Abstract
Objective:
Inflammation is a key driver of excessive neointimal hyperplasia within vein grafts. Recent work demonstrates that specialized proresolving lipid mediators biosynthesized from omega-3 polyunsaturated fatty acids, such as resolvin D1 (RvD1), actively orchestrate the process of inflammation resolution. We investigated the effects of local perivascular delivery of RvD1 in a rabbit vein graft model.
Methods:
Ipsilateral jugular veins were implanted as carotid interposition grafts through an anastomotic cuff technique in New Zealand white rabbits (3–4 kg; N = 80). RvD1 (1 μg) was delivered to the vein bypass grafts in a perivascular fashion, using either 25% Pluronic F127 gel (Sigma-Aldrich, St. Louis, Mo) or a thin bilayered poly(lactic-co-glycolic acid) (PLGA) film. No treatment (bypass only) and vehicle-loaded Pluronic gels or PLGA films served as controls. Delivery of RvD1 to venous tissue was evaluated 3 days later by liquid chromatography-tandem mass spectrometry. Total leukocyte infiltration, macrophage infiltration, and cell proliferation were evaluated by immunohistochemistry. Elastin and trichrome staining was performed on grafts harvested at 28 days after bypass to evaluate neointimal hyperplasia and vein graft remodeling.
Results:
Perivascular treatments did not influence rates of graft thrombosis (23%), major wound complications (4%), or death (3%). Leukocyte (CD45) and macrophage (RAM11) infiltration was significantly reduced in the RvD1 treatment groups vs controls at 3 days (60%−72% reduction; P < .01). Cellular proliferation (Ki67 index) was also significantly lower in RvD1-treated vs control grafts at 3 days (40%−50% reduction; P < .01). Treatment of vein grafts with RvD1-loaded gels reduced neointimal thickness at 28 days by 61% vs bypass only (P < .001) and by 63% vs vehicle gel (P < .001). RvD1-loaded PLGA films reduced neointimal formation at 28 days by 50% vs bypass only (P < .001). RvD1 treatment was also associated with reduced collagen deposition in vein grafts at 28 days.
Conclusions:
Local perivascular delivery of RvD1 attenuates vein graft hyperplasia without associated toxicity in a rabbit carotid bypass model. This effect appears to be mediated by both reduced leukocyte recruitment and decreased cell proliferation within the graft. Perivascular PLGA films may also impart protection through biomechanical scaffolding in this venous arterialization model. Our studies provide further support for the potential therapeutic role of specialized proresolving lipid mediators such as D-series resolvins in modulating vascular injury and repair. (J Vasc Surg 2018;■:1–12.)
Clinical Relevance:
Autologous vein bypass grafts are the most durable means for revascularization in peripheral vascular disease; however, midterm and long-term outcomes are limited by vein graft hyperplasia with associated vein graft failure. Endogenous proresolving lipid mediators such as resolvin D1 have the potential to attenuate vein graft hyperplasia by accelerating repair. This study provides proof of concept for local delivery of resolvin D1 to reduce inflammation and to improve the healing response after vein bypass grafting.
Keywords: Inflammation, Resolution, Resolvins, Lipid mediator, Neointimal hyperplasia, Vein graft
Peripheral arterial disease (PAD) currently affects approximately 8 to 10 million Americans, with an increasing incidence and burden on the health care system each year.1,2 Revascularization is often required for advanced stages of the disease, with autologous vein grafts providing the most durable bypass conduit.3,4 Despite advancements in treatment options, significant midterm failure rates greatly limit clinical benefit for patients with advanced PAD.5,6 Whereas moderate wall thickening is necessary as vein conduits adapt to arterial pressures, persistent inflammation leads to excessive neointimal hyperplasia, stenosis, and associated risk of failure.7–9
Because chronic inflammation plays a causal role in both atherosclerosis and restenosis after vascular intervention, targeting the inflammatory response is of wide interest from a therapeutic perspective. Acute inflammation is normally self-limited and is actively resolved by mediators biosynthesized in a temporal manner within local inflammatory exudates. These include the specialized proresolving lipid mediators (SPMs) generated enzymatically from polyunsaturated fatty acids.10 Several structurally unique families of SPMs have been elucidated, including the E- and D-series resolvins derived from eicosapentaenoic acid and docosahexaenoic acid, respectively.11–13 SPMs have been demonstrated to orchestrate the process of resolution while enhancing host defense,10,14,15 presenting distinct advantages over anti-inflammatory agents that are immunosuppressive. Recent work has demonstrated that SPMs have biologic activity relevant to vascular disease, with specific actions that attenuate inflammatory pathways and vascular smooth muscle cell (VSMC) phenotypic changes critical to the pathobiology of neointimal hyperplasia.16–18 Previous studies have also shown that intra-arterial, sub-cutaneous, or intraperitoneal administration of SPMs attenuates neointimal hyperplasia in animal models of vascular injury and promotes revascularization of ischemic limbs19–22; however, these delivery methods might not be suitable for most clinical situations.
Local perivascular administration of bioactive compounds may allow greater pharmacokinetic efficiency and relevance, particularly for surgical applications. Biomaterials such as polycaprolactone and poly(lactic-co-glycolic acid) (PLGA) have been well described for drug delivery, with variable degradation and drug elution kinetics.23–25 Among the available biomaterials, PLGA is particularly appealing as it is biodegradable and Food and Drug Administration approved for use as a drug delivery device.26 Another option for perivascular drug delivery in animal models is Pluronic F127 gel (Sigma-Aldrich, St. Louis, Mo), which has established utility in proof-of-concept studies.27 Previous attempts at local delivery of therapeutic agents to sites of vascular intervention have been limited by toxicity associated with the candidate drugs themselves, which generally delay rather than accelerate healing.28 For example, the delivery of potent antiproliferative agents to sites of vascular graft implantation has led to infection-related complications when advanced to clinical trials in humans.29
We have recently demonstrated sustained and unidirectional release of the SPM resolvin D1 (7S,8R,17S-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid; RvD1) from a thin PLGA film (“wrap”) construct.30 In a rat model of arterial injury, local perivascular delivery of RvD1 was effective in reducing inflammation and intimal hyperplasia.31 Here, we demonstrate the safety and efficacy of local perivascular delivery of RvD1 in a rabbit vein graft model. Our results provide further evidence for the vasculoprotective effects of SPMs and suggest their utility in modulating healing after vein bypass grafting.
METHODS
Perivascular drug delivery constructs.
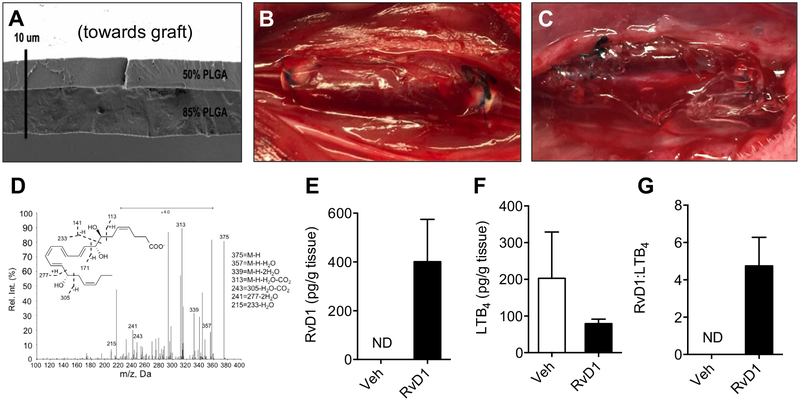
Polymer thin films were spun-cast from solutions of PLGA (Poly-SciTech, West Lafayette, Ind) in 2,2,2-trifluoroethanol (Sigma-Aldrich). PLGA compositions of varying ratios of lactic acid-glycolic acid were used for each layer and are reported as the ratio of lactic acid to glycolic acid (eg, 85:15 PLGA). A total of 1 mg of RvD1 (7S,8R,17S-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid; Cayman, Ann Arbor, Mich) was loaded between layers of 85:15 and 50:50 PLGA to create a bilayered construct. The asymmetry of the PLGA layers facilitated unidirectional release toward the layer with a relatively lower concentration of lactic vs glycolic acid (50:50 PLGA). Vehicle-loaded wraps were processed identically, except pure ethanol (10 μL) was applied during the load step instead of RvD1 (stock diluted in ethanol). Full details of the fabrication procedure for the PLGA films were published previously.30 Total thickness of each device was kept under 10 mm to maintain compliance, which is especially important in the setting of application around a vein graft (Fig 1, A). In comparison, the average thickness of ungrafted contralateral jugular veins was 27 μm (range, 22–38 μm; n = 4; data not shown). Wraps (RvD1 and vehicle) were sized at 20×20 mm to accommodate rabbit jugular vein grafts after carotid interposition (Fig 1, B). A second adventitial drug delivery approach used Pluronic F127 gel. A total of 1 μg of RvD1 was dissolved into 500 μL of 25% Pluronic F127 gel for delivery to vein grafts after carotid interposition. Vehicle gel constructs were processed identically to RvD1-loaded constructs, except pure ethanol was applied during the load step instead of RvD1 (final gel concentration, 2% ethanol). Pluronic gels were kept on ice (4°C) until use to allow application in a liquid form. To ensure circumferential contact with the vein graft, a layer of Pluronic gel was placed posteriorly, followed by a second layer anteriorly (Fig 1, C).
Fig 1.
Perivascular delivery of resolvin D1 (RvD1) to vein grafts in a rabbit carotid bypass model. Poly(lactic-coglycolic acid) (PLGA) films (“wraps”) were constructed using a thin bilayered design, with 1 μg of RvD1 loaded between layers of 50% and 85% PLGA. A, A representative scanning electron microscopy film is shown. B, The thin (<10 μm) design facilitated compliance to the vein graft wall in vivo. C, Alternatively, 1 mg of RvD1 was delivered through a Pluronic gel construct. In vitro studies demonstrated directional drug release from the wrap construct but not from the gels (data not shown). D, Liquid chromatography-tandem mass spectrometry (LC-MS/MS) from vein grafts harvested 3 days after bypass demonstrated delivery of RvD1 to the vessel wall with RvD1-loaded wraps. E, There was no detectable RvD1 at 3 days after bypass with vehicle (Veh) wraps. F and G, There was no detectable RvD1 from vein grafts treated with vehicle and RvD1 gels (n = 1; data not shown). RvD1-loaded wrap treatment resulted in an increase in the ratio of RvD1 to leukotriene B4 (LTB4; n = 3). ND, Not detected.
Rabbit carotid interposition model.
Female New Zealand white rabbits (3–4 kg) were used in compliance with an Institutional Animal Care and Use Committee-approved protocol (UCSF #AN109633–02). The left jugular vein was harvested for use as a carotid interposition graft, and heparin (100 units/kg) was administered before carotid cross-clamping. The bypass was performed using an anastomotic cuff model, as previously described.27,32 Specifically, 2-mm cuffs were made from 4F sheaths (Terumo Medical, Somerset, NJ). The proximal and distal ends of the vein were then pulled through the cuff, everted, and secured to the cuff with 6–0 polypropylene suture. Care was taken to reverse the vein grafts and to avoid valves within the cuff. Following an arteriotomy in the left (ipsilateral) common carotid artery, the cuffs were inserted into and secured to the artery with 6–0 polypropylene suture. The artery was then divided to allow longitudinal extension of the vein interposition graft. Treatments were applied to the external surface of the graft after unclamping. Both vehicle wraps and RvD1-loaded wraps were oriented with the 50:50 PLGA side facing “inward” toward the target vessel. Additional groups underwent perivascular application of 500 mL of vehicle gel or RvD1 gel. Gels were kept on ice until perivascular application and subsequently allowed to solidify at body temperature, as previously described.27 Rabbits were euthanized at 3 or 28 days after bypass. Specimens were harvested and immediately frozen after perfusion with heparinized saline (for 3-day analyses) or fixed after perfusion with heparinized saline followed by 4% formaldehyde (for 28-day analyses). Grafts found to be thrombosed at time of sacrifice (23%) were excluded from all subsequent analyses.
Solid-phase extraction and liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis of lipid mediators.
Vein grafts were harvested at 3 days for LC-MS/MS analysis of targeted lipid mediators. For this, visible PLGA material was gently and completely removed from the outside of the grafts and discarded. Tissues were rinsed with cold saline and snap frozen. Before extraction, frozen tissues were finely minced in ice-cold methanol. Internal deuterium-labeled standards, including d5−resolvin D2, d4−leukotriene B4 (LTB4), and d8−5-hydroxyeicosatetraenoic acid (Cayman, Ann Arbor, MI), were then added to assess extraction recovery. Solid-phase extraction and LC-MS/MS analysis were carried out as previously described.33 Briefly, lipid mediators were extracted by C18 column chromatography, and methyl formate fractions were taken to dryness under a stream of N2 gas before suspension in methanol-water (50:50). Samples were analyzed using a high-performance liquid chromatograph (Shimadzu, Kyoto, Japan) coupled to a QTrap 5500 mass spectrometer (AB Sciex, Framingham, Mass). The instrument was operated in negative ionization mode, and lipid mediators were identified and quantified using multiple reaction monitoring transitions, information-dependent acquisition, and enhanced product ion scanning after normalization to extraction recovery based on internal deuterium-labeled standards and calibration curves for external authentic standards for each mediator.
Immunohistochemistry.
Frozen specimens (3-day grafts) or paraffin-embedded tissues (28-day grafts) were processed, and 6-μm sections were taken throughout the graft, excluding the regions immediately adjacent to the anastomotic cuffs. Frozen sections were fixed in either 4% formaldehyde or acetone for 10 minutes before staining. Paraffin sections were first deparaffinized in xylene, then rehydrated before antigen retrieval with a sodium citrate buffer (Sigma-Aldrich) using a commercial microwave to heat to achieve >95°C for 20 minutes. Sections were then permeabilized with 0.2% Tween 20 (Sigma-Aldrich) and blocked in 5% goat serum (Sigma-Aldrich). Blocking of endogenous avidin-biotin was performed with a commercial kit (Vector, Burlingame, Calif) before incubation with one of the following primary antibodies: mouse-anti-CD45 (1:50; AbD Serotec, Hercules, Calif), mouse-anti-RAM11 (1:50 for frozen samples and 1:200 for paraffin-embedded samples; Dako, Santa Clara, Calif), mouse-anti-Ki67 (1:50; Dako), or mouse-anti-a-smooth muscle actin (1:200; Sigma-Aldrich). This was followed by incubation with a biotin-goat-anti-mouse secondary (1:200; BioLegends, San Diego, Calif), then a fluorescein isothiocyanate-conjugated streptavidin (1:200; Dako). Sections were then mounted in a 4’,6-diamidino-2-phenylindole (DAPI) mounting solution (Southern Biotech, Birmingham, Ala). Staining for apoptosis (terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling [TUNEL]; Sigma-Aldrich) was performed using commercially available kits per the manufacturer’s protocol. Additional staining was also performed for neutrophils (mouse-anti-rabbit RPN 3/57; Bio-Rad, Hercules, Calif) with a goat-anti-mouse Alexa Fluor 488 secondary (Molecular Probes, Waltham, Mass). Three separate sections per graft were analyzed for all markers.
Reverse transcription-polymerase chain reaction (PCR).
Control veins and 3-day vein grafts were immediately stored in RNAlater after harvest. Total RNA was isolated using an RNeasy Micro Kit (Qiagen, Germantown, Md) with RNase-free DNase treatment according to the manufacturer’s protocol. Total RNA from each specimen was used to generate complementary DNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, Calif) for subsequent reverse transcription-PCR reactions. SYBR Green-amplified DNA was detected by incorporation of SYBR Green (Applied Biosystems). Dissociation curve analyses were performed to confirm the specificity of the SYBR Green signal. Data were normalized to two reference genes and subsequently to untreated bypass (bypass-only) grafts. PCR parameters included an initial 10-minute denaturation step at 95°C, followed by a cycling program of 95°C for 10 seconds and 60°C for 30 seconds for 40 cycles (CFX96 Real-Time System; Bio-Rad).
Morphometric and collagen analysis.
After fixation in 4% formaldehyde and processing in 70% ethanol, specimens harvested at 28 days were paraffin embedded; 6-μm sections were taken throughout the graft, excluding the regions immediately adjacent to the anastomotic cuffs. Sections were stained with an Elastin Kit (Thermo Fisher Scientific, Waltham, Mass), and analysis was performed using ImageJ (National Institutes of Health, Bethesda, Md). At least five equally spaced sections of the bypass grafts were analyzed for each specimen. Standard morphometric measurements were recorded, including luminal area, neointimal area (area inside the innermost elastic lamina minus luminal area), and medial area (area inside the outermost elastic lamina minus area inside the innermost elastic lamina). Luminal radius, neointimal thickness, and medial thickness were then calculated from the respective area measurements using standard formulas.32 Total vessel thickness was calculated as the neointimal thickness plus the medial thickness and excluded the adventitia. Collagen deposition was evaluated by trichrome stain (American MasterTech, Lodi, Calif), with total blue (collagen) staining quantified using ImageJ software. Positive cell markers were normalized to either total DAPI-positive cells or vessel area as noted.
Statistical analysis.
Initial comparisons between groups were performed using one-way analysis of variance on all data sets. Nonpaired, two-tailed Student t−tests were used for individual group comparisons, with Bonferroni correction for multiple comparisons.
RESULTS
Adventitial delivery of RvD1 to vein grafts is enhanced by PLGA films.
A rabbit model of carotid bypass was used to study the effects of local perivascular delivery of RvD1 to vein grafts. Delivery of RvD1 was achieved through either a thin bilayered PLGA wrap or a 25% Pluronic gel immediately after completion of the vein bypass (Fig 1, A–C). Rabbits (total N = 80) were divided into five different groups: bypass only, bypass with vehicle wrap, bypass with RvD1 wrap, bypass with vehicle gel, and bypass with RvD1 gel. There were no observed differences in postoperative mortality (3%), thrombosis (23%), or major wound complications (4%) across treatment groups. A total of 60 patent bypass grafts were explanted for the analyses described (Supplementary Table I, online only).
LC-MS/MS analysis of vein grafts harvested 3 days after bypass demonstrated increased levels of RvD1 in the vessel wall for the RvD1-loaded perivascular wrap group (Fig 1, D and E). There were no detectable tissue levels of RvD1 3 days after vehicle-loaded wrap treatment vs a mean of 401.2 pg RvD1 per gram of tissue 3 days after application of the RvD1-loaded wraps (n = 3). Of note, RvD1 was not detected in the graft tissue 3 days after Pluronic gel treatment (n = 1; data not shown). The ratio of RvD1 to the proinflammatory lipid mediator LTB4 in graft tissue was increased in the RvD1 wrap treatment group (n = 3; Fig 1, F and G).
Local perivascular delivery of RvD1 decreases early inflammatory cell infiltration into vein grafts.
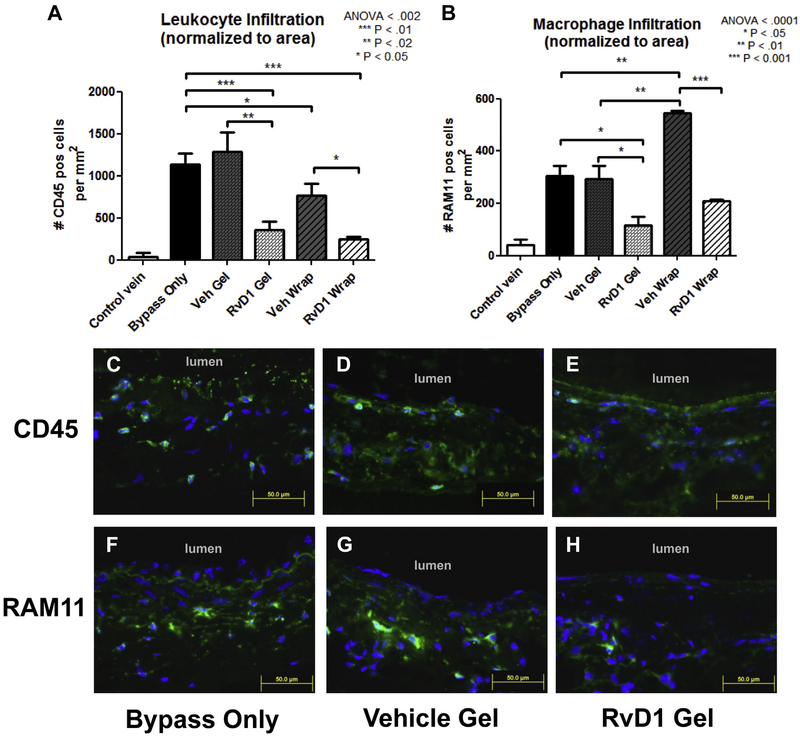
Infiltration of total leukocytes and macrophages at 3 days was analyzed by immunostaining for CD45 and RAM11, respectively (Fig 2, A and B). Cell infiltration was normalized to total vessel area and compared between treatment groups. Ungrafted (control) veins had minimal leukocytes and macrophages (<40 positive cells/mm2 for both CD45 and RAM11). Untreated (bypass-only) vein bypass grafts demonstrated increased CD45+ cellularity (1133 cells/mm2) and RAM11+ cellularity (304 cells/mm2) at 3 days after bypass. CD45+ cellularity was 1282 cells/mm2 in the vehicle gel group, 360 cells/mm2 in the RvD1 gel group, 766 cells/mm2 in the vehicle wrap group, and 247 cells/mm2 in the RvD1 wrap group (Fig 2, A). Leukocyte infiltration was demonstrated in all layers of the vessel wall (Fig 2, C–E). RAM11+ cellularity was 239 cells/mm2 in the vehicle gel group, 117 cells/mm2 in the RvD1 gel group, 545 cells/mm2 in the vehicle wrap group, and 209 cells/mm2 in the RvD1 wrap group (Fig 2, B). Macrophage infiltration was demonstrated in all layers of the vessel wall (Fig 2, F–H). Neutrophil infiltration was assessed with staining for RPN 3/57. RPN 3/57+ cellularity was 36 cells/mm2 for ungrafted (control) veins, 80 cells/mm2 in the untreated (bypass-only) graft group, 119 cells/mm2 in the vehicle gel group, and 27 cells/mm2 in the RvD1 gel group (P < .05 for RvD1 gel vs vehicle gel; Supplementary Fig 1, A, online only). Neutrophil infiltration was demonstrated in all layers of the vessel wall (Supplementary Fig 1, B-D, online only).
Fig 2.
Perivascular delivery of resolvin D1 (RvD1) decreases inflammatory cell infiltration into vein grafts 3 days after bypass. Jugular veins were harvested and used as ipsilateral carotid interposition grafts in a rabbit model. RvD1 or vehicle (Veh) was delivered to the vein graft at time of bypass through perivascular application of a thin bilayered poly(lactic-co-glycolic acid) (PLGA) wrap or 25% Pluronic F127 gel. Treatment groups consisted of no treatment (bypass only) and perivascular application of vehicle gels, RvD1 gels, vehicle wraps, or RvD1 wraps (n = 3–5). A total of 1 μg of RvD1 was delivered for each drug-loaded group (gel and wrap). Vein grafts were harvested at 3 days after bypass and stained for CD45 and RAM11 to detect leukocyte and macrophage infiltration into the vessel wall. 4’,6-Diamidino-2-phenylindole (DAPI) nuclear counterstaining was also performed. A and B, Quantification of leukocyte and macrophage infiltration was performed using three sections from each graft and normalized to wall area. Inflammatory cells were demonstrated throughout the vessel wall. C-H, Representative images (CD45 and RAM11, green; DAPI, blue). ANOVA, Analysis of variance.
Local perivascular delivery of RvD1 does not alter proinflammatory gene expression in early vein grafts.
Gene expression at 3 days after injury was investigated in control and gel-treated bypass grafts, with specific attention to interleukin (IL) 1α, IL-1β, IL-RA, IL-6, IL-8, IL-10, heme oxygenase 1, monocyte chemotactic protein 1, tumor necrosis factor α, transforming growth factor β, and endothelial nitric oxide synthase (Supplementary Table II, online only). Target gene expression was normalized to two housekeeping genes (HPRT, GAPDH) and subsequently to untreated (bypass-only) vein grafts. No signifi-cant effects on expression of these target genes were noted across treatment groups (Supplementary Fig 2, online only). There was significant variability in expression levels noted between specimens within each group.
Local perivascular delivery of RvD1 decreases cell proliferation after vein grafting.
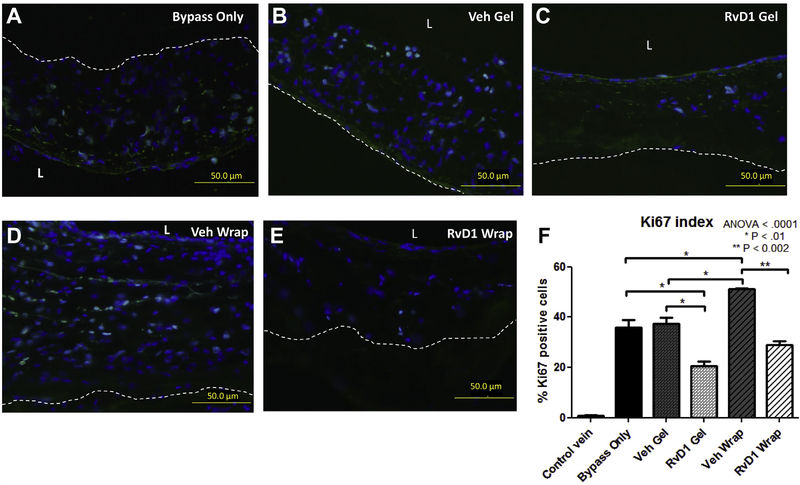
Proliferation was quanti-fied by the Ki67 index (total number of Ki67-positive cells divided by the total number of nucleated cells) 3 days after bypass (Fig 3, A–F). Ungrafted (control) veins demonstrated minimal proliferation (<1%). Proliferation was pronounced in vein grafts 3 days after bypass, with no difference between the bypass-only group (36%) and either the vehicle gel group (37%) or the vehicle wrap group (41%). Perivascular application of RvD1 gels demonstrated 20% proliferation at 3 days after bypass (P < .01 vs bypass only and vehicle gel). Perivascular application of RvD1 wraps demonstrated 24% proliferation at 3 days after bypass (P < .05 vs vehicle wrap). Proliferation was less evident at 28 days after bypass (Ki67 index <1% across all groups, not shown). There were no significant differences in proliferation between the groups at 28 days after bypass.
Fig 3.
Perivascular delivery of resolvin D1 (RvD1) inhibits proliferation within vein grafts 3 days after bypass. Vein grafts were harvested at 3 days after bypass and stained for Ki67 to detect proliferation (green). 4’,6-Diamidino-2-phenylindole (DAPI) counterstaining was also performed (blue). The Ki67 index was calculated by the number of Ki67-positive cells in the vessel wall divided by the total number of nucleated (DAPI-positive) cells. A-E, Representative images. F, Quantitative analysis was performed using three sections from each graft. ANOVA, Analysis of variance; L, lumen; Veh, vehicle.
Apoptosis in early grafts (3 days after bypass; control and gel-treated groups) was assessed by TUNEL staining. The percentage of TUNEL-positive nuclei was 4.7% for ungrafted (control) veins, 4.0% in the untreated (bypass-only) graft group, 5.1% in the vehicle gel group, and 14.9% in the RvD1 gel group (P < .05; Supplementary Fig 3, A, online only). A significant fraction of apoptotic cells in the RvD1-treated grafts were double stained for RPN 3/57, indicating the appearance of apoptotic neutrophils (Supplementary Fig 3, B, online only).
Local perivascular delivery of RvD1 attenuates vein graft neointimal hyperplasia.
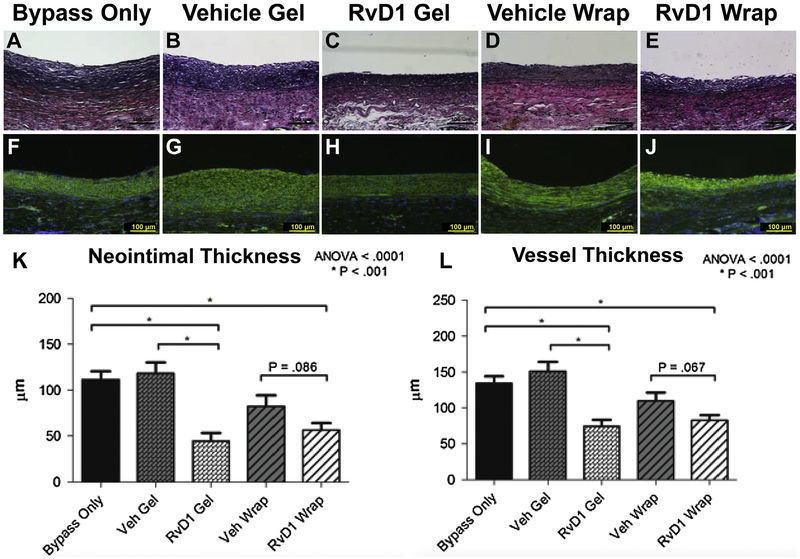
Morphometric analysis of grafts explanted 28 days after bypass (Fig 4, A–E) demonstrated decreased neointimal formation after perivascular delivery of RvD1. α-Smooth muscle cell actin and DAPI staining were used to further characterize the neointimal lesions (Fig 4, F–J). The cellular composition of the neointima appeared similar for all groups, predominantly actin-positive VSMCs. Quantification of the neointimal thickness, medial thickness, and total vessel thickness was performed using at least five equally spaced sections along the nonanastomotic vein for each bypass graft. Mean neointimal thickness at 28 days after bypass was 112 μm in the bypass-only group, 119 mm in the vehicle gel group, 44 μm in the RvD1 gel group, 82 μm in the vehicle wrap group, and 56 μm in the RvD1 wrap group (Fig 4, K). The medial thickness was similar for all groups. Total vessel thickness (intima and media) at 28 days after bypass was 144 μm in the bypass-only group, 154 μm in the vehicle gel group, 78 μm in the RvD1 gel group, 112 μm in the vehicle wrap group, and 84 μm in the RvD1 wrap group (Fig 4, L).
Fig 4.
Perivascular delivery of resolvin D1 (RvD1) attenuates neointimal hyperplasia within rabbit vein grafts. Treatment groups consisted of no treatment (bypass only, n = 7) and perivascular application of vehicle (Veh) gels (n = 7), RvD1 gels (n = 7), vehicle wraps (n = 9). A total of 1 μg of RvD1 was delivered for each group (gel or wrap). A-E, Vein grafts were harvested at 28 days after injury, and elastin staining was performed to facilitate morphometric analysis. F-J, Further characterization of the neointima was performed by staining for α-smooth muscle actin (green), followed by nuclear counterstaining with 4’,6-diamidino-2-phenylindole (DAPI; blue). The cellular composition of the neointima appeared similar for all groups. K and L, Quantification of the neointimal thickness, medial thickness, and total vessel thickness was performed. ANOVA, Analysis of variance.
Delivery of RvD1 attenuates host response to PLGA wraps at 28 days.
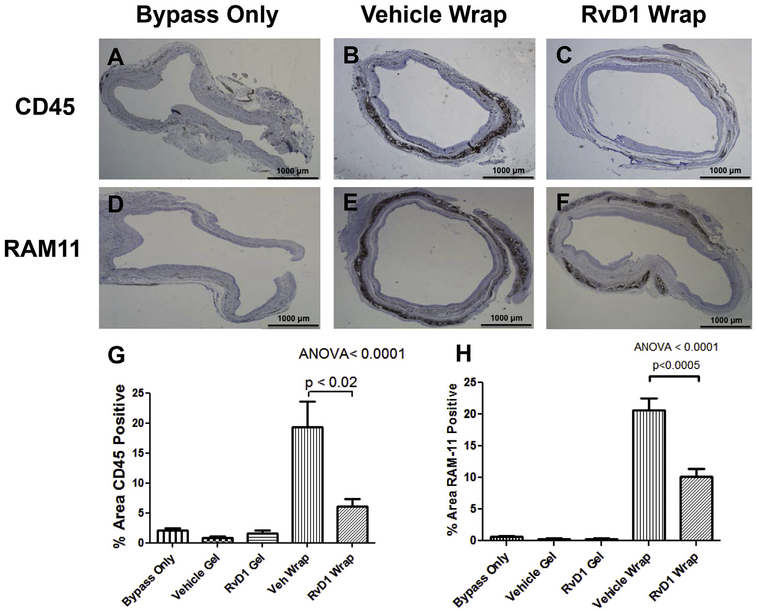
Grafts explanted 28 days after bypass were also stained for CD45 and RAM11 to detect leukocyte and macrophage infiltration, respectively (Fig 5, A–F). There was minimal leukocyte infiltration at 28 days in control bypass grafts and grafts treated with Pluronic gel (RvD1 or vehicle). Leukocyte infiltration was noted at 28 days with both PLGA device groups, with significantly less infiltration observed in the RvD1 wrap group compared with the vehicle wrap group (19% vs 6%; P < .02; Fig 5, G). Similarly, there was minimal macrophage infiltration in control bypass grafts and grafts treated with Pluronic gel (RvD1 or vehicle). Macrophage infiltration was noted at 28 days with both PLGA device groups, with significantly less infiltration observed in the RvD1 wrap group compared with the vehicle wrap group (10% vs 20%; P < .0005; Fig 5, H).
Fig 5.
Delivery of resolvin D1 (RvD1) attenuates host response to poly(lactic-co-glycolic acid) (PLGA) films at 28 days. Control and treated vein grafts were harvested at 28 days after bypass and stained for CD45 to detect leukocyte infiltration (brown, A-C) and RAM11 to detect macrophage infiltration (brown, D-F). The percentage area of CD45 and RAM11 positivity was calculated by dividing the positively stained area by the total area of the vessel wall. G and H, Quantitative analysis demonstrated significantly reduced leukocyte and macrophage infiltration in response to RvD1 wraps compared with vehicle (Veh) wraps, with minimal staining in control bypass grafts and grafts treated with Pluronic gel (RvD1 or vehicle). ANOVA, Analysis of variance.
Local perivascular delivery of RvD1 after vein bypass reduces collagen deposition and does not impair positive graft remodeling.
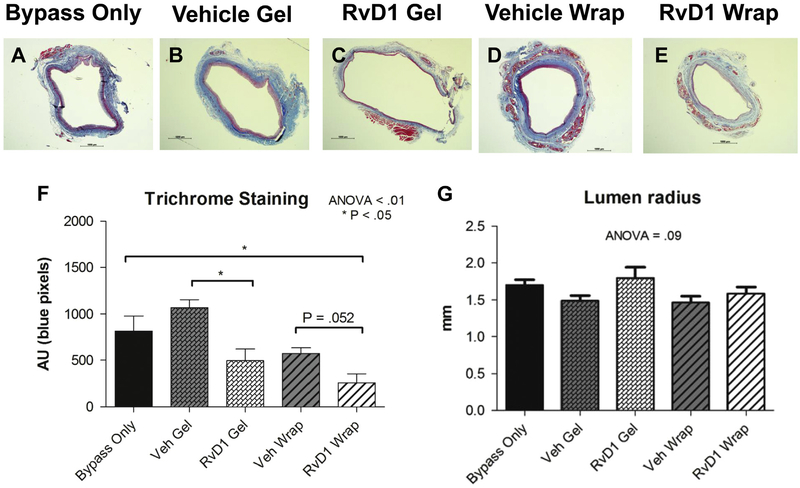
The perivascular fibrotic response was quantified by trichrome (collagen) staining in grafts explanted 28 days after bypass (Fig 6, A–F). There was no difference in collagen content between the bypass-only (813 arbitrary units [AU]), vehicle gel (1068 AU), and vehicle wrap (573 AU) groups. Mean collagen deposition in the RvD1 gel group was 493 AU (P < .05 vs vehicle gel). Mean collagen deposition in RvD1 wraps was 260 AU (P < .05 vs bypass only). Positive (outward) lumen remodeling of the vein bypass grafts was not compromised by perivascular delivery of RvD1 or by any of the adventitial treatments. At 28 days, the mean lumen radius was 1.71 mm for the bypass-only group, 1.49 mm for the vehicle gel group, 1.80 mm for the RvD1 gel group, 1.47 mm for the vehicle wrap group, and 1.59 mm for the RvD1 wrap group (analysis of variance = 0.09; Fig 6, G).
Fig 6.
Perivascular delivery of resolvin D1 (RvD1) after vein bypass reduces collagen deposition and fibrosis. A-E, Vein grafts were harvested at 28 days after implantation, and trichrome staining was performed to assess collagen (fibrosis) response. F, Quantification of the collagen staining (blue) was performed using ImageJ. Vein grafts demonstrate positive (outward) remodeling within the first few weeks of implantation. G, This positive remodeling was not compromised by perivascular delivery of RvD1 or vehicle (Veh). ANOVA, Analysis of variance; AU, arbitrary units.
DISCUSSION
Despite advances in drug elution technology, restenosis continues to plague vascular intervention, and thus considerable interest remains in identifying new approaches to reduce vessel injury and to improve healing. In particular, venous and prosthetic bypass grafts are still frequently employed to treat PAD, with no currently available treatment that prevents restenosis in these surgical settings. Recent investigations suggest that the evolving pharmacobiology of resolution may offer new candidate vascular therapeutics.34 SPMs are naturally occurring autacoids and exert homeostatic effects across a range of disease models with biologic activity in picomolar to nanomolar concentrations.10 In this study, we found that RvD1 delivered to the external surface of rabbit vein grafts at the time of implantation through either a Pluronic gel or a PLGA film reduced neointimal hyperplasia at 28 days by 50% to 61% compared with bypass-only controls. Whereas the molecular mechanisms require further elucidation, our results suggest that reduced acute inflammatory responses as well as antiproliferative activity in the graft wall are likely to be involved in this effect.
Protective effects of SPMs have been previously demonstrated in various models of acute vascular injury. SPMs decrease leukocyte-endothelial cell interactions, potentially through upregulation of endothelial cell nitric oxide production and decreased expression of adhesion molecules on both leukocytes and endothelial cells.14,16,18,35 SPMs attenuate inflammatory cytokine-induced activation of both the nuclear factor kB pathway and reactive oxygen species within endothelial cells.18,36,37 Similar effects have been reported in VSMCs.18–20,31 Attenuation of growth factor-stimulated VSMC migration by SPMs has been observed in vitro.17,19–21,31 RvD1 exposure induces cytoskel etal changes in VSMCs corresponding to an antimigratory phenotype,17,19,31 probably through the cyclic adenosine monophosphate-protein kinase A pathway.38 Furthermore, SPMs have shown modest antiproliferative effects on VSMCs both in vitro19–21,31 and in vivo.19,20,31
Whereas previous studies have investigated effects of SPMs on neointimal hyperplasia after acute arterial injury, this study demonstrates potential efficacy of local delivery of SPMs in inhibiting neointimal hyperplasia within vein bypass grafts.19–21,31 The role of inflammation in vein graft disease has been well described.8,9,39 Trauma during vein harvest and hemodynamic stresses associated with arterialization lead to activation of inflammatory pathways, local expression of cytokines, and infiltration of neutrophils and monocytes. This inflammatory milieu stimulates VSMC dedifferentiation, migration into the intima, and proliferation within the neointima. Although a certain degree of vein graft hyperplasia is adaptive for the arterial circulation, excessive inflammation results in exuberant hyperplasia in the vessel wall and subsequent graft stenosis.8,9,39 An antiproliferative strategy targeting the transcription factor E2F failed to reduce vein graft failure in either the coronary or peripheral circulation.5,40 Therapeutic approaches to prevent bypass graft failure remain an important unmet need, and there are significant risks associated with cytotoxic or anti-inflammatory therapies within a surgical environment.28,29 Thus, the potential for pharmacologic agents that promote the resolution of inflammation is of particular interest in this arena.
This study and others provide a foundation for continued evaluation of SPMs in the modulation of vascular injury and repair. Recent work has described biosynthetic pathways of SPMs in vascular tissues, signaling mechanisms, and vascular cell-specific responses to various SPMs.34,38,41 Definition of a “resolution index” biomarker after acute vascular injury, similar to that employed in other models of sterile inflammation (eg, peritonitis), could also be useful as a surrogate end point for translational studies.13,42,43 Along these lines, we observed an increase in the ratio of RvD1 to the proinflammatory lipid mediator LTB4 in vein grafts treated with RvD1-loaded films. We and others have previously found that the ratio of RvD1 to LTB4 is associated with features of stable atherosclerotic plaques in humans and that this ratio may be a useful systemic biomarker of vascular disease (eg, carotid intima-media thickness).44,45
This study provides only limited insight into the mechanisms through which RvD1 reduced vein graft neointima in this model. However, our data, taken in context with previous studies, suggest effects of RvD1 on both early inflammation and cellular proliferation within the vessel wall. We were not able to reliably determine the specific cell types undergoing proliferation within the grafts, nor were we able to characterize macrophage phenotype (M1/M2). Of note, whereas SPMs have not previously demonstrated toxicity either in vitro17,19,20,31 or in vivo,31 we observed increased apoptosis with adventitial RvD1 treatment in early vein grafts in this study. A significant fraction of the TUNEL-positive cells were neutrophils (Supplementary Fig 3, B, online only), consistent with previous reports demonstrating that SPMs stimulate neutrophil apoptosis.46–48 Neutrophil apoptosis is a characteristic feature of transition to the resolution phase. To gain further insight into both endogenous and pharmacologic resolution, a better understanding of tissue- specific expression of SPM receptors, such as those for RvD1 (ALX/FPR2 and GPR3210,19,35,49,50), and how they are regulated after vascular injury is needed. Receptors and specific mechanisms through which RvD1 affects rabbit vein grafts after bypass require further study. The pharmacokinetics of perivascular delivery of SPMs also needs to be better defined in future translational studies.
Optimizing a perivascular drug delivery strategy for surgical applications such as bypass grafting is a challenge. The ideal approach would provide adequate pharmacokinetics of the therapeutic agent in the target cells, with minimal adverse effects on the vessel and surrounding tissues. The perivascular PLGA film used in this study is thinner and allows better surgical handling and compliance with the vessel wall than the first-generation device used in our previously published study.31 Of note, we observed a modest but significant host inflammatory response to the films at 28 days after implantation. The release of RvD1 attenuated this response; however, continued attention to optimization of the biodegradable delivery system will be important moving forward. In subsequent development of this approach, we have transitioned production of the films to a medical-grade PLGA source material and identified significantly reduced macrophage responses in a rabbit perivascular model (Supplementary Fig 4, online only). Further work is needed to optimize the delivery platform for SPMs in the cardiovascular arena.
The external scaffold provided by the PLGA wrap was potentially a confounding factor in the study as we observed a 24% decrease in neointimal formation at 28 days in comparing the vehicle wrap group with the bypass-only group (P = .081). Although this finding was not statistically significant, decreased neointimal formation with an external polymer scaffold has been well described in the experimental vein graft literature and is thought to be due to reduction in hemodynamic stresses (ie, decreased wall tension).51–53
The decreased neointimal response observed in the vehicle wrap group likely explains the absence of statistical significance in comparing the RvD1-loaded wrap with the vehicle wrap treatments (32% reduction; P = .085). This response was not seen with Pluronic gel-treated grafts, in which RvD1 gel treatment demonstrated significant attenuation of neointimal hyperplasia compared with the vehicle gel group (63% reduction; P < .001). Whereas external scaffolding may provide hemodynamic benefit during vein graft remodeling, restriction of positive remodeling and inflammation associated with Dacron and nitinol scaffolds investigated to date have rendered them ineffective when advanced to human trials.9,54,55 In contrast, the thin-film PLGA devices used in this study did not compromise positive remodeling of the vein grafts, and further development of this approach is justified.
In summary, these data demonstrate biologic efficacy of RvD1 in a rabbit model of vein graft hyperplasia; however, the pharmacokinetic requirements remain unclear. Pluronic gel is rapidly degraded in vivo, yet a significant beneficial effect for RvD1 gel treatment was seen on vein graft neointimal thickness at 28 days, suggesting that early bioavailability of RvD1 in high concentration may be adequate in this model. We hypothesize that more sustained delivery will be needed in larger animal preclinical models of bypass grafting. Moreover, the thin PLGA film device described provides consistent, controllable dosing and direct application in a surgical field, such as at a vascular anastomosis. Further studies are needed to define the best formulation and delivery approach for SPM-based therapeutic strategies in venous or prosthetic bypass grafting.
CONCLUSIONS
Perivascular delivery of RvD1 using either a gel matrix or a biodegradable film significantly attenuated neointimal hyperplasia in a rabbit model of autologous vein bypass. The short-term results observed in this study provide optimism to further evaluate perivascular delivery of SPMs in larger animal models of vascular injury and bypass grafting. We hope that these studies will eventually translate to clinical application in the prevention of restenosis and bypass graft failure.
Supplementary Material
ARTICLE HIGHLIGHTS.
Type of Research: Experimental study using a rabbit carotid vein graft model
Take Home Message: The authors found that perivascular delivery of resolvin D1 reduced leukocyte recruitment and cell proliferation to inhibit vein graft hyperplasia.
Recommendation: Proresolving lipid mediators such as resolvin D1 may be a new class of therapeutics to inhibit the response to vascular injury.
Acknowledgments
This work was supported by the U.S. National Institutes of Health Vascular Interventions/Innovations and Therapeutic Advances Program (VITA) Award HHSN268201400005C (M.S.C. and T.A.D.) and U.S. National Institutes of Health Grants HL119508 (M.S.C.), HL106173 (M.S.), GM095467 (M.S.), HL136044 (B.E.S.), T32AI125222 (E.C.W.), and F32HL123318 (B.W.).
Footnotes
Additional material for this article may be found online at www.jvascsurg.org.
Author conflict of interest: M.S.C. is an inventor on a patent assigned to UCSF relevant to this work.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
REFERENCES
- 1.Dua A, Lee CJ. Epidemiology of peripheral arterial disease and critical limb ischemia. Tech Vasc Interv Radiol 2016;19:91–5. [DOI] [PubMed] [Google Scholar]
- 2.Rundback JH, Armstrong EJ, Contos B, Iida O, Jacobs D, Jaff MR, et al. Key concepts in critical limb ischemia: selected proceedings from the 2015 Vascular Interventional Advances meeting. Ann Vasc Surg 2017;38:191–205. [DOI] [PubMed] [Google Scholar]
- 3.Causey MW, Ahmed A, Wu B, Gasper WJ, Reyzelman A, Vartanian SM, et al. Society for Vascular Surgery limb stage and patient risk correlate with outcomes in an amputation prevention program. J Vasc Surg 2016;63:1563–73.e2. [DOI] [PubMed] [Google Scholar]
- 4.Schanzer A, Hevelone N, Owens CD, Belkin M, Bandyk DF, Clowes AW, et al. Technical factors affecting autogenous vein graft failure: observations from a large multicenter trial. J Vasc Surg 2007;46:1180–90; discussion: 1190. [DOI] [PubMed] [Google Scholar]
- 5.Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, et al. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg 2006;43:742–51; discussion: 751. [DOI] [PubMed] [Google Scholar]
- 6.Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: analysis of amputation free and overall survival by treatment received. J Vasc Surg 2010;51(Suppl):18S–31S. [DOI] [PubMed] [Google Scholar]
- 7.Shah PK. Inflammation, neointimal hyperplasia, and restenosis: as the leukocytes roll, the arteries thicken. Circulation 2003;107:2175–7. [DOI] [PubMed] [Google Scholar]
- 8.Muto A, Model L, Ziegler K, Eghbalieh SD, Dardik A. Mechanisms of vein graft adaptation to the arterial circulation: insights into the neointimal algorithm and management strategies. Circ J 2010;74:1501–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Vries MR, Simons KH, Jukema JW, Braun J, Quax PH. Vein graft failure: from pathophysiology to clinical outcomes. Nat Rev Cardiol 2016;13:451–70. [DOI] [PubMed] [Google Scholar]
- 10.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014;510:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem 2003;278: 14677–87. [DOI] [PubMed] [Google Scholar]
- 12.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiin-flammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med 2000;192:1197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med 2002;196:1025–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, et al. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 2009;461:1287–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, Schmidt BA, et al. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 2012;484:524–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J 2008;22:3595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho KJ, Spite M, Owens CD, Lancero H, Kroemer AH, Pande R, et al. Aspirin-triggered lipoxin and resolvin E1 modulate vascular smooth muscle phenotype and correlate with peripheral atherosclerosis. Am J Pathol 2010;177:2116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chatterjee A, Sharma A, Chen M, Toy R, Mottola G, Conte MS. The pro-resolving lipid mediator maresin 1 (MaR1) attenuates inflammatory signaling pathways in vascular smooth muscle and endothelial cells. PLoS One 2014;9:e113480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyahara T, Runge S, Chatterjee A, Chen M, Mottola G, Fitzgerald JM, et al. D-series resolvin attenuates vascular smooth muscle cell activation and neointimal hyperplasia following vascular injury. FASEB J 2013;27:2220–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akagi D, Chen M, Toy R, Chatterjee A, Conte MS. Systemic delivery of proresolving lipid mediators resolvin D2 and maresin 1 attenuates intimal hyperplasia in mice. FASEB J 2015;29:2504–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petri MH, Laguna-Fernandez A, Tseng CN, Hedin U, Perretti M, Back M. Aspirin-triggered 15-epi-lipoxin A4 signals through FPR2/ALX in vascular smooth muscle cells and protects against intimal hyperplasia after carotid ligation. Int J Cardiol 2015;179:370–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang MJ, Sansbury BE, Hellmann J, Baker JF, Guo L, Parmer CM, et al. Resolvin D2 enhances postischemic revascularization while resolving inflammation. Circulation 2016;134:666–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohler TR, Toleikis PM, Gravett DM, Avelar RL. Inhibition of neointimal hyperplasia in a sheep model of dialysis access failure with the bioabsorbable Vascular Wrap paclitaxeleluting mesh. J Vasc Surg 2007;45:1029–37; discussion: 1037–8. [DOI] [PubMed] [Google Scholar]
- 24.Sanders WG, Hogrebe PC, Grainger DW, Cheung AK, Terry CM. A biodegradable perivascular wrap for controlled, local and directed drug delivery. J Control Release 2012;161:81–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu X, Takayama T, Goel SA, Shi X, Zhou Y, Kent KC, et al. A rapamycin-releasing perivascular polymeric sheath produces highly effective inhibition of intimal hyperplasia. J Control Release 2014;191:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makadia HK, Siegel SJ. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers (Basel) 2011;3:1377–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang GJ, Sui XX, Simosa HF, Jain MK, Altieri DC, Conte MS. Regulation of vein graft hyperplasia by survivin, an inhibitor of apoptosis protein. Arterioscler Thromb Vasc Biol 2005;25: 2081–7. [DOI] [PubMed] [Google Scholar]
- 28.Wessely R New drug-eluting stent concepts. Nat Rev Cardiol 2010;7:194–203. [DOI] [PubMed] [Google Scholar]
- 29.Ostrovsky G Angiotech suspends Vascular Wrap trial enrollment. Medgadget 2008. Available at: https://www.medgadget.com/2008/04/angiotech_suspends_vascular_wrap_trial_enrollment.html. Accessed July 7, 2018.
- 30.Lance KD, Chatterjee A, Wu B, Mottola G, Nuhn H, Lee PP, et al. Unidirectional and sustained delivery of the pro-resolving lipid mediator resolvin D1 from a biodegradable thin film device. J Biomed Mater Res A 2017;105:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu B, Mottola G, Chatterjee A, Lance KD, Chen M, Siguenza IO, et al. Perivascular delivery of resolvin D1 inhibits neointimal hyperplasia in a rat model of arterial injury. J Vasc Surg 2017;65:207–17.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang Z, Wu L, Miller BL, Goldman DR, Fernandez CM, Abouhamze ZS, et al. Novel vein graft model: adaptation to different flow environments. Am J Physiol Heart Circ Physiol 2004;286:H240–5. [DOI] [PubMed] [Google Scholar]
- 33.Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN. Identification and signature profiles for pro-resolving and in-flammatory lipid mediators in human tissue. Am J Physiol Cell Physiol 2014;307:C39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu B, Mottola G, Schaller M, Upchurch GR Jr, Conte MS. Resolution of vascular injury: specialized lipid mediators and their evolving therapeutic implications. Mol Aspects Med 2017;58:72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norling LV, Dalli J, Flower RJ, Serhan CN, Perretti M. Resolvin D1 limits polymorphonuclear leukocyte recruitment to in-flammatory loci: receptor-dependent actions. Arterioscler Thromb Vasc Biol 2012;32:1970–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nascimento-Silva V, Augusta Arruda M, Barja-Fidalgo C, Fierro IM. Aspirin-triggered lipoxin A4 blocks reactive oxygen species generation in endothelial cells: a novel antioxidative mechanism. Thromb Haemost 2007;97:88–98. [PubMed] [Google Scholar]
- 37.Zhang X, Wang T, Gui P, Yao C, Sun W, Wang L, et al. Resolvin D1 reverts lipopolysaccharide-induced TJ proteins disruption and the increase of cellular permeability by regulating IkBa signaling in human vascular endothelial cells. Oxid Med Cell Longev 2013;2013:185715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mottola G, Chatterjee A, Wu B, Chen M, Conte MS. Aspirin-triggered resolvin D1 attenuates PDGF-induced vascular smooth muscle cell migration via the cyclic adenosine monophosphate/protein kinase A (cAMP/PKA) pathway. PLoS One 2017;12:e0174936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Owens CD, Gasper WJ, Rahman AS, Conte MS. Vein graft failure. J Vasc Surg 2015;61:203–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alexander J, Hafley G, Harrington R, Peterson E, Ferguson TJ, Lorenz T, et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA 2005;294:2446–54. [DOI] [PubMed] [Google Scholar]
- 41.Chatterjee A, Komshian S, Sansbury BE, Wu B, Mottola G, Chen M, et al. Biosynthesis of proresolving lipid mediators by vascular cells and tissues. FASEB J 2017;31:3393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, et al. Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol 2005;174:4345–55. [DOI] [PubMed] [Google Scholar]
- 43.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 2007;447:869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thul S, Labat C, Temmar M, Benetos A, Back M. Low salivary resolvin D1 to leukotriene B4 ratio predicts carotid intima media thickness: a novel biomarker of non-resolving vascular inflammation. Eur J Prev Cardiol 2017;24:903–6. [DOI] [PubMed] [Google Scholar]
- 45.Fredman G, Hellmann J, Proto JD, Kuriakose G, Colas RA, Dorweiler B, et al. An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat Commun 2016;7:12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.El Kebir D, Filep JG. Targeting neutrophil apoptosis for enhancing the resolution of inflammation. Cells 2013;2:330–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El Kebir D, Filep JG. Modulation of neutrophil apoptosis and the resolution of inflammation through β2 integrins. Front Immunol 2013;4:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang L, Yuan R, Yao C, Wu Q, Christelle M, Xie W, et al. Effects of resolvin D1 on inflammatory responses and oxidative stress of lipopolysaccharide-induced acute lung injury in mice. Chin Med J (Engl) 2014;127:803–9. [PubMed] [Google Scholar]
- 49.Petri MH, Laguna-Fernandez A, Gonzalez-Diez M, Paulsson-Berne G, Hansson GK, Back M. The role of the FPR2/ALX receptor in atherosclerosis development and plaque stability. Cardiovasc Res 2015;105:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, et al. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U S A 2010;107:1660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kohler TR, Kirkman TR, Clowes AW. The effect of rigid external support on vein graft adaptation to the arterial circulation. J Vasc Surg 1989;9:277–85. [PubMed] [Google Scholar]
- 52.You Q, Duan L, Wang F, Du X, Xiao M. Characterization of the inhibition of vein graft intimal hyperplasia by a biodegradable vascular stent. Cell Biochem Biophys 2011;59:99–107. [DOI] [PubMed] [Google Scholar]
- 53.Dai L, Gao M, Gu C, Zhang F, Yu Y. Perivenous application of cyanoacrylate tissue sealants reduces intimal and medial thickening of the vein graft and inflammatory responses in a rabbit model of carotid artery bypass grafting. Eur J Cardiothorac Surg 2016;49:675–81. [DOI] [PubMed] [Google Scholar]
- 54.Murphy GJ, Newby AC, Jeremy JY, Baumbach A, Angelini GD. A randomized trial of an external Dacron sheath for the prevention of vein graft disease: the Extent study. J Thorac Cardiovasc Surg 2007;134:504–5. [DOI] [PubMed] [Google Scholar]
- 55.Schoettler J, Jussli-Melchers J, Grothusen C, Stracke L, Schoeneich F, Stohn S, et al. Highly flexible nitinol mesh to encase aortocoronary saphenous vein grafts: first clinical experiences and angiographic results nine months postoperatively. Interact Cardiovasc Thorac Surg 2011;13:396–400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.