeding was present with an elevated INR. Invasive endoscopy was performed in most cases, with the addition of capsule endoscopy, push enteroscopy, or mesenteric angiography when the diagnosis was not clear. Clipping, argon plasma coagulation, or infrared radiation embolization were performed when indicated.
When active GIB resolved, anticoagulation was slowly reintroduced using the previous INR target, while antiplatelet therapy was held indefinitely. In cases of recurrent bleeding, patients were started on long-acting octreotide as an outpatient,11 and in select cases, the INR target was reduced.
Variables Evaluated
All clinical variables of both groups were obtained from the electronic medical record. The primary end point was GIB events rates during the observational period. Blood products, length of hospitalization, and amount of GI procedures were collected. Aspirin dose, INR, LVAD speed, hemoglobin levels, and anti-HF medication prescription data were obtained at the time of follow-up start, at first month, third months, and sixth months.
Statistical Analyses
Statistics were performed using SPSS Statistics 22 (SPSS Inc, Chicago, IL). Variables with P<0.05 were considered as significant. Continuous data were described as mean±SD when normally distributed or described as median (25% quartile, 75% quartile) when non-normally distributed and compared between 2 groups using unpaired t test or Mann-Whitney U test as appropriate. Categorical data were compared between 2 groups by χ2 test or Fischer exact test as appropriate. Clinical events were calculated as per patient-year or patient-event and expressed as mean±SD. The 1-year GIB-free rates were analyzed by Kaplan-Meier analyses and compared between the 2 groups by using log-rank test. Univariate Cox proportional hazard ratio (HR) analyses were performed to investigate significant factors associated with any GIB events. Variables with P<0.05 in these analyses were enrolled into the multivariate analysis model.
We also performed a propensity score analysis, matching for age, sex, device type, and aspirin dose at discharge to collect 1:2 of omega-3 group and control group because of the impact of these 4 variables on GIB. A propensity score was calculated using logistic regression modeling, including all 4 of the above variables and paired participants were selected based on the propensity scoring.
RESULTS
Baseline Characteristics
One hundred sixty-six patients (101 axial flow pumps and 65 centrifugal flow pump) were enrolled. Patients were 57 (48, 65) years old and 137 were male. Most patients received an LVAD as destination therapy (87%), and 57 (34%) had an ischemic HF cause (Table 1). No patients experienced GIB before the follow-up.
Table 1.
Comparison of Baseline Characteristics
| Total (N=166) | Omega-3 (+) (N=30) |
Omega-3 (−) (N=136) |
P Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 57 (48, 65) | 49 (40, 57) | 58 (51,67) | <0.001* |
| Body mass index | 30.0 (25.4, 36.2) | 32.8 (28.1, 35.8) | 29.9 (25.4, 36.2) | 0.36 |
| Male | 137 (83%) | 26 (87%) | 111 (82%) | 0.07 |
| LVAD duration before the enrollment, d | 20 (14, 32) | 25 (5, 64) | 20 (14, 28) | 0.06 |
| Destination therapy | 145 (87%) | 26 (87%) | 119 (88%) | 0.18 |
| Device | ||||
| Axial LVAD | 100 (60%) | 17 (57%) | 83 (61%) | 0.40 |
| Centrifugal LVAD | 66 (40%) | 13 (43%) | 53 (39%) | 0.84 |
| Temporary RVAD use | 12 (7%) | 2 (7%) | 10 (7%) | 0.90 |
| Comorbidity | ||||
| Ischemic cause | 57 (34%) | 7 (23%) | 50 (37%) | 0.22 |
| Hypertension | 95 (57%) | 18 (60%) | 77 (57%) | 0.45 |
| Diabetes mellitus | 58 (35%) | 8 (27%) | 50 (37%) | 0.20 |
| Atrial fibrillation | 60 (36%) | 10 (33%) | 50 (37%) | 0.45 |
| History of stroke | 28 (17%) | 3 (10%) | 25 (18%) | 0.20 |
| History of ventricular tachyarrhythmia | 61 (37%) | 10 (33%) | 51 (38%) | 0.42 |
| Hemoglobin, g/dL | 9.56±1.25 | 9.80±1.35 | 9.50±1.23 | 0.32 |
LVAD indicates left ventricular assist device; and RVAD, right ventricular assist device.
P<0.05 by unpaired t test or Mann-Whitney U test.
Thirty patients received omega-3, starting 25 (5, 64) days after LVAD implantation, with therapy continuing for 310.4±87.3 days. None of the patients in the omega-3 arm experienced any side effects leading to discontinuation of the medication. As a control group, 136 patients who did not receive omega-3 were followed from the first discharge of the index hospitalization (20 [14, 28] days after LVAD implantation) and observed for 302.0±101.5 days. Eighteen patients who died during the index hospitalization were excluded. None of the excluded patients received omega-3 before their death.
Patients in the omega-3 group were younger (49 [40, 57] years old versus 58 [51, 67] years old, P<0.001), while other baseline characteristics were identical between the groups (P>0.05 for all). There were no statistical differences between the 2 groups in anti-thrombotic therapy, LVAD speed, or anti-HF medications during the observational period (Table I in the Data Supplement).
GIB Events
Only 1 patient (3%) in the omega-3 group experienced GIB (2 events at 52 and 270 days). In the control group, 30 patients (22%) experienced 42 GIB events. Among them, the duration between baseline and the first event was 60 days (27, 272).
The 1-year freedom from GIB was 97% in the omega-3 group as compared with 73% in the control group (P=0.019; Figure 1A). The frequency of GIB was 0.08±0.42 events/y as compared with 0.37±0.93 events/y in the control group (P=0.010; Figure 1B).
Figure 1. Comparison of freedom from gastrointestinal (GI) bleeding (A) and GI bleeding rate (B) between the omega-3 group and control group.
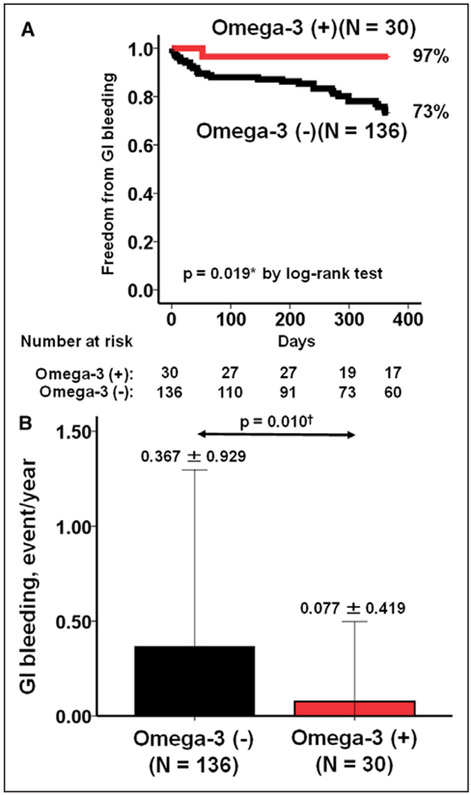
*P<0.05 by log-rank test and †P<0.05 by Mann-Whitney U test.
Among the patients aged <60 years old (N=96), the omega-3 group had significantly higher freedom from GIB compared with the control group (100% versus 82%, P=0.049; Figure IA in the Data Supplement). Among those aged ≥60 years old (N=70), the omega-3 group had a numerically higher freedom from GIB compared with the control group, whereas the difference was not statistically significant (83% versus 64%; Figure IB in the Data Supplement).
In the multivariate Cox proportional HR analysis, age (per 10 years) had an HR of 1.51 (95% CI, 1.11–2.05; P=0.008) and omega-3 use had an HR of 0.13 (95% CI, 0.02–0.98; P=0.047) in association with GIB (Table 2). The use of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker at discharge was significant only in the univariate analysis (HR, 0.39; 95% CI, 0.17–0.86; P=0.019).
Table 2.
Cox Proportional Hazard Ratio Analyses for any GI Bleeding Events
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) |
P Value | Hazard Ratio (95% CI) |
P Value | |
| Demographics | ||||
| Age, per 10 y | 1.62 (1.22–2.16) | 0.001* | 1.51 (1.11–2.05) | 0.008* |
| Body mass index | 0.97 (0.92–1.03) | 0.29 | ||
| Female | 1.00 (0.45–2.21) | 0.97 | ||
| Destination therapy | 1.25 (0.52–3.02) | 0.63 | ||
| Axial LVAD vs centrifugal LVAD | 0.95 (0.47–1.92) | 0.89 | ||
| Temporary RVAD use | 0.39 (0.05–2.82) | 0.35 | ||
| Omega-3 administration | 0.12 (0.02–0.91) | 0.040* | 0.13 (0.02–0.98) | 0.047* |
| Comorbidity | ||||
| Ischemic cause | 1.67 (0.84–3.33) | 0.15 | ||
| Hypertension | 0.75 (0.38–1.49) | 0.42 | ||
| Diabetes mellitus | 0.79 (0.38–1.66) | 0.53 | ||
| Atrial fibrillation | 1.31 (0.65–2.63) | 0.46 | ||
| Hemoglobin, g/dL | 0.81 (0.59–1.11) | 0.19 | ||
| Baseline therapeutic parameters | ||||
| PT-INR | 1.12 (0.57–2.21) | 0.75 | ||
| PT-INR >3.0 | 1.84 (0.56–6.04) | 0.31 | ||
| Aspirin dose, per 100 mg | 0.84 (0.52–1.28) | 0.38 | ||
| Aspirin administration | 0.77 (0.33–1.77) | 0.53 | ||
| LVAD speed, per 100 rpm | ||||
| Axial LVAD | 0.99 (0.97–1.01) | 0.38 | ||
| Centrifugal LVAD | 0.94 (0.51–1.75) | 0.85 | ||
| ACE or ARB | 0.39 (0.17–0.86) | 0.019* | 0.48 (0.18–1.16) | 0.12 |
| β-blocker | 0.57 (0.26–1.22) | 0.15 | ||
| Aldosterone antagonist | 0.49 (0.22–1.14) | 0.10 | ||
ACE indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; GI, gastrointestinal; LVAD, left ventricular assist device; PT-INR, prothrombin time with international normalized ratio; and RVAD, right ventricular assist device.
P<0.05 by Cox proportional hazard ratio analyses.
Clinical Outcomes
Hemoglobin levels were comparable at baseline and 1 month but were significantly higher at 3 and 6 months in the omega-3 group compared with the control group (P=0.001 for both; Figure 2).
Figure 2. Trends of hemoglobin level during the observational period in both groups.
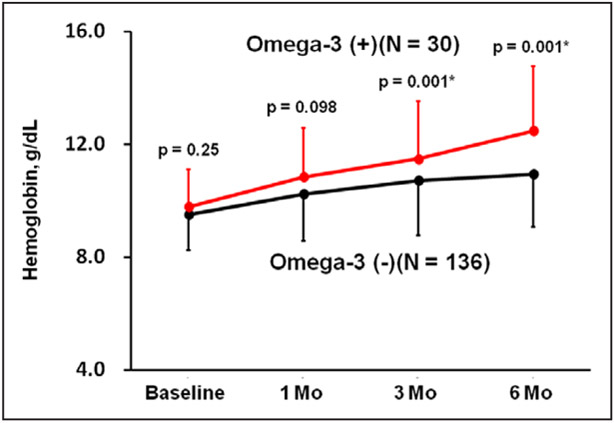
*P<0.05 by unpaired t test between the groups. Vertical bars indicate SDs.
The blood product utilization and days in hospital were significantly lower in the omega-3 group (Table 3; P<0.05 for all). There were no adverse events associated with omega-3, and no patients in the omega-3 group died during the study period, whereas 20 patients (17%) in the control group died.
Table 3.
Comparison of Parameters Associating With GI Bleeding
| Omega-3 (+) (N=30) |
Omega-3 (−) (N=136) |
P Value | |
|---|---|---|---|
| Per patient-year | |||
| RBC, units | 0.2±0.8 | 0.8±2.8 | 0.028* |
| FFP, units | 0 | 0.2±0.9 | 0.011* |
| LOS, d | 0.6±3.4 | 3.9±11.4 | 0.005* |
| GI procedures | 0.08±0.42 | 0.23±0.72 | 0.088 |
| Per patient-event | |||
| RBC, units | 0.07±0.37 | 0.4±1.1 | 0.008* |
| FFP, units | 0 | 0.1±0.4 | 0.009* |
| LOS, d | 0.3±1.5 | 2.1±4.8 | <0.001* |
| GI procedures | 0.03±0.18 | 0.14±0.35 | 0.015* |
FFP indicates fresh frozen plasma; GI, gastrointestinal; LOS, length of stay; and RBC, red blood cell.
P<0.05 by unpaired t test or Mann-Whitney U test.
Comparison Among Background-Matched Populations
Using propensity analysis, 27 patients in the omega-3 group were matched with 54 patients in the control group. There are no significant differences in background characteristics between the 2 groups (Table II in the Data Supplement). We found that the omega-3 group continued to demonstrate a higher 1-year freedom from GIB (Figure 3A; 96% versus 73%, P=0.044) and a lower GIB rate (Figure 3B; 0.09±0.44 versus 0.39±0.87 event/y; P=0.028) as compared with the background-matched control group, although Cox HR regression analysis did not show statistical significance (HR, 0.16; 95% CI, 0.02–1.24; P=0.083).
Figure 3. Comparison of freedom from gastrointestinal (GI) bleeding (A) and GI bleeding rate (B) between the omega-3 group and propensity-matched control group.
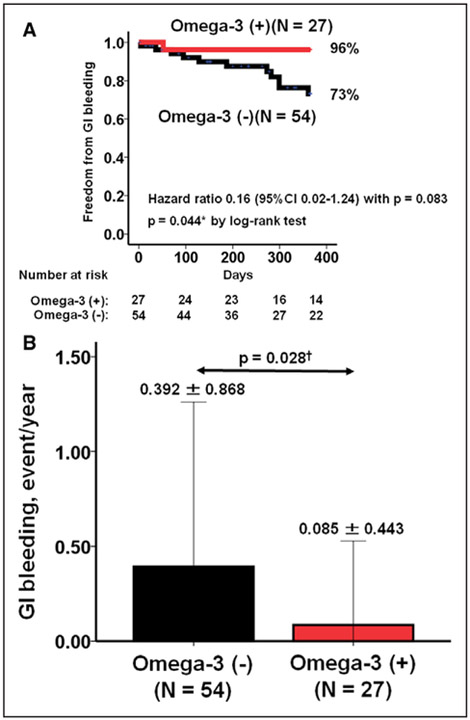
*P<0.05 by log-rank test and †P<0.05 by Mann-Whitney U test. Background was matched between 2 groups in age, sex, device types, and aspirin dose at discharge by using the propensity score matching analysis. Hazard ratio was calculated by Cox proportional hazard ratio analysis.
DISCUSSION
In this study, we analyzed the efficacy of omega-3 therapy on the primary prevention of GIB in LVAD patients.GIB rate was lower in the omega-3 group, and these findings remained despite propensity-adjusted analysis. Consistent with these findings was that patients receiving omega-3 therapy required fewer blood product transfusions, days in the hospital, and GI procedures.
Pathophysiology of GIB, Current Therapy, and Omega-3
GIB is a leading cause of readmissions among LVAD patients, occurring in 15% of the MOMENTUM 3 study (Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy With HeartMate 3) population at 6 months12 and 35% of the ENDURANCE study (Clinical Trial to Evaluate the HeartWare Ventricular Assist System) population at 2 years.13 Previously, the use of anticoagulation and antiplatelet therapy in combination with the development of acquired von Willebrand disease was thought to be the leading causes of GIB events in LVAD patients.4
However, recent data have revealed the critical role of abnormal angiogenesis and the development of GI angiodysplasia. We previously reported that patients supported by LVADs have elevated thrombin levels because of the expression of tissue factor, causing an increase in the levels of angiopoietin-2, which promotes abnormal angiogenesis by proliferation of endothelial cells.6 Furthermore, we also demonstrated that elevated TNF-α levels lead to an increase in tissue factors, which promotes thrombin generation. Elevated TNF-α also facilitates pericyte apoptosis, reduces angiopoietin-1, and makes endothelial cells destabilized, which also result in abnormal angiogenesis.7 In LVAD patients who had high concentrations of both angiopoietin-2 and TNF-α before the surgery, there was a highly significant increase in the risk of GIB.7
These inflammatory and angiogenesis activation are already observed in patients with advanced HF before LVAD implantation. Consistently, the prevalence of nasal hypervascularity, which is associated with GIB, in patients with advanced HF was as high as in those with LVAD support.14 However, the severity of nasal hypervascularity was lower in HF group compared with the LVAD group. LVAD-induced activation of TNF-α would more enhance the angiogenesis cascade.7
After a GIB event, alteration of anticoagulation and antiplatelet therapy is recommended.4 The European TRACE study (Study of Reduced Anti-Coagulation/AntiPlatelet Therapy in Patients With the HeartMate II LVAS) demonstrated that anticoagulation therapy alone (without antiplatelet therapy) reduced the incidence of major bleeding without increasing the risk of thromboembolic events.15 Nevertheless, optimal magnitude of anticoagulation and antiplatelet therapies remains uncertain. Reduced pulsatility has also been associated with GIB, and reduction in LVAD speed has been recommended to enhance arterial pulsatility and thereby suppress the formation of AVMs.16,17 However, the role of pulsatility in GIB has been questioned by the results of partial LV support device, which continued to have a relatively high GIB rate, despite sufficient preservation of pulse pressure.18 Furthermore, lowering LVAD speeds may run counter to the goals of LV unloading and hemodynamic optimization, which often required an increase in LVAD speed.19
In the arena of therapeutics, the use of octreotide, a somatostatin analog that induces splanchnic arterial vasoconstriction, was assessed in LVAD patients and found to reduce the rate of GIB in a secondary prevention population.11,20 Other therapies, such as danazol and thalidomide, have recently been proposed for the management of GIB.21,22 However, their clinical use may be restricted to the secondary prevention of GIB, considering their significant adverse effect profiles. Strict patient selection and careful monitoring are required for thalidomide therapy because of pancytopenia and neuropathy,21 and patients may not tolerate long-term danazol therapy because of its androgenic side effects.22
In the current study, omega-3 therapy prevented the development of GIB in LVAD patients in both an unadjusted and propensity-matched adjusted analysis. The GIB rate in the omega-3 group was 0.08 events/y, which was 79% lower than the rate of 0.37 events/y in the control group and 84% lower than the rate of 0.49 events/y recently reported by Interagency Registry for Mechanically Assisted Circulatory Support (at 3–12 months after LVAD implantation).1 The rate of GIB in the omega-3 group was surprisingly similar to the rate of all-cause bleeding reported by the Japanese registry for Mechanically Assisted Circulatory Support (0.12 events/y).23 One potential mechanism for the lower rates of GIB Japan may be related to the Japanese diet, which is rich in fish oil containing omega-3.24 Further studies that compare angiogenesis-related biomarkers and GIB rates in patients receiving a same device (ie, HeartMate II) between United States and Japan are warranted.
Why Does Omega-3 Therapy Suppress GIB?
Omega-3 suppresses inflammation, angiogenesis, and cell proliferation and invasion via its effects on cyclooxygenase, lipoxygenase, and cytochrome P450 enzymes.8 Mehra et al25 demonstrated that omega-3 decreased TNF-α production in HF patients. We demonstrated that the reduction in TNF-α and angiopoietin-2 levels at 3 months after LVAD implantation was associated with lower GIB occurrence.26 Thus, the mechanism of action of omega-3 may be related to reductions in TNF-α, with subsequent suppression of angiopoietin-2 expression, although nobody has any direct pieces of evidence for their relationship.
The omega-3 inhibits the aggregation of platelet and may provoke the efficacy of antiplatelet therapy. The omega-3 group had numerically higher dose of antiplatelet agent during the observational period, probably because of less occurrence of GIB in this group. Nevertheless, the omega-3 therapy was associated with the reduced GIB rate, which may strengthen our hypothesis that the major cause of GIB in LVAD patients is the development of AVM via angiogenesis-related cascade instead of anticoagulation and antiplatelet therapies. The prophylactic efficacy of omega-3 therapy on GIB in patients without LVAD is also uncertain or rather may be unexpected because AVM may not be a major cause of bleeding in such situation.
Future Direction and Study Limitations
This study is a proof of concept, and as such is an observational analysis that was not randomized. The indication for omega-3 administration was determined by the attending physicians and inclusion bias may exist. Particularly, there was a significant difference in age, which was also a significant risk factor of GIB, between 2 groups. Furthermore, the timing of initiation of omega-3 therapy varied among patients. We started follow-up of the control group at discharge from the index hospitalization, and the timings of follow-up were comparable between 2 groups. We used propensity score matching technique to manage these biases. Also, we performed sub-analyses among age <60 years old population and age ≥60 years old population and confirmed a prophylactic efficacy of omega-3 among <60 years old population. In contrast, it did not reach statistical significance among age ≥60 years old. Our results may not be adopted in the elderly population, and the efficacy of omega-3 therapy should be validated also in such population. Despite these statistical procedures, we cannot completely delete the confounding of age in this study. We are planning a prospective, randomized, placebo-controlled study to overcome such confounding.
In the current study, we did not measure angiogenesis and inflammatory parameters, and the theoretical effect of omega-3 on angiogenesis and inflammation was not proven. Longitudinal biomarker assessment is essential to build a stronger foundation for the current hypothesis. In this study, patients were administered omega-3 at a high dose of 4 g daily; whether this is the optimal dose to suppress GIB is unknown. We studied the efficacy of omega-3 therapy for the primary prevention of GIB, but the patients with refractory GIBs may also be good candidates for the omega-3 therapy. Those who receive intensified anticoagulation and antiplatelet therapies to manage pump thrombosis are at high-risk of GIB and may also be good candidates of the omega-3 therapy. The indication of omega-3 therapy on such high-risk populations are also next concerns. Last, using next-generation sequencing, the gut microbiome has been described and implicated in multiple disease processes, including GIB,27 and its fingerprint can be modified by omega-3 administration.28 Assessment of changes in the microbiome during omega-3 therapy may further elucidate the mechanism by which omega-3 suppresses GIB.
Conclusions
Omega-3 therapy was associated with significantly less GIB, as well as better clinical outcomes during LVAD support, even after the adjustment with key background factors. A randomized placebo-controlled study and a biomarker study to approach the mechanism of omega-3 therapy are warranted to support the current results.
Supplementary Material
WHAT IS NEW?
Omega-3, an unsaturated fatty acid (ie, fish oil) that may possess anti-inflammatory and antiangiogenic properties, had a primary preventive efficacy of gastrointestinal bleeding in patients with left ventricular assist device.
The preventive efficacy of omega-3 remained, despite background-adjusted analysis.
WHAT ARE THE CLINICAL IMPLICATIONS?
Omega-3 may be considered as a standard therapy to prevent gastrointestinal bleeding in patients with left ventricular assist device in the near future.
The next concern is a comparison study with other countries such as Japan, where high load of fish is taken with low gastrointestinal bleeding rate during left ventricular assist device therapy.
Another concern is a prospective randomized control study to investigate primary and secondary preventive efficacy of omega-3 on gastrointestinal bleeding.
Footnotes
Disclosures
Dr Imamura receives financial funding from Fukuda Foundation for Medical Technology and Postdoctoral Fellowship for Research Abroad of Japan Society for the Promotion of Science. Dr Uriel receives consultant fee and grants support from Abbott and Medtronic; Dr Sayer receives consultant fees from Medtronic; Dr Jeevanandam receives consultant fee from Abbott. Dr Burkhoff receives consultant fee from Medtronic, Corvia Medical, Sensible Medical, Impulse Dynamics, Cardiac Implants, and educational grant support from Abiomed. The other authors report no conflicts.
Contributor Information
Teruhiko Imamura, Department of Medicine, University of Chicago Medical Center, IL..
Ann Nguyen, Department of Medicine, University of Chicago Medical Center, IL..
Daniel Rodgers, Department of Medicine, University of Chicago Medical Center, IL..
Gene Kim, Department of Medicine, University of Chicago Medical Center, IL..
Jayant Raikhelkar, Department of Medicine, University of Chicago Medical Center, IL..
Nitasha Sarswat, Department of Medicine, University of Chicago Medical Center, IL..
Sara Kalantari, Department of Medicine, University of Chicago Medical Center, IL..
Bryan Smith, Department of Medicine, University of Chicago Medical Center, IL..
Ben Chung, Department of Medicine, University of Chicago Medical Center, IL..
Nikhil Narang, Department of Medicine, University of Chicago Medical Center, IL..
Colleen Juricek, Department of Surgery, University of Chicago Medical Center, IL..
Daniel Burkhoff, Columbia University Medical Center, Cardiovascular Research Foundation, New York, NY.
Tae Song, Department of Surgery, University of Chicago Medical Center, IL..
Takeyoshi Ota, Department of Surgery, University of Chicago Medical Center, IL..
Valluvan Jeevanandam, Department of Surgery, University of Chicago Medical Center, IL..
Gabriel Sayer, Department of Medicine, University of Chicago Medical Center, IL..
Nir Uriel, Department of Medicine, University of Chicago Medical Center, IL..
REFERENCES
- 1.Kirklin JK, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB, Naftel DC. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant. 2017;36:1080–1086. doi: 10.1016/j.healun.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 2.Forest SJ, Bello R, Friedmann P, Casazza D, Nucci C, Shin JJ, D’Alessandro D, Stevens G, Goldstein DJ. Readmissions after ventricular assist device: etiologies, patterns, and days out of hospital. Ann Thorac Surg. 2013;95:1276–1281. doi: 10.1016/j.athoracsur.2012.12.039 [DOI] [PubMed] [Google Scholar]
- 3.Maltais S, Kilic A, Nathan S, Keebler M, Emani S, Ransom J, Katz JN, Sheridan B, Brieke A, Egnaczyk G, Entwistle JW III, Adamson R, Stulak J, Uriel N, O’Connell JB, Farrar DJ, Sundareswaran KS, Gregoric I; PREVENT Study Investigators. PREVENtion of HeartMate II Pump Thrombosis Through Clinical Management: The PREVENT multi-center study. J Heart Lung Transplant. 2017;36:1–12. doi: 10.1016/j.healun.2016.10.001 [DOI] [PubMed] [Google Scholar]
- 4.Eckman PM, John R. Bleeding and thrombosis in patients with continuous-flow ventricular assist devices. Circulation. 2012;125:3038–3047. doi: 10.1161/CIRCULATIONAHA.111.040246 [DOI] [PubMed] [Google Scholar]
- 5.Uriel N, Pak SW, Jorde UP, Jude B, Susen S, Vincentelli A, Ennezat PV, Cappleman S, Naka Y, Mancini D. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J Am Coll Cardiol. 2010;56:1207–1213. doi: 10.1016/j.jacc.2010.05.016 [DOI] [PubMed] [Google Scholar]
- 6.Tabit CE, Chen P, Kim GH, Fedson SE, Sayer G, Coplan MJ, Jeevanandam V, Uriel N, Liao JK. Elevated Angiopoietin-2 level in patients with continuous-flow left ventricular assist devices leads to altered angiogenesis and is associated with higher nonsurgical bleeding. Circulation. 2016;134:141–152. doi: 10.1161/CIRCULATIONAHA.115.019692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabit CE, Coplan MJ, Chen P, Jeevanandam V, Uriel N, Liao JK. Tumor necrosis factor-α levels and non-surgical bleeding in continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2018;37:107–115. doi: 10.1016/j.healun.2017.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang W, Zhu J, Lyu F, Panigrahy D, Ferrara KW, Hammock B, Zhang G. ω-3 polyunsaturated fatty acids-derived lipid metabolites on angiogenesis, inflammation and cancer. Prostaglandins Other Lipid Mediat. 2014;113–115:13–20. doi: 10.1016/j.prostaglandins.2014.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang G, Kodani S, Hammock BD. Stabilized epoxygenated fatty acids regulate inflammation, pain, angiogenesis and cancer. Prog Lipid Res. 2014;53:108–123. doi: 10.1016/j.plipres.2013.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lau JY, Leung WK, Wu JC, Chan FK, Wong VW, Chiu PW, Lee VW, Lee KK, Cheung FK, Siu P, Ng EK, Sung JJ. Omeprazole before endoscopy in patients with gastrointestinal bleeding. N Engl J Med. 2007;356:1631–1640. doi: 10.1056/NEJMoa065703 [DOI] [PubMed] [Google Scholar]
- 11.Juricek C, Imamura T, Nguyen A, Chung B, Rodgers D, Sarswat N, Kim G, Raikhelkar J, Ota T, Song T, Burkhoff D, Sayer G, Jeevanandam V, Uriel N. Long-acting octreotide reduces the recurrence of gastrointestinal bleeding in patients with a continuous-flow left ventricular assist device. J Card Fail. 2018;24:249–254. doi: 10.1016/j.cardfail.2018.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehra MR, Naka Y, Uriel N, Goldstein DJ, Cleveland JC Jr, Colombo PC, Walsh MN, Milano CA, Patel CB, Jorde UP, Pagani FD, Aaronson KD, Dean DA, McCants K, Itoh A, Ewald GA, Horstmanshof D, Long JW, Salerno C; MOMENTUM 3 Investigators. A fully magnetically levitated circulatory pump for advanced heart failure. N Engl J Med. 2017;376:440–450. doi: 10.1056/NEJMoa1610426 [DOI] [PubMed] [Google Scholar]
- 13.Rogers JG, Pagani FD, Tatooles AJ, Bhat G, Slaughter MS, Birks EJ, Boyce SW, Najjar SS, Jeevanandam V, Anderson AS, Gregoric ID, Mallidi H, Lead-ley K, Aaronson KD, Frazier OH, Milano CA. Intrapericardial left ventricular assist device for advanced heart failure. N Engl J Med. 2017;376:451–460. doi: 10.1056/NEJMoa1602954 [DOI] [PubMed] [Google Scholar]
- 14.Patel SR, Madan S, Saeed O, Algodi M, Luke A, Gibber M, Goldstein DJ, Jorde UP. Association of nasal mucosal vascular alterations, gastrointestinal arteriovenous malformations, and bleeding in patients with continuous-flow left ventricular assist devices. JACC Heart Fail. 2016;4:962–970. doi: 10.1016/j.jchf.2016.08.005 [DOI] [PubMed] [Google Scholar]
- 15.Netuka I, Litzler PY, Berchtold-Herz M, Flecher E, Zimpfer D, Damme L, Sundareswaran KS, Farrar DJ, Schmitto JD; EU TRACE Investigators. Outcomes in HeartMate II Patients With No Antiplatelet Therapy: 2-year results from the European TRACE Study. Ann Thorac Surg. 2017;103:1262–1268. doi: 10.1016/j.athoracsur.2016.07.072 [DOI] [PubMed] [Google Scholar]
- 16.Feldman D, Pamboukian SV, Teuteberg JJ, Birks E, Lietz K, Moore SA, Morgan JA, Arabia F, Bauman ME, Buchholz HW, Deng M, Dickstein ML, El-Banayosy A, Elliot T, Goldstein DJ, Grady KL, Jones K, Hryniewicz K, John R, Kaan A, Kusne S, Loebe M, Massicotte MP, Moazami N, Mohacsi P, Mooney M, Nelson T, Pagani F, Perry W, Potapov EV, Eduardo Rame J, Russell SD, Sorensen EN, Sun B, Strueber M, Mangi AA, Petty MG, Rogers J; International Society for Heart and Lung Transplantation. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant. 2013;32:157–187. doi: 10.1016/j.healun.2012.09.013 [DOI] [PubMed] [Google Scholar]
- 17.Wever-Pinzon O, Selzman CH, Drakos SG, Saidi A, Stoddard GJ, Gilbert EM, Labedi M, Reid BB, Davis ES, Kfoury AG, Li DY, Stehlik J, Bader F. Pulsatility and the risk of nonsurgical bleeding in patients supported with the continuous-flow left ventricular assist device HeartMate II. Circ Heart Fail. 2013;6:517–526. doi: 10.1161/CIRCHEARTFAILURE.112.000206 [DOI] [PubMed] [Google Scholar]
- 18.Meyns B, Rega F, Ector J, Droogne W, Vanhaecke J, Van Hemelrijck J, Griffith B, Dowling R, Zucker M, Burkhoff D. Partial left ventricular support implanted through minimal access surgery as a bridge to cardiac transplant. J Thorac Cardiovasc Surg. 2009;137:243–245. doi: 10.1016/j.jtcvs.2008.02.010 [DOI] [PubMed] [Google Scholar]
- 19.Uriel N, Sayer G, Addetia K, Fedson S, Kim GH, Rodgers D, Kruse E, Collins K, Adatya S, Sarswat N, Jorde UP, Juricek C, Ota T, Jeevanandam V, Burkhoff D, Lang RM. Hemodynamic ramp tests in patients with left ventricular assist devices. JACC Heart Fail. 2016;4:208–217. doi: 10.1016/j.jchf.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 20.Shah KB, Gunda S, Emani S, Kanwar MK, Uriel N, Colombo PC, Uber PA, Sears ML, Chuang J, Farrar DJ, Brophy DF, Smallfield GB. Multicenter evaluation of octreotide as secondary prophylaxis in patients with left ventricular assist devices and gastrointestinal bleeding. Circ Heart Fail. 2017;10:e004500. doi: 10.1161/CIRCHEARTFAILURE.117.004500 [DOI] [PubMed] [Google Scholar]
- 21.Draper K, Kale P, Martin B, Cordero K, Ha R, Banerjee D. Thalidomide for treatment of gastrointestinal angiodysplasia in patients with left ventricular assist devices: case series and treatment protocol. J Heart Lung Transplant. 2015;34:132–134. doi: 10.1016/j.healun.2014.09.013 [DOI] [PubMed] [Google Scholar]
- 22.Schettle SD, Pruthi RK, Pereira NL. Continuous-flow left ventricular assist devices and gastrointestinal bleeding: potential role of danazol. J Heart Lung Transplant. 2014;33:549–550. doi: 10.1016/j.healun.2014.01.922 [DOI] [PubMed] [Google Scholar]
- 23.Nakatani T, Sase K, Oshiyama H, Akiyama M, Horie M, Nawata K, Nishinaka T, Tanoue Y, Toda K, Tozawa M, Yamazaki S, Yanase M, Ohtsu H, Ishida M, Hiramatsu A, Ishii K, Kitamura S; J-MACS investigators. Japanese registry for mechanically assisted circulatory support: first report. J Heart Lung Transplant. 2017;36:1087–1096. doi: 10.1016/j.healun.2017.08.002 [DOI] [PubMed] [Google Scholar]
- 24.Petrova S, Dimitrov P, Willett WC, Campos H. The global availability of n-3 fatty acids. Public Health Nutr. 2011;14:1157–1164. doi: 10.1017/S1368980010003678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehra MR, Lavie CJ, Ventura HO, Milani RV. Fish oils produce anti-inflammatory effects and improve body weight in severe heart failure. J Heart Lung Transplant. 2006;25:834–838. doi: 10.1016/j.healun.2006.03.005 [DOI] [PubMed] [Google Scholar]
- 26.Kim G, Sayer G, Ransom J, Keebler M, Katz J, Kilic A, Lindenfeld J, Egnaczyk G, Shah P, Brieke A, Walenga J, Crandall D, Farrar D, Sundareswaram K, Uriel N. Increased bleeding risk in LVAD patients with elevated Angiopoietin-2 and TNF-a: analysis of the PREVENT multicenter study. J Heart Lung Transplant. 2018;37:S71. [Google Scholar]
- 27.Costantini L, Molinari R, Farinon B, Merendino N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int J Mol Sci. 2017;18:E2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noriega BS, Sanchez-Gonzalez MA, Salyakina D, Coffman J. Understanding the impact of Omega-3 rich diet on the Gut Microbiota. Case Rep Med. 2016;2016:3089303. doi: 10.1155/2016/3089303 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


