Abstract
Sexually selected weapons are among the most exaggerated traits in nature. Sexual selection theory frequently assumes a high cost of this exaggeration; yet, those costs are rarely measured. We know very little about the energetic resources required to maintain these traits at rest and the difference in energetic costs for the largest individuals relative to the smallest individuals. Knowledge in this area is crucial; resting metabolic rate can account for 30–40% of daily energy expenditure in wild animals. Here, we capitalized on the phenomenon of autotomy to take a unique look at weapon maintenance costs. Using Leptoscelis tricolor (Hemiptera: Coreidae), we measured CO2 production rates before and after a weapon was shed. Males in this insect species use enlarged hind femora as weapons in male–male combat, and yet can shed them readily, without regeneration, upon entrapment. We found that metabolic rate decreased by an average of 23.5% in males after leg loss and by 7.9% in females. Notably, larger males had less of a drop in metabolic rate per gram of weapon lost. Our findings suggest that sexually selected weapons contribute to a large portion of resting metabolic rate in males, but these costs do not scale in direct proportion to size; larger males can have larger weapons for a reduced metabolic cost. These energetic maintenance costs may be integral to the evolution of the allometries of sexually selected weapons, and yet they remain largely unexplored.
Keywords: sexually selected weapons, resting metabolic rate, hypermetric allometry, energy budgets, tissue-specific metabolic rate, honest signalling
1. Introduction
In many species, sexually selected weapons become disproportionately larger as individuals scale up in size [1,2]. For example, the largest deer develop especially large antlers for their body size, and the largest elephants develop especially large tusks relative to smaller individuals [3,4]. Much attention has been paid to the relative size of weapons; yet, we still know remarkably little about the energetic resources required to maintain these traits at rest, and how these energetic costs change for the largest individuals relative to the smallest individuals [5,6]. Such costs could fundamentally shape or limit the exaggeration and diversification of sexually selected weapons. Metabolic theory provides a general prediction across animal taxa that larger organisms use less energy per gram of tissue than smaller organisms at rest [7–9], yet the extent to which this pattern is true for sexually selected traits has only begun to be tested.
Sexually selected traits provide some of the most extreme examples of traits that increase in relative size with body size [1,4,10]. Traits that get disproportionately larger (slope > 1) as individuals increase in size are said to scale hypermetrically or with positive allometry [2,10–12]. Positive allometries in sexually selected traits are likely to be driven by size-dependent costs and benefits of these traits [13–16]. For example, larger individuals may benefit more from amplifying signals of high competitive ability [17–20]. Furthermore, high-quality individuals may be able to express or carry proportionally larger sexual structures [21–23]. While the absolute cost of a proportionally larger weapon may be high, its marginal cost may be lower [24]. To examine the costs of sexually selected traits, many studies focus on the costs of sexual displays or contests [25–27]. Other studies focus on the cost of growth, and such studies have sometimes revealed growth costs manifesting as developmental trade-offs with other traits [28–30]. In addition, some studies have measured the energetic cost of routine behaviours, such as swimming and running, with sexually selected traits [26,31,32]. However, the costs of sexually selected traits are not limited to displays, contests or routine behaviour and do not end with growth. Sexually selected structures often contain metabolically active tissue that can contribute significantly to whole-organism resting metabolic rate during adulthood, even when the trait is no longer growing [26,32]. In fact, theory often implicitly assumes that the relative resting maintenance costs of sexually selected traits scale in direct proportion to size [33,34], which, metabolic theory suggests, may not be the case for other energetically expensive organs such as nervous system and muscle [35–37]. Indeed, the relative energetic expenses incurred by different organs may change with body size [38]. This effect may come from allocating disproportionate materials and energy to certain expensive tissues such as muscle or nervous tissue within exaggerated traits. Measuring the resting energetic maintenance costs for these structures may be crucial for understanding their evolution and exaggeration. This energetic perspective is likely to provide insight into why steep allometric scaling of sexually selected traits can be so common in nature.
Here, we examine the resting metabolic cost of maintaining a sexual weapon in a sexually dimorphic insect, Leptoscelis tricolor (Hemiptera: Coreidae). Males have enlarged hind femora, which they use as weapons in combat against rivals for mating opportunities [39,40]. The weapons scale positively with body size and are composed of soft tissues surrounded by a hardened cuticle. The steep scaling slope of weapon size means that larger males dedicate a larger proportion of their body mass to their weapons. Dissections of the hind femora and antibody staining revealed evidence that the legs are filled with metabolically active muscle fibres (figure 1). Furthermore, L. tricolor can autotomize (drop) hind legs that become entrapped (e.g. during a moult or by predators), a feature common to many insects in this family [42]. For these reasons, we realized that this species provides an unusual opportunity to disentangle the relationship between relative size of weapons and the metabolic expenses they incur. We compared CO2 production before and after autotomy to infer changes in aerobic metabolic rates in response to leg (weapon) loss among males that differ in relative leg size. If weapons are costly to maintain, we predicted a drop in metabolic rate after leg autotomy. In addition, if weapon energetic costs scale with the size of the sexual weapon, we predicted metabolic rates to drop in proportion to the mass of weapon that is lost. We present four hypotheses, as follows. (i) Isometry—the metabolic cost of the hind leg scales in direct proportion to its mass, in this case the mass of the hind leg is directly indicative of its metabolic expense. This hypothesis is often implicitly assumed by sexual selection theory [17,43]. (ii) Typical allometry—the metabolic cost of the hind leg scales with a slope of 0.75, equivalent to the slope of metabolic rate predicted by metabolic theory [44,45]. (iii) Cost minimization—the metabolic cost of weapons is lower per gram of tissue for larger individuals. This is consistent with the hypothesis that there is strong selection for the largest males to minimize the ongoing, resting energetic expense of their giant weapons. (iv) Expensive signals—the metabolic cost of weapons is higher per gram of tissue for larger individuals (electronic supplementary material, figure S3). This suggests that larger traits are disproportionately expensive to maintain and may act as signals for high competitive ability [46].
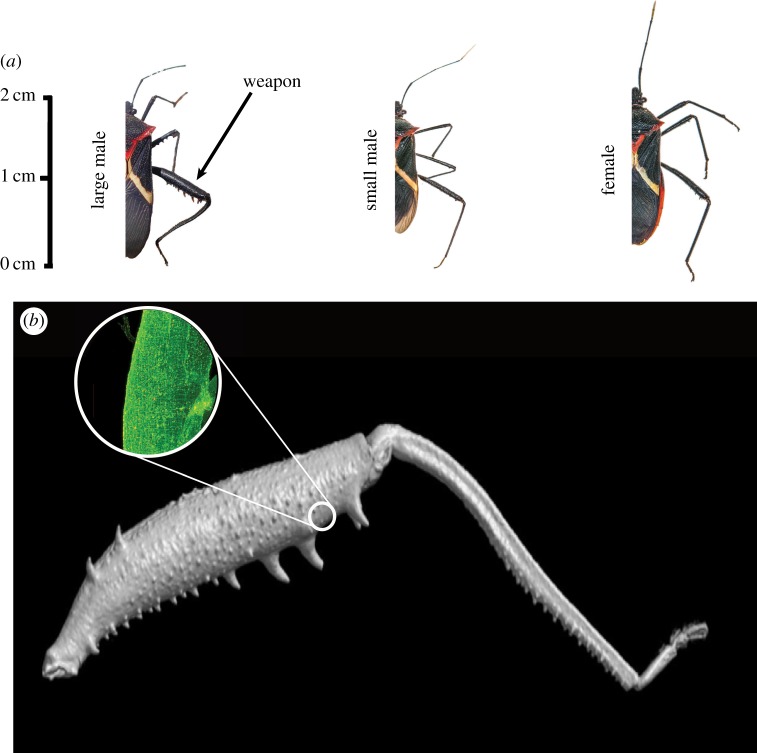
Figure 1.
(a) The hind legs of males are larger than those of females and are modified as weapons. Large males carry proportionally larger weapons than small males (photos: Geena Hill). (b) A scan of a hind leg from a large male (Image: Joshua Yarrow). Prior to this study, we dissected the tissue from the hind femora of a sample of six individuals and used antigen staining techniques to visualize tissue structure (i.e. sarcomeres of striated muscle). To identify this as metabolically active muscular tissue, we stained for an enzyme subunit critical in mitochondrial oxidative phosphorylation, COX IV [41]. Our phosphorescent staining (green) revealed sarcomeres, a distinctive feature of metabolically active muscular tissue. (Online version in colour.)
Our primary goal was to compare CO2 production across males after the loss of a sexually selected weapon. However, we also compared males to a small sample of females for added inference. For females that carry relatively smaller hind legs, we expected a smaller drop in metabolic rate compared to males after leg loss. This study allowed us to ask a critical question underlying many theoretical predictions about the scaling of sexually selected traits: do larger males pay a lower relative energetic cost of maintaining a proportionally larger sexual weapon?
2. Methods
(a). Collection
Leptoscelis tricolor were collected from Gamboa, Panama, transported to the laboratory in plastic containers with moist paper towels, and held in outdoor cages for 24 h before measurements. We collected males (mean body mass ± s.d. = 142.7 ± 27.3 mg, mean single hind leg mass = 9.16 ± 2.99 mg, n = 43) and females (mean body mass ± s.d. = 162.6 ± 31.8 mg, mean single hind leg mass = 3.39 ± 0.480 mg, n = 15). To minimize stress for these liquid feeders, all insects were fed ad libitum with host plant inflorescences from the area in which they were collected. We recorded body mass and transferred individual bugs to respirometry chambers 15–30 min prior to measurement to allow them to become accustomed to the chamber and laboratory environment.
(b). Respirometry
We used a flow-through respirometry system consisting of an air pump, Omega flow meter, and Licor LI 6252 CO2 analyzer in differential mode with a resolution of approximately 0.2 ppm with hardware and software time-averaging of 1 s. The system was calibrated and spanned using a certified gas containing 1010 ppm CO2 in N2. The zero span was reset each time the flow rate was changed, and the system re-zeroed before and after each insect was measured. Incurrent air was scrubbed of CO2 and moisture using Drierite and Ascarite/Soda Lime columns prior to entering the animal chamber. Airflow rates were between 50 and 180 ml min−1. Lower flow rates were used for smaller animals and higher flow rates for larger animals. Excurrent CO2 (ppm) was recorded at a sampling rate 15 samples per second, using Sable Systems Expedata 1.7.3 software version interfaced with a Sable Systems UI1 analogue-digital converter for a period of 10–20 min to ensure capture of resting metabolic rates (see below on assessment of resting metabolic rate).
Excurrent CO2 at rest ranged from 6 to 85 ppm, with a signal-to-noise ratio of 10.37. CO2 emission rate was calculated by dividing ppm CO2 by 1 million, multiplying by the air flow rate (in ml min−1) and then dividing by 60 min h−1. To calculate the metabolic rate in Watts, this value was divided by a standardized RQ (assuming RQ = 1) and then multiplied by the conversion factor 21.1 joules per millilitre O2 [47].
We used 30 ml animal chambers made of airtight plastic syringes. Each chamber was kept in a dark box with red LED bulb during measurement. We recorded activity with a Panasonic High definition HC-V10 camera. We later coded the level of activity by visually interpreting activity level (leg, antennae and body movement) every 3 s from these videos. Activity was coded from a scale of 0 (no activity) to 4 (intense activity); time periods where individuals exhibited no activity (scale = 0) were matched to areas of low and stable CO2 production to determine our measure of resting metabolic rate.
For each individual, we first measured CO2 emission rates before we removed the leg. Immediately after the whole body metabolic rate was measured, we induced leg loss by holding the right hind femur with reverse pressure forceps until the insect made a stereotyped movement and detached its leg [42]. Twenty-four hours later, we again measured CO2 emission rates for each individual. Care was taken to minimize stress on the insects. In coreids, autotomy occurs at specialized break-points and appears to heal quickly with little loss of haemolymph. Indeed, in other arthropod species, autotomy is coupled with rapid wound-healing; the immune response typically lasts for just a few hours and autotomy does not appear to impair survival [48–50]. We attempted to reduce stress by inducing leg loss of only a single leg. Loss of a single hind leg occurs often in nature; on average 12% of L. tricolor individuals in the wild are missing a single hind leg [51].
We examined a possible short-term stress response of leg loss. For a subset of males, we measured CO2 emission at two additional time points after leg loss, 1 min and 1 h, and then followed with the routine 24 h measurement. We found CO2 production increased non-significantly 1 min after leg loss and decreased within 24 h after leg loss (GLM: Walds χ² = 3.14, d.f. = 3,39, p = 0.035, electronic supplementary material, figure S2); These findings are consistent with previous findings that reveal a heightened metabolic rate only for a few hours after autotomy [48,49]. Once all metabolic measurements were completed, we induced autotomy of the second hind leg to measure average wet hind leg mass.
(c). Statistical analysis
(i). Comparisons among males
To test our hypothesis that the metabolic rate required to maintain a weapon scales in direct proportion to the size of the weapon, we measured metabolic rates for each individual male before and after leg loss. This allowed us to calculate the drop in mass due to leg loss, and to determine how this drop in mass is related to the drop in metabolic rate. We calculated mass-specific metabolic rate by dividing the change in metabolic rate by the mass of the leg lost. We also accounted for the mass of tissue lost by subtracting leg mass from total body mass when calculating our mass-specific metabolic rate after leg loss. We calculated drop or change in mass-specific metabolic rate between treatments by subtracting mass-specific metabolic rate measured before leg loss from mass-specific metabolic rate after leg loss for each individual. To examine the effect of treatment (leg loss) on log10 mass-specific metabolic rate, we constructed a GLM, with log10 change in mass-specific metabolic rate as the response variable and body mass as the predictor variable. This analysis allowed us to determine if larger individuals pay a lower or higher energetic cost of maintaining a proportionally larger weapon. All measurements were log10 transformed to control for non-normality and unequal variances. We tested the assumption of homogeneity of slopes using a GLM, and we found this assumption fulfilled before we proceeded with each analysis. All statistical analyses were conducted in the R v. 0.99.893 statistical software (R Development Core Team 2016).
(ii). Comparisons between males and females
To examine the allometry of hind leg mass for males and females, we used a general linear model (GLM), with log10 body mass and sex as continuous and categorical explanatory variables respectively, and the interaction between body mass and sex. Second, to examine the allometry of resting metabolic rate for males and females, a separate GLM was constructed with log10 body mass, sex and their interaction as explanatory variables. In addition, to examine the slope of log10 body mass to log10 leg mass in males and females separately, we used ordinary least squares (OLS) regression [52]. Similarly, OLS regression was used to examine the slope of log10 body mass to log10 resting metabolic rate in males and females. We compared slopes and intercepts of males and females using a simple linear model and tested male and female allometry against a slope of 1 (isometry).
3. Results
(a). Leg mass allometry
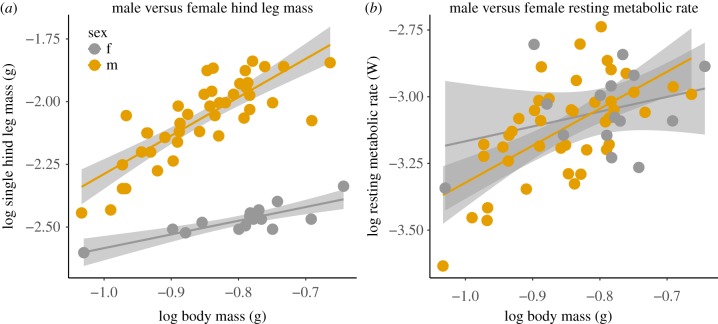
Steep allometries (hypermetric scaling) of weapon mass were confirmed. Larger males had heavier hind legs for their body size than did smaller males (figure 2a: y = 1.53x − 0.75, OLS regression: R = 0.64, p < 0.001, OLS against slope = 1: F1,41 = 77.26, p < 0.001). The opposite was found in females, where larger females had proportionally lighter hind legs than did smaller females (figure 2a: y = 0.54x − 2.03, OLS regression: R = 0.67, p < 0.001, OLS against slope = 1: F1,13 = 29.18, p < 0.001). Body mass significantly predicted leg mass, as revealed by a GLM with log10 body mass and sex as predictor variables and log10 leg mass as a response variable (GLM: Wald χ² = 29.27, d.f. = 1,54, p = 0.029). However, this scaling relationship was different for males and for females, as shown by a significant interaction between log10 leg mass and sex (figure 1a: GLM: Wald χ² = 11.58, d.f. = 1,53, p = 0.001).
Figure 2.
(a) Males (orange, n = 43) exhibit positive allometries; larger males produced proportionally heavier weapons for their body size than do small males. In comparison, females (grey, n = 15) did not exhibit positive allometries in hind leg mass. (b) RMR for insects with all legs intact exhibited positive allometries in males, while female RMR had no significant relationship with body mass. The mass of a single hind leg was used in all estimates of hind leg mass. All lines were generated from OLS regression linear models. Each point on the graph represents a single measurement from each individual. (Online version in colour.)
(b). Metabolic rate allometry
We also found positive allometry of resting metabolic rate in males. Males with greater body mass had higher metabolic rates in proportion to their size compared to small males (figure 2b: y = 1.39x − 1.93, OLS regression: R = 0.38, p < 0.001, OLS against slope = 1: F1,41 = 26.48, p < 0.001). Female resting metabolic rate had no significant relationship with body mass (figure 2b: y = 0.55x − 2.61, OLS regression: R = 0.04, p = 0.24, OLS against slope = 1: F1,13 = 1.51, p = 0.24). We found no relationship between log10 body mass (GLM: Wald χ² = 2.62, d.f. = 1, 54, p = 0.20), sex (GLM: Wald χ² = 1.66, d.f. = 1, 54, p = 0.11) or their interaction on resting metabolic rate (figure 2b).
(c). Effects of leg loss on mass and metabolic rate
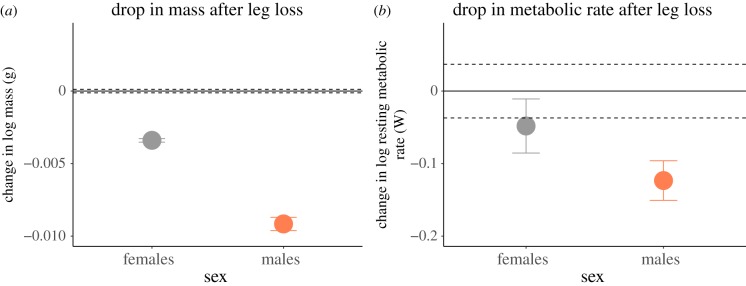
Males had a larger drop in mass than did females after leg loss (GLM: Wald χ² = 29.27, d.f. = 1,54, p = 0.029; figure 3a). Males also had a larger drop in mass-specific metabolic rate compared to females after leg loss, revealed by a significant effect of sex on log10 change in metabolic rate (GLM: Wald χ² = 6.07, d.f. = 1,54, p = 0.017; figure 3b).
Figure 3.
(a) Males had a greater change in mass after leg loss compared to females; mass lost corresponded to mass of leg lost (GLM: Wald χ² = 29.27, d.f. = 1,54, p = 0.029, n males = 43, n females = 15). (b) Female metabolic rate dropped after leg loss, but not significantly, while male metabolic rate dropped significantly after leg loss (GLM: Wald χ² = 6.07, d.f. = 1,54, p = 0.017). Each point on the graph represents mean values for females (grey) and males (orange); dashed lines and error bars show standard errors. (Online version in colour.)
(d). Allometry of resting energetic cost of the weapon
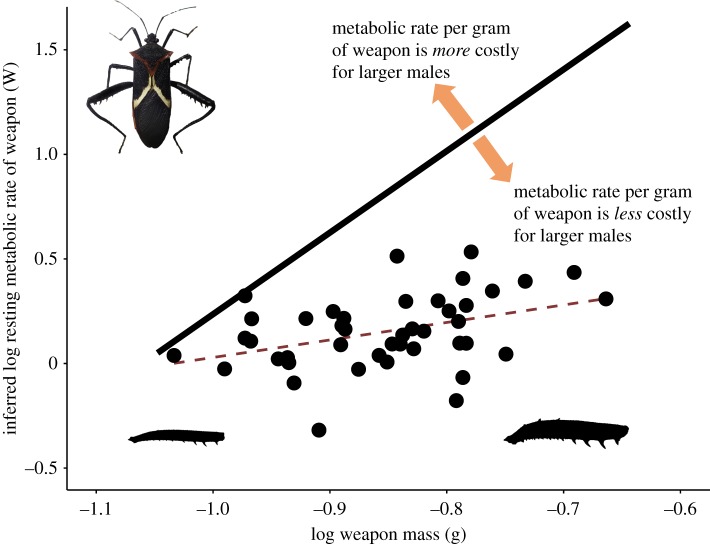
For males, the amount of energy used to carry a proportionally larger weapon did not scale in direct proportion to weapon mass as indicated by the slope of the scaling relationship between mass of the lost leg and change in metabolic rate (figure 4, OLS regression: slope = 0.36, p = 0.036). Specifically, for males we found a significant relationship between log10 body mass (y = 0.84x + 0.86, OLS regression: R = 0.13, p < 0.001, OLS against slope = 1: F1,41 = 7.54, p < 0.01) and log10 leg mass (figure 4: y = 0.36x + 0.89, OLS regression: R = 0.08, p < 0.001) and change in resting metabolic rate.
Figure 4.
To visualize the prediction that weapon mass scales in direct proportion to metabolic rate, we constructed a line that depicts an equivalent metabolic rate for every gram of weapon mass (hypothesis 1, isometry; solid black line, slope = 1). Our results (red dashed line) fall below this prediction, showing that the inferred metabolic rate per gram of tissue lost is lower than predicted if weapons cost the same for large and small males. (Test against isometry: OLS against slope = 1: F1,41 = 4.71, p = 0.036. Test against typical allometry: OLS against slope = 0.75, F1,41 = 4.70, p = 0.024, n = 43). These findings suggest that larger males pay a lower cost per gram to maintain their weapons. Each point represents inferred metabolic rate of a single hind leg from measured values of each individual male. (Online version in colour.)
After leg loss, males no longer exhibited a positive allometry in resting metabolic rate (y = 0.59x − 2.74, OLS regression: R = 0.03, p = 0.13, OLS against slope = 1: F1,41 = 2.36, p = 0.13). However, comparisons between the largest (n = 12) and smallest (n = 13) quartiles of males revealed that the largest males had the largest drop in mass-specific resting metabolic rate (RMR) after leg loss compared to the small males (GLM: Walds χ2 = 4.29, d.f. = 1, 23, p = 0.03), but still not as large as predicted based on the relatively larger size of the leg (see electronic supplementary material, figure S1).
In comparison, for females the mass of the hind leg showed a non-significant relationship with change in metabolic rate (OLS regression slope = 0.54, R < 0.01, p = 0.36); we found no significant relationship between log10 body mass (y = −0.35x − 0.23, OLS regression: R < 0.01, p = 0.37) or log10 leg mass (y = 0.54x − 1.29, OLS regression: R < 0.01, p = 0.36) and change in resting metabolic rate in females.
4. Discussion
Sexually selected weapons often vary greatly in size, yet we know little about how these traits vary in energy consumption at rest. We have experimentally demonstrated that resting metabolic rate declines in males after the loss of a hind leg weapon. Furthermore, we find that the largest drop in metabolic rate occurs in the largest males, which carry proportionally the largest weapons. Our data indicate that large males, bearing large weapons, pay higher overall energetic costs. Weapon cost, however, does not scale 1 : 1 with weapon size as predicted by isometry, but rather with a lower exponent. In contrast to the expensive signals hypothesis (electronic supplementary material, figure S3), our result suggests that the largest weapons are not as energetically costly as their size might imply; the largest weapons found in large males are proportionately (but not absolutely) cheaper to metabolically maintain. The inferred cost of maintaining a weapon scales with a lower exponent than typical allometry (lower than slope: 0.75: figure 4); this is consistent with cost minimization, where males lessen the relative costs of these structures as weapons get larger.
We also compared CO2 production in males relative to females. In contrast to large males, large females exhibit proportionally smaller hind legs and the loss of these structures results in non-significant change in metabolic rate. This weak negative allometry in the size of female hind legs is consistent with a large number of previous studies of the scaling of naturally selected traits in females [53]. We did not find a significant relationship between metabolic rate and body size in females, which suggests that different organs other than hind legs might play a larger role in the resting energy budget of females compared to males, an interesting area for future research.
Our results suggest that sexually selected traits can account for a large proportion of resting metabolic rate in males, and that larger males pay a lower proportional energetic cost for maintaining these structures. It is, however, possible that additional unmeasured size-dependent responses to leg loss may contribute to our observed trends and further studies are needed to investigate the ways in which tissue-specific metabolic rates of weapons directly contribute to a male's total resting energy expenditure. One open question is whether the proportionately smaller cost of large male weapons we report here reflects the same processes that underlie metabolic scaling more generally, or whether it reflects specifically the effects of selection to minimize the cost of a large, sexually selected trait. Investigating this question is an important next step in this line of inquiry.
Maintaining metabolically active tissues can impose significant energetic costs on organisms even during periods of inactivity. For example, flying insects have higher resting metabolic rates than do non-flying insects, probably because of the upkeep of metabolically active flight muscles [54–56]. The resting metabolic costs of sexually selected muscles are much less studied. Yet, the descriptive studies that do exist, and the results we show here, suggest that sexually selected traits, and the muscles needed to support them, incur substantial maintenance costs (figure 3) [26,57]. In the sexually dimorphic horned isopod, Deto echinata, males with proportionally larger horns exhibited higher resting metabolic rates than do juveniles or females [57]. In the fiddler crab, Uca pugilator, resting metabolic rates of males were 17% higher than those of females of equivalent mass [58], potentially because males had more striated muscle in their enlarged weapons [59].
Multiple studies have focused on the energetic costs that sexually selected traits might impose during routine behaviour such as walking or swimming. Indeed, large and exaggerated traits seem well poised to increase locomotory costs. Energetic costs of sexually selected traits on locomotion have been examined in fiddler crabs [26,31], stag beetles [60,61] and swordtail fish [32]. Not all results are as expected. In fiddler crabs for example, males that had their major claw removed did not consume less oxygen during rest or during sustainable locomotion; yet an increase in lactic acid was found in crabs with claws during strenuous exercise which may suggest metabolic costs can continue to accrue during rest after strenuous exercise [31,62].
In addition to the cost of routine behaviours, several studies have examined the costs of sexual displays or contests, for example, the energetic cost of mate calling in birds [25,27], frogs [63,64] and leafhoppers [65], and courtship in fish [32] and spiders [66]. While some studies show that sexually selected behaviours can carry high energetic costs over short periods of time [67–69], others studies reveal that such behaviours are not energetically demanding [25,70]. However, many of these behaviours require specialized muscles and metabolically active tissue, the energetic costs of which continue to accrue over periods of inactivity. In addition, most organisms spend much more time at rest than they do in sexual displays or contests. Small maintenance costs incurred during periods of inactivity can lead to large amounts of energy expended over longer periods of time [26,32]. Indeed, resting metabolic rate can account for 30–40% of daily energy expenditure in free living animals [71–73]. Thus, the daily energy expenditure of maintaining sexual traits might impose a larger energetic burden on an organism than sexual contests. In other words, the persistent costs of maintaining the metabolic machinery required to perform high-energy behaviours might contribute to a large energetic cost over time [74,75] and ultimately shape the evolution of sexually selected traits.
Energetic costs may be more pronounced for those traits containing metabolically active tissue, yet many sexual traits may not contain such tissue, for example, the air-filled horns of the rhinoceros beetle, Trypoxylus dichotomus [76], the mature antlers of elk, Cervus canadensis [77], the tusks of tusked wasps, Synagris cornuta [78] or the elytral projections of tortoise beetles, Acromis sparsa [79]. We would not expect the expression of such traits to be limited by metabolic maintenance costs of the weapon itself [80–84]. By contrast, L. tricolor males have muscular tissue within their weapons which appear to contribute to resting metabolic rates. Indeed, we found the flexor muscle (used in moving the tibia towards the femur in a ‘squeezing’ motion typical of the motion used in male–male combat in this species) is greatly enlarged in large male weapons (electronic supplementary material, figure S3), and the fibres in these muscles show a high occurrence of enzymes involved in mitochondrial phosphorylation, indicative of high metabolic activity (figure 1). However, species with even greater muscle masses associated with their weapons might pay larger metabolic costs [85]. One potential explanation for why larger males pay a lower metabolic cost for a larger trait is that the proportion of tissue with low metabolic rates such as cuticle, trachea and connective tissue may differ with overall body size, such that large individuals have a higher proportion of these tissues. Indeed, a recent study looking at 23 insect species across 5 orders suggests that different body tissue often scales with unique slopes with body size [86]; however, the role that energetics plays in shaping these relationships is far less studied [87].
In addition to weapons, it is likely that other supportive traits and musculature are expressed to allow L. tricolor to carry and use these structures. We have not specifically identified such traits yet; however, examples in other taxa include increased investment in flight musculature to carry weapons during flight in stag beetles [60], increased neck musculature in Irish elk [88] and moose [89], or other muscles to support the costs of locomotion, such as running in fiddler crabs with large claws [26] or swimming in swordtail fish with elongated tails [32]. In our study, we did not account for potential supportive traits that might increase the cost of bearing sexually selected structures, and therefore we probably underestimate the full metabolic cost of bearing these weapons.
5. Conclusion
The positive allometries so common to sexually selected traits have long been a source of interest and puzzlement to evolutionary biologists, and most early studies focused on the relative size of these traits, not on the proportion of the energy budget these traits consume [1,10,90]. Via an experimental manipulation, we found that larger males pay a reduced metabolic cost for the enlarged sexually selected weapon than predicted from the size of the trait. In other words, the size of a sexually selected weapon does not scale in direct proportion to its metabolic maintenance cost. We also found that the energetic maintenance costs are not as clear for the female homologue, a much reduced version of the male weapon. These findings inspire a more integrated perspective on the factors that shape and constrain weapon exaggeration based on the cost of maintaining these structures and the specific energy budgets that individual organisms experience.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Rachel Watson and Rebecca Perry for many hours of field collecting and respirometry trials required for this study; Armando Castillo provided assistance with enzyme assays. Jamie F. Giloolly, William Wcislo, William Eberhard and Leigh Simmons provided advice during various stages of this study. We thank Claudio Monteza, Lourdes Hernandez, Erin Welsh, Erin Allman Updyke, Salvatore Anzaldo, May Dixon, Peter Marting and Sara Fern Leitman for the assistance with data collection during different phases of this project. We thank Geena Hill for photographs and Marguerite Mauritz and Harlan Gough for the assistance with figures. Colette St Mary and Stephen M. Shuster provided helpful comments on previous drafts of this manuscript. We thank Ministerio de Ambiente, Panamá for permits to conduct this research.
Data accessibility
Data available in Dryad Digital Repository at: https://doi.org/10.5061/dryad.757v3h2 [91].
Authors' contributions
U.S. and M.D. carried out the experiments and participated in the design of the study. U.S. conceived of the study, coordinated the study and drafted the manuscript. H.A.W. and C.W.M. guided in data analysis, writing and structure of the paper. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by a University of Florida Graduate Student Fellowship through the UF School of Natural Resources and Environment (U.S.), Smithsonian Tropical Research Institute Short-term Fellowship 2016 (U.S.), Society for the Study of Evolution Rosemary Grant Award (U.S.) and National Science Foundation Grant, NSF IOS-1553100 (C.W.M.).
References
- 1.Gould SJ. 1974. The origin and function of ‘bizarre’ structures: antler size and skull size in the ‘Irish elk’, Megaloceros giganteus . Evolution 28, 191–220. [DOI] [PubMed] [Google Scholar]
- 2.Kodric-Brown A, Sibly RM, Brown JH. 2006. The allometry of ornaments and weapons. Proc. Natl Acad. Sci. USA 103, 8733–8738. ( 10.1073/pnas.0602994103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clutton-Brock TH. 1982. The functions of antlers. Behavior 79, 108–125. ( 10.1163/156853982X00201) [DOI] [Google Scholar]
- 4.Emlen DJ. 2008. The evolution of animal weapons. Annu. Rev. Ecol. Evol. Syst. 39, 387–413. ( 10.1146/annurev.ecolsys.39.110707.173502) [DOI] [Google Scholar]
- 5.Biro PA, Stamps JA. 2010. Do consistent individual differences in metabolic rate promote consistent individual differences in behavior? Trends Ecol. Evol. 25, 653–659. ( 10.1016/j.tree.2010.08.003) [DOI] [PubMed] [Google Scholar]
- 6.Burton T, Killen S, Armstrong J, Metcalfe N. 2011. What causes intraspecific variation in resting metabolic rate and what are its ecological consequences? Proc. R. Soc. B 278, 3444–3451. ( 10.1098/rspb.201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kleiber M. 1932. Body size and metabolism. Hilgardia A J. Agric. Sci. 6, 315–353. ( 10.1017/CBO9781107415324.004) [DOI] [Google Scholar]
- 8.Glazier DS. 2014. Is metabolic rate a universal ‘pacemaker’ for biological processes? Biol. Rev. Camb. Phil. Soc. 90, 377–407. ( 10.1111/brv.12115) [DOI] [PubMed] [Google Scholar]
- 9.West GB. 2005. The origin of allometric scaling laws in biology from genomes to ecosystems: towards a quantitative unifying theory of biological structure and organization. J. Exp. Biol. 208, 1575–1592. ( 10.1242/jeb.01589) [DOI] [PubMed] [Google Scholar]
- 10.Huxley JS. 1932. Problems of relative growth. London, UK: Methuen. [Google Scholar]
- 11.Bonduriansky R, Day T. 2003. The evolution of static allometry in sexually selected traits. Evolution 57, 2450–2458. ( 10.1111/j.0014-3820.2003.tb01490.x) [DOI] [PubMed] [Google Scholar]
- 12.Bonduriansky R. 2007. Sexual selection and allometry: a critical reappraisal of the evidence and ideas. Evolution 61, 838–849. ( 10.1111/j.1558-5646.2007.00081.x) [DOI] [PubMed] [Google Scholar]
- 13.Kotiaho JS. 2001. Costs of sexual traits: a mismatch between theoretical considerations and empirical evidence. Biol. Rev. 76, 365–376. ( 10.1017/S1464793101005711) [DOI] [PubMed] [Google Scholar]
- 14.Unrug J, Tomkins JL, Radwan J. 2004. Alternative phenotypes and sexual selection: can dichotomous handicaps honestly signal quality? Proc. R. Soc. B 271, 1401–1406. ( 10.1098/rspb.2004.2729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zollman KJS, Bergstrom CT, Huttegger SM. 2012. Between cheap and costly signals: the evolution of partially honest communication. Proc. R. Soc. B 280, 20121878 ( 10.1098/rspb.2012.1878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biernaskie JM, Grafen A, Perry JC. 2014. The evolution of index signals to avoid the cost of dishonesty. Proc. R. Soc. B 281, 20140876 ( 10.1098/rspb.2014.0876) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green AJ. 1992. Positive allometry is likely with mate choice, competitive display and other functions. Anim. Behav. 43, 170–172. ( 10.1016/S0003-3472(05)80086-7) [DOI] [Google Scholar]
- 18.Petrie M. 1992. Are all secondary sexual display structures positively allometric and, if so, why? Anim. Behav. 43, 173–175. ( 10.1016/S0003-3472(05)80087-9) [DOI] [Google Scholar]
- 19.Simmons LW, Tomkins JL. 1996. Sexual selection and the allometry of earwig forceps. Evol. Ecol. 10, 97–104. ( 10.1007/BF01239350) [DOI] [Google Scholar]
- 20.Eberhard WG, Rodríguez RL, Huber BA. 2018. Sexual selection and static allometry: the importance of function. Quart. Rev. Biol. 93, 207–250. ( 10.1086/699410) [DOI] [Google Scholar]
- 21.Zahavi A. 1975. Mate selection: a selection for a handicap. J. Theor. Biol. 53, 205–214. ( 10.1016/0022-5193(75)90111-3) [DOI] [PubMed] [Google Scholar]
- 22.Andersson M, Simmons LW. 2006. Sexual selection and mate choice. Trends Ecol. Evol. 21, 296–302. ( 10.1016/j.tree.2006.03.015) [DOI] [PubMed] [Google Scholar]
- 23.Grafen A. 1990. Biological signals as handicaps. J. Theor. Biol. 144, 517–546. ( 10.1016/S0022-5193(05)80088-8) [DOI] [PubMed] [Google Scholar]
- 24.Getty T. 2006. Sexually selected signals are not similar to sports handicaps. Trends Ecol. Evol. 21, 83–88. ( 10.1016/j.tree.2005.10.016) [DOI] [PubMed] [Google Scholar]
- 25.Ward S, Speakman JR, Slater PJ. B. 2003. The energy cost of song in the canary, Serinus canaria. Anim. Behav. 66, 893–902. ( 10.1006/anbe.2003.2250) [DOI] [Google Scholar]
- 26.Allen BJ, Levinton JS. 2007. Costs of bearing a sexually selected ornamental weapon in a fiddler crab. Funct. Ecol. 21, 154–161. ( 10.1111/j.1365-2435.2006.01219.x) [DOI] [Google Scholar]
- 27.Eberhardt LS. 1994. Oxygen consumption during singing by male Carolina wrens (Thryothorus ludovicianus). Auk 111, 124–130. ( 10.2307/4088511) [DOI] [Google Scholar]
- 28.Nijhout HF, Emlen DJ. 1998. Competition among body parts in the development and evolution of insect morphology. Proc. Natl Acad. Sci. USA 95, 3685–3689. ( 10.1073/pnas.95.7.3685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simmons LW, Emlen DJ. 2006. Evolutionary trade-off between weapons and testes. Proc. Natl Acad. Sci. USA 103, 16 346–16 351. ( 10.1073/pnas.0603474103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Somjee U, Miller CW, Tatarnic NJ, Simmons LW. 2017. Experimental manipulation reveals a trade-off between weapons and testes. J. Evol. Biol. 31, 57–65. ( 10.1111/jeb.13193) [DOI] [PubMed] [Google Scholar]
- 31.Bywater CL, White CR, Wilson RS. 2014. Metabolic incentives for dishonest signals of strength in the fiddler crab Uca vomeris. J. Exp. Biol. 217, 2848–2850. ( 10.1242/jeb.099390) [DOI] [PubMed] [Google Scholar]
- 32.Basolo AL, Alcaraz G. 2003. The turn of the sword: length increases male swimming costs in swordtails. Proc. R. Soc. Lond. B 270, 1631–1636. ( 10.1098/rspb.2003.2388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maynard SJ, Parker GA. 1976. The logic of asymmetric contests. Anim. Behav. 24, 159–175. ( 10.1016/S0003-3472(76)80110-8) [DOI] [Google Scholar]
- 34.Smith MJ, Harper DG. C. 1995. Animal signals: models and terminology. J. Theor. Biol. 177, 305–311. ( 10.1006/jtbi.1995.0248) [DOI] [Google Scholar]
- 35.Crnokrak P, Roff DA. 2002. Trade-offs to flight capability in Gryllus firmus: the influence of whole-organism respiration rate on fitness. J. Evol. Biol. 15, 388–398. ( 10.1046/j.1420-9101.2002.00401.x) [DOI] [Google Scholar]
- 36.Nespolo RF, Roff DA, Fairbairn DJ. 2008. Energetic trade-off between maintenance costs and flight capacity in the sand cricket (Gryllus firmus). Funct. Ecol. 22, 624–631. ( 10.1111/j.1365-2435.2008.01394.x) [DOI] [Google Scholar]
- 37.Wiersma P, Nowak B, Williams JB. 2012. Small organ size contributes to the slow pace of life in tropical birds. J. Exp. Biol. 215, 1662–1669. ( 10.1242/jeb.065144) [DOI] [PubMed] [Google Scholar]
- 38.Savage VM, Allen AP, Brown JH, Gillooly JF, Herman AB, Woodruff WH, West GB. 2007. Scaling of number, size, and metabolic rate of cells with body size in mammals. Proc. Natl Acad. Sci. USA 104, 4718–4723. ( 10.1073/pnas.0611235104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller CW, Emlen DJ. 2010. Dynamic effects of oviposition site on offspring sexually-selected traits and scaling relationships. Evol. Ecol. 24, 375–390. ( 10.1007/s10682-009-9312-6) [DOI] [Google Scholar]
- 40.Miller CW, Emlen DJ. 2010. Across- and within-population differences in the size and scaling relationship of a sexually selected trait in Leptoscelis tricolor (Hemiptera: Coreidae). Ann. Entomol. Soc. Am. 103, 209–215. ( 10.1603/AN09039) [DOI] [Google Scholar]
- 41.Tanji K, Bonilla E. 2001. Optical imaging techniques (histochemical, immunohistochemical, and in situ hybridization staining methods) to visualize mitochondria. In Methods in cell biology (eds Pon LA, Schon EA), pp. 311–332. San Diego: Academic Press. [DOI] [PubMed] [Google Scholar]
- 42.Emberts Z, St Mary CM, Miller CW. 2016. Coreidae (Insecta: Hemiptera) limb loss and autotomy. Ann. Entomol. Soc. Am. 109, 1–6. ( 10.1093/aesa/saw037) [DOI] [Google Scholar]
- 43.Johnstone RA. 1995. Sexual selection, honest advertisement and the handicap principle: reviewing the evidence. Biol. Rev. 70, 1–65. ( 10.1111/j.1469-185X.1995.tb01439.x) [DOI] [PubMed] [Google Scholar]
- 44.Brown JH, Gillooly JF, Allen AP, Savage VM, West GB. 2004. Toward a metabolic theory of ecology. Ecology 85, 1771–1789. ( 10.1890/03-9000) [DOI] [Google Scholar]
- 45.Glazier DS. 2006. The 3/4-power law Is not universal: evolution of isometric, ontogenetic metabolic scaling in pelagic animals. Bioscience 56, 325–332. ( 10.1641/0006-3568(2006)56%5B325:TPLINU%5D2.0.CO;2) [DOI] [Google Scholar]
- 46.Pomiankowski A. 1987. Sexual selection: The handicap principle does work—sometimes. Proc. R. Soc. Lond. B 231, 123–145. ( 10.1098/rspb.1987.0038) [DOI] [Google Scholar]
- 47.Lighton JRB. 2008. Measuring metabolic rates: a manual for scientists. Oxford, UK: Oxford University Press. [Google Scholar]
- 48.Slos S, De Block M, Stoks R. 2009. Autotomy reduces immune function and antioxidant defence. Biol. Lett. 5, 90–92. ( 10.1098/rsbl.2008.0501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang X, Zhang C, Huang G, Xu M, Cheng Y, Yang Z, Zhang Q, Wang Y. 2018. Cellular and biochemical parameters following autotomy and ablation-mediated cheliped loss in the Chinese mitten crab, Eriocheir sinensis. Dev. Comp. Immunol. 81, 33–43. ( 10.1016/j.dci.2017.11.003) [DOI] [PubMed] [Google Scholar]
- 50.Emberts Z, Miller CW, Kiehl D, St. Mary CM. 2017. Cut your losses: self-amputation of injured limbs increases survival. Behav. Ecol. 28, 1047–1054. ( 10.1093/beheco/arx063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Somjee U. 2014. Environmental effects on sexual selection in a wild insect population of Leptoscelis tricolor (Hemiptera: Coreidae). MS thesis, University of Florida, Gainesville, FL. [Google Scholar]
- 52.Kilmer JT, Rodríguez RL. 2017. Ordinary least squares (OLS) regression is indicated for studies of allometry. J. Evol. Biol. 30, 4–12. ( 10.1111/JEB.12986) [DOI] [PubMed] [Google Scholar]
- 53.Voje KL. 2016. Scaling of morphological characters across trait type, sex, and environment. Am. Nat. 187, 89–98. ( 10.1086/684159) [DOI] [PubMed] [Google Scholar]
- 54.Zera AJ, Mole S. 1994. The physiological costs of flight capability in wing-dimorphic crickets. Res. Popul. Ecol. 36, 151–156. ( 10.1007/BF02514930) [DOI] [Google Scholar]
- 55.Niven JE, Scharlemann JP. W. 2005. Do insect metabolic rates at rest and during flight scale with body mass? Biol. Lett. 1, 346–349. ( 10.1098/rsbl.2005.0311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reinhold K. 1999. Energetically costly behaviour and the evolution of resting metabolic rate in insects. Funct. Ecol. 13, 217–224. ( 10.1046/j.1365-2435.1999.00300.x) [DOI] [Google Scholar]
- 57.Glazier DS, Clusella-Trullas S, Terblanche JS. 2016. Sexual dimorphism and physiological correlates of horn length in a South African isopod crustacean. J. Zool. 300, 99–110. ( 10.1111/jzo.12338) [DOI] [Google Scholar]
- 58.Weissburg M. 1993. Sex and the single forager: gender-specific energy maximization strategies in fiddler crabs. Ecology 74, 279–291. ( 10.2307/1939292) [DOI] [Google Scholar]
- 59.Levinton JS, Judge ML. 1993. The relationship of closing force to body size for the major claw of Uca pugnax (Decapoda: Ocypodidae). Funct. Ecol. 7, 339–345. ( 10.2307/2390214) [DOI] [Google Scholar]
- 60.Goyens J, Van Wassenbergh S, Dirckx J, Aerts P. 2015. Cost of flight and the evolution of stag beetle weaponry. J. R. Soc. Interface 12, 20150222 ( 10.1098/rsif.2015.0222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goyens J, Dirckx J, Aerts P. 2015. Costly sexual dimorphism in Cyclommatus metallifer stag beetles. Funct. Ecol. 29, 35–43. ( 10.1111/1365-2435.12294) [DOI] [PubMed] [Google Scholar]
- 62.Tullis A, Straube CHT. 2017. The metabolic cost of carrying a sexually selected trait in the male fiddler crab Uca pugilator. J. Exp. Biol. 220, 3641–3648. ( 10.1242/jeb.163816) [DOI] [PubMed] [Google Scholar]
- 63.Ryan MJ. 1988. Energy, calling and selection. Am. Zool. 11, 545–564. ( 10.1093/icb/28.3.885) [DOI] [Google Scholar]
- 64.Grafe TU, Schmuck R, Linsenmair KE. 1992. Reproductive energetics of the African reed frogs, Hyperolius viridiflavus and Hyperolius marmoratus. Physiol. Zool. 65, 153–171. ( 10.1086/physzool.65.1.30158244) [DOI] [Google Scholar]
- 65.Kuhelj A, de Groot M, Pajk F, Simčič T, Virant-Doberlet M. 2015. Energetic cost of vibrational signalling in a leafhopper. Behav. Ecol. Sociobiol. 69, 815–828. ( 10.1007/s00265-015-1898-9) [DOI] [Google Scholar]
- 66.Cady AB, Delaney KJ, Uetz GW. 2011. Contrasting energetic costs of courtship signaling in two wolf spiders having divergent courtship behaviors. J. Arachnol. 39, 161–165. ( 10.1636/Hi09-70.1) [DOI] [Google Scholar]
- 67.Briffa M, Sneddon LU. 2007. Physiological constraints on contest behaviour. Funct. Ecol. 21, 627–637. ( 10.1111/j.1365-2435.2006.01188.x) [DOI] [Google Scholar]
- 68.Taigen TL, Wells KD, Marsh RL. 1985. The enzymatic basis of high metabolic rates in calling frogs. Physiol. Zool. 58, 719–726. ( 10.1086/physzool.58.6.30156075) [DOI] [Google Scholar]
- 69.Oberweger K, Goller F. 2001. The metabolic cost of birdsong production. J. Exp. Bol. 204, 3379–3388. [DOI] [PubMed] [Google Scholar]
- 70.Stoddard PK, Salazar VL. 2011. Energetic cost of communication. J. Exp. Biol. 214, 200–205. ( 10.1242/jeb.047910) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sears MW. 2005. Resting metabolic expenditure as a potential source of variation in growth rates of the sagebrush lizard. Comp. Biochem. Physiol. 140, 171–177. ( 10.1016/j.cbpb.2004.12.003) [DOI] [PubMed] [Google Scholar]
- 72.Ricklefs R, Konarzewski M, Daan S. 1996. The relationship between basal metabolic rate and daily energy expenditure in birds and mammals. Am. Nat. 147, 1047–1071. ( 10.1086/285892) [DOI] [Google Scholar]
- 73.Speakman JR, Ergon T, Cavanagh R, Reid K, Scantlebury DM, Lambin X. 2003. Resting and daily energy expenditures of free-living field voles are positively correlated but reflect extrinsic rather than intrinsic effects. Proc. Natl. Acad. Sci. USA 100, 14 057–14 062. ( 10.1073/pnas.2235671100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hill GE. 2011. Condition-dependent traits as signals of the functionality of vital cellular processes. Ecol. Lett. 14, 625–634. ( 10.1111/j.1461-0248.2011.01622.x) [DOI] [PubMed] [Google Scholar]
- 75.Hill GE. 2014. Cellular respiration: the nexus of stress, condition, and ornamentation. Integr. Comp. Biol. 54, 645–657. ( 10.1093/icb/icu029) [DOI] [PubMed] [Google Scholar]
- 76.McCullough EL. 2014. Mechanical limits to maximum weapon size in a giant rhinoceros beetle. Proc. R. Soc. B 281, 20140696 ( 10.1098/rspb.2014.0696) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Price JS, Allen S, Faucheux C, Althnaian T, Mount JG. 2005. Deer antlers: a zoological curiosity or the key to understanding organ regeneration in mammals? J. Anat. 207, 603–618. ( 10.1111/j.1469-7580.2005.00478.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Longair RW. 2004. Tusked Males, male dimorphism and nesting behavior in a subsocial Afrotropical wasp Synagris cornuta, and weapons and dimorphism in the genus (Hymenoptera: Vespidae: Eumeninae). J. Kansas Entomol. Soc. 77, 528–557. ( 10.2317/E-38.1) [DOI] [Google Scholar]
- 79.Trillo PA. 2008. Pre- and post-copulatory sexual selection in the tortoise beetle. PhD dissertation, University of Montana, Missoula, MN. [Google Scholar]
- 80.McCullough EL, Weingarden P, Emlen D. 2012. Costs of elaborate weapons in a rhinoceros beetle: how difficult is it to fly with a big horn? Behav. Ecol. 23, 1042–1048. ( 10.1093/beheco/ars069) [DOI] [Google Scholar]
- 81.Mccullough EL, Tobalske BW. 2013. Elaborate horns in a giant rhinoceros beetle incur negligible aerodynamic costs. Proc. R. Soc. B 280, 933–958. ( 10.1098/rspb.2013.0197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McCullough EL, Emlen DJ. 2013. Evaluating the costs of a sexually selected weapon: big horns at a small price. Anim. Behav. 86, 977–985. ( 10.1016/j.anbehav.2013.08.017) [DOI] [Google Scholar]
- 83.Knell RJ, Pomfret JC, Tomkins JL. 2004. The limits of elaboration: curved allometries reveal the constraints on mandible size in stag beetles. Proc. R. Soc. B 271, 523–528. ( 10.1098/rspb.2003.2641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kram R. 1996. Inexpensive load carrying by rhinoceros beetles. J. Exp. Biol. 612, 609–612. [DOI] [PubMed] [Google Scholar]
- 85.O'Brien DM, Boisseau RP, Duell M, McCullough E, Powell E, Somjee U, Solie S, Painting C, Emlen DJ. In preparation. Muscle mass drives cost in sexually selected arthropod weapons. [DOI] [PMC free article] [PubMed]
- 86.Polilov AA, Makarova AA. 2017. The scaling and allometry of organ size associated with miniaturization in insects: A case study for Coleoptera and Hymenoptera. Sci. Rep. 7, 1–7. ( 10.1038/srep43095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eberhard WG, Wcislo WT. 2011. Grade changes in brain–body allometry: morphological and behavioural correlates of brain size in miniature spiders, insects and other invertebrates. Adv. Insect Physiol. 60, 155e214. [Google Scholar]
- 88.Moen RA, Pastor J, Cohen Y. 1999. Antler growth and extinction of Irish elk. Evol. Ecol. Res. 1, 235–249. [Google Scholar]
- 89.Solberg EJ, Sæther B-E. 1994. Fluctuating asymmetry in the antlers of moose (Alces alces). Proc. R. Soc. Lond. B 254, 251–255. ( 10.1098/rspb.1993.0154) [DOI] [Google Scholar]
- 90.Darwin C. 1871. The descent of man, and selection in relation to sex. London, UK: Murray. [Google Scholar]
- 91.Somjee U, Woods HA, Duell M, Miller CW. 2018. Data from: The hidden cost of sexually selected traits: the metabolic expense of maintaining a sexually selected weapon. Dyrad Digital Repository. ( 10.5061/dryad.757v3h2) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Somjee U, Woods HA, Duell M, Miller CW. 2018. Data from: The hidden cost of sexually selected traits: the metabolic expense of maintaining a sexually selected weapon. Dyrad Digital Repository. ( 10.5061/dryad.757v3h2) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available in Dryad Digital Repository at: https://doi.org/10.5061/dryad.757v3h2 [91].