Abstract
Reactions of (4S,5R)-1-(3,4-Dimethyl-2-oxo-5-phenylimidazolidine)carbonyl-isocyanate (4) with appropriate Baylis-Hillman adducts 5 gave the corresponding acyl carbamates 6,7 as equimolar diastereomeric mixtures. These mixtures were treated with DABCO, to afford with moderate diastereoselection easily separable [2-(3",4"-dimethyl-2"-oxo-5"-phenylimidazolidine-1-carboxamido)(aryl)methyl]acrylates 8 and 9.
Keywords: chiral auxiliary, aza-Baylis-Hillman, rearrangement
Introduction
Biological processes are mainly governed by the interaction of peptides with macromolecular receptors. For these interactions to be successful, the three-dimensional conformation of the peptide chain is of crucial importance, and considerable efforts have been directed towards improving the pharmacological properties of natural peptides by structure modifications of the amino acid constituents [1,2,3]. Thus, in recent years research has largely focused on the incorporation of stereochemically constrained amino acids into peptides, with the aim of effectively reducing the populations of possible peptide chain conformations, enhancing in turn potency, receptor selectivity and pharmacokinetic properties [4,5,6]. In addition, modifications which decrease conformational mobility are useful in order to gain insight into the relationship between the biological activity and the three dimensional topology, new procedures aimed to enforce conformational restrictions in peptides are welcome. In this field, we sought to prepare enantiomerically pure derivatives of α-methylene β-amino acids [7,8,9,10], which can be also obtained by aza-Baylis-Hillman reactions [11,12]. In fact, these compounds deserve interest in their own right due to the conformational restriction of the double bond [13]. In addition, the functionalization of the double bond leads to an α-quaternary centre, also able to induce conformational restrictions [14].
Results and Discussion
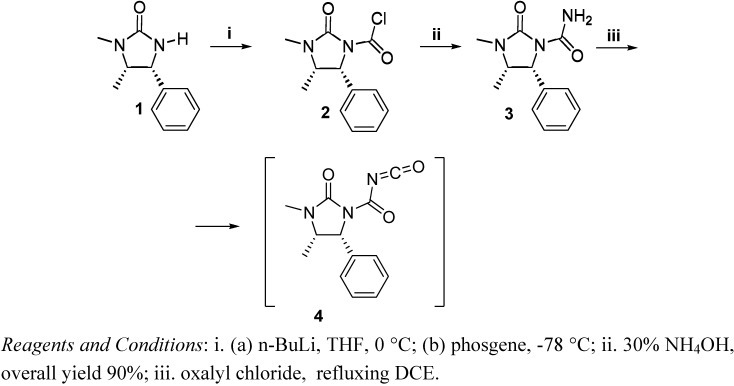
The use of chiral auxiliaries for controlling the stereochemistry of numerous reactions is well established. Thus, in recent years imidazolidin-2-one 1 and its enantiomer, easily obtained starting from either enantiomer of ephedrine [15], were employed as versatile chiral auxiliaries in a lot of synthetically useful reactions [16,17,18]. In fact, on addition of a solution of the lithium anion of the compound 1 in THF to a solution of phosgene in toluene at -78 °C, the corresponding chloride 2 was obtained [18], which was subsequently converted into the derivative 3 by reaction with a 30% solution of ammonia (Scheme 1) [19]. Following the literature method, compound 3 was converted into the corresponding acyl isocyanate 4 [20].
Scheme 1.
Synthesis of (4S,5R)-1-(3,4-Dimethyl-2-oxo-5-phenylimidazolidine)carbonyl-isocyanate (4).
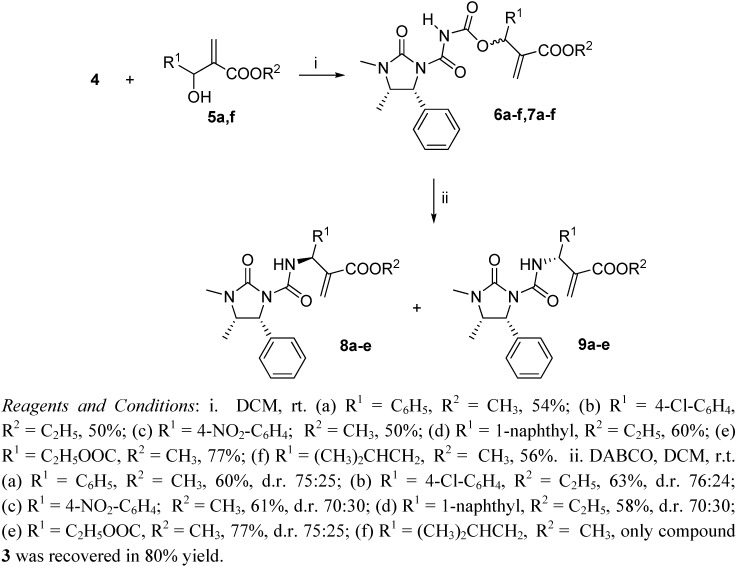
The solution containing compound 4 was immediately treated with the Baylis-Hillman adducts 5a-f, leading in good yield to the acyl carbamates 6,7 as nearly equimolar diastereomeric mixtures. Since these compounds were hard to separate, only small samples could be isolated for analytical purposes and they were assigned as 6 or 7 on the basis of their Rf, exclusively (Scheme 2). Then, the mixtures of acyl carbamates 6,7 were treated with DABCO [21], affording in moderate yield diastereomeric mixtures of compounds 8 and 9 which in turn were easily separated by silica gel chromatography. The configurations at the newly formed stereogenic centre were assigned as S for the major component of the reaction mixture and as R for the minor one, on the basis of both mechanistic considerations and 1H-NMR data.
Scheme 2.
Synthesis of [2-(3",4"-dimethyl-2"-oxo-5"-phenylimidazolidine-1-carbox-amido)(aryl) methyl] acrylates 8 and 9.
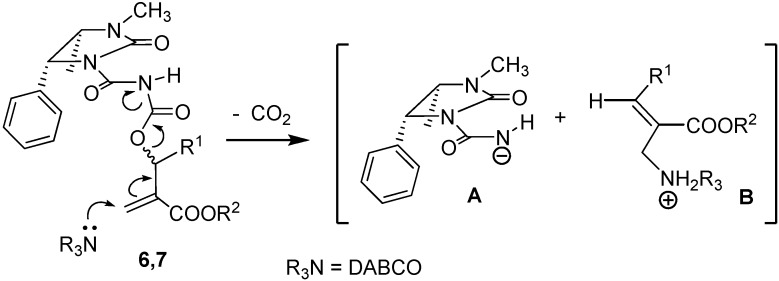
Thus, having observed for the reaction leading to both 8 and 9 an interesting asymmetric induction, that is attractive owing to the easy separation of the diastereomeric products 8 and 9, we first assigned the configuration of the newly formed stereogenic centre on the basis of mechanistic considerations. In fact, in the first step of the reaction of acylcarbamates 6,7, DABCO attacks the conjugate double bond and the first products are the anionic species A and the cationic species B, both resulting from the initial SN′ pathway, involving loss of carbon dioxide, irrespectively from the configuration at C-1' of the starting acyl carbamate (Scheme 3).
Scheme 3.
Formation of the intermediates A and B in the first SN' pathway.
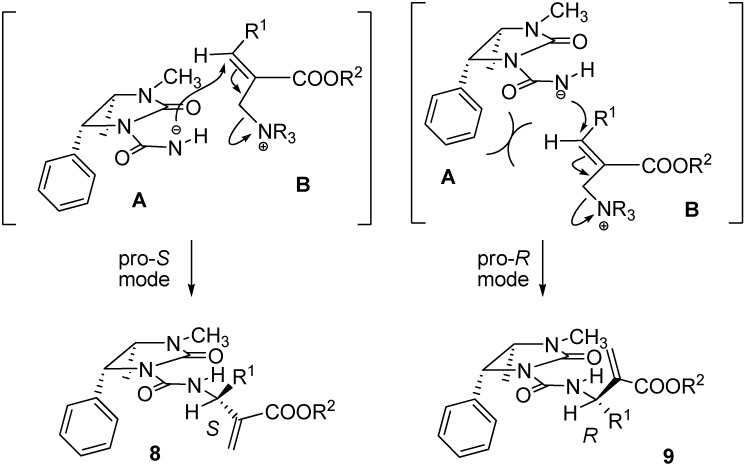
The asymmetric induction arises from the second SN' pathway, in which the anion A attacks the cationic species B leading to the ureas 8 and 9. As it appears from Scheme 4, the attack proceeding via a pro-S mode is free from steric interactions, whereas in the attack occurring in the pro-R mode steric interaction results from the phenyl group and B. Thus, the two transition states leading to 8 and 9, respectively, are different in energy and the less demanding 8 is favoured with respect to 9.
Scheme 4.
Preferential formation of compound 8 due to steric interaction in the second SN' pathway.
Mechanistic considerations are supported also by the chemical shift pattern observed for the olefinic protons for both 8 and 9. In fact, since H-1' in the preferential conformation must be co-planar with the sp2 urea system, the C-3 olefinic protons in 8, displaying the S-configuration at carbon atom of methine group attached to C-2, experience the shielding effect of the phenyl group of the auxiliary, and are observed upfield with respect to the same protons in 9, having the R-configuration, where this effect is missing. On the other hand, the chemical shift of H-1' remains unchanged in both diastereomers (Table 1). Eventually, the configurational assignment at C-1' is in agreement with the Rf values, observed for compounds 8 and 9, these latter being more polar owing to the less compact conformation.
Table 1.
Significant 1H-NMR data for both olefinic protons and H-1' in compounds 8 and 9.
| Compound | δ(Hcis) | δ(Htrans) | δ(H-1') |
|---|---|---|---|
| 8a | 6.29 | 5.82 | 5.26 |
| 9a | 6.36 | 5.91 | 5.28 |
| 8b | 6.31 | 5.81 | 5.26 |
| 9b | 6.37 | 5.89 | 5.27 |
| 8c | 6.38 | 5.92 | 5.26 |
| 9c | 6.43 | 5.97 | 5.28 |
| 8d | 6.38 | 5.90 | 5.28 |
| 9d | 6.46 | 6.00 | 5.32 |
| 8e | 6.31 | 5.85 | 5.26 |
| 9e | 6.37 | 5.92 | 5.28 |
We can also tentatively explain the total lack of reactivity observed for the acylcarbamates 6f and 7f. In this case, the intermediate A surely forms, owing to the recover of compound 3, but further SN' process cannot occur owing to the low electrophilicity of C-3, in which the alkyl group significantly decreases the electron-withdrawing effect of both the ester and the ammonium ion, thus preventing the second reaction.
In conclusion, starting from the Baylis-Hillman adducts 5, we were able to prepare chiral derivatives of the aza-Baylis-Hillman adducts by using two successive SN' steps. These compounds can be further elaborated by removing the chiral auxiliary, leading to monomers useful for preparing new β-foldamers, and work along this line is currently underway.
Experimental
General
Melting points (uncorrected) were measured on a Electrothermal IA 9000 apparatus. NMR spectra (200 MHz for 1H, 50 MHz for 13C, chemical shifts as ppm in the δ scale, coupling constants J in Hertz) were recorded at 25 °C in CDCl3 solutions on a Varian Gemini 200 spectrometer. Diastereomeric ratios were determined with a liquid chromatography Agilent Technologies HP1100 equipped with a Zorbax Eclipse XDB-C8 Agilent Technologies column (flow rate 0.5 mL/min) and equipped with a diode-array UV detector (220 and 254 nm). Enantiomeric purity of all compounds was determined ≥98% by using a Chiralcel OD column. Acetonitrile and methanol for HPLC were purchased from a commercial supplier. Samples were prepared by diluting 1 mg in 5 mL of a 1:1 mixture of H2O and acetonitrile in pure acetonitrile or in pure methanol. The MSD1100 mass detector was utilized under the following conditions: mass range 100-2500 uma, positive scanning, energy of fragmentor 50 V, drying gas flow (nitrogen) 10.0 mL/min, nebulizer pressure 45 psig, drying gas temperature 350 °C, capillary voltage 4500 V. Optical rotations were measured on a Perkin Elmer 341 polarimeter. Column chromatography was performed using Kieselgel 60 Merck (230-400 mesh ASTM).
(4S,5R)-1-(3,4-Dimethyl-2-oxo-5-phenylimidazolidine)carboxamide (3): The imidazolidin-2-one 1 (0.38 g; 2.0 mmol) was dissolved in dry THF (3 mL) and n-BuLi (0.74 mL of 2.7 M solution in hexanes; 2.0 mmol) was added at 0 °C and after 1 h the solution was slowly added dropwise at -78 °C to phosgene (1.98 mL of 1.98 M solution in toluene; 4.0 mmol) to give compound 2, which was not isolated. After 3 h 30% aqueous NH4OH (1.2 mL) was added to the mixture which after 30 min was extracted with DCM (2 × 40 mL). After drying (Na2SO4), the solvent was removed under reduced pressure, to give compound 3 as a white solid (0.42 g; 90% yield), which was used without further purification. 1H-NMR: δ 0.80 (d, J = 6.6 Hz, 3H, 4-CH3), 2.80 (s, 3H, 3-CH3), 3.91 (dq, J = 6.6 Hz, J = 9.3 Hz, 1H, H-4), 4.99 (br s, 1H, NH), 5.27 (d, J = 9.3 Hz, 1H, H-5), 7.13 - 7.17 (m, 2 ArH), 7.22 - 7.38 (m, 3 ArH) 8.11 (br s, 1H, NH); 13C-NMR: δ 14.8, 28.2, 54.5, 59.1, 126.8, 128.1, 128.5, 136.7, 153.1 157.4; [α]D -3.7 (c 0.5, CHCl3) (Lit. [19]: -2.3 c 1, CH2Cl2); MS (ESI): m/z 233.1 [M]+, 256.1 [M+Na]+; Anal. calcd. for C12H15N3O2: C, 61.79; H, 6.48; N, 18.01. Found: C, 61.74; H, 6.54; N, 17.96.
Preparation of derivatives 6 and 7
To a solution containing compound 3 (1.17 g; 5.0 mmol) in dichloroethane (10 mL), oxalyl chloride (0.66 g; 5.2 mmol) was added and the mixture was refluxed for 4 h. The solvent was partially removed and then the Baylis-Hillman adducts 5a-f (3.3 mmol) were added, dissolved in DCM (10 mL). The reaction was stirred for 12 h, volatiles were removed under reduced pressure and the products 6 and 7 were obtained as equimolar mixtures hard to separate by silica gel chromatography (cyclohexane-ethyl acetate 1:1), although small amounts could be obtained pure enough for analytical determinations.
Methyl(1'S,4"S,5"R)-2-[(3",4"-dimethyl-2"-oxo-5"-phenylimidazolidine-1"-carbonylcarbamoyloxy)-(phenyl)methyl]acrylate (6a) and its (1'R,4"S,5"R)-isomer 7a: The title compounds were obtained in 54% overall yield as an equimolar diastereomeric mixture. White solid; MS (ESI): m/z 451.2 [M+], 474.2 [M+Na]+; Anal. Calcd. for C24H25N3O6: C, 63.85; H, 5.58; N, 9.31; Found: C, 63.79; H, 5.54; N, 9.35. Isomer 6a: Rf 0.63 (cyclohexane-AcOEt 1:1); 1H-NMR: δ 0.82 (d, J = 6.6 Hz, 3H, 4"-CH3), 2.82 (s, 3H, 3"-CH3), 3.68 (s, 3H, OCH3), 3.95 (dq, J = 6.6 Hz, J = 8.7 Hz, 1H, H-4"), 5.29 (d, J = 8.7 Hz, 1H, H-5"), 5.97 (bs, 1H, H-1'), 6.41 (s, 1H, =CH2), 6.67 (s, 1H, =CH2), 7.07 - 7.18 (m, 2 ArH), 7.24 - 7.42 (m, 8 ArH), 10.98 (s, 1H, NH); 13C-NMR: δ 14.5, 28.0, 51.9, 54.3, 59.0, 74.4, 126.2, 126.8, 126.9, 127.7, 127.8, 128.3, 128.5, 128.7, 135.6, 137.3, 139.0, 147.2, 149.3, 156.8, 165.2. Isomer 7a: Rf 0.58 (cyclohexane-AcOEt 1:1); 1H-NMR: δ 0.82 (d, J = 6.6, 3H, 4"-CH3), 2.84 (s, 3H, 3"-CH3), 3.68 (s, 3H, OCH3), 3.96 (dq, J = 6.6 Hz, J = 8.8 Hz, 1H, H-4"), 5.31 (d, J = 8.8 Hz, 1H, H-5"), 5.97 (bs s, 1H, H-1'), 6.41 (s, 1H, =CH2), 6.68 (s, 1H, =CH2), 7.07 - 7.18 (m, 2 ArH), 7.23 - 7.44 (m, 8 ArH), 10.97 (s, 1H, NH); 13C-NMR: δ 14.6, 28.0, 51.9, 54.3, 59.1, 74.4, 126.0, 126.8, 126.9, 127.8, 128.4, 128.5, 128.6, 135.6, 139.0, 147.3, 149.3, 156.9, 165.2.
Ethyl(1'S,4"S,5"R)-2-[(3",4"-dimethyl-2"-oxo-5"-phenylimidazolidine-1"-carbonylcarbamoy-loxy)(4-chlorophenyl)methyl]acrylate (6b) and its (1'R,4"S,5"R)-isomer 7b: The title compounds were obtained in 50% overall yield as an equimolar diastereomeric mixture. White solid; MS (ESI): m/z 499.2 [M]+, 522.2 [M+Na]+; Anal. Calcd. for C25H26ClN3O6: C, 60.06; H, 5.24; N, 8.40; Found: C, 60.02; H, 5.19; N, 8.35. Isomer 6b: Rf 0.67 (cyclohexane-AcOEt 1:1); 1H-NMR: δ 0.82 (d, J = 6.7 Hz, 3H, 3"-CH3), 1.19 (t, J = 7.2 Hz, 3H, CH3CH2O), 2.83 (s, 3H, 3"-CH3), 3.96 (dq, J = 6.7 Hz, J = 8.8 Hz, 1H, H-4"), 4.13 (q, J = 7.2 Hz, 2H, CH3CH2O), 5.29 (d, J = 8.8 Hz, 1H, H-5"), 5.98 (s, 1H, H-1'), 6.42 (s, 1H, =CH2), 6.62 (s, 1H, =CH2), 7.07 - 7.17 (m, 2 ArH), 7.21 - 7.86 (m, 8 ArH), 11.00 (s, 1H, NH); 13C-NMR: δ 13.9, 14.6, 28.0, 54.4, 59.1, 61.0, 73.9, 126.0, 126.9, 128.4, 128.5, 128.6, 129.3, 134.3, 135.6, 136.1, 136.9, 147.2, 149.2, 156.9, 164.6. Isomer 7b: Rf 0.61 (cyclohexane-AcOEt 1:1); 1H-NMR: δ 0.83 (d, J = 6.6 Hz, 3H, 3"-CH3), 1.23 (t, J = 7.1 Hz, 3H, CH3CH2O), 2.84 (s, 3H, 3"-CH3), 3.97 (dq, J = 6.6 Hz, J = 8.7 Hz, 1H, H-4"), 4.13 (q, J = 7.1 Hz, 2H, CH3CH2O), 5.30 (d, J = 8.7 Hz, 1H, H-5"), 5.97 (s, 1H, H-1'), 6.41 (s, 1H, =CH2), 6.63 (s, 1H, =CH2), 7.05 - 7.17 (m, 2 ArH), 7.21 - 7.38 (m, 8 ArH), 10.99 (s, 1H, NH); 13C-NMR: δ 14.0, 14.6, 28.1, 54.4, 59.2, 61.0, 73.9, 125.9, 126.9, 128.4, 128.6, 129.4, 134.4, 135.6, 136.0, 139.0, 147.3, 149.3, 156.9, 164.6.
Methyl (1'S,4"S,5"R)-2-[(3",4"-dimethyl-2"-oxo-5"-phenylimidazolidine-1"-carbonylcarbamoyloxy)-(4-nitrophenylmethyl)]acrylate (6c) and its (1'R,4"S,5"R)-isomer (7c): The title compounds were obtained in 50% overall yield as an equimolar diastereomeric mixture. White solid; MS (ESI): m/z 496.2 [M]+, 519.2 [M+Na]+; Anal. Calcd. for C24H24N4O8: C, 58.06; H, 4.87; N, 11.28; Found: C, 58.00; H, 4.84; N, 11.33. Isomer 6c: Rf 0.56 (cyclohexane-AcOEt 1:1); 1H-NMR: δ 0.83 (d, J = 6.6 Hz, 3H, 4"-CH3), 2.84 (s, 3H, 3"-CH3), 3.69 (s, 3H, OCH3), 3.97 (dq, J = 6.6 Hz, J = 8.8 Hz, 1H, H-4"), 5.30 (d, J = 8.8 Hz, 1H, H-5"), 6.08 (d, J = 1.3 Hz, 1H, H-1'), 6.47 (s, 1H, =CH2), 6.70 (s, 1H, =CH2), 7.08 - 7.18 (m, 2 ArH), 7.25 - 7.35 (m, 3 ArH), 7.58 (d, J = 8.9 Hz, 2 ArH), 8.16 (d, J = 8.9 Hz, 2 ArH), 11.09 (s, 1H, NH); 13C-NMR: δ 14.6, 26.9, 28.1, 52.1, 54.4, 59.2, 73.5, 123.7, 126.9, 127.3, 128.5, 128.7, 135.6, 138.1, 144.7, 147.2, 147.9, 149.2, 156.9, 164.8. Isomer 7c: Rf 0.49 (cyclohexane-AcOEt 1:1); 1H-NMR: δ 0.83 (d, J = 6.6 Hz, 3H, 4"-CH3), 2.84 (s, 3H, 3"-CH3), 3.69 (s, 3H, OCH3), 3.98 (dq, J = 6.6 Hz, J = 8.8 Hz, 1H, H-4"), 5.30 (d, J = 8.8 Hz, 1H, H-5"), 6.06 (d, J = 1.1 Hz, 1H, H-1'), 6.46 (s, 1H, =CH2), 6.71 (s, 1H, =CH2), 7.08 - 7.18 (m, 2 ArH), 7.21 - 7.35 (m, 3 ArH), 7.58 (d, J = 8.8 Hz, 2 ArH), 8.17 (d, J = 8.8 Hz, 2 ArH), 11.09 (s, 1H, NH); 13C-NMR: δ 14.6, 26.9, 28.1, 52.1, 54.4, 59.2, 73.5, 123.7, 126.8, 126.9, 127.2, 127.8, 128.1, 128.5, 128.7, 135.5, 138.1, 144.6, 147.2, 147.8, 149.2, 156.9, 164.8.
Ethyl (1'S,4"S,5"R)-2-[(3",4"-dimethyl-2"-oxo-5"-phenylimidazolidine-1"-carbonylcarbamoyloxy)-(naphthalen-1-ylmethyl)]acrylate (6d) and its (1'R,4"S,5"R)-isomer (7d): The title compounds were obtained in 60% overall yield as an equimolar diastereomeric mixture. White solid; MS (ESI): m/z 515.2 [M]+, 538.2 [M+Na]+; Anal. Calcd. for C29H29N3O6: C, 67.56; H, 5.67; N, 8.15; Found: C, 67.51; H, 5.62; N, 8.19. Isomer 6d: Rf 0.62 (cyclohexane-AcOEt 1:1); 1H-NMR: δ 0.80 (d, J = 6.6 Hz, 3H, 4"-CH3), 1.17 (t, J = 7.1 Hz, 3H, CH3CH2O), 2.81 (s, 3H, 3"-CH3), 3.92 (dq, J = 6.6 Hz, J = 8.8 Hz, 1H, H-4"), 4.12 (q, J = 7.1 Hz, 2H, CH3CH2O), 5.28 (d, J = 8.8 Hz, H-5"), 6.02 (d, J = 1.3 Hz, 1H, H-1'), 6.46 (s, 1H, =CH2), 6.85 (s, 1H, =CH2), 7.09 - 7.15 (m, 2 ArH), 7.21 - 7.38 (m, 3ArH), 7.40 - 7.51 (m, 3ArH), 7.74 - 7.87 (m, 4ArH), 11.02 (s, 1H, NH); 13C-NMR: δ 14.0, 14.6, 26.9, 28.0, 54.4, 59.1, 60.9, 74.7, 125.3, 126.1, 126.3, 127.0, 127.4, 127.6, 128.1, 128.2, 128.4, 128.6, 133.0, 133.3, 134.8, 135.7, 139.3, 147.3, 149.4, 156.9, 164.8. Isomer 7d: Rf 0.56 (cyclohexane-AcOEt 1:1); 1H- NMR: δ 0.76 (d, J = 6.6 Hz, 3H, 4"-CH3), 1.18 (t, J = 7.1 Hz, 3H, CH3CH2O), 2.79 (s, 3H, 3"-CH3), 3.90 (dq, J= 6.6 Hz, J = 8.8 Hz, 1H, H-4"), 4.12 (q, J = 7.1 Hz, 2H, CH3CH2O), 5.28 (d, J = 8.8 Hz, 1H, H-5"), 6.05 (s, 1H, H-1'), 6.47 (s, 1H, =CH2), 6.88 (s, 1H, =CH2), 7.05 -7.16 (m, 2 ArH), 7.20 - 7.32 (m, 3 ArH), 7.40 - 7.55 (m, 3 ArH), 7.75 - 7.91 (m, 4 ArH), 11.07 (s, 1H, NH); 13C-NMR: δ 13.8, 14.4, 26.8, 27.9, 54.2, 59.0, 60.8, 74.6, 125.2, 125.8, 126.0, 126.2, 126.8, 127.3, 127.5, 128.1, 128.2, 128.4, 132.9, 133.2, 134.6, 135.6, 139.2, 147.2, 149.3, 156.7, 164.6.
Methyl (1'R,4"S,5"R)-2-[(3",4"-dimethyl-2"-oxo-5"-phenylimidazolidine-1"-carbonylcarbamoyloxy)-(ethoxycarbonylmethyl]acrylate (6e) and its (1'S,4"S,5"R)-isomer (7e): The title compounds were obtained in 77% overall yield as an equimolar diastereomeric mixture. White solid; MS (ESI): m/z 447.2 [M]+, 470.2 [M+Na]+; Anal. Calcd. for C21H25N3O8: C, 56.37; H, 5.63; N, 9.39; Found: C, 56.33; H, 5.57; N, 9.43. Isomer 6e: Rf 0.57 (cyclohexane-AcOEt 1:1); 1H-NMR: δ 0.80 (d, J = 6.6, 3H, 4"-CH3), 1.21 (t, J = 7.0 Hz, 3H, CH3CH2O), 2.80 (s, 3H, 3"-CH3), 3.77 (s, 3H, OCH3), 3.93 (dq, J = 6.6, J = 8.7, 1 H, H-4"), 4.17 (q, J = 7.0 Hz, 2H, CH3CH2O), 5.29 (d, J = 8.7, 1H, H-5"), 5.94 (s, 1H, H-1'), 6.00 (s, 1H, =CH2), 6.47 (s, 1H, =CH2), 7.06 – 7.21 (m, 3 ArH), 7.22 – 7.93 (m, 7 ArH + NH); + NH); 13C-NMR:: δ 14.5, 28.0, 51.9, 54.3, 59.0, 74.4, 126.2, 126.9, 127.7, 127.8, 128.3, 128.5, 128.7, 135.6, 137.3, 139.0, 147.2, 149.3, 156.8, 165.2. Isomer 7e: Rf 0.51 (cyclohexane-AcOEt 1:1); 1H-NMR: δ 0.83 (d, J = 6.6, 3H, 4"-CH3), 1.22 (t, J = 7.0 Hz, 3H, CH3CH2O), 2.81 (s, 3H, 3"-CH3), 3.76 (s, 3H, OCH3), 3.95 (dq, J = 6.6, J = 8.8, 1H, H-4"), 4.19 (q, J = 7.0 Hz, 2H, CH3CH2O), 5.31 (d, J = 8.8, 1H, H-5"), 5.95 (s, 1H, H-1'), 5.99 (s, 1H, =CH2), 6.48 (s, 1H, =CH2), 7.07 – 7.20 (m, 3 ArH), 7.26 – 7.47 (m, 7 ArH + NH); 13C-NMR: δ 14.6, 28.0, 51.9, 54.3, 59.1, 74.4, 126.0, 126.8, 126.9, 127.8, 128.4, 128.5, 128.6, 135.6, 139.0, 147.3, 149.3, 156.8, 165.2.
Methyl (3S,4'S,5'R)-3-(3',4'-dimethyl-2'-oxo-5'-phenylimidazolidine-1'-carbonylcarbamoyloxy)-5-methyl-2-methylenehexanoate (6f) and its (3R,4'S,5'R)-isomer (7f): The title compounds were obtained in 56% overall yield as an equimolar diastereomeric mixture. White solid; MS (ESI): m/z 431.2 [M]+, 454.2 [M+Na]+; Anal. Calcd. for C22H29N3O6: C, 61.24; H, 6.77; N, 9.74. Found: C, 61.18; H, 6.73; N, 9.69. Isomer 6f: Rf 0.53 (cyclohexane-AcOEt 1:1); 1H-NMR: δ 0.81 (d, J = 6.6, 3H, CH3), 0.90 (d, J = 6.6, 3H, CH3), 0.93 (d, J = 6.6, 3H, 4'-CH3), 1.45 – 1.73 (m, 3H, CH-CH2), 2.83 (s, 3H, 3'-CH3), 3.73 (s, 3H, OCH3), 3.96 (dq, J = 6.6, J = 8.8, 1H, H-4'), 5.30 (d, J = 8.8, 1H, H-5'), 5.66 (dd, J = 4.0, J = 8.8, 1H, H-3), 5.82 (s, 1H, =CH2), 6.25 (s, 1H, =CH2), 7.09 – 7.18 (m, 3 ArH), 7.26 – 7.35 (m, 2 ArH + NH); 13C NMR (50 MHz, CDCl3) δ 14.5, 21.6, 23.1, 24.7, 26.8, 29.1, 43.7, 51.8, 54.3, 59.1, 72.0, 125.3, 126.9, 128.3, 128.5, 135.7, 140.3, 147.3, 149.7, 156.9, 165.5. Isomer 7f: Rf 0.44 (cyclohexane-AcOEt 1:1); 1H-NMR: δ 0.79 (d, J = 6.6, 3H, CH3), 0.88 (d, J = 5.3, 3H, CH3), 0.91 (d, J = 5.3, 3H, 4'-CH3), 1.41 - 1.71 (m, 3H, CH-CH2), 2.81 (s, 3H, 3'-CH3), 3.73 (s, 3H, OCH3), 3.95 (dq, J = 5.3, J = 8.8, 1H, H-4'), 5.28 (d, J = 8.8, 1H, ), 5.65 (dd, J = 3.3, J = 8.9, 1H, H-3), 5.79 (s, 1H, =CH2), 6.24 (s, 1H, =CH2), 7.07 – 7.15 (m, 3 ArH), 7.23 – 7.35 (m, 2 ArH + NH); 13C-NMR: δ 14.6, 21.5, 23.1, 24.6, 26.8, 27.9, 43.7, 51.7, 54.3, 59.0, 71.8, 125.0, 126.9, 128.2, 128.5, 135.7, 140.4, 147.3, 149.6, 158.9, 165.5.
Preparation of compounds 8 and 9
To a solution containing an equimolar mixture of acyl carbamates 6 and 7 (3.0 mmol) in dry DCM (8.0 mL), DABCO (0.07 g; 0.6 mmol) was slowly added at r.t. After 20 min, 2 M HCl (10 mL) was added and the mixture was extracted with DCM (2 × 35 mL). After drying (Na2SO4) and removal of the volatiles, the residue was purified by silica gel chromatography (cyclohexane:ethyl acetate 60:40), to give pure compounds 8 and 9.
Methyl (1'S,4"S,5"R)-2-[(3",4"-dimethyl-2"-oxo-5"-phenylimidazolidine-1"-carboxamido)(phenyl)-methyl]acrylate (8a) and its (1'R,4"S,5"R)-isomer (9a): The title compounds were obtained in 60% overall yield as 75:25 diastereomeric mixture. MS (ESI): m/z 407.2 [M]+, 430.2 [M+Na]+; Anal. Calcd. for C23H25N3O4: C, 67.80; H, 6.18; N, 10.31; Found: C, 67.84; H, 6.13; N, 10.27. Isomer 8a: Rf 0.47 (cyclohexane-AcOEt 1:1); White crystals; Mp 50-52 °C; 1H-NMR: δ 0.80 (d, J = 6.6 Hz, 3H, 4"-CH3), 2.80 (s, 3H, 3"-CH3), 3.64 (s, 3H, OCH3), 3.91 (dq, J = 6.6 Hz, J= 8.8 Hz, 1H, H-4"), 5.26 (d, J = 8.7 Hz, 1H, H-1'), 5.82 (s, 1H, =CH2), 5.89 (d, J = 8.8 Hz, 1H, H-5"), 6.29 (s, 1H, =CH2), 7.11 - 7.19 (m, 2 ArH), 7.20 - 7.41 (m, 8 ArH), 9.21 (d, J = 8.7, 1H, NH); 13C-NMR: δ 14.7, 26.9, 28.0, 51.8, 54.5, 54.8, 59.3, 125.7, 126.8, 126.9, 127.4, 128.0, 128.5, 136.8, 140.1, 140.3, 151.6, 157.9, 165.9; [α]D -122.0 (c 0.5, CHCl3). Isomer 9a: Rf 0.27 (cyclohexane-AcOEt 1:1); White crystals; Mp 123-125 °C; 1H-NMR: δ 0.81 (d, J = 6.6 Hz, 3H, 4"-CH3), 2.81 (s, 3H, 3"-CH3), 3.66 (s, 3H, OCH3), 3.94 (dq, J = 6.6 Hz, J = 8.8 Hz, 1H, H-4"), 5.28 (d, J = 8.5 Hz, 1H, H-1'), 5.89 (d, J = 8.8 Hz, 1H, H-5"), 5.91 (s, 1H, =CH2), 6.36 (s, 1H, =CH2), 7.08 - 7.17 (m, 2 ArH), 7.20 - 7.39 (m, 8 ArH), 9.15 (d, J = 8.5 Hz, 1H, NH); 13C- NMR: δ 14.8, 26.9, 28.0, 51.8, 54.6, 54.9, 59.4, 126.1, 126.7, 126.8, 127.0, 127.3, 127.8, 128.1, 128.3, 128.5, 128.6, 136.8, 139.6, 140.6, 151.8, 157.9, 165.9; [α]D 93.0 (c 0.5, CHCl3).
Ethyl (1'S,4"S,5"R)-2-[(3",4"-dimethyl-2"-oxo-5"-phenylimidazolidine-1"-carboxamido)(4-chloro-phenyl)methyl]acrylate (8b) and its (1'R,4"S,5"R)-isomer (9b): The title compounds were obtained in 63% overall yield as 76:24 diastereomeric mixture. MS (ESI): m/z 455.2 [M] +, 478.2 [M+Na]+; Anal. Calcd. for C24H26ClN3O4: C, 63.22; H, 5.75; N, 9.22; Found: C, 63.17; H, 5.71; N, 9.26. Isomer 8b: Rf 0.47 (cyclohexane-AcOEt 1:1); White crystals; Mp 46-48 °C; 1H-NMR: δ 0.80 (d, J = 6.6 Hz, 3H, 4"-CH3), 1.16 (t, J = 7.2 Hz, 3H, CH3CH2O), 2.80 (s, 3H, 3"-CH3), 3.94 (dq, J = 6.6 Hz, J= 8.8 Hz, 1H, H-4"), 4.09 (50% q, J = 7.2 Hz, 2H, CH3CH2O), 4.10 (50% q, J = 7.2 Hz, 2H, CH3CH2O), 5.26 (d, J = 8.3 Hz, 1H, H-1'), 5.81 (s, 1H, =CH2), 5.86 (d, J = 8.8 Hz, 1H, H-5"), 6.31 (s, 1H, =CH2), 7.10 - 7.18 (m, 2 ArH), 7.22 - 7.38 (m, 7 ArH), 9.22 (d, J = 8.3 Hz, 1H, NH); 13C-NMR: δ 13.9, 14.8, 26.9, 28.1, 54.4 (50%), 54.6 (50%), 59.3, 61.0, 125.9, 126.8, 128.1, 128.3, 128.4, 128.5, 128.6, 133.2, 136.8, 139.0, 140.2, 151.7, 157.9, 165.3; [α]D -125.0 (c 0.5, CHCl3). Isomer 9b: Rf 0.29 (cyclohexane-AcOEt 1:1); Low melting white solid; 1H-NMR: δ 0.80 (d, J = 6.6 Hz, 3H, 4"-CH3), 1.17 (t, J = 7.2 Hz, 3H, CH3CH2O), 2.80 (s, 3H, 3"-CH3), 3.93 (dq, J = 6.6 Hz, J = 8.8 Hz, 1H, H-4"), 4.10 (q, J = 7.2 Hz, 3H, CH3CH2O), 5.26 (d, J = 8.8 Hz, 1H, H-1'), 5.86 (d, 1H, J = 8.8 Hz, 1H, H-5"), 5.89 (s, 1H, =CH2), 6.37 (s, 1H, =CH2), 7.09 - 7.18 (m, 2 ArH), 7.19 - 7.38 (m, 7 ArH), 9.17 (d, J = 8.8, 1H, NH); 13C-NMR: δ 13.9, 14.8, 28.0, 29.7, 54.4 (50%), 54.6 (50%), 59.4, 60.9, 126.4, 126.8, 128.1, 128.2, 128.5, 133.1, 136.8, 138.5, 140.3, 151.8, 157.9, 165.3; [α]D 155.0 (c 0.5, CHCl3).
Methyl (1'S,4"S,5"R)-2-[(3",4"-dimethyl-2"-oxo-5"-phenylimidazolidine-1"-carboxamido)(4-nitro-phenyl)methyl]acrylate (8c) and its (1'R,4"S,5"R)-isomer (9c): The title compounds were obtained in 61% overall yield as 70:30 diastereomeric mixture. MS (ESI): m/z 452.2 [M]+, 475.2 [M+Na]+; Anal. Calcd. for C23H24N4O6: C, 61.05; H, 5.35; N, 12.38; Found: C, 61.00; H, 5.31; N, 12.35. Isomer 8c: Rf 0.50 (cyclohexane-AcOEt 1:1); White crystals; Mp. 55-57 °C; 1H-NMR: δ 0.82 (d, J = 6.6 Hz, 3H, 4"-CH3), 2.83 (s, 3H, 3"-CH3), 3.68 (s, 3H, OCH3), 3.95 (dq, J = 6.6 Hz, J = 8.8 Hz, 1H, H-4"), 5.26 (d, J = 8.7 Hz, 1H, H-1'), 5.92 (s, 1H, =CH2), 5.95 (d, J = 8.8 Hz, 1H, H-5"), 6.38 (s, 1H, =CH2), 7.10 - 7.22 (m, 2 ArH), 7.25 - 7.41 (m, 3 ArH), 7.51 (d, J= 8.5 Hz, 2 ArH), 8.16 (d, J = 8.5 Hz, 2 ArH), 9.44 (d, J = 8.7 Hz, 1H, NH); 13C-NMR: δ 14.8, 28.1, 52.1 (50%), 52.2 (50%), 54.6 (50%), 54.8 (50%), 59.4, 76.4, 123.8, 126.8, 127.5, 127.8, 128.2, 128.6, 136.7, 139.1, 147.8, 151.9, 157.9, 165.5; [α]D -107.0 (c 0.5, CHCl3). Isomer 9c: Rf 0.20 (cyclohexane-AcOEt 1:1); White crystals; Mp. 62-64 °C; 1H-NMR: δ 0.83 (d, J = 6.6 Hz, 3H, 4"-CH3), 2.83 (s, 3H, 3"-CH3), 3.70 (s, 3H, OCH3), 3.96 (dq, J = 6.6 Hz, J = 8.8 Hz, 1H, H-4"), 5.28 (d, J = 8.8 Hz, 1H, H-1'), 5.97 (s, 1H, =CH2), 5.97 (d, J = 8.5 Hz, 1H, H-5"), 6.43 (s, 1H, =CH2), 7.11 - 7.18 (m, 2 ArH), 7.25 - 7.38 (m, 3 ArH), 7.44 (d, J = 8.5 Hz, 2 ArH), 8.13 (d, J = 8.5 Hz, 2 ArH), 9.37 (d, J = 8.8 Hz, 1H, NH); 13C-NMR: δ 14.8, 28.1, 52.1, 54.6 (50%), 54.8 (50%), 59.5, 76.4, 123.7, 126.8, 127.4, 128.0, 128.3, 128.6, 136.7, 139.3, 147.4, 152.0, 156.2 (50%), 157.8 (50%), 165.4; [α]D 78.9 (c 0.5, CHCl3).
Ethyl (1'S,4"S,5"R)-2-[(3",4"-dimethyl-2"-oxo-5"-phenylimidazolidine-1"-carboxamido) (naphthalen-1-yl)methyl]acrylate (8d) and its (1'R,4"S,5"R)-isomer (9d): The title compounds were obtained in 58% overall yield as 70:30 diastereomeric mixture. MS (ESI): m/z 471.2 [M]+, 494.2 [M+Na]+; Anal. Calcd. for C28H29N3O4: C, 71.32; H, 6.20; N, 8.91. Found: C, 71.36; H, 6.15; N, 8.87. Isomer 8d: Rf 0.50 (cyclohexane-AcOEt 1:1); White crystals; Mp. 53-55 °C; 1H-NMR: δ 0.79 (d, J = 6.6 Hz, 3H, 4"-CH3), 1.13 (t, J = 7.1 Hz, 3H, CH3CH2O), 2.79 (s, 3H, 3"-CH3), 3.88 (dq, J = 6.6 Hz, J = 8.8 Hz, 1H, H-4"), 4.08 (q, J = 7.1 Hz, 2H, CH3CH2O), 5.28 (d, J = 8.5 Hz, 1H, H-1'), 5.90 (s, 1H, =CH2), 6.10 (d, J = 8.8 Hz, 1H, H-5"), 6.38 (s, 1H, =CH2), 7.15 - 7.21 (m, 2 ArH), 7.25 - 7.52 (m, 6 ArH), 7.74 - 7.86 (m, 4 ArH), 9.31 (d, J = 8.5, 1H, NH); 13C-NMR: δ 13.9, 14.7, 28.0, 54.5, 55.0, 59.3, 60.8, 125.3, 125.6, 125.7, 125.8, 126.0, 126.8, 127.4, 127.6, 127.7, 128.0, 128.3, 128.5, 132.8, 133.4, 137.0, 137.8, 140.6, 151.7, 157.9, 165.5; [α]D -106.0 (c 0.5, CHCl3). Isomer 9d: Rf 0.35 (cyclohexane-AcOEt 1:1); White crystals; Mp. 56-57 °C; 1H-NMR: δ 0.79 (d, J = 6.6 Hz, 3H, 4"-CH3), 1.16 (t, J = 7.1 Hz, 3H, CH3CH2O), 2.80 (s, 3H, 3"-CH3), 3.91 (dq, J = 6.6 Hz, J = 8.8 Hz, 1H, H-4"), 5.32 (d, J = 8.8 Hz, 1H, H-1'), 6.00 (s, 1H, =CH2), 6.11 (d, J = 8.7 Hz, 1H, H-5"), 6.46 (s, 1H, =CH2), 7.14 - 7.20 (m, 2 ArH), 7.24 - 7.33 (m, 3 ArH), 7.39 - 7.49 (m, 3 ArH), 7.73 - 7.84 (m, 4 ArH), 9.29 (d, J = 8.7 Hz, 1H, NH); 13C-NMR: δ 13.8, 4.6, 27.9, 54.4, 54.8, 59.3, 60.7, 124.9, 125.4, 125.7, 125.9, 126.0, 126.7, 127.4, 127.9, 128.1, 128.4, 128.6, 132.6, 133.2, 136.8, 137.2, 140.7, 151.8 157.8, 165.4; [α]D 54.0 (c 0.5, CHCl3).
Methyl (1'R,4"S,5"R)-2-[(3",4"-dimethyl-2"-oxo-5"-phenylimidazolidine-1"-carboxamido) (ethoxy-carbonyl)methyl]acrylate (8e) and its (1'S,4"S,5"R)-isomer (9e): The title compounds were obtained in 77% overall yield as 75:25 diastereomeric mixture. MS (ESI): m/z 403.2 [M]+, 426.2 [M+Na]+; Anal. Calcd. for C20H25N3O6: C, 59.54; H, 6.25; N, 10.42; Found: C, 59.50; H, 6.21; N, 10.46. Isomer 8e: Rf 0.35 (cyclohexane-AcOEt 1:1); White crystals; Mp. 78-80 °C; 1H-NMR: δ 0.78 (d, J = 6.6 Hz, 3H, 4"-CH3), 1.21 (t, J = 7.1 Hz, 3H, CH3CH2O), 2.80 (s, 3H, 3"-CH3), 3.78 (s, 3H, OCH3), 3.88 (dq, J = 6.6 Hz, J = 8.4 Hz, 1H, H-4"), 4.15 (q, J = 7.1 Hz, 2H, CH3CH2O), 5.26 (d, J = 8.7 Hz, 1H, H-1'), 5.33 (d, J = 8.4 Hz, 1H, H-5"), 5.85 (s, 1H, =CH2), 6.31 (s, 1H, =CH2), 7.09 - 7.18 (m, 2 ArH), 7.22 - 7.35 (m, 3 ArH), 9.22 (d, J = 8.7 Hz, 1H, NH); 13C-NMR: δ 14.0, 14.7, 28.1, 52.1, 54.5, 54.6, 59.2, 61.7, 126.8, 128.0, 128.4, 129.3, 136.5, 136.8, 152.2, 157.6, 165.5, 169.7; [α]D 17.8 (c 0.5, CHCl3). Isomer 9e: Rf 0.25 (cyclohexane-AcOEt 1:1); Low melting white solid; 1H-NMR: δ 0.80 (d, J = 6.6 Hz, 3H, 4"-CH3), 1.21 (t, J = 7.1 Hz, 3H, CH3CH2O), 2.81 (s, 3H, 3"-CH3), 3.79 (s, 3H, OCH3), 3.91 (dq, J = 6.6 Hz, J= 8.8 Hz, 1H, H-4"), 4.18 (q, J = 7.1 Hz, 2H, CH3CH2O), 5.23 (d, J = 8.8 Hz, 1H, H-5"), 5.28 (d, J = 8.2 Hz, 1H, H-1'), 5.92 (s, 1H, =CH2), 6.37 (s, 1H, =CH2), 7.10 - 7.21 (m, 2 ArH), 7.25 - 7.38 (m, 3 ArH), 9.25 (d, J = 8.2 Hz, 1H, NH); 13C-NMR: δ 14.0, 14.8, 28.1, 52.1, 54.5, 54.8, 59.4, 61.8, 126.9, 128.1, 128.5, 129.6, 136.8, 152.1, 157.6, 165.5, 169.5; [α]D - 41.7 (c 0.5, CHCl3).
Acknowledgements
We thank Diatech s.r.l. and Nanodream s.r.l., Iesi (Ancona, Italy) for financial support.
Footnotes
Sample Availability: Samples of all the compounds are available from the authors.
References and Notes
- 1.Martin S.F., Dwyer M.P., Hartmann B., Knight K.S. Cyclopropane-derived peptidomimetics. Design, synthesis, and evaluation of novel enkephalin analogues. J. Org. Chem. 2000;65:1305–1318. doi: 10.1021/jo991288h. [DOI] [PubMed] [Google Scholar]
- 2.Wang S., Tang X., Hruby V.J. First stereoselective synthesis of an optically pure β-substituted histidine: (2S,3S)-β-methylhistidine. Tetrahedron Lett. 2000;41:1307–1310. [Google Scholar]
- 3.Hruby V.J., Balse P.M. Conformational and topographical considerations in designing agonist peptidomimetics from peptide leads. Curr. Med. Chem. 2000;7:945–970. doi: 10.2174/0929867003374499. [DOI] [PubMed] [Google Scholar]
- 4.Goodman M., Moroder L., Toniolo C., editors. Peptides and Peptidomimetics. Houben-Weyl E22c; Thieme Verlag; Stuttgart, Germany: 2004. [Google Scholar]
- 5.Che Y., Brooks B.R., Marshall G.R. Protein recognition motifs: Design of peptidomimetics of helix surfaces. Biopolymers. 2007;86:288–297. doi: 10.1002/bip.20744. [DOI] [PubMed] [Google Scholar]
- 6.Sillerud L.O., Larson L.S. Design and structure of peptide and peptidomimetic antagonists of protein-protein interaction. Curr. Prot. Pept. Sci. 2005;6:151–169. doi: 10.2174/1389203053545462. [DOI] [PubMed] [Google Scholar]
- 7.Schmid N., Zagrovic B., van Gunsteren W.F. Folding-unfolding equilibrium ofmethylidene - substituted β-peptide. Helv. Chim. Acta. 2007;90:1966–1979. [Google Scholar]
- 8.Abele S., Seebach D. Preparation of achiral and enantiopure geminally disubstituted β-amino acids for β-peptide synthesis. Eur. J. Org. Chem. 2000:1–15. doi: 10.1002/(SICI)1099-0690(200001)2000:1<1::AID-EJOC1>3.0.CO;2-6. [DOI] [Google Scholar]
- 9.Abele S., Seiler P., Seebach D. Synthesis, crystal structures, and modelling of β-oligopeptides consisting of 1-(aminomethyl)cyclopropanecarboxylic acid: ribbon-type arrangement of eight-membered H-bonded rings. Helv. Chim. Acta. 1999;82:1559–1571. doi: 10.1002/(SICI)1522-2675(19991006)82:10<1559::AID-HLCA1559>3.0.CO;2-A. [DOI] [Google Scholar]
- 10.Seebach D., Abele S., Sifferlen T., Hänggi M., Gruner S., Seiler P. Preparation and structure of β-peptides consisting of geminally disubstituted β2,2- and β3,3-amino acids: a turn motif for β−peptides. Helv. Chim. Acta. 1998;81:2218–2243. doi: 10.1002/(SICI)1522-2675(19981216)81:12<2218::AID-HLCA2218>3.0.CO;2-0. [DOI] [Google Scholar]
- 11.Shi Y.-L., Shi M. Aza-Baylis-Hillman reactions and their synthetic applications. Eur. J. Org. Chem. 2007:2905–2916. [Google Scholar]
- 12.Declerck V., Martinez J., Lamaty F. Aza-Baylis−Hillman reaction. Chem. Rev. 2009;109:1–48. doi: 10.1021/cr068057c. [DOI] [PubMed] [Google Scholar]
- 13.Bierbaum D.J., Seebach D. Synthesis and spectroscopic characterization of β-di-, β-tri-, and β-hexapeptides built with (S)-2-methylene-3-aminoalkanoic acids derived from alanine, valine, and leucine. Aust. J. Chem. 2004;57:859–863. [Google Scholar]
- 14.Galeazzi R., Martelli G., Mobbili G., Orena M., Rinaldi S. Stereoselective iodocyclization of 3-acylamino-2-methylene alkanoates: synthesis of analogues of N-benzoyl-syn-phenylisoserine. Org. Lett. 2004;6:2571–2574. doi: 10.1021/ol049146j. [DOI] [PubMed] [Google Scholar]
- 15.Close W. J. The conformation of the ephedrines. J. Org. Chem. 1950;15:1131–1134. doi: 10.1021/jo01151a034. [DOI] [Google Scholar]
- 16.Cardillo G., D'Amico A., Orena M., Sandri S. Diastereoselective alkylation of 3-acylimidazolidin-2-ones: synthesis of (R)- and (S)-lavandulol. J. Org. Chem. 1988;53:2354–2356. [Google Scholar]
- 17.Cardillo G., Orena M., Romero M., Sandri S. Enantioselective synthesis of 2-benzyloxyalcohols and 1,2-diols via alkylation of chiral glycolate imides. A convenient approach to optically active glycerol derivatives. Tetrahedron. 1989;45:1501–1508. [Google Scholar]
- 18.Cardillo B., Galeazzi R., Mobbili G., Orena M., Rossetti M. Diastereoselective hetero-Diels-Alder cycloaddition of a C-nitroso compound prepared starting from a homochiral imidazolidin-2-one. Tetrahedron Asymmetry. 1994;5:1535–1540. [Google Scholar]
- 19.Roos G.H.P., Balasubramaniam S. Synthesis of α-amino phosphonates under diastereocontrol by imidazolidin-2-one auxiliaries. Synth. Comm. 1998;28:3877–3884. [Google Scholar]
- 20.Speziale A.J., Smith L.R. The reaction of oxalyl chloride with amides. II. Oxazolidinediones and acyl isocyanates. J. Org. Chem. 1963;28:1805–1811. doi: 10.1021/jo01042a016. [DOI] [Google Scholar]
- 21.Ciclosi M., Fava C., Galeazzi R., Orena M., Sepulveda-Arques J. Synthesis of unsaturated β-amino acid derivatives from carbamates of the Baylis–Hillman products. Tetrahedron Lett. 2002;43:2199–2202. [Google Scholar]