Abstract
Background
Despite the abundance of measures to assess medication adherence by persons suffering schizophrenia, few studies have evaluated their concordance and validity against a reference standard in resource-poor community settings. We explored the concordance and validity of several measures to assess antipsychotic medication adherence in a resource-poor community.
Method
Based on a random sample of 278 villagers diagnosed with schizophrenia from Liuyang, Hunan Province, China, we used a concordance correlation coefficient (rc) and Kappa statistic to assess agreement among pill counts, refill records, clinician rating, Drug Attitude Inventory (DAI), and the Brief Adherence Rating Scale (BARS). The validity of various measures was evaluated by their concordance and sensitivity/specificity to home-based unannounced pill count (UPC) as the reference standard.
Results
The estimated proportion of adherent patients according to all measures (41%~88%) was substantially higher than identified by UPC (35%). Concordance between any two measures was poor (rc /Kappa mostly < 0.30). Validity of various measures also was poor against the UPC (rc<0.20; Kappa < 0.16), although refill records and the structured instruments (BARS) performed better than office-based pill counts and clinician impression. BARS, DAI and clinician rating were not sensitive to changes in adherence and would likely underestimate any program effect.
Conclusion
In resource-poor community settings, most measures assessed in this study should not be used alone as they overestimated adherence, underestimated program effect, and had poor validity. A combination of UPC and several other measures may provide more insight into clinical trials and programmatic management.
Keywords: medication adherence, pill-count, concordance of adherence measures, antipsychotic medication adherence, “686 Program”
1 Background
Most people with schizophrenia are prescribed long-term antipsychotic treatment.(Leucht et al., 2012a; Leucht et al., 2012b; Tiihonen et al., 2017) Nonadherence to antipsychotics is associated with higher risks for relapse, re-hospitalization, violence in society, and suicide, and increased costs and resource use for health systems.(Cutler et al., 2018; Dilla et al., 2013; Higashi et al., 2013; Leucht and Heres, 2005) Accurate measurement of adherence is important for effective management of persons diagnosed with schizophrenia,(Shafrin et al., 2017) yet reported rates of adherence vary widely. Two meta-analyses reported pooled rates of adherence of 70%(Nose et al., 2003) and 60%,(Lacro et al., 2002) respectively, and nonadherence is a common finding challenging the meaning of large drug trials.(Keefe et al., 2007) Rates of adherence between individual studies vary even more widely, ranging from 47–95%.(Sendt et al., 2015) The great disparity depends on the population under study and the definition of adherence and measurement methods.(Farmer, 1999; Velligan et al., 2006; Velligan et al., 2017)
While no single measure can be applied to all settings, numerous methods have been used to measure adherence.(Farmer, 1999; Velligan et al., 2010; Williams et al., 2013; Zullig et al., 2017) These include self-report and informant-report measures (interviews, patient/family diaries, survey instruments) and a variety of so-called “objective” numerical indices (drug level in biologic fluids, direct observation, electronic monitoring, pill counts, pharmacy records).(Farmer, 1999; Lam and Fresco, 2015; Sajatovic et al., 2010; Velligan et al., 2010; Williams et al., 2013; Zullig et al., 2017) Self and informant-report measures are most commonly used (77% of all antipsychotic adherence studies used subjective measures only). (Velligan et al., 2006) The available tools can also be categorized as direct measures of pill-taking (e.g., drug level, direct observation) versus indirect measures (e.g., office pill counts, self-report tools) that potentially are prone to manipulation. In recent decades, adherence assessment has become more sophisticated. Structured and standardized scales have been developed to improve patient interviews and self-reports, including the Brief Adherence Rating Scale (BARS), (Byerly et al., 2008) the Medication Adherence Rating Scale (MARS), (Fialko et al., 2008) the Brief Evaluation of Medication Influences and Beliefs (BEMIB), (Dolder et al., 2004) and the Drug Attitude Inventory (DAI). (Awad, 1993) As a presumably more objective measure electronic monitoring via a microchip-imbedded cap to capture each pill bottle opening appeared in the literature during the 1980s(Cramer et al., 1989; Spector et al., 1986) and has been used increasingly in addition to pill counts.(Cramer and Rosenheck, 1999; Nakonezny et al., 2008)
Despite advances in measurement methodologies, there has been scant research examining the concordance among measures of adherence to antipsychotics or appraisal of their validity versus a reference standard. In the broader biomedical literature, most studies (68%) reported high or moderate concordance among various measures or against a reference standard.(Garber et al., 2004; Shi et al., 2010b) Those related to schizophrenia were few: We identified only 7 relevant studies. (Brain et al., 2014; Byerly et al., 2005; Byerly et al., 2008; Byerly et al., 2007; Cassidy et al., 2010; Remington et al., 2007; Velligan et al., 2007; Yang et al., 2012) They reported poor to excellent concordance among adherence measures. All were conducted in high-income countries (US 4; Canada 2; Sweden 1; South Korea 1), with small, convenience samples (64 participants on average ranging from N = 25 to 131). Most were conducted in urban hospital settings and failed to include often-used adherence scales such as BARS and DAI in the comparison.
In this analysis, conducted in the context of a large, prospective, community-based intervention, we aimed to understand (1) concordance between various antipsychotic adherence measures, and (2) the validity of these measures in measuring adherence among people with schizophrenia. Home-based unannounced pill counts (UPC) was used as the reference standard. Our random sample included 278 Chinese consenting villagers who suffered schizophrenia.
2 Methods
2.1 Setting and Participants
Our analysis of adherence measures was part of a randomized controlled trial (Xu et al., 2016) designed to test the efficacy of texting reminders to improve medication adherence within the 686 Program, a national community-based program for people with psychosis. (Liu et al., 2011) By 2016, the 686 Program had covered 5.4 million people (75% of whom had schizophrenia) (Dandan, 2017) and 96% of China’s counties were running this program. (Wang et al., 2016) In Liuyang County, the program provides its participants at no charge with consultation, prescription and dispensing of antipsychotic medications every two months by psychiatrists traveling to township health centers, follow-up visits quarterly by community health workers (CHWs), and yearly physical exams. (Xu et al., 2016) The study was conducted in 9 rural townships of Liuyang County, Hunan Province, November 2015–July 2016.
The 686 Program registry included almost all known villagers with a diagnosis of schizophrenia. We selected 400 names from the registry with simple random sampling. To reflect real-world context, we applied minimal inclusion/exclusion on program participants, mainly excluding those who were currently institutionalized or not residing in Liuyang, or those not able to provide informed consent. Among the 400, 56 did not satisfy our inclusion/exclusion, 12 declined to participate, and 54 were not successfully contacted for the consent due to various reasons (e.g., wrong contact information in the registry; not available/present at the time of our recruitment visits). In the end, we recruited 278 consenting participants. The study received approvals from the institutional review boards of both University of Washington in Seattle, US (49464 G), and Central South University in Hunan, China (CTXY-150002-6).
2.2 Measures and Definition of Adherence
We selected 3 commonly used self-report or informant-based measures (“686” clinician impression, DAI, and BARS) as well as three presumably less subjective measures (home-based pill counts; office-based pill counts, and refill record).(Kreyenbuhl et al., 2016; Nieuwlaat et al., 2014) Unless otherwise specified, adherence was defined as a continuous variable (percentage of dosages taken (0–100%) in the past month). For patients on multiple antipsychotics, we took the average of adherence to all antipsychotic medications. Those continuous 0–100% variables also were dichotomized as adherent versus non-adherent at a cut-point of 75%, an informal convention often used in the schizophrenia literature. (Acosta et al., 2009; Byerly et al., 2008; Byerly et al., 2007; Farmer, 1999; Hansen et al., 2009; Remington et al., 2007; Yang et al., 2012)
2.2.1 Home-Based Unannounced Pill Counts (UPC): Double Counts
The pill count refers to counting dosage units (e.g., tablets, capsules) that remain in pill containers. We followed the best practices for pill counts.(Farmer, 1999; Grymonpre et al., 1998; Sajatovic et al., 2010) Evaluators made two home-based pill counts 30-days apart; they asked family caregivers and patients to report the “number of additional pills obtained” and “number of pills discarded” intentionally and unintendedly over the same period. The number of pills prescribed for that period was obtained from the 686 Program prescribing system. Adherence was calculated as the ratio of “(# of 1st count − # of 2nd count + # of additional pills obtained - # of pills discarded) ÷ (# of pills prescribed).” To verify that all pills were revealed by the family, evaluators inspected the family’s usual sites for medication storage with their consent. The counts were unannounced in the sense that although patients knew the general purpose of our study, they did not know on which home visit we would count pills, given that CHWs scheduled those visits for evaluators as part of their routine “686” home visits. The UPC was used as the reference standard for this study. (Cassidy et al., 2010)
2.2.2 Office-based Pill Counts: Double and Single Count(s)
On two consecutive visits between the “686” traveling psychiatrist and patients every two months, we asked patients and their family caregivers to bring in their medicine bottles to the prescription site. Pills counts were performed as described above, except that the pill count form posed the additional question, “How many pills have you forgotten to bring in?” The adherence formula was adjusted from the above slightly by the ratio of “(# of 1st count − # of 2nd count + # of additional pills obtained - # of pills discarded - # of pills not brought in) ÷ (# of pills prescribed).” For comparing methods of double count versus single count, we also regarded the second count as if it were the only count and used the following formula for single-count-based adherence: (# of pills prescribed - # of 2nd count) ÷ (# of pills prescribed).
2.2.3 Refill Record
We used the refill record from the “686” psychiatrists bi-monthly visits to the township health centers to calculate a cumulative medication possession ratio (CMPR) (Lam and Fresco, 2015) (0–100%) over half a year: (# of days medication obtained) ÷ (182 days).
2.2.4 Brief Adherence Rating Scale (BARS), Drug Attitude Inventory (DAI-10) and clinician impression
The BARS is a 4-item scale specifically developed to measure adherence to antipsychotics in schizophrenia. (Byerly et al., 2008) The evaluator marked the dosage taken over the past month on a 0–100% visual analog scale based informant provided information. The DAI-10 is a 10-item scale that assesses subjective experiences, attitude, and beliefs toward antipsychotic medications. (Dolder et al., 2004; Hogan et al., 1983) Patients respond to 10 true/false items. Scores range from −10 to +10 with positive scores interpreted as a positive subjective response (hence adherence) and a negative score interpreted as a negative subjective response (suggesting nonadherence). The 686 Program has a nationally standardized patient follow-up form, which includes a rating of patient adherence as “routinely taking medicine,” “intermittently taking medicine,” and “not taking medicine.” The 686 mental health workers rate patients based on their impressions.
2.3 Other Outcome Measures
Patients’ symptoms were assessed by “686” psychiatrists using the Clinical Global Impression in Schizophrenia scale (CGI-SCH), which consists of two categories: severity of illness and degree of change. (Haro et al., 2003) Each category covers five different aspects of symptoms (positive, negative, depressive, cognitive and global). Scores range from 1–7 with higher scores indicative of greater severity. Patient functions were evaluated by the 12-item proxy-administered WHO Disability Assessment Schedule 2.0 (WHODAS 2.0). (Üstün et al., 2010) WHODAS scores indicate percent of functions lost.
2.4 Data Collection
The afore-mentioned clinical trial collected data on patient adherence, symptoms and functions at baseline, mid-point (3 months after the launch of the intervention) and end-point (6 months after the launch). Baseline and mid-point data were collected during patients’ routine visits with psychiatrists at the township health center, while final data were collected at patients’ homes. All evaluators of adherence were Master of Public Health students from Central South University. They received intensive training on the related methods of assessing medication adherence.
2.5 Analysis
Concordance among continuous measures was evaluated with Lin’s concordance correlation coefficient (rc) (Lin, 1989), which indicates how closely pairs of observation fell on a 45° line (the perfect concordance line) through the origin in addition to their correlation. (Lawrence and Lin, 1992; Lin, 1989; Steichen and Cox, 2002) The Pearson correlation coefficient (rp) and Spearman correlation coefficient (rs) are inappropriate measures of agreement as high correlation does not mean that the two methods agree. (Bland and Altman, 1986; Shi et al., 2010a) We calculated rp and rs for comparisons with previous studies. The Kappa statistic (Cohen, 1960), which assesses agreement in assessment beyond what is expected by chance alone, was used to evaluate agreement of the dichotomized measurements.
In addition to assessing concordance of measures among one another, we investigated the criterion validity of the measures by assessing their concordance (rc or Kappa statistics) against UPC as the reference standard. For the continuous measures, a Bland-Altman plot was used to visualize the concordance. (Bland and Altman, 1986; Kwiecien et al., 2011) For the dichotomous measures, we also analyzed their sensitivity (strength to detect adherence) and specificity (strength to detect non-adherence) with UPC as the reference.
3 Results
3.1 Patient Characteristics
Among the 278 participants, 6 were lost to follow up (2 dropped out; 2 died, and 1 relocated to an unknown address). Table 1 shows that participants had a median age of 45 years; more women, unemployed, poorly educated, with low income, and living with their families. The median duration of illness was 16 years; the median CGI-severity was 3.0, with a median 20% loss of full functions. Eleven antipsychotic medications were prescribed to the participants (Table 1); 79% were taking multiple antipsychotics.
Table 1.
Patient and Family Characteristics
| Variable | Count(%)/Median(IQR) |
|---|---|
| Sex | |
| Female | 154 (55.60%) |
| Male | 122 (44.04) |
| Missing | 1 (0.36%) |
| Marriage | |
| Married | 177 (63.90%) |
| Unmarried | 99 (35.74%) |
| Missing | 1 (0.36%) |
| Employment | |
| Employed | 92 (33.21%) |
| Unemployed | 184 (66.43%) |
| Missing | 1 (0.36%) |
| Living | |
| Living alone | 13 (4.69%) |
| Living with family/friends | 262 (94.58%) |
| Missing | 2 (0.72%) |
| Age (years) | 45 (36–54) |
| Education (years) | 8 (6–9) |
| Patient income last month (RMB) | 80 (0–600) |
| Family annual income (RMB) | 20000 (10000–50000) |
| Duration of Schizophrenia (years) | 16 (10–25) |
| Clinical Global Impression-severitya | |
| Overall severity | 3 (2–4) |
| Positive symptoms | 2 (1–4) |
| Negative symptoms | 3 (2–4) |
| Depressive symptoms | 2 (1–3) |
| Cognitive symptoms | 3 (1–4) |
| WHO Disability Assessment Schedule 2.0b | 0.13 (0.04–0.29) |
| Antipsychotics Prescribed | |
| Clozapine | 84 (30.32%) |
| Risperidone | 74 (26.71%) |
| Quetiapine | 38 (13.72%) |
| Sulpiride | 36 (13.00%) |
| Perphenazine | 22 (7.94%) |
| Aripiprazole | 15 (5.42%) |
| Chlorpromazine | 10 (3.61%) |
| Penfluridol | 4 (1.44%) |
| Olanzapine | 2 (0.72%) |
| Other antipsychotics | 3 (1.08%) |
Higher scores indicate worse symptoms (possible range 1–7 for both total and domain scores).
Scores indicate percent of functions lost
3.2 Adherence
Table 2 shows adherence by different measures. The percent of adherent patients identified by UPC (34.9%) was substantially lower than that identified by other measures BARS 68.0%, DAI 41.9%, refill record 58.1%, office pill counts (55.6% for the 1-count method and 68.6% for the 2-count method).
Table 2.
Rates of Adherence as Assessed by Various Measures and Sensitivity and Specificity of the Measures Against Home-based Unannounced Pill-count
| Measures | ||
|---|---|---|
| Mean Adherenceb (SD) | No. Adherent Subjects (%) | |
| Home-based Unannounced Pill-counta | 0.55 (0.35) | 76/228 (34.86%) |
| Office-based Pill-count (1 count)a | 0.41 (1.14) | 94/169 (55.62%) |
| Office-based Pill-count (2 counts)a | 0.96(1.39) | 116/169(68.64%) |
| Refill recorda | 0.79 (0.31) | 158/272 (58.09%) |
| Brief Adherence Rating Scale (BARS)a | 0.70 (0.22) | 147/216 (68.06%) |
| Drug Attitude Inventory (DAI)b | 3.55 (4.20) | 80/191 (41.88%) |
| Clinician Impressionc | 1.15 (0.42) | 227/258 (87.98%) |
| Sensitivityd | Specificityd | |
| Objective Measures (Cut-point ≥0.75) | ||
| Office-based Pill Count (1 count) | 55.77% | 47.25% |
| Office-based Pill Count (2 counts) | 73.08% | 29.67% |
| Refill Record | 75.00% | 44.29% |
| Subjective Measures | ||
| Brief Adherence Rating Scale (BARS) (Cut-point ≥0.75) | 74.65% | 34.88% |
| Drug Attitude Inventory (DAI) (Cut-point ≥6) | 52.45% | 59.46% |
| Clinician Impression (Cut-point at ≤2) | 93.15% | 9.85% |
For pill-counts, refill and BARS, adherences are percentage of dosages taken over the past month or dichotomized as adherent and non-adherent at the cut-point of 0.75
DAI adherence is from −10 to +10 (higher score=more positive attitude toward medication). Also dichotomized at ≥6
Clinician impression is from 1 to 3 (1=routinely taking medicine, 2=intermittently taking medicine, 3=not taking medicine); Also dichotomized at ≤2.
Reference standard: home-based Unannounced Pill-Count (Cut-point at ≥0.75).
3.3 Concordance and Correlation Among Measures
Overall, there was poor concordance and correlation between any two measures (rc/rs/rp mostly = 0.20–0.30; Kappa mostly < 0.30) (Figure 1). Office-based pill counts were least concordant with other measures.
Figure 1.
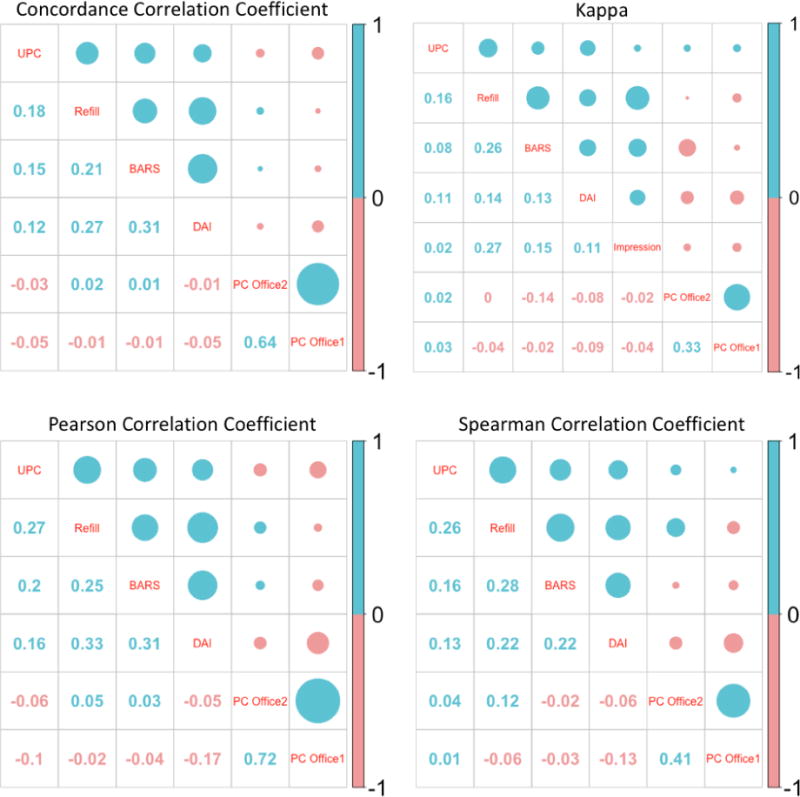
Concordance and Correlation of Measuring Adherence between Different Measures
*UPC: Home-based unannounced pill-count; Refill: refill record; BARS: Brief Adherence Rating Scale; DAI: Drug Attitude Inventory; PC Office 1: Office-based pill-count (1 count); PC Office 2: Office-based pill count (2-count); Impression: Clinician Impression
**For Kappa, following cut-points were used: ≥0.75 for pill-counts, refill and BARS; ≥6 for DAI.
***Size of the circle indicates the strength of the association; blue color indicates positive association while red indicates inverse association.
3.4 Validity (Concordance with UPC)
Criterion validity was poor, with all measures poorly concordant with the UPC as the reference standard (rc <0.20; Kappa mostly < 0.2) (Figure 1). Among all measures, refill record had relatively better concordance with UPCs (rc=0.18; Kappa=0.16); followed by BARS (rc=0.15; Kappa=0.08). Office-based pill counts and clinician rating performed most poorly. The analysis of sensitivity and specificity revealed similar patterns of the overall strength of the measures (Table 2).
The Bland-Altman plot confirmed the low validity of all studied measures and revealed several patterns (Figure 2): (1) the distributions of the points were diamond-shaped, suggesting various measures were better at correctly detecting the most adherent and most non-adherent patients; (2) the dotted lines (mean-of-all-differences) are all below zero, suggesting a systematic bias of overestimating adherence of those measures; and (3) the distance between the two dashed lines (limits of agreement) was wide, indicating poor agreement between the measures and the UPC. Note that although our data were not normally distributed as assumed by the Bland-Altman plot, non-normality generally does not have a great impact on the limits-of-agreement.(Bland and Altman, 1999)
Figure 2. Bland-Altman Plot.
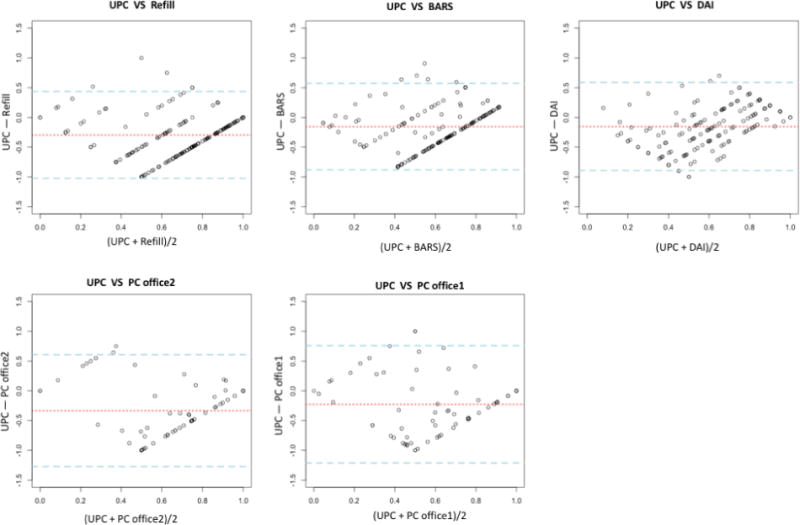
*UPC: Home-based unannounced pill-count; Refill: refill record; BARS: Brief Adherence Rating Scale; DAI: Drug Attitude Inventory; PC Office 1: Office-based pill-count (1 count); PC Office 2: Office-based pill count (2-count)
**X-axis: mean of the adherence rates assessed by the two measures for each subject; Y-axis: differences of the adherence rates between the two measurements for each subject; Dotted red line: “mean-of-all-differences” line; Dashed blue lines: “limits of agreement” lines indicating 1.96 standard deviations of the measured differences between the two measurements above or below the mean-of-all-difference line
***DAI scores were rescaled to range from 0–1 for comparative purposes.
4 Discussion
Although adherence to antipsychotics was studied in the low and middle income settings, (Farooq et al., 2011; Kane et al., 2013) there is lack of studies validating those measures in those settings. In this study of measures of adherence to antipsychotic medications in a random community sample in China, concordance between any two measures was poor (rc/rs/rp < 0.3 in general). More important, all measures had low validity as assessed by their concordance and sensitivity/specificity against UPCs as the reference standard.
4.1 Self-report Measures
Despite the widespread use of self-report and informant measures of adherence, (Velligan et al., 2017) we found poor validity compared to our unannounced home pill count. Several previous studies reported stronger correlation or concordance (rs/rc=0.54 ~ 0.93) between subjective measures (patient/family/clinician reports) and pill counts /electronic monitoring in patients with schizophrenia in Canada, (Cassidy et al., 2010) Sweden, (Brain et al., 2014) and the US. (Byerly et al., 2008) However, all three studies involved self-selected patients, small samples (n = 60~80), intensive case management, and a high-income urban setting, which could bias toward participants who may self-report adherence more accurately. Our results were comparable, however, to those of a Korean study in schizophrenia (rc= 0.14 between self-report measures and the reference standard). (Yang et al., 2012) Another Canadian study reported a negative correlation between self-report and the electronic cap. (Remington et al., 2007) In the general medical literature, the correlation or concordance between subjective measures and a reference standard tended to be much higher (pooled rs=0.45 according to a meta-analysis). (Garber et al., 2004; Shi et al., 2010a) Patient-reported measures involving persons with schizophrenia are likely less reliable than in other groups, considering the poor insight and impaired cognition linked to schizophrenia.
Few earlier studies compare the validity of structured instruments with simpler ratings such as the clinician impression used in this study. In this study, BARS and DAI minimally improved the validity of measuring adherence over clinician impression: Kappa improved from 0.02 in clinician impression to 0.08 in BARS and 0.11 for DAI. However, clinician impression had much lower specificity (10%) than DAI (60%) and BARS (35%), suggesting that using simple structured instrument is much more effective than clinician impression in detecting non-adherence. Although clinician impression had better sensitivity (i.e., less false negative) than DAI and BARS, false negative (people adherent rated as non-adherent) at worst strengthens already strong adherence. Thus, having patients or family members complete DAI while waiting for the clinical consultation may greatly improve clinician’s understanding of patient adherence to their medications.
4.2 “Objective” Measures
Pill counts are widely used in adherence studies as a simple and low-cost method of assessment. (Nieuwlaat et al., 2014) We provided the details of UPC earlier, which we believe minimized the Hawthorne effect and issues with residual pills and pills not brought in. However, office-based pill counts were affected by these problems: despite two text reminders, only about 75% of participants appeared for the office-based count, among whom approximately 73% failed to bring in their pills for count. It is also likely that a large portion of patients did not bring in all pills. Because of these irregularities, office-based pill counts proved to be the least valid approach in our study. The UPC was logistically more complicated than office-based counts, but if routine home visits were already a program component, pill counts only took 6–10 minutes once at the patient’s home.
The UPC was used as the reference standard in this study despite its limitations to be discussed later. Recent literature proposed electronic caps as the gold standard. We deem electronic caps, while prohibitively expensive, not superior to UPC for this study. In our pilot, we found it common for our patients to transfer pills between bottles, to use original paper boxes rather than bottles, and to take partial or multiple dose (s) for each cap opening. Other studies have reported lost caps (USD 85/cap), “curiosity” cap opening, leaving caps open for a long time, (Sajatovic et al., 2010) and increased patient anxiety. (Elixhauser et al., 1990) Electronic caps have an advantage of tracking adherence patterns, (Farmer, 1999; Velligan et al., 2006) but our focus is on overall consumption of dosage over a monitoring period. Finally, though drug level in blood or urine is the most direct reflection of patient adherence, assessment by these methods can be highly affected by patient behavior before the blood draw, and there is considerable individual variability in half-lives or detectability across medications and patients. (Kinon et al., 2003)
4.3 Sensitivity to Change
Medication adherence is often used in clinical trials as an outcome. In the parent study, we detected an improvement of 12 percentage points in the intervention group as measured by the UPC, while the self-report measures showed < 4 percentage points change (Error! Reference source not found.). This finding suggests that by all self-report measures self-report ratings, when used in our rural setting in China, were relatively insensitive to change, and potentially obscured an intervention effect. Of note, self-report measures such as DAI provided additional information regarding patient attitudes toward their medicine, which complemented what we found in our UPC data.
4.4 Limitations
Several limitations may exist in this study. First, we recognize limitations using UPC as the reference standard:(Cassidy et al., 2010) (1) there is always the possibility of pills not correctly counted or additional pills not reported; (2) missing either of the 2 counts may lead to missing data; and (3) pills counted may not be equated with pills taken. Earlier (see 4.2 “Objective” Measures) we discussed the possibility of using electronic caps as the reference standard, although it is not necessarily superior to pill-counts if adherence assessment rather than continuous monitoring is the primary concern. Second, the level of adherence, concordance, and sensitivity/specificity among dichotomized measures are affected by cut-points. While the conventional choice of 70–80% as cut-points in schizophrenia-related research, (Acosta et al., 2009; Byerly et al., 2008; Byerly et al., 2007; Farmer, 1999; Hansen et al., 2009; Remington et al., 2007; Yang et al., 2012) little evidence supports the clinical efficacy those cuts imply. (Sendt et al., 2015; Velligan et al., 2009) We chose a 75% cut-point as a convention benchmark, used continuous adherence whenever possible, and recognize that it may be possible to examine various cut-points to assess whether there is a level of adherence that is symptomatically and functionally meaningful. This will be a subject for future examination but is beyond the scope of this paper. We suggest that future studies include an estimate of the percentage of dosages taken to improve comparability across studies on a common scale. Third, the refill adherence was calculated over a 6-month period rather than 1-month as other measures. In the 686 Program, patients get their prescription every two months. Adherence as measured by refill records at shorter intervals may improve its accuracy. Forth, as approximately half of our patients get medication support from their family members, we should have included adherence instruments that are specifically designed for the caregivers such as The Adherencia Terapéutica en la Esquizofrenia (ADHES) carers’ survey (Svettini et al., 2015) and Medication Adherence Rating Scale (MARS-5) (Thompson et al., 2000). Fifth, we did not adapt the structured instruments used in this study in any significant fashion to better suit the local cultural context as we wanted to maintain comparability with previous studies. We did identify some items of the instruments that was difficult to be understood in the Chinese culture such as the concept of “feeling like a zombie” in DAI. Adapted versions may help improve the validity of those instruments. Lastly, our random sample was drawn from the 686 Program participants in 9 townships in Liuyang County. Extrapolation of the results to the entire 686 Program population in Hunan or other parts of China requires caution. While we cannot generalize these results to other studies of populations prescribed antipsychotic medications, our results raise important issues regarding the measurement of adherence among persons suffering schizophrenia, especially among less educated individuals.
4.5 Policy and clinical implications
The 686 Program shifted the care from medical centers to communities and provided free basic medications in many program sites. This low-cost community-based program provided valuable experiences for other developing countries in integrating psychoses care in universal health coverage. (Patel, 2015) One lesson from our study is that even though the free medication program removed some access barriers, it did not guarantee medication adherence. It is critical to identify feasible and accurate assessment tools for the benefit of patient and program management. However, although some studies assessed adherence in schizophrenia in developing countries, (Farooq and Naeem, 2014; Farooq et al., 2011; Kane et al., 2013) there is lack of efforts to validate the various adherence measures in those settings. In the 686 Program, it is the standard procedure to report patient adherence based on the clinician impression; and the reported result was used for patient management as well as the program evaluation. In view of our earlier discussion on sensitivity and specificity of the different measures, replacing clinician impression with simple structured instrument such as DAI may improve identification of non-adherence at little marginal cost. In other settings, many projects used pill counts in clinicians’ offices with a single count due to its convenience. Our study suggests that those office-based pill-counts produce highly inaccurate assessment. The method leads to several methodological limitations, including:1) patients’ intentional or unintentional failure to bring in all or any pill bottles; 2) lack of a baseline count to incorporate residual pills from the last prescription cycle; 3) no consideration of extra refills and pills discarded over the monitoring period; and 4) the Hawthorne effect that participants may change their normal behavior when knowingly under observation. (Farmer, 1999; Lam and Fresco, 2015; Williams et al., 2013) Our study reinforced the consensus guideline that assessing adherence should include both objective and subjective measures.(Bellack et al., 2009; Velligan et al., 2006; Velligan et al., 2010)
4.6 Conclusion
We conclude that, in a resource-poor community setting in rural China, the concordance among various measures for documenting adherence to antipsychotic medications was poor, and the validity of various measures was low when assessed by their concordance and sensitivity/specificity against UPC as the reference standard. Office-based pill counts were misleading, and for the community-based patients we studied, they could adversely affect patient management by over-estimating the amount of prescribed medications patients are consuming. Given our results, self-report measures should not be used alone. They overestimated adherence, underestimated program effect, and showed poor validity. Simple structured measures such as DAI may be used in routine practice instead of clinician impression as they better detect non-adherence. A combination of UPC and self-report measures may prove more useful for future clinical trials and for assessing the impact of mental health programs.
Supplementary Material
Acknowledgments
We would like to thank many people who have contributed to the field implementation of this study including Yunfang Wang and Hui Wang from Central South University, Di Liu from Peking Union Medical College, Yeqing Yuan from New York University, and Meng Dai from Liuyang Mental Health Hospital.
Role of funding source
The project received grant support from the China Medical Board (grant number 12-114, Wenjie Gong, PI) and NIH research training grant (#R25 TW009345, Dong Xu, Fogarty fellowship). However, our funders had no role in the design, execution, or analysis of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions
DX and WG designed the study with advice from SX, SG, EC, JS, and JH. ML and JN managed the field work and data. WH and BD prepared the data and implemented the data analysis under the guidance of DX and HH. DX drafted the first manuscript, and all other members contributed amendments and critical reviews.
Conflict of interest
All authors declare no conflict of interest in this study.
References
- Acosta FJ, Bosch E, Sarmiento G, Juanes N, Caballero-Hidalgo A, Mayans T. Evaluation of noncompliance in schizophrenia patients using electronic monitoring (MEMS®) and its relationship to sociodemographic, clinical and psychopathological variables. Schizophrenia Research. 2009;107(2):213–217. doi: 10.1016/j.schres.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Awad AG. Subjective response to neuroleptics in schizophrenia. Schizophrenia bulletin. 1993;19(3):609. doi: 10.1093/schbul/19.3.609. [DOI] [PubMed] [Google Scholar]
- Bellack AS, Bowden CL, Bowie CR, Byerly MJ, Carpenter WT, Copeland LA, Dassori AM, Davis JM, Depp CA, Diaz E. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. Journal of Clinical Psychiatry. 2009;70(SUPPL. 4):1–48. [PubMed] [Google Scholar]
- Bland JM, Altman D. Statistical methods for assessing agreement between two methods of clinical measurement. The lancet. 1986;327(8476):307–310. [PubMed] [Google Scholar]
- Bland JM, Altman DG. Measuring agreement in method comparison studies. Statistical methods in medical research. 1999;8(2):135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- Brain C, Sameby B, Allerby K, Lindström E, Eberhard J, Burns T, Waern M. Twelve months of electronic monitoring (MEMS®) in the Swedish COAST-study: a comparison of methods for the measurement of adherence in schizophrenia. European Neuropsychopharmacology. 2014;24(2):215–222. doi: 10.1016/j.euroneuro.2013.11.013. [DOI] [PubMed] [Google Scholar]
- Byerly M, Fisher R, Whatley K, Holland R, Varghese F, Carmody T, Magouirk B, Rush AJ. A comparison of electronic monitoring vs. clinician rating of antipsychotic adherence in outpatients with schizophrenia. Psychiatry research. 2005;133(2):129–133. doi: 10.1016/j.psychres.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Byerly MJ, Nakonezny PA, Rush AJ. The Brief Adherence Rating Scale (BARS) validated against electronic monitoring in assessing the antipsychotic medication adherence of outpatients with schizophrenia and schizoaffective disorder. Schizophrenia research. 2008;100(1):60–69. doi: 10.1016/j.schres.2007.12.470. [DOI] [PubMed] [Google Scholar]
- Byerly MJ, Thompson A, Carmody T, Bugno R, Erwin T, Kashner M, Rush AJ. Validity of electronically monitored medication adherence and conventional adherence measures in schizophrenia. Psychiatric Services. 2007;58(6):844–847. doi: 10.1176/ps.2007.58.6.844. [DOI] [PubMed] [Google Scholar]
- Cassidy CM, Rabinovitch M, Schmitz N, Joober R, Malla A. A comparison study of multiple measures of adherence to antipsychotic medication in first-episode psychosis. Journal of clinical psychopharmacology. 2010;30(1):64–67. doi: 10.1097/JCP.0b013e3181ca03df. [DOI] [PubMed] [Google Scholar]
- Cohen J. A coefficient of agreement for nominal scales. Educational and psychological measurement. 1960;20(1):37–46. [Google Scholar]
- Cramer JA, Mattson RH, Prevey ML, Scheyer RD, Ouellette VL. How often is medication taken as prescribed?: A novel assessment technique. Jama. 1989;261(22):3273–3277. [PubMed] [Google Scholar]
- Cramer JA, Rosenheck R. Enhancing medication compliance for people with serious mental illness. The Journal of nervous and mental disease. 1999;187(1):53–55. doi: 10.1097/00005053-199901000-00009. [DOI] [PubMed] [Google Scholar]
- Cutler RL, Fernandez-Llimos F, Frommer M, Benrimoj C, Garcia-Cardenas V. Economic impact of medication non-adherence by disease groups: a systematic review. BMJ open. 2018;8(1):e016982. doi: 10.1136/bmjopen-2017-016982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandan L. Ministry of Health and Family Planning: Number of People with Psychosis under Management Reached 5.4 Million in 2016 in China. The Beijing News 2017 [Google Scholar]
- Dilla T, Ciudad A, Alvarez M. Systematic review of the economic aspects of nonadherence to antipsychotic medication in patients with schizophrenia. Patient preference and adherence. 2013;7:275. doi: 10.2147/PPA.S41609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolder CR, Lacro JP, Warren KA, Golshan S, Perkins DO, Jeste DV. Brief evaluation of medication influences and beliefs: development and testing of a brief scale for medication adherence. Journal of clinical psychopharmacology. 2004;24(4):404–409. doi: 10.1097/01.jcp.0000130554.63254.3a. [DOI] [PubMed] [Google Scholar]
- Elixhauser A, Eisen SA, Romeis JC, Homan SM. The effects of monitoring and feedback on compliance. Medical care. 1990:882–893. doi: 10.1097/00005650-199010000-00003. [DOI] [PubMed] [Google Scholar]
- Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clinical therapeutics. 1999;21(6):1074–1090. doi: 10.1016/S0149-2918(99)80026-5. [DOI] [PubMed] [Google Scholar]
- Farooq S, Naeem F. Tackling nonadherence in psychiatric disorders: current opinion. Neuropsychiatric disease and treatment. 2014;10:1069. doi: 10.2147/NDT.S40777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq S, Nazar Z, Irfan M, Akhter J, Gul E, Irfan U, Naeem F. Schizophrenia medication adherence in a resource-poor setting: randomised controlled trial of supervised treatment in outpatients for schizophrenia (STOPS) The British Journal of Psychiatry. 2011;199(6):467–472. doi: 10.1192/bjp.bp.110.085340. [DOI] [PubMed] [Google Scholar]
- Fialko L, Garety PA, Kuipers E, Dunn G, Bebbington PE, Fowler D, Freeman D. A large-scale validation study of the Medication Adherence Rating Scale (MARS) Schizophrenia research. 2008;100(1):53–59. doi: 10.1016/j.schres.2007.10.029. [DOI] [PubMed] [Google Scholar]
- Garber MC, Nau DP, Erickson SR, Aikens JE, Lawrence JB. The concordance of self-report with other measures of medication adherence: a summary of the literature. Medical care. 2004;42(7):649–652. doi: 10.1097/01.mlr.0000129496.05898.02. [DOI] [PubMed] [Google Scholar]
- Grymonpre RE, Didur CD, Montgomery PR, Sitar DS. Pill count, self-report, and pharmacy claims data to measure medication adherence in the elderly. Annals of Pharmacotherapy. 1998;32(7–8):749–754. doi: 10.1345/aph.17423. [DOI] [PubMed] [Google Scholar]
- Hansen RA, Kim MM, Song L, Tu W, Wu J, Murray MD. Adherence: Comparison of Methods to Assess Medication Adherence and Classify Nonadherence. Annals of Pharmacotherapy. 2009;43(3):413–422. doi: 10.1345/aph.1L496. [DOI] [PubMed] [Google Scholar]
- Haro J, Kamath S, Ochoa S, Novick D, Rele K, Fargas A, Rodriguez M, Rele R, Orta J, Kharbeng A. The Clinical Global Impression–Schizophrenia scale: a simple instrument to measure the diversity of symptoms present in schizophrenia. Acta Psychiatrica Scandinavica. 2003;107(s416):16–23. doi: 10.1034/j.1600-0447.107.s416.5.x. [DOI] [PubMed] [Google Scholar]
- Higashi K, Medic G, Littlewood KJ, Diez T, Granström O, De Hert M. Medication adherence in schizophrenia: factors influencing adherence and consequences of nonadherence, a systematic literature review. Therapeutic advances in psychopharmacology. 2013;3(4):200–218. doi: 10.1177/2045125312474019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan TP, Awad A, Eastwood R. A self-report scale predictive of drug compliance in schizophrenics: reliability and discriminative validity. Psychological medicine. 1983;13(1):177–183. doi: 10.1017/s0033291700050182. [DOI] [PubMed] [Google Scholar]
- Kane JM, Perlis RH, DiCarlo LA, Au-Yeung K, Duong J, Petrides G. First experience with a wireless system incorporating physiologic assessments and direct confirmation of digital tablet ingestions in ambulatory patients with schizophrenia or bipolar disorder. The Journal of clinical psychiatry. 2013;74(6):e533–540. doi: 10.4088/JCP.12m08222. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Bilder RM, Davis SM, Harvey PD, Palmer BW, Gold JM, Meltzer HY, Green MF, Capuano G, Stroup TS. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Archives of general psychiatry. 2007;64(6):633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- Kinon BJ, Hill AL, Liu H, Kollack-Walker S. Olanzapine orally disintegrating tablets in the treatment of acutely ill non-compliant patients with schizophrenia. The The International Journal of Neuropsychopharmacology. 2003;6(2):97–102. doi: 10.1017/S1461145703003389. [DOI] [PubMed] [Google Scholar]
- Kreyenbuhl J, Record EJ, Palmer-Bacon J. A review of behavioral tailoring strategies for improving medication adherence in serious mental illness. Dialogues in clinical neuroscience. 2016;18(2):191. doi: 10.31887/DCNS.2016.18.2/jkreyenbuhl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krousel-Wood M, Islam T, Muntner P, Holt E, Joyce C, Morisky DE, Webber LS, Frohlich ED. Association of depression with antihypertensive medication adherence in older adults: cross-sectional and longitudinal findings from CoSMO. Annals of Behavioral Medicine. 2010;40(3):248–257. doi: 10.1007/s12160-010-9217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krousel-Wood M, Islam T, Webber LS, Re R, Morisky DE, Muntner P. New medication adherence scale versus pharmacy fill rates in hypertensive seniors. The American journal of managed care. 2009;15(1):59. [PMC free article] [PubMed] [Google Scholar]
- Kwiecien R, Kopp-Schneider A, Blettner M. Concordance analysis: part 16 of a series on evaluation of scientific publications. Deutsches Ärzteblatt International. 2011;108(30):515. doi: 10.3238/arztebl.2011.0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacro JP, Dunn LB, Dolder CR, Leckband SG, Jeste DV. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. The Journal of clinical psychiatry. 2002;63(10):892–909. doi: 10.4088/jcp.v63n1007. [DOI] [PubMed] [Google Scholar]
- Lam WY, Fresco P. Medication adherence measures: an overview. BioMed research international. 2015;2015 doi: 10.1155/2015/217047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence I, Lin K. Assay validation using the concordance correlation coefficient. Biometrics. 1992:599–604. [Google Scholar]
- Leucht S, Heres S. Epidemiology, clinical consequences, and psychosocial treatment of nonadherence in schizophrenia. The Journal of clinical psychiatry. 2005;67:3–8. [PubMed] [Google Scholar]
- Leucht S, Tardy M, Komossa K, Heres S, Kissling W, Davis JM. Maintenance treatment with antipsychotic drugs for schizophrenia. The Cochrane Library. 2012a doi: 10.1002/14651858.CD008016.pub2. [DOI] [PubMed] [Google Scholar]
- Leucht S, Tardy M, Komossa K, Heres S, Kissling W, Salanti G, Davis JM. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. The Lancet. 2012b;379(9831):2063–2071. doi: 10.1016/S0140-6736(12)60239-6. [DOI] [PubMed] [Google Scholar]
- Lin L. A Concordance Correlation Coefficient to Evaluate Reproducibility. Biometric. 1989;45:255–268. [PubMed] [Google Scholar]
- Liu J, Ma H, HE YL, Xie B, XU YF, TANG HY, Li M, Hao W, WANG XD, ZHANG MY. Mental health system in China: history, recent service reform and future challenges. World Psychiatry. 2011;10(3):210–216. doi: 10.1002/j.2051-5545.2011.tb00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisky DE, DiMatteo MR. Improving the measurement of self-reported medication nonadherence: response to authors. Journal of clinical epidemiology. 2011;64(3):255. doi: 10.1016/j.jclinepi.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Medical care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- Nakonezny PA, Byerly MJ, Rush AJ. Electronic monitoring of antipsychotic medication adherence in outpatients with schizophrenia or schizoaffective disorder: an empirical evaluation of its reliability and predictive validity. Psychiatry research. 2008;157(1):259–263. doi: 10.1016/j.psychres.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, Agoritsas T, Mistry N, Iorio A, Jack S. Interventions for enhancing medication adherence. The Cochrane Library. 2014 doi: 10.1002/14651858.CD000011.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose M, Barbui C, Tansella M. How often do patients with psychosis fail to adhere to treatment programmes? A systematic review. Psychological medicine. 2003;33(7):1149–1160. doi: 10.1017/s0033291703008328. [DOI] [PubMed] [Google Scholar]
- Novick D, Haro JM, Suarez D, Perez V, Dittmann RW, Haddad PM. Predictors and clinical consequences of non-adherence with antipsychotic medication in the outpatient treatment of schizophrenia. Psychiatry research. 2010;176(2):109–113. doi: 10.1016/j.psychres.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Patel V. Universal health coverage for schizophrenia: a global mental health priority. Schizophrenia bulletin. 2015;42(4):885–890. doi: 10.1093/schbul/sbv107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington G, Kwon J, Collins A, Laporte D, Mann S, Christensen B. The use of electronic monitoring (MEMS®) to evaluate antipsychotic compliance in outpatients with schizophrenia. Schizophrenia research. 2007;90(1):229–237. doi: 10.1016/j.schres.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Rudd P. In search of the gold standard for compliance measurement. Archives of Internal Medicine. 1979;139(6):627–628. [PubMed] [Google Scholar]
- Sajatovic M, Velligan DI, Weiden PJ, Valenstein MA, Ogedegbe G. Measurement of psychiatric treatment adherence. Journal of psychosomatic research. 2010;69(6):591–599. doi: 10.1016/j.jpsychores.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendt KV, Tracy DK, Bhattacharyya S. A systematic review of factors influencing adherence to antipsychotic medication in schizophrenia-spectrum disorders. Psychiatry research. 2015;225(1):14–30. doi: 10.1016/j.psychres.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Shafrin J, May SG, Shrestha A, Ruetsch C, Gerlanc N, Forma F, Hatch A, Lakdawalla DN, Lindenmayer JP. Access to credible information on schizophrenia patients’ medication adherence by prescribers can change their treatment strategies: evidence from an online survey of providers. Patient preference and adherence. 2017;11:1071. doi: 10.2147/PPA.S135957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Liu J, Fonseca V, Walker P, Kalsekar A, Pawaskar M. Correlation between adherence rates measured by MEMS and self-reported questionnaires: a meta-analysis. Health and Quality of Life Outcomes. 2010a;8(1):99. doi: 10.1186/1477-7525-8-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Liu J, Koleva Y, Fonseca V, Kalsekar A, Pawaskar M. Concordance of adherence measurement using self-reported adherence questionnaires and medication monitoring devices. Pharmacoeconomics. 2010b;28(12):1097. doi: 10.2165/11537400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Spector SL, Kinsman R, Mawhinney H, Siegel SC, Rachelefsky GS, Katz RM, Rohr AS. Compliance of patients with asthma with an experimental aerosolized medication: implications for controlled clinical trials. Journal of Allergy and Clinical Immunology. 1986;77(1):65–70. doi: 10.1016/0091-6749(86)90325-8. [DOI] [PubMed] [Google Scholar]
- Spilker B. Methods of assessing and improving patient compliance in clinical trials. IRB: Ethics & Human Research. 1992;14(3):1–6. [Google Scholar]
- Steichen TJ, Cox NJ. A note on the concordance correlation coefficient. Stata J. 2002;2(2):183–189. [Google Scholar]
- Svettini A, Johnson B, Magro C, Saunders J, Jones K, Silk S, Hargarter L, Schreiner A. Schizophrenia through the carers’ eyes: results of a European cross‐sectional survey. Journal of psychiatric and mental health nursing. 2015;22(7):472–483. doi: 10.1111/jpm.12209. [DOI] [PubMed] [Google Scholar]
- Thompson K, Kulkarni J, Sergejew A. Reliability and validity of a new Medication Adherence Rating Scale (MARS) for the psychoses. Schizophrenia research. 2000;42(3):241–247. doi: 10.1016/s0920-9964(99)00130-9. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Mittendorfer-Rutz E, Majak M, Mehtälä J, Hoti F, Jedenius E, Enkusson D, Leval A, Sermon J, Tanskanen A. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29 823 patients with schizophrenia. JAMA psychiatry. 2017;74(7):686–693. doi: 10.1001/jamapsychiatry.2017.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Üstün TB, Chatterji S, Kostanjsek N, Rehm J, Kennedy C, Epping-Jordan J, Saxena S, Korff MV, Pull C. Developing the World Health Organization disability assessment schedule 2.0. Bulletin of the World Health Organization. 2010;88(11):815–823. doi: 10.2471/BLT.09.067231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velligan D, Weiden P, Sajatovic M, Scott J, Carpenter D, Ross R, Docherty J. Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(suppl 4):1–46. [PubMed] [Google Scholar]
- Velligan DI, Lam YWF, Glahn DC, Barrett JA, Maples NJ, Ereshefsky L, Miller AL. Defining and assessing adherence to oral antipsychotics: a review of the literature. Schizophrenia Bulletin. 2006;32(4):724–742. doi: 10.1093/schbul/sbj075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velligan DI, Sajatovic M, Hatch A, Kramata P, Docherty JP. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient preference and adherence. 2017;11:449. doi: 10.2147/PPA.S124658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velligan DI, Wang M, Diamond P, Glahn DC, Castillo D, Bendle S, Lam YF, Ereshefsky L, Miller AL. Relationships among subjective and objective measures of adherence to oral antipsychotic medications. Psychiatric Services. 2007;58(9):1187–1192. doi: 10.1176/ps.2007.58.9.1187. [DOI] [PubMed] [Google Scholar]
- Velligan DI, Weiden PJ, Sajatovic M, Scott J, Carpenter D, Ross R, Docherty JP. Assessment of adherence problems in patients with serious and persistent mental illness: recommendations from the Expert Consensus Guidelines. Journal of Psychiatric Practice®. 2010;16(1):34–45. doi: 10.1097/01.pra.0000367776.96012.ca. [DOI] [PubMed] [Google Scholar]
- Wang X, Ma N, Wang L, Yan J, Jin T, Wu X. Management and services for psychosis in People’s Republic of China in 2014. Zhong Hua Jing Shen Ke Za Zhi. 2016;49(3):182–188. [Google Scholar]
- Williams AB, Amico KR, Bova C, Womack JA. A proposal for quality standards for measuring medication adherence in research. AIDS and Behavior. 2013:1–14. doi: 10.1007/s10461-012-0172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu DR, Gong W, Caine ED, Xiao S, Hughes JP, Ng M, Simoni J, He H, Smith KL, Brown HS. Lay health supporters aided by a mobile phone messaging system to improve care of villagers with schizophrenia in Liuyang, China: protocol for a randomised control trial. BMJ open. 2016;6(1):e010120. doi: 10.1136/bmjopen-2015-010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Ko YH, Paik JW, Lee MS, Han C, Joe SH, Jung IK, Jung HG, Kim SH. Symptom severity and attitudes toward medication: impacts on adherence in outpatients with schizophrenia. Schizophrenia research. 2012;134(2):226–231. doi: 10.1016/j.schres.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Zullig LL, Mendys P, Bosworth HB. Medication adherence: A practical measurement selection guide using case studies. Patient education and counseling. 2017;100(7):1410–1414. doi: 10.1016/j.pec.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


