Abstract
Plant defense inducers that mimic functions of the plant immune hormone salicylic acid (SA) often affect plant growth. Although benzothiadiazole (BTH), a synthetic analog of SA, has been widely used to protect crops from diseases by inducing plant defense responses, we recently demonstrated that SA, but not BTH, confers resistance against Rhizoctonia solani, the causal agent of sheath blight disease, in Brachypodium distachyon. Here, we demonstrated that BTH compromised the resistance of Bd3-1 and Gaz4, the two sheath blight-resistant accessions of B. distachyon, which activate SA-dependent signaling following challenge by R. solani. Moreover, upon analyzing our published RNA-seq data from B. distachyon treated with SA or BTH, we found that BTH specifically induces expression of genes related to chloroplast function and jasmonic acid (JA) signaling, suggesting that BTH attenuates R. solani resistance by perturbing growth-defense trade-offs and/or by inducing a JA response that may increase susceptibility to R. solani. Our findings demonstrated that BTH does not work as a simple mimic of SA in B. distachyon, and consequently may presumably cause unfavorable side effects through the transcriptional alteration, particularly with respect to R. solani resistance.
Introduction
Salicylic acid (SA) is a phytohormone that plays a central role in plant disease resistance against fungi, oomycetes, bacteria, and viruses1. It is rapidly biosynthesized in response to pathogen challenges and invokes various defense responses, including hypersensitive response, defense gene expression, phytoalexin biosynthesis, and systemic acquired resistance1. Mutations in genes involved in SA biosynthesis or in the signaling pathway downstream of SA enhance the susceptibility of plants to diseases2,3. Exogenous application of SA also induces defense responses and confers disease resistance to treated plants. The effects of SA on plant disease resistance often depend on the plant species and the pathogen’s lifestyle. For examples, in tomato, SA increases susceptibility to the necrotrophic pathogen Botrytis cinerea by antagonizing the signaling pathway of jasmonic acid (JA), another defense-related phytohormone4, whereas in Arabidopsis, both SA and JA are required for B. cinerea resistance5. To capitalize on the properties of SA, chemical inducers of the SA-signaling pathway have been developed and used as plant defense inducers in practical crop protection6,7. Benzothiadiazole (BTH), also referred to as acibenzolar-S-methyl, is a synthetic chemical analog of SA that has been used for protection against diseases in various agronomically important crops, such as rice, wheat, potato, and tomato8–11. BTH is believed to mimic the effects of SA, because transcriptional changes induced by SA closely mirror those induced by BTH, which are both under the control of Nonexpressor of Pathogenesis-Related 1 (NPR1), a master regulator of the SA-signaling pathway, in Arabidopsis12,13.
Care needs to be taken with respect to the use of plant defense inducers because SA has been shown to affect plant growth14. In particular, marked activation of the defense responses induced by SA is often associated with negative effects on plant growth. For example, cpr and slh1 mutants of Arabidopsis, which accumulate high levels of SA, show severe growth defects15,16. Moreover, high doses of SA and related plant defense inducers negatively affect photosynthesis, growth, and seed production in plants17,18. Conversely, Arabidopsis mutants and transgenic lines that are deficient in endogenous SA show increased growth and seed production19. These examples demonstrate that growth-defense trade-offs often influence plant defense responses resulting from the allocation of limited resources. Because plant defense requires massive amounts of energy to regulate the various cellular processes involved in immune responses, plants presumably deploy a trade-off system between defense and growth to effectively combat pathogens using limited resources20,21.
Sheath blight, caused by the soil-borne plant pathogenic fungus Rhizoctonia solani AG-1, IA, is one of the most destructive diseases of cultivated rice22. It occurs in areas of rice production areas worldwide and is particularly prevalent in the paddy fields of East Asia as well as the southern United States22. We previously reported that SA-dependent plant immunity contributes to sheath blight resistance in rice and the small model grass species Brachypodium distachyon23. Exogenous SA treatment induced resistance to R. solani resistance in a sheath blight-susceptible B. distachyon accession, Bd21. In contrast, treatment with JA enhanced the susceptibility of Bd21 to R. solani. Surprisingly, treatment with BTH could not increase the level of R. solani resistance in Bd2123. Our comparative transcriptome analysis of Bd21 leaves after treatment with SA or BTH revealed that their transcriptional changes involved a number of common genes but also included very large differences although the corresponding effects on gene expression in Arabidopsis following treatment with either SA or BTH were very similar13. Specifically, SA induced the expression of 89 genes that were not induced by BTH in B. distachyon, including a number of genes related to secondary cell wall (SCW) biosynthesis. SCW reinforcement, such as lignification and suberization, acts as a physical barrier that prevents the access of pathogens to host cells24. Therefore, we speculated that the lack of transcriptional activation of SCW-related genes may explain why BTH cannot confer R. solani resistance in B. distachyon23. On the other hand, the number of differentially expressed genes (DEGs) in B. distachyon following treatment with BTH was 2.8 times greater than that with SA, and BTH specifically regulated the expression of more than 1,300 genes at the same BTH concentration as did SA23. Of the DEGs for SA, 82% were also regulated by BTH but this figure corresponds to only 29% of the total number of DEGs detected following treatment with BTH23. These findings indicate that, in B. distachyon, BTH affects various other cellular responses in addition to the SA-dependent signaling in B. distachyon. It is also possible that the BTH-specific DEGs include genes that negatively regulate resistance of B. distachyon to R. solani.
In this study, we further investigated the reasons why SA, but not BTH, induces resistance to R. solani in B. distachyon. We found that BTH treatment induced susceptibility to R. solani in two sheath blight-resistant accessions of B. distachyon, indicating that BTH-induced cellular responses included negative impacts on R. solani resistance. Analysis of our previously published RNA-seq data of B. distachyon treated with SA or BTH identified potential host cellular responses that were induced by BTH that could attenuate the defense responses to R. solani. Our results demonstrated that, whereas BTH works as a simple mimic of SA in Arabidopsis, this is not the situation in B. distachyon, with BTH treatment of B. distachyon being associated with unfavorable side effects, particularly with respect to R. solani resistance.
Results
BTH does not induce R. solani resistance in the sheath blight-susceptible B. distachyon accession Bd21 at low concentrations
In our previous study23, we demonstrated that treatment of B. distachyon accession Bd21 with 500 μM BTH treatment did not induce resistance to R. solani. To investigate whether this lack of effect was due to the high BTH concentration used, we assessed the effect of a range of low concentrations of BTH on resistance to R. solani. We treated detached Bd21 leaves with 1, 10, 100, or 500 μM BTH and 24 h later inoculated them with R. solani. The results showed that BTH did not induce resistance at any of the concentrations tested (Supplementary Fig. S1a,b), although the expression of a SA-marker gene BdWRKY45L125 was induced even at the lowest concentration of 1 μM (Supplementary Fig. S1c). These results indicate that the absence of an ability of BTH to induce R. solani resistance is not due to a side effect observed only at a high BTH concentration.
BTH compromises R. solani resistance in two sheath blight-resistant accessions of B. distachyon
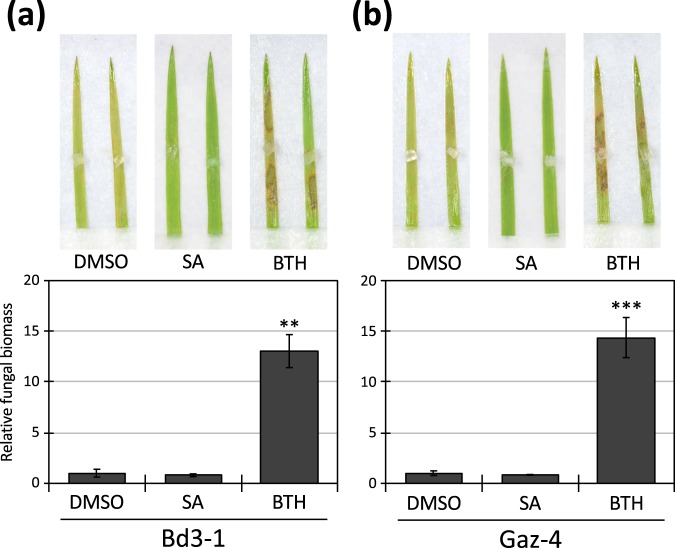
To investigate the cause of the difference between SA and BTH in terms of inducing resistance to R. solani, we used B. distachyon accessions Bd3-1 and Gaz-4. Bd21 is susceptible to R. solani, whereas Bd3-1 and Gaz-4 are resistant to this pathogen and activate SA-dependent signaling following R. solani inoculation23. The detached leaves of Bd3-1 and Gaz-4 were treated with SA or BTH and subsequently inoculated with R. solani. We found that SA treatment did not significantly affect the R. solani resistance in either accession at 3 d post-inoculation (dpi) (Fig. 1). This suggests that R. solani resistance in these accessions could be conferred by a gene set similar to those induced by SA treatment or that SA-induced responses may be able to work additively with the R. solani resistance in these accessions. Intriguingly, BTH treatment compromised R. solani resistance in both accessions, as evidenced by increases in both lesion formation and foliar fungal biomass (Fig. 1). To test whether BTH directly promoted fungal growth, we measured the diameter of colonies of R. solani on PDA medium containing either SA or BTH. As we reported earlier23, SA did not affect the growth of R. solani on PDA medium, but BTH inhibited its growth under our experimental conditions (Supplementary Fig. S2). BTH has been shown to have no antimicrobial activity against various plant pathogenic fungi in vitro8, so these observed effects on colony growth may be due to the experimental conditions. Indeed, BTH itself did not promote hyphal growth of R. solani on PDA medium, indicating that some of the cellular responses in B. distachyon triggered by BTH act negatively on the R. solani resistance even in accessions that express the SA-dependent defense phenotype against R. solani, such as Bd3-1 and Gaz-4.
Figure 1.
BTH compromises Rhizoctonia solani resistance in Brachypodium distachyon accessions Bd3-1 and Gaz-4. Detached leaves of B. distachyon Bd3-1 and Gaz-4 were spray-treated with 0.5% (v/v) DMSO, 0.5 mM SA, or 0.5 mM BTH and 24 h later inoculated with R. solani mycelial plugs (2–3 mm3). Lesion formation (upper panel) and relative biomass of R. solani (lower panel) in (a) Bd3-1 and (b) Gaz-4 leaves were evaluated at 3 d post-inoculation. Data are presented as means ± SEM of values relative to the DMSO treatment, n = 9; **P < 0.01, ***P < 0.001 using Student’s t-tests. The experiments were performed twice with similar results and a representative result is shown.
BTH induces biological functions related to chloroplast properties and jasmonic acid response in B. distachyon
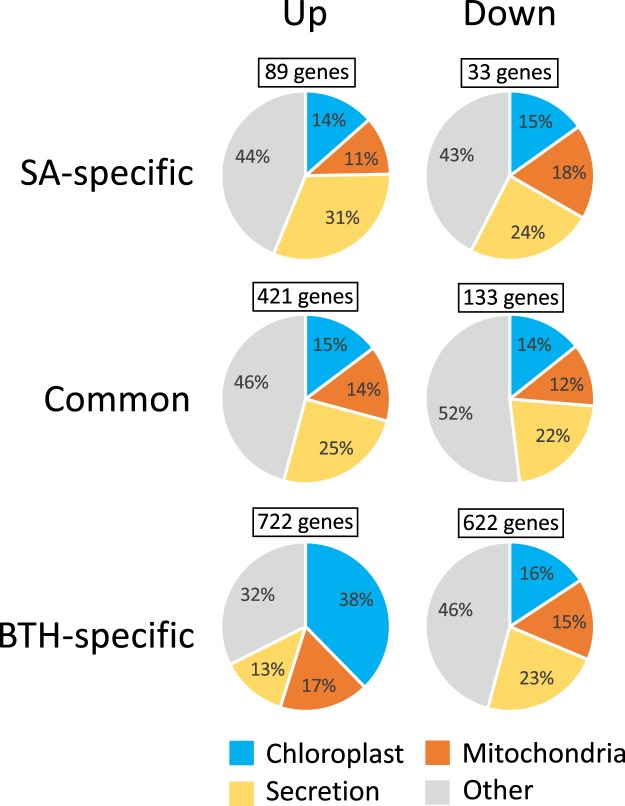
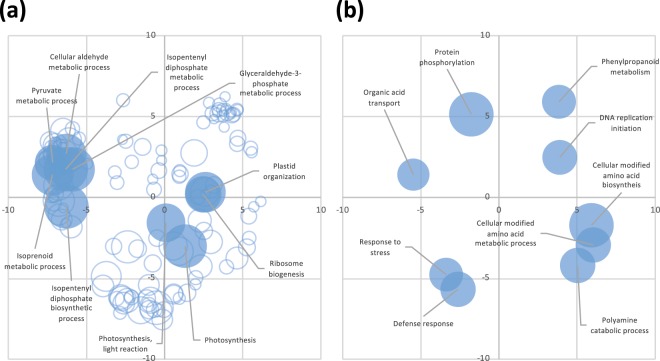
To understand which host responses account for the suppressive effect of BTH on R. solani resistance, we analyzed the published RNA-seq data from B. distachyon leaves of the sheath blight-susceptible accession Bd21 treated with SA or BTH for 24 h (DDBJ accession no. DRP003562)23. In our previous study, we had classified the DEGs in response to SA or BTH into six gene sets: SA-specific genes, commonly regulated genes (i.e., SA and BTH), and BTH-specific genes, with each subdivided into either up- or down-regulated genes (Fig. 2). In the current study, we further subdivided each set by the subcellular localizations of the proteins that they encode, which were predicted using the TargetP program26. A comparison of the proportions determined by these analyses clearly demonstrated that the BTH-specific upregulated genes contained a larger number of genes encoding chloroplast-targeted proteins (38% of the BTH-specific genes) than did the other gene sets (14–16%) (Fig. 2). No characteristic patterns with respect to subcellular localization were found in the BTH-specific downregulated genes (Fig. 2). In addition, we performed a Gene Ontology (GO) enrichment analysis for the BTH-specific upregulated and downregulated genes and identified 323 and 36 significantly over-represented GO terms, respectively, when applying a threshold false discovery rate (FDR) value lower than 0.05 (Supplementary Tables S1 and S2). To represent the functional properties of the BTH-specific genes, the markedly over-represented GO terms categorized to biological process were summarized and depicted in scatter plots based on their semantic similarities (Fig. 3), using REVIGO27. Consistent with the high proportion of chloroplast-localized proteins shown in Fig. 2, GO terms related to chloroplast properties, such as photosynthesis (GO:0015979, FDR value: 5.3E−25; GO:0019684, FDR value: 8.5E−20) and the non-mevalonate pathway (GO:0046490, FDR value: 5.3E−26; GO:0009240, FDR value: 5.3E−26; GO:0019682, FDR value: 5.3E−26; GO:0006090, FDR value: 8.8E−17) were over-represented in the BTH-specific upregulated genes (Fig. 3a). These results imply that BTH treatment could upregulate chloroplast activities, including photosynthesis, in B. distachyon. In the BTH-specific downregulated genes, GO terms related to cellular amino acid modification (GO:0042398, FDR value: 1.3E−03; GO:0006468, FDR value: 1.3E−03; GO:0006575, FDR value: 1.5E−02), and stress responses (GO:0006952, FDR value: 1.5E−02; GO:0006950, FDR value: 2.2E−02) were over-represented (Fig. 3b).
Figure 2.
Predicted subcellular localization of proteins corresponding to the sets of differentially expressed genes after treatment with SA or BTH in Brachypodium distachyon. Subcellular localization of the proteins corresponding to each gene set was predicted using TargetP analysis. Proteins were predicted to localize to chloroplasts, mitochondria, secretion pathways, or other locations.
Figure 3.
The GO enrichment analysis for the sets of genes specifically regulated by BTH in Brachypodium distachyon. GO enrichment analysis for the BTH-specific upregulated and downregulated genes were performed using AgriGO47. The significantly over-represented GO terms categorized to biological process in each gene set were summarized and visualized in two-dimensional semantic similarity-based scatter plots using REVIGO27. Panel a shows the summarized GO terms for the “BTH-specific upregulated genes.” Panel b shows the summarized GO terms for the “BTH-specific downregulated genes.” The circle size represents the −log10 transformed FDR value. Circles depicted by filled color with references show the top 10 significantly over-represented GO terms for each gene set.
We also focused on the phytohormone-related GO terms and found that biological functions related to SA, JA, ethylene (ET), abscisic acid (ABA), and auxin (AUX) were all over-represented in the BTH-specific upregulated genes (Table 1). This indicates that BTH can affect biological processes related to not only SA but also other phytohormones in B. distachyon. Five GO terms related to JA were over-represented in this gene set: biosynthetic process (GO:0009695, 15 genes, FDR value: 5.70E−04), metabolic process (GO:0009694, 15 genes, FDR value: 2.50E−03), and the downstream signaling pathway (GO:0009753, 29 genes, FDR value: 7.50E−04; GO:0009867, 23 genes, FDR value: 3.00E−04; GO:0071395, FDR value: 3.00E−04). Moreover, two GO terms related to oxylipins were also over-represented in this gene set: biosynthetic process (GO:0031408, 15 genes, FDR value: 9.80E−04) and metabolic process (GO:0031407, 16 genes, FDR value: 1.60E−03) (Supplementary Table S1). The numbers of GO terms and annotated genes related to SA (6 GO terms, 134 genes) and JA (5 GO terms, 105 genes) were clearly greater than those related to ET (1 GO term, 19 genes), ABA (2 GO terms, 33 genes), and AUX (1 GO term, 20 genes) in this gene set (Table 1). Although signal crosstalk between SA and ET, ABA, or AUX are known in rice and Arabidopsis28,29, GO terms related to the phytohormones ET, ABA and AUX were not detected when a threshold FDR value lower than 0.01 was applied (Table 1). These results indicated that BTH activates certain signaling pathways of both SA and JA in B. distachyon. In contrast, no GO terms related to phytohormones were identified in the BTH-specific downregulated genes.
Table 1.
Phytohormone-related functions (biological process) over-represented in the BTH-specific upregulated genes of Brachypodium distachyon.
| GO accession | GO name | FDR | Number of annoteted genes in the query set | Number of annoteted genes in the background set | Percentage of annoteted genes in the query set | Percentage of annotated genes in the background set | Related phytohormones |
|---|---|---|---|---|---|---|---|
| GO:0009696 | salicylic acid metabolic process | 8.10E-05 | 20 | 113 | 3.34 | 1.00 | SA |
| GO:0009862 | systemic acquired resistance, salicylic acid mediated signaling pathway | 1.70E-04 | 21 | 129 | 3.51 | 1.14 | SA |
| GO:0009867 | jasmonic acid mediated signaling pathway | 3.00E-04 | 23 | 155 | 3.84 | 1.37 | JA |
| GO:0071395 | cellular response to jasmonic acid stimulus | 3.00E-04 | 23 | 155 | 3.84 | 1.37 | JA |
| GO:0009697 | salicylic acid biosynthetic process | 3.10E-04 | 18 | 104 | 3.01 | 0.92 | SA |
| GO:0009751 | response to salicylic acid stimulus | 3.50E-04 | 29 | 224 | 4.84 | 1.97 | SA |
| GO:0009695 | jasmonic acid biosynthetic process | 5.70E-04 | 15 | 80 | 2.50 | 0.70 | JA |
| GO:0009753 | response to jasmonic acid stimulus | 7.50E-04 | 29 | 234 | 4.84 | 2.06 | JA |
| GO:0009863 | salicylic acid mediated signaling pathway | 1.90E-03 | 23 | 176 | 3.84 | 1.55 | SA |
| GO:0071446 | cellular response to salicylic acid stimulus | 1.90E-03 | 23 | 176 | 3.84 | 1.55 | SA |
| GO:0009694 | jasmonic acid metabolic process | 2.50E-03 | 15 | 92 | 2.50 | 0.81 | JA |
| GO:0009733 | response to auxin stimulus | 2.60E-02 | 20 | 178 | 3.34 | 1.57 | AUX |
| GO:0071215 | cellular response to abscisic acid stimulus | 3.00E-02 | 17 | 143 | 2.84 | 1.26 | ABA |
| GO:0009723 | response to ethylene stimulus | 3.40E-02 | 19 | 170 | 3.17 | 1.50 | ET |
| GO:0009738 | abscisic acid mediated signaling pathway | 3.90E-02 | 16 | 135 | 2.67 | 1.19 | ABA |
BTH induces the expression of chloroplast- and JA-related genes in B. distachyon
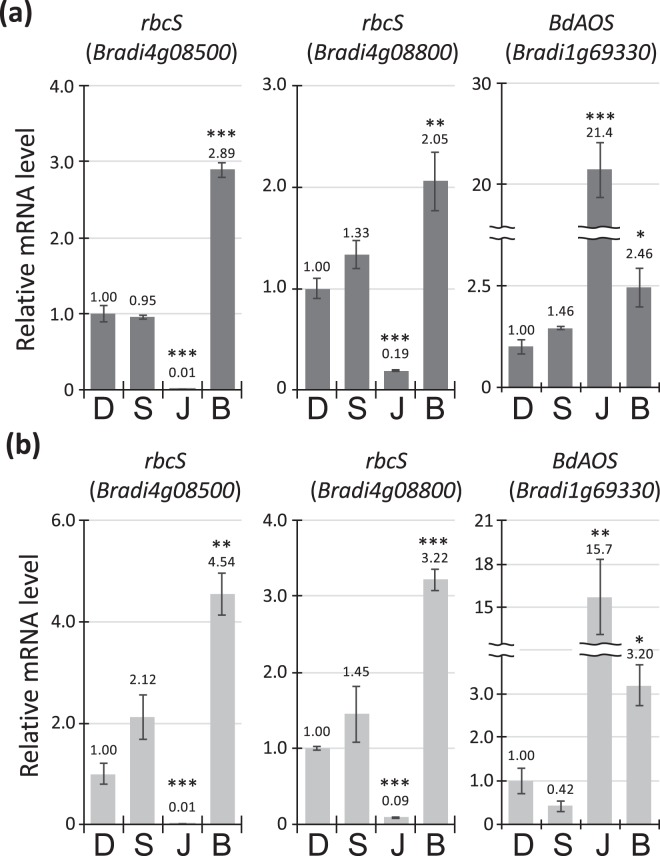
To confirm our observations from the transcriptome analysis, that BTH can induce the expression of chloroplast- and JA-related genes in B. distachyon, we investigated the expression of genes encoding allene oxide synthase (AOS) and the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase (rbcS) in response to BTH, SA, or JA. The rbcS proteins are localized to the chloroplast and play key roles in plant photosynthesis30. In B. distachyon, two rbcS proteins encoded by Bradi4g08500 and Bradi4g08800, have been identified in abundance in the soluble proteins extracted from young leaves31. AOS is required for the plant JA biosynthetic pathway32, and expression of BdAOS (Bradi1g69330), the closest homolog of rice AOS2 in B. distachyon, is strongly responsive to JA25. In accordance with the results from our GO analysis of the BTH-specific upregulated genes, we found that transcription of these genes in accession Bd21 was significantly upregulated by BTH, but not by SA, in Bd21 (Fig. 4a). Similar to a previous observation in barley33,34, we also found that JA treatment decreased expression of the rbcS genes (Fig. 4a). Moreover, our gene expression analysis demonstrated that the induction of the genes was retained in the sheath blight-resistant accession Bd3-1 (Fig. 4b). These results support the hypothesis that BTH treatment can activate genes related to both chloroplast and JA in B. distachyon.
Figure 4.
BTH induces expression of chloroplast- and JA-related genes in Brachypodium distachyon Bd21 and Bd3-1. Detached leaves of B. distachyon were spray-treated with 0.5% (v/v) DMSO, 0.5 mM SA, 0.5 mM JA, or 0.5 mM BTH for 24 h. Expression levels of rbcS genes and BdAOS were evaluated by qRT-PCR analysis in Bd21 (a) and Bd3-1 (b). Data are presented as means ± SEM of values relative to the DMSO treatment, n = 4; *P < 0.05, **P < 0.01, ***P < 0.001 using Student’s t-tests. The experiments were performed twice with similar results and a representative result is shown.
Discussion
Our previous study had demonstrated that treatment with SA, but not BTH, induced R. solani resistance in a sheath blight-susceptible B. distachyon accession, Bd21, although BTH is widely used as a SA analog and it regulated more than 80% of the DEGs identified following SA treatment in Bd2123. Here we showed that BTH treatment also suppressed the R. solani resistance in two sheath blight-resistant accessions of B. distachyon, Bd3-1 and Gaz-4, possibly by affecting the host-dependent cellular responses (Fig. 1 and Supplementary Fig. S2). From this result, we conclude that the DEGs specifically regulated in response to BTH treatment include genes that decrease the disease resistance of B. distachyon to R. solani. Because BTH did not enhance susceptibility to R. solani at any tested concentration in Bd21 that did not activate SA-dependent signaling against R. solani inoculation (Supplementary Fig. S1), the BTH-specific DEGs may particularly disturb the SA-dependent resistance against R. solani, which is exhibited by the B. distachyon accessions Bd3-1 and Gaz-4. Indeed, SA-dependent signaling is rapidly activated following inoculation of Bd3-1 and Gaz-4, but not Bd21with R. solani23.
Our analysis of the RNA-seq data of Bd21 leaves treated with SA or BTH provided two possible reasons why BTH did not confer R. solani resistance in sheath blight-susceptible B. distachyon. One is related to chloroplast activities. The BTH-specific upregulated genes include a large number of genes encoding chloroplast-targeted proteins (Fig. 2) and are significantly over-represented with respect to functions related to chloroplast activities such as photosynthesis (Fig. 3). These findings are supported by our qRT-PCR-based expression analysis of the rbcS genes in response to BTH treatment of Bd21 and Bd3-1 (Fig. 4). In contrast to our findings, BTH had previously been reported to downregulate genes involved in photosynthesis via an NPR1-dependent pathway in Arabidopsis and rice13,35. Rice plants reduced the maximum quantum yield of photosystem II (Fv/Fm) after treatment with BTH35. This down-regulation of photosynthesis could be caused by a trade-off between defense and growth. The transfer of resources from growth to defense is known to be a part of plant defense responses21,36,37. An example of this has been reported with respect to photosynthetic electron transport, which was reduced within a few hours in response to infection of tobacco with an incompatible strain of Phytophthora nicotianae38. Moreover, reduction in photosynthetic activities was found to be more rapid and pronounced after inoculation of Arabidopsis with an incompatible strain of Pseudomonas syringae pv. tomato DC3000 than that with a compatible strain39. Considering the switch in resource allocation from growth to defense during disease resistance responses21, it is possible that the BTH-induced up-regulation of chloroplast activities negatively affects the expression of disease resistance in B. distachyon, particularly against R. solani infection. The question remains as to whether the BTH-induced stimulation of chloroplast activity is specific to only B. distachyon because BTH upregulated the expression of chloroplast-related genes in B. distachyon but not in rice35. The plant immune system is known to differ between monocots and dicots25,28,40. In Arabidopsis, the transcriptional changes in response to BTH treatment are similar to those which occur in response to SA12,13. However, we demonstrated that BTH affects the expression of more genes in B. distachyon than SA does23. As far as we know, the only comparative transcriptome analysis of monocot in response to both SA and BTH was reported in B. distachyon23, although transcriptome profiling in response to BTH has been reported in rice11,35. Rice plants naturally contain a much higher level of endogenous SA than do other monocots, including B. distachyon and barley23,41–43. Therefore, it is possible that rice has acquired unique features, particularly with respect to biotic defense response, during domestication and breeding, compared with other monocots. Further analysis is needed to clarify whether the BTH-induced up-regulation of chloroplast-related genes is specific to B. distachyon or is conserved in other monocots.
The other possible reason why BTH does not induce R. solani resistance in B. distachyon is JA-related responses. The functional enrichment analysis of the BTH-specific upregulated genes demonstrated that BTH can affect a broad range of phytohormone signaling pathways in B. distachyon, particularly those associated with SA and JA (Table 1). In fact, BdAOS, a marker gene for JA response25, was significantly upregulated by BTH treatment in both susceptible and resistant accessions Bd21 and Bd3-1 (Fig. 4). In accordance with our findings, BTH has been reported to activate JA signaling in rice40. A comparative transcriptome analysis of BTH- and JA-treated rice demonstrated that more than half of the BTH-induced genes are also upregulated by JA40, suggesting that the induction of JA-related biological functions by BTH should be a common feature in rice and B. distachyon. In rice, BTH treatment enhanced the resistance against both Magnaporthe oryzae and Xanthomonas oryzae pv. oryzae (Xoo)11,44, which are the causal agents of blast disease and bacterial leaf blight, respectively. This is consistent with the result that exogenously applied JA also improves rice resistance against these pathogens45,46. SA and JA are known to induce the expression of the same gene set, designated as a common defense pathway25,28,40, and this gene set should contribute to defense against these pathogens. In contrast, in B. distachyon, treatment with either JA or BTH increases susceptibility to R. solani in both a sheath blight-susceptible accession23 as well as two sheath blight-resistant accessions (Fig. 1). Taking these findings together, the JA-related biological functions induced by BTH should act positively on the resistance against M. oryzae and Xoo, but negatively on the resistance against R. solani. These differences may be attributable to the infection strategy of each pathogen.
There are some common transcriptional changes in the responses of the model monocot plant B. distachyon to SA and BTH, but many more different ones, a finding which contrasts with the situation in Arabidopsis. Although the transcriptome profile in response to SA remains unclear in another model monocot, rice, our results showed that the actions of plant defense inducers as well as phytohormones are not always the same among different plant species. We need to be careful about such differences because they could be associated with unexpected effects on non-target pathogens. Characterization of plant defense inducers by comparative transcriptome analyses will facilitate the evidence-based design of crop protection chemicals for durable and sustainable crop protection, as well as providing new insights into the evolution of the SA signaling pathway in plants.
Methods
Plant and fungal materials and growth conditions
Dry seeds of the B. distachyon accessions Bd21, Bd3-1, and Gaz-4 were provided by the National Plant Germplasm System of USDA-ARS. The seeds were sown on moist filter paper, incubated at 4 °C in the dark for 3 d, and then germinated in a growth chamber (MLR-350HT; Sanyo, Osaka, Japan) at 25 °C under a photoperiod of 16 h light and 8 h dark for 3 d. The germinated seedlings were transplanted to 24-well microplates filled with soil (Sakata Supermix-A; Sakata Seed, Yokohama, Kanagawa, Japan). The seedlings were subsequently grown in a growth chamber (Nippon Medical & Chemical Instruments, Osaka, Japan) at 23 °C under a photoperiod of 20 h light and 4 h dark for 3 wk until the experiments carried out.
The R. solani AG-1, IA strain MAFF305230 was obtained from the Genebank of the National Agricultural Research Organization (NARO) in Japan and grown on potato dextrose agar (PDA; BD, Franklin Lakes, NJ, USA) plates at 23 °C for 3–5 d. To examine the effect of SA and BTH on fungal growth, mycelial plugs (3 mm diameter) bored from the edge of the fungal mycelia growing on PDA plates were inoculated onto the center of PDA plates containing 0.5% (v/v) dimethyl sulfoxide (DMSO), 0.1 mM SA in 0.5% (v/v) DMSO, or 0.1 mM BTH in 0.5% (v/v) DMSO and incubated at 23 °C for 24 h.
Chemicals
Sodium salicylate (SA) (Wako, Osaka, Japan) and methyl jasmonate (JA) (Wako) were used as phytohormones. Benzothiadiazole (acibenzolar-S-methyl, BTH) (Wako) was used as a functional analog of SA. These were diluted with DMSO (Wako).
Analysis of RNA-seq data
The previously published RNA-seq data from B. distachyon Bd21 leaves obtained 24 h after treatment with SA or BTH (DDBJ accession no. DRP003562)23 were used. The subcellular localization of the proteins corresponding to a set of DEGs was analyzed using TargetP software26. The proteins were predicted to be located in mitochondria, chloroplasts, secretory pathways, or other sites based on their N-terminal amino acid sequence. GO enrichment analysis for the set of DEGs was performed as follows. GO terms were assigned to B. distachyon genes based on the GO annotations of homologs most similar to those of Arabidopsis, as identified in a blastp analysis. Significantly enriched GO terms categorized to biological processes with a threshold of FDR < 0.05 from Fisher’s exact test were identified by AgriGO analysis47. Summarization of GO terms according to their semantic similarities and the representation of summarized GO terms in 2D semantic space were carried out using the REVIGO web service27.
Inoculation tests
Detached leaves of B. distachyon were placed on moist filter paper in a square plate. After 1 d wound acclimation, the leaves were spray-treated with chemical solution (0.5% [v/v] DMSO, SA in 0.5% [v/v] DMSO, or BTH in 0.5% [v/v] DMSO) containing 0.04% (v/v) Tween 20 and incubated at 23 °C under a photoperiod of 20 h light and 4 h dark for 24 h. Before subsequent experiments were carried out, droplets of the chemical solution attached to the leaf surface were removed. The leaves were then inoculated with cubic mycelial plugs (2–3 mm3) of R. solani and incubated at 23 °C under a photoperiod of 20 h light and 4 h dark for 3 d. Disease severity was evaluated from the amounts of fungal DNAs in the inoculated leaves, expressed as fungal biomass. Total DNAs were extracted from the inoculated leaves using a Nucleospin Plant II Kit (Takara Bio, Shiga, Japan), and qPCR for fungal DNAs (28 S rDNA) was performed using a SYBR Premix Ex Taq II (Takara Bio) employing the Applied Biosystems 7500 System (Thermo Fisher Scientific, Waltham, MA, USA). The B. distachyon BdFIM gene (Bradi2g13800) was used to normalize the data. The primers used for amplification are listed in Supplementary Table S3.
Gene expression analysis
Total RNAs were extracted from B. distachyon leaves following 24 h treatment with chemical solution (0.5% [v/v] DMSO, SA in 0.5% [v/v] DMSO, JA in 0.5% [v/v] DMSO, or BTH in 0.5% [v/v] DMSO) using a RNeasy Plant Mini Kit (Qiagen, Hilden, Germany) and cDNAs were synthesized using an Omniscript RT Kit (Qiagen). Gene expression analysis for BdWRKY45L1, BdAOS, and rbcS genes was performed by qRT-PCR using SYBR Premix Ex Taq II (Takara Bio) in conjunction with the Applied Biosystems 7500 System (Thermo Fisher Scientific). The BdUbi4 gene (Bradi3g04730) was used to normalize the data. The primers used for amplification are listed in Supplementary Table S3.
Statistical analysis
Statistical differences between populations were analyzed using Student’s t-tests. Data are presented as the mean value and error bars show the standard error (SE).
Electronic supplementary material
Acknowledgements
We would like to thank Risa Nakayama, Toshie Kita, and Tomoko Okachi for their support regarding plant cultivation, and Yukiko Yamaguchi-Uehara for her valuable suggestions and comments on the manuscript. This research was supported by Grants-in-Aid for Research Activity Start-up (grant No. 16H07452) and Early-Career Scientists (grant No. 18K14469) from the Japan Society for the Promotion of Science, and the Special Postdoctoral Researchers Program from RIKEN to Y. Kouzai.
Author Contributions
Y.K., Y.N. and K.M. conceived the research and designed the experiments. Y.K. and M.S. performed the experiments, bioinformatics analysis, and the statistical analysis. K.I. performed the TargetP analysis. Y.K., Y.N., Y.O. and K.M. wrote the manuscript. All authors read and approved the final manuscript.
Data Availability
DNA Data bank of Japan (DDBJ) DRP003562 (2017). https://trace.ddbj.nig.ac.jp/DRASearch/study?acc=DRP003562.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-35790-w.
References
- 1.Vlot AC, Dempsey DA, Klessig DF. Salicylic Acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol. 2009;47:177–206. doi: 10.1146/annurev.phyto.050908.135202. [DOI] [PubMed] [Google Scholar]
- 2.Cao H, Glazebrook J, Clarke JD, Volko S, Dong X. The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell. 1997;88:57–63. doi: 10.1016/S0092-8674(00)81858-9. [DOI] [PubMed] [Google Scholar]
- 3.Wildermuth MC, Dewdney J, Wu G, Ausubel FM. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature. 2001;414:562–565. doi: 10.1038/35107108. [DOI] [PubMed] [Google Scholar]
- 4.Rahman TA, Oirdi ME, Gonzalez-Lamothe R, Bouarab K. Necrotrophic pathogens use the salicylic acid signaling pathway to promote disease development in tomato. Mol Plant Microbe Interact. 2012;25:1584–1593. doi: 10.1094/MPMI-07-12-0187-R. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari S, Plotnikova JM, De Lorenzo G, Ausubel FM. Arabidopsis local resistance to Botrytis cinerea involves salicylic acid and camalexin and requires EDS4 and PAD2, but not SID2, EDS5 or PAD4. Plant J. 2003;35:193–205. doi: 10.1046/j.1365-313X.2003.01794.x. [DOI] [PubMed] [Google Scholar]
- 6.Noutoshi Y, et al. Novel plant immune-priming compounds identified via high-throughput chemical screening target salicylic acid glucosyltransferases in Arabidopsis. Plant Cell. 2012;24:3795–3804. doi: 10.1105/tpc.112.098343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schreiber K, Desveaux D. Message in a bottle: chemical biology of induced disease resistance in plants. Plant Pathol. J. 2008;24:245–268. doi: 10.5423/PPJ.2008.24.3.245. [DOI] [Google Scholar]
- 8.Friedrich L, et al. A benzothiadiazole derivative induces systemic acquired resistance in tobacco. The Plant Journal. 1996;10:61–70. doi: 10.1046/j.1365-313X.1996.10010061.x. [DOI] [Google Scholar]
- 9.Gorlach J, et al. Benzothiadiazole, a novel class of inducers of systemic acquired resistance, activates gene expression and disease resistance in wheat. Plant Cell. 1996;8:629–643. doi: 10.1105/tpc.8.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawton KA, et al. Benzothiadiazole induces disease resistance in Arabidopsis by activation of the systemic acquired resistance signal transduction pathway. Plant J. 1996;10:71–82. doi: 10.1046/j.1365-313X.1996.10010071.x. [DOI] [PubMed] [Google Scholar]
- 11.Shimono M, et al. Rice WRKY45 plays a crucial role in benzothiadiazole-inducible blast resistance. Plant Cell. 2007;19:2064–2076. doi: 10.1105/tpc.106.046250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanco F, et al. Early genomic responses to salicylic acid in Arabidopsis. Plant Mol Biol. 2009;70:79–102. doi: 10.1007/s11103-009-9458-1. [DOI] [PubMed] [Google Scholar]
- 13.Wang D, Amornsiripanitch N, Dong X. A genomic approach to identify regulatory nodes in the transcriptional network of systemic acquired resistance in plants. PLoS Pathog. 2006;2:e123. doi: 10.1371/journal.ppat.0020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rivas-San Vicente M, Plasencia J. Salicylic acid beyond defence: its role in plant growth and development. J Exp Bot. 2011;62:3321–3338. doi: 10.1093/jxb/err031. [DOI] [PubMed] [Google Scholar]
- 15.Clarke JD, Volko SM, Ledford H, Ausubel FM, Dong X. Roles of salicylic acid, jasmonic acid, and ethylene in cpr-induced resistance in Arabidopsis. Plant Cell. 2000;12:2175–2190. doi: 10.1105/tpc.12.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noutoshi Y, et al. A single amino acid insertion in the WRKY domain of the Arabidopsis TIR-NBS-LRR-WRKY-type disease resistance protein SLH1 (sensitive to low humidity 1) causes activation of defense responses and hypersensitive cell death. Plant J. 2005;43:873–888. doi: 10.1111/j.1365-313X.2005.02500.x. [DOI] [PubMed] [Google Scholar]
- 17.Heil M, Hilpert A, Kaiser W, Linsenmair KE. Reduced growth and seed set following chemical induction of pathogen defence: does systemic acquired resistance (SAR) incur allocation costs? Journal of Ecology. 2000;88:645–654. doi: 10.1046/j.1365-2745.2000.00479.x. [DOI] [Google Scholar]
- 18.Pancheva T, Popova L, Uzunova A. Effects of salicylic acid on growth and photosynthesis in barley plants. Journal of Plant Physiology. 1996;149:57–63. doi: 10.1016/S0176-1617(96)80173-8. [DOI] [Google Scholar]
- 19.Abreu ME, Munne-Bosch S. Salicylic acid deficiency in NahG transgenic lines andsid2 mutants increases seed yield in the annual plant Arabidopsis thaliana. J Exp Bot. 2009;60:1261–1271. doi: 10.1093/jxb/ern363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolton MD. Primary metabolism and plant defense—fuel for the fire. Mol Plant Microbe Interact. 2009;22:487–497. doi: 10.1094/MPMI-22-5-0487. [DOI] [PubMed] [Google Scholar]
- 21.Takatsuji H. Regulating tradeoffs to improve rice production. Front Plant Sci. 2017;8:171. doi: 10.3389/fpls.2017.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rush M, Lee F. Rice sheath blight: a major rice disease. Plant Dis. 1983;67:829–832. doi: 10.1094/PD-67-829. [DOI] [Google Scholar]
- 23.Kouzai Y, et al. Salicylic acid-dependent immunity contributes to resistance against Rhizoctonia solani, a necrotrophic fungal agent of sheath blight, in rice and Brachypodium distachyon. New Phytol. 2018;217:771–783. doi: 10.1111/nph.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miedes E, Vanholme R, Boerjan W, Molina A. The role of the secondary cell wall in plant resistance to pathogens. Front Plant Sci. 2014;5:358. doi: 10.3389/fpls.2014.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kouzai Y, et al. Expression profiling of marker genes responsive to the defence-associated phytohormones salicylic acid, jasmonic acid and ethylene in Brachypodium distachyon. BMC Plant Biol. 2016;16:59. doi: 10.1186/s12870-016-0749-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emanuelsson O, Nielsen H, Brunak S, von Heijne G. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol. 2000;300:1005–1016. doi: 10.1006/jmbi.2000.3903. [DOI] [PubMed] [Google Scholar]
- 27.Supek F, Bosnjak M, Skunca N, Smuc T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS One. 2011;6:e21800. doi: 10.1371/journal.pone.0021800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Vleesschauwer D, Gheysen G, Hofte M. Hormone defense networking in rice: tales from a different world. Trends Plant Sci. 2013;18:555–565. doi: 10.1016/j.tplants.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Robert-Seilaniantz A, Navarro L, Bari R, Jones JD. Pathological hormone imbalances. Curr Opin Plant Biol. 2007;10:372–379. doi: 10.1016/j.pbi.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Hauser T, Popilka L, Hartl FU, Hayer-Hartl M. Role of auxiliary proteins in Rubisco biogenesis and function. Nat Plants. 2015;1:15065. doi: 10.1038/nplants.2015.65. [DOI] [PubMed] [Google Scholar]
- 31.Douche T, et al. Brachypodium distachyon as a model plant toward improved biofuel crops: Search for secreted proteins involved in biogenesis and disassembly of cell wall polymers. Proteomics. 2013;13:2438–2454. doi: 10.1002/pmic.201200507. [DOI] [PubMed] [Google Scholar]
- 32.Mueller MJ. Enzymes involved in jasmonic acid biosynthesis. Physiologia Plantarum. 1997;100:653–663. doi: 10.1111/j.1399-3054.1997.tb03072.x. [DOI] [Google Scholar]
- 33.Parthier B. Jasmonates: Hormonal regulators or stress factors in leaf senescence? Journal of Plant Growth Regulation. 1990;9:57. doi: 10.1007/BF02041942. [DOI] [Google Scholar]
- 34.Weidhase RA, et al. Methyljasmonate-induced changes in the polypeptide pattern of senescing barley leaf segments. Plant Science. 1987;51:177–186. doi: 10.1016/0168-9452(87)90191-9. [DOI] [Google Scholar]
- 35.Sugano S, et al. Role of OsNPR1 in rice defense program as revealed by genome-wide expression analysis. Plant Mol Biol. 2010;74:549–562. doi: 10.1007/s11103-010-9695-3. [DOI] [PubMed] [Google Scholar]
- 36.Rojas CM, Senthil-Kumar M, Tzin V, Mysore KS. Regulation of primary plant metabolism during plant-pathogen interactions and its contribution to plant defense. Front Plant Sci. 2014;5:17. doi: 10.3389/fpls.2014.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Somssich IE, Hahlbrock K. Pathogen defence in plants—a paradigm of biological complexity. Trends in Plant Science. 1998;3:86–90. doi: 10.1016/S1360-1385(98)01199-6. [DOI] [Google Scholar]
- 38.Scharte J, SCHÖN H, Weis E. Photosynthesis and carbohydrate metabolism in tobacco leaves during an incompatible interaction with Phytophthora nicotianae. Plant, Cell & Environment. 2005;28:1421–1435. doi: 10.1111/j.1365-3040.2005.01380.x. [DOI] [Google Scholar]
- 39.Bonfig KB, Schreiber U, Gabler A, Roitsch T, Berger S. Infection with virulent and avirulent P. syringae strains differentially affects photosynthesis and sink metabolism in Arabidopsis leaves. Planta. 2006;225:1–12. doi: 10.1007/s00425-006-0303-3. [DOI] [PubMed] [Google Scholar]
- 40.Tamaoki D, et al. Jasmonic acid and salicylic acid activate a common defense system in rice. Plant Signal Behav. 2013;8:e24260. doi: 10.4161/psb.24260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ayliffe M, Singh D, Park R, Moscou M, Pryor T. Infection of Brachypodium distachyon with selected grass rust pathogens. Mol Plant Microbe Interact. 2013;26:946–957. doi: 10.1094/MPMI-01-13-0017-R. [DOI] [PubMed] [Google Scholar]
- 42.Huckelhoven R, Fodor J, Preis C, Kogel KH. Hypersensitive cell death and papilla formation in barley attacked by the powdery mildew fungus are associated with hydrogen peroxide but not with salicylic acid accumulation. Plant Physiol. 1999;119:1251–1260. doi: 10.1104/pp.119.4.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang Y, Qi M, Mei C. Endogenous salicylic acid protects rice plants from oxidative damage caused by aging as well as biotic and abiotic stress. Plant J. 2004;40:909–919. doi: 10.1111/j.1365-313X.2004.02267.x. [DOI] [PubMed] [Google Scholar]
- 44.Babu R, Velazhahan R, Vidhyasekaran P, Seetharaman K, Sajeena A. Induction of resistance to Xanthomonas oryzae pv. oryzae in rice by benzothiadiazole (BTH) Acta phytopathologica et entomologica hungarica. 2003;38:73–78. doi: 10.1556/APhyt.38.2003.1-2.9. [DOI] [Google Scholar]
- 45.Mei C, Qi M, Sheng G, Yang Y. Inducible overexpression of a rice allene oxide synthase gene increases the endogenous jasmonic acid level, PR gene expression, and host resistance to fungal infection. Mol Plant Microbe Interact. 2006;19:1127–1137. doi: 10.1094/MPMI-19-1127. [DOI] [PubMed] [Google Scholar]
- 46.Yamada S, et al. Involvement of OsJAZ8 in jasmonate-induced resistance to bacterial blight in rice. Plant Cell Physiol. 2012;53:2060–2072. doi: 10.1093/pcp/pcs145. [DOI] [PubMed] [Google Scholar]
- 47.Du Z, Zhou X, Ling Y, Zhang Z, Su Z. agriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Res. 2010;38:W64–70. doi: 10.1093/nar/gkq310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
DNA Data bank of Japan (DDBJ) DRP003562 (2017). https://trace.ddbj.nig.ac.jp/DRASearch/study?acc=DRP003562.